Therapeutic Targeting of Ovarian Cancer Stem Cells Using Estrogen Receptor Beta Agonist
Abstract
1. Introduction
2. Results
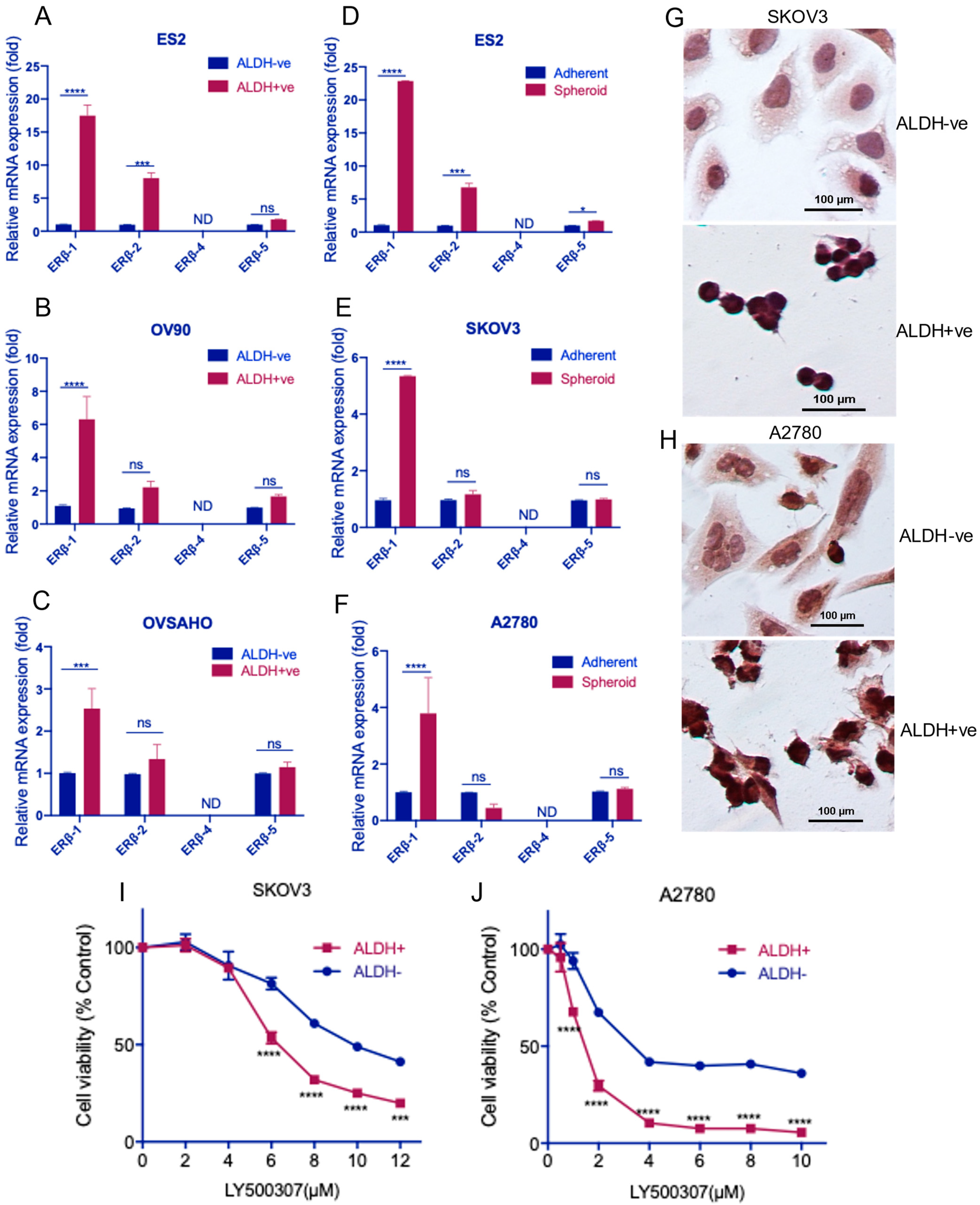
2.1. Estrogen Receptor β Agonist Reduces the Viability of OCSCs
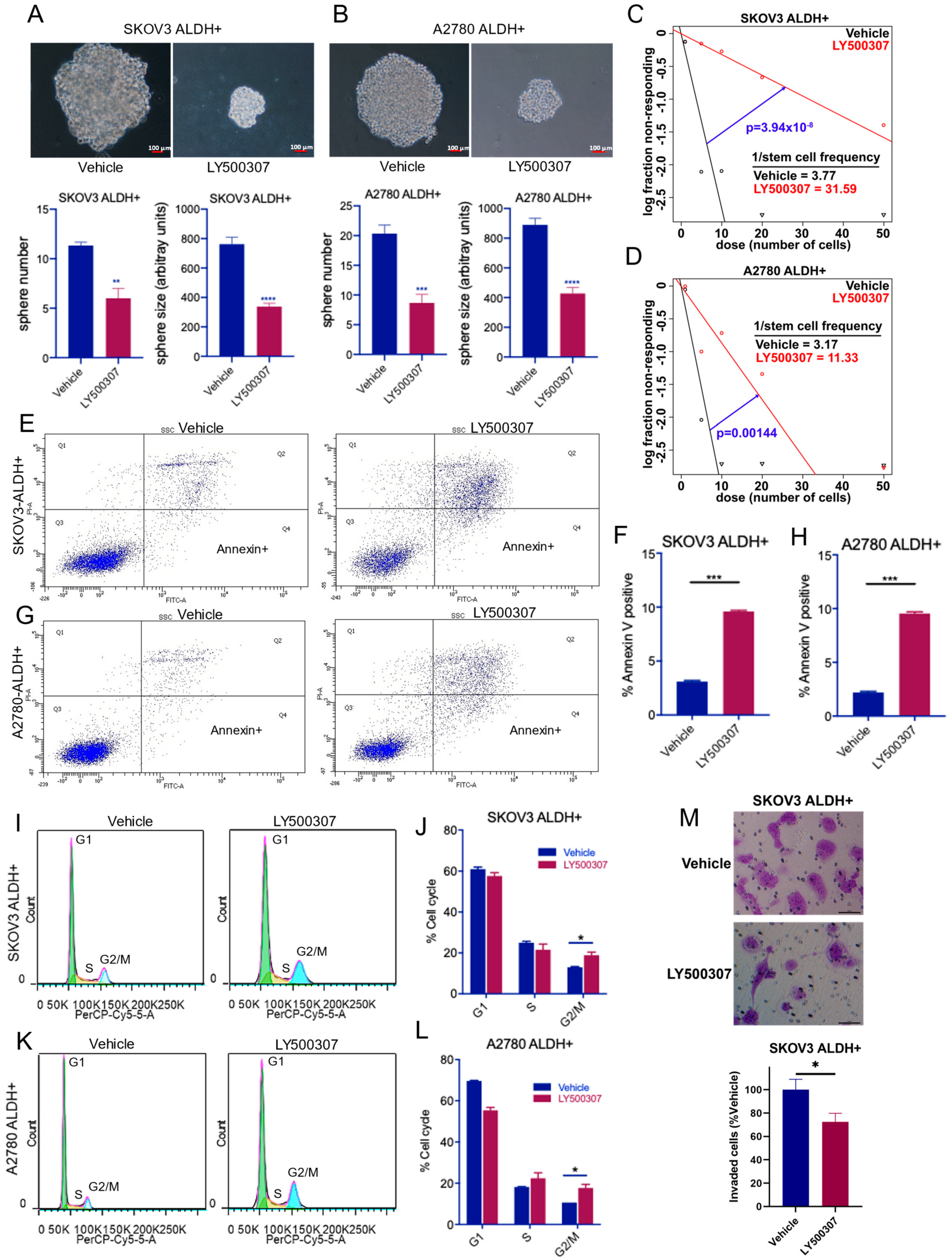
2.2. ERβ Agonist LY500307 Reduces Sphere Formation, Self-Renewal, and Invasion and Induces Apoptosis and G2/M Cell Cycle Arrest in OCSCs
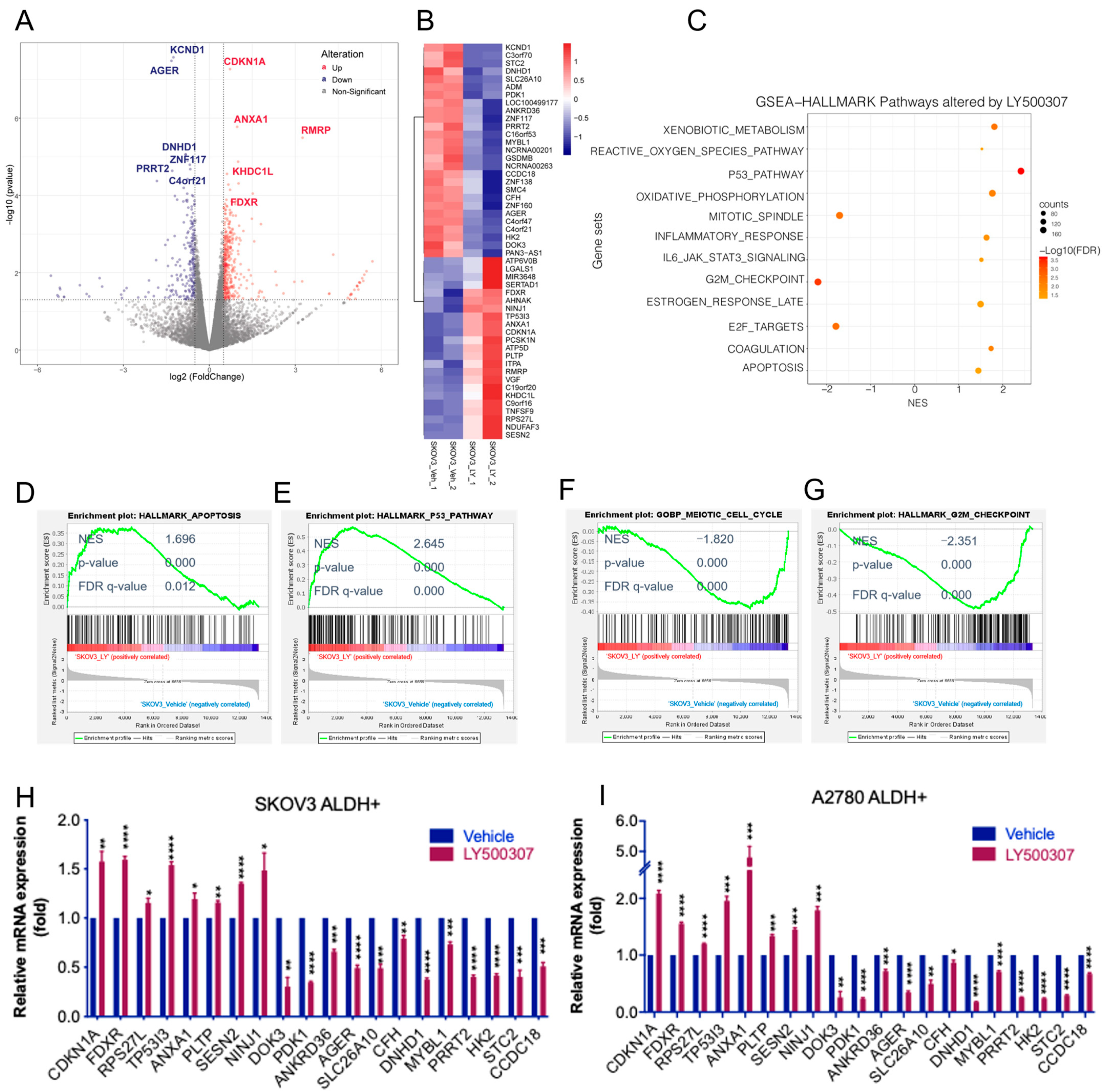
2.3. Analysis of Transcriptional Changes Altered by LY500307 Treatment in OCSCs
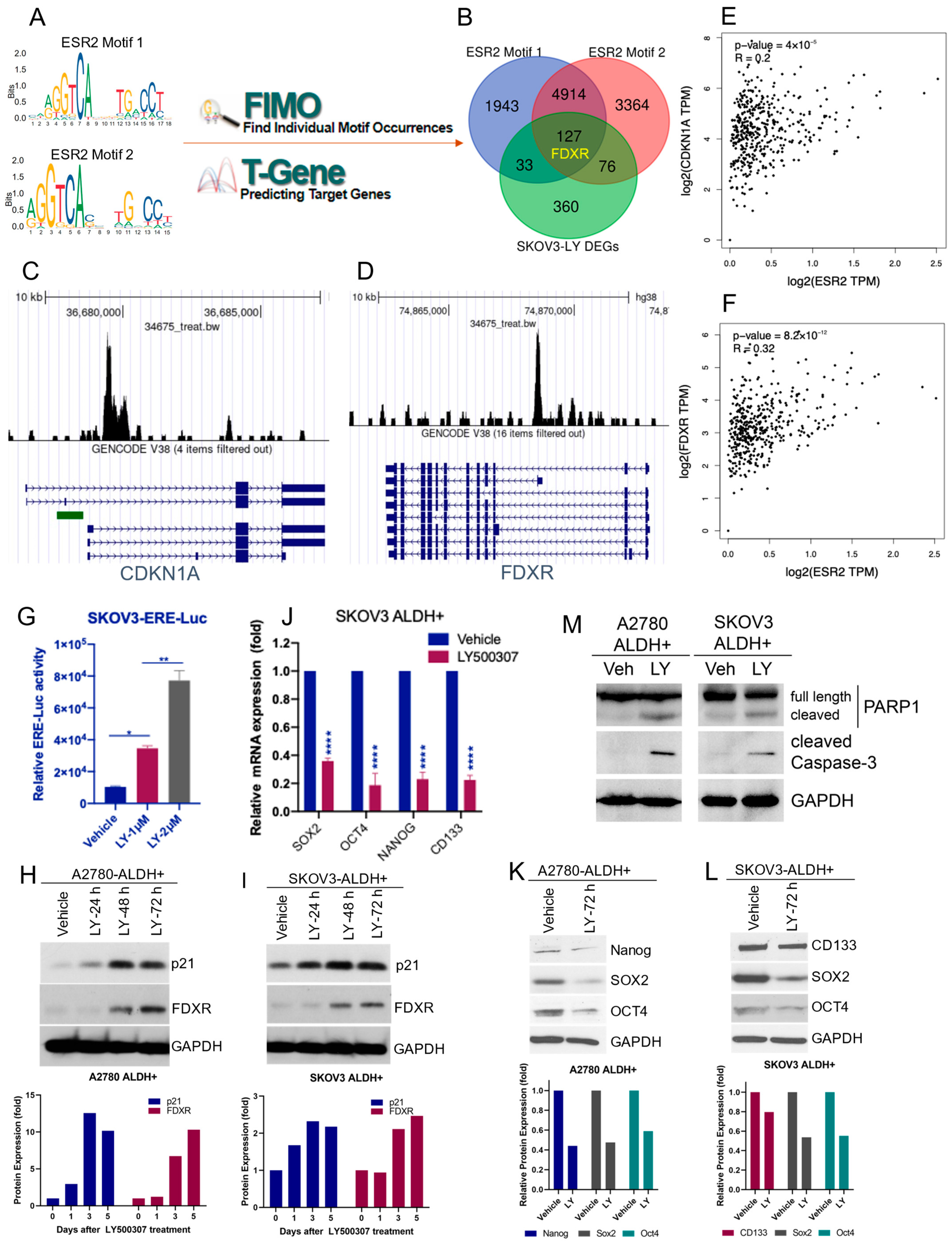
2.4. ERβ Agonist Induces the Expression of FDXR and CDKN1A in OCSCs
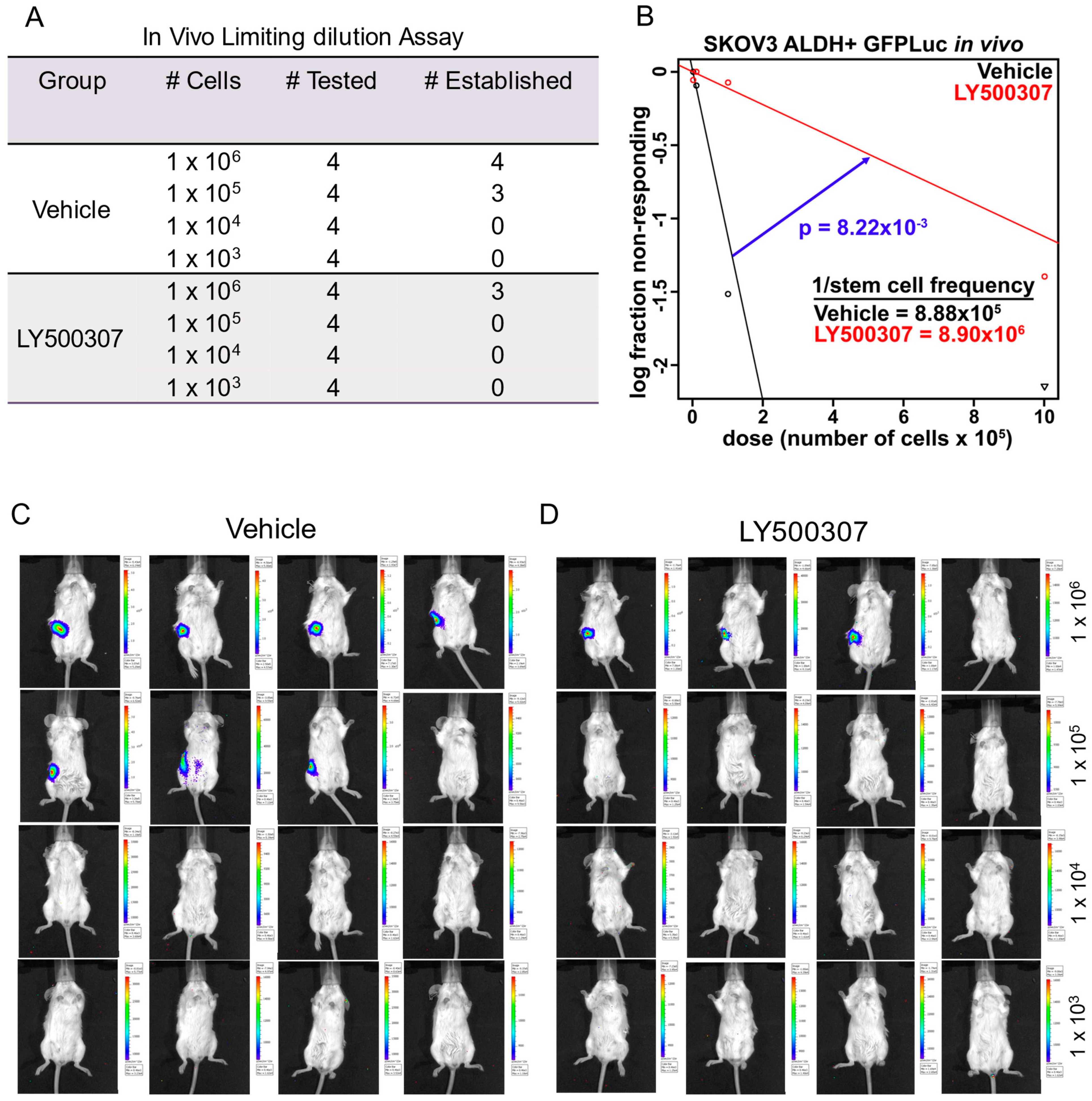
2.5. ERβ Agonist Treatment Suppresses Tumor Initiation Capacity of OCSCs in Orthotopic Models
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Reagents
4.2. Isolation of OCSCs and Cell Viability Assays
4.3. Spheroid Formation and Extreme Limiting Dilution Assays
4.4. Annexin V, Cell Cycle, and Cell Invasion Assays
4.5. Cell Lysis and Western Blotting
4.6. RNA-Sequencing
4.7. RT-qPCR
4.8. Reporter Gene Assays
4.9. Immunocytochemistry (ICC)
4.10. In Silico Exploration of ESR2 Target Genes
4.11. Mice Studies
4.12. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petrillo, M.; Nero, C.; Amadio, G.; Gallo, D.; Fagotti, A.; Scambia, G. Targeting the hallmarks of ovarian cancer: The big picture. Gynecol. Oncol. 2016, 142, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA A Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Giusti, I.; Bianchi, S.; Nottola, S.A.; Macchiarelli, G.; Dolo, V. Clinical electron microscopy in the study of human ovarian tissues. EuroMediterranean Biomed. J. 2019, 14, 145–151. [Google Scholar]
- Matulonis, U.A.; Sood, A.K.; Fallowfield, L.; Howitt, B.E.; Sehouli, J.; Karlan, B.Y. Ovarian cancer. Nat. Rev. Dis. Primers 2016, 2, 16061. [Google Scholar] [CrossRef]
- Marchetti, C.; Ledermann, J.A.; Benedetti Panici, P. An overview of early investigational therapies for chemoresistant ovarian cancer. Expert Opin. Investig. Drugs 2015, 24, 1163–1183. [Google Scholar] [CrossRef]
- Hu, L.; McArthur, C.; Jaffe, R.B. Ovarian cancer stem-like side-population cells are tumourigenic and chemoresistant. Br. J. Cancer 2010, 102, 1276–1283. [Google Scholar] [CrossRef]
- Zong, X.; Nephew, K.P. Ovarian Cancer Stem Cells: Role in Metastasis and Opportunity for Therapeutic Targeting. Cancers 2019, 11, 934. [Google Scholar] [CrossRef]
- Zhang, S.; Balch, C.; Chan, M.W.; Lai, H.C.; Matei, D.; Schilder, J.M.; Yan, P.S.; Huang, T.H.; Nephew, K.P. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008, 68, 4311–4320. [Google Scholar] [CrossRef]
- Keyvani, V.; Farshchian, M.; Esmaeili, S.-A.; Yari, H.; Moghbeli, M.; Nezhad, S.-R.K.; Abbaszadegan, M.R. Ovarian cancer stem cells and targeted therapy. J. Ovarian Res. 2019, 12, 120. [Google Scholar] [CrossRef]
- Brown, J.R.; Chan, D.K.; Shank, J.J.; Griffith, K.A.; Fan, H.; Szulawski, R.; Yang, K.; Reynolds, R.K.; Johnston, C.; McLean, K.; et al. Phase II clinical trial of metformin as a cancer stem cell-targeting agent in ovarian cancer. JCI Insight 2020, 5, e133247. [Google Scholar] [CrossRef]
- Nacarelli, T.; Fukumoto, T.; Zundell, J.A.; Fatkhutdinov, N.; Jean, S.; Cadungog, M.G.; Borowsky, M.E.; Zhang, R. NAMPT Inhibition Suppresses Cancer Stem-like Cells Associated with Therapy-Induced Senescence in Ovarian Cancer. Cancer Res. 2020, 80, 890–900. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Gallardo, C.; Rutledge, E.C.; Martínez-Arroyo, A.M.; Hidalgo, J.J.; Domingo, S.; Simón, C. Overcoming challenges of ovarian cancer stem cells: Novel therapeutic approaches. Stem Cell Rev. Rep. 2012, 8, 994–1010. [Google Scholar] [CrossRef] [PubMed]
- Saygin, C.; Matei, D.; Majeti, R.; Reizes, O.; Lathia, J.D. Targeting Cancer Stemness in the Clinic: From Hype to Hope. Cell Stem Cell 2019, 24, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Cunat, S.; Hoffmann, P.; Pujol, P. Estrogens and epithelial ovarian cancer. Gynecol. Oncol. 2004, 94, 25–32. [Google Scholar] [CrossRef]
- Paruthiyil, S.; Cvoro, A.; Zhao, X.; Wu, Z.; Sui, Y.; Staub, R.E.; Baggett, S.; Herber, C.B.; Griffin, C.; Tagliaferri, M.; et al. Drug and cell type-specific regulation of genes with different classes of estrogen receptor beta-selective agonists. PLoS ONE 2009, 4, e6271. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, S.; Gustafsson, J. Estrogen receptors: Therapies targeted to receptor subtypes. Clin. Pharmacol. Ther. 2011, 89, 44–55. [Google Scholar] [CrossRef]
- Chakraborty, S.; Willett, H.; Biswas, P.K. Insight into estrogen receptor beta-beta and alpha-beta homo- and heterodimerization: A combined molecular dynamics and sequence analysis study. Biophys. Chem. 2012, 170, 42–50. [Google Scholar] [CrossRef]
- Mal, R.; Magner, A.; David, J.; Datta, J.; Vallabhaneni, M.; Kassem, M.; Manouchehri, J.; Willingham, N.; Stover, D.; Vandeusen, J.; et al. Estrogen Receptor Beta (ERβ): A Ligand Activated Tumor Suppressor. Front. Oncol. 2020, 10, 587386. [Google Scholar] [CrossRef]
- Lindberg, M.K.; Movérare, S.; Skrtic, S.; Gao, H.; Dahlman-Wright, K.; Gustafsson, J.A.; Ohlsson, C. Estrogen receptor (ER)-beta reduces ERalpha-regulated gene transcription, supporting a “ying yang” relationship between ERalpha and ERbeta in mice. Mol. Endocrinol. 2003, 17, 203–208. [Google Scholar] [CrossRef]
- Hamilton, K.J.; Hewitt, S.C.; Arao, Y.; Korach, K.S. Estrogen Hormone Biology. Curr. Top. Dev. Biol. 2017, 125, 109–146. [Google Scholar] [CrossRef]
- Sareddy, G.R.; Vadlamudi, R.K. Cancer therapy using natural ligands that target estrogen receptor beta. Chin. J. Nat. Med. 2015, 13, 801–807. [Google Scholar] [CrossRef]
- Palmieri, C.; Cheng, G.J.; Saji, S.; Zelada-Hedman, M.; Wärri, A.; Weihua, Z.; Van Noorden, S.; Wahlstrom, T.; Coombes, R.C.; Warner, M.; et al. Estrogen receptor beta in breast cancer. Endocr. Relat. Cancer 2002, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mak, P.; Leav, I.; Pursell, B.; Bae, D.; Yang, X.; Taglienti, C.A.; Gouvin, L.M.; Sharma, V.M.; Mercurio, A.M. ERbeta impedes prostate cancer EMT by destabilizing HIF-1alpha and inhibiting VEGF-mediated snail nuclear localization: Implications for Gleason grading. Cancer Cell 2010, 17, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Konstantinopoulos, P.A.; Kominea, A.; Vandoros, G.; Sykiotis, G.P.; Andricopoulos, P.; Varakis, I.; Sotiropoulou-Bonikou, G.; Papavassiliou, A.G. Oestrogen receptor beta (ERbeta) is abundantly expressed in normal colonic mucosa, but declines in colon adenocarcinoma paralleling the tumour’s dedifferentiation. Eur. J. Cancer 2003, 39, 1251–1258. [Google Scholar] [CrossRef]
- Sareddy, G.R.; Nair, B.C.; Gonugunta, V.K.; Zhang, Q.G.; Brenner, A.; Brann, D.W.; Tekmal, R.R.; Vadlamudi, R.K. Therapeutic significance of estrogen receptor β agonists in gliomas. Mol. Cancer Ther. 2012, 11, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Lazennec, G. Estrogen receptor beta, a possible tumor suppressor involved in ovarian carcinogenesis. Cancer Lett. 2006, 231, 151–157. [Google Scholar] [CrossRef]
- Rutherford, T.; Brown, W.D.; Sapi, E.; Aschkenazi, S.; Muñoz, A.; Mor, G. Absence of estrogen receptor-beta expression in metastatic ovarian cancer. Obstet. Gynecol. 2000, 96, 417–421. [Google Scholar] [CrossRef]
- Halon, A.; Nowak-Markwitz, E.; Maciejczyk, A.; Pudelko, M.; Gansukh, T.; Györffy, B.; Donizy, P.; Murawa, D.; Matkowski, R.; Spaczynski, M.; et al. Loss of estrogen receptor beta expression correlates with shorter overall survival and lack of clinical response to chemotherapy in ovarian cancer patients. Anticancer. Res. 2011, 31, 711–718. [Google Scholar]
- Lo, R.; Matthews, J. A new class of estrogen receptor beta-selective activators. Mol. Interv. 2010, 10, 133–136. [Google Scholar] [CrossRef]
- Norman, B.H.; Dodge, J.A.; Richardson, T.I.; Borromeo, P.S.; Lugar, C.W.; Jones, S.A.; Chen, K.; Wang, Y.; Durst, G.L.; Barr, R.J.; et al. Benzopyrans are selective estrogen receptor beta agonists with novel activity in models of benign prostatic hyperplasia. J. Med. Chem. 2006, 49, 6155–6157. [Google Scholar] [CrossRef]
- Roehrborn, C.G.; Spann, M.E.; Myers, S.L.; Serviss, C.R.; Hu, L.; Jin, Y. Estrogen receptor beta agonist LY500307 fails to improve symptoms in men with enlarged prostate secondary to benign prostatic hypertrophy. Prostate Cancer Prostatic Dis. 2015, 18, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Sareddy, G.R.; Li, X.; Liu, J.; Viswanadhapalli, S.; Garcia, L.; Gruslova, A.; Cavazos, D.; Garcia, M.; Strom, A.M.; Gustafsson, J.A.; et al. Selective Estrogen Receptor β Agonist LY500307 as a Novel Therapeutic Agent for Glioblastoma. Sci. Rep. 2016, 6, 24185. [Google Scholar] [CrossRef]
- Sareddy, G.R.; Pratap, U.P.; Venkata, P.P.; Zhou, M.; Alejo, S.; Viswanadhapalli, S.; Tekmal, R.R.; Brenner, A.J.; Vadlamudi, R.K. Activation of estrogen receptor beta signaling reduces stemness of glioma stem cells. Stem Cells 2021, 39, 536–550. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Huang, S.; Mei, S.; Yang, Z.; Xu, L.; Zhou, N.; Yang, Q.; Shen, Q.; Wang, W.; Le, X.; et al. Pharmacological activation of estrogen receptor beta augments innate immunity to suppress cancer metastasis. Proc. Natl. Acad. Sci USA 2018, 115, E3673–E3681. [Google Scholar] [CrossRef]
- Chan, K.K.; Wei, N.; Liu, S.S.; Xiao-Yun, L.; Cheung, A.N.; Ngan, H.Y. Estrogen receptor subtypes in ovarian cancer: A clinical correlation. Obstet. Gynecol. 2008, 111, 144–151. [Google Scholar] [CrossRef]
- Pujol, P.; Rey, J.M.; Nirde, P.; Roger, P.; Gastaldi, M.; Laffargue, F.; Rochefort, H.; Maudelonde, T. Differential expression of estrogen receptor-alpha and -beta messenger RNAs as a potential marker of ovarian carcinogenesis. Cancer Res. 1998, 58, 5367–5373. [Google Scholar] [PubMed]
- Bossard, C.; Busson, M.; Vindrieux, D.; Gaudin, F.; Machelon, V.; Brigitte, M.; Jacquard, C.; Pillon, A.; Balaguer, P.; Balabanian, K.; et al. Potential role of estrogen receptor beta as a tumor suppressor of epithelial ovarian cancer. PLoS ONE 2012, 7, e44787. [Google Scholar] [CrossRef] [PubMed]
- Meng, E.; Mitra, A.; Tripathi, K.; Finan, M.A.; Scalici, J.; McClellan, S.; Madeira da Silva, L.; Reed, E.; Shevde, L.A.; Palle, K.; et al. ALDH1A1 maintains ovarian cancer stem cell-like properties by altered regulation of cell cycle checkpoint and DNA repair network signaling. PLoS ONE 2014, 9, e107142. [Google Scholar] [CrossRef]
- Wang, Y.C.; Yo, Y.T.; Lee, H.Y.; Liao, Y.P.; Chao, T.K.; Su, P.H.; Lai, H.C. ALDH1-bright epithelial ovarian cancer cells are associated with CD44 expression, drug resistance, and poor clinical outcome. Am. J. Pathol. 2012, 180, 1159–1169. [Google Scholar] [CrossRef]
- Li, Y.; Chen, T.; Zhu, J.; Zhang, H.; Jiang, H.; Sun, H. High ALDH activity defines ovarian cancer stem-like cells with enhanced invasiveness and EMT progress which are responsible for tumor invasion. Biochem. Biophys. Res. Commun. 2018, 495, 1081–1088. [Google Scholar] [CrossRef]
- Landen, C.N., Jr.; Goodman, B.; Katre, A.A.; Steg, A.D.; Nick, A.M.; Stone, R.L.; Miller, L.D.; Mejia, P.V.; Jennings, N.B.; Gershenson, D.M.; et al. Targeting aldehyde dehydrogenase cancer stem cells in ovarian cancer. Mol. Cancer Ther. 2010, 9, 3186–3199. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, S.; Rinaldi, J.C.; Malhotra, N.R.; Xie, L.; Hu, D.P.; Gauntner, T.D.; Grewal, H.S.; Hu, W.Y.; Kim, S.H.; Katzenellenbogen, J.A.; et al. Differential Actions of Estrogen Receptor α and β via Nongenomic Signaling in Human Prostate Stem and Progenitor Cells. Endocrinology 2019, 160, 2692–2708. [Google Scholar] [CrossRef]
- Hussain, S.; Lawrence, M.G.; Taylor, R.A.; Lo, C.Y.; Frydenberg, M.; Ellem, S.J.; Furic, L.; Risbridger, G.P. Estrogen receptor beta activation impairs prostatic regeneration by inducing apoptosis in murine and human stem/progenitor enriched cell populations. PLoS ONE 2012, 7, e40732. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Karthik, G.-M.; Lövrot, J.; Haglund, F.; Rosin, G.; Katchy, A.; Zhang, X.; Viberg, L.; Frisell, J.; Williams, C.; et al. Estrogen Receptor β as a Therapeutic Target in Breast Cancer Stem Cells. J. Natl. Cancer Inst. 2017, 109, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rota, S.G.; Roma, A.; Dude, I.; Ma, C.; Stevens, R.; MacEachern, J.; Graczyk, J.; Espiritu, S.M.G.; Rao, P.N.; Minden, M.D.; et al. Estrogen Receptor β Is a Novel Target in Acute Myeloid Leukemia. Mol. Cancer Ther. 2017, 16, 2618–2626. [Google Scholar] [CrossRef]
- Leung, Y.-K.; Mak, P.; Hassan, S.; Ho, S.-M. Estrogen receptor (ER)-beta isoforms: A key to understanding ER-beta signaling. Proc. Natl. Acad. Sci. USA 2006, 103, 13162–13167. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-T.; Ouyang, B.; Ho, S.-M.; Leung, Y.-K. Differential expression of estrogen receptor beta isoforms in prostate cancer through interplay between transcriptional and translational regulation. Mol. Cell. Endocrinol. 2013, 376, 125–135. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thomas, C.; Gustafsson, J.-Å. The different roles of ER subtypes in cancer biology and therapy. Nat. Rev. Cancer 2011, 11, 597–608. [Google Scholar] [CrossRef]
- Leung, Y.-K.; Lam, H.-M.; Wu, S.; Song, D.; Levin, L.; Cheng, L.; Wu, C.-L.; Ho, S.-M. Estrogen receptor beta2 and beta5 are associated with poor prognosis in prostate cancer, and promote cancer cell migration and invasion. Endocr. Relat. Cancer 2010, 17, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Mandusic, V.; Dimitrijevic, B.; Nikolic-Vukosavljevic, D.; Neskovic-Konstantinovic, Z.; Kanjer, K.; Hamann, U. Different associations of estrogen receptor β isoforms, ERβ1 and ERβ2, expression levels with tumor size and survival in early- and late-onset breast cancer. Cancer Lett. 2012, 321, 73–79. [Google Scholar] [CrossRef]
- Buttarelli, M.; Mascilini, F.; Zannoni, G.F.; Ciucci, A.; Martinelli, E.; Filippetti, F.; Scambia, G.; Ferrandina, G.; Gallo, D. Hormone receptor expression profile of low-grade serous ovarian cancers. Gynecol. Oncol. 2017, 145, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Faria, M.; Shepherd, P.; Pan, Y.; Chatterjee, S.S.; Navone, N.; Gustafsson, J.; Strom, A. The estrogen receptor variants β2 and β5 induce stem cell characteristics and chemotherapy resistance in prostate cancer through activation of hypoxic signaling. Oncotarget 2018, 9, 36273–36288. [Google Scholar] [CrossRef] [PubMed]
- Bogush, T.A.; Basharina, A.A.; Bogush, E.A.; Scherbakov, A.M.; Davydov, M.M.; Kosorukov, V.S. The expression and clinical significance of ERβ/ERα in ovarian cancer: Can we predict the effectiveness of platinum plus taxane therapy? Ir. J. Med. Sci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Davie, J.R. Estrogen regulated expression of the p21 Waf1/Cip1 gene in estrogen receptor positive human breast cancer cells. J. Cell Physiol. 2010, 224, 28–32. [Google Scholar] [CrossRef]
- Dey, P.; Barros, R.P.; Warner, M.; Ström, A.; Gustafsson, J. Insight into the mechanisms of action of estrogen receptor β in the breast, prostate, colon, and CNS. J. Mol. Endocrinol. 2013, 51, T61–T74. [Google Scholar] [CrossRef]
- Matsumura, K.; Tanaka, T.; Kawashima, H.; Nakatani, T. Involvement of the estrogen receptor beta in genistein-induced expression of p21(waf1/cip1) in PC-3 prostate cancer cells. Anticancer Res. 2008, 28, 709–714. [Google Scholar]
- Rizza, P.; Barone, I.; Zito, D.; Giordano, F.; Lanzino, M.; De Amicis, F.; Mauro, L.; Sisci, D.; Catalano, S.; Wright, K.D.; et al. Estrogen receptor beta as a novel target of androgen receptor action in breast cancer cell lines. Breast Cancer Res. 2014, 16, R21. [Google Scholar] [CrossRef]
- Dey, P.; Jonsson, P.; Hartman, J.; Williams, C.; Ström, A.; Gustafsson, J.-Å. Estrogen Receptors β1 and β2 Have Opposing Roles in Regulating Proliferation and Bone Metastasis Genes in the Prostate Cancer Cell Line PC3. Mol. Endocrinol. 2012, 26, 1991–2003. [Google Scholar] [CrossRef]
- Pratap, U.P.; Sareddy, G.R.; Liu, Z.; Venkata, P.P.; Liu, J.; Tang, W.; Altwegg, K.A.; Ebrahimi, B.; Li, X.; Tekmal, R.R.; et al. Histone deacetylase inhibitors enhance estrogen receptor beta expression and augment agonist-mediated tumor suppression in glioblastoma. Neuro-Oncol. Adv. 2021, 3. [Google Scholar] [CrossRef]
- Marqués-Torrejón, M.Á.; Porlan, E.; Banito, A.; Gómez-Ibarlucea, E.; Lopez-Contreras, A.J.; Fernández-Capetillo, Ó.; Vidal, A.; Gil, J.; Torres, J.; Fariñas, I. Cyclin-Dependent Kinase Inhibitor p21 Controls Adult Neural Stem Cell Expansion by Regulating Sox2 Gene Expression. Cell Stem Cell 2013, 12, 88–100. [Google Scholar] [CrossRef]
- Cheng, T.; Rodrigues, N.; Shen, H.; Yang, Y.; Dombkowski, D.; Sykes, M.; Scadden, D.T. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science 2000, 287, 1804–1808. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Casimiro, M.C.; Wang, C.; Shirley, L.A.; Jiao, X.; Katiyar, S.; Ju, X.; Li, Z.; Yu, Z.; Zhou, J.; et al. p21CIP1 attenuates Ras- and c-Myc-dependent breast tumor epithelial mesenchymal transition and cancer stem cell-like gene expression in vivo. Proc. Natl. Acad. Sci. USA 2009, 106, 19035–19039. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ghosh, M.; Kovtunovych, G.; Crooks, D.R.; Rouault, T.A. Both human ferredoxins 1 and 2 and ferredoxin reductase are important for iron-sulfur cluster biogenesis. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2012, 1823, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Drecourt, A.; Petit, F.; Deguine, D.D.; Vasnier, C.; Oufadem, M.; Masson, C.; Bonnet, C.; Masmoudi, S.; Mosnier, I.; et al. FDXR Mutations Cause Sensorial Neuropathies and Expand the Spectrum of Mitochondrial Fe-S-Synthesis Diseases. Am. J. Hum. Genet. 2017, 101, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Slone, J.D.; Yang, L.; Peng, Y.; Queme, L.F.; Harris, B.; Rizzo, S.J.S.; Green, T.; Ryan, J.L.; Jankowski, M.P.; Reinholdt, L.G.; et al. Integrated analysis of the molecular pathogenesis of FDXR-associated disease. Cell Death Dis. 2020, 11, 423. [Google Scholar] [CrossRef]
- Slone, J.; Peng, Y.; Chamberlin, A.; Harris, B.; Kaylor, J.; McDonald, M.T.; Lemmon, M.; El-Dairi, M.A.; Tchapyjnikov, D.; Gonzalez-Krellwitz, L.A.; et al. Biallelic mutations in FDXR cause neurodegeneration associated with inflammation. J. Hum. Genet. 2018, 63, 1211–1222. [Google Scholar] [CrossRef]
- Hwang, P.M.; Bunz, F.; Yu, J.; Rago, C.; Chan, T.A.; Murphy, M.P.; Kelso, G.F.; Smith, R.A.J.; Kinzler, K.W.; Vogelstein, B. Ferredoxin reductase affects p53-dependent, 5-fluorouracil–induced apoptosis in colorectal cancer cells. Nat. Med. 2001, 7, 1111–1117. [Google Scholar] [CrossRef]
- Liu, G.; Chen, X. The ferredoxin reductase gene is regulated by the p53 family and sensitizes cells to oxidative stress-induced apoptosis. Oncogene 2002, 21, 7195–7204. [Google Scholar] [CrossRef]
- Sareddy, G.R.; Viswanadhapalli, S.; Surapaneni, P.; Suzuki, T.; Brenner, A.; Vadlamudi, R.K. Novel KDM1A inhibitors induce differentiation and apoptosis of glioma stem cells via unfolded protein response pathway. Oncogene 2017, 36, 2423–2434. [Google Scholar] [CrossRef]
- Venkata, P.P.; Chen, Y.; Alejo, S.; He, Y.; Palacios, B.E.; Loeffel, I.; Liu, J.; Pratap, U.P.; Gray, G.; Achuthan Pillai, S.M.; et al. KDM1A inhibition augments the efficacy of rapamycin for the treatment of endometrial cancer. Cancer Lett. 2022, 524, 219–231. [Google Scholar] [CrossRef]
- Castro-Mondragon, J.A.; Riudavets-Puig, R.; Rauluseviciute, I.; Berhanu Lemma, R.; Turchi, L.; Blanc-Mathieu, R.; Lucas, J.; Boddie, P.; Khan, A.; Manosalva Pérez, N.; et al. JASPAR 2022: The 9th release of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 2021, 50, D165–D173. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Alejo, S.; Venkata, P.P.; Johnson, J.D.; Loeffel, I.; Pratap, U.P.; Zou, Y.; Lai, Z.; Tekmal, R.R.; Kost, E.R.; et al. Therapeutic Targeting of Ovarian Cancer Stem Cells Using Estrogen Receptor Beta Agonist. Int. J. Mol. Sci. 2022, 23, 7159. https://doi.org/10.3390/ijms23137159
He Y, Alejo S, Venkata PP, Johnson JD, Loeffel I, Pratap UP, Zou Y, Lai Z, Tekmal RR, Kost ER, et al. Therapeutic Targeting of Ovarian Cancer Stem Cells Using Estrogen Receptor Beta Agonist. International Journal of Molecular Sciences. 2022; 23(13):7159. https://doi.org/10.3390/ijms23137159
Chicago/Turabian StyleHe, Yi, Salvador Alejo, Prabhakar Pitta Venkata, Jessica D. Johnson, Ilanna Loeffel, Uday P. Pratap, Yi Zou, Zhao Lai, Rajeshwar R. Tekmal, Edward R. Kost, and et al. 2022. "Therapeutic Targeting of Ovarian Cancer Stem Cells Using Estrogen Receptor Beta Agonist" International Journal of Molecular Sciences 23, no. 13: 7159. https://doi.org/10.3390/ijms23137159
APA StyleHe, Y., Alejo, S., Venkata, P. P., Johnson, J. D., Loeffel, I., Pratap, U. P., Zou, Y., Lai, Z., Tekmal, R. R., Kost, E. R., & Sareddy, G. R. (2022). Therapeutic Targeting of Ovarian Cancer Stem Cells Using Estrogen Receptor Beta Agonist. International Journal of Molecular Sciences, 23(13), 7159. https://doi.org/10.3390/ijms23137159






