Hydrogen Sulfide Plays an Important Role by Regulating Endoplasmic Reticulum Stress in Diabetes-Related Diseases
Abstract
:1. Introduction
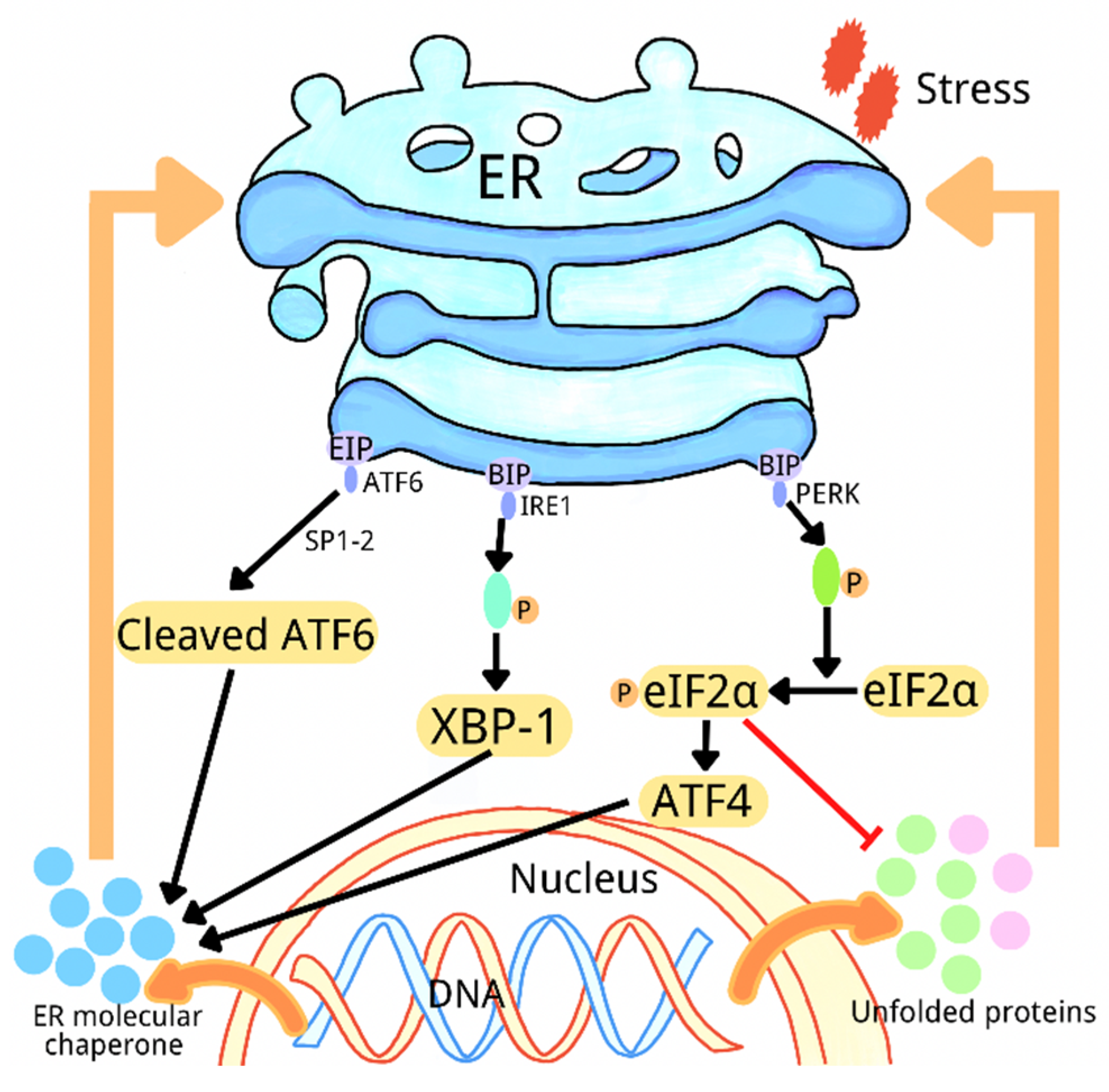
2. Overview of Endoplasmic Reticulum Stress
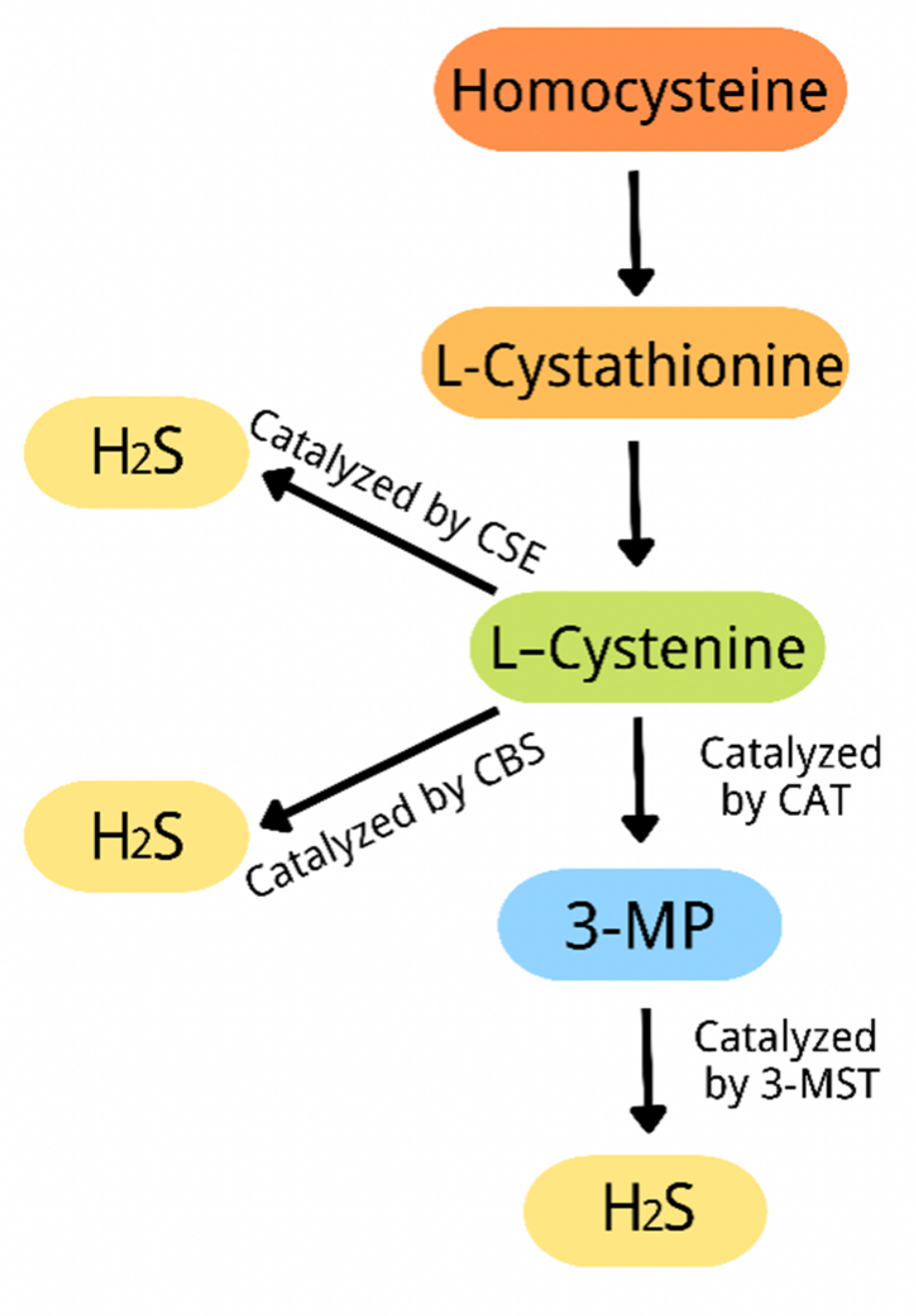
3. Overview of Hydrogen Sulfide
4. Hydrogen Sulfide Improves Diabetes-Associated Cognitive Dysfunction by Regulating Endoplasmic Reticulum Stress
5. Hydrogen Sulfide Improves Diabetic Cardiomyopathy by Regulating Endoplasmic Reticulum Stress
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, Z.; Wang, H.; Fang, S.; Xu, C. Roles of endoplasmic reticulum stress and autophagy on H2O2-induced oxidative stress injury in HepG2 cells. Mol. Med. Rep. 2018, 18, 4163–4174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.J.; Kim, Y.; Chen, Y.M. Endoplasmic reticulum stress and monogenic kidney diseases in precision nephrology. Pediatr. Nephrol. 2019, 34, 1493–1500. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shi, X.; Qiu, M.; Lv, S.; Liu, H. Hydrogen Sulfide Plays an Important Protective Role through Influencing Endoplasmic Reticulum Stress in Diseases. Int. J. Biol. Sci. 2020, 16, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Han, Y.; Yang, W.; Xu, B.; Sun, M.; Jiang, S.; Yu, Y.; Jin, Z.; Ma, Z.; Yang, Y.; et al. Endoplasmic reticulum stress and NLRP3 inflammasome: Crosstalk in cardiovascular and metabolic disorders. J. Cell Physiol. 2019, 234, 14773–14782. [Google Scholar] [CrossRef]
- Landry, A.P.; Ballou, D.P.; Banerjee, R. Hydrogen Sulfide Oxidation by Sulfide Quinone Oxidoreductase. ChemBioChem 2021, 22, 949–960. [Google Scholar] [CrossRef]
- Sun, H.J.; Wu, Z.Y.; Cao, L.; Zhu, M.Y.; Liu, T.T.; Guo, L.; Lin, Y.; Nie, X.W.; Bian, J.S. Hydrogen Sulfide: Recent Progression and Perspectives for the Treatment of Diabetic Nephropathy. Molecules 2019, 24, 2857. [Google Scholar] [CrossRef] [Green Version]
- Qian, L.L.; Liu, X.Y.; Chai, Q.; Wang, R.X. Hydrogen Sulfide in Diabetic Complications: Focus on Molecular Mechanisms. Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 470–476. [Google Scholar] [CrossRef]
- Chen, Y.H.; Teng, X.; Hu, Z.J.; Tian, D.Y.; Jin, S.; Wu, Y.M. Hydrogen Sulfide Attenuated Sepsis-Induced Myocardial Dysfunction Through TLR4 Pathway and Endoplasmic Reticulum Stress. Front. Physiol. 2021, 12, 653601. [Google Scholar] [CrossRef]
- He, J.; Chen, Z.; Kang, X.; Wu, L.; Jiang, J.M.; Liu, S.M.; Wei, H.J.; Chen, Y.J.; Zou, W.; Wang, C.Y.; et al. SIRT1 Mediates H2S-Ameliorated Diabetes-Associated Cognitive Dysfunction in Rats: Possible Involvement of Inhibiting Hippocampal Endoplasmic Reticulum Stress and Synaptic Dysfunction. Neurochem. Res. 2021, 46, 611–623. [Google Scholar] [CrossRef]
- Chen, L.; Ma, K.; Fan, H.; Wang, X.; Cao, T. Exogenous hydrogen sulfide protects against hepatic ischemia/reperfusion injury by inhibiting endoplasmic reticulum stress and cell apoptosis. Exp. Ther. Med. 2021, 22, 799. [Google Scholar] [CrossRef]
- Li, Y.; Liu, M.; Yi, J.; Song, X.; Zheng, X.; Liu, D.; Wang, S.; Chu, C.; Yang, J. Exogenous hydrogen sulfide inhibits apoptosis by regulating endoplasmic reticulum stress-autophagy axis and improves myocardial reconstruction after acute myocardial infarction. Acta Biochim. Biophys. Sin. 2020, 52, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.; Lee, C.; Li, B.; Pierro, A. Endoplasmic reticulum stress in the acute intestinal epithelial injury of necrotizing enterocolitis. Pediatr. Surg. Int. 2021, 37, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Mandl, J.; Meszaros, T.; Banhegyi, G.; Csala, M. Minireview: Endoplasmic reticulum stress: Control in protein, lipid, and signal homeostasis. Mol. Endocrinol. 2013, 27, 384–393. [Google Scholar] [CrossRef] [Green Version]
- Rangel-Aldao, R. The unfolded protein response, inflammation, oscillators, and disease: A systems biology approach. Endoplasmic Reticulum Stress Dis. 2015, 2, 30–52. [Google Scholar] [CrossRef]
- Xia, S.W.; Wang, Z.M.; Sun, S.M.; Su, Y.; Li, Z.H.; Shao, J.J.; Tan, S.Z.; Chen, A.P.; Wang, S.J.; Zhang, Z.L.; et al. Endoplasmic reticulum stress and protein degradation in chronic liver disease. Pharmacol. Res. 2020, 161, 105218. [Google Scholar] [CrossRef]
- Marciniak, S.J.; Chambers, J.E.; Ron, D. Pharmacological targeting of endoplasmic reticulum stress in disease. Nat. Rev. Drug Discov. 2022, 21, 115–140. [Google Scholar] [CrossRef]
- So, J.S. Roles of Endoplasmic Reticulum Stress in Immune Responses. Mol. Cells 2018, 41, 705–716. [Google Scholar] [CrossRef]
- Oakes, S.A. Endoplasmic Reticulum Stress Signaling in Cancer Cells. Am. J. Pathol. 2020, 190, 934–946. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Li, X.; Lu, P.; Li, X.; Sun, M.; Wang, H. The Role of the Signaling Pathways Involved in the Effects of Hydrogen Sulfide on Endoplasmic Reticulum Stress. Front. Cell Dev. Biol. 2021, 9, 646723. [Google Scholar] [CrossRef]
- Hu, H.; Tian, M.; Ding, C.; Yu, S. The C/EBP Homologous Protein (CHOP) Transcription Factor Functions in Endoplasmic Reticulum Stress-Induced Apoptosis and Microbial Infection. Front. Immunol. 2018, 9, 3083. [Google Scholar] [CrossRef] [Green Version]
- Kong, F.J.; Ma, L.L.; Guo, J.J.; Xu, L.H.; Li, Y.; Qu, S. Endoplasmic reticulum stress/autophagy pathway is involved in diabetes-induced neuronal apoptosis and cognitive decline in mice. Clin. Sci. 2018, 132, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Menikdiwela, K.R.; Torres Guimaraes, J.P.; Ramalingam, L.; Kalupahana, N.S.; Dufour, J.M.; Washburn, R.L.; Moustaid-Moussa, N. Mechanisms linking endoplasmic reticulum (ER) stress and microRNAs to adipose tissue dysfunction in obesity. Crit. Rev. Biochem. Mol. Biol. 2021, 56, 455–481. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Xu, X.; Zhou, S.; Chen, Y.; Ding, G.; Cao, L. Fisetin induces autophagy in pancreatic cancer cells via endoplasmic reticulum stress- and mitochondrial stress-dependent pathways. Cell Death Dis. 2019, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Barrera, M.J.; Aguilera, S.; Castro, I.; Gonzalez, S.; Carvajal, P.; Molina, C.; Hermoso, M.A.; Gonzalez, M.J. Endoplasmic reticulum stress in autoimmune diseases: Can altered protein quality control and/or unfolded protein response contribute to autoimmunity? A critical review on Sjogren’s syndrome. Autoimmun. Rev. 2018, 17, 796–808. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, W.; Wang, Y. STING is an essential regulator of heart inflammation and fibrosis in mice with pathological cardiac hypertrophy via endoplasmic reticulum (ER) stress. Biomed. Pharmacother. 2020, 125, 110022. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, M.N.; Min, S.H.; Ham, D.S.; Kim, J.W.; Yoon, K.H.; Park, K.S.; Jung, H.S. Specific PERK inhibitors enhanced glucose-stimulated insulin secretion in a mouse model of type 2 diabetes. Metabolism 2019, 97, 87–91. [Google Scholar] [CrossRef]
- Herlea-Pana, O.; Eeda, V.; Undi, R.B.; Lim, H.Y.; Wang, W. Pharmacological Inhibition of Inositol-Requiring Enzyme 1alpha RNase Activity Protects Pancreatic Beta Cell and Improves Diabetic Condition in Insulin Mutation-Induced Diabetes. Front. Endocrinol. 2021, 12, 749879. [Google Scholar] [CrossRef]
- Roubenne, L.; Marthan, R.; Le Grand, B.; Guibert, C. Hydrogen Sulfide Metabolism and Pulmonary Hypertension. Cells 2021, 10, 1477. [Google Scholar] [CrossRef]
- Zaorska, E.; Tomasova, L.; Koszelewski, D.; Ostaszewski, R.; Ufnal, M. Hydrogen Sulfide in Pharmacotherapy, Beyond the Hydrogen Sulfide-Donors. Biomolecules 2020, 10, 323. [Google Scholar] [CrossRef] [Green Version]
- Powell, C.R.; Dillon, K.M.; Matson, J.B. A review of hydrogen sulfide (H2S) donors: Chemistry and potential therapeutic applications. Biochem. Pharmacol. 2018, 149, 110–123. [Google Scholar] [CrossRef]
- Rose, P.; Moore, P.K.; Zhu, Y.Z. H2S biosynthesis and catabolism: New insights from molecular studies. Cell Mol. Life Sci. 2017, 74, 1391–1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.; Ding, Y.; Liu, H.; Sun, M.; Chen, C.; Yang, Y.; Wang, H. The Role of Hydrogen Sulfide Regulation of Autophagy in Liver Disorders. Int. J. Mol. Sci. 2022, 23, 4035. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Liu, H.; Wang, H. Exogenous Hydrogen Sulfide Plays an Important Role by Regulating Autophagy in Diabetic-Related Diseases. Int. J. Mol. Sci. 2021, 22, 6715. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, D.; Wang, H. Hydrogen sulfide plays an important protective role by influencing autophagy in diseases. Physiol. Res. 2019, 68, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H. Signaling by hydrogen sulfide (H2S) and polysulfides (H2Sn) in the central nervous system. Neurochem. Int. 2019, 126, 118–125. [Google Scholar] [CrossRef]
- Kabil, O.; Banerjee, R. Enzymology of H2S biogenesis, decay and signaling. Antioxid. Redox Signal 2014, 20, 770–782. [Google Scholar] [CrossRef] [Green Version]
- Baskar, R.; Sparatore, A.; Del Soldato, P.; Moore, P.K. Effect of S-diclofenac, a novel hydrogen sulfide releasing derivative inhibit rat vascular smooth muscle cell proliferation. Eur. J. Pharmacol. 2008, 594, 1–8. [Google Scholar] [CrossRef]
- Sun, Y.; Teng, Z.; Sun, X.; Zhang, L.; Chen, J.; Wang, B.; Lu, F.; Liu, N.; Yu, M.; Peng, S.; et al. Exogenous H2S reduces the acetylation levels of mitochondrial respiratory enzymes via regulating the NAD+-SIRT3 pathway in cardiac tissues of db/db mice. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E284–E297. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, R.; Wu, L.; Yang, G. Hydrogen sulfide signaling in regulation of cell behaviors. Nitric Oxide 2020, 103, 9–19. [Google Scholar] [CrossRef]
- Yuan, C.L.; Yi, R.; Dong, Q.; Yao, L.F.; Liu, B. The relationship between diabetes-related cognitive dysfunction and leukoaraiosis. Acta Neurol. Belg. 2021, 121, 1101–1110. [Google Scholar] [CrossRef]
- Biessels, G.J.; Despa, F. Cognitive decline and dementia in diabetes mellitus: Mechanisms and clinical implications. Nat. Rev. Endocrinol. 2018, 14, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Ennis, G.E.; Saelzler, U.; Umpierrez, G.E.; Moffat, S.D. Prediabetes and working memory in older adults. Brain Neurosci. Adv. 2020, 4, 2398212820961725. [Google Scholar] [CrossRef] [PubMed]
- Willmann, C.; Brockmann, K.; Wagner, R.; Kullmann, S.; Preissl, H.; Schnauder, G.; Maetzler, W.; Gasser, T.; Berg, D.; Eschweiler, G.W.; et al. Insulin sensitivity predicts cognitive decline in individuals with prediabetes. BMJ Open Diabetes Res. Care 2020, 8, e001741. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Liu, Z.; Chen, Y.; Xu, Y.; Qin, J.; Guo, S.; Huang, J.; Tao, J. The prevalence of mild cognitive impairment in type 2 diabetes mellitus patients: A systematic review and meta-analysis. Acta Diabetol. 2021, 58, 671–685. [Google Scholar] [CrossRef] [PubMed]
- Biessels, G.J.; Kerssen, A.; de Haan, E.H.; Kappelle, L.J. Cognitive dysfunction and diabetes: Implications for primary care. Prim. Care Diabetes 2007, 1, 187–193. [Google Scholar] [CrossRef]
- Wu, X.L.; Deng, M.Z.; Gao, Z.J.; Dang, Y.Y.; Li, Y.C.; Li, C.W. Neferine alleviates memory and cognitive dysfunction in diabetic mice through modulation of the NLRP3 inflammasome pathway and alleviation of endoplasmic-reticulum stress. Int. Immunopharmacol. 2020, 84, 106559. [Google Scholar] [CrossRef]
- Ye, T.; Meng, X.; Zhai, Y.; Xie, W.; Wang, R.; Sun, G.; Sun, X. Gastrodin Ameliorates Cognitive Dysfunction in Diabetes Rat Model via the Suppression of Endoplasmic Reticulum Stress and NLRP3 Inflammasome Activation. Front. Pharmacol. 2018, 9, 1346. [Google Scholar] [CrossRef]
- Zou, W.; Yuan, J.; Tang, Z.J.; Wei, H.J.; Zhu, W.W.; Zhang, P.; Gu, H.F.; Wang, C.Y.; Tang, X.Q. Hydrogen sulfide ameliorates cognitive dysfunction in streptozotocin-induced diabetic rats: Involving suppression in hippocampal endoplasmic reticulum stress. Oncotarget 2017, 8, 64203–64216. [Google Scholar] [CrossRef] [Green Version]
- Balakumar, M.; Raji, L.; Prabhu, D.; Sathishkumar, C.; Prabu, P.; Mohan, V.; Balasubramanyam, M. High-fructose diet is as detrimental as high-fat diet in the induction of insulin resistance and diabetes mediated by hepatic/pancreatic endoplasmic reticulum (ER) stress. Mol. Cell Biochem. 2016, 423, 93–104. [Google Scholar] [CrossRef]
- Doyle, K.M.; Kennedy, D.; Gorman, A.M.; Gupta, S.; Healy, S.J.; Samali, A. Unfolded proteins and endoplasmic reticulum stress in neurodegenerative disorders. J. Cell Mol. Med. 2011, 15, 2025–2039. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Xu, L.; He, D.; Ling, S. Endoplasmic reticulum stress-mediated hippocampal neuron apoptosis involved in diabetic cognitive impairment. BioMed Res. Int. 2013, 2013, 924327. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Yang, W.; Wu, L.; Wang, R. H2S, endoplasmic reticulum stress, and apoptosis of insulin-secreting beta cells. J. Biol. Chem. 2007, 282, 16567–16576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trujillo-Estrada, L.; Nguyen, C.; da Cunha, C.; Cai, L.; Forner, S.; Martini, A.C.; Ager, R.R.; Prieto, G.A.; Cotman, C.W.; Baglietto-Vargas, D.; et al. Tau underlies synaptic and cognitive deficits for type 1, but not type 2 diabetes mouse models. Aging Cell 2019, 18, e12919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, X.; Wang, X.; Wu, Y.; Hu, J.; Li, Y.; Jin, S.; Wu, X. Activation of GPR55 attenuates cognitive impairment, oxidative stress, neuroinflammation, and synaptic dysfunction in a streptozotocin-induced Alzheimer’s mouse model. Pharmacol. Biochem. Behav. 2022, 214, 173340. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.J.; Lin, C.C.; Yeh, C.M.; Chien, M.E.; Tsao, M.C.; Tseng, P.; Huang, C.W.; Hsu, K.S. Repeated transcranial direct current stimulation improves cognitive dysfunction and synaptic plasticity deficit in the prefrontal cortex of streptozotocin-induced diabetic rats. Brain Stimul. 2017, 10, 1079–1087. [Google Scholar] [CrossRef]
- Tian, X.; Liu, Y.; Ren, G.; Yin, L.; Liang, X.; Geng, T.; Dang, H.; An, R. Resveratrol limits diabetes-associated cognitive decline in rats by preventing oxidative stress and inflammation and modulating hippocampal structural synaptic plasticity. Brain Res. 2016, 1650, 1–9. [Google Scholar] [CrossRef]
- Kassab, S.; Begley, P.; Church, S.J.; Rotariu, S.M.; Chevalier-Riffard, C.; Dowsey, A.W.; Phillips, A.M.; Zeef, L.A.H.; Grayson, B.; Neill, J.C.; et al. Cognitive dysfunction in diabetic rats is prevented by pyridoxamine treatment. A multidisciplinary investigation. Mol. Metab. 2019, 28, 107–119. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Liu, C.; Li, M. Silent Information Regulator 1 Promotes Proliferation, Migration, and Invasion of Cervical Cancer Cells and Is Upregulated by Human Papillomavirus 16 E7 Oncoprotein. Gynecol. Obstet. Investig. 2022, 87, 22–29. [Google Scholar] [CrossRef]
- Zhang, J.; Peng, J.; Kong, D.; Wang, X.; Wang, Z.; Liu, J.; Yu, W.; Wu, H.; Long, Z.; Zhang, W.; et al. Silent information regulator 1 suppresses epithelial-to-mesenchymal transition in lung cancer cells via its regulation of mitochondria status. Life Sci. 2021, 280, 119716. [Google Scholar] [CrossRef]
- Ma, L.H.; Wan, J.; Yan, J.; Wang, N.; Liu, Y.P.; Wang, H.B.; Zhou, C.H.; Wu, Y.Q. Hippocampal SIRT1-Mediated Synaptic Plasticity and Glutamatergic Neuronal Excitability Are Involved in Prolonged Cognitive Dysfunction of Neonatal Rats Exposed to Propofol. Mol. Neurobiol. 2022, 59, 1938–1953. [Google Scholar] [CrossRef]
- Peng, X.; Wang, J.; Peng, J.; Jiang, H.; Le, K. Resveratrol Improves Synaptic Plasticity in Hypoxic-Ischemic Brain Injury in Neonatal Mice via Alleviating SIRT1/NF-kappaB Signaling-Mediated Neuroinflammation. J. Mol. Neurosci. 2022, 72, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Palomer, X.; Aguilar-Recarte, D.; Garcia, R.; Nistal, J.F.; Vazquez-Carrera, M. Sirtuins: To Be or Not To Be in Diabetic Cardiomyopathy. Trends Mol. Med. 2021, 27, 554–571. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Guan, Y.; Raja, R.; Ruiz-Velasco, A.; Liu, W. Mechanisms and Therapeutic Prospects of Diabetic Cardiomyopathy Through the Inflammatory Response. Front. Physiol. 2021, 12, 694864. [Google Scholar] [CrossRef]
- Wan, H.; Zhao, S.; Zeng, Q.; Tan, Y.; Zhang, C.; Liu, L.; Qu, S. CircRNAs in diabetic cardiomyopathy. Clin. Chim. Acta 2021, 517, 127–132. [Google Scholar] [CrossRef]
- Kumric, M.; Ticinovic Kurir, T.; Borovac, J.A.; Bozic, J. Role of novel biomarkers in diabetic cardiomyopathy. World J. Diabetes 2021, 12, 685–705. [Google Scholar] [CrossRef]
- Wu, M.X.; Wang, S.H.; Xie, Y.; Chen, Z.T.; Guo, Q.; Yuan, W.L.; Guan, C.; Xu, C.Z.; Huang, Y.N.; Wang, J.F.; et al. Interleukin-33 alleviates diabetic cardiomyopathy through regulation of endoplasmic reticulum stress and autophagy via insulin-like growth factor-binding protein 3. J. Cell Physiol. 2021, 236, 4403–4419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, F.; Wang, Y. Mitofusin-2 Enhances Mitochondrial Contact with the Endoplasmic Reticulum and Promotes Diabetic Cardiomyopathy. Front. Physiol. 2021, 12, 707634. [Google Scholar] [CrossRef]
- Park, I.H.; Shen, G.Y.; Song, Y.S.; Jong Cho, Y.; Kim, B.S.; Lee, Y.; Lim, Y.H.; Shin, J.H.; Kim, K.S. Granulocyte colony-stimulating factor reduces the endoplasmic reticulum stress in a rat model of diabetic cardiomyopathy. Endocr. J. 2021, 68, 1293–1301. [Google Scholar] [CrossRef]
- Barr, L.A.; Shimizu, Y.; Lambert, J.P.; Nicholson, C.K.; Calvert, J.W. Hydrogen sulfide attenuates high fat diet-induced cardiac dysfunction via the suppression of endoplasmic reticulum stress. Nitric Oxide 2015, 46, 145–156. [Google Scholar] [CrossRef] [Green Version]
- Achari, A.E.; Jain, S.K. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int. J. Mol. Sci. 2017, 18, 1321. [Google Scholar] [CrossRef] [Green Version]
- Zhu, K.; Guo, J.; Yu, X.; Wang, Q.; Yan, C.; Qiu, Q.; Tang, W.; Huang, X.; Mu, H.; Dou, L.; et al. Polypeptide Globular Adiponectin Ameliorates Hypoxia/Reoxygenation-Induced Cardiomyocyte Injury by Inhibiting Both Apoptosis and Necroptosis. J. Immunol. Res. 2021, 2021, 1815098. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lim, J.H.; Kim, E.N.; Hong, Y.A.; Park, H.J.; Chung, S.; Choi, B.S.; Kim, Y.S.; Park, J.Y.; Kim, H.W.; et al. Adiponectin receptor agonist ameliorates cardiac lipotoxicity via enhancing ceramide metabolism in type 2 diabetic mice. Cell Death Dis. 2022, 13, 282. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Jia, Q.; Liu, X.F.; Gao, Q.; Wang, L.; Ma, S.F. [Effect of hydrogen sulfide on oxidative stress and endoplasmic reticulum stress in diabetic cardiomyopathy]. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2016, 32, 8–12. [Google Scholar] [PubMed]
- Li, F.; Luo, J.; Wu, Z.; Xiao, T.; Zeng, O.; Li, L.; Li, Y.; Yang, J. Hydrogen sulfide exhibits cardioprotective effects by decreasing endoplasmic reticulum stress in a diabetic cardiomyopathy rat model. Mol. Med. Rep. 2016, 14, 865–873. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Zhen, J.; Yang, Y.; Gu, J.; Wu, S.; Liu, Q. Ginsenoside Rg1 ameliorates diabetic cardiomyopathy by inhibiting endoplasmic reticulum stress-induced apoptosis in a streptozotocin-induced diabetes rat model. J. Cell Mol. Med. 2016, 20, 623–631. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Q.; Chen, S.; Ren, W.; Liu, G.; Yao, K.; Wu, G.; Yin, Y. Escherichia coli aggravates endoplasmic reticulum stress and triggers CHOP-dependent apoptosis in weaned pigs. Amino Acids 2017, 49, 2073–2082. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, Y.; Xiang, Y.; Xie, Y.Q.; Huang, X.H.; Zhang, Y.C. Shengmai injection improved doxorubicin-induced cardiomyopathy by alleviating myocardial endoplasmic reticulum stress and caspase-12 dependent apoptosis. BioMed Res. Int. 2015, 2015, 952671. [Google Scholar] [CrossRef]
- Wu, H.; Guo, J.; Yao, Y.; Xu, S. Polystyrene nanoplastics induced cardiomyocyte apoptosis and myocardial inflammation in carp by promoting ROS production. Fish Shellfish Immunol. 2022, 125, 1–8. [Google Scholar] [CrossRef]
- Che, Y.; Tian, Y.; Chen, R.; Xia, L.; Liu, F.; Su, Z. IL-22 ameliorated cardiomyocyte apoptosis in cardiac ischemia/reperfusion injury by blocking mitochondrial membrane potential decrease, inhibiting ROS and cytochrome C. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166171. [Google Scholar] [CrossRef]
- Yang, F.; Yu, X.; Li, T.; Wu, J.; Zhao, Y.; Liu, J.; Sun, A.; Dong, S.; Wu, J.; Zhong, X.; et al. Exogenous H2S regulates endoplasmic reticulum-mitochondria cross-talk to inhibit apoptotic pathways in STZ-induced type I diabetes. Am. J. Physiol. Endocrinol. Metab. 2017, 312, E190–E203. [Google Scholar] [CrossRef] [Green Version]
- Khodzhaeva, V.; Schreiber, Y.; Geisslinger, G.; Brandes, R.P.; Brune, B.; Namgaladze, D. Mitofusin 2 Deficiency Causes Pro-Inflammatory Effects in Human Primary Macrophages. Front. Immunol. 2021, 12, 723683. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.Y.; Song, J.; Kim, Y.C.; Kim, M.H.; Hyun, Y.M. Mitofusin-2 Promotes the Epithelial-Mesenchymal Transition-Induced Cervical Cancer Progression. Immune Netw. 2021, 21, e30. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, V.; Menzies, K.J.; Auwerx, J. Repairing Mitochondrial Dysfunction in Disease. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 353–389. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Zheng, M.; Cao, C.; Chen, C.; Tang, J.; Zhang, W.; Cheng, H.; Chen, K.H.; Xiao, R.P. Mitofusin-2 is a major determinant of oxidative stress-mediated heart muscle cell apoptosis. J. Biol. Chem. 2007, 282, 23354–23361. [Google Scholar] [CrossRef] [Green Version]
- Cantoni, O.; Zito, E.; Fiorani, M.; Guidarelli, A. Arsenite impinges on endoplasmic reticulum-mitochondria crosstalk to elicit mitochondrial ROS formation and downstreAm. toxicity. Semin. Cancer Biol. 2021, 76, 132–138. [Google Scholar] [CrossRef]
- Hartwick Bjorkman, S.; Oliveira Pereira, R. The Interplay between Mitochondrial Reactive Oxygen Species, Endoplasmic Reticulum Stress, and Nrf2 Signaling in Cardiometabolic Health. Antioxid. Redox Signal. 2021, 35, 252–269. [Google Scholar] [CrossRef]
- Guo, R.; Wu, Z.; Jiang, J.; Liu, C.; Wu, B.; Li, X.; Li, T.; Mo, H.; He, S.; Li, S.; et al. New mechanism of lipotoxicity in diabetic cardiomyopathy: Deficiency of Endogenous H2S Production and ER stress. Mech. Ageing Dev. 2017, 162, 46–52. [Google Scholar] [CrossRef]
- Li, M.; Xu, C.; Shi, J.; Ding, J.; Wan, X.; Chen, D.; Gao, J.; Li, C.; Zhang, J.; Lin, Y.; et al. Fatty acids promote fatty liver disease via the dysregulation of 3-mercaptopyruvate sulfurtransferase/hydrogen sulfide pathway. Gut 2018, 67, 2169–2180. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, M.; Fan, M.; Pan, S.; Li, S.; Chen, M.; Wang, H. Metabolomic-proteomic combination analysis reveals the targets and molecular pathways associated with hydrogen sulfide alleviating NAFLD. Life Sci. 2021, 264, 118629. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, B.; Meng, M.; Zhao, W.; Wang, D.; Yuan, Y.; Zheng, Y.; Qiu, J.; Li, Y.; Li, G.; et al. FOXA3 induction under endoplasmic reticulum stress contributes to non-alcoholic fatty liver disease. J. Hepatol. 2021, 75, 150–162. [Google Scholar] [CrossRef]
- Zhao, T.; Wu, K.; Hogstrand, C.; Xu, Y.H.; Chen, G.H.; Wei, C.C.; Luo, Z. Lipophagy mediated carbohydrate-induced changes of lipid metabolism via oxidative stress, endoplasmic reticulum (ER) stress and ChREBP/PPARgamma pathways. Cell Mol. Life Sci. 2020, 77, 1987–2003. [Google Scholar] [CrossRef] [PubMed]
- Falcao-Pires, I.; Leite-Moreira, A.F. Diabetic cardiomyopathy: Understanding the molecular and cellular basis to progress in diagnosis and treatment. Heart Fail Rev. 2012, 17, 325–344. [Google Scholar] [CrossRef] [PubMed]
- Strong, V.; Moittie, S.; Sheppard, M.N.; Liptovszky, M.; White, K.; Redrobe, S.; Cobb, M.; Baiker, K. Idiopathic Myocardial Fibrosis in Captive Chimpanzees (Pan troglodytes). Vet. Pathol. 2020, 57, 183–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, J.; Liu, M.; Liu, S.; Tang, F.; Tan, W.; Xiao, T.; Chu, C.; Yang, J. H2S attenuates the myocardial fibrosis in diabetic rats through modulating PKC-ERK1/2MAPK signaling pathway. Technol. Health Care 2019, 27 (Suppl. S1), 307–316. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Chandra, T.P.; Song, X.; Nie, L.; Liu, M.; Yi, J.; Zheng, X.; Chu, C.; Yang, J. H2S improves doxorubicin-induced myocardial fibrosis by inhibiting oxidative stress and apoptosis via Keap1-Nrf2. Technol. Health Care 2021, 29 (Suppl. S1), 195–209. [Google Scholar] [CrossRef]
- Liu, M.; Li, Y.; Liang, B.; Li, Z.; Jiang, Z.; Chu, C.; Yang, J. Hydrogen sulfide attenuates myocardial fibrosis in diabetic rats through the JAK/STAT signaling pathway. Int. J. Mol. Med. 2018, 41, 1867–1876. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.C.; Ge, L.S.; Yang, P.L.; Tang, J.F.; Lin, J.F.; Chen, P.; Guan, X.Q. Carvedilol treatment ameliorates acute coxsackievirus B3-induced myocarditis associated with oxidative stress reduction. Eur. J. Pharmacol. 2010, 640, 112–116. [Google Scholar]
- Montero, P.; Milara, J.; Roger, I.; Cortijo, J. Role of JAK/STAT in Interstitial Lung Diseases; Molecular and Cellular Mechanisms. Int. J. Mol. Sci. 2021, 22, 6211. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Zhao, J.; Su, K.; He, K.; Da, Y.; Xia, H. Endoplasmic Reticulum Stress Regulates Cardiomyocyte Apoptosis in Myocardial Fibrosis Development via PERK-Mediated Autophagy. Cardiovasc. Toxicol. 2020, 20, 618–626. [Google Scholar] [CrossRef]
- Lv, S.; Li, X.; Wang, H. The Role of the Effects of Endoplasmic Reticulum Stress on NLRP3 Inflammasome in Diabetes. Front. Cell Dev. Biol. 2021, 9, 663528. [Google Scholar] [CrossRef]
| The Type of Diabetes-Related Diseases | The Role H2S and Endoplasmic Reticulum (ER) Stress | Experimental Model | References |
|---|---|---|---|
| diabetes-associated cognitive dysfunction | Exogenous H2S ameliorates diabetes-associated cognitive dysfunction, most likely through the inhibition of hippocampal ER stress. | Streptozotocin (STZ)-induced diabetic rats | [48] |
| diabetes-associated cognitive dysfunction | Exogenous H2S ameliorates cognitive dysfunction through the inhibition of hippocampal ER stress and improvement of the synaptic dysfunction by upregulating SIRT1. | STZ-induced diabetic rats | [9] |
| diabetes cardiomyopathy (DC) | Exogenous H2S improves high fat diet (HFD)-induced cardiac dysfunction by inhibiting ER stress and by restoring HFD-suppressed circulating and cardiac H2S. | HFD-induced diabetic rats | [69] |
| DC | Exogenous H2S improves DC probably through the reduction in oxidative stress injury and ER stress-induced apoptosis. | STZ-induced diabetic rats | [73] |
| DC | Exogenous H2S improves DC through the inhibition of ER stress-mediated apoptosis by suppressing CHOP and caspase-12. | STZ-induced diabetic rats | [74] |
| DC | Exogenous H2S improves myocardial tissue damage by suppressing hyperglycemia-induced ER stress and mitochondrial apoptosis of cardiomyocyte through inhibiting Mfn-2. | STZ-induced diabetic rats | [80] |
| DC | Exogenous H2S alleviates myocardial injury by suppressing ER stress. | STZ/palmitic acid-induced diabetic rat/cardiac cells | [87] |
| DC | Exogenous H2S improved myocardial fibrosis in diabetic rats through suppressing ER stress by inhibiting the JAK/STAT pathway, which needs further confirmation with the inhibitors. | STZ-induced diabetic rats | [96] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Liu, H.; Yang, Y.; Lan, T.; Wang, H.; Wu, D. Hydrogen Sulfide Plays an Important Role by Regulating Endoplasmic Reticulum Stress in Diabetes-Related Diseases. Int. J. Mol. Sci. 2022, 23, 7170. https://doi.org/10.3390/ijms23137170
Zhao H, Liu H, Yang Y, Lan T, Wang H, Wu D. Hydrogen Sulfide Plays an Important Role by Regulating Endoplasmic Reticulum Stress in Diabetes-Related Diseases. International Journal of Molecular Sciences. 2022; 23(13):7170. https://doi.org/10.3390/ijms23137170
Chicago/Turabian StyleZhao, Huijie, Huiyang Liu, Yihan Yang, Tianyue Lan, Honggang Wang, and Dongdong Wu. 2022. "Hydrogen Sulfide Plays an Important Role by Regulating Endoplasmic Reticulum Stress in Diabetes-Related Diseases" International Journal of Molecular Sciences 23, no. 13: 7170. https://doi.org/10.3390/ijms23137170
APA StyleZhao, H., Liu, H., Yang, Y., Lan, T., Wang, H., & Wu, D. (2022). Hydrogen Sulfide Plays an Important Role by Regulating Endoplasmic Reticulum Stress in Diabetes-Related Diseases. International Journal of Molecular Sciences, 23(13), 7170. https://doi.org/10.3390/ijms23137170






