Cannabidiol Interacts Antagonistically with Cisplatin and Additively with Mitoxantrone in Various Melanoma Cell Lines—An Isobolographic Analysis
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Drugs
4.3. Cell Viability Assessment
4.4. Cell Proliferation Assay
4.5. Cytotoxicity Assessment—LDH Assay
4.6. Isobolographic Analysis of Interactions
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Memon, A.; Bannister, P.; Rogers, I.; Sundin, J.; Al-Ayadhy, B.; James, P.W.; McNally, R.J.Q. Changing Epidemiology and Age-Specific Incidence of Cutaneous Malignant Melanoma in England: An Analysis of the National Cancer Registration Data by Age, Gender and Anatomical Site, 1981–2018. Lancet Reg. Health Eur. 2021, 2, 100024. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Alfonzetti, T.; Yasmin-Karim, S.; Ngwa, W.; Avery, S. Phytoradiotherapy: An Integrative Approach to Cancer Treatment by Combining Radiotherapy with Phytomedicines. Front. Oncol. 2021, 10, 624663. [Google Scholar] [CrossRef] [PubMed]
- Dall’Stella, P.B.; Docema, M.F.L.; Maldaun, M.V.C.; Feher, O.; Lancellotti, C.L.P. Case Report: Clinical Outcome and Image Response of Two Patients with Secondary High-Grade Glioma Treated with Chemoradiation, PCV, and Cannabidiol. Front. Oncol. 2019, 9, 643. [Google Scholar] [CrossRef] [Green Version]
- Tímár, J.; Ladányi, A. Molecular Pathology of Skin Melanoma: Epidemiology, Differential Diagnostics, Prognosis and Therapy Prediction. Int. J. Mol. Sci. 2022, 23, 5384. [Google Scholar] [CrossRef]
- Garbe, C.; Amaral, T.; Peris, K.; Hauschild, A.; Arenberger, P.; Basset-Seguin, N.; Bastholt, L.; Bataille, V.; Del Marmol, V.; Dréno, B.; et al. European Consensus-Based Interdisciplinary Guideline for Melanoma. Part 2: Treatment—Update 2022. Eur. J. Cancer, 2022; in press. [Google Scholar] [CrossRef]
- Vukadin, S.; Khaznadar, F.; Kizivat, T.; Vcev, A.; Smolic, M. Molecular Mechanisms of Resistance to Immune Checkpoint Inhibitors in Melanoma Treatment: An Update. Biomedicines 2021, 9, 835. [Google Scholar] [CrossRef]
- Turner, S.E.; Williams, C.M.; Iversen, L.; Whalley, B.J. Molecular Pharmacology of Phytocannabinoids. Prog. Chem. Org. Nat. Prod. 2017, 103, 61–101. [Google Scholar]
- Zuardi, A.W. Cannabidiol: From an Inactive Cannabinoid to a Drug with Wide Spectrum of Action. Rev. Bras. Psiquiatr. 2008, 30, 271–280. [Google Scholar] [CrossRef] [Green Version]
- Crippa, J.A.; Guimarães, F.S.; Campos, A.C.; Zuardi, A.W. Translational Investigation of the Therapeutic Potential of Cannabidiol (CBD): Toward a New Age. Front. Immunol. 2018, 9, 2009. [Google Scholar] [CrossRef] [Green Version]
- Ożarowski, M.; Karpiński, T.M.; Zielińska, A.; Souto, E.B.; Wielgus, K. Cannabidiol in Neurological and Neoplastic Diseases: Latest Developments on the Molecular Mechanism of Action. Int. J. Mol. Sci. 2021, 22, 4294. [Google Scholar] [CrossRef] [PubMed]
- Marzęda, P.; Drozd, M.; Wróblewska-Łuczka, P.; Łuszczki, J.J. Cannabinoids and Their Derivatives in Struggle against Melanoma. Pharmacol. Rep. 2021, 73, 1485–1496. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, J.; Liu, W.; Dalgleish, A. Report of Objective Clinical Responses of Cancer Patients to Pharmaceutical-Grade Synthetic Cannabidiol. Anticancer Res. 2018, 38, 5831–5835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seltzer, E.S.; Watters, A.K.; Mackenzie, D.; Granat, L.M.; Zhang, D. Cannabidiol (CBD) as a Promising Anti-Cancer Drug. Cancers 2020, 12, 3203. [Google Scholar] [CrossRef]
- Kis, B.; Ifrim, F.C.; Buda, V.; Avram, S.; Pavel, I.Z.; Antal, D.; Paunescu, V.; Dehelean, C.A.; Ardelean, F.; Diaconeasa, Z.; et al. Cannabidiol—From Plant to Human Body: A Promising Bioactive Molecule with Multi-Target Effects in Cancer. Int. J. Mol. Sci. 2019, 20, 5905. [Google Scholar] [CrossRef] [Green Version]
- Rock, E.M.; Bolognini, D.; Limebeer, C.L.; Cascio, M.G.; Anavi-Goffer, S.; Fletcher, P.J.; Mechoulam, R.; Pertwee, R.G.; Parker, L.A. Cannabidiol, a Nonpsychotropic Component of Cannabis, Attenuates Vomiting and Nausea-like Behaviour via Indirect Agonism of 5-HT 1A Somatodendritic Autoreceptors in the Dorsal Raphe Nucleus. Br. J. Pharmacol. 2012, 165, 2620–2634. [Google Scholar] [CrossRef] [Green Version]
- Amin, M.R.; Ali, D.W. Pharmacology of Medical Cannabis. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2019; Volume 1162, pp. 151–165. [Google Scholar]
- Zanelati, T.V.; Biojone, C.; Moreira, F.A.; Guimarães, F.S.; Joca, S.R.L. Antidepressant-like Effects of Cannabidiol in Mice: Possible Involvement of 5-HT 1A Receptors. Br. J. Pharmacol. 2010, 159, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Shannon, S.; Lewis, N.; Lee, H.; Hughes, S. Cannabidiol in Anxiety and Sleep: A Large Case Series. Perm. J. 2019, 23, 18–41. [Google Scholar] [CrossRef] [Green Version]
- Jarocka-Karpowicz, I.; Biernacki, M.; Wroński, A.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol Effects on Phospholipid Metabolism in Keratinocytes from Patients with Psoriasis Vulgaris. Biomolecules 2020, 10, 367. [Google Scholar] [CrossRef] [Green Version]
- Milando, R.; Friedman, A. Cannabinoids: Potential Role in Inflammatory and Neoplastic Skin Diseases. Am. J. Clin. Dermatol. 2019, 20, 167–180. [Google Scholar] [CrossRef]
- Vimal, D.; D’Souza, L.C.; Rai, V.; Lal, S.; Sharma, A.; Gupta, S.C. Efficacy of Cannabis and Its Constituents in Disease Management: Insights from Clinical Studies. Curr. Med. Chem. 2022; 29, in press. [Google Scholar] [CrossRef] [PubMed]
- Javid, F.A.; Phillips, R.M.; Afshinjavid, S.; Verde, R.; Ligresti, A. Cannabinoid Pharmacology in Cancer Research: A New Hope for Cancer Patients? Eur. J. Pharmacol. 2016, 775, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Preet, A.; Ganju, R.K.; Groopman, J.E. Delta9-Tetrahydrocannabinol Inhibits Epithelial Growth Factor-Induced Lung Cancer Cell Migration in Vitro as Well as Its Growth and Metastasis in Vivo. Oncogene 2008, 27, 339–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramer, R.; Wendt, F.; Wittig, F.; Schäfer, M.; Boeckmann, L.; Emmert, S.; Hinz, B. Impact of Cannabinoid Compounds on Skin Cancer. Cancers 2022, 14, 1769. [Google Scholar] [CrossRef]
- Hinz, B.; Ramer, R. Cannabinoids as Anticancer Drugs: Current Status of Preclinical Research. Br. J. Cancer 2022, 1–3. [Google Scholar] [CrossRef]
- Blázquez, C.; Casanova, M.L.; Planas, A.; Gómez Del Pulgar, T.; Villanueva, C.; Fernández-Aceñero, M.J.; Aragonés, J.; Huffman, J.W.; Jorcano, J.L.; Guzmán, M. Inhibition of Tumor Angiogenesis by Cannabinoids. FASEB J. 2003, 17, 529–531. [Google Scholar] [CrossRef]
- Pagano, C.; Navarra, G.; Coppola, L.; Avilia, G.; Bifulco, M.; Laezza, C. Cannabinoids: Therapeutic Use in Clinical Practice. Int. J. Mol. Sci. 2022, 23, 3344. [Google Scholar] [CrossRef]
- Salazar, M.; Carracedo, A.; Salanueva, I.J.; Hernández-Tiedra, S.; Egia, A.; Lorente, M.; Vázquez, P.; Torres, S.; Iovanna, J.L.; Guzmán, M.; et al. TRB3 Links ER Stress to Autophagy in Cannabinoid Anti-Tumoral Action. Autophagy 2009, 5, 1048–1049. [Google Scholar] [CrossRef] [Green Version]
- Bisogno, T.; Hanus, L.; De Petrocellis, L.; Tchilibon, S.; Ponde, D.E.; Brandi, I.; Moriello, A.S.; Davis, J.B.; Mechoulam, R.; Di Marzo, V. Molecular Targets for Cannabidiol and Its Synthetic Analogues: Effect on Vanilloid VR1 Receptors and on the Cellular Uptake and Enzymatic Hydrolysis of Anandamide. Br. J. Pharmacol. 2001, 134, 845–852. [Google Scholar] [CrossRef]
- Shrivastava, A.; Kuzontkoski, P.M.; Groopman, J.E.; Prasad, A. Cannabidiol Induces Programmed Cell Death in Breast Cancer Cells by Coordinating the Cross-Talk between Apoptosis and Autophagy. Mol. Cancer Ther. 2011, 10, 1161–1172. [Google Scholar] [CrossRef] [Green Version]
- Petrovici, A.R.; Simionescu, N.; Sandu, A.I.; Paraschiv, V.; Silion, M.; Pinteala, M. New Insights on Hemp Oil Enriched in Cannabidiol: Decarboxylation, Antioxidant Properties and In Vitro Anticancer Effect. Antioxidants 2021, 10, 738. [Google Scholar] [CrossRef]
- Burch, R.; Mortuza, A.; Blumenthal, E.; Mustafa, A. Effects of Cannabidiol (CBD) on the Inhibition of Melanoma Cells in Vitro. J. Immunoass. Immunochem. 2021, 42, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Siddik, Z.H. Cisplatin: Mode of Cytotoxic Action and Molecular Basis of Resistance. Oncogene 2003, 22, 7265–7279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krajewski, A.; Gagat, M.; Żuryń, A.; Hałas-Wiśniewska, M.; Grzanka, D.; Grzanka, A. Cyclin F Is Involved in Response to Cisplatin Treatment in Melanoma Cell Lines. Oncol. Rep. 2020, 43, 765–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makino, E.; Gutmann, V.; Kosnopfel, C.; Niessner, H.; Forschner, A.; Garbe, C.; Sinnberg, T.; Schittek, B. Melanoma Cells Resistant towards MAPK Inhibitors Exhibit Reduced TAp73 Expression Mediating Enhanced Sensitivity to Platinum-Based Drugs. Cell Death Dis. 2018, 9, 930. [Google Scholar] [CrossRef]
- Lim, S.Y.; Menzies, A.M.; Rizos, H. Mechanisms and Strategies to Overcome Resistance to Molecularly Targeted Therapy for Melanoma. Cancer 2017, 123, 2118–2129. [Google Scholar] [CrossRef] [Green Version]
- Soengas, M.S.; Lowe, S.W. Apoptosis and Melanoma Chemoresistance. Oncogene 2003, 22, 3138–3151. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S. Cisplatin: The First Metal Based Anticancer Drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef]
- Kapuscinski, J.; Darzynkiewicz, Z. Interactions of Antitumor Agents Ametantrone and Mitoxantrone (Novatrone) with Double-Stranded DNA. Biochem. Pharmacol. 1985, 34, 4203–4213. [Google Scholar] [CrossRef]
- Mazerski, J.; Martelli, S.; Borowski, E. The Geometry of Intercalation Complex of Antitumor Mitoxantrone and Ametantrone with DNA: Molecular Dynamics Simulations. Acta Biochim. Pol. 1998, 45, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Lenk, H.; Müller, U.; Tanneberger, S. Mitoxantrone: Mechanism of Action, Antitumor Activity, Pharmacokinetics, Efficacy in the Treatment of Solid Tumors and Lymphomas, and Toxicity. Anticancer Res. 1987, 7, 1257–1264. [Google Scholar] [PubMed]
- Faulds, D.; Balfour, J.A.; Chrisp, P.; Langtry, H.D. Mitoxantrone. A Review of Its Pharmacodynamic and Pharmacokinetic Properties, and Therapeutic Potential in the Chemotherapy of Cancer. Drugs 1991, 41, 400–449. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Pandey, V.P.; Yadav, K.; Yadav, A.; Dwivedi, U.N. Natural Products as Anti-Cancerous Therapeutic Molecules Targeted towards Topoisomerases. Curr. Protein Pept. Sci. 2020, 21, 1103–1142. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.Y.; Pei, L.; Liu, Q.; Chen, S.; Dou, H.; Shu, G.; Yuan, Z.X.; Lin, J.; Peng, G.; Zhang, W.; et al. Isobologram Analysis: A Comprehensive Review of Methodology and Current Research. Front. Pharmacol. 2019, 10, 1222. [Google Scholar] [CrossRef] [PubMed]
- Wawruszak, A.; Luszczki, J.J.; Grabarska, A.; Gumbarewicz, E.; Dmoszynska-Graniczka, M.; Polberg, K.; Stepulak, A. Assessment of Interactions between Cisplatin and Two Histone Deacetylase Inhibitors in MCF7, T47D and MDA-MB-231 Human Breast Cancer Cell Lines—An Isobolographic Analysis. PLoS ONE 2015, 10, e0143013. [Google Scholar] [CrossRef] [Green Version]
- Gumbarewicz, E.; Luszczki, J.J.; Wawruszak, A.; Dmoszynska-Graniczka, M.; Grabarska, A.J.; Jarzab, A.M.; Polberg, K.; Stepulak, A. Isobolographic Analysis Demonstrates Additive Effect of Cisplatin and HDIs Combined Treatment Augmenting Their Anti-Cancer Activity in Lung Cancer Cell Lines. Am. J. Cancer Res. 2016, 6, 2831–2845. [Google Scholar]
- Lawrence, M.S.; Stojanov, P.; Polak, P.; Kryukov, G.V.; Cibulskis, K.; Sivachenko, A.; Carter, S.L.; Stewart, C.; Mermel, C.H.; Roberts, S.A.; et al. Mutational Heterogeneity in Cancer and the Search for New Cancer-Associated Genes. Nature 2013, 499, 214–218. [Google Scholar] [CrossRef]
- Wolf, Y.; Bartok, O.; Patkar, S.; Eli, G.B.; Cohen, S.; Litchfield, K.; Levy, R.; Jiménez-Sánchez, A.; Trabish, S.; Lee, J.S.; et al. UVB-Induced Tumor Heterogeneity Diminishes Immune Response in Melanoma. Cell 2019, 179, 219–235.e21. [Google Scholar] [CrossRef] [Green Version]
- Simmerman, E.; Qin, X.; Yu, J.C.; Baban, B. Cannabinoids as a Potential New and Novel Treatment for Melanoma: A Pilot Study in a Murine Model. J. Surg. Res. 2019, 235, 210–215. [Google Scholar] [CrossRef]
- Deng, L.; Ng, L.; Ozawa, T.; Stella, N. Quantitative Analyses of Synergistic Responses between Cannabidiol and DNA-Damaging Agents on the Proliferation and Viability of Glioblastoma and Neural Progenitor Cells in Culture. J. Pharmacol. Exp. Ther. 2017, 360, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Wróblewska-Łuczka, P.; Grabarska, A.; Łuszczki, J.J.; Florek-Łuszczki, M.; Plewa, Z. Synergy, Additivity, and Antagonism between Cisplatin and Selected Coumarins in Human Melanoma Cells. Int. J. Mol. Sci. 2021, 22, 537. [Google Scholar] [CrossRef] [PubMed]
- Inkol, J.M.; Hocker, S.E.; Mutsaers, A.J. Combination Therapy with Cannabidiol and Chemotherapeutics in Canine Urothelial Carcinoma Cells. PLoS ONE 2021, 16, e0255591. [Google Scholar] [CrossRef]
- Holland, M.L.; Lau, D.T.T.; Allen, J.D.; Arnold, J.C. The Multidrug Transporter ABCG2 (BCRP) Is Inhibited by Plant-Derived Cannabinoids. Br. J. Pharmacol. 2007, 152, 815–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrosino, S.; Verde, R.; Vaia, M.; Allará, M.; Iuvone, T.; Di Marzo, V. Anti-Inflammatory Properties of Cannabidiol, a Nonpsychotropic Cannabinoid, in Experimental Allergic Contact Dermatitis. J. Pharmacol. Exp. Ther. 2018, 365, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.L.; Hill, D.S.; McKee, C.S.; Hernandez-Tiedra, S.; Lorente, M.; Lopez-Valero, I.; Anagnostou, M.E.; Babatunde, F.; Corazzari, M.; Redfern, C.P.F.; et al. Exploiting Cannabinoid-Induced Cytotoxic Autophagy to Drive Melanoma Cell Death. J. Investig. Dermatol. 2015, 135, 1629–1637. [Google Scholar] [CrossRef] [Green Version]
- Hardy, J.; Haywood, A.; Gogna, G.; Martin, J.; Yates, P.; Greer, R.; Good, P. Oral Medicinal Cannabinoids to Relieve Symptom Burden in the Palliative Care of Patients with Advanced Cancer: A Double-Blind, Placebo-Controlled, Randomised Clinical Trial of Efficacy and Safety of 1:1 Delta-9-Tetrahydrocannabinol (THC) and Cannabidiol. Trials 2020, 21, 611. [Google Scholar] [CrossRef]
- Yasmin-Karim, S.; Moreau, M.; Mueller, R.; Sinha, N.; Dabney, R.; Herman, A.; Ngwa, W. Enhancing the Therapeutic Efficacy of Cancer Treatment with Cannabinoids. Front. Oncol. 2018, 8, 114. [Google Scholar] [CrossRef] [Green Version]
- Spałek, M.; Czarnecka, A.M. The Role of Radiotherapy in Melanoma. Oncol. Clin. Pract. 2020, 15, 310–319. [Google Scholar] [CrossRef]
- Sühnel, J. Parallel Dose-Response Curves in Combination Experiments. Bull. Math. Biol. 1998, 60, 197–213. [Google Scholar] [CrossRef] [Green Version]
- Tallarida, R.J. Drug Synergism: Its Detection and Applications. J. Pharmacol. Exp. Ther. 2001, 298, 865–872. [Google Scholar]
- Berenbaum, M.C. What Is Synergy? Pharmacol. Rev. 1989, 41, 93–141. [Google Scholar] [PubMed]
- Luszczki, J.J. Isobolographic Analysis of Interaction between Drugs with Nonparallel Dose-Response Relationship Curves: A Practical Application. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2007, 375, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Luszczki, J.J.; Czuczwar, S.J. Biphasic Characteristic of Interactions between Stiripentol and Carbamazepine in the Mouse Maximal Electroshock-Induced Seizure Model: A Three-Dimensional Isobolographic Analysis. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2006, 374, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Pöch, G.; Vychodil-Kahr, S.; Petru, E. Sigmoid Model versus Median-Effect Analysis for Obtaining Dose-Response Curves for in Vitro Chemosensitivity Testing. Int. J. Clin. Pharmacol. Ther. 1999, 37, 189–192. [Google Scholar] [PubMed]
- Bobiński, M.; Okła, K.; Łuszczki, J.; Bednarek, W.; Wawruszak, A.; Moreno-Bueno, G.; Dmoszyńska-Graniczka, M.; Tarkowski, R.; Kotarski, J. Isobolographic Analysis Demonstrates the Additive and Synergistic Effects of Gemcitabine Combined with Fucoidan in Uterine Sarcomas and Carcinosarcoma Cells. Cancers 2019, 12, 107. [Google Scholar] [CrossRef] [Green Version]
- Litchfield, J.T.; Wilcoxon, F. A Simplified Method of Evaluating Dose-Effect Experiments. J. Pharmacol. Exp. Ther. 1949, 96, 99–113. [Google Scholar]
- Tallarida, R.J.; Porreca, F.; Cowan, A. Statistical Analysis of Drug-Drug and Site-Site Interactions with Isobolograms. Life Sci. 1989, 45, 947–961. [Google Scholar] [CrossRef]
- Loewe, S. The Problem of Synergism and Antagonism of Combined Drugs. Arzneimittelforschung 1953, 3, 285–290. [Google Scholar]
- Tallarida, R.J. Quantitative methods for assessing drug synergism. Genes Cancer. 2011, 2, 1003–1008. [Google Scholar] [CrossRef] [Green Version]


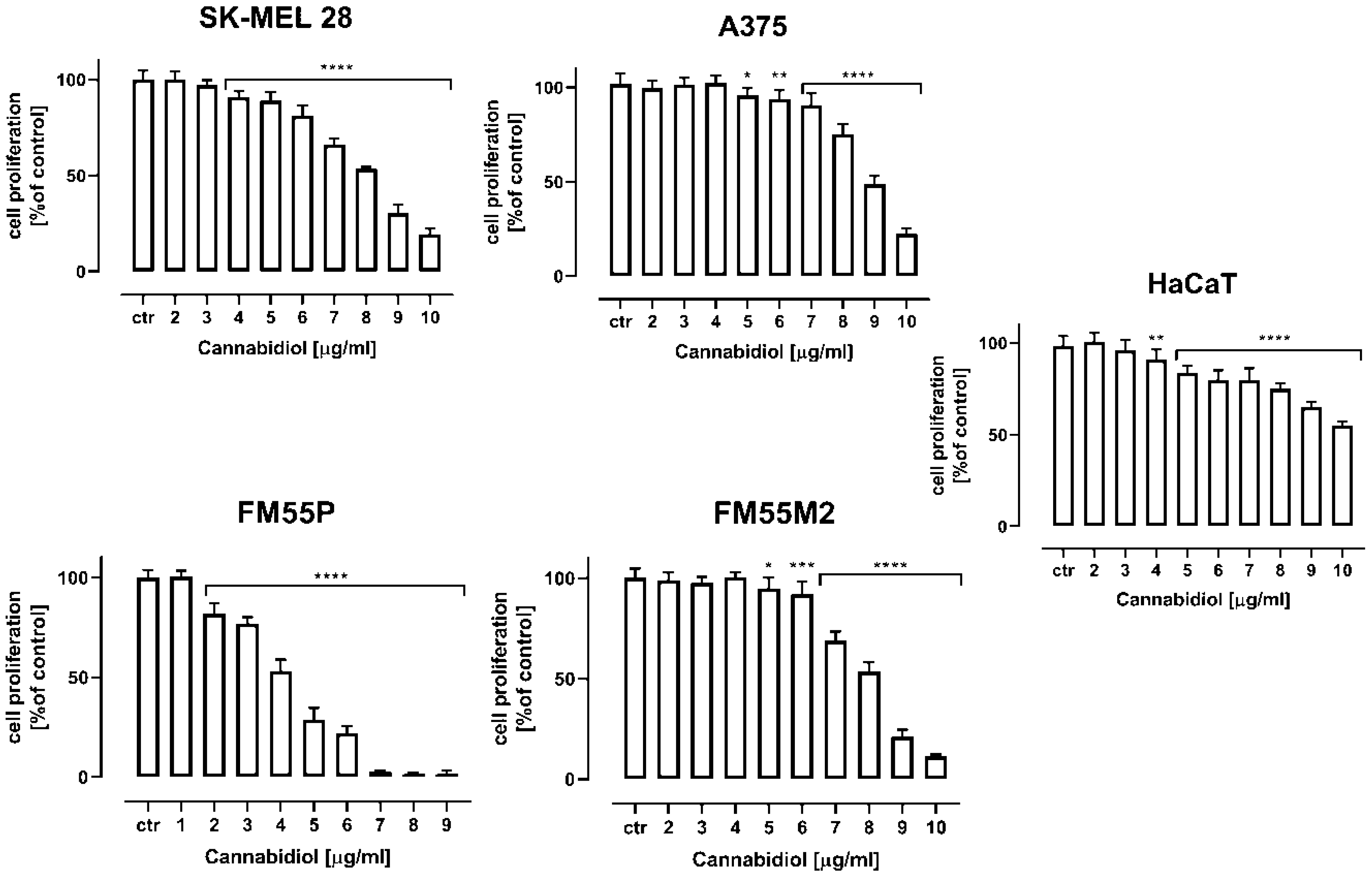
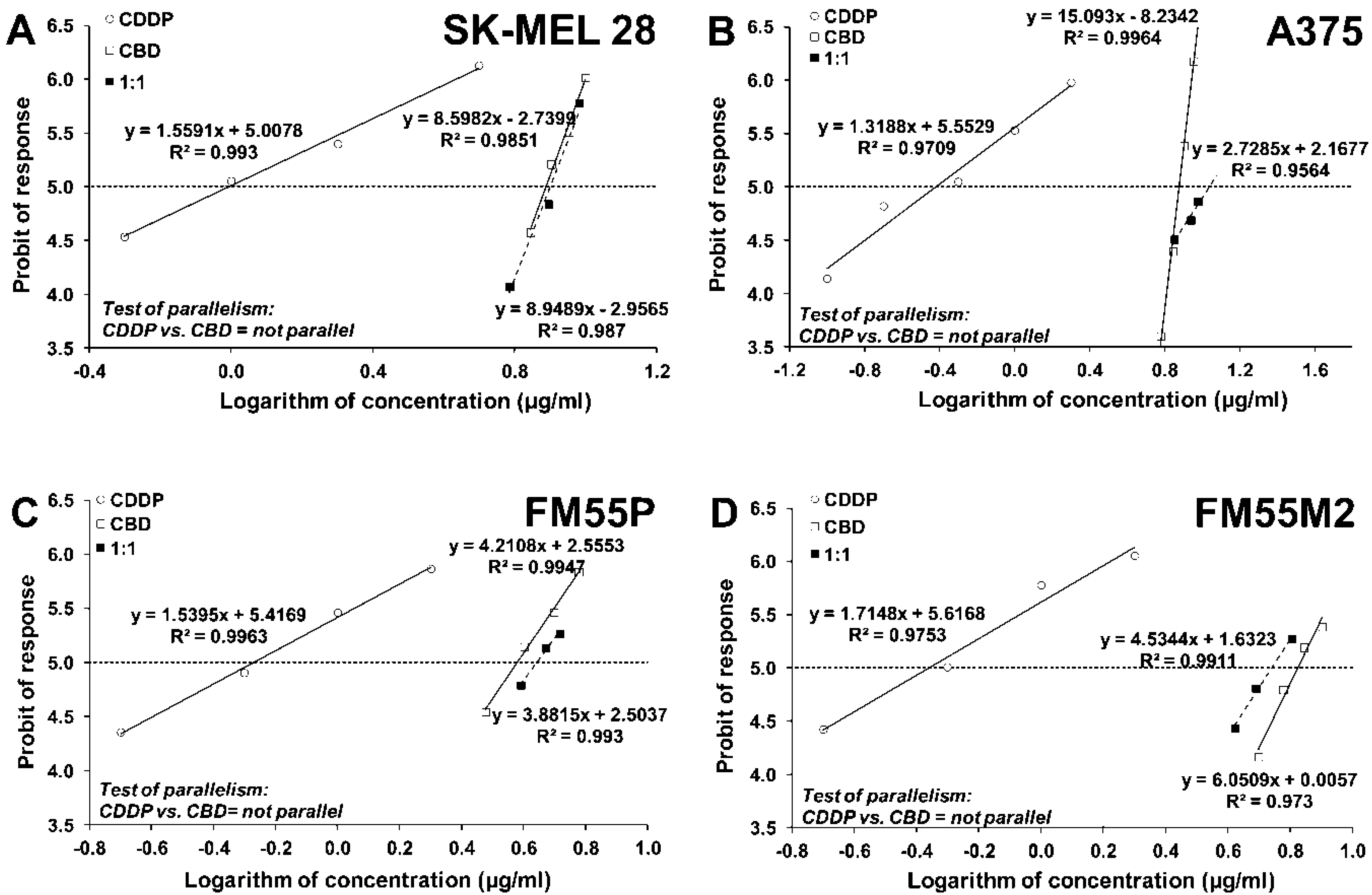
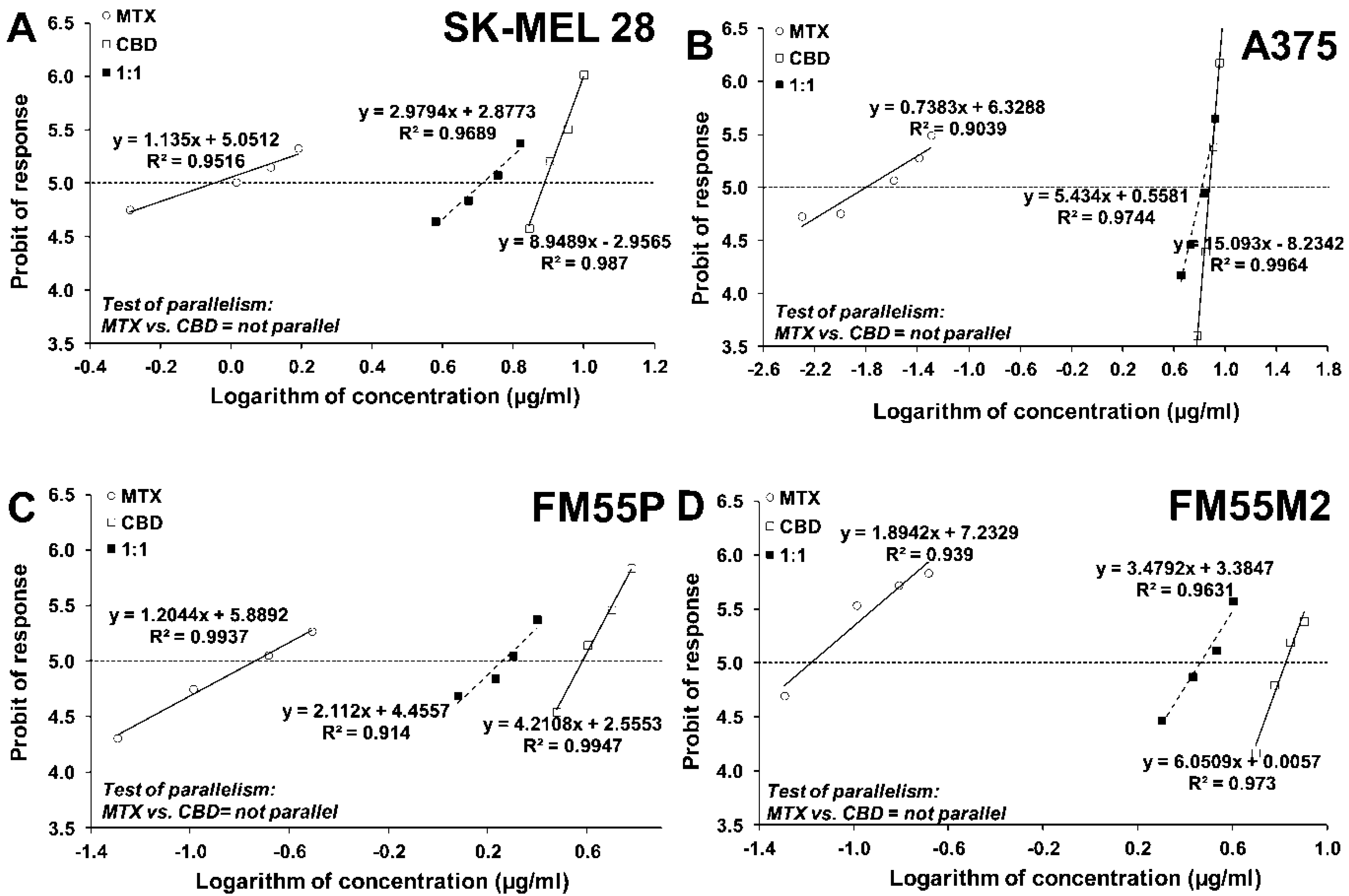
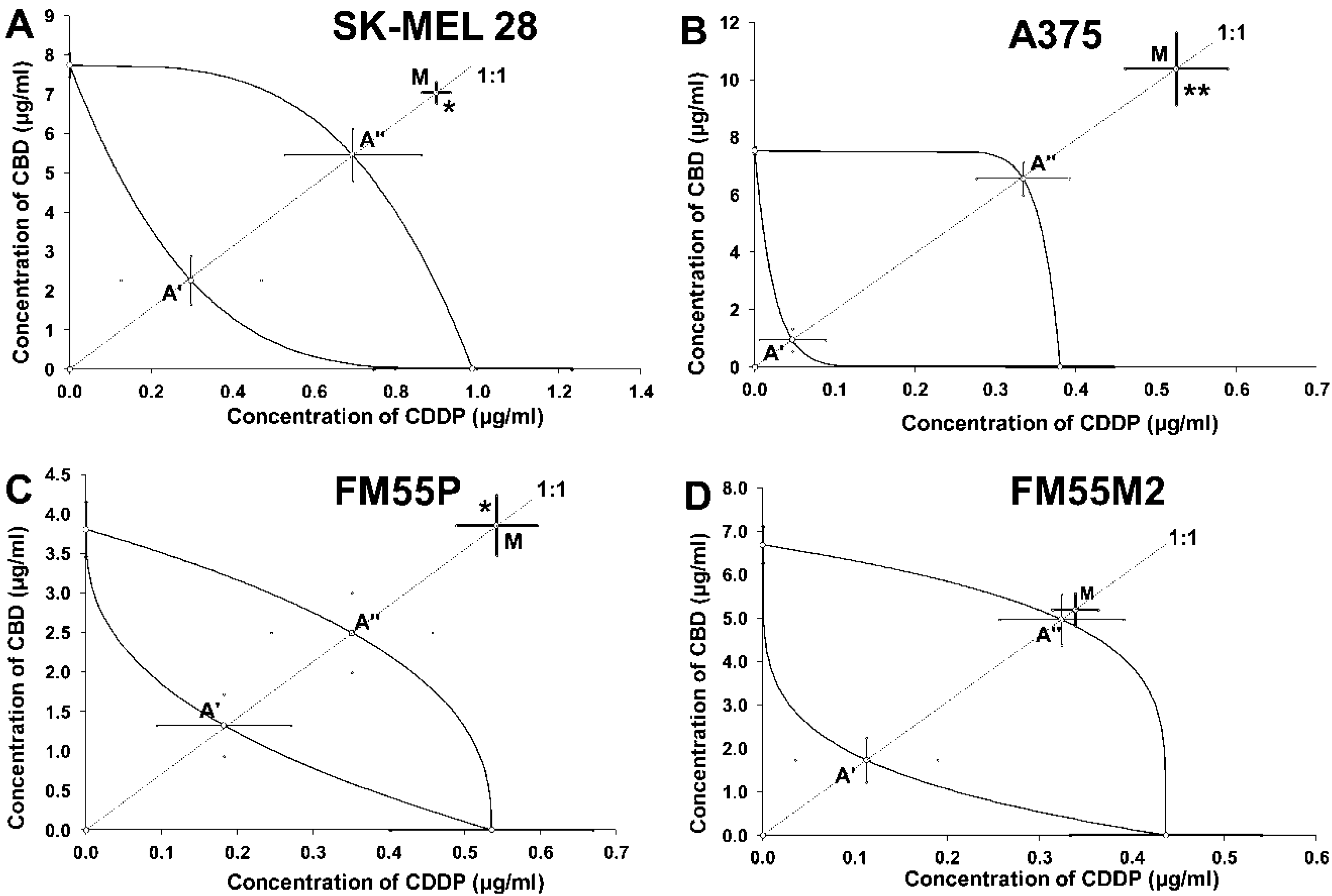
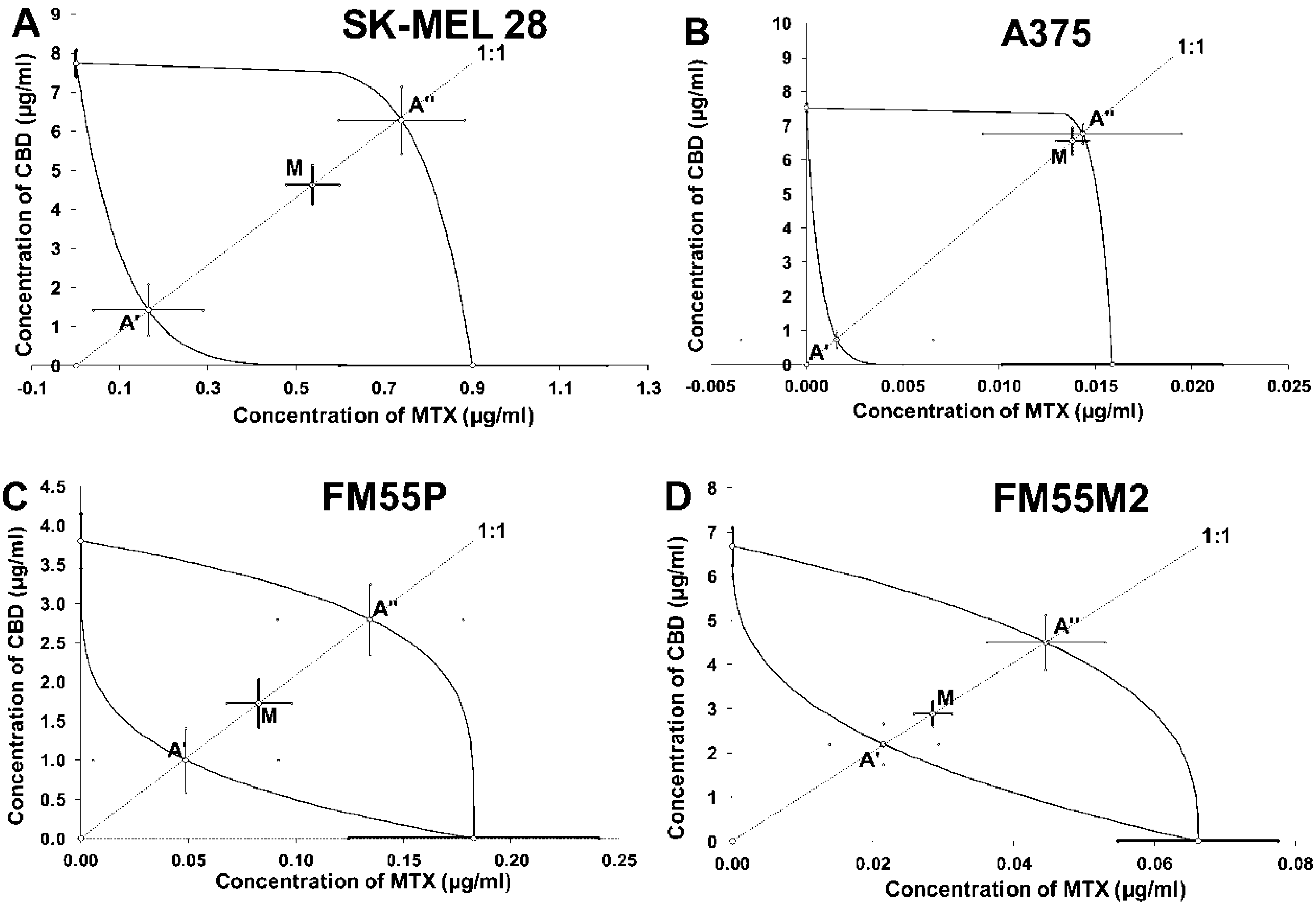
| Drug | SK-MEL 28 | A375 | FM55P | FM55M2 |
|---|---|---|---|---|
| CBD | 7.75 ± 0.29 | 7.53 ± 0.13 | 3.81 ± 0.35 | 6.69 ± 0.42 |
| MTX | 0.90 ± 0.30 | 0.016 ± 0.005 | 0.18 ± 0.06 | 0.07 ± 0.01 |
| CDDP | 0.99 ± 0.21 | 0.38 ± 0.07 | 0.54 ± 0.13 | 0.44 ± 0.10 |
| Cell Line | IC50 mix (μg/mL) | n mix | Lower IC50 add (μg/mL) | n add | Upper IC50 add (μg/mL) | Interaction |
|---|---|---|---|---|---|---|
| SK-MEL 28 | 7.95 ± 0.31 * | 96 | 2.56 ± 0.78 | 158 | 6.15 ± 0.84 | Antagonism |
| A375 | 10.92 ± 1.33 ** | 96 | 0.98 ± 0.44 | 356 | 6.90 ± 0.63 | Antagonism |
| FM55P | 4.40 ± 0.43 * | 72 | 1.51 ± 0.48 | 140 | 2.85 ± 0.61 | Antagonism |
| FM55M2 | 5.53 ± 0.41 | 96 | 1.84 ± 0.59 | 132 | 5.29 ± 0.66 | Additivity |
| Cell Line | IC50 mix (μg/mL) | n mix | Lower IC50 add (μg/mL) | n add | Upper IC50 add (μg/mL) | Interaction |
|---|---|---|---|---|---|---|
| SK-MEL 28 | 5.16 ± 0.57 | 96 | 1.59 ± 0.78 | 140 | 7.03 ± 1.00 | Additivity |
| A375 | 6.57 ± 0.40 | 96 | 0.73 ± 0.25 | 306 | 6.79 ± 0.30 | Additivity |
| FM55P | 1.81 ± 0.33 | 72 | 1.04 ± 0.46 | 140 | 2.93 ± 0.49 | Additivity |
| FM55M2 | 2.91 ± 0.28 | 96 | 2.22 ± 0.48 | 168 | 4.55 ± 0.64 | Additivity |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marzęda, P.; Wróblewska-Łuczka, P.; Drozd, M.; Florek-Łuszczki, M.; Załuska-Ogryzek, K.; Łuszczki, J.J. Cannabidiol Interacts Antagonistically with Cisplatin and Additively with Mitoxantrone in Various Melanoma Cell Lines—An Isobolographic Analysis. Int. J. Mol. Sci. 2022, 23, 6752. https://doi.org/10.3390/ijms23126752
Marzęda P, Wróblewska-Łuczka P, Drozd M, Florek-Łuszczki M, Załuska-Ogryzek K, Łuszczki JJ. Cannabidiol Interacts Antagonistically with Cisplatin and Additively with Mitoxantrone in Various Melanoma Cell Lines—An Isobolographic Analysis. International Journal of Molecular Sciences. 2022; 23(12):6752. https://doi.org/10.3390/ijms23126752
Chicago/Turabian StyleMarzęda, Paweł, Paula Wróblewska-Łuczka, Małgorzata Drozd, Magdalena Florek-Łuszczki, Katarzyna Załuska-Ogryzek, and Jarogniew J. Łuszczki. 2022. "Cannabidiol Interacts Antagonistically with Cisplatin and Additively with Mitoxantrone in Various Melanoma Cell Lines—An Isobolographic Analysis" International Journal of Molecular Sciences 23, no. 12: 6752. https://doi.org/10.3390/ijms23126752
APA StyleMarzęda, P., Wróblewska-Łuczka, P., Drozd, M., Florek-Łuszczki, M., Załuska-Ogryzek, K., & Łuszczki, J. J. (2022). Cannabidiol Interacts Antagonistically with Cisplatin and Additively with Mitoxantrone in Various Melanoma Cell Lines—An Isobolographic Analysis. International Journal of Molecular Sciences, 23(12), 6752. https://doi.org/10.3390/ijms23126752






