Nitric Oxide Signalling in Descending Vasa Recta after Hypoxia/Re-Oxygenation
Abstract
1. Introduction
2. Results
2.1. Pharmacological Characterization of NO-sGC-System in Rat DVR
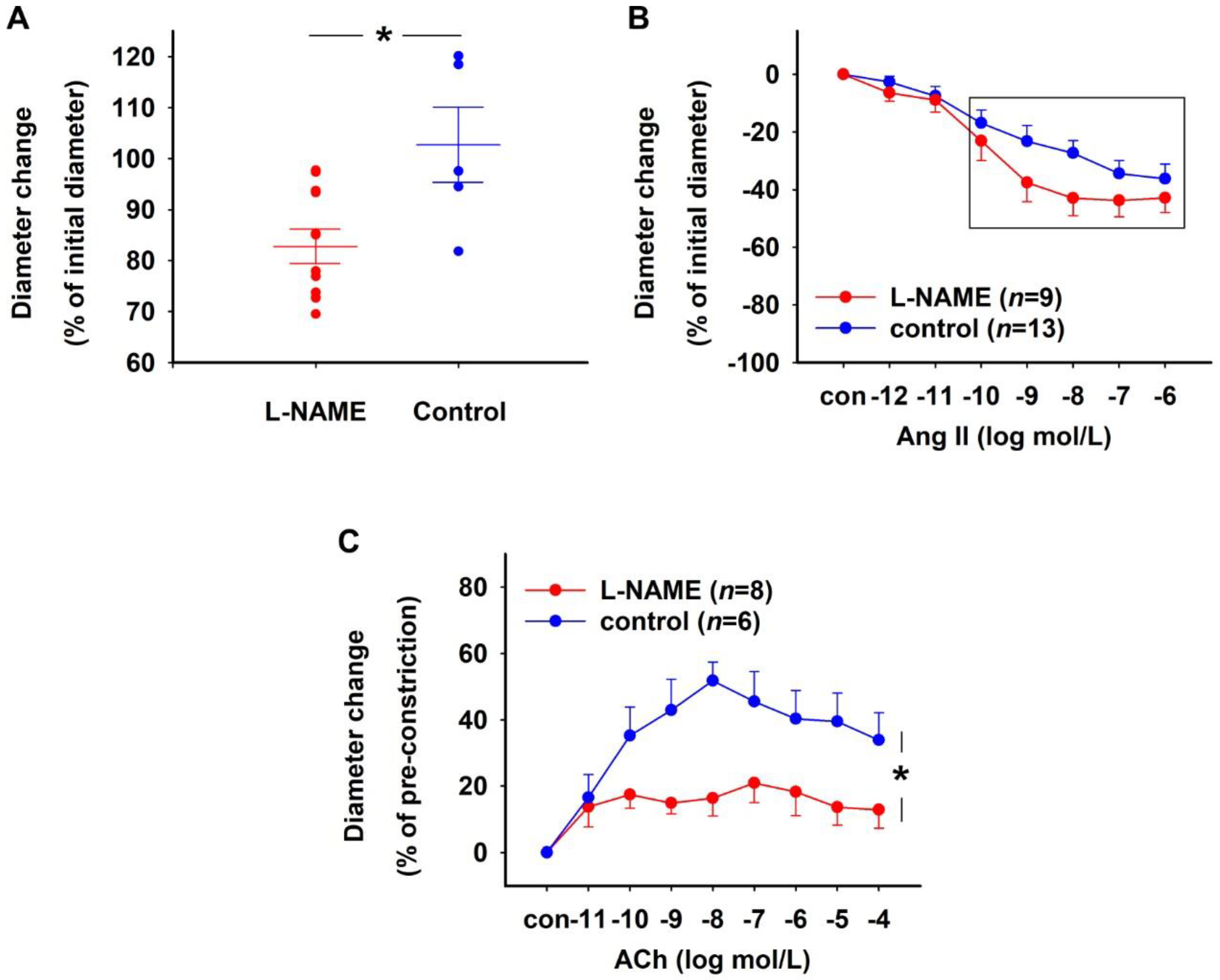
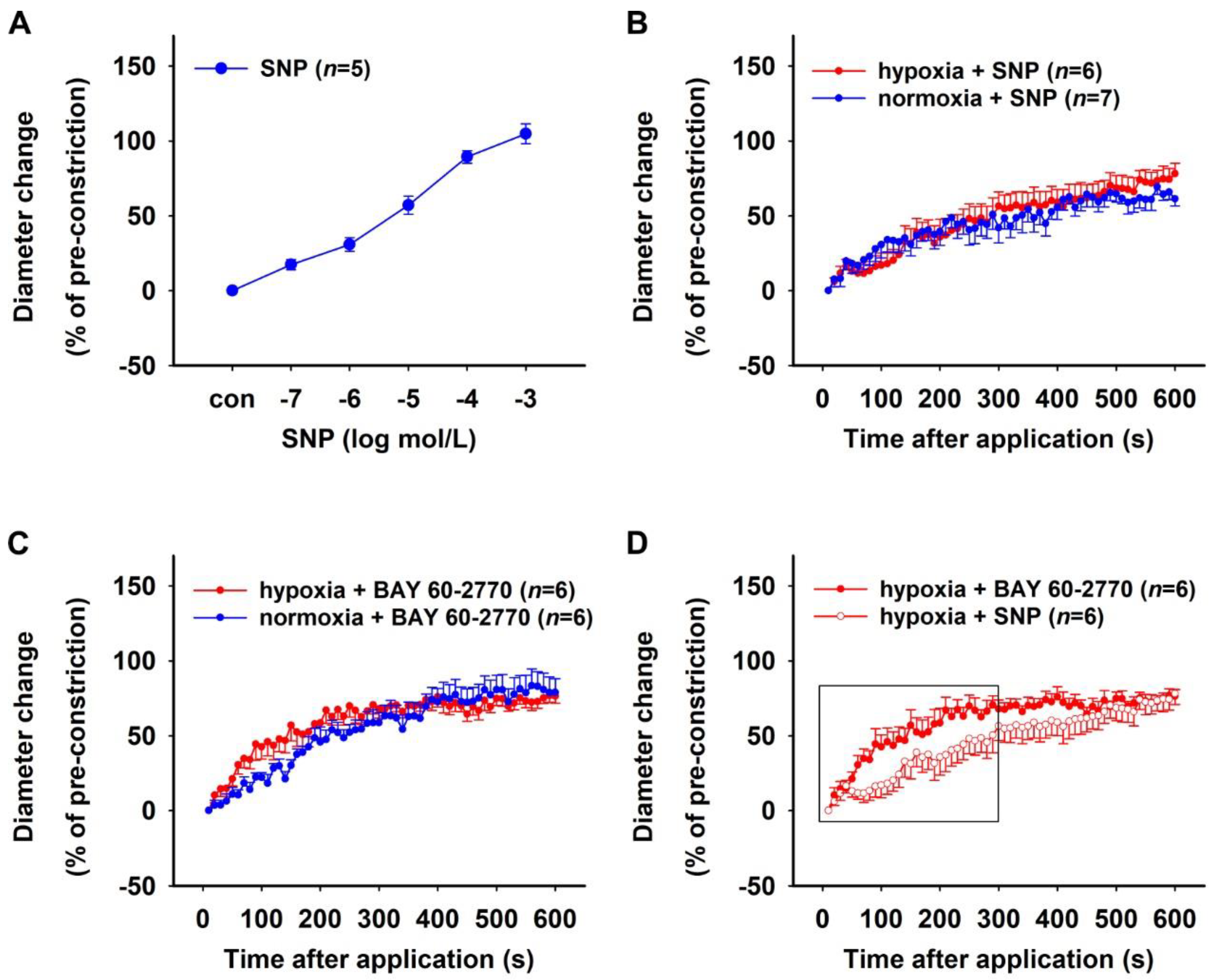
2.1.1. Effect of NOS Inhibition
2.1.2. Effect of PDE5 Inhibition
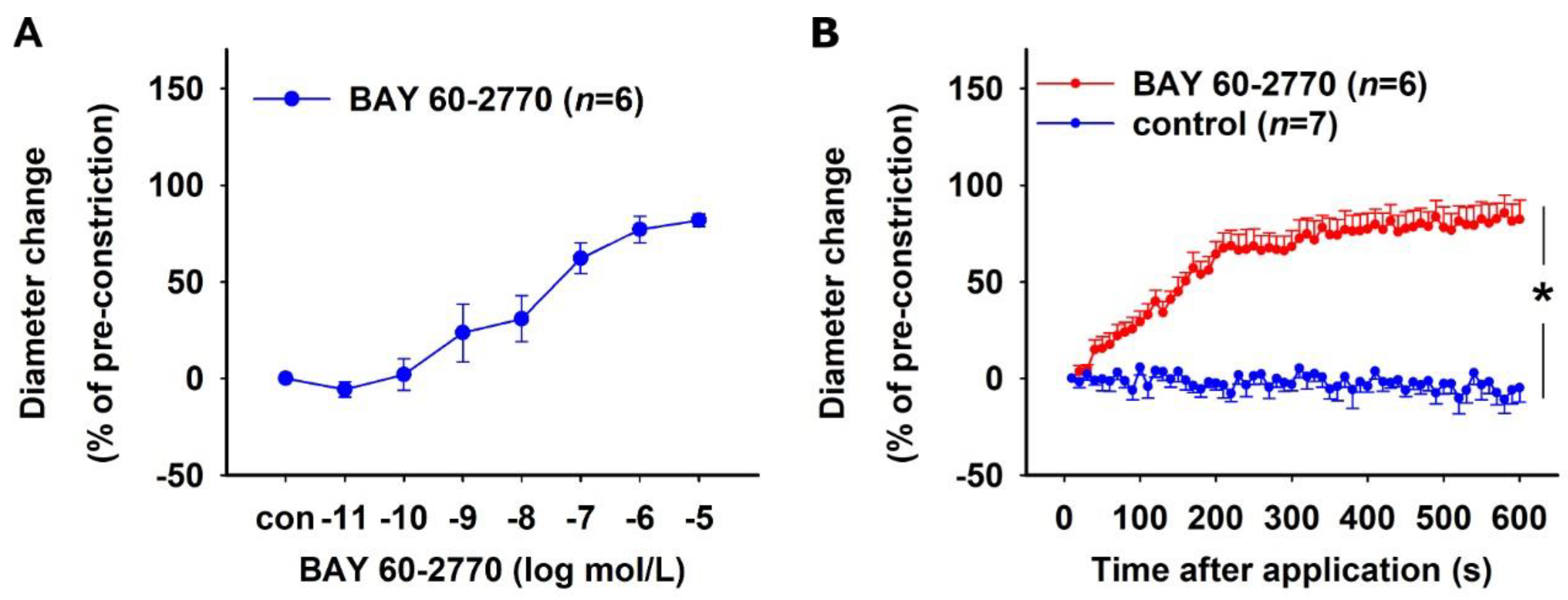
2.1.3. Effect of sGC Activation in NO-Deficient Vessels
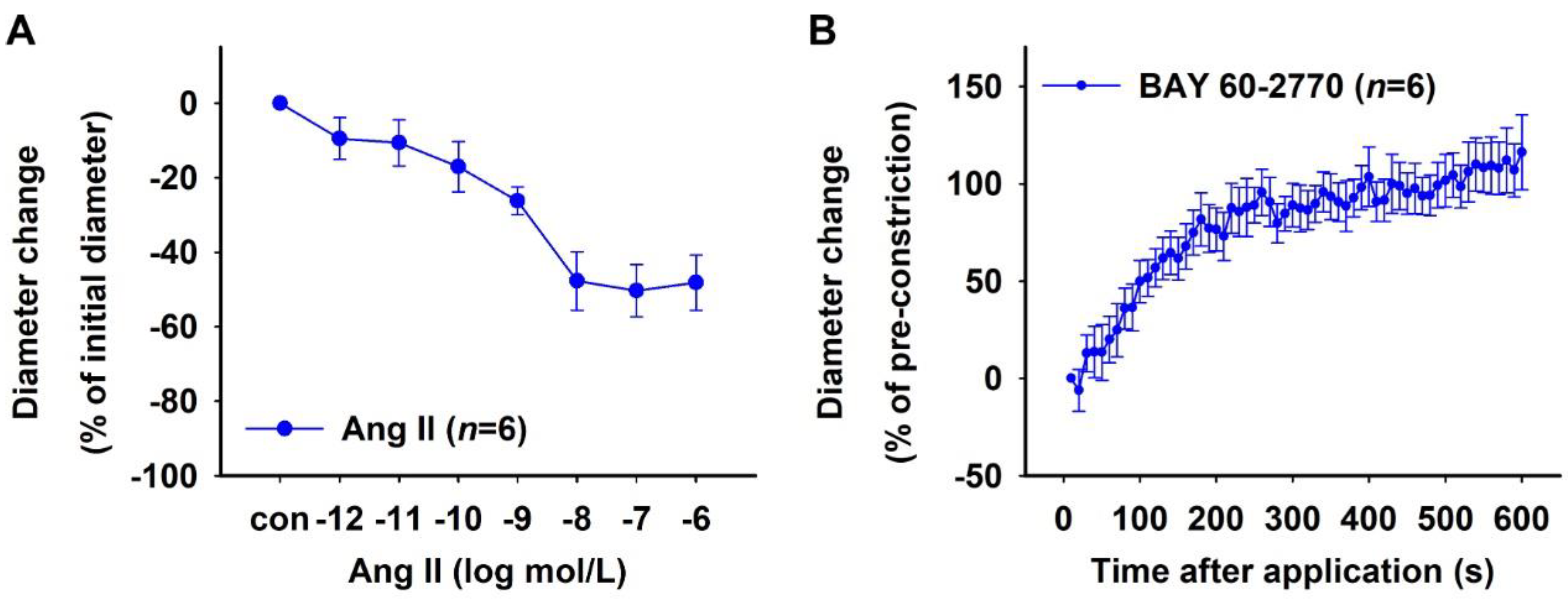
2.2. Characterization of Human DVR
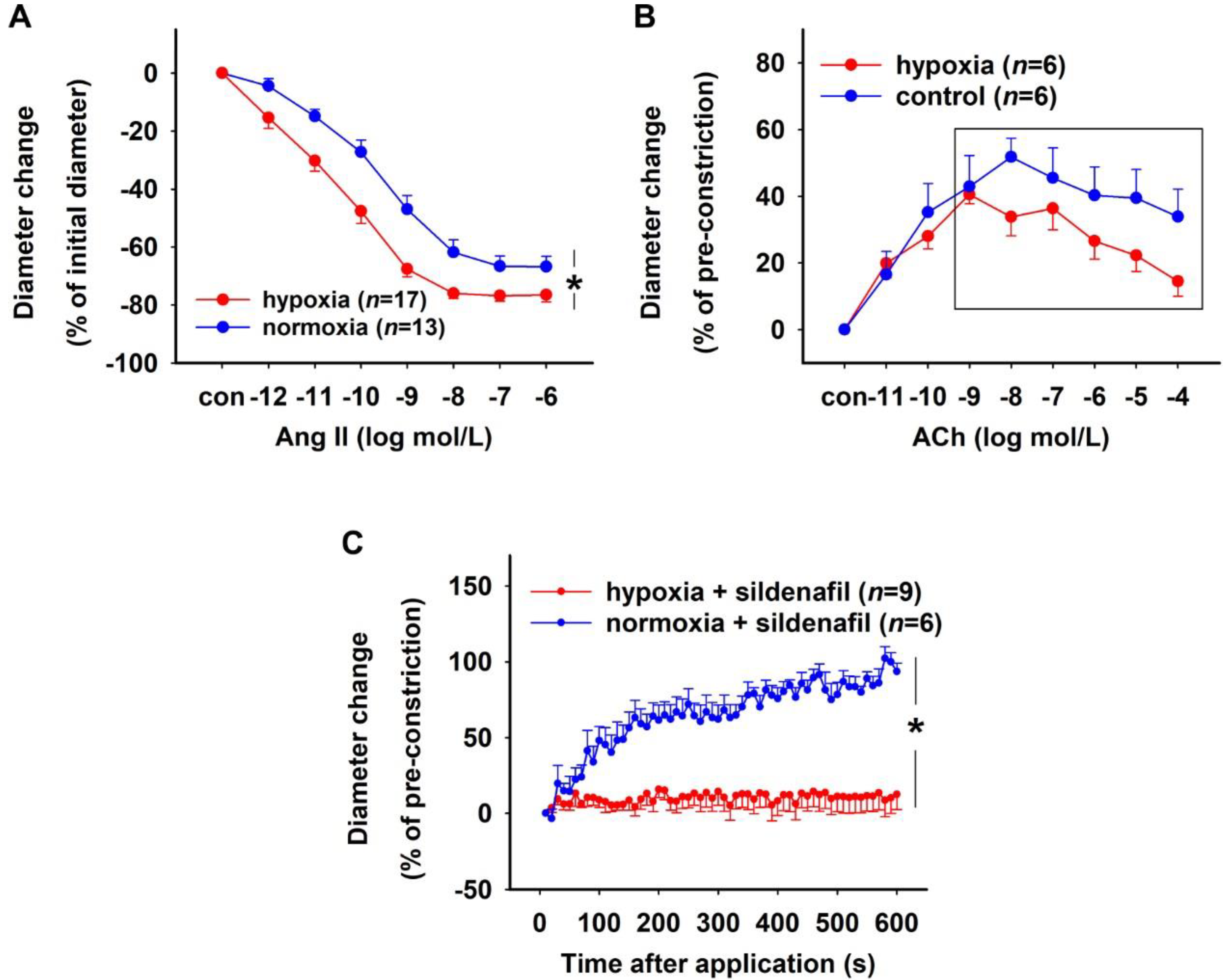
2.3. Effect of H/R on Rat DVR
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
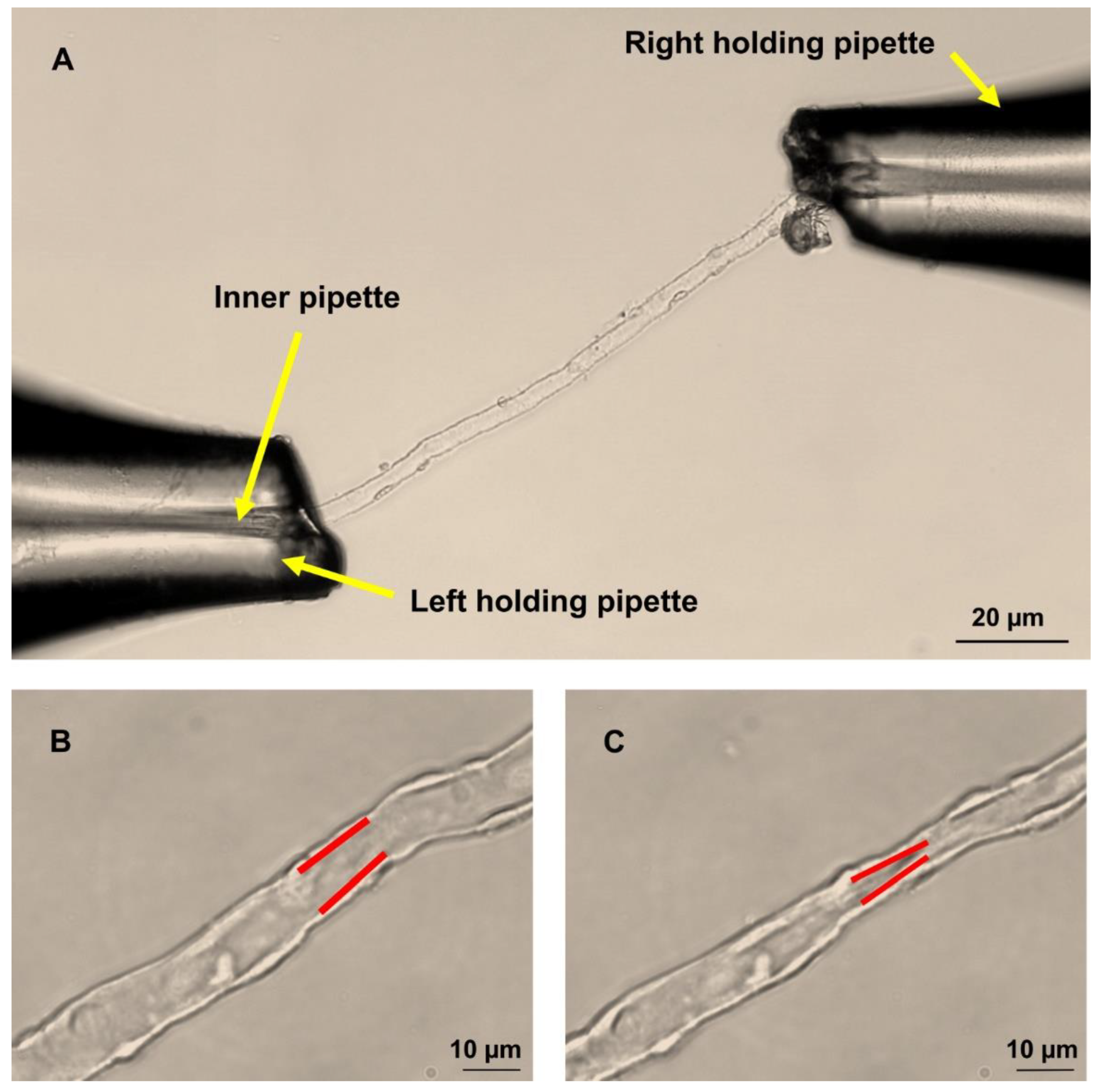
4.2. Dissection of DVR
4.3. Human DVR
4.4. Perfusion of Isolated DVR
4.5. Measurement of DVR Diameters
4.6. Protocols
4.6.1. Pharmacological Characterization of NO-sGC System
4.6.2. Human DVR
4.6.3. Effect of Hypoxia on the NO System
4.7. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kriz, W. Structural Organization of the Renal Medulla: Comparative and Functional Aspects. Am. J. Physiol. 1981, 241, R3–RS16. [Google Scholar] [CrossRef] [PubMed]
- Pallone, T.L.; Silldorff, E.P.; Turner, M.R. Intrarenal Blood Flow: Microvascular Anatomy and the Regulation of Medullary Perfusion. Clin. Exp. Pharmacol. Physiol. 1998, 25, 383–392. [Google Scholar] [CrossRef]
- Ren, H.; Gu, L.; Andreasen, A.; Thomsen, J.S.; Cao, L.; Christensen, E.I.; Zhai, X.Y. Spatial Organization of the Vascular Bundle and the Interbundle Region: Three-Dimensional Reconstruction at the Inner Stripe of the Outer Medulla in the Mouse Kidney. Am. J. Physiol. Ren. Physiol. 2014, 306, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Heyman, S.N.; Brezis, M.; Reubinoff, C.A.; Greenfeld, Z.; Lechene, G.; Epstein, F.H.; Rosen, S. Acute Renal Failure with Selective Medullary Injury in the Rat. J. Clin. Investig. 1988, 82, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Kennedy-Lydon, T.M.; Crawford, C.; Wildman, S.S.P.; Peppiatt-Wildman, C.M. Renal Pericytes: Regulators of Medullary Blood Flow. Acta Physiol. 2013, 207, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Pallone, T.L.; Edwards, A.; Mattson, D.L. Renal Medullary Circulation. Compr. Physiol. 2012, 2, 97–140. [Google Scholar] [CrossRef]
- Pallone, T.L.; Silldorff, E.P. Pericyte Regulation of Renal Medullary Blood Flow. Exp. Nephrol. 2001, 9, 165–170. [Google Scholar] [CrossRef]
- Crawford, C.; Wildman, S.S.P.; Kelly, M.C.; Kennedy-Lydon, T.M.; Peppiatt-Wildman, C.M. Sympathetic Nerve-Derived ATP Regulates Renal Medullary Vasa Recta Diameter via Pericyte Cells: A Role for Regulating Medullary Blood Flow? Front. Physiol. 2013, 4, 307. [Google Scholar] [CrossRef]
- Crawford, C.; Kennedy-Lydon, T.; Sprott, C.; Desai, T.; Sawbridge, L.; Munday, J.; Unwin, R.J.; Wildman, S.S.P.; Peppiatt-Wildman, C.M. An Intact Kidney Slice Model to Investigate Vasa Recta Properties and Function in Situ. Nephron Physiol. 2012, 120, 17–31. [Google Scholar] [CrossRef]
- Pohlmann, A.; Hentschel, J.; Fechner, M.; Hoff, U.; Bubalo, G.; Arakelyan, K.; Cantow, K.; Seeliger, E.; Flemming, B.; Waiczies, H.; et al. High Temporal Resolution Parametric MRI Monitoring of the Initial Ischemia/Reperfusion Phase in Experimental Acute Kidney Injury. PLoS ONE 2013, 8, e57411. [Google Scholar] [CrossRef]
- Cantow, K.; Arakelyan, K.; Seeliger, E.; Niendorf, T.; Pohlmann, A. Assessment of Renal Hemodynamics and Oxygenation by Simultaneous Magnetic Resonance Imaging (MRI) and Quantitative Invasive Physiological Measurements. In Kidney Research: Experimental Protocols; Hewitson, T.D., Smith, E.R., Holt, S.G., Eds.; Springer: New York, NY, USA, 2016; pp. 129–154. ISBN 978-1-4939-3353-2. [Google Scholar]
- Moore, J.K.; Chen, J.; Pan, H.; Gaut, J.P.; Jain, S.; Wickline, S.A. Quantification of Vascular Damage in Acute Kidney Injury with Fluorine Magnetic Resonance Imaging and Spectroscopy. Magn. Reson. Med. 2018, 79, 3144–3153. [Google Scholar] [CrossRef] [PubMed]
- Emans, T.W.; Janssen, B.J.; Joles, J.A.; Krediet, C.T.P. Nitric Oxide Synthase Inhibition Induces Renal Medullary Hypoxia in Conscious Rats. J. Am. Heart Assoc. 2018, 7, e009501. [Google Scholar] [CrossRef] [PubMed]
- Baumann, J.E.; Persson, P.B.; Ehmke, H.; Nafz, B.; Kirchheim, H.R. Role of Endothelium-Derived Relaxing Factor in Renal Autoregulation in Conscious Dogs. Am. J. Physiol. 1992, 263, F208–F213. [Google Scholar] [CrossRef] [PubMed]
- Stegbauer, J.; Kuczka, Y.; Vonend, O.; Quack, I.; Sellin, L.; Patzak, A.; Steege, A.; Langnaese, K.; Rump, L.C. Endothelial Nitric Oxide Synthase Is Predominantly Involved in Angiotensin II Modulation of Renal Vascular Resistance and Norepinephrine Release. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R421–R428. [Google Scholar] [CrossRef]
- Patzak, A.; Mrowka, R.; Storch, E.; Hocher, B.; Persson, P.B. Interaction of Angiotensin II and Nitric Oxide in Isolated Perfused Afferent Arterioles of Mice. J. Am. Soc. Nephrol. 2001, 12, 1122–1127. [Google Scholar] [CrossRef]
- Hu, L.; Sealey, J.E.; Chen, R.; Zhou, Y.; Merali, C.; Shi, Y.; Laragh, J.H.; Catanzaro, D.F. Nitric Oxide Synthase Inhibition Accelerates the Pressor Response to Low-Dose Angiotensin II, Exacerbates Target Organ Damage, and Induces Renin Escape. Am. J. Hypertens. 2004, 17, 395–403. [Google Scholar] [CrossRef][Green Version]
- Navarro-Cid, J.; Maeso, R.; Rodrigo, E.; Muñoz-García, R.; Ruilope, L.M.; Lahera, V.; Cachofeiro, V. Renal and Vascular Consequences of the Chronic Nitric Oxide Synthase Inhibition: Effects of Antihypertensive Drugs. Am. J. Hypertens. 1996, 9, 1077–1083. [Google Scholar] [CrossRef]
- Araujo, M.; Welch, W.J. Oxidative Stress and Nitric Oxide in Kidney Function. Curr. Opin. Nephrol. Hypertens. 2006, 15, 72–77. [Google Scholar] [CrossRef]
- Breitenstein, S.; Roessig, L.; Sandner, P.; Lewis, K.S. Novel SGC Stimulators and SGC Activators for the Treatment of Heart Failure. Handb. Exp. Pharmacol. 2017, 243, 225–247. [Google Scholar] [CrossRef]
- Stasch, J.P.; Schlossmann, J.; Hocher, B. Renal Effects of Soluble Guanylate Cyclase Stimulators and Activators: A Review of the Preclinical Evidence. Curr. Opin. Pharmacol. 2015, 21, 95–104. [Google Scholar] [CrossRef]
- Sandner, P.; Zimmer, D.P.; Milne, G.T.; Follmann, M.; Hobbs, A.; Stasch, J.-P. Soluble Guanylate Cyclase Stimulators and Activators. Handb. Exp. Pharmacol. 2021, 264, 355–394. [Google Scholar] [CrossRef] [PubMed]
- Stehle, D.; Xu, M.Z.; Schomber, T.; Hahn, M.G.; Schweda, F.; Feil, S.; Kraehling, J.R.; Eitner, F.; Patzak, A.; Sandner, P.; et al. Novel Soluble Guanylyl Cyclase Activators Increase Glomerular CGMP, Induce Vasodilation and Improve Blood Flow in the Murine Kidney. Br. J. Pharmacol. 2022, 179, 2476–2489. [Google Scholar] [CrossRef] [PubMed]
- Wennysia, I.C.; Zhao, L.; Schomber, T.; Braun, D.; Golz, S.; Summer, H.; Benardeau, A.; Lai, E.Y.; Lichtenberger, F.B.; Schubert, R.; et al. Role of Soluble Guanylyl Cyclase in Renal Afferent and Efferent Arterioles. Am. J. Physiol. Ren. Physiol. 2021, 320, F193–F202. [Google Scholar] [CrossRef] [PubMed]
- Pallone, T.L. Microdissected Perfused Vessels. In Renal Disease: Techniques and Protocols; Goligorsky, M.S., Ed.; Humana Press: Totowa, NJ, USA, 2003; pp. 443–456. ISBN 978-1-59259-392-7. [Google Scholar]
- Zhang, Z.; Payne, K.; Pallone, T.L. Adaptive Responses of Rat Descending Vasa Recta to Ischemia. Am. J. Physiol. Renal Physiol. 2018, 314, F373–F380. [Google Scholar] [CrossRef]
- Sendeski, M.; Patzak, A.; Pallone, T.L.; Cao, C.; Persson, A.E.; Persson, P.B. Iodixanol, Constriction of Medullary Descending Vasa Recta, and Risk for Contrast Medium-Induced Nephropathy. Radiology 2009, 251, 697–704. [Google Scholar] [CrossRef]
- Sendeski, M.M.; Liu, Z.Z.; Perlewitz, A.; Busch, J.F.; Ikromov, O.; Weikert, S.; Persson, P.B.; Patzak, A. Functional Characterization of Isolated, Perfused Outermedullary Descending Human Vasa Recta. Acta Physiol. 2013, 208, 50–56. [Google Scholar] [CrossRef]
- Yang, S.; Silldorff, E.P.; Pallone, T.L. Effect of Norepinephrine and Acetylcholine on Outer Medullary Descending Vasa Recta. Am. J. Physiol. 1995, 269, H710–H716. [Google Scholar] [CrossRef]
- McDonald, L.J.; Murad, F. Nitric Oxide and Cyclic GMP Signaling. Proc. Soc. Exp. Biol. Med. 1996, 211, 1–6. [Google Scholar] [CrossRef]
- Dautzenberg, M.; Kahnert, A.; Stasch, J.-P.; Just, A. Role of Soluble Guanylate Cyclase in Renal Hemodynamics and Autoregulation in the Rat. Am. J. Physiol. Renal Physiol. 2014, 307, F1003–F1012. [Google Scholar] [CrossRef][Green Version]
- Wilck, N.; Markó, L.; Balogh, A.; Kräker, K.; Herse, F.; Bartolomaeus, H.; Szijártó, I.A.; Gollasch, M.; Reichhart, N.; Strauss, O.; et al. Nitric Oxide-Sensitive Guanylyl Cyclase Stimulation Improves Experimental Heart Failure with Preserved Ejection Fraction. JCI Insight 2018, 3, e96006. [Google Scholar] [CrossRef]
- Legrand, M.; Mik, E.G.; Johannes, T.; Payen, D.; Ince, C. Renal Hypoxia and Dysoxia after Reperfusion of the Ischemic Kidney. Mol. Med. 2008, 14, 502–516. [Google Scholar] [CrossRef] [PubMed]
- Freitas, F.; Attwell, D. Pericyte-Mediated Constriction of Renal Capillaries Evokes No-Reflow and Kidney Injury Following Ischaemia. Elife 2022, 11, e74211. [Google Scholar] [CrossRef] [PubMed]
- Cantow, K.; Flemming, B.; Ladwig-wiegard, M.; Persson, P.B. Low Dose Nitrite Improves Reoxygenation Following Renal Ischemia in Rats. Sci. Rep. 2017, 7, 14597. [Google Scholar] [CrossRef] [PubMed]
- Patzak, A.; Lai, E.Y.; Mrowka, R.; Steege, A.; Persson, P.B.; Persson, A.E.G. AT1 Receptors Mediate Angiotensin II-Induced Release of Nitric Oxide in Afferent Arterioles. Kidney Int. 2004, 66, 1949–1958. [Google Scholar] [CrossRef] [PubMed]
- Elias-Miró, M.; Jiménez-Castro, M.B.; Rodés, J.; Peralta, C. Current Knowledge on Oxidative Stress in Hepatic Ischemia/Reperfusion. Free Radic. Res. 2013, 47, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Just, A.; Olson, A.J.M.; Whitten, C.L.; Arendshorst, W.J. Superoxide Mediates Acute Renal Vasoconstriction Produced by Angiotensin II and Catecholamines by a Mechanism Independent of Nitric Oxide. Am. J. Physiol. Hear. Circ. Physiol. 2007, 292, H83–H92. [Google Scholar] [CrossRef]
- Carlström, M.; Lai, E.Y.; Ma, Z.; Patzak, A.; Brown, R.D.; Persson, A.E.G. Role of NOX2 in the Regulation of Afferent Arteriole Responsiveness. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R72–R79. [Google Scholar] [CrossRef]
- Braun, D.; Zollbrecht, C.; Dietze, S.; Schubert, R.; Golz, S.; Summer, H.; Persson, P.B.; Carlström, M.; Ludwig, M.; Patzak, A. Hypoxia/Reoxygenation of Rat Renal Arteries Impairs Vasorelaxation via Modulation of Endothelium-Independent SGC/CGMP/PKG Signaling. Front. Physiol. 2018, 9, 480. [Google Scholar] [CrossRef]
- Krishnan, S.M.; Kraehling, J.R.; Eitner, F.; Sandner, P.; Ignarro, L. The Impact of the Nitric Oxide (NO)/Soluble Guanylyl Cyclase (SGC) Signaling Cascade on Kidney Health and Disease: A Preclinical Perspective. Int. J. Mol. Sci. 2018, 19, 1712. [Google Scholar] [CrossRef]
- Braun, D.; Dietze, S.; Pahlitzsch, T.M.J.; Wennysia, I.C.; Persson, P.B.; Ludwig, M.; Patzak, A. Short-Term Hypoxia and Vasa Recta Function in Kidney Slices. Clin. Hemorheol. Microcirc. 2017, 67, 475–484. [Google Scholar] [CrossRef]
- Zhao, Y.; Brandish, P.E.; Di Valentin, M.; Schelvis, J.P.; Babcock, G.T.; Marletta, M.A. Inhibition of Soluble Guanylate Cyclase by ODQ. Biochemistry 2000, 39, 10848–10854. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014; Available online: http://www.r-project.org/ (accessed on 15 April 2022).

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Lichtenberger, F.-B.; Erdoǧan, C.; Lai, E.; Persson, P.B.; Patzak, A.; Khedkar, P.H. Nitric Oxide Signalling in Descending Vasa Recta after Hypoxia/Re-Oxygenation. Int. J. Mol. Sci. 2022, 23, 7016. https://doi.org/10.3390/ijms23137016
Xu M, Lichtenberger F-B, Erdoǧan C, Lai E, Persson PB, Patzak A, Khedkar PH. Nitric Oxide Signalling in Descending Vasa Recta after Hypoxia/Re-Oxygenation. International Journal of Molecular Sciences. 2022; 23(13):7016. https://doi.org/10.3390/ijms23137016
Chicago/Turabian StyleXu, Minze, Falk-Bach Lichtenberger, Cem Erdoǧan, Enyin Lai, Pontus B. Persson, Andreas Patzak, and Pratik H. Khedkar. 2022. "Nitric Oxide Signalling in Descending Vasa Recta after Hypoxia/Re-Oxygenation" International Journal of Molecular Sciences 23, no. 13: 7016. https://doi.org/10.3390/ijms23137016
APA StyleXu, M., Lichtenberger, F.-B., Erdoǧan, C., Lai, E., Persson, P. B., Patzak, A., & Khedkar, P. H. (2022). Nitric Oxide Signalling in Descending Vasa Recta after Hypoxia/Re-Oxygenation. International Journal of Molecular Sciences, 23(13), 7016. https://doi.org/10.3390/ijms23137016





