Anxiety-like Behavior and GABAAR/BDZ Binding Site Response to Progesterone Withdrawal in a Stress-Vulnerable Strain, the Wistar Kyoto Rats
Abstract
:1. Introduction
2. Results
2.1. Progesterone Withdrawal Induced Anxiety-like Behaviors
2.2. Locomotor Activity Was Not Altered Due to PW or Diazepam Administration
2.3. Corticosterone Serum Levels
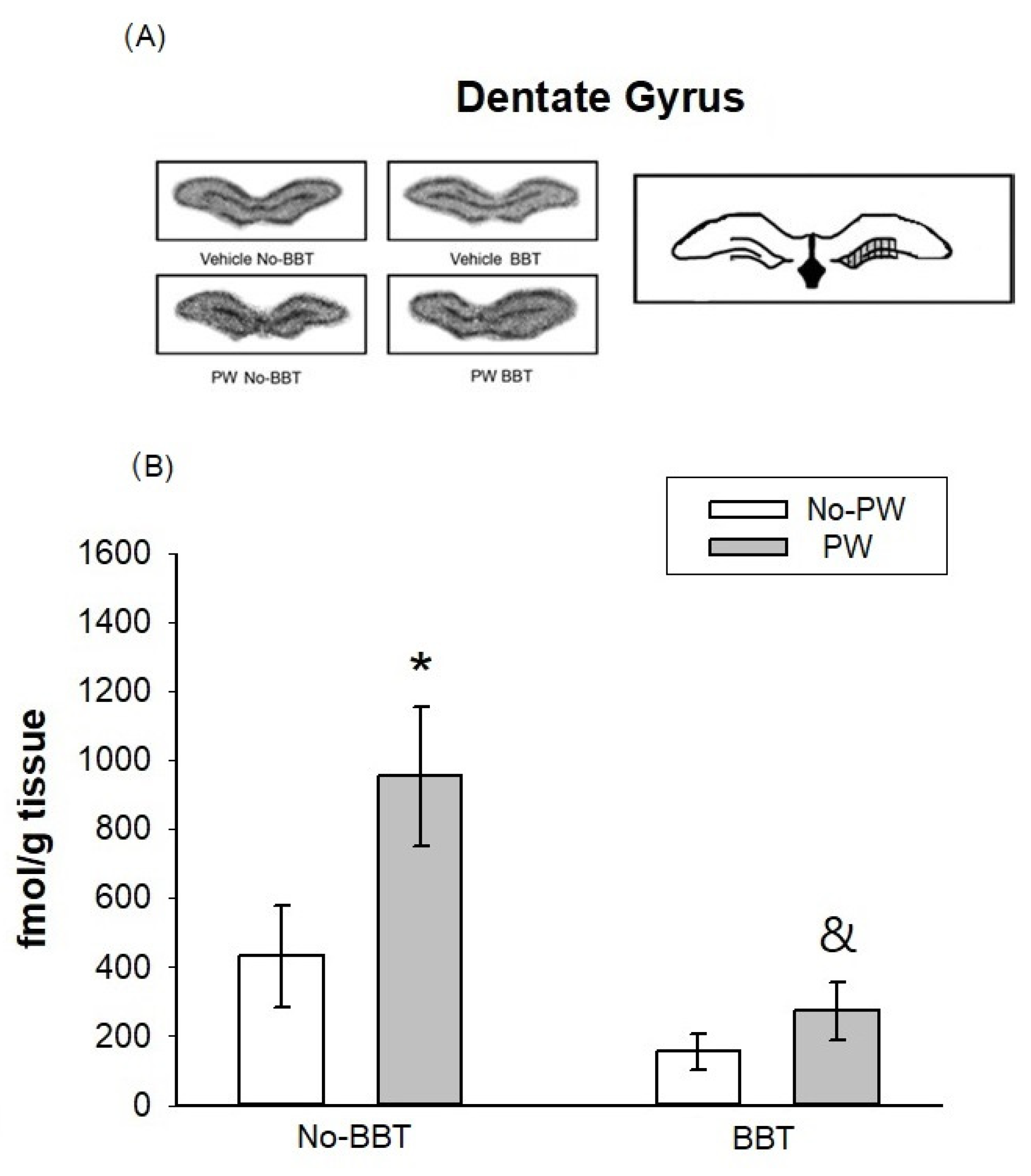
2.4. [3H]Flunitrazepam Binding Autoradiography in WKY Female Rats
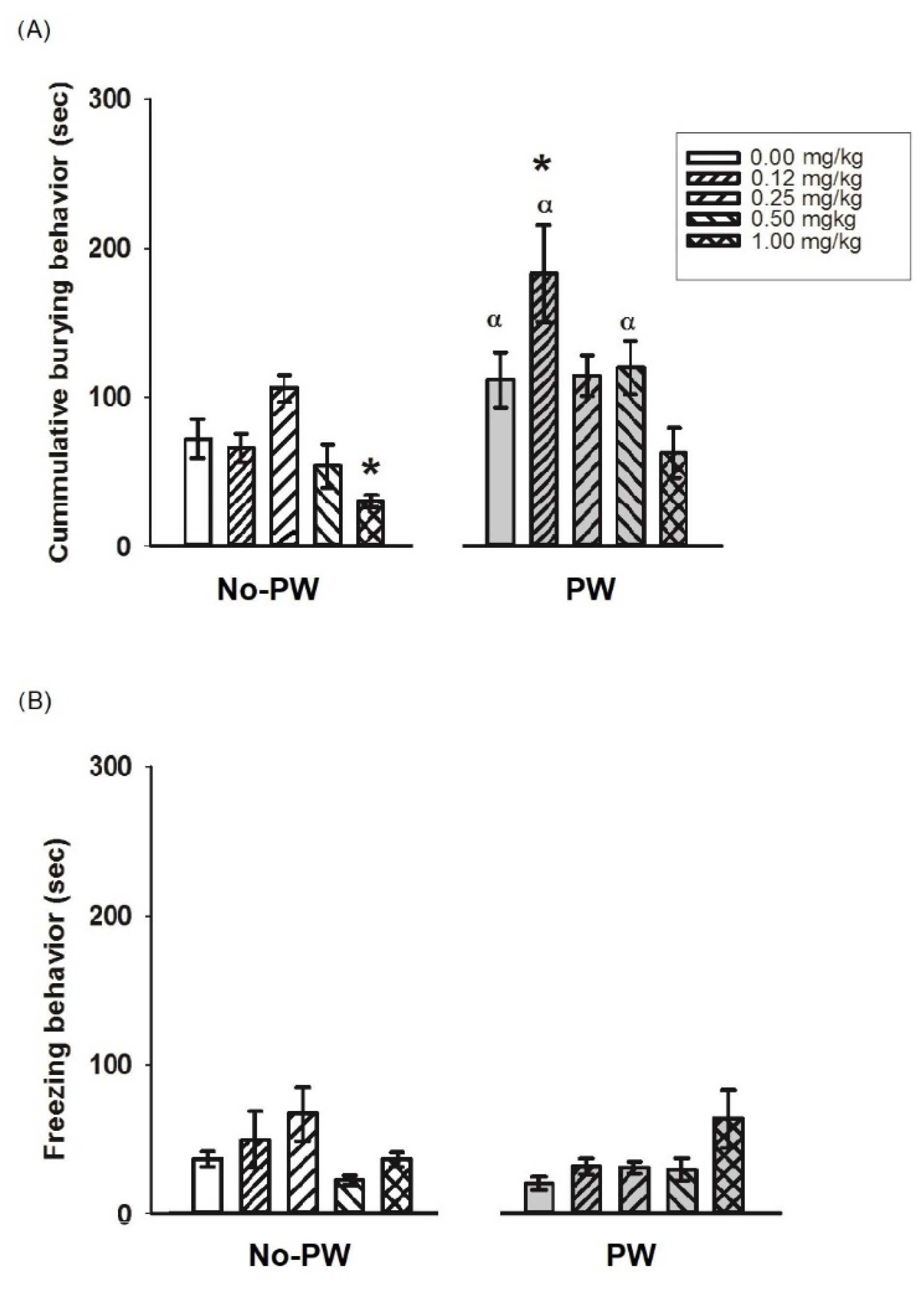
2.5. Diazepam Effects on Anxiety-like Behavior in WKY Rats
3. Discussion
3.1. Progesterone Withdrawal Induced Anxiety-like Behaviors in Wistar Kyoto Rats
3.2. [3H]Flunitrazepam Receptor Binding in WKY Rats Subjected to the Burying Behavioral Test and/or Progesterone Withdrawal Challenge
3.3. Behavioral Effect of Diazepam on Anxiety-like Behaviors Induced by PW
4. Materials and Methods
4.1. Animals
4.2. Ovariectomy
4.3. Progesterone Withdrawal (PW)
4.4. Behavioral Models
4.4.1. Locomotor Activity Test
4.4.2. Elevated Plus-Maze Test
4.4.3. Defensive Burying Behavior Test (BBT)
4.5. Determination of Corticosterone Levels
4.6. Evaluation of BDZ Binding Site in the GABAA Receptor Using [3H]-Flunitrazepam Autoradiography
4.7. Effects of Diazepam on Anxiety-like Behavior Induced by PW
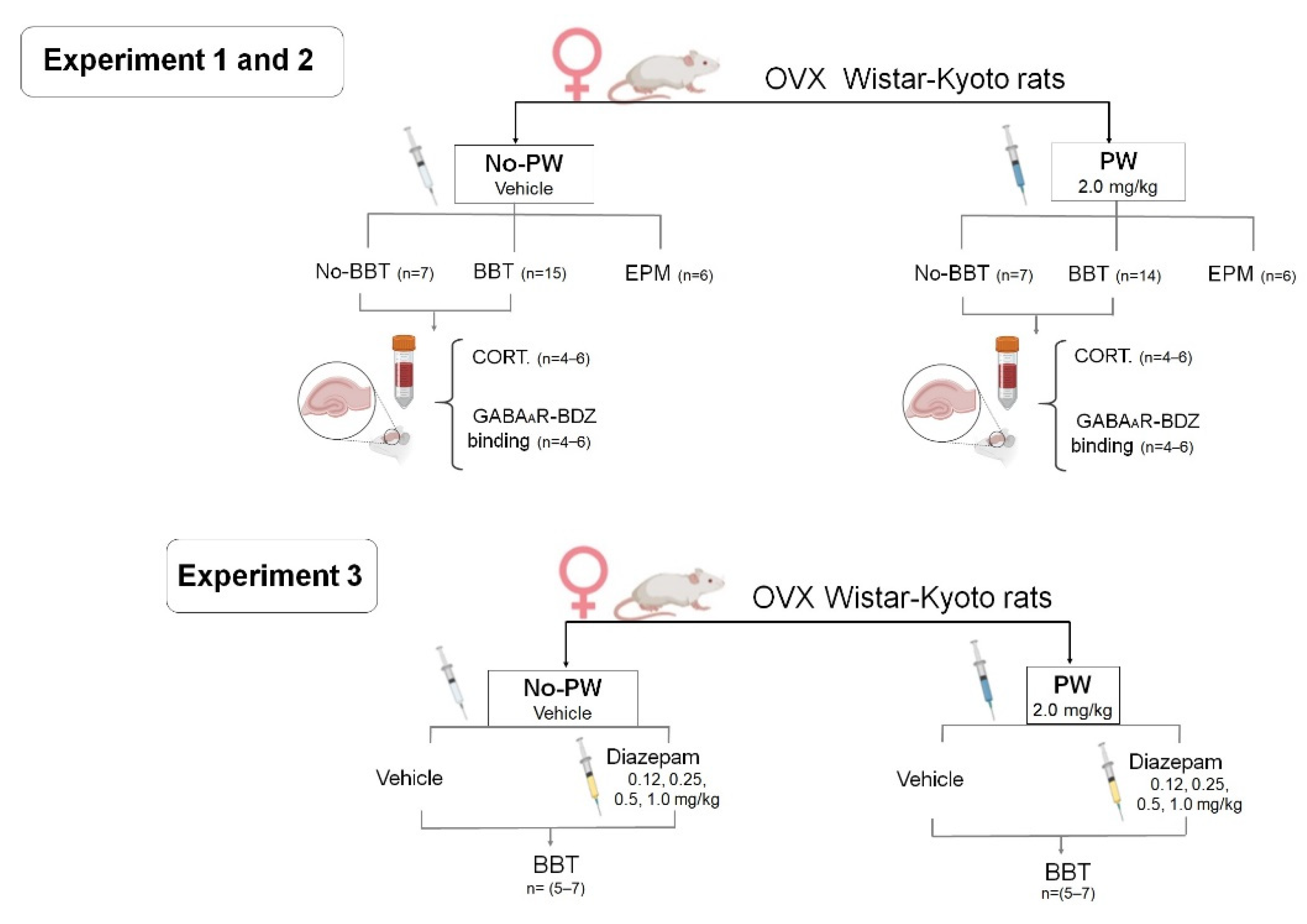
4.8. Experimental Design
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bäckström, T.; Bixo, M.; Johansson, M.; Nyberg, S.; Ossewaarde, L.; Ragagnin, G.; Savic, I.; Strömberg, J.; Timby, E.; van Broekhoven, F.; et al. Allopregnanolone and Mood Disorders. Prog. Neurobiol. 2014, 113, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Rapkin, A.J.; Akopians, A.L. Pathophysiology of Premenstrual Syndrome and Premenstrual Dysphoric Disorder. Menopause Int. 2012, 18, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Andreen, L.; Bixo, M.; Nyberg, S.; Sundstrom-Poromaa, I.; Backstrom, T. Progesterone Effects during Sequential Hormone Replacement Therapy. Eur. J. Endocrinol. 2003, 148, 571–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craner, J.; Sigmon, S.; Martinson, A.; McGillicuddy, M. Perceptions of Health and Somatic Sensations in Women Reporting Premenstrual Syndrome and Premenstrual Dysphoric Disorder. J. Nerv. Ment. Dis. 2013, 201, 780–785. [Google Scholar] [CrossRef]
- Yen, J.-Y.; Lin, P.-C.; Huang, M.-F.; Chou, W.-P.; Long, C.-Y.; Ko, C.-H. Association between Generalized Anxiety Disorder and Premenstrual Dysphoric Disorder in a Diagnostic Interviewing Study. Int. J. Environ. Res. Public. Health 2020, 17, 988. [Google Scholar] [CrossRef] [Green Version]
- Lombardi, I.; Luisi, S.; Quirici, B.; Monteleone, P.; Bernardi, F.; Liut, M.; Casarosa, E.; Palumbo, M.; Petraglia, F.; Genazzani, A.R. Adrenal Response to Adrenocorticotropic Hormone Stimulation in Patients with Premenstrual Syndrome. Gynecol. Endocrinol. 2004, 18, 79–87. [Google Scholar] [CrossRef]
- Lovick, T.A.; Guapo, V.G.; Anselmo-Franci, J.A.; Loureiro, C.M.; Faleiros, M.C.M.; Del Ben, C.M.; Brandão, M.L. A Specific Profile of Luteal Phase Progesterone Is Associated with the Development of Premenstrual Symptoms. Psychoneuroendocrinology 2017, 75, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Monteleone, P.; Luisi, S.; Tonetti, A.; Bernardi, F.; Genazzani, A.; Luisi, M.; Petraglia, F.; Genazzani, A. Allopregnanolone Concentrations and Premenstrual Syndrome. Eur. J. Endocrinol. 2000, 142, 269–273. [Google Scholar] [CrossRef] [Green Version]
- Rabin, D.S.; Schmidt, P.J.; Campbell, G.; Gold, P.W.; Jensvold, M.; Rubinow, D.R.; Chrousos, G.P. Hypothalamic-Pituitary-Adrenal Function in Patients with the Premenstrual Syndrome. J. Clin. Endocrinol. Metab. 1990, 71, 1158–1162. [Google Scholar] [CrossRef]
- Roca, C.A.; Schmidt, P.J.; Altemus, M.; Deuster, P.; Danaceau, M.A.; Putnam, K.; Rubinow, D.R. Differential Menstrual Cycle Regulation of Hypothalamic-Pituitary-Adrenal Axis in Women with Premenstrual Syndrome and Controls. J. Clin. Endocrinol. Metab. 2003, 88, 3057–3063. [Google Scholar] [CrossRef] [Green Version]
- Segebladh, B.; Bannbers, E.; Moby, L.; Nyberg, S.; Bixo, M.; Bäckström, T.; Sundström Poromaa, I. Allopregnanolone Serum Concentrations and Diurnal Cortisol Secretion in Women with Premenstrual Dysphoric Disorder. Arch. Womens Ment. Health 2013, 16, 131–137. [Google Scholar] [CrossRef]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. In Comprehensive Physiology; Terjung, R., Ed.; Wiley: Hoboken, NJ, USA, 2016; pp. 603–621. [Google Scholar] [CrossRef] [Green Version]
- Gjerstad, J.K.; Lightman, S.L.; Spiga, F. Role of Glucocorticoid Negative Feedback in the Regulation of HPA Axis Pulsatility. Stress 2018, 21, 403–416. [Google Scholar] [CrossRef] [Green Version]
- Almeida, F.B.; Barros, H.M.T.; Pinna, G. Neurosteroids and Neurotrophic Factors: What Is Their Promise as Biomarkers for Major Depression and PTSD? Int. J. Mol. Sci. 2021, 22, 1758. [Google Scholar] [CrossRef]
- Jankord, R.; Herman, J.P. Limbic Regulation of Hypothalamo-Pituitary-Adrenocortical Function during Acute and Chronic Stress. Ann. N. Y. Acad. Sci. 2008, 1148, 64–73. [Google Scholar] [CrossRef]
- Redei, E.; Freeman, E.W. Preliminary Evidence for Plasma Adrenocorticotropin Levels as Biological Correlates of Premenstrual Symptoms. Acta Endocrinol. 1993, 128, 536–542. [Google Scholar] [CrossRef]
- Girdler, S.S.; Pedersen, C.A.; Straneva, P.A.; Leserman, J.; Stanwyck, C.L.; Benjamin, S.; Light, K.C. Dysregulation of Cardiovascular and Neuroendocrine Responses to Stress in Premenstrual Dysphoric Disorder. Psychiatry Res. 1998, 81, 163–178. [Google Scholar] [CrossRef]
- Girdler, S.S.; Straneva, P.A.; Light, K.C.; Pedersen, C.A.; Morrow, A.L. Allopregnanolone Levels and Reactivity to Mental Stress in Premenstrual Dysphoric Disorder. Biol. Psychiatry 2001, 49, 788–797. [Google Scholar] [CrossRef]
- Beddig, T.; Reinhard, I.; Kuehner, C. Stress, Mood, and Cortisol during Daily Life in Women with Premenstrual Dysphoric Disorder (PMDD). Psychoneuroendocrinology 2019, 109, 104372. [Google Scholar] [CrossRef]
- Hou, L.; Huang, Y.; Zhou, R. Premenstrual Syndrome Is Associated with Altered Cortisol Awakening Response. Stress 2019, 22, 640–646. [Google Scholar] [CrossRef]
- Namavar Jahromi, B.; Pakmehr, S.; Hagh-Shenas, H. Work Stress, Premenstrual Syndrome and Dysphoric Disorder: Are There Any Associations? Iran. Red Crescent Med. J. 2011, 13, 199–202. [Google Scholar]
- Deuster, P.A.; Adera, T.; South-Paul, J. Biological, Social, and Behavioral Factors Associated With Premenstrual Syndrome. Arch. Fam. Med. 1999, 8, 122–128. [Google Scholar] [CrossRef]
- Takeda, T.; Tadakawa, M.; Koga, S.; Nagase, S.; Yaegashi, N. Premenstrual Symptoms and Posttraumatic Stress Disorder in Japanese High School Students 9 Months after the Great East-Japan Earthquake. Tohoku J. Exp. Med. 2013, 230, 151–154. [Google Scholar] [CrossRef] [Green Version]
- Gangisetty, O.; Reddy, D.S. Neurosteroid Withdrawal Regulates GABA-A Receptor A4-Subunit Expression and Seizure Susceptibility by Activation of Progesterone Receptor-Independent Early Growth Response Factor-3 Pathway. Neuroscience 2010, 170, 865–880. [Google Scholar] [CrossRef] [Green Version]
- Gulinello, M.; Gong, Q.H.; Smith, S.S. Progesterone Withdrawal Increases the A4 Subunit of the GABAA Receptor in Male Rats in Association with Anxiety and Altered Pharmacology—A Comparison with Female Rats. Neuropharmacology 2002, 43, 701–714. [Google Scholar] [CrossRef]
- Skórzewska, A.; Lehner, M.; Wisłowska-Stanek, A.; Krząścik, P.; Ziemba, A.; Płaźnik, A. The Effect of Chronic Administration of Corticosterone on Anxiety- and Depression-like Behavior and the Expression of GABA-A Receptor Alpha-2 Subunits in Brain Structures of Low- and High-Anxiety Rats. Horm. Behav. 2014, 65, 6–13. [Google Scholar] [CrossRef]
- Smith, S.S.; Gong, Q.H.; Hsu, F.-C.; Markowitz, R.S.; ffrench-Mullen, J.M.H.; Li, X. GABAA Receptor A4 Subunit Suppression Prevents Withdrawal Properties of an Endogenous Steroid. Nature 1998, 392, 926–929. [Google Scholar] [CrossRef]
- Dubol, M.; Epperson, C.N.; Lanzenberger, R.; Sundström-Poromaa, I.; Comasco, E. Neuroimaging Premenstrual Dysphoric Disorder: A Systematic and Critical Review. Front. Neuroendocrinol. 2020, 57, 100838. [Google Scholar] [CrossRef]
- Gingnell, M.; Morell, A.; Bannbers, E.; Wikström, J.; Sundström Poromaa, I. Menstrual Cycle Effects on Amygdala Reactivity to Emotional Stimulation in Premenstrual Dysphoric Disorder. Horm. Behav. 2012, 62, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Gingnell, M.; Ahlstedt, V.; Bannbers, E.; Wikström, J.; Sundström-Poromaa, I.; Fredrikson, M. Social Stimulation and Corticolimbic Reactivity in Premenstrual Dysphoric Disorder: A Preliminary Study. Biol. Mood Anxiety Disord. 2014, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Wang, G.; Gao, D.; Gao, F.; Zhao, B.; Qiao, M.; Yang, H.; Yu, Y.; Ren, F.; Yang, P.; et al. Alterations of GABA and Glutamate–Glutamine Levels in Premenstrual Dysphoric Disorder: A 3T Proton Magnetic Resonance Spectroscopy Study. Psychiatry Res. Neuroimaging 2015, 231, 64–70. [Google Scholar] [CrossRef] [Green Version]
- Timby, E.; Bäckström, T.; Nyberg, S.; Stenlund, H.; Wihlbäck, A.-C.N.; Bixo, M. Women with Premenstrual Dysphoric Disorder Have Altered Sensitivity to Allopregnanolone over the Menstrual Cycle Compared to Controls—a Pilot Study. Psychopharmacology 2016, 233, 2109–2117. [Google Scholar] [CrossRef] [PubMed]
- Zezula, J.; Cortés, R.; Probst, A.; Palacios, J.M. Benzodiazepine Receptor Sites in the Human Brain: Autoradiographic Mapping. Neuroscience 1988, 25, 771–795. [Google Scholar] [CrossRef]
- O’ Mahony, S.M.; Clarke, G.; McKernan, D.P.; Bravo, J.A.; Dinan, T.G.; Cryan, J.F. Differential Visceral Nociceptive, Behavioural and Neurochemical Responses to an Immune Challenge in the Stress-Sensitive Wistar Kyoto Rat Strain. Behav. Brain Res. 2013, 253, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Pardon, M.-C.; Ma, S.; Morilak, D.A. Chronic Cold Stress Sensitizes Brain Noradrenergic Reactivity and Noradrenergic Facilitation of the HPA Stress Response in Wistar Kyoto Rats. Brain Res. 2003, 971, 55–65. [Google Scholar] [CrossRef]
- Pearson, K.A.; Stephen, A.; Beck, S.G.; Valentino, R.J. Identifying Genes in Monoamine Nuclei That May Determine Stress Vulnerability and Depressive Behavior in Wistar–Kyoto Rats. Neuropsychopharmacology 2006, 31, 2449–2461. [Google Scholar] [CrossRef]
- Islas-Preciado, D.; López-Rubalcava, C.; González-Olvera, J.; Gallardo-Tenorio, A.; Estrada-Camarena, E. Environmental Enrichment Prevents Anxiety-like Behavior Induced by Progesterone Withdrawal in Two Strains of Rats. Neuroscience 2016, 336, 123–132. [Google Scholar] [CrossRef]
- Marti, J.; Armario, A. Forced Swimming Behavior Is Not Related to the Corticosterone Levels Ain the Test: A Study with Four Inbred Rat Strains. Physiol. Behav. 1996, 59, 369–373. [Google Scholar] [CrossRef]
- Paré, W.P.; Redei, E. Depressive Behavior and Stress Ulcer in Wistar Kyoto Rats. J. Physiol. 1993, 87, 229–238. [Google Scholar] [CrossRef]
- Rittenhouse, P.A.; López-Rubalcava, C.; Stanwood, G.D.; Lucki, I. Amplified Behavioral and Endocrine Responses to Forced Swim Stress in the Wistar–Kyoto Rat. Psychoneuroendocrinology 2002, 27, 303–318. [Google Scholar] [CrossRef]
- Rogel-Salazar, G.; López-Rubalcava, C. Evaluation of the Anxiolytic-like Effects of Clomipramine in Two Rat Strains with Different Anxiety Vulnerability (Wistar and Wistar-Kyoto Rats): Participation of 5-HT1A Receptors. Behav. Pharmacol. 2011, 22, 136–146. [Google Scholar] [CrossRef]
- Mileva, G.R.; Rooke, J.; Ismail, N.; Bielajew, C. Corticosterone and Immune Cytokine Characterization Following Environmental Manipulation in Female WKY Rats. Behav. Brain Res. 2017, 316, 197–204. [Google Scholar] [CrossRef]
- Hernandez, M.E.; Martinez-Mota, L.; Salinas, C.; Marquez-Velasco, R.; Hernandez-Chan, N.G.; Morales-Montor, J.; Pérez-Tapia, M.; Streber, M.L.; Granados-Camacho, I.; Becerril, E.; et al. Chronic Stress Induces Structural Alterations in Splenic Lymphoid Tissue That Are Associated with Changes in Corticosterone Levels in Wistar-Kyoto Rats. BioMed Res. Int. 2013, 2013, 868742. [Google Scholar] [CrossRef]
- Girdler, S.S.; Leserman, J.; Bunevicius, R.; Klatzkin, R.; Pedersen, C.A.; Light, K.C. Persistent Alterations in Biological Profiles in Women with Abuse Histories: Influence of Premenstrual Dysphoric Disorder. Health Psychol. 2007, 26, 201–213. [Google Scholar] [CrossRef] [Green Version]
- Pare, D.; Duvarci, S. Amygdala Microcircuits Mediating Fear Expression and Extinction. Curr. Opin. Neurobiol. 2012, 22, 717–723. [Google Scholar] [CrossRef] [Green Version]
- Ghasemi, M.; Navidhamidi, M.; Rezaei, F.; Azizikia, A.; Mehranfard, N. Anxiety and Hippocampal Neuronal Activity: Relationship and Potential Mechanisms. Cogn. Affect. Behav. Neurosci. 2022, 22, 431–449. [Google Scholar] [CrossRef]
- Dayas, C.V.; Buller, K.M.; Crane, J.W.; Xu, Y.; Day, T.A. Stressor Categorization: Acute Physical and Psychological Stressors Elicit Distinctive Recruitment Patterns in the Amygdala and in Medullary Noradrenergic Cell Groups: Categorization of Stressors by the Brain. Eur. J. Neurosci. 2001, 14, 1143–1152. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Guasti, A.; Picazo, O. The Actions of Diazepam and Serotonergic Anxiolytics Vary According to the Gender and the Estrous Cycle Phase. Pharmacol. Biochem. Behav. 1990, 37, 77–81. [Google Scholar] [CrossRef]
- Olvera-Hernández, S.; Fernández-Guasti, A. Sex Differences in the Burying Behavior Test in Middle-Aged Rats: Effects of Diazepam. Pharmacol. Biochem. Behav. 2011, 99, 532–539. [Google Scholar] [CrossRef]
- De Boer, S.F.; Koolhaas, J.M. Defensive Burying in Rodents: Ethology, Neurobiology and Psychopharmacology. Eur. J. Pharmacol. 2003, 463, 145–161. [Google Scholar] [CrossRef]
- Paré, W.P.; Tejani-Butt, S.; Kluczynski, J. The Emergence Test: Effects of Psychotropic Drugs on Neophobic Disposition in Wistar Kyoto (WKY) and Sprague Dawley Rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2001, 25, 1615–1628. [Google Scholar] [CrossRef]
- Estrada-Camarena, E.; Contreras, C.M.; Saavedra, M.; Luna-Baltazar, I.; López-Rubalcava, C. Participation of the Lateral Septal Nuclei (LSN) in the Antidepressant-like Actions of Progesterone in the Forced Swimming Test (FST). Behav. Brain Res. 2002, 134, 175–183. [Google Scholar] [CrossRef]
- Gallo, M.A.; Smith, S.S. Progesterone Withdrawal Decreases Latency to and Increases Duration of Electrified Prod Burial: A Possible Rat Model of PMS Anxiety. Pharmacol. Biochem. Behav. 1993, 46, 897–904. [Google Scholar] [CrossRef]
- Saavedra, M.; Contreras, C.M.; Azamar-Arizmendi, G.; Hernández-Lozano, M. Differential Progesterone Effects on Defensive Burying and Forced Swimming Tests Depending upon a Gradual Decrease or an Abrupt Suppression Schedules. Pharmacol. Biochem. Behav. 2006, 83, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Lovick, T. SSRIs and the Female Brain—Potential for Utilizing Steroid-Stimulating Properties to Treat Menstrual Cycle-Linked Dysphorias. J. Psychopharmacol. 2013, 27, 1180–1185. [Google Scholar] [CrossRef] [PubMed]
- Yonkers, K.A.; O’Brien, P.S.; Eriksson, E. Premenstrual Syndrome. The Lancet 2008, 371, 1200–1210. [Google Scholar] [CrossRef]
- Facchinetti, F.; Fioroni, L.; Martignoni, E.; Sances, G.; Costa, A.; Genazzani, A.R. Changes of opioid modulation of the hypothalamo-pituitary-adrenal axis in patients with severe premenstrual syndrome. Psychosom. Med. 1994, 56, 418–422. [Google Scholar] [PubMed]
- Huang, Y.; Zhou, R.; Wu, M.; Wang, Q.; Zhao, Y. Premenstrual Syndrome Is Associated with Blunted Cortisol Reactivity to the TSST. Stress 2015, 18, 160–168. [Google Scholar] [CrossRef]
- Girdler, S.S.; Sherwood, A.; Hinderliter, A.L.; Leserman, J.; Costello, N.L.; Straneva, P.A.; Pedersen, C.A.; Light, K.C. Biological Correlates of Abuse in Women with Premenstrual Dysphoric Disorder and Healthy Controls. Psychosom. Med. 2003, 65, 849–856. [Google Scholar] [CrossRef]
- Engin, E.; Smith, K.S.; Gao, Y.; Nagy, D.; Foster, R.A.; Tsvetkov, E.; Keist, R.; Crestani, F.; Fritschy, J.-M.; Bolshakov, V.Y.; et al. Modulation of Anxiety and Fear via Distinct Intrahippocampal Circuits. eLife 2016, 5, e14120. [Google Scholar] [CrossRef] [Green Version]
- Godoy, L.D.; Rossignoli, M.T.; Delfino-Pereira, P.; Garcia-Cairasco, N.; de Lima Umeoka, E.H. A Comprehensive Overview on Stress Neurobiology: Basic Concepts and Clinical Implications. Front. Behav. Neurosci. 2018, 12, 127. [Google Scholar] [CrossRef] [Green Version]
- Craft, R.M.; Howard, J.L.; Pollard, G.T. Conditioned Defensive Burying as a Model for Identifying Anxiolytics. Pharmacol. Biochem. Behav. 1988, 30, 775–780. [Google Scholar] [CrossRef]
- Anisman, H.; Matheson, K. Stress, Depression, and Anhedonia: Caveats Concerning Animal Models. Neurosci. Biobehav. Rev. 2005, 29, 525–546. [Google Scholar] [CrossRef]
- Redei, E.; Pare, W.P.; Aird, F.; Kluczynski, J. Strain Differences in Hypothalamic-Pituitary-Adrenal Activity and Stress Ulcer. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1994, 266, R353–R360. [Google Scholar] [CrossRef]
- Locci, A.; Pinna, G. Neurosteroid Biosynthesis Down-Regulation and Changes in GABA A Receptor Subunit Composition: A Biomarker Axis in Stress-Induced Cognitive and Emotional Impairment: Neurosteroids and GABA: Biomarkers for Emotions. Br. J. Pharmacol. 2017, 174, 3226–3241. [Google Scholar] [CrossRef] [Green Version]
- Cagetti, E.; Liang, J.; Spigelman, I.; Olsen, R.W. Withdrawal from Chronic Intermittent Ethanol Treatment Changes Subunit Composition, Reduces Synaptic Function, and Decreases Behavioral Responses to Positive Allosteric Modulators of GABA A Receptors. Mol. Pharmacol. 2003, 63, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Deutsch, S.I.; Park, C.H.; Hitri, A. Allosteric Effects of a GABA Receptor-Active Steroid Are Altered by Stress. Pharmacol. Biochem. Behav. 1994, 47, 913–917. [Google Scholar] [CrossRef]
- Drugan, R.C.; Paul, S.M.; Crawley, J.N. Decreased Forebrain [35S]TBPS Binding and Increased [3H]Muscimol Binding in Rats That Do Not Develop Stress-Induced Behavioral Depression. Brain Res. 1993, 631, 270–276. [Google Scholar] [CrossRef]
- Frye, C.A.; Bayon, L.E. Prenatal Stress Reduces the Effectiveness of the Neurosteroid 3 Alpha,5 Alpha-THP to Block Kainic-Acid-Induced Seizures. Dev. Psychobiol. 1999, 34, 227–234. [Google Scholar] [CrossRef]
- Gruen, R.J.; Wenberg, K.; Elahi, R.; Friedhoff, A.J. Alterations in GABAA Receptor Binding in the Prefrontal Cortex Following Exposure to Chronic Stress. Brain Res. 1995, 684, 112–114. [Google Scholar] [CrossRef]
- Orchinik, M.; Carroll, S.S.; Li, Y.-H.; McEwen, B.S.; Weiland, N.G. Heterogeneity of Hippocampal GABA A Receptors: Regulation by Corticosterone. J. Neurosci. 2001, 21, 330–339. [Google Scholar] [CrossRef] [Green Version]
- Serra, M.; Pisu, M.G.; Littera, M.; Papi, G.; Sanna, E.; Tuveri, F.; Usala, L.; Purdy, R.H.; Biggio, G. Social Isolation-Induced Decreases in Both the Abundance of Neuroactive Steroids and GABAA Receptor Function in Rat Brain. J. Neurochem. 2002, 75, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, S.; Wahlström, G.; Bäckström, T.; Poromaa, I.S. Altered Sensitivity to Alcohol in the Late Luteal Phase among Patients with Premenstrual Dysphoric Disorder. Psychoneuroendocrinology 2004, 29, 767–777. [Google Scholar] [CrossRef]
- Bixo, M.; Johansson, M.; Timby, E.; Michalski, L.; Bäckström, T. Effects of GABA Active Steroids in the Female Brain with a Focus on the Premenstrual Dysphoric Disorder. J. Neuroendocrinol. 2018, 30, e12553. [Google Scholar] [CrossRef] [PubMed]
- Bäckström, T.; Haage, D.; Löfgren, M.; Johansson, I.M.; Strömberg, J.; Nyberg, S.; Andréen, L.; Ossewaarde, L.; van Wingen, G.A.; Turkmen, S.; et al. Paradoxical Effects of GABA-A Modulators May Explain Sex Steroid Induced Negative Mood Symptoms in Some Persons. Neuroscience 2011, 191, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Paré, W.P.; Kluczynski, J. Differences in the Stress Response of Wistar-Kyoto (WKY) Rats from Different Vendors. Physiol. Behav. 1997, 62, 643–648. [Google Scholar] [CrossRef]
- Estrada-Camarena, E.; Sollozo-Dupont, I.; Islas-Preciado, D.; González-Trujano, M.E.; Carro-Juárez, M.; López-Rubalcava, C. Anxiolytic- and Anxiogenic-like Effects of Montanoa Tomentosa (Asteraceae): Dependence on the Endocrine Condition. J. Ethnopharmacol. 2019, 241, 112006. [Google Scholar] [CrossRef]
- Pellow, S.; Chopin, P.; File, S.E.; Briley, M. Validation of Open: Closed Arm Entries in an Elevated plus-Maze as a Measure of Anxiety in the Rat. J. Neurosci. Methods 1985, 14, 149–167. [Google Scholar] [CrossRef]
- Lister, R.G. Ethologically-Based Animal Models of Anxiety Disorders. Pharmacol. Ther. 1990, 46, 321–340. [Google Scholar] [CrossRef]
- Picazo, O.; Ferna´ndez-Guasti, A. Anti-Anxiety Effects of Progesterone and Some of Its Reduced Metabolites: An Evaluation Using the Burying Behavior Test. Brain Res. 1995, 680, 135–141. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 4th ed.; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Belzung, C.; Turiault, M.; Griebel, G. Optogenetics to Study the Circuits of Fear- and Depression-like Behaviors: A Critical Analysis. Pharmacol. Biochem. Behav. 2014, 122, 144–157. [Google Scholar] [CrossRef]
- Carvalho, M.C.; Moreira, C.M.; Zanoveli, J.M.; Brandão, M.L. Central, but Not Basolateral, Amygdala Involvement in the Anxiolytic-like Effects of Midazolam in Rats in the Elevated plus Maze. J. Psychopharmacol. 2012, 26, 543–554. [Google Scholar] [CrossRef]
- Marrocco, J.; Mairesse, J.; Ngomba, R.T.; Silletti, V.; Van Camp, G.; Bouwalerh, H.; Summa, M.; Pittaluga, A.; Nicoletti, F.; Maccari, S.; et al. Anxiety-Like Behavior of Prenatally Stressed Rats Is Associated with a Selective Reduction of Glutamate Release in the Ventral Hippocampus. J. Neurosci. 2012, 32, 17143–17154. [Google Scholar] [CrossRef] [Green Version]



| Treatment | Squares Crossed (n) |
|---|---|
| No-PW | 31.6 ± 1.8 |
| PW | 34.8 ± 1.7 |
| DZ 0.0 | 20.2 ± 0.7 |
| DZ 0.125 | 19.6 ± 1.6 |
| DZ 0.25 | 21.8 ± 2.4 |
| DZ 0.50 | 22.0 ± 2.7 |
| DZ 1.0 | 18.6 ± 1.4 |
| PW + DZ 0.0 | 19.4 ± 0.8 |
| PW + DZ 0.125 | 20.4 ± 1.3 |
| PW + DZ 0.25 | 21.4 ± 1.5 |
| PW + DZ 0.50 | 21.6 ± 1.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islas-Preciado, D.; Ugalde-Fuentes, G.; Sollozo-Dupont, I.; González Trujano, M.E.; Cervantes-Anaya, N.; Estrada-Camarena, E.; López-Rubalcava, C. Anxiety-like Behavior and GABAAR/BDZ Binding Site Response to Progesterone Withdrawal in a Stress-Vulnerable Strain, the Wistar Kyoto Rats. Int. J. Mol. Sci. 2022, 23, 7259. https://doi.org/10.3390/ijms23137259
Islas-Preciado D, Ugalde-Fuentes G, Sollozo-Dupont I, González Trujano ME, Cervantes-Anaya N, Estrada-Camarena E, López-Rubalcava C. Anxiety-like Behavior and GABAAR/BDZ Binding Site Response to Progesterone Withdrawal in a Stress-Vulnerable Strain, the Wistar Kyoto Rats. International Journal of Molecular Sciences. 2022; 23(13):7259. https://doi.org/10.3390/ijms23137259
Chicago/Turabian StyleIslas-Preciado, Dannia, Gabriela Ugalde-Fuentes, Isabel Sollozo-Dupont, María Eva González Trujano, Nancy Cervantes-Anaya, Erika Estrada-Camarena, and Carolina López-Rubalcava. 2022. "Anxiety-like Behavior and GABAAR/BDZ Binding Site Response to Progesterone Withdrawal in a Stress-Vulnerable Strain, the Wistar Kyoto Rats" International Journal of Molecular Sciences 23, no. 13: 7259. https://doi.org/10.3390/ijms23137259
APA StyleIslas-Preciado, D., Ugalde-Fuentes, G., Sollozo-Dupont, I., González Trujano, M. E., Cervantes-Anaya, N., Estrada-Camarena, E., & López-Rubalcava, C. (2022). Anxiety-like Behavior and GABAAR/BDZ Binding Site Response to Progesterone Withdrawal in a Stress-Vulnerable Strain, the Wistar Kyoto Rats. International Journal of Molecular Sciences, 23(13), 7259. https://doi.org/10.3390/ijms23137259








