Effects of Ultrafine Single-Nanometer Oxygen Bubbles on Radiation Sensitivity in a Tumor-Bearing Mouse Model
Abstract
:1. Introduction
2. Results
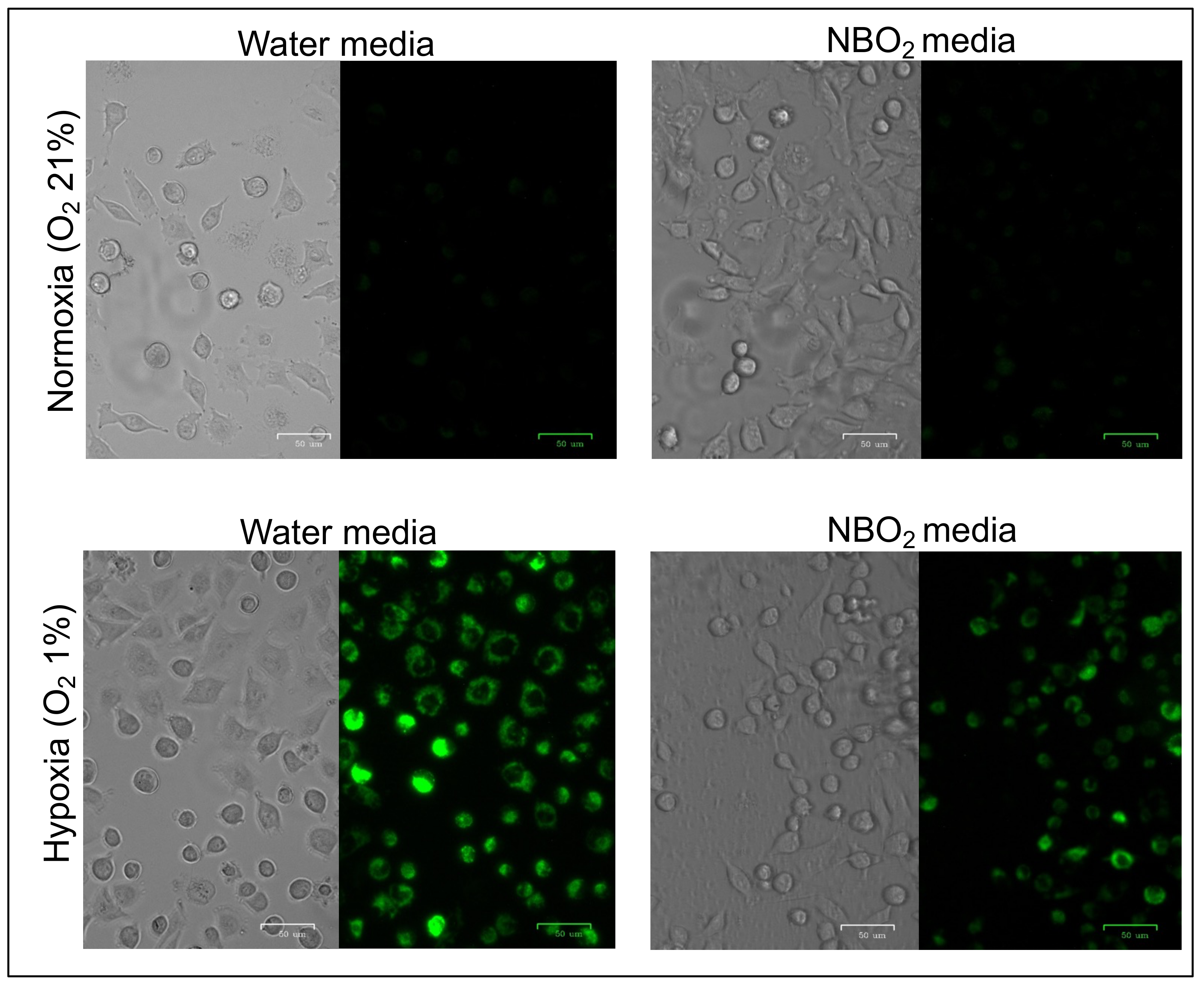
2.1. Suppression of Hypoxic Conditions in Cancer Cell Lines Treated by the NBO2 Media
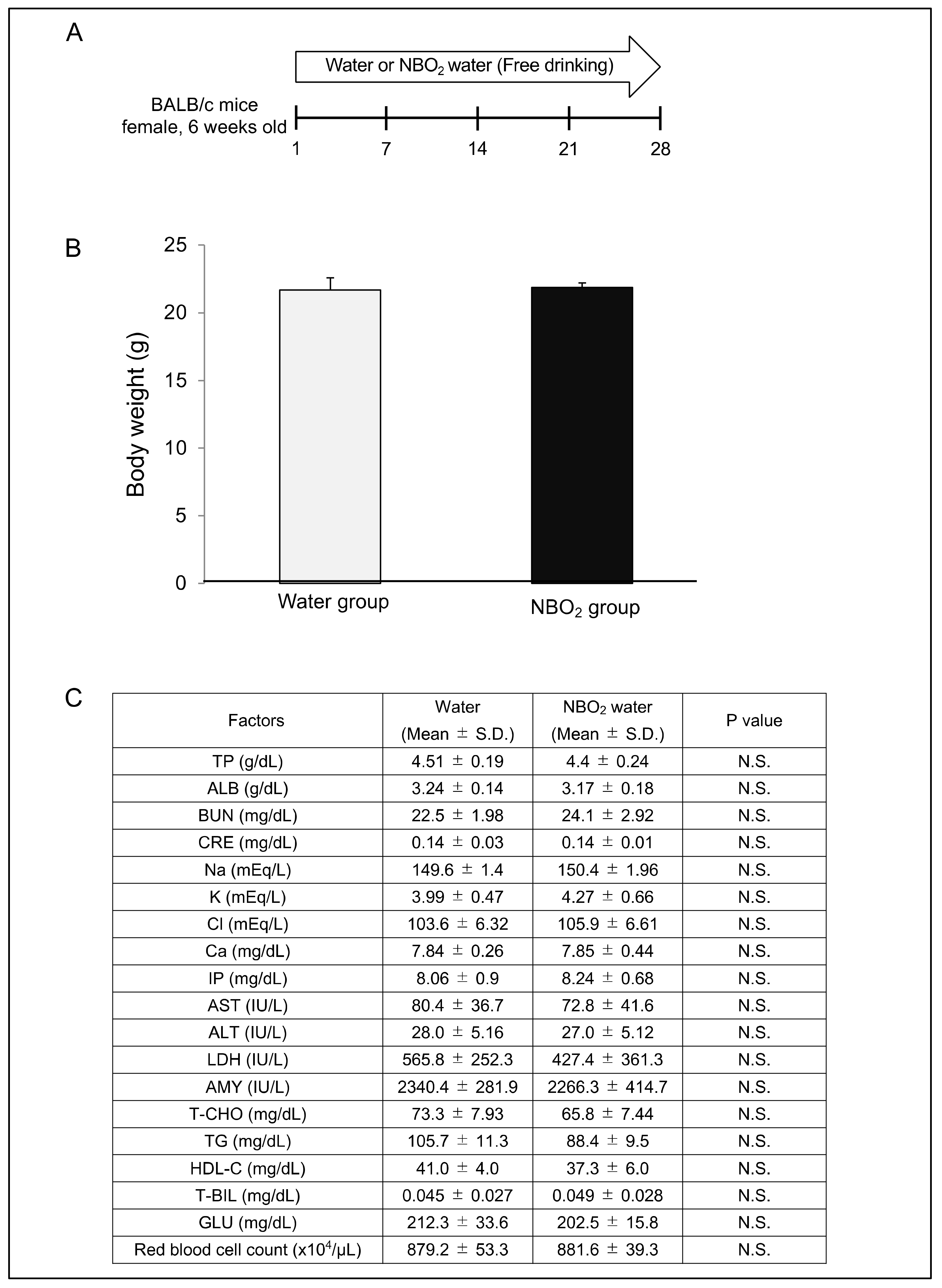
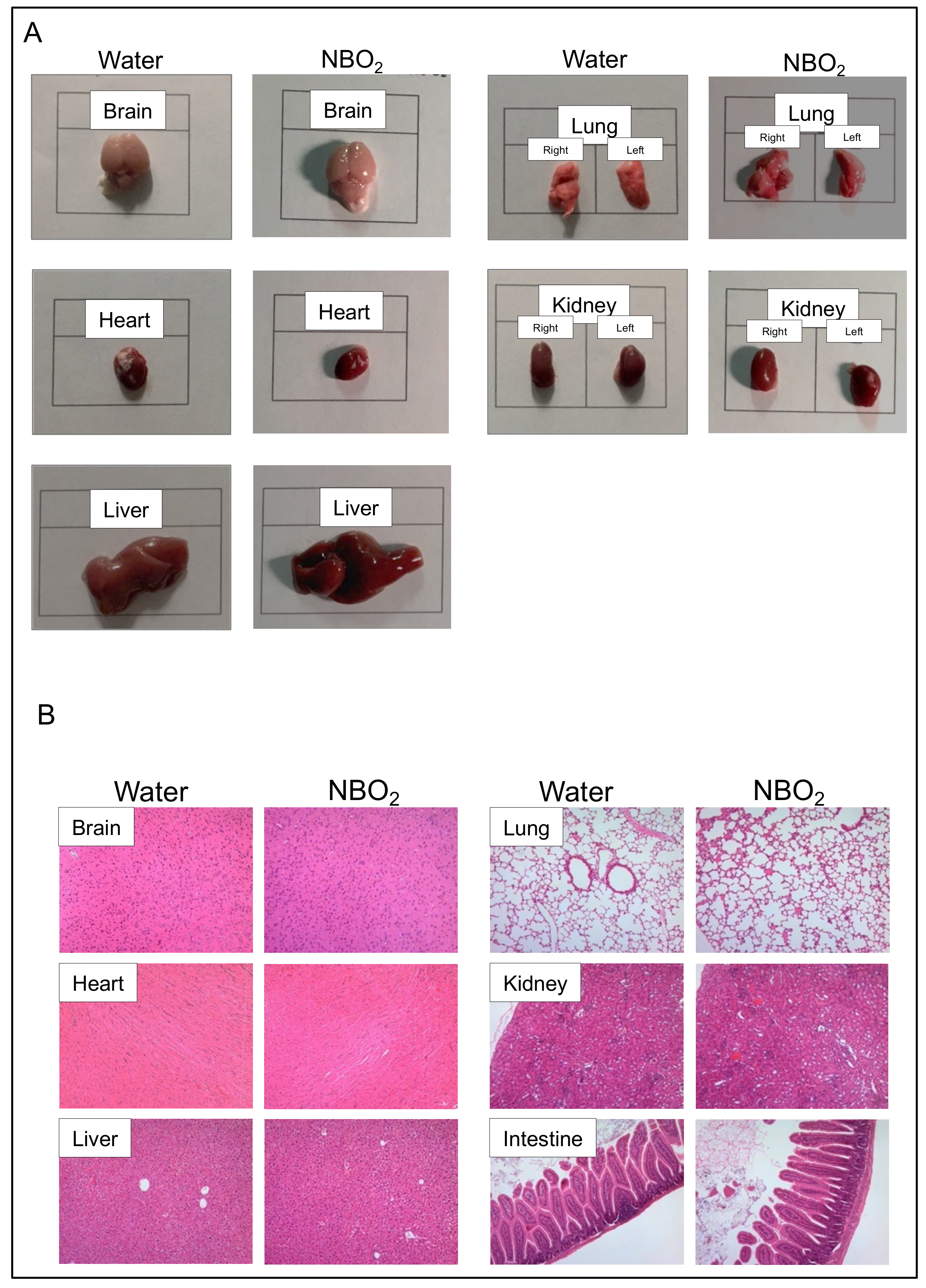
2.2. Safety Verification of NBO2 Water in Mice
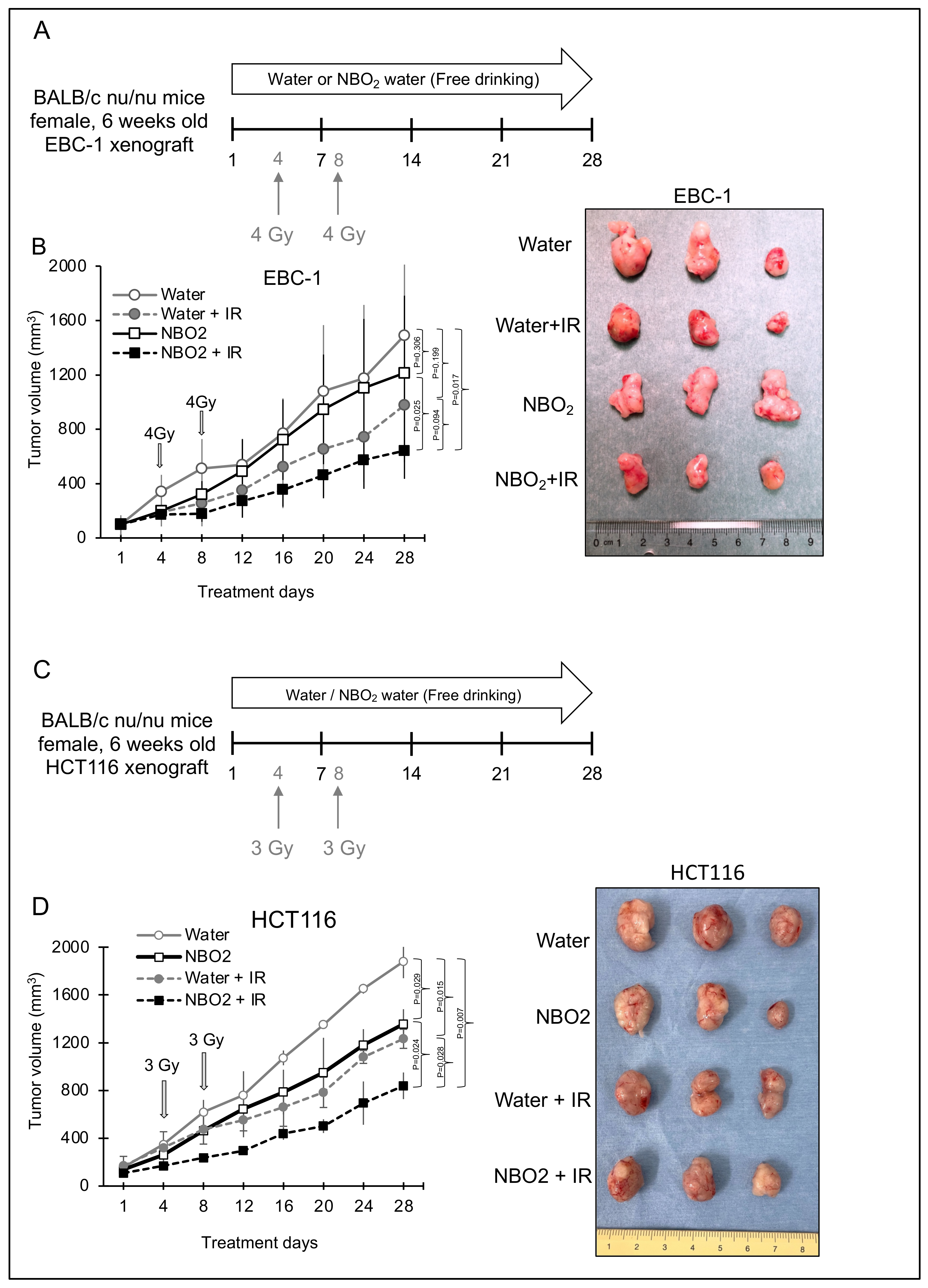
2.3. Effect of NBO2 Water with Radiation in a Xenograft Mouse Model
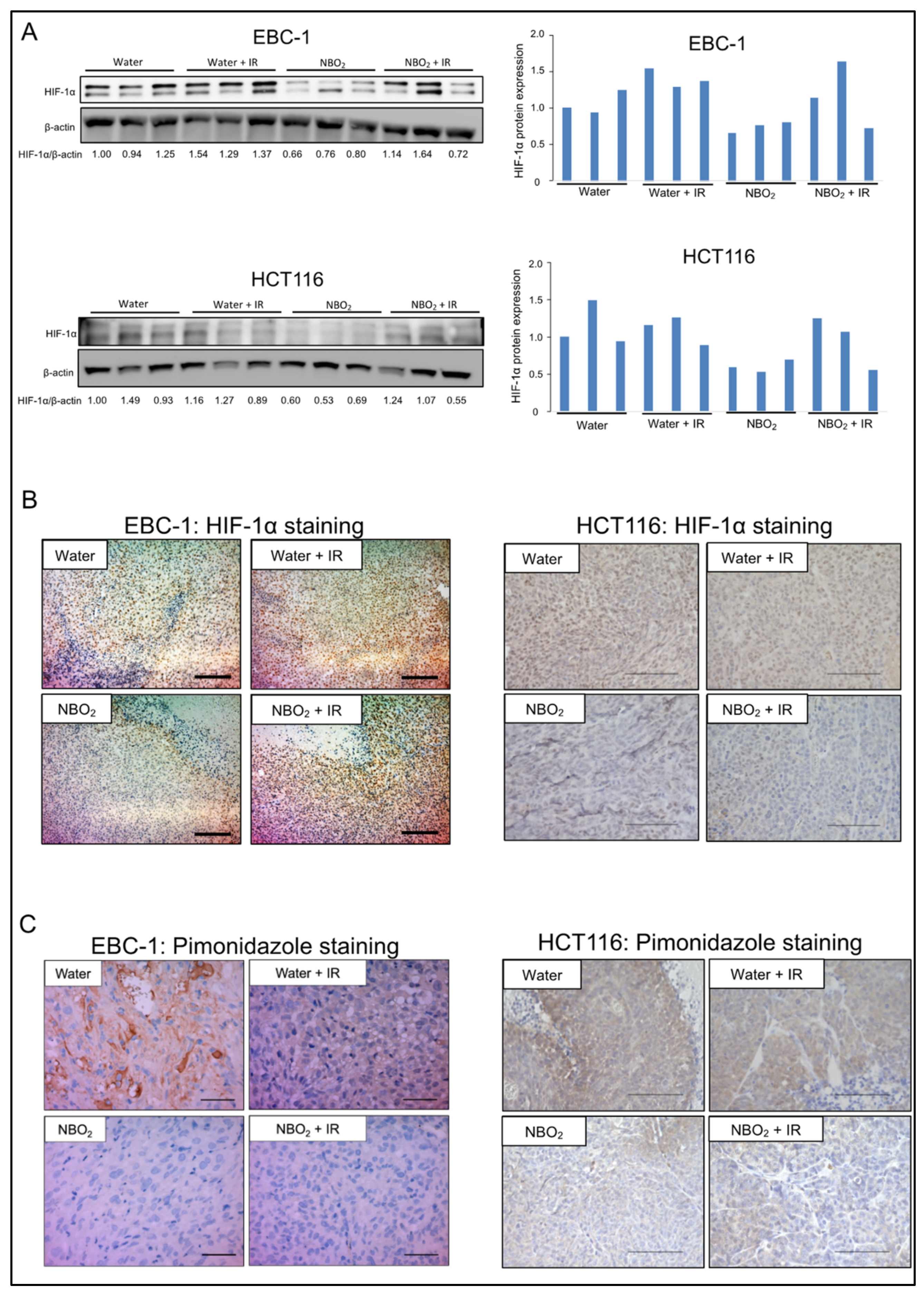
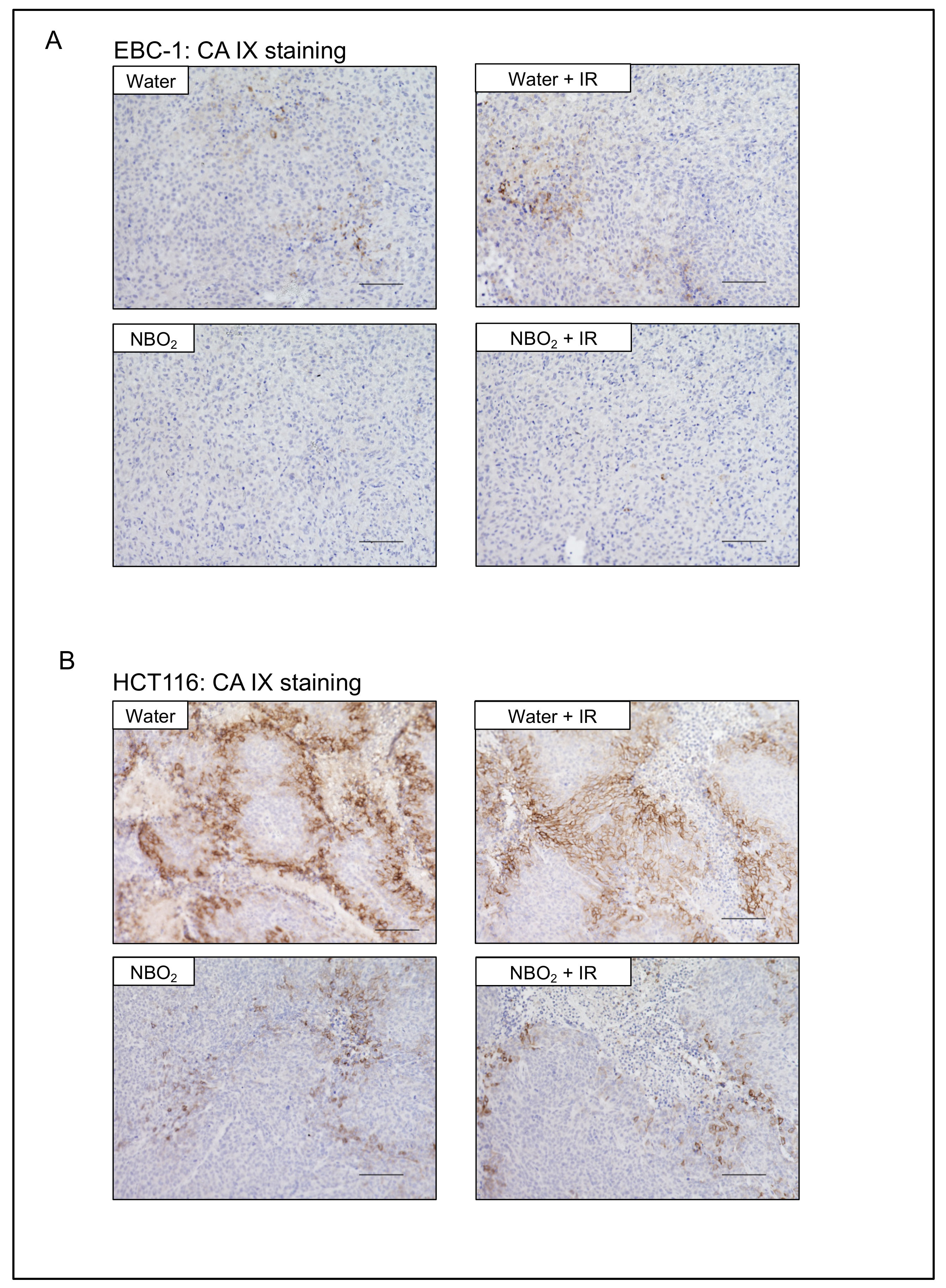
2.4. Suppression of Hypoxic Condition and HIF-1α Expression by NBO2 Administration in Xenografted Tumors
3. Discussion
4. Materials and Methods
4.1. Production of NBO2 Water
4.2. Cell Lines
4.3. Hypoxia Detection by Fluorescent Hypoxic Probe
4.4. Protein Extraction and Western Blotting
4.5. Safety Assessment of the NBO2 Water in Mice
4.6. Nude Mouse Xenograft Model
4.7. Radiation Treatment against Xenograft Tumors
4.8. Detection of Hypoxic Conditions in Xenograft Tumors
4.9. Immunohistochemistry
4.10. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Felice, F.; Piccioli, A.; Musio, D.; Tombolini, V. The role of radiation therapy in bone metastases management. Oncotarget 2017, 8, 25691–25699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sampath, S. Treatment: Radiation therapy. Cancer Treat. Res. 2016, 170, 105–118. [Google Scholar] [PubMed]
- Purkayastha, A.; Sharma, N.; Sarin, A.; Bhatnagar, S.; Chakravarty, N.; Mukundan, H.; Suhag, V.; Singh, S. Radiation fibrosis syndrome: The evergreen menace of radiation therapy. Asia Pac. J. Oncol. Nurs. 2019, 6, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Barker, H.E.; Paget, J.T.; Khan, A.A.; Harrington, K.J. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat. Rev. Cancer 2015, 15, 409–425. [Google Scholar] [CrossRef]
- Moeller, B.J.; Cao, Y.; Vujaskovic, Z.; Li, C.Y.; Haroon, Z.A.; Dewhirst, M.W. The relationship between hypoxia and angiogenesis. Semin. Radiat. Oncol. 2004, 14, 215–221. [Google Scholar] [CrossRef]
- Simonsen, T.G.; Gaustad, J.V.; Leinaas, M.N.; Rofstad, E.K. Vascular abnormalities associated with acute hypoxia in human melanoma xenografts. Radiother. Oncol. 2012, 105, 72–78. [Google Scholar] [CrossRef]
- Weidemann, A.; Johnson, R.S. Biology of HIF-1alpha. Cell Death Differ. 2008, 15, 621–627. [Google Scholar] [CrossRef] [Green Version]
- Schito, L.; Semenza, G.L. Hypoxia-inducible factors: Master regulators of cancer progression. Trends Cancer 2016, 2, 758–770. [Google Scholar] [CrossRef] [Green Version]
- Kunz, M.; Ibrahim, S.M. Molecular responses to hypoxia in tumor cells. Mol Cancer 2003, 2, 23. [Google Scholar] [CrossRef] [Green Version]
- Koh, M.Y.; Powis, G. Passing the baton: The HIF switch. Trends Biochem. Sci. 2012, 37, 364–372. [Google Scholar] [CrossRef] [Green Version]
- Albadari, N.; Deng, S.; Li, W. The transcriptional factors HIF-1 and HIF-2 and their novel inhibitors in cancer therapy. Expert Opin. Drug Discov. 2019, 14, 667–682. [Google Scholar] [CrossRef]
- Shirai, Y.; Chow, C.C.T.; Kambe, G.; Suwa, T.; Kobayashi, M.; Takahashi, I.; Harada, H.; Nam, J.M. An overview of the recent development of anticancer agents targeting the HIF-1 transcription factor. Cancers 2021, 13, 2813. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Tang, B.; Sun, X. Development of inhibitors targeting hypoxia-inducible factor 1 and 2 for cancer therapy. Yonsei Med. J. 2017, 58, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Burroughs, S.K.; Kaluz, S.; Wang, D.; Wang, K.; Van Meir, E.G.; Wang, B. Hypoxia inducible factor pathway inhibitors as anticancer therapeutics. Future Med. Chem. 2013, 5, 553–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iijima, M.; Gombodorj, N.; Tachibana, Y.; Tachibana, K.; Yokobori, T.; Honma, K.; Nakano, T.; Asao, T.; Kuwahara, R.; Aoyama, K.; et al. Development of single nanometer-sized ultrafine oxygen bubbles to overcome the hypoxia-induced resistance to radiation therapy via the suppression of hypoxia-inducible factor1alpha. Int. J. Oncol. 2018, 52, 679–686. [Google Scholar] [CrossRef] [Green Version]
- Ward, C.; Meehan, J.; Gray, M.; Kunkler, I.H.; Langdon, S.P.; Argyle, D.J. Carbonic anhydrase IX (CAIX), cancer, and radiation responsiveness. Metabolites 2018, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Xiong, A.; Liu, Y. Targeting hypoxia inducible factors-1alpha as a novel therapy in fibrosis. Front. Pharmacol. 2017, 8, 326. [Google Scholar] [CrossRef]
- Epstein Shochet, G.; Bardenstein-Wald, B.; McElroy, M.; Kukuy, A.; Surber, M.; Edelstein, E.; Pertzov, B.; Kramer, M.R.; Shitrit, D. Hypoxia inducible factor 1a supports a pro-fibrotic phenotype loop in idiopathic pulmonary fibrosis. Int. J. Mol. Sci. 2021, 22, 3331. [Google Scholar] [CrossRef]
- King, M.; Joseph, S.; Albert, A.; Thomas, T.V.; Nittala, M.R.; Woods, W.C.; Vijayakumar, S.; Packianathan, S. Use of amifostine for cytoprotection during radiation therapy: A review. Oncology 2020, 98, 61–80. [Google Scholar] [CrossRef]
- Liu, J.; Pandya, P.; Afshar, S. Therapeutic advances in oncology. Int. J. Mol. Sci. 2021, 22, 2008. [Google Scholar] [CrossRef]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Johnston, F.M.; Beckman, M. Updates on management of gastric cancer. Curr. Oncol. Rep. 2019, 21, 67. [Google Scholar] [CrossRef] [PubMed]
- Huh, G.; Ahn, S.H.; Suk, J.G.; Lee, M.H.; Kim, W.S.; Kwon, S.K.; Ock, C.Y.; Keam, B.; Heo, D.S.; Kim, J.H.; et al. Severe late dysphagia after multimodal treatment of stage III/IV laryngeal and hypopharyngeal cancer. Jpn. J. Clin. Oncol. 2020, 50, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Kodama, H.; Hatakeyama, S.; Momota, M.; Togashi, K.; Hamaya, T.; Hamano, I.; Fujita, N.; Kojima, Y.; Okamoto, T.; Yoneyama, T.; et al. Effect of frailty and comorbidity on surgical contraindication in patients with localized prostate cancer (FRART-PC Study). Urol. Oncol. 2021, 39, 191.e1–191.e8. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.R.; Hay, M.P. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer 2011, 11, 393–410. [Google Scholar] [CrossRef]
- Sorensen, B.S.; Horsman, M.R. Tumor hypoxia: Impact on radiation therapy and molecular pathways. Front. Oncol. 2020, 10, 562. [Google Scholar] [CrossRef]
- Shannon, A.M.; Bouchier-Hayes, D.J.; Condron, C.M.; Toomey, D. Tumour hypoxia, chemotherapeutic resistance and hypoxia-related therapies. Cancer Treat. Rev. 2003, 29, 297–307. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gombodorj, N.; Yokobori, T.; Mutsuki, N.; Erkhem-Ochir, B.; Okami, H.; Asao, T.; Saeki, H.; Shirabe, K.; Yamanouchi, D. Effects of Ultrafine Single-Nanometer Oxygen Bubbles on Radiation Sensitivity in a Tumor-Bearing Mouse Model. Int. J. Mol. Sci. 2022, 23, 6838. https://doi.org/10.3390/ijms23126838
Gombodorj N, Yokobori T, Mutsuki N, Erkhem-Ochir B, Okami H, Asao T, Saeki H, Shirabe K, Yamanouchi D. Effects of Ultrafine Single-Nanometer Oxygen Bubbles on Radiation Sensitivity in a Tumor-Bearing Mouse Model. International Journal of Molecular Sciences. 2022; 23(12):6838. https://doi.org/10.3390/ijms23126838
Chicago/Turabian StyleGombodorj, Navchaa, Takehiko Yokobori, Nobutoshi Mutsuki, Bilguun Erkhem-Ochir, Haruka Okami, Takayuki Asao, Hiroshi Saeki, Ken Shirabe, and Dai Yamanouchi. 2022. "Effects of Ultrafine Single-Nanometer Oxygen Bubbles on Radiation Sensitivity in a Tumor-Bearing Mouse Model" International Journal of Molecular Sciences 23, no. 12: 6838. https://doi.org/10.3390/ijms23126838
APA StyleGombodorj, N., Yokobori, T., Mutsuki, N., Erkhem-Ochir, B., Okami, H., Asao, T., Saeki, H., Shirabe, K., & Yamanouchi, D. (2022). Effects of Ultrafine Single-Nanometer Oxygen Bubbles on Radiation Sensitivity in a Tumor-Bearing Mouse Model. International Journal of Molecular Sciences, 23(12), 6838. https://doi.org/10.3390/ijms23126838






