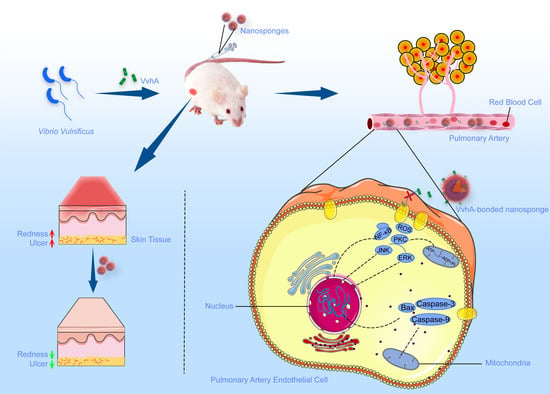
Biomimetic Nanosponges Enable the Detoxification of Vibrio vulnificus Hemolysin
Abstract
:1. Introduction
2. Results
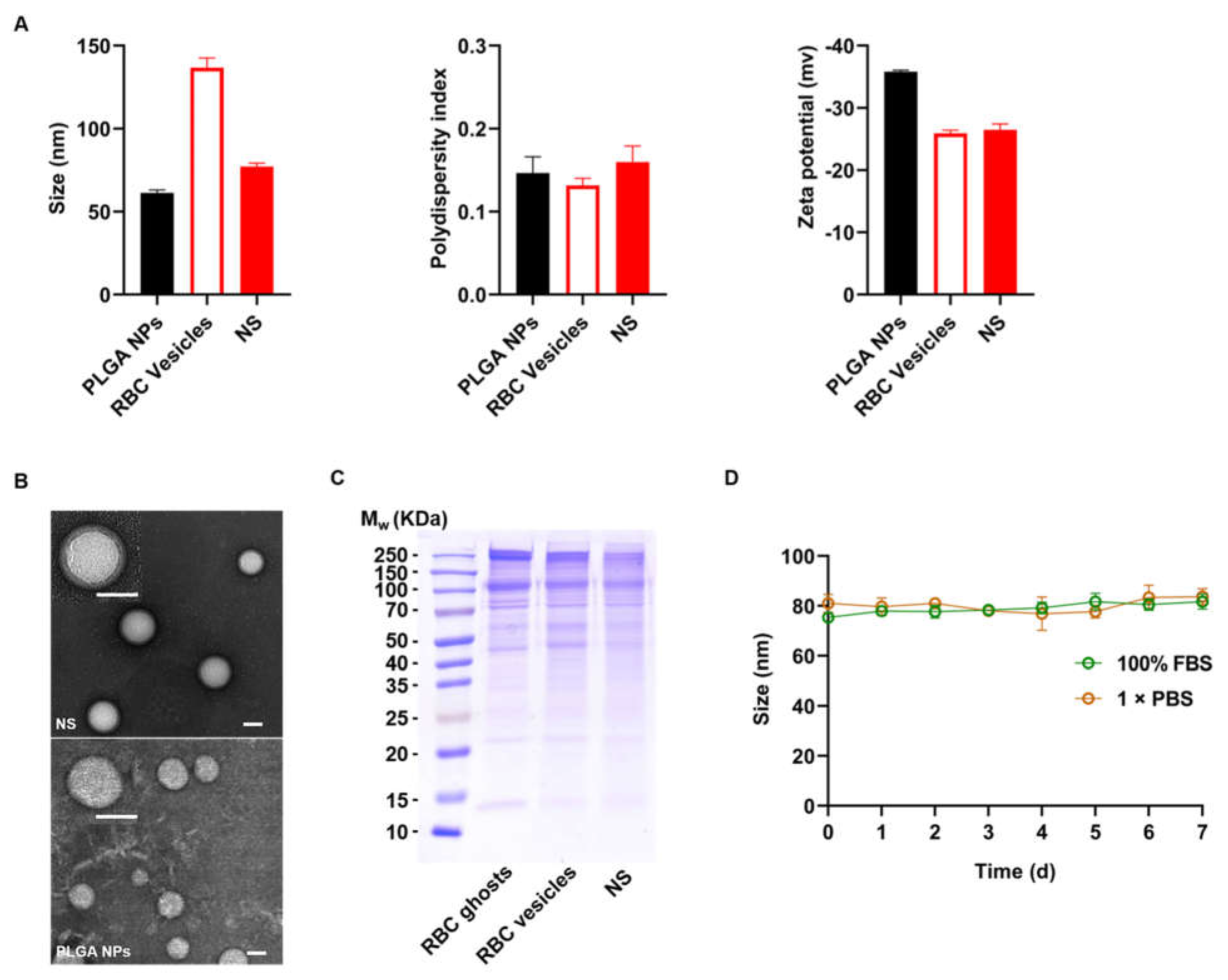
2.1. Characterization of NSs
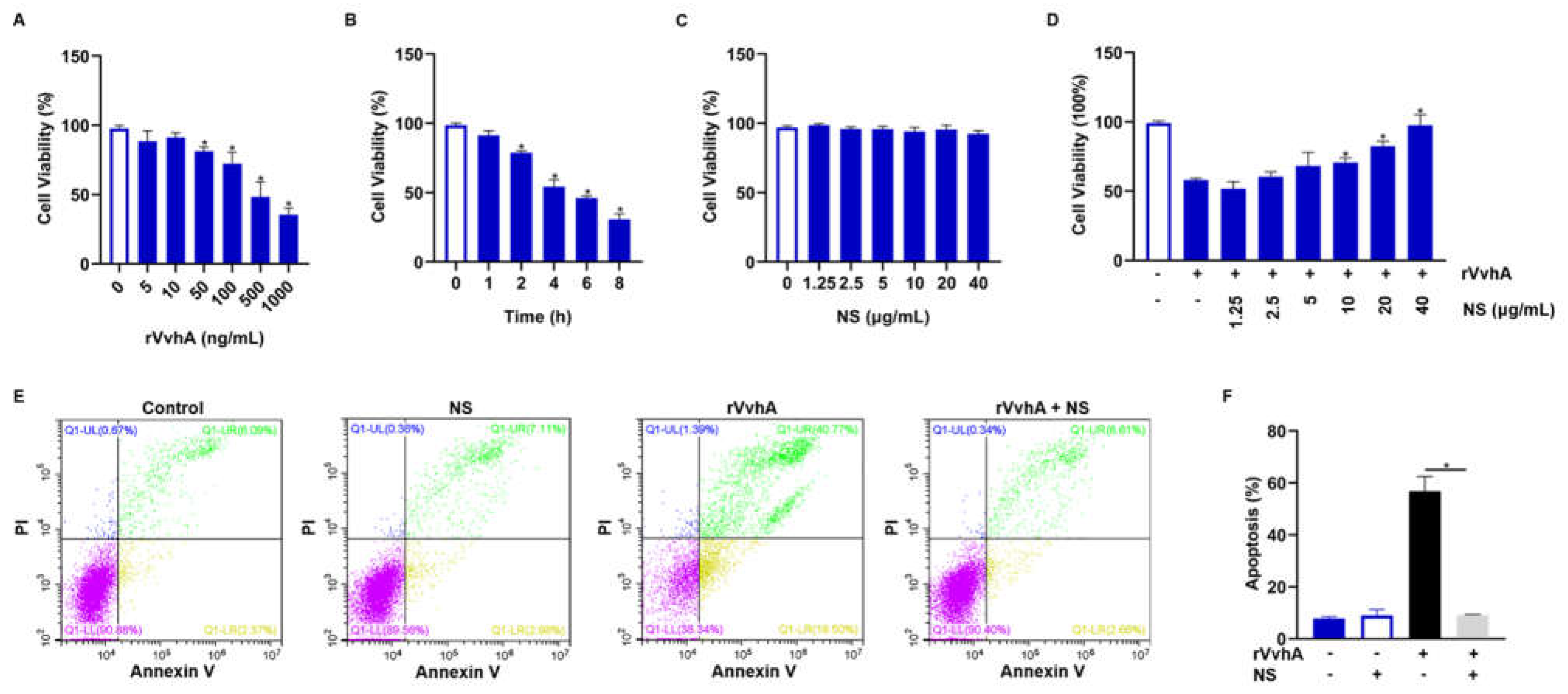
2.2. Inhibitory Effect of NSs on Cell Viability and Apoptosis Induced by Recombinant VvhA (rVvhA)
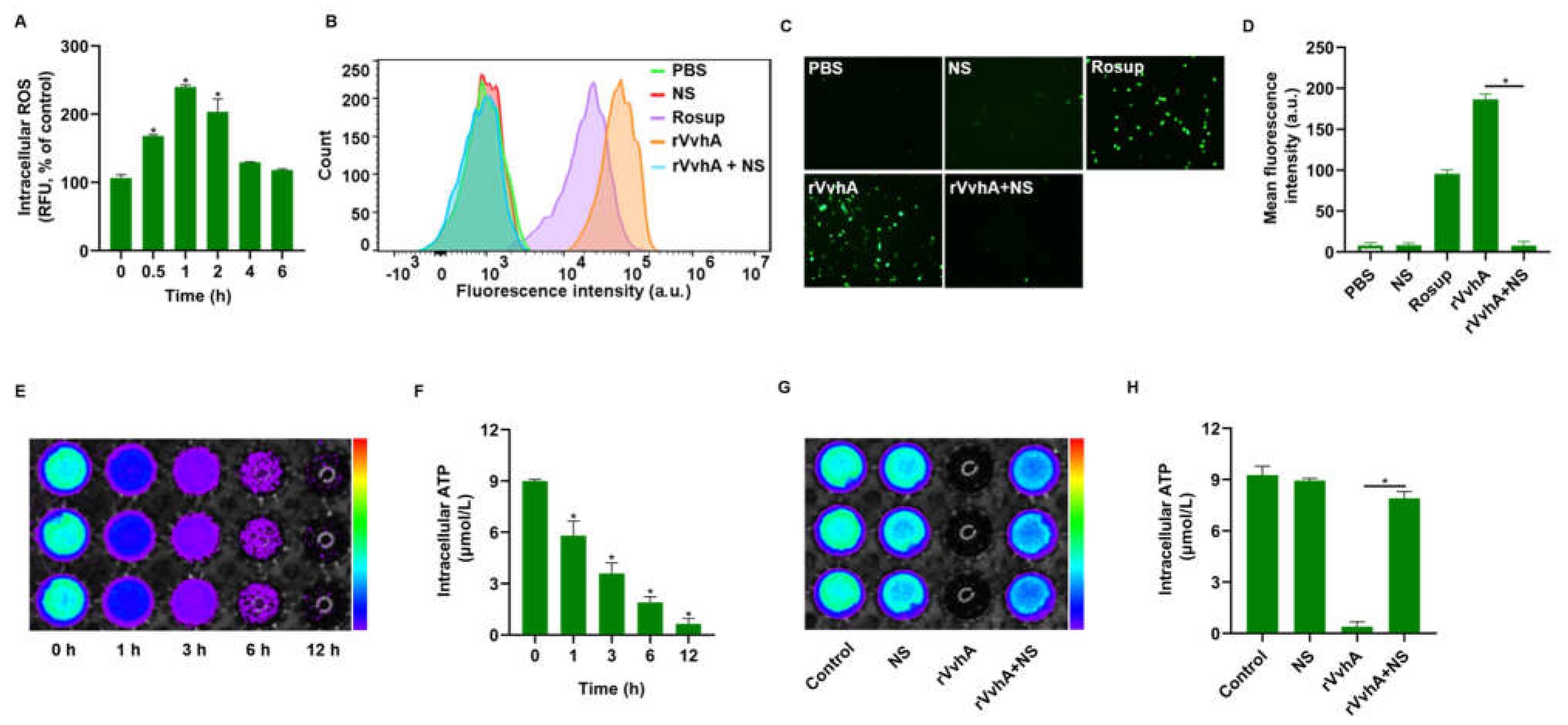
2.3. NSs Inhibited ROS Production and ATP Depletion Induced by rVvhA
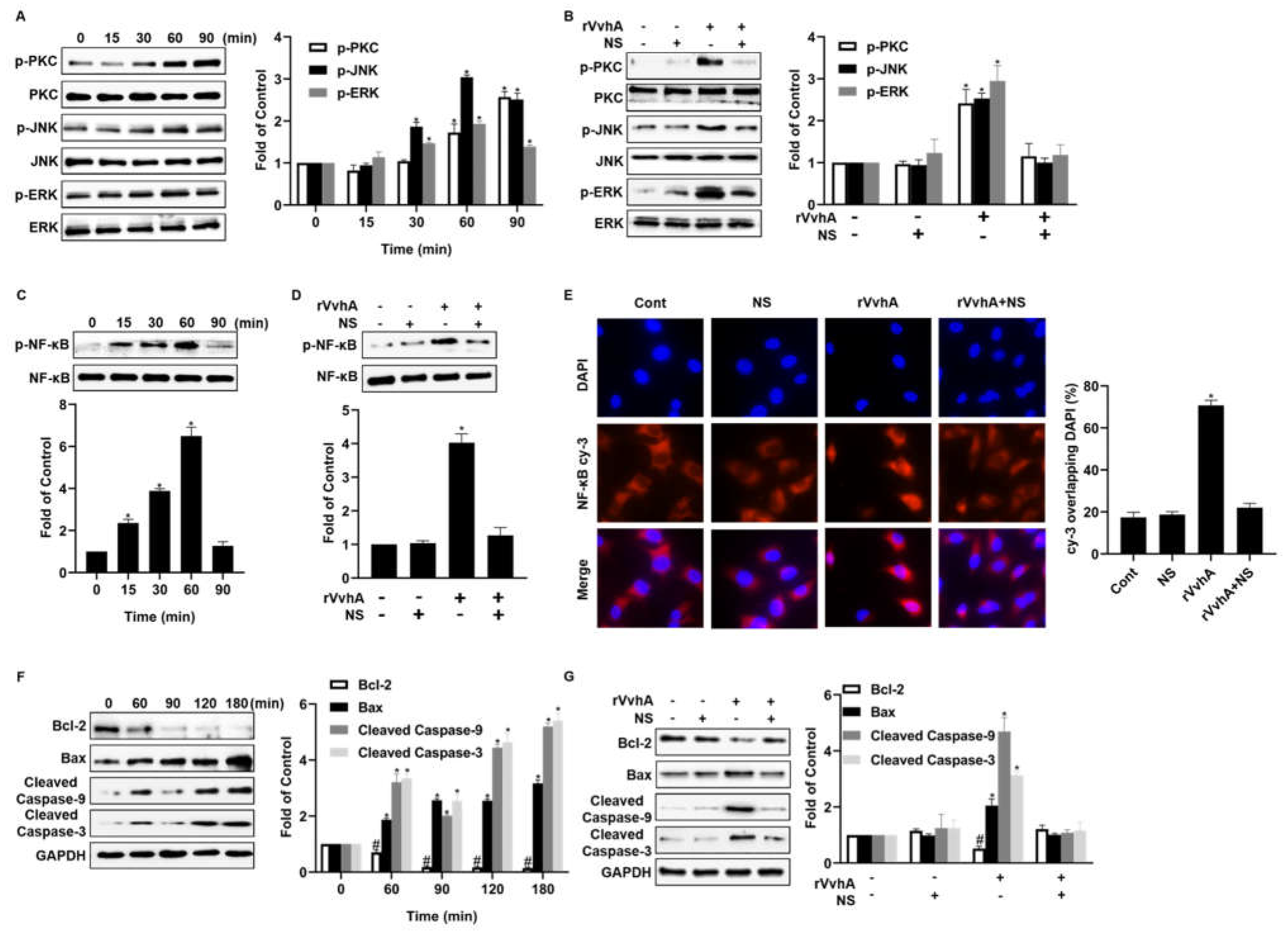
2.4. NSs Inhibit PKC/ERK/JNK and NF-κB Pathway Activation Induced by rVvhA
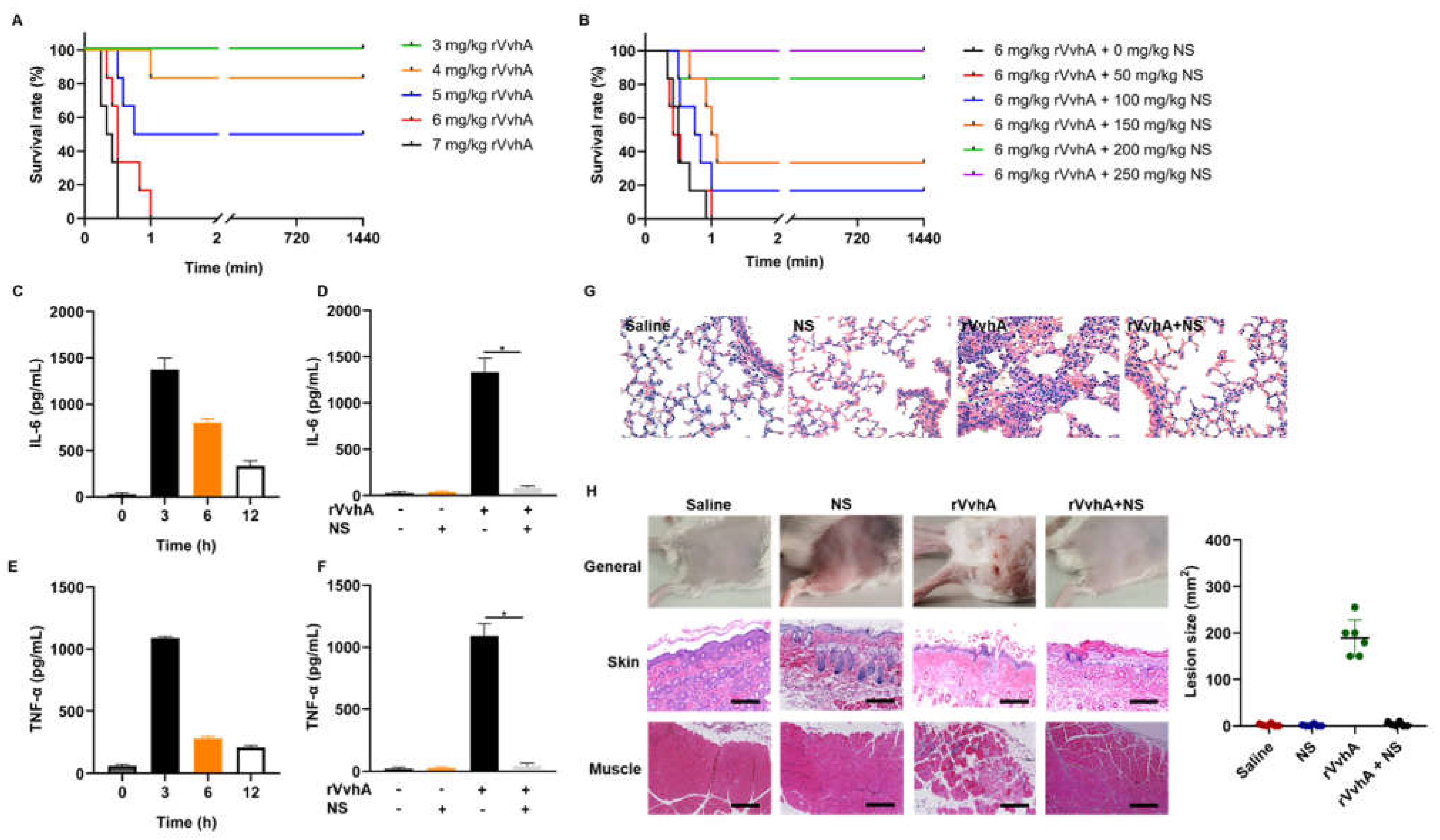
2.5. In Vivo Neutralization of rVvhA by NSs
3. Discussion
4. Materials and Methods
4.1. Cells, Animals and Reagents
4.2. Preparation and Characterization of NSs
4.2.1. Preparation of RBC Ghosts and Vesicles
4.2.2. Preparation of PLGA Cores and NSs
4.2.3. Characterization of NSs
4.3. Cell Viability and Apoptosis/Necrosis Detection in HPAECs
4.4. Intracellular ROS and ATP Detection
4.5. Analysis of PKC/ERK/JNK and NF-κB Pathway in HPAECs
4.6. In Vivo rVvhA Detoxification by NSs
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, H.; Xu, L.; Dong, H.; Hu, J.; Gao, H.; Yang, M.; Zhang, X.; Chen, X.; Fan, J.; Ma, W. Correlations between clinical features and mortality in patients with Vibrio vulnificus infection. PLoS ONE 2015, 10, e0136019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, N.R.; Kim, D.M. Vibrio vulnificus infection: A persistent threat to public health. Korean J. Intern. Med. 2018, 33, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, C.S. Vibrio vulnificus infection. N. Engl. J. Med. 2018, 379, 375. [Google Scholar] [CrossRef] [PubMed]
- Horseman, M.A.; Surani, S. A comprehensive review of Vibrio vulnificus: An important cause of severe sepsis and skin and soft-tissue infection. Int. J. Infect. Dis. 2011, 15, e157–e166. [Google Scholar] [CrossRef] [Green Version]
- Bhat, P.; Bhaskar, M.; Sistla, S.; Kadhiravan, T. Fatal case of necrotising fasciitis due to Vibrio vulnificus in a patient with alcoholic liver disease and diabetes mellitus. BMJ Case Rep. 2019, 12, bcr-2018-227851. [Google Scholar] [CrossRef] [PubMed]
- Baker-Austin, C.; Oliver, J.D.; Alam, M.; Ali, A.; Waldor, M.K.; Qadri, F.; Martinez-Urtaza, J. Vibrio spp. infections. Nat. Rev. Dis. Primers 2018, 4, 8. [Google Scholar] [CrossRef]
- Lee, Y.C.; Hor, L.I.; Chiu, H.Y.; Lee, J.W.; Shieh, S.J. Prognostic factor of mortality and its clinical implications in patients with necrotizing fasciitis caused by Vibrio vulnificus. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1011–1018. [Google Scholar] [CrossRef]
- King, M.; Rose, L.; Fraimow, H.; Nagori, M.; Danish, M.; Doktor, K. Vibrio vulnificus infections from a previously nonendemic area. Ann. Intern. Med. 2019, 171, 520–521. [Google Scholar] [CrossRef]
- Blake, P.A.; Merson, M.H.; Weaver, R.E.; Hollis, D.G.; Heublein, P.C. Disease caused by a marine Vibrio—clinical characteristics and epidemiology. N. Engl. J. Med. 1979, 300, 1–5. [Google Scholar] [CrossRef]
- Elmahdi, S.; DaSilva, L.V.; Parveen, S. Antibiotic resistance of Vibrio parahaemolyticus and Vibrio vulnificus in various countries: A review. Food Microbiol. 2016, 57, 128–134. [Google Scholar] [CrossRef] [Green Version]
- Mok, J.S.; Ryu, A.; Kwon, J.Y.; Park, K.; Shim, K.B. Abundance, antimicrobial resistance, and virulence of pathogenic Vibrio strains from molluscan shellfish farms along the Korean coast. Mar. Pollut. Bull. 2019, 149, 110559. [Google Scholar] [CrossRef] [PubMed]
- Larry, D.; Gray, A.S.K. Mouse skin damage caused by cytolysin from Vibrio vulniflcus and by V. vulnificus infection. J. Infect. Dis. 1987, 155, 236–241. [Google Scholar]
- Park, J.W.; Ma, S.N.; Song, E.S.; Park, B.H.; Rho, H.W.; Song, C.H.; Park, S.D.; Kim, H.R. Pulmonary damage by Vibrio vulnificus cytolysin. Infect. Immun. 1996, 64, 2873–2876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, H.G.; Satchell, K.J. Additive function of Vibrio vulnificus MARTXVv and VvhA cytolysins promotes rapid growth and epithelial tissue necrosis during intestinal infection. PLoS Pathog. 2012, 8, e1002581. [Google Scholar] [CrossRef]
- Lee, S.E.; Ryu, P.Y.; Kim, S.Y.; Kim, Y.R.; Koh, J.T.; Kim, O.J.; Chung, S.S.; Choy, H.E.; Rhee, J.H. Production of Vibrio vulnificus hemolysin in vivo and its pathogenic significance. Biochem. Biophys. Res. Commun. 2004, 324, 86–91. [Google Scholar] [CrossRef]
- Yuan, Y.; Feng, Z.; Wang, J. Vibrio vulnificus hemolysin: Biological activity, regulation of VvhA expression, and role in pathogenesis. Front. Immunol. 2020, 11, 599439. [Google Scholar] [CrossRef]
- Leng, F.; Lin, S.; Wu, W.; Zhang, J.; Song, J.; Zhong, M. Epidemiology, pathogenetic mechanism, clinical characteristics, and treatment of Vibrio vulnificus infection: A case report and literature review. Eur. J. Clin. Microbiol. Infect. Dis 2019, 38, 1999–2004. [Google Scholar] [CrossRef]
- Song, E.J.; Lee, S.J.; Lim, H.S.; Kim, J.S.; Jang, K.K.; Choi, S.H.; Han, H.J. Vibrio vulnificus VvhA induces autophagy-related cell death through the lipid raft-dependent c-Src/NOX signaling pathway. Sci. Rep. 2016, 6, 27080. [Google Scholar] [CrossRef]
- Shinoda, S.; Miyoshi, S.I.; Yamanaka, H.; Miyoshi-Nakahara, N. Some properties of Vibrio Vulnificus hemolysin. Microbiol. Immunol. 1985, 29, 583–590. [Google Scholar] [CrossRef]
- Lee, S.J.; Jung, Y.H.; Oh, S.Y.; Song, E.J.; Choi, S.H.; Han, H.J. Vibrio vulnificus VvhA induces NF-kappaB-dependent mitochondrial cell death via lipid raft-mediated ROS production in intestinal epithelial cells. Cell Death Dis. 2015, 6, 1655. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.M.; Fang, R.H.; Copp, J.; Luk, B.T.; Zhang, L. A biomimetic nanosponge that absorbs pore-forming toxins. Nat. Nanotechnol. 2013, 8, 336–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, C.M.; Zhang, L.; Aryal, S.; Cheung, C.; Fang, R.H.; Zhang, L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl. Acad. Sci. USA 2011, 108, 10980–10985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, R.H.; Hu, C.M.; Zhang, L. Nanoparticles disguised as red blood cells to evade the immune system. Expert Opin. Biol. Ther. 2012, 12, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Distler, U.; Tenzer, S. Tools for pathogen proteomics: Fishing with biomimetic nanosponges. ACS Nano 2017, 11, 11768–11772. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, M.; Zhang, Y.; Lee, J.H.; Escajadillo, T.; Gong, H.; Fang, R.H.; Gao, W.; Nizet, V.; Zhang, L. Broad-spectrum neutralization of pore-forming toxins with human erythrocyte membrane-coated nanosponges. Adv. Healthc. Mater. 2018, 7, e1701366. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Yu, F.; Ao, M.; Zheng, X.; Li, N.; Xia, J.; Li, Y.; Li, D.; Hou, Z.; Qi, Z.; Chen, X.D. PEG-lipid-PLGA hybrid nanoparticles loaded with berberine–phospholipid complex to facilitate the oral delivery efficiency. Drug Deliv. 2017, 24, 825–833. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.J.; Wang, J.H.; Wang, C.Y.; Wu, Y.C. The preparation and characteristic of poly(lactide-co-glycolide) microspheres as novel antigen delivery systems. Int. J. Nanotechnol. 2013, 10, 870–890. [Google Scholar] [CrossRef]
- Zohra, M.; Fawzia, A. Hemolytic activity of different herbal extracts used in Algeria. Int. J. Pharm. Sci. Res. 2014, 5, 495–500. [Google Scholar]
- Kannan, K.; Jain, S.K. Oxidative stress and apoptosis. Pathophysiology 2000, 7, 153–163. [Google Scholar] [CrossRef]
- Tsujimoto, Y. Apoptosis and necrosis: Intracellular ATP level as a determinant for cell death modes. Cell Death Differ. 1997, 4, 429–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.J.; Lee, H.J.; Jung, Y.H.; Kim, J.S.; Choi, S.H.; Han, H.J. Melatonin inhibits apoptotic cell death induced by Vibrio vulnificus VvhA via melatonin receptor 2 coupling with NCF-1. Cell Death Dis. 2018, 9, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osunla, C.A.; Okoh, A.I. Vibrio pathogens: A public health concern in rural water resources in sub-Saharan Africa. Int. J. Environ. Res. Public Health 2017, 14, 1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, S.J.; Jung, S.I.; Peck, K.R. Historical and clinical perspective of Vibrio vulnificus infections in Korea. Infect. Chemother. 2020, 52, 245–251. [Google Scholar] [CrossRef]
- Lu, Z.; Li, M.; Liang, H.; Qiu, Q.; Yang, G.; Zhou, T.; Hong, G. Dynamic expressions of liver tissue apoptosis-related genes of Vibrio vulnificus sepsis rats and the effects of antibacterial agents. J. Huazhong Univ. Sci. Technolog. Med. Sci. 2009, 29, 193–197. [Google Scholar] [CrossRef]
- Liu, C.C.; Wang, S.M.; Chiou, Y.Y.; Chen, C.T. Vibrio vulnificus infection complicated by acute respiratory distress syndrome in a child with nephrotic syndrome. Pediatr. Pulm. 2000, 29, 400–403. [Google Scholar]
- Rho, H.W.; Choi, M.J.; Lee, J.N.; Park, J.W.; Kim, J.S.; Park, B.H.; Sohn, H.S.; Kim, H.R. Cytotoxic mechanism of Vibrio vulnificus cytolysin in CPAE cells. Life Sci. 2002, 70, 1923–1934. [Google Scholar] [CrossRef]
- Víctor, V.M.; Esplugues, J.V.; Hernández-Mijares, A.; Rocha, M. Oxidative stress and mitochondrial dysfunction in sepsis: A potential therapy with mitochondria-targeted antioxidants. Infect. Disord.-Drug Targets 2009, 9, 376–389. [Google Scholar] [CrossRef]
- Lou, Y.L.; Xie, D.L.; Huang, X.H.; Zheng, M.M.; Zhang, T.; Xu, J. Vibrio vulnificus hemolysin induces TNF-α independent ROS production by primary murine macrophages. Res. Sq. 2021. [Google Scholar]
- Prauchner, C.A. Oxidative stress in sepsis: Pathophysiological implications justifying antioxidant co-therapy. Burns 2017, 43, 471–485. [Google Scholar] [CrossRef]
- Fang, R.H.; Luk, B.T.; Hu, C.M.; Zhang, L. Engineered nanoparticles mimicking cell membranes for toxin neutralization. Adv. Drug Deliv. Rev. 2015, 90, 69–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Fang, R.H.; Luk, B.T.; Hu, C.J.; Thamphiwatana, S.; Dehaini, D.; Angsantikul, P.; Kroll, A.V.; Pang, Z.; Gao, W.; et al. Nanoparticle-based antivirulence vaccine for the management of methicillin-resistant Staphylococcus aureus skin infection. Adv. Funct. Mater. 2016, 26, 1628–1635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bricarello, D.A.; Patel, M.A.; Parikh, A.N. Inhibiting host-pathogen interactions using membrane-based nanostructures. Trends Biotechnol. 2012, 30, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.C.; Weng, H.H.; Yang, T.Y.; Chang, T.S.; Huang, T.W.; Lee, M.S. Distribution of fatal Vibrio vulnificus necrotizing skin and soft-tissue infections: A systematic review and meta-analysis. Medicine 2016, 95, e2627. [Google Scholar] [CrossRef]
- Xia, Q.; Zhang, Y.; Li, Z.; Hou, X.; Feng, N. Red blood cell membrane-camouflaged nanoparticles: A novel drug delivery system for antitumor application. Acta. Pharm. Sin. B 2019, 9, 675–689. [Google Scholar] [CrossRef]
- Yang, J.; Wang, F.; Lu, Y.; Qi, J.; Deng, L.; Sousa, F.; Sarmento, B.; Xu, X.; Cui, W. Recent advance of erythrocyte-mimicking nanovehicles: From bench to bedside. J. Control. Release 2019, 314, 81–91. [Google Scholar] [CrossRef]
- Kotb, S.; Piraquive, J.; Lamberton, F.; Lux, F.; Verset, M.; Di Cataldo, V.; Contamin, H.; Tillement, O.; Canet-Soulas, E.; Sancey, L. Safety evaluation and imaging properties of gadolinium-based nanoparticles in nonhuman primates. Sci. Rep. 2016, 6, 35053. [Google Scholar] [CrossRef] [Green Version]
- Passow, G.S.H. Preparation and properties of human erythrocyte ghosts. Mol. Cell Biochem. 1973, 2, 197–218. [Google Scholar]
- Kaur, H.; Fisher, K.; Othman, M. Thromboelastography testing in mice following blood collection from facial vein and cardiac puncture. Blood Coagul. Fibrinolysis 2019, 30, 366–369. [Google Scholar] [CrossRef]
- Govender, T.; Stolnik, S.; Garnett, M.C.; Illum, L.; Davis, S.S. PLGA nanoparticles prepared by nanoprecipitation: Drug loading and release studies of a water-soluble drug. J. Control. Release 1999, 57, 171–185. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, S.; Wang, Q.; Zhang, P.; Wang, B.; Liu, G.; Zhang, F.; Li, J.; Wang, F.; Wang, B.; Zhang, L. Biomimetic Nanosponges Enable the Detoxification of Vibrio vulnificus Hemolysin. Int. J. Mol. Sci. 2022, 23, 6821. https://doi.org/10.3390/ijms23126821
Zou S, Wang Q, Zhang P, Wang B, Liu G, Zhang F, Li J, Wang F, Wang B, Zhang L. Biomimetic Nanosponges Enable the Detoxification of Vibrio vulnificus Hemolysin. International Journal of Molecular Sciences. 2022; 23(12):6821. https://doi.org/10.3390/ijms23126821
Chicago/Turabian StyleZou, Shuaijun, Qianqian Wang, Peipei Zhang, Bo Wang, Guoyan Liu, Fuhai Zhang, Jie Li, Fan Wang, Beilei Wang, and Liming Zhang. 2022. "Biomimetic Nanosponges Enable the Detoxification of Vibrio vulnificus Hemolysin" International Journal of Molecular Sciences 23, no. 12: 6821. https://doi.org/10.3390/ijms23126821
APA StyleZou, S., Wang, Q., Zhang, P., Wang, B., Liu, G., Zhang, F., Li, J., Wang, F., Wang, B., & Zhang, L. (2022). Biomimetic Nanosponges Enable the Detoxification of Vibrio vulnificus Hemolysin. International Journal of Molecular Sciences, 23(12), 6821. https://doi.org/10.3390/ijms23126821