Enterohemorrhagic Escherichia coli and a Fresh View on Shiga Toxin-Binding Glycosphingolipids of Primary Human Kidney and Colon Epithelial Cells and Their Toxin Susceptibility
Abstract
1. Introduction
2. Clinical Impact of Colonic EHEC Infections, Stx-Mediated Extraintestinal Complications, and Organ Damage
2.1. EHEC Zoonotic Infections and Reservoir
2.2. Epidemiology and Virulence Potency of EHEC
2.3. EHEC Colonization of the Gut
2.4. Colonic Outer-Membrane Vesicles of EHEC
2.5. Translocation of Shiga Toxin and Toxin Carriers in the Circulation
2.6. EHEC-Caused Systemic Complications
3. Shiga Toxin Structure and Glycosphingolipid Receptor Lipoforms
3.1. Shiga Toxin Structure
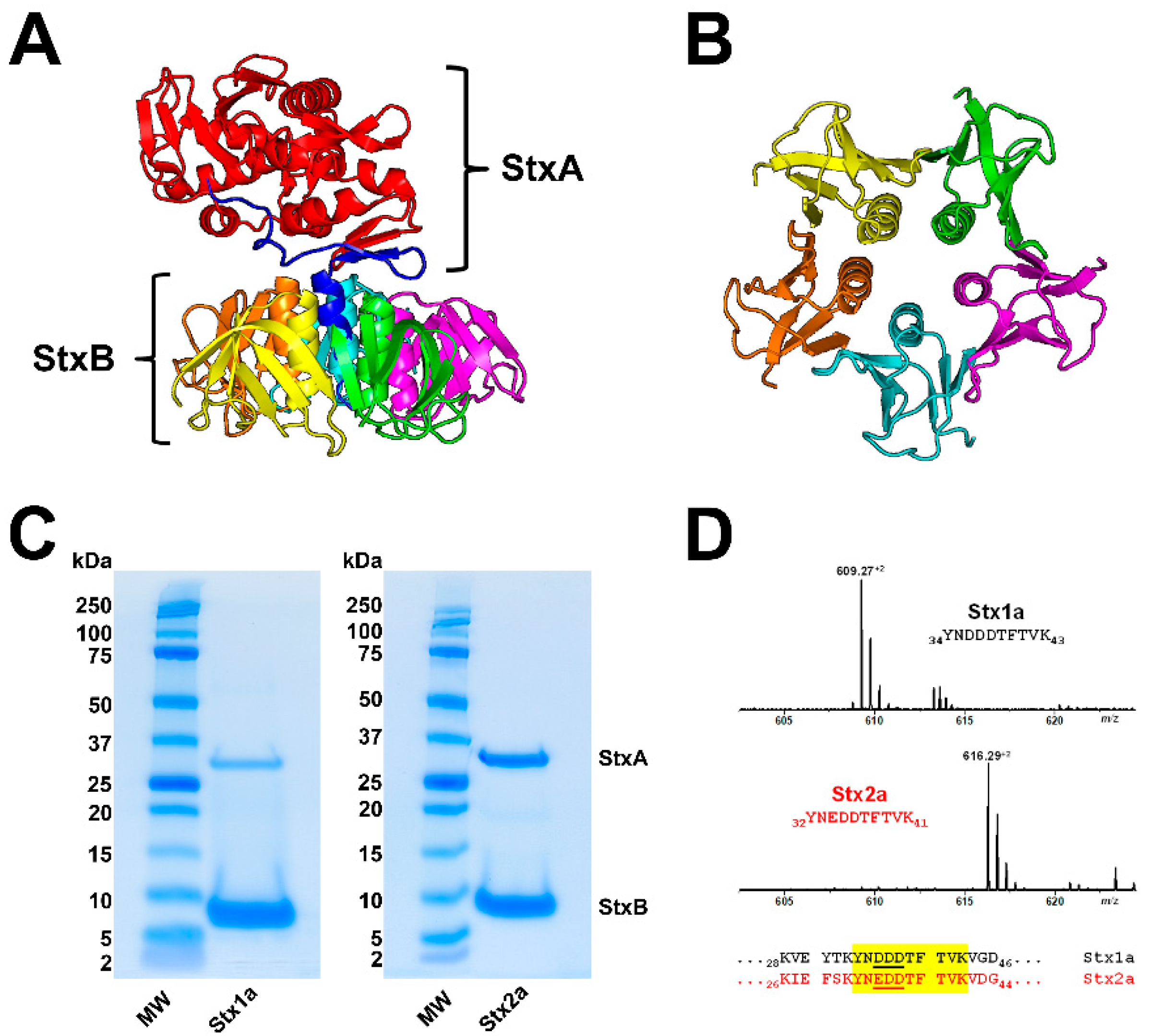
3.2. Glycosphingolipid Receptor Lipoforms
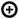 ), weak binding to Gb4Cer (
), weak binding to Gb4Cer (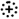 ), and no binding at all to Gb5Cer (
), and no binding at all to Gb5Cer ( ).
).
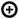 ), weak binding to Gb4Cer (
), weak binding to Gb4Cer (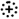 ), and no binding at all to Gb5Cer (
), and no binding at all to Gb5Cer ( ).
).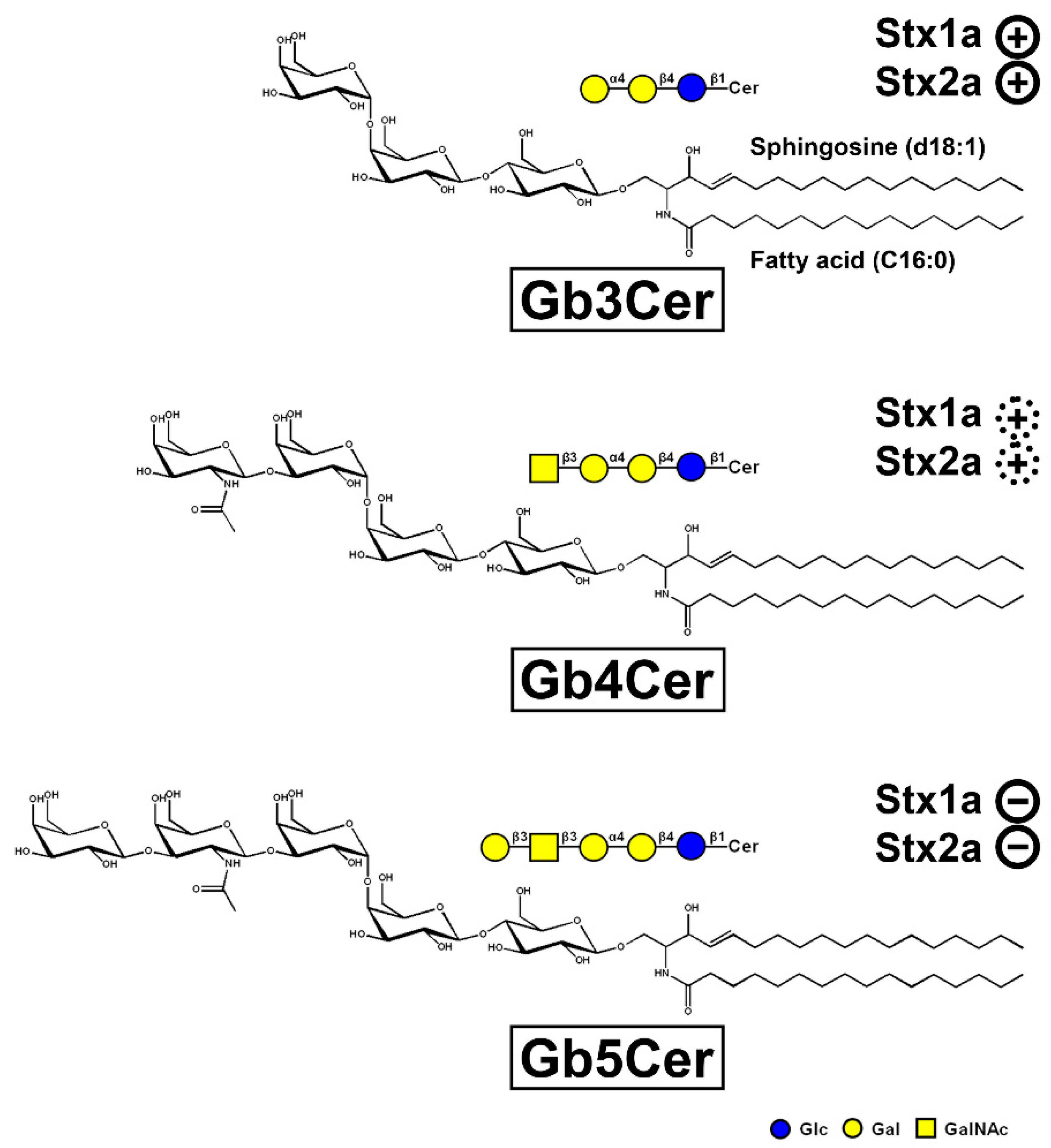
4. Shiga Toxin Receptor Glycosphingolipids of Primary Human Kidney and Colon Epithelial Cells
5. Liquid-Ordered and Liquid-Disordered Membrane Phases
5.1. The Lipid Raft Concept
5.2. Lipid Raft Association of Stx-Binding GSLs
5.3. Detergent-Resistant Membranes as Membrane Analog Tools
5.4. Membrane Distribution of Stx Receptor GSLs in pHRPTEpiCs and pHCoEpiCs
6. Different Susceptibility of Human Kidney and Colon Epithelial Cells toward Stx1a and Stx2a
7. Therapeutic Options of EHEC Infections
7.1. Application of Antibiotics or Not That’s the Question
7.2. Development of Non-Antibiotic Therapeutics
7.2.1. Inhibitors of Glycosphingolipid Biosynthesis and Stx-Neutralizing Glycoconjugates
7.2.2. Monoclonal Antibodies
7.2.3. Further Alternative Therapeutic Concepts
7.2.4. Current Situation
8. Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bock, K.; Karlsson, K.A.; Strömberg, N.; Teneberg, S. Interaction of viruses, bacteria and bacterial toxins with host cell surface glycolipids. Aspects on receptor identification and dissection of binding epitopes. Adv. Exp. Med. Biol. 1988, 228, 153–186. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, K.A. Microbial recognition of target-cell glycoconjugates. Curr. Opin. Struct. Biol. 1995, 5, 622–635. [Google Scholar] [CrossRef]
- Lencer, W.I.; Saslowsky, D. Raft trafficking of AB5 subunit bacterial toxins. Biochim. Biophys. Acta 2005, 1746, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Beddoe, T.; Paton, A.W.; Le Nours, J.; Rossjohn, J.; Paton, J.C. Structure, biological functions and applications of the AB5 toxins. Trends Biochem. Sci. 2010, 35, 411–418. [Google Scholar] [CrossRef]
- Cho, J.A.; Chinnapen, D.J.F.; Aamar, E.; Te Welscher, Y.M.; Lencer, W.I.; Massol, R. Insights on the trafficking and retro-translocation of glycosphingolipid-binding bacterial toxins. Front. Cell. Infect. Microbiol. 2012, 2, 51. [Google Scholar] [CrossRef]
- Kavaliauskiene, S.; Lingelem, A.B.D.; Skotland, T.; Sandvig, K. Protection against Shiga toxins. Toxins 2017, 9, 44. [Google Scholar] [CrossRef]
- Fishman, P.H. Role of membrane gangliosides in the binding and action of bacterial toxins. J. Membr. Biol. 1982, 69, 85–97. [Google Scholar] [CrossRef]
- Baldauf, K.J.; Royal, J.M.; Hamorsky, K.T.; Matoba, N. Cholera toxin B: One subunit with many pharmaceutical applications. Toxins 2015, 7, 974–996. [Google Scholar] [CrossRef]
- Kenworthy, A.K.; Schmieder, S.S.; Raghunathan, K.; Tiwari, A.; Wang, T.; Kelly, C.V.; Lencer, W.I. Cholera toxin as a probe for membrane biology. Toxins 2021, 13, 543. [Google Scholar] [CrossRef]
- Berenson, C.S.; Nawar, H.F.; Kruzel, R.L.; Mandell, L.M.; Connell, T.D. Ganglioside-binding specificities of E. coli enterotoxin LT-IIc: Importance of long-chain fatty acyl ceramide. Glycobiology 2013, 23, 23–31. [Google Scholar] [CrossRef][Green Version]
- Zalem, D.; Ribeiro, J.P.; Varrot, A.; Lebens, M.; Imberty, A.; Teneberg, S. Biochemical and structural characterization of the novel sialic acid-binding site of Escherichia coli heat-labile enterotoxin LT-IIb. Biochem. J. 2016, 473, 3923–3936. [Google Scholar] [CrossRef]
- Patry, R.T.; Stahl, M.; Perez-Munoz, M.E.; Nothaft, H.; Wenzel, C.Q.; Sacher, J.C.; Coros, C.; Walter, J.; Vallance, B.A.; Szymanski, C.M. Bacterial AB5 toxins inhibit the growth of gut bacteria by targeting ganglioside-like glycoconjugates. Nat. Commun. 2019, 10, 1390. [Google Scholar] [CrossRef]
- Byres, E.; Paton, A.W.; Paton, J.C.; Löfling, J.C.; Smith, D.F.; Wilce, M.C.J.; Talbot, U.M.; Chong, D.C.; Yu, H.; Huang, S.; et al. Incorporation of non-human glycan mediates human susceptibility to a bacterial toxin. Nature 2008, 456, 648–652. [Google Scholar] [CrossRef]
- Paton, A.W.; Paton, J.C. Escherichia coli subtilase cytotoxin. Toxins 2010, 2, 215–228. [Google Scholar] [CrossRef]
- Amaral, M.M.; Sacerdoti, F.; Jancic, C.; Repetto, H.A.; Paton, A.W.; Paton, J.C.; Ibarra, C. Action of Shiga toxin type-2 and subtilase cytotoxin on human microvascular endothelial cells. PLoS ONE 2013, 8, e70431. [Google Scholar] [CrossRef]
- Le Nours, J.; Paton, A.W.; Byres, E.; Troy, S.; Herdman, B.P.; Johnson, M.D.; Paton, J.C.; Rossjohn, J.; Beddoe, T. Structural basis of subtilase cytotoxin SubAB assembly. J. Biol. Chem. 2013, 288, 27505–27516. [Google Scholar] [CrossRef]
- Funk, J.; Biber, N.; Schneider, M.; Hauser, E.; Enzenmüller, S.; Förtsch, C.; Barth, H.; Schmidt, H. Cytotoxic and apoptotic effects of recombinant subtilase cytotoxin variants of Shiga toxin- producing Escherichia coli. Infect. Immun. 2015, 83, 2338–2349. [Google Scholar] [CrossRef]
- Hauser, E.; Bruederle, M.; Reich, C.; Bruckbauer, A.; Funk, J.; Schmidt, H. Subtilase contributes to the cytotoxicity of Shiga toxin-producing Escherichia coli strain encoding three different toxins. Int. J. Food Microbiol. 2016, 217, 156–161. [Google Scholar] [CrossRef]
- Bergan, J.; Dyve Lingelem, A.B.; Simm, R.; Skotland, T.; Sandvig, K. Shiga toxins. Toxicon 2012, 60, 1085–1107. [Google Scholar] [CrossRef]
- Melton-Celsa, A.R. Shiga toxin (Stx) classification, structure, and function. Microbiol. Spectr. 2014, 2, EHEC-0024-2013. [Google Scholar] [CrossRef]
- Lee, M.S.; Koo, S.; Jeong, D.G.; Tesh, V.L. Shiga toxins as multi-functional proteins: Induction of host cellular stress responses, role in pathogenesis and therapeutic applications. Toxins 2016, 8, 77. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, S.; Thaker, H.; Dong, M. Shiga toxins: An update on host factors and biomedical applications. Toxins 2021, 13, 222. [Google Scholar] [CrossRef]
- Piérard, D.; De Greve, H.; Haesebrouck, F.; Mainil, J. O157:H7 and O104:H4 Vero/Shiga toxin-producing Escherichia coli outbreaks: Respective role of cattle and humans. Vet. Res. 2012, 43, 13. [Google Scholar] [CrossRef]
- Beutin, L.; Fach, P. Detection of Shiga toxin-producing Escherichia coli from nonhuman sources and strain typing. Microbiol. Spectr. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Menge, C. The role of Escherichia coli Shiga toxins in STEC colonization of cattle. Toxins 2020, 12, 607. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.L.; Kase, J.A.; Harrison, L.M.; Balan, K.V.; Babu, U.; Chen, Y.; Macarisin, D.; Kwon, H.J.; Zheng, J.; Stevens, E.L.; et al. The persistence of bacterial pathogens in surface water and its impact on global food safety. Pathogens 2021, 10, 1391. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, M.S.; Kim, J.H. Recent updates on outbreaks of Shiga toxin-producing Escherichia coli and its potential reservoirs. Front. Cell. Infect. Microbiol. 2020, 10, 273. [Google Scholar] [CrossRef] [PubMed]
- Chase-Topping, M.; Gally, D.; Low, C.; Matthews, L.; Woolhouse, M. Super-shedding and the link between human infection and livestock carriage of Escherichia coli O157. Nat. Rev. Microbiol. 2008, 6, 904–912. [Google Scholar] [CrossRef]
- Moxley, R.A.; Acuff, G.R. Peri- and postharvest factors in the control of Shiga toxin-producing Escherichia coli in beef. Microbiol. Spectr. 2014, 2, 2–6. [Google Scholar] [CrossRef]
- Kolenda, R.; Burdukiewicz, M.; Schierack, P. A systematic review and meta-analysis of the epidemiology of pathogenic Escherichia coli of calves and the role of calves as reservoirs for human pathogenic E. coli. Front. Cell. Infect Microbiol. 2015, 5, 23. [Google Scholar] [CrossRef]
- Munns, K.D.; Selinger, L.B.; Stanford, K.; Guan, L.; Callaway, T.R.; McAllister, T.A. Perspectives on super-shedding of Escherichia coli O157:H7 by cattle. Foodborne Pathog. Dis. 2015, 12, 89–103. [Google Scholar] [CrossRef]
- Barth, S.A.; Bauerfeind, R.; Berens, C.; Menge, C. Shiga toxin-producing E. coli in animals: Detection, characterization, and virulence assessment. Methods Mol. Biol. 2021, 2291, 19–86. [Google Scholar] [CrossRef]
- Caprioli, A.; Morabito, S.; Brugère, H.; Oswald, E. Enterohaemorrhagic Escherichia coli: Emerging issues on virulence and modes of transmission. Vet. Res. 2005, 36, 289–311. [Google Scholar] [CrossRef]
- Tseng, M.; Fratamico, P.M.; Manning, S.D.; Funk, J.A. Shiga toxin-producing Escherichia coli in swine: The public health perspective. Anim. Health Res. Rev. 2014, 15, 63–75. [Google Scholar] [CrossRef]
- Moxley, R.A. Edema disease. Vet. Clin. N. Am. Food Anim. Pract. 2000, 16, 175–185. [Google Scholar] [CrossRef]
- Ercoli, L.; Farneti, S.; Ranucci, D.; Scuota, S.; Branciari, R. Role of verocytotoxigenic Escherichia coli in the swine production chain. Ital. J. Food Sci. 2015, 4, 5156. [Google Scholar] [CrossRef]
- Casanova, N.A.; Redondo, L.M.; Dailoff, G.C.; Arenas, D.; Miyakawa, M.E.F. Overview of the role of Shiga toxins in porcine edema disease pathogenesis. Toxicon 2018, 148, 149–154. [Google Scholar] [CrossRef]
- Barth, S.A.; Menge, C.; Eichhorn, I.; Semmler, T.; Wieler, L.H.; Pickard, D.; Belka, A.; Berens, C.; Geue, L. The accessory genome of Shiga toxin-producing Escherichia coli defines a persistent colonization type in cattle. Appl. Environ. Microbiol. 2016, 82, 5455–5464. [Google Scholar] [CrossRef]
- Hamm, K.; Barth, S.A.; Stalb, S.; Geue, L.; Liebler-Tenorio, E.; Teifke, J.P.; Lange, E.; Tauscher, K.; Kotterba, G.; Bielaszewska, M.; et al. Experimental infection of calves with Escherichia coli O104:H4 outbreak strain. Sci. Rep. 2016, 6, 32812. [Google Scholar] [CrossRef]
- Moxley, R.A. Escherichia coli O157:H7: An update on intestinal colonization and virulence mechanisms. Anim. Health Res. Rev. 2004, 5, 15–33. [Google Scholar] [CrossRef]
- Moxley, A.R.; Smith, D.R. Attaching-effacing Escherichia coli infections in cattle. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 29–56. [Google Scholar] [CrossRef]
- Menge, C.; Stamm, I.; Wuhrer, M.; Geyer, R.; Wieler, L.H.; Baljer, G. Globotriaosylceramide (Gb(3)/CD77) is synthesized and surface expressed by bovine lymphocytes upon activation in vitro. Vet. Immunol. Immunopathol. 2001, 83, 19–36. [Google Scholar] [CrossRef]
- Stamm, I.; Wuhrer, M.; Geyer, R.; Baljer, G.; Menge, C. Bovine lymphocytes express functional receptors for Escherichia coli Shiga toxin 1. Microb. Pathog. 2002, 33, 251–264. [Google Scholar] [CrossRef]
- Stamm, I.; Mohr, M.; Bridger, P.S.; Schröpfer, E.; König, M.; Stoffregen, W.C.; Dean-Nystrom, E.A.; Baljer, G.; Menge, C. Epitheila and mesenchymal cells in the bovine colonic mucosa differ in their responsiveness to Escherichia coli Shiga toxin 1. Infect. Immun. 2008, 76, 5381–5391. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Menge, C.; Loos, D.; Bridger, P.S.; Barth, S.; Werling, D.; Baljer, G. Bovine macrophages sense Escherichia coli Shiga toxin 1. Innate Immun. 2015, 21, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Hoey, D.E.; Currie, C.; Else, R.W.; Nutikka, A.; Lingwood, C.A.; Gally, D.L.; Smith, D.G. Expression of receptors for verotoxin 1 from Escherichia coli O157 on bovine intestinal epithelium. J. Med. Microbiol. 2002, 5, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Valdivieso-Garcia, A.; MacLeod, D.L.; Clarke, R.C.; Gyles, C.L.; Lingwood, C.; Boyd, B.; Durette, A. Comparative cytotoxicity of purified Shiga-like toxin-IIe on porcine and bovine aortic endothelial and human colonic adenocarcinoma cells. J. Med. Microbiol. 1996, 45, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Duvar, S.; Peter-Katalinić, J.; Hanisch, F.G.; Müthing, J. Isolation and structural characterization of glycosphingolipids of in vitro propagated bovine aortic endothelial cells. Glycobiology 1997, 7, 1099–1109. [Google Scholar] [CrossRef][Green Version]
- FAO/WHO STEC EXPERT GROUP. Hazard identification and characterization: Criteria for categorizing Shiga toxin-producing Escherichia coli on a risk basis. J. Food Prot. 2019, 82, 7–21. [Google Scholar] [CrossRef]
- Scheutz, F.; Teel, L.D.; Beutin, L.; Piérard, D.; Buvens, G.; Karch, H.; Mellmann, A.; Caprioli, A.; Tozzoli, R.; Morabito, S.; et al. Multicenter evaluation of a sequence-based protocol for subtyping Shiga toxins and standardizing Stx nomenclature. J. Clin. Microbiol. 2012, 50, 2951–2963. [Google Scholar] [CrossRef]
- Verweyen, H.M.; Karch, H.; Brandis, M.; Zimmerhackl, L.B. Enterohemorrhagic Escherichia coli infections: Following transmission routes. Pediatr. Nephrol. 2000, 14, 73–83. [Google Scholar] [CrossRef]
- Omer, M.K.; Álvarez-Ordoñez, A.; Prieto, M.; Skjerve, E.; Asehun, T.; Alvseike, O.A. A systematic review of bacterial foodborne outbreaks related to red meat and meat products. Foodborne Pathog. Dis. 2018, 15, 598–611. [Google Scholar] [CrossRef]
- Tarr, G.A.M.; Stokowski, T.; Shringi, S.; Tarr, P.I.; Freedman, S.B.; Oltean, H.N.; Rabinowitz, P.M.; Chui, L. Contribution and interaction of Shiga toxin genes to Escherichia coli O157:H7 virulence. Toxins 2019, 11, 607. [Google Scholar] [CrossRef]
- Bielaszewska, M.; Mellmann, A.; Zhang, W.; Köck, R.; Fruth, A.; Bauwens, A.; Peters, G.; Karch, H. Characterisation of the Escherichia coli strain associated with an outbreak of haemolytic uraemic syndrome in Germany, 2011: A microbiological study. Lancet Infect. 2011, 11, 671–676. [Google Scholar] [CrossRef]
- Mellmann, A.; Harmsen, D.; Cummings, C.A.; Zentz, E.B.; Leopold, S.R.; Rico, A.; Prior, K.; Szczepanowski, R.; Ji, Y.; Zhang, W.; et al. Prospective genome characterization of the German enterohemorrhagic Escherichia coli O104:H4 outbreak by rapid next generation sequencing technology. PLoS ONE 2011, 6, e22751. [Google Scholar] [CrossRef]
- Karch, H.; Denamur, E.; Dobrindt, U.; Finlay, B.B.; Hengge, R.; Johannes, L.; Ron, E.Z.; Tønjum, T.; Sansonetti, P.J.; Vicente, M. The enemy within us: Lessons from the 2011 European Escherichia coli O104:H4 outbreak. EMBO Mol. Med. 2012, 4, 841–848. [Google Scholar] [CrossRef]
- Muniesa, M.; Hammerl, J.A.; Hertwig, S.; Appel, B.; Brüssow, H. Shiga toxin-producing Escherichia coli O104:H4: A new challenge for microbiology. Appl. Environ. Microbiol. 2012, 78, 4065–4073. [Google Scholar] [CrossRef]
- Page, A.V.; Liles, W.C. Enterohemorrhagic Escherichia coli infections and the hemolytic-uremic syndrome. Med. Clin. N. Am. 2013, 97, 681–695. [Google Scholar] [CrossRef]
- Navarro-Garcia, F. Escherichia coli O104:H4 pathogenesis: An enteroaggregative E. coli/Shiga toxin-producing E. coli explosive cocktail of high virulence. Microbiol. Spectr. 2014, 2, 2–6. [Google Scholar] [CrossRef]
- Boisen, N.; Melton-Celsa, A.R.; Scheutz, F.; O’Brien, A.D.; Nataro, J.P. Shiga toxin 2a and enteroaggregative Escherichia coli—A deadly combination. Gut Microbes 2015, 6, 272–278. [Google Scholar] [CrossRef]
- Kampmeier, S.; Berger, M.; Mellmann, A.; Karch, H.; Berger, P. The 2011 German enterohemorrhagic Escherichia coli O104:H4 outbreak—The danger is still out there. Curr. Top. Microbiol. Immunol. 2018, 416, 117–148. [Google Scholar] [CrossRef]
- Karch, H.; Tarr, P.I.; Bielaszewska, M. Enterohaemorrhagic Escherichia coli in human medicine. Int. J. Med. Microbiol. 2005, 295, 405–418. [Google Scholar] [CrossRef]
- Tarr, P.I.; Gordon, C.A.; Chandler, W.L. Shiga-toxin-producing Escherichia coli and haemolytic urameic syndrome. Lancet 2005, 365, 1073–1086. [Google Scholar] [CrossRef]
- Davis, T.K.; Van De Kar, N.C.; Tarr, P.I. Shiga toxin/verocytotoxin-producing Escherichia coli infections: Practical clinical perspectives. Microbiol. Spectr. 2014, 2, EHEC-0025-2014. [Google Scholar] [CrossRef]
- Friedrich, A.W.; Bielaszewska, M.; Zhang, W.L.; Pulz, M.; Kuczius, T.; Ammon, A.; Karch, H. Escherichia coli harboring Shiga toxin 2 gene variants: Frequency and association with clinical symptoms. J. Infect. Dis. 2002, 185, 74–84. [Google Scholar] [CrossRef]
- Sonntag, A.K.; Bielaszewska, M.; Mellmann, A.; Dierksen, N.; Schierack, P.; Wieler, L.H.; Schmidt, M.A.; Karch, H. Shiga toxin 2e-producing Escherichia coli isolates from humans and pigs differ in their virulence profiles and interactions with intestinal epithelial cells. Appl. Environ. Microbiol. 2005, 71, 8855–8863. [Google Scholar] [CrossRef]
- Fuller, C.A.; Pellino, C.A.; Flagler, M.J.; Strasser, J.E.; Weiss, A.A. Shiga toxin subtypes display dramatic differences in potency. Infect. Immun. 2011, 79, 1329–1337. [Google Scholar] [CrossRef]
- Fruth, A.; Prager, R.; Tietze, E.; Rabsch, W.; Flieger, A. Molecular epidemiological view on Shiga toxin-producing Escherichia coli causing human disease in Germany: Diversity, prevalence, and outbreaks. Int. J. Med. Microbiol. 2015, 305, 697–704. [Google Scholar] [CrossRef]
- Nakao, H.; Takeda, T. Escherichia coli Shiga toxin. J. Nat. Toxins 2000, 9, 299–313. [Google Scholar]
- Basu, D.; Tumer, N.E. Do the A subunits contribute to the differences in the toxicity of Shiga toxin 1 and Shiga toxin 2? Toxins 2015, 7, 1467–1485. [Google Scholar] [CrossRef] [PubMed]
- Bitzan, M.; Schaefer, F.; Reymond, D. Treatment of typical (enteropathic) hemolytic uremic syndrome. Semin. Thromb. Hemost. 2010, 36, 594–610. [Google Scholar] [CrossRef] [PubMed]
- Basu, D.; Li, X.P.; Kahn, J.N.; May, K.L.; Kahn, P.C.; Tumer, N.E. The A1 subunit of Shiga toxin 2 has higher affinity for ribosomes and higher catalytic activity than the A1 subunit of Shiga toxin 1. Infect. Immun. 2015, 84, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Orth, D.; Grif, K.; Khan, A.B.; Naim, A.; Dierich, M.P.; Würzner, R. The Shiga toxin genotype rather than the amount of Shiga toxin or the cytotoxicity of Shiga toxin in vitro correlates with the appearance of the hemolytic uremic syndrome. Diagn. Microbiol. Infect. Dis. 2007, 59, 235–242. [Google Scholar] [CrossRef]
- Ogura, Y.; Mondal, S.I.; Islam, M.R.; Mako, T.; Arisawa, K.; Katsura, K.; Ooka, T.; Gotoh, Y.; Murase, K.; Ohnishi, M.; et al. The Shiga toxin 2 production level in enterohemorrhagic Escherichia coli O157:H7 is correlated with the subtypes of toxin-encoding phage. Sci. Rep. 2015, 5, 16663. [Google Scholar] [CrossRef]
- Fagerlund, A.; Aspholm, M.; Węgrzyn, G.; Lindbäck, T. High diversity in the regulatory region of Shiga toxin encoding bacteriophages. BMC Genom. 2022, 23, 230. [Google Scholar] [CrossRef]
- Janka, A.; Bielaszewska, M.; Dobrindt, U.; Greune, L.; Schmidt, M.A.; Karch, H. Cytolethal distending toxin gene cluster in enterohemorrhagic Escherichia coli O157:H- and O157:H7: Characterization and evolutionary considerations. Infect. Immun. 2003, 71, 3634–3638. [Google Scholar] [CrossRef]
- Bielaszewska, M.; Fell, M.; Greune, L.; Prager, R.; Fruth, A.; Tschäpe, H.; Schmidt, M.A.; Karch, H. Characterization of cytolethal distending toxin genes and expression in Shiga toxin-producing Escherichia coli strains of non-O157 serogroups. Infect. Immun. 2004, 72, 1812–1816. [Google Scholar] [CrossRef]
- Bielaszewska, M.; Sinha, B.; Kuczius, T.; Karch, H. Cytolethal distending toxin from Shiga toxin-producing Escherichia coli O157 causes irreversible G2/M arrest, inhibition of proliferation, and death of human endothelial cells. Infect. Immun. 2005, 73, 552–562. [Google Scholar] [CrossRef]
- Friedrich, A.W.; Lu, S.; Bielaszewska, M.; Prager, R.; Bruns, P.; Xu, J.G.; Tschäpe, H.; Karch, H. Cytolethal distending toxin in Escherichia coli O157:H7: Spectrum of conservation, structure, and endothelial toxicity. J. Clin. Microbiol. 2006, 44, 1844–1846. [Google Scholar] [CrossRef]
- Orth, D.; Grif, K.; Dierich, M.P.; Würzner, R. Cytolethal distending toxins in Shiga toxin-producing Escherichia coli: Alleles, serotype distribution and biological effects. J. Med. Microbiol. 2006, 55, 1487–1492. [Google Scholar] [CrossRef][Green Version]
- Bielaszewska, M.; Stoewe, F.; Fruth, A.; Zhang, W.; Prager, R.; Brockmeyer, J.; Mellmann, A.; Karch, H.; Friedrich, A.W. Shiga toxin, cytolethal distending toxin, and hemolysin repertoires in clinical Escherichia coli O91 isolates. J. Clin. Microbiol. 2009, 47, 2061–2066. [Google Scholar] [CrossRef]
- Schmidt, H.; Benz, R. Detection and characterization of EHEC-hemolysin. Methods Mol. Med. 2003, 73, 151–163. [Google Scholar] [CrossRef]
- Bielaszewska, M.; Aldick, T.; Bauwens, A.; Karch, H. Hemolysin of enterohemorrhagic Escherichia coli: Structure, transport, biological activity and putative role in virulence. Int. J. Med. Microbiol. 2014, 304, 521–529. [Google Scholar] [CrossRef]
- Schwidder, M.; Heinisch, L.; Schmidt, H. Genetics, toxicity, and distribution of enterohemorrhagic Escherichia coli hemolysin. Toxins 2019, 11, 502. [Google Scholar] [CrossRef]
- Funk, J.; Stoeber, H.; Hauser, E.; Schmidt, H. Molecular analysis of subtilase cytotoxin genes of food-borne Shiga toxin-producing Escherichia coli reveals a new allelic subAB variant. BMC Microbiol. 2013, 13, 230. [Google Scholar] [CrossRef]
- Krause, M.; Sessler, K.; Kaziales, A.; Grahl, R.; Noettger, S.; Barth, H.; Schmidt, H. Variants of Escherichia coli subtilase cytotoxin subunits show differences in complex formation in vitro. Toxins 2019, 11, 703. [Google Scholar] [CrossRef]
- Sessler, K.; Papatheodorou, P.; Wondany, F.; Krause, M.; Noettger, S.; Bernhard, D.; Michaelis, J.; Schmidt, H.; Barth, H. The enzyme subunit SubA of Shiga toxin-producing E. coli strains demonstrates comparable intracellular transport and cytotoxic activity as the holotoxin SubAB in HeLa and HCT116 cells in vitro. Arch. Toxicol. 2021, 95, 975–983. [Google Scholar] [CrossRef]
- Álvarez, R.S.; Gómez, F.D.; Zotta, E.; Paton, A.W.; Paton, J.C.; Ibarra, C.; Sacerdoti, F.; Amaral, M.M. Combined action of Shiga toxin type 2 and subtilase cytotoxin in the pathogenesis of hemolytic uremic syndrome. Toxins 2021, 13, 536. [Google Scholar] [CrossRef]
- Schüller, S. Shiga toxin interaction with human intestinal epithelium. Toxins 2011, 3, 626–639. [Google Scholar] [CrossRef]
- Lai, Y.; Rosenshine, I.; Leong, J.M.; Frankel, G. Intimate host attachment: Enteropathogenic and enterohaemorrhagic Escherichia coli. Cell. Microbiol. 2013, 15, 1796–1808. [Google Scholar] [CrossRef]
- Monteiro, R.; Ageorges, V.; Rojas-Lopez, M.; Schmidt, H.; Weiss, A.; Bertin, Y.; Forano, E.; Jubelin, G.; Henderson, I.R.; Livrelli, V.; et al. A secretome view of colonisation factors in Shiga toxin-encoding Escherichia coli (STEC): From enterohaemorrhagic E. coli (EHEC) to related enteropathotypes. FEMS Microbiol. Lett. 2016, 363, fnw179. [Google Scholar] [CrossRef] [PubMed]
- Pifer, R.; Sperandio, V. The interplay between the microbiota and enterohemorrhagic Escherichia coli. Microbiol. Spectr. 2014, 2, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Ducarmon, Q.R.; Zwittink, R.D.; Hornung, B.V.H.; van Schaik, W.; Young, V.B.; Kuijper, E.J. Gut microbiota and colonization resistance against bacterial enteric infection. Microbiol. Mol. Biol. Rev. 2019, 83, e00007-19. [Google Scholar] [CrossRef] [PubMed]
- Tovaglieri, A.; Sontheimer-Phelps, A.; Geirnaert, A.; Prantil-Baun, R.; Camacho, D.M.; Chou, D.B.; Jalili-Firoozinezhad, S.; De Wouters, T.; Kasendra, M.; Super, M.; et al. Species-specific enhancement of enterohemorhhagic E. coli pathogenesis mediated by microbiome metabolites. Microbiome 2019, 7, 43. [Google Scholar] [CrossRef]
- Josenhans, C.; Müthing, J.; Elling, L.; Bartfeld, S.; Schmidt, H. How bacterial pathogens of the gastrointestinal tract use the mucosal glyco-code to harness mucus and microbiota: New ways to study an ancient bag of tricks. Int. J. Med. Microbiol. 2020, 310, 151392. [Google Scholar] [CrossRef]
- Nawrocki, E.M.; Mosso, H.M.; Dudley, E.G. A toxic environment: A growing understanding of how microbial communities affect Escherichia coli O157:H7 Shiga toxin expression. Appl. Environ. Microbiol. 2020, 86, e00509-20. [Google Scholar] [CrossRef]
- Lee, K.S.; Jeong, Y.J.; Lee, M.S. Escherichia coli Shiga toxins and gut microbiota interactions. Toxins 2021, 13, 416. [Google Scholar] [CrossRef]
- Jores, J.; Rumer, L.; Wieler, L.H. Impact of the locus of enterocyte effacement pathogenicity island on the evolution of pathogenic Escherichia coli. Int. J. Med. Microbiol. 2004, 294, 103–113. [Google Scholar] [CrossRef]
- Kendall, M.M. Interkingdom chemical signaling in enterohemorrhagic Escherichia coli O157:H7. Adv. Exp. Med. Biol. 2016, 874, 201–213. [Google Scholar] [CrossRef]
- Schmidt, M.A. LEEways: Tales of EPEC, ATEC and EHEC. Cell. Microbiol. 2010, 12, 1544–1552. [Google Scholar] [CrossRef]
- Stevens, M.P.; Frankel, G.M. The locus of enterocyte effacement and associated virulence factors of enterohemorrhagic Escherichia coli. Microbiol. Spectr. 2014, 2, EHEC-0007-2013. [Google Scholar] [CrossRef]
- Conolly, J.P.R.; Finlay, B.B.; Roe, A.J. From ingestion to colonization: The influence of the host environment on regulation of the LEE encoded type III secretion system in enterohaemorrhagic Escherichia coli. Front. Microbiol. 2015, 6, 568. [Google Scholar] [CrossRef]
- Furniss, R.C.; Clements, A. Regulation of the locus of enterocyte effacement in attaching and effacing pathogens. J. Bacteriol. 2017, 200, e00336-17. [Google Scholar] [CrossRef]
- Melton-Celsa, A.; Mohawk, K.; Teel, L.; O’Brien, A. Pathogenesis of Shiga toxin-producing Escherichia coli. Curr. Top. Microbiol. Immunol. 2012, 357, 67–103. [Google Scholar] [CrossRef]
- McWilliams, B.D.; Torres, A.G. Enterohemorrhagic Escherichia coli adhesins. Microbiol. Spectr. 2014, 2. [Google Scholar] [CrossRef]
- Gaytán, M.O.; Martínez, V.I.; Soto, E.; González-Pedrajo, B. Type three secretion system in attaching and effacing pathogens. Front. Cell. Infect. Microbiol. 2016, 6, 129. [Google Scholar] [CrossRef]
- Frankel, G.; Phillips, A.D. Attaching effacing Escherichia coli and paradigms of Tir-triggered actin polymerization: Getting off the pedestal. Cell. Microbiol. 2008, 10, 549–556. [Google Scholar] [CrossRef]
- Campellone, K.G. Cytoskeleton-modulating effectors of enteropathogenic and enterohaemorrhagic Escherichia coli: Tir, EspFU and actin pedestal assembly. FEBS J. 2010, 277, 2390–2402. [Google Scholar] [CrossRef]
- Franzin, F.M.; Sircili, M.P. Locus for enterocyte effacement: A pathogenicity island involved in the virulence of enteropathogenic and enterohemorrhagic Escherichia coli subjected to a complex network of gene regulation. Biomed. Res. Int. 2015, 2015, 534738. [Google Scholar] [CrossRef]
- Thorpe, C.M.; Pulsifer, M.S.; Osburne, M.S.; Vanaja, S.K.; Leong, J.M. Citrobacter rodentium (ΦStx2dact), a murine infection model for enterohemorrhagic Escherichia coli. Curr. Opin. Microbiol. 2022, 65, 183–190. [Google Scholar] [CrossRef]
- Herold, S.; Karch, H.; Schmidt, H. Shiga toxin-encoding bacteriophages—Genomes in motion. Int. J. Med. Microbiol. 2004, 294, 115–121. [Google Scholar] [CrossRef]
- Allison, H.E. Stx-phages: Drivers and mediators of the evolution of STEC and STEC-like pathogens. Future Microbiol. 2007, 2, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Krüger, A.; Lucchesi, P.M.A. Shiga toxins and stx phages: Highly diverse entities. Microbiology 2015, 161, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Wagner, P.I.; Livny, J.; Neely, M.N.; Acheson, D.W.K.; Friedman, D.I.; Waldor, M.K. Bacteriophage control of Shiga toxin 1 production and release by Escherichia coli. Mol. Microbiol. 2002, 44, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Iversen, H.; Abée-Lund, T.M.; Aspholm, M.; Arnesen, L.P.; Lindbäck, T. Commensal E. coli Stx2 lysogens produce high levels of phages after spontaneous prophage induction. Front. Cell. Infect. Microbiol. 2015, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rubio, L.; Haarmann, N.; Schwidder, M.; Muniesa, M.; Schmidt, H. Bacteriophages of Shiga toxin-producing Escherichia coli and their contribution to pathogenicity. Pathogens 2021, 10, 404. [Google Scholar] [CrossRef]
- Kolling, G.L.; Matthews, K.R. Export of virulence genes and Shiga toxin by membrane vesicles of Escherichia coli O157:H7. Appl. Environ. Microbiol. 1999, 65, 1843–1848. [Google Scholar] [CrossRef]
- Kunsmann, L.; Rüter, C.; Bauwens, A.; Greune, L.; Glüder, M.; Kemper, B.; Fruth, A.; Wai, S.N.; He, X.; Lloubes, R.; et al. Virulence from vesicles: Novel mechanisms of host cell injury by Escherichia coli O104:H4 outbreak strain. Sci. Rep. 2015, 5, 13252. [Google Scholar] [CrossRef]
- Bielaszewska, M.; Rüter, C.; Bauwens, A.; Greune, L.; Jarosch, K.A.; Steil, D.; Zhang, W.; He, X.; Lloubes, R.; Fruth, A.; et al. Host cell interactions of outer membrane vesicle-associated virulence factors of enterohemorrhagic Escherichia coli O157: Intracellular delivery, trafficking and mechanisms of cell injury. PLoS Pathog. 2017, 3, e1006159. [Google Scholar] [CrossRef]
- Bielaszewska, M.; Greune, L.; Bauwens, A.; Dersch, P.; Mellmann, A.; Rüter, C. Virulence factor cargo and host cell interactions of Shiga toxin-producing Escherichia coli outer membrane vesicles. Methods Mol. Biol. 2021, 2291, 177–205. [Google Scholar] [CrossRef]
- Bauwens, A.; Kunsmann, L.; Karch, H.; Mellmann, A.; Bielaszewska, M. Antibiotic-mediated modulations of outer membrane vesicles in enterohemorrhagic Escherichia coli O104:H4 and O157:H7. Antimicrob. Agents Chemother. 2017, 61, e00937-17. [Google Scholar] [CrossRef]
- Bauwens, A.; Kunsmann, L.; Mareijková, M.; Zhang, W.; Karch, H.; Bielaszewska, M.; Mellmann, A. Intrahost milieu modulates production of outer membrane vesicles, vesicle-associated Shiga toxin 2a and cytotoxicity in Escherichia coli O157:H7 and O104:H4. Environ. Microbiol. 2017, 9, 626–634. [Google Scholar] [CrossRef]
- Rueter, C.; Bielaszewska, M. Secretion and delivery of intestinal pathogenic Escherichia coli virulence factors via outer membrane vesicles. Front. Cell. Infect. Microbiol. 2020, 10, 91. [Google Scholar] [CrossRef]
- Acheson, D.W.; Moore, R.; De Breucker, S.; Lincicome, L.; Jacewicz, M.; Skutelsky, E.; Keusch, G.T. Translocation of Shiga toxin across polarized intestinal cells in tissue culture. Infect. Immun. 1996, 64, 3294–3300. [Google Scholar] [CrossRef]
- Hurley, B.P.; Thorpe, C.M.; Acheson, D.W. Shiga toxin translocation across intestinal epithelial cells is enhanceds by neutrophil transmigration. Infect. Immun. 2001, 69, 6148–6155. [Google Scholar] [CrossRef]
- Schüller, S.; Frankel, G.; Phillips, A.D. Interaction of Shiga toxin from Escherichia coli with human intestinal epithelial cell lines and explants: Stx2 induces epithelial damage in organ culture. Cell. Microbiol. 2004, 6, 289–301. [Google Scholar] [CrossRef]
- Maluykova, I.; Gutsal, O.; Laiko, M.; Kane, A.; Donowitz, M.; Kovbasnjuk, O. Latrunculin B facilitates Shiga toxin 1 transcellular transcytosis across T84 intestinal epithelial cells. Biochim. Biophys. Acta 2008, 1782, 370–377. [Google Scholar] [CrossRef]
- Lukyanenko, V.; Malyukova, I.; Hubbard, A.; Delannoy, M.; Boedeker, E.; Zhu, C.; Cebotaru, L.; Kovbasnjuk, O. Enterohemorrhagic Escherichia coli infection stimulates Shiga toxin 1 macropinocytosis and transcytosis across intestinal epithelial cells. Am. J. Physiol. Cell Physiol. 2011, 301, C1140–C1149. [Google Scholar] [CrossRef]
- Boisen, N.; Hansen, A.M.; Melton-Celsa, A.R.; Zangari, T.; Mortensen, N.P.; Kaper, A.D.; O’Brien, A.D.; Nataro, J.P. The presence of the pAA plasmid in the German O104:H4 Shiga toxin type 2a (Stx2a)-producing enteroaggregative Escherichia coli strain promotes the translocation of Stx2a across an epithelial cell monolayer. J. Infect. Dis. 2014, 210, 1909–1919. [Google Scholar] [CrossRef]
- Garimano, N.; Amaral, M.M.; Ibarra, C. Endocytosis, cytotoxicity, and translocation of Shiga toxin-2 are stimulated by infection of human intestinal (HCT-8) monolayers with an hypervirulent E. coli O157:H7 lacking stx2 gene. Front. Cell. Infect. Microbiol. 2019, 9, 396. [Google Scholar] [CrossRef]
- Philpott, D.J.; Ackerley, C.A.; Kiliaan, A.J.; Karmali, M.A.; Perdue, M.H.; Sherman, P.M. Translocation of verotoxin-1 across T84 monolayers: Mechanism of bacterial toxin penetration of epithelium. Am. J. Physiol. 1997, 273, G1349–G1358. [Google Scholar] [CrossRef] [PubMed]
- McGrath, C.J.; Schüller, S. Determining Shiga toxin-producing Escherichia coli interactions with human intestinal epithelium in a microaerobic vertical diffusion chamber. Methods Mol. Biol. 2021, 2291, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Tran, S.L.; Billoud, L.; Lewis, S.B.; Phillips, A.D.; Schüller, S. Shiga toxin production and translocation during microaerobic human colonic infection with Shiga toxin-producing E. coli O157:H7 and O104:H4. Cell. Microbiol. 2014, 16, 1255–1266. [Google Scholar] [CrossRef] [PubMed]
- Te Loo, D.M.; Monnens, L.A.; Van Der Velden, T.J.; Vermeer, M.A.; Preyers, F.; Demacker, P.N.; Van Den Heuvel, L.P.; Van Hinsbergh, V.W. Binding and transfer of verocytotoxin by polymorphonuclear leukocytes in hemolytic uremic syndrome. Blood 2000, 95, 3396–3402. [Google Scholar] [CrossRef]
- Brigotti, M.; Carnicelli, D.; Ravanelli, E.; Barbieri, S.; Ricci, F.; Bontadini, A.; Tozzi, A.E.; Scavia, G.; Caprioloi, A.; Tazzari, P.L. Interactions between Shiga toxins and human polymorphonuclear leukocytes. J. Leukoc. Biol. 2008, 84, 1019–1027. [Google Scholar] [CrossRef]
- Te Loo, D.M.; Van Hinsbergh, V.W.; Van den Heuvel, L.P.; Monnens, L.A. Detection of verocytotoxin bound to circulating polymorphonuclear leukocytes of patients with hemolytic uremic syndrome. J. Am. Soc. Nephrol. 2001, 12, 800–806. [Google Scholar] [CrossRef]
- Te Loo, D.M.; Heuvelink, A.E.; De Boer, E.; Nauta, J.; Van der Walle, J.; Schröder, C.; Van Hinsbergh, V.W.; Chart, H.; Van de Kar, N.C.; Van den Heuvel, L.P. Vero cytotoxin binding to polymorphonuclear leukocytes among households with children with hemolytic uremic syndrome. J. Infect. Dis. 2001, 184, 446–450. [Google Scholar] [CrossRef]
- Brigotti, M.; Caprioli, A.; Tozzi, A.E.; Tazzari, P.L.; Ricci, F.; Conte, R.; Carnicelli, D.; Procaccino, M.A.; Minelli, F.; Ferretti, A.V.; et al. Shiga toxins present in the gut and in the polymorphonuclear leukocytes circulating in the blood of children with hemolytic-uremic syndrome. J. Clin. Microbiol. 2006, 44, 313–317. [Google Scholar] [CrossRef]
- Brigotti, M.; Tazzari, P.L.; Ravanelli, E.; Carnicelli, D.; Barbieri, S.; Rocchi, L.; Arfilli, V.; Scavia, G.; Ricci, F.; Bontadini, A.; et al. Endothelial damage induced by Shiga toxins delivered by neutrophils during transmigration. J. Leukoc. Biol. 2010, 88, 201–210. [Google Scholar] [CrossRef]
- Brigotti, M.; Carnicelli, D.; Arfilli, V.; Rocchi, L.; Ricci, F.; Pagliaro, P.; Tazzari, P.L.; Vara, A.G.; Amelia, M.; Manoli, F.; et al. Change in conformation with reduction of α-helix content causes loss of neutrophil binding activity in fully cytotoxic Shiga toxin 1. J. Biol. Chem. 2011, 286, 34514–34521. [Google Scholar] [CrossRef]
- Brigotti, M.; Carnicelli, D.; Arfilli, V.; Tamassia, N.; Borsetti, F.; Fabbri, E.; Tazzari, P.L.; Ricci, F.; Pagliaro, P.; Spisni, E.; et al. Identification of TLR4 as the receptor that recognizes Shiga toxins in human neutrophils. J. Immunol. 2013, 191, 4748–4758. [Google Scholar] [CrossRef] [PubMed]
- Torgersen, M.L.; Engedal, N.; Pedersen, A.M.G.; Husebye, H.; Espevik, T.; Sandvig, K. Toll-like receptor 4 facilitates binding of Shiga toxin to colon carcinoma and pulmonary umbilical vein endothelial cells. FEMS Immunol. Med. Microbiol. 2011, 61, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Brigotti, M.; Arfilli, V.; Carnicelli, D.; Ricci, F.; Tazzari, P.L.; Ardissino, G.; Scavia, G.; Morabito, S.; He, X. Soluble toll-like receptor 4 impairs the interaction of Shiga toxin 2a with human serum amyloid P component. Toxins 2018, 10, 379. [Google Scholar] [CrossRef] [PubMed]
- Villysson, A.; Tontanahal, A.; Karpman, D. Microvesicle involvement in Shiga toxin-associated infection. Toxins 2017, 9, 376. [Google Scholar] [CrossRef]
- Johansson, K.; Willysson, A.; Kristoffersson, A.C.; Tontanahal, A.; Gillet, D.; Ståhl, A.L.; Karpman, D. Shiga toxin-bearing microvesicles exert a cytotoxic effect on recipient cells only when the cells express the toxin receptor. Front. Cell. Infect. Microbiol. 2020, 10, 212. [Google Scholar] [CrossRef]
- Brigotti, M.; He, X.; Carnicelli, D.; Arfilli, V.; Porcellini, E.; Galassi, E.; Tazzari, P.L.; Ricci, F.; Patfield, S.A.; Testa, S.; et al. Particulate Shiga toxin 2 in blood is associated to the development of hemolytic uremic syndrome in children. Thromb. Haemost. 2020, 120, 107–120. [Google Scholar] [CrossRef]
- Varrone, E.; Carnicelli, D.; Brigotti, M. Extracellular vesicles and renal endothelial cells: A fatal attraction in hemolytic uremic syndrome. Am. J. Pathol. 2021, 191, 795–804. [Google Scholar] [CrossRef]
- Kravitz, G.R.; Smith, K.; Wagstrom, L. Colonic necrosis and perforation secondary to Escherichia coli O157:H7 gastroenteritis in an adult patient without hemolytic uremic syndrome. Clin. Infect. Dis. 2002, 35, e103–e105. [Google Scholar] [CrossRef]
- Hagiwara, S.I.; Tobayama, H.; Kagimoto, S. Sucessful colonoscopic approach in a child with intussusception associated with enterohemorrhagic Escherichia coli O157 infection. Pediatr. Rep. 2012, 4, e33. [Google Scholar] [CrossRef]
- Chang, H.J.; Kim, H.Y.; Choi, J.H.; Choi, H.J.; Ko, J.S.; Ha, I.S.; Cheong, H.I.; Choi, Y.; Kang, H.G. Shiga toxin-associated hemolytic uremic syndrome complicated by intestinal perforation in a child with typical hemolytic uremic syndrome. Korean J. Pediatr. 2014, 57, 96–99. [Google Scholar] [CrossRef][Green Version]
- Cha, P.I.; Gurland, B.; Forrester, J.D. First reported case of intussusception caused by Escherichia coli O157:H7 in an adult: Literature review and case report. Surg. Infect. 2019, 20, 95–99. [Google Scholar] [CrossRef]
- Khalid, M.; Andreoli, S. Extrarenal manifestation of the hemolytic uremic syndrome associated with Shiga toxin-produicing Escherichia coli (STEC HUS). Pediatr. Nephrol. 2019, 34, 2495–2507. [Google Scholar] [CrossRef]
- Obata, F. Influence of Escherichia coli Shiga toxin on the mammalian central nervous system. Adv. Appl. Microbiol. 2010, 71, 1–19. [Google Scholar] [CrossRef]
- Karpman, D.; Ståhl, A.L. Enterohemorrhagic Escherichia coli pathogenesis and the host response. Microbiol. Spectr. 2014, 2, 2–5. [Google Scholar] [CrossRef]
- Costigan, C.; Raftery, T.; Carroll, A.G.; Wildes, D.; Reynolds, C.; Cunney, R.; Dolan, N.; Drew, R.J.; Lynch, B.J.; O’Rourke, D.J.; et al. Neurological involvement in children with hemolytic uremic syndrome. Eur. J. Pediatr. 2022, 181, 501–512. [Google Scholar] [CrossRef]
- Bitzan, M. Treatment options for HUS secondary to Escherichia coli O157:H7. Kidney Int. 2009, 75, S62–S66. [Google Scholar] [CrossRef]
- Karmali, M.A. Host and pathogen determinants of verocytotoxin-producing Escherichia coli-associated hemolytic uremic syndrome. Kidney Int. 2009, 75, S4–S7. [Google Scholar] [CrossRef]
- Tarr, P.I. Shiga toxin-associated hemolytic uremic syndrome and thrombotic thrombocytopenic purpura: Distinct mechanisms of pathogenesis. Kidney Int. 2009, 75, S29–S32. [Google Scholar] [CrossRef]
- Karpman, D.; Sartz, L.; Johnson, S. Pathophysiology of typical hemolytic uremic syndrome. Semin. Thromb. Hemost. 2010, 36, 575–585. [Google Scholar] [CrossRef]
- Karpman, D.; Loos, S.; Tati, R.; Arvidsson, I. Haemolytic uraemic syndrome. J. Intern. Med. 2017, 281, 123–148. [Google Scholar] [CrossRef]
- Trachtman, H.; Austin, C.; Lewinski, M.; Stahl, R.A. Renal and neurological involvement in typical Shiga toxin-associated HUS. Nat. Rev. Nephrol. 2012, 8, 658–669. [Google Scholar] [CrossRef] [PubMed]
- Rosales, A.; Hofer, J.; Zimmerhackl, L.B.; Jungraithmayr, T.C.; Riedl, M.; Giner, T.; Strasak, A.; Orth-Höller, D.; Würzner, R.; Karch, H.; et al. Need for long-term follow-up in enterohemorrhagic Escherichia coli-associated hemolytic uremic syndrome due to late-emerging sequelae. Clin. Infect. Dis. 2012, 54, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Spinale, J.M.; Ruebner, R.L.; Copelovitch, L.; Kaplan, B.S. Long-term outcome of Shiga toxin hemolytic uremic syndrome. Pediatr. Nephrol. 2013, 28, 2097–2105. [Google Scholar] [CrossRef] [PubMed]
- Siukstaite, L.; Imberty, A.; Römer, W. Structural diversities of lectins binding to the glycosphingolipid Gb3. Front. Mol. Biosci. 2021, 8, 70485. [Google Scholar] [CrossRef]
- Müthing, J.; Schweppe, C.H.; Karch, H.; Friedrich, A.W. Shiga toxins, glycosphingolipid diversity, and endothelial cell injury. Thromb. Haemost. 2009, 101, 252–264. [Google Scholar]
- Legros, N.; Pohlentz, G.; Steil, D.; Müthing, J. Shiga toxin-glycosphingolipid interaction: Status quo of research with focus on primary human brain and kidney endothelial cells. Int. J. Med. Microbiol. 2018, 308, 1073–1084. [Google Scholar] [CrossRef]
- Lingwood, C. Therapeutic uses of baterial subunit toxins. Toxins 2021, 13, 378. [Google Scholar] [CrossRef]
- Kale, R.R.; McGannon, C.M.; Fuller-Schaefer, C.; Hatch, D.M.; Flagler, M.J.; Gamage, S.D.; Weiss, A.A.; Iyer, S.S. Differentiation between structurally homologous Shiga 1 and Shiga 2 toxins by using synthetic glycoconjugates. Angew. Chem. Int. Ed. Engl. 2008, 47, 1265–1268. [Google Scholar] [CrossRef]
- Hughes, A.C.; Zhang, Y.; Bai, X.; Xiong, Y.; Wang, Y.; Yang, X.; Xu, Q.; He, X. Structural and functional characterization of Stx2k, a new subtype of Shiga toxin 2. Microorganisms 2019, 8, 4. [Google Scholar] [CrossRef]
- Steil, D.; Pohlentz, G.; Legros, N.; Mormann, M.; Mellmann, A.; Karch, H.; Müthing, J. Combining mass spectrometry, surface acoustic wave interaction analysis, and cell viability assays for characterization of Shiga toxin subtypes of pathogenic Escherichia coli bacteria. Anal. Chem. 2018, 90, 8989–8997. [Google Scholar] [CrossRef]
- Fraser, M.E.; Fujinaga, M.; Cherney, M.M.; Melton-Celsa, A.R.; Twiddy, E.M.; O’Brien, A.D.; James, M.N. Structure of Shiga toxin type 2 (Stx2) from Escherichia coli O157:H7. J. Biol. Chem. 2004, 279, 27511–27517. [Google Scholar] [CrossRef]
- Wennekes, T.; Van den Berg, R.J.; Boot, R.G.; Van der Marel, G.A.; Overkleeft, H.S.; Aerts, J.M. Glycosphingolipids—Nature, function, and pharmacological modulation. Angew. Chem. Int. Ed. Engl. 2009, 48, 8848–8869. [Google Scholar] [CrossRef]
- Müthing, J.; Distler, U. Advances on the compositional analysis of glycosphingolipids combining thin-layer chromatography with mass spectrometry. Mass Spectrom. Rev. 2010, 29, 425–479. [Google Scholar] [CrossRef]
- Merrill, A.H., Jr. Sphingolipid and glycosphingolipid metabolic pathways in the era of sphingolipidomics. Chem. Rev. 2011, 111, 6387–6422. [Google Scholar] [CrossRef]
- Kolter, T. Ganglioside biochemistry. ISRN Biochem. 2012, 2012, 506160. [Google Scholar] [CrossRef]
- Jennemann, R.; Gröne, H.J. Cell-specific in vivo functions of glycosphingolipids: Lessons from genetic deletions of enzymes involved in glycosphingolipid synthesis. Prog. Lipid Res. 2013, 52, 231–248. [Google Scholar] [CrossRef]
- D’Angelo, G.; Capasso, S.; Sticco, L.; Russo, D. Glycosphingolipids: Synthesis and functions. FEBS J. 2013, 280, 6338–6353. [Google Scholar] [CrossRef]
- Zhang, T.; De Waard, A.A.; Wuhrer, M.; Spaapen, R.M. The role of glycosphingolipids in immune cell functions. Front. Immunol. 2019, 10, 90. [Google Scholar] [CrossRef]
- Merrill, A.H., Jr.; Schmelz, E.M.; Dillehay, D.L.; Spiegel, S.; Shayman, J.A.; Schroeder, J.J.; Riley, R.T.; Voss, K.A.; Wang, E. Sphingolipids—The enigmatic class: Biochemistry, physiology, and pathophysiology. Toxicol. Appl. Pharmacol. 1997, 142, 208–225. [Google Scholar] [CrossRef]
- Bauwens, A.; Betz, J.; Meisen, I.; Kemper, B.; Karch, H.; Müthing, J. Facing glycosphingolipid-Shiga toxin interaction: Dire straits for endothelial cells of the human vasculature. Cell. Mol. Life Sci. 2013, 70, 425–457. [Google Scholar] [CrossRef]
- Betz, J.; Dorn, I.; Kouzel, I.U.; Bauwens, A.; Meisen, I.; Kemper, B.; Bielaszewska, M.; Mormann, M.; Weymann, L.; Sibrowski, W.; et al. Shiga toxin of enterohaemorrhagic Escherichia coli directly injures developing human erythrocytes. Cell. Microbiol. 2016, 18, 1339–1348. [Google Scholar] [CrossRef]
- Legros, N.; Dusny, S.; Humpf, H.U.; Pohlentz, G.; Karch, H.; Müthing, J. Shiga toxin glycosphingolipid receptors and their lipid membrane ensemble in primary human blood-brain-barrier endothelial cells. Glycobiology 2017, 27, 99–109. [Google Scholar] [CrossRef]
- Legros, N.; Pohlentz, G.; Runde, J.; Dusny, S.; Humpf, H.U.; Karch, H.; Müthing, J. Colocalization of receptors for Shiga toxins with lipid rafts in primary human renal glomerular endothelial cells and influence of D-PDMP on synthesis and distribution of glycosphingolipid receptors. Glycobiology 2017, 27, 947–965. [Google Scholar] [CrossRef]
- Detzner, J.; Pohlentz, G.; Müthing, J. Valid presumption of Shiga toxin-mediated damage of developing erythrocytes in EHEC-associated hemolytic uremic syndrome. Toxins 2020, 12, 373. [Google Scholar] [CrossRef]
- Iwabuchi, K.; Prinetti, A.; Sonnino, S.; Mauri, L.; Kobayashi, T.; Ishii, K.; Kaga, N.; Murayama, K.; Kurihara, H.; Nakayama, H.; et al. Involvement of very long fatty acid-containing lactosylceramide in lactosylceramide-mediated superoxide generation and migration in neutrophils. Glycoconj. J. 2008, 25, 357–374. [Google Scholar] [CrossRef]
- Chiricozzi, E.; Ciampa, M.G.; Brasile, G.; Compostella, F.; Prinetti, A.; Nakayama, H.; Ekyalongo, R.; Iwabuchi, K.; Sonnino, S.; Mauri, L. Direct interaction, instrumental for signalling processes, between LacCer and Lyn in the lipid rafts of neutrophil-like cells. J. Lipid Res. 2015, 56, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Skotland, T.; Sandvig, K.; Llorente, A. Lipids in exosomes: Current knowledge and the way forward. Prog. Lipid Res. 2017, 66, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Skotland, T.; Sandvig, K. The role of PS 18:0/18:1 in membrane function. Nat. Commun. 2019, 10, 2752. [Google Scholar] [CrossRef]
- Bielaszewska, M.; Karch, H. Consequences of enterohaemohhagic Escherichia coli infection for the vascular endothelium. Thromb. Haemost. 2005, 94, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.S.; Ng, T.B. Shiga toxins: From structure and mechanism to applications. Appl. Microbiol. Biotechnol. 2016, 100, 1597–1610. [Google Scholar] [CrossRef]
- Lingwood, C. Verotoxin receptor-based pathology and therapies. Front. Cell. Infect. Microbiol. 2020, 10, 123. [Google Scholar] [CrossRef]
- Detzner, J.; Krojnewski, E.; Pohlentz, G.; Steil, D.; Humpf, H.U.; Mellmann, A.; Karch, H.; Müthing, J. Shiga toxin (Stx)-binding glycosphingolipids of primary human renal cortical epithelial cells (pHRCEpiCs) and Stx-mediated cytotoxicity. Toxins 2021, 13, 139. [Google Scholar] [CrossRef]
- Detzner, J.; Klein, A.L.; Pohlentz, G.; Krojnewski, E.; Humpf, H.U.; Mellmann, A.; Karch, H.; Müthing, J. Primary human renal proximal tubular epithelial cells (pHRPTEpiCs): Shiga toxin (Stx) glycosphingolipid receptors, Stx susceptibility, and interaction with membrane microdomains. Toxins 2021, 13, 529. [Google Scholar] [CrossRef]
- Detzner, J.; Püttmann, C.; Pohlentz, G.; Humpf, H.-U.; Mellmann, A.; Karch, H.; Müthing, J. Primary human colon epithelial cells (pHCoEpiCs) do express the Shiga toxin (Stx) receptor glycosphingolipids Gb3Cer and Gb4Cer and are largely refractory but not resistant towards Stx. Int. J. Mol. Sci. 2021, 22, 10002. [Google Scholar] [CrossRef]
- Detzner, J.; Pohlentz, G.; Müthing, J. Thin-layer chromatography in structure and recognition studies of Shiga toxin glycosphingolipid receptors. Methods Mol. Biol. 2021, 2291, 229–252. [Google Scholar] [CrossRef]
- Legros, N.; Ptascheck, S.; Pohlentz, G.; Karch, H.; Dobrindt, U.; Müthing, J. PapG subtype-specific binding characteristics of Escherichia coli towards globo-series glycosphingolipids of human kidney and bladder uroepithelial cells. Glycobiology 2019, 29, 789–802. [Google Scholar] [CrossRef]
- Singer, S.J.; Nicolson, G.L. The fluid mosaic model of the structure of cell membranes. Science 1972, 175, 720–731. [Google Scholar] [CrossRef]
- Quinn, P.J.; Wolf, C. The liquid-ordered phase in membranes. Biochim. Biophys. Acta 2009, 1788, 33–46. [Google Scholar] [CrossRef]
- Marquardt, D.; Kučerka, N.; Wassall, S.R.; Harroun, T.A.; Katsaras, J. Cholesterol’s location in lipid bilayers. Chem. Phys. Lipids 2016, 199, 17–25. [Google Scholar] [CrossRef]
- Nicolson, G.L. Update of the 1972 Singer-Nicolson fluid-mosaic model of membrane structure. Discoveries 2013, 1, e3. [Google Scholar] [CrossRef][Green Version]
- Owen, D.M.; Gaus, K. Imaging lipid domains in cell membranes: The advent of super-resolution fluorescence microscopy. Front. Plant Sci. 2013, 4, 503. [Google Scholar] [CrossRef] [PubMed]
- Nyholm, T.K.M. Lipid-protein interplay and lateral organization in biomembranes. Chem. Phys. Lipids 2015, 189, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Morigaki, K.; Tanimoto, Y. Evolution and development of model membranes for physicochemical and functional studies of the membrane lateral heterogeneity. Biochim. Biophys. Acta Biomembr. 2018, 1860, 2012–2017. [Google Scholar] [CrossRef] [PubMed]
- Kusumi, A.; Fujiwara, T.K.; Tsunoyama, T.A.; Kasai, R.S.; Liu, A.A.; Hirosawa, K.M.; Kinoshita, M.; Matsumori, N.; Komura, N.; Ando, H.; et al. Defining raft domains in the plasma membrane. Traffic 2020, 21, 106–137. [Google Scholar] [CrossRef] [PubMed]
- Bieberich, E. Sphingolipids and lipid rafts: Novel concepts and methods of analysis. Chem. Phys Lipids 2018, 216, 114–131. [Google Scholar] [CrossRef]
- Bodosa, J.; Iyer, S.S.; Srivastava, A. Preferential protein partitioning in biological membrane with coexisting liquid ordered and liquid disordered phase behavior: Underlying design principles. J. Membr. Biol. 2020, 253, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Surolia, A. Glycosphingolipids in microdomain formation and their spatial organization. FEBS Lett. 2010, 584, 1634–1641. [Google Scholar] [CrossRef]
- Sonnino, S.; Prinetti, A. Membrane domains and the “lipid raft” concept. Curr. Med. Chem. 2013, 20, 4–21. [Google Scholar]
- Barenholz, Y. Sphingomyelin and cholesterol: From membrane biophysics and rafts to potential medical applications. Subcell. Biochem. 2004, 37, 167–215. [Google Scholar] [CrossRef]
- Rao, M.; Mayor, S. Use of Forster’s resonance energy transfer microscopy to study lipid rafts. Biochim. Biophys. Acta 2005, 1746, 221–233. [Google Scholar] [CrossRef]
- Ishitsuka, R.; Sato, S.B.; Kobayashi, T. Imaging lipid rafts. J. Biochem. 2005, 137, 249–254. [Google Scholar] [CrossRef]
- Pike, L.J. The challenge of lipid rafts. J. Lipid Res. 2009, 50, S323–S328. [Google Scholar] [CrossRef]
- Quinn, P.J. A lipid matrix model of membrane raft structure. Prog. Lipid Res. 2010, 49, 390–406. [Google Scholar] [CrossRef]
- Owen, D.M.; Magenau, A.; Williamson, D.; Gaus, K. The lipid raft hypothesis revisited—New insights on raft composition and function from super-resolution fluorescence microscopy. Bioessays 2012, 34, 739–747. [Google Scholar] [CrossRef]
- Róg, T.; Vattulainen, I. Cholesterol, sphingolipids, and glycolipids: What do we know about their role in raft-like membranes? Chem. Phys. Lipids 2014, 184, 82–104. [Google Scholar] [CrossRef]
- Kinoshita, M.; Suzuki, K.G.N.; Murata, M.; Matsumori, N. Evidence of lipid rafts based on the partition and dynamic behavior of sphingomyelins. Chem. Phys. Lipids 2018, 215, 84–95. [Google Scholar] [CrossRef]
- Simons, K.; Toomre, D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000, 1, 31–39. [Google Scholar] [CrossRef]
- Helms, J.B.; Zurzolo, C. Lipids as targeting signals: Lipid rafts and intracellular trafficking. Traffic 2004, 5, 247–254. [Google Scholar] [CrossRef]
- Lingwood, D.; Simons, K. Lipid rafts as a membrane-organizing principle. Science 2010, 327, 46–50. [Google Scholar] [CrossRef]
- Simons, K.; Gerl, M. Revitalizing membrane rafts: New tools and insights. Nat. Rev. Mol. Cell Biol. 2010, 11, 688–699. [Google Scholar] [CrossRef]
- Kasahara, K.; Sanai, Y. Functional roles of glycosphingolipids in signal transduction via lipid rafts. Glycoconj. J. 2000, 17, 153–162. [Google Scholar] [CrossRef]
- Hoessli, D.C.; Ilangumaran, S.; Soltermann, A.; Robinson, P.J.; Borisch, B.; Ud-Din, N. Signaling through sphingolipid microdomains of the plasma membrane: The concept of sigbaling platform. Glycoconj. J. 2000, 17, 191–197. [Google Scholar] [CrossRef]
- George, K.S.; Wu, S. Lipid raft: A floating island of death or survival. Toxicol. Appl. Pharmacol. 2012, 259, 311–319. [Google Scholar] [CrossRef]
- Head, B.P.; Patel, H.H.; Insel, P.A. Interaction of membrane/lipid rafts with the cytoskeleton: Impact on signaling and function: Membrane/lipid rafts, mediators of cytoskeletal arrangement and cell signaling. Biochim. Biophys. Acta 2014, 1838, 532–545. [Google Scholar] [CrossRef]
- Sonnino, S.; Aureli, M.; Mauri, L.; Ciampa, M.G.; Prinetti, A. Membrane lipid domains in the nervous system. Front. Biosci. Landmark Ed. 2015, 20, 280–302. [Google Scholar] [CrossRef] [PubMed]
- Egawa, J.; Pearn, M.L.; Lemkuil, B.P.; Patel, P.M.; Head, B.P. Membrane lipid rafts and neurobiology: Age-related changes in membrane lipids and loss of neuronal function. J. Physiol. 2016, 594, 4565–4579. [Google Scholar] [CrossRef]
- Bian, F.; Xiong, B.; Yang, X.; Jin, S. Lipid rafts, ceramide and molecular transcytosis. Front. Biosci. Landmark Ed. 2016, 21, 806–838. [Google Scholar] [CrossRef]
- Varshney, P.; Yadav, V.; Saini, N. Lipid rafts in immune signalling: Current progress and future perspective. Immunology 2016, 149, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Laurenzana, A.; Fibbi, G.; Chillà, A.; Margheri, G.; Del Rosso, T.; Rovida, E.; Del Rosso, M.; Margheri, F. Lipid rafts: Integrated platforms for vascular organization offering therapeutic opportunities. Cell. Mol. Life Sci. 2015, 72, 1537–1557. [Google Scholar] [CrossRef] [PubMed]
- Filippini, A.; D’Alessio, A. Caveolae and lipid rafts in endothelium: Valuable organelles for multiple functions. Biomolecules 2020, 10, 1218. [Google Scholar] [CrossRef] [PubMed]
- Danielsen, E.M.; Hansen, G.H. Lipid rafts in epithelial brush borders: Atypical membrane microdomains with specialized functions. Biochim. Biophys. Acta 2003, 1617, 1–9. [Google Scholar] [CrossRef]
- Danielsen, E.M.; Hansen, G.H. Lipid raft organization and function in brush borders of epithelial cells. Mol. Membr. Biol. 2006, 23, 71–79. [Google Scholar] [CrossRef]
- Chinnapen, D.J.; Chinnapen, H.; Saslowsky, D.; Lencer, W.I. Rafting with cholera toxin: Endocytosis and trafficking from plasma membrane to ER. FEMS Microbiol. Lett. 2007, 266, 129–137. [Google Scholar] [CrossRef]
- Lingwood, C.A.; Binnington, B.; Manis, A.; Branch, D.R. Globotriaosyl ceramide receptor function—Where membrane structure and pathology intersect. FEBS Lett. 2010, 584, 1879–1886. [Google Scholar] [CrossRef]
- Sandvig, K.; Bergan, J.; Kavaliauskiene, S.; Skotland, T. Lipid requirements for entry of protein toxins into cells. Prog. Lipid Res. 2014, 54, 1–13. [Google Scholar] [CrossRef]
- Römer, W.; Berland, L.; Chambon, V.; Gaus, K.; Windschiegl, B.; Tenza, D.; Aly, M.R.; Fraisier, V.; Florent, J.C.; Perrais, D.; et al. Shiga toxin induces tubular membrane invaginations for its uptake into cells. Nature 2007, 450, 670–675. [Google Scholar] [CrossRef]
- Aigal, S.; Claudinon, J.; Römer, W. Plasma membrane reorganization: A glycolipid gateway for microbes. Biochim. Biophys. Acta 2015, 1853, 858–871. [Google Scholar] [CrossRef]
- Sens, P.; Johannes, L.; Bassereau, P. Biophysical approaches to protein-induced membrane deformations in trafficking. Curr. Opin. Cell Biol. 2008, 20, 476–482. [Google Scholar] [CrossRef]
- Khan, F.; Proulx, F.; Lingwood, C.A. Detergent-resistant globotriaosyl ceramide may define verotoxin/glomeruli-restricted hemolytic uremic syndrome pathology. Kidney Int. 2009, 75, 1209–1216. [Google Scholar] [CrossRef]
- Ray, P.E. Shiga-like toxins and HIV-1 ‘go through’ glycosphingolipids and lipid rafts in renal cells. Kidney Int. 2009, 75, 1135–11337. [Google Scholar] [CrossRef]
- Kovbasnjuk, O.; Edidin, M.; Donowitz, M. Role of lipid rafts in Shiga toxin 1 interaction with the apical surface of Caco-2 cells. J. Cell Sci. 2001, 114, 4025–4031. [Google Scholar] [CrossRef]
- Hanashima, T.; Miyake, M.; Yahiro, K.; Iwamaru, Y.; Ando, A.; Morinaga, N.; Noda, M. Effect of Gb3 in lipid rafts in resistance to Shiga-like toxin of mutant Vero cells. Microb. Pathog. 2008, 45, 124–133. [Google Scholar] [CrossRef]
- Brown, D.A.; Rose, J.K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 1992, 68, 533–544. [Google Scholar] [CrossRef]
- London, E.; Brown, D.A. Insolubility of lipids in Triton X-100: Physical origin and relationship to sphingolipid/cholesterol membrane domains (rafts). Biochim. Biophys. Acta 2000, 1508, 182–195. [Google Scholar] [CrossRef]
- Brown, D.A. Lipid rafts, detergent-resistant membranes, and raft targeting signals. Physiology 2006, 21, 430–439. [Google Scholar] [CrossRef]
- Lingwood, D.; Simons, K. Detergent resistance as a tool in membrane research. Nat. Protoc. 2007, 2, 2159–2165. [Google Scholar] [CrossRef]
- Brown, D.A. Preparation of detergent-resistant membranes (DRMs) from cultured mammalian cells. Methods Mol. Biol. 2015, 1232, 55–64. [Google Scholar] [CrossRef]
- Dawson, G. Isolation of lipid rafts (detergent-resistant microdomains) and comparison to extracellular vesicles (exosomes). Methods Mol. Biol. 2021, 2187, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.C.; Sillence, D.J.; Falguières, T.; Jarvis, R.M.; Johannes, L.; Lord, J.M.; Platt, F.M.; Roberts, L.M. The association of Shiga-like toxin with detergent-resistant membranes is modulated by glucosylceramide and is an essential requirement in the endoplasmic reticulum for a cytotoxic effect. Mol. Biol. Cell 2006, 17, 1375–1387. [Google Scholar] [CrossRef] [PubMed]
- Kiyokawa, N.; Taguchi, T.; Mori, T.; Uchida, H.; Sato, N.; Takeda, T.; Fujimoto, J. Induction of apoptosis in normal human renal tubular epithelial cells by Escherichia coli Shiga toxins 1 and 2. J. Infect. Dis. 1998, 178, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Kodama, T.; Nagayama, K.; Yamada, K.; Ohba, Y.; Akeda, Y.; Honda, T. Induction of apoptosis in human renal proximal tubular epithelial cells by Escherichia coli verocytotoxin 1 in vitro. Med. Microbiol. Immunol. 1999, 188, 73–78. [Google Scholar] [CrossRef]
- Williams, J.M.; Boyd, B.; Nutikka, A.; Lingwood, C.A.; Barnett Foster, D.E.; Milford, D.V.; Taylor, C.M. A comparison of the effects of verocytotoxin-1 on primary human renal cell cultures. Toxicol. Lett. 1999, 105, 47–57. [Google Scholar] [CrossRef]
- Hughes, A.K.; Stricklett, P.K.; Schmidt, D.; Kohan, D.E. Cytotoxic effect of Shiga toxin-1 on human glomerular epithelial cells. Kidney Int. 2000, 57, 2350–2359. [Google Scholar] [CrossRef]
- Creydt, V.P.; Silberstein, C.; Zotta, E.; Ibarra, C. Cytotoxic effect of Shiga toxin-2 holotoxin and its B subunit on human renal tubular epithelial cells. Microbes Infect. 2006, 8, 410–419. [Google Scholar] [CrossRef]
- Silberstein, C.; Copeland, D.P.; Chiang, W.L.; Repetto, H.A.; Ibarra, C. A glucosylceramide synthase inhibitor prevents the cytotoxic effects of Shiga toxin-2 on human renal tubular epithelial cells. J. Epithel. Biol. Pharmacol. 2008, 1, 71–75. [Google Scholar] [CrossRef]
- Márquez, L.B.; Araoz, A.; Repetto, H.A.; Ibarra, C.; Silberstein, C. Effects of Shiga toxin 2 on cellular regeneration mechanisms in primary and three-dimensional cultures of human renal tubular epithelial cells. Microb. Pathog. 2016, 99, 87–94. [Google Scholar] [CrossRef]
- Karpman, D.; Håkansson, A.; Perez, M.T.; Isaksson, C.; Carlemalm, E.; Caprioli, A.; Svanborg, C. Apoptosis of renal cortical cells in the hemolytic-uremic syndrome: In vivo and in vitro studies. Infect. Immun. 1998, 66, 636–644. [Google Scholar] [CrossRef]
- Kaneko, K.; Kiyokawa, N.; Ohtomo, Y.; Nagaoka, R.; Yamashiro, Y.; Taguchi, T.; Mori, T.; Fujimoto, J.; Takeda, T. Apoptosis of renal tubular cells in Shiga toxin-mediated hemolytic uremic syndrome. Nephron 2001, 87, 182–185. [Google Scholar] [CrossRef]
- Menge, C. Molecular biology of Escherichia coli Shiga toxins’ effects on mammalian cells. Toxins 2020, 12, 345. [Google Scholar] [CrossRef]
- Porubsky, S.; Federico, G.; Müthing, J.; Jennemann, R.; Gretz, N.; Büttner, S.; Obermüller, N.; Jung, O.; Hauser, I.A.; Gröne, E.; et al. Direct acute tubular damage contributes to Shigatoxin-mediated kidney failure. J. Pathol. 2014, 234, 120–133. [Google Scholar] [CrossRef]
- Morace, I.; Pilz, R.; Federico, G.; Jennemann, R.; Krunic, D.; Nordström, V.; Von Gerichten, J.; Marsching, C.; Schießl, I.M.; Müthing, J.; et al. Renal globotriaosylceramide facilitates tubular albumin absorption and its inhibition protects against acute kidney injury. Kidney Int. 2019, 96, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Melby, E.L.; Jacobsen, J.; Olsnes, S.; Sandvig, K. Entry of protein toxins in polarized epithelial cells. Cancer Res. 1993, 53, 1755–1760. [Google Scholar] [PubMed]
- Jacewicz, M.S.; Acheson, D.W.; Mobassaleh, M.; Donohue-Rolfe, A.; Balasubramanian, K.A.; Keusch, G.T. Maturational regulation of globotriaosylceramide, the Shiga-like toxin 1 receptor, in cultured human gut epithelial cells. J. Clin. Investig. 1995, 96, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- Olano-Martin, E.; Williams, M.R.; Gibson, G.R.; Rastall, R.A. Pectins and pectic-oligosaccharides inhibit Escherichia coli O157:H7 Shiga toxin as directed towards the human colonic cell line HT29. FEMS Microbiol. Lett. 2003, 218, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Tran, S.L.; Jenkins, C.; Livrelli, V.; Schüller, S. Shiga toxin 2 translocation across intestinal epithelium is linked to virulence of Shiga toxin-producing Escherichia coli in humans. Microbiology 2018, 164, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Kovbasnjuk, O.; Mourtazina, R.; Baibakov, B.; Wang, T.; Elowsky, C.; Choti, M.A.; Kane, A.; Donowitz, M. The glycosphingolipid globotriaosylceramide in the metastatic transformation of colon cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 19087–19092. [Google Scholar] [CrossRef] [PubMed]
- Laiko, M.; Murtazina, R.; Malyukova, I.; Zhu, C.; Boedeker, E.C.; Gutsal, O.; O’Malley, R.; Cole, R.N.; Tarr, P.I.; Murray, K.F.; et al. Shiga toxin 1 interaction with enterocytes causes apical protein mistargeting through the depletion of intracellular galectin-3. Exp. Cell Res. 2010, 316, 657–666. [Google Scholar] [CrossRef]
- Zumbrun, S.D.; Hanson, L.; Sinclair, J.F.; Freedy, J.; Melton-Celsa, A.R.; Rodriguez-Canales, J.; Hanson, J.C.; O’Brien, A.D. Human intestinal tissue and cultured colonic cells contain globotriaosylceramide synthase mRNA and the alternate Shiga toxin receptor globotetraosylceramide. Infect. Immun. 2010, 78, 4488–4499. [Google Scholar] [CrossRef]
- Kouzel, I.U.; Pohlentz, G.; Schmitz, J.S.; Steil, D.; Humpf, H.U.; Karch, H.; Müthing, J. Shiga toxin glycosphingolipid receptors in human Caco-2 and HCT-8 colon epithelial cell lines. Toxins 2017, 9, 338. [Google Scholar] [CrossRef]
- Schüller, S.; Heuschkel, R.; Torrente, F.; Kaper, J.B.; Phillips, A.D. Shiga toxin binding in normal and inflamed human intestinal mucosa. Microbes Infect. 2007, 9, 35–39. [Google Scholar] [CrossRef]
- Sato, J.D.; Kan, M. Media for culture of mammalian cells. Curr. Protoc. Cell Biol. 2001, 1, 1–2. [Google Scholar] [CrossRef]
- Price, P.J. Best practices for media selection for mammalian cells. In Vitro Cell Dev. Biol. Anim. 2017, 53, 673–681. [Google Scholar] [CrossRef]
- Iijima, K.; Kamioka, I.; Nozu, K. Management of diarrhea-associated hemolytic uremic syndrome in children. Clin. Exp. Nephrol. 2008, 12, 16–19. [Google Scholar] [CrossRef]
- Orth, D.; Grif, K.; Zimmerhackl, L.B.; Würzner, R. Prevention and treatment of enterohemorrhagic Escherichia coli infections in humans. Exp. Rev. Anti-Infect. Ther. 2008, 6, 101–108. [Google Scholar] [CrossRef]
- Palermo, M.S.; Exeni, R.A.; Fernández, G.C. Hemolytic uremic syndrome: Pathogenesis and update interventions. Exp. Rev. Anti-Infect. Ther. 2009, 7, 697–797. [Google Scholar] [CrossRef]
- Travert, B.; Rafat, C.; Mariani, P.; Cointe, A.; Dossier, A.; Coppo, P.; Joseph, A. Shiga toxin-associated hemolytic uremic syndrome: Specificities of adult patients and implications for critical care management. Toxins 2021, 13, 306. [Google Scholar] [CrossRef]
- Puentes, S.S.; Dunstan, M. Escherichia coli complications in pediatric critical care. Crit. Care Nurs. Clin. N. Am. 2018, 30, 149–156. [Google Scholar] [CrossRef]
- Mayer, C.L.; Leibowitz, C.S.; Kurosawa, S.; Stearns-Kurosawa, D.J. Shiga toxins and the pathophysiology of hemolytic uremic syndrome in humans and animals. Toxins 2012, 4, 1261–1287. [Google Scholar] [CrossRef]
- Bielaszewska, M.; Mellmann, A.; Bletz, S.; Zhang, W.; Köck, R.; Kossow, A.; Prager, R.; Fruth, A.; Orth-Höller, D.; Marejková, M.; et al. Enterohemorrhagic Escherichia coli O26:H11/H−: A new virulent clone emerges in Europe. Clin. Infect. Dis. 2013, 56, 1373–1381. [Google Scholar] [CrossRef]
- Van Overbeek, L.S.; Van Doorn, J.; Wichers, J.H.; Van Amerongen, A.; Van Roermund, H.J.W.; Willemsen, P.T.J. The arable ecosystem as battleground for emergence of new human pathogens. Front. Microbiol. 2014, 5, 104. [Google Scholar] [CrossRef]
- Sadiq, S.M.; Hazen, T.H.; Rasko, D.A.; Eppinger, M. EHEC genomics: Past, present, and future. Microbiol. Spectr. 2014, 2. [Google Scholar] [CrossRef]
- Karmali, M.A. Factors in the emergence of serious human infections associated with highly pathogenic strains of Shiga toxin-producing Escherichia coli. Int. J. Med. Microbiol. 2018, 308, 1067–1072. [Google Scholar] [CrossRef]
- Cointe, A.; Birgy, A.; Bridier-Nahmias, A.; Mariani-Kurkdjian, P.; Walewski, V.; Lévy, C.; Cohen, R.; Fach, P.; Delannoy, S.; Bidet, P.; et al. Escherichia coli O80 hybrid pathotype strains producing Shiga toxin and ESBL: Molecular characterization and potential therapeutic options. J. Antimicrob. Chemother. 2020, 75, 537–542. [Google Scholar] [CrossRef]
- Hwang, S.B.; Chelliah, R.; Kang, J.E.; Rubab, M.; Banan-MwineDaliri, E.; Elahi, F.; Oh, D.H. Role of recent therapeutic applications and the infection strategies of Shiga toxin-producing Escherichia coli. Front. Cell. Infect. Microbiol. 2021, 11, 614963. [Google Scholar] [CrossRef]
- Kakoullis, L.; Papachristodoulou, E.; Chra, P.; Panos, G. Shiga toxin-induced haemolytic uraemic syndrome and the role of antibiotics: A global overview. J. Infect. 2019, 79, 75–94. [Google Scholar] [CrossRef]
- Biernbaum, E.N.; Kudva, I.T. AB5 enterotoxin-mediated pathogenesis: Perspectives gleaned from Shiga toxins. Toxins 2022, 14, 62. [Google Scholar] [CrossRef]
- Tarr, P.I.; Freedman, S.B. Why antibiotics should not be used to treat Shiga toxin-producing Escherichia coli infections. Curr. Opin. Gastroenterol. 2022, 38, 30–38. [Google Scholar] [CrossRef]
- Loś, J.M.; Loś, M.; Węgrzyn, G. Bacteriophages carrying Shiga toxin genes: Genomic variations, detection and potential treatment of pathogenic bacteria. Future Microbiol. 2011, 6, 909–924. [Google Scholar] [CrossRef]
- Ochoa, T.J.; Chen, J.; Walker, C.M.; Gonzales, E.; Cleary, T.G. Rifaximin does not induce toxin production or phage-mediated lysis of Shiga toxin-producing Escherichia coli. Antimicrob. Agents Chemother. 2007, 51, 2837–2841. [Google Scholar] [CrossRef]
- Bielaszewska, M.; Idelevich, E.A.; Zhang, W.; Bauwens, A.; Schaumburg, F.; Mellmann, A.; Peters, G.; Karch, K. Effects of antibiotics on Shiga toxin 2 production and bacteriophage induction by epidemic Escherichia coli O104:H4 strain. Antimicrob. Agents Chemother. 2012, 56, 3277–3282. [Google Scholar] [CrossRef]
- Corogeanu, D.; Willmes, R.; Wolke, M.; Plum, G.; Utermöhlen, O.; Krönke, M. Therapeutic concentrations of antibiotics inhibit Shiga toxin release from enterohemorrhagic E. coli O104:H4 from the 2011 German outbreak. BMC Microbiol. 2012, 12, 160. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.E.; Elliott, E.J. Interventions for preventing diarrhea-associated hemolytic uremic syndrome: Systematic review. BMC Public Health 2013, 13, 799. [Google Scholar] [CrossRef] [PubMed]
- Melton-Celsa, A.R.; O’Brien, A.D. New therapeutic developments against Shiga toxin-producing Escherichia coli. Microbiol. Spectr. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Tarr, G.A.M.; Oltean, H.N.; Phipps, A.I.; Rabinowitz, P.; Tarr, P.I. Strength of the association between antibiotic use and hemolytic uremic syndrome following Escherichia coli O157H7 infection varies with case definition. Int. J. Med. Microbiol. 2018, 308, 921–926. [Google Scholar] [CrossRef]
- Mühlen, S.; Ramming, I.; Pils, M.C.; Koeppel, M.; Glaser, J.; Leong, J.; Flieger, A.; Stecher, B.; Dersch, P. Identification of antibiotics that diminish disease in a murine model of enterohemorrhagic Escherichia coli infection. Antimicrob. Agents Chemother. 2020, 64, e02159-19. [Google Scholar] [CrossRef]
- Ramstad, S.N.; Taxt, A.M.; Naseer, U.; Wasteson, Y.; Bjørnholt, J.V.; Brandal, L.T. Effects of antimicrobials on Shiga toxin production in high-virulent Shiga toxin-producing Escherichia coli. Microb. Pathog. 2021, 152, 104636. [Google Scholar] [CrossRef]
- Mühlen, S.; Dersch, P. Treatment strategies for infections with Shiga toxin-producing Escherichia coli. Front. Cell. Infect. Microbiol. 2020, 10, 169. [Google Scholar] [CrossRef]
- Imdad, A.; Mackoff, S.P.; Urciuoli, D.; Syed, T.; Tanner-Smith, E.E.; Huang, D.; Gomez-Duarte, O.G. Interventions for preventing diarrhoea-associated haemolytic uraemic syndrome. Cochrane Database Syst. Rev. 2021, 7, CD012997. [Google Scholar] [CrossRef]
- Nishikawa, K. Recent progress of Shiga toxin neutralizer for treatment of infections by Shiga toxin-producing Escherichia coli. Arch. Immunol. Ther. Exp. 2011, 59, 239–247. [Google Scholar] [CrossRef]
- Hall, G.; Kurosawa, S.; Stearns-Kurosawa, D.J. Shiga toxin therapeutics: Beyond neutralization. Toxins 2017, 9, 291. [Google Scholar] [CrossRef]
- Robert, A.; Wiels, J. Shiga toxins as antitumor tools. Toxins 2021, 13, 690. [Google Scholar] [CrossRef]
- Shimizu, M. Pathogenic functions and diagnostic utility of cytokines/chemokines in EHEC-HUS. Pediatr. Int. 2020, 62, 308–315. [Google Scholar] [CrossRef]
- Sandhoff, K.; Kolter, T. Biosynthesis and degradation of mammalian glycosphingolipids. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2003, 358, 847–861. [Google Scholar] [CrossRef]
- Abe, A.; Wild, S.R.; Lee, W.L.; Shayman, J.A. Agents for the treatment of glycosphingolipid storage disorders. Curr. Drug Metab. 2001, 2, 331–338. [Google Scholar] [CrossRef]
- Jeyakumar, M.; Butters, T.D.; Dwek, R.A.; Platt, F.M. Glycosphingolipid lysosomal storage diseases: Therapy and pathogenesis. Neuropathol. Appl. Neurobiol. 2002, 28, 343–357. [Google Scholar] [CrossRef]
- Aerts, J.M.F.G.; Hollak, C.E.M.; Boot, R.G.; Groener, J.E.M.; Maas, M. Substrate reduction therapy of glycosphingolipid storage disorders. J. Inherit. Metab. Dis. 2006, 29, 449–456. [Google Scholar] [CrossRef]
- Larsen, S.D.; Wilson, M.W.; Abe, A.; Shu, L.; George, C.H.; Kirchhoff, P.; Showalter, H.D.H.; Xiang, J.; Keep, R.F.; Shayman, J.A. Property-based design of glucosylceramide synthase inhibitor that reduces glucosylceramide in the brain. J. Lipid Res. 2012, 53, 282–291. [Google Scholar] [CrossRef]
- Shayman, J.A. Eliglustat tartrate, a prototypic glucosylceramide synthase inhibitor. Exp. Rev. Endocrinol. Metab. 2013, 8, 491–504. [Google Scholar] [CrossRef]
- Raa, H.; Grimmer, S.; Schwudke, D.; Bergan, J.; Wälchli, S.; Skotland, T.; Shevchenko, A.; Sandvig, K. Glycosphingolipid requirements for endosome-to-Golgi transport of Shiga toxin. Traffic 2009, 10, 868–882. [Google Scholar] [CrossRef]
- Cox, T.M. Eliglustat tartrate, an orally active glucocerebroside synthase inhibitor for the potential treatment of Gaucher disease and other lysosomal storage diseases. J. Curr. Opin. Investig. Drugs 2010, 11, 1169–1181. [Google Scholar]
- Sánchez, D.S.; Fischer Sigel, L.K.; Balestracci, A.; Ibarra, C.; Amaral, M.M.; Silberstein, C. Eliglustat prevents Shiga toxin 2 cytotoxic effects in human renal tubular epithelial cells. Pediatr. Res. 2022, 91, 1121–1129. [Google Scholar] [CrossRef]
- Feitz, W.J.C.; Bouwmeester, R.; Van der Velden, T.J.A.M.; Goorden, S.; Licht, C.; Van den Heuvel, L.P.J.W.; Van de Kar, N.C.A.J. The Shiga toxin receptor globotriaosylceramide as therapeutic target in Shiga toxin E. coli mediated HUS. Microorganisms 2021, 9, 2157. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, M.; Lucero, M.S.; Zotta, E.; Copeland, D.P.; Lingyun, L.; Repetto, H.A.; Iberra, C. A glucosylceramide synthase inhibitor protects rats against the cytotoxic effects of Shiga toxin 2. Pediatr. Res. 2011, 69, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Kavaliauskiene, S.; Skotland, T.; Sylvänne, T.; Simolin, H.; Klokk, T.I.; Torgersen, M.L.; Lingelem, A.B.D.; Simm, R.; Ekroos, K.; Sandvig, K. Novel actions of 2-deoxy-D-glucose: Protection against Shiga toxins and changes in cellular lipids. Biochem. J. 2015, 470, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Kavaliauskiene, S.; Torgersen, M.L.; Lingelem, A.B.D.; Klokk, T.I.; Lintonen, T.; Simolin, H.; Ekroos, K.; Skotland, T.; Sandvig, K. Cellular effects of fluorodeoxyglucose: Global changes in the lipidome and alteration in intracellular transport. Oncotarget 2016, 7, 79885–79900. [Google Scholar] [CrossRef]
- Girard, M.C.; Sacerdoti, F.; Rivera, F.P.; Repetto, H.A.; Ibarra, C.; Amaral, M.M. Prevention of renal damage caused by Shiga toxin type 2: Action of Miglustat on human endothelial and epithelial cells. Toxicon 2015, 105, 27–33. [Google Scholar] [CrossRef]
- Ivarsson, M.E.; Leroux, J.C.; Castagner, B. Targeting bacterial toxins. Angew. Chem. Int. Ed. Engl. 2012, 51, 4024–4045. [Google Scholar] [CrossRef]
- MacConnachie, A.A.; Todd, W.T.A. Potential therapeutic agents for the prevention and treatment of haemolytic uraemic syndrome in Shiga toxin producing Escherichia coli infections. Curr. Opin. Infect. Dis. 2004, 17, 479–482. [Google Scholar] [CrossRef]
- Trachtman, H.; Cnaan, A.; Christen, E.; Gibbs, K.; Zhao, S.; Acheson, D.W.K.; Weiss, R.; Kaskel, F.J.; Spitzer, A.; Hirschman, G.H.; et al. Effect of an oral Shiga toxin-binding agent on diarrhea-associated hemolytric uremic syndrome in children: A randomized controlled trial. JAMA 2003, 290, 1337–1344. [Google Scholar] [CrossRef]
- Kitov, P.I.; Sadowska, J.M.; Mulvey, G.; Armstrong, G.D.; Ling, H.; Pannu, N.S.; Read, R.J.; Bundle, D.R. Shiga-like toxins are neutralized by tailored multivalent carbohydrate ligands. Nature 2000, 403, 669–672. [Google Scholar] [CrossRef]
- Mulvey, G.L.; Marcato, P.; Kitov, P.I.; Sadowska, J.; Bundle, D.R.; Armstrong, G.D. Assessment in mice of the therapeutic potential of tailored, multivalent Shiga toxin carbohydrate ligands. J. Infect. Dis. 2003, 187, 640–649. [Google Scholar] [CrossRef]
- Nishikawa, K.; Matsuoka, K.; Kita, E.; Okabe, N.; Mizuguchi, M.; Hino, K.; Miyazawa, S.; Yamasaki, C.; Aoki, J.; Takashima, S.; et al. A therapeutic agent with oriented carbohydrates for treatment of infections by Shiga toxin-producing Escherichia coli O157:H7. Proc. Natl. Acad. Sci. USA 2002, 99, 7669–7674. [Google Scholar] [CrossRef]
- Nishikawa, K.; Matsuoka, K.; Watanabe, M.; Igai, K.; Hino, K.; Hatano, K.; Yamada, A.; Abe, N.; Terunuma, D.; Kuzuhara, H.; et al. Identification of the optimal structure required for a Shiga toxin neutralizer with oriented carbohydrates to function in the circulation. J. Infect. Dis. 2005, 191, 2097–2105. [Google Scholar] [CrossRef]
- Watanabe, M.; Matsuoka, K.; Kita, E.; Igai, K.; Higashi, N.; Miyagawa, A.; Watanabe, T.; Yanoshita, R.; Samejima, Y.; Terunuma, D.; et al. Oral therapeutic agents with highly clustered globotriose for treatment of Shiga toxigenic Escherichia coli infections. J. Infect. Dis. 2004, 189, 360–368. [Google Scholar] [CrossRef]
- Kulkarni, A.A.; Weiss, A.A.; Iyer, S.S. Glycan-based high-affinity ligands for toxins and pathogen receptors. Med. Res. Rev. 2010, 30, 327–393. [Google Scholar] [CrossRef]
- Kulkarni, A.A.; Fuller, C.; Korman, H.; Weiss, A.A.; Iyer, S.S. Glycan encapsulated gold nanoparticles selectively inhibit Shiga toxins 1 and 2. Bioconjug. Chem. 2010, 21, 1486–1493. [Google Scholar] [CrossRef]
- Pohlentz, G.; Steil, D.; Rubin, D.; Mellmann, A.; Karch, H.; Müthing, J. Pectin-derived neoglycolipids: Tools for differentiation of Shiga toxin-subtypes and inhibitors of Shiga toxin-mediated cellular injury. Carbohydr. Polym. 2019, 212, 323–333. [Google Scholar] [CrossRef]
- Detzner, J.; Gloerfeld, C.; Pohlentz, G.; Legros, N.; Humpf, H.U.; Mellmann, A.; Karch, H.; Müthing, J. Structural insights into Escherichia coli Shiga toxin (Stx) glycosphingolipid receptors of porcine renal epithelial cells and inhibition of Stx-mediated cellular injury using neoglycolipid-spiked glycovesicles. Microorganisms 2019, 7, 582. [Google Scholar] [CrossRef]
- Hostetter, S.J.; Helgerson, A.F.; Paton, J.C.; Paton, A.W.; Cornick, N.A. Therapeutic use of a receptor mimic probiotic reduces intestinal Shiga toxin levels in a piglet model of hemolytic uremic syndrome. BMC Res. Notes 2014, 7, 331. [Google Scholar] [CrossRef]
- Tzipori, S.; Sheoran, A.; Akiyoshi, D.; Donohue-Rolfe, A.; Trachtman, H. Antibody therapy in the management of Shiga toxin-induced hemolytic uremic syndrome. Clin. Microbiol. Rev. 2004, 17, 926–941. [Google Scholar] [CrossRef]
- Arimitsu, H.; Sasaki, K.; Iba, Y.; Kurosawa, Y.; Shimizu, T.; Tsuji, T. Isolation of B subunit-specific monoclonal antibody clones that strongly neutralize the toxicity of Shiga toxin 2. Microbiol. Immunol. 2015, 59, 71–81. [Google Scholar] [CrossRef]
- Moxley, R.A.; Francis, D.H.; Tamura, M.; Marx, D.B.; Santiago-Mateo, K.; Zhao, M. Efficacy of Urtoxazumab (TMA-15 humanized monoclonal antibody specific for Shiga toxin 2) against post-diarrheal neurological sequelae caused by Escherichia coli O157:H7 infection in the neonatal gnotobiotic piglet model. Toxins 2017, 9, 49. [Google Scholar] [CrossRef]
- De Macedo Henrique, I.; Sacerdoti, F.; Ferreira, R.L.; Henrique, C.; Amaral, M.M.; Piazza, R.M.F.; Luz, D. Therapeutic antibodies against Shiga toxins: Trends and perspectives. Front. Cell. Infect. Microbiol. 2022, 12, 825856. [Google Scholar] [CrossRef]
- Würzner, R.; Riedl, M.; Rosales, A.; Orth-Höller, D. Treatment of enterohemorrhagic Escherichia coli-induced hemolytic uremic syndrome (eHUS). Semin. Thromb. Hemost. 2014, 40, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Salvadori, M.; Bertoni, E. Update on hemolytic uremic syndrome: Diagnostic and therapeutic recommendations. World J. Nephrol. 2013, 2, 56–76. [Google Scholar] [CrossRef] [PubMed]
- Pape, L.; Hartmann, H.; Bange, F.C.; Suerbaum, S.; Bueltmann, E.; Ahlenstiel-Grunow, T. Eculizumab in typical hemolytic uremic syndrome (HUS) with neurological involvement. Medicine 2015, 94, e1000. [Google Scholar] [CrossRef] [PubMed]
- Walsh, P.R.; Johnson, S. Eculizumab in the treatment of Shiga toxin haemolytic uraemic syndrome. Pediatr. Nephrol. 2019, 34, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Mahat, U.; Matac, R.B.; Rotz, S.J. Use of complement monoclonal antibody eculizumab in Shiga toxin producing Escherichia coli associated hemolytic uremic syndrome: A review of current evidence. Pediatr. Blood Cancer 2019, 66, e27913. [Google Scholar] [CrossRef]
- Eaton, K.A.; Honkala, A.; Auchtung, T.A.; Britton, R.A. Probiotic Lactobacillus reuteri ameliorates disease due to enterohemorrhagic Escherichia coli in germfree mice. Infect. Immun. 2011, 79, 185–191. [Google Scholar] [CrossRef]
- Rund, S.A.; Rohde, H.; Sonnenborn, U.; Oelschlaeger, T.A. Antagonistic effects of probiotic Escherichia coli Nissle 1917 on EHEC strains of serotype O104:H4 and O157:H7. Int. J. Med. Microbiol. 2013, 303, 1–8. [Google Scholar] [CrossRef]
- Cordonnier, C.; Thévenot, J.; Etienne-Mesmin, L.; Alric, M.; Livrelli, V.; Blanquet-Diot, S. Probiotic and enterohemorrhagic Escherichia coli: An effective strategy against a deadly enemy? Crit. Rev. Microbiol. 2017, 43, 116–132. [Google Scholar] [CrossRef]
- Szu, S.C.; Ahmed, A. Clinical studies of Escherichia coli O157:H7 conjugate vaccines in adults and young children. Microbiol. Spectr. 2014, 2, 2–6. [Google Scholar] [CrossRef]
- Rojas-Lopez, M.; Monterio, R.; Pizza, M.; Desvaux, M.; Rosini, R. Intestinal pathogenic Escherichia coli insights for vaccine development. Front. Microbiol. 2018, 9, 440. [Google Scholar] [CrossRef]
- Sabouri, S.; Sepehrizadeh, Z.; Amirpour-Rostami, S.; Skumik, M. A minireview on the in vitro and in vivo experiments with anti-Escherichia coli O157:H7 phages as potential biocontrol and phage therapy agents. Int. J. Food Microbiol. 2017, 243, 52–57. [Google Scholar] [CrossRef]
- Lino, M.; Kus, J.V.; Tran, S.I.; Naqvi, Z.; Binnington, B.; Goodman, S.D.; Segall, A.M.; Foster, D.B. A novel antimicrobial peptide significantly enhances acid-induced killing of Shiga toxin-producing Escherichia coli O157 and non-O157 serotypes. Microbiology 2011, 157, 1768–1775. [Google Scholar] [CrossRef]
- Puño-Sarmiento, J.; Anderson, E.M.; Park, A.J.; Khursigara, C.M.; Foster, D.E.B. Potentiation of antibiotics by a novel antimicrobial peptide against Shiga toxin producing E. coli O157:H7. Sci. Rep. 2020, 10, 10029. [Google Scholar] [CrossRef]
- Johannes, L.; Römer, W. Shiga toxins—From cell biology to biomedical applications. Nat. Rev. Microbiol. 2010, 8, 105–116. [Google Scholar] [CrossRef]
- Spooner, R.A.; Lord, J.M. How ricin and Shiga toxin reach the cytosol of target cells: Retranslocation from the endoplasmic reticulum. Curr. Top. Microbiol. Immunol. 2012, 357, 19–40. [Google Scholar] [CrossRef]
- Johannes, L. Shiga toxin—A model for glycolipid-dependent and lectin-driven endocytosis. Toxins 2017, 9, 340. [Google Scholar] [CrossRef]
- Sandvig, K.; Kavaliauskiene, S.; Skotland, T. The protein toxins ricin and Shiga toxin as tools to explore cellular mechanisms of internalization and intracellular transport. Toxins 2021, 13, 377. [Google Scholar] [CrossRef]
- Li, D.; Selyunin, A.; Mukhopadhyay, S. Targeting the endosome-to-Golgi transport of Shiga toxins as a therapeutic strategy. Toxins 2020, 12, 342. [Google Scholar] [CrossRef]
- Kouzel, I.U.; Kehl, A.; Berger, P.; Liashkovich, I.; Steil, D.; Makalowski, W.; Suzuki, Y.; Pohlentz, G.; Karch, H.; Mellmann, A.; et al. RAB5A and TRAPPC6B are novel targets for Shiga toxin 2a inactivation in kidney epithelial cells. Sci. Rep. 2020, 10, 4945. [Google Scholar] [CrossRef]
- Fuchs, B.; Süss, R.; Schiller, J. An update of MALDI-TOF mass spectrometry in lipid research. Prog. Lipid Res. 2010, 49, 450–475. [Google Scholar] [CrossRef]
- Gode, D.; Volmer, D.A. Lipid imaging by mass spectrometry. Analyst 2013, 138, 1289–1315. [Google Scholar] [CrossRef]
- Soltwisch, J.; Kettling, H.; Vens-Cappell, S.; Wiegelmann, M.; Müthing, J.; Dreisewerd, K. Mass spectrometry imaging with laser-induced postionization. Science 2015, 348, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Dreisewerd, K.; Bien, T.; Soltwisch, J. MALDI-2 and t-MALDI-2 mass spectrometry. Methods Mol. Biol. 2022, 2437, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Baker, T.C.; Han, J.; Borchers, C.H. Recent advancements in matrix-assisted laser desorption/ionization mass spectrometry imaging. Curr. Opin. Biotechnol. 2017, 43, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Passarelli, M.K.; Ewing, A.G. Single-cell imaging mass spectrometry. Curr. Opin. Chem. Biol. 2013, 17, 854–859. [Google Scholar] [CrossRef]
- Römpp, A.; Spengler, B. Mass spectrometry imaging with high resolution in mass and space. Histochem. Cell Biol. 2013, 139, 759–783. [Google Scholar] [CrossRef]
- Niehaus, M.; Soltwisch, J.; Belov, M.E.; Dreisewerd, K. Transmission-mode MALDI-2 mass spectrometry imaging of cells and tissues at subcellular resolution. Nat. Methods 2019, 16, 925–931. [Google Scholar] [CrossRef]
- Bien, T.; Bessler, S.; Dreisewerd, K.; Soltwisch, J. Transmission-mode MALDI mass spectrometry imaging of single cells: Optimizing sample preparation protocols. Anal. Chem. 2021, 93, 4513–4520. [Google Scholar] [CrossRef] [PubMed]
- Vens-Cappell, S.; Kouzel, I.U.; Kettling, H.; Soltwisch, J.; Bauwens, A.; Porubsky, S.; Müthing, J.; Dreisewerd, K. On-tissue phospholipase C digestion for enhanced MALDI MS imaging of neutral glycosphingolipids. Anal. Chem. 2016, 88, 5595–5599. [Google Scholar] [CrossRef] [PubMed]
- Bednařík, A.; Bölsker, S.; Soltwisch, J.; Dreisewerd, K. An on-tissue Paternò-Büchi reaction for localization of carbon-carbon double bonds in phospholipids and glycolipids by matrix-assisted laser-desorption-ionization mass-spectrometry imaging. Angew. Chem. Int. Ed. Engl. 2018, 57, 12092–12096. [Google Scholar] [CrossRef] [PubMed]
- Bednařik, A.; Preisler, J.; Bezdeková, D.; Machálková, M.; Hendrych, M.; Navrátilová, J.; Knopfová, L.; Moskovets, E.; Soltwisch, J.; Dreisewerd, K. Ozonization of tissue sections for MALDI MS imaging of carbon-carbon double bond positional isomers of phospholipids. Anal. Chem. 2020, 92, 6245–6250. [Google Scholar] [CrossRef] [PubMed]
- Lalowski, M.; Magni, F.; Mainini, V.; Monogioudi, E.; Gotsopoulos, A.; Soliymani, R.; Chinello, C.; Baumann, M. Imaging mass spectrometry: A new tool for kidney disease investigations. Nephrol. Dial. Transplant. 2013, 28, 1648–1656. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, E.B.; De la Monte, S.M. Review of matrix-assisted laser desorption ionization-imaging mass spectrometry for lipid biochemical histopathology. J. Histochem. Cytochem. 2015, 63, 762–771. [Google Scholar] [CrossRef]
- Bien, T.; Perl, M.; Machmüller, A.C.; Nitsche, U.; Conrad, A.; Johannes, L.; Müthing, J.; Soltwisch, J.; Janssen, K.P.; Dreisewerd, K. MALDI-2 mass spectrometry and immunohistochemistry imaging of Gb3Cer, Gb4Cer, and further glycosphingolipids in human colorectal cancer tissue. Anal. Chem. 2020, 92, 7096–7105. [Google Scholar] [CrossRef]
- Kettling, H.; Vens-Cappell, S.; Soltwisch, J.; Pirkl, A.; Haier, J.; Müthing, J.; Dreisewerd, K. MALDI mass spectrometry imaging of bioactive lipids in mouse brain with a Synapt G2-S mass spectrometer operated at elevated pressure: Improving the analytical sensitivity and the lateral resolution to ten micrometers. Anal. Chem. 2014, 86, 7798–7805. [Google Scholar] [CrossRef]
- Kompauer, M.; Heiles, S.; Spengler, B. Autofocusing MALDI mass spectrometry imaging of tissue sections and 3D chemical topography of nonflat surfaces. Nat. Methods 2017, 14, 1156–1158. [Google Scholar] [CrossRef]
- Passarelli, M.; Pirkl, A.; Moellers, R.; Grinfeld, D.; Kollmer, F.; Havelund, R.; Newman, C.F.; Marshall, P.S.; Arlinghaus, H.; Alexander, M.R.; et al. The 3D OrbiSIMS-label-free metabolic imaging with subcellular lateral resolution and high mass-resolving power. Nat. Methods 2017, 14, 1175–1183. [Google Scholar] [CrossRef]
- Dreisewerd, K.; Yew, J.Y. Mass spectrometry imaging goes three dimensional. Nat. Methods 2017, 14, 1139–1140. [Google Scholar] [CrossRef]
- Capolupo, L.; Khven, I.; Lederer, A.R.; Mazzeo, L.; Glousker, G.; Ho, S.; Russo, F.; Montoya, J.P.; Bhandari, D.R.; Bowman, A.P.; et al. Sphingolipids control dermal fibroblast heterogeneity. Science 2022, 376, eabh1623. [Google Scholar] [CrossRef]
- Karlsson, K.A. Animal glycosphingolipids as membrane attachment sites for bacteria. Annu. Rev. Biochem. 1989, 58, 309–350. [Google Scholar] [CrossRef]
- Sharon, N.; Ofek, I. Fighting infectious diseases with inhibitors of microbial adhesion to host tissues. Crit. Rev. Food Sci. Nutr. 2002, 42 (Suppl. 3), 267–272. [Google Scholar] [CrossRef]
- Kanda, V.; Kitov, P.; Bundle, D.R.; McDermott, M.T. Surface plasmon resonance imaging measurements of the inhibition of Shiga-like toxin by synthetic multivalent inhibitors. Anal. Chem. 2005, 77, 7497–7504. [Google Scholar] [CrossRef]
- Sharon, N. Carbohydrates as future anti-adhesion drugs for infectious diseases. Biochim. Biophys. Acta 2006, 1760, 527–537. [Google Scholar] [CrossRef]
- Jacobson, J.M.; Kitov, P.I.; Bundle, D.R. The synthesis of a multivalent heterobifunctiponal ligand for specific interaction with Shiga toxin 2 produced by E. coli O157:H7. Carbohydr. Res. 2013, 378, 4–14. [Google Scholar] [CrossRef]
- Di, R.; Vakkalanka, M.S.; Onumpai, C.; Chau, H.K.; White, A.; Rastall, R.A.; Yam, K.; Hotchkiss, A.T., Jr. Pectic oligosaccharide structure-function relationships: Prebiotics, inhibitors of Escherichia coli O157:H7 adhesion and reduction of Shiga toxin cytotoxicity in HT29 cells. Food Chem. 2017, 227, 245–254. [Google Scholar] [CrossRef]
- Zhang, P.; Paszkiewicz, E.; Wang, Q.; Sadowska, J.M.; Kitov, P.I.; Bundle, D.R.; Ling, C.C. Clustering of Pk-trisaccharides on amphiphilic cyclodextrin reveals unprecedented affinity for the Shiga-like toxin Stx2. Chem. Commun. 2017, 53, 10528–10531. [Google Scholar] [CrossRef]
- Detzner, J.; Steil, D.; Pohlentz, G.; Legros, N.; Humpf, H.U.; Mellmann, A.; Karch, H.; Müthing, J. Real-time interaction analysis of Shiga toxins and membrane microdomains of primary human brain microvascular endothelial cells. Glycobiology 2020, 30, 174–185. [Google Scholar] [CrossRef]
- Detzner, J.; Steil, D.; Pohlentz, G.; Legros, N.; Müthing, J. Surface acoustic wave (SAW) real-time interaction analysis of influenza A virus hemagglutinins with sialylated neoglycolipids. Glycobiology 2021, 31, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Aerts, J.M.F.G.; Artola, M.; Van Eijk, M.; Ferraz, M.J.; Boot, R.G. Glycosphingolipids and infection. Potential new therapeutic avenues. Front. Cell Dev. Biol. 2019, 7, 324. [Google Scholar] [CrossRef] [PubMed]
- Spaulding, C.N.; Klein, R.D.; Ruer, S.; Kau, A.L.; Schreiber, H.L.; Cusumano, Z.T.; Dodson, K.W.; Pinkner, J.S.; Fremont, D.H.; Janetka, J.W.; et al. Selective depletion of uropathogenic E. coli from the gut by a FimH antagonist. Nature 2017, 546, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Uribe, A.; Roe, A.J. Disarming the enemy: Targeting bacterial toxins with small molecules. Emerg. Top. Life Sci. 2017, 1, 31–39. [Google Scholar] [CrossRef]
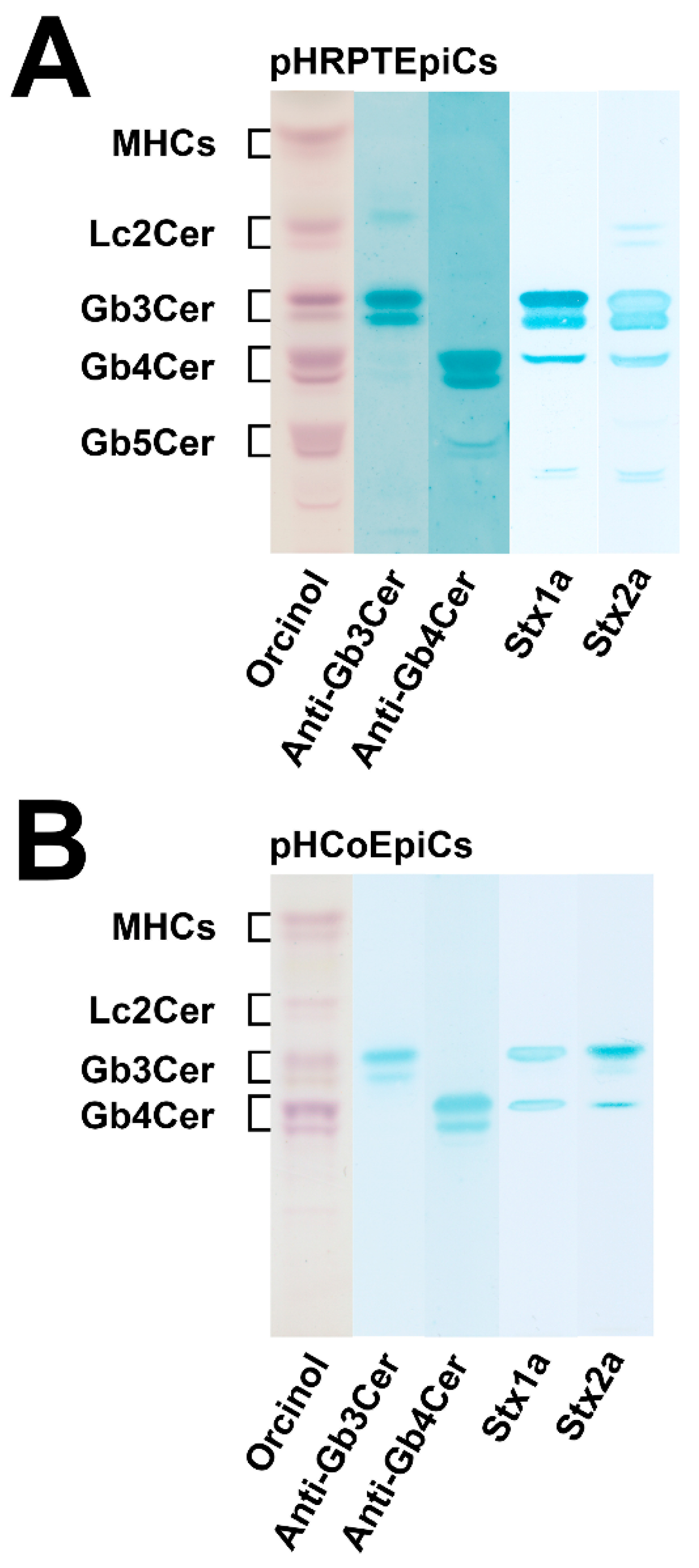

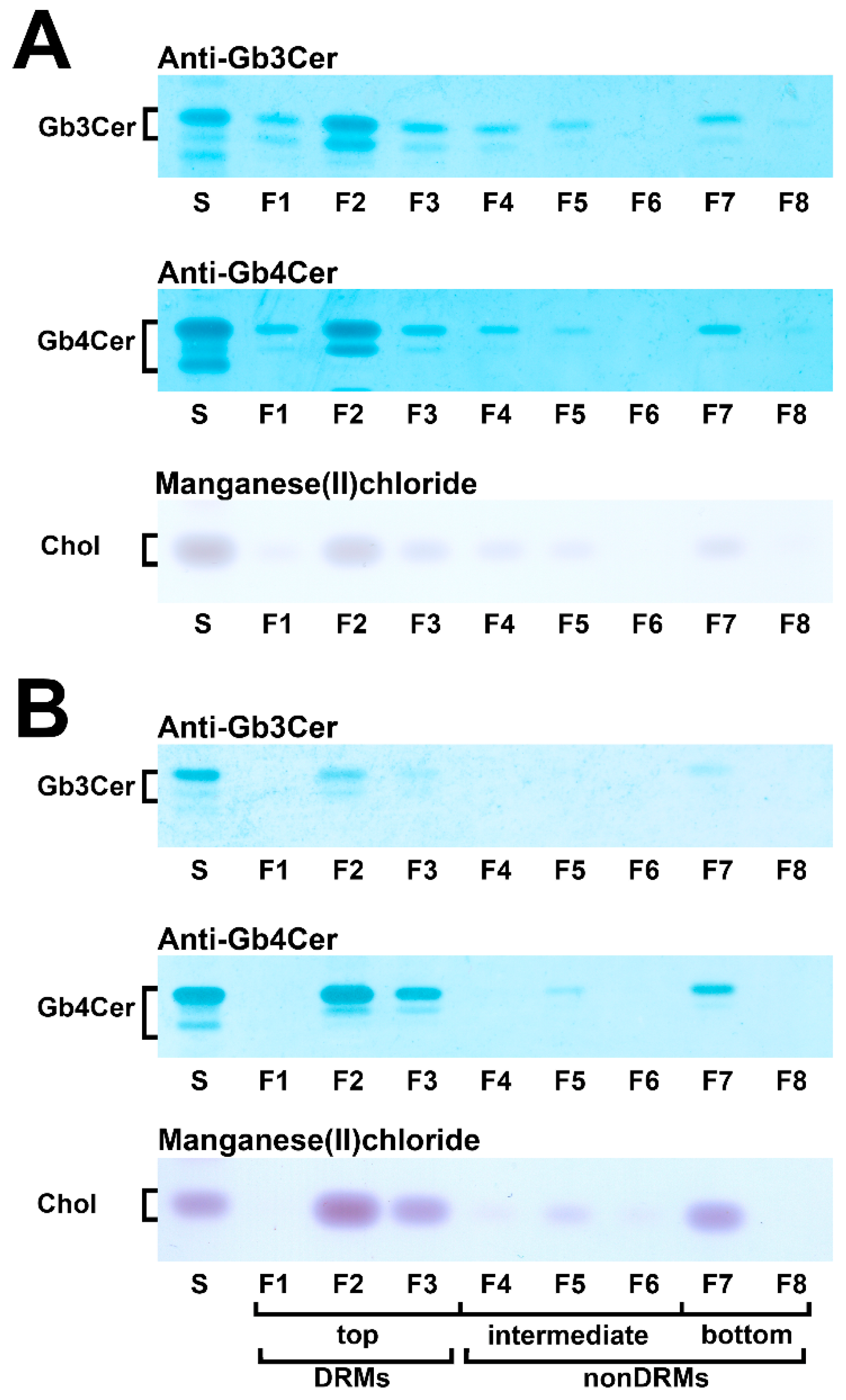

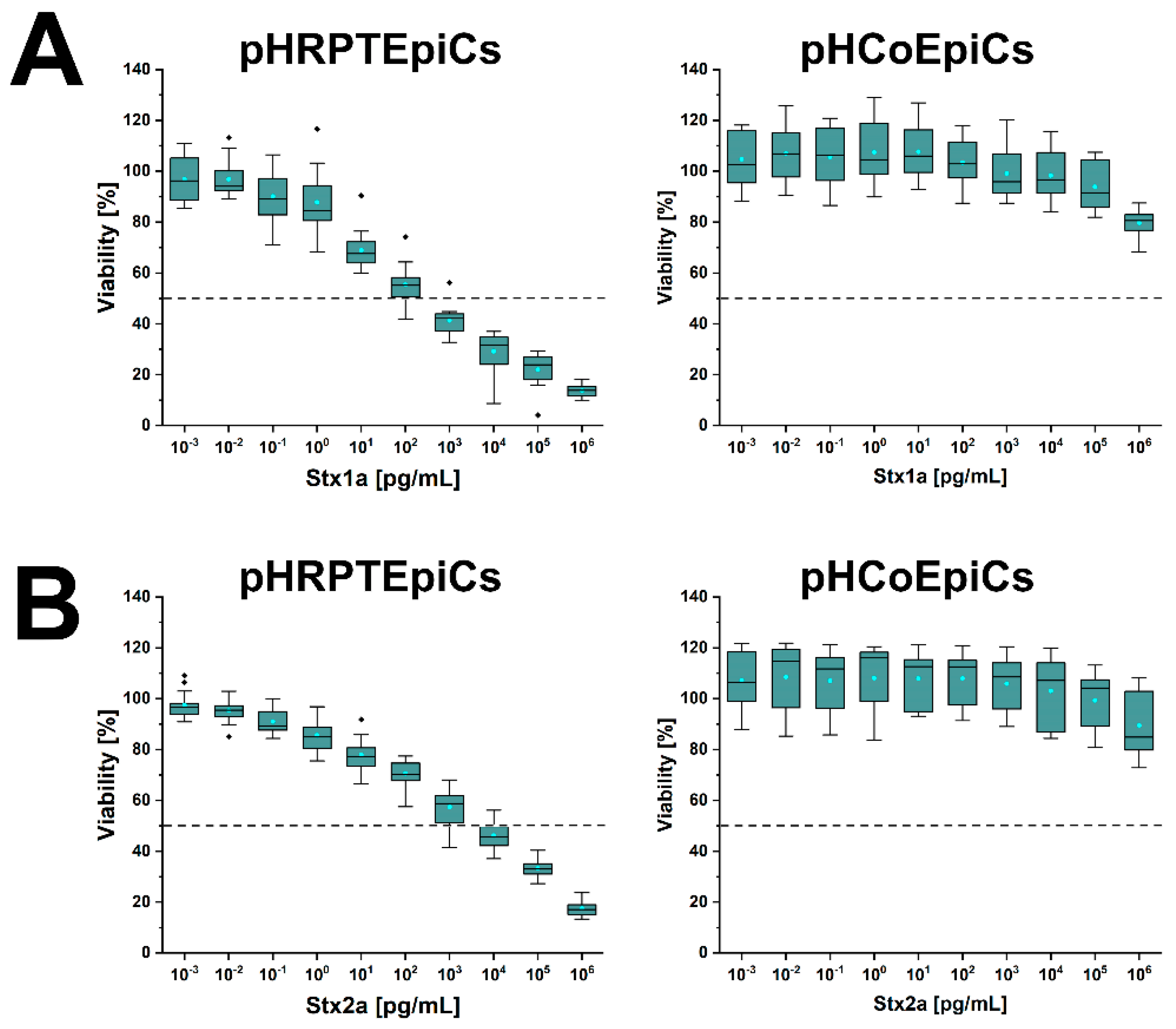
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Detzner, J.; Pohlentz, G.; Müthing, J. Enterohemorrhagic Escherichia coli and a Fresh View on Shiga Toxin-Binding Glycosphingolipids of Primary Human Kidney and Colon Epithelial Cells and Their Toxin Susceptibility. Int. J. Mol. Sci. 2022, 23, 6884. https://doi.org/10.3390/ijms23136884
Detzner J, Pohlentz G, Müthing J. Enterohemorrhagic Escherichia coli and a Fresh View on Shiga Toxin-Binding Glycosphingolipids of Primary Human Kidney and Colon Epithelial Cells and Their Toxin Susceptibility. International Journal of Molecular Sciences. 2022; 23(13):6884. https://doi.org/10.3390/ijms23136884
Chicago/Turabian StyleDetzner, Johanna, Gottfried Pohlentz, and Johannes Müthing. 2022. "Enterohemorrhagic Escherichia coli and a Fresh View on Shiga Toxin-Binding Glycosphingolipids of Primary Human Kidney and Colon Epithelial Cells and Their Toxin Susceptibility" International Journal of Molecular Sciences 23, no. 13: 6884. https://doi.org/10.3390/ijms23136884
APA StyleDetzner, J., Pohlentz, G., & Müthing, J. (2022). Enterohemorrhagic Escherichia coli and a Fresh View on Shiga Toxin-Binding Glycosphingolipids of Primary Human Kidney and Colon Epithelial Cells and Their Toxin Susceptibility. International Journal of Molecular Sciences, 23(13), 6884. https://doi.org/10.3390/ijms23136884





