Exopolysaccharide ID1 Improves Post-Warming Outcomes after Vitrification of In Vitro-Produced Bovine Embryos
Abstract
1. Introduction
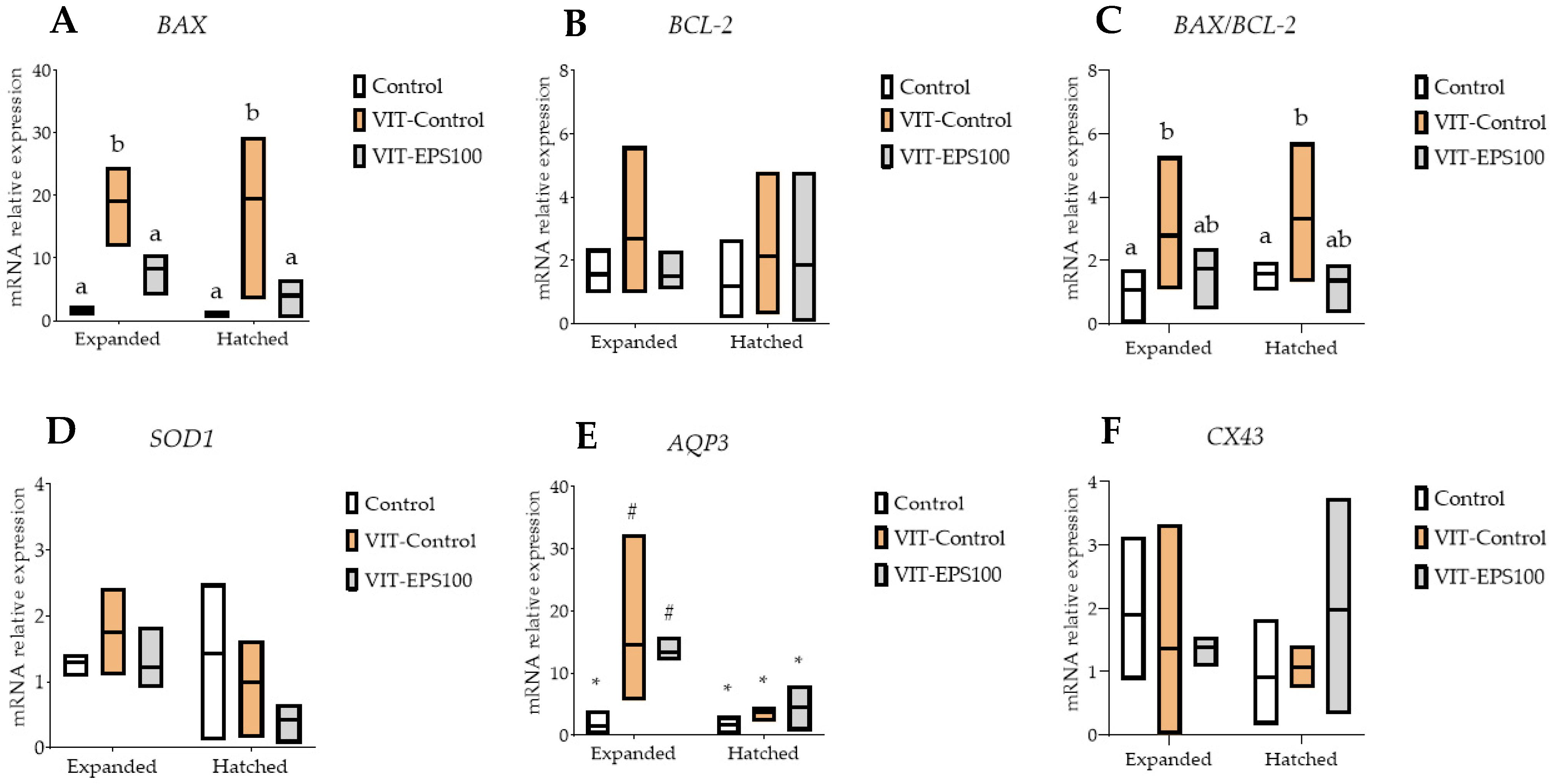
2. Results
3. Discussion
4. Materials and Methods
4.1. Chemicals and Suppliers
4.2. In Vitro Embryo Production
4.3. Supplementation with EPS ID1
4.4. Embryo Vitrification and Warming
4.4.1. Vitrification Protocol
4.4.2. Warming Protocol
4.5. Immunostaining for Differential Cell Count and DNA Fragmentation
4.6. Gene Expression Analysis by Reverse Transcription and Quantitative PCR (rt-qPCR)
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Viana, J.H.M. 2020 Statistics of embryo production and transfer in domestic farm animals. World embryo industry grows despite the pandemic. Embryo Technol. Newsl. 2021, 39, 24–38. [Google Scholar]
- Ferre, L.B.; Kjelland, M.E.; Taiyeb, A.M.; Campos-Chillon, F.; Ross, P.J. Recent progress in bovine in vitro-derived embryo cryotolerance: Impact of in vitro culture systems, advances in cryopreservation and future considerations. Reprod. Domest. Anim. 2020, 55, 659–676. [Google Scholar] [CrossRef] [PubMed]
- Mogas, T. Update on the vitrification of bovine oocytes and invitro-produced embryos. Reprod. Fertil. Dev. 2018, 31, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Rizos, D.; Ward, F.; Boland, F.; Lonergan, P. Effect of culture system on the yield and quality of bovine blastocysts as assessed by survival after vitrification. Theriogenology 2001, 56, 1–16. [Google Scholar] [CrossRef]
- Bojic, S.; Murray, A.; Bentley, B.L.; Spindler, R.; Pawlik, P.; Cordeiro, J.L.; Bauer, R.; de Magalhaes, J.P. Winter is coming: The future of cryopreservation. BMC Biol. 2021, 19, 56. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.H.; Hur, Y.B.; Lee, C.W.; Park, S.H.; Koo, B.W. Marine Antifreeze Proteins: Structure, Function, and Application to Cryopreservation as a Potential Cryoprotectant. Mar. Drugs 2017, 15, 27. [Google Scholar] [CrossRef]
- Robles, V.; Valcarce, D.G.; Riesco, M.F. The Use of Antifreeze Proteins in the Cryopreservation of Gametes and Embryos. Biomolecules 2019, 9, 181. [Google Scholar] [CrossRef]
- Arav, A.; Rubinsky, B.; Fletcher, G.; Seren, E. Cryogenic protection of oocytes with antifreeze proteins. Mol. Reprod. Dev. 1993, 36, 488–493. [Google Scholar] [CrossRef]
- Chaves, D.F.; Campelo, I.S.; Silva, M.M.; Bhat, M.H.; Teixeira, D.I.; Melo, L.M.; Souza-Fabjan, J.M.; Mermillod, P.; Freitas, V.J. The use of antifreeze protein type III for vitrification of in vitro matured bovine oocytes. Cryobiology 2016, 73, 324–328. [Google Scholar] [CrossRef]
- Lee, H.H.; Lee, H.J.; Kim, H.J.; Lee, J.H.; Ko, Y.; Kim, S.M.; Lee, J.R.; Suh, C.S.; Kim, S.H. Effects of antifreeze proteins on the vitrification of mouse oocytes: Comparison of three different antifreeze proteins. Hum. Reprod. 2015, 30, 2110–2119. [Google Scholar] [CrossRef]
- Liang, S.; Yuan, B.; Kwon, J.-W.; Ahn, M.; Cui, X.-S.; Bang, J.K.; Kim, N.-H. Effect of antifreeze glycoprotein 8 supplementation during vitrification on the developmental competence of bovine oocytes. Theriogenology 2016, 86, 485–494.e1. [Google Scholar] [CrossRef] [PubMed]
- O’neil, L.; Paynter, S.; Fuller, B.; Shaw, R.; DeVries, A. Vitrification of mature mouse oocytes in a 6 M Me2SO solution supplemented with antifreeze glycoproteins: The effect of temperature. Cryobiology 1998, 37, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Zhao, S.; Chao, L.; Yu, H.; Song, C.; Shen, Y.; Chen, H.; Deng, X. The protective role of antifreeze protein 3 on the structure and function of mature mouse oocytes in vitrification. Cryobiology 2014, 69, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Ideta, A.; Aoyagi, Y.; Tsuchiya, K.; Nakamura, Y.; Hayama, K.; Shirasawa, A.; Sakaguchi, K.; Tominaga, N.; Nishimiya, Y.; Tsuda, S. Prolonging hypothermic storage (4 C) of bovine embryos with fish antifreeze protein. J. Reprod. Dev. 2015, 61, 1–6. [Google Scholar] [CrossRef]
- Karanova, M.; Mezhevikina, L.; Petropavlov, N. Study of cryoprotective properties of antifreeze glycoproteins from the white sea cod Gadus morhua on low temperature freezing of mouse embryos. Biofizika 1995, 40, 1341–1347. [Google Scholar]
- Li, X.; Wang, L.; Yin, C.; Lin, J.; Wu, Y.; Chen, D.; Qiu, C.; Jia, B.; Huang, J.; Jiang, X.; et al. Antifreeze protein from Anatolia polita (ApAFP914) improved outcome of vitrified in vitro sheep embryos. Cryobiology 2020, 93, 109–114. [Google Scholar] [CrossRef]
- Martínez-Páramo, S.; Barbosa, V.; Pérez-Cerezales, S.; Robles, V.; Herráez, M. Cryoprotective effects of antifreeze proteins delivered into zebrafish embryos. Cryobiology 2009, 58, 128–133. [Google Scholar] [CrossRef]
- Martínez-Páramo, S.; Pérez-Cerezales, S.; Robles, V.; Anel, L.; Herráez, M. Incorporation of antifreeze proteins into zebrafish embryos by a non-invasive method. Cryobiology 2008, 56, 216–222. [Google Scholar] [CrossRef]
- Robles, V.; Barbosa, V.; Herráez, M.; Martínez-Páramo, S.; Cancela, M. The antifreeze protein type I (AFP I) increases seabream (Sparus aurata) embryos tolerance to low temperatures. Theriogenology 2007, 68, 284–289. [Google Scholar] [CrossRef]
- Robles, V.; Cabrita, E.; Anel, L.; Herráez, M. Microinjection of the antifreeze protein type III (AFPIII) in turbot (Scophthalmus maximus) embryos: Toxicity and protein distribution. Aquaculture 2006, 261, 1299–1306. [Google Scholar] [CrossRef]
- Shaw, J.; Ward, C.; Trounson, A. Evaluation of propanediol, ethylene glycol, sucrose and antifreeze proteins on the survival of slow-cooled mouse pronuclear and 4-cell embryos. Hum. Reprod. (Oxf. Engl.) 1995, 10, 396–402. [Google Scholar] [CrossRef]
- Casillo, A.; Lanzetta, R.; Parrilli, M.; Corsaro, M.M. Exopolysaccharides from Marine and Marine Extremophilic Bacteria: Structures, Properties, Ecological Roles and Applications. Mar. Drugs 2018, 16, 69. [Google Scholar] [CrossRef] [PubMed]
- Carillo, S.; Casillo, A.; Pieretti, G.; Parrilli, E.; Sannino, F.; Bayer-Giraldi, M.; Cosconati, S.; Novellino, E.; Ewert, M.; Deming, J.W.; et al. A Unique Capsular Polysaccharide Structure from the Psychrophilic Marine Bacterium Colwellia psychrerythraea 34H That Mimics Antifreeze (Glyco)proteins. J. Am. Chem. Soc. 2015, 137, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Yim, J.H. Cryoprotective properties of exopolysaccharide (P-21653) produced by the Antarctic bacterium, Pseudoalteromonas arctica KOPRI 21653. J. Microbiol. 2007, 45, 510–514. [Google Scholar] [PubMed]
- Liu, S.-B.; Chen, X.-L.; He, H.-L.; Zhang, X.-Y.; Xie, B.-B.; Yu, Y.; Chen, B.; Zhou, B.-C.; Zhang, Y.-Z. Structure and Ecological Roles of a Novel Exopolysaccharide from the Arctic Sea Ice Bacterium Pseudoalteromonas sp. Strain SM20310. Appl. Environ. Microbiol. 2013, 79, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Arcarons, N.; Vendrell-Flotats, M.; Yeste, M.; Mercade, E.; Lopez-Bejar, M.; Mogas, T. Cryoprotectant role of exopolysaccharide of Pseudomonas sp. ID1 in the vitrification of IVM cow oocytes. Reprod. Fertil. Dev. 2019, 31, 1507–1519. [Google Scholar] [CrossRef]
- Carrion, O.; Delgado, L.; Mercade, E. New emulsifying and cryoprotective exopolysaccharide from Antarctic Pseudomonas sp. ID1. Carbohydr. Polym. 2015, 117, 1028–1034. [Google Scholar] [CrossRef]
- Rizos, D.; Clemente, M.; Bermejo-Alvarez, P.; de La Fuente, J.; Lonergan, P.; Gutierrez-Adan, A. Consequences of in vitro culture conditions on embryo development and quality. Reprod. Domest. Anim. 2008, 43 (Suppl. S4), 44–50. [Google Scholar] [CrossRef]
- Madura, J.D.; Baran, K.; Wierzbicki, A. Molecular recognition and binding of thermal hysteresis proteins to ice. J. Mol. Recognit. 2000, 13, 101–113. [Google Scholar] [CrossRef]
- Liang, S.; Yuan, B.; Jin, Y.X.; Zhang, J.B.; Bang, J.K.; Kim, N.H. Effects of antifreeze glycoprotein 8 (AFGP8) supplementation during vitrification on the in vitro developmental capacity of expanded bovine blastocysts. Reprod. Fertil. Dev. 2017, 29, 2140–2148. [Google Scholar] [CrossRef]
- Nishijima, K.; Tanaka, M.; Sakai, Y.; Koshimoto, C.; Morimoto, M.; Watanabe, T.; Fan, J.; Kitajima, S. Effects of type III antifreeze protein on sperm and embryo cryopreservation in rabbit. Cryobiology 2014, 69, 22–25. [Google Scholar] [CrossRef]
- Lagneaux, D.; Huhtinen, M.; Koskinen, E.; Palmer, E. Effect of anti-freeze protein (AFP) on the cooling and freezing of equine embryos as measured by DAPI-staining. Equine Vet. J. Suppl. 1997, 29, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Baguisi, A.; Arav, A.; Crosby, T.F.; Roche, J.F.; Boland, M.P. Hypothermic storage of sheep embryos with antifreeze proteins: Development in vitro and in vivo. Theriogenology 1997, 48, 1017–1024. [Google Scholar] [CrossRef]
- Rubinsky, B.; Arav, A.; Devries, A.L. The cryoprotective effect of antifreeze glycopeptides from antarctic fishes. Cryobiology 1992, 29, 69–79. [Google Scholar] [CrossRef]
- Wang, J.H. A comprehensive evaluation of the effects and mechanisms of antifreeze proteins during low-temperature preservation. Cryobiology 2000, 41, 1–9. [Google Scholar] [CrossRef]
- Morato, R.; Izquierdo, D.; Paramio, M.T.; Mogas, T. Survival and apoptosis rates after vitrification in cryotop devices of in vitro-produced calf and cow blastocysts at different developmental stages. Reprod. Fertil. Dev. 2010, 22, 1141–1147. [Google Scholar] [CrossRef]
- Martinez-Rodero, I.; Garcia-Martinez, T.; Ordonez-Leon, E.A.; Vendrell-Flotats, M.; Olegario Hidalgo, C.; Esmoris, J.; Mendibil, X.; Azcarate, S.; Lopez-Bejar, M.; Yeste, M.; et al. A Shorter Equilibration Period Improves Post-Warming Outcomes after Vitrification and in Straw Dilution of In Vitro-Produced Bovine Embryos. Biology 2021, 10, 142. [Google Scholar] [CrossRef]
- Gutiérrez-Adan, A.; Rizos, D.; Fair, T.; Moreira, P.; Pintado, B.; de la Fuente, J.; Boland, M.; Lonergan, P. Effect of speed of development on mRNA expression pattern in early bovine embryos cultured in vivo or in vitro. Mol. Reprod. Dev. 2004, 68, 441–448. [Google Scholar] [CrossRef]
- Van Soom, A.; Ysebaert, M.T.; de Kruif, A.J.M.R.; Research, D.I.G. Relationship between timing of development, morula morphology, and cell allocation to inner cell mass and trophectoderm in in vitro-produced bovine embryos. Mol. Reprod. Dev. Inc. Gamete Res. 1997, 47, 47–56. [Google Scholar] [CrossRef]
- Lonergan, P.; Khatir, H.; Piumi, F.; Rieger, D.; Humblot, P.; Boland, M.J.R. Effect of time interval from insemination to first cleavage on the developmental characteristics, sex ratio and pregnancy rate after transfer of bovine embryos. Reproduction 1999, 117, 159–167. [Google Scholar] [CrossRef]
- Wrenzycki, C.; Herrmann, D.; Niemann, H. Timing of blastocyst expansion affects spatial messenger RNA expression patterns of genes in bovine blastocysts produced in vitro. Biol. Reprod. 2003, 68, 2073–2080. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hasler, J.; Henderson, W.; Hurtgen, P.; Jin, Z.; McCauley, A.; Mower, S.; Neely, B.; Shuey, L.; Stokes, J.; Trimmer, S.J.T. Production, freezing and transfer of bovine IVF embryos and subsequent calving results. Theriogenology 1995, 43, 141–152. [Google Scholar] [CrossRef]
- Gustafsson, H.; Larsson, B.; Shamsuddin, M.; Jaakma, U.; Emanuelson, U. Factors affecting the survival of frozen thawed bovine in vitro produced blastocysts. Asian-Australas. J. Anim. Sci. 2001, 14, 7–12. [Google Scholar] [CrossRef]
- Havlicek, V.; Kuzmany, A.; Cseh, S.; Brem, G.; Besenfelder, U. The effect of long-term in vivo culture in bovine oviduct and uterus on the development and cryo-tolerance of in vitro produced bovine embryos. Reprod. Domest. Anim. 2010, 45, 832–837. [Google Scholar] [PubMed]
- Saha, S.; Suzuki, T.J.R. Vitrication of in vitro produced bovine embryos at different ages using one-and three-step addition of cryoprotective additives. Reprod. Fertil. Dev. 1997, 9, 741–746. [Google Scholar] [CrossRef]
- Mahmoudzadeh, A.; Van Soom, A.; Bols, P.; Ysebaert, M.; de Kruif, A.J.R. Optimization of a simple vitrification procedure for bovine embryos produced in vitro: Effect of developmental stage, two-step addition of cryoprotectant and sucrose dilution on embryonic survival. Reproduction 1995, 103, 33–39. [Google Scholar] [CrossRef]
- Salamone, D.F.; Damiani, P.; Fissore, J.M.; Robl; Duby, R.T. Biochemical an developmental Evidence that ooplasmic maturation of prepubertal bovine oocyte is compromised. Biol. Reprod. 2001, 64, 1761–1768. [Google Scholar] [CrossRef]
- Presicce, G.A.; Jiang, S.; Simkin, M.; Zhang, L.; Looney, C.R.; Godke, R.A.; Yang, X. Age and hormonal dependence of acquisition of oocyte competence for embryogenesis in prepubertal calves. Biol. Reprod. 1997, 56, 386–392. [Google Scholar] [CrossRef]
- Diederich, M.; Hansmann, T.; Heinzmann, J.; Barg-Kues, B.; Herrmann, D.; Aldag, P.; Baulain, U.; Reinhard, R.; Kues, W.; Weissgerber, C.; et al. DNA methylation and mRNA expression profiles in bovine oocytes derived from prepubertal and adult donors. Reproduction 2012, 144, 319–330. [Google Scholar] [CrossRef]
- Zaraza, J.; Oropeza, A.; Velazquez, M.A.; Korsawe, K.; Herrmann, D.; Carnwath, J.W.; Niemann, H. Developmental competence and mRNA expression of preimplantation in vitro-produced embryos from prepubertal and postpubertal cattle and their relationship with apoptosis after intraovarian administration of IGF-1. Theriogenology 2010, 74, 75–89. [Google Scholar] [CrossRef]
- Baldassarre, H.; Currin, L.; Michalovic, L.; Bellefleur, A.M.; Gutierrez, K.; Mondadori, R.G.; Glanzner, W.G.; Schuermann, Y.; Bohrer, R.C.; Dicks, N.; et al. Interval of gonadotropin administration for in vitro embryo production from oocytes collected from Holstein calves between 2 and 6 months of age by repeated laparoscopy. Theriogenology 2018, 116, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Khatir, H.; Lonergan, P.; Touzé, J.L.; Mermillod, P. The characterization of bovine embryos obtained from prepubertal calf oocytes and their viability after non surgical embryo transfer. Theriogenology 1998, 50, 1201–1210. [Google Scholar] [CrossRef]
- Gómez, E.; Carrocera, S.; Martín, D.; Pérez-Jánez, J.J.; Prendes, J.; Prendes, J.M.; Vázquez, A.; Murillo, A.; Gimeno, I.; Muñoz, M. Efficient one-step direct transfer to recipients of thawed bovine embryos cultured in vitro and frozen in chemically defined medium. Theriogenology 2020, 146, 39–47. [Google Scholar] [CrossRef]
- Gómez, E.; Muñoz, M.; Rodríguez, A.; Caamaño, J.; Facal, N.; Díez, C. Vitrification of bovine blastocysts produced in vitro inflicts selective damage to the inner cell mass. Reprod. Domest. Anim. 2009, 44, 194–199. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, J.; Cao, S.; Zhang, J.; Heng, B.C.; Huang, M.; Ling, X.; Duan, T.; Tong, G.Q. Vitrified-warmed blastocyst transfer cycles yield higher pregnancy and implantation rates compared with fresh blastocyst transfer cycles--time for a new embryo transfer strategy? Fertil. Steril. 2011, 95, 1691–1695. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Ibeas, P.; Gimeno, I.; Cañon-Beltran, K.; Gutierrez Adan, A.; Rizos, D.; Gomez, E. Senescence and Apoptosis in In Vitro Produced Embryos in the Bovine Model. Front. Cell Dev. Biol. 2020, 8, 1646. [Google Scholar] [CrossRef]
- Paula-Lopes, F.; Hansen, P.J.B. Apoptosis is an adaptive response in bovine preimplantation embryos that facilitates survival after heat shock. Biochem. Biophys. Res. Commun. 2002, 295, 37–42. [Google Scholar] [CrossRef]
- Neuber, E.; Luetjens, C.; Chan, A.; Schatten, G.J.T. Analysis of DNA fragmentation of in vitro cultured bovine blastocysts using TUNEL. Theriogenology 2002, 57, 2193–2202. [Google Scholar] [CrossRef]
- Yang, M.Y.; Rajamahendran, R. Expression of Bcl-2 and Bax proteins in relation to quality of bovine oocytes and embryos produced in vitro. Anim. Reprod. Sci. 2002, 70, 159–169. [Google Scholar] [CrossRef]
- Iwayama, H.; Hochi, S.; Yamashita, M. In vitro and in vivo viability of human blastocysts collapsed by laser pulse or osmotic shock prior to vitrification. J. Assist. Reprod. Genet. 2011, 28, 355–361. [Google Scholar] [CrossRef]
- Bó, G.; Mapletoft, R. Evaluation and classification of bovine embryos. Anim. Reprod. AR 2018, 10, 344–348. [Google Scholar]
- Walton, S.; Catt, S.; Taylor-Robinson, A.W.J.C. A comparative analysis of the efficacy of three cryopreservation protocols on the survival of in vitro-derived cattle embryos at pronuclear and blastocyst stages. Cryobiology 2017, 77, 58–63. [Google Scholar]
- García-Martínez, T.; Vendrell-Flotats, M.; Martínez-Rodero, I.; Ordóñez-León, E.A.; Álvarez-Rodríguez, M.; López-Béjar, M.; Yeste, M.; Mogas, T. Glutathione ethyl ester protects in vitro-maturing bovine oocytes against oxidative stress induced by subsequent vitrification/warming. Int. J. Mol. Sci. 2020, 21, 7547. [Google Scholar] [CrossRef] [PubMed]

| Blastocyst Derived from Cow Oocytes | ||||||||
|---|---|---|---|---|---|---|---|---|
| Day 7 Blastocysts | Day 8 Blastocysts | |||||||
| n | Post-Warming | n | Post-Warming | |||||
| Re-Expansion Rate (%) (3 h) | Re-Expansion Rate (%) (24 h) | Hatching Rate (%) (24 h) | Re-Expansion Rate (%) (3 h) | Re-Expansion Rate (%) (24 h) | Hatching Rate (%) (24 h) | |||
| Control | 101 | 100 a | 100 a | 34.2 ± 1.7 a | 40 | 100 a | 100 a | 52.5 ± 8.5 |
| VIT-Control | 97 | 34.7 ± 2.4 b | 74.9 ± 3.3 b,* | 22.7 ± 5.7 b | 40 | 39.5 ± 8.5 b | 52.1 ± 4.1 b,# | 32.5 ± 2.5 b |
| VIT-EPS10 | 40 | 50.0 ± 7.1 b | 70.0 ± 2.4 b,* | 20.0 ± 5.7 b | 45 | 35.0 ± 2.9 b | 47.9 ± 4.3 b,# | 28.2 ± 5.4 b |
| VIT-EPS100 | 92 | 49.1 ± 3.7 b | 86.4 ± 3.7 c,* | 46.5 ± 5.1 a | 30 | 46.7 ± 6.6 b | 66.7 ± 5.7 c,# | 56.7 ± 8.8 a |
| Blastocyst Derived from Calf Oocytes | ||||||||
|---|---|---|---|---|---|---|---|---|
| Day 7 Blastocysts | Day 8 Blastocysts | |||||||
| n | Post-Warming | n | Post-Warming | |||||
| Re-Expansion Rate (%) (3 h) | Re-Expansion Rate (%) (24 h) | Hatching Rate (%) (24 h) | Re-Expansion Rate (%) (3 h) | Re-Expansion Rate (%) (24 h) | Hatching Rate (%) (24 h) | |||
| Control | 34 | 100 a | 100 a | 57.5 ± 13.2 a | 40 | 100 a | 100 a | 52.5 ± 8.5 a |
| VIT-Control | 40 | 29.5 ± 7.5 b | 57.5 ± 4.7 b | 22.5 ± 7.5 b | 43 | 32.1 ± 4.6 b | 57.3 ± 7.1 b | 20.2 ± 4.3 b |
| VIT-EPS10 | 38 | 31.7 ± 2.4 b | 55.0 ± 2.8 b | 18.7 ± 6.5 b | 40 | 37.5 ± 4.8 b | 50.0 ± 4.1 b | 15.0 ± 6.4 b |
| VIT-EPS100 | 32 | 34.1 ± 6.7 b | 71.5 ± 3.8 c | 33.3 ± 12.0 c | 30 | 46.7 ± 8.8 b | 70.0 ± 5.7 c | 40.0 ± 5.4 a |
| Blastocyst Derived from Cow Oocytes | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 7 Blastocysts | |||||||||
| TCN ± SEM | ICM Cell Number ± SEM | TE Cell Number ± SEM | AR ± SEM | ||||||
| n | Expanded | Hatched | Expanded | Hatched | Expanded | Hatched | Expanded | Hatched | |
| Control | 44 | 134.6 ± 6.4 1 | 219.3 ± 4.8 2 | 32.1 ± 1.4 1 | 43.6 ± 1.7 2 | 102.5 ± 5.4 1 | 175.7 ± 4.9 2 | 6.9 ± 0.5 a,1 | 4.5 ± 0.8 a,2 |
| VIT-Control | 42 | 140.1 ± 5.8 1 | 214.1 ± 2.9 2 | 22.7 ± 3.2 1 | 47.5 ± 1.9 2 | 117.4 ± 14.8 1 | 166.6 ± 2.2 2 | 15.6 ± 1.0 b,2 | 13.1 ± 0.6 b,2 |
| VIT-EPS10 | 40 | 123.7 ± 6.4 1 | 205.6 ± 3.4 2 | 26.2 ± 1.5 1 | 42.3 ± 2.4 2 | 97.5 ± 11.0 1 | 163.3 ± 10.7 2 | 18.8 ± 2.3 c,2 | 16.4 ± 0.8 c,2 |
| VIT-EPS100 | 36 | 133.8 ± 8.2 1 | 224.1 ± 2.4 2 | 33.6 ± 3.9 1 | 44.3 ± 2.6 2 | 100.2 ± 6.5 1 | 179.8 ± 2.5 2 | 13.5 ± 1.2 d,2 | 11.5 ± 0.6 d,2 |
| Day 8 blastocysts | |||||||||
| TCN ± SEM | ICM cell number ± SEM | TE cell number ± SEM | AR ± SEM | ||||||
| n | Expanded | Hatched | Expanded | Hatched | Expanded | Hatched | Expanded | Hatched | |
| Control | 40 | 145.0 ± 8.1 1 | 216.5 ± 3.4 2 | 41.2 ± 3.4 1 | 53.7 ± 0.8 2 | 103.8 ± 6.0 1 | 207.8 ± 3.6 2 | 5.3 ± 0.6 a,1 | 4.7 ± 0.3 a,2 |
| VIT-Control | 40 | 121.8 ± 5.6 1 | 206.4 ± 5.9 2 | 27.7 ± 3.2,1 | 49.5 ± 1.2 2 | 94.1 ± 14.8 1 | 156.9 ± 2.9 2 | 13.6 ± 0.5 b,1 | 11.1 ± 0.9 b,2 |
| VIT-EPS10 | 45 | 137.9 ± 10.8 1 | 203.6 ± 7.8 2 | 33.2 ± 4.0 1 | 48.1 ± 3.5 2 | 104.7 ± 8.3 1 | 155.5 ± 7.2 2 | 20.3 ± 1.3 c,1 | 17.9 ± 1.6 c,2 |
| VIT-EPS100 | 30 | 143.0 ± 6.2 1 | 209.8 ± 4.5 2 | 38.0 ± 2.9 1 | 52.3 ± 2.8 2 | 105.0 ± 12.3 1 | 157.5 ± 4.6 2 | 9.2 ± 1.4 d,1 | 7.8 ± 0.6 d,2 |
| Blastocyst Derived from Calf Oocytes | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 7 Blastocysts | |||||||||
| TCN ± SEM | ICM Cell Number ± SEM | TE Cell Number ± SEM | AR ± SEM | ||||||
| n | Expanded | Hatched | Expanded | Hatched | Expanded | Hatched | Expanded | Hatched | |
| Control | 34 | 120.5 ± 5.3 a,1 | 190.2 ± 4.3 a,2 | 34.5 ± 1.9 a,1 | 49.1 ± 2.2 a,2 | 86.0 ± 5.3 a,1 | 141.1 ± 4.3 a,2 | 7.2 ± 0.6 a,1 | 5.2 ± 0.5 a,2 |
| VIT-Control | 40 | 134.4 ± 7.1 a,1 | 211.3 ± 3.6 a,2 | 40.3 ± 7.2 a,1 | 56.7 ± 3.2 a,2 | 94.1 ± 4.8 a,1 | 154.6 ± 4.5 a,2 | 13.6 ± 0.9 b,1 | 11.2 ± 1.2 b,2 |
| VIT-EPS10 | 38 | 138.0 ± 6.4 a,1 | 206.1 ± 5.8 a,2 | 33.0 ± 3.7 a,1 | 47.6 ± 4.0 a,2 | 105.0 ± 6.1 a,1 | 158.5 ± 6.0 a,2 | 17.9 ± 1.2 c,1 | 14.3 ± 1.5 c,2 |
| Day 8 blastocysts | |||||||||
| TCN ± SEM | ICM cell number ± SEM | TE cell number ± SEM | AR ± SEM | ||||||
| n | Expanded | Hatched | Expanded | Hatched | Expanded | Hatched | Expanded | Hatched | |
| Control | 40 | 113.2 ± 6.8 a,1 | 215.4 ± 2.9 a,2 | 32.5 ± 1.8 a,1 | 50.1 ± 1.4 a,2 | 80.7 ± 5.8 a,1 | 165.3 ± 3.3 a,2 | 6.2 ± 0.5 a,1 | 5.1 ± 0.4 a,2 |
| VIT-Control | 43 | 98.2 ± 5.3 a,1 | 209.5 ± 1.6 a,2 | 22.6 ± 0.8 a,1 | 44.3 ± 2.5 a,2 | 75.6 ± 2.9 a,1 | 165.2 ± 8.3 a,2 | 15.1 ± 1.2 b,1 | 12.1 ± 0.7 b,2 |
| VIT-EPS10 | 40 | 129.8 ± 6.9 a,1 | 204.2 ± 2.1 a,2 | 35.3 ± 3.2 a,1 | 44.5 ± 2.9 a,2 | 94.5 ± 10.1 a,1 | 159.7 ± 1.8 a,2 | 21.6 ± 0.7 c,1 | 15.4 ± 0.6 c,2 |
| VIT-EPS100 | 30 | 120.8 ± 5.7 a,1 | 213.3 ± 3.1 a,2 | 32.8 ± 5.3 a,1 | 53.7 ± 1.6 a,2 | 88.0 ± 10.1 a,1 | 159.6 ± 2.7 a,2 | 10.5 ± 1.5 d,1 | 8.4 ± 0.5 d,2 |
| Symbol | Primer Sequences (5′-3′) | Amplicon Size (bp) | GenBank Accession No. |
|---|---|---|---|
| BCL2 associated X apoptosis regulator (BAX) | F: ACCAAGAAGCTGAGCGAGTG | 116 | NM_173894.1 |
| R: CGGAAAAAGACCTCTCGGGG | |||
| BCL2-like 1 (BCL-2) | F: GAGTTCGGAGGGGTCATGTG | 211 | NM_001166486.1 |
| R: TGAGCAGTGCCTTCAGAGAC | |||
| Superoxide dismutase 1 (SOD1) | F: ACACAAGGCTGTACCAGTGC | 102 | NM_174615.2 |
| R: CACATTGCCCAGGTCTCCAA | |||
| Aquaporin 3 (AQP3) | F: GTGGACCCCTACAACAACCC | 222 | NM_001079794.1 |
| R: CAGGAGCGGAGAGACAATGG | |||
| Connexin 43 (CX43) | F:TGGAATGCAAGAGAGGTTGAAGAGG | 294 | NM_174068.2 |
| R: AACACTCTCCAGAACACATGATCG | |||
| Peptidylprolyl isomerase A (PPIA) | F: CATACAGGTCCTGGCATCTTGTCC | 108 | NM_178320.2 |
| R: CACGTGCTTGCCATCCAACC | |||
| H3.3 histone A (H3F3A) | F: CATGGCTCGTACAAAGCAGA | 136 | NM_001014389.2 |
| R: ACCAGGCCTGTAACGATGAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ordóñez-León, E.A.; Martínez-Rodero, I.; García-Martínez, T.; López-Béjar, M.; Yeste, M.; Mercade, E.; Mogas, T. Exopolysaccharide ID1 Improves Post-Warming Outcomes after Vitrification of In Vitro-Produced Bovine Embryos. Int. J. Mol. Sci. 2022, 23, 7069. https://doi.org/10.3390/ijms23137069
Ordóñez-León EA, Martínez-Rodero I, García-Martínez T, López-Béjar M, Yeste M, Mercade E, Mogas T. Exopolysaccharide ID1 Improves Post-Warming Outcomes after Vitrification of In Vitro-Produced Bovine Embryos. International Journal of Molecular Sciences. 2022; 23(13):7069. https://doi.org/10.3390/ijms23137069
Chicago/Turabian StyleOrdóñez-León, Erika Alina, Iris Martínez-Rodero, Tania García-Martínez, Manel López-Béjar, Marc Yeste, Elena Mercade, and Teresa Mogas. 2022. "Exopolysaccharide ID1 Improves Post-Warming Outcomes after Vitrification of In Vitro-Produced Bovine Embryos" International Journal of Molecular Sciences 23, no. 13: 7069. https://doi.org/10.3390/ijms23137069
APA StyleOrdóñez-León, E. A., Martínez-Rodero, I., García-Martínez, T., López-Béjar, M., Yeste, M., Mercade, E., & Mogas, T. (2022). Exopolysaccharide ID1 Improves Post-Warming Outcomes after Vitrification of In Vitro-Produced Bovine Embryos. International Journal of Molecular Sciences, 23(13), 7069. https://doi.org/10.3390/ijms23137069








