The Influence of Proteins on Fate and Biological Role of Circulating DNA
Abstract
1. Introduction
2. Origins of cirDNA
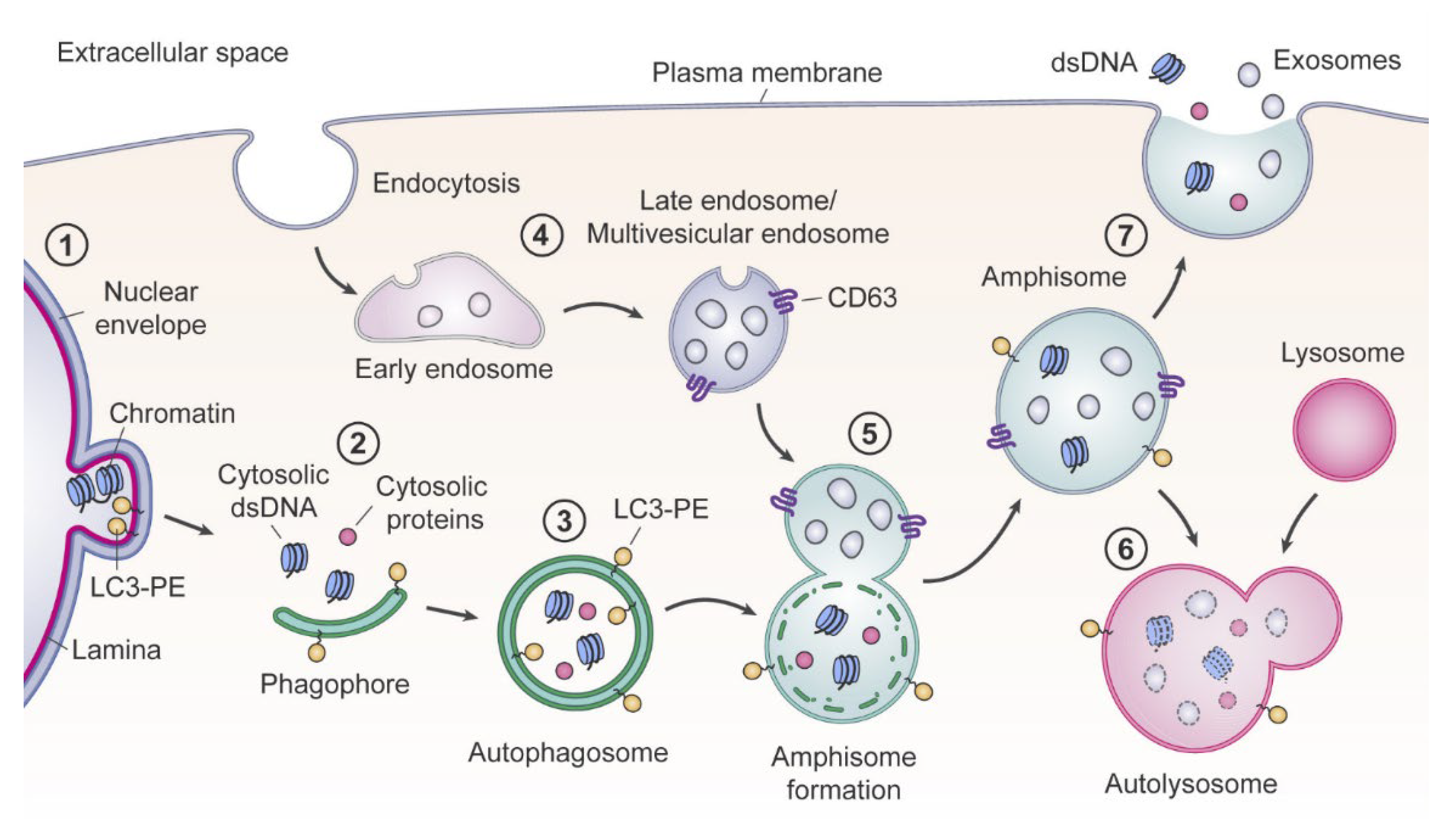
2.1. Cell Death Origin of cirDNA (Apoptosis, Necrosis, and Autophagy)
2.2. Appearance of Blood cirDNA during Secretion by Normal and Tumor Cells
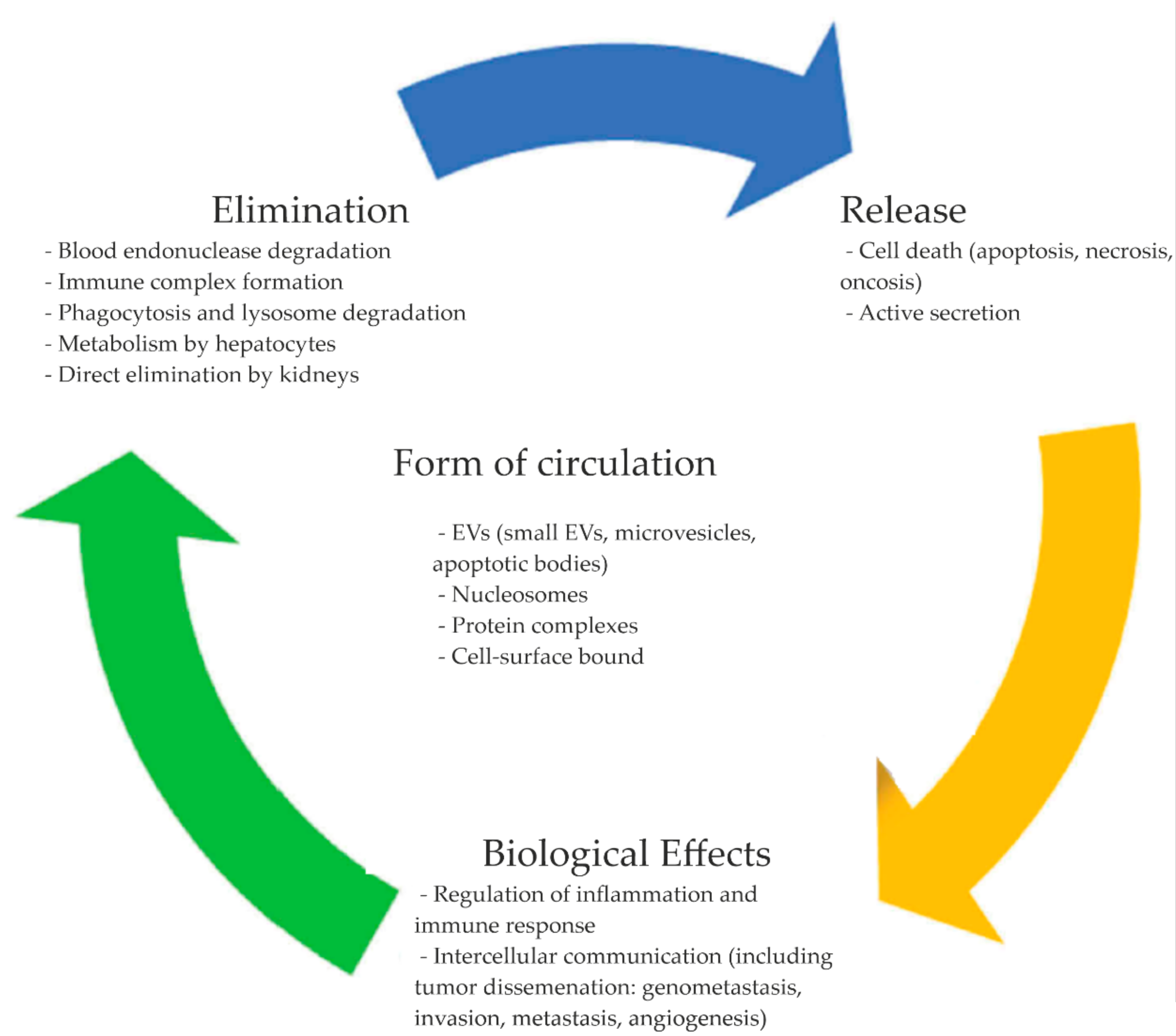
3. CirDNA Forms of Circulation
4. CirDNA Metabolism and Biological Role
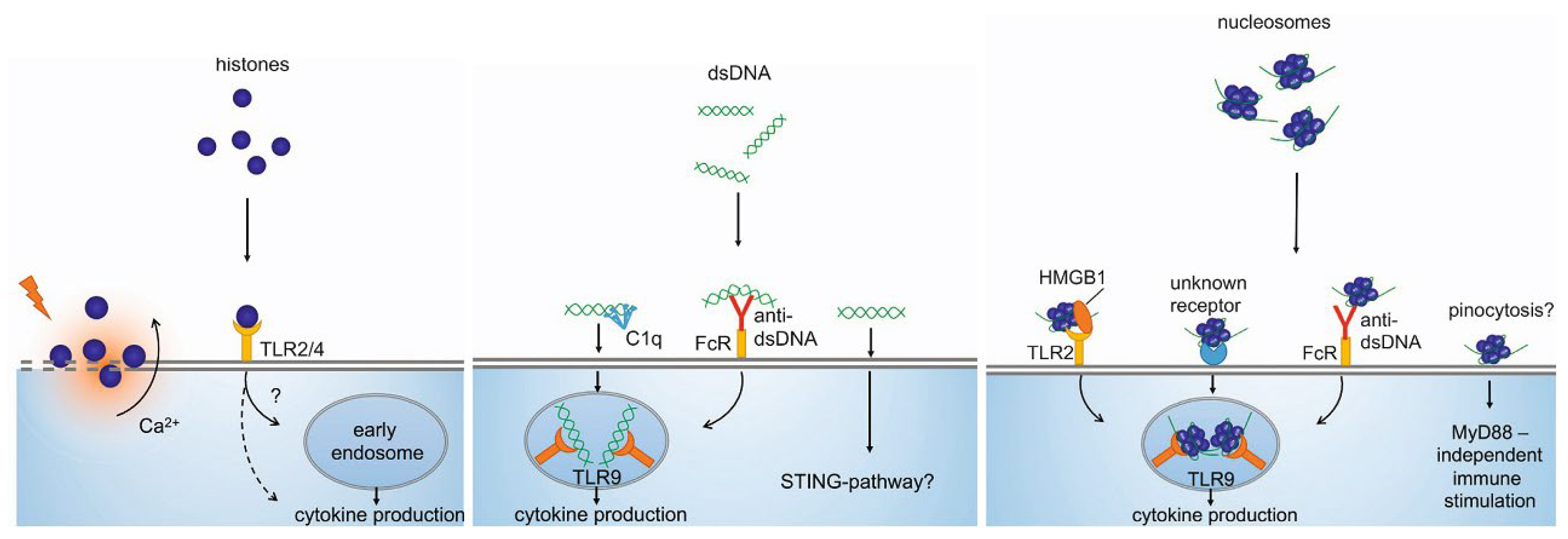
4.1. Immunostimulatory Characteristics of cirDNA
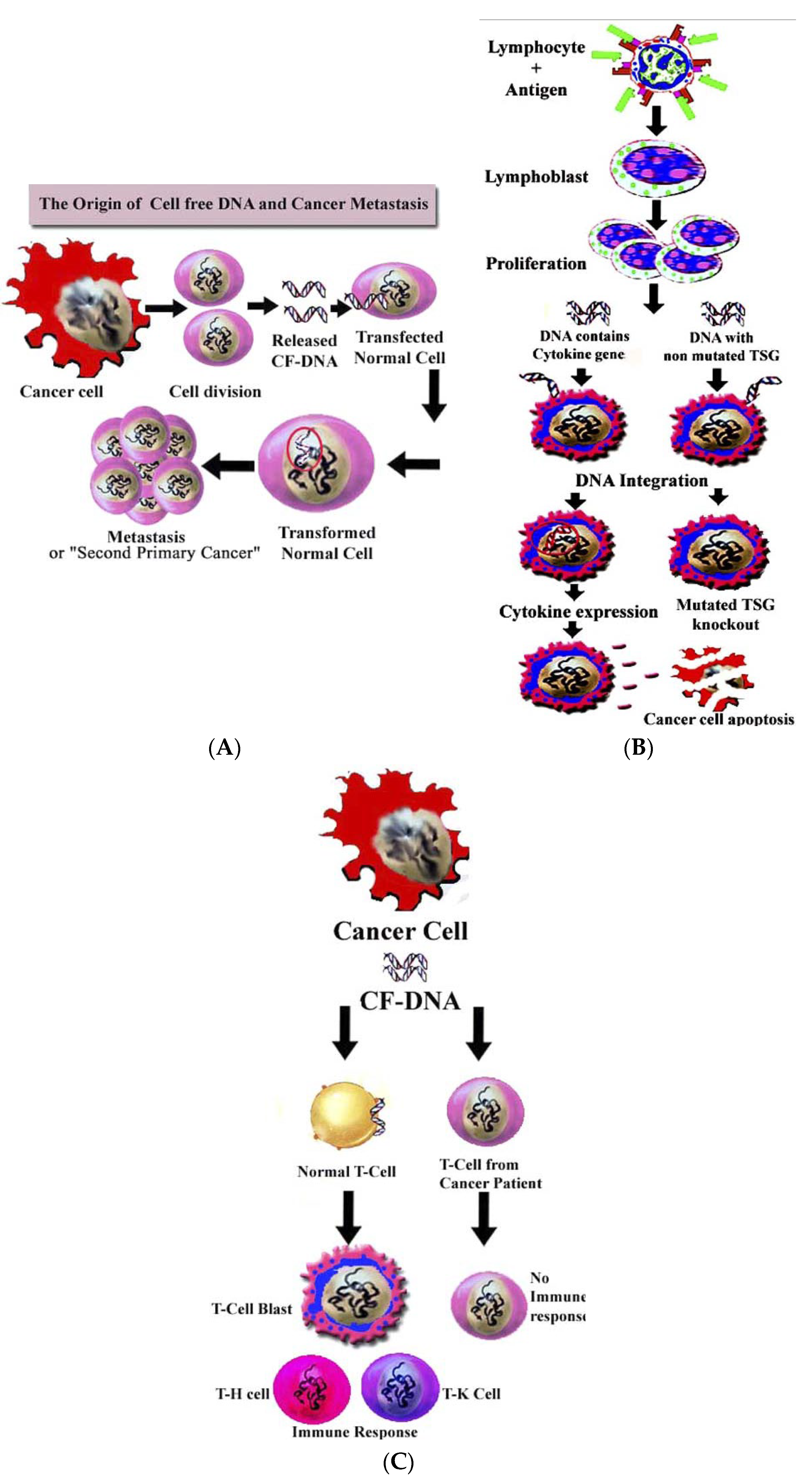
4.2. Role of cirDNA in Horizontal Gene Transfer
4.3. Role of cirDNA in Angiogenesis and Blood Coagulation
4.4. Blood cirDNA Clearance
5. CirDNA Perspectives in Liquid Biopsy
Author Contributions
Funding
Conflicts of Interest
References
- Thierry, A.R.; El Messaoudi, S.; Gahan, P.B.; Anker, P.; Stroun, M. Origins, structures, and functions of circulating DNA in oncology. Cancer Metastasis Rev. 2016, 35, 347–376. [Google Scholar] [CrossRef] [PubMed]
- Mandel, P.; Métais, P. Les acides nucléiques du plasma sanguin chez l’Homme. Comptes Rendus Des Seances De La Soc. De Biol. Et De Ses Fil. 1948, 142, 241–243. [Google Scholar]
- Tan, E.; Schur, P.; Carr, R.; Kunkel, H. Deoxybonucleic acid (DNA) and antibodies to DNA in the serum of patients with systemic lupus erythematosus. J. Clin. Investig. 1966, 45, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Lehmann-Werman, R.; Neiman, D.; Zemmour, H.; Moss, J.; Magenheim, J.; Vaknin-Dembinsky, A.; Rubertsson, S.; Nellgard, B.; Blennow, K.; Zetterberg, H.; et al. Identification of tissue-specific cell death using methylation patterns of circulating DNA. Proc. Natl Acad. Sci. USA 2016, 113, 1826–1834. [Google Scholar] [CrossRef]
- Bryzgunova, O.E.; Laktionov, P.P. Generation of blood circulating DNAs: Sources, features of struction and circulation. Biochem. (Mosc.) Suppl. Ser. B Biomed. Chem. 2014, 8, 203–219. [Google Scholar] [CrossRef]
- Grabuschnig, S.; Bronkhorst, A.J.; Holdenrieder, S.; Rosales Rodriguez, I.; Schliep, K.P.; Schwendenwein, D.; Ungerer, V.; Sensen, C.W. Putative Origins of Cell-Free DNA in Humans: A Review of Active and Passive Nucleic Acid Release Mechanisms. Int. J. Mol. Sci. 2020, 21, 8062. [Google Scholar] [CrossRef]
- Umansky, S. The genetic program of cell death. Hypothesis and some applications: Transformation, carcinogenesis, ageing. J. Theor. Biol. 1982, 97, 591–602. [Google Scholar] [CrossRef]
- Giacona, M.; Ruben, G.; Iczkowski, K.; Roos, T.; Porter, D.; Sorenson, G. Cell-free DNA in human blood plasma: Length measurements in patients with pancreatic cancer and healthy controls. Pancreas 1998, 17, 89–97. [Google Scholar] [CrossRef]
- Tamkovich, S.N.; Kirushina, N.A.; Voytsitskiy, V.E.; Tkachuk, V.A.; Laktionov, P.P. Features of Circulating DNA Fragmentation in Blood of Healthy Females and Breast Cancer Patients. Adv. Exp. Med. Biol. 2016, 24, 47–51. [Google Scholar]
- Jahr, S.; Hentze, H.; Englisch, S.; Hardt, D.; Fackelmayer, F.; Hesch, R.; Knippers, R. DNA fragments in the blood plasma of cancer patients: Quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 2001, 61, 1659–1665. [Google Scholar]
- Deligezer, U.; Yaman, F.; Erten, N.; Dalay, N. Frequent copresence of methylated DNA and fragmented nucleosomal DNA in plasma of lymphoma patients. Clin. Chim. Acta 2003, 335, 89–94. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, D.; Chia, J.; Tsao, K.; Sun, C.; Wu, J. Cell-free DNA: Measurement in various carcinomas and establishment of normal reference range. Clin. Chim. Acta 2002, 321, 77–87. [Google Scholar] [CrossRef]
- Pisetsky, D.; Fairhurst, A. The origin of extracellular DNA during the clearance of dead and dying cells. Autoimmunity 2007, 40, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Reich, C., III; Pisetsky, D. The role of macrophages in the in vitro generation of extracellular DNA from apoptotic and necrotic cells. Immunology 2005, 115, 55–62. [Google Scholar] [CrossRef]
- Choi, J.; Reich, C., III; Pisetsky, D. Release of DNA from Dead and Dying Lymphocyte and Monocyte Cell Lines In Vitro. Scand. J. Immunol. 2004, 60, 159–166. [Google Scholar] [CrossRef]
- Boone, B.A.; Orlichenko, L.; Schapiro, N.E.; Loughran, P.; Gianfrate, G.C.; Ellis, J.T.; Zeh, H.J. The receptor for advanced glycation end products (RAGE) enhances autophagy and neutrophil extracellular traps in pancreatic cancer. Cancer Gene Ther. 2015, 22, 326–334. [Google Scholar] [CrossRef]
- Zhu, H.; Guariglia, S.; Yu, R.Y.; Li, W.; Brancho, D.; Peinado, H.; Lyden, D.; Salzer, J.; Bennett, C.; Chow, C.W. Mutation of SIMPLE in Charcot-Marie-Tooth 1C alters production of exosomes. Mol. Biol. Cell 2013, 24, 1619–1637. [Google Scholar] [CrossRef]
- Soehnlein, O.; Lindbom, L. Phagocyte partnership during the onset and resolution of inflammation. Nat. Rev. Immunol. 2010, 10, 427–439. [Google Scholar] [CrossRef]
- Blankenberg, F.; Tait, J.; Ohtsuki, K.; Strauss, H. Apoptosis: The importance of nuclear medicine. Nucl. Med. Commun. 2000, 21, 241–250. [Google Scholar] [CrossRef]
- Jiang, N.; Reich, C., III; Pisetsky, D. Role of macrophages in the generation of circulating blood nucleosomes from dead and dying cells. Blood 2003, 102, 2243–2250. [Google Scholar] [CrossRef][Green Version]
- Leon, S.; Shapiro, B.; Sklaroff, D.; Yaros, M. Free DNA in the serum of cancer patients and the effect of therapy. Cancer Res. 1977, 37, 646–650. [Google Scholar] [PubMed]
- Abe, T.; Nakashima, C.; Sato, A.; Harada, Y.; Sueoka, E.; Kimura, S.; Kawaguchi, A.; Sueoka-Aragane, N. Origin of circulating free DNA in patients with lung cancer. PLoS ONE 2020, 15, e0235611. [Google Scholar] [CrossRef] [PubMed]
- Elshimali, Y.I.; Khaddour, H.; Sarkissyan, M.; Wu, Y.; Vadgama, J.V. The clinical utilization of circulating cell free DNA (CCFDNA) in blood of cancer patients. Int. J. Mol. Sci. 2013, 14, 18925–18958. [Google Scholar] [CrossRef]
- Fiala, C.; Diamandis, E.P. Utility of circulating tumor DNA in cancer diagnostics with emphasis on early detection. BMC Med. 2018, 16, 166. [Google Scholar] [CrossRef] [PubMed]
- Sumbal, S.; Javed, A.; Afroze, B.; Zulfiqar, H.F.; Javed, F.; Noreen, S.; Ijaz, B. Circulating tumor DNA in blood: Future genomic biomarkers for cancer detection. Exp. Hematol. 2018, 65, 17–28. [Google Scholar] [CrossRef]
- Sato, A.; Nakashima, C.; Abe, T.; Kato, J.; Hirai, M.; Nakamura, T.; Komiya, K.; Kimura, S.; Sueoka, E.; Sueoka-Aragane, N. Investigation of appropriate pre-analytical procedure for circulating 161 free DNA from liquid biopsy. Oncotarget 2018, 9, 31904–31914. [Google Scholar] [CrossRef]
- Morozkin, E.S.; Laktionov, P.P.; Rykova, E.Y.; Vlassov, V.V. Extracellular nucleic acids in cultures of long-term cultivated eukaryotic cells. Ann. N. Y. Acad. Sci. 2004, 1022, 244–249. [Google Scholar] [CrossRef]
- Nakashima, C.; Sato, A.; Abe, T.; Kato, J.; Hirai, M.; Nakamura, T.; Komiya, K.; Sueoka, E.; Kimura, S.; Sueoka-Aragane, N. Automated DNA extraction using cellulose magnetic beads can improve EGFR point mutation detection with liquid biopsy by efficiently recovering short and long DNA fragments. Oncotarget 2018, 9, 25181–25192. [Google Scholar] [CrossRef]
- Bryzgunova, O.E.; Tamkovich, S.N.; Cherepanova, A.V.; Yarmoshchuk, S.V.; Permyakova, V.I.; Anykeeva, O.Y.; Laktionov, P.P. Redistribution of Free- and Cell-Surface-Bound DNA in Blood of Benign and Malignant Prostate Tumor Patients. Acta Nat. 2015, 7, 115–118. [Google Scholar] [CrossRef]
- Tutanov, O.; Shtam, T.; Grigoreva, A.; Tupikin, A.; Tsentalovich, Y.; Tamkovich, S. Blood Plasma Exosomes Contain Circulating DNA in Their Crown. Diagnostics 2022, 12, 854. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445. [Google Scholar] [CrossRef] [PubMed]
- Tamkovich, S.N.; Tutanov, O.S.; Serdukov, D.S.; Belenikin, M.S.; Shlikht, A.G.; Kirushina, N.A.; Voytsitskiy, V.E.; Tsentalovich, Y.P.; Tkachuk, V.A.; Laktionov, P.P. Protein Content of Circulating Nucleoprotein Complexes. Adv. Exp. Med. Biol. 2016, 924, 133–136. [Google Scholar] [PubMed]
- Holdenrieder, S.; Nagel, D.; Schalhorn, A.; Heinemann, V.; Wilkowski, R.; von Pawel, J.; Raith, H.; Feldmann, K.; Kremer, A.E.; Muller, S.; et al. Clinical Relevance of Circulating Nucleosomes in Cancer. Ann. N. Y. Acad. Sci. 2008, 1137, 180–189. [Google Scholar] [CrossRef]
- Tamkovich, S.; Laktionov, P. Cell-surface-bound circulating DNA in the blood: Biology and clinical application. IUBMB Life 2019, 71, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Skvortsova, T.E.; Bryzgunova, O.E.; Lebedeva, A.O.; Mak, V.V.; Vlassov, V.V.; Laktionov, P.P. Methylated Cell-Free DNA In Vitro and In Vivo. Circ. Nucleic Acids Plasma Serum 2011, 185–194. [Google Scholar] [CrossRef]
- Kustanovich, A.; Schwartz, R.; Peretz, T.; Grinshpun, A. Life and death of circulating cell-free DNA. Cancer Biol. Ther. 2019, 20, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Jeppesen, D.K.; Higginbotham, J.N.; Graves-Deal, R.; Trinh, V.Q.; Ramirez, M.A.; Sohn, Y.; Neininger, A.C.; Taneja, N.; McKinley, E.T.; et al. Supermeres are functional extracellular nanoparticles replete with disease biomarkers and therapeutic targets. Nat. Cell. Biol. 2021, 23, 1240–1254. [Google Scholar] [CrossRef]
- Zhang, H.; Freitas, D.; Kim, H.S.; Fabijanic, K.; Li, Z.; Chen, H.; Mark, M.T.; Molina, H.; Martin, A.B.; Bojmar, L.; et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell. Biol. 2018, 20, 332–343. [Google Scholar] [CrossRef]
- Balaj, L.; Lessard, R.; Dai, L.; Cho, Y.; Pomeroy, S.L.; Breakefield, X.O.; Skog, J. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat. Commun. 2011, 2, 180. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Chekhonin, V.P. Extracellular vesicles shed by glioma cells: Pathogenic role and clinical value. Tumour Biol. 2014, 35, 8425–8438. [Google Scholar] [CrossRef]
- Vlassov, V.V.; Laktionov, P.P.; Rykova, E.Y. Extracellular nucleic acids. BioEssays 2007, 29, 654–667. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, P.; Widlak, P.; Zou, H.; Luo, X.; Garrard, W.T.; Wang, X. The 40-kDa subunit of DNA fragmentation factor induces DNA fragmentation and chromatin condensation during apoptosis. Proc. Natl. Acad. Sci. USA 1998, 95, 8461–8466. [Google Scholar] [CrossRef] [PubMed]
- Butler, P.; Tennent, G.; Pepys, M. Pentraxin-chromatin interactions: Serum amyloid P component specifically displaces H1-type histones and solubilizes native long chromatin. J. Exp. Med. 1990, 172, 13–18. [Google Scholar] [CrossRef]
- Holdenrieder, S.; Stieber, P.; Bodenmüller, H.; Busch, M.; Von Pawel, J.; Schalhorn, A.; Seidel, D. Circulating Nucleosomes in Serum. Ann. N. Y. Acad. Sci. 2001, 945, 93–102. [Google Scholar] [CrossRef]
- Holdenrieder, S.; Stieber, P. Clinical use ofcirculating nucleosomes. Crit. Rev. Clin. Lab. Sci. 2009, 46, 1–24. [Google Scholar] [CrossRef]
- Kiroi, K.; Tanaka, C.; Toi, M. Plasma Nucleosome Levels in Node-Negative Breast Cancer Patients. Breast Cancer 1999, 6, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, A.V.; Melkonyan, H.S.; Tomei, L.D.; Umansky, S.R. Circulating nucleic acids and apoptosis. Ann. N. Y. Acad. Sci. 2001, 945, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Stroun, M.; Maurice, P.; Vasioukhin, V.; Lyautey, J.; Lederrey, C.; Lefort, F.; Rossier, A.; Chen, X.Q.; Anker, P. The origin and mechanism of circulating DNA. Ann. N. Y. Acad. Sci. 2000, 906, 161–168. [Google Scholar] [CrossRef]
- Holdenrieder, S.; Kolligs, F.T.; Braess, J.; Manukyan, D.; Stieber, P. Nature and dynamics of nucleosome release from neoplastic and non-neoplastic cells. Anticancer Res. 2012, 32, 2179–2183. [Google Scholar]
- Gezer, U.; Holdenrieder, S. Post-translational histone modifications in circulating nucleosomes as new biomarkers in colorectal cancer. In Vivo 2014, 28, 287–292. [Google Scholar]
- Lo Re, O.; Maugeri, A.; Hruskova, J.; Jakubik, J.; Kucera, J.; Bienertova-Vasku, J.; Oben, J.A.; Kubala, L.; Dvorakova, A.; Ciz, M.; et al. Obesity-induced nucleosome release predicts poor cardio-metabolic health. Clin. Epigenetics 2019, 12, 2. [Google Scholar] [CrossRef]
- Tamkovich, S.N.; Vlassov, V.V.; Laktionov, P.P. Circulating DNA in the blood and its application in medical diagnosis. Mol. Biol. 2008, 42, 9–19. [Google Scholar] [CrossRef]
- Yan, X.; Scherphof, G.L.; Kamps, J.A. Liposome opsonization. J. Liposome Res. 2005, 15, 109–139. [Google Scholar] [CrossRef]
- Liu, F.; Frick, A.; Yuan, X.; Huang, L. Dysopsonin Activity of Serum DNA-Binding Proteins Favorable for Gene Delivery. J. Pharmacol. Exp. Ther. 2010, 332, 500–504. [Google Scholar] [CrossRef]
- Kanyshkova, T.G.; Semenov, D.V.; Buneva, V.N.; Nevinsky, G.A. Human milk lactoferrin binds two DNA molecules with different affinities. FEBS Lett. 1999, 451, 235–237. [Google Scholar] [CrossRef]
- Baker, E.N.; Baker, H.M. A structural framework for understanding the multifunctional character of lactoferrin. Biochimie. 2009, 91, 3–10. [Google Scholar] [CrossRef]
- Van Berkel, P.H.; Geerts, M.E.; Van Veen, H.A.; Mericskay, M.; de Boer, H.A.; Nuijens, J.H. N-termina1 stretch Arg2, Arg3, Arg4 and Arg5 of human lactoferrin is essential for binding to heparin, bacterial lipopolysaccharide, human lysozyme and DNA. Biochem. J. 1997, 328, 145–151. [Google Scholar] [CrossRef]
- Steinrauf, L.K.; Shiuan, D.; Yang, W.J.; Chiang, M.Y. Lysozyme association with nucleic acid. Biochem. Biophys. Res. Commun. 1999, 266, 366–370. [Google Scholar] [CrossRef]
- Lee-Huang, S.; Maiorov, V.; Huang, P.L.; Ng, A.; Lee, H.C.; Chang, Y.T.; Kallenbach, N.; Huang, P.L.; Chen, H.C. Structural and functional modeling of human lysozyme reveals a unique nonapeptide, HL9, with anti-HIV activity. Biochemistry 2005, 44, 4648–4655. [Google Scholar] [CrossRef]
- Tomita, H.; Sato, S.; Matsuda, R.; Sugiura, Y.; Kawaguchi, H.; Niimi, T.; Yoshida, S.; Morishita, M. Serum lysozyme levels and clinical features of sarcoidosis. Lung 1999, 177, 161–167. [Google Scholar] [CrossRef]
- Kononova, I.V.; Mamaeva, S.N.; Alekseev, V.A.; Nikolaeva, N.A.; Afanasyeva, L.N.; Nikiforov, P.V.; Vasilyeva, N.A.; Vasiliev, I.V.; Maximov, G.V. Simultaneous Detection of the HPV L1 Gene and the Human β-Globin Gene in the Blood Components of Cervical Cancer Patients Living in Yakutia. Int. J. Biomed. 2022, 12, 109–114. [Google Scholar] [CrossRef]
- Schwarzenbach, H.; Hoon, D.; Pantel, K. Cell-free nucleic acids as biomarkers in cancer patients. Nat. Rev. Cancer. 2011, 11, 426–437. [Google Scholar] [CrossRef]
- Watson, K.; Gooderham, N.; Davies, D.; Edwards, R. Nucleosomes bind to cell surface proteoglycans. J. Biol. Chem. 1999, 274, 21707–21713. [Google Scholar] [CrossRef]
- Kubota, T.; Kanai, Y.; Miyasaka, N. Interpretation of the cross-reactivity of anti-DNA antibodies with cell surface proteins: The role of cell surface histones. Immunol. Lett. 1990, 23, 187–194. [Google Scholar] [CrossRef]
- Koutouzov, S.; Cabrespines, A.; Amoura, Z.; Chabre, H.; Lotton, C.; Bach, J. Binding of nucleosomes to a cell surface receptor: Redistribution and endocytosis in the presence of lupus antibodies. Eur. J. Immunol. 1996, 26, 472–486. [Google Scholar] [CrossRef]
- Gahan, P.B.; Swaminathan, R. Circulating nucleic acids in plasma and serum. Recent developments. Ann. N. Y. Acad. Sci. 2008, 1137, 1–6. [Google Scholar] [CrossRef]
- Mittra, I.; Nair, N.K.; Mishra, P.K. Nucleic acids in circulation: Are they harmful to the host? J. Biosci. 2012, 37, 301–312. [Google Scholar] [CrossRef]
- Pisetsky, D.S. The origin and properties of extracellular DNA: From PAMP to DAMP. Clin. Immunol. 2012, 144, 32–40. [Google Scholar] [CrossRef]
- Bhagirath, V.C.; Dwivedi, D.J.; Liaw, P.C. Comparison of the proinflammatory and procoagulant properties of nuclear, mitochondrial, and bacterial DNA. Shock 2015, 44, 265–271. [Google Scholar] [CrossRef]
- Mouliere, F.; Thierry, A.R. The importance of examining the proportion of circulating DNA originating from tumor, microenvironment and normal cells in colorectal cancer patients. Exp. Opin. Biol. Ther. 2012, 12, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Marsman, G.; Zeerleder, S.; Luken, B.M. Extracellular histones, cell-free DNA, or nucleosomes: Differences in immunostimulation. Cell Death Dis. 2016, 7, e2518. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, X.; Pelayo, R.; Monestier, M.; Ammollo, C.T.; Semeraro, F.; Taylor, F.B.; Esmon, N.L.; Lupu, F.; Esmon, C.T. Extracellular histones are major mediators of death in sepsis. Nat. Med. 2009, 15, 1318–1321. [Google Scholar] [CrossRef]
- Tian, J.; Avalos, A.M.; Mao, S.; Chen, B.; Senthil, K.; Wu, H.; Parroche, P.; Drabic, S.; Golenbock, D.; Sirois, C.; et al. Toll-like receptor 9- dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat. Immunol. 2007, 8, 487–496. [Google Scholar] [CrossRef]
- Scaffidi, P.; Misteli, T.; Bianchi, M.E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002, 418, 191–195. [Google Scholar] [CrossRef]
- Bendich, A.; Wilczok, T.; Borenfreund, E. Circulating DNA as a possible factor in oncogenesis. Science 1965, 148, 374–376. [Google Scholar] [CrossRef]
- Garcia-Olmo, D.C.; Picazo, M.G.; Garcia-Olmo, D. Transformation of non-tumor host cells during tumor progression: Theories and evidence. Expert Opin. Biol. Ther. 2012, 12, 199–207. [Google Scholar] [CrossRef]
- Anker, P.; Lyautey, J.; Lefort, F.; Lederrey, C.; Stroun, M. Transformation de cellules NIH/3 T3 et cellules SW480 porteuses d’une mutation K-ras. Comptes Rendus De L’academie Des Sci. Ser. III Sci. De La Vie 1994, 317, 869–874. [Google Scholar]
- Garcia-Olmo, D.C.; Dominguez, C.; Garcia-Arranz, M.; Anker, P.; Stroun, M.; Garcia-Verdugo, J.M.; Garcia-Olmo, D. Cell-free nucleic acids circulating in the plasma of colorectal cancer patients induce the oncogenic transformation of susceptible cultured cells. Cancer Res. 2010, 70, 560–567. [Google Scholar] [CrossRef]
- Wang, K.; Shan, S.; Wang, S.; Gu, X.; Zhou, X.; Ren, T. HMGB1-containing nucleosome mediates chemotherapy-induced metastasis of human lung cancer. Biochem. Biophys. Res. Commun. 2018, 500, 758–764. [Google Scholar] [CrossRef]
- Chen, Z.; Fadiel, A.; Naftolin, F.; Eichenbaum, K.D.; Xia, Y. Circulation DNA: Biological implications for cancer metastasis and immunology. Med. Hypot. 2005, 65, 956–961. [Google Scholar] [CrossRef] [PubMed]
- Beltinger, C.; Saragovi, H.; Smith, R.; LeSauteur, L.; Shah, N.; DeDionisio, L.; Christensen, L.; Raible, A.; Jarett, L.; Gewirtz, A. Binding, uptake, and intracellular trafficking of phosphorothioatemodified oligodeoxynucleotides. J. Clin. Investig. 1995, 95, 1814–1823. [Google Scholar] [CrossRef] [PubMed]
- Huss, R. A 42 kD erythrocyte surface membrane protein with binding capacity to polynucleotides shows functional lack in systemic lupus erythematosus. Immunobiology 1988, 178, 141–142. [Google Scholar]
- Laktionov, P.P.; Tamkovich, S.N.; Rykova, E.Y.; Bryzgunova, O.E.; Starikov, A.V.; Kuznetsova, N.P.; Vlassov, V.V. Free and cell surface bound nucleic acids in blood of healthy donors and breast cancer patients. Ann. N. Y. Acad. Sci. 2004, 1022, 221–227. [Google Scholar] [CrossRef]
- Fozza, C.; Bellizzi, S.; Bonfigli, S.; Campus, P.M.; Dore, F. Cytogenetic and hematological spontaneous remission in a case of acute myelogeneous leukemia. Eur. J. Haematol. 2004, 73, 219–222. [Google Scholar] [CrossRef]
- Tanner, J.E. Nucleosomes activate NF-kappaB in endothelial cells for induction of the proangiogenic cytokine IL-8. Int. J. Cancer 2004, 1, 155–160. [Google Scholar] [CrossRef]
- Tanner, J.E.; Forte, A.; Panchal, C. Nucleosomes bind fibroblast growth factor-2 for increased angiogenesis in vitro and in vivo. Mol. Cancer Res. 2004, 2, 281–288. [Google Scholar] [CrossRef]
- Fernandez-Dominguez, I.J.; Manzo-Merino, J.; Taja-Chayeb, L.; Duenas-Gonzalez, A.; Perez-Cardenas, E.; Trejo-Becerril, C. The role of extracellular DNA (exDNA) in cellular processes. Cancer Biol. Ther. 2021, 22, 267–278. [Google Scholar] [CrossRef]
- Celec, P.; Vlkova, B.; Laukova, L.; Babíckova, J.; Boor, P. Cell-free DNA: The role in pathophysiology and as a biomarker in kidney diseases. Expert Rev. Mol. 2018, 20, e1. [Google Scholar] [CrossRef]
- Diehl, F.; Schmidt, K.; Choti, M.A.; Romans, K.; Goodman, S.; Li, M.; Thornton, K.; Agrawal, N.; Sokoll, L.; Szabo, S.A.; et al. Circulating mutant DNA to assess tumor dynamics. Nat. Med. 2008, 14, 985–990. [Google Scholar] [CrossRef]
- Leung, F.; Kulasingam, V.; Diamandis, E.P.; Hoon, D.S.B.; Kinzler, K.; Pantel, K.; Alix-Panabières, C. Circulating tumor DNA as a cancer biomarker: Fact or fiction? Clin. Chem. 2016, 62, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Sallai, K.; Nagy, E.; Derfalvy, B.; Muzes, G.; Gergely, P. Antinucleosome antibodies and decreased deoxyribonuclease activity in sera of patients with systemic lupus erythematosus. Clin. Diagn. Lab. Immunol. 2005, 12, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Pisetsky, D.S. DNA as amarker of cell death in systemic lupus erythematosus. Rheum. Dis. Clin. N. Am. 2004, 30, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Odaka, C.; Mizuochi, T. Role of macrophage lysosomal enzymes in the degradation of nucleosomes of apoptotic cells. J. Immunol. 1999, 163, 5346–5352. [Google Scholar] [PubMed]
- Savill, J.; Fadok, V. Corpse clearance defines the meaning of cell death. Nature 2000, 407, 784–788. [Google Scholar] [CrossRef]
- Bryzgunova, O.E.; Laktionov, P.P. Extracellular Nucleic Acids in Urine: Sources, Structure, Diagnostic Potential. Acta Nat. 2015, 7, 48–54. [Google Scholar] [CrossRef]
- Stephan, F.; Marsman, G.; Bakker, L.M.; Bulder, I.; Stavenuiter, F.; Aarden, L.A.; Zeerleder, S. Cooperation of factor vii-activating protease and serum DNase I in the release of nucleosomes from necrotic cells. Arthritis. Rheumatol. 2014, 66, 686–693. [Google Scholar] [CrossRef]
- Martin, M.; Leffler, J.; Smolag, K.I.; Mytych, J.; Bjork, A.; Chaves, L.D.; Alexander, J.J.; Quigg, R.J.; Blom, A.M. Factor H uptake regulates intracellular C3 activation during apoptosis and decreases the inflammatory potential of nucleosomes. Cell Death Differ. 2016, 23, 903–911. [Google Scholar] [CrossRef]
- Amoura, Z.; Piette, J.C.; Chabre, H.; Cacoub, P.; Papo, T.; Wechsler, B.; Bach, J.F.; Koutouzov, S. Circulating plasma levels of nucleosomes in patients with systemic lupus erythematosus: Correlation with serum antinucleosome antibody titers and absence of clear association with disease activity. Arthritis. Rheum. 1997, 40, 2217–2225. [Google Scholar] [CrossRef]
- Emlen, W.; Burdick, G. Clearance and organ localization of small DNA anti-DNA immune complexes in mice. J. Immunol. 1988, 140, 1816–1822. [Google Scholar]
- Macanovic, M.; Lachmann, P.J. Measurement of deoxyribonuclease I (DNase) in the serum and urine of systemic lupus erythematosus (SLE)-prone NZB/NZW mice by a new radial enzyme diffusion assay. Clin. Exp. Immunol. 1997, 108, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Blood Sample Monitoring of Patients With EGFR Mutated Lung Cancer//US National Library of Medicine. Available online: https://clinicaltrials.gov/ct2/show/NCT02284633 (accessed on 25 May 2021).
- Monitoring Plasma Tumor DNA in Early—Stage Breast Cancer//US National Library of Medicine. 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02743910 (accessed on 25 May 2021).
- Naik, S.H.; Schumacher, T.N.; Perie, L. Cellular barcoding: A technical appraisal. Exp. Hematol. 2014, 42, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Jiang, P.; Chan, K.C.A.; Wong, J.; Cheng, Y.K.Y.; Liang, R.H.S.; Chan, W.; Ma, E.S.K.; Chan, S.L.; Cheng, S.H.; et al. Plasma DNA tissue mapping by genome-wide methylation sequencing for noninvasive prenatal, cancer, and transplantation assessments. Proc. Natl. Acad. Sci. USA 2015, 112, 5503–5512. [Google Scholar] [CrossRef]
- Mouliere, F.; Robert, F.B.; Peyrotte, E.A.; Del Rio, M.; Ychou, M.; Molina, F.; Gongora, C.; Thierry, A.R. High fragmentation characterizes tumour-derived circulating DNA. PLoS ONE 2011, 6, e23418. [Google Scholar] [CrossRef] [PubMed]
- Dawson, S.; Tsui, D.W.Y.; Murtaza, M.; Biggs, H.; Rueda, O.M.; Chin, S.; Dunning, M.J.; Gale, D.; Forshew, T.; Mahler-Araujo, B.; et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N. Engl. J. Med. 2013, 368, 1199–1209. [Google Scholar] [CrossRef]
- Thierry, A.R.; Mouliere, F.; El Messaoudi, S.; Mollevi, C.; Lopez-Crapez, E.; Rolet, F.; Gillet, B.; Gongora, C.; Dechelotte, P.; Robert, B.; et al. Clinical validation of the detection of KRAS and BRAF mutations from circulating tumor DNA. Nat. Med. 2014, 20, 430–435. [Google Scholar] [CrossRef]
- Mouliere, F.; El Messaoudi, S.; Pang, D.; Dritschilo, A.; Thierry, A.R. Multi-marker analysis of circulating cell-free DNA toward personalized medicine for colorectal cancer. Mol. Oncol. 2014, 8, 927–941. [Google Scholar] [CrossRef]
- Spindler, K.L.G.; Pallisgaard, N.; Andersen, R.F.; Jakobsen, A. Changes in mutational status during third-line treatment for metastatic colorectal cancer—Results of consecutive measurement of cell free DNA, KRAS and BRAF in the plasma. Int. J. Cancer 2014, 135, 2215–2222. [Google Scholar] [CrossRef]
- Snyder, M.W.; Kircher, M.; Hill, A.J.; Daza, R.M.; Shendure, J. Cell-free DNA comprises an in vivo nucleosome footprint that informs its tissues-of-origin. Cell 2016, 164, 57–68. [Google Scholar] [CrossRef]
- Sullivan, R.J.; O’Neill, V.J.; Brinkmann, K.; Enderle, D.; Koestler, T.; Spiel, A.; Emenegger, J.; Noerholm, M.; Skog, J.; Berking, C.; et al. Plasma-based monitoring of BRAF mutations during therapy for malignant melanoma using combined exosomal RNA and cell-free DNA analysis. J. Clin. Oncol. 2015, 33, 9017. [Google Scholar] [CrossRef][Green Version]
- Ulz, P.; Thallinger, G.G.; Auer, M.; Graf, R.; Kashofer, K.; Jahn, S.W.; Abete, L.; Pristauz, G.; Petru, E.; Geigl, J.B.; et al. Inferring expressed genes by whole-genome sequencing of plasma DNA. Nat. Genet. 2016, 48, 1273–1278. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, E.; Davilas, E.; Sotiriou, V.; Georgakopoulos, E.; Georgakopoulou, S.; Koliopanos, A.; Aggelakis, F.; Dardoufas, K.; Agnanti, N.J.; Karydas, I.; et al. Cell-free DNA and RNA in plasma as a new molecular marker for prostate and breast cancer. Ann. N. Y. Acad. Sci. 2006, 1075, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Adrianzen, O.A.; Koutouzov, S.; Mota, R.M.; das Chagas Medeiros, M.M.; Bach, J.F.; de Holanda Campos, H. Diagnostic value of anti-nucleosome antibodies in the assessment of disease activity of systemic lupus erythematosus: A prospective study comparing anti-nucleosome with antidsDNA antibodies. J. Rheumat. 2006, 33, 1538–1544. [Google Scholar]
- Holdenrieder, S.; Stieber, P.; von Pawel, J.; Raith, H.; Nagel, D.; Feldmann, K.; Seidel, D. Early and specific prediction of the therapeutic efficacy in lung cancer by nucleosomal DNA and cytokeratin 19 fragments. Ann. N. Y. Acad. Sci. 2006, 1075, 244–275. [Google Scholar] [CrossRef] [PubMed]
- Waldron, D. A nucleosome footprint reveals the source of cfDNA. Nat. Rev. Genet. 2016, 17, 125. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.S.; Webster, M. Nucleosome Spacing—The Baggage Tag for Cell-Free DNA. Clin. Chem. 2016, 62, 1545–1547. [Google Scholar] [CrossRef] [PubMed]
- Ulz, P.; Perakis, S.; Zhou, Q.; Moser, T.; Belic, J.; Lazzeri, I.; Wolfler, A.; Zebisch, A.; Gerger, A.; Pristauz, G.; et al. Inference of transcription factor binding from cellfree DNA enables tumor subtype prediction and early detection. Nat. Comm. 2019, 10, 4666. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tutanov, O.; Tamkovich, S. The Influence of Proteins on Fate and Biological Role of Circulating DNA. Int. J. Mol. Sci. 2022, 23, 7224. https://doi.org/10.3390/ijms23137224
Tutanov O, Tamkovich S. The Influence of Proteins on Fate and Biological Role of Circulating DNA. International Journal of Molecular Sciences. 2022; 23(13):7224. https://doi.org/10.3390/ijms23137224
Chicago/Turabian StyleTutanov, Oleg, and Svetlana Tamkovich. 2022. "The Influence of Proteins on Fate and Biological Role of Circulating DNA" International Journal of Molecular Sciences 23, no. 13: 7224. https://doi.org/10.3390/ijms23137224
APA StyleTutanov, O., & Tamkovich, S. (2022). The Influence of Proteins on Fate and Biological Role of Circulating DNA. International Journal of Molecular Sciences, 23(13), 7224. https://doi.org/10.3390/ijms23137224





