An Altered Sphingolipid Profile as a Risk Factor for Progressive Neurodegeneration in Long-Chain 3-Hydroxyacyl-CoA Deficiency (LCHADD)
Abstract
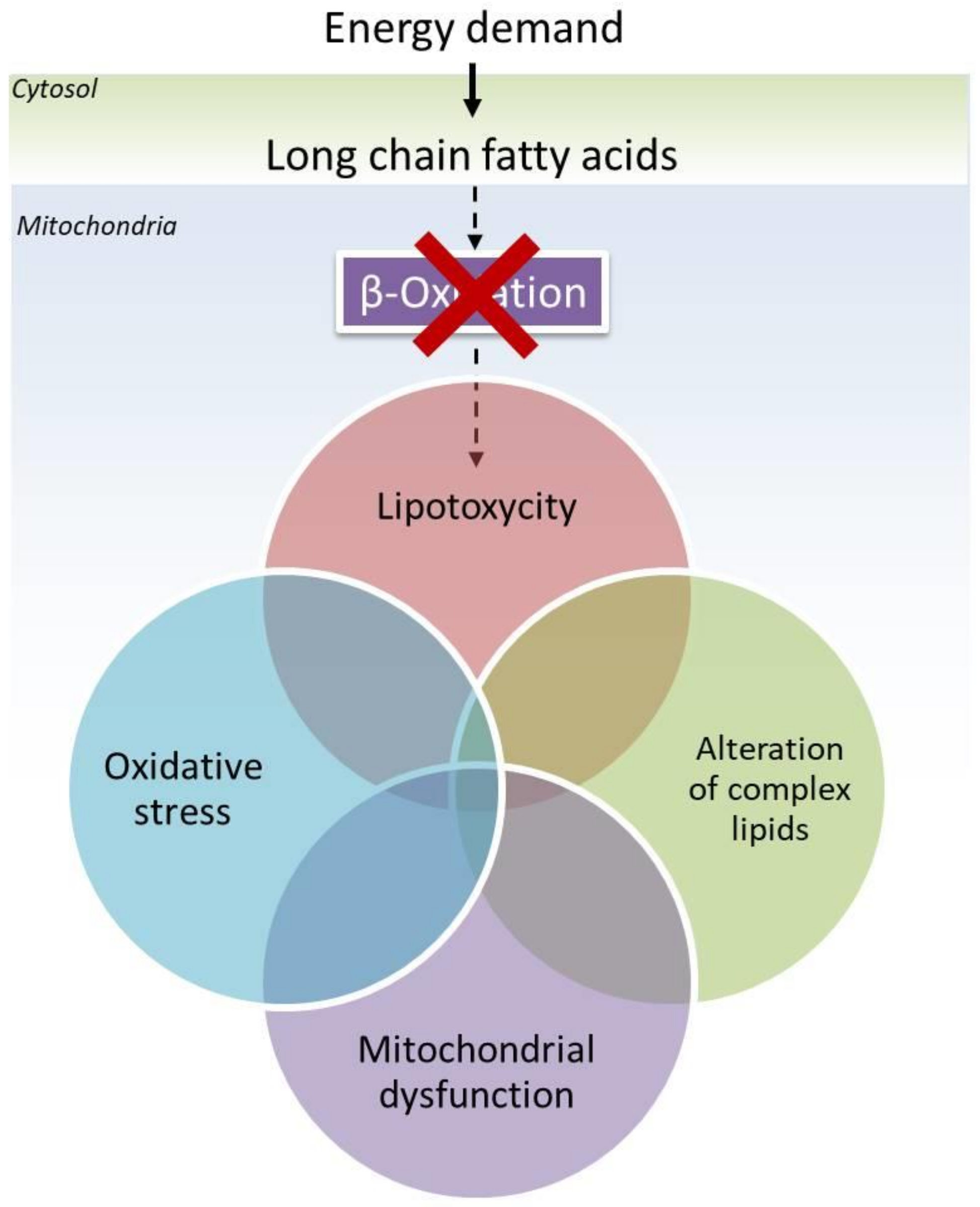
1. Introduction
2. Mitochondrial Trifunctional Protein (MTP) and Cardiolipins
3. Sphingomyelins and Ceramides: New Co-Player for Neurodegeneration in LCHADD?
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, K.; Li, N.; Wang, X.; Dai, J.; Liu, P.; Wang, C.; Chen, X.W.; Gao, N.; Xiao, J. Cryo-EM structure of human mitochondrial trifunctional protein. Proc. Natl. Acad. Sci. USA 2018, 115, 7039–7044. [Google Scholar] [CrossRef]
- Xia, C.; Fu, Z.; Battaile, K.P.; Kim, J.P. Crystal structure of human mitochondrial trifunctional protein, a fatty acid beta-oxidation metabolon. Proc. Natl. Acad. Sci. USA 2019, 116, 6069–6074. [Google Scholar] [CrossRef]
- Merritt, J.L., 2nd; Norris, M.; Kanungo, S. Fatty acid oxidation disorders. Ann. Transl. Med. 2018, 6, 473. [Google Scholar] [CrossRef]
- Arnold, G.L.; Van Hove, J.; Freedenberg, D.; Strauss, A.; Longo, N.; Burton, B.; Garganta, C.; Ficicioglu, C.; Cederbaum, S.; Harding, C.; et al. A Delphi clinical practice protocol for the management of very long chain acyl-CoA dehydrogenase deficiency. Mol. Genet. Metab. 2009, 96, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Spiekerkoetter, U.; Lindner, M.; Santer, R.; Grotzke, M.; Baumgartner, M.R.; Boehles, H.; Das, A.; Haase, C.; Hennermann, J.B.; Karall, D.; et al. Treatment recommendations in long-chain fatty acid oxidation defects: Consensus from a workshop. J. Inherit. Metab. Dis. 2009, 32, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Zand, D.; Doan, J.; Yi, S.; Wang, J.; Ma, L.; Akinshola, E.; Chakder, S.; Meyer, J.; Pacanowski, M.; Johnson, L.L.; et al. Regulatory news: Dojolvi (triheptanoin) as a source of calories and fatty acids in long-chain fatty acid oxidation disorders: FDA approval summary. J. Inherit. Metab. Dis. 2021, 44, 515–517. [Google Scholar] [CrossRef] [PubMed]
- Alatibi, K.I.; Hagenbuchner, J.; Wehbe, Z.; Karall, D.; Ausserlechner, M.J.; Vockley, J.; Spiekerkoetter, U.; Grunert, S.C.; Tucci, S. Different Lipid Signature in Fibroblasts of Long-Chain Fatty Acid Oxidation Disorders. Cells 2021, 10, 1239. [Google Scholar] [CrossRef]
- Alatibi, K.I.; Tholen, S.; Wehbe, Z.; Hagenbuchner, J.; Karall, D.; Ausserlechner, M.J.; Schilling, O.; Grunert, S.C.; Vockley, J.; Tucci, S. Lipidomic and Proteomic Alterations Induced by Even and Odd Medium-Chain Fatty Acids on Fibroblasts of Long-Chain Fatty Acid Oxidation Disorders. Int. J. Mol. Sci. 2021, 22, 10556. [Google Scholar] [CrossRef]
- Boese, E.A.; Jain, N.; Jia, Y.; Schlechter, C.L.; Harding, C.O.; Gao, S.S.; Patel, R.C.; Huang, D.; Weleber, R.G.; Gillingham, M.B.; et al. Characterization of Chorioretinopathy Associated with Mitochondrial Trifunctional Protein Disorders: Long-Term Follow-up of 21 Cases. Ophthalmology 2016, 123, 2183–2195. [Google Scholar] [CrossRef]
- Ibdah, J.A.; Bennett, M.J.; Rinaldo, P.; Zhao, Y.; Gibson, B.; Sims, H.F.; Strauss, A.W. A fetal fatty-acid oxidation disorder as a cause of liver disease in pregnant women. N. Engl. J. Med. 1999, 340, 1723–1731. [Google Scholar] [CrossRef]
- Liu, J.; Ghaziani, T.T.; Wolf, J.L. Acute Fatty Liver Disease of Pregnancy: Updates in Pathogenesis, Diagnosis, and Management. Am. J. Gastroenterol. 2017, 112, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Tyni, T.; Johnson, M.; Eaton, S.; Pourfarzam, M.; Andrews, R.; Turnbull, D.M. Mitochondrial fatty acid beta-oxidation in the retinal pigment epithelium. Pediatr. Res. 2002, 52, 595–600. [Google Scholar] [CrossRef]
- Boutron, A.; Acquaviva, C.; Vianey-Saban, C.; de Lonlay, P.; de Baulny, H.O.; Guffon, N.; Dobbelaere, D.; Feillet, F.; Labarthe, F.; Lamireau, D.; et al. Comprehensive cDNA study and quantitative analysis of mutant HADHA and HADHB transcripts in a French cohort of 52 patients with mitochondrial trifunctional protein deficiency. Mol. Genet. Metab. 2011, 103, 341–348. [Google Scholar] [CrossRef]
- Scheuerman, O.; Wanders, R.J.; Waterham, H.R.; Dubnov-Raz, G.; Garty, B.Z. Mitochondrial trifunctional protein deficiency with recurrent rhabdomyolysis. Pediatr. Neurol. 2009, 40, 465–467. [Google Scholar] [CrossRef] [PubMed]
- Spiekerkoetter, U.; Sun, B.; Khuchua, Z.; Bennett, M.J.; Strauss, A.W. Molecular and phenotypic heterogeneity in mitochondrial trifunctional protein deficiency due to beta-subunit mutations. Hum. Mutat. 2003, 21, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Ushikubo, S.; Aoyama, T.; Kamijo, T.; Wanders, R.J.; Rinaldo, P.; Vockley, J.; Hashimoto, T. Molecular characterization of mitochondrial trifunctional protein deficiency: Formation of the enzyme complex is important for stabilization of both alpha- and beta-subunits. Am. J. Hum. Genet. 1996, 58, 979–988. [Google Scholar]
- Taylor, W.A.; Mejia, E.M.; Mitchell, R.W.; Choy, P.C.; Sparagna, G.C.; Hatch, G.M. Human trifunctional protein alpha links cardiolipin remodeling to beta-oxidation. PLoS ONE 2012, 7, e48628. [Google Scholar] [CrossRef] [PubMed]
- Ban, T.; Ishihara, T.; Kohno, H.; Saita, S.; Ichimura, A.; Maenaka, K.; Oka, T.; Mihara, K.; Ishihara, N. Molecular basis of selective mitochondrial fusion by heterotypic action between OPA1 and cardiolipin. Nat. Cell Biol. 2017, 19, 856–863. [Google Scholar] [CrossRef]
- Kameoka, S.; Adachi, Y.; Okamoto, K.; Iijima, M.; Sesaki, H. Phosphatidic Acid and Cardiolipin Coordinate Mitochondrial Dynamics. Trends Cell Biol. 2018, 28, 67–76. [Google Scholar] [CrossRef]
- Musatov, A.; Sedlak, E. Role of cardiolipin in stability of integral membrane proteins. Biochimie 2017, 142, 102–111. [Google Scholar] [CrossRef]
- Jheng, H.F.; Tsai, P.J.; Guo, S.M.; Kuo, L.H.; Chang, C.S.; Su, I.J.; Chang, C.R.; Tsai, Y.S. Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Mol. Cell Biol. 2012, 32, 309–319. [Google Scholar] [CrossRef]
- Alessenko, A.V.; Albi, E. Exploring Sphingolipid Implications in Neurodegeneration. Front. Neurol. 2020, 11, 437. [Google Scholar] [CrossRef] [PubMed]
- Orvisky, E.; Park, J.K.; LaMarca, M.E.; Ginns, E.I.; Martin, B.M.; Tayebi, N.; Sidransky, E. Glucosylsphingosine accumulation in tissues from patients with Gaucher disease: Correlation with phenotype and genotype. Mol. Genet. Metab. 2002, 76, 262–270. [Google Scholar] [CrossRef]
- Ehlert, K.; Frosch, M.; Fehse, N.; Zander, A.; Roth, J.; Vormoor, J. Farber disease: Clinical presentation, pathogenesis and a new approach to treatment. Pediatr. Rheumatol. Online J. 2007, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Sugita, M.; Dulaney, J.T.; Moser, H.W. Ceramidase deficiency in Farber’s disease (lipogranulomatosis). Science 1972, 178, 1100–1102. [Google Scholar] [CrossRef] [PubMed]
- Trompetero, A.; Gordillo, A.; Del Pilar, M.C.; Cristina, V.M.; Bustos Cruz, R.H. Alzheimer’s Disease and Parkinson’s Disease: A Review of Current Treatment Adopting a Nanotechnology Approach. Curr. Pharm. Des. 2018, 24, 22–45. [Google Scholar] [CrossRef]
- Olsen, A.S.B.; Faergeman, N.J. Sphingolipids: Membrane microdomains in brain development, function and neurological diseases. Open Biol. 2017, 7, 170069. [Google Scholar] [CrossRef]
- Bouscary, A.; Quessada, C.; Rene, F.; Spedding, M.; Turner, B.J.; Henriques, A.; Ngo, S.T.; Loeffler, J.P. Sphingolipids metabolism alteration in the central nervous system: Amyotrophic lateral sclerosis (ALS) and other neurodegenerative diseases. Semin. Cell Dev. Biol. 2020, 112, 82–91. [Google Scholar] [CrossRef]
- Bandet, C.L.; Tan-Chen, S.; Bourron, O.; Le Stunff, H.; Hajduch, E. Sphingolipid Metabolism: New Insight into Ceramide-Induced Lipotoxicity in Muscle Cells. Int. J. Mol. Sci. 2019, 20, 479. [Google Scholar] [CrossRef]
- Castro, B.M.; Prieto, M.; Silva, L.C. Ceramide: A simple sphingolipid with unique biophysical properties. Prog. Lipid Res. 2014, 54, 53–67. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef]
- Moskot, M.; Bochenska, K.; Jakobkiewicz-Banecka, J.; Banecki, B.; Gabig-Ciminska, M. Abnormal Sphingolipid World in Inflammation Specific for Lysosomal Storage Diseases and Skin Disorders. Int. J. Mol. Sci. 2018, 19, 247. [Google Scholar] [CrossRef] [PubMed]
- Hussain, G.; Wang, J.; Rasul, A.; Anwar, H.; Imran, A.; Qasim, M.; Zafar, S.; Kamran, S.K.S.; Razzaq, A.; Aziz, N.; et al. Role of cholesterol and sphingolipids in brain development and neurological diseases. Lipids Health Dis. 2019, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- McCoin, C.S.; Piccolo, B.D.; Knotts, T.A.; Matern, D.; Vockley, J.; Gillingham, M.B.; Adams, S.H. Unique plasma metabolomic signatures of individuals with inherited disorders of long-chain fatty acid oxidation. J. Inherit. Metab. Dis. 2016, 39, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Koczwara, J.B.; Gallelli, C.A.; Vergara, D.; Di Bonaventura, M.V.; Gaetani, S.; Giudetti, A.M. Fats for thoughts: An update on brain fatty acid metabolism. Int. J. Biochem. Cell Biol. 2017, 84, 40–45. [Google Scholar] [CrossRef]
- Erecinska, M.; Silver, I.A. ATP and brain function. J. Cereb. Blood Flow Metab. 1989, 9, 2–19. [Google Scholar] [CrossRef]
- Schonfeld, P.; Reiser, G. Why does brain metabolism not favor burning of fatty acids to provide energy? Reflections on disadvantages of the use of free fatty acids as fuel for brain. J. Cereb. Blood Flow Metab. 2013, 33, 1493–1499. [Google Scholar] [CrossRef]
- Siesjo, B.K. Brain energy metabolism and catecholaminergic activity in hypoxia, hypercapnia and ischemia. J. Neural Transm. Suppl. 1978, 17–22. [Google Scholar]
- Simón, M.V.; Prado Spalm, F.H.; Vera, M.S.; Rotstein, N.P. Sphingolipids as Emerging Mediators in Retina Degeneration. Front. Cell. Neurosci. 2019, 13, 246. [Google Scholar] [CrossRef]
- Brownstein, S.; Carpenter, S.; Polomeno, R.C.; Little, J.M. Sandhoff’s disease (GM2 gangliosidosis type 2). Histopathology and ultrastructure of the eye. Arch. Ophthalmol. 1980, 98, 1089–1097. [Google Scholar] [CrossRef]
- Brownstein, S.; Meagher-Villemure, K.; Polomeno, R.C.; Little, J.M. Optic nerve in globoid leukodystrophy (Krabbe’s disease). Ultrastructural changes. Arch. Ophthalmol. 1978, 96, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chan, A.Y.; Stone, D.U.; Mandal, N.A. Beyond the cherry-red spot: Ocular manifestations of sphingolipid-mediated neurodegenerative and inflammatory disorders. Surv. Ophthalmol. 2014, 59, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Harcourt, B.; Ashton, N. Ultrastructure of the optic nerve in Krabbe’s leucodystrophy. Br. J. Ophthalmol. 1973, 57, 885–891. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Thayyullathil, F. Tumor suppressive functions of ceramide: Evidence and mechanisms. Apoptosis 2015, 20, 689–711. [Google Scholar] [CrossRef]
- Dasgupta, U.; Bamba, T.; Chiantia, S.; Karim, P.; Tayoun, A.N.; Yonamine, I.; Rawat, S.S.; Rao, R.P.; Nagashima, K.; Fukusaki, E.; et al. Ceramide kinase regulates phospholipase C and phosphatidylinositol 4, 5, bisphosphate in phototransduction. Proc. Natl. Acad. Sci. USA 2009, 106, 20063–20068. [Google Scholar] [CrossRef]
- Fan, J.; Wu, B.X.; Crosson, C.E. Suppression of Acid Sphingomyelinase Protects the Retina from Ischemic Injury. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4476–4484. [Google Scholar] [CrossRef]
- Ranty, M.L.; Carpentier, S.; Cournot, M.; Rico-Lattes, I.; Malecaze, F.; Levade, T.; Delisle, M.B.; Quintyn, J.C. Ceramide production associated with retinal apoptosis after retinal detachment. Graefes Arch. Clin. Exp. Ophthalmol. 2009, 247, 215–224. [Google Scholar] [CrossRef]
- Sugano, E.; Edwards, G.; Saha, S.; Wilmott, L.A.; Grambergs, R.C.; Mondal, K.; Qi, H.; Stiles, M.; Tomita, H.; Mandal, N. Overexpression of acid ceramidase (ASAH1) protects retinal cells (ARPE19) from oxidative stress. J. Lipid Res. 2019, 60, 30–43. [Google Scholar] [CrossRef]
- Tomita, H.; Abe, T.; Tamai, M. Ceramide-induced cell death in cultured rat retinal pigment epithelial cells. Tohoku J. Exp. Med. 2000, 190, 223–229. [Google Scholar] [CrossRef][Green Version]
- Abed Rabbo, M.; Khodour, Y.; Kaguni, L.S.; Stiban, J. Sphingolipid lysosomal storage diseases: From bench to bedside. Lipids Health Dis. 2021, 20, 44. [Google Scholar] [CrossRef] [PubMed]
- Traversier, M.; Gaslondes, T.; Milesi, S.; Michel, S.; Delannay, E. Polar lipids in cosmetics: Recent trends in extraction, separation, analysis and main applications. Phytochem. Rev. 2018, 17, 1179–1210. [Google Scholar] [CrossRef]
- Jurevics, H.; Hostettler, J.; Muse, E.D.; Sammond, D.W.; Matsushima, G.K.; Toews, A.D.; Morell, P. Cerebroside synthesis as a measure of the rate of remyelination following cuprizone-induced demyelination in brain. J. Neurochem. 2001, 77, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Yanagihara, T.; Cumings, J.N. Fatty acid composition of cerebrosides and cerebroside sulphatides in cerebral oedema. Acta Neuropathol. 1968, 12, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Pujol-Lereis, L.M. Alteration of Sphingolipids in Biofluids: Implications for Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 3564. [Google Scholar] [CrossRef] [PubMed]
- Mielke, M.M.; Maetzler, W.; Haughey, N.J.; Bandaru, V.V.; Savica, R.; Deuschle, C.; Gasser, T.; Hauser, A.K.; Graber-Sultan, S.; Schleicher, E.; et al. Plasma ceramide and glucosylceramide metabolism is altered in sporadic Parkinson’s disease and associated with cognitive impairment: A pilot study. PLoS ONE 2013, 8, e73094. [Google Scholar] [CrossRef] [PubMed]
- Pujol-Lereis, L.M.; Liebisch, G.; Schick, T.; Lin, Y.; Grassmann, F.; Uchida, K.; Zipfel, P.F.; Fauser, S.; Skerka, C.; Weber, B.H.F. Evaluation of serum sphingolipids and the influence of genetic risk factors in age-related macular degeneration. PLoS ONE 2018, 13, e0200739. [Google Scholar] [CrossRef] [PubMed]
- Vidaurre, O.G.; Haines, J.D.; Katz Sand, I.; Adula, K.P.; Huynh, J.L.; McGraw, C.A.; Zhang, F.; Varghese, M.; Sotirchos, E.; Bhargava, P.; et al. Cerebrospinal fluid ceramides from patients with multiple sclerosis impair neuronal bioenergetics. Brain 2014, 137, 2271–2286. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Gal, J.; Kwinter, D.M.; Liu, X.; Zhu, H. Mitochondrial dysfunction in amyotrophic lateral sclerosis. Biochim. Biophys. Acta 2010, 1802, 45–51. [Google Scholar] [CrossRef]
- McCombe, P.A.; Henderson, R.D. The Role of immune and inflammatory mechanisms in ALS. Curr. Mol. Med. 2011, 11, 246–254. [Google Scholar] [CrossRef]
- Vissing, C.R.; Duno, M.; Wibrand, F.; Christensen, M.; Vissing, J. Hydroxylated Long-Chain Acylcarnitines are Biomarkers of Mitochondrial Myopathy. J. Clin. Endocrinol. Metab. 2019, 104, 5968–5976. [Google Scholar] [CrossRef]
- Tonin, A.M.; Grings, M.; Busanello, E.N.; Moura, A.P.; Ferreira, G.C.; Viegas, C.M.; Fernandes, C.G.; Schuck, P.F.; Wajner, M. Long-chain 3-hydroxy fatty acids accumulating in LCHAD and MTP deficiencies induce oxidative stress in rat brain. Neurochem. Int. 2010, 56, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, S.K.; Ibdah, J.A. Role of 3-Hydroxy Fatty Acid-Induced Hepatic Lipotoxicity in Acute Fatty Liver of Pregnancy. Int. J. Mol. Sci. 2018, 19, 322. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen. Res. 2013, 8, 2003–2014. [Google Scholar] [PubMed]
- Islam, M.T. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol Res. 2017, 39, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Ayasolla, K.; Khan, M.; Singh, A.K.; Singh, I. Inflammatory mediator and beta-amyloid (25-35)-induced ceramide generation and iNOS expression are inhibited by vitamin E. Free Radic. Biol. Med. 2004, 37, 325–338. [Google Scholar] [CrossRef]
- Bello, R.I.; Gomez-Diaz, C.; Buron, M.I.; Alcain, F.J.; Navas, P.; Villalba, J.M. Enhanced anti-oxidant protection of liver membranes in long-lived rats fed on a coenzyme Q10-supplemented diet. Exp. Gerontol. 2005, 40, 694–706. [Google Scholar] [CrossRef]
- Summers, S.A.; Chaurasia, B.; Holland, W.L. Metabolic Messengers: Ceramides. Nat. Metab. 2019, 1, 1051–1058. [Google Scholar] [CrossRef]
- Amaral, A.U.; Cecatto, C.; da Silva, J.C.; Wajner, A.; Wagner, M. Mechanistic Bases of Neurotoxicity Provoked by Fatty Acids Accumulating in MCAD and LCHAD Deficiencies. J. Inborn Errors Metab. Screen. 2017, 5, 2326409817701472. [Google Scholar] [CrossRef][Green Version]
- Hagenbuchner, J.; Scholl-Buergi, S.; Karall, D.; Ausserlechner, M.J. Very long-/and long Chain-3-Hydroxy Acyl CoA Dehydrogenase Deficiency correlates with deregulation of the mitochondrial fusion/fission machinery. Sci. Rep. 2018, 8, 3254. [Google Scholar] [CrossRef]
- Francis, J.S.; Markov, V.; Leone, P. Dietary triheptanoin rescues oligodendrocyte loss, dysmyelination and motor function in the nur7 mouse model of Canavan disease. J. Inherit. Metab. Dis. 2014, 37, 369–381. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tucci, S. An Altered Sphingolipid Profile as a Risk Factor for Progressive Neurodegeneration in Long-Chain 3-Hydroxyacyl-CoA Deficiency (LCHADD). Int. J. Mol. Sci. 2022, 23, 7144. https://doi.org/10.3390/ijms23137144
Tucci S. An Altered Sphingolipid Profile as a Risk Factor for Progressive Neurodegeneration in Long-Chain 3-Hydroxyacyl-CoA Deficiency (LCHADD). International Journal of Molecular Sciences. 2022; 23(13):7144. https://doi.org/10.3390/ijms23137144
Chicago/Turabian StyleTucci, Sara. 2022. "An Altered Sphingolipid Profile as a Risk Factor for Progressive Neurodegeneration in Long-Chain 3-Hydroxyacyl-CoA Deficiency (LCHADD)" International Journal of Molecular Sciences 23, no. 13: 7144. https://doi.org/10.3390/ijms23137144
APA StyleTucci, S. (2022). An Altered Sphingolipid Profile as a Risk Factor for Progressive Neurodegeneration in Long-Chain 3-Hydroxyacyl-CoA Deficiency (LCHADD). International Journal of Molecular Sciences, 23(13), 7144. https://doi.org/10.3390/ijms23137144





