EST79232 and EST79376, Two Novel Sigma-1 Receptor Ligands, Exert Neuroprotection on Models of Motoneuron Degeneration
Abstract
:1. Introduction
2. Results
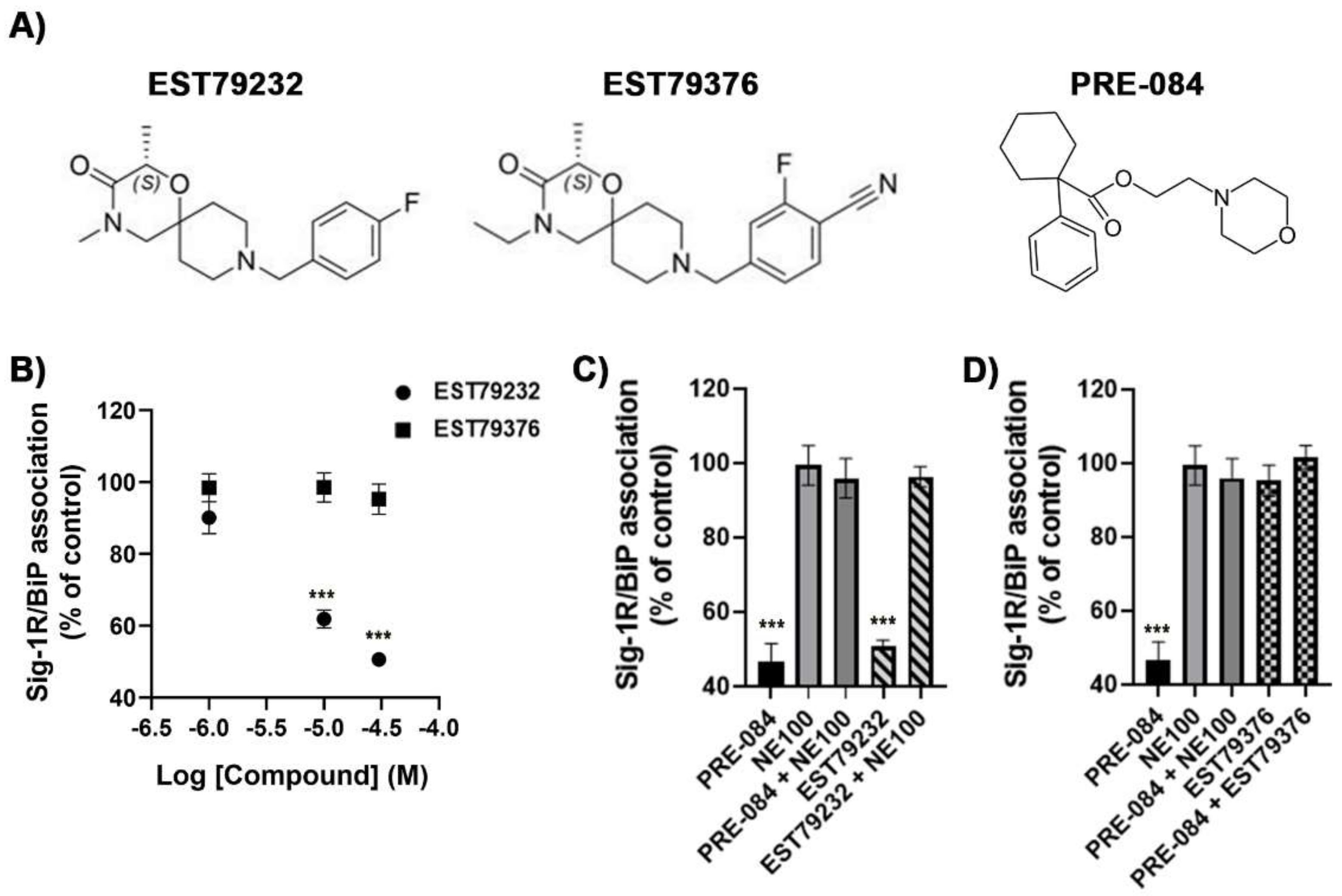
2.1. In Vitro Pharmacological Profile of EST79232 and EST79376
2.2. EST79232 and EST79376 Prevent MN Death in SCOC under Chronic Excitotoxic Stress
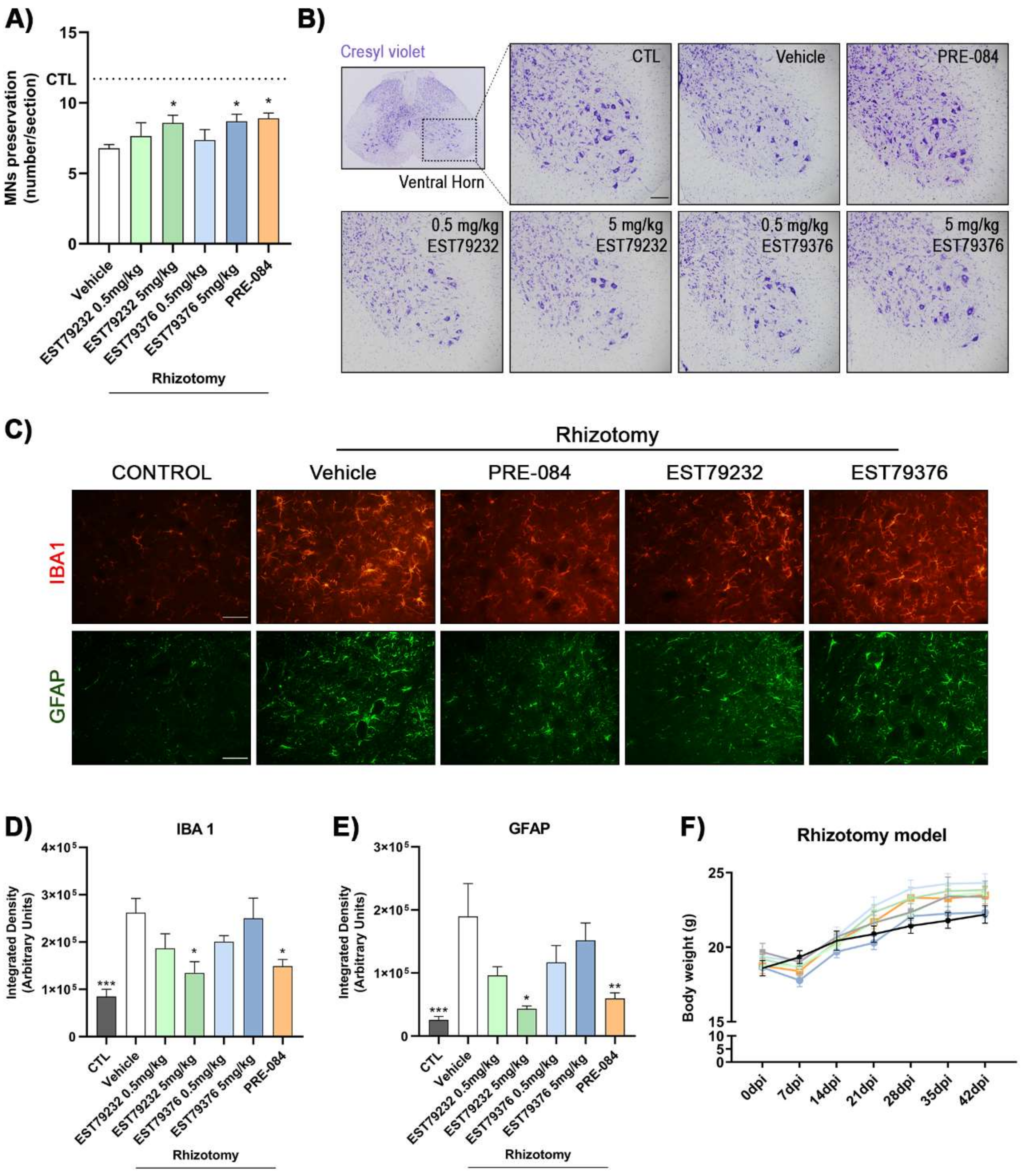
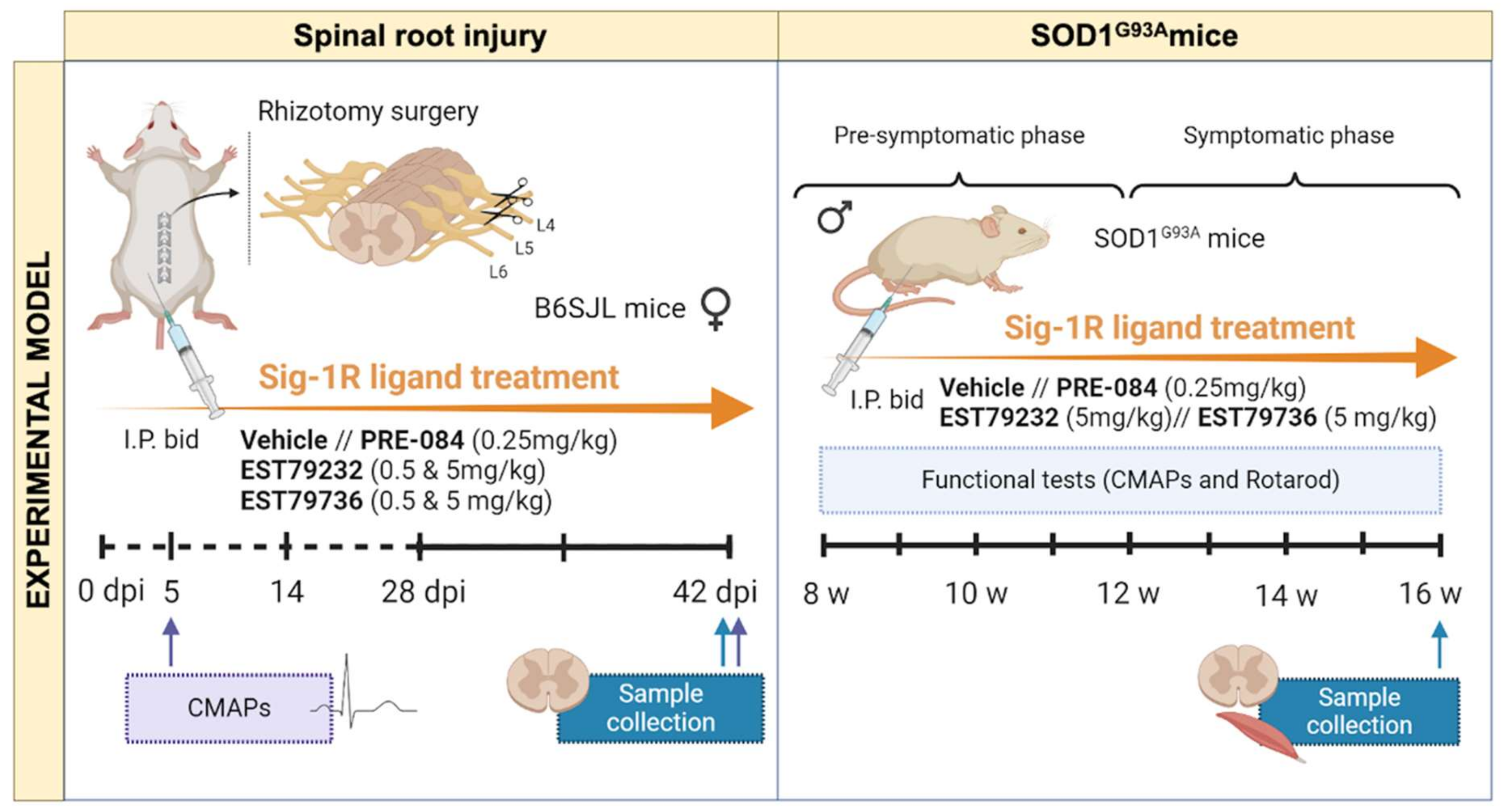
2.3. EST79232 and EST79376 Enhance MN Survival after Rhizotomy
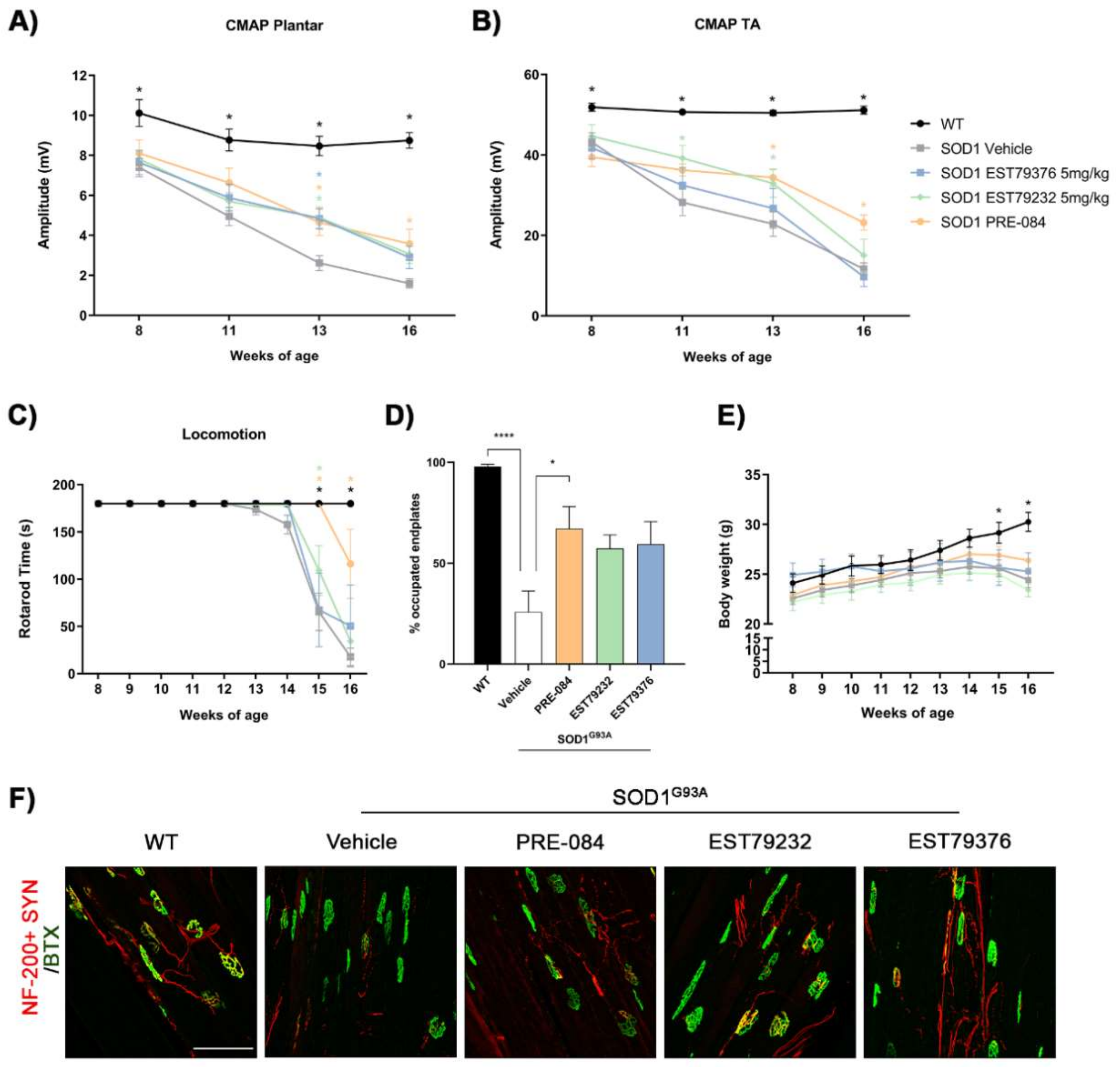
2.4. EST79232 Treatment Slows Disease Progression in SOD1G93A Mice
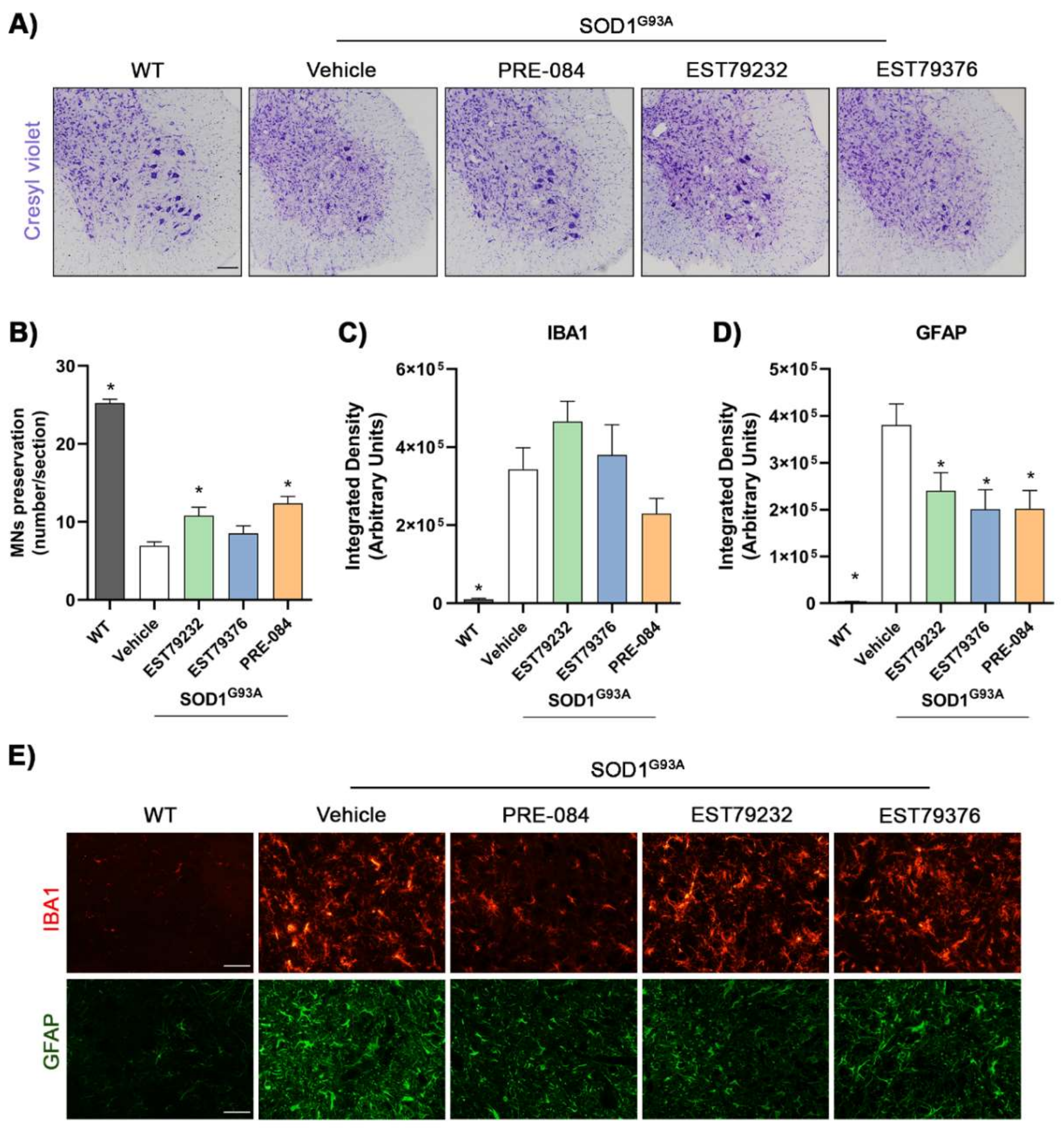
2.5. EST79232 Treatment Protects Spinal MNs and Reduces Astroglial Activation in SOD1G93A Mice
3. Discussion
4. Materials and Methods
4.1. Human Sig-1R Radioligand Assay
4.2. Human Sig-2R Radioligand Assay
4.3. Selectivity Profile
4.4. BiP/Sig-1R Association Assay
4.5. Spinal Cord Organotypic Cultures (SCOCs)
4.6. Animals
4.7. Rhizotomy Procedure
4.8. Drug Administration
4.9. Plasma Levels Associated with Pharmacological Activity
4.10. Electrophysiological Tests
4.11. Locomotion Tests
4.12. Histological and Immunofluorescence Analyses
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Edaravone (MCI-186) ALS 19 Study Group. Safety and Efficacy of Edaravone in Well Defined Patients with Amyotrophic Lateral Sclerosis: A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Neurol. 2017, 16, 505–512. [Google Scholar] [CrossRef]
- Ludolph, A.C.; Jesse, S. Review: Evidence-Based Drug Treatment in Amyotrophic Lateral Sclerosis and Upcoming Clinical Trials. Ther. Adv. Neurol. Disord. 2009, 2, 319–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancuso, R.; Navarro, X. Amyotrophic Lateral Sclerosis: Current Perspectives from Basic Research to the Clinic. Prog. Neurobiol. 2015, 133, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Langa, F.; Codony, X.; Tovar, V.; Lavado, A.; Giménez, E.; Cozar, P.; Cantero, M.; Dordal, A.; Hernández, E.; Pérez, R.; et al. Generation and Phenotypic Analysis of Sigma Receptor Type I (Σ1) Knockout Mice. Eur. J. Neurosci. 2003, 18, 2188–2196. [Google Scholar] [CrossRef]
- Mavlyutov, T.A.; Epstein, M.L.; Andersen, K.A.; Ziskind-Conhaim, L.; Ruoho, A.E. The Sigma-1 Receptor Is Enriched in Postsynaptic Sites of C-Terminals in Mouse Motoneurons. An Anatomical and Behavioral Study. Neuroscience 2010, 167, 247–255. [Google Scholar] [CrossRef] [Green Version]
- Al-Saif, A.; Al-Mohanna, F.; Bohlega, S. A Mutation in Sigma-1 Receptor Causes Juvenile Amyotrophic Lateral Sclerosis. Ann. Neurol. 2011, 70, 913–919. [Google Scholar] [CrossRef]
- Luty, A.A.; Kwok, J.B.J.; Dobson-Stone, C.; Loy, C.T.; Coupland, K.G.; Karlström, H.; Sobow, T.; Tchorzewska, J.; Maruszak, A.; Barcikowska, M.; et al. Sigma Nonopioid Intracellular Receptor 1 Mutations Cause Frontotemporal Lobar Degeneration-Motor Neuron Disease. Ann. Neurol. 2010, 68, 639–649. [Google Scholar] [CrossRef]
- Almendra, L.; Laranjeira, F.; Fernández-Marmiesse, A.; Negrão, L. SIGMAR1 Gene Mutation Causing Distal Hereditary Motor Neuropathy in a Portuguese Family. Acta Myol. 2018, 37, 2–4. [Google Scholar]
- Ververis, A.; Dajani, R.; Koutsou, P.; Aloqaily, A.; Nelson-Williams, C.; Loring, E.; Arafat, A.; Mubaidin, A.F.; Horany, K.; Bader, M.B.; et al. Distal Hereditary Motor Neuronopathy of the Jerash Type Is Caused by a Novel SIGMAR1 c.500A>T Missense Mutation. J. Med. Genet. 2020, 57, 178–186. [Google Scholar] [CrossRef] [Green Version]
- Brimson, J.M.; Brimson, S.; Chomchoei, C.; Tencomnao, T. Using Sigma-Ligands as Part of a Multi-Receptor Approach to Target Diseases of the Brain. Expert Opin. Ther. Targets 2020, 24, 1009–1028. [Google Scholar] [CrossRef]
- Guzmán-Lenis, M.-S.; Navarro, X.; Casas, C. Selective Sigma Receptor Agonist 2-(4-Morpholinethyl)1-Phenylcyclohexanecarboxylate (PRE084) Promotes Neuroprotection and Neurite Elongation through Protein Kinase C (PKC) Signaling on Motoneurons. Neuroscience 2009, 162, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Tanaka, H.; Takata, M.; Nagahara, Y.; Noda, Y.; Tsuruma, K.; Shimazawa, M.; Hozumi, I.; Hara, H. SA4503, a Sigma-1 Receptor Agonist, Suppresses Motor Neuron Damage in in Vitro and in Vivo Amyotrophic Lateral Sclerosis Models. Neurosci. Lett. 2014, 559, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, A.; Gradus, T.; Altman, T.; Maimon, R.; Saraf Avraham, N.; Geva, M.; Hayden, M.; Perlson, E. Targeting the Sigma-1 Receptor via Pridopidine Ameliorates Central Features of ALS Pathology in a SOD1G93A Model. Cell Death Dis. 2019, 10, 210. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, R.; Oliván, S.; Rando, A.; Casas, C.; Osta, R.; Navarro, X. Sigma-1R Agonist Improves Motor Function and Motoneuron Survival in ALS Mice. Neurotherapeutics 2012, 9, 814–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaja-Capdevila, N.; Hernández, N.; Navarro, X.; Herrando-Grabulosa, M. Sigma-1 Receptor Is a Pharmacological Target to Promote Neuroprotection in the SOD1G93A ALS Mice. Front. Pharmacol. 2021, 12, 780588. [Google Scholar] [CrossRef]
- Penas, C.; Pascual-Font, A.; Mancuso, R.; Forés, J.; Casas, C.; Navarro, X. Sigma Receptor Agonist 2-(4-Morpholinethyl)1 Phenylcyclohexanecarboxylate (Pre084) Increases GDNF and BiP Expression and Promotes Neuroprotection after Root Avulsion Injury. J. Neurotrauma 2011, 28, 831–840. [Google Scholar] [CrossRef]
- Gaja-Capdevila, N.; Hernández, N.; Zamanillo, D.; Vela, J.M.; Merlos, M.; Navarro, X.; Herrando-Grabulosa, M. Neuroprotective Effects of Sigma 1 Receptor Ligands on Motoneuron Death after Spinal Root Injury in Mice. Int. J. Mol. Sci. 2021, 22, 6956. [Google Scholar] [CrossRef]
- Peviani, M.; Salvaneschi, E.; Bontempi, L.; Petese, A.; Manzo, A.; Rossi, D.; Salmona, M.; Collina, S.; Bigini, P.; Curti, D. Neuroprotective Effects of the Sigma-1 Receptor (S1R) Agonist PRE-084, in a Mouse Model of Motor Neuron Disease Not Linked to SOD1 Mutation. Neurobiol. Dis. 2014, 62, 218–232. [Google Scholar] [CrossRef]
- Hayashi, T.; Su, T.P. Sigma-1 Receptor Chaperones at the ER- Mitochondrion Interface Regulate Ca2+ Signaling and Cell Survival. Cell 2007, 131, 596–610. [Google Scholar] [CrossRef] [Green Version]
- Herrando-Grabulosa, M.; Gaja-Capdevila, N.; Vela, J.M.; Navarro, X. Sigma 1 Receptor as a Therapeutic Target for Amyotrophic Lateral Sclerosis. Br. J. Pharmacol. 2021, 178, 1336–1352. [Google Scholar] [CrossRef]
- Weng, T.-Y.; Tsai, S.-Y.A.; Su, T.-P. Roles of Sigma-1 Receptors on Mitochondrial Functions Relevant to Neurodegenerative Diseases. J. Biomed. Sci. 2017, 24, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haga, H.; Matsuo, K.; Yabuki, Y.; Zhang, C.; Han, F.; Fukunaga, K. Enhancement of ATP Production Ameliorates Motor and Cognitive Impairments in a Mouse Model of MPTP−induced Parkinson’s Disease. Neurochem. Int. 2019, 129, 104492. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Williams, C.; Etcheto, A.; Goodsaid, F.; Parmentier, F.; Sallantin, J.; Kaufmann, W.E.; Missling, C.U.; Afshar, M. A Precision Medicine Framework Using Artificial Intelligence for the Identification and Confirmation of Genomic Biomarkers of Response to an Alzheimer’s Disease Therapy: Analysis of the Blarcamesine (ANAVEX2-73) Phase 2a Clinical Study. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2020, 6, e12013. [Google Scholar] [CrossRef] [PubMed]
- Reilmann, R.; McGarry, A.; Grachev, I.D.; Savola, J.M.; Borowsky, B.; Eyal, E.; Gross, N.; Langbehn, D.; Schubert, R.; Wickenberg, A.T.; et al. Safety and Efficacy of Pridopidine in Patients with Huntington’s Disease (PRIDE-HD): A Phase 2, Randomised, Placebo-Controlled, Multicentre, Dose-Ranging Study. Lancet Neurol. 2019, 18, 165–176. [Google Scholar] [CrossRef]
- Zhao, X.; Zhu, L.; Liu, D.; Chi, T.; Ji, X.; Liu, P.; Yang, X.; Tian, X.; Zou, L. Sigma-1 Receptor Protects against Endoplasmic Reticulum Stress-Mediated Apoptosis in Mice with Cerebral Ischemia/Reperfusion Injury. Apoptosis 2019, 24, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Bruna, J.; Videla, S.; Argyriou, A.A.; Velasco, R.; Villoria, J.; Santos, C.; Nadal, C.; Cavaletti, G.; Alberti, P.; Briani, C.; et al. Efficacy of a Novel Sigma-1 Receptor Antagonist for Oxaliplatin-Induced Neuropathy: A Randomized, Double-Blind, Placebo-Controlled Phase IIa Clinical Trial. Neurotherapeutics 2018, 15, 178–189. [Google Scholar] [CrossRef]
- Herrando-Grabulosa, M.; Mulet, R.; Pujol, A.; Mas, J.M.; Navarro, X.; Aloy, P.; Coma, M.; Casas, C. Novel Neuroprotective Multicomponent Therapy for Amyotrophic Lateral Sclerosis Designed by Networked Systems. PLoS ONE 2016, 11, e0147626. [Google Scholar] [CrossRef] [Green Version]
- Rothstein, J.D.; Jin, L.; Dykes-Hoberg, M.; Kuncl, R.W. Chronic Inhibition of Glutamate Uptake Produces a Model of Slow Neurotoxicity. Proc. Natl. Acad. Sci. USA 1993, 90, 6591–6595. [Google Scholar] [CrossRef] [Green Version]
- Maurice, T. Bi-Phasic Dose Response in the Preclinical and Clinical Developments of Sigma-1 Receptor Ligands for the Treatment of Neurodegenerative Disorders. Expert Opin. Drug Discov. 2021, 16, 373–389. [Google Scholar] [CrossRef]
- Tadić, V.; Malci, A.; Goldhammer, N.; Stubendorff, B.; Sengupta, S.; Prell, T.; Keiner, S.; Liu, J.; Guenther, M.; Frahm, C.; et al. Sigma 1 Receptor Activation Modifies Intracellular Calcium Exchange in the G93AhSOD1 ALS Model. Neuroscience 2017, 359, 105–118. [Google Scholar] [CrossRef]
- Koliatsos, V.E.; Price, W.L.; Pardo, C.A.; Price, D.L. Ventral Root Avulsion: An Experimental Model of Death of Adult Motor Neurons. J. Comp. Neurol. 1994, 342, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Cheng, J.; Wang, C.; Zhen, X. Sigma-1 Receptor-Modulated Neuroinflammation in Neurological Diseases. Front. Cell. Neurosci. 2018, 12, 314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brites, D.; Vaz, A.R. Microglia Centered Pathogenesis in ALS: Insights in Cell Interconnectivity. Front. Cell. Neurosci. 2014, 8, 117. [Google Scholar] [CrossRef] [PubMed]
- Filipi, T.; Hermanova, Z.; Tureckova, J.; Vanatko, O.; Anderova, M. Glial Cells—The Strategic Targets in Amyotrophic Lateral Sclerosis Treatment. J. Clin. Med. 2020, 9, 261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pehar, M.; Harlan, B.A.; Killoy, K.M.; Vargas, M.R. Role and Therapeutic Potential of Astrocytes in Amyotrophic Lateral Sclerosis. Curr. Pharm. Des. 2017, 23, 5010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerveró, C.; Blasco, A.; Tarabal, O.; Casanovas, A.; Piedrafita, L.; Navarro, X.; Esquerda, J.E.; Calderó, J. Glial Activation and Central Synapse Loss, but Not Motoneuron Degeneration, Are Prevented by the Sigma-1 Receptor Agonist PRE-084 in the Smn2B/- Mouse Model of Spinal Muscular Atrophy. J. Neuropathol. Exp. Neurol. 2018, 77, 577–597. [Google Scholar] [CrossRef] [Green Version]
- Díaz, J.L.; Cuevas, F.; Oliva, A.I.; Font, D.; Sarmentero, M.Á.; Álvarez-Bercedo, P.; López-Valbuena, J.M.; Pericàs, M.A.; Enrech, R.; Montero, A.; et al. Tricyclic Triazoles as σ 1 Receptor Antagonists for Treating Pain. J. Med. Chem. 2021, 64, 5157–5170. [Google Scholar] [CrossRef]
- Ortega-Roldan, J.L.; Ossa, F.; Schnell, J.R. Characterization of the Human Sigma-1 Receptor Chaperone Domain Structure and Binding Immunoglobulin Protein (BiP) Interactions. J. Biol. Chem. 2013, 288, 21448–21457. [Google Scholar] [CrossRef] [Green Version]
- Mòdol-Caballero, G.; Santos, D.; Navarro, X.; Herrando-Grabulosa, M. Neuregulin 1 Reduces Motoneuron Cell Death and Promotes Neurite Growth in an in Vitro Model of Motoneuron Degeneration. Front. Cell. Neurosci. 2017, 11, 431. [Google Scholar] [CrossRef]
- Mancuso, R.; Oliván, S.; Mancera, P.; Pastén-Zamorano, A.; Manzano, R.; Casas, C.; Osta, R.; Navarro, X. Effect of Genetic Background on Onset and Disease Progression in the SOD1-G93A Model of Amyotrophic Lateral Sclerosis. Amyotroph. Lateral Scler. 2012, 13, 302–310. [Google Scholar] [CrossRef]
- Mancuso, R.; Santos-Nogueira, E.; Osta, R.; Navarro, X. Electrophysiological Analysis of a Murine Model of Motoneuron Disease. Clin. Neurophysiol. 2011, 122, 1660–1670. [Google Scholar] [CrossRef] [PubMed]
- Miana-Mena, F.J.; Muñoz, M.J.; Yagüe, G.; Mendez, M.; Moreno, M.; Ciriza, J.; Zaragoza, P.; Osta, R. Optimal methods to characterize the G93A mouse model of ALS. Amyotroph. Lateral Scler. 2005, 6, 55–62. [Google Scholar] [CrossRef] [PubMed]

| Model | Compound | Dose (mg/kg) | Plasma Concentration (ng/mL)15 min Post-Administration | |
|---|---|---|---|---|
| Mean | SD | |||
| Spinal Nerve Injury female mouse | EST79232 | 0.5 | 20 | 8 |
| 5 | 265 | 53 | ||
| EST79376 | 0.5 | 21 | 7 | |
| 5 | 592 | 176 | ||
| PRE-084 a | 0.25 | 3.6 | 0.8 | |
| Transgenic SOD1G93A male mouse | EST79232 | 5 | 425 | 129 |
| EST79376 | 5 | 692 | 206 | |
| PRE-084 a | 0.25 | 2.6 | 1.3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaja-Capdevila, N.; Hernández, N.; Yeste, S.; Reinoso, R.F.; Burgueño, J.; Montero, A.; Merlos, M.; Vela, J.M.; Herrando-Grabulosa, M.; Navarro, X. EST79232 and EST79376, Two Novel Sigma-1 Receptor Ligands, Exert Neuroprotection on Models of Motoneuron Degeneration. Int. J. Mol. Sci. 2022, 23, 6737. https://doi.org/10.3390/ijms23126737
Gaja-Capdevila N, Hernández N, Yeste S, Reinoso RF, Burgueño J, Montero A, Merlos M, Vela JM, Herrando-Grabulosa M, Navarro X. EST79232 and EST79376, Two Novel Sigma-1 Receptor Ligands, Exert Neuroprotection on Models of Motoneuron Degeneration. International Journal of Molecular Sciences. 2022; 23(12):6737. https://doi.org/10.3390/ijms23126737
Chicago/Turabian StyleGaja-Capdevila, Núria, Neus Hernández, Sandra Yeste, Raquel F. Reinoso, Javier Burgueño, Ana Montero, Manuel Merlos, José M. Vela, Mireia Herrando-Grabulosa, and Xavier Navarro. 2022. "EST79232 and EST79376, Two Novel Sigma-1 Receptor Ligands, Exert Neuroprotection on Models of Motoneuron Degeneration" International Journal of Molecular Sciences 23, no. 12: 6737. https://doi.org/10.3390/ijms23126737
APA StyleGaja-Capdevila, N., Hernández, N., Yeste, S., Reinoso, R. F., Burgueño, J., Montero, A., Merlos, M., Vela, J. M., Herrando-Grabulosa, M., & Navarro, X. (2022). EST79232 and EST79376, Two Novel Sigma-1 Receptor Ligands, Exert Neuroprotection on Models of Motoneuron Degeneration. International Journal of Molecular Sciences, 23(12), 6737. https://doi.org/10.3390/ijms23126737






