Abstract
Microcirculation homeostasis depends on several channels permeable to ions and/or small molecules that facilitate the regulation of the vasomotor tone, hyperpermeability, the blood–brain barrier, and the neurovascular coupling function. Connexin (Cxs) and Pannexin (Panxs) large-pore channel proteins are implicated in several aspects of vascular physiology. The permeation of ions (i.e., Ca2+) and key metabolites (ATP, prostaglandins, D-serine, etc.) through Cxs (i.e., gap junction channels or hemichannels) and Panxs proteins plays a vital role in intercellular communication and maintaining vascular homeostasis. Therefore, dysregulation or genetic pathologies associated with these channels promote deleterious tissue consequences. This review provides an overview of current knowledge concerning the physiological role of these large-pore molecule channels in microcirculation (arterioles, capillaries, venules) and in the neurovascular coupling function.
1. Introduction
The cardiovascular system is constituted by the heart, arteries, arterioles, capillaries, postcapillary venules, venules, and veins. The heart pumps the blood to all tissues, allowing the continuous supply of oxygen and crucial metabolites to support tissue homeostasis. Elastic arteries form the systemic circulation, such as the aorta, work under high pressure, and carry the blood to the arterioles. Arterioles play a crucial role in regulating blood flow and peripheral resistance. The blood then flows into the capillary bed to supply nutrients and meet the metabolic demands of tissues. Subsequently, the blood returns to the heart via venules and veins.
In the microcirculation, which includes arterioles, capillaries, and postcapillary venules, connexin and pannexin channels play a vital role in maintaining the activity of microcirculation homeostasis. This review will focus on Cxs and Panxs large channels in microcirculation and neurovascular coupling regulation.
1.1. Connexin Channels
Connexin channels form two different functional channels: gap junctional (GJ) channels and hemichannels. GJ channels directly connect the cytoplasm of adjacent cells [1,2,3]. Connexin (Cxs) hemichannels (also termed connexons) are the hexameric single-membrane subunits of GJ contributed by each cell (Figure 1). GJ channels and Cxs hemichannels are permeable to ions and small molecules (<1.5 kDa) [4,5,6,7,8,9,10]. Twenty connexin isoforms are expressed in mammalian cells, and each connexin isoform is referred to according to its molecular weight (i.e., Cx43 has a molecular weight of 43 kDa) [11]. Tissues and cells, including the vascular microcirculation, often express more than one connexin isoform [12,13,14]. Hemichannels can be organized by an association of one (homomeric channels) or a combination (heteromeric channels) of additional connexin isoforms, which normally differ in unitary conductance, ion/molecule permeability, and regulation [4,6,8,11,15,16]. The activity of connexin proteins is regulated by several factors including: voltage, pH, carbon monoxide, and extracellular–intracellular Ca2+ [4,11,17,18,19,20,21,22]. Connexin post-translational modifications such as phosphorylation or S-nitrosylation play a role in keeping the function and homeostasis of the tissue [23,24,25,26,27]. Cxs hemichannels also promote ion permeation (i.e., Ca2+) [6,9,15,28,29,30] and molecule release (ATP, D-serine, prostaglandins, nitric oxide, etc.) to the milieu, playing a vital role in cell function activity [15,31,32,33,34,35].
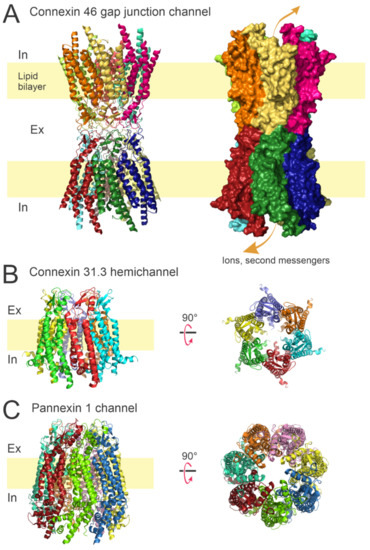
Figure 1.
Overall architecture of gap junction channels, connexin hemichannels, and Panx-1 channels. (A) Ribbon and surface representations of the Cx46 GJ channel viewed from the lateral side. The dodecameric assembly allows the permeation of ions and small metabolites between adjacent cells. (B) Ribbon model for the Cx31.3 hemichannel viewed from lateral side (left) and from the extracellular side (right). Hemichannels assemble in hexamers and provide a conduction pathway for ions and small molecules between extracellular and intracellular compartments. (C) Ribbon representations of the Panx-1 channel. Panx-1 channels assemble as heptamers and provide a pathway for paracrine/autocrine signaling. Protein data bank (PDB) codes: 7JKC (Cx46) [36], 6L3U (Cx31.3) [37], and 6WBF (Panx-1) [38].
Under some circumstances, mutant, and even wild type (WT) hemichannels contribute to pathologies. For example, several connexin mutations that cause human pathologies result from an exacerbated hemichannel opening at the plasma membrane, with deleterious consequences [39,40,41,42,43,44,45]. Similarly, in ischemia and inflammation, there is strong evidence that the opening of hemichannels enhances tissue damage in astrocytes, hepatocytes, and cardiac and skeletal myocytes [5,15,46,47,48,49,50,51,52,53]. In contrast to pathology, the physiological role of connexin hemichannels is not well defined.
1.2. Pannexin Channels
Panxs, similar to Cxs hemichannels, represent another family of large pore channels. The major structural difference is that pannexin (i.e., Panx-1) channels are heptameric channels [54,55,56] compared to connexins, that are hexameric channels [36,37,38,57,58,59] (Figure 1). Three members of this protein family have been described (Panx-1, Panx-2, and Panx-3) [60]. Evidence reveals that Panx-1 channels cannot anchor with additional pannexins from adjacent cells probably because of several glycosylations located in extracellular loops [61]. However, recent studies demonstrated that Panx-1 overexpressed in HeLa cells form GJ channels [62]. Nevertheless, it is still unclear whether or not Panx-1 proteins dock with adjacent cells under physiological conditions.
Panx-1 is the most studied pannexin because its expression is detected in several cells and tissues. Similar to connexin hemichannels, Panx-1 facilitates the release of small metabolites to extracellular space, for instance, purines (i.e., ATP), that afterward signal through the activation of membrane receptors (i.e., purinergic receptors) in neighboring cells or even at some distance [63,64,65].
2. Connexin Expression in Arterioles
Connexin and pannexin channels are usually evaluated through the manipulation of rodent models or human primary cells. Here, we briefly summarize the expression of connexin proteins in peripheral small vessels (Table 1).

Table 1.
Connexin and Pannexin protein distribution in arterioles (+ expression/− no expression).
Interactions of one or more connexin isoforms (Cx37, Cx40, Cx43, and Cx45) expressed in the vessel wall [12,66,72,73,74,75,76] mediate intercellular communication via gap junctions in the vascular system. The pattern of connexin expression in the vasculature is variable and depends on vessel size, vascular territory, and species [66,67,73,76,77] (Table 1). Cx37, Cx40, and Cx43 are expressed in endothelial cells (ECs) and smooth muscle cells (SMCs) [12,66,68,75,78,79,80]. Cx45 has been observed in the SMCs of cerebral vessels [81,82,83,84]. The goal of the cited studies [12,66,68,73,75,76,77,78,79,80,81,82,83,84] was to evaluate the role of gap junction channels in microcirculation homeostasis. However, the direct contribution of Cxs hemichannels to the regulation of the vasomotor tone was not evaluated and is incompletely understood.
ECs in resistance arteries are a critical component in the tonic regulation of vascular homeostasis by mainly the Ca2+-dependent production of vasorelaxation signaling as nitric oxide (NO) and prostaglandins [69,85,86,87]. However, another NO- and prostaglandin-independent response has been observed only in arterioles, which is associated with SMCs hyperpolarization [12,66,68,75,78,79,80]. This smooth muscle hyperpolarization signaling produced in ECs is transmitted to SMCs, leading to relaxation by the consequent reduction in the open probability of L-type voltage-dependent Ca2+ channels. The contraction intensity of SMCs is determined by the intracellular Ca2+ concentration and the Ca2+ sensitivity of the contractile apparatus. L-type voltage-dependent Ca2+ channels of vascular smooth muscle play a central role in the control of the vasomotor tone by changes in the membrane potential: depolarization produces a Ca2+ influx that leads to vasoconstriction, while hyperpolarization results in a decrease in the intracellular Ca2+ concentration that leads to vasodilation. In this context, connexin gap junction channels have an essential role in the regulation of the blood flow distribution because these channels allow a direct cell-to-cell communication [12,66,68,75,78,79,80] and participate in the regulation of the vessels diameter. Therefore, cell communication in the vessel wall has emerged as a critical and relevant signaling pathway to coordinate vascular wall function in resistance arteries by the radial (among ECs and SMCs) and longitudinal (along the vessel length) conduction of vasomotor signals.
ECs and SMCs are physically divided by the internal elastic lamina in arterioles; these cells are in contact via cell promontories that skewer the internal elastic lamina and match the other cell type at points comprehended as myoendothelial junctions [12,66,68,73,75,76,77,78,79,80,81,82,83,84]. These points of connection seem to form approvingly specialized subcellular signaling microdomains, and gap junctions located at myoendothelial junctions (i.e., myoendothelial gap junctions) supply an essential pathway for a key regulation of the vasomotor tone through the radial transmission of current and molecules as we described above (i.e., Ca2+ and small signaling metabolites). However, whether connexin hemichannels facilitate the Ca2+ influx or metabolite release through the endothelial or smooth muscle membrane is not completely understood.
Role of Connexin Protein Function in Arterioles
As we described above, there is more than one connexin type in the vascular wall from small vessels (Table 1). Here we describe the participation and the main contribution of each connexin to the vascular homeostasis function.
Cx40 knockout mice show irregular arteriolar vasomotion affecting gap junction communication in ECs and a reduction of conducted vasodilation along the vessel [12,88]. The cellular pathway of conducted vasomotor signals depends on the cell type that starts the vasomotion response. For example, vasoconstrictor agonist responses (i.e., phenylephrine [PE]), which activates SMCs, are invariably conducted by SMCs, not by the endothelium layer [89,90]. Vasodilator signals evoked by acetylcholine (ACh) or bradykinin (BK) are spread exclusively by ECs in feed arteries [91,92] or by both SMCs and ECs in arterioles [89,90], which shows that the cellular conduction pathway depends on the functional location of the particular vessel in the microvascular network. However, the cellular path of vasodilator signals may also depend on the stimulus that initiated the response because, in contrast to ACh, selective damage of the endothelium precludes the vasodilation induced by BK in arterioles [90,93].
Deletion of Cx37 does not affect vasomotor function or arterial blood pressure [12], suggesting that Cx37 is not involved in the vasomotor tone. The endothelial-specific knockout of Cx43 (EC-Cx43−/−), promotes hypotension by reducing gap junction communication between ECs and SMCs [94]. The molecular mechanisms of this hypotensive phenotype are elusive. It is most likely that the disruption of Cx43 GJ channels increases eNOS activity, promoting hypotension. The colocalization of eNOS and myoendothelial Cx43 GJ channels coordinate the vasomotor tone [95]. Therefore, a reduction of Cx43 GJ channels’ formation in the myoendothelial space promotes more cytosolic eNOS activation, exacerbating endothelial NO production, leading to a hypotensive phenotype. In ECs, eNOS is predominantly localized in caveolae and released by inhibition via caveolin-1 (Cav-1) upon the stretching of the vascular wall (as reviewed in [96]). In Cav-1 knockout (KO) mice, eNOS is constitutively activated, leading to enhanced NO production. Saliez et al. showed that these mice have fewer gap junctions in vessels due to a lower expression of Cxs 37, 40, and 43, and the fact that TRPV4 channels and Cxs colocalize with Cav-1 in caveolae [97]. Cav-1 KO mice were reported to develop cardiac hypertrophy, pulmonary hypertension, and systemic hypotension [98]. Since this knockout eliminates Cx43 in both GJ channels and hemichannels, the specific contribution of Cx43 hemichannels in regulating the vasomotor tone is not clear. Cxs hemichannels function has not been explored directly in arterioles, in contrast with other tissues or cells where Cx43 hemichannels in physiological conditions regulate metabolite release (ATP, D-serine), electrical activity behavior (i.e., action potentials, resting membrane potential), or the potential redox balance in tanycytes, cardiac cells, astrocytes, neurons, and mouse lenses, respectively [15,29,63,64,65,99,100,101].
The endothelium is critical in mediating vascular function by the Ca2+-dependent generation of vasodilator signals such as nitric oxide (NO) and prostaglandins [69,85,86,87]. Although NO is the major endothelium-dependent vasodilator signal in large conduit vessels, NO or prostaglandin production inhibition solely attenuates the vasorelaxation initiated by endothelium-dependent vasodilators in small resistance arteries [86]. The vasodilatory process in arterioles involves the hyperpolarization of SMCs [13,102,103,104]. This process is closely associated with the endothelium-derived hyperpolarizing factor (EDHF), which plays a fundamental role controlling the vasomotor tone in small arteries [13,102,103,104].
Several EDHF candidates have been proposed, such as K+ ions [105], NO, epoxyeicosatrienoic acids (EETs) [106,107], hydrogen peroxide [108], and C-type natriuretic peptide (CNP) [109,110], but the identity of EDHF has not been established. EDHF-mediated vasodilation is paralleled by the hyperpolarization of the endothelium. Endothelial hyperpolarization signaling is completely prevented by the simultaneous inhibition of Ca2+-activated K+ channels (KCa) of small (SKCa) and intermediate conductance (IKCa) [103,111,112], indicating that KCa channels mediate this endothelial electrical signal. These K+ channels are expressed in ECs but not in SMCs [112,113]. The endothelial hyperpolarization triggered directly by K+ channel activation is called endothelium-derived hyperpolarization (EDH) [114]. As the longitudinal and radial transmission of endothelial hyperpolarization depends on connexin gap junction communication between ECs and SMCs to evoke vessel dilation, EDH-associated vasodilatory signaling is prevented by pharmacological approaches that block both GJ channels and hemichannels [115,116,117]. As this methodology blocks GJ channels and hemichannels, these studies [115,116,117] do not distinguish the effects of blocking the connexin GJ channels from the effects of blocking the Cxs-hemichannels with regards to endothelium hyperpolarization.
3. Pannexin Proteins in Arterioles
Panx-1 is almost ubiquitously expressed in murine ECs and SMCs of large and small vessels, while Panx-3 is only found in arterioles [65,70,71,118,119]. In addition, Panx-2 is expressed in SMCs of the pulmonary artery of mice and in the SMCs of the rat middle cerebral artery (MCA) [71].
Several studies about pannexin function, focusing mainly on Panx-1, have been performed in SMCs. For example, Panx-1 and the α1-adrenergic receptor (AR) are coupled in the regulation of vasoconstriction processes [119,120,121]. SMCs-specific Panx-1 deletion, as well as the use of multiple pannexin inhibitors (such as mimetic peptides, trovafloxacin, and spironolactone), blunts the noradrenaline- and phenylephrine-mediated vasoconstriction of resistance arteries [122,123].
These studies strongly support the concept that smooth muscle Panx-1 channels regulate the vasomotor tone in arterioles. In addition, the regulation of Panx-1 could play a key role in the vasomotor tone in endothelial cells. NO potently inhibits endothelial Panx-1 channels by S-nitrosylation at amino acids cysteine 40 and cysteine 346 to prevent channel opening and ATP release [124], which could be associated with peripheral resistance homeostasis.
Independent of the NO signaling pathway, the activation of endothelial Panx-1 channel-initiated purinergic signaling mediates EDH-dependent vasodilation by the endothelium-dependent vasodilator ACh [125]. Nevertheless, although the EDH-mediated vasodilator pathway is reduced in Panx-1 knockout mice, the NO-dependent vasodilator component is enhanced in these animals, indicating that endothelial Panx-1 may be involved in the tonic regulation of NO production by eNOS. Similar results have been found in mesenteric rat vessels, where general blockers of Panx-1 channels promote endothelial superoxide production by activating endothelial NADP(H)oxidase [70]. Endothelial TTX-sensitive Nav channels and Cav3.2 channels mediate endothelial depolarization and this superoxide production, promoting an increase in NO-mediated vasorelaxation via NADPH oxidase-dependent eNOS phosphorylation at serine 1177 [70]. On the other hand, the prolonged activation of Panx-1 channels by Calcitonin Gene-Related Peptide (CGRP) receptor stimulation leads to a Panx-1-formed channel opening and the ensuing superoxide-dependent connexin-based hemichannel activation exclusively in ECs [69]. The lengthy opening of endothelial Panx-1 channels by perivascular sensory nerves results in the progressive inhibition of NO production by reducing the eNOS phosphorylation status at serine 1177 [69]. These studies, [69] and [70], indicate that endothelial Panx-1 signaling seems to be more complex and is associated to regulate NO production through the eNOS phosphorylation status by a NADPH oxidase/O2⋅–-mediated signaling. These results also indicate that Panx-1 may participate in the endothelium-dependent control of arterial blood pressure.
4. Postcapillary Venules Hyperpermeability
The endothelial barrier controls nutrients and solute exchange between blood and tissues. However, during inflammation, ECs from postcapillary venules are susceptible to the disruption of their barrier and increased permeability to macromolecules, also called hyperpermeability [126,127]. The rearrangement in tight junctions (TJs), adherent junctions (AJs), and cytoskeletal alterations induce contractile forces leading to form gaps between ECs, so that macromolecules and even immune cells can pass through [128,129,130]. Takeuchi et al. showed that cell extrusion is driven by actomyosin contraction and triggered by a Ca2+ wave involving IP3 receptors, GJ, and TRPC1 [131]. In oncogenically transformed or apoptotic cells, this Ca2+ wave induces the polarized movement of the surrounding cells toward the extruding cells and facilitates apical extrusion by inducing actin rearrangement in the nearest neighboring cells (reviewed in [132]). Normally, the inflammatory environment is resolved and hyperpermeability is transient, contributing to repair and wound healing. However, prolonged hyperpermeability leads to the impairment of tissues and constitutes a hallmark of several inflammatory diseases such lung injury, ischemic stroke, and sepsis [133,134,135,136].
4.1. Connexin Proteins in Postcapillary Venules Hyperpermeability
Connexin protein expression is mainly described in large veins (Table 2). Cx40 and Cx43 are found in ECs from the vena cava, femoral veins, portal veins, and postcapillary venules; however, Cx37 is less detected [137,138,139,140,141]. These connexins are also found in ECs in in vitro models such as human saphenous vein culture, human umbilical vein endothelial cells (HUVEC), and primary cultures or cell lines from microvascular vessels [142,143,144]. In contrast, Cx37, Cx43, and Cx47 are commonly expressed in ECs vein valves [139,140,145]. Cx37 and Cx40 knockout mice show localized hemorrhages, but permeability is not affected in basal conditions. However, this model was not evaluated in the inflammatory response [80]. The Cx37-deficient mouse lacks vein valves [139] and the ECs-specific deletion of Cx40 induces an increase in leucocyte adhesion [146].

Table 2.
Connexin and Pannexin protein distribution in in vivo veins, venules, and in vitro cell models (+ expression/− not expression).
GJ channels could contribute to the hyperpermeability mechanism because of their proximity and interactions with other junction proteins [170,171,172,173]. GJ channels and a hemichannel opening could be involved in hyperpermeability because the development of hyperpermeability is associated with an agonist-induced increment in the endothelial intracellular calcium concentration ([Ca2+]i), and maintaining hyperpermeability over time depends on an extracellular Ca2+ influx [174,175,176,177,178,179,180], while ATP signaling is related to hyperpermeability mainly through purinergic receptor activation [181,182].
In addition, the relationship between Cxs and hyperpermeability depends on the vascular territory, including in vivo or in vitro models, and agonists used. For example, in the pulmonary barrier, studies show that during sepsis, lipopolysaccharide (LPS)-induced hyperpermeability is related to increased Cx43 expression, which is associated to TJs and AJs decreased protein expression, such as ZO-1, claudin, and vascular endothelial (VE)-cadherin, respectively, or increased Cx43 GJ channels improving the spread of signaling molecules or ions such as IP3 and Ca2+, which affects vascular hyperpermeability [152,153,154,161,183,184,185]. There is no consensus explaining the involved mechanisms, but Zhang and others, describe a Rho-associated protein kinase (Rock)1-myosin light chain (MLC)2 phosphorylation pathway that relates to contractile forces’ disruption or activation of transcription factor (Tcf)-4/β-catenin when glycoprotein osteopontin (OPN) is involved [153,154]. Interestingly, in Idiopathic Pulmonary Hypertension, asymmetric dimethylarginine (ADMA), a nitric oxide synthase inhibitor, increased, and there was a reduced expression and phosphorylation of Cx43 mediated by the NO–cyclic guanosine monophosphate (cGMP) pathway [186]. The contribution of Cx40 GJ channels in the hyperpermeability response is ambiguous. In the gun-shot-induced acute lung injury (ALI) model, TNF-α and IL-8 increased hyperpermeability by a Cx40 expression decrease [187,188]. However, in the acid (HCl)-induced ALI model, thrombin and PAF produced Cx40 GJ channels’ inhibition and genetic deficiency mitigated vascular endothelial permeability via the upregulation of Rock1, causing the subsequent phosphorylation of Myosin Phosphatase target subunit (MYPT)1 and MLC20 [189]. Furthermore, the specific Cx43 hemichannel blocking by the P5 mimetic peptide in acute respiratory distress syndrome (ARDS), associated with ALI, leads to a reduction of the alveolar infiltration of innate immune cells and protection against LPS-induced lung injury [190].
In the blood–brain barrier (BBB), Cx43 GJ channels’ contribution in familial cerebral cavernous malformations type III hyperpermeability is related to increasing its expression and debilitating TJs’ structure, because of its interaction with ZO-1 [191]. Moreover, BBB disruption caused by cerebral ischemia increased Cx43 phosphorylation [151]. Phagocytosis of myelin debris by microvascular endothelial cells (MECs) impaired TJs and Cx43 GJ channels after spinal cord injury (SCI) and led to increased permeability. Cx43 downregulation was found to reduce both the extravasation of intravenously injected FITC-BSA (bovine serum albumin) and the recruitment of neutrophil leukocytes into the injured cord [192,193]. Interestingly, Cx43 downregulation is also induced by SARS-CoV-2 Spike and is accompanied by decreased AJs proteins, resulting in hyperpermeability in primary brain vascular ECs, and is more pronounced in diabetic brain vascular ECs [194]. Since the endothelial barrier also depends on other cells, the perivascular organization of Cx43 GJ channels in astrocytes contributes to maintaining vascular communication and the BBB equilibrium [195]. Cx43 hemichannel participation was evaluated by the short-time exposure of Cx43 peptide Gap27, inhibiting BK-triggered [Ca2+]i oscillations and BBB permeability in mouse brain ECs [147] and in human brain endothelial cells [159]. Multi-walled carbon nanotube induces BBB barrier disruption mainly by ATP release [31]. Oxygen/glucose deprivation decreases Ca2+ in rat brain capillary endothelial cells, inducing a Cx43 hemichannel opening and, subsequently, ATP release, contributing to BBB damage [164]. In the retinal barrier, ischemia-reperfusion (I/R) induces the upregulation of Cx43 and increases vascular leakage. However, high glucose-induced hyperpermeability in retinal ECs provokes Cx43 downregulation accompanied with ZO-1 and occludin downregulation, which is associated with Cx43 GJ channels uncoupling and a loss of interaction of the C-terminus of Cx43 with the second PDZ domain of ZO-1 [155,156,196].
In other inflammatory models, there is evidence that α1AMP-activated protein kinase (AMPK) protects against LPS-induced ECs barrier disruption. AMPK deficiency leads to a substantial loss of Cx43 in ECs and is correlated with TJs and AJs disruption [162]. Treatment with a Cx43 carboxyl-terminal mimetic peptide protects against thrombin-induced hyperpermeability by stabilizing ZO-1 and VE-cadherin [197]. Cxs hemichannel contribution to the ATP-related mechanism is described in acute inflammation, where Cx43 hemichannel-mediated ATP release from ECs mediates leucocyte recruitment during a foreign body response to sterile implants [198]. In addition, an in vitro study in human EaHy 926 cells report that in high-glucose and IL-1β/TNF-α conditions, Cx43 hemichannel activity increases and is associated with ATP release and the subsequent activation of purinergic receptors and a [Ca2+]i increase [163]. In systemic inflammatory response syndrome (SIRS), TNF-α activates and promotes the Cx43 hemichannel opening in a Ca2+-dependent manner, leading to renal vascular permeability and mortality; however, in the presence of Gap19, a specific Cx43 hemichannel inhibitor, there is a protective effect. Interestingly, electrophysiological evidence demonstrated a fast stimulatory effect of TNF on the Cx43 hemichannel opening, which was linked to [Ca2+]i-dynamics [28].
In the context of atherosclerosis, Cx43 participation is related to leukocyte migration, which is part of the inflammatory condition where hyperpermeability is involved. Then, oxidized low-density lipoprotein (ox-LDL) induced an increase in Cx43, JAM-A, and VE-cadherin protein expression [158]. In addition, statin drug Simvastatin suppresses the TNF-α-induced inhibition of GJ channels’ activity in an in vitro ECs model, using HUVEC, by upregulating Cx37 and Cx40 expression but downregulating Cx43 [199]. Moreover, in line with leucocyte-ECs interactions, in a hamster cheek pouch, the TNF-α inflammatory effect on leucocyte adhesion is suppressed in ECs Cx43-deficient mice as well as by a pharmacological blockade of GJ [200]; thus, Cx43 is predominantly involved in atherosclerosis-related leucocyte adhesion, this is consistent with its contribution to hyperpermeability.
There is evidence of connexin contribution to the hyperpermeability response, inasmuch as agonist-induced eNOS translocation from the plasma membrane to cytosol and subsequent cytosolic NO production is crucial to agonist-induced hyperpermeability [126,201,202,203,204,205], influencing S-nitrosylation and the disassembly of AJs proteins and VASP [206,207,208,209,210,211,212,213]. The participation of GJ channels or Cxs-hemichannels in hyperpermeability may be related to a NO-cGMP-PKG pathway. This could possibly be linked to S-nitrosylation of connexin proteins. For instance, there is evidence that NO induces the upregulation of Cx40 NO-cGMP-PKG pathway-dependent alleviating cerebral vasospasm [214]. On the other hand, β-adrenergic cardiac stress and NO donors evoke S-nitrosylation of Cx43 proteins in mouse cardiac cells [15,27] and in Cx43 expressing Xenopus oocytes at cysteine 271 [15], respectively. Additionally, Cx43 is closely associated with eNOS in heart subsarcolemmal mitochondria [215], regulating Cx43 open probability by post-translational modifications. However, the mechanisms by which GJ channels or Cxs-hemichannels and Cx-S-nitrosylation contribute to hyperpermeability are not fully evaluated yet.
At the present time, few of these studies have raised the question of whether GJ channels may contribute to maintaining the normal ECs barrier. In fact, in normal conditions, monolayers of brain or pulmonary ECs cultures treated with Cx43 or Cx40 blockers lead to an unstable barrier [149]. This issue is discussed by Ange et al., 2020 [162]; however, there is no evidence regarding inflammatory conditions.
4.2. Pannexin Proteins in Postcapillary Venules Hyperpermeability
Pannexin channels are less documented in veins and venules (Table 2). Panx-1 is the most evaluated in inflammatory conditions [65,159,165,168,169]. The relationship between Panx-1 channels and the hyperpermeability response was first approached in HUVEC where the knockdown of Panx-1 blocks’ ATP release was induced by thrombin [168]. The Panx-1 channel openings may contribute to the BBB disruption by ATP release under mimicked ischemic stroke conditions by using Ca2+-free media in a BBB cell line or oxygen/glucose deprivation in a rat brain capillary endothelial cell line [159,164]. The increasing infiltration of leukocytes in mouse cerebral ischemic sections is blunted in Panx-1 inducible knockout mice [166]. Moreover, multi-walled carbon nanotube-induced hyperpermeability is a result of ATP release associated to the Panx-1 channel opening [31]. In lung ischemia reperfusion injury, endothelial-specific Panx-1 inducible knockout mice demonstrate a protective phenotype after I/R with reduced endothelial permeability, edema, and inflammation [167]. Studies in ECs postcapillary venules describe that Panx-1 channels are involved in TNF-α-induced hyperpermeability and leucocyte adhesion and migration in vivo, mediated by subsequent ATP hydrolyzation and purine signaling that could lead to a [Ca2+]i increase by a transient receptor potential vanilloid 4 (TRPV4) channel opening [65,165,216]. In HUVEC, long exposure to TNF-α may lead to Panx-1 channel opening and a [Ca2+]i increase and is associated with IL-1β production [169]. The Panx-1 channel contribution to the hyperpermeability response may be related to oxidative stress conditions or purinergic signaling [65,165,167]; however, NO signaling participation is not considered, since Panx-1 channel activation by S-nitrosylation is ambiguous [124,217,218] or NO seems to inhibit the Panx-1 channel opening in a cGMP-PKC pathway [219].
5. Neurovascular Coupling
Neurovascular coupling (NVC) is a mechanism that applies to changes in cerebral blood flow in response to the increase in neuronal activity to ensure the glucose and oxygen supply necessary for cerebral functions [220,221,222]. NVC depends on the coordinated cell communication between neurons, astrocytes, and microvascular cells (endothelium, SMCs, and/or pericytes). All of them, including the extracellular matrix (ECM) components, form a functional unit called the Neurovascular Unit (NVU) [223,224,225]. NVU plays a vital role in regulating vasomotor tone and vascular homeostasis in the brain [220,226,227]. Astrocyte cells are critical in NVC, functioning as transductors between neurons and brain blood vessels through specialized structures called astrocytic endfeet, allowing several vasoactive mediators (including ATP, glutamate, D-serine, or other neurotransmitters) to enter the perivascular space [32,220,228,229,230,231]. This mediator release depends on the intracellular Ca2+ increase propagated as Ca2+ waves between neighboring astrocytes, and is coordinated by adenosine triphosphate (ATP) release [220,232,233,234,235,236,237,238,239].
5.1. NVC Mediators in Astrocytes and Vascular Cells
Neuronal activity is especially associated with glutamate, the main excitatory neurotransmitter in the central nervous system (CNS) [240,241,242]. Glutamate can activate ionotropic and metabotropic receptors in astrocytes [243,244,245,246], even though astrocytes express receptors to many neurotransmitters. Group I metabotropic glutamate receptors’ (mGluR) activation, especially mGluR5 expressed in astrocytes, correlates with intracellular Ca2+ oscillations observed during NVC through the phospholipase C (PLC) and inositol triphosphate (IP3) signaling [240,247,248]. Interestingly, mGluR5 expression in astrocytes was reported to decrease during development, being absent in the adult; thus the role of astrocytic mGluR5 in NVC might depend on developmental age (as reviewed in [249]). Arachidonic acid (AA) production under the phospholipase C A2 (PLCA2) signaling pathway in astrocytes constitutes the substrate of cytochrome P450 epoxygenases (CYP450) or cyclooxygenase (COX), allowing the synthesis of SMCs vasodilators such as EET and prostaglandins (PGs), respectively [230,250,251].
Astrocyte studies using primary cultures, brain slices, and in vivo murine models have shown an autocrine role of EET, triggering Ca2+-activated potassium channels (KCa), increasing the intracellular Ca2+, and as a paracrine signal in SMCs, producing hyperpolarization and vasodilation [251,252,253,254]. This response is activated after EET binding to G-protein coupled receptors (GPCR) expressed in SMC brain vasculature, and the large conductance of Ca2+-activated potassium channel (BK) activation [255,256]. Furthermore, it has been reported that prostaglandin E2 (PGE-2) correlates with parenchymal vasodilation after direct astrocytic stimulation in a rat cortical slices model [251,257,258]. The activation of the E-prostanoid receptors family, probably EP4, coupled to adenylyl cyclase via Gs-proteins has been related to SMCs’ relaxation in the brain during NVC [259,260,261].
K+ released from the astrocyte endfeet is an essential astrocyte signaling ion associated with vasodilation in brain blood vessels. The astrocytic KCa allows a K+ increase in the perivascular space, activating inwardly rectifying K+ (Kir) channels in brain capillaries/arterioles, inducing hyperpolarization and conducting vasodilation so that feed arteries provide sufficient blood to satisfy the metabolic demand [220]. Nevertheless, the K+ perivascular concentration can determine switches between vasodilation and vasoconstriction [235,260,262,263]. The production of 20-hydroxyeicosatetraenoic acid downstream of the AA pathway in astrocytes has been related to the vasoconstriction of parenchymal brain vessels [258,263,264,265,266]. These observations show the complex and regulated interactive signaling produced during the astrocytic Ca2+ increase associated with the AA metabolism and K+ released from astrocytes during NVC.
Another essential component during NVC is the astrocytic ATP release, which coordinates Ca2+ waves in an autocrine manner and has vasomotor activity effects [233,261,267,268,269]. ATP activates metabotropic purinergic receptors (P2Y) evoking an IP3 signaling pathway in astrocytes [268,269], and it has also been observed in pericytes and brain SMCs, capillaries, and pial arterioles, where the P2Y receptors activation (and apparently not through P2X) leads to an intracellular Ca2+ increase and vasoconstriction [268,270]. Additionally, in vitro and in vivo experiments using SMCs from rat brain arterioles suggest coupling between TRPV4 and P2Y receptors, leading to the constriction of cerebral parenchymal arterioles [271]. However, due to the short half-life of ATP (by the extensive expression of its hydrolyzing ectonucleotidases), the potent brain vasculature vasodilator adenosine production is consistent with the responses mediated by astrocytes during NVC [239,272,273,274,275]. Brain ectonucleotidases are expressed in microglia, ECs, SMCs, and astrocytes membranes, permitting to consolidate an adenosine source in the brain [276,277,278].
There are four types of adenosine receptors: A1, A2A, A2B, and A3. A1/A3 activate inhibitory signaling through Gi-PLC signaling, while A2A/A2B is associated with an activating pathway through Gs-cAMP [279]. Studies in isolated brain arterioles have shown the association between vasodilation and A2A/A2B receptors’ activation [280,281]. SMCs in cerebral arteries and arterioles express adenosine receptors A2B, activating the GPCR adenylate cyclase/cAMP/PKA pathway and KATP channels, which hyperpolarize the SMCs [282,283]. This pathway has been associated with vasodilation in brain pial arterioles during in vivo and in vitro studies, in ECs’ nitric oxide production-dependent manner, under adenosine receptors’ activation [284,285,286].
Despite the relevance of ATP release from astrocytes during NVC, there is no consensus about the ATP release mechanism. Several channels have been proposed in purine release and their possible contribution to the intracellular Ca2+ signaling pathway in NVC. Thus, the Cxs hemichannels and the Panxs channels are promising candidates due to their permeation properties and expression patterns in astrocytes [287].
5.2. Cxs Expression in Astrocytes and Vascular Brain Cells, and Possible Role in NVC
Astrocytes express Cx43 predominantly; fewer levels of Cx26, Cx30, Cx40, and Cx45 have been described as hemichannels, while astrocyte GJ channels are formed by Cx26, Cx30, and Cx43 [288,289,290,291,292,293]. The high coupling between astrocytes through Cx43 GJ channels permits Ca2+ propagation in the astrocytic network, developing an extensive brain territory signaling coordinated by ATP release [294,295,296]. However, whether Cx26, Cx30, and Cx43 hemichannels contribute to an intracellular Ca2+ influx directly or by ATP release (which activates purinergic receptors) during NVC has not been completely established. Based on two observations, it is possible to hypothesize that Cxs hemichannels participate in the astrocytic Ca2+ signaling during NVC: (1) the inhibition of the intracellular Ca2+ signaling using GPCR blockers does not abolish the Ca2+ increase in astrocytes altogether [297], which suggests that the dominant Ca2+ entry is via another Ca2+-permeable channel, and (2) an intracellular Ca2+ increase in astrocytes mediate Cx43 hemichannels opening [298,299]. Furthermore, a Cx43 hemichannel-mediated ATP release has been described in astrocytic cell lines and primary cultures, associating it to the intracellular Ca2+ waves by P2Y receptors’ activation [298,300,301]. However, these events have not been completely elucidated in NVC.
The Cx37/Cx40/Cx43 expression in ECs and SMCs has been reported in brain basilar arteries and pial branches [302,303]. Cx45 is only expressed in brain SMCs arterioles [303,304], and Cx37/Cx40 have been identified in myoendothelial junctions [302,305]. The Cxs expression in ECs and SMCs in brain blood vessels acquires relevance according to the study by Figueroa et al., where NO diffusion through Cx43, Cx40, and Cx37 hemichannels and myoendothelial GJ channels was established [85]. NO diffusion through these channels could correlate with NO signaling in the NVC. Nevertheless, it is unknown if these events are involved in vasodilation during NVC.
In addition to the Cxs expression in astrocytes and vascular brain cells, Panx-1 and Panx-2 are abundantly expressed in many regions of the CNS [306,307], without a defined role in NVC.
5.3. Panx-1 Expression in Astrocytes and Vascular Brain Cells, and Possible Role in NVC
Only Panx-1 expression has been consistently reported in astrocytes [308,309,310]. In vitro studies, using mice astrocytic primary cultures, have shown that Panx-1 channels can release ATP after depolarization or the downstream of P2X7R activation [311,312]. The participation of P2XRs in NVC has not been entirely accepted. It is possible that the high extracellular concentration of the P2X7R agonist, BzATP, used in these studies may permit these receptors’ activation, which do not participate under physiological conditions. Nonetheless, these studies support the possible release of ATP from astrocytes through Panx-1 channels [313], and establish it as a candidate for this signaling during NVC.
Consistent with Girouard et al., 2010, studies, where the extracellular K+ concentration in the perivascular space regulates the vasomotor responses in murine brains, Scemes and Spray showed that the increase of extracellular K+ observed during NVC is associated with an increase in the astrocytic coupling through Cxs GJ channels, leading to the activation of Panx-1 channels and ATP release from astrocytes [314], which is in line with the observations during NVC, and could establish the differential contribution of these types of channels in this mechanism.
Finally, Panx-1 is expressed in ECs and SMCs from mice cerebral arteries. According to recent observations, ECs regulate the myogenic tone in small brain vessels in a P2YRs-dependent manner [166]. In the same study, Good et al. found that endothelial Panx-1 did not control the myogenic tone in small peripheral vessels. These data suggest the territorial-space activity of Panx-1 channels mediating the arterial vasomotor tone. These functional activities of Panx-1 channels may be considered to determine whether pannexin proteins are cardiovascular targets to prevent/improve vascular dysfunction.
6. Conclusions
Cxs and Panxs channel activity and their differential expression in distinct cells of the blood vessels have a crucial role in the physiological and pathophysiological processes associated with vascular homeostasis. Cxs and Panxs contribute to microcirculatory regulation as their participation has been observed during the vasomotor control exerted by arterioles as well as during hyperpermeability (at postcapillary venules) observed under inflammatory conditions. We discussed also neurovascular coupling, an essential mechanism for adequate brain function, where primarily Cx43 and Panx-1 participate actively in mediating complex signaling pathways associated with NVC regulation.
The role and participation of Cxs and Panxs large-pore channels are still under experimental observation. For instance, recent results display a transport behavior with a molecular permeability at a negative potential (i.e., resting membrane potential) in the absence or reduced atomic ion conductance [6]. The physiological and pathophysiological roles of Cxs and Panxs could be essential in homeostatic functions of the microcirculation.
We believe Cxs and Panxs might be considered a robust therapeutic target for controlling physiological and pathophysiological functions in microcirculation and NVC. However, mechanistic studies and novel technical approaches are required to establish firmly the physiological role of these large-pore channels, which presents a potentially fruitful line of research to be addressed in the near future.
Author Contributions
Conceptualization, M.A.L.; Methodology: P.C.B., M.P. and M.A.L.; Software: P.S.G.; Investigation, P.C.B., M.P. and M.A.L.; Writing—original draft preparation, P.C.B., M.P. and M.A.L.; writing—review and editing, P.C.B., M.P., W.N.D. and M.A.L.; Visualization: P.S.G., W.N.D. and M.A.L.; Supervision, M.A.L.; funding acquisition: W.N.D. and M.A.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by NIH-R01 HL 146539 to W.N.D. and by the American Heart Association (AHA) Career Development Award Number 932684 to M.A.L.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Evans, W.H.; Martin, P.E. Gap junctions: Structure and function (Review). Mol. Membr. Biol. 2002, 19, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Isakson, B.E.; Ramos, S.I.; Duling, B.R. Ca2+ and inositol 1,4,5-trisphosphate-mediated signaling across the myoendothelial junction. Circ. Res. 2007, 100, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Saez, J.C.; Berthoud, V.M.; Branes, M.C.; Martinez, A.D.; Beyer, E.C. Plasma membrane channels formed by connexins: Their regulation and functions. Physiol. Rev. 2003, 83, 1359–1400. [Google Scholar] [CrossRef] [PubMed]
- Contreras, J.E.; Saez, J.C.; Bukauskas, F.F.; Bennett, M.V. Gating and regulation of connexin 43 (Cx43) hemichannels. Proc. Natl. Acad. Sci. USA 2003, 100, 11388–11393. [Google Scholar] [CrossRef]
- Contreras, J.E.; Sanchez, H.A.; Eugenin, E.A.; Speidel, D.; Theis, M.; Willecke, K.; Bukauskas, F.F.; Bennett, M.V.; Saez, J.C. Metabolic inhibition induces opening of unapposed connexin 43 gap junction hemichannels and reduces gap junctional communication in cortical astrocytes in culture. Proc. Natl. Acad. Sci. USA 2002, 99, 495–500. [Google Scholar] [CrossRef]
- Gaete, P.S.; Lillo, M.A.; Lopez, W.; Liu, Y.; Jiang, W.; Luo, Y.; Harris, A.L.; Contreras, J.E. A novel voltage-clamp/dye uptake assay reveals saturable transport of molecules through CALHM1 and connexin channels. J. Gen. Physiol. 2020, 152, 202–206. [Google Scholar] [CrossRef]
- Harris, A.L.; Contreras, J.E. Motifs in the permeation pathway of connexin channels mediate voltage and Ca(2+) sensing. Front. Physiol. 2014, 5, 113. [Google Scholar] [CrossRef]
- Lopez, W.; Ramachandran, J.; Alsamarah, A.; Luo, Y.; Harris, A.L.; Contreras, J.E. Mechanism of gating by calcium in connexin hemichannels. Proc. Natl. Acad. Sci. USA 2016, 113, E7986–E7995. [Google Scholar] [CrossRef]
- Tong, X.; Lopez, W.; Ramachandran, J.; Ayad, W.A.; Liu, Y.; Lopez-Rodriguez, A.; Harris, A.L.; Contreras, J.E. Glutathione release through connexin hemichannels: Implications for chemical modification of pores permeable to large molecules. J. Gen. Physiol. 2015, 146, 245–254. [Google Scholar] [CrossRef]
- Contreras, J.E.; Saez, J.C.; Bukauskas, F.F.; Bennett, M.V. Functioning of cx43 hemichannels demonstrated by single channel properties. Cell Commun. Adhes. 2003, 10, 245–249. [Google Scholar] [CrossRef]
- Saez, J.C.; Retamal, M.A.; Basilio, D.; Bukauskas, F.F.; Bennett, M.V. Connexin-based gap junction hemichannels: Gating mechanisms. Biochim. Biophys. Acta 2005, 1711, 215–224. [Google Scholar] [CrossRef]
- Figueroa, X.F.; Duling, B.R. Dissection of two Cx37-independent conducted vasodilator mechanisms by deletion of Cx40: Electrotonic versus regenerative conduction. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H2001–H2007. [Google Scholar] [CrossRef]
- Figueroa, X.F.; Duling, B.R. Gap junctions in the control of vascular function. Antioxid. Redox Signal. 2009, 11, 251–266. [Google Scholar] [CrossRef]
- Gaete, P.S.; Lillo, M.A.; Figueroa, X.F. Functional role of connexins and pannexins in the interaction between vascular and nervous system. J. Cell. Physiol. 2014, 229, 1336–1345. [Google Scholar] [CrossRef]
- Lillo, M.A.; Himelman, E.; Shirokova, N.; Xie, L.H.; Fraidenraich, D.; Contreras, J.E. S-nitrosylation of connexin43 hemichannels elicits cardiac stress-induced arrhythmias in Duchenne muscular dystrophy mice. JCI Insight 2019, 4, 130–136. [Google Scholar] [CrossRef]
- Vargas, A.A.; Cisterna, B.A.; Saavedra-Leiva, F.; Urrutia, C.; Cea, L.A.; Vielma, A.H.; Gutierrez-Maldonado, S.E.; Martin, A.J.; Pareja-Barrueto, C.; Escalona, Y.; et al. On Biophysical Properties and Sensitivity to Gap Junction Blockers of Connexin 39 Hemichannels Expressed in HeLa Cells. Front. Physiol. 2017, 8, 38. [Google Scholar] [CrossRef]
- Gonzalez-Nieto, D.; Gomez-Hernandez, J.M.; Larrosa, B.; Gutierrez, C.; Munoz, M.D.; Fasciani, I.; O’Brien, J.; Zappala, A.; Cicirata, F.; Barrio, L.C. Regulation of neuronal connexin-36 channels by pH. Proc. Natl. Acad. Sci. USA 2008, 105, 17169–17174. [Google Scholar] [CrossRef]
- Skeberdis, V.A.; Rimkute, L.; Skeberdyte, A.; Paulauskas, N.; Bukauskas, F.F. pH-dependent modulation of connexin-based gap junctional uncouplers. J. Physiol. 2011, 589 Pt 14, 3495–3506. [Google Scholar] [CrossRef]
- Yamaguchi, D.T.; Ma, D. Mechanism of pH regulation of connexin 43 expression in MC3T3-E1 cells. Biochem. Biophys. Res. Commun. 2003, 304, 736–739. [Google Scholar] [CrossRef]
- Retamal, M.A.; Schalper, K.A.; Shoji, K.F.; Bennett, M.V.; Saez, J.C. Opening of connexin 43 hemichannels is increased by lowering intracellular redox potential. Proc. Natl. Acad. Sci. USA 2007, 104, 8322–8327. [Google Scholar] [CrossRef]
- Bennett, B.C.; Purdy, M.D.; Baker, K.A.; Acharya, C.; McIntire, W.E.; Stevens, R.C.; Zhang, Q.; Harris, A.L.; Abagyan, R.; Yeager, M. An electrostatic mechanism for Ca(2+)-mediated regulation of gap junction channels. Nat. Commun. 2016, 7, 8770. [Google Scholar] [CrossRef]
- Leon-Paravic, C.G.; Figueroa, V.A.; Guzman, D.J.; Valderrama, C.F.; Vallejos, A.A.; Fiori, M.C.; Altenberg, G.A.; Reuss, L.; Retamal, M.A. Carbon monoxide (CO) is a novel inhibitor of connexin hemichannels. J. Biol. Chem. 2014, 289, 36150–36157. [Google Scholar] [CrossRef]
- Hirschhauser, C.; Lissoni, A.; Gorge, P.M.; Lampe, P.D.; Heger, J.; Schluter, K.D.; Leybaert, L.; Schulz, R.; Boengler, K. Connexin 43 phosphorylation by casein kinase 1 is essential for the cardioprotection by ischemic preconditioning. Basic Res. Cardiol. 2021, 116, 21. [Google Scholar] [CrossRef]
- Johnstone, S.R.; Kroncke, B.M.; Straub, A.C.; Best, A.K.; Dunn, C.A.; Mitchell, L.A.; Peskova, Y.; Nakamoto, R.K.; Koval, M.; Lo, C.W.; et al. MAPK phosphorylation of connexin 43 promotes binding of cyclin E and smooth muscle cell proliferation. Circ. Res. 2012, 111, 201–211. [Google Scholar] [CrossRef]
- Slavi, N.; Toychiev, A.H.; Kosmidis, S.; Ackert, J.; Bloomfield, S.A.; Wulff, H.; Viswanathan, S.; Lampe, P.D.; Srinivas, M. Suppression of connexin 43 phosphorylation promotes astrocyte survival and vascular regeneration in proliferative retinopathy. Proc. Natl. Acad. Sci. USA 2018, 115, E5934–E5943. [Google Scholar] [CrossRef]
- Retamal, M.A.; Cortes, C.J.; Reuss, L.; Bennett, M.V.; Saez, J.C. S-nitrosylation and permeation through connexin 43 hemichannels in astrocytes: Induction by oxidant stress and reversal by reducing agents. Proc. Natl. Acad. Sci. USA 2006, 103, 4475–4480. [Google Scholar] [CrossRef]
- Vielma, A.Z.; Boric, M.P.; Gonzalez, D.R. Apocynin Treatment Prevents Cardiac Connexin 43 Hemichannels Hyperactivity by Reducing Nitroso-Redox Stress in Mdx Mice. Int. J. Mol. Sci. 2020, 21, 5415. [Google Scholar] [CrossRef]
- Delvaeye, T.; De Smet, M.A.J.; Verwaerde, S.; Decrock, E.; Czekaj, A.; Vandenbroucke, R.E.; Lemeire, K.; Goncalves, A.; Declercq, W.; Vandenabeele, P.; et al. Blocking connexin43 hemichannels protects mice against tumour necrosis factor-induced inflammatory shock. Sci. Rep. 2019, 9, 16623. [Google Scholar] [CrossRef]
- De Smet, M.A.; Lissoni, A.; Nezlobinsky, T.; Wang, N.; Dries, E.; Perez-Hernandez, M.; Lin, X.; Amoni, M.; Vervliet, T.; Witschas, K.; et al. Cx43 hemichannel microdomain signaling at the intercalated disc enhances cardiac excitability. J. Clin. Investig. 2021, 131, 173–179. [Google Scholar] [CrossRef]
- Kim, J.C.; Perez-Hernandez, M.; Alvarado, F.J.; Maurya, S.R.; Montnach, J.; Yin, Y.; Zhang, M.; Lin, X.; Vasquez, C.; Heguy, A.; et al. Disruption of Ca(2+)i Homeostasis and Connexin 43 Hemichannel Function in the Right Ventricle Precedes Overt Arrhythmogenic Cardiomyopathy in Plakophilin-2-Deficient Mice. Circulation 2019, 140, 1015–1030. [Google Scholar] [CrossRef]
- Yang, D.; Shen, J.; Fan, J.; Chen, Y.; Guo, X. Paracellular permeability changes induced by multi-walled carbon nanotubes in brain endothelial cells and associated roles of hemichannels. Toxicology 2020, 440, 152491. [Google Scholar] [CrossRef] [PubMed]
- Meunier, C.; Wang, N.; Yi, C.; Dallerac, G.; Ezan, P.; Koulakoff, A.; Leybaert, L.; Giaume, C. Contribution of Astroglial Cx43 Hemichannels to the Modulation of Glutamatergic Currents by D-Serine in the Mouse Prefrontal Cortex. J. Neurosci. 2017, 37, 9064–9075. [Google Scholar] [CrossRef] [PubMed]
- Dosch, M.; Zindel, J.; Jebbawi, F.; Melin, N.; Sanchez-Taltavull, D.; Stroka, D.; Candinas, D.; Beldi, G. Connexin-43-dependent ATP release mediates macrophage activation during sepsis. Elife 2019, 8, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Anselmi, F.; Hernandez, V.H.; Crispino, G.; Seydel, A.; Ortolano, S.; Roper, S.D.; Kessaris, N.; Richardson, W.; Rickheit, G.; Filippov, M.A.; et al. ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc. Natl. Acad. Sci. USA 2008, 105, 18770–18775. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Riquelme, M.A.; Guda, T.; Tu, C.; Xu, H.; Gu, S.; Jiang, J.X. Connexin hemichannels with prostaglandin release in anabolic function of bone to mechanical loading. Elife 2022, 11, e74365. [Google Scholar] [CrossRef]
- Flores, J.A.; Haddad, B.G.; Dolan, K.A.; Myers, J.B.; Yoshioka, C.C.; Copperman, J.; Zuckerman, D.M.; Reichow, S.L. Connexin-46/50 in a dynamic lipid environment resolved by CryoEM at 1.9 A. Nat. Commun. 2020, 11, 4331. [Google Scholar] [CrossRef]
- Lee, H.J.; Jeong, H.; Hyun, J.; Ryu, B.; Park, K.; Lim, H.H.; Yoo, J.; Woo, J.S. Cryo-EM structure of human Cx31.3/GJC3 connexin hemichannel. Sci. Adv. 2020, 6, eaba4996. [Google Scholar] [CrossRef]
- Ruan, Z.; Orozco, I.J.; Du, J.; Lu, W. Structures of human pannexin 1 reveal ion pathways and mechanism of gating. Nature 2020, 584, 646–651. [Google Scholar] [CrossRef]
- Garcia, I.E.; Maripillan, J.; Jara, O.; Ceriani, R.; Palacios-Munoz, A.; Ramachandran, J.; Olivero, P.; Perez-Acle, T.; Gonzalez, C.; Saez, J.C.; et al. Keratitis-ichthyosis-deafness syndrome-associated Cx26 mutants produce nonfunctional gap junctions but hyperactive hemichannels when co-expressed with wild type Cx43. J. Investig. Dermatol 2015, 135, 1338–1347. [Google Scholar] [CrossRef]
- Sanchez, H.A.; Verselis, V.K. Aberrant Cx26 hemichannels and keratitis-ichthyosis-deafness syndrome: Insights into syndromic hearing loss. Front. Cell. Neurosci. 2014, 8, 354. [Google Scholar] [CrossRef]
- Sanchez, H.A.; Villone, K.; Srinivas, M.; Verselis, V.K. The D50N mutation and syndromic deafness: Altered Cx26 hemichannel properties caused by effects on the pore and intersubunit interactions. J. Gen. Physiol. 2013, 142, 3–22. [Google Scholar] [CrossRef]
- Xu, J.; Nicholson, B.J. The role of connexins in ear and skin physiology—Functional insights from disease-associated mutations. Biochim. Biophys. Acta 2013, 1828, 167–178. [Google Scholar] [CrossRef]
- Lee, J.R.; Derosa, A.M.; White, T.W. Connexin mutations causing skin disease and deafness increase hemichannel activity and cell death when expressed in Xenopus oocytes. J. Investig. Dermatol. 2009, 129, 870–878. [Google Scholar] [CrossRef]
- Lee, J.R.; White, T.W. Connexin-26 mutations in deafness and skin disease. Expert Rev. Mol. Med. 2009, 11, e35. [Google Scholar] [CrossRef]
- Beyer, E.C.; Ebihara, L.; Berthoud, V.M. Connexin mutants and cataracts. Front. Pharmacol. 2013, 4, 43. [Google Scholar] [CrossRef]
- Vinken, M.; Decrock, E.; De Vuyst, E.; De Bock, M.; Vandenbroucke, R.E.; De Geest, B.G.; Demeester, J.; Sanders, N.N.; Vanhaecke, T.; Leybaert, L.; et al. Connexin32 hemichannels contribute to the apoptotic-to-necrotic transition during Fas-mediated hepatocyte cell death. Cell. Mol. Life Sci. 2010, 67, 907–918. [Google Scholar] [CrossRef]
- Cea, L.A.; Cisterna, B.A.; Puebla, C.; Frank, M.; Figueroa, X.F.; Cardozo, C.; Willecke, K.; Latorre, R.; Saez, J.C. De novo expression of connexin hemichannels in denervated fast skeletal muscles leads to atrophy. Proc. Natl. Acad. Sci. USA 2013, 110, 16229–16234. [Google Scholar] [CrossRef]
- Contreras, J.E.; Sanchez, H.A.; Veliz, L.P.; Bukauskas, F.F.; Bennett, M.V.; Saez, J.C. Role of connexin-based gap junction channels and hemichannels in ischemia-induced cell death in nervous tissue. Brain Res. Rev. 2004, 47, 290–303. [Google Scholar] [CrossRef]
- Li, T.; Niu, J.; Yu, G.; Ezan, P.; Yi, C.; Wang, X.; Koulakoff, A.; Gao, X.; Chen, X.; Saez, J.C.; et al. Connexin 43 deletion in astrocytes promotes CNS remyelination by modulating local inflammation. Glia 2020, 68, 1201–1212. [Google Scholar] [CrossRef]
- Himelman, E.; Lillo, M.A.; Nouet, J.; Gonzalez, J.P.; Zhao, Q.; Xie, L.H.; Li, H.; Liu, T.; Wehrens, X.H.; Lampe, P.D.; et al. Prevention of connexin-43 remodeling protects against Duchenne muscular dystrophy cardiomyopathy. J. Clin. Investig. 2020, 130, 1713–1727. [Google Scholar] [CrossRef]
- Fernandez, G.; Arias-Bravo, G.; Bevilacqua, J.A.; Castillo-Ruiz, M.; Caviedes, P.; Saez, J.C.; Cea, L.A. Myofibers deficient in connexins 43 and 45 expression protect mice from skeletal muscle and systemic dysfunction promoted by a dysferlin mutation. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165800. [Google Scholar] [CrossRef]
- Cisterna, B.A.; Vargas, A.A.; Puebla, C.; Fernandez, P.; Escamilla, R.; Lagos, C.F.; Matus, M.F.; Vilos, C.; Cea, L.A.; Barnafi, E.; et al. Active acetylcholine receptors prevent the atrophy of skeletal muscles and favor reinnervation. Nat. Commun. 2020, 11, 1073. [Google Scholar] [CrossRef]
- Almad, A.A.; Taga, A.; Joseph, J.; Gross, S.K.; Welsh, C.; Patankar, A.; Richard, J.P.; Rust, K.; Pokharel, A.; Plott, C.; et al. Cx43 hemichannels contribute to astrocyte-mediated toxicity in sporadic and familial ALS. Proc. Natl. Acad. Sci. USA 2022, 119, 154–169. [Google Scholar] [CrossRef]
- Qu, R.; Dong, L.; Zhang, J.; Yu, X.; Wang, L.; Zhu, S. Cryo-EM structure of human heptameric Pannexin 1 channel. Cell Res. 2020, 30, 446–448. [Google Scholar] [CrossRef]
- Michalski, K.; Syrjanen, J.L.; Henze, E.; Kumpf, J.; Furukawa, H.; Kawate, T. The Cryo-EM structure of pannexin 1 reveals unique motifs for ion selection and inhibition. Elife 2020, 9, e54670. [Google Scholar] [CrossRef]
- Deng, Z.; He, Z.; Maksaev, G.; Bitter, R.M.; Rau, M.; Fitzpatrick, J.A.J.; Yuan, P. Cryo-EM structures of the ATP release channel pannexin 1. Nat. Struct. Mol. Biol. 2020, 27, 373–381. [Google Scholar] [CrossRef]
- Unger, V.M.; Kumar, N.M.; Gilula, N.B.; Yeager, M. Three-dimensional structure of a recombinant gap junction membrane channel. Science 1999, 283, 1176–1180. [Google Scholar] [CrossRef]
- Myers, J.B.; Haddad, B.G.; O’Neill, S.E.; Chorev, D.S.; Yoshioka, C.C.; Robinson, C.V.; Zuckerman, D.M.; Reichow, S.L. Structure of native lens connexin 46/50 intercellular channels by cryo-EM. Nature 2018, 564, 372–377. [Google Scholar] [CrossRef]
- Maeda, S.; Nakagawa, S.; Suga, M.; Yamashita, E.; Oshima, A.; Fujiyoshi, Y.; Tsukihara, T. Structure of the connexin 26 gap junction channel at 3.5 A resolution. Nature 2009, 458, 597–602. [Google Scholar] [CrossRef]
- Penuela, S.; Gehi, R.; Laird, D.W. The biochemistry and function of pannexin channels. Biochim. Biophys. Acta 2013, 1828, 15–22. [Google Scholar] [CrossRef]
- Penuela, S.; Bhalla, R.; Gong, X.Q.; Cowan, K.N.; Celetti, S.J.; Cowan, B.J.; Bai, D.; Shao, Q.; Laird, D.W. Pannexin 1 and pannexin 3 are glycoproteins that exhibit many distinct characteristics from the connexin family of gap junction proteins. J. Cell Sci. 2007, 120 Pt 21, 3772–3783. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Prado, N.; Soto, P.A.; Lopez, X.; Choi, E.J.; Marquez-Miranda, V.; Rojas, M.; Duarte, Y.; Lee, J.; Gonzalez-Nilo, F.D.; Saez, J.C. Endogenous pannexin1 channels form functional intercellular cell-cell channels with characteristic voltage-dependent properties. Proc. Natl. Acad. Sci. USA 2022, 119, 315–365. [Google Scholar] [CrossRef] [PubMed]
- Beckel, J.M.; Daugherty, S.L.; Tyagi, P.; Wolf-Johnston, A.S.; Birder, L.A.; Mitchell, C.H.; de Groat, W.C. Pannexin 1 channels mediate the release of ATP into the lumen of the rat urinary bladder. J. Physiol. 2015, 593, 1857–1871. [Google Scholar] [CrossRef] [PubMed]
- Narahari, A.K.; Kreutzberger, A.J.; Gaete, P.S.; Chiu, Y.H.; Leonhardt, S.A.; Medina, C.B.; Jin, X.; Oleniacz, P.W.; Kiessling, V.; Barrett, P.Q.; et al. ATP and large signaling metabolites flux through caspase-activated Pannexin 1 channels. Elife 2021, 10, e64787. [Google Scholar] [CrossRef] [PubMed]
- Lohman, A.W.; Leskov, I.L.; Butcher, J.T.; Johnstone, S.R.; Stokes, T.A.; Begandt, D.; DeLalio, L.J.; Best, A.K.; Penuela, S.; Leitinger, N.; et al. Pannexin 1 channels regulate leukocyte emigration through the venous endothelium during acute inflammation. Nat. Commun. 2015, 6, 7965. [Google Scholar] [CrossRef]
- Van Kempen, M.J.; Jongsma, H.J. Distribution of connexin37, connexin40 and connexin43 in the aorta and coronary artery of several mammals. Histochem. Cell Biol. 1999, 112, 479–486. [Google Scholar] [CrossRef]
- Gustafsson, F.; Mikkelsen, H.B.; Arensbak, B.; Thuneberg, L.; Neve, S.; Jensen, L.J.; Holstein-Rathlou, N.H. Expression of connexin 37, 40 and 43 in rat mesenteric arterioles and resistance arteries. Histochem. Cell Biol. 2003, 119, 139–148. [Google Scholar] [CrossRef]
- Figueroa, X.F.; Paul, D.L.; Simon, A.M.; Goodenough, D.A.; Day, K.H.; Damon, D.N.; Duling, B.R. Central role of connexin40 in the propagation of electrically activated vasodilation in mouse cremasteric arterioles in vivo. Circ. Res. 2003, 92, 793–800. [Google Scholar] [CrossRef]
- Gaete, P.S.; Lillo, M.A.; Puebla, M.; Poblete, I.; Figueroa, X.F. CGRP signalling inhibits NO production through pannexin-1 channel activation in endothelial cells. Sci. Rep. 2019, 9, 7932. [Google Scholar] [CrossRef]
- Lillo, M.A.; Gaete, P.S.; Puebla, M.; Burboa, P.C.; Poblete, I.; Figueroa, X.F. Novel Pannexin-1-Coupled Signaling Cascade Involved in the Control of Endothelial Cell Function and NO-Dependent Relaxation. Oxid. Med. Cell. Longev. 2021, 2021, 2678134. [Google Scholar] [CrossRef]
- Lohman, A.W.; Billaud, M.; Straub, A.C.; Johnstone, S.R.; Best, A.K.; Lee, M.; Barr, K.; Penuela, S.; Laird, D.W.; Isakson, B.E. Expression of pannexin isoforms in the systemic murine arterial network. J. Vasc. Res. 2012, 49, 405–416. [Google Scholar] [CrossRef]
- Haefliger, J.A.; Nicod, P.; Meda, P. Contribution of connexins to the function of the vascular wall. Cardiovasc. Res. 2004, 62, 345–356. [Google Scholar] [CrossRef]
- Hill, C.E.; Rummery, N.; Hickey, H.; Sandow, S.L. Heterogeneity in the distribution of vascular gap junctions and connexins: Implications for function. Clin. Exp. Pharmacol. Physiol. 2002, 29, 620–625. [Google Scholar] [CrossRef]
- Okamoto, T.; Akiyama, M.; Takeda, M.; Gabazza, E.C.; Hayashi, T.; Suzuki, K. Connexin32 is expressed in vascular endothelial cells and participates in gap-junction intercellular communication. Biochem. Biophys. Res. Commun. 2009, 382, 264–268. [Google Scholar] [CrossRef]
- Severs, N.J.; Rothery, S.; Dupont, E.; Coppen, S.R.; Yeh, H.I.; Ko, Y.S.; Matsushita, T.; Kaba, R.; Halliday, D. Immunocytochemical analysis of connexin expression in the healthy and diseased cardiovascular system. Microsc. Res. Tech. 2001, 52, 301–322. [Google Scholar] [CrossRef]
- Van Kempen, M.J.; ten Velde, I.; Wessels, A.; Oosthoek, P.W.; Gros, D.; Jongsma, H.J.; Moorman, A.F.; Lamers, W.H. Differential connexin distribution accommodates cardiac function in different species. Microsc. Res. Tech. 1995, 31, 420–436. [Google Scholar] [CrossRef]
- Figueroa, X.F.; Isakson, B.E.; Duling, B.R. Connexins: Gaps in our knowledge of vascular function. Physiology 2004, 19, 277–284. [Google Scholar] [CrossRef]
- Gabriels, J.E.; Paul, D.L. Connexin43 is highly localized to sites of disturbed flow in rat aortic endothelium but connexin37 and connexin40 are more uniformly distributed. Circ. Res. 1998, 83, 636–643. [Google Scholar] [CrossRef]
- Kwak, B.R.; Mulhaupt, F.; Veillard, N.; Gros, D.B.; Mach, F. Altered pattern of vascular connexin expression in atherosclerotic plaques. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 225–230. [Google Scholar] [CrossRef]
- Simon, A.M.; McWhorter, A.R. Vascular abnormalities in mice lacking the endothelial gap junction proteins connexin37 and connexin40. Dev. Biol. 2002, 251, 206–220. [Google Scholar] [CrossRef]
- Kruger, O.; Plum, A.; Kim, J.S.; Winterhager, E.; Maxeiner, S.; Hallas, G.; Kirchhoff, S.; Traub, O.; Lamers, W.H.; Willecke, K. Defective vascular development in connexin 45-deficient mice. Development 2000, 127, 4179–4193. [Google Scholar] [CrossRef]
- Li, X.; Simard, J.M. Connexin45 gap junction channels in rat cerebral vascular smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H1890–H1898. [Google Scholar] [CrossRef]
- Li, X.; Simard, J.M. Increase in Cx45 gap junction channels in cerebral smooth muscle cells from SHR. Hypertension 2002, 40, 940–946. [Google Scholar] [CrossRef][Green Version]
- Moller, S.; Jacobsen, J.C.B.; Holstein-Rathlou, N.H.; Sorensen, C.M. Lack of Connexins 40 and 45 Reduces Local and Conducted Vasoconstrictor Responses in the Murine Afferent Arterioles. Front. Physiol. 2020, 11, 961. [Google Scholar] [CrossRef]
- Figueroa, X.F.; Lillo, M.A.; Gaete, P.S.; Riquelme, M.A.; Saez, J.C. Diffusion of nitric oxide across cell membranes of the vascular wall requires specific connexin-based channels. Neuropharmacology 2013, 75, 471–478. [Google Scholar] [CrossRef]
- Gaete, P.S.; Lillo, M.A.; Ardiles, N.M.; Perez, F.R.; Figueroa, X.F. Ca2+-activated K+ channels of small and intermediate conductance control eNOS activation through NAD(P)H oxidase. Free Radic. Biol. Med. 2012, 52, 860–870. [Google Scholar] [CrossRef]
- Lillo, M.A.; Gaete, P.S.; Puebla, M.; Ardiles, N.M.; Poblete, I.; Becerra, A.; Simon, F.; Figueroa, X.F. Critical contribution of Na(+)-Ca(2+) exchanger to the Ca(2+)-mediated vasodilation activated in endothelial cells of resistance arteries. FASEB J. 2018, 32, 2137–2147. [Google Scholar] [CrossRef]
- De Wit, C.; Roos, F.; Bolz, S.S.; Pohl, U. Lack of vascular connexin 40 is associated with hypertension and irregular arteriolar vasomotion. Physiol. Genom. 2003, 13, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, I.S.; Segal, S.S. Resolution of smooth muscle and endothelial pathways for conduction along hamster cheek pouch arterioles. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H604–H612. [Google Scholar] [CrossRef] [PubMed]
- Budel, S.; Bartlett, I.S.; Segal, S.S. Homocellular conduction along endothelium and smooth muscle of arterioles in hamster cheek pouch: Unmasking an NO wave. Circ. Res. 2003, 93, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Emerson, G.G.; Segal, S.S. Endothelial cell pathway for conduction of hyperpolarization and vasodilation along hamster feed artery. Circ. Res. 2000, 86, 94–100. [Google Scholar] [CrossRef]
- Segal, S.S.; Jacobs, T.L. Role for endothelial cell conduction in ascending vasodilatation and exercise hyperaemia in hamster skeletal muscle. J. Physiol. 2001, 536 Pt 3, 937–946. [Google Scholar] [CrossRef]
- Welsh, D.G.; Segal, S.S. Endothelial and smooth muscle cell conduction in arterioles controlling blood flow. Am. J. Physiol. 1998, 274, H178–H186. [Google Scholar] [CrossRef]
- Liao, Y.; Day, K.; Damon, D.; Duling, B.J.P. Endothelial cell-specific knockout of connexin 43 causes hypotension and bradycardia in mice. Proc. Natl. Acad. Sci. USA 2001, 98, 9989–9994. [Google Scholar] [CrossRef]
- Dora, K.; Doyle, M.; Duling, B.J.P. Elevation of intracellular calcium in smooth muscle causes endothelial cell generation of NO in arterioles. Proc. Natl. Acad. Sci. USA 1997, 94, 6529–6534. [Google Scholar] [CrossRef]
- Lourenco, C.F.; Laranjinha, J. Nitric Oxide Pathways in Neurovascular Coupling Under Normal and Stress Conditions in the Brain: Strategies to Rescue Aberrant Coupling and Improve Cerebral Blood Flow. Front. Physiol. 2021, 12, 729201. [Google Scholar] [CrossRef]
- Saliez, J.; Bouzin, C.; Rath, G.; Ghisdal, P.; Desjardins, F.; Rezzani, R.; Rodella, L.F.; Vriens, J.; Nilius, B.; Feron, O.; et al. Role of caveolar compartmentation in endothelium-derived hyperpolarizing factor-mediated relaxation: Ca2+ signals and gap junction function are regulated by caveolin in endothelial cells. Circulation 2008, 117, 1065–1074. [Google Scholar] [CrossRef]
- Murata, T.; Lin, M.I.; Huang, Y.; Yu, J.; Bauer, P.M.; Giordano, F.J.; Sessa, W.C. Reexpression of caveolin-1 in endothelium rescues the vascular, cardiac, and pulmonary defects in global caveolin-1 knockout mice. J. Exp. Med. 2007, 204, 2373–2382. [Google Scholar] [CrossRef]
- Orellana, J.A.; Saez, P.J.; Cortes-Campos, C.; Elizondo, R.J.; Shoji, K.F.; Contreras-Duarte, S.; Figueroa, V.; Velarde, V.; Jiang, J.X.; Nualart, F.; et al. Glucose increases intracellular free Ca(2+) in tanycytes via ATP released through connexin 43 hemichannels. Glia 2012, 60, 53–68. [Google Scholar] [CrossRef]
- Quan, Y.; Du, Y.; Wu, C.; Gu, S.; Jiang, J.X. Connexin hemichannels regulate redox potential via metabolite exchange and protect lens against cellular oxidative damage. Redox Biol. 2021, 46, 102102. [Google Scholar] [CrossRef]
- Moore, A.R.; Zhou, W.L.; Sirois, C.L.; Belinsky, G.S.; Zecevic, N.; Antic, S.D. Connexin hemichannels contribute to spontaneous electrical activity in the human fetal cortex. Proc. Natl. Acad. Sci. USA 2014, 111, E3919–E3928. [Google Scholar] [CrossRef]
- Feletou, M.; Vanhoutte, P.M. Endothelium-derived hyperpolarizing factor. Clin. Exp. Pharmacol. Physiol. 1996, 23, 1082–1090. [Google Scholar] [CrossRef]
- Morin, E.E.; Salbato, S.; Walker, B.R.; Naik, J.S. Endothelial cell membrane cholesterol content regulates the contribution of TRPV4 channels in ACh-induced vasodilation in rat gracilis arteries. Microcirculation 2022, e12774. [Google Scholar] [CrossRef]
- Vanhoutte, P.M. Endothelium-dependent hyperpolariz.zations: The history. Pharmacol. Res. 2004, 49, 503–508. [Google Scholar] [CrossRef]
- Edwards, G.; Dora, K.A.; Gardener, M.J.; Garland, C.J.; Weston, A.H. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature 1998, 396, 269–272. [Google Scholar] [CrossRef]
- Archer, S.L.; Gragasin, F.S.; Wu, X.; Wang, S.; McMurtry, S.; Kim, D.H.; Platonov, M.; Koshal, A.; Hashimoto, K.; Campbell, W.B.; et al. Endothelium-derived hyperpolarizing factor in human internal mammary artery is 11,12-epoxyeicosatrienoic acid and causes relaxation by activating smooth muscle BK(Ca) channels. Circulation 2003, 107, 769–776. [Google Scholar] [CrossRef]
- Fleming, I. Cytochrome P450 epoxygenases as EDHF synthase(s). Pharmacol. Res. 2004, 49, 525–533. [Google Scholar] [CrossRef]
- Shimokawa, H.; Morikawa, K. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in animals and humans. J. Mol. Cell. Cardiol. 2005, 39, 725–732. [Google Scholar] [CrossRef]
- Ahluwalia, A.; Hobbs, A.J. Endothelium-derived C-type natriuretic peptide: More than just a hyperpolarizing factor. Trends Pharmacol. Sci. 2005, 26, 162–167. [Google Scholar] [CrossRef]
- Chauhan, S.D.; Nilsson, H.; Ahluwalia, A.; Hobbs, A.J. Release of C-type natriuretic peptide accounts for the biological activity of endothelium-derived hyperpolarizing factor. Proc. Natl. Acad. Sci. USA 2003, 100, 1426–1431. [Google Scholar] [CrossRef]
- Busse, R.; Edwards, G.; Feletou, M.; Fleming, I.; Vanhoutte, P.M.; Weston, A.H. EDHF: Bringing the concepts together. Trends Pharmacol. Sci. 2002, 23, 374–380. [Google Scholar] [CrossRef]
- Doughty, J.M.; Plane, F.; Langton, P.D. Charybdotoxin and apamin block EDHF in rat mesenteric artery if selectively applied to the endothelium. Am. J. Physiol. 1999, 276 Pt 2, H1107–H1112. [Google Scholar] [CrossRef] [PubMed]
- Eichler, I.; Wibawa, J.; Grgic, I.; Knorr, A.; Brakemeier, S.; Pries, A.R.; Hoyer, J.; Kohler, R. Selective blockade of endothelial Ca2+-activated small- and intermediate-conductance K+-channels suppresses EDHF-mediated vasodilation. Br. J. Pharmacol. 2003, 138, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Félétou, M.; Vanhoutte, P.M. Endothelium-dependent hyperpolarization: No longer an f-word! J. Cardiovasc. Pharmacol. 2013, 61, 91–92. [Google Scholar] [CrossRef] [PubMed]
- Chaytor, A.T.; Bakker, L.M.; Edwards, D.H.; Griffith, T.M. Connexin-mimetic peptides dissociate electrotonic EDHF-type signalling via myoendothelial and smooth muscle gap junctions in the rabbit iliac artery. Br. J. Pharmacol. 2005, 144, 108–114. [Google Scholar] [CrossRef] [PubMed]
- De Vriese, A.S.; Van de Voorde, J.; Lameire, N.H. Effects of connexin-mimetic peptides on nitric oxide synthase- and cyclooxygenase-independent renal vasodilation. Kidney Int. 2002, 61, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Karagiannis, J.; Rand, M.; Li, C.G. Role of gap junctions in endothelium-derived hyperpolarizing factor-mediated vasodilatation in rat renal artery. Acta Pharmacol. Sin. 2004, 25, 1031–1037. [Google Scholar]
- Begandt, D.; Good, M.E.; Keller, A.S.; DeLalio, L.J.; Rowley, C.; Isakson, B.E.; Figueroa, X.F. Pannexin channel and connexin hemichannel expression in vascular function and inflammation. BMC Cell Biol 2017, 18 (Suppl. 1), 2. [Google Scholar] [CrossRef]
- Billaud, M.; Sandilos, J.K.; Isakson, B.E. Pannexin 1 in the regulation of vascular tone. Trends Cardiovasc. Med. 2012, 22, 68–72. [Google Scholar] [CrossRef]
- Billaud, M.; Lohman, A.W.; Straub, A.C.; Looft-Wilson, R.; Johnstone, S.R.; Araj, C.A.; Best, A.K.; Chekeni, F.B.; Ravichandran, K.S.; Penuela, S.; et al. Pannexin1 regulates alpha1-adrenergic receptor- mediated vasoconstriction. Circ. Res. 2011, 109, 80–85. [Google Scholar] [CrossRef]
- Isakson, B.E.; Thompson, R.J. Pannexin-1 as a potentiator of ligand-gated receptor signaling. Channels 2014, 8, 118–123. [Google Scholar] [CrossRef]
- Good, M.E.; Chiu, Y.H.; Poon, I.K.H.; Medina, C.B.; Butcher, J.T.; Mendu, S.K.; DeLalio, L.J.; Lohman, A.W.; Leitinger, N.; Barrett, E.; et al. Pannexin 1 Channels as an Unexpected New Target of the Anti-Hypertensive Drug Spironolactone. Circ. Res. 2018, 122, 606–615. [Google Scholar] [CrossRef]
- Angus, J.A.; Wright, C.E. Novel alpha1-adrenoceptor antagonism by the fluroquinolone antibiotic trovafloxacin. Eur. J. Pharmacol. 2016, 791, 179–184. [Google Scholar] [CrossRef]
- Lohman, A.W.; Weaver, J.L.; Billaud, M.; Sandilos, J.K.; Griffiths, R.; Straub, A.C.; Penuela, S.; Leitinger, N.; Laird, D.W.; Bayliss, D.A.; et al. S-nitrosylation inhibits pannexin 1 channel function. J. Biol. Chem. 2012, 287, 39602–39612. [Google Scholar] [CrossRef]
- Gaynullina, D.; Shestopalov, V.I.; Panchin, Y.; Tarasova, O.S. Pannexin 1 facilitates arterial relaxation via an endothelium-derived hyperpolarization mechanism. FEBS Lett. 2015, 589, 1164–1170. [Google Scholar] [CrossRef]
- Sanchez, F.A.; Rana, R.; Kim, D.D.; Iwahashi, T.; Zheng, R.; Lal, B.K.; Gordon, D.M.; Meininger, C.J.; Durán, W.N. Internalization of eNOS and NO delivery to subcellular targets determine agonist-induced hyperpermeability. Proc. Natl. Acad. Sci. USA 2009, 106, 6849–6853. [Google Scholar] [CrossRef]
- Claesson-Welsh, L. Vascular permeability—The essentials. Ups. J. Med. Sci. 2015, 120, 135–143. [Google Scholar] [CrossRef]
- Shen, Q.; Rigor, R.R.; Pivetti, C.D.; Wu, M.H.; Yuan, S.Y. Myosin light chain kinase in microvascular endothelial barrier function. Cardiovasc. Res. 2010, 87, 272–280. [Google Scholar] [CrossRef]
- Tietz, S.; Engelhardt, B. Brain barriers: Crosstalk between complex tight junctions and adherens junctions. J. Cell Biol. 2015, 209, 493–506. [Google Scholar] [CrossRef]
- Lai, Y.; Huang, Y. Mechanisms of Mechanical Force Induced Pulmonary Vascular Endothelial Hyperpermeability. Front. Physiol. 2021, 12, 714064. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Narumi, R.; Akiyama, R.; Vitiello, E.; Shirai, T.; Tanimura, N.; Kuromiya, K.; Ishikawa, S.; Kajita, M.; Tada, M.; et al. Calcium Wave Promotes Cell Extrusion. Curr. Biol. 2020, 30, 670–681e6. [Google Scholar] [CrossRef]
- Matsui, T. Calcium wave propagation during cell extrusion. Curr. Opin. Cell Biol. 2022, 76, 102083. [Google Scholar] [CrossRef]
- Nagy, J.A.; Benjamin, L.; Zeng, H.; Dvorak, A.M.; Dvorak, H.F. Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis 2008, 11, 109–119. [Google Scholar] [CrossRef]
- Joffre, J.; Hellman, J.; Ince, C.; Ait-Oufella, H. Endothelial Responses in Sepsis. Am. J. Respir. Crit. Care Med. 2020, 202, 361–370. [Google Scholar] [CrossRef]
- Claesson-Welsh, L.; Dejana, E.; McDonald, D.M. Permeability of the Endothelial Barrier: Identifying and Reconciling Controversies. Trends Mol. Med. 2021, 27, 314–331. [Google Scholar] [CrossRef]
- Colunga Biancatelli, R.M.L.; Solopov, P.A.; Sharlow, E.R.; Lazo, J.S.; Marik, P.E.; Catravas, J.D. The SARS-CoV-2 spike protein subunit S1 induces COVID-19-like acute lung injury in Kappa18-hACE2 transgenic mice and barrier dysfunction in human endothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 321, L477–L484. [Google Scholar] [CrossRef]
- Inai, T.; Shibata, Y. Heterogeneous expression of endothelial connexin (Cx) 37, Cx40, and Cx43 in rat large veins. Anat. Sci. Int. 2009, 84, 237–245. [Google Scholar] [CrossRef]
- Chang, C.J.; Wu, L.S.; Hsu, L.A.; Chang, G.J.; Chen, C.F.; Yeh, H.I.; Ko, Y.S. Differential endothelial gap junction expression in venous vessels exposed to different hemodynamics. J. Histochem. Cytochem. 2010, 58, 1083–1092. [Google Scholar] [CrossRef][Green Version]
- Munger, S.J.; Kanady, J.D.; Simon, A.M. Absence of venous valves in mice lacking Connexin37. Dev. Biol. 2013, 373, 338–348. [Google Scholar] [CrossRef]
- Munger, S.J.; Geng, X.; Srinivasan, R.S.; Witte, M.H.; Paul, D.L.; Simon, A.M. Segregated Foxc2, NFATc1 and Connexin expression at normal developing venous valves, and Connexin-specific differences in the valve phenotypes of Cx37, Cx43, and Cx47 knockout mice. Dev. Biol. 2016, 412, 173–190. [Google Scholar] [CrossRef]
- Zhou, H.S.; Li, M.; Sui, B.D.; Wei, L.; Hou, R.; Chen, W.S.; Li, Q.; Bi, S.H.; Zhang, J.Z.; Yi, D.H. Lipopolysaccharide impairs permeability of pulmonary microvascular endothelial cells via Connexin40. Microvasc. Res. 2018, 115, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Deglise, S.; Martin, D.; Probst, H.; Saucy, F.; Hayoz, D.; Waeber, G.; Nicod, P.; Ris, H.B.; Corpataux, J.M.; Haefliger, J.A. Increased connexin43 expression in human saphenous veins in culture is associated with intimal hyperplasia. J. Vasc. Surg. 2005, 41, 1043–1052. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bol, M.; Van Geyt, C.; Baert, S.; Decrock, E.; Wang, N.; De Bock, M.; Gadicherla, A.K.; Randon, C.; Evans, W.H.; Beele, H.; et al. Inhibiting connexin channels protects against cryopreservation-induced cell death in human blood vessels. Eur. J. Vasc. Endovasc. Surg. 2013, 45, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Longchamp, A.; Allagnat, F.; Alonso, F.; Kuppler, C.; Dubuis, C.; Ozaki, C.K.; Mitchell, J.R.; Berceli, S.; Corpataux, J.M.; Deglise, S.; et al. Connexin43 Inhibition Prevents Human Vein Grafts Intimal Hyperplasia. PLoS ONE 2015, 10, e0138847. [Google Scholar] [CrossRef]
- Lyons, O.; Saha, P.; Seet, C.; Kuchta, A.; Arnold, A.; Grover, S.; Rashbrook, V.; Sabine, A.; Vizcay-Barrena, G.; Patel, A.; et al. Human venous valve disease caused by mutations in FOXC2 and GJC2. J. Exp. Med. 2017, 214, 2437–2452. [Google Scholar] [CrossRef]
- Chadjichristos, C.E.; Scheckenbach, K.E.; van Veen, T.A.; Richani Sarieddine, M.Z.; de Wit, C.; Yang, Z.; Roth, I.; Bacchetta, M.; Viswambharan, H.; Foglia, B.; et al. Endothelial-specific deletion of connexin40 promotes atherosclerosis by increasing CD73-dependent leukocyte adhesion. Circulation 2010, 121, 123–131. [Google Scholar] [CrossRef]
- De Bock, M.; Culot, M.; Wang, N.; Bol, M.; Decrock, E.; De Vuyst, E.; da Costa, A.; Dauwe, I.; Vinken, M.; Simon, A.M.; et al. Connexin channels provide a target to manipulate brain endothelial calcium dynamics and blood-brain barrier permeability. J. Cereb. Blood Flow Metab. 2011, 31, 1942–1957. [Google Scholar] [CrossRef]
- Shiojiri, N.; Niwa, T.; Sugiyama, Y.; Koike, T. Preferential expression of connexin37 and connexin40 in the endothelium of the portal veins during mouse liver development. Cell Tissue Res. 2006, 324, 547. [Google Scholar] [CrossRef]
- Nagasawa, K.; Chiba, H.; Fujita, H.; Kojima, T.; Saito, T.; Endo, T.; Sawada, N. Possible involvement of gap junctions in the barrier function of tight junctions of brain and lung endothelial cells. J. Cell. Physiol. 2006, 208, 123–132. [Google Scholar] [CrossRef]
- Hwan Seul, K.; Beyer, E.C. Heterogeneous localization of connexin40 in the renal vasculature. Microvasc. Res. 2000, 59, 140–148. [Google Scholar] [CrossRef]
- Yang, X.; Chu, H.; Tang, Y.; Dong, Q. The role of connexin43 in hemorrhagic transformation after thrombolysis in vivo and in vitro. Neuroscience 2016, 329, 54–65. [Google Scholar] [CrossRef]
- Kandasamy, K.; Escue, R.; Manna, J.; Adebiyi, A.; Parthasarathi, K. Changes in endothelial connexin 43 expression inversely correlate with microvessel permeability and VE-cadherin expression in endotoxin-challenged lungs. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 309, L584–L592. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, G.M.; Zhu, Y.; Peng, X.Y.; Li, T.; Liu, L.M. Role of connexin 43 in vascular hyperpermeability and relationship to Rock1-MLC20 pathway in septic rats. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 309, L1323–L1332. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, G.; Zhu, Y.; Peng, X.; Li, T.; Liu, L. Relationship of Cx43 regulation of vascular permeability to osteopontin-tight junction protein pathway after sepsis in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R1–R11. [Google Scholar] [CrossRef]
- Tien, T.; Barrette, K.F.; Chronopoulos, A.; Roy, S. Effects of high glucose-induced Cx43 downregulation on occludin and ZO-1 expression and tight junction barrier function in retinal endothelial cells. Invest. Ophthalmol. Vis. Sci. 2013, 54, 6518–6525. [Google Scholar] [CrossRef]
- Kim, D.; Mouritzen, U.; Larsen, B.D.; Roy, S. Inhibition of Cx43 gap junction uncoupling prevents high glucose-induced apoptosis and reduces excess cell monolayer permeability in retinal vascular endothelial cells. Exp. Eye Res. 2018, 173, 85–90. [Google Scholar] [CrossRef]
- Ivanova, E.; Kovacs-Oller, T.; Sagdullaev, B.T. Domain-specific distribution of gap junctions defines cellular coupling to establish a vascular relay in the retina. J. Comp. Neurol. 2019, 527, 2675–2693. [Google Scholar] [CrossRef]
- Liu, X.; Sun, W.; Zhao, Y.; Chen, B.; Wu, W.; Bao, L.; Qi, R. Ginkgolide B Inhibits JAM-A, Cx43, and VE-Cadherin Expression and Reduces Monocyte Transmigration in Oxidized LDL-Stimulated Human Umbilical Vein Endothelial Cells. Oxid. Med. Cell. Longev. 2015, 2015, 907926. [Google Scholar] [CrossRef]
- Kaneko, Y.; Tachikawa, M.; Akaogi, R.; Fujimoto, K.; Ishibashi, M.; Uchida, Y.; Couraud, P.O.; Ohtsuki, S.; Hosoya, K.; Terasaki, T. Contribution of pannexin 1 and connexin 43 hemichannels to extracellular calcium-dependent transport dynamics in human blood-brain barrier endothelial cells. J. Pharmacol. Exp. Ther. 2015, 353, 192–200. [Google Scholar] [CrossRef]
- Kim, Y.; Griffin, J.M.; Harris, P.W.; Chan, S.H.; Nicholson, L.F.; Brimble, M.A.; O’Carroll, S.J.; Green, C.R. Characterizing the mode of action of extracellular Connexin43 channel blocking mimetic peptides in an in vitro ischemia injury model. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 68–78. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, W.; Hu, X. Different concentrations of lipopolysaccharide regulate barrier function through the PI3K/Akt signalling pathway in human pulmonary microvascular endothelial cells. Sci. Rep. 2018, 8, 9963. [Google Scholar] [CrossRef]
- Ange, M.; Castanares-Zapatero, D.; De Poortere, J.; Dufeys, C.; Courtoy, G.E.; Bouzin, C.; Quarck, R.; Bertrand, L.; Beauloye, C.; Horman, S. alpha1AMP-Activated Protein Kinase Protects against Lipopolysaccharide-Induced Endothelial Barrier Disruption via Junctional Reinforcement and Activation of the p38 MAPK/HSP27 Pathway. Int. J. Mol. Sci. 2020, 21, 5581. [Google Scholar] [CrossRef]
- Saez, J.C.; Contreras-Duarte, S.; Gomez, G.I.; Labra, V.C.; Santibanez, C.A.; Gajardo-Gomez, R.; Avendano, B.C.; Diaz, E.F.; Montero, T.D.; Velarde, V.; et al. Connexin 43 Hemichannel Activity Promoted by Pro-Inflammatory Cytokines and High Glucose Alters Endothelial Cell Function. Front. Immunol. 2018, 9, 1899. [Google Scholar] [CrossRef]
- Tachikawa, M.; Murakami, K.; Akaogi, R.; Akanuma, S.I.; Terasaki, T.; Hosoya, K.I. Polarized hemichannel opening of pannexin 1/connexin 43 contributes to dysregulation of transport function in blood-brain barrier endothelial cells. Neurochem. Int. 2020, 132, 104600. [Google Scholar] [CrossRef]
- Maier-Begandt, D.; Comstra, H.S.; Molina, S.A.; Kruger, N.; Ruddiman, C.A.; Chen, Y.L.; Chen, X.; Biwer, L.A.; Johnstone, S.R.; Lohman, A.W.; et al. A venous-specific purinergic signaling cascade initiated by Pannexin 1 regulates TNFalpha-induced increases in endothelial permeability. Sci. Signal. 2021, 14, eaba2940. [Google Scholar] [CrossRef]
- Good, M.E.; Eucker, S.A.; Li, J.; Bacon, H.M.; Lang, S.M.; Butcher, J.T.; Johnson, T.J.; Gaykema, R.P.; Patel, M.K.; Zuo, Z.; et al. Endothelial cell Pannexin1 modulates severity of ischemic stroke by regulating cerebral inflammation and myogenic tone. JCI Insight 2018, 3, e96272. [Google Scholar] [CrossRef]
- Sharma, A.K.; Charles, E.J.; Zhao, Y.; Narahari, A.K.; Baderdinni, P.K.; Good, M.E.; Lorenz, U.M.; Kron, I.L.; Bayliss, D.A.; Ravichandran, K.S.; et al. Pannexin-1 channels on endothelial cells mediate vascular inflammation during lung ischemia-reperfusion injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 315, L301–L312. [Google Scholar] [CrossRef]
- Godecke, S.; Roderigo, C.; Rose, C.R.; Rauch, B.H.; Godecke, A.; Schrader, J. Thrombin-induced ATP release from human umbilical vein endothelial cells. Am. J. Physiol. Cell Physiol. 2012, 302, C915–C923. [Google Scholar] [CrossRef]
- Yang, Y.; Delalio, L.J.; Best, A.K.; Macal, E.; Milstein, J.; Donnelly, I.; Miller, A.M.; McBride, M.; Shu, X.; Koval, M.; et al. Endothelial Pannexin 1 Channels Control Inflammation by Regulating Intracellular Calcium. J. Immunol. 2020, 204, 2995–3007. [Google Scholar] [CrossRef]
- Soon, A.S.; Chua, J.W.; Becker, D.L. Connexins in endothelial barrier function—Novel therapeutic targets countering vascular hyperpermeability. Thromb. Haemost. 2016, 116, 852–867. [Google Scholar] [CrossRef]
- Stamatovic, S.M.; Johnson, A.M.; Keep, R.F.; Andjelkovic, A.V. Junctional proteins of the blood-brain barrier: New insights into function and dysfunction. Tissue Barriers 2016, 4, e1154641. [Google Scholar] [CrossRef] [PubMed]
- Hautefort, A.; Pfenniger, A.; Kwak, B.R. Endothelial connexins in vascular function. Vasc. Biol. 2019, 1, H117–H124. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Xu, C.; Wang, S.; Zhang, Y.; Li, W. The Role of Connexin Hemichannels in Inflammatory Diseases. Biology 2022, 11, 237. [Google Scholar] [CrossRef] [PubMed]
- Oshiro, H.; Kobayashi, I.; Kim, D.; Takenaka, H.; Hobson, R.W., 2nd; Durán, W.N. L-type calcium channel blockers modulate the microvascular hyperpermeability induced by platelet-activating factor in vivo. J. Vasc. Surg. 1995, 22, 732–739, discussion 739–741. [Google Scholar] [CrossRef]
- De Bock, M.; Wang, N.; Decrock, E.; Bol, M.; Gadicherla, A.K.; Culot, M.; Cecchelli, R.; Bultynck, G.; Leybaert, L. Endothelial calcium dynamics, connexin channels and blood-brain barrier function. Prog. Neurobiol. 2013, 108, 1–20. [Google Scholar] [CrossRef]
- Vinet, R.; Cortes, M.P.; Alvarez, R.; Delpiano, M.A. Bradykinin and histamine-induced cytosolic calcium increase in capillary endothelial cells of bovine adrenal medulla. Cell Biol. Int. 2014, 38, 1023–1031. [Google Scholar] [CrossRef]
- Brailoiu, E.; Shipsky, M.M.; Yan, G.; Abood, M.E.; Brailoiu, G.C. Mechanisms of modulation of brain microvascular endothelial cells function by thrombin. Brain Res. 2017, 1657, 167–175. [Google Scholar] [CrossRef]
- Brailoiu, E.; Barlow, C.L.; Ramirez, S.H.; Abood, M.E.; Brailoiu, G.C. Effects of Platelet-Activating Factor on Brain Microvascular Endothelial Cells. Neuroscience 2018, 377, 105–113. [Google Scholar] [CrossRef]
- Obata, Y.; Takeuchi, K.; Wei, J.; Hakamata, A.; Odagiri, K.; Nakajima, Y.; Watanabe, H. Interactions between bradykinin and plasmin in the endothelial Ca(2+) response. Mol. Cell. Biochem. 2018, 445, 179–186. [Google Scholar] [CrossRef]
- Dalal, P.J.; Muller, W.A.; Sullivan, D.P. Endothelial Cell Calcium Signaling during Barrier Function and Inflammation. Am. J. Pathol. 2020, 190, 535–542. [Google Scholar] [CrossRef]
- Pocock, T.M.; Williams, B.; Curry, F.E.; Bates, D.O. VEGF and ATP act by different mechanisms to increase microvascular permeability and endothelial [Ca(2+)](i). Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H1625–H1634. [Google Scholar] [CrossRef]
- Aslam, M.; Gunduz, D.; Troidl, C.; Heger, J.; Hamm, C.W.; Schulz, R. Purinergic Regulation of Endothelial Barrier Function. Int. J. Mol. Sci. 2021, 22, 1207. [Google Scholar] [CrossRef]
- Parthasarathi, K.; Ichimura, H.; Monma, E.; Lindert, J.; Quadri, S.; Issekutz, A.; Bhattacharya, J. Connexin 43 mediates spread of Ca2+-dependent proinflammatory responses in lung capillaries. J. Clin. Investig. 2006, 116, 2193–2200. [Google Scholar] [CrossRef]
- Parthasarathi, K. Endothelial connexin43 mediates acid-induced increases in pulmonary microvascular permeability. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 303, L33–L42. [Google Scholar] [CrossRef]
- O’Donnell, J.J., 3rd; Birukova, A.A.; Beyer, E.C.; Birukov, K.G. Gap junction protein connexin43 exacerbates lung vascular permeability. PLoS ONE 2014, 9, e100931. [Google Scholar] [CrossRef]
- Tsang, H.; Leiper, J.; Hou Lao, K.; Dowsett, L.; Delahaye, M.W.; Barnes, G.; Wharton, J.; Howard, L.; Iannone, L.; Lang, N.N.; et al. Role of asymmetric methylarginine and connexin 43 in the regulation of pulmonary endothelial function. Pulm. Circ. 2013, 3, 675–691. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, J.; Wang, W.; Liu, J.; Zhu, H.; Chen, W.; Chen, T.; Yu, S.; Wang, H.; Sun, G.; et al. Connexin40 modulates pulmonary permeability through gap junction channel in acute lung injury after thoracic gunshot wounds. J. Trauma 2010, 68, 802–809. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, W.; Sun, J.; Li, Q.; Liu, J.; Zhu, H.; Chen, T.; Wang, H.; Yu, S.; Sun, G.; et al. Gap junction channel modulates pulmonary vascular permeability through calcium in acute lung injury: An experimental study. Respiration 2010, 80, 236–245. [Google Scholar] [CrossRef]
- Yin, J.; Lv, L.; Zhai, P.; Long, T.; Zhou, Q.; Pan, H.; Botwe, G.; Wang, L.; Wang, Q.; Tan, L.; et al. Connexin 40 regulates lung endothelial permeability in acute lung injury via the ROCK1-MYPT1- MLC20 pathway. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 316, L35–L44. [Google Scholar] [CrossRef]
- Wang, S.; Sun, Y.; Bai, Y.; Zhou, N.; Chen, N.; Miller, E.J.; Zhang, Y.; Li, W. Contribution of Connexin Hemichannels to the Pathogenesis of Acute Lung Injury. Mediat. Inflamm. 2020, 2020, 8094347. [Google Scholar] [CrossRef]
- Johnson, A.M.; Roach, J.P.; Hu, A.; Stamatovic, S.M.; Zochowski, M.R.; Keep, R.F.; Andjelkovic, A.V. Connexin 43 gap junctions contribute to brain endothelial barrier hyperpermeability in familial cerebral cavernous malformations type III by modulating tight junction structure. FASEB J. 2018, 32, 2615–2629. [Google Scholar] [CrossRef]
- Cronin, M.; Anderson, P.N.; Cook, J.E.; Green, C.R.; Becker, D.L. Blocking connexin43 expression reduces inflammation and improves functional recovery after spinal cord injury. Mol. Cell. Neurosci. 2008, 39, 152–160. [Google Scholar] [CrossRef]
- Yao, F.; Luo, Y.; Chen, Y.; Li, Y.; Hu, X.; You, X.; Li, Z.; Yu, S.; Tian, D.; Zheng, M.; et al. Myelin Debris Impairs Tight Junctions and Promotes the Migration of Microvascular Endothelial Cells in the Injured Spinal Cord. Cell. Mol. Neurobiol. 2022. [Google Scholar] [CrossRef]
- Raghavan, S.; Kenchappa, D.B.; Leo, M.D. SARS-CoV-2 Spike Protein Induces Degradation of Junctional Proteins That Maintain Endothelial Barrier Integrity. Front. Cardiovasc. Med. 2021, 8, 687783. [Google Scholar] [CrossRef]
- Cibelli, A.; Stout, R.; Timmermann, A.; de Menezes, L.; Guo, P.; Maass, K.; Seifert, G.; Steinhauser, C.; Spray, D.C.; Scemes, E. Cx43 carboxyl terminal domain determines AQP4 and Cx30 endfoot organization and blood brain barrier permeability. Sci. Rep. 2021, 11, 24334. [Google Scholar] [CrossRef]
- Tien, T.; Muto, T.; Barrette, K.; Challyandra, L.; Roy, S. Downregulation of Connexin 43 promotes vascular cell loss and excess permeability associated with the development of vascullar lesions in the diabetic retina. Mol. Vis. 2014, 20, 732–741. [Google Scholar]
- Strauss, R.E.; Mezache, L.; Veeraraghavan, R.; Gourdie, R.G. The Cx43 Carboxyl-Terminal Mimetic Peptide alphaCT1 Protects Endothelial Barrier Function in a ZO1 Binding-Competent Manner. Biomolecules 2021, 11, 1192. [Google Scholar] [CrossRef]
- Calder, B.W.; Matthew Rhett, J.; Bainbridge, H.; Fann, S.A.; Gourdie, R.G.; Yost, M.J. Inhibition of connexin 43 hemichannel-mediated ATP release attenuates early inflammation during the foreign body response. Tissue Eng. Part A 2015, 21, 1752–1762. [Google Scholar] [CrossRef]
- Ling, X.; Peng, S.; Xu, Y.; Chu, F. Beneficial effect of simvastatin on human umbilical vein endothelial cells gap junctions induced by TNF-alpha. Anim. Cells Syst. 2022, 26, 10–18. [Google Scholar] [CrossRef]
- Veliz, L.P.; Gonzalez, F.G.; Duling, B.R.; Saez, J.C.; Boric, M.P. Functional role of gap junctions in cytokine-induced leukocyte adhesion to endothelium in vivo. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H1056–H1066. [Google Scholar] [CrossRef]
- Fulton, D.; Babbitt, R.; Zoellner, S.; Fontana, J.; Acevedo, L.; McCabe, T.J.; Iwakiri, Y.; Sessa, W.C. Targeting of endothelial nitric-oxide synthase to the cytoplasmic face of the Golgi complex or plasma membrane regulates Akt- versus calcium-dependent mechanisms for nitric oxide release. J. Biol. Chem. 2004, 279, 30349–30357. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, T.; Pappas, P.J.; Hobson, R.W., 2nd; Boric, M.P.; Sessa, W.C.; Durán, W.N. Endothelial nitric oxide synthase regulates microvascular hyperpermeability in vivo. J. Physiol. 2006, 574 Pt 1, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, F.A.; Savalia, N.B.; Durán, R.G.; Lal, B.K.; Boric, M.P.; Durán, W.N. Functional significance of differential eNOS translocation. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1058–H1064. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, F.A.; Kim, D.D.; Durán, R.G.; Meininger, C.J.; Durán, W.N. Internalization of eNOS via caveolae regulates PAF-induced inflammatory hyperpermeability to macromolecules. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H1642–H1648. [Google Scholar] [CrossRef]
- Durán, W.N.; Breslin, J.W.; Sanchez, F.A. The NO cascade, eNOS location, and microvascular permeability. Cardiovasc. Res. 2010, 87, 254–261. [Google Scholar] [CrossRef]
- Marin, N.; Zamorano, P.; Carrasco, R.; Mujica, P.; Gonzalez, F.G.; Quezada, C.; Meininger, C.J.; Boric, M.P.; Durán, W.N.; Sanchez, F.A. S-Nitrosation of beta-catenin and p120 catenin: A novel regulatory mechanism in endothelial hyperpermeability. Circ. Res. 2012, 111, 553–563. [Google Scholar] [CrossRef]
- Durán, W.N.; Beuve, A.V.; Sanchez, F.A. Nitric oxide, S-nitrosation, and endothelial permeability. IUBMB Life 2013, 65, 819–826. [Google Scholar] [CrossRef]
- Sanchez, F.A.; Ehrenfeld, I.P.; Durán, W.N. S-nitrosation of proteins: An emergent regulatory mechanism in microvascular permeability and vascular function. Tissue Barriers 2013, 1, e23896. [Google Scholar] [CrossRef]
- Guequen, A.; Carrasco, R.; Zamorano, P.; Rebolledo, L.; Burboa, P.; Sarmiento, J.; Boric, M.P.; Korayem, A.; Durán, W.N.; Sanchez, F.A. S-nitrosylation regulates VE-cadherin phosphorylation and internalization in microvascular permeability. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H1039–H1044. [Google Scholar] [CrossRef][Green Version]
- Zamorano, P.; Marin, N.; Cordova, F.; Aguilar, A.; Meininger, C.; Boric, M.P.; Golenhofen, N.; Contreras, J.E.; Sarmiento, J.; Durán, W.N.; et al. S-nitrosylation of VASP at cysteine 64 mediates the inflammation-stimulated increase in microvascular permeability. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H66–H71. [Google Scholar] [CrossRef]
- Guequen, A.; Zamorano, P.; Cordova, F.; Koning, T.; Torres, A.; Ehrenfeld, P.; Boric, M.P.; Salazar-Onfray, F.; Gavard, J.; Durán, W.N.; et al. Interleukin-8 Secreted by Glioblastoma Cells Induces Microvascular Hyperpermeability through NO Signaling Involving S-Nitrosylation of VE-Cadherin and p120 in Endothelial Cells. Front. Physiol. 2019, 10, 988. [Google Scholar] [CrossRef]
- Zamorano, P.; Koning, T.; Oyanadel, C.; Mardones, G.A.; Ehrenfeld, P.; Boric, M.P.; Gonzalez, A.; Soza, A.; Sanchez, F.A. Galectin-8 induces endothelial hyperpermeability through the eNOS pathway involving S-nitrosylation-mediated adherens junction disassembly. Carcinogenesis 2019, 40, 313–323. [Google Scholar] [CrossRef]
- Aguilar, G.; Cordova, F.; Koning, T.; Sarmiento, J.; Boric, M.P.; Birukov, K.; Cancino, J.; Varas-Godoy, M.; Soza, A.; Alves, N.G.; et al. TNF-alpha-activated eNOS signaling increases leukocyte adhesion through the S-nitrosylation pathway. Am. J. Physiol. Heart Circ. Physiol. 2021, 321, H1083–H1095. [Google Scholar] [CrossRef]
- Lan, S.H.; Lai, W.T.; Zheng, S.Y.; Yang, L.; Fang, L.C.; Zhou, L.; Tang, B.; Duan, J.; Hong, T. Upregulation of Connexin 40 Mediated by Nitric Oxide Attenuates Cerebral Vasospasm After Subarachnoid Hemorrhage via the Nitric Oxide-Cyclic Guanosine Monophosphate-Protein Kinase G Pathway. World Neurosurg. 2020, 136, e476–e486. [Google Scholar] [CrossRef]
- Sun, J.; Nguyen, T.; Aponte, A.M.; Menazza, S.; Kohr, M.J.; Roth, D.M.; Patel, H.H.; Murphy, E.; Steenbergen, C. Ischaemic preconditioning preferentially increases protein S-nitrosylation in subsarcolemmal mitochondria. Cardiovasc. Res. 2015, 106, 227–236. [Google Scholar] [CrossRef]
- Kameritsch, P.; Pogoda, K. The Role of Connexin 43 and Pannexin 1 During Acute Inflammation. Front. Physiol. 2020, 11, 594097. [Google Scholar] [CrossRef]
- Bunse, S.; Schmidt, M.; Prochnow, N.; Zoidl, G.; Dermietzel, R. Intracellular cysteine 346 is essentially involved in regulating Panx1 channel activity. J. Biol. Chem. 2010, 285, 38444–38452. [Google Scholar] [CrossRef]
- Penuela, S.; Simek, J.; Thompson, R.J. Regulation of pannexin channels by post-translational modifications. FEBS Lett. 2014, 588, 1411–1415. [Google Scholar] [CrossRef]
- Poornima, V.; Vallabhaneni, S.; Mukhopadhyay, M.; Bera, A.K. Nitric oxide inhibits the pannexin 1 channel through a cGMP-PKG dependent pathway. Nitric Oxide 2015, 47, 77–84. [Google Scholar] [CrossRef]
- Filosa, J.A.; Blanco, V.M. Neurovascular coupling in the mammalian brain. Exp. Physiol. 2007, 92, 641–646. [Google Scholar] [CrossRef]
- Phillips, A.A.; Chan, F.H.; Zheng, M.M.Z.; Krassioukov, A.V.; Ainslie, P.N. Neurovascular coupling in humans: Physiology, methodological advances and clinical implications. J. Cereb. Blood Flow Metab. 2016, 36, 647–664. [Google Scholar] [CrossRef]
- Zheng, Y.; Pan, Y.; Harris, S.; Billings, S.; Coca, D.; Berwick, J.; Jones, M.; Kennerley, A.; Johnston, D.; Martin, C.; et al. A dynamic model of neurovascular coupling: Implications for blood vessel dilation and constriction. NeuroImage 2010, 52, 1135–1147. [Google Scholar] [CrossRef]
- Bell, A.H.; Miller, S.L.; Castillo-Melendez, M.; Malhotra, A. The Neurovascular Unit: Effects of Brain Insults During the Perinatal Period. Front. Neurosci. 2020, 13, 1452. [Google Scholar] [CrossRef]
- Muoio, V.; Persson, P.B.; Sendeski, M.M. The neurovascular unit—Concept review. Acta Physiol. 2014, 210, 790–798. [Google Scholar] [CrossRef]
- Netto, J.P.; Iliff, J.; Stanimirovic, D.; Krohn, K.A.; Hamilton, B.; Varallyay, C.; Gahramanov, S.; Daldrup-Link, H.; D’Esterre, C.; Zlokovic, B.; et al. Neurovascular unit: Basic and clinical imaging with emphasis on advantages of ferumoxytol. Neurosurgery 2018, 82, 770–780. [Google Scholar] [CrossRef]
- Filosa, J.A. Vascular tone and neurovascular coupling: Considerations toward an improved in vitro model. Front. Neuroenergetics 2010, 2, 16. [Google Scholar] [CrossRef][Green Version]
- Girouard, H.; Iadecola, C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J. Appl. Physiol. 2006, 100, 328–335. [Google Scholar] [CrossRef]
- Filosa, J.A.; Iddings, J.A. Astrocyte regulation of cerebral vascular tone. Am. J. Physiol.-Heart Circ. Physiol. 2013, 305, H609–H619. [Google Scholar] [CrossRef]
- Gordon, G.R.J.; Mulligan, S.J.; MacVicar, B.A. Astrocyte Control of the Cerebrovasculature GRANT. Glia 2007, 55, 1214–1221. [Google Scholar] [CrossRef]
- Haydon, P.G.; Carmignoto, G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol. Rev. 2006, 86, 1009–1031. [Google Scholar] [CrossRef]
- Nuriya, M.; Hirase, H. Involvement of astrocytes in neurovascular communication. Prog. Brain Res. 2016, 225, 41–62. [Google Scholar] [PubMed]
- Bazargani, N.; Attwell, D. Astrocyte calcium signaling: The third wave. Nat. Neurosci. 2016, 19, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.M. ATP: A ubiquitous gliotransmitter integrating neuron-glial networks. Semin. Cell Dev. Biol. 2011, 22, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Filosa, J.A.; Bonev, A.D.; Nelson, M.T. Calcium dynamics in cortical astrocytes and arterioles during neurovascular coupling. Circ. Res. 2004, 95, e73–e81. [Google Scholar] [CrossRef] [PubMed]
- Girouard, H.; Bonev, A.D.; Hannah, R.M.; Meredith, A.; Aldrich, R.W.; Nelson, M.T. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc. Natl. Acad. Sci. USA 2010, 107, 3811–3816. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, M.; Perea, G.; Maglio, L.; Pastor, J.; García De Sola, R.; Araque, A. Astrocyte calcium signal and gliotransmission in human brain tissue. Cereb. Cortex 2013, 23, 1240–1246. [Google Scholar] [CrossRef]
- Otsu, Y.; Couchman, K.; Lyons, D.G.; Collot, M.; Agarwal, A.; Mallet, J.-M.; Pfrieger, F.W.; Bergles, D.E.; Charpak, S. Calcium dynamics in astrocyte processes during neurovascular coupling. Nat. Neurosci. 2015, 18, 210–218. [Google Scholar] [CrossRef]
- Straub, S.V.; Nelson, M.T. Astrocytic Calcium Signaling: The Information Currency Coupling Neuronal Activity to the Cerebral Microcirculation. Trends Cardiovasc. Med. 2007, 17, 183–190. [Google Scholar] [CrossRef][Green Version]
- Xu, H.L.; Pelligrino, D.A. ATP release and hydrolysis contribute to rat pial arteriolar dilatation elicited by neuronal activation. Exp. Physiol. 2007, 92, 647–651. [Google Scholar] [CrossRef]
- Niswender, C.M.; Conn, P.J. Metabotropic glutamate receptors: Physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 295–322. [Google Scholar] [CrossRef]
- Petroff, O.A.C. Book Review: GABA and Glutamate in the Human Brain. Neuroscience 2002, 8, 562–573. [Google Scholar] [CrossRef]
- Zhou, Y.; Danbolt, N.C. Glutamate as a neurotransmitter in the healthy brain. J. Neural Transm. 2014, 121, 799–817. [Google Scholar] [CrossRef]
- Bradley, S.J.; Challiss, R.A.J. G protein-coupled receptor signalling in astrocytes in health and disease: A focus on metabotropic glutamate receptors. Biochem. Pharmacol. 2012, 84, 249–259. [Google Scholar] [CrossRef]
- D’Antoni, S.; Berretta, A.; Bonaccorso, C.M.; Bruno, V.; Aronica, E.; Nicoletti, F.; Catania, M.V. Metabotropic Glutamate Receptors in Glial Cells. Neurochem. Res. 2008, 33, 2436–2443. [Google Scholar] [CrossRef]
- Seifert, G.; Steinhäuser, C. Ionotropic glutamate receptors in astrocytes. Prog. Brain Res. 2001, 132, 287–299. [Google Scholar]
- Verkhratsky, A.; Chvátal, A. NMDA Receptors in Astrocytes. Neurochem. Res. 2020, 45, 122–133. [Google Scholar] [CrossRef]
- He, L.; Linden, D.J.; Sapirstein, A. Astrocyte Inositol Triphosphate Receptor Type 2 and Cytosolic Phospholipase A2 Alpha Regulate Arteriole Responses in Mouse Neocortical Brain Slices. PLoS ONE 2012, 7, e42194. [Google Scholar] [CrossRef]
- Zur Nieden, R.; Deitmer, J.W. The Role of Metabotropic Glutamate Receptors for the Generation of Calcium Oscillations in Rat Hippocampal Astrocytes in situ. Cereb. Cortex 2006, 16, 676–687. [Google Scholar] [CrossRef]
- Stackhouse, T.L.; Mishra, A. Neurovascular Coupling in Development and Disease: Focus on Astrocytes. Front. Cell Dev. Biol. 2021, 9, 1745. [Google Scholar] [CrossRef]
- Dunn, K.M.; Nelson, M.T. Potassium channels and neurovascular coupling. Circ. J. 2010, 74, 608–616. [Google Scholar] [CrossRef]
- Gordon, G.R.J.; Howarth, C.; Macvicar, B.A. Bidirectional control of arteriole diameter by astrocytes. Exp. Physiol. 2011, 96, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, C.; Gebremedhin, D.; Hwang, S.H.; Hammock, B.D.; Falck, J.R.; Roman, R.J.; Harder, D.R.; Koehler, R.C. Epoxyeicosatrienoic acid-dependent cerebral vasodilation evoked by metabotropic glutamate receptor activation in vivo. Am. J. Physiol.-Heart Circ. Physiol. 2011, 301, H373–H381. [Google Scholar] [CrossRef] [PubMed]
- Medhora, M. Dual Regulation of the Cerebral Microvasculature by Epoxyeicosatrienoic Acids. Trends Cardiovasc. Med. 2001, 11, 38–42. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, X.; Gebremedhin, D.; Falck, J.R.; Harder, D.R.; Koehler, R.C. Interaction of Mechanisms Involving Epoxyeicosatrienoic Acids, Adenosine Receptors, and Metabotropic Glutamate Receptors in Neurovascular Coupling in Rat Whisker Barrel Cortex. J. Cereb. Blood Flow Metab. 2008, 28, 111–125. [Google Scholar] [CrossRef]
- Earley, S.; Brayden, J.E. Transient Receptor Potential Channels in the Vasculature. Physiol. Rev. 2015, 95, 645–690. [Google Scholar] [CrossRef]
- Iliff, J.J.; Jia, J.; Nelson, J.; Goyagi, T.; Klaus, J.; Alkayed, N.J. Epoxyeicosanoid signaling in CNS function and disease. Prostaglandins Other Lipid Mediat. 2010, 91, 68–84. [Google Scholar] [CrossRef]
- Filosa, J.A.; Morrison, H.W.; Iddings, J.A.; Du, W.; Kim, K.J. Beyond neurovascular coupling, role of astrocytes in the regulation of vascular tone. Neuroscience 2016, 323, 96–109. [Google Scholar] [CrossRef]
- Imig, J.D.; Simpkins, A.N.; Renic, M.; Harder, D.R. Cytochrome P450 eicosanoids and cerebral vascular function. Expert Rev. Mol. Med. 2011, 13, e7. [Google Scholar] [CrossRef]
- Attwell, D.; Buchan, A.M.; Charpak, S.; Lauritzen, M.; MacVicar, B.A.; Newman, E.A. Glial and neuronal control of brain blood flow. Nature 2010, 468, 232–243. [Google Scholar] [CrossRef]
- Filosa, J.A.; Bonev, A.D.; Straub, S.V.; Meredith, A.L.; Wilkerson, M.K.; Aldrich, R.W.; Nelson, M.T. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat. Neurosci. 2006, 9, 1397–1403. [Google Scholar] [CrossRef]
- Koizumi, S. Synchronization of Ca2+ oscillations: Involvement of ATP release in astrocytes. FEBS J 2010, 277, 286–292. [Google Scholar] [CrossRef]
- Farr, H.; David, T. Models of neurovascular coupling via potassium and EET signalling. J. Theor. Biol. 2011, 286, 13–23. [Google Scholar] [CrossRef]
- Macvicar, B.A.; Newman, E.A. Astrocyte regulation of blood flow in the brain. Cold Spring Harb. Perspect. Biol. 2015, 7, a020388. [Google Scholar] [CrossRef]
- Gordon, G.R.J.; Choi, H.B.; Rungta, R.L.; Ellis-Davies, G.C.R.; MacVicar, B.A. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature 2008, 456, 745–750. [Google Scholar] [CrossRef]
- Metea, M.R. Glial Cells Dilate and Constrict Blood Vessels: A Mechanism of Neurovascular Coupling. J. Neurosci. 2006, 26, 2862–2870. [Google Scholar] [CrossRef]
- Roman, R.J. Molecular mechanisms and cell signaling of 20-hydroxyeicosatetraenoic acid in vascular pathophysiology. Front. Biosci. 2016, 21, 4465. [Google Scholar] [CrossRef]
- Newman, E.A. Glial cell regulation of neuronal activity and blood flow in the retina by release of gliotransmitters. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140195. [Google Scholar] [CrossRef]
- Pelligrino, D.A.; Vetri, F.; Xu, H.L. Purinergic mechanisms in gliovascular coupling. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2011; Volume 22, pp. 229–236. [Google Scholar]
- Wells, J.A.; Christie, I.N.; Hosford, P.S.; Huckstepp, R.T.R.; Angelova, P.R.; Vihko, P.; Cork, S.C.; Abramov, A.Y.; Teschemacher, A.G.; Kasparov, S.; et al. A Critical Role for Purinergic Signalling in the Mechanisms Underlying Generation of BOLD fMRI Responses. J. Neurosci. 2015, 35, 5284–5292. [Google Scholar] [CrossRef]
- Hørlyck, S.; Cai, C.; Helms, H.C.C.; Lauritzen, M.; Brodin, B. ATP induces contraction of cultured brain capillary pericytes via activation of P2Y-type purinergic receptors. Am. J. Physiol.-Heart Circ. Physiol. 2021, 320, H699–H712. [Google Scholar] [CrossRef]
- Li, Y.; Baylie, R.L.; Tavares, M.J.; Brayden, J.E. TRPM4 Channels Couple Purinergic Receptor Mechanoactivation and Myogenic Tone Development in Cerebral Parenchymal Arterioles. J. Cereb. Blood Flow Metab. 2014, 34, 1706–1714. [Google Scholar] [CrossRef]
- Beamer, E.; Conte, G.; Engel, T. ATP release during seizures—A critical evaluation of the evidence. Brain Res. Bull. 2019, 151, 65–73. [Google Scholar] [CrossRef]
- Dunn, K.M.; Nelson, M.T. Neurovascular signaling in the brain and the pathological consequences of hypertension. Am. J. Physiol.-Heart Circ. Physiol. 2014, 306, H1–H14. [Google Scholar] [CrossRef]
- Fitz, J.G. Regulation of cellular ATP release. Trans. Am. Clin. Climatol. Assoc. 2007, 118, 199–208. [Google Scholar]
- Vetri, F.; Xu, H.; Mao, L.; Paisansathan, C.; Pelligrino, D.A. ATP hydrolysis pathways and their contributions to pial arteriolar dilation in rats. Am. J. Physiol.-Heart Circ. Physiol. 2011, 301, H1369–H1377. [Google Scholar] [CrossRef]
- Braun, N.; Sévigny, J.; Robson, S.C.; Enjyoji, K.; Guckelberger, O.; Hammer, K.; Di Virgilio, F.; Zimmermann, H. Assignment of ecto-nucleoside triphosphate diphosphohydrolase-1/cd39 expression to microglia and vasculature of the brain. Eur. J. Neurosci. 2000, 12, 4357–4366. [Google Scholar]
- Grković, I.; Drakulić, D.; Martinović, J.; Mitrović, N. Role of Ectonucleotidases in Synapse Formation During Brain Development: Physiological and Pathological Implications. Curr. Neuropharmacol. 2018, 17, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.M.; Buchakjian, M.R.; Dubyak, G.R. Colocalization of ATP Release Sites and Ecto-ATPase Activity at the Extracellular Surface of Human Astrocytes. J. Biol. Chem. 2003, 278, 23331–23342. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, K.A.; Gao, Z.-G. Adenosine receptors as therapeutic targets. Nat. Rev. Drug Discov. 2006, 5, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Kusano, Y.; Echeverry, G.; Miekisiak, G.; Kulik, T.B.; Aronhime, S.N.; Chen, J.F.; Winn, H.R. Role of Adenosine A2 Receptors in Regulation of Cerebral Blood Flow during Induced Hypotension. J. Cereb. Blood Flow Metab. 2010, 30, 808–815. [Google Scholar] [CrossRef]
- Ngai, A.C.; Coyne, E.F.; Meno, J.R.; West, G.A.; Winn, H.R. Receptor subtypes mediating adenosine-induced dilation of cerebral arterioles. Am. J. Physiol.-Heart Circ. Physiol. 2001, 280, H2329–H2335. [Google Scholar] [CrossRef]
- Paisansathan, C.; Xu, H.; Vetri, F.; Hernandez, M.; Pelligrino, D.A. Interactions between adenosine and K+ channel-related pathways in the coupling of somatosensory activation and pial arteriolar dilation. Am. J. Physiol.-Heart Circ. Physiol. 2010, 299, H2009–H2017. [Google Scholar] [CrossRef]
- Rosenblum, W.I. ATP-Sensitive Potassium Channels in the Cerebral Circulation. Stroke 2003, 34, 1547–1552. [Google Scholar] [CrossRef]
- Hein, T.W.; Xu, W.; Ren, Y.; Kuo, L. Cellular signalling pathways mediating dilation of porcine pial arterioles to adenosine A2A receptor activation. Cardiovasc. Res. 2013, 99, 156–163. [Google Scholar] [CrossRef]
- Mills, J.H.; Alabanza, L.; Weksler, B.B.; Couraud, P.-O.; Romero, I.A.; Bynoe, M.S. Human brain endothelial cells are responsive to adenosine receptor activation. Purinergic Signal. 2011, 7, 265–273. [Google Scholar] [CrossRef]
- Shin, H.K.; Shin, Y.W.; Hong, K.W. Role of adenosine A 2B receptors in vasodilation of rat pial artery and cerebral blood flow autoregulation. Am. J. Physiol.-Heart Circ. Physiol. 2000, 278, H339–H344. [Google Scholar] [CrossRef]
- Sáez, J.C.; Nicholson, B. Connexin and Pannexin Based Channels in the Nervous System. In From Molecules to Networks; Elsevier: Amsterdam, The Netherlands, 2014; pp. 257–283. [Google Scholar]
- Kar, R.; Batra, N.; Riquelme, M.A.; Jiang, J.X. Biological role of connexin intercellular channels and hemichannels. Arch Biochem Biophys 2012, 524, 2–15. [Google Scholar] [CrossRef]
- Giaume, C.; Theis, M. Pharmacological and genetic approaches to study connexin-mediated channels in glial cells of the central nervous system. Brain Res. Rev. 2010, 63, 160–176. [Google Scholar] [CrossRef] [PubMed]
- Nagy, J.I.; Ionescu, A.V.; Lynn, B.D.; Rash, J.E. Coupling of astrocyte connexins Cx26, Cx30, Cx43 to oligodendrocyte Cx29, Cx32, Cx47: Implications from normal and connexin32 knockout mice. Glia 2003, 44, 205–218. [Google Scholar] [CrossRef]
- Nagy, J.I.; Lynn, B.D.; Tress, O.; Willecke, K.; Rash, J.E. Connexin26 expression in brain parenchymal cells demonstrated by targeted connexin ablation in transgenic mice. Eur. J. Neurosci. 2011, 34, 263–271. [Google Scholar] [CrossRef]
- Nielsen, B.S.; Hansen, D.B.; Ransom, B.R.; Nielsen, M.S.; MacAulay, N. Connexin Hemichannels in Astrocytes: An Assessment of Controversies Regarding Their Functional Characteristics. Neurochem. Res. 2017, 42, 2537–2550. [Google Scholar] [CrossRef]
- Xing, L.; Yang, T.; Cui, S.; Chen, G. Connexin Hemichannels in Astrocytes: Role in CNS Disorders. Front. Mol. Neurosci. 2019, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- De Bock, M.; Decrock, E.; Wang, N.; Bol, M.; Vinken, M.; Bultynck, G.; Leybaert, L. The dual face of connexin-based astroglial Ca2+ communication: A key player in brain physiology and a prime target in pathology. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2014, 1843, 2211–2232. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Kiyoshi, C.M.; Terman, D.; Zhou, M. Analysis of the Functional States of an Astrocyte Syncytium. In Basic Neurobiology Techniques; Wright, N., Ed.; Neuromethods; Springer: New York, NY, USA, 2020; Volume 152, pp. 285–313. [Google Scholar]
- Pacholko, A.G.; Wotton, C.A.; Bekar, L.K. Astrocytes—The Ultimate Effectors of Long-Range Neuromodulatory Networks? Front. Cell. Neurosci. 2020, 14, 581075. [Google Scholar] [CrossRef]
- Ahmadpour, N.; Kantroo, M.; Stobart, J.L. Extracellular Calcium Influx Pathways in Astrocyte Calcium Microdomain Physiology. Biomolecules 2021, 11, 1467. [Google Scholar] [CrossRef]
- Lagos-Cabré, R.; Brenet, M.; Díaz, J.; Pérez, R.; Pérez, L.; Herrera-Molina, R.; Quest, A.; Leyton, L. Intracellular Ca2+ Increases and Connexin 43 Hemichannel Opening Are Necessary but Not Sufficient for Thy-1-Induced Astrocyte Migration. Int. J. Mol. Sci. 2018, 19, 2179. [Google Scholar] [CrossRef]
- Turovsky, E.A.; Braga, A.; Yu, Y.; Esteras, N.; Korsak, A.; Theparambil, S.M.; Hadjihambi, A.; Hosford, P.S.; Teschemacher, A.G.; Marina, N.; et al. Mechanosensory Signaling in Astrocytes. J. Neurosci. 2020, 40, 9364–9371. [Google Scholar] [CrossRef]
- Kang, J.; Kang, N.; Lovatt, D.; Torres, A.; Zhao, Z.; Lin, J.; Nedergaard, M. Connexin 43 Hemichannels Are Permeable to ATP. J. Neurosci. 2008, 28, 4702–4711. [Google Scholar] [CrossRef]
- Stout, C.E.; Costantin, J.L.; Naus, C.C.G.; Charles, A.C. Intercellular Calcium Signaling in Astrocytes via ATP Release through Connexin Hemichannels. J. Biol. Chem. 2002, 277, 10482–10488. [Google Scholar] [CrossRef]
- Haddock, R.E.; Grayson, T.H.; Brackenbury, T.D.; Meaney, K.R.; Neylon, C.B.; Sandow, S.L.; Hill, C.E. Endothelial coordination of cerebral vasomotion via myoendothelial gap junctions containing connexins 37 and 40. Am. J. Physiol.-Heart Circ. Physiol. 2006, 291, 2047–2056. [Google Scholar] [CrossRef]
- Avila, M.A.; Sell, S.L.; Hawkins, B.E.; Hellmich, H.L.; Boone, D.R.; Crookshanks, J.M.; Prough, D.S.; DeWitt, D.S. Cerebrovascular connexin expression: Effects of traumatic brain injury. J. Neurotrauma 2011, 28, 1803–1811. [Google Scholar] [CrossRef]
- Brisset, A.C.; Isakson, B.E.; Kwak, B.R. Connexins in vascular physiology and pathology. Antioxid. Redox Signal. 2009, 11, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xin, Y.; He, Z.; Hu, W. Function of Connexins in the Interaction between Glial and Vascular Cells in the Central Nervous System and Related Neurological Diseases. Neural Plast. 2018, 2018, 6323901. [Google Scholar] [CrossRef] [PubMed]
- Bruzzone, R.; Hormuzdi, S.G.; Barbe, M.T.; Herb, A.; Monyer, H. Pannexins, a family of gap junction proteins expressed in brain. Proc. Natl. Acad. Sci. USA 2003, 100, 13644–13649. [Google Scholar] [CrossRef]
- Yeung, A.K.; Patil, C.S.; Jackson, M.F. Pannexin-1 in the CNS: Emerging concepts in health and disease. J. Neurochem. 2020, 154, 468–485. [Google Scholar] [CrossRef]
- Giaume, C.; Naus, C.C.; Sáez, J.C.; Leybaert, L. Glial Connexins and Pannexins in the Healthy and Diseased Brain. Physiol. Rev. 2021, 101, 93–145. [Google Scholar] [CrossRef]
- Huang, Y.; Grinspan, J.B.; Abrams, C.K.; Scherer, S.S. Pannexin1 is expressed by neurons and glia but does not form functional gap junctions. Glia 2007, 55, 46–56. [Google Scholar] [CrossRef]
- MacVicar, B.A.; Thompson, R.J. Non-junction functions of pannexin-1 channels. Trends Neurosci. 2010, 33, 93–102. [Google Scholar] [CrossRef]
- Iglesias, R.; Dahl, G.; Qiu, F.; Spray, D.C.; Scemes, E. Pannexin 1: The Molecular Substrate of Astrocyte “Hemichannels”. J. Neurosci. 2009, 29, 7092–7097. [Google Scholar] [CrossRef]
- Suadicani, S.O.; Iglesias, R.; Wang, J.; Dahl, G.; Spray, D.C.; Scemes, E. ATP signaling is deficient in cultured pannexin1-null mouse astrocytes. Glia 2012, 60, 1106–1116. [Google Scholar] [CrossRef]
- Dahl, G. ATP release through pannexon channels. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140191. [Google Scholar] [CrossRef]
- Scemes, E.; Spray, D.C. Extracellular K+ and Astrocyte Signaling via Connexin and Pannexin Channels. Neurochem. Res. 2012, 37, 2310–2316. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).