Metformin Attenuates UVA-Induced Skin Photoaging by Suppressing Mitophagy and the PI3K/AKT/mTOR Pathway
Abstract
:1. Introduction
2. Results
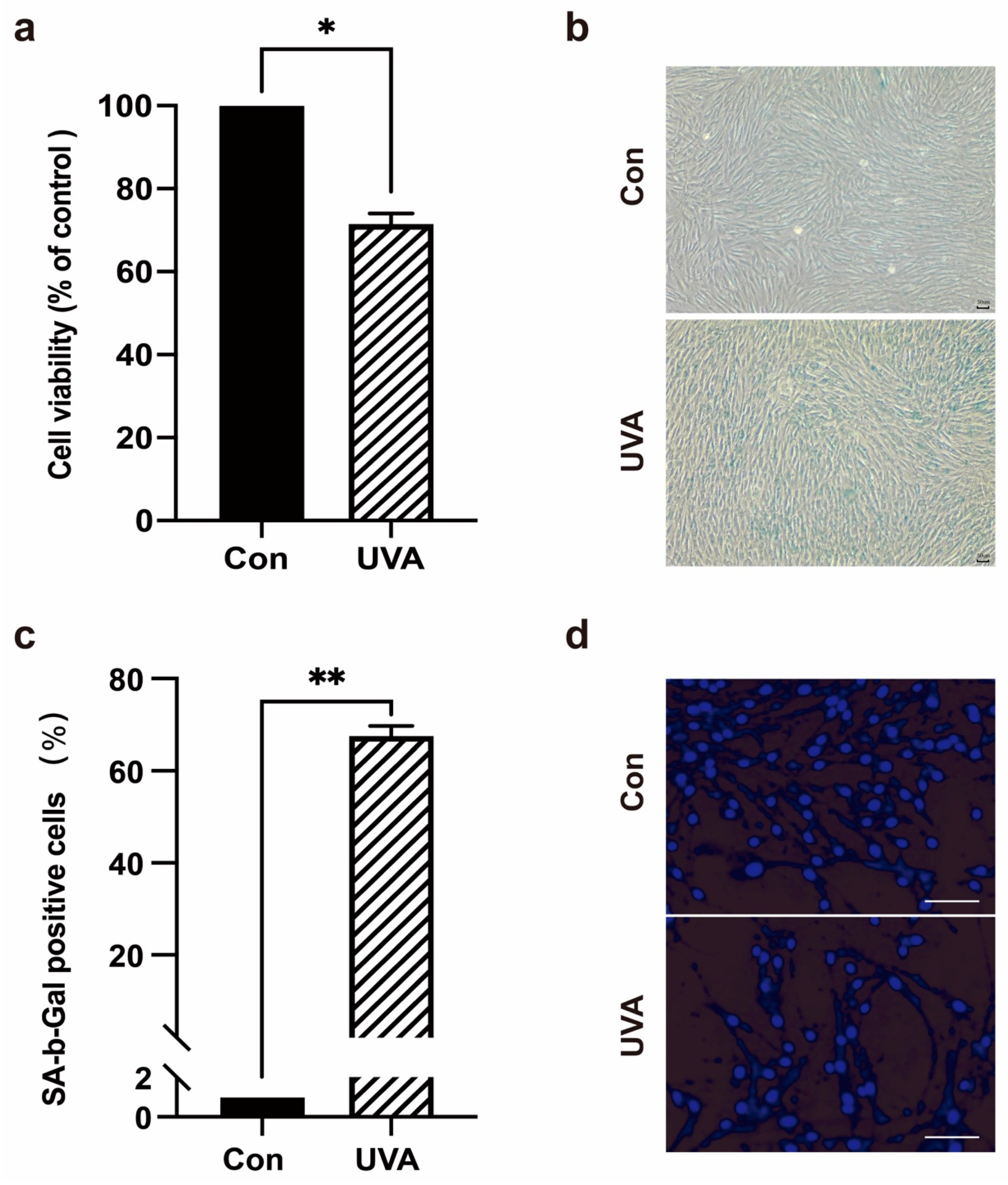
2.1. UVA Can Induce Photoaging in Foreskin Dermal Fibroblasts
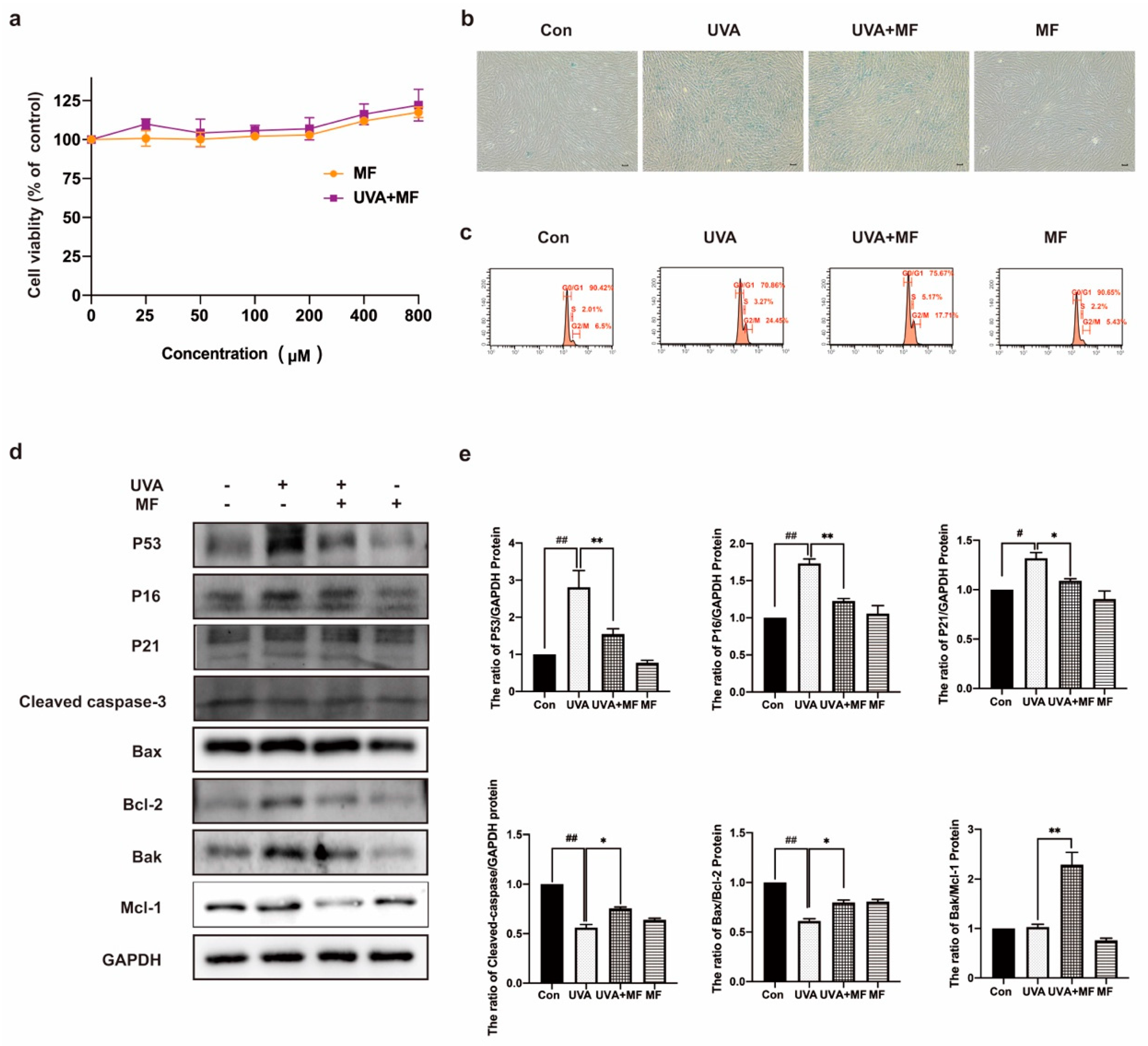
2.2. Metformin Can Ameliorate UVA-Induced Photoaging on HFFs
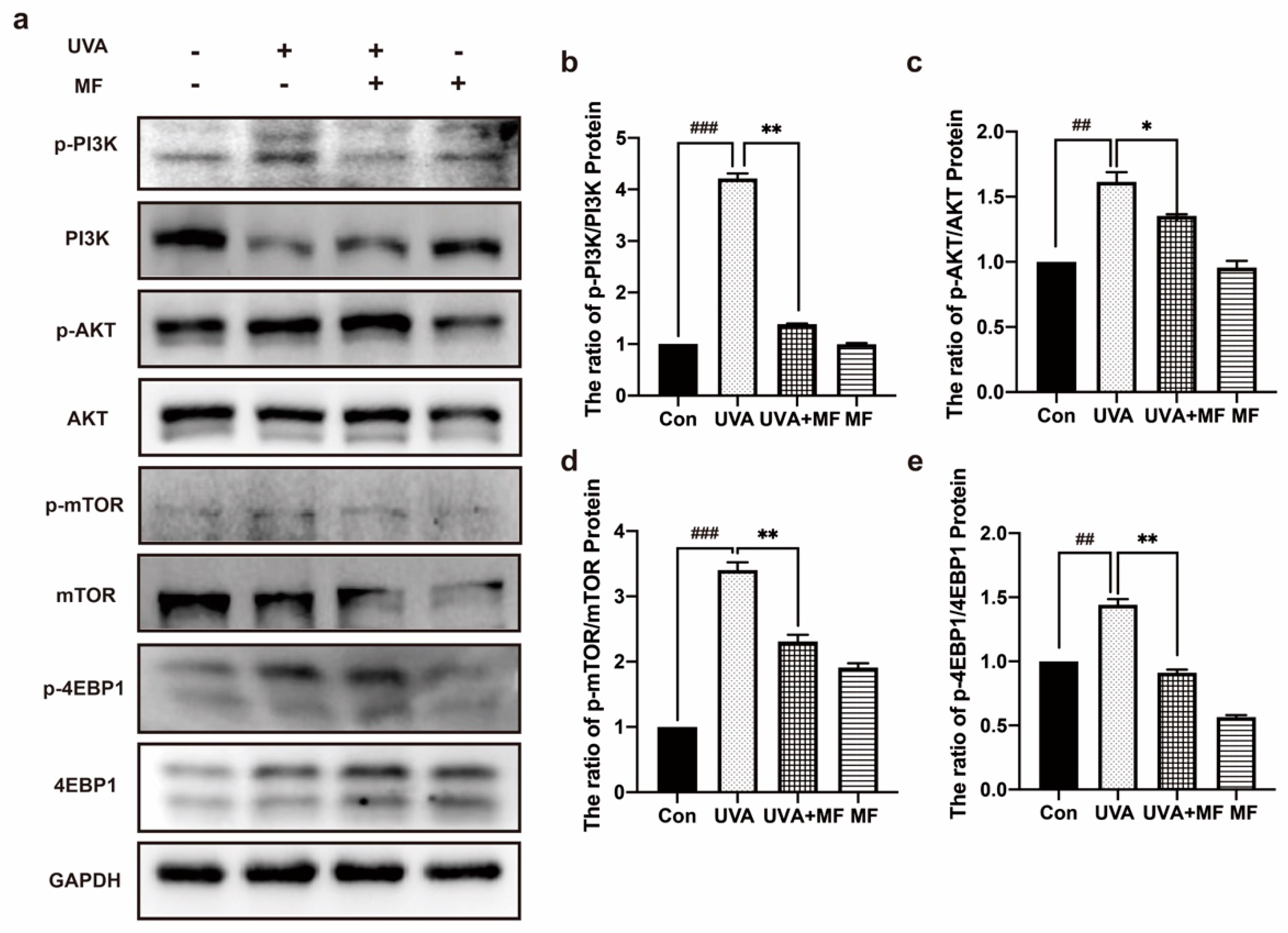
2.3. Metformin Inhibits PI3K/AKT/mTOR Signaling Pathway of HFFs
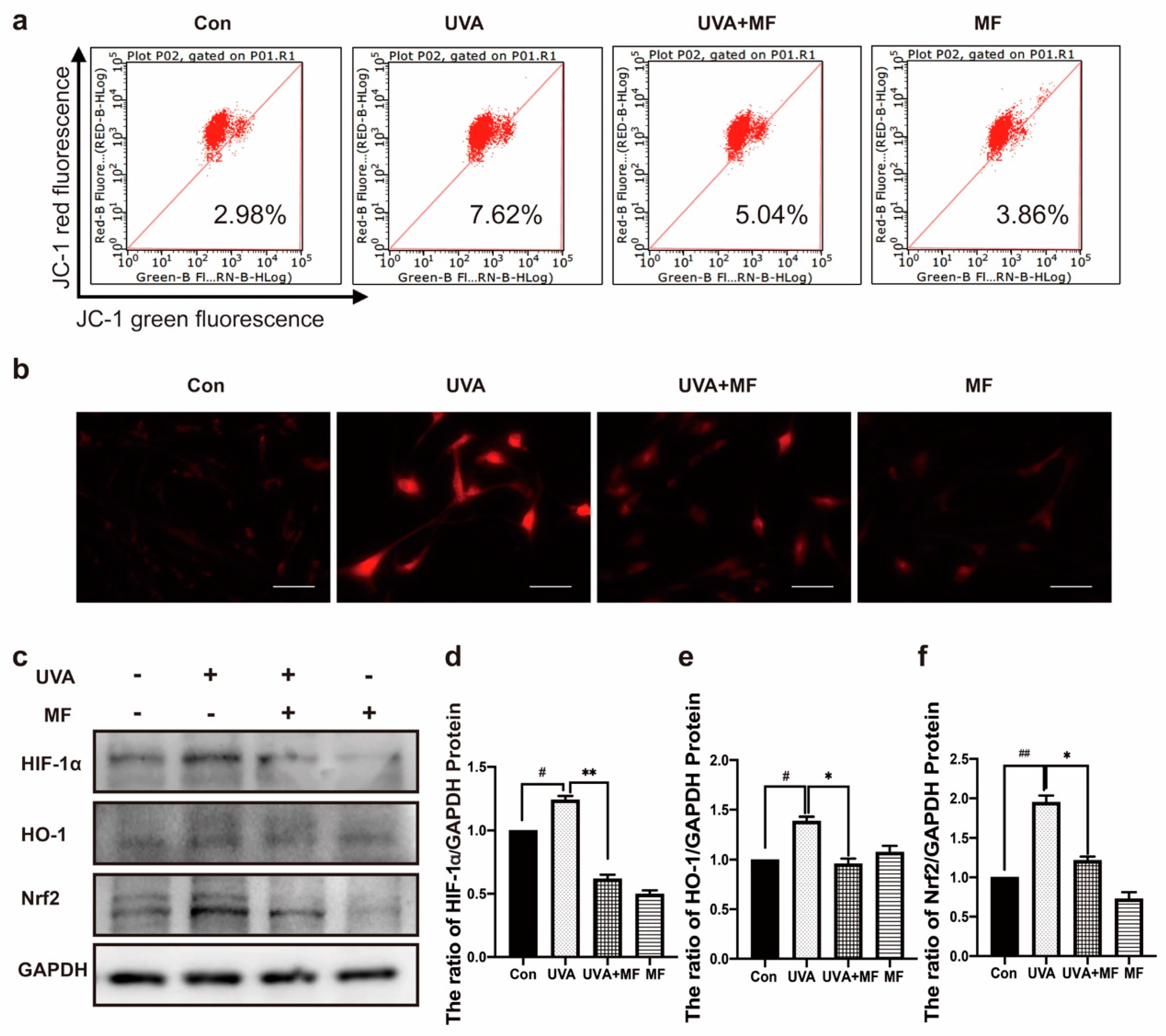
2.4. Metformin Reduces UVA-Induced Oxidative Stress
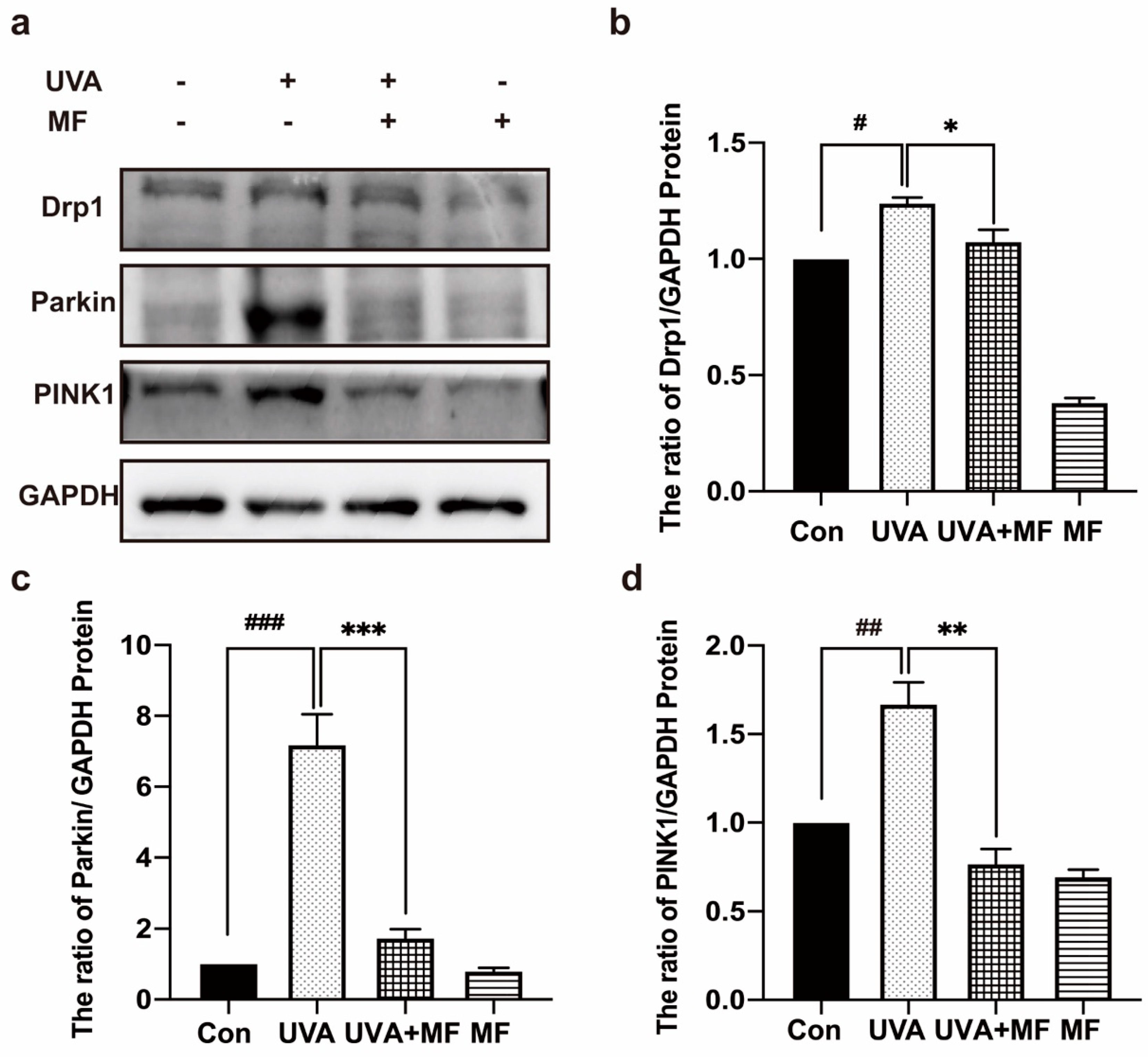
2.5. Metformin Can Reduce UVA-Induced Mitophagy in HFFs
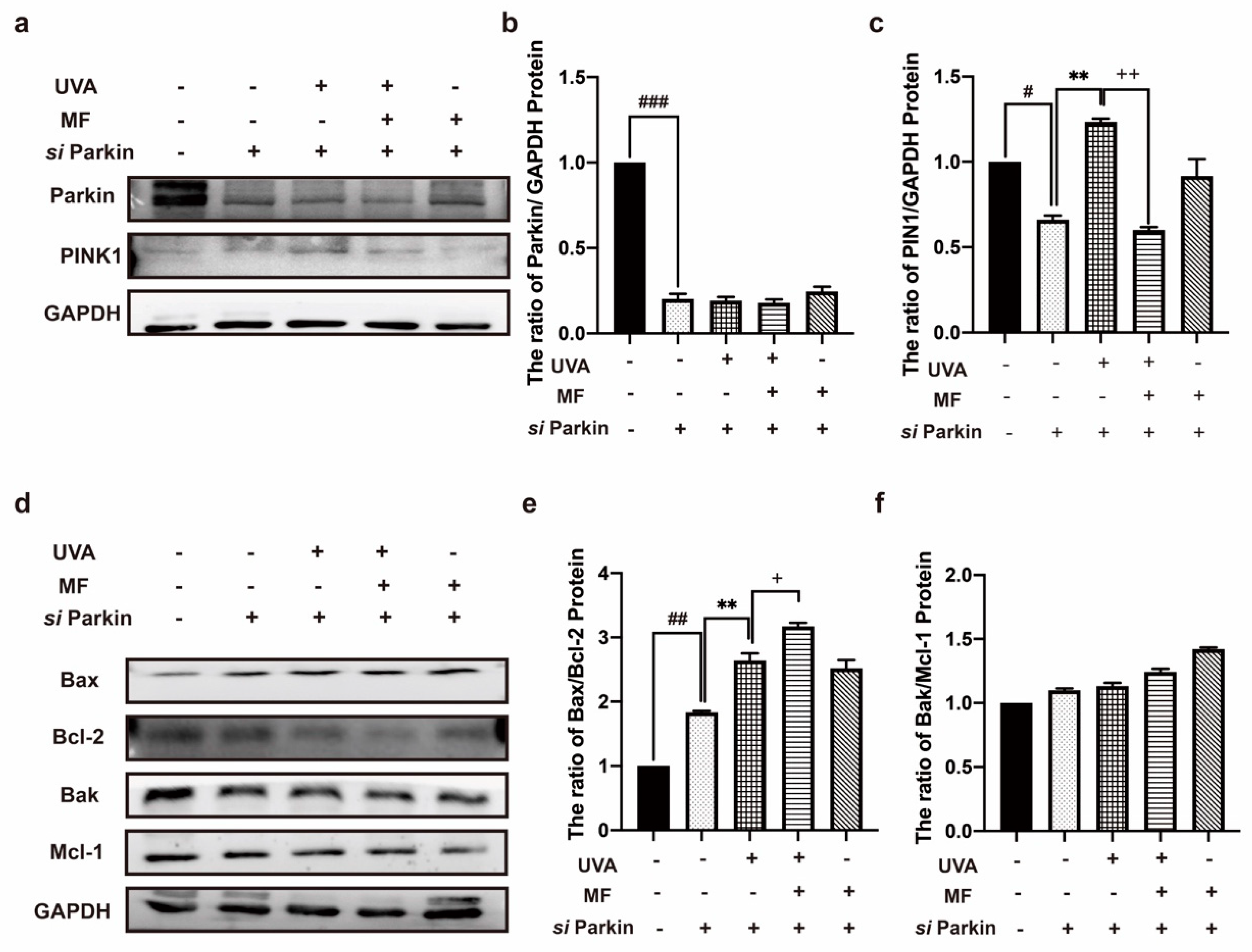
2.6. Alteration of Parkin Expression Can Regulate Apoptosis in HFF Cells after UVA Irradiation
2.7. Metformin Can Improve UVA-Induced Skin Photoaging in Mice
3. Discussion
4. Materials and Methods
4.1. Extraction and Culture of Foreskin Fibroblasts
4.2. Human Foreskin Fibroblasts Photoaging Model
4.3. Reagents and Antibodies
4.4. SA-β-Galactosidase Staining
4.5. Western Blotting Analysis
4.6. Nuclear Staining by Hoechst 33342
4.7. Cell Viability Assay
4.8. Cell Cycle Assay
4.9. Mitochondrial Membrane Potential (MMP) Assay
4.10. Mitochondrial Reactive Oxygen Species (ROS) Detection
4.11. siRNA Transient Transfection
4.12. Experiment Animals and Treatment Protocols
4.13. Hematoxylin and Eosin (HE) Staining
4.14. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ogrodnik, M. Cellular aging beyond cellular senescence: Markers of senescence prior to cell cycle arrest in vitro and in vivo. Aging Cell 2021, 20, e13338. [Google Scholar] [CrossRef] [PubMed]
- Summer, R.; Shaghaghi, H.; Schriner, D.; Roque, W.; Sales, D.; Cuevas-Mora, K.; Desai, V.; Bhushan, A.; Ramirez, M.I.; Romero, F. Activation of the mTORC1/PGC-1 axis promotes mitochondrial biogenesis and induces cellular senescence in the lung epithelium. Am. J. Physiol. Cell. Mol. Physiol. 2019, 316, L1049–L1060. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.C.; Aveleira, C.; Cavadas, C. Skin senescence: Mechanisms and impact on whole-body aging. Trends Mol. Med. 2022, 28, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.C.-S.; Lin, C.-L.; Yu, H.-S. Dermoscopic assessment of xerosis severity, pigmentation pattern and vascular morphology in subjects with physiological aging and photoaging. Eur. J. Dermatol. 2019, 29, 274–280. [Google Scholar] [PubMed]
- D’Uva, G.; Baci, D.; Albini, A.; Noonan, D.M. Cancer chemoprevention revisited: Cytochrome P450 family 1B1 as a target in the tumor and the microenvironment. Cancer Treat. Rev. 2018, 63, 1–18. [Google Scholar] [CrossRef]
- Banach, K.; Kowalska, J.; Rzepka, Z.; Beberok, A.; Rok, J.; Wrześniok, D. The role of UVA radiation in ketoprofen-mediated BRAF-mutant amelanotic melanoma cells death—A study at the cellular and molecular level. Toxicol. In Vitro Int. J. Publ. Assoc. BIBRA 2021, 72, 105108. [Google Scholar] [CrossRef]
- Karthikeyan, R.; Kanimozhi, G.; Prasad, N.R.; Agilan, B.; Ganesan, M.; Srithar, G. Alpha pinene modulates UVA-induced oxidative stress, DNA damage and apoptosis in human skin epidermal keratinocytes. Life Sci. 2018, 212, 150–158. [Google Scholar] [CrossRef]
- Salama, S.A.; Arab, H.; Omar, H.; Gad, H.S.; Abd-Allah, G.M.; Maghrabi, I.A.; Al Robaian, M.M. L-carnitine mitigates UVA-induced skin tissue injury in rats through downregulation of oxidative stress, p38/c-Fos signaling, and the proinflammatory cytokines. Chem. Biol. Interact. 2018, 285, 40–47. [Google Scholar] [CrossRef]
- Yagura, T.; Schuch, A.P.; Garcia, C.C.M.; Rocha, C.R.R.; Moreno, N.C.; Angeli, J.P.F.; Mendes, D.; Severino, D.; Sanchez, A.B.; Di Mascio, P.; et al. Direct participation of DNA in the formation of singlet oxygen and base damage under UVA irradiation. Free Radic. Biol. Med. 2017, 108, 86–93. [Google Scholar] [CrossRef]
- Xie, C.; Yi, J.; Lu, J.; Nie, M.; Huang, M.; Rong, J.; Zhu, Z.; Chen, J.; Zhou, X.; Li, B.; et al. N-Acetylcysteine Reduces ROS-Mediated Oxidative DNA Damage and PI3K/Akt Pathway Activation Induced by Infection. Oxidative Med. Cell. Longev. 2018, 2018, 1874985. [Google Scholar] [CrossRef] [Green Version]
- Karimian, A.; Mir, S.M.; Parsian, H.; Refieyan, S.; Mirza-Aghazadeh-Attari, M.; Yousefi, B.; Majidinia, M. Crosstalk between Phosphoinositide 3-kinase/Akt signaling pathway with DNA damage response and oxidative stress in cancer. J. Cell. Biochem. 2019, 120, 10248–10272. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Vassetzky, Y.; Dokudovskaya, S. mTORC1 pathway in DNA damage response. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 1293–1311. [Google Scholar] [CrossRef] [PubMed]
- Mohiuddin, M.; Kasahara, K. Paclitaxel Impedes EGFR-mutated PC9 Cell Growth via Reactive Oxygen Species-mediated DNA Damage and EGFR/PI3K/AKT/mTOR Signaling Pathway Suppression. Cancer Genom. Proteom. 2021, 18, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Alemi, F.; Sadigh, A.R.; Malakoti, F.; Elhaei, Y.; Ghaffari, S.H.; Maleki, M.; Asemi, Z.; Yousefi, B.; Targhazeh, N.; Majidinia, M. Molecular mechanisms involved in DNA repair in human cancers: An overview of PI3k/Akt signaling and PIKKs crosstalk. J. Cell. Physiol. 2022, 237, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Barzegari, A.; Dehnad, A.; Barar, J.; Omidi, Y. Astaxanthin protects mesenchymal stem cells from oxidative stress by direct scavenging of free radicals and modulation of cell signaling. Chem. Biol. Interact. 2021, 333, 109324. [Google Scholar] [CrossRef]
- Barodia, S.K.; Creed, R.B.; Goldberg, M.S. Parkin and PINK1 functions in oxidative stress and neurodegeneration. Brain Res. Bull. 2017, 133, 51–59. [Google Scholar] [CrossRef]
- Wu, Q.; Gao, C.; Wang, H.; Zhang, X.; Li, Q.; Gu, Z.; Shi, X.; Cui, Y.; Wang, T.; Chen, X.; et al. Mdivi-1 alleviates blood-brain barrier disruption and cell death in experimental traumatic brain injury by mitigating autophagy dysfunction and mitophagy activation. Int. J. Biochem. Cell Biol. 2018, 94, 44–55. [Google Scholar] [CrossRef]
- Kulkarni, A.S.; Gubbi, S.; Barzilai, N. Benefits of Metformin in Attenuating the Hallmarks of Aging. Cell Metab. 2020, 32, 15–30. [Google Scholar] [CrossRef]
- Sunjaya, A.P.; Sunjaya, A.F. Targeting ageing and preventing organ degeneration with metformin. Diabetes Metab. 2021, 47, 101203. [Google Scholar] [CrossRef]
- Kim, H.; Yu, M.R.; Lee, H.; Kwon, S.H.; Jeon, J.S.; Han, D.C.; Noh, H. Metformin inhibits chronic kidney disease-induced DNA damage and senescence of mesenchymal stem cells. Aging Cell 2021, 20, e13317. [Google Scholar] [CrossRef]
- Song, Y.; Wu, Z.; Zhao, P. The Function of Metformin in Aging-Related Musculoskeletal Disorders. Front. Pharmacol. 2022, 13, 865524. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Yu, X.; Zou, Z.; Zheng, W.; Deng, X.; Guo, L.; Jiang, W.; Zhan, Q.; Lu, S.-H. Metformin suppresses the esophageal carcinogenesis in rats treated with NMBzA through inhibiting AMPK/mTOR signaling pathway. Carcinogenesis 2019, 40, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Gaddameedhi, S. Solar ultraviolet-induced DNA damage response: Melanocytes story in transformation to environmental melanomagenesis. Environ. Mol. Mutagen. 2020, 61, 736–751. [Google Scholar] [CrossRef]
- Zawrotniak, M.; Bartnicka, D.; Rapala-Kozik, M. UVA and UVB radiation induce the formation of neutrophil extracellular traps by human polymorphonuclear cells. J. Photochem. Photobiol. B Biol. 2019, 196, 111511. [Google Scholar] [CrossRef] [PubMed]
- Mimeault, M.; Batra, S.K. Recent advances on skin-resident stem/progenitor cell functions in skin regeneration, aging and cancers and novel anti-aging and cancer therapies. J. Cell. Mol. Med. 2010, 14, 116–134. [Google Scholar] [CrossRef] [Green Version]
- Favas, R.; Morone, J.; Martins, R.; Vasconcelos, V.; Lopes, G. Cyanobacteria Secondary Metabolites as Biotechnological Ingredients in Natural Anti-Aging Cosmetics: Potential to Overcome Hyperpigmentation, Loss of Skin Density and UV Radiation-Deleterious Effects. Mar. Drugs 2022, 20, 183. [Google Scholar] [CrossRef]
- Squier, T.C. Oxidative stress and protein aggregation during biological aging. Exp. Gerontol. 2001, 36, 1539–1550. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, B.; Zang, W.; Wang, X.; Liu, Z.; Li, W.; Jia, J. Resveratrol Inhibits the Growth of Gastric Cancer by Inducing G1 Phase Arrest and Senescence in a Sirt1-Dependent Manner. PLoS ONE 2013, 8, e70627. [Google Scholar] [CrossRef]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; di Fagagna, F.D. Cellular senescence in ageing: From mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef]
- Ma, C.; Sun, Y.; Pi, C.; Wang, H.; Sun, H.; Yu, X.; Shi, Y.; He, X. Sirt3 Attenuates Oxidative Stress Damage and Rescues Cellular Senescence in Rat Bone Marrow Mesenchymal Stem Cells by Targeting Superoxide Dismutase 2. Front. Cell Dev. Biol. 2020, 8, 599376. [Google Scholar] [CrossRef]
- Swalwell, H.; Latimer, J.; Haywood, R.M.; Birch-Machin, M.A. Investigating the role of melanin in UVA/UVB- and hydrogen peroxide-induced cellular and mitochondrial ROS production and mitochondrial DNA damage in human melanoma cells. Free Radic. Biol. Med. 2012, 52, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Shikata, M.; Toyooka, T.; Komaki, Y.; Ibuki, Y. 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone-Induced Histone Acetylation via α7nAChR-Mediated PI3K/Akt Activation and Its Impact on γ-H2AX Generation. Chem. Res. Toxicol. 2021, 34, 2512–2521. [Google Scholar] [CrossRef] [PubMed]
- Demel, H.-R.; Feuerecker, B.; Piontek, G.; Seidl, C.; Blechert, B.; Pickhard, A.; Essler, M. Effects of topoisomerase inhibitors that induce DNA damage response on glucose metabolism and PI3K/Akt/mTOR signaling in multiple myeloma cells. Am. J. Cancer Res. 2015, 5, 1649–1664. [Google Scholar] [PubMed]
- Hwang, H.-T.V.; Lin, Y.; Rebuffatti, M.N.; Tran, D.T.; Lee, L.; Gomes, A.V.; Li, C.-S.; Knowlton, A.A. Impaired proteostasis in senescent vascular endothelial cells: A perspective on estrogen and oxidative stress in the aging vasculature. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H421–H429. [Google Scholar] [CrossRef]
- Jandova, J.; Janda, J.; Sligh, J.E. Cyclophilin 40 alters UVA-induced apoptosis and mitochondrial ROS generation in keratinocytes. Exp. Cell Res. 2013, 319, 750–760. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, T.; Zhou, Y.; Wang, S.; Zhou, X.; Wang, L.; Ou, T.; Chen, Y.; Zhou, Y.; Zhang, H.; et al. Carnitine palmitoyltransferase 1C contributes to progressive cellular senescence. Aging 2020, 12, 6733–6755. [Google Scholar] [CrossRef]
- Mengus, C.; Neutzner, M.; Bento, A.C.P.F.; Bippes, C.C.; Kohler, C.; Decembrini, S.; Häusel, J.; Hemion, C.; Sironi, L.; Frank, S.; et al. VCP/p97 cofactor UBXN1/SAKS1 regulates mitophagy by modulating MFN2 removal from mitochondria. Autophagy 2022, 18, 171–190. [Google Scholar] [CrossRef]
- Ko, H.-J.; Tsai, C.-Y.; Chiou, S.-J.; Lai, Y.-L.; Wang, C.-H.; Cheng, J.-T.; Chuang, T.-H.; Huang, C.-Y.; Kwan, A.-L.; Loh, J.-K.; et al. The Phosphorylation Status of Drp1-Ser637 by PKA in Mitochondrial Fission Modulates Mitophagy via PINK1/Parkin to Exert Multipolar Spindles Assembly during Mitosis. Biomolecules 2021, 11, 424. [Google Scholar] [CrossRef]
- Barazzuol, L.; Giamogante, F.; Brini, M.; Calì, T. PINK1/Parkin Mediated Mitophagy, Ca2+ Signalling, and ER–Mitochondria Contacts in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 1772. [Google Scholar] [CrossRef] [Green Version]
- Sarraf, S.A.; Sideris, D.P.; Giagtzoglou, N.; Ni, L.; Kankel, M.W.; Sen, A.; Bochicchio, L.E.; Huang, C.-H.; Nussenzweig, S.C.; Worley, S.H.; et al. PINK1/Parkin Influences Cell Cycle by Sequestering TBK1 at Damaged Mitochondria, Inhibiting Mitosis. Cell Rep. 2019, 29, 225–235.e5. [Google Scholar] [CrossRef] [Green Version]
- Lyu, J.-L.; Liu, Y.-J.; Wen, K.-C.; Chiu, C.-Y.; Lin, Y.-H.; Chiang, H.-M. Protective Effect of Djulis (Chenopodium formosanum) Extract against UV- and AGEs-Induced Skin Aging via Alleviating Oxidative Stress and Collagen Degradation. Molecules 2022, 27, 2332. [Google Scholar] [CrossRef] [PubMed]
- Shih, M.-F.; Cherng, J.-Y. Potential protective effect of fresh grown unicellular green algae component (resilient factor) against PMA- and UVB-induced MMP1 expression in skin fibroblasts. Eur. J. Dermatol. 2008, 18, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Heiden, M.G.V.; McCormick, F. The metabolic landscape of RAS-driven cancers from biology to therapy. Nat. Cancer 2021, 2, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Wang, X.-J. TGFβ Signaling in Photoaging and UV-Induced Skin Cancer. J. Investig. Dermatol. 2021, 141, 1104–1110. [Google Scholar] [CrossRef]
- Marks, D.; Belov, L.; Davey, M.W.; Davey, R.A.; Kidman, A.D. The MTT cell viability assay for cytotoxicity testing in multidrug-resistant human leukemic cells. Leuk. Res. 1992, 16, 1165–1173. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Zhang, H.; Yang, Y.; Zhang, S.; Wang, J.; Zhang, D.; Yu, H. Metformin Attenuates UVA-Induced Skin Photoaging by Suppressing Mitophagy and the PI3K/AKT/mTOR Pathway. Int. J. Mol. Sci. 2022, 23, 6960. https://doi.org/10.3390/ijms23136960
Chen Q, Zhang H, Yang Y, Zhang S, Wang J, Zhang D, Yu H. Metformin Attenuates UVA-Induced Skin Photoaging by Suppressing Mitophagy and the PI3K/AKT/mTOR Pathway. International Journal of Molecular Sciences. 2022; 23(13):6960. https://doi.org/10.3390/ijms23136960
Chicago/Turabian StyleChen, Qiuyan, Haiying Zhang, Yimeng Yang, Shuming Zhang, Jing Wang, Dawei Zhang, and Huimei Yu. 2022. "Metformin Attenuates UVA-Induced Skin Photoaging by Suppressing Mitophagy and the PI3K/AKT/mTOR Pathway" International Journal of Molecular Sciences 23, no. 13: 6960. https://doi.org/10.3390/ijms23136960
APA StyleChen, Q., Zhang, H., Yang, Y., Zhang, S., Wang, J., Zhang, D., & Yu, H. (2022). Metformin Attenuates UVA-Induced Skin Photoaging by Suppressing Mitophagy and the PI3K/AKT/mTOR Pathway. International Journal of Molecular Sciences, 23(13), 6960. https://doi.org/10.3390/ijms23136960





