A Quantitative Assay for Ca2+ Uptake through Normal and Pathological Hemichannels
Abstract
1. Introduction
2. Results
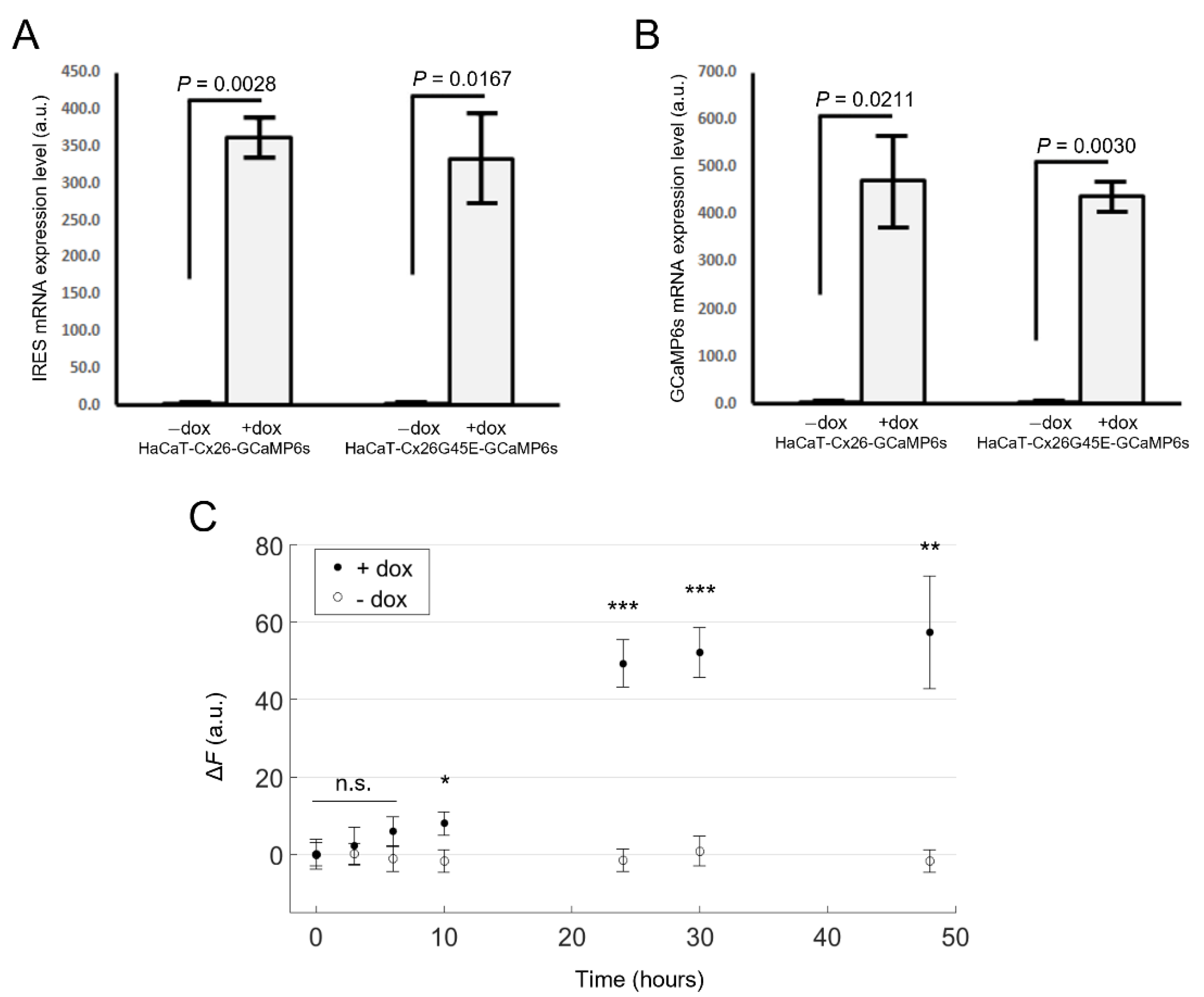
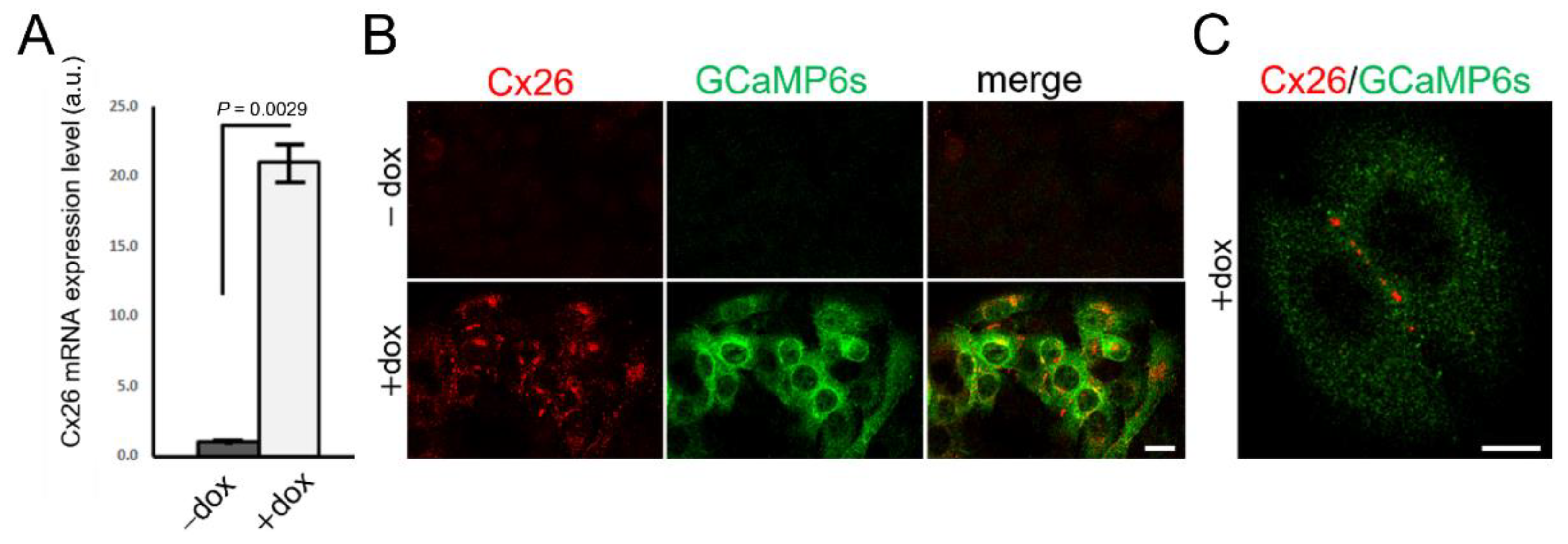
2.1. Generation of Stable Cell Pools Overexpressing the Cx of Interest and GCaMP6s
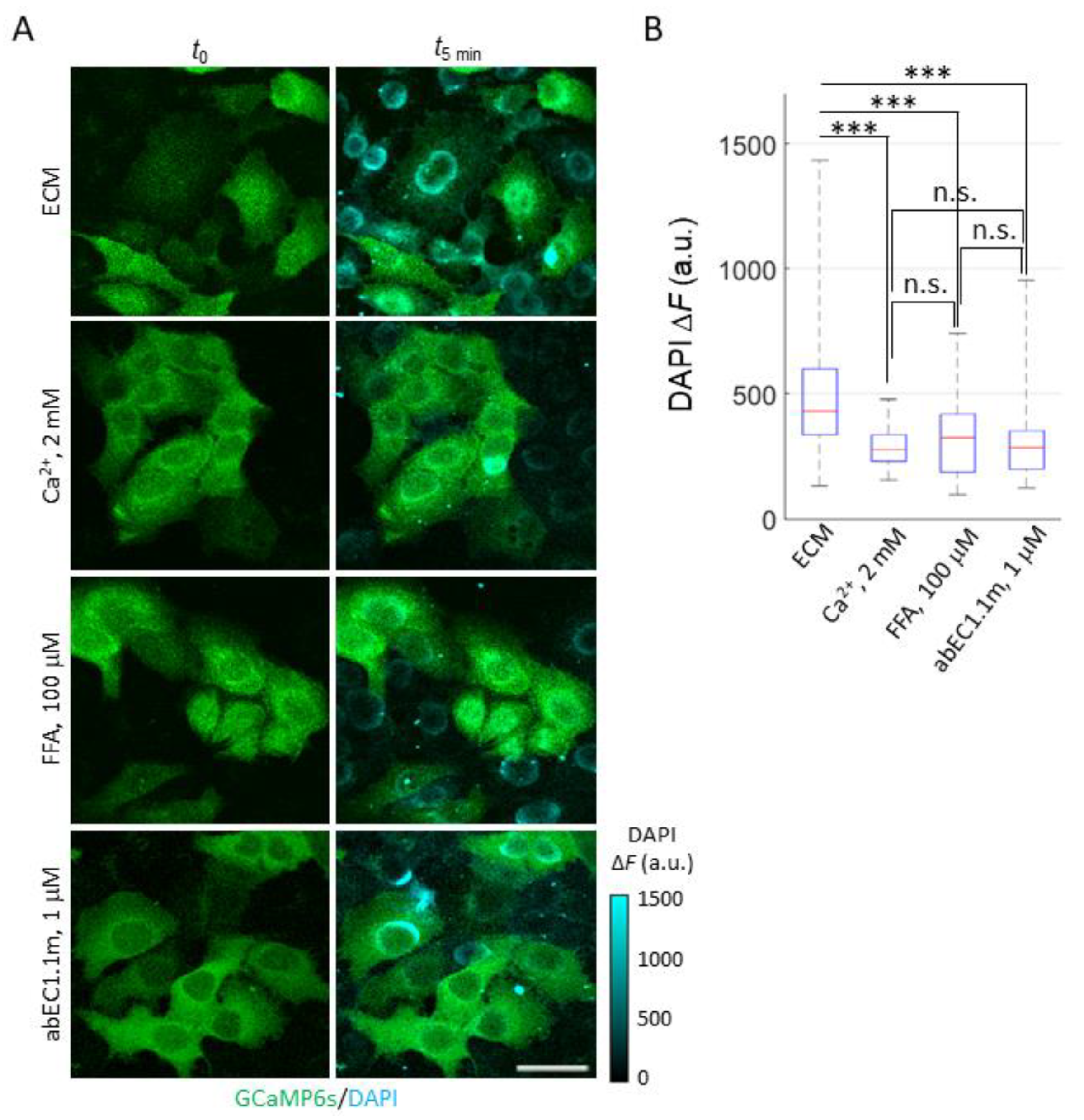
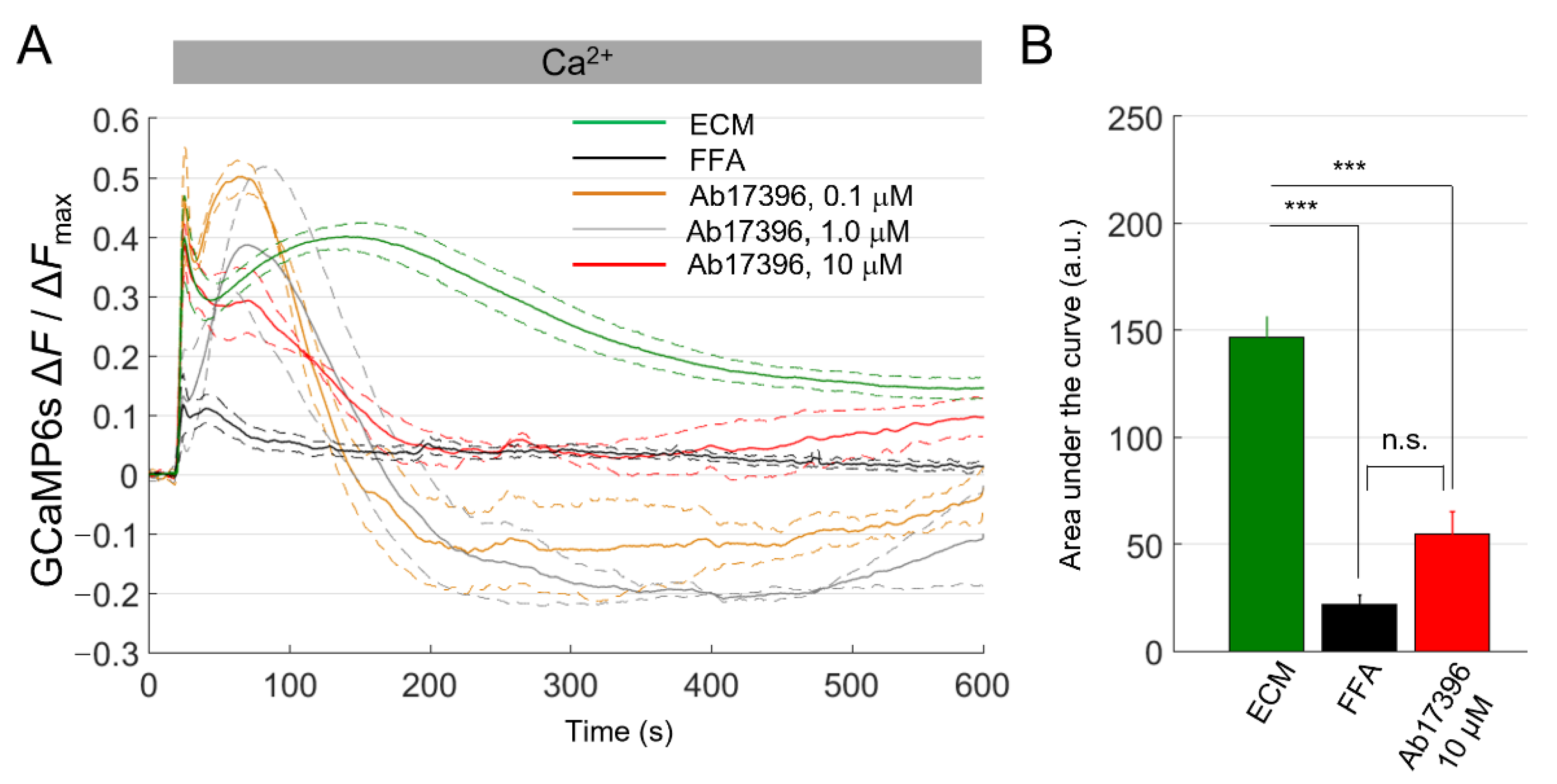
2.2. Development and Validation of the Ca2+ Uptake Assay
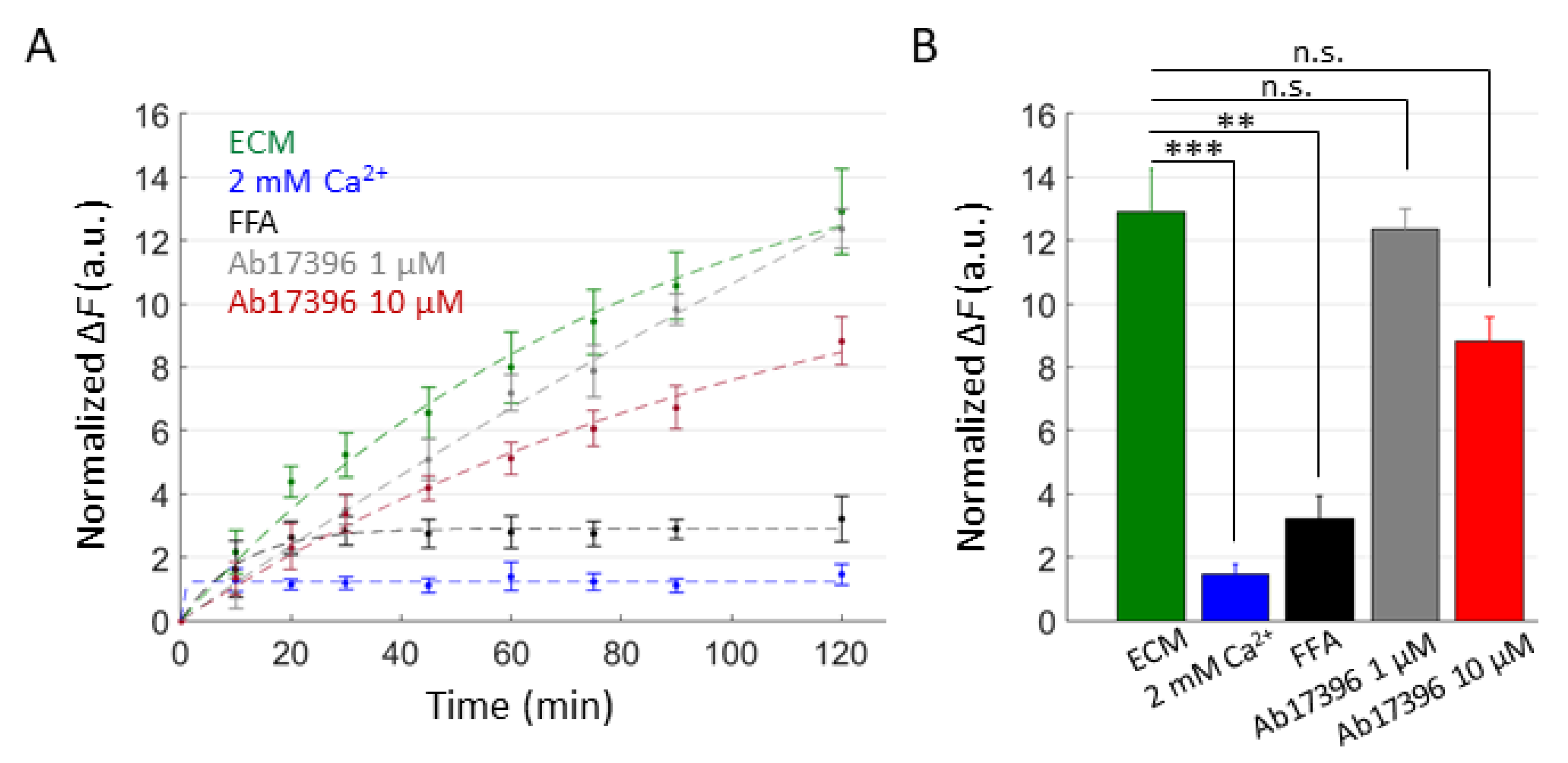
2.3. mAbs Targeting the Extracellular Domain of Cx26 Inhibit Ca2+ Uptake through Leaky HCs Implicated in KID Syndrome
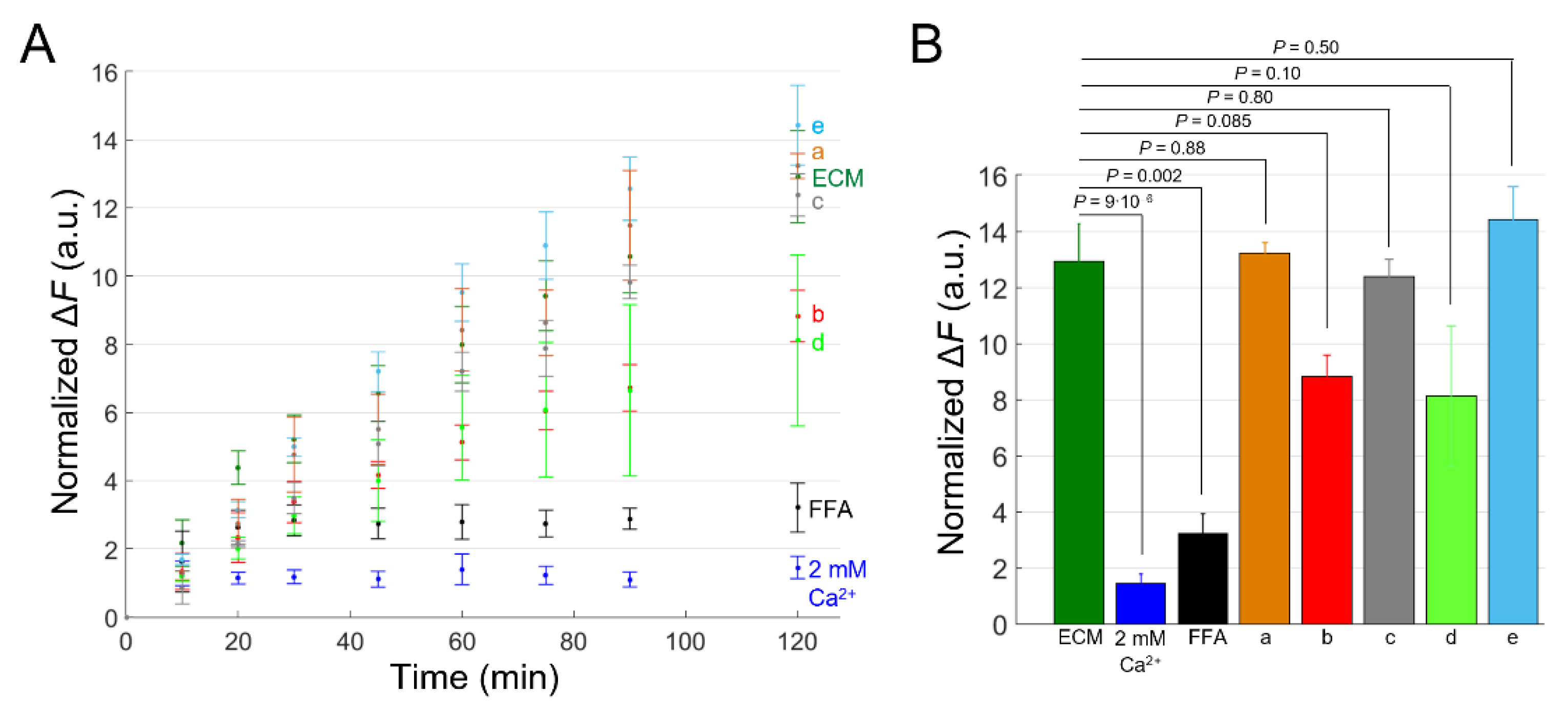
2.4. Effects of Uncharacterized mAbs, Targeting the Extracellular Domain of Cx46, on Ca2+ and DAPI Uptake
3. Discussion
4. Materials and Methods
4.1. Viruses
4.2. Establishment of Stable Cell Pools
4.3. qPCR
- The primers used for these experiments are listed here.
- Cx26 (Cx26 wt and Cx26G45E) primers:
- EMCV-IRES primers:
- GCaMP6S primers:
- GAPDH primers:
4.4. Immunofluorescence
4.5. Dye Uptake
4.6. Ca2+ Uptake
4.7. Selection of Anti-Cx46 mAbs
4.8. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maeda, S.; Nakagawa, S.; Suga, M.; Yamashita, E.; Oshima, A.; Fujiyoshi, Y.; Tsukihara, T. Structure of the connexin 26 gap junction channel at 3.5 A resolution. Nature 2009, 458, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.B.; Haddad, B.G.; O’Neill, S.E.; Chorev, D.S.; Yoshioka, C.C.; Robinson, C.V.; Zuckerman, D.M.; Reichow, S.L. Structure of native lens connexin 46/50 intercellular channels by cryo-EM. Nature 2018, 564, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Jeong, H.; Hyun, J.; Ryu, B.; Park, K.; Lim, H.H.; Yoo, J.; Woo, J.S. Cryo-EM structure of human Cx31.3/GJC3 connexin hemichannel. Sci. Adv. 2020, 6, eaba4996. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.K.; Jagielnicki, M.; Bennett, B.C.; Purdy, M.D.; Yeager, M. Cryo-EM structure of an open conformation of a gap junction hemichannel in lipid bilayer nanodiscs. Structure 2021, 29, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Esseltine, J.L.; Laird, D.W. Next-Generation Connexin and Pannexin Cell Biology. Trends Cell Biol. 2016, 26, 944–955. [Google Scholar] [CrossRef]
- Schalper, K.A.; Palacios-Prado, N.; Retamal, M.A.; Shoji, K.F.; Martinez, A.D.; Saez, J.C. Connexin hemichannel composition determines the FGF-1-induced membrane permeability and free [Ca2+]i responses. Mol. Biol. Cell 2008, 19, 3501–3513. [Google Scholar] [CrossRef]
- Schalper, K.A.; Sanchez, H.A.; Lee, S.C.; Altenberg, G.A.; Nathanson, M.H.; Saez, J.C. Connexin 43 hemichannels mediate the Ca2+ influx induced by extracellular alkalinization. Am. J. Physiology. Cell Physiol. 2010, 299, C1504–C1515. [Google Scholar] [CrossRef]
- Fiori, M.C.; Figueroa, V.; Zoghbi, M.E.; Saez, J.C.; Reuss, L.; Altenberg, G.A. Permeation of calcium through purified connexin 26 hemichannels. J. Biol. Chem. 2012, 287, 40826–40834. [Google Scholar] [CrossRef]
- Sanchez, H.A.; Orellana, J.A.; Verselis, V.K.; Saez, J.C. Metabolic inhibition increases activity of connexin-32 hemichannels permeable to Ca2+ in transfected HeLa cells. Am. J. Physiol. Cell Physiol. 2009, 297, C665–C678. [Google Scholar] [CrossRef]
- Mandal, A.; Shahidullah, M.; Delamere, N.A. Calcium entry via connexin hemichannels in lens epithelium. Exp. Eye Res. 2015, 132, 52–58. [Google Scholar] [CrossRef][Green Version]
- Paul, D.L.; Ebihara, L.; Takemoto, L.J.; Swenson, K.I.; Goodenough, D.A. Connexin46, a novel lens gap junction protein, induces voltage-gated currents in nonjunctional plasma membrane of Xenopus oocytes. J. Cell Biol. 1991, 115, 1077–1089. [Google Scholar] [CrossRef] [PubMed]
- Ebihara, L.; Steiner, E. Properties of a nonjunctional current expressed from a rat connexin46 cDNA in Xenopus oocytes. J. Gen. Physiol. 1993, 102, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Contreras, J.E.; Saez, J.C.; Bukauskas, F.F.; Bennett, M.V. Gating and regulation of connexin 43 (Cx43) hemichannels. Proc. Natl. Acad. Sci. USA 2003, 100, 11388–11393. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.V.; Contreras, J.E.; Bukauskas, F.F.; Saez, J.C. New roles for astrocytes: Gap junction hemichannels have something to communicate. Trends Neurosci. 2003, 26, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, D.A.; Paul, D.L. Beyond the gap: Functions of unpaired connexon channels. Nat. Rev. Mol. Cell Biol. 2003, 4, 285–294. [Google Scholar] [CrossRef]
- Saez, J.C.; Retamal, M.A.; Basilio, D.; Bukauskas, F.F.; Bennett, M.V. Connexin-based gap junction hemichannels: Gating mechanisms. Biochim. Biophys. Acta 2005, 1711, 215–224. [Google Scholar] [CrossRef]
- Saez, J.C.; Schalper, K.A.; Retamal, M.A.; Orellana, J.A.; Shoji, K.F.; Bennett, M.V. Cell membrane permeabilization via connexin hemichannels in living and dying cells. Exp. Cell Res. 2010, 316, 2377–2389. [Google Scholar] [CrossRef]
- Fasciani, I.; Temperan, A.; Perez-Atencio, L.F.; Escudero, A.; Martinez-Montero, P.; Molano, J.; Gomez-Hernandez, J.M.; Paino, C.L.; Gonzalez-Nieto, D.; Barrio, L.C. Regulation of connexin hemichannel activity by membrane potential and the extracellular calcium in health and disease. Neuropharmacology 2013, 75, 479–490. [Google Scholar] [CrossRef]
- Saez, J.C.; Leybaert, L. Hunting for connexin hemichannels. FEBS Lett. 2014, 588, 1205–1211. [Google Scholar] [CrossRef]
- Leybaert, L.; Lampe, P.D.; Dhein, S.; Kwak, B.R.; Ferdinandy, P.; Beyer, E.C.; Laird, D.W.; Naus, C.C.; Green, C.R.; Schulz, R. Connexins in Cardiovascular and Neurovascular Health and Disease: Pharmacological Implications. Pharmacol. Rev. 2017, 69, 396–478. [Google Scholar] [CrossRef]
- Cotrina, M.L.; Lin, J.H.; Alves-Rodrigues, A.; Liu, S.; Li, J.; Azmi-Ghadimi, H.; Kang, J.; Naus, C.C.; Nedergaard, M. Connexins regulate calcium signaling by controlling ATP release. Proc. Natl. Acad. Sci. USA 1998, 95, 15735–15740. [Google Scholar] [CrossRef] [PubMed]
- Stout, C.E.; Costantin, J.L.; Naus, C.C.; Charles, A.C. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J. Biol. Chem. 2002, 277, 10482–10488. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.A.; Dale, N.; Llaudet, E.; Mobbs, P. ATP released via gap junction hemichannels from the pigment epithelium regulates neural retinal progenitor proliferation. Neuron 2005, 46, 731–744. [Google Scholar] [CrossRef]
- Anselmi, F.; Hernandez, V.H.; Crispino, G.; Seydel, A.; Ortolano, S.; Roper, S.D.; Kessaris, N.; Richardson, W.; Rickheit, G.; Filippov, M.A.; et al. ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc. Natl. Acad. Sci. USA 2008, 105, 18770–18775. [Google Scholar] [CrossRef]
- Mazzarda, F.; D’Elia, A.; Massari, R.; De Ninno, A.; Bertani, F.R.; Businaro, L.; Ziraldo, G.; Zorzi, V.; Nardin, C.; Peres, C.; et al. Organ-on-chip model shows that ATP release through connexin hemichannels drives spontaneous Ca(2+) signaling in non-sensory cells of the greater epithelial ridge in the developing cochlea. Lab A Chip 2020, 20, 3011–3023. [Google Scholar] [CrossRef] [PubMed]
- Nardin, C.; Peres, C.; Putti, S.; Orsini, T.; Colussi, C.; Mazzarda, F.; Raspa, M.; Scavizzi, F.; Salvatore, A.M.; Chiani, F.; et al. Connexin Hemichannel Activation by S-Nitrosoglutathione Synergizes Strongly with Photodynamic Therapy Potentiating Anti-Tumor Bystander Killing. Cancers 2021, 13, 5062. [Google Scholar] [CrossRef]
- Orellana, J.A.; Froger, N.; Ezan, P.; Jiang, J.X.; Bennett, M.V.; Naus, C.C.; Giaume, C.; Saez, J.C. ATP and glutamate released via astroglial connexin 43 hemichannels mediate neuronal death through activation of pannexin 1 hemichannels. J. Neurochem. 2011, 118, 826–840. [Google Scholar] [CrossRef]
- Cherian, P.P.; Siller-Jackson, A.J.; Gu, S.; Wang, X.; Bonewald, L.F.; Sprague, E.; Jiang, J.X. Mechanical strain opens connexin 43 hemichannels in osteocytes: A novel mechanism for the release of prostaglandin. Mol. Biol. Cell 2005, 16, 3100–3106. [Google Scholar] [CrossRef]
- De Flora, A.; Zocchi, E.; Guida, L.; Franco, L.; Bruzzone, S. Autocrine and paracrine calcium signaling by the CD38/NAD+/cyclic ADP-ribose system. Ann. N. Y. Acad. Sci. 2004, 1028, 176–191. [Google Scholar] [CrossRef]
- Rana, S.; Dringen, R. Gap junction hemichannel-mediated release of glutathione from cultured rat astrocytes. Neurosci. Lett. 2007, 415, 45–48. [Google Scholar] [CrossRef]
- Tong, X.; Lopez, W.; Ramachandran, J.; Ayad, W.A.; Liu, Y.; Lopez-Rodriguez, A.; Harris, A.L.; Contreras, J.E. Glutathione release through connexin hemichannels: Implications for chemical modification of pores permeable to large molecules. J. Gen. Physiol. 2015, 146, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; De Bock, M.; Decrock, E.; Bol, M.; Gadicherla, A.; Vinken, M.; Rogiers, V.; Bukauskas, F.F.; Bultynck, G.; Leybaert, L. Paracrine signaling through plasma membrane hemichannels. Biochim. Biophys. Acta 2013, 1828, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Vénéreau, E.; Ceriotti, C.; Bianchi, M.E. DAMPs from Cell Death to New Life. Front. Immunol. 2015, 6, 422. [Google Scholar] [CrossRef] [PubMed]
- Decrock, E.; Vinken, M.; De Vuyst, E.; Krysko, D.V.; D’Herde, K.; Vanhaecke, T.; Vandenabeele, P.; Rogiers, V.; Leybaert, L. Connexin-related signaling in cell death: To live or let die? Cell Death Differ. 2009, 16, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, A.; Bera, A.K. Hemichannels: Permeants and their effect on development, physiology and death. Cell Biochem. Funct. 2012, 30, 89–100. [Google Scholar] [CrossRef]
- Vinken, M. Connexin hemichannels: Novel mediators of toxicity. Arch. Toxicol. 2015, 89, 143–145. [Google Scholar] [CrossRef]
- Dosch, M.; Gerber, J.; Jebbawi, F.; Beldi, G. Mechanisms of ATP Release by Inflammatory Cells. Int. J. Mol. Sci. 2018, 19, 1222. [Google Scholar] [CrossRef]
- Retamal, M.A.; Reyes, E.P.; Garcia, I.E.; Pinto, B.; Martinez, A.D.; Gonzalez, C. Diseases associated with leaky hemichannels. Front. Cell. Neurosci. 2015, 9, 267. [Google Scholar] [CrossRef]
- Laird, D.W.; Lampe, P.D. Cellular mechanisms of connexin-based inherited diseases. Trends Cell Biol. 2022, 32, 58–69. [Google Scholar] [CrossRef]
- Garcia, I.E.; Bosen, F.; Mujica, P.; Pupo, A.; Flores-Munoz, C.; Jara, O.; Gonzalez, C.; Willecke, K.; Martinez, A.D. From Hyperactive Connexin26 Hemichannels to Impairments in Epidermal Calcium Gradient and Permeability Barrier in the Keratitis-Ichthyosis-Deafness Syndrome. J. Investig. Dermatol. 2016, 136, 574–583. [Google Scholar] [CrossRef]
- Willebrords, J.; Maes, M.; Crespo Yanguas, S.; Vinken, M. Inhibitors of connexin and pannexin channels as potential therapeutics. Pharmacol. Ther. 2017, 180, 144–160. [Google Scholar] [CrossRef] [PubMed]
- Delvaeye, T.; Vandenabeele, P.; Bultynck, G.; Leybaert, L.; Krysko, D.V. Therapeutic Targeting of Connexin Channels: New Views and Challenges. Trends Mol. Med. 2018, 24, 1036–1053. [Google Scholar] [CrossRef] [PubMed]
- Laird, D.W.; Lampe, P.D. Therapeutic strategies targeting connexins. Nat. Rev. Drug Discov. 2018, 17, 905–921. [Google Scholar] [CrossRef]
- Retamal, M.A.; Fernandez-Olivares, A.; Stehberg, J. Over-activated hemichannels: A possible therapeutic target for human diseases. Biochim. Biophys. Acta. Mol. Basis Dis. 2021, 1867, 166232. [Google Scholar] [CrossRef] [PubMed]
- Van Campenhout, R.; Gomes, A.R.; De Groof, T.W.M.; Muyldermans, S.; Devoogdt, N.; Vinken, M. Mechanisms Underlying Connexin Hemichannel Activation in Disease. Int. J. Mol. Sci. 2021, 22, 3503. [Google Scholar] [CrossRef]
- Giaume, C.; Naus, C.C.; Saez, J.C.; Leybaert, L. Glial Connexins and Pannexins in the Healthy and Diseased Brain. Physiol. Rev. 2021, 101, 93–145. [Google Scholar] [CrossRef]
- Walrave, L.; Pierre, A.; Albertini, G.; Aourz, N.; De Bundel, D.; Van Eeckhaut, A.; Vinken, M.; Giaume, C.; Leybaert, L.; Smolders, I. Inhibition of astroglial connexin43 hemichannels with TAT-Gap19 exerts anticonvulsant effects in rodents. Glia 2018, 66, 1788–1804. [Google Scholar] [CrossRef]
- Crespo Yanguas, S.; da Silva, T.C.; Pereira, I.V.A.; Willebrords, J.; Maes, M.; Sayuri Nogueira, M.; Alves de Castro, I.; Leclercq, I.; Romualdo, G.R.; Barbisan, L.F.; et al. TAT-Gap19 and Carbenoxolone Alleviate Liver Fibrosis in Mice. Int. J. Mol. Sci. 2018, 19, 817. [Google Scholar] [CrossRef]
- Zhang, C.; Yan, Z.; Maknojia, A.; Riquelme, M.A.; Gu, S.; Booher, G.; Wallace, D.J.; Bartanusz, V.; Goswami, A.; Xiong, W.; et al. Inhibition of astrocyte hemichannel improves recovery from spinal cord injury. JCI Insight 2021, 6, e134611. [Google Scholar] [CrossRef]
- Johnson, R.G.; Le, H.C.; Evenson, K.; Loberg, S.W.; Myslajek, T.M.; Prabhu, A.; Manley, A.M.; O’Shea, C.; Grunenwald, H.; Haddican, M.; et al. Connexin Hemichannels: Methods for Dye Uptake and Leakage. J. Membr. Biol. 2016, 249, 713–741. [Google Scholar] [CrossRef]
- Lilly, E.; Sellitto, C.; Milstone, L.M.; White, T.W. Connexin channels in congenital skin disorders. Semin. Cell Dev. Biol. 2016, 50, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Sybert, V.P. Genetic Skin Disorders; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Hitomi, M.; Deleyrolle, L.P.; Mulkearns-Hubert, E.E.; Jarrar, A.; Li, M.; Sinyuk, M.; Otvos, B.; Brunet, S.; Flavahan, W.A.; Hubert, C.G.; et al. Differential connexin function enhances self-renewal in glioblastoma. Cell Rep. 2015, 11, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Mulkearns-Hubert, E.E.; Torre-Healy, L.A.; Silver, D.J.; Eurich, J.T.; Bayik, D.; Serbinowski, E.; Hitomi, M.; Zhou, J.; Przychodzen, B.; Zhang, R.; et al. Development of a Cx46 Targeting Strategy for Cancer Stem Cells. Cell Rep. 2019, 27, 1062–1072.e1065. [Google Scholar] [CrossRef] [PubMed]
- Acuna, R.A.; Varas-Godoy, M.; Berthoud, V.M.; Alfaro, I.E.; Retamal, M.A. Connexin-46 Contained in Extracellular Vesicles Enhance Malignancy Features in Breast Cancer Cells. Biomolecules 2020, 10, 676. [Google Scholar] [CrossRef] [PubMed]
- Orellana, V.P.; Tittarelli, A.; Retamal, M.A. Connexins in melanoma: Potential role of Cx46 in its aggressiveness. Pigment. Cell Melanoma Res. 2021, 34, 853–868. [Google Scholar] [CrossRef] [PubMed]
- Acuna, R.A.; Varas-Godoy, M.; Herrera-Sepulveda, D.; Retamal, M.A. Connexin46 Expression Enhances Cancer Stem Cell and Epithelial-to-Mesenchymal Transition Characteristics of Human Breast Cancer MCF-7 Cells. Int. J. Mol. Sci. 2021, 22, 12604. [Google Scholar] [CrossRef]
- Shi, Y.; Li, X.; Yang, J. Mutations of CX46/CX50 and Cataract Development. Front. Mol. Biosci. 2022, 9, 842399. [Google Scholar] [CrossRef]
- Xu, L.; Carrer, A.; Zonta, F.; Qu, Z.; Ma, P.; Li, S.; Ceriani, F.; Buratto, D.; Crispino, G.; Zorzi, V.; et al. Design and Characterization of a Human Monoclonal Antibody that Modulates Mutant Connexin 26 Hemichannels Implicated in Deafness and Skin Disorders. Front. Mol. Neurosci. 2017, 10, 298. [Google Scholar] [CrossRef]
- Ziraldo, G.; Buratto, D.; Kuang, Y.; Xu, L.; Carrer, A.; Nardin, C.; Chiani, F.; Salvatore, A.M.; Paludetti, G.; Lerner, R.A.; et al. A Human-Derived Monoclonal Antibody Targeting Extracellular Connexin Domain Selectively Modulates Hemichannel Function. Front. Physiol. 2019, 10, 392. [Google Scholar] [CrossRef]
- Kuang, Y.; Zorzi, V.; Buratto, D.; Ziraldo, G.; Mazzarda, F.; Peres, C.; Nardin, C.; Salvatore, A.M.; Chiani, F.; Scavizzi, F.; et al. A potent antagonist antibody targeting connexin hemichannels alleviates Clouston syndrome symptoms in mutant mice. EBioMedicine 2020, 57, 102825. [Google Scholar] [CrossRef]
- Buratto, D.; Donati, V.; Zonta, F.; Mammano, F. Harnessing the therapeutic potential of antibodies targeting connexin hemichannels. Biochim. Biophys. Acta. Mol. Basis Dis. 2021, 1867, 166047. [Google Scholar] [CrossRef] [PubMed]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [PubMed]
- Schoop, V.M.; Mirancea, N.; Fusenig, N.E. Epidermal organization and differentiation of HaCaT keratinocytes in organotypic coculture with human dermal fibroblasts. J. Investig. Dermatol. 1999, 112, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Maas-Szabowski, N.; Starker, A.; Fusenig, N.E. Epidermal tissue regeneration and stromal interaction in HaCaT cells is initiated by TGF-alpha. J. Cell Sci. 2003, 116, 2937–2948. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, R.; Wang, Z.; Zhou, S.; Song, Y.; Chen, L.; Chen, N.; Liu, W.; Ji, C.; Wu, W.; et al. Mechanistic effect of the human GJB6 gene and its mutations in HaCaT cell proliferation and apoptosis. Braz. J. Med. Biol. Res. 2018, 51, e7560. [Google Scholar] [CrossRef]
- Essenfelder, G.M.; Bruzzone, R.; Lamartine, J.; Charollais, A.; Blanchet-Bardon, C.; Barbe, M.T.; Meda, P.; Waksman, G. Connexin30 mutations responsible for hidrotic ectodermal dysplasia cause abnormal hemichannel activity. Hum. Mol. Genet. 2004, 13, 1703–1714. [Google Scholar]
- Stong, B.C.; Chang, Q.; Ahmad, S.; Lin, X. A novel mechanism for connexin 26 mutation linked deafness: Cell death caused by leaky gap junction hemichannels. Laryngoscope 2006, 116, 2205–2210. [Google Scholar] [CrossRef]
- Lee, J.R.; Derosa, A.M.; White, T.W. Connexin mutations causing skin disease and deafness increase hemichannel activity and cell death when expressed in Xenopus oocytes. J. Investig. Dermatol. 2009, 129, 870–878. [Google Scholar] [CrossRef]
- Sanchez, H.A.; Mese, G.; Srinivas, M.; White, T.W.; Verselis, V.K. Differentially altered Ca2+ regulation and Ca2+ permeability in Cx26 hemichannels formed by the A40V and G45E mutations that cause keratitis ichthyosis deafness syndrome. J. Gen. Physiol. 2010, 136, 47–62. [Google Scholar] [CrossRef]
- Terrinoni, A.; Codispoti, A.; Serra, V.; Didona, B.; Bruno, E.; Nistico, R.; Giustizieri, M.; Alessandrini, M.; Campione, E.; Melino, G. Connexin 26 (GJB2) mutations, causing KID Syndrome, are associated with cell death due to calcium gating deregulation. Biochem. Biophys. Res. Commun. 2010, 394, 909–914. [Google Scholar] [CrossRef]
- Sanchez, H.A.; Villone, K.; Srinivas, M.; Verselis, V.K. The D50N mutation and syndromic deafness: Altered Cx26 hemichannel properties caused by effects on the pore and intersubunit interactions. J. Gen. Physiol. 2013, 142, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.C.; Kelly, J.J.; Lajoie, P.; Shao, Q.; Laird, D.W. Mutations in Cx30 that are linked to skin disease and non-syndromic hearing loss exhibit several distinct cellular pathologies. J. Cell Sci. 2014, 127, 1751–1764. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.W.; Wardill, T.J.; Sun, Y.; Pulver, S.R.; Renninger, S.L.; Baohan, A.; Schreiter, E.R.; Kerr, R.A.; Orger, M.B.; Jayaraman, V.; et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 2013, 499, 295–300. [Google Scholar] [CrossRef]
- Gerido, D.A.; DeRosa, A.M.; Richard, G.; White, T.W. Aberrant hemichannel properties of Cx26 mutations causing skin disease and deafness. Am. J. Physiol. Cell Physiol. 2007, 293, C337–C345. [Google Scholar] [CrossRef] [PubMed]
- Mese, G.; Sellitto, C.; Li, L.; Wang, H.Z.; Valiunas, V.; Richard, G.; Brink, P.R.; White, T.W. The Cx26-G45E mutation displays increased hemichannel activity in a mouse model of the lethal form of keratitis-ichthyosis-deafness syndrome. Mol. Biol. Cell 2011, 22, 4776–4786. [Google Scholar] [CrossRef] [PubMed]
- Sellitto, C.; Li, L.; White, T.W. Connexin hemichannel inhibition ameliorates epidermal pathology in a mouse model of keratitis ichthyosis deafness syndrome. Sci. Rep. 2021, 11, 24118. [Google Scholar] [CrossRef]
- Agha-Mohammadi, S.; O’Malley, M.; Etemad, A.; Wang, Z.; Xiao, X.; Lotze, M.T. Second-generation tetracycline-regulatable promoter: Repositioned tet operator elements optimize transactivator synergy while shorter minimal promoter offers tight basal leakiness. J. Gene Med. 2004, 6, 817–828. [Google Scholar] [CrossRef]
- Donati, V.; Peres, C.; Nardin, C.; Scavizzi, F.; Raspa, M.; Ciubotaru, C.D.; Bortolozzi, M.; Pedersen, M.G.; Mammano, F. Calcium Signaling in the Photodamaged Skin: In Vivo Experiments and Mathematical Modeling. Function 2022, 3, zqab064. [Google Scholar] [CrossRef]
- Hennings, H.; Michael, D.; Cheng, C.; Steinert, P.; Holbrook, K.; Yuspa, S.H. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell 1980, 19, 245–254. [Google Scholar] [CrossRef]
- Watt, F.M.; Green, H. Stratification and terminal differentiation of cultured epidermal cells. Nature 1982, 295, 434–436. [Google Scholar] [CrossRef]
- Bikle, D.D.; Xie, Z.; Tu, C.L. Calcium regulation of keratinocyte differentiation. Expert Rev. Endocrinol. Metab. 2012, 7, 461–472. [Google Scholar] [CrossRef]
- Garcia-Vega, L.; O’Shaughnessy, E.M.; Jan, A.; Bartholomew, C.; Martin, P.E. Connexin 26 and 43 play a role in regulating proinflammatory events in the epidermis. J. Cell. Physiol. 2019, 234, 15594–15606. [Google Scholar] [CrossRef]
- Fitzgerald, D.J.; Fusenig, N.E.; Boukamp, P.; Piccoli, C.; Mesnil, M.; Yamasaki, H. Expression and function of connexin in normal and transformed human keratinocytes in culture. Carcinogenesis 1994, 15, 1859–1865. [Google Scholar] [CrossRef]
- Nugent, L.; Ofori-Frimpong, B.; Martin, P.E.; Green, C.R.; Wright, C.S. Cx31.1 expression is modulated in HaCaT cells exposed to UV-induced damage and scrape-wounding. J. Cell. Physiol. 2021, 236, 911–920. [Google Scholar] [CrossRef]
- Ceriani, F.; Ciubotaru, C.D.; Bortolozzi, M.; Mammano, F. Design and Construction of a Cost-Effective Spinning Disk System for Live Imaging of Inner Ear Tissue. Methods Mol. Biol. 2016, 1427, 223–241. [Google Scholar] [CrossRef]
- Mammano, F.; Bortolozzi, M. Ca2+ imaging: Principles of analysis and enhancement. In Calcium Measurement Methods; Verkhratsky, A., Petersen, O., Eds.; Humana Press: New York, NY, USA, 2010; Volume 43, pp. 57–80. [Google Scholar]
- Woszczek, G.; Fuerst, E.; Maguire, T.J.A. FLIPR Calcium Mobilization Assays in GPCR Drug Discovery. Methods Mol. Biol. 2021, 2268, 193–205. [Google Scholar] [CrossRef]
- Vetter, I.; Carter, D.; Bassett, J.; Deuis, J.R.; Tay, B.; Jami, S.; Robinson, S.D. High-Throughput Fluorescence Assays for Ion Channels and GPCRs. Adv. Exp. Med. Biol. 2020, 1131, 27–72. [Google Scholar] [CrossRef]
- Bikle, D.D.; Ratnam, A.; Mauro, T.; Harris, J.; Pillai, S. Changes in calcium responsiveness and handling during keratinocyte differentiation. Potential role of the calcium receptor. J. Clin. Investig. 1996, 97, 1085–1093. [Google Scholar] [CrossRef]
- Elsholz, F.; Harteneck, C.; Muller, W.; Friedland, K. Calcium—A central regulator of keratinocyte differentiation in health and disease. Eur. J. Dermatol. EJD 2014, 24, 650–661. [Google Scholar] [CrossRef]
- Bezprozvanny, I.; Watras, J.; Ehrlich, B.E. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature 1991, 351, 751–754. [Google Scholar] [CrossRef]
- Woll, K.A.; Van Petegem, F. Calcium-release channels: Structure and function of IP3 receptors and ryanodine receptors. Physiol. Rev. 2022, 102, 209–268. [Google Scholar] [CrossRef]
- Glover, J.D.; Taylor, L.; Sherman, A.; Zeiger-Poli, C.; Sang, H.M.; McGrew, M.J. A novel piggyBac transposon inducible expression system identifies a role for AKT signalling in primordial germ cell migration. PLoS ONE 2013, 8, e77222. [Google Scholar] [CrossRef]
- Choi, E.J.; Palacios-Prado, N.; Saez, J.C.; Lee, J. Identification of Cx45 as a Major Component of GJs in HeLa Cells. Biomolecules 2020, 10, 1389. [Google Scholar] [CrossRef]
- Zonta, F.; Polles, G.; Zanotti, G.; Mammano, F. Permeation pathway of homomeric connexin 26 and connexin 30 channels investigated by molecular dynamics. J. Biomol. Struct. Dyn. 2012, 29, 985–998. [Google Scholar] [CrossRef]
- Zonta, F.; Polles, G.; Sanasi, M.F.; Bortolozzi, M.; Mammano, F. The 3.5 angstrom X-ray structure of the human connexin26 gap junction channel is unlikely that of a fully open channel. Cell Commun. Signal. CCS 2013, 11, 15. [Google Scholar] [CrossRef]
- Cribbs, A.P.; Kennedy, A.; Gregory, B.; Brennan, F.M. Simplified production and concentration of lentiviral vectors to achieve high transduction in primary human T cells. BMC Biotechnol. 2013, 13, 98. [Google Scholar] [CrossRef]
- Persson, H.; Ye, W.; Wernimont, A.; Adams, J.J.; Koide, A.; Koide, S.; Lam, R.; Sidhu, S.S. CDR-H3 diversity is not required for antigen recognition by synthetic antibodies. J. Mol. Biol. 2013, 425, 803–811. [Google Scholar] [CrossRef]


| Type of Disease (Acronym/OMIM § No.) | P | C | E | Cx (Gene) | Amino Acid Mutations [62] |
|---|---|---|---|---|---|
| Keratitis-ichthyosis-deafness syndrome, autosomal dominant (KID/148210) | <1/1,000,000 †, | <100 † | 1–300 ╬ | Cx26 (GJB2) | G12R, N14K, N14Y, S17F, I30N, A40V, G45E, D50A, D50N, D50Y, A88V |
| Palmoplantar keratoderma with deafness (PPKDFN/148350) | <1/1,000,000 †, | 5 § | 1–300╬ | H73R, S183F | |
| Ectodermal Dysplasia 2, Clouston type (ECTD2/129500) | 1–9/100,000 †, | >150 ‡ | 3000–30,000 ╬ | Cx30 (GJB6) | G11R, A88V |
| Palmoplantar keratoderma and congenital alopecia 1 (PPKCA1/104100) | <1/1,000,000 †, | 10 † | NA # | Cx43 (GJA1) | G8V |
| Erythrokeratodermia variabilis et progressiva 3 (EKVP3/617525) | <1/2,000,000 † | 3 § | NA # | A44V, E227D |
| Cell Pool | Average Percentage |
|---|---|
| Cx26 −dox | 1.86 ± 0.21 |
| Cx26 +dox | 3.4 ± 0.15 |
| Cx26G45E −dox | 3.11 ± 0.23 |
| Cx26G45E +dox | 6.65 ± 0.86 |
| Cell Pools | p-Value |
|---|---|
| Cx26 −dox vs. Cx26 +dox | 0.7703 |
| Cx26G45E −dox vs. Cx26G45E +dox | 0.1574 |
| Cx26 −dox vs. Cx26G45E −dox | 0.8642 |
| Cx26 +dox vs. Cx26G45E +dox | 0.2172 |
| Cx26 −dox vs. Cx26G45E +dox | 0.0346 |
| Cx26 +dox vs. Cx26G45E −dox | 0.9975 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nardin, C.; Tettey-Matey, A.; Donati, V.; Marazziti, D.; Di Pietro, C.; Peres, C.; Raspa, M.; Zonta, F.; Yang, G.; Gorelik, M.; et al. A Quantitative Assay for Ca2+ Uptake through Normal and Pathological Hemichannels. Int. J. Mol. Sci. 2022, 23, 7337. https://doi.org/10.3390/ijms23137337
Nardin C, Tettey-Matey A, Donati V, Marazziti D, Di Pietro C, Peres C, Raspa M, Zonta F, Yang G, Gorelik M, et al. A Quantitative Assay for Ca2+ Uptake through Normal and Pathological Hemichannels. International Journal of Molecular Sciences. 2022; 23(13):7337. https://doi.org/10.3390/ijms23137337
Chicago/Turabian StyleNardin, Chiara, Abraham Tettey-Matey, Viola Donati, Daniela Marazziti, Chiara Di Pietro, Chiara Peres, Marcello Raspa, Francesco Zonta, Guang Yang, Maryna Gorelik, and et al. 2022. "A Quantitative Assay for Ca2+ Uptake through Normal and Pathological Hemichannels" International Journal of Molecular Sciences 23, no. 13: 7337. https://doi.org/10.3390/ijms23137337
APA StyleNardin, C., Tettey-Matey, A., Donati, V., Marazziti, D., Di Pietro, C., Peres, C., Raspa, M., Zonta, F., Yang, G., Gorelik, M., Singh, S., Cardarelli, L., Sidhu, S. S., & Mammano, F. (2022). A Quantitative Assay for Ca2+ Uptake through Normal and Pathological Hemichannels. International Journal of Molecular Sciences, 23(13), 7337. https://doi.org/10.3390/ijms23137337








