Gal-3BP in Viral Infections: An Emerging Role in Severe Acute Respiratory Syndrome Coronavirus 2
Abstract
1. Introduction
2. Gal-3BP and Viral Infections
3. Gal-3BP and SARS-CoV-2 Infection
3.1. Role of Gal-3BP in Severe COVID-19 Disease and Pulmonary Fibrosis
3.2. Interaction of Gal-3BP with SARS-CoV-2 Components and Spike Protein
3.3. Clinical Significance of Gal-3BP in SARS-CoV-2 Infection
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koths, K.; Taylor, E.; Halenbeck, R.; Casipit, C.; Wang, A. Cloning and characterization of a human Mac-2-binding protein, a new member of the superfamily defined by the macrophage scavenger receptor cysteine-rich domain. J. Biol. Chem. 1993, 268, 14245–14249. [Google Scholar] [CrossRef]
- Calabrese, G.; Sures, I.; Pompetti, F.; Natoli, G.; Palka, G.; Iacobelli, S. The gene (LGALS3BP) encoding the serum protein 90K, associated with cancer and infection by the human immunodeficiency virus, maps at 17q25. Cytogenet. Cell. Genet. 1995, 69, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Inohara, H.; Akahani, S.; Koths, K.; Raz, A. Interactions between galectin-3 and Mac-2-binding protein mediate cell-cell adhesion. Cancer Res. 1996, 56, 4530–4534. [Google Scholar] [PubMed]
- Sasaki, T.; Brakebusch, C.; Engel, J.; Timpl, R. Mac-2 binding protein is a cell-adhesive protein of the extracellular matrix which self-assembles into ring-like structures and binds beta1 integrins, collagens and fibronectin. EMBO J. 1998, 17, 1606–1613. [Google Scholar] [CrossRef]
- Iacobelli, S.; Arnò, E.; D’Orazio, A.; Coletti, G. Detection of antigens recognized by a novel monoclonal antibody in tissue and serum from patients with breast cancer. Cancer Res. 1986, 46, 3005–3010. [Google Scholar]
- Linsley, P.S.; Horn, D.; Marquardt, H.; Brown, J.P.; Hellstroem, I.; Hellstroem, K.E.; Ochs, V.; Tolentino, E. Identification of a novel serum protein secreted by lung carcinoma cells. Biochemistry 1986, 25, 2978–2986. [Google Scholar] [CrossRef]
- Fornarini, B.; Iacobelli, S.; Tinari, N.; Natoli, C.; De Martino, M.; Sabatino, G. Human milk 90K (Mac-2 BP): Possible protective effects against acute respiratory infections. Clin. Exp. Immunol. 1999, 115, 91–94. [Google Scholar] [CrossRef]
- Grassadonia, A.; Tinari, N.; Iurisci, I.; Piccolo, E.; Cumashi, A.; Innominato, P.; D’Egidio, M.; Natoli, C.; Piantelli, M.; Iacobelli, S. 90K (Mac-2 BP) and galectins in tumor progression and metastasis. Glycoconj. J. 2002, 19, 551–556. [Google Scholar] [CrossRef]
- Bair, E.L.; Nagle, R.B.; Ulmer, T.A.; Laferté, S.; Bowden, G.T. 90K/Mac-2 binding protein is expressed in prostate cancer and induces promatrilysin expression. Prostate 2006, 66, 283–293. [Google Scholar] [CrossRef]
- Dumic, J.; Dabelic, S.; Flögel, M. Galectin-3: An open-ended story. Biochim. Biophys. Acta 2006, 1760, 616–635. [Google Scholar] [CrossRef]
- Laferté, S.; Loh, L.C.; Keeler, V. Monoclonal antibodies specific for human tumor-associated antigen 90K/Mac-2 binding protein: Tools to examine protein conformation and function. J. Cell. Biochem. 2000, 77, 540–559. [Google Scholar] [CrossRef]
- Hellstern, S.; Sasaki, T.; Fauser, C.; Lustig, A.; Timpl, R.; Engel, J. Functional studies on recombinant domains of Mac-2-binding protein. J. Biol. Chem. 2002, 277, 15690–15696. [Google Scholar] [CrossRef]
- Yu, B.; Wright, S.D. LPS-dependent interaction of Mac-2-binding protein with immobilized CD14. J. Inflamm. 1995, 45, 115–125. [Google Scholar]
- Loimaranta, V.; Hepojoki, J.; Laaksoaho, O.; Pulliainen, A.T. Galectin-3-binding protein: A multitask glycoprotein with innate immunity functions in viral and bacterial infections. J. Leukoc. Biol. 2018, 104, 777–786. [Google Scholar] [CrossRef]
- Sarrias, M.R.; Grønlund, J.; Padilla, O.; Madsen, J.; Holmskov, U.; Lozano, F. The Scavenger Receptor Cysteine-Rich (SRCR) domain: An ancient and highly conserved protein module of the innate immune system. Crit. Rev. Immunol. 2004, 24, 1–37. [Google Scholar] [CrossRef]
- Martínez, V.G.; Moestrup, S.K.; Holmskov, U.; Mollenhauer, J.; Lozano, F. The conserved scavenger receptor cysteine-rich superfamily in therapy and diagnosis. Pharmacol Rev. 2011, 63, 967–1000. [Google Scholar] [CrossRef]
- Stogios, P.J.; Downs, G.S.; Jauhal, J.J.; Nandra, S.K.; Privé, G.G. Sequence and structural analysis of BTB domain proteins. Genome Biol. 2005, 6, R82. [Google Scholar] [CrossRef]
- Bardwell, V.J.; Treisman, R. The POZ domain: A conserved protein-protein interaction motif. Genes Dev. 1994, 8, 1664–1677. [Google Scholar] [CrossRef]
- Müller, S.A.; Sasaki, T.; Bork, P.; Wolpensinger, B.; Schulthess, T.; Timpl, R.; Engel, A.; Engel, J. Domain organization of Mac-2 binding protein and its oligomerization to linear and ring-like structures. J. Mol. Biol. 1999, 291, 801–813. [Google Scholar] [CrossRef]
- Nonaka, M.; Ma, B.Y.; Imaeda, H.; Kawabe, K.; Kawasaki, N.; Hodohara, K.; Kawasaki, N.; Andoh, A.; Fujiyama, Y.; Kawasaki, T. Dendritic cell-specific intercellular adhesion molecule 3-grabbing non-integrin (DC-SIGN) recognizes a novel ligand, Mac-2-binding protein, characteristically expressed on human colorectal carcinomas. J. Biol. Chem. 2011, 286, 22403–22413. [Google Scholar] [CrossRef]
- Anthony, R.M.; Wermeling, F.; Karlsson, M.C.; Ravetch, J.V. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc. Natl. Acad. Sci. USA 2008, 105, 19571–19578. [Google Scholar] [CrossRef] [PubMed]
- Natoli, C.; Iacobelli, S.; Ghinelli, F. Unusually high level of a tumor-associated antigen in the serum of human immunodeficiency virus-seropositive individuals. J. Infect. Dis. 1991, 164, 616–617. [Google Scholar] [CrossRef]
- Rea, A.; Palmieri, G.; Tinari, N.; Natoli, C.; Tagliaferri, P.; Morabito, A.; Grassadonia, A.; Bianco, A.; Iacobelli, S. 90k is a serum marker of poor-prognosis in non-hodgkins-lymphoma patients. Oncol. Rep. 1994, 1, 723–725. [Google Scholar] [CrossRef]
- Marchetti, A.; Tinari, N.; Buttitta, F.; Chella, A.; Angeletti, C.A.; Sacco, R.; Mucilli, F.; Ullrich, A.; Iacobelli, S. Expression of 90K (Mac-2 BP) correlates with distant metastasis and predicts survival in stage I non-small cell lung cancer patients. Cancer Res. 2002, 62, 2535–2539. [Google Scholar]
- Ostergaard, O.; Nielsen, C.T.; Iversen, L.V.; Tanassi, J.T.; Knudsen, S.; Jacobsen, S.; Heegaard, N.H.H. Unique protein signature of circulating microparticles in systemic lupus erythematosus. Arthritis Rheum. 2013, 65, 2680–2690. [Google Scholar] [CrossRef]
- Liu, K.T.; Liu, Y.H.; Chen, Y.H.; Lin, C.Y.; Huang, C.H.; Yen, M.C.; Kuo, P. Serum Galectin-9 and Galectin-3-Binding Protein in Acute Dengue Virus Infection. Int. J. Mol. Sci. 2016, 17, 832. [Google Scholar] [CrossRef]
- Cecamore, C.; Marsili, M.; Salvatore, R.; Troiani, R.; D’Egidio, M.; Tinari, N.; Pelliccia, P.; Chiarelli, F.; Marcovecchio, M.L.; Breda, L. 90K immunostimulatory glycoprotein in children with juvenile idiopathic arthritis. Mod. Rheumatol. 2018, 28, 637–641. [Google Scholar] [CrossRef]
- Briggs, N.C.; Natoli, C.; Tinari, N.; D’Egidio, M.; Goedert, J.J.; Iacobelli, S. A 90-kDa protein serum marker for the prediction of progression to AIDS in a cohort of HIV-1+ homosexual men. AIDS Res. Hum. Retrovir. 1993, 9, 811–816. [Google Scholar] [CrossRef]
- Natoli, C.; Dianzani, F.; Mazzotta, F.; Balocchini, E.; Pierotti, P.; Antonelli, G.; Iacobelli, S. 90K protein: A new predictor marker of disease progression in human immunodeficiency virus infection. J. Acquir. Immune Defic. Syndr. 1993, 6, 370–375. [Google Scholar]
- Cesinaro, A.M.; Natoli, C.; Grassadonia, A.; Tinari, N.; Iacobelli, S.; Trentini, G.P. Expression of the 90K tumor-associated protein in benign and malignant melanocytic lesions. J. Investig. Dermatol. 2002, 119, 187–190. [Google Scholar] [CrossRef]
- Mbeunkui, F.; Metge, B.J.; Shevde, L.A.; Pannell, L.K. Identification of differentially secreted biomarkers using LC-MS/MS in isogenic cell lines representing a progression of breast cancer. J. Proteome Res. 2007, 6, 2993–3002. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.K.; Jang, J.E.; Kang, J.H.; Seong, J.K.; Yoon, Y.S.; Kim, H.C.; Lee, S.J.; Oh, S.H. Lectin, Galactoside-Binding Soluble 3 Binding Protein Promotes 17-N-Allylamino-17-demethoxygeldanamycin Resistance through PI3K/Akt Pathway in Lung Cancer Cell Line. Mol. Cancer Ther. 2017, 16, 1355–1365. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, S.; Chen, J.; Li, D. Increased LGALS3 expression independently predicts shorter overall survival in patients with the proneural subtype of glioblastoma. Cancer Med. 2019, 8, 2031–2040. [Google Scholar] [CrossRef] [PubMed]
- Capone, E.; Iacobelli, S.; Sala, G. Role of galectin 3 binding protein in cancer progression: A potential novel therapeutic target. J. Transl. Med. 2021, 26, 405. [Google Scholar] [CrossRef]
- Iacovazzi, P.A.; Cozzolongo, R.; Lanzillotta, E.; Frisullo, S.; Guerra, V.; Correale, M. Serum 90K/Mac-2 binding protein (Mac-2BP) as a response predictor to peginterferon and ribavirin combined treatment in HCV chronic patients. Immunopharmacol. Immunotoxicol. 2008, 30, 687–700. [Google Scholar] [CrossRef]
- Artini, M.; Natoli, C.; Tinari, N.; Costanzo, A.; Marinelli, R.; Balsano, C.; Porcari, P.; Angelucci, D.; D’Egidio, M.; Levrero, M.; et al. Elevated serum levels of 90K/MAC-2 BP predict unresponsiveness to alpha-interferon therapy in chronic HCV hepatitis patients. J. Hepatol. 1996, 25, 212–217. [Google Scholar] [CrossRef]
- Hepojoki, J.; Strandin, T.; Hetzel, U.; Sironen, T.; Klingström, J.; Sane, J.; Mäkelä, S.; Mustonen, J.; Meri, S.; Lundkvist, A.; et al. Acute hantavirus infection induces galectin-3-binding protein. J. Gen. Virol. 2014, 95, 2356–2364. [Google Scholar] [CrossRef]
- Filer, A.; Bik, M.; Parsonage, G.N.; Fitton, J.; Trebilcock, E.; Howlett, K.; Cook, M.; Raza, K.; Simmons, D.L.; Thomas, A.M.C.; et al. Galectin 3 induces a distinctive pattern of cytokine and chemokine production in rheumatoid synovial fibroblasts via selective signaling pathways. Arthritis Rheum. 2009, 60, 1604–1614. [Google Scholar] [CrossRef]
- Kuśnierz-Cabala, B.; Maziarz, B.; Dumnicka, P.; Dembiński, M.; Kapusta, M.; Bociąga-Jasik, M.; Winiarski, M.; Garlicki, A.; Grodzicki, T.; Kukla, M. Diagnostic Significance of Serum Galectin-3 in Hospitalized Patients with COVID-19-A Preliminary Study. Biomolecules 2021, 11, 1136. [Google Scholar] [CrossRef]
- Gallo, V.; ISERC-Team; Gentile, R.; Antonini, G.; Iacobelli, S. Increased Gal-3BP plasma levels in hospitalized patients infected with SARS-CoV-2. Clin. Exp. Med. 2022, 25, 1–5. [Google Scholar] [CrossRef]
- Portacci, A.; Diaferia, F.; Santomasi, C.; Dragonieri, S.; Boniello, E.; Serio, F.D.; Carpagnano, G.E. Galectin-3 as prognostic biomarker in patients with COVID-19 acute respiratory failure. Respir. Med. 2021, 187, 106556. [Google Scholar] [CrossRef]
- Xu, G.; Xia, Z.; Deng, F.; Liu, L.; Wang, Q.; Yu, Y.; Wang, F.; Zhu, C.; Liu, W.; Cheng, Z.; et al. Inducible LGALS3BP/90K activates antiviral innate immune responses by targeting TRAF6 and TRAF3 complex. PLoS Pathog. 2019, 15, e1008002. [Google Scholar] [CrossRef]
- Zhang, X.L.; Topley, N.; Ito, T.; Phillips, A. Interleukin-6 regulation of transforming growth factor (TGF)-beta receptor compartmentalization and turnover enhances TGF-beta1 signaling. J. Biol. Chem. 2005, 280, 12239–12245. [Google Scholar] [CrossRef]
- Luckett-Chastain, L.R.; Gallucci, R.M. Interleukin (IL)-6 modulates transforming growth factor-beta expression in skin and dermal fibroblasts from IL-6-deficient mice. Br. J. Dermatol. 2009, 161, 237–248. [Google Scholar] [CrossRef]
- Iacobelli, S.; Natoli, C.; D’Egidio, M.; Tamburrini, E.; Antinori, A.; Ortona, L. Lipoprotein 90K in human immunodeficiency virus-infected patients: A further serologic marker of progression. J. Infect. Dis. 1991, 164, 819. [Google Scholar] [CrossRef]
- Pelliccia, P.; Galli, L.; de Martino, M.; Chiarelli, F.; Verrotti, A.; Sabatino, G.; Fornarini, B.; Iacobelli, S.; Natoli, C. Lack of mother-to-child HIV-1 transmission is associated with elevated serum levels of 90 K immune modulatory protein. AIDS 2000, 14, F41–F45. [Google Scholar] [CrossRef]
- Lodermeyer, V.; Suhr, K.; Schrott, N.; Kolbe, C.; Stürzel, C.M.; Krnavek, D.; Münch, J.; Dietz, C.; Waldmann, T.; Kirchhoff, F.; et al. 90K, an interferon-stimulated gene product, reduces the infectivity of HIV-1. Retrovirology 2013, 10, 111. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, X.; Han, Y.; Wang, X.; Gao, G. M2BP inhibits HIV-1 virion production in a vimentin filaments-dependent manner. Sci. Rep. 2016, 6, 32736. [Google Scholar] [CrossRef]
- Iacobelli, S.; Scambia, G.; Natoli, C.; Panici, P.B.; Baiocchi, G.; Perrone, L.; Mancuso, S. Recombinant human leukocyte interferon-alpha 2b stimulates the synthesis and release of a 90K tumor-associated antigen in human breast cancer cells. Int. J. Cancer 1988, 42, 182–184. [Google Scholar] [CrossRef]
- Marth, C.; Dreps, A.; Natoli, C.; Zeimet, A.G.; Lang, T.; Widschwendter, M.; Daxenbichler, G.; Ullrich, A.; Iacobelli, S. Effects of type-I and -II interferons on 90K antigen expression in ovarian carcinoma cells. Int. J. Cancer 1994, 59, 808–813. [Google Scholar] [CrossRef]
- D’Ostilio, N.; Natoli, C.; Grassadonia, A.; Rossi, N. Prognostic value of a novel interferon-inducible 90K tumor antigen. Ann. N. Y. Acad. Sci. 1996, 784, 288–293. [Google Scholar] [CrossRef]
- Grassadonia, A.; Tinari, N.; Fiorentino, B.; Nakazato, M.; Chung, H.K.; Giuliani, C.; Napolitano, G.; Iacobelli, S.; Howcroft, T.K.; Singer, D.S.; et al. Upstream stimulatory factor regulates constitutive expression and hormonal suppression of the 90K (Mac-2BP) protein. Endocrinology 2007, 148, 3507–3517. [Google Scholar] [CrossRef][Green Version]
- Park, Y.P.; Choi, S.C.; Kim, B.Y.; Kim, J.; Song, E.Y.; Kang, S.O.; Yoon, D.; Paik, S.; Kim, K.D.; Kim, J.W.; et al. Induction of Mac-2BP by nerve growth factor is regulated by the PI3K/Akt/NF-kappaB-dependent pathway in the HEK293 cell line. BMB Rep. 2008, 41, 784–789. [Google Scholar] [CrossRef]
- Noma, N.; Simizu, S.; Kambayashi, Y.; Kabe, Y.; Suematsu, M.; Umezawa, K. Involvement of NF-κB-mediated expression of galectin-3-binding protein in TNF-α-induced breast cancer cell adhesion. Oncol. Rep. 2012, 27, 2080–2084. [Google Scholar] [CrossRef]
- Grassadonia, A.; Tinari, N.; Fiorentino, B.; Suzuki, K.; Nakazato, M.; De Tursi, M.; Giuliani, C.; Napolitano, G.; Singer, D.S.; Iacobelli, S. The 90K protein increases major histocompatibility complex class I expression and is regulated by hormones, gamma-interferon, and double-strand polynucleotides. Endocrinology 2004, 145, 4728–4736. [Google Scholar] [CrossRef][Green Version]
- Fukaya, Y.; Shimada, H.; Wang, L.C.; Zandi, E.; DeClerck, Y.A. Identification of galectin-3-binding protein as a factor secreted by tumor cells that stimulates interleukin-6 expression in the bone marrow stroma. J. Biol. Chem. 2008, 283, 18573–18581. [Google Scholar] [CrossRef]
- Song, L.; Asgharzadeh, S.; Salo, J.; Engell, K.; Wu, H.W.; Sposto, R.; Ara, T.; Silverman, A.M.; DeClerck, Y.A.; Seeger, R.C.; et al. Valpha24-invariant NKT cells mediate antitumor activity via killing of tumor-associated macrophages. J. Clin. Investig. 2009, 119, 1524–1536. [Google Scholar] [CrossRef]
- Silverman, A.M.; Nakata, R.; Shimada, H.; Sposto, R.; DeClerck, Y.A. A galectin-3-dependent pathway upregulates interleukin-6 in the microenvironment of human neuroblastoma. Cancer Res. 2012, 72, 2228–2238. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, A.; Sures, I.; D’Egidio, M.; Jallal, B.; Powell, T.J.; Herbst, R.; Dreps, A.; Azam, M.; Rubinstein, M.; Natoli, C.; et al. The secreted tumor-associated antigen 90K is a potent immune stimulator. J. Biol. Chem. 1994, 269, 18401–18407. [Google Scholar] [CrossRef]
- Kalayci, O.; Birben, E.; Tinari, N.; Oguma, T.; Iacobelli, S.; Lilly, C.M. Role of 90K protein in asthma and TH2-type cytokine expression. Ann. Allergy Asthma Immunol. 2004, 93, 485–492. [Google Scholar] [CrossRef]
- Powell, T.J.; Schreck, R.; McCall, M.; Hui, T.; Rice, A.; App, H.; Azam, M.; Ullrich, A.; Shawver, L.K. A tumor-derived protein which provides T-cell costimulation through accessory cell activation. J. Immunother. Emphas. Tumor Immunol. 1995, 17, 209–221. [Google Scholar] [CrossRef]
- Gleissner, C.A.C.; Erbel, F.; Linden, G.; Domschke, M.; Akhavanpoor, C.M.; Helmes, C.M.; Doesch, A.O.; Kleber, M.E.; Katus, H.A.; Maerz, W. Galectin-3 binding protein, coronary artery disease and cardiovascular mortality: Insights from the LURIC study Atherosclerosis. J. Atheroscler. 2017, 260, 121–129. [Google Scholar] [CrossRef]
- Lin, T.W.; Chang, H.T.; Chen, C.H.; Chen, C.H.; Lin, S.W.; Hsu, T.L.; Wong, C.H. Galectin-3 Binding Protein and Galectin-1 Interaction in Breast Cancer Cell Aggregation and Metastasis. J. Am. Chem. Soc. 2015, 137, 9685–9693. [Google Scholar] [CrossRef]
- White, M.J.; Roife, D.; Gomer, R.H. Galectin-3 binding protein secreted by breast cancer cells inhibits monocyte-derived fibrocyte differentiation. J. Immunol. 2015, 195, 1858–1867. [Google Scholar] [CrossRef]
- Hong, C.S.; Park, M.; Sun, E.; Choi, W.; Hwang, J.; Bae, W.; Rhee, J.H.; Cho, S.; Chung, I. Gal-3BP Negatively Regulates NF-κB Signaling by Inhibiting the Activation of TAK1. Front. Immunol. 2019, 10, 1760. [Google Scholar] [CrossRef]
- Denard, J.; Beley, C.; Kotin, R.; Lai-Kuen, R.; Blot, S.; Leh, H.; Asokan, A.; Samulski, R.J.; Moullier, P.; Voit, T.; et al. Human galectin 3 binding protein interacts with recombinant adeno-associated virus type 6. J. Virol. 2012, 86, 6620–6631. [Google Scholar] [CrossRef]
- Caniglia, J.L.; Guda, M.R.; Asuthkar, S.; Tsung, A.J.; Velpula, K.K. A potential role for Galectin-3 inhibitors in the treatment of COVID-19. PeerJ 2020, 15, e9392. [Google Scholar] [CrossRef]
- Fantini, J.; Di Scala, C.; Chahinian, H.; Yahi, N. Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int. J. Antimicrob. Agents 2020, 55, 105960. [Google Scholar] [CrossRef]
- Ou, X.; Liu, Y.; Lei, X.; Li, P.; Mi, D.; Ren, L.; Guo, L.; Guo, R.; Chen, T.; Hu, J.; et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020, 11, 1620. [Google Scholar] [CrossRef]
- Braga, L.; Ali, H.; Secco, I.; Chiavacci, E.; Neves, G.; Goldhill, D.; Penn, R.; Jimenez-Guardeño, J.M.; Ortega-Prieto, A.M.; Bussani, R.; et al. Drugs that inhibit TMEM16 proteins block SARS-CoV-2 spike-induced syncytia. Nature 2021, 594, 88–93. [Google Scholar] [CrossRef]
- Messner, C.B.; Demichev, V.; Wendisch, D.; Michalick, L.; White, M.; Freiwald, A.; Textoris-Taube, K.; Vernardis, S.I.; Egger, A.S.; Kreidl, M. Ultra-High-Throughput Clinical Proteomics Reveals Classifiers of COVID-19 Infection. Cell Syst. 2020, 11, 11–24.e4. [Google Scholar] [CrossRef]
- Gutmann, C.; Takov, K.; Burnap, S.A.; Singh, B.; Ali, H.; Theofilatos, K.; Reed, E.; Hasman, M.; Nabeebaccus, A.; Fish, M.; et al. SARS-CoV-2 RNAemia and proteomic trajectories inform prognostication in COVID-19 patients admitted to intensive care. Nat. Commun. 2021, 12, 3406. [Google Scholar] [CrossRef]
- Geyer, P.E.; Arend, F.M.; Doll, S.; Louiset, M.L.; Virreira Winter, S.; Müller-Reif, J.B.; Torun, F.M.; Weigand, M.; Eichhorn, P.; Bruegel, M.; et al. High-resolution serum proteome trajectories in COVID-19 reveal patient-specific seroconversion. EMBO Mol. Med. 2021, 9, e14167. [Google Scholar] [CrossRef]
- Zeng, F.; Huang, Y.; Guo, Y.; Yin, M.; Chen, X.; Xiao, L.; Deng, G. Association of inflammatory markers with the severity of COVID-19: A meta-analysis. Int. J. Infect. Dis. 2020, 96, 467–474. [Google Scholar] [CrossRef]
- Zhang, J.; Hao, Y.; Ou, W.; Ming, F.; Liang, G.; Qian, Y.; Cai, Q.; Dong, S.; Hu, S.; Wang, W.; et al. Serum interleukin-6 is an indicator for severity in 901 patients with SARS-CoV-2 infection: A cohort study. J. Transl. Med. 2020, 18, 406. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; the Northwell COVID-19 Research Consortium; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; et al. The Northwell COVID-19 Research Consortium Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Osuchowski, M.F.; Winkler, M.S.; Skirecki, T.; Cajander, S.; Shankar-Hari, M.; Lachmann, G.; Monneret, G.; Venet, F.; Bauer, M.; Brunkhorst, F.M.; et al. The COVID-19 puzzle: Deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir. Med. 2021, 9, 622–642. [Google Scholar] [CrossRef]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease. J. Clin. Investig. 2019, 130, 2620–2629. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.Z. SARS-CoV-2 Virology. Infect. Dis. Clin. N. Am. 2022, 36, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.G.; Homer, R.J.; Zhu, Z.; Lanone, S.; Wang, X.; Koteliansky, V.; Shipley, J.M.; Gotwals, P.; Noble, P.; Chen, Q.; et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta(1). J. Exp. Med. 2001, 194, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Thompson, B.T.; Chambers, R.C.; Liu, K.D. Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2017, 377, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Gomes, M.; Kruglov, A.; Durek, P.; Heinrich, F.; Tizian, C.; Heinz, G.A.; Pascual-Reguant, A.; Du, W.; Mothes, R.; Fan, C.; et al. SARS-CoV-2 in severe COVID-19 induces a TGF-β-dominated chronic immune response that does not target itself. Nat. Commun. 2021, 12, 1961. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.X.; Luo, R.C.; Wang, J.Q.; Chen, Z.S. Features of Cytokine Storm Identified by Distinguishing Clinical Manifestations in COVID-19. Front. Public. Health 2021, 9, 671788. [Google Scholar] [CrossRef]
- Windisch, D.; Dietrich, O.; Mari, T.; von Stillfried, S.; Ibarra, I.L.; Mittermaier, M.; Mache, C.; Chua, R.L.; Knoll, R.; Timm, S.; et al. SARS-CoV-2 infection triggers profibrotic macrophage responses and lung fibrosis. Cell 2021, 184, 6243–6261.e27. [Google Scholar] [CrossRef]
- Haudek, K.C.; Spronk, K.J.; Voss, P.G.; Patterson, R.J.; Wang, J.L.; Arnoys, E.J. Dynamics of galectin-3 in the nucleus and cytoplasm. Biochim. Biophys. Acta 2010, 1800, 181–189. [Google Scholar] [CrossRef]
- Nakahara, S.; Raz, A. On the role of galectins in signal transduction. Methods Enzymol. 2006, 417, 273–289. [Google Scholar] [CrossRef]
- Mackinnon, A.C.; Gibbons, M.A.; Farnworth, S.L.; Leffler, H.; Nilsson, U.J.; Delaine, T.; Simpson, A.J.; Forbes, S.J.; Hirani, N.; Gauldie, J.; et al. Regulation of transforming growth factor-β1-driven lung fibrosis by galectin-3. Am. J. Respir. Crit. Care Med. 2012, 185, 537–546. [Google Scholar] [CrossRef]
- Gawlik, K.I.; Holmberg, J.; Svensson, M.; Einerborg, M.; Oliveira, B.M.; Deierborg, T.; Durbeej, M. Potent pro-inflammatory and pro-fibrotic molecules, osteopontin and galectin-3, are not major disease modulators of laminin α2 chain-deficient muscular dystrophy. Sci. Rep. 2017, 7, 44059. [Google Scholar] [CrossRef]
- Hirani, N.; MacKinnon, A.C.; Nicol, L.; Ford, P.; Schambye, H.; Pedersen, A.; Nilsson, U.J.; Leffler, H.; Sethi, T.; Tantawi, S. Target inhibition of galectin-3 by inhaled TD139 in patients with idiopathic pulmonary fibrosis. Eur. Respir. J. 2021, 57, 2002559. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, Y.; Cao, L.; Wang, D.; Guo, M.; Jiang, A.; Guo, D.; Hu, W.; Yang, J.; Tang, Z.; et al. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg. Microbes Infect. 2020, 9, 761–770. [Google Scholar] [CrossRef]
- Schroeder, J.T.; Bieneman, A.P. The S1 Subunit of the SARS-CoV-2 Spike Protein Activates Human Monocytes to Produce Cytokines Linked to COVID-19: Relevance to Galectin-3. Front. Immunol. 2022, 13, 831766. [Google Scholar] [CrossRef]
- Lenza, M.P.; Oyenarte, I.; Diercks, T.; Quintana, J.I.; Gimeno, A.; Coelho, H.; Diniz, A.; Peccati, F.; Delgado, S.; Bosch, A.; et al. Structural characterization of the N-linked glycans in the receptor binding domain of the SARS-CoV-2 spike protein and their interactions with human lectins using NMR spectroscopy. Angew. Chem. Int. Ed. Engl. 2020, 59, 23763–23771. [Google Scholar] [CrossRef]
- Caniglia, J.L.; Asuthkar, S.; Tsung, A.J.; Guda, M.R.; Velpula, K.K. Immunopathology of galectin-3: An increasingly promising target in COVID-19. F1000Research 2020, 9, 1078. [Google Scholar] [CrossRef]
- Trbojević-Akmačić, I.; Petrović, T.; Lauc, G. SARS-CoV-2 S glycoprotein binding to multiple host receptors enables cell entry and infection. Glycoconj. J. 2021, 38, 611–623. [Google Scholar] [CrossRef]
- Wu, G.; Lu, Z.H.; Andre, S.; Gabius, H.J.; Ledeen, R.W. Functional interplay between ganglioside GM1 and cross-linking galectin-1 induces axon-like neuritogenesis via integrin-based signaling and TRPC5-dependent Ca2+ influx. J. Neurochem. 2016, 136, 550–563. [Google Scholar] [CrossRef]
- Kim, P.S.; Read, S.W.; Fauci, A.S. Therapy for early COVID-19a critical need. JAMA 2020, 324, 2149–2150. [Google Scholar] [CrossRef]
- Qi, J.; He, D.; Yang, D.; Wang, M.; Ma, W.; Cui, H.; Ye, F.; Wang, F.; Xu, J.; Li, Z.; et al. Severity-associated markers and assessment model for predicting the severity of COVID-19: A retrospective study in Hangzhou, China. BMC Infect. Dis. 2021, 21, 774. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Cervantes-Alvarez, E.; la Rosa, N.L.; la Mora, M.S.; Valdez-Sandoval, P.; Palacios-Jimenez, M.; Rodriguez-Alvarez, F.; Vera-Maldonado, B.I.; Aguirre-Aguilar, E.; Escobar-Valderrama, J.M.; Alanis-Mendizabal, J.; et al. Galectin-3 as a potential prognostic biomarker of severe COVID-19 in SARS-CoV-2 infected patients. Sci. Rep. 2022, 12, 1856. [Google Scholar] [CrossRef]
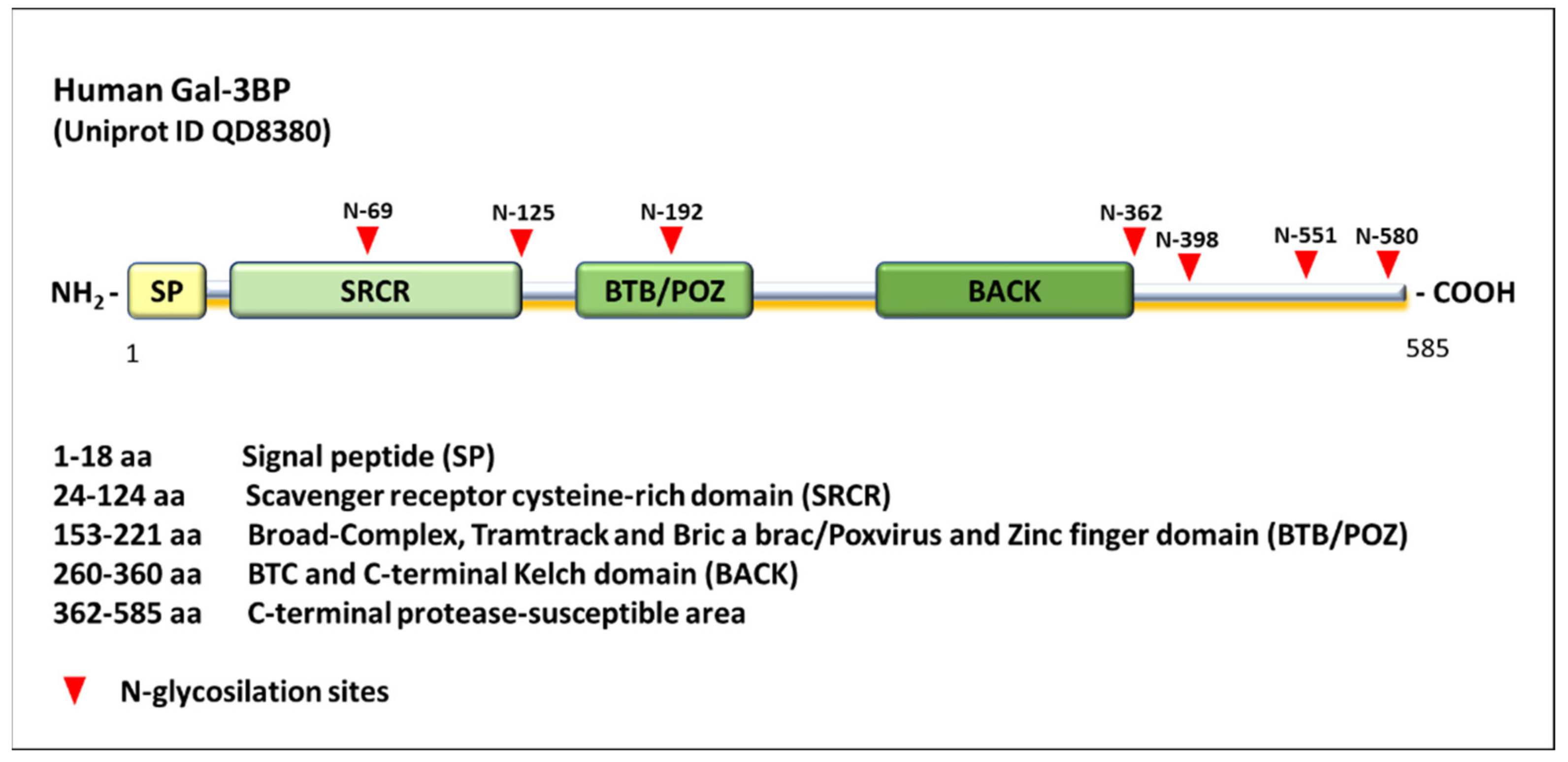
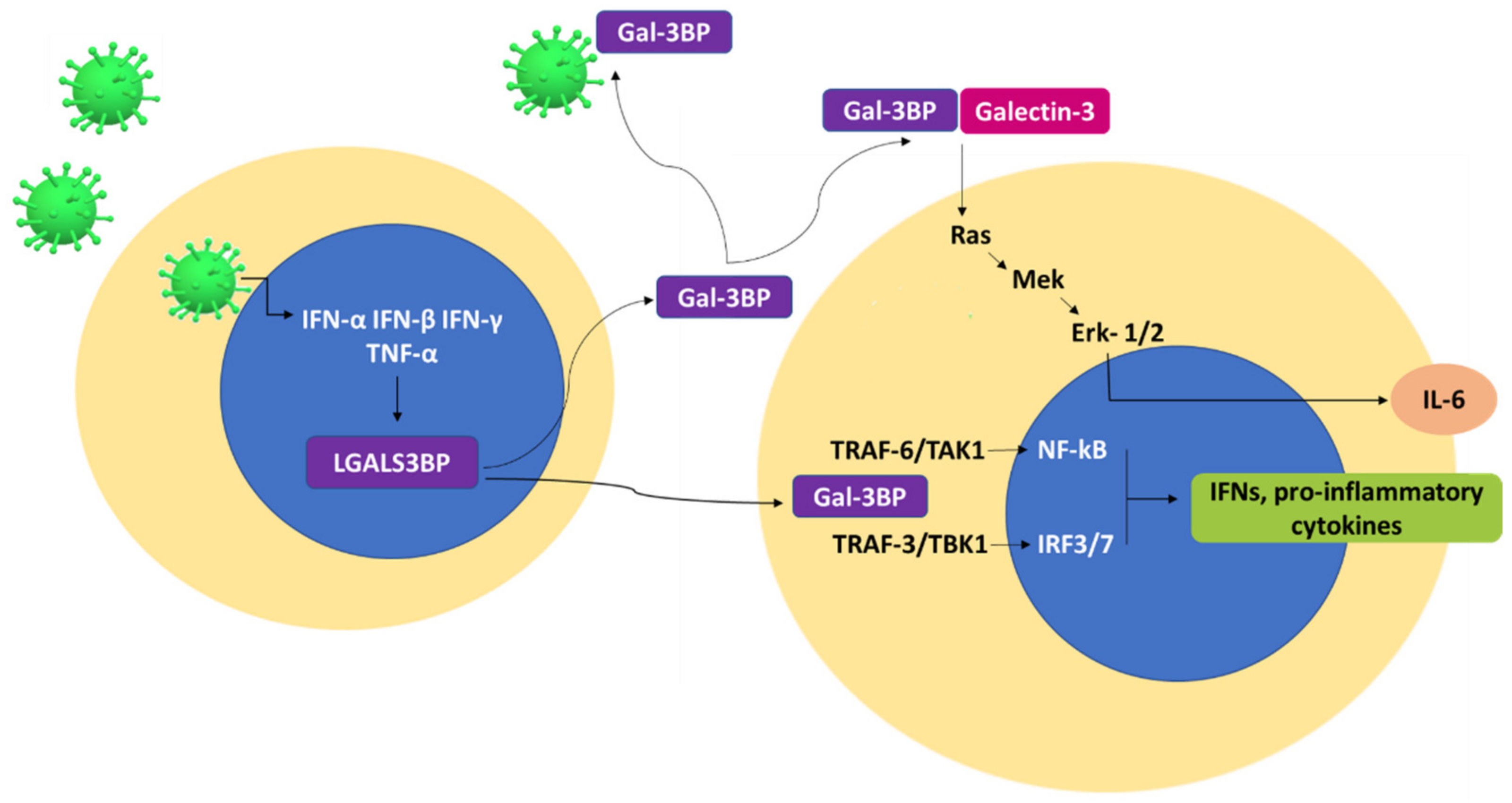
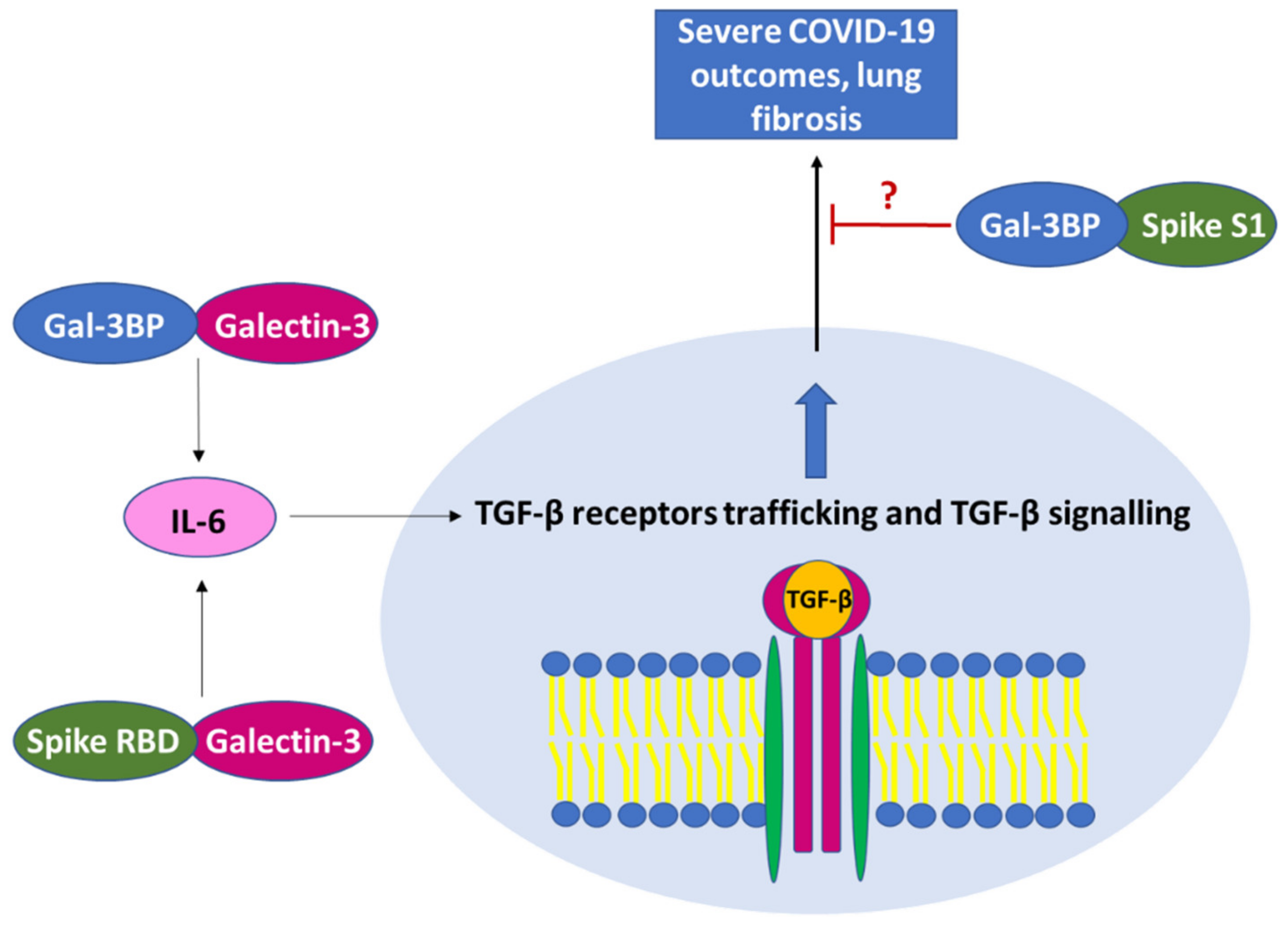
| Interaction Partner | Mechanism of Action | Effect | References |
| Galectin-3 | Induction of Ras-Mek-Erk1/2-signaling pathway. | IL-6 expression and secretion. | [56,57,58] |
| TRAF6/TAK1 | Translocation of NF-κB to the nucleus. | Expression of IFN and inflammatory cytokines. | [42] |
| TRAF3/TBK1 | Translocation of IRF3/7 to the nucleus. | Expression of IFN and inflammatory cytokines. | [42] |
| Viruses | Direct binding with adeno-associated Viruses. | Induces viral particle aggregation and impairment of transduction. | [66] |
| Direct binding with HIV-1. | Prevents transportation of HIV-1 Gag to the plasma membrane, inhibiting HIV-1 virion production; reduces the amount of gp120 and gp41 at the plasma membrane. | [14,48] | |
| Direct binding with SARS-CoV-2 components (i.e., spike protein). | Reduction in spike-mediated syncytia formation, and to a decreased spike-pseudo-particle entry. | [67,68,69,70] | |
| DC-SIGN | DC maturation and fibrocyte differentiation. | Immunosuppression. | [14,20,64] |
| Unknown | Unknown | Induces Ca2+ mobilization; increases the amount of ICAM-I and MHC-I on the cell surface. | [14] |
| Unknown | Induction of IL-1; IL-2; GM-CSF; and TNF-α. | Immunostimulatory effects. | [56,58,59,61] |
| Unknown | Suppression of IL-4, IL-5, and IL-13. | Immunosuppressive effects. | [60] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallo, V.; Arienzo, A.; Iacobelli, S.; Iacobelli, V.; Antonini, G. Gal-3BP in Viral Infections: An Emerging Role in Severe Acute Respiratory Syndrome Coronavirus 2. Int. J. Mol. Sci. 2022, 23, 7314. https://doi.org/10.3390/ijms23137314
Gallo V, Arienzo A, Iacobelli S, Iacobelli V, Antonini G. Gal-3BP in Viral Infections: An Emerging Role in Severe Acute Respiratory Syndrome Coronavirus 2. International Journal of Molecular Sciences. 2022; 23(13):7314. https://doi.org/10.3390/ijms23137314
Chicago/Turabian StyleGallo, Valentina, Alyexandra Arienzo, Stefano Iacobelli, Valentina Iacobelli, and Giovanni Antonini. 2022. "Gal-3BP in Viral Infections: An Emerging Role in Severe Acute Respiratory Syndrome Coronavirus 2" International Journal of Molecular Sciences 23, no. 13: 7314. https://doi.org/10.3390/ijms23137314
APA StyleGallo, V., Arienzo, A., Iacobelli, S., Iacobelli, V., & Antonini, G. (2022). Gal-3BP in Viral Infections: An Emerging Role in Severe Acute Respiratory Syndrome Coronavirus 2. International Journal of Molecular Sciences, 23(13), 7314. https://doi.org/10.3390/ijms23137314







