Improved Generation of Human Induced Pluripotent Stem Cell-Derived Cardiac Pacemaker Cells Using Novel Differentiation Protocols
Abstract
1. Introduction
2. Results
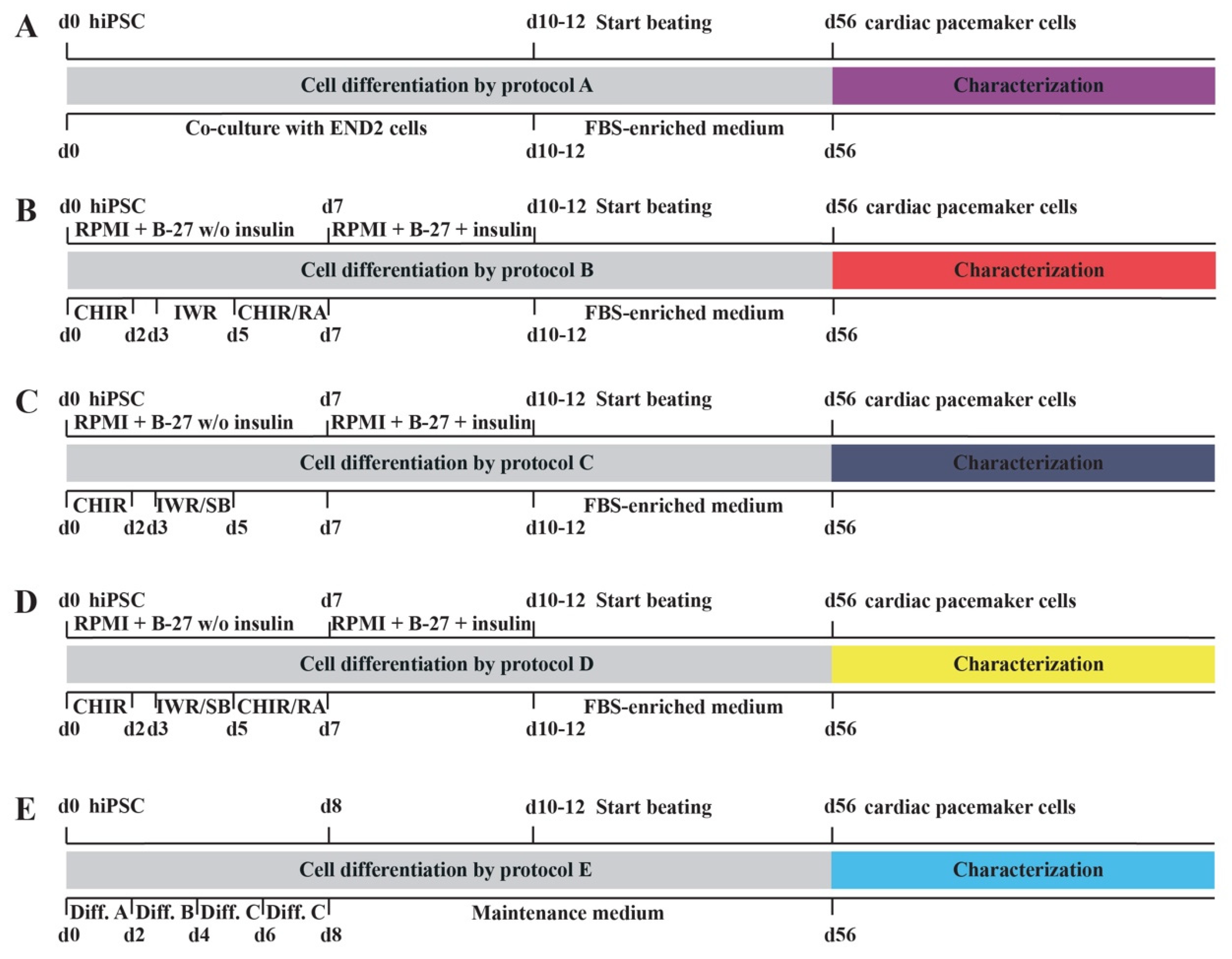
2.1. Novel Cell Culture Protocols for the Differentiation of hiPSC into Cardiac Pacemaker Cells
2.2. Proliferation and Survival Analysis of Cardiac Pacemaker Cells
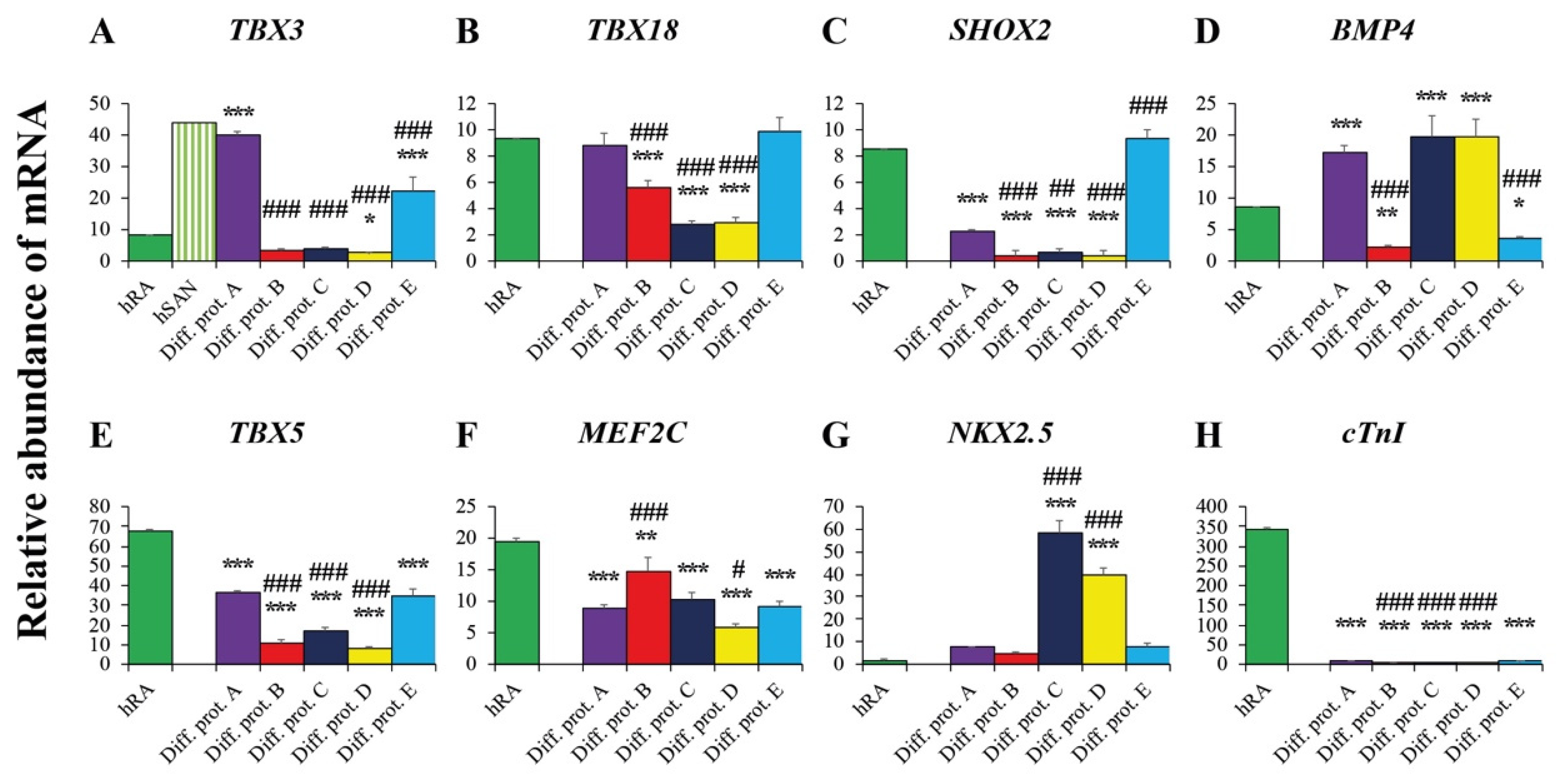
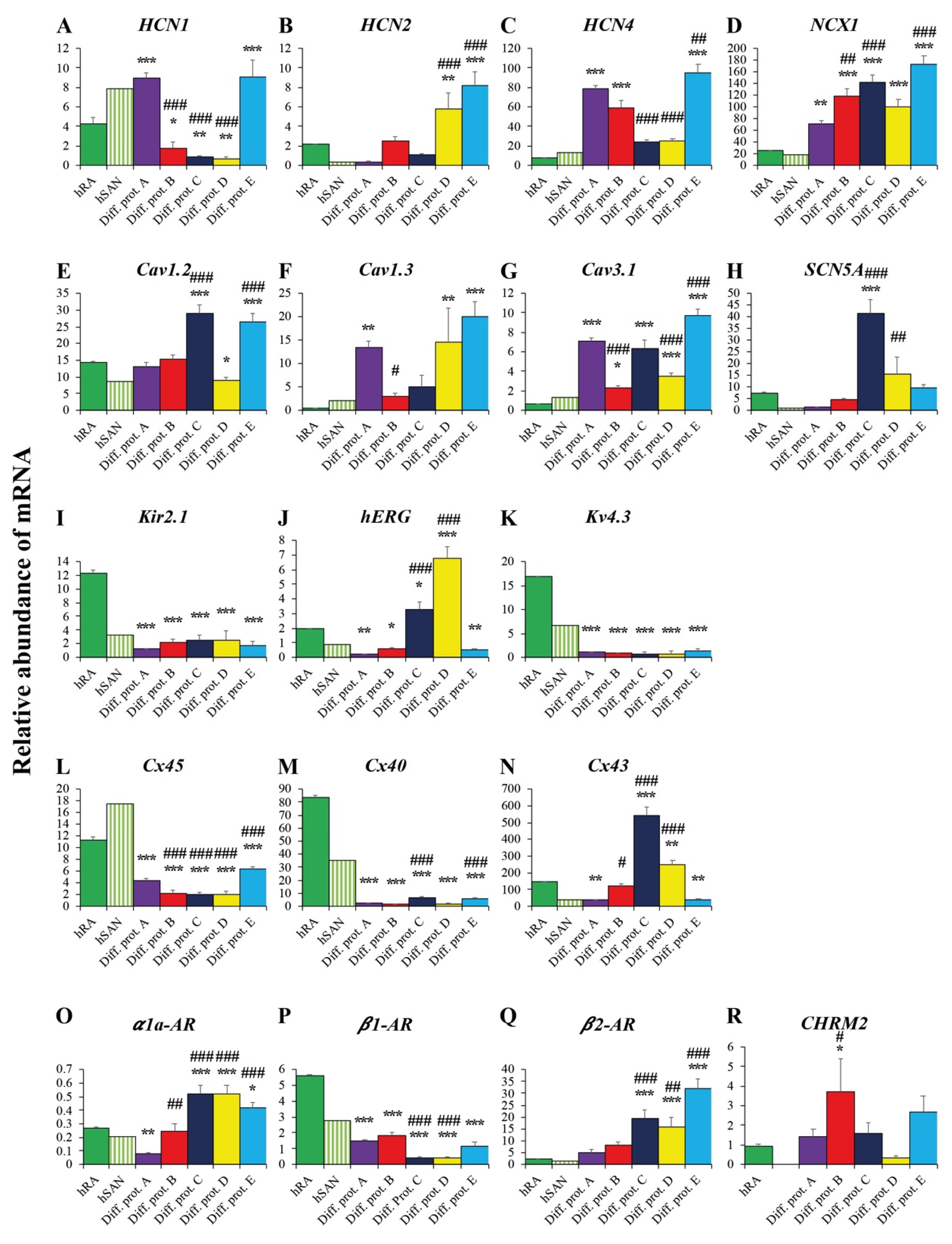
2.3. Valuation of the New Protocols’ Differentiation Efficiency toward Cardiac Pacemaker Cells by qRT-PCR
2.3.1. Pacemaker-Specific Transcription Factors
2.3.2. Transcription Factors and Markers of the Working Myocardium
2.3.3. Ion Channels and Transporters
2.3.4. Connexins
2.3.5. Adrenergic and Cholinergic Receptors
2.4. Comparison of the Differentiation Protocols Regarding the Transcription of Cardiac Pacemaker-Specific Genes
2.5. Immunocytochemical Analysis of Protocol E Derived Cardiac Pacemaker Cells
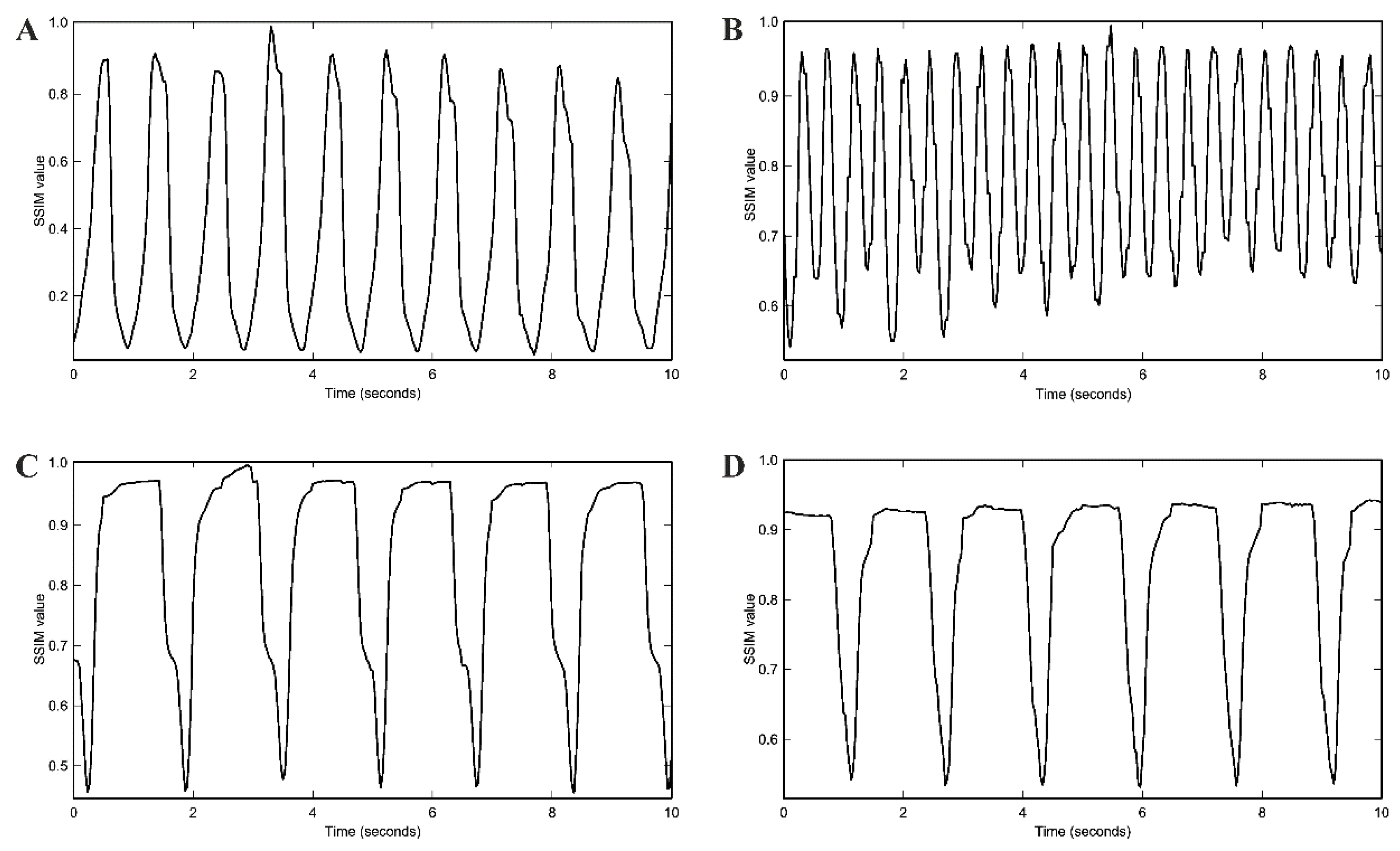
2.6. Spontaneous Beating Rate and Pharmacological Testing
3. Discussion
4. Materials and Methods
4.1. Differentiation of hiPSC by Novel Cell Culture Protocols
4.2. Cell Proliferation Assays
4.3. RNA Isolation and cDNA Synthesis
4.4. Quantitative Real-Time Polymerase Chain Reaction
4.5. Immunocytochemistry of hiPSC-Derived Cardiac Pacemaker Cells
4.6. Assessment of Spontaneous Beating Rate of Cardiac Pacemaker Cells by Movement Frequency Analysis with Structural Similarity Approach
4.7. Pharmacological Testing
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosen, M.R.; Robinson, R.B.; Brink, P.R.; Cohen, I.S. The road to biological pacing. Nat. Rev. Cardiol. 2011, 8, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Rosen, M.R. Gene therapy and biological pacing. N. Engl. J. Med. 2014, 371, 1158–1159. [Google Scholar] [CrossRef] [PubMed]
- Cingolani, E.; Goldhaber, J.I.; Marbán, E. Next-generation pacemakers: From small devices to biological pacemakers. Nat. Rev. Cardiol. 2018, 15, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, P.A.; Darche, F.F.; Ullrich, N.D.; Geschwill, P.; Greber, B.; Rivinius, R.; Seyler, C.; Müller-Decker, K.; Draguhn, A.; Utikal, J.; et al. Subtype-specific differentiation of cardiac pacemaker cell clusters from human induced pluripotent stem cells. Stem Cell Res. Ther. 2017, 8, 229. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.C.; Marbán, E. Biological therapies for cardiac arrhythmias: Can genes and cells replace drugs and devices? Circ. Res. 2010, 106, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, N.; Liang, W.; Marbán, E.; Cho, H.C. Direct conversion of quiescent cardiomyocytes to pacemaker cells by expression of Tbx18. Nat. Biotechnol. 2013, 31, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.F.; Dawkins, J.F.; Cho, H.C.; Marbán, E.; Cingolani, E. Biological pacemaker created by minimally invasive somatic reprogramming in pigs with complete heart block. Sci. Transl. Med. 2014, 6, 245ra94. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Matsa, E.; Burridge, P.W.; Wu, J.C. Human stem cells for modeling heart disease and for drug discovery. Sci. Transl. Med. 2014, 6, 239ps6. [Google Scholar] [CrossRef] [PubMed]
- Mummery, C.; Ward-van Oostwaard, D.; Doevendans, P.; Spijker, R.; van den Brink, S.; Hassink, R.; van der Heyden, M.; Opthof, T.; Pera, M.; de la Riviere, A.B.; et al. Differentiation of human embryonic stem cells to cardiomyocytes: Role of coculture with visceral endoderm-like cells. Circulation 2003, 107, 2733–2740. [Google Scholar] [CrossRef] [PubMed]
- Laflamme, M.A.; Chen, K.Y.; Naumova, A.V.; Muskheli, V.; Fugate, J.A.; Dupras, S.K.; Reinecke, H.; Xu, C.; Hassanipour, M.; Police, S.; et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat. Biotechnol. 2007, 25, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Soonpaa, M.H.; Adler, E.D.; Roepke, T.K.; Kattman, S.J.; Kennedy, M.; Henckaerts, E.; Bonham, K.; Abbott, G.W.; Linden, R.M.; et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stemcell-derived population. Nature 2008, 453, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wilson, G.F.; Soerens, A.G.; Koonce, C.H.; Yu, J.; Palecek, S.P.; Thomson, J.A.; Kamp, T.J. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ. Res. 2009, 104, e30–e41. [Google Scholar] [CrossRef] [PubMed]
- Zwi, L.; Caspi, O.; Arbel, G.; Huber, I.; Gepstein, A.; Park, I.H.; Gepstein, L. Cardiomyocyte differentiation of human induced pluripotent stem cells. Circulation 2009, 120, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Hsiao, C.; Wilson, G.; Zhu, K.; Hazeltine, L.B.; Azarin, S.M.; Raval, K.K.; Zhang, J.; Kamp, T.J.; Palecek, S.P. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc. Natl. Acad. Sci. USA 2012, 109, E1848–E1857. [Google Scholar] [CrossRef]
- Lian, X.; Zhang, J.; Azarin, S.M.; Zhu, K.; Hazeltine, L.B.; Bao, X.; Hsiao, C.; Kamp, T.J.; Palecek, S.P. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/beta-catenin signaling under fully defined conditions. Nat. Protoc. 2013, 8, 162–175. [Google Scholar] [CrossRef]
- Yechikov, S.; Copaciu, R.; Gluck, J.M.; Deng, W.; Chiamvimonvat, N.; Chan, J.W.; Lieu, D.K. Same-single-cell analysis of pacemaker-specific markers in human induced pluripotent stem cell-derived cardiomyocyte subtypes classified by electrophysiology. Stem Cells 2016, 34, 2670–2680. [Google Scholar] [CrossRef]
- Xu, X.Q.; Graichen, R.; Soo, S.Y.; Balakrishnan, T.; Rahmat, S.N.; Sieh, S.; Tham, S.C.; Freund, C.; Moore, J.; Mummery, C.; et al. Chemically defined medium supporting cardiomyocyte differentiation of human embryonic stem cells. Differentiation 2008, 76, 958–970. [Google Scholar] [CrossRef]
- Graichen, R.; Xu, X.; Braam, S.R.; Balakrishnan, T.; Norfiza, S.; Sieh, S.; Soo, S.Y.; Tham, S.C.; Mummery, C.; Colman, A.; et al. Enhanced cardiomyogenesis of human embryonic stem cells by a small molecular inhibitor of p38 MAPK. Differentiation 2008, 76, 357–370. [Google Scholar] [CrossRef]
- Yechikov, S.; Kao, H.K.J.; Chang, C.W.; Pretto, D.; Zhang, X.D.; Sun, Y.H.; Smithers, R.; Sirish, P.; Nolta, J.A.; Chan, J.W.; et al. NODAL inhibition promotes differentiation of pacemaker-like cardiomyocytes from human induced pluripotent stem cells. Stem Cell Res. 2020, 49, 102043. [Google Scholar] [CrossRef]
- Protze, S.I.; Liu, J.; Nussinovitch, U.; Ohana, L.; Backx, P.H.; Gepstein, L.; Keller, G.M. Sinoatrial node cardiomyocytes derived from human pluripotent cells function as a biological pacemaker. Nat. Biotechnol. 2017, 35, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Cao, H.; Tian, L.; Huo, W.; Zhai, K.; Wang, P.; Ji, G.; Ma, Y. Efficient Differentiation of TBX18+/WT1+ Epicardial-Like Cells from Human Pluripotent Stem Cells Using Small Molecular Compounds. Stem Cells Dev. 2017, 26, 528–540. [Google Scholar] [CrossRef] [PubMed]
- Christoffels, V.M.; Mommersteeg, M.T.; Trowe, M.O.; Prall, O.W.; de Gier-de Vries, C.; Soufan, A.T.; Bussen, M.; Schuster-Gossler, K.; Harvey, R.P.; Moorman, A.F.; et al. Formation of the venous pole of the heart from an Nkx2-5-negative precursor population requires Tbx18. Circ. Res. 2006, 98, 1555–1563. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, H.; Quan, D.; Tang, Y.; Wang, X.; Huang, C. Tbx18 promoted the conversion of human-induced pluripotent stem cell-derived cardiomyocytes into sinoatrial node-like pacemaker cells. Cell Biol. Int. 2022, 46, 403–414. [Google Scholar] [CrossRef]
- Chandler, N.J.; Greener, I.D.; Tellez, J.O.; Inada, S.; Musa, H.; Molenaar, P.; Difrancesco, D.; Baruscotti, M.; Longhi, R.; Anderson, R.H.; et al. Molecular architecture of the human sinus node: Insights into the function of the cardiac pacemaker. Circulation 2009, 119, 1562–1575. [Google Scholar] [CrossRef] [PubMed]
- Sizarov, A.; Devalla, H.D.; Anderson, R.H.; Passier, R.; Christoffels, V.M.; Moorman, A.F. Molecular analysis of patterning of conduction tissues in the developing human heart. Circ. Arrhythm. Electrophysiol. 2011, 4, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Puskaric, S.; Schmitteckert, S.; Mori, A.D.; Glaser, A.; Schneider, K.U.; Bruneau, B.G.; Blaschke, R.J.; Steinbeisser, H.; Rappold, G. Shox2 mediates Tbx5 activity by regulating Bmp4 in the pacemaker region of the developing heart. Hum. Mol. Genet. 2010, 19, 4625–4633. [Google Scholar] [CrossRef]
- Olson, E.N. Gene regulatory networks in the evolution and development of the heart. Science 2006, 313, 1922–1927. [Google Scholar] [CrossRef]
- Moskowitz, I.P.; Kim, J.B.; Moore, M.L.; Wolf, C.M.; Peterson, M.A.; Shendure, J.; Nobrega, M.A.; Yokota, Y.; Berul, C.; Izumo, S.; et al. A molecular pathway including Id2, Tbx5, and Nkx2-5 required for cardiac conduction system development. Cell 2007, 129, 1365–1376. [Google Scholar] [CrossRef]
- Espinoza-Lewis, R.A.; Liu, H.; Sun, C.; Chen, C.; Jiao, K.; Chen, Y. Ectopic expression of Nkx2.5 suppresses the formation of the sinoatrial node in mice. Dev. Biol. 2011, 356, 359–369. [Google Scholar] [CrossRef]
- DiFrancesco, D.; Tortora, P. Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature 1991, 351, 145–147. [Google Scholar] [CrossRef]
- DiFrancesco, D. The role of the funny current in pacemaker activity. Circ. Res. 2010, 106, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Baruscotti, M.; Bucchi, A.; Viscomi, C.; Mandelli, G.; Consalez, G.; Gnecchi-Rusconi, T.; Montano, N.; Casali, K.R.; Micheloni, S.; Barbuti, A.; et al. Deep bradycardia and heart block caused by inducible cardiac-specific knockout of the pacemaker channel gene Hcn4. Proc. Natl. Acad. Sci. USA 2011, 108, 1705–1710. [Google Scholar] [CrossRef] [PubMed]
- Später, D.; Abramczuk, M.K.; Buac, K.; Zangi, L.; Stachel, M.W.; Clarke, J.; Sahara, M.; Ludwig, A.; Chien, K.R. A HCN4+ cardiomyogenic progenitor derived from the first heart field and human pluripotent stem cells. Nat. Cell. Biol. 2013, 15, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, P.A.; Schröter, J.; Greiner, S.; Haas, J.; Yampolsky, P.; Mereles, D.; Buss, S.J.; Seyler, C.; Bruehl, C.; Draguhn, A.; et al. The symptom complex of familial sinus node dysfunction and myocardial noncompaction is associated with mutations in the HCN4 channel. J. Am. Coll. Cardiol. 2014, 64, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, P.A.; Yampolsky, P.; Malik, R.; Thomas, D.; Zehelein, J.; Katus, H.A.; Koenen, M. Transcription profiling of HCN-channel isotypes throughout mouse cardiac development. Basic Res. Cardiol. 2009, 104, 621–629. [Google Scholar] [CrossRef]
- Ludwig, A.; Budde, T.; Stieber, J.; Moosmang, S.; Wahl, C.; Holthoff, K.; Langebartels, A.; Wotjak, C.; Munsch, T.; Zong, X.; et al. Absence epilepsy and sinus dysrhythmia in mice lacking the pacemaker channel HCN2. EMBO J. 2003, 22, 216–224. [Google Scholar] [CrossRef]
- Li, N.; Csepe, T.A.; Hansen, B.J.; Dobrzynski, H.; Higgins, R.S.; Kilic, A.; Mohler, P.J.; Janssen, P.M.; Rosen, M.R.; Biesiadecki, B.J.; et al. Molecular Mapping of Sinoatrial Node HCN Channel Expression in the Human Heart. Circ. Arrhythm. Electrophysiol. 2015, 8, 1219–1227. [Google Scholar] [CrossRef]
- Herrmann, S.; Lipp, P.; Wiesen, K.; Stieber, J.; Nguyen, H.; Kaiser, E.; Ludwig, A. The cardiac sodium-calcium exchanger NCX1 is a key player in the initiation and maintenance of a stable heart rhythm. Cardiovasc. Res. 2013, 99, 780–788. [Google Scholar] [CrossRef]
- Maltsev, V.A.; Lakatta, E.G. Synergism of coupled subsarcolemmal Ca2+ clocks and sarcolemmal voltage clocks confers robust and flexible pacemaker function in a novel pacemaker cell model. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H594–H615. [Google Scholar] [CrossRef]
- Lakatta, E.G.; Maltsev, V.A.; Vinogradova, T.M. A coupled SYSTEM of intracellular Ca2+ clocks and surface membrane voltage clocks controls the timekeeping mechanism of the heart’s pacemaker. Circ. Res. 2010, 106, 659–673. [Google Scholar] [CrossRef] [PubMed]
- Rook, M.B.; Evers, M.M.; Vos, M.A.; Bierhuizen, M.F. Biology of cardiac sodium channel Nav1.5 expression. Cardiovasc. Res. 2012, 93, 12–23. [Google Scholar] [CrossRef]
- Jansen, J.A.; van Veen, T.A.; de Bakker, J.M.; van Rijen, H.V. Cardiac connexins and impulse propagation. J. Mol. Cell. Cardiol. 2010, 48, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Gaborit, N.; Le Bouter, S.; Szuts, V.; Varro, A.; Escande, D.; Nattel, S.; Demolombe, S. Regional and tissue specific transcript signatures of ion channel genes in the non-diseased human heart. J. Physiol. 2007, 582, 675–693. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, V.V.; Schuessler, R.B.; Hemphill, M.L.; Ambrosi, C.M.; Chang, R.; Voloshina, A.S.; Brown, K.; Hucker, W.J.; Efimov, I.R. Structural and functional evidence for discrete exit pathways that connect the canine sinoatrial node and atria. Circ. Res. 2009, 104, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Butters, T.; Adeniran, I.; Higham, J.; Holden, A.V.; Boyett, M.R.; Hancox, J.C. Modeling the chronotropic effect of isoprenaline on rabbit sinoatrial node. Front. Physiol. 2012, 3, 241. [Google Scholar] [CrossRef][Green Version]
- Behar, J.; Ganesan, A.; Zhang, J.; Yaniv, Y. The Autonomic Nervous System Regulates the Heart Rate through cAMP-PKA Dependent and Independent Coupled-Clock Pacemaker Cell Mechanisms. Front. Physiol. 2016, 7, 419. [Google Scholar] [CrossRef]
- Yaniv, Y.; Maltsev, V.A.; Ziman, B.D.; Spurgeon, H.A.; Lakatta, E.G. The “funny” current (I(f)) inhibition by ivabradine at membrane potentials encompassing spontaneous depolarization in pacemaker cells. Molecules 2012, 17, 8241–8254. [Google Scholar] [CrossRef]
- Jung, J.J.; Husse, B.; Rimmbach, C.; Krebs, S.; Stieber, J.; Steinhoff, G.; Dendorfer, A.; Franz, W.M.; David, R. Programming and isolation of highly pure physiologically and pharmacologically functional sinus-nodal bodies from pluripotent stem cells. Stem Cell Rep. 2014, 2, 592–605. [Google Scholar] [CrossRef]
- Ionta, V.; Liang, W.; Kim, E.H.; Rafie, R.; Giacomello, A.; Marbán, E.; Cho, H.C. SHOX2 overexpression favors differentiation of embryonic stem cells into cardiac pacemaker cells, improving biological pacing ability. Stem Cell Rep. 2015, 4, 129–142. [Google Scholar] [CrossRef]
- Scavone, A.; Capilupo, D.; Mazzocchi, N.; Crespi, A.; Zoia, S.; Campostrini, G.; Bucchi, A.; Milanesi, R.; Baruscotti, M.; Benedetti, S.; et al. Embryonic stem cell-derived CD166+ precursors develop into fully functional sinoatrial-like cells. Circ. Res. 2013, 113, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Lewis, R.A.; Yu, L.; He, F.; Liu, H.; Tang, R.; Shi, J.; Sun, X.; Martin, J.F.; Wang, D.; Yang, J.; et al. Shox2 is essential for the differentiation of cardiac pacemaker cells by repressing Nkx2-5. Dev. Biol. 2009, 327, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Wiese, C.; Grieskamp, T.; Airik, R.; Mommersteeg, M.T.; Gardiwal, A.; de Gier-de Vries, C.; Schuster-Gossler, K.; Moorman, A.F.; Kispert, A.; Christoffels, V.M. Formation of the sinus node head and differentiation of sinus node myocardium are independently regulated by Tbx18 and Tbx3. Circ. Res. 2009, 104, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Hoogaars, W.M.; Tessari, A.; Moorman, A.F.; de Boer, P.A.; Hagoort, J.; Soufan, A.T.; Campione, M.; Christoffels, V.M. The transcriptional repressor Tbx3 delineates the developing central conduction system of the heart. Cardiovasc. Res. 2004, 62, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Hoogaars, W.M.; Engel, A.; Brons, J.F.; Verkerk, A.O.; de Lange, F.J.; Wong, L.Y.; Bakker, M.L.; Clout, D.E.; Wakker, V.; Barnett, P.; et al. Tbx3 controls the sinoatrial node gene program and imposes pacemaker function on the atria. Genes Dev. 2007, 21, 1098–1112. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Wang, J.; Song, Y.; Yu, D.; Sun, C.; Liu, C.; Chen, F.; Zhang, Y.; Wang, F.; Harvey, R.P.; et al. A common Shox2-Nkx2-5 antagonistic mechanism primes the pacemaker cell fate in the pulmonary vein myocardium and sinoatrial node. Development 2015, 142, 2521–2532. [Google Scholar]
- Blaschke, R.J.; Hahurij, N.D.; Kuijper, S.; Just, S.; Wisse, L.J.; Deissler, K.; Maxelon, T.; Anastassiadis, K.; Spitzer, J.; Hardt, S.E.; et al. Targeted mutation reveals essential functions of the homeodomain transcription factor Shox2 in sinoatrial and pacemaking development. Circulation 2007, 115, 1830–1838. [Google Scholar] [CrossRef]
- Mommersteeg, M.T.; Hoogaars, W.M.; Prall, O.W.; de Gier-de Vries, C.; Wiese, C.; Clout, D.E.; Papaioannou, V.E.; Brown, N.A.; Harvey, R.P.; Moorman, A.F.; et al. Molecular pathway for the localized formation of the sinoatrial node. Circ. Res. 2007, 100, 3541–3562. [Google Scholar] [CrossRef]
- Stennard, F.A.; Harvey, R.P. T-box transcription factors and their roles in regulatory hierarchies in the developing heart. Development 2005, 132, 4897–4910. [Google Scholar] [CrossRef]
- Miake, J.; Marbán, E.; Nuss, H.B. Biological pacemaker created by gene transfer. Nature 2002, 419, 132–133. [Google Scholar] [CrossRef]
- Rosen, M.R.; Brink, P.R.; Cohen, I.S.; Robinson, R.B. Cardiac pacing: From biological to electronic … to biological? Circ. Arrhythm. Electrophysiol. 2008, 1, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Yang, L.; Lin, B.; Zhu, X.; Sun, B.; Kaplan, A.D.; Bett, G.C.; Rasmusson, R.L.; London, B.; Salama, G. Mechanism of automaticity in cardiomyocytes derived from human induced pluripotent stem cells. J. Mol. Cell. Cardiol. 2015, 81, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Harrell, M.D.; Harbi, S.; Hoffman, J.F.; Zavadil, J.; Coetzee, W.A. Large-scale analysis of ion channel gene expression in the mouse heart during perinatal development. Physiol. Genom. 2007, 28, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Bovik, A.C.; Sheikh, H.R.; Simoncelli, E.P. Image quality assessment: From error visibility to structural similarity. IEEE Trans Image Process. 2004, 13, 600–612. [Google Scholar] [CrossRef]


| Diff. Prot. A | Diff. Prot. B | Diff. Prot. C | Diff. Prot. D | Diff. Prot. E | |
|---|---|---|---|---|---|
| TBX3 | + | + | + | − | + |
| TBX18 | + | − | − | − | + |
| SHOX2 | − | − | − | − | + |
| BMP4 | + | − | + | + | − |
| HCN1 | + | − | − | − | + |
| HCN4 | + | + | + | + | + |
| NCX1 | + | + | + | + | + |
| Cav1.2 | + | + | + | − | + |
| Cav1.3 | + | + | + | + | + |
| Cav3.1 | + | + | + | + | + |
| Cx45 | − | − | − | − | − |
| α1a-AR | − | + | + | + | + |
| β1-AR | − | − | − | − | − |
| β2-AR | + | + | + | + | + |
| CHRM2 | + | + | + | + | + |
| Total | 11/15 | 9/15 | 10/15 | 8/15 | 12/15 |
| Analyzed Gene | Primer Accession Number (TaqMan Probe and Primer) | |
|---|---|---|
| Abbreviation | Written-Out Form | |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase | Hs99999905_m1 |
| HPRT1 | hypoxanthine phosphoribosyltransferase 1 | Hs01003270_m1 |
| ACTB | actin beta | Hs010606665_m1 |
| TBX3 | T-box transcription factor 3 | Hs00195612_m1 |
| TBX18 | T-box transcription factor 18 | Hs01385458_m1 |
| SHOX2 | short stature homeobox 2 | Hs00243203_m1 |
| BMP4 | bone morphogenetic protein 4 | Hs00370078_m1 |
| TBX5 | T-box transcription factor 5 | Hs01052563_m1 |
| MEF2C | myocyte enhancer factor 2C | Hs01554602_g1 |
| NKX2.5 | NK2 homeobox 5 | Hs00231763_m1 |
| cTnI | troponin I3, cardiac type | Hs01036382_g1 |
| HCN1 | hyperpolarization activated cyclic nucleotide gated potassium channel 1 | Hs00395037_m1 |
| HCN2 | hyperpolarization activated cyclic nucleotide gated potassium channel 2 | Hs00606903_m1 |
| HCN4 | hyperpolarization activated cyclic nucleotide gated potassium channel 4 | Hs00175760_m1 |
| NCX1 | sodium/calcium exchanger protein | Hs01062258_m1 |
| Cav1.2 | calcium voltage-gated channel subunit alpha1 C | Hs00167681_m1 |
| Cav1.3 | calcium voltage-gated channel subunit alpha1 D | Hs00167753_m1 |
| Cav3.1 | calcium voltage-gated channel subunit alpha1 G | Hs00367969_m1 |
| SCN5A | sodium voltage-gated channel alpha subunit 5 | Hs00165693_m1 |
| Kir2.1 | potassium inwardly rectifying channel subfamily J member 2 | Hs00542478_m1 |
| hERG | potassium voltage-gated channel subfamily H member 2 | Hs00265315_m1 |
| Kv4.3 | potassium voltage-gated channel subfamily D member 3 | Hs00542597_m1 |
| Cx45 | gap junction protein gamma 1 | Hs01087407_s1 |
| Cx40 | gap junction protein alpha 5 | Hs99999170_s1 |
| Cx43 | gap junction protein alpha 1 | Hs00748445_s1 |
| α1a-AR | adrenoceptor alpha 1A | Hs00169124_m1 |
| β1-AR | adrenoceptor beta 1 | Hs02330048_s1 |
| β2-AR | adrenoceptor beta 2 | Hs00240532_s1 |
| CHRM2 | cholinergic receptor muscarinic 2 | Hs00265208_s1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darche, F.F.; Ullrich, N.D.; Huang, Z.; Koenen, M.; Rivinius, R.; Frey, N.; Schweizer, P.A. Improved Generation of Human Induced Pluripotent Stem Cell-Derived Cardiac Pacemaker Cells Using Novel Differentiation Protocols. Int. J. Mol. Sci. 2022, 23, 7318. https://doi.org/10.3390/ijms23137318
Darche FF, Ullrich ND, Huang Z, Koenen M, Rivinius R, Frey N, Schweizer PA. Improved Generation of Human Induced Pluripotent Stem Cell-Derived Cardiac Pacemaker Cells Using Novel Differentiation Protocols. International Journal of Molecular Sciences. 2022; 23(13):7318. https://doi.org/10.3390/ijms23137318
Chicago/Turabian StyleDarche, Fabrice F., Nina D. Ullrich, Ziqiang Huang, Michael Koenen, Rasmus Rivinius, Norbert Frey, and Patrick A. Schweizer. 2022. "Improved Generation of Human Induced Pluripotent Stem Cell-Derived Cardiac Pacemaker Cells Using Novel Differentiation Protocols" International Journal of Molecular Sciences 23, no. 13: 7318. https://doi.org/10.3390/ijms23137318
APA StyleDarche, F. F., Ullrich, N. D., Huang, Z., Koenen, M., Rivinius, R., Frey, N., & Schweizer, P. A. (2022). Improved Generation of Human Induced Pluripotent Stem Cell-Derived Cardiac Pacemaker Cells Using Novel Differentiation Protocols. International Journal of Molecular Sciences, 23(13), 7318. https://doi.org/10.3390/ijms23137318









