Molecular Basis Underlying the Therapeutic Potential of Vitamin D for the Treatment of Depression and Anxiety
Abstract
1. Introduction
2. Literature Data Searching
3. Neuroinflammation as a Key Pathophysiological Mechanism Related to Mood Disorders
4. Metabolism and Biological Functions of Vitamin D
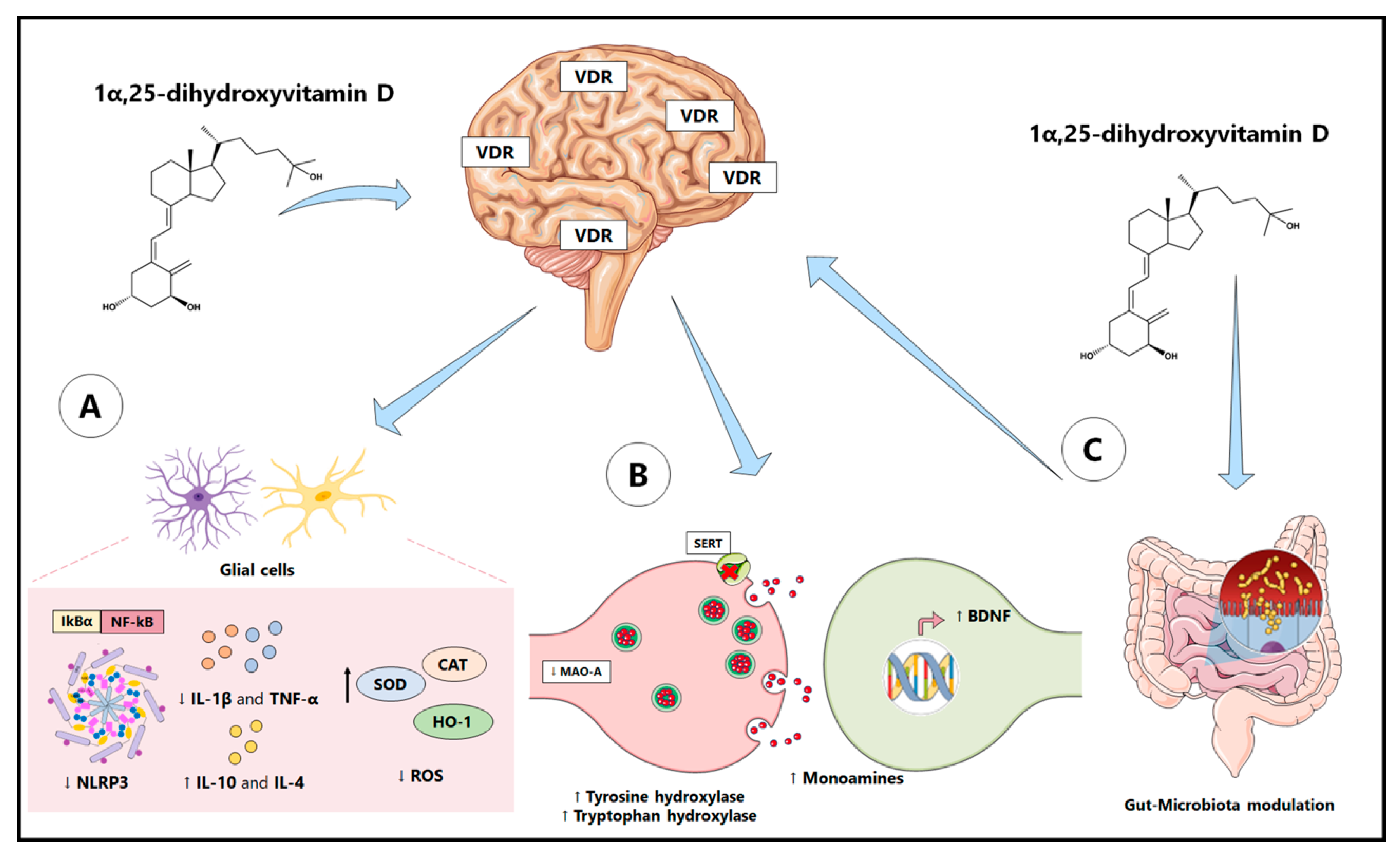
4.1. Antioxidant and Anti-Inflammatory Properties of Vitamin D
4.2. Pro-Neurogenic and Neuromodulatory Properties of Vitamin D
4.3. Vitamin D: Modulation of Gut Microbiota
5. Preclinical Studies: Effects of Vitamin D in Models of Depression and Anxiety
6. Clinical Studies: Effects of Vitamin D in Depression and Anxiety
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Craske, M.G.; Stein, M.B.; Eley, T.C.; Milad, M.R.; Holmes, A.; Rapee, R.M.; Wittchen, H.U. Anxiety Disorders. Nat. Rev. Dis. Primers 2017, 3, 17024. [Google Scholar] [CrossRef] [PubMed]
- Otte, C.; Gold, S.M.; Penninx, B.W.; Pariante, C.M.; Etkin, A.; Fava, M.; Mohr, D.C.; Schatzberg, A.F. Major Depressive Disorder. Nat. Rev. Dis. Primers 2016, 2, 16065. [Google Scholar] [CrossRef] [PubMed]
- Lamers, F.; Van Oppen, P.; Comijs, H.C.; Smit, J.H.; Spinhoven, P.; van Balkom, A.J.L.M.; Nolen, W.A.; Zitman, F.G.; Beekman, A.T.F.; Penninx, B.W.J.H. Comorbidity Patterns of Anxiety and Depressive Disorders in a Large Cohort Study: The Netherlands Study of Depression and Anxiety (NESDA). J. Clin. Psychiatry 2011, 72, 341–348. [Google Scholar] [CrossRef]
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef]
- Schiele, M.A.; Domschke, K. Epigenetics at the Crossroads between Genes, Environment and Resilience in Anxiety Disorders: Epigenetics in Anxiety Disorders. Genes Brain Behav. 2018, 17, e12423. [Google Scholar] [CrossRef]
- De Haan, P.; Klein, H.C.; ’t Hart, B.A. Autoimmune Aspects of Neurodegenerative and Psychiatric Diseases: A Template for Innovative Therapy. Front. Psychiatry 2017, 8, 46. [Google Scholar] [CrossRef]
- Carlessi, A.S.; Borba, L.A.; Zugno, A.I.; Quevedo, J.; Réus, G.Z. Gut Microbiota-Brain Axis in Depression: The Role of Neuroinflammation. Eur. J. Neurosci. 2021, 53, 222–235. [Google Scholar] [CrossRef]
- Kim, Y.K.; Jeon, S.W. Neuroinflammation and the Immune-Kynurenine Pathway in Anxiety Disorders. Curr. Neuropharmacol. 2018, 16, 574–582. [Google Scholar] [CrossRef]
- Stevens, B.R.; Goel, R.; Seungbum, K.; Richards, E.M.; Holbert, R.C.; Pepine, C.J.; Raizada, M.K. Increased Human Intestinal Barrier Permeability Plasma Biomarkers Zonulin and FABP2 Correlated with Plasma LPS and Altered Gut Microbiome in Anxiety or Depression. Gut 2018, 67, 1555–1557. [Google Scholar] [CrossRef]
- Fedotova, J.; Zarembo, D.; Dragasek, J.; Caprnda, M.; Kruzliak, P.; Dudnichenko, T. Modulating Effects of Cholecalciferol Treatment on Estrogen Deficiency-Induced Anxiety-Like Behavior of Adult Female Rats. Folia Med. 2017, 59, 139–158. [Google Scholar] [CrossRef]
- Khairy, E.Y.; Attia, M.M. Protective Effects of Vitamin D on Neurophysiologic Alterations in Brain Aging: Role of Brain-Derived Neurotrophic Factor (BDNF). Nutr. Neurosci. 2021, 24, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre d’Hellencourt, C.; Montero-Menei, C.N.; Bernard, R.; Couez, D. Vitamin D3 Inhibits Proinflammatory Cytokines and Nitric Oxide Production by the EOC13 Microglial Cell Line. J. Neurosci. Res. 2003, 71, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Morello, M.; Landel, V.; Lacassagne, E.; Baranger, K.; Annweiler, C.; Féron, F.; Millet, P. Vitamin D Improves Neurogenesis and Cognition in a Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2018, 55, 6463–6479. [Google Scholar] [CrossRef] [PubMed]
- Yong, H.Y.F.; Rawji, K.S.; Ghorbani, S.; Xue, M.; Yong, V.W. The Benefits of Neuroinflammation for the Repair of the Injured Central Nervous System. Cell. Mol. Immunol. 2019, 16, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Fullerton, J.N.; Gilroy, D.W. Resolution of Inflammation: A New Therapeutic Frontier. Nat. Rev. Drug Discov. 2016, 15, 551–567. [Google Scholar] [CrossRef]
- Barton, G.M. A Calculated Response: Control of Inflammation by the Innate Immune System. J. Clin. Investig. 2008, 118, 413–420. [Google Scholar] [CrossRef]
- Herman, F.J.; Pasinetti, G.M. Principles of Inflammasome Priming and Inhibition: Implications for Psychiatric Disorders. Brain. Behav. Immun. 2018, 73, 66–84. [Google Scholar] [CrossRef]
- Orihuela, R.; McPherson, C.A.; Harry, G.J. Microglial M1/M2 Polarization and Metabolic States: Microglia Bioenergetics with Acute Polarization. Br. J. Pharmacol. 2016, 173, 649–665. [Google Scholar] [CrossRef]
- Rudzki, L.; Maes, M. The Microbiota-Gut-Immune-Glia (MGIG) Axis in Major Depression. Mol. Neurobiol. 2020, 57, 4269–4295. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Song, N.; Li, T. Regulation of NLRP3 Inflammasome by Phosphorylation. Front. Immunol. 2018, 9, 2305. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, X.; Ding, Y.; Zhou, W.; Tao, L.; Lu, P.; Wang, Y.; Hu, R. Nuclear Factor E2-Related Factor-2 Negatively Regulates NLRP3 Inflammasome Activity by Inhibiting Reactive Oxygen Species-Induced NLRP3 Priming. Antioxid. Redox Signal. 2017, 26, 28–43. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, L.; Ye, X.; Hao, Q.; Zhang, T.; Cui, G.; Yu, M. Nrf2/ARE Pathway Inhibits ROS-Induced NLRP3 Inflammasome Activation in BV2 Cells after Cerebral Ischemia Reperfusion. Inflamm. Res. 2018, 67, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Sutterwala, F.S.; Haasken, S.; Cassel, S.L. Mechanism of NLRP3 Inflammasome Activation. Ann. N. Y. Acad. Sci. 2014, 1319, 82–95. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic Reactive Astrocytes Are Induced by Activated Microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Tejeda, G.; Díaz-Guerra, M. Integral Characterization of Defective BDNF/TrkB Signalling in Neurological and Psychiatric Disorders Leads the Way to New Therapies. Int. J. Mol. Sci. 2017, 18, 268. [Google Scholar] [CrossRef]
- Farina, C.; Aloisi, F.; Meinl, E. Astrocytes Are Active Players in Cerebral Innate Immunity. Trends Immunol. 2007, 28, 138–145. [Google Scholar] [CrossRef]
- Troubat, R.; Barone, P.; Leman, S.; Desmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; El Hage, W.; Surget, A.; Belzung, C.; et al. Neuroinflammation and Depression: A Review. Eur. J. Neurosci. 2021, 53, 151–171. [Google Scholar] [CrossRef]
- Bansal, Y.; Singh, R.; Parhar, I.; Kuhad, A.; Soga, T. Quinolinic Acid and Nuclear Factor Erythroid 2-Related Factor 2 in Depression: Role in Neuroprogression. Front. Pharmacol. 2019, 10, 452. [Google Scholar] [CrossRef]
- Braidy, N.; Grant, R.; Brew, B.J.; Adams, S.; Jayasena, T.; Guillemin, G.J. Effects of Kynurenine Pathway Metabolites on Intracellular NAD + Synthesis and Cell Death in Human Primary Astrocytes and Neurons. Int. J. Tryptophan Res. 2009, 2, 61–69. [Google Scholar] [CrossRef]
- Pierozan, P.; Biasibetti, H.; Schmitz, F.; Ávila, H.; Parisi, M.M.; Barbe-Tuana, F.; Wyse, A.T.S.; Pessoa-Pureur, R. Quinolinic Acid Neurotoxicity: Differential Roles of Astrocytes and Microglia via FGF-2-Mediated Signaling in Redox-Linked Cytoskeletal Changes. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2016, 1863, 3001–3014. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.M.; Bao, C.H.; Wu, Y.; Liang, S.H.; Wang, D.; Wu, L.Y.; Huang, Y.; Liu, H.R.; Wu, H.G. Tryptophan-Kynurenine Metabolism: A Link between the Gut and Brain for Depression in Inflammatory Bowel Disease. J. Neuroinflammation 2021, 18, 135. [Google Scholar] [CrossRef] [PubMed]
- Siopi, E.; Chevalier, G.; Katsimpardi, L.; Saha, S.; Bigot, M.; Moigneu, C.; Eberl, G.; Lledo, P.M. Changes in Gut Microbiota by Chronic Stress Impair the Efficacy of Fluoxetine. Cell Rep. 2020, 30, 3682–3690.e6. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S. Neuronal Damage and Protection in the Pathophysiology and Treatment of Psychiatric Illness: Stress and Depression. Dialogues Clin. Neurosci. 2009, 11, 239–255. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Shirvani, A.; Holick, M.F. Vitamin D for Skeletal and Non-Skeletal Health: What We Should Know. J. Clin. Orthop. Trauma 2019, 10, 1082–1093. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D: A D-Lightful Solution for Health. J. Investig. Med. 2011, 59, 872–880. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Zhu, J.; DeLuca, H.F. Vitamin D 25-Hydroxylase—Four Decades of Searching, Are We There Yet? Arch. Biochem. Biophys. 2012, 523, 30–36. [Google Scholar] [CrossRef]
- Hossein-nezhad, A.; Holick, M.F. Vitamin D for Health: A Global Perspective. Mayo Clin. Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef] [PubMed]
- Wacker, M.; Holick, M. Vitamin D—Effects on Skeletal and Extraskeletal Health and the Need for Supplementation. Nutrients 2013, 5, 111–148. [Google Scholar] [CrossRef] [PubMed]
- Haussler, M.R.; Whitfield, G.K.; Kaneko, I.; Haussler, C.A.; Hsieh, D.; Hsieh, J.-C.; Jurutka, P.W. Molecular Mechanisms of Vitamin D Action. Calcif. Tissue Int. 2013, 92, 77–98. [Google Scholar] [CrossRef] [PubMed]
- Alshahrani, F.; Aljohani, N. Vitamin D: Deficiency, Sufficiency and Toxicity. Nutrients 2013, 5, 3605–3616. [Google Scholar] [CrossRef]
- Eyles, D.W.; Smith, S.; Kinobe, R.; Hewison, M.; McGrath, J.J. Distribution of the Vitamin D Receptor and 1α-Hydroxylase in Human Brain. J. Chem. Neuroanat. 2005, 29, 21–30. [Google Scholar] [CrossRef]
- Casseb, G.A.S.; Kaster, M.P.; Rodrigues, A.L.S. Potential Role of Vitamin D for the Management of Depression and Anxiety. CNS Drugs 2019, 33, 619–637. [Google Scholar] [CrossRef]
- Hoogendijk, W.J.G.; Lips, P.; Dik, M.G.; Deeg, D.J.H.; Beekman, A.T.F.; Penninx, B.W.J.H. Depression Is Associated With Decreased 25-Hydroxyvitamin D and Increased Parathyroid Hormone Levels in Older Adults. Arch. Gen. Psychiatry 2008, 65, 508. [Google Scholar] [CrossRef]
- Pu, D.; Luo, J.; Wang, Y.; Ju, B.; Lv, X.; Fan, P.; He, L. Prevalence of Depression and Anxiety in Rheumatoid Arthritis Patients and Their Associations with Serum Vitamin D Level. Clin. Rheumatol. 2018, 37, 179–184. [Google Scholar] [CrossRef]
- Baeke, F.; Takiishi, T.; Korf, H.; Gysemans, C.; Mathieu, C. Vitamin D: Modulator of the Immune System. Curr. Opin. Pharmacol. 2010, 10, 482–496. [Google Scholar] [CrossRef]
- Nakajo, T.; Katayoshi, T.; Kitajima, N.; Tsuji-Naito, K. 1,25-Dihydroxyvitamin D3 Attenuates IL-1β Secretion by Suppressing NLRP1 Inflammasome Activation by Upregulating the NRF2-HO-1 Pathway in Epidermal Keratinocytes. Redox Biol. 2021, 48, 102203. [Google Scholar] [CrossRef]
- Yamini, P.; Ray, R.S.; Chopra, K. Vitamin D3 Attenuates Cognitive Deficits and Neuroinflammatory Responses in ICV-STZ Induced Sporadic Alzheimer’s Disease. Inflammopharmacology 2018, 26, 39–55. [Google Scholar] [CrossRef]
- Cui, C.; Xu, P.; Li, G.; Qiao, Y.; Han, W.; Geng, C.; Liao, D.; Yang, M.; Chen, D.; Jiang, P. Vitamin D Receptor Activation Regulates Microglia Polarization and Oxidative Stress in Spontaneously Hypertensive Rats and Angiotensin II-Exposed Microglial Cells: Role of Renin-Angiotensin System. Redox Biol. 2019, 26, 101295. [Google Scholar] [CrossRef] [PubMed]
- Boontanrart, M.; Hall, S.D.; Spanier, J.A.; Hayes, C.E.; Olson, J.K. Vitamin D3 Alters Microglia Immune Activation by an IL-10 Dependent SOCS3 Mechanism. J. Neuroimmunol. 2016, 292, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Calvello, R.; Cianciulli, A.; Nicolardi, G.; De Nuccio, F.; Giannotti, L.; Salvatore, R.; Porro, C.; Trotta, T.; Panaro, M.A.; Lofrumento, D.D. Vitamin D Treatment Attenuates Neuroinflammation and Dopaminergic Neurodegeneration in an Animal Model of Parkinson’s Disease, Shifting M1 to M2 Microglia Responses. J. Neuroimmune Pharmacol. 2017, 12, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Jung, J.H.; Kim, J.Y. 1,25-Dihydroxyvitamin D3 up-Regulates TLR10 While down-Regulating TLR2, 4, and 5 in Human Monocyte THP-1. J. Steroid Biochem. Mol. Biol. 2014, 141, 1–6. [Google Scholar] [CrossRef]
- Verma, R.; Kim, J.Y. 1,25-Dihydroxyvitamin D3 Facilitates M2 Polarization and Upregulates TLR10 Expression on Human Microglial Cells. Neuroimmunomodulation 2016, 23, 75–80. [Google Scholar] [CrossRef]
- Camargo, A.; Dalmagro, A.P.; Platt, N.; Rosado, A.F.; Neis, V.B.; Zeni, A.L.B.; Kaster, M.P.; Rodrigues, A.L.S. Cholecalciferol Abolishes Depressive-like Behavior and Hippocampal Glucocorticoid Receptor Impairment Induced by Chronic Corticosterone Administration in Mice. Pharmacol. Biochem. Behav. 2020, 196, 172971. [Google Scholar] [CrossRef]
- Xin, L.; Che, B.; Zhai, B.; Luo, Q.; Zhang, C.; Wang, J.; Wang, S.; Fan, G.; Liu, Z.; Feng, J.; et al. 1,25-Dihydroxy Vitamin D3 Attenuates the Oxidative Stress-Mediated Inflammation Induced by PM2.5via the P38/NF-ΚB/NLRP3 Pathway. Inflammation 2019, 42, 702–713. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, H.; Li, X.; Yi, B.; Huang, L.; Hu, Z.; Li, A.; Du, J.; Li, Y.; Zhang, W. Vitamin D/VDR Attenuate Cisplatin-Induced AKI by down-Regulating NLRP3/Caspase-1/GSDMD Pyroptosis Pathway. J. Steroid Biochem. Mol. Biol. 2021, 206, 105789. [Google Scholar] [CrossRef]
- Cohen-Lahav, M.; Shany, S.; Tobvin, D.; Chaimovitz, C.; Douvdevani, A. Vitamin D Decreases NFκB Activity by Increasing IκBα Levels. Nephrol. Dial. Transplant. 2006, 21, 889–897. [Google Scholar] [CrossRef]
- Huang, H.; Hong, J.Y.; Wu, Y.J.; Wang, E.Y.; Liu, Z.Q.; Cheng, B.H.; Mei, L.; Liu, Z.G.; Yang, P.C.; Zheng, P.Y. Vitamin D Receptor Interacts with NLRP3 to Restrict the Allergic Response. Clin. Exp. Immunol. 2018, 194, 17–26. [Google Scholar] [CrossRef]
- Rao, Z.; Chen, X.; Wu, J.; Xiao, M.; Zhang, J.; Wang, B.; Fang, L.; Zhang, H.; Wang, X.; Yang, S.; et al. Vitamin D Receptor Inhibits NLRP3 Activation by Impeding Its BRCC3-Mediated Deubiquitination. Front. Immunol. 2019, 10, 2783. [Google Scholar] [CrossRef]
- Cui, C.; Wang, C.; Jin, F.; Yang, M.; Kong, L.; Han, W.; Jiang, P. Calcitriol Confers Neuroprotective Effects in Traumatic Brain Injury by Activating Nrf2 Signaling through an Autophagy-Mediated Mechanism. Mol. Med. 2021, 27, 118. [Google Scholar] [CrossRef] [PubMed]
- Fão, L.; Mota, S.I.; Rego, A.C. Shaping the Nrf2-ARE-Related Pathways in Alzheimer’s and Parkinson’s Diseases. Ageing Res. Rev. 2019, 54, 100942. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Shah, S.A.; Zaman, N.; Uddin, M.N.; Khan, W.; Ali, A.; Riaz, M.; Kamil, A. Vitamin D Exerts Neuroprotection via SIRT1/Nrf-2/NF-KB Signaling Pathways against D-Galactose-Induced Memory Impairment in Adult Mice. Neurochem. Int. 2021, 142, 104893. [Google Scholar] [CrossRef] [PubMed]
- Mehrabadi, S.; Sadr, S.S. Administration of Vitamin D3 and E Supplements Reduces Neuronal Loss and Oxidative Stress in a Model of Rats with Alzheimer’s Disease. Neurol. Res. 2020, 42, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Shinpo, K.; Kikuchi, S.; Sasaki, H.; Moriwaka, F.; Tashiro, K. Effect of 1,25-Dihydroxyvitamin D3 on Cultured Mesencephalic Dopaminergic Neurons to the Combined Toxicity Caused by L-Buthionine Sulfoximine and 1-Methyl-4-Phenylpyridine. J. Neurosci. Res. 2000, 62, 374–382. [Google Scholar] [CrossRef]
- Cui, X.; McGrath, J.J.; Burne, T.H.J.; Mackay-Sim, A.; Eyles, D.W. Maternal Vitamin D Depletion Alters Neurogenesis in the Developing Rat Brain. Int. J. Dev. Neurosci. 2007, 25, 227–232. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, R.; Yang, R.; Zhang, Z.; Bai, Y.; Chang, F.; Li, L.; Sokabe, M.; Goltzman, D.; Miao, D.; et al. Abnormal Neurogenesis in the Dentate Gyrus of Adult Mice Lacking 1,25-Dihydroxy Vitamin D3 (1,25-(OH)2D3). Hippocampus 2012, 22, 421–433. [Google Scholar] [CrossRef]
- Eyles, D.; Almeras, L.; Benech, P.; Patatian, A.; Mackay-Sim, A.; McGrath, J.; Féron, F. Developmental Vitamin D Deficiency Alters the Expression of Genes Encoding Mitochondrial, Cytoskeletal and Synaptic Proteins in the Adult Rat Brain. J. Steroid Biochem. Mol. Biol. 2007, 103, 538–545. [Google Scholar] [CrossRef]
- Almeras, L.; Eyles, D.; Benech, P.; Laffite, D.; Villard, C.; Patatian, A.; Boucraut, J.; Mackay-Sim, A.; McGrath, J.; Féron, F. Developmental Vitamin D Deficiency Alters Brain Protein Expression in the Adult Rat: Implications for Neuropsychiatric Disorders. PROTEOMICS 2007, 7, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.; Brown, J.; Mackay-Sim, A.; McGrath, J.; Feron, F. Vitamin D3 and Brain Development. Neuroscience 2003, 118, 641–653. [Google Scholar] [CrossRef]
- Féron, F.; Burne, T.H.J.; Brown, J.; Smith, E.; McGrath, J.J.; Mackay-Sim, A.; Eyles, D.W. Developmental Vitamin D3 Deficiency Alters the Adult Rat Brain. Brain Res. Bull. 2005, 65, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Naveilhan, P.; Neveu, I.; Wion, D.; Brachet, P. 1,25-Dihydroxyvitamin D3, an Inducer of Glial Cell Line-Derived Neurotrophic Factor. NeuroReport 1996, 7, 2171–2175. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liang, L. Vitamin D3/Vitamin D Receptor Signaling Mitigates Symptoms of Post-Stroke Depression in Mice by Upregulating Hippocampal BDNF Expression. Neurosci. Res. 2021, 170, 306–313. [Google Scholar] [CrossRef]
- Koshkina, A.; Dudnichenko, T.; Baranenko, D.; Fedotova, J.; Drago, F. Effects of Vitamin D3 in Long-Term Ovariectomized Rats Subjected to Chronic Unpredictable Mild Stress: BDNF, NT-3, and NT-4 Implications. Nutrients 2019, 11, 1726. [Google Scholar] [CrossRef]
- Neveu, I.; Naveilhan, P.; Baudet, C.; Brachet, P.; Metsis, M. 1,25-Dihydroxyvitamin D3 Regulates NT-3, NT-4 but Not BDNF MRNA in Astrocytes. NeuroReport 1994, 6, 124–126. [Google Scholar] [CrossRef]
- Scott-Solomon, E.; Kuruvilla, R. Mechanisms of Neurotrophin Trafficking via Trk Receptors. Mol. Cell. Neurosci. 2018, 91, 25–33. [Google Scholar] [CrossRef]
- Atif, F.; Yousuf, S.; Sayeed, I.; Ishrat, T.; Hua, F.; Stein, D.G. Combination Treatment with Progesterone and Vitamin D Hormone Is More Effective than Monotherapy in Ischemic Stroke: The Role of BDNF/TrkB/Erk1/2 Signaling in Neuroprotection. Neuropharmacology 2013, 67, 78–87. [Google Scholar] [CrossRef]
- Abdallah, C.G.; Sanacora, G.; Duman, R.S.; Krystal, J.H. Ketamine and Rapid-Acting Antidepressants: A Window into a New Neurobiology for Mood Disorder Therapeutics. Annu. Rev. Med. 2015, 66, 509–523. [Google Scholar] [CrossRef]
- Li, N.; Lee, B.; Liu, R.J.; Banasr, M.; Dwyer, J.M.; Iwata, M.; Li, X.Y.; Aghajanian, G.; Duman, R.S. MTOR-Dependent Synapse Formation Underlies the Rapid Antidepressant Effects of NMDA Antagonists. Science 2010, 329, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, J.N.; Czernik, A.J.; Fienberg, A.A.; Greengard, P.; Sihra, T.S. Synapsins as Mediators of BDNF-Enhanced Neurotransmitter Release. Nat. Neurosci. 2000, 3, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, S.; Alsulami, N.; Khoja, S.; Alsufiani, H.; Tayeb, H.O.; Tarazi, F.I. Vitamin D Supplementation Ameliorates Severity of Major Depressive Disorder. J. Mol. Neurosci. 2020, 70, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Seyedi, M.; Gholami, F.; Samadi, M.; Djalali, M.; Effatpanah, M.; Yekaninejad, M.S.; Hashemi, R.; Abdolahi, M.; Chamari, M.; Honarvar, N.M. The Effect of Vitamin D3 Supplementation on Serum BDNF, Dopamine, and Serotonin in Children with Attention-Deficit/Hyperactivity Disorder. CNS Neurol. Disord.-Drug Targets 2019, 18, 496–501. [Google Scholar] [CrossRef]
- Jiang, P.; Zhang, L.H.; Cai, H.L.; Li, H.D.; Liu, Y.P.; Tang, M.M.; Dang, R.L.; Zhu, W.Y.; Xue, Y.; He, X. Neurochemical Effects of Chronic Administration of Calcitriol in Rats. Nutrients 2014, 6, 6048–6059. [Google Scholar] [CrossRef] [PubMed]
- Pertile, R.A.N.; Cui, X.; Eyles, D.W. Vitamin D Signaling and the Differentiation of Developing Dopamine Systems. Neuroscience 2016, 333, 193–203. [Google Scholar] [CrossRef]
- Sabir, M.S.; Haussler, M.R.; Mallick, S.; Kaneko, I.; Lucas, D.A.; Haussler, C.A.; Whitfield, G.K.; Jurutka, P.W. Optimal Vitamin D Spurs Serotonin: 1,25-Dihydroxyvitamin D Represses Serotonin Reuptake Transport (SERT) and Degradation (MAO-A) Gene Expression in Cultured Rat Serotonergic Neuronal Cell Lines. Genes Nutr. 2018, 13, 19. [Google Scholar] [CrossRef]
- Luthold, R.V.; Fernandes, G.R.; Franco-de-Moraes, A.C.; Folchetti, L.G.D.; Ferreira, S.R.G. Gut Microbiota Interactions with the Immunomodulatory Role of Vitamin D in Normal Individuals. Metabolism 2017, 69, 76–86. [Google Scholar] [CrossRef]
- Assa, A.; Vong, L.; Pinnell, L.J.; Avitzur, N.; Johnson-Henry, K.C.; Sherman, P.M. Vitamin D Deficiency Promotes Epithelial Barrier Dysfunction and Intestinal Inflammation. J. Infect. Dis. 2014, 210, 1296–1305. [Google Scholar] [CrossRef]
- Ooi, J.H.; Li, Y.; Rogers, C.J.; Cantorna, M.T. Vitamin D Regulates the Gut Microbiome and Protects Mice from Dextran Sodium Sulfate–Induced Colitis. J. Nutr. 2013, 143, 1679–1686. [Google Scholar] [CrossRef]
- Wu, S.; Liao, A.P.; Xia, Y.; Chun Li, Y.; Li, J.D.; Sartor, R.B.; Sun, J. Vitamin D Receptor Negatively Regulates Bacterial-Stimulated NF-ΚB Activity in Intestine. Am. J. Pathol. 2010, 177, 686–697. [Google Scholar] [CrossRef] [PubMed]
- Fedotova, J.; Dudnichenko, T.; Kruzliak, P.; Puchavskaya, Z. Different Effects of Vitamin D Hormone Treatment on Depression-like Behavior in the Adult Ovariectomized Female Rats. Biomed. Pharmacother. 2016, 84, 1865–1872. [Google Scholar] [CrossRef] [PubMed]
- Fedotova, J.O. Vitamin D3 Treatment Differentially Affects Anxiety-like Behavior in the Old Ovariectomized Female Rats and Old Ovariectomized Female Rats Treated with Low Dose of 17β-Estradiol. BMC Med. Genet. 2019, 20, 49. [Google Scholar] [CrossRef] [PubMed]
- Camargo, A.; Dalmagro, A.P.; Rikel, L.; da Silva, E.B.; Simão da Silva, K.A.B.; Zeni, A.L.B. Cholecalciferol Counteracts Depressive-like Behavior and Oxidative Stress Induced by Repeated Corticosterone Treatment in Mice. Eur. J. Pharmacol. 2018, 833, 451–461. [Google Scholar] [CrossRef]
- Da Silva Souza, S.V.; da Rosa, P.B.; Neis, V.B.; Moreira, J.D.; Rodrigues, A.L.S.; Moretti, M. Effects of Cholecalciferol on Behavior and Production of Reactive Oxygen Species in Female Mice Subjected to Corticosterone-Induced Model of Depression. Naunyn. Schmiedebergs Arch. Pharmacol. 2020, 393, 111–120. [Google Scholar] [CrossRef]
- Neis, V.B.; Werle, I.; Moretti, M.; Rosa, P.B.; Camargo, A.; Dalsenter, Y.D.O.; Platt, N.; Rosado, A.F.; Engel, W.D.; de Almeida, G.R.L.; et al. Involvement of Serotonergic Neurotransmission in the Antidepressant-like Effect Elicited by Cholecalciferol in the Chronic Unpredictable Stress Model in Mice. Metab. Brain Dis. 2022, 37, 1597–1608. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, Y.; Wang, K.; Chen, L.; Jiang, P. Neuroprotective Effects of Vitamin D and 17ß-Estradiol against Ovariectomy-Induced Neuroinflammation and Depressive-like State: Role of the AMPK/NF-ΚB Pathway. Int. Immunopharmacol. 2020, 86, 106734. [Google Scholar] [CrossRef]
- Bakhtiari-Dovvombaygi, H.; Izadi, S.; Zare Moghaddam, M.; Hashemzehi, M.; Hosseini, M.; Azhdari-Zarmehri, H.; Dinpanah, H.; Beheshti, F. Beneficial Effects of Vitamin D on Anxiety and Depression-like Behaviors Induced by Unpredictable Chronic Mild Stress by Suppression of Brain Oxidative Stress and Neuroinflammation in Rats. Naunyn. Schmiedebergs Arch. Pharmacol. 2021, 394, 655–667. [Google Scholar] [CrossRef]
- Jiang, P.; Zhang, W.Y.; Li, H.D.; Cai, H.L.; Liu, Y.P.; Chen, L.Y. Stress and Vitamin D: Altered Vitamin D Metabolism in Both the Hippocampus and Myocardium of Chronic Unpredictable Mild Stress Exposed Rats. Psychoneuroendocrinology 2013, 38, 2091–2098. [Google Scholar] [CrossRef]
- Groves, N.J.; Bradford, D.; Sullivan, R.K.P.; Conn, K.A.; Aljelaify, R.F.; McGrath, J.J.; Burne, T.H.J. Behavioural Effects of Adult Vitamin D Deficiency in BALB/c Mice Are Not Associated with Proliferation or Survival of Neurons in the Adult Hippocampus. PLoS ONE 2016, 11, e0152328. [Google Scholar] [CrossRef]
- Jorde, R.; Sneve, M.; Figenschau, Y.; Svartberg, J.; Waterloo, K. Effects of Vitamin D Supplementation on Symptoms of Depression in Overweight and Obese Subjects: Randomized Double-Blind Trial. J. Intern. Med. 2008, 264, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Narula, N.; Cooray, M.; Anglin, R.; Muqtadir, Z.; Narula, A.; Marshall, J.K. Impact of High-Dose Vitamin D3 Supplementation in Patients with Crohn’s Disease in Remission: A Pilot Randomized Double-Blind Controlled Study. Dig. Dis. Sci. 2017, 62, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Penckofer, S.; Byrn, M.; Adams, W.; Emanuele, M.A.; Mumby, P.; Kouba, J.; Wallis, D.E. Vitamin D Supplementation Improves Mood in Women with Type 2 Diabetes. J. Diabetes Res. 2017, 2017, 8232863. [Google Scholar] [CrossRef]
- Sharifi, A.; Vahedi, H.; Nedjat, S.; Mohamadkhani, A.; Hosseinzadeh Attar, M.J. Vitamin D Decreases Beck Depression Inventory Score in Patients with Mild to Moderate Ulcerative Colitis: A Double-Blind Randomized Placebo-Controlled Trial. J. Diet. Suppl. 2019, 16, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Yosaee, S.; Soltani, S.; Esteghamati, A.; Motevalian, S.A.; Tehrani-Doost, M.; Clark, C.C.T.; Jazayeri, S. Effects of Zinc, Vitamin D, and Their Co-Supplementation on Mood, Serum Cortisol, and Brain-Derived Neurotrophic Factor in Patients with Obesity and Mild to Moderate Depressive Symptoms: A Phase II, 12-Wk, 2 × 2 Factorial Design, Double-Blind, Randomized, Placebo-Controlled Trial. Nutrition 2020, 71, 110601. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, Y.; Wang, T.; Lin, Y.; Yu, J.; Xia, Q.; Zhu, P.; Zhu, D. Vitamin D Supplementation Improves Anxiety but Not Depression Symptoms in Patients with Vitamin D Deficiency. Brain Behav. 2020, 10, e01760. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.P.; Pareek, M.; Hvolby, A.; Schmedes, A.; Toft, T.; Dahl, E.; Nielsen, C.T. Vitamin D3 Supplementation and Treatment Outcomes in Patients with Depression (D3-Vit-Dep). BMC Res. Notes 2019, 12, 203. [Google Scholar] [CrossRef]
- Kaviani, M.; Nikooyeh, B.; Zand, H.; Yaghmaei, P.; Neyestani, T.R. Effects of Vitamin D Supplementation on Depression and Some Involved Neurotransmitters. J. Affect. Disord. 2020, 269, 28–35. [Google Scholar] [CrossRef]
- Alavi, N.M.; Khademalhoseini, S.; Vakili, Z.; Assarian, F. Effect of Vitamin D Supplementation on Depression in Elderly Patients: A Randomized Clinical Trial. Clin. Nutr. 2019, 38, 2065–2070. [Google Scholar] [CrossRef]
- Bertone-Johnson, E.R.; Powers, S.I.; Spangler, L.; Larson, J.; Michael, Y.L.; Millen, A.E.; Bueche, M.N.; Salmoirago-Blotcher, E.; Wassertheil-Smoller, S.; Brunner, R.L.; et al. Vitamin D Supplementation and Depression in the Women’s Health Initiative Calcium and Vitamin D Trial. Am. J. Epidemiol. 2012, 176, 1–13. [Google Scholar] [CrossRef]
- Mozaffari-Khosravi, H.; Nabizade, L.; Yassini-Ardakani, S.M.; Hadinedoushan, H.; Barzegar, K. The Effect of 2 Different Single Injections of High Dose of Vitamin D on Improving the Depression in Depressed Patients With Vitamin D Deficiency: A Randomized Clinical Trial. J. Clin. Psychopharmacol. 2013, 33, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Vellekkatt, F.; Menon, V.; Rajappa, M.; Sahoo, J. Effect of Adjunctive Single Dose Parenteral Vitamin D Supplementation in Major Depressive Disorder with Concurrent Vitamin D Deficiency: A Double-Blind Randomized Placebo-Controlled Trial. J. Psychiatr. Res. 2020, 129, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Khoraminya, N.; Tehrani-Doost, M.; Jazayeri, S.; Hosseini, A.; Djazayery, A. Therapeutic Effects of Vitamin D as Adjunctive Therapy to Fluoxetine in Patients with Major Depressive Disorder. Aust. N. Z. J. Psychiatry 2013, 47, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Eid, A.; Khoja, S.; AlGhamdi, S.; Alsufiani, H.; Alzeben, F.; Alhejaili, N.; Tayeb, H.O.; Tarazi, F.I. Vitamin D Supplementation Ameliorates Severity of Generalized Anxiety Disorder (GAD). Metab. Brain Dis. 2019, 34, 1781–1786. [Google Scholar] [CrossRef] [PubMed]
- Valdivielso, J.M.; Fernandez, E. Vitamin D receptor polymorphisms and diseases. Clin. Chim. Acta 2006, 371, 1–12. [Google Scholar] [CrossRef]
- Lye, M.S.; Tor, Y.S.; Tey, Y.Y.; Shahabudin, A.; Loh, S.P.; Ibrahim, N.; Stanslas, J.; Rosli, R.; Ling, K.H. BsmI-ApaI-TaqI TAC (BAt) Haplotype of Vitamin D Receptor Gene Is Associated with Increased Risk of Major Depressive Disorder. J. Mol. Neurosci. 2020, 71, 981–990. [Google Scholar] [CrossRef]
- Usategui-Martín, R.; De Luis-Román, D.A.; Fernández-Gómez, J.M.; Ruiz-Mambrilla, M.; Pérez-Castrillón, J.L. Vitamin D Receptor (VDR) Gene Polymorphisms Modify the Response to Vitamin D Supplementation: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 360. [Google Scholar] [CrossRef]
| Study | Animal Model | Treatment | Behavioral Alterations | Biochemical Alterations |
|---|---|---|---|---|
| Fedotova et al., 2016 [92] | Ovariectomized Wistar rats | Cholecalciferol (5 mg/kg for 14 days; s.c.) | Antidepressant-like effect in the FST | Not evaluated |
| Fedotova et al., 2017 [10] | Ovariectomized Wistar rats | Cholecalciferol (5 mg/kg for 14 days; s.c.) | Anxiolytic-like effect in EPM and LDT | Not evaluated |
| Fedotova 2019 [93] | Ovariectomized Wistar rats | Cholecalciferol (5 mg/kg for 14 days; s.c.) | Anxiolytic-like effect in EPM and LDT | Not evaluated |
| Camargo et al., 2018 [94] | Corticosterone (21 days) in male Swiss mice | Cholecalciferol (2.5 µg/kg, for 7 days; p.o.) | Antidepressant-like effect in the splash test and TST | ↓ Protein carbonyl and nitrite levels |
| da Silva Souza et al., 2020 [95] | Corticosterone (21days) in female Swiss mice | Cholecalciferol (100 IU/kg, p.o.) for 7 days | Antidepressant-like effect in the TST | ↓ ROS |
| Camargo et al., 2020 [57] | Corticosterone (21 days) in male Swiss mice | Cholecalciferol (2.5 μg/kg, p.o.) for 7 days | Antidepressant-like effect in the TST and splash test | ↓ ASC, caspase-1, and TXNIP |
| Neis et al., 2022 [96] | CUMS in female Swiss mice | Cholecalciferol (2.5 μg/kg, p.o.) for 7 days | Antidepressant-like effect in the TST | ↑ serotonin levels in the prefrontal cortex |
| Zhang et al., 2020 [97] | Ovariectomized female Sprague-Dawley rats | Calcitriol (100 ng/kg, p.o.) for 10 weeks | Antidepressant-like effect in the FST and novelty-suppressed feeding test | ↓ IL-1β, IL-6, and TNF-α, iNOS and COX-2 |
| Bakhtiari-Dovvombaygi et al., 2021 [98] | CUMS in male Wistar rats | Vitamin D3 (10,000 IU/kg) for 28 days | Anxiolytic and antidepressant-like effect in EPM and FST | ↓ Malondialdehyde and IL-6 ↑ Total thiols, SOD, and CAT |
| Jiang et al., 2013 [99] | CUMS (4 weeks) in male Sprague-Dawley rats | Without treatment | Depressive-like behavior in sucrose preference test | ↑ 1,25(OH)2D and VDR |
| Koshkina et al., 2019 [76] | CUMS in ovariectomized Wistar rat | Vitamin D3 (5 mg/kg for 4 weeks; s.c.) | Antidepressant-like effect in FST and sucrose preference test | ↑BDNF and NT-3/NT-4 |
| Xu and Liang 2021 [75] | post-stroke depression model (male C57BL/6 mice) | Calcitriol (25 μg/kg/day for 4 weeks; i.c.v.) | Antidepressant-like effect in FST and sucrose preference test | ↑ VDR and BDNF |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kouba, B.R.; Camargo, A.; Gil-Mohapel, J.; Rodrigues, A.L.S. Molecular Basis Underlying the Therapeutic Potential of Vitamin D for the Treatment of Depression and Anxiety. Int. J. Mol. Sci. 2022, 23, 7077. https://doi.org/10.3390/ijms23137077
Kouba BR, Camargo A, Gil-Mohapel J, Rodrigues ALS. Molecular Basis Underlying the Therapeutic Potential of Vitamin D for the Treatment of Depression and Anxiety. International Journal of Molecular Sciences. 2022; 23(13):7077. https://doi.org/10.3390/ijms23137077
Chicago/Turabian StyleKouba, Bruna R., Anderson Camargo, Joana Gil-Mohapel, and Ana Lúcia S. Rodrigues. 2022. "Molecular Basis Underlying the Therapeutic Potential of Vitamin D for the Treatment of Depression and Anxiety" International Journal of Molecular Sciences 23, no. 13: 7077. https://doi.org/10.3390/ijms23137077
APA StyleKouba, B. R., Camargo, A., Gil-Mohapel, J., & Rodrigues, A. L. S. (2022). Molecular Basis Underlying the Therapeutic Potential of Vitamin D for the Treatment of Depression and Anxiety. International Journal of Molecular Sciences, 23(13), 7077. https://doi.org/10.3390/ijms23137077









