Electroconvulsive Therapy in Psychiatric Disorders: A Narrative Review Exploring Neuroendocrine–Immune Therapeutic Mechanisms and Clinical Implications
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Molecular Mechanisms Involved in Electroconvulsive Therapy
3.1.1. Neurobiological Effects of Electroconvulsive Therapy
3.1.2. Impact of ECT on the Endocrine System
3.1.3. Effects of ECT on the Immune System
3.2. Unifying Neuroendocrine–Immune Hypothesis of ECT in Psychiatric Disorders
3.3. From Molecular Mechanisms to Clinical Evidence: Impact of ECT on Psychiatric Diseases
3.3.1. Refractory Major Depressive Disorder
3.3.2. Schizophrenia
3.3.3. Bipolar Disorder
3.3.4. Other Disorders
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fink, M. Meduna and the Origins of Convulsive Therapy. Am. J. Psychiatry 1984, 141, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Gazdag, G.; Ungvari, G.S.; Czech, H. Mass Killing under the Guise of ECT: The Darkest Chapter in the History of Biological Psychiatry. Hist. Psychiatry 2017, 28, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Duxbury, A.; Smith, I.; Mair-Edwards, B.; Bennison, G.; Irving, K.; Hodge, S.; Anderson, I.; Weatherhead, S. What Is the Process by Which a Decision to Administer Electroconvulsive Therapy (ECT) or Not Is Made? A Grounded Theory Informed Study of the Multi-Disciplinary Professionals Involved. Soc. Psychiatry Psychiatr. Epidemiol. 2018, 53, 785–793. [Google Scholar] [CrossRef]
- Chávez, M.E. Propuesta de protocolización para la práctica de la Terapia Electroconvulsiva en el Hospital de Clínicas de la Universidad Nacional de Asunción, Paraguay: Protocol’s proposal for the practice of Electroconvulsive Therapy at Hospital De Clínicas of the National University of Asunción, Paraguay. Med. Clín. Y Soc. 2017, 1, 126–142. [Google Scholar] [CrossRef]
- Cortez-Vergara, C.; Cruzado, L.; Rojas-Rojas, I.G.; Sánchez-Fernández, M.; Ladd-Huarachi, G. Características clínicas de pacientes tratados con terapia electroconvulsiva en un hospital público de Perú. Rev. Peru. Med. Exp. Salud Publica 2016, 33, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, V.; Li, A.; Oxley, C.; Waite, S.; De Felice, N.; Hadzi-Pavlovic, D.; Kumar, D.; Page, A.C.; Hooke, G.; Loo, C.K. Health Related Quality of Life after ECT for Depression: A Study Exploring the Role of Different Electrode-Placements and Pulse-Widths. J. Affect. Disord. 2016, 206, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Subedi, S.; Aich, T.K.; Sharma, N. Use of ECT in Nepal: A One Year Study from the Country’s Largest Psychiatric Facility. J. Clin. Diagn. Res. 2016, 10, VC01–VC04. [Google Scholar] [CrossRef]
- Bernardo i Arroyo, M.; Gonzalez-Pinto, A.; Urretavizcaya, M. Consenso Español Sobre la Terapia Electroconvulsiva; TEC: Madrid, Spain, 2018; ISBN 978-84-15734-12-3. [Google Scholar]
- Ueda, S.; Koyama, K.; Okubo, Y. Marked Improvement of Psychotic Symptoms after Electroconvulsive Therapy in Parkinson Disease. J. ECT 2010, 26, 111–115. [Google Scholar] [CrossRef]
- Trollor, J.N.; Sachdev, P.S. Electroconvulsive Treatment of Neuroleptic Malignant Syndrome: A Review and Report of Cases. Aust. N. Z. J. Psychiatry 1999, 33, 650–659. [Google Scholar] [CrossRef]
- Maletzky, B.; McFarland, B.; Burt, A. Refractory Obsessive Compulsive Disorder and ECT. Convuls. Ther. 1994, 10, 34–42. [Google Scholar]
- Margoob, M.A.; Ali, Z.; Andrade, C. Efficacy of ECT in Chronic, Severe, Antidepressant- and CBT-Refractory PTSD: An Open, Prospective Study. Brain Stimul. 2010, 3, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Hermida, A.P.; Glass, O.M.; Shafi, H.; McDonald, W.M. Electroconvulsive Therapy in Depression: Current Practice and Future Direction. Psychiatr. Clin. N. Am. 2018, 41, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, M.; Urretavizcaya, M. Dignificando una terapia electroconvulsiva basada en la evidencia. Rev. Psiquiatr. Salud Ment. 2015, 8, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmy, S.; Rolfes, V.; Grözinger, M.; Chikere, Y.; Schöttle, S.; Groß, D. Knowledge and Attitudes on Electroconvulsive Therapy in Germany: A Web Based Survey. Psychiatry Res. 2018, 262, 407–412. [Google Scholar] [CrossRef] [PubMed]
- van Diermen, L.; van den Ameele, S.; Kamperman, A.M.; Sabbe, B.C.G.; Vermeulen, T.; Schrijvers, D.; Birkenhäger, T.K. Prediction of Electroconvulsive Therapy Response and Remission in Major Depression: Meta-Analysis. Br. J. Psychiatry 2018, 212, 71–80. [Google Scholar] [CrossRef]
- Sienaert, P.; Peuskens, J. Electroconvulsive Therapy: An Effective Therapy of Medication-Resistant Bipolar Disorder. Bipolar. Disord. 2006, 8, 304–306. [Google Scholar] [CrossRef]
- Santos Pina, L.; Bouckaert, F.; Obbels, J.; Wampers, M.; Simons, W.; Wyckaert, S.; Sienaert, P. Maintenance Electroconvulsive Therapy in Severe Bipolar Disorder: A Retrospective Chart Review. J. ECT 2016, 32, 23–28. [Google Scholar] [CrossRef]
- Ahmed, S.; Khan, A.M.; Mekala, H.M.; Venigalla, H.; Ahmed, R.; Etman, A.; Esang, M.; Qureshi, M. Combined Use of Electroconvulsive Therapy and Antipsychotics (Both Clozapine and Non-Clozapine) in Treatment Resistant Schizophrenia: A Comparative Meta-Analysis. Heliyon 2017, 3, e00429. [Google Scholar] [CrossRef]
- Rosenquist, P.B.; Miller, B.; Pillai, A. The Antipsychotic Effects of ECT: A Review of Possible Mechanisms. J. ECT 2014, 30, 125–131. [Google Scholar] [CrossRef]
- Singh, A.; Kar, S.K. How Electroconvulsive Therapy Works? Understanding the Neurobiological Mechanisms. Clin. Psychopharmacol. Neurosci. 2017, 15, 210–221. [Google Scholar] [CrossRef]
- Baldinger, P.; Lotan, A.; Frey, R.; Kasper, S.; Lerer, B.; Lanzenberger, R. Neurotransmitters and Electroconvulsive Therapy. J. ECT 2014, 30, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Hirano, J.; Takamiya, A.; Yamagata, B.; Hotta, S.; Miyasaka, Y.; Pu, S.; Iwanami, A.; Uchida, H.; Mimura, M. Frontal and Temporal Cortical Functional Recovery after Electroconvulsive Therapy for Depression: A Longitudinal Functional near-Infrared Spectroscopy Study. J. Psychiatr. Res. 2017, 91, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Bolwig, T.G. Neuroimaging and Electroconvulsive Therapy: A Review. J. ECT 2014, 30, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Berggren, Å.; Gustafson, L.; Höglund, P.; Johanson, A. A Long-Term Follow-up of Clinical Response and Regional Cerebral Blood Flow Changes in Depressed Patients Treated with ECT. J. Affect. Disord. 2014, 167, 235–243. [Google Scholar] [CrossRef]
- Deng, Z.-D.; McClinctock, S.M.; Lisanby, S.H. Brain Network Properties in Depressed Patients Receiving Seizure Therapy: A Graph Theoretical Analysis of Peri-Treatment Resting EEG. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2015, 2015, 2203–2206. [Google Scholar] [CrossRef]
- Lythe, K.E.; Moll, J.; Gethin, J.A.; Workman, C.I.; Green, S.; Lambon Ralph, M.A.; Deakin, J.F.W.; Zahn, R. Self-Blame–Selective Hyperconnectivity Between Anterior Temporal and Subgenual Cortices and Prediction of Recurrent Depressive Episodes. JAMA Psychiatry 2015, 72, 1119–1126. [Google Scholar] [CrossRef]
- Leaver, A.M.; Wade, B.; Vasavada, M.; Hellemann, G.; Joshi, S.H.; Espinoza, R.; Narr, K.L. Fronto-Temporal Connectivity Predicts ECT Outcome in Major Depression. Front. Psychiatry 2018, 9, 92. [Google Scholar] [CrossRef]
- Joshi, S.H.; Espinoza, R.T.; Pirnia, T.; Shi, J.; Wang, Y.; Ayers, B.; Leaver, A.; Woods, R.P.; Narr, K.L. Structural Plasticity of the Hippocampus and Amygdala Induced by Electroconvulsive Therapy in Major Depression. Biol. Psychiatry 2016, 79, 282–292. [Google Scholar] [CrossRef]
- Oltedal, L.; Narr, K.L.; Abbott, C.; Anand, A.; Argyelan, M.; Bartsch, H.; Dannlowski, U.; Dols, A.; van Eijndhoven, P.; Emsell, L.; et al. Volume of the Human Hippocampus and Clinical Response Following Electroconvulsive Therapy. Biol. Psychiatry 2018, 84, 574–581. [Google Scholar] [CrossRef]
- Cao, B.; Luo, Q.; Fu, Y.; Du, L.; Qiu, T.; Yang, X.; Chen, X.; Chen, Q.; Soares, J.C.; Cho, R.Y.; et al. Predicting Individual Responses to the Electroconvulsive Therapy with Hippocampal Subfield Volumes in Major Depression Disorder. Sci. Rep. 2018, 8, 5434. [Google Scholar] [CrossRef]
- Gandy, K.; Kim, S.; Sharp, C.; Dindo, L.; Maletic-Savatic, M.; Calarge, C. Pattern Separation: A Potential Marker of Impaired Hippocampal Adult Neurogenesis in Major Depressive Disorder. Front. Neurosci. 2017, 11, 571. [Google Scholar] [CrossRef]
- Yun, S.; Reynolds, R.P.; Masiulis, I.; Eisch, A.J. Re-Evaluating the Link between Neuropsychiatric Disorders and Dysregulated Adult Neurogenesis. Nat. Med. 2016, 22, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.T.; Sanacora, G.; Bloch, M.H. Hippocampal Volume Changes Following Electroconvulsive Therapy: A Systematic Review and Meta-Analysis. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2017, 2, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Atzori, M.; Cuevas-Olguin, R.; Esquivel-Rendon, E.; Garcia-Oscos, F.; Salgado-Delgado, R.C.; Saderi, N.; Miranda-Morales, M.; Treviño, M.; Pineda, J.C.; Salgado, H. Locus Ceruleus Norepinephrine Release: A Central Regulator of CNS Spatio-Temporal Activation? Front. Synaptic Neurosci. 2016, 8, 25. [Google Scholar] [CrossRef]
- Maletic, V.; Eramo, A.; Gwin, K.; Offord, S.J.; Duffy, R.A. The Role of Norepinephrine and Its α-Adrenergic Receptors in the Pathophysiology and Treatment of Major Depressive Disorder and Schizophrenia: A Systematic Review. Front. Psychiatry 2017, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Landau, A.M.; Phan, J.-A.; Iversen, P.; Lillethorup, T.P.; Simonsen, M.; Wegener, G.; Jakobsen, S.; Doudet, D.J. Decreased in Vivo A2 Adrenoceptor Binding in the Flinders Sensitive Line Rat Model of Depression. Neuropharmacology 2015, 91, 97–102. [Google Scholar] [CrossRef]
- Lillethorup, T.P.; Iversen, P.; Wegener, G.; Doudet, D.J.M.; Landau, A.M. A2-Adrenoceptor Binding in Flinders-Sensitive Line Compared with Flinders-Resistant Line and Sprague-Dawley Rats. Acta Neuropsychiatr. 2015, 27, 345–352. [Google Scholar] [CrossRef]
- Madeira, C.; Vargas-Lopes, C.; Brandão, C.O.; Reis, T.; Laks, J.; Panizzutti, R.; Ferreira, S.T. Elevated Glutamate and Glutamine Levels in the Cerebrospinal Fluid of Patients with Probable Alzheimer’s Disease and Depression. Front. Psychiatry 2018, 9, 561. [Google Scholar] [CrossRef]
- Galińska-Skok, B.; Małus, A.; Konarzewska, B.; Rogowska-Zach, A.; Milewski, R.; Tarasów, E.; Szulc, A.; Waszkiewicz, N. Choline Compounds of the Frontal Lobe and Temporal Glutamatergic System in Bipolar and Schizophrenia Proton Magnetic Resonance Spectroscopy Study. Dis. Markers 2018, 2018, 3654894. [Google Scholar] [CrossRef]
- Njau, S.; Joshi, S.H.; Espinoza, R.; Leaver, A.M.; Vasavada, M.; Marquina, A.; Woods, R.P.; Narr, K.L. Neurochemical Correlates of Rapid Treatment Response to Electroconvulsive Therapy in Patients with Major Depression. J. Psychiatry Neurosci. 2017, 42, 6–16. [Google Scholar] [CrossRef]
- Cano, M.; Martínez-Zalacaín, I.; Bernabéu-Sanz, Á.; Contreras-Rodríguez, O.; Hernández-Ribas, R.; Via, E.; de Arriba-Arnau, A.; Gálvez, V.; Urretavizcaya, M.; Pujol, J.; et al. Brain Volumetric and Metabolic Correlates of Electroconvulsive Therapy for Treatment-Resistant Depression: A Longitudinal Neuroimaging Study. Transl. Psychiatry 2017, 7, e1023. [Google Scholar] [CrossRef] [PubMed]
- Abbott, C.C.; Gallegos, P.; Rediske, N.; Lemke, N.T.; Quinn, D.K. A Review of Longitudinal Electroconvulsive Therapy: Neuroimaging Investigations. J. Geriatr. Psychiatry Neurol. 2014, 27, 33–46. [Google Scholar] [CrossRef]
- Xia, M.; Wang, J.; Sheng, J.; Tang, Y.; Li, C.; Lim, K.; He, B.; Li, C.; Xu, Y.; Wang, J. Effect of Electroconvulsive Therapy on Medial Prefrontal γ-Aminobutyric Acid among Schizophrenia Patients: A Proton Magnetic Resonance Spectroscopy Study. J. ECT 2018, 34, 227–232. [Google Scholar] [CrossRef]
- Knudsen, M.K.; Near, J.; Blicher, A.B.; Videbech, P.; Blicher, J.U. Magnetic Resonance (MR) Spectroscopic Measurement of γ-Aminobutyric Acid (GABA) in Major Depression before and after Electroconvulsive Therapy. Acta Neuropsychiatr. 2019, 31, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Belujon, P.; Grace, A.A. Restoring Mood Balance in Depression: Ketamine Reverses Deficit in Dopamine-Dependent Synaptic Plasticity. Biol. Psychiatry 2014, 76, 927–936. [Google Scholar] [CrossRef]
- Grace, A.A. Dysregulation of the Dopamine System in the Pathophysiology of Schizophrenia and Depression. Nat. Rev. Neurosci. 2016, 17, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Williams, N.R.; Bentzley, B.S.; Sahlem, G.L.; Pannu, J.; Korte, J.E.; Revuelta, G.; Short, E.B.; George, M.S. Unilateral Ultra-Brief Pulse Electroconvulsive Therapy for Depression in Parkinson’s Disease. Acta Neurol. Scand. 2017, 135, 407–411. [Google Scholar] [CrossRef]
- Grover, S.; Chakrabarti, S.; Hazari, N.; Avasthi, A. Effectiveness of Electroconvulsive Therapy in Patients with Treatment Resistant Schizophrenia: A Retrospective Study. Psychiatry Res. 2017, 249, 349–353. [Google Scholar] [CrossRef]
- Quintana, C.; Beaulieu, J.-M. A Fresh Look at Cortical Dopamine D2 Receptor Expressing Neurons. Pharmacol. Res. 2019, 139, 440–445. [Google Scholar] [CrossRef]
- Cumper, S.K.; Ahle, G.M.; Liebman, L.S.; Kellner, C.H. Electroconvulsive Therapy (ECT) in Parkinson’s Disease: ECS and Dopamine Enhancement. J. ECT 2014, 30, 122–124. [Google Scholar] [CrossRef]
- Saijo, T.; Takano, A.; Suhara, T.; Arakawa, R.; Okumura, M.; Ichimiya, T.; Ito, H.; Okubo, Y. Electroconvulsive Therapy Decreases Dopamine D2 receptor Binding in the Anterior Cingulate in Patients with Depression: A Controlled Study Using Positron Emission Tomography with Radioligand [11C]FLB 457. J. Clin. Psychiatry 2010, 71, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Imoto, Y.; Yamamoto, F.; Kawasaki, M.; Ueno, M.; Segi-Nishida, E.; Suzuki, H. Rapid and Lasting Enhancement of Dopaminergic Modulation at the Hippocampal Mossy Fiber Synapse by Electroconvulsive Treatment. J. Neurophysiol. 2017, 117, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Landau, A.M.; Alstrup, A.K.; Audrain, H.; Jakobsen, S.; Simonsen, M.; Møller, A.; Videbech, P.; Wegener, G.; Gjedde, A.; Doudet, D.J. Elevated Dopamine D1 Receptor Availability in Striatum of Göttingen Minipigs after Electroconvulsive Therapy. J. Cereb. Blood Flow Metab. 2018, 38, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Wise, T.; Radua, J.; Nortje, G.; Cleare, A.J.; Young, A.H.; Arnone, D. Voxel-Based Meta-Analytical Evidence of Structural Disconnectivity in Major Depression and Bipolar Disorder. Biol. Psychiatry 2016, 79, 293–302. [Google Scholar] [CrossRef]
- Kraus, C.; Castrén, E.; Kasper, S.; Lanzenberger, R. Serotonin and Neuroplasticity—Links between Molecular, Functional and Structural Pathophysiology in Depression. Neurosci. Biobehav. Rev. 2017, 77, 317–326. [Google Scholar] [CrossRef]
- Underwood, M.D.; Kassir, S.A.; Bakalian, M.J.; Galfalvy, H.; Dwork, A.J.; Mann, J.J.; Arango, V. Serotonin Receptors and Suicide, Major Depression, Alcohol Use Disorder and Reported Early Life Adversity. Transl. Psychiatry 2018, 8, 279. [Google Scholar] [CrossRef]
- Saijo, T.; Takano, A.; Suhara, T.; Arakawa, R.; Okumura, M.; Ichimiya, T.; Ito, H.; Okubo, Y. Effect of Electroconvulsive Therapy on 5-HT1A Receptor Binding in Patients with Depression: A PET Study with [11C]WAY 100635. Int. J. Neuropsychopharmacol. 2010, 13, 785–791. [Google Scholar] [CrossRef]
- Lanzenberger, R.; Baldinger, P.; Hahn, A.; Ungersboeck, J.; Mitterhauser, M.; Winkler, D.; Micskei, Z.; Stein, P.; Karanikas, G.; Wadsak, W.; et al. Global Decrease of Serotonin-1A Receptor Binding after Electroconvulsive Therapy in Major Depression Measured by PET. Mol. Psychiatry 2013, 18, 93–100. [Google Scholar] [CrossRef]
- Kronenberg, G.; Petermann, M.; Dormann, C.; Bader, M.; Gass, P.; Hellweg, R.; Klempin, F. Brain Serotonin Critically Contributes to the Biological Effects of Electroconvulsive Seizures. Eur. Arch. Psychiatry Clin. Neurosci. 2018, 268, 861–864. [Google Scholar] [CrossRef]
- Kronenberg, G.; Mosienko, V.; Gertz, K.; Alenina, N.; Hellweg, R.; Klempin, F. Increased Brain-Derived Neurotrophic Factor (BDNF) Protein Concentrations in Mice Lacking Brain Serotonin. Eur. Arch. Psychiatry Clin. Neurosci. 2016, 266, 281–284. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, W.; Hashimoto, K. Brain-Derived Neurotrophic Factor (BDNF)-TrkB Signaling in Inflammation-Related Depression and Potential Therapeutic Targets. Curr. Neuropharmacol. 2016, 14, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Dong, E.; Dzitoyeva, S.G.; Matrisciano, F.; Tueting, P.; Grayson, D.R.; Guidotti, A. Brain-Derived Neurotrophic Factor Epigenetic Modifications Associated with Schizophrenia-like Phenotype Induced by Prenatal Stress in Mice. Biol. Psychiatry 2015, 77, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Krivoy, A.; Hochman, E.; Sendt, K.-V.; Hollander, S.; Vilner, Y.; Selakovic, M.; Weizman, A.; Taler, M. Association between Serum Levels of Glutamate and Neurotrophic Factors and Response to Clozapine Treatment. Schizophr. Res. 2018, 192, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Rocha, R.B.; Dondossola, E.R.; Grande, A.J.; Colonetti, T.; Ceretta, L.B.; Passos, I.C.; Quevedo, J.; da Rosa, M.I. Increased BDNF Levels after Electroconvulsive Therapy in Patients with Major Depressive Disorder: A Meta-Analysis Study. J. Psychiatr. Res. 2016, 83, 47–53. [Google Scholar] [CrossRef]
- Li, J.; Ye, F.; Xiao, W.; Tang, X.; Sha, W.; Zhang, X.; Wang, J. Increased Serum Brain-Derived Neurotrophic Factor Levels Following Electroconvulsive Therapy or Antipsychotic Treatment in Patients with Schizophrenia. Eur. Psychiatry 2016, 36, 23–28. [Google Scholar] [CrossRef]
- Zhang, F.; Luo, J.; Min, S.; Ren, L.; Qin, P. Propofol Alleviates Electroconvulsive Shock-Induced Memory Impairment by Modulating ProBDNF/MBDNF Ratio in Depressive Rats. Brain Res. 2016, 1642, 43–50. [Google Scholar] [CrossRef]
- Jiang, H.; Li, X.; Chen, S.; Lu, N.; Yue, Y.; Liang, J.; Zhang, Z.; Yuan, Y. Plasminogen Activator Inhibitor-1 in Depression: Results from Animal and Clinical Studies. Sci. Rep. 2016, 6, 30464. [Google Scholar] [CrossRef]
- Ryan, K.M.; Dunne, R.; McLoughlin, D.M. BDNF Plasma Levels and Genotype in Depression and the Response to Electroconvulsive Therapy. Brain Stimul. 2018, 11, 1123–1131. [Google Scholar] [CrossRef]
- Sorri, A.; Järventausta, K.; Kampman, O.; Lehtimäki, K.; Björkqvist, M.; Tuohimaa, K.; Hämäläinen, M.; Moilanen, E.; Leinonen, E. Effect of Electroconvulsive Therapy on Brain-Derived Neurotrophic Factor Levels in Patients with Major Depressive Disorder. Brain Behav. 2018, 8, e01101. [Google Scholar] [CrossRef]
- van Zutphen, E.M.; Rhebergen, D.; van Exel, E.; Oudega, M.L.; Bouckaert, F.; Sienaert, P.; Vandenbulcke, M.; Stek, M.; Dols, A. Brain-Derived Neurotrophic Factor as a Possible Predictor of Electroconvulsive Therapy Outcome. Transl. Psychiatry 2019, 9, 155. [Google Scholar] [CrossRef]
- Vanicek, T.; Kranz, G.S.; Vyssoki, B.; Fugger, G.; Komorowski, A.; Höflich, A.; Saumer, G.; Milovic, S.; Lanzenberger, R.; Eckert, A.; et al. Acute and Subsequent Continuation Electroconvulsive Therapy Elevates Serum BDNF Levels in Patients with Major Depression. Brain Stimul. 2019, 12, 1041–1050. [Google Scholar] [CrossRef]
- Polyakova, M.; Schroeter, M.L.; Elzinga, B.M.; Holiga, S.; Schoenknecht, P.; de Kloet, E.R.; Molendijk, M.L. Brain-Derived Neurotrophic Factor and Antidepressive Effect of Electroconvulsive Therapy: Systematic Review and Meta-Analyses of the Preclinical and Clinical Literature. PLoS ONE 2015, 10, e0141564. [Google Scholar] [CrossRef]
- Juruena, M.F.; Bocharova, M.; Agustini, B.; Young, A.H. Atypical Depression and Non-Atypical Depression: Is HPA Axis Function a Biomarker? A Systematic Review. J. Affect. Disord. 2018, 233, 45–67. [Google Scholar] [CrossRef]
- Spiga, F.; Walker, J.J.; Terry, J.R.; Lightman, S.L. HPA Axis-Rhythms. Compr. Physiol. 2014, 4, 1273–1298. [Google Scholar] [CrossRef]
- O’Donovan, S.; Dalton, V.; Harkin, A.; McLoughlin, D.M. Effects of Brief Pulse and Ultrabrief Pulse Electroconvulsive Stimulation on Rodent Brain and Behaviour in the Corticosterone Model of Depression. Int. J. Neuropsychopharmacol. 2014, 17, 1477–1486. [Google Scholar] [CrossRef]
- Tournikioti, K.; Dikeos, D.; Alevizaki, M.; Michopoulos, I.; Ferentinos, P.; Porichi, E.; Soldatos, C.R.; Douzenis, A. Hypothalamus-Pituitary-Adrenal (HPA) Axis Parameters and Neurocognitive Evaluation in Patients with Bipolar Disorder. Psychiatriki 2018, 29, 199–208. [Google Scholar] [CrossRef][Green Version]
- Bolhuis, K.; Tiemeier, H.; Jansen, P.R.; Muetzel, R.L.; Neumann, A.; Hillegers, M.H.J.; van den Akker, E.T.L.; van Rossum, E.F.C.; Jaddoe, V.W.V.; Vernooij, M.W.; et al. Interaction of Schizophrenia Polygenic Risk and Cortisol Level on Pre-Adolescent Brain Structure. Psychoneuroendocrinology 2019, 101, 295–303. [Google Scholar] [CrossRef]
- Keller, J.; Gomez, R.; Williams, G.; Lembke, A.; Lazzeroni, L.; Murphy, G.M.; Schatzberg, A.F. HPA Axis in Major Depression: Cortisol, Clinical Symptomatology and Genetic Variation Predict Cognition. Mol. Psychiatry 2017, 22, 527–536. [Google Scholar] [CrossRef]
- Sharma, A.; Sawant, N.; Shah, N. A Study on Psychiatric Disorders, Body Image Disturbances, and Self-Esteem in Patients of Cushing’s Disease. Indian J. Endocrinol. Metab. 2018, 22, 445–450. [Google Scholar] [CrossRef]
- Streit, F.; Memic, A.; Hasandedić, L.; Rietschel, L.; Frank, J.; Lang, M.; Witt, S.H.; Forstner, A.J.; Degenhardt, F.; Wüst, S.; et al. Perceived Stress and Hair Cortisol: Differences in Bipolar Disorder and Schizophrenia. Psychoneuroendocrinology 2016, 69, 26–34. [Google Scholar] [CrossRef]
- Saunders, T.S.; Mondelli, V.; Cullen, A.E. Pituitary Volume in Individuals at Elevated Risk for Psychosis: A Systematic Review and Meta-Analysis. Schizophr. Res. 2019, 213, 23–31. [Google Scholar] [CrossRef]
- Delvecchio, G.; Mandolini, G.M.; Perlini, C.; Barillari, M.; Marinelli, V.; Ruggeri, M.; Altamura, A.C.; Bellani, M.; Brambilla, P. Pituitary Gland Shrinkage in Bipolar Disorder: The Role of Gender. Compr. Psychiatry 2018, 82, 95–99. [Google Scholar] [CrossRef]
- Burgese, D.F.; Bassitt, D.P. Variation of Plasma Cortisol Levels in Patients with Depression after Treatment with Bilateral Electroconvulsive Therapy. Trends Psychiatry Psychother. 2015, 37, 27–36. [Google Scholar] [CrossRef]
- Mickey, B.J.; Ginsburg, Y.; Sitzmann, A.F.; Grayhack, C.; Sen, S.; Kirschbaum, C.; Maixner, D.F.; Abelson, J.L. Cortisol Trajectory, Melancholia, and Response to Electroconvulsive Therapy. J. Psychiatr. Res. 2018, 103, 46–53. [Google Scholar] [CrossRef]
- Kyeremanteng, C.; MacKay, J.C.; James, J.S.; Kent, P.; Cayer, C.; Anisman, H.; Merali, Z. Effects of Electroconvulsive Seizures on Depression-Related Behavior, Memory and Neurochemical Changes in Wistar and Wistar-Kyoto Rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 54, 170–178. [Google Scholar] [CrossRef]
- Choi, H.J.; Byun, M.S.; Yi, D.; Sohn, B.K.; Lee, J.H.; Lee, J.-Y.; Kim, Y.K.; Lee, D.Y. KBASE Research Group Associations of Thyroid Hormone Serum Levels with In-Vivo Alzheimer’s Disease Pathologies. Alzheimers Res. Ther. 2017, 9, 64. [Google Scholar] [CrossRef]
- Jose, J.; Nandeesha, H.; Kattimani, S.; Meiyappan, K.; Sarkar, S.; Sivasankar, D. Association between Prolactin and Thyroid Hormones with Severity of Psychopathology and Suicide Risk in Drug Free Male Schizophrenia. Clin. Chim. Acta 2015, 444, 78–80. [Google Scholar] [CrossRef]
- Loh, H.H.; Lim, L.L.; Yee, A.; Loh, H.S. Association between Subclinical Hypothyroidism and Depression: An Updated Systematic Review and Meta-Analysis. BMC Psychiatry 2019, 19, 12. [Google Scholar] [CrossRef]
- Fugger, G.; Dold, M.; Bartova, L.; Kautzky, A.; Souery, D.; Mendlewicz, J.; Serretti, A.; Zohar, J.; Montgomery, S.; Frey, R.; et al. Comorbid Thyroid Disease in Patients with Major Depressive Disorder—Results from the European Group for the Study of Resistant Depression (GSRD). Eur. Neuropsychopharmacol. 2018, 28, 752–760. [Google Scholar] [CrossRef]
- Esel, E.; Turan, T.; Kula, M.; Reyhancan, M.; Gonul, A.; Basturk, M.; Sofuoglu, S. Effects of Electroconvulsive Therapy on Hypothalamic-Pituitary-Thyroid Axis Activity in Depressed Patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 2002, 26, 1171–1175. [Google Scholar] [CrossRef]
- Papakostas, Y.G.; Markianos, M.; Pehlivanidis, A.; Zervas, I.M.; Papadimitriou, G.N.; Stefanis, C. Blunted TSH Response to TRH and Seizure Duration in ECT. Acta Psychiatr. Scand. 1999, 99, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Papakostas, Y.; Markianos, M.; Papadimitriou, G.; Lykouras, L.; Stefanis, C. Thyrotropin and Prolactin Responses to ECT in Schizophrenia and Depression. Psychiatry Res. 1991, 37, 5–10. [Google Scholar] [CrossRef]
- Decina, P.; Sackeim, H.A.; Kahn, D.A.; Pierson, D.; Hopkins, N.; Malitz, S. Effects of ECT on the TRH Stimulation Test. Psychoneuroendocrinology 1987, 12, 29–34. [Google Scholar] [CrossRef]
- Dykes, S.; Scott, A.I.; Gow, S.M.; Whalley, L.J. Effects of Seizure Duration on Serum TSH Concentration after ECT. Psychoneuroendocrinology 1987, 12, 477–482. [Google Scholar] [CrossRef]
- Ríos, B.; Vicente, N. Mecanismo de acción de la terapia electroconvulsiva en la depresión. Actas Esp. Psiquiatr. 2001, 29, 199–207. [Google Scholar]
- Parmentier, T.; Sienaert, P. The Use of Triiodothyronine (T3) in the Treatment of Bipolar Depression: A Review of the Literature. J. Affect. Disord. 2018, 229, 410–414. [Google Scholar] [CrossRef]
- van Buel, E.M.; Patas, K.; Peters, M.; Bosker, F.J.; Eisel, U.L.M.; Klein, H.C. Immune and Neurotrophin Stimulation by Electroconvulsive Therapy: Is Some Inflammation Needed after All? Transl. Psychiatry 2015, 5, e609. [Google Scholar] [CrossRef]
- Yrondi, A.; Sporer, M.; Péran, P.; Schmitt, L.; Arbus, C.; Sauvaget, A. Electroconvulsive Therapy, Depression, the Immune System and Inflammation: A Systematic Review. Brain Stimul. 2018, 11, 29–51. [Google Scholar] [CrossRef]
- Zincir, S.; Öztürk, P.; Bilgen, A.E.; İzci, F.; Yükselir, C. Levels of Serum Immunomodulators and Alterations with Electroconvulsive Therapy in Treatment-Resistant Major Depression. NDT 2016, 12, 1389–1396. [Google Scholar] [CrossRef]
- Rush, G.; O’Donovan, A.; Nagle, L.; Conway, C.; McCrohan, A.; O’Farrelly, C.; Lucey, J.V.; Malone, K.M. Alteration of Immune Markers in a Group of Melancholic Depressed Patients and Their Response to Electroconvulsive Therapy. J. Affect. Disord. 2016, 205, 60–68. [Google Scholar] [CrossRef]
- Järventausta, K.; Sorri, A.; Kampman, O.; Björkqvist, M.; Tuohimaa, K.; Hämäläinen, M.; Moilanen, E.; Leinonen, E.; Peltola, J.; Lehtimäki, K. Changes in Interleukin-6 Levels during Electroconvulsive Therapy May Reflect the Therapeutic Response in Major Depression. Acta Psychiatr. Scand. 2017, 135, 87–92. [Google Scholar] [CrossRef]
- Sorri, A.; Järventausta, K.; Kampman, O.; Lehtimäki, K.; Björkqvist, M.; Tuohimaa, K.; Hämäläinen, M.; Moilanen, E.; Leinonen, E. Low Tumor Necrosis Factor-α Levels Predict Symptom Reduction during Electroconvulsive Therapy in Major Depressive Disorder. Brain Behav. 2018, 8, e00933. [Google Scholar] [CrossRef]
- Kartalci, S.; Karabulut, A.B.; Erbay, L.G.; Acar, C. Effects of Electroconvulsive Therapy on Some Inflammatory Factors in Patients with Treatment-Resistant Schizophrenia. J. ECT 2016, 32, 174–179. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Chadda, R.K.; Rusia, U.; Jain, N. Effect of Electroconvulsive Therapy on Hematological Parameters. Psychiatry Res. 2001, 104, 265–268. [Google Scholar] [CrossRef]
- Kronfol, Z.; Nair, M.P.; Weinberg, V.; Young, E.A.; Aziz, M. Acute Effects of Electroconvulsive Therapy on Lymphocyte Natural Killer Cell Activity in Patients with Major Depression. J. Affect. Disord. 2002, 71, 211–215. [Google Scholar] [CrossRef]
- Roman, A.; Nawrat, D.; Nalepa, I. Chronic Treatment with Electroconvulsive Shock May Modulate the Immune Function of Macrophages. J. ECT 2008, 24, 260–267. [Google Scholar] [CrossRef]
- Roman, A.; Nalepa, I. Effect of Repeated Administration of Paroxetine and Electroconvulsive Shock on the Proliferative Response of Lymphocytes and the Synthesis of Nitric Oxide by Macrophages in Rats. J. ECT 2005, 21, 111–117. [Google Scholar] [CrossRef]
- Sepulveda-Rodriguez, A.; Li, P.; Khan, T.; Ma, J.D.; Carlone, C.A.; Bozzelli, P.L.; Conant, K.E.; Forcelli, P.A.; Vicini, S. Electroconvulsive Shock Enhances Responsive Motility and Purinergic Currents in Microglia in the Mouse Hippocampus. eNeuro 2019, 6, ENEURO.0056-19.2019. [Google Scholar] [CrossRef]
- Kranaster, L.; Hoyer, C.; Aksay, S.S.; Bumb, J.M.; Müller, N.; Zill, P.; Schwarz, M.J.; Sartorius, A. Antidepressant Efficacy of Electroconvulsive Therapy Is Associated with a Reduction of the Innate Cellular Immune Activity in the Cerebrospinal Fluid in Patients with Depression. World J. Biol. Psychiatry 2018, 19, 379–389. [Google Scholar] [CrossRef]
- Jansson, L.; Orre, K.; Tingström, A. Repeated Electroconvulsive Seizures Increase the Number of Vessel-Associated Macrophages in Rat Hippocampus. J. ECT 2012, 28, 174–179. [Google Scholar] [CrossRef]
- Yirmiya, R.; Rimmerman, N.; Reshef, R. Depression as a Microglial Disease. Trends Neurosci. 2015, 38, 637–658. [Google Scholar] [CrossRef] [PubMed]
- Arauchi, R.; Hashioka, S.; Tsuchie, K.; Miyaoka, T.; Tsumori, T.; Limoa, E.; Azis, I.A.; Oh-Nishi, A.; Miura, S.; Otsuki, K.; et al. Gunn Rats with Glial Activation in the Hippocampus Show Prolonged Immobility Time in the Forced Swimming Test and Tail Suspension Test. Brain Behav. 2018, 8, e01028. [Google Scholar] [CrossRef] [PubMed]
- Limoa, E.; Hashioka, S.; Miyaoka, T.; Tsuchie, K.; Arauchi, R.; Azis, I.A.; Wake, R.; Hayashida, M.; Araki, T.; Furuya, M.; et al. Electroconvulsive Shock Attenuated Microgliosis and Astrogliosis in the Hippocampus and Ameliorated Schizophrenia-like Behavior of Gunn Rat. J. Neuroinflamm. 2016, 13, 230. [Google Scholar] [CrossRef] [PubMed]
- Soria, V.; Uribe, J.; Salvat-Pujol, N.; Palao, D.; Menchón, J.M.; Labad, J. Psychoneuroimmunology of Mental Disorders. Rev. Psiquiatr. Salud. Ment. Engl. Ed. 2018, 11, 115–124. [Google Scholar] [CrossRef]
- Li, Q.; Liu, S.; Guo, M.; Yang, C.-X.; Xu, Y. The Principles of Electroconvulsive Therapy Based on Correlations of Schizophrenia and Epilepsy: A View from Brain Networks. Front. Neurol. 2019, 10, 688. [Google Scholar] [CrossRef]
- Sinha, P.; Reddy, R.V.; Srivastava, P.; Mehta, U.M.; Bharath, R.D. Network Neurobiology of Electroconvulsive Therapy in Patients with Depression. Psychiatry Res. Neuroimaging 2019, 287, 31–40. [Google Scholar] [CrossRef]
- Pettinati, H.M.; Mathisen, K.S.; Rosenberg, J.; Lynch, J.F. Meta-Analytical Approach to Reconciling Discrepancies in Efficacy between Bilateral and Unilateral Electroconvulsive Therapy. Convuls. Ther. 1986, 2, 7–17. [Google Scholar]
- Takamiya, A.; Seki, M.; Kudo, S.; Yoshizaki, T.; Nakahara, J.; Mimura, M.; Kishimoto, T. Electroconvulsive Therapy for Parkinson’s Disease: A Systematic Review and Meta-Analysis. Mov. Disord. 2021, 36, 50–58. [Google Scholar] [CrossRef]
- Dos Santos-Ribeiro, S.; de Salles Andrade, J.B.; Quintas, J.N.; Baptista, K.B.; Moreira-de-Oliveira, M.E.; Yücel, M.; Fontenelle, L.F. A Systematic Review of the Utility of Electroconvulsive Therapy in Broadly Defined Obsessive-Compulsive-Related Disorders. Prim. Care Companion CNS Disord. 2018, 20, 18r02342. [Google Scholar] [CrossRef]
- Ahmadi, N.; Moss, L.; Hauser, P.; Nemeroff, C.; Atre-Vaidya, N. Clinical Outcome of Maintenance Electroconvulsive Therapy in Comorbid Posttraumatic Stress Disorder and Major Depressive Disorder. J. Psychiatr. Res. 2018, 105, 132–136. [Google Scholar] [CrossRef]
- Song, G.-M.; Tian, X.; Shuai, T.; Yi, L.-J.; Zeng, Z.; Liu, S.; Zhou, J.-G.; Wang, Y. Treatment of Adults with Treatment-Resistant Depression: Electroconvulsive Therapy Plus Antidepressant or Electroconvulsive Therapy Alone? Evidence From an Indirect Comparison Meta-Analysis. Medicine 2015, 94, e1052. [Google Scholar] [CrossRef] [PubMed]
- Janicak, P.G.; Davis, J.M.; Gibbons, R.D.; Ericksen, S.; Chang, S.; Gallagher, P. Efficacy of ECT: A Meta-Analysis. Am. J. Psychiatry 1985, 142, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Overall, J.E.; Rhoades, H.M. A Comment on the Efficacy of Unilateral Versus Bilateral ECT. Convuls. Ther. 1986, 2, 245–251. [Google Scholar] [PubMed]
- Kho, K.H.; van Vreeswijk, M.F.; Simpson, S.; Zwinderman, A.H. A Meta-Analysis of Electroconvulsive Therapy Efficacy in Depression. J. ECT 2003, 19, 139–147. [Google Scholar] [CrossRef]
- National Collaborating Centre for Mental Health (UK). Depression: The Treatment and Management of Depression in Adults (Updated Edition); National Institute for Health and Clinical Excellence: Guidance; British Psychological Society: Leicester, UK, 2010; ISBN 978-1-904671-85-5. [Google Scholar]
- Ellis, P. Royal Australian and New Zealand College of Psychiatrists Clinical Practice Guidelines Team for Depression Australian and New Zealand Clinical Practice Guidelines for the Treatment of Depression. Aust. N. Z. J. Psychiatry 2004, 38, 389–407. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.H.; Lam, R.W.; McIntyre, R.S.; Tourjman, S.V.; Bhat, V.; Blier, P.; Hasnain, M.; Jollant, F.; Levitt, A.J.; MacQueen, G.M.; et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: Section 3. Pharmacological Treatments. Can. J. Psychiatry 2016, 61, 540–560. [Google Scholar] [CrossRef]
- Bauer, M.; Pfennig, A.; Severus, E.; Whybrow, P.C.; Angst, J.; Möller, H.-J.; World Federation of Societies of Biological Psychiatry. Task Force on Unipolar Depressive Disorders World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Unipolar Depressive Disorders, Part 1: Update 2013 on the Acute and Continuation Treatment of Unipolar Depressive Disorders. World J. Biol. Psychiatry 2013, 14, 334–385. [Google Scholar] [CrossRef]
- Haq, A.U.; Sitzmann, A.F.; Goldman, M.L.; Maixner, D.F.; Mickey, B.J. Response of Depression to Electroconvulsive Therapy: A Meta-Analysis of Clinical Predictors. J. Clin. Psychiatry 2015, 76, 1374–1384. [Google Scholar] [CrossRef]
- Pagnin, D.; de Queiroz, V.; Pini, S.; Cassano, G.B. Efficacy of ECT in Depression: A Meta-Analytic Review. J. ECT 2004, 20, 13–20. [Google Scholar] [CrossRef]
- Jelovac, A.; Kolshus, E.; McLoughlin, D.M. Relapse Following Successful Electroconvulsive Therapy for Major Depression: A Meta-Analysis. Neuropsychopharmacology 2013, 38, 2467–2474. [Google Scholar] [CrossRef]
- Rasmussen, K.G.; Mueller, M.; Rummans, T.A.; Husain, M.M.; Petrides, G.; Knapp, R.G.; Fink, M.; Sampson, S.M.; Bailine, S.H.; Kellner, C.H. Is Baseline Medication Resistance Associated with Potential for Relapse after Successful Remission of a Depressive Episode with ECT? Data from the Consortium for Research on Electroconvulsive Therapy (CORE). J. Clin. Psychiatry 2009, 70, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Sackeim, H.A.; Haskett, R.F.; Mulsant, B.H.; Thase, M.E.; Mann, J.J.; Pettinati, H.M.; Greenberg, R.M.; Crowe, R.R.; Cooper, T.B.; Prudic, J. Continuation Pharmacotherapy in the Prevention of Relapse Following Electroconvulsive Therapy: A Randomized Controlled Trial. JAMA 2001, 285, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- Elias, A.; Phutane, V.H.; Clarke, S.; Prudic, J. Electroconvulsive Therapy in the Continuation and Maintenance Treatment of Depression: Systematic Review and Meta-Analyses. Aust. N. Z. J. Psychiatry 2018, 52, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, R. The Practice of Electroconvulsive Therapy: Recommendations for Treatment, Training, and Privileging: A Task Force Report of the American Psychiatric Association. Am. J. Psychiatry 2002, 159, 331. [Google Scholar] [CrossRef]
- Bahji, A.; Hawken, E.R.; Sepehry, A.A.; Cabrera, C.A.; Vazquez, G. ECT beyond Unipolar Major Depression: Systematic Review and Meta-Analysis of Electroconvulsive Therapy in Bipolar Depression. Acta Psychiatr. Scand. 2019, 139, 214–226. [Google Scholar] [CrossRef]
- Conseglieri, A.; Villasante, O. Shock Therapies in Spain (1939–1952) after the Civil War: Santa Isabel National Mental Asylum in Leganés. Hist. Psychiatry 2021, 32, 402–418. [Google Scholar] [CrossRef]
- Sanghani, S.N.; Petrides, G.; Kellner, C.H. Electroconvulsive Therapy (ECT) in Schizophrenia: A Review of Recent Literature. Curr. Opin. Psychiatry 2018, 31, 213–222. [Google Scholar] [CrossRef]
- Tharyan, P.; Adams, C.E. Electroconvulsive Therapy for Schizophrenia. Cochrane Database Syst. Rev. 2005, CD000076. [Google Scholar] [CrossRef]
- Scott, A.I.F. College Guidelines on Electroconvulsive Therapy: An Update for Prescribers. Adv. Psychiatr. Treat. 2005, 11, 150–156. [Google Scholar] [CrossRef][Green Version]
- American Psychiatric Association. The American Psychiatric Association Practice Guideline for the Treatment of Patients with Schizophrenia, 3rd ed.; American Psychiatric Association Publishing: Washington, DC, USA, 2020; ISBN 978-0-89042-484-1. [Google Scholar]
- Gazdag, G.; Sebestyén, G.; Zsargó, E.; Tolna, J.; Ungvari, G.S. Survey of Referrals to Electroconvulsive Therapy in Hungary. World J. Biol. Psychiatry 2009, 10, 900–904. [Google Scholar] [CrossRef]
- Phutane, V.H.; Thirthalli, J.; Muralidharan, K.; Naveen Kumar, C.; Keshav Kumar, J.; Gangadhar, B.N. Double-Blind Randomized Controlled Study Showing Symptomatic and Cognitive Superiority of Bifrontal over Bitemporal Electrode Placement during Electroconvulsive Therapy for Schizophrenia. Brain Stimul. 2013, 6, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Thirthalli, J.; Phutane, V.H.; Muralidharan, K.; Kumar, C.N.; Munishwar, B.; Baspure, P.; Gangadhar, B.N. Does Catatonic Schizophrenia Improve Faster with Electroconvulsive Therapy than Other Subtypes of Schizophrenia? World J. Biol. Psychiatry 2009, 10, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Awata, S.; Matsuoka, H. One-Year Outcome after Response to ECT in Middle-Aged and Elderly Patients with Intractable Catatonic Schizophrenia. J. ECT 2004, 20, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Awata, S.; Takano, T.; Ebina, Y.; Iwasaki, H.; Matsuoka, H. Continuation Electroconvulsive Therapy for Relapse Prevention in Middle-Aged and Elderly Patients with Intractable Catatonic Schizophrenia. Psychiatry Clin. Neurosci. 2005, 59, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Awata, S.; Takano, T.; Ebina, Y.; Shindo, T.; Harada, N.; Matsuoka, H. Adjusting the Frequency of Continuation and Maintenance Electroconvulsive Therapy to Prevent Relapse of Catatonic Schizophrenia in Middle-Aged and Elderly Patients Who Are Relapse-Prone. Psychiatry Clin. Neurosci. 2006, 60, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Awata, S.; Takano, T.; Ebina, Y.; Takamatsu, K.; Kajiwara, T.; Ito, K.; Shindo, T.; Funakoshi, S.; Matsuoka, H. Improvement of Psychiatric Symptoms after Electroconvulsive Therapy in Young Adults with Intractable First-Episode Schizophrenia and Schizophreniform Disorder. Tohoku J. Exp. Med. 2006, 210, 213–220. [Google Scholar] [CrossRef] [PubMed]
- de la Serna, E.; Flamarique, I.; Castro-Fornieles, J.; Pons, A.; Puig, O.; Andrés-Perpiña, S.; Lázaro, L.; Garrido, J.M.; Bernardo, M.; Baeza, I. Two-Year Follow-up of Cognitive Functions in Schizophrenia Spectrum Disorders of Adolescent Patients Treated with Electroconvulsive Therapy. J. Child. Adolesc. Psychopharmacol. 2011, 21, 611–619. [Google Scholar] [CrossRef]
- Zhang, Z.-J.; Chen, Y.-C.; Wang, H.-N.; Wang, H.-H.; Xue, Y.-Y.; Feng, S.-F.; Tan, Q.-R. Electroconvulsive Therapy Improves Antipsychotic and Somnographic Responses in Adolescents with First-Episode Psychosis--a Case-Control Study. Schizophr. Res. 2012, 137, 97–103. [Google Scholar] [CrossRef]
- Versiani, M.; Cheniaux, E.; Landeira-Fernandez, J. Efficacy and Safety of Electroconvulsive Therapy in the Treatment of Bipolar Disorder: A Systematic Review. J. ECT 2011, 27, 153–164. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE) Guidance on the Use of Electroconvulsive Therapy. Available online: https://www.nice.org.uk/guidance/ta59 (accessed on 20 January 2022).
- Yatham, L.N.; Kennedy, S.H.; Parikh, S.V.; Schaffer, A.; Bond, D.J.; Frey, B.N.; Sharma, V.; Goldstein, B.I.; Rej, S.; Beaulieu, S.; et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 Guidelines for the Management of Patients with Bipolar Disorder. Bipolar. Disord. 2018, 20, 97–170. [Google Scholar] [CrossRef]
- Goodwin, G.M.; Haddad, P.M.; Ferrier, I.N.; Aronson, J.K.; Barnes, T.; Cipriani, A.; Coghill, D.R.; Fazel, S.; Geddes, J.R.; Grunze, H.; et al. Evidence-Based Guidelines for Treating Bipolar Disorder: Revised Third Edition Recommendations from the British Association for Psychopharmacology. J. Psychopharmacol. 2016, 30, 495–553. [Google Scholar] [CrossRef] [PubMed]
- Hirschfeld, R.M.A. Guideline Watch (November 2005): Practice Guideline for the Treatment of Patients with Bipolar Disorder. Focus 2007, 5, 34–39. [Google Scholar] [CrossRef]
- Nivoli, A.M.A.; Colom, F.; Murru, A.; Pacchiarotti, I.; Castro-Loli, P.; González-Pinto, A.; Fountoulakis, K.N.; Vieta, E. New Treatment Guidelines for Acute Bipolar Depression: A Systematic Review. J. Affect. Disord. 2011, 129, 14–26. [Google Scholar] [CrossRef] [PubMed]
- UK ECT Review Group. Efficacy and Safety of Electroconvulsive Therapy in Depressive Disorders: A Systematic Review and Meta-Analysis. Lancet 2003, 361, 799–808. [Google Scholar] [CrossRef]
- Liang, C.-S.; Chung, C.-H.; Ho, P.-S.; Tsai, C.-K.; Chien, W.-C. Superior Anti-Suicidal Effects of Electroconvulsive Therapy in Unipolar Disorder and Bipolar Depression. Bipolar. Disord. 2018, 20, 539–546. [Google Scholar] [CrossRef]
- Schoeyen, H.K.; Kessler, U.; Andreassen, O.A.; Auestad, B.H.; Bergsholm, P.; Malt, U.F.; Morken, G.; Oedegaard, K.J.; Vaaler, A. Treatment-Resistant Bipolar Depression: A Randomized Controlled Trial of Electroconvulsive Therapy versus Algorithm-Based Pharmacological Treatment. Am. J. Psychiatry 2015, 172, 41–51. [Google Scholar] [CrossRef]
- Danivas, V.; Behere, R.V.; Varambally, S.; Rao, N.P.; Venkatasubramanian, G.; Gangadhar, B.N. Electroconvulsive Therapy in the Treatment of Delirious Mania: A Report of 2 Patients. J. ECT 2010, 26, 278–279. [Google Scholar] [CrossRef]
- Jacobowski, N.L.; Heckers, S.; Bobo, W.V. Delirious Mania: Detection, Diagnosis, and Clinical Management in the Acute Setting. J. Psychiatr. Pract. 2013, 19, 15–28. [Google Scholar] [CrossRef]
- Barekatain, M.; Jahangard, L.; Haghighi, M.; Ranjkesh, F. Bifrontal versus Bitemporal Electroconvulsive Therapy in Severe Manic Patients. J. ECT 2008, 24, 199–202. [Google Scholar] [CrossRef]
- Hiremani, R.M.; Thirthalli, J.; Tharayil, B.S.; Gangadhar, B.N. Double-Blind Randomized Controlled Study Comparing Short-Term Efficacy of Bifrontal and Bitemporal Electroconvulsive Therapy in Acute Mania. Bipolar. Disord. 2008, 10, 701–707. [Google Scholar] [CrossRef]
- Jahangard, L.; Haghighi, M.; Bigdelou, G.; Bajoghli, H.; Brand, S. Comparing Efficacy of ECT with and without Concurrent Sodium Valproate Therapy in Manic Patients. J. ECT 2012, 28, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Mohan, T.S.P.; Tharyan, P.; Alexander, J.; Raveendran, N.S. Effects of Stimulus Intensity on the Efficacy and Safety of Twice-Weekly, Bilateral Electroconvulsive Therapy (ECT) Combined with Antipsychotics in Acute Mania: A Randomised Controlled Trial. Bipolar. Disord. 2009, 11, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Perugi, G.; Medda, P.; Toni, C.; Mariani, M.G.; Socci, C.; Mauri, M. The Role of Electroconvulsive Therapy (ECT) in Bipolar Disorder: Effectiveness in 522 Patients with Bipolar Depression, Mixed-State, Mania and Catatonic Features. Curr. Neuropharmacol. 2017, 15, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Palma, M.; Ferreira, B.; Borja-Santos, N.; Trancas, B.; Monteiro, C.; Cardoso, G. Efficacy of Electroconvulsive Therapy in Bipolar Disorder with Mixed Features. Depress. Res. Treat. 2016, 2016, 8306071. [Google Scholar] [CrossRef]
- Strömgren, L.S. Electroconvulsive Therapy in Aarhus, Denmark, in 1984: Its Application in Nondepressive Disorders. Convuls. Ther. 1988, 4, 306–313. [Google Scholar]
- Gruber, N.P.; Dilsaver, S.C.; Shoaib, A.M.; Swann, A.C. ECT in Mixed Affective States: A Case Series. J. ECT 2000, 16, 183–188. [Google Scholar] [CrossRef]
- Valentí, M.; Benabarre, A.; García-Amador, M.; Molina, O.; Bernardo, M.; Vieta, E. Electroconvulsive Therapy in the Treatment of Mixed States in Bipolar Disorder. Eur. Psychiatry 2008, 23, 53–56. [Google Scholar] [CrossRef]
- Ciapparelli, A.; Dell’Osso, L.; Tundo, A.; Pini, S.; Chiavacci, M.C.; Di Sacco, I.; Cassano, G.B. Electroconvulsive Therapy in Medication-Nonresponsive Patients with Mixed Mania and Bipolar Depression. J. Clin. Psychiatry 2001, 62, 552–555. [Google Scholar] [CrossRef]
- Medda, P.; Perugi, G.; Zanello, S.; Ciuffa, M.; Rizzato, S.; Cassano, G.B. Comparative Response to Electroconvulsive Therapy in Medication-Resistant Bipolar I Patients with Depression and Mixed State. J. ECT 2010, 26, 82–86. [Google Scholar] [CrossRef]
- Vanelle, J.M.; Loo, H.; Galinowski, A.; de Carvalho, W.; Bourdel, M.C.; Brochier, P.; Bouvet, O.; Brochier, T.; Olie, J.P. Maintenance ECT in Intractable Manic-Depressive Disorders. Convuls. Ther. 1994, 10, 195–205. [Google Scholar]
- Fregni, F.; Simon, D.K.; Wu, A.; Pascual-Leone, A. Non-Invasive Brain Stimulation for Parkinson’s Disease: A Systematic Review and Meta-Analysis of the Literature. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1614–1623. [Google Scholar] [CrossRef] [PubMed]
- Narang, P.; Glowacki, A.; Lippmann, S. Electroconvulsive Therapy Intervention for Parkinson’s Disease. Innov. Clin. Neurosci. 2015, 12, 25–28. [Google Scholar] [PubMed]
- Calderón-Fajardo, H.; Cervantes-Arriaga, A.; Llorens-Arenas, R.; Ramírez-Bermudez, J.; Ruiz-Chow, Á.; Rodríguez-Violante, M. Electroconvulsive Therapy in Parkinson’s Disease. Arq. Neuropsiquiatr. 2015, 73, 856–860. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sadananda, S.K.; Holla, B.; Viswanath, B.; Narasimha, A.; Sebastian, A.; Math, S.B.; Chandrashekar, C.R. Effectiveness of Electroconvulsive Therapy for Drug-Induced Parkinsonism in the Elderly. J. ECT 2013, 29, e6-7. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, K.; Tanaka, R.; Shimura, H.; Hirano, K.; Hatano, T.; Miyakawa, K.; Arai, H.; Hattori, N.; Urabe, T. Quantitative Evaluation of Electroconvulsive Therapy for Parkinson’s Disease with Refractory Psychiatric Symptoms. J. Neural Transm. 2014, 121, 1405–1410. [Google Scholar] [CrossRef] [PubMed]
- Usui, C.; Hatta, K.; Doi, N.; Kubo, S.; Kamigaichi, R.; Nakanishi, A.; Nakamura, H.; Hattori, N.; Arai, H. Improvements in Both Psychosis and Motor Signs in Parkinson’s Disease, and Changes in Regional Cerebral Blood Flow after Electroconvulsive Therapy. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 1704–1708. [Google Scholar] [CrossRef]
- Borisovskaya, A.; Bryson, W.C.; Buchholz, J.; Samii, A.; Borson, S. Electroconvulsive Therapy for Depression in Parkinson’s Disease: Systematic Review of Evidence and Recommendations. Neurodegener. Dis. Manag. 2016, 6, 161–176. [Google Scholar] [CrossRef]
- National Collaborating Centre for Mental Health (UK). Obsessive-Compulsive Disorder: Core Interventions in the Treatment of Obsessive-Compulsive Disorder and Body Dysmorphic Disorder; National Institute for Health and Clinical Excellence: Guidance; British Psychological Society: Leicester, UK, 2006; ISBN 978-1-85433-430-5. [Google Scholar]
- Koran, L.M.; Hanna, G.L.; Hollander, E.; Nestadt, G.; Simpson, H.B. American Psychiatric Association Practice Guideline for the Treatment of Patients with Obsessive-Compulsive Disorder. Am. J. Psychiatry 2007, 164, 5–53. [Google Scholar]
- Fontenelle, L.F.; Coutinho, E.S.F.; Lins-Martins, N.M.; Fitzgerald, P.B.; Fujiwara, H.; Yücel, M. Electroconvulsive Therapy for Obsessive-Compulsive Disorder: A Systematic Review. J. Clin. Psychiatry 2015, 76, 949–957. [Google Scholar] [CrossRef]
- Bikson, M.; Inoue, M.; Akiyama, H.; Deans, J.K.; Fox, J.E.; Miyakawa, H.; Jefferys, J.G.R. Effects of Uniform Extracellular DC Electric Fields on Excitability in Rat Hippocampal Slices in Vitro. J. Physiol. 2004, 557, 175–190. [Google Scholar] [CrossRef]
- Casey, D.A.; Davis, M.H. Obsessive-Compulsive Disorder Responsive to Electroconvulsive Therapy in an Elderly Woman. South. Med. J. 1994, 87, 862–864. [Google Scholar] [CrossRef] [PubMed]
- Bülbül, F.; Copoglu, U.S.; Alpak, G.; Unal, A.; Tastan, M.F.; Savas, H.A. Maintenance Therapy with Electroconvulsive Therapy in a Patient with a Codiagnosis of Bipolar Disorder and Obsessive-Compulsive Disorder. J. ECT 2013, 29, e21–e22. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.G.; Kellner, C.H. Remission of Major Depression and Obsessive-Compulsive Disorder after a Single Unilateral ECT. J. ECT 2003, 19, 50–51. [Google Scholar] [CrossRef] [PubMed]
- Strassnig, M.; Riedel, M.; Müller, N. Electroconvulsive Therapy in a Patient with Tourette’s Syndrome and Co-Morbid Obsessive Compulsive Disorder. World J. Biol. Psychiatry 2004, 5, 164–166. [Google Scholar] [CrossRef]
- Sutor, B.; Rasmussen, K.G. Electroconvulsive Therapy for Agitation in Alzheimer Disease: A Case Series. J. ECT 2008, 24, 239–241. [Google Scholar] [CrossRef]
- Isserles, M.; Daskalakis, Z.J.; Kumar, S.; Rajji, T.K.; Blumberger, D.M. Clinical Effectiveness and Tolerability of Electroconvulsive Therapy in Patients with Neuropsychiatric Symptoms of Dementia. J. Alzheimers Dis. 2017, 57, 45–51. [Google Scholar] [CrossRef]
- Forester, B.P.; Mellen, E.; Mathias, L.; Monette, P.; Rahman, A.; Harper, D.G.; Mueller, M.; Knapp, R.; Hermida, A.; Nykamp, L.; et al. Electroconvulsive Therapy for the Treatment of Acute Agitation and Aggression in Alzheimer’s Dementia (ECT-AD). Am. J. Geriatr. Psychiatry 2019, 27, S166–S167. [Google Scholar] [CrossRef]
- Central Institute of Mental Health, Mannheim. Electroconvulsive Therapy for Treatment of Alzheimer’s Disease (ECTAD); NCBI: Bethesda, MD, USA, 2015. [Google Scholar]
- University of British Columbia. Safety and Efficacy of Electroconvulsive Therapy (ECT) for Behavioural and Psychological Symptoms of Dementia (BPSD) (ECTBPSD); NIH: Bethesda, MD, USA, 2016.
- Scheftner, W.A.; Shulman, R.B. Treatment Choice in Neuroleptic Malignant Syndrome. Convuls. Ther. 1992, 8, 267–279. [Google Scholar]
- Hermesh, H.; Aizenberg, D.; Weizman, A. A Successful Electroconvulsive Treatment of Neuroleptic Malignant Syndrome. Acta Psychiatr. Scand. 1987, 75, 237–239. [Google Scholar] [CrossRef]
- Morcos, N.; Rosinski, A.; Maixner, D.F. Electroconvulsive Therapy for Neuroleptic Malignant Syndrome: A Case Series. J. ECT 2019, 35, 225–230. [Google Scholar] [CrossRef]
- Hanretta, A.T.; Malek-Ahmadi, P. Combined Use of ECT with Duloxetine and Olanzapine: A Case Report. J. ECT 2006, 22, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Helsley, S.; Sheikh, T.; Kim, K.Y.; Park, S.K. ECT Therapy in PTSD. Am. J. Psychiatry 1999, 156, 494–495. [Google Scholar] [CrossRef] [PubMed]
- Watts, B.V. Electroconvulsive Therapy for Comorbid Major Depressive Disorder and Posttraumatic Stress Disorder. J. ECT 2007, 23, 93–95. [Google Scholar] [CrossRef]
- Watts, B.V.; Groft, A. Retrospective Evaluation of the Dexamethasone Suppression Test as a Predictor of Response to Electroconvulsive Therapy in Patients with Comorbid Major Depressive Disorder and Posttraumatic Stress Disorder. J. ECT 2010, 26, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Centre for Addiction and Mental Health. Electroconvulsive Therapy for Traumatic Memories; NIH: Bethesda, MD, USA, 2019.
- Imperial College London. Magnetic Seizure Therapy (MST) for Treatment Resistant Depression, Schizophrenia, and Obsessive Compulsive Disorder; NIH: Bethesda, MD, USA, 2012.
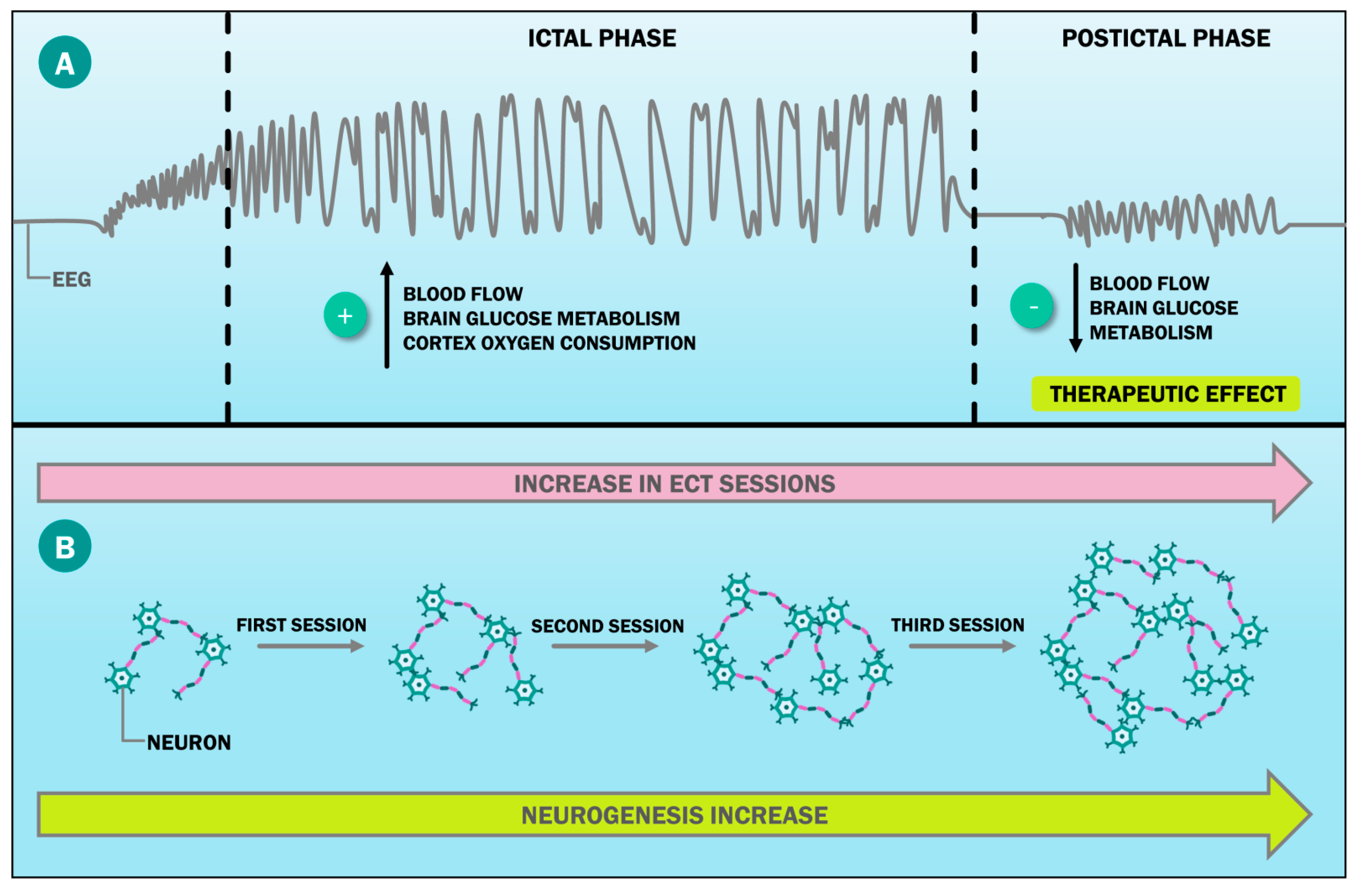
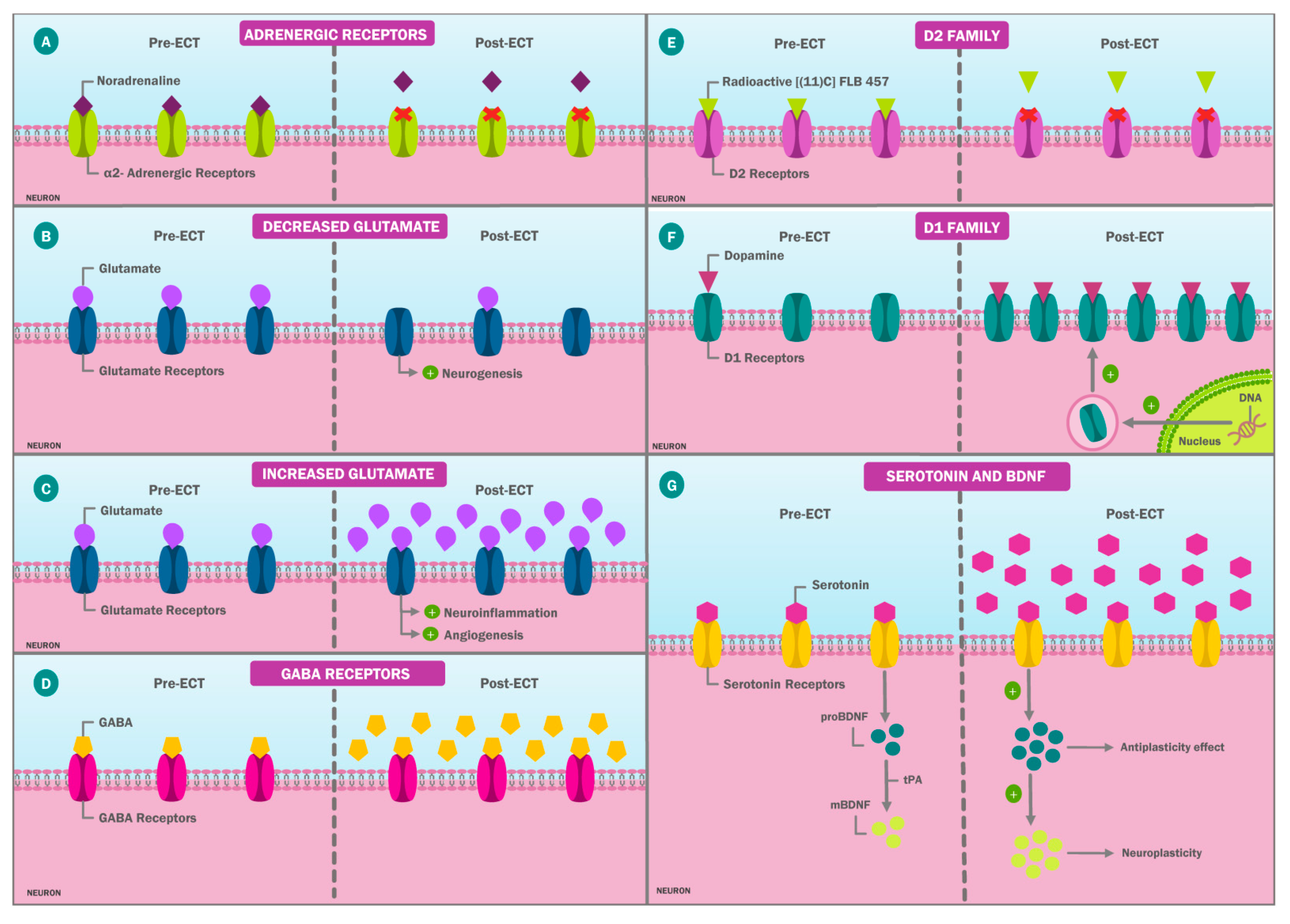

| Human Studies * | |||
| Author | Mechanism | Methodology | Results |
| Berggren et al. [25] | Brain interconnectivity | A total of 49 patients underwent ECT. A total of 41 patients grading improvement after the initial ECT series were compared with 8, grading no improvement. The patients underwent neuropsychiatric ratings, the measure of clinical response (defined as ≥50% reduction of pretreatment depression score), and the measure of rCBF. | The responder group had an initial 60–82%, and the nonresponder group a 30–64% clinical response throughout the follow-up. The nonresponder group showed more reported depression (p = 0.003) and vegetative anxiety (p = 0.024), with a generally higher left temporal rCBF (p = 0.045). |
| Joshi et al. [28] | Neurogenesis | Longitudinal changes in hippocampal and amygdala structures were examined in 43 patients with major depression, referred for ECT as part of their standard clinical care. Cross-sectional comparisons with 32 demographically similar controls established diagnosis effects. | Patients showed smaller hippocampal volumes than controls at baseline (p < 0.04). Both the hippocampal and the amygdala volumes increased with ECT (p < 0.001) and in relation to the symptom improvement (p < 0.01). Hippocampal volume at baseline predicted subsequent clinical response (p < 0.05). All structural measurements remained stable across time in controls. |
| Saijo et al. [52] | Dopaminergic system | A total of 7 patients with depression underwent PET scans before and after a series of 6–7 treatments with the bilateral ECT. The [(11)C]FLB 457 binding parametric images were generated on the basis of a simplified reference tissue model. Voxel-based methods were used to assess the ECT effect on D(2) receptor binding. | There were no significant differences in D(2) receptor binding between patients with depression and controls. Significant changes in D(2) receptor binding, a mean of 25.2% reduction, were found in the right rostral anterior cingulate following ECT (p < 0.001). |
| Burgese et al. [84] | Endocrine effects | Blood cortisol levels were measured before the beginning of treatment with ECT, at the seventh session, at the last session, and at treatment completion. Depression symptoms were assessed using the BDI. | Cortisol levels remained stable between the seventh and the last sessions of ECT; values ranged at 0.686 ± 9.6330 g/dL for women, and there was a mean decrease of 5.825 ± 6.0780 g/dL (p = 0.024). After the seventh and the last ECT sessions, patients with depression and individuals in the control group had similar cortisol levels, whereas the BDI scores remained different. |
| Animal Studies * | |||
| Author | Mechanism | Methodology | Results |
| Roman et al. [107] | Immunological effects | Wistar rats received single or chronic treatment with ECS, once a day for 10 consecutive days, or sham ECS was administered likewise. The rats were killed 24 h after the last treatment, and peritoneal macrophages were cultured in vitro for a subsequent metabolic activity determination. | We found statistically significant changes in the biological properties of macrophages. Rats receiving chronic 10-fold ECS showed an increase in the macrophages’ metabolic activity, increased arginase activity, and a marked but statistically insignificant decrease in nitric oxide synthesis compared with the respective controls. |
| Author * | Psychiatric Disorder | Methodology | Results |
|---|---|---|---|
| Diermen et al. [16] | Depression | Meta-analysis with 34 randomized controlled clinical trials evaluating the effects of ECT in patients with major depression. | The presence of psychotic features is a predictor of ECT remission (OR = 1.47, p = 0.001) and response (OR = 1.69, p < 0.001), as is older age (SMD = 0.26 for remission and 0.35 for response p < 0.001). The severity of depression predicts response (SMD = 0.19, p = 0.001) but not remission. |
| Elias et al. [135] | Depression | Meta-analysis with 5 randomized controlled clinical trials that assessed the efficacy of continuation ECT and maintenance ECT in preventing relapse and recurrence of depression. | Continuation ECT and maintenance ECT with pharmacotherapy were associated with significantly fewer relapses and recurrences than pharmacotherapy. |
| Ahmed et al. [19] | Schizophrenia | Meta-analysis with 9 randomized controlled clinical trials evaluating the effects of TEC in patients with resistant schizophrenia. | The ECT augmentation technique was found to be effective in the reduction of psychometric scale scores, and the resulting improvement was significant. |
| Bahji et al. [137] | Bipolar depression | Meta-analysis with 19 randomized controlled clinical trials evaluating the effects of TEC in patients with bipolar disorder in a resistant depressive episode. | The pooled response and remission rates with TEC in bipolar depression were 77.1% (n = 437/567) and 52.3% (n = 275/377), respectively. Response rates to TEC were statistically higher in bipolar depression than in unipolar depression (OR = 0.73, 95% CI: 0.56–0.95, p = 0.02). |
| Ueda et al. [9] | PDP | Retrospective study evaluating the influence of acute ECT on PDP. | The psychosis scores after ECT improved significantly compared with those before ECT. |
| Maletzky et al. [11] | OCD | Systematic review of 50 articles reporting the efficacy of the acute treatment of ECT for OCD. | A positive response was reported in 60.4% of the 265 cases that were studied. |
| Margoob et al. [12] | PTSD | An open, prospective study evaluating the influence of ECT in patients with severe, chronic, extensive antidepressant-refractory PTSD. | Scores evaluating PTSD significantly decreased by a mean of 34.4%. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas, M.; Ariza, D.; Ortega, Á.; Riaño-Garzón, M.E.; Chávez-Castillo, M.; Pérez, J.L.; Cudris-Torres, L.; Bautista, M.J.; Medina-Ortiz, O.; Rojas-Quintero, J.; et al. Electroconvulsive Therapy in Psychiatric Disorders: A Narrative Review Exploring Neuroendocrine–Immune Therapeutic Mechanisms and Clinical Implications. Int. J. Mol. Sci. 2022, 23, 6918. https://doi.org/10.3390/ijms23136918
Rojas M, Ariza D, Ortega Á, Riaño-Garzón ME, Chávez-Castillo M, Pérez JL, Cudris-Torres L, Bautista MJ, Medina-Ortiz O, Rojas-Quintero J, et al. Electroconvulsive Therapy in Psychiatric Disorders: A Narrative Review Exploring Neuroendocrine–Immune Therapeutic Mechanisms and Clinical Implications. International Journal of Molecular Sciences. 2022; 23(13):6918. https://doi.org/10.3390/ijms23136918
Chicago/Turabian StyleRojas, Milagros, Daniela Ariza, Ángel Ortega, Manuel E. Riaño-Garzón, Mervin Chávez-Castillo, José Luis Pérez, Lorena Cudris-Torres, María Judith Bautista, Oscar Medina-Ortiz, Joselyn Rojas-Quintero, and et al. 2022. "Electroconvulsive Therapy in Psychiatric Disorders: A Narrative Review Exploring Neuroendocrine–Immune Therapeutic Mechanisms and Clinical Implications" International Journal of Molecular Sciences 23, no. 13: 6918. https://doi.org/10.3390/ijms23136918
APA StyleRojas, M., Ariza, D., Ortega, Á., Riaño-Garzón, M. E., Chávez-Castillo, M., Pérez, J. L., Cudris-Torres, L., Bautista, M. J., Medina-Ortiz, O., Rojas-Quintero, J., & Bermúdez, V. (2022). Electroconvulsive Therapy in Psychiatric Disorders: A Narrative Review Exploring Neuroendocrine–Immune Therapeutic Mechanisms and Clinical Implications. International Journal of Molecular Sciences, 23(13), 6918. https://doi.org/10.3390/ijms23136918







