In Vitro Effect of Δ9-Tetrahydrocannabinol and Cannabidiol on Cancer-Associated Fibroblasts Isolated from Lung Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Cell Lines and Cell Isolation
2.3. Conditioned Medium (CM) Obtaining
2.4. Cell Viability
2.5. F-actin Fluorescence Staining and Evaluation of Cell Density
2.6. Determination of COL1A1, VIM, FSP1, CDH1, CDH2 and VIM Expression Levels
2.7. Detection of Type I Collagen, Vimentin, FSP1 and Pankeratin by Immunofluorescence
2.8. Data Presentation and Analysis
3. Results
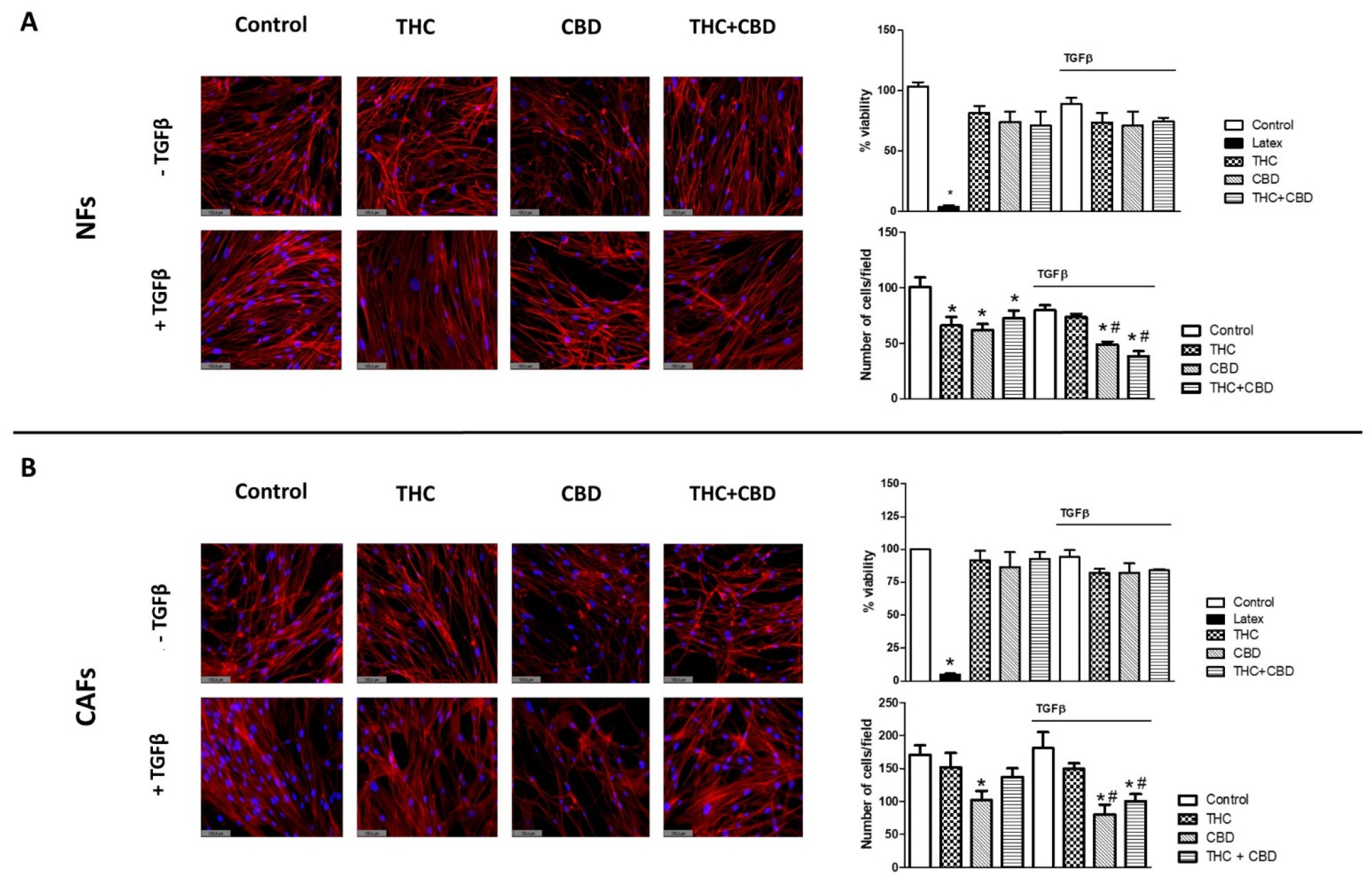
3.1. Effect of Cannabinoids on Non-Tumor Fibroblasts’ (NFs) and Cancer-Associated Fibroblasts’ (CAFs) Morphology, Viability and Cell Density
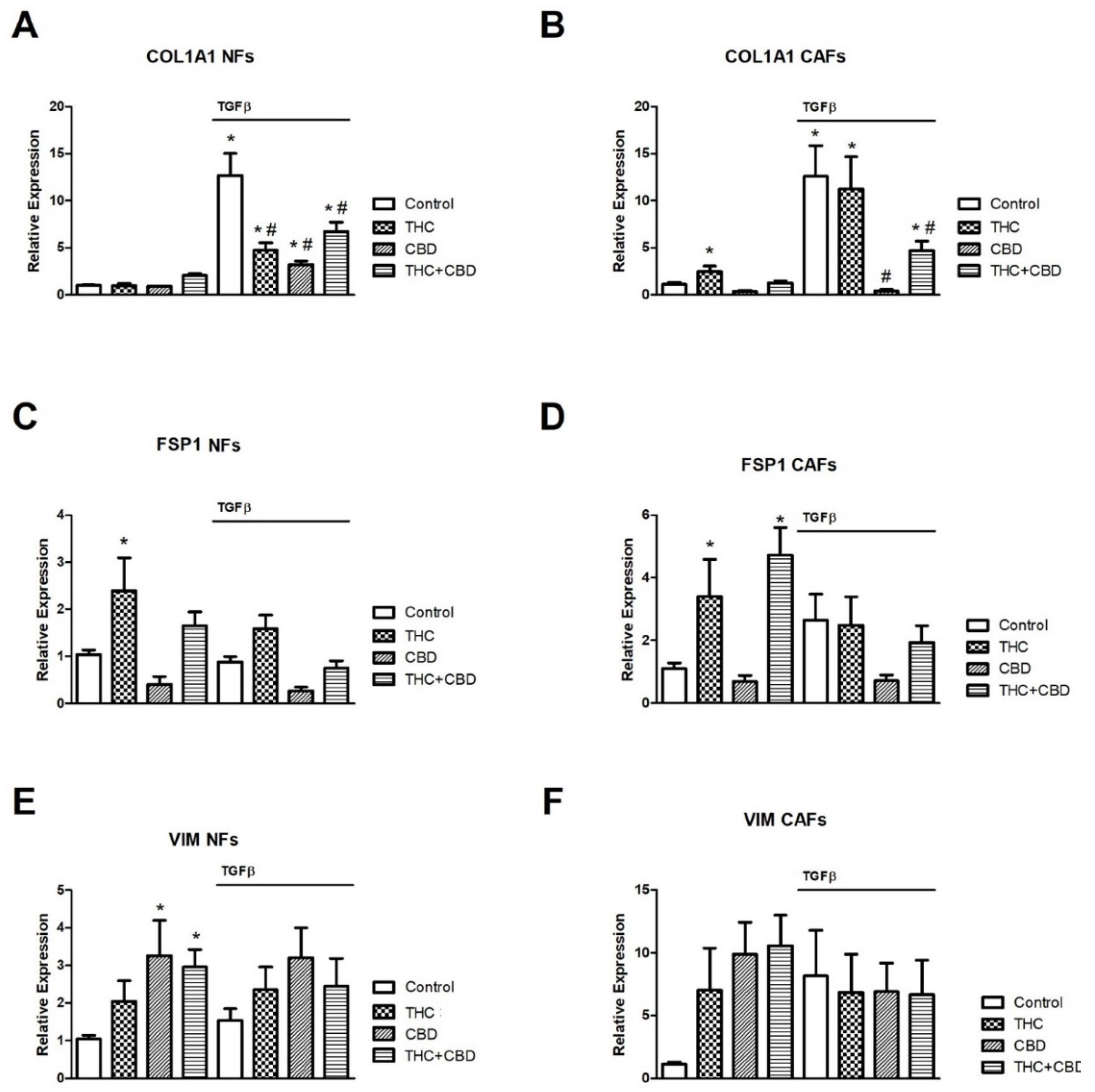
3.2. Effect of Cannabinoids on NF and CAF Gene Expression Levels of COL1A1, FSP1 and VIM
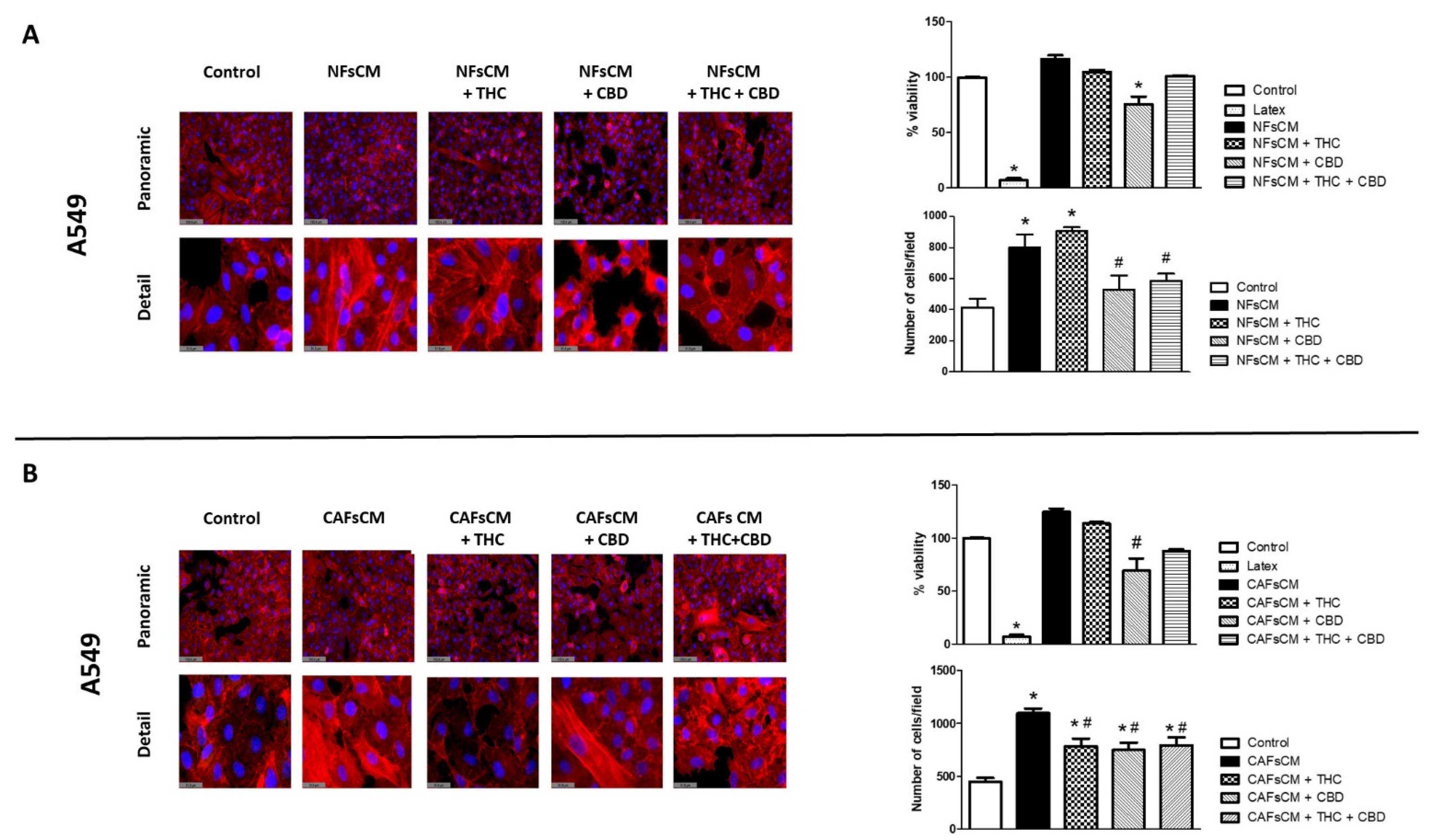
3.3. Stromal Cell CM Induces Changes in A549 Cells’ Morphology and Density and Efficacy of Treatment with THC and/or CBD
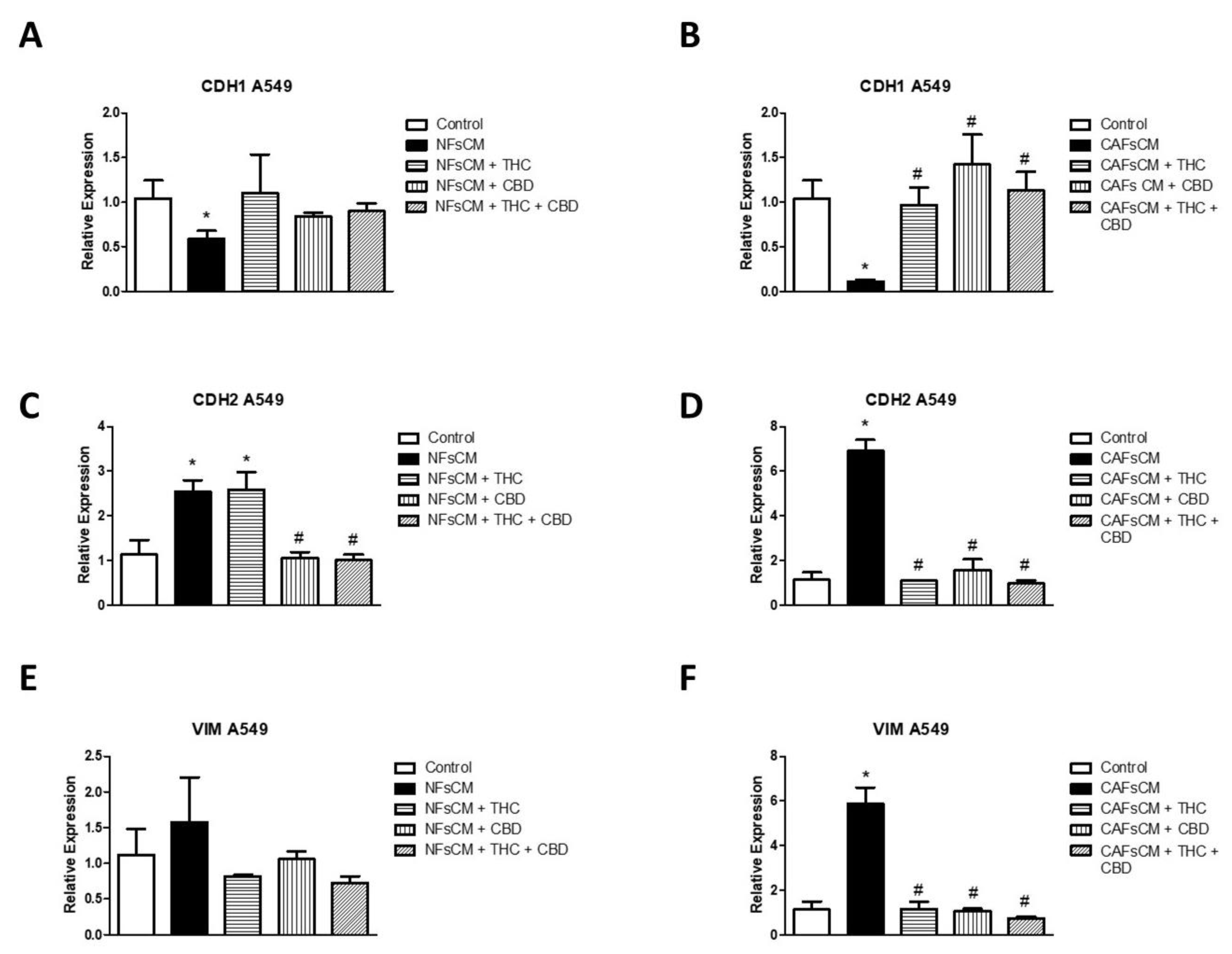
3.4. Changes in EMT Gene Expression in A549 Cells Cultured with Stromal Cell CM and Effect of THC and CBD
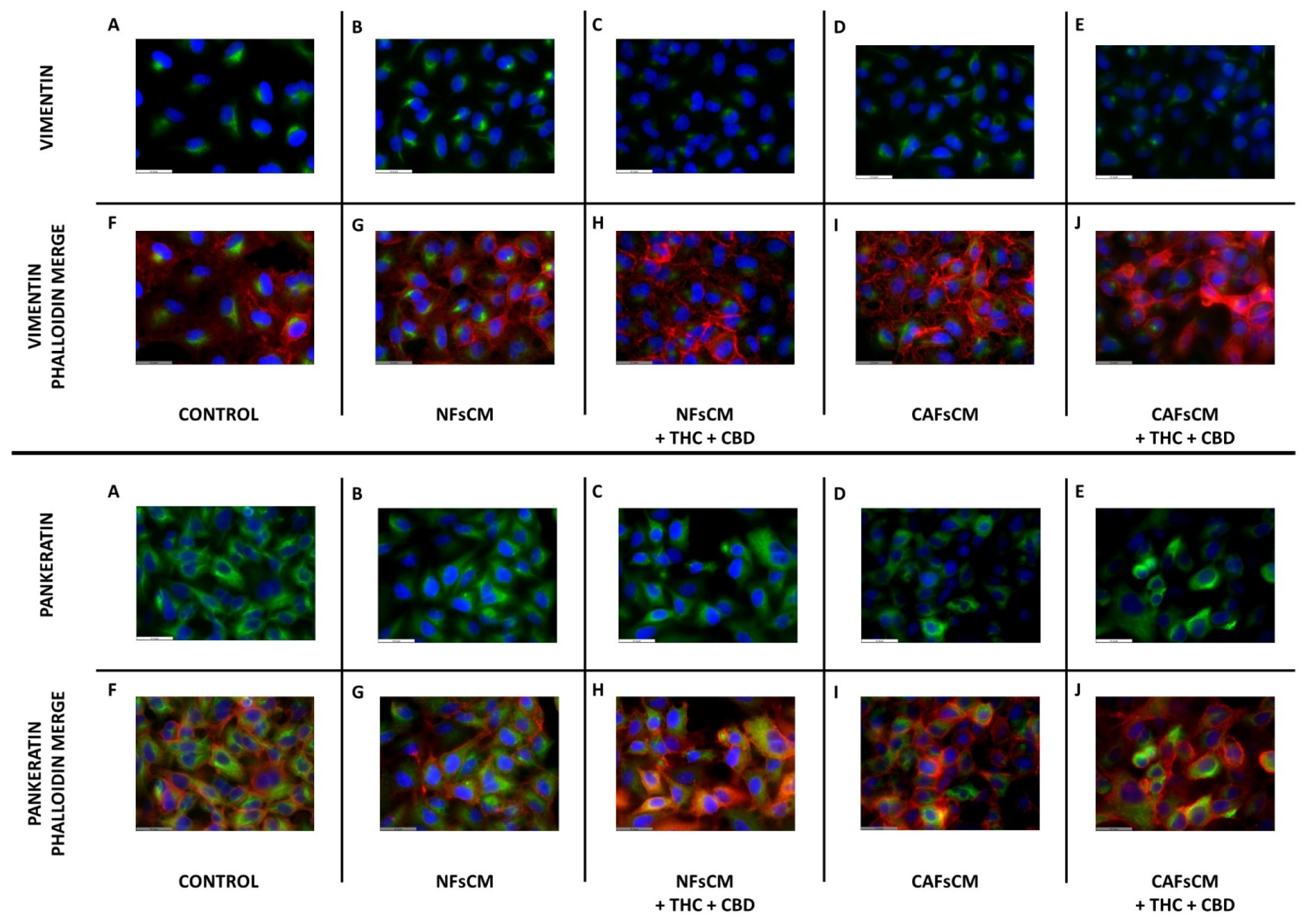
3.5. Effect of Stromal Cell CM and Cannabinoid Agonists on A549 Cell Expression and Distribution of Vimentin and Pankeratin Proteins
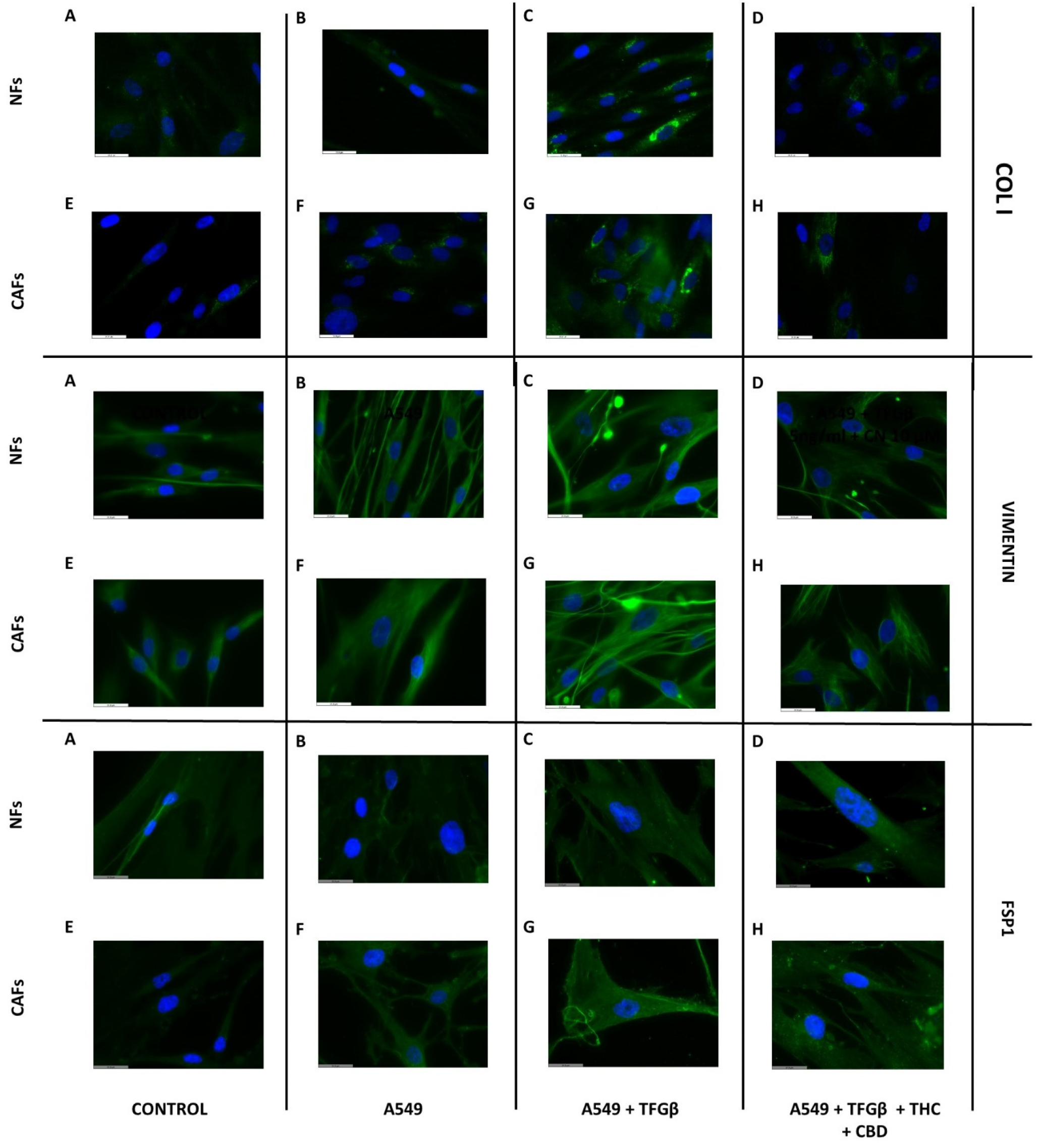
3.6. A549 Cell CM Stimulates CAF and NF Expression of Type I Collagen, Vimentin and FSP1 and Effect of Cannabinoids
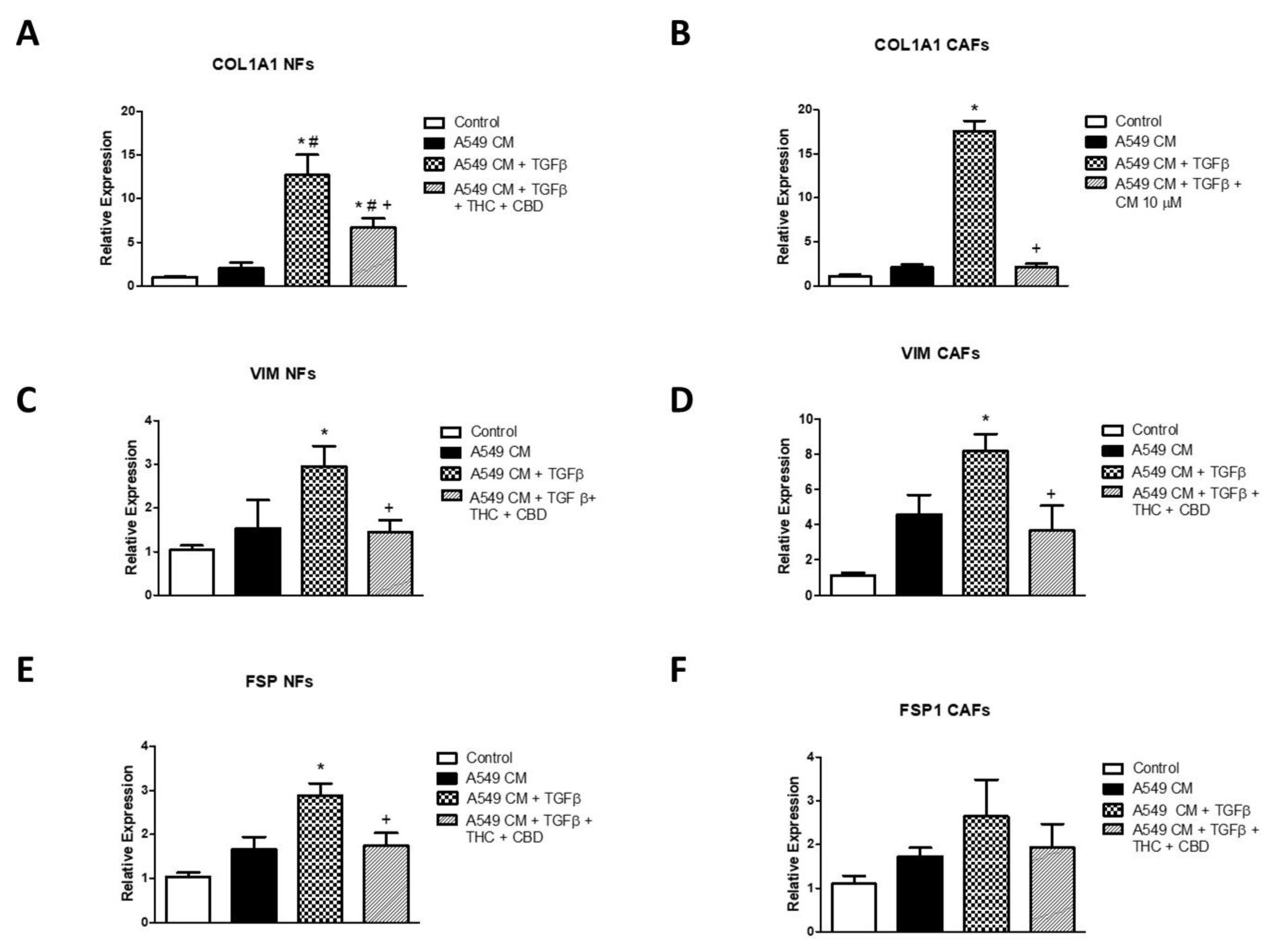
3.7. Effects of A549 Cell CM on Gene Expression of COL1A1, VIM and FSP1 in NFs and CAFs and Effects of Cannabinoids
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hsu, C.-L.; Chen, K.-Y.; Shih, J.-Y.; Ho, C.-C.; Yang, C.-H.; Yu, C.-J.; Yang, P.-C. Advanced non-small cell lung cancer in patients aged 45 years or younger: Outcomes and prognostic factors. BMC Cancer 2012, 12, 241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Wu, H.; Zhang, M.; Ding, L.; Meng, F.; Fan, X. Expression of the epithelial-mesenchymal transition-related proteins and their clinical significance in lung adenocarcinoma. Diagn. Pathol. 2013, 8, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, B.; Zhang, W.; Zhang, M.; Wang, X.; Peng, S.; Zhang, R. KRT6A Promotes EMT and Cancer Stem Cell Transformation in Lung Adenocarcinoma. Technol. Cancer Res. Treat. 2020, 19, 1533033820921248. [Google Scholar] [CrossRef] [PubMed]
- Alfarouk, K.O.; Stock, C.-M.; Taylor, S.; Walsh, M.; Muddathir, A.K.; Verduzco, D.; Bashir, A.H.H.; Mohammed, O.Y.; O ElHassan, G.; Harguindey, S.; et al. Resistance to cancer chemotherapy: Failure in drug response from ADME to P-gp. Cancer Cell Int. 2015, 15, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sosa Iglesias, V.; Giuranno, L.; Dubois, L.J.; Theys, J.; Vooijs, M. Drug Resistance in Non-Small Cell Lung Cancer: A Potential for NOTCH Targeting? Front. Oncol. 2018, 8, 267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittal, V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu. Rev. Pathol. 2018, 13, 395–412. [Google Scholar] [CrossRef]
- Joyce, J.A.; Pollard, J.W. Microenvironmental regulation of metastasis. Nat. Cancer 2008, 9, 239–252. [Google Scholar] [CrossRef]
- Hanahan, D.; Coussens, L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Yan, Y.; Yang, Y.; Hong, X.; Wang, M.; Yang, Z.; Liu, B.; Ye, L. MiR-210 in exosomes derived from CAFs promotes non-small cell lung cancer migration and invasion through PTEN/PI3K/AKT pathway. Cell. Signal. 2020, 73, 109675. [Google Scholar] [CrossRef]
- Choe, C.; Shin, Y.-S.; Choi, S.-J.; Lee, J.; Kim, S.Y.; Cho, Y.B.; Kim, J.; Kim, C. Crosstalk with cancer-associated fibroblasts induces resistance of non-small cell lung cancer cells to epidermal growth factor receptor tyrosine kinase inhibition. OncoTargets Ther. 2015, 8, 3665–3678. [Google Scholar] [CrossRef] [Green Version]
- Linares, J.; Marín-Jiménez, J.A.; Badia-Ramentol, J.; Calon, A. Determinants and Functions of CAFs Secretome during Cancer Progression and Therapy. Front. Cell Dev. Biol. 2021, 8, 621070. [Google Scholar] [CrossRef] [PubMed]
- Seltzer, E.S.; Watters, A.K.; MacKenzie, J.D., Jr.; Granat, L.M.; Zhang, D. Cannabidiol (CBD) as a Promising Anti-Cancer Drug. Cancers 2020, 12, 3203. [Google Scholar] [CrossRef] [PubMed]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zheng, W.; Shen, K.; Shen, W. ∆9-tetrahydrocannabinol inhibits epithelial-mesenchymal transition and metastasis by targeting matrix metalloproteinase-9 in endometrial cancer. Oncol. Lett. 2018, 15, 8527–8535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacher, P.; Kogan, N.M.; Mechoulam, R. Beyond THC and Endocannabinoids. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 637–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellati, F.; Borgonetti, V.; Brighenti, V.; Biagi, M.; Benvenuti, S.; Corsi, L. Cannabis sativa L. and Nonpsychoactive Cannabinoids: Their Chemistry and Role against Oxidative Stress, Inflammation, and Cancer. BioMed Res. Int. 2018, 2018, 1691428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munson, A.E.; Harris, L.S.; Friedman, M.A.; Dewey, W.L.; Carchman, R.A. Antineoplastic Activity of Cannabinoids. JNCI J. Natl. Cancer Inst. 1975, 55, 597–602. [Google Scholar] [CrossRef]
- Velasco, G.; Sánchez, C.; Guzmán, M. Towards the use of cannabinoids as antitumour agents. Nat. Rev. Cancer 2012, 12, 436–444. [Google Scholar] [CrossRef]
- García-Morales, L.; Castillo, A.M.; Ramírez, J.T.; Zamudio-Meza, H.; Domínguez-Robles, M.D.C.; Meza, I. CBD Reverts the Mesenchymal Invasive Phenotype of Breast Cancer Cells Induced by the Inflammatory Cytokine IL-1β. Int. J. Mol. Sci. 2020, 21, 2429. [Google Scholar] [CrossRef] [Green Version]
- Milian, L.; Mata, M.; Alcacer, J.; Oliver, M.; Sancho-Tello, M.; De Llano, J.J.M.; Camps, C.; Galbis, J.; Carretero, J.; Carda, C. Cannabinoid receptor expression in non-small cell lung cancer. Effectiveness of tetrahydrocannabinol and cannabidiol inhibiting cell proliferation and epithelial-mesenchymal transition in vitro. PLoS ONE 2020, 15, e0228909. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Piotrowska, Z.; Hare, P.J.; Chen, H.; Mulvey, H.E.; Mayfield, A.; Noeen, S.; Kattermann, K.; Greenberg, M.; Williams, A.; et al. Three subtypes of lung cancer fibroblasts define distinct therapeutic paradigms. Cancer Cell 2021, 39, 1531–1547.e10. [Google Scholar] [CrossRef] [PubMed]
- Salvador-Clavell, R.; de Llano, J.J.M.; Milián, L.; Oliver, M.; Mata, M.; Carda, C.; Sancho-Tello, M. Chondrogenic Potential of Human Dental Pulp Stem Cells Cultured as Microtissues. Stem Cells Int. 2021, 2021, 7843798. [Google Scholar] [CrossRef] [PubMed]
- Oliver-Ferrándiz, M.; Milián, L.; Sancho-Tello, M.; de Llano, J.M.; Roca, F.G.; Martínez-Ramos, C.; Carda, C.; Mata, M. Alginate-Agarose Hydrogels Improve the In Vitro Differentiation of Human Dental Pulp Stem Cells in Chondrocytes. A Histological Study. Biomedicines 2021, 9, 834. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [Green Version]
- Youlden, D.R.; Cramb, S.; Baade, P. The International Epidemiology of Lung Cancer: Geographical Distribution and Secular Trends. J. Thorac. Oncol. 2008, 3, 819–831. [Google Scholar] [CrossRef]
- Zappa, C.; Mousa, S.A. Non-small cell lung cancer: Current treatment and future advances. Transl. Lung Cancer Res. 2016, 5, 288–300. [Google Scholar] [CrossRef] [Green Version]
- Bethune, G.; Bethune, D.; Ridgway, N.; Xu, Z. Epidermal growth factor receptor (EGFR) in lung cancer: An overview and update. J. Thorac. Dis. 2010, 2, 48–51. [Google Scholar]
- Hermanson, D.J.; Marnett, L.J. Cannabinoids, endocannabinoids, and cancer. Cancer Metastasis Rev. 2011, 30, 599–612. [Google Scholar] [CrossRef]
- Hinz, B.; Ramer, R. Cannabinoids as anticancer drugs: Current status of preclinical research. Br. J. Cancer 2022, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Conte, A.; Silván, C.V. Review of available data for the efficacy and effectiveness of nabiximols oromucosal spray (Sativex®) in multiple sclerosis patients with moderate to severe spasticity. Neurodegener. Dis. 2021, 21, 55–62. [Google Scholar] [CrossRef]
- Altorki, N.K.; Markowitz, G.J.; Gao, D.; Port, J.L.; Saxena, A.; Stiles, B.; McGraw, T.; Mittal, V. The lung microenvironment: An important regulator of tumour growth and metastasis. Nat. Rev. Cancer 2019, 19, 9–31. [Google Scholar] [CrossRef] [Green Version]
- You, J.; Li, M.; Cao, L.M.; Gu, Q.H.; Deng, P.B.; Tan, Y.; Hu, C.P. Snail1-dependent cancer-associated fibroblasts induce epithelial-mesenchymal transition in lung cancer cells via exosomes. QJM Int. J. Med. 2019, 112, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Hamad, H.; Olsen, B.B. Cannabidiol Induces Cell Death in Human Lung Cancer Cells and Cancer Stem Cells. Pharmaceuticals 2021, 14, 1169. [Google Scholar] [CrossRef] [PubMed]
- Angioni, R.; Sánchez-Rodríguez, R.; Viola, A.; Molon, B. TGF-β in Cancer: Metabolic Driver of the Tolerogenic Crosstalk in the Tumor Microenvironment. Cancers 2021, 13, 401. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, A.; Funaki, S.; Fukui, E.; Kimura, K.; Kanou, T.; Ose, N.; Minami, M.; Shintani, Y. Effects of pirfenidone targeting the tumor microenvironment and tumor-stroma interaction as a novel treatment for non-small cell lung cancer. Sci. Rep. 2020, 10, 10900. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, B.; Webb, D.J. Cancer-associated fibroblasts modulate growth factor signaling and extracellular matrix remodeling to regulate tumor metastasis. Biochem. Soc. Trans. 2017, 45, 229–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, X.; Chen, C.; Wu, F.; Qiu, L.; Ke, Q.; Sun, R.; Duan, Q.; Luo, M.; Luo, Z. Curcumin Inhibits the Migration and Invasion of Non-Small-Cell Lung Cancer Cells Through Radiation-Induced Suppression of Epithelial-Mesenchymal Transition and Soluble E-Cadherin Expression. Technol. Cancer Res. Treat. 2020, 19. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Kanugula, S.; Sudhir, S.; Pereira, M.; Jain, S.; Aghi, M. The Role of Cancer-Associated Fibroblasts in Tumor Progression. Cancers 2021, 13, 1399. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milián, L.; Monleón-Guinot, I.; Sancho-Tello, M.; Galbis, J.M.; Cremades, A.; Almenar-Ordaz, M.; Peñaroja-Martinez, J.; Farras, R.; Martín de Llano, J.J.; Carda, C.; et al. In Vitro Effect of Δ9-Tetrahydrocannabinol and Cannabidiol on Cancer-Associated Fibroblasts Isolated from Lung Cancer. Int. J. Mol. Sci. 2022, 23, 6766. https://doi.org/10.3390/ijms23126766
Milián L, Monleón-Guinot I, Sancho-Tello M, Galbis JM, Cremades A, Almenar-Ordaz M, Peñaroja-Martinez J, Farras R, Martín de Llano JJ, Carda C, et al. In Vitro Effect of Δ9-Tetrahydrocannabinol and Cannabidiol on Cancer-Associated Fibroblasts Isolated from Lung Cancer. International Journal of Molecular Sciences. 2022; 23(12):6766. https://doi.org/10.3390/ijms23126766
Chicago/Turabian StyleMilián, Lara, Irene Monleón-Guinot, María Sancho-Tello, José Marcelo Galbis, Antonio Cremades, María Almenar-Ordaz, Josep Peñaroja-Martinez, Rosa Farras, José Javier Martín de Llano, Carmen Carda, and et al. 2022. "In Vitro Effect of Δ9-Tetrahydrocannabinol and Cannabidiol on Cancer-Associated Fibroblasts Isolated from Lung Cancer" International Journal of Molecular Sciences 23, no. 12: 6766. https://doi.org/10.3390/ijms23126766
APA StyleMilián, L., Monleón-Guinot, I., Sancho-Tello, M., Galbis, J. M., Cremades, A., Almenar-Ordaz, M., Peñaroja-Martinez, J., Farras, R., Martín de Llano, J. J., Carda, C., & Mata, M. (2022). In Vitro Effect of Δ9-Tetrahydrocannabinol and Cannabidiol on Cancer-Associated Fibroblasts Isolated from Lung Cancer. International Journal of Molecular Sciences, 23(12), 6766. https://doi.org/10.3390/ijms23126766








