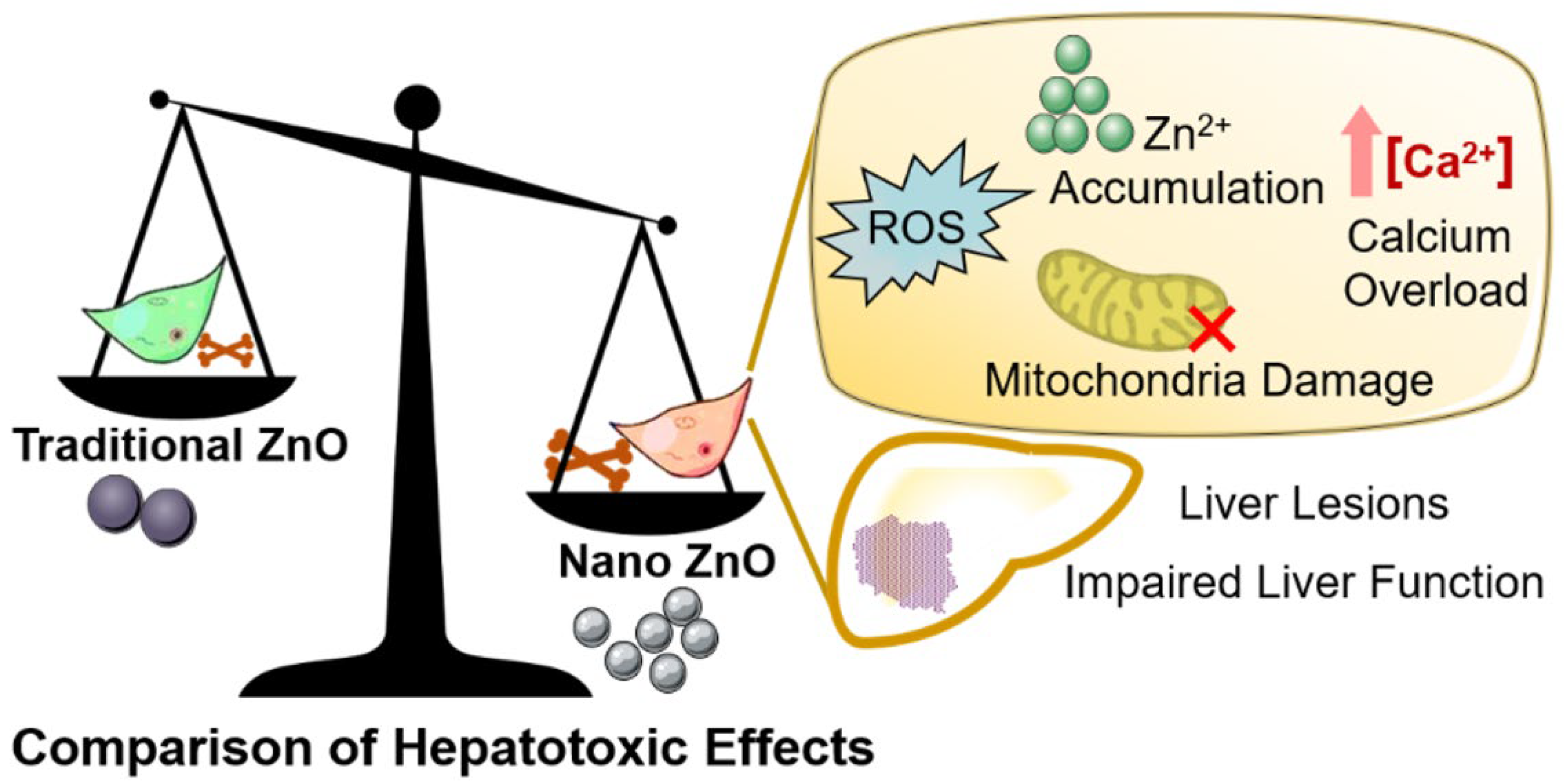
Lethality of Zinc Oxide Nanoparticles Surpasses Conventional Zinc Oxide via Oxidative Stress, Mitochondrial Damage and Calcium Overload: A Comparative Hepatotoxicity Study
Abstract
:1. Introduction
2. Results
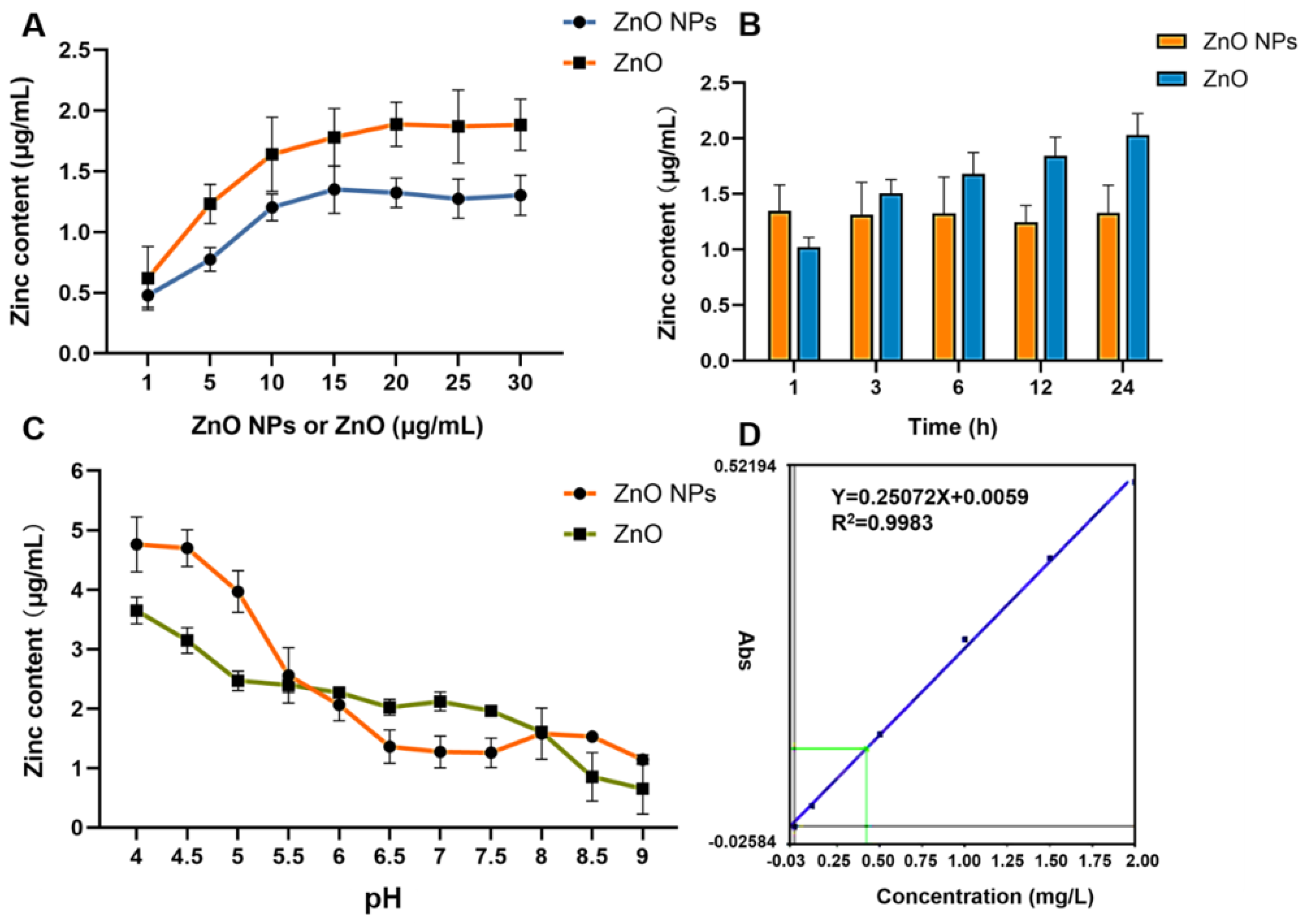
2.1. Physicochemical Properties and Comparisons of Zn Ions
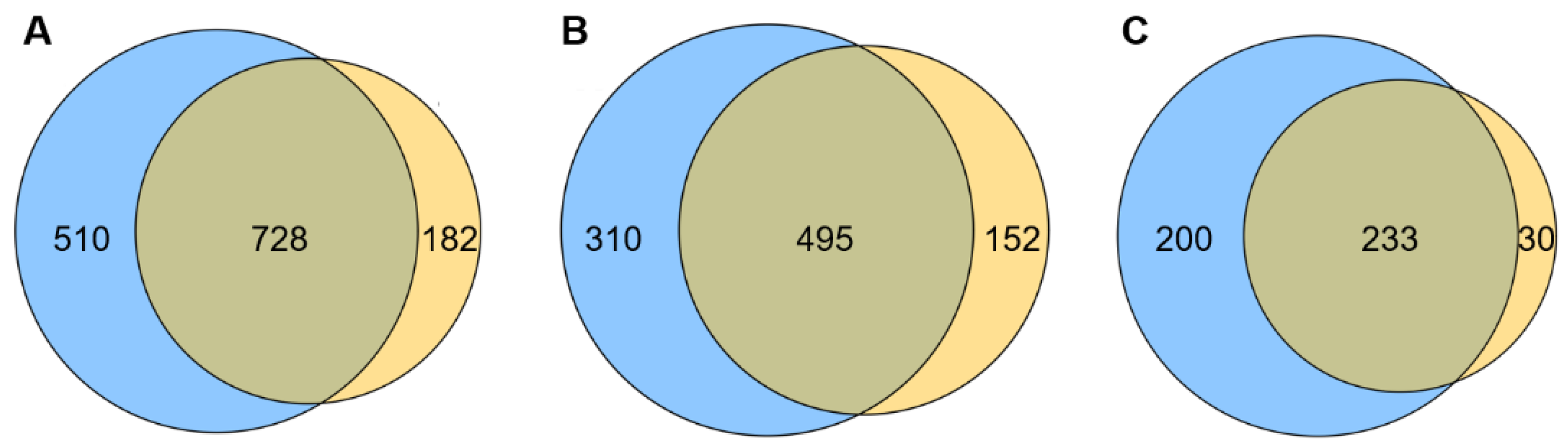
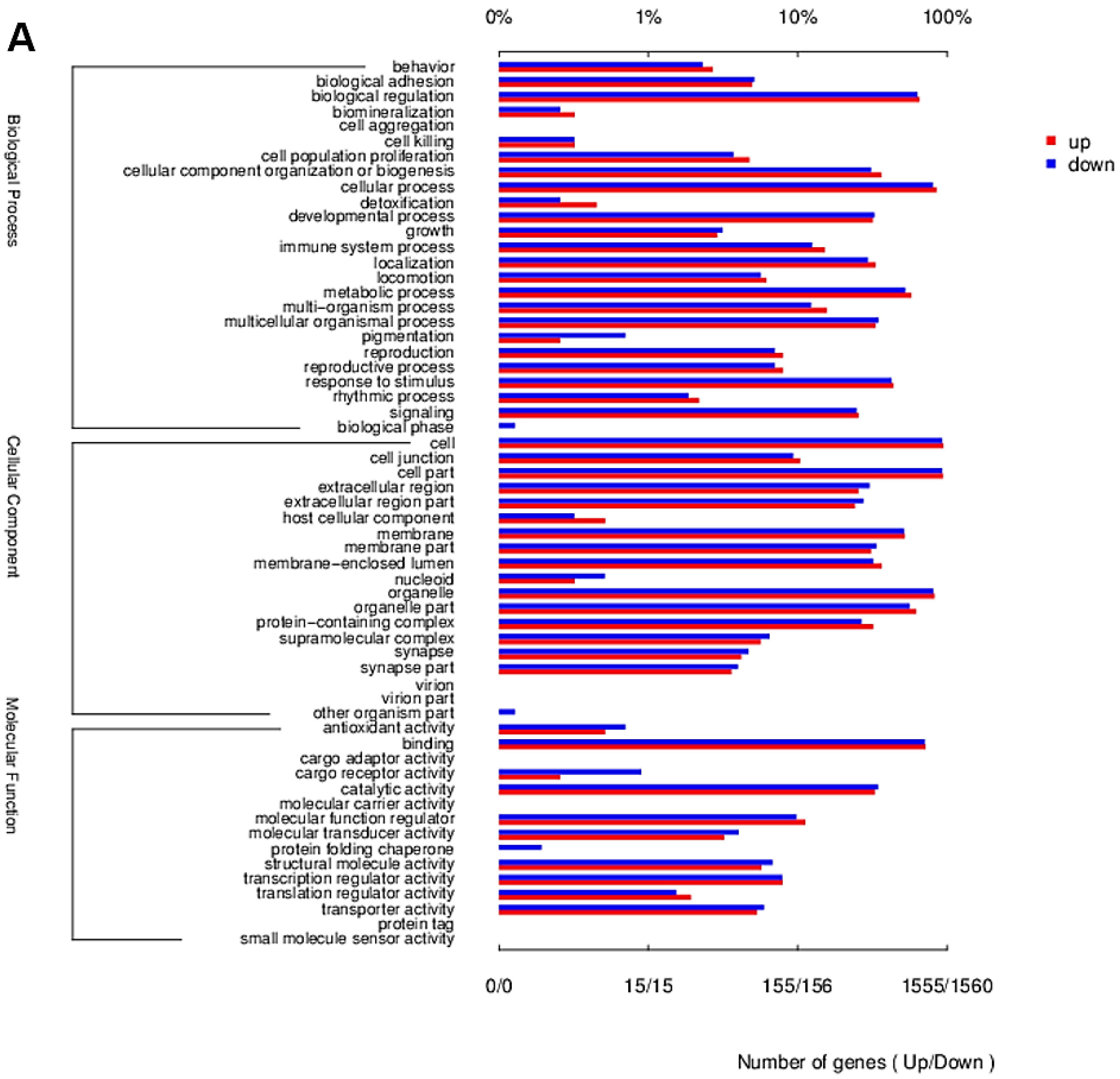
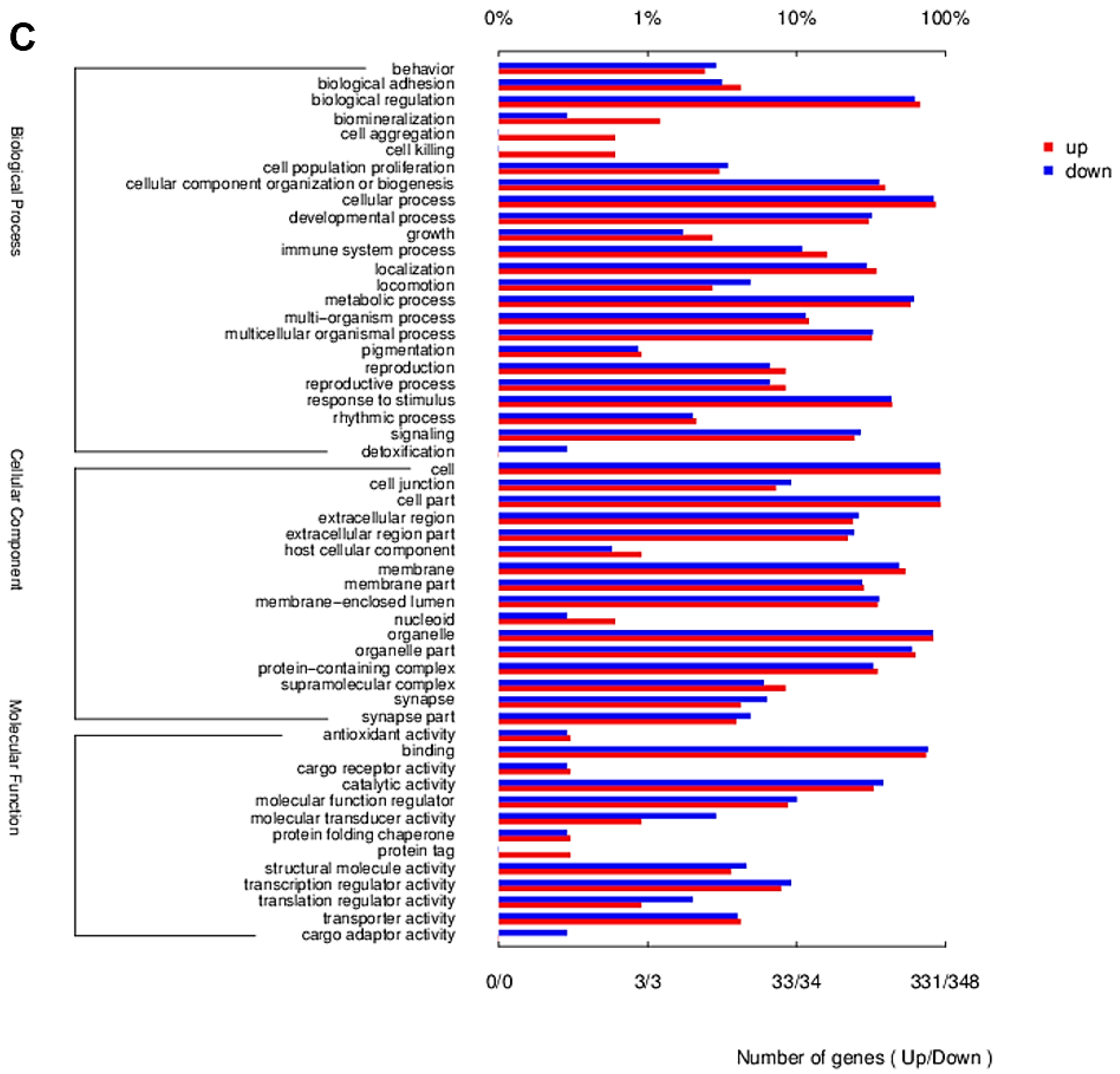
2.2. Transcriptome Contrast
2.3. Cell Inactivation Caused by ZnO NPs and ZnO
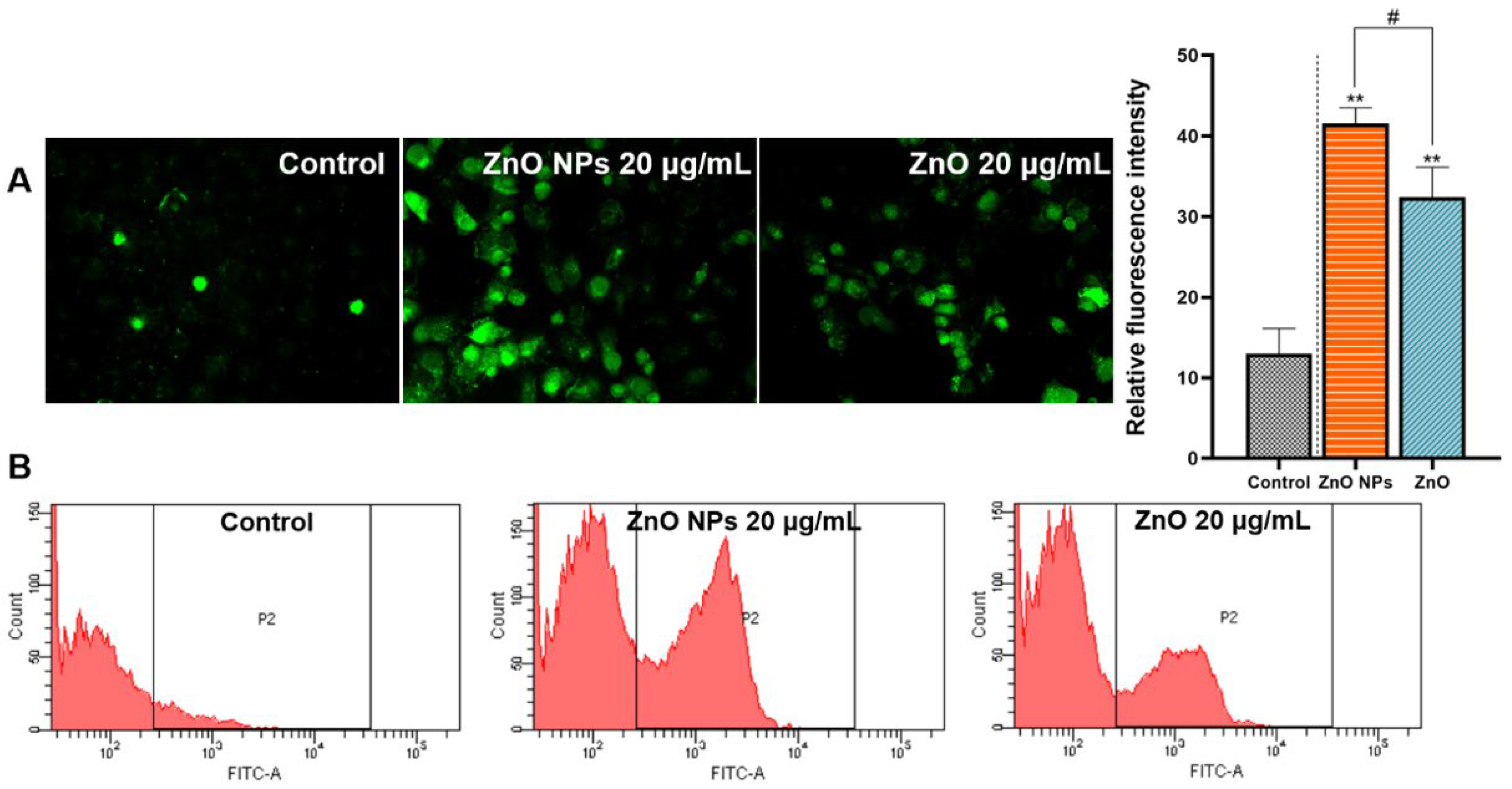
2.4. Comparison of Cellular Oxidative Stress
2.5. Mitochondria Damage Triggered by ZnO NPs and ZnO
2.6. The Different Levels of Ca2+ Accumulation
2.7. Effects on Biochemical Indices and Antioxidant Levels in a Rat Model
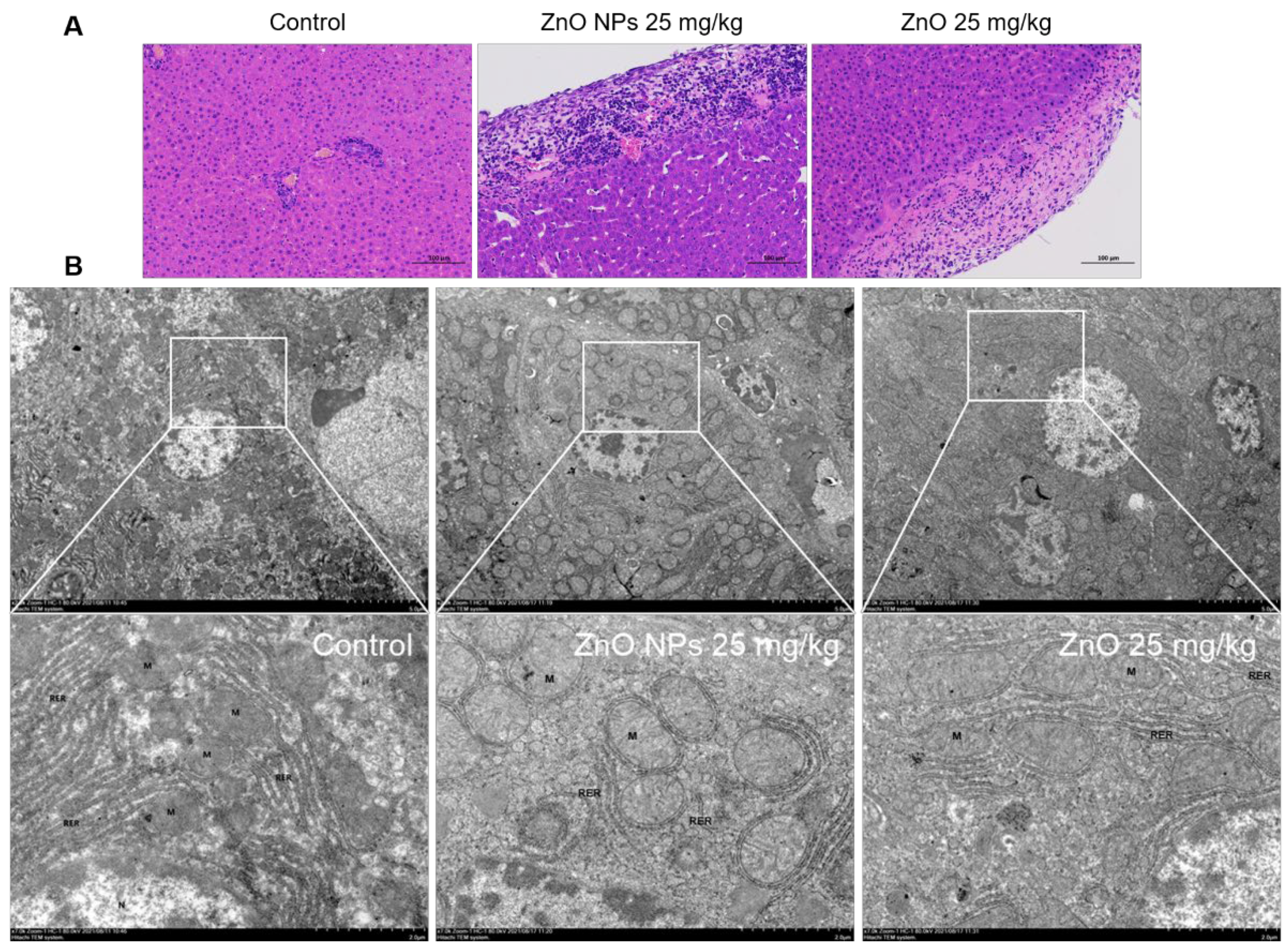
2.8. Comparison of Rat Liver Damage Induced by ZnO NPs and ZnO
3. Discussion
4. Materials and Methods
4.1. Reagents and Chemicals
4.2. ZnO NPs Characterization and Quantitative Analysis
4.3. Animal Exposure
4.4. Histopathological Detection
4.5. Ultrastructural Analyses
4.6. Liver Function Indicator and Oxidative Stress Index
4.7. Cell Culture
4.8. Cell Viability Examination
4.9. Oxidative Stress Measurement
4.10. Mitochondrial Staining
4.11. Ca2+ Flow Assessment
4.12. RNA-Sequencing (RNA-seq) Analysis
4.13. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siddiqi, K.S.; Ur Rahman, A.; Tajuddin; Husen, A. Properties of Zinc Oxide Nanoparticles and Their Activity Against Microbes. Nanoscale Res. Lett. 2018, 13, 141. [Google Scholar] [CrossRef] [PubMed]
- Yusof, H.M.; Mohamad, R.; Zaidan, U.H.; Rahman, N.A.A. Microbial synthesis of zinc oxide nanoparticles and their potential application as an antimicrobial agent and a feed supplement in animal industry: A review. J. Anim. Sci. Biotechnol. 2019, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Swain, P.S.; Rao, S.B.N.; Rajendran, D.; Dominic, G.; Selvaraju, S. Nano zinc, an alternative to conventional zinc as animal feed supplement: A review. Anim. Nutr. 2016, 2, 134–141. [Google Scholar] [CrossRef]
- Madhumitha, G.; Elango, G.; Roopan, S.M. Biotechnological aspects of ZnO nanoparticles: Overview on synthesis and its applications. Appl. Microbiol. Biotechnol. 2016, 100, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, J.; Tongu, M.; Hashimoto, H.; Yamada, T.; Kimura-Kataoka, K.; Yasuda, T.; Fujita, Y.; Takeshita, H. Distribution and toxicity evaluation of ZnO dispersion nanoparticles in single intravenously exposed mice. J. Med. Investig. 2015, 62, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Ogunsuyi, O.; Ogunsuyi, O.; Akanni, O.; Alabi, O.; Alimba, C.; Adaramoye, O.; Cambier, S.; Eswara, S.; Gutleb, A.C.; Bakare, A. Physiological and histopathological alterations in male Swiss mice after exposure to titanium dioxide (anatase) and zinc oxide nanoparticles and their binary mixture. Drug Chem. Toxicol. 2022, 45, 1188–1213. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.R.; Park, J.I.; Lee, E.J.; Park, S.H.; Seong, N.W.; Kim, J.H.; Kim, G.Y.; Meang, E.H.; Hong, J.S.; Kim, S.H.; et al. Toxicity of 100 nm zinc oxide nanoparticles: A report of 90-day repeated oral administration in Sprague Dawley rats. Int. J. Nanomed. 2014, 9, 109–126. [Google Scholar]
- Majeed, A.; Javed, F.; Akhtar, S.; Saleem, U.; Anwar, F.; Ahmad, B.; Nadhman, A.; Shahnaz, G.; Hussain, I.; Hussain, S.Z.; et al. Green synthesized selenium doped zinc oxide nano-antibiotic: Synthesis, characterization and evaluation of antimicrobial, nanotoxicity and teratogenicity potential. J. Mater. Chem. 2020, 8, 8444–8458. [Google Scholar] [CrossRef]
- de Andrade Vieira, J.E.; de Oliveira Ferreira, R.; Dos Reis Sampaio, D.M.; da Costa Araujo, A.P.; Malafaia, G. An insight on the mutagenicity and cytotoxicity of zinc oxide nanoparticles in Gallus gallus domesticus (Phasianidae). Chemosphere 2019, 231, 10–19. [Google Scholar] [CrossRef]
- Barguilla, I.; Barszczewska, G.; Annangi, B.; Domenech, J.; Velazquez, A.; Marcos, R.; Hernandez, A. MTH1 is involved in the toxic and carcinogenic long-term effects induced by zinc oxide and cobalt nanoparticles. Arch. Toxicol. 2020, 94, 1973–1984. [Google Scholar] [CrossRef]
- Lin, Y.F.; Chiu, I.J.; Cheng, F.Y.; Lee, Y.H.; Wang, Y.J.; Hsu, Y.H.; Chiu, H.W. The role of hypoxia-inducible factor-1alpha in zinc oxide nanoparticle-induced nephrotoxicity in vitro and in vivo. Part. Fibre Toxicol. 2016, 13, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baky, N.A.; Faddah, L.M.; Al-Rasheed, N.M.; Al-Rasheed, N.M.; Fatani, A.J. Induction of inflammation, DNA damage and apoptosis in rat heart after oral exposure to zinc oxide nanoparticles and the cardioprotective role of alpha-lipoic acid and vitamin E. Drug Res. 2013, 63, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Amer, M.G.; Karam, R.A. Morphological and Biochemical Features of Cerebellar Cortex After Exposure to Zinc Oxide Nanoparticles: Possible Protective Role of Curcumin. Anat. Rec. 2018, 301, 1454–1466. [Google Scholar]
- Abass, M.A.; Selim, S.A.; Selim, A.O.; El-Shal, A.S.; Gouda, Z.A. Effect of orally administered zinc oxide nanoparticles on albino rat thymus and spleen. IUBMB Life 2017, 69, 528–539. [Google Scholar] [CrossRef]
- Kuang, H.; Zhang, W.; Yang, L.; Aguilar, Z.P.; Xu, H. Reproductive organ dysfunction and gene expression after orally administration of ZnO nanoparticles in murine. Environ. Toxicol. 2021, 36, 550–561. [Google Scholar] [CrossRef]
- Chen, A.; Feng, X.; Sun, T.; Zhang, Y.; An, S.; Shao, L. Evaluation of the effect of time on the distribution of zinc oxide nanoparticles in tissues of rats and mice: A systematic review. IET Nanobiotechnol. 2016, 10, 97–106. [Google Scholar] [CrossRef]
- Watson, C.Y.; Molina, R.M.; Louzada, A.; Murdaugh, K.M.; Donaghey, T.C.; Brain, J.D. Effects of zinc oxide nanoparticles on Kupffer cell phagosomal motility, bacterial clearance, and liver function. Int. J. Nanomed. 2015, 10, 4173–4184. [Google Scholar] [CrossRef] [Green Version]
- Park, H.S.; Shin, S.S.; Meang, E.H.; Hong, J.S.; Park, J.I.; Kim, S.H.; Koh, S.B.; Lee, S.Y.; Jang, D.H.; Lee, J.Y.; et al. A 90-day study of subchronic oral toxicity of 20 nm, negatively charged zinc oxide nanoparticles in Sprague Dawley rats. Int. J. Nanomed. 2014, 9, 79–92. [Google Scholar]
- Nazir, S.; Rabbani, A.; Mehmood, K.; Maqbool, F.; Shah, G.M.; Khan, M.F.; Sajid, M. Antileishmanial activity and cytotoxicity of ZnO-based nano-formulations. Int. J. Nanomed. 2019, 14, 7809–7822. [Google Scholar] [CrossRef] [Green Version]
- Beegam, A.; Lopes, M.; Fernandes, T.; Jose, J.; Barreto, A.; Oliveira, M.; Soares, A.; Trindade, T.; Thomas, S.; Pereira, M.L. Multiorgan histopathological changes in the juvenile seabream Sparus aurata as a biomarker for zinc oxide particles toxicity. Environ. Sci. Pollut. Res. Int. 2020, 27, 30907–30917. [Google Scholar] [CrossRef]
- Germain, R.N.; Margulies, D.H. The biochemistry and cell biology of antigen processing and presentation. Annu. Rev. Immunol. 1993, 11, 403–450. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Singh, S.K.; Das, M.; Tripathi, A.; Dwivedi, P.D. Toll-like receptor 6 mediated inflammatory and functional responses of zinc oxide nanoparticles primed macrophages. Immunology 2014, 142, 453–464. [Google Scholar] [CrossRef]
- Srivastav, A.K.; Dhiman, N.; Tiwari, R.; Arjaria, N.; Prakash, J.; Jagdale, P.; Ayanur, A.; Singh, D.; Patnaik, S.; Kumar, M. Sub-acute oral exposure of zinc oxide nanoparticles causes alteration in iron homeostasis through acute phase response: A protective effect by surface modification. J. Trace Elem. Med. Biol. 2019, 52, 270–287. [Google Scholar] [CrossRef] [PubMed]
- Esmaeillou, M.; Moharamnejad, M.; Hsankhani, R.; Tehrani, A.A.; Maadi, H. Toxicity of ZnO nanoparticles in healthy adult mice. Environ. Toxicol. Pharmacol. 2013, 35, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Al-Rasheed, N.M.; Al-Rasheed, N.M.; Baky, N.A.A.; Faddah, L.M.; Fatani, A.J.; Hasan, I.H.; Mohamad, R.A. Prophylactic role of alpha-lipoic acid and vitamin E against zinc oxide nanoparticles induced metabolic and immune disorders in rat’s liver. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 1813–1828. [Google Scholar]
- Yousef, M.I.; Mutar, T.F.; Kamel, M.A.E. Hepato-renal toxicity of oral sub-chronic exposure to aluminum oxide and/or zinc oxide nanoparticles in rats. Toxicol. Rep. 2019, 6, 336–346. [Google Scholar] [CrossRef]
- Jarrar, Y.; Al-Doaiss, A.; Alfaifi, M.; Shati, A.; Al-Kahtani, M.; Jarrar, B. The influence of five metallic nanoparticles on the expression of major drug-metabolizing enzyme genes with correlation of inflammation in mouse livers. Environ. Toxicol. Pharmacol. 2020, 80, 103449. [Google Scholar] [CrossRef]
- Ezhuthupurakkal, P.B.; Ariraman, S.; Arumugam, S.; Subramaniyan, N.; Muthuvel, S.K.; Kumpati, P.; Rajamani, B.; Chinnasamy, T. Anticancer potential of ZnO nanoparticle-ferulic acid conjugate on Huh-7 and HepG2 cells and diethyl nitrosamine induced hepatocellular cancer on Wistar albino rat. Nanomedicine 2018, 14, 415–428. [Google Scholar] [CrossRef]
- Li, J.; Song, Y.; Vogt, R.D.; Liu, Y.; Luo, J.; Li, T. Bioavailability and cytotoxicity of Cerium- (IV), Copper- (II), and Zinc oxide nanoparticles to human intestinal and liver cells through food. Sci. Total Environ. 2020, 702, 134700. [Google Scholar] [CrossRef]
- El-Shorbagy, H.M.; Eissa, S.M.; Sabet, S.; El-Ghor, A.A. Apoptosis and oxidative stress as relevant mechanisms of antitumor activity and genotoxicity of ZnO-NPs alone and in combination with N-acetyl cysteine in tumor-bearing mice. Int. J. Nanomed. 2019, 14, 3911–3928. [Google Scholar] [CrossRef] [Green Version]
- Nel, A.; Xia, T.; Madler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seok, S.H.; Cho, W.S.; Park, J.S.; Na, Y.; Jang, A.; Kim, H.; Cho, Y.; Kim, T.; You, J.R.; Ko, S.; et al. Rat pancreatitis produced by 13-week administration of zinc oxide nanoparticles: Biopersistence of nanoparticles and possible solutions. J. Appl. Toxicol. 2013, 33, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.W.; Mudunkotuwa, I.A.; Rupasinghe, T.; Grassian, V.H. Aggregation and dissolution of 4 nm ZnO nanoparticles in aqueous environments: Influence of pH, ionic strength, size, and adsorption of humic acid. Langmuir 2011, 27, 6059–6068. [Google Scholar] [CrossRef] [PubMed]
- Bellmann, S.; Carlander, D.; Fasano, A.; Momcilovic, D.; Scimeca, J.A.; Waldman, W.J.; Gombau, L.; Tsytsikova, L.; Canady, R.; Pereira, D.I.; et al. Mammalian gastrointestinal tract parameters modulating the integrity, surface properties, and absorption of food-relevant nanomaterials. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 609–622. [Google Scholar] [CrossRef]
- Yan, X.; Rong, R.; Zhu, S.; Guo, M.; Gao, S.; Wang, S.; Xu, X. Effects of ZnO Nanoparticles on Dimethoate-Induced Toxicity in Mice. J. Agric. Food. Chem. 2015, 63, 8292–8298. [Google Scholar] [CrossRef]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef]
- Huang, L.; Tepaamorndech, S. The SLC30 family of zinc transporters—A review of current understanding of their biological and pathophysiological roles. Mol. Asp. Med. 2013, 34, 548–560. [Google Scholar] [CrossRef]
- Singh, S. Zinc oxide nanoparticles impacts: Cytotoxicity, genotoxicity, developmental toxicity, and neurotoxicity. Toxicol. Mech. Methods 2019, 29, 300–311. [Google Scholar] [CrossRef]
- Vandebriel, R.J.; De Jong, W.H. A review of mammalian toxicity of ZnO nanoparticles. Nanotechnol. Sci. Appl. 2012, 5, 61–71. [Google Scholar] [CrossRef] [Green Version]
- Jia, L.; Yiyuan, K.; Wei, Z.; Bin, S.; Limin, W.; Liangjiao, C.; Longquan, S. Ion-shedding zinc oxide nanoparticles induce microglial BV2 cell proliferation via the ERK and Akt signaling pathways. Toxicol. Sci. 2017, 156, 167–178. [Google Scholar] [CrossRef] [Green Version]
- Sudhakaran, S.; Athira, S.S.; Mohanan, P.V. Zinc oxide nanoparticle induced neurotoxic potential upon interaction with primary astrocytes. Neurotoxicology 2019, 73, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Chen, L.; Hao, J.; Zhong, N. Bioaccumulation and sub-acute toxicity of zinc oxide nanoparticles in juvenile carp (Cyprinus carpio): A comparative study with its bulk counterparts. Ecotoxicol. Environ. Saf. 2013, 91, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Kielbik, P.; Kaszewski, J.; Rosowska, J.; Wolska, E.; Witkowski, B.S.; Gralak, M.A.; Gajewski, Z.; Godlewski, M.; Godlewski, M.M. Biodegradation of the ZnO:Eu nanoparticles in the tissues of adult mouse after alimentary application. Nanomedicine 2017, 13, 843–852. [Google Scholar] [CrossRef]
- Czyzowska, A.; Barbasz, A. A review: Zinc oxide nanoparticles—Friends or enemies? Int. J. Environ. Health Res. 2022, 32, 885–901. [Google Scholar] [CrossRef] [PubMed]
- Sruthi, S.; Ashtami, J.; Mohanan, P.V. Biomedical application and hidden toxicity of Zinc oxide nanoparticles. Mater. Today Chem. 2018, 10, 175–186. [Google Scholar] [CrossRef]
- Hou, J.; Wu, Y.; Li, X.; Wei, B.; Li, S.; Wang, X. Toxic effects of different types of zinc oxide nanoparticles on algae, plants, invertebrates, vertebrates and microorganisms. Chemosphere 2018, 193, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Rajput, V.; Minkina, T.; Sushkova, S.; Behal, A.; Maksimov, A.; Blicharska, E.; Ghazaryan, K.; Movsesyan, H.; Barsova, N. ZnO and CuO nanoparticles: A threat to soil organisms, plants, and human health. Environ. Geochem. Health 2020, 42, 147–158. [Google Scholar] [CrossRef]
- Ma, H.; Williams, P.L.; Diamond, S.A. Ecotoxicity of manufactured ZnO nanoparticles—A review. Environ. Pollut. 2013, 172, 76–85. [Google Scholar] [CrossRef]
- Abedini, M.; Shariatmadari, F.; Torshizi, M.A.K.; Ahmadi, H. Effects of zinc oxide nanoparticles on the egg quality, immune response, zinc retention, and blood parameters of laying hens in the late phase of production. J. Anim. Physiol. Anim. Nutr. 2018, 102, 736–745. [Google Scholar] [CrossRef]
- Long, L.; Chen, J.; Zhang, Y.; Liang, X.; Ni, H.; Zhang, B.; Yin, Y. Comparison of porous and nano zinc oxide for replacing high-dose dietary regular zinc oxide in weaning piglets. PLoS ONE 2017, 12, e0182550. [Google Scholar]
- Pei, X.; Xiao, Z.; Liu, L.; Wang, G.; Tao, W.; Wang, M.; Zou, J.; Leng, D. Effects of dietary zinc oxide nanoparticles supplementation on growth performance, zinc status, intestinal morphology, microflora population, and immune response in weaned pigs. J. Sci. Food Agric. 2019, 99, 1366–1374. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, L.; Ying, Z.; He, J.; Zhou, L.; Zhang, L.; Zhong, X.; Wang, T. Effects of Dietary Zinc Oxide Nanoparticles on Growth, Diarrhea, Mineral Deposition, Intestinal Morphology, and Barrier of Weaned Piglets. Biol. Trace Elem. Res. 2018, 185, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, L.; Su, W.; Ying, Z.; He, J.; Zhang, L.; Zhong, X.; Wang, T. Zinc oxide nanoparticles as a substitute for zinc oxide or colistin sulfate: Effects on growth, serum enzymes, zinc deposition, intestinal morphology and epithelial barrier in weaned piglets. PLoS ONE 2017, 12, e0181136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Song, W.; Guo, J.; Zhang, J.; Sun, Z.; Ding, F.; Gao, M. Toxic effect of different ZnO particles on mouse alveolar macrophages. J. Hazard. Mater. 2012, 219, 148–155. [Google Scholar] [CrossRef]
- Tian, L.; Wang, K.; Liu, H.; Li, K.; Lin, B.; Fang, Z.; Han, J.; Li, N.; Yang, H.; Bian, L.; et al. UCH-L1 mitigates neurotoxicity induced by ZnO particles via stabilizing the inhibitor of NF-kappa B signaling, IkappaB-alpha. Ecotoxicol. Environ. Saf. 2019, 180, 259–268. [Google Scholar] [CrossRef]
- Jin, M.; Li, N.; Sheng, W.; Ji, X.; Liang, X.; Kong, B.; Yin, P.; Li, Y.; Zhang, X.; Liu, K. Toxicity of different zinc oxide nanomaterials and dose-dependent onset and development of Parkinson’s disease-like symptoms induced by zinc oxide nanorods. Environ. Int. 2021, 146, 106179. [Google Scholar] [CrossRef]
- Mittag, A.; Hoera, C.; Kampfe, A.; Westermann, M.; Kuckelkorn, J.; Schneider, T.; Glei, M. Cellular Uptake and Toxicological Effects of Differently Sized Zinc Oxide Nanoparticles in Intestinal Cells. Toxics 2021, 9, 96. [Google Scholar] [CrossRef]
- Hsiao, I.L.; Huang, Y.J. Effects of various physicochemical characteristics on the toxicities of ZnO and TiO nanoparticles toward human lung epithelial cells. Sci. Total Environ. 2011, 409, 1219–1228. [Google Scholar] [CrossRef]
- Fan, W.; Li, Q.; Yang, X.; Zhang, L. Zn subcellular distribution in liver of goldfish (Carassius auratus) with exposure to zinc oxide nanoparticles and mechanism of hepatic detoxification. PLoS ONE 2013, 8, e78123. [Google Scholar]
- Kteeba, S.M.; El-Ghobashy, A.E.; El-Adawi, H.I.; El-Rayis, O.A.; Sreevidya, V.S.; Guo, L.; Svoboda, K.R. Exposure to ZnO nanoparticles alters neuronal and vascular development in zebrafish: Acute and transgenerational effects mitigated with dissolved organic matter. Environ. Pollut. 2018, 242, 433–448. [Google Scholar] [CrossRef]
- Park, E.J.; Jeong, U.; Yoon, C.; Kim, Y. Comparison of distribution and toxicity of different types of zinc-based nanoparticles. Environ. Toxicol. 2017, 32, 1363–1374. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Luo, Y.; Zhang, P.; Chetwynd, A.J.; Xie, H.Q.; Monikh, F.A.; Tao, W.; Xie, C.; Liu, Y.; Xu, L.; et al. Deciphering the particle specific effects on metabolism in rat liver and plasma from ZnO nanoparticles versus ionic Zn exposure. Environ. Int. 2020, 136, 105437. [Google Scholar] [CrossRef] [PubMed]







| ZnO NPs Dosage | Toxicity Classification | Species/Cell Type | Exposure/Assay | Observations | Ref. |
|---|---|---|---|---|---|
| 0.05, 0.2 mg/kg | Acute toxicity | Mice | Single intravenous exposure | Accumulation to spleen, liver and lungs; increase of 8-OHdG. LD50: 0.3 mg/kg | [5] |
| 9.38, 18.75, 37.50, 75.00, 150.00 mg/kg | Acute toxicity | Mice | Intraperitoneal injection for 5 d and 10 d | Histopathological damage to kidneys, spleen, heart, and brain; effects on hematological and biochemical parameters. LD50: 299.9 mg/kg | [6] |
| 31.25, 125, 500 mg/kg | Subchronic toxicity | Rat | Oral gavage for 90 d | Inflammatory damage to stomach, pancreas, eye, prostate gland tissues. | [7] |
| 2000 mg/kg | Teratogenicity | Rat | Oral gavage | Fetal malformation, skeletal dysplasia and spinal insufficiency. | [8] |
| 0.245, 245.26 mg/kg | Mutagenicity | Chicks | Intraperitoneal injection for 2 d | Induction of micronucleus, binucleus and heteromorphus in erythrocytes. | [9] |
| 1 µg/mL | Carcinogenic risk | Mice embryonic fibroblasts | Exposure for 2 and 12 weeks | Upregulation of the early biomarkers of carcinogenesis, MTH1. | [10] |
| 10 mg/kg | Nephrotoxicity | Mice | Single intraperitoneal injection | Renal tubule and glomerulus damage, increase of serum creatinine and blood urea nitrogen; HIF-1α-mediated apoptosis and autophagy. | [11] |
| 600, 1000 mg/kg | Cardiotoxicity | Rat | Oral gavage for 5 d | Increase of troponin-T, creatine kinase-MB, myoglobin, TNF-α, IL-6, cardiac calcium concentration, DNA damage and Caspase-3 activity. | [12] |
| 5.6 mg/kg | Neurotoxicity | Mice | Intraperitoneal injection 3 times per week for 28 d | Damage to blood brain barrier; disorder of nerve cell arrangement; neuronal degeneration; nistenite loss. | [13] |
| 350 mg/kg | Immunotoxicity | Rat | Oral gavage for 28 d | Increase of MDA, IL-1β, TNF-α, TLR4 and TLR6; increase of apoptotic bodies and tingible body macrophages; appearance of abnormal thymocytes; | [14] |
| 100 mg/kg | Reproductive toxicity | Mice | Oral gavage for 3 d | Induction of oxidative stress and apoptosis; weight loss of male mice testicular; activation of Shh pathway in ovaries; Caspase-related uteri injury. | [15] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, X.; Jiang, H.; Xu, G.; Li, C.; Li, D.; Tang, S. Lethality of Zinc Oxide Nanoparticles Surpasses Conventional Zinc Oxide via Oxidative Stress, Mitochondrial Damage and Calcium Overload: A Comparative Hepatotoxicity Study. Int. J. Mol. Sci. 2022, 23, 6724. https://doi.org/10.3390/ijms23126724
Pei X, Jiang H, Xu G, Li C, Li D, Tang S. Lethality of Zinc Oxide Nanoparticles Surpasses Conventional Zinc Oxide via Oxidative Stress, Mitochondrial Damage and Calcium Overload: A Comparative Hepatotoxicity Study. International Journal of Molecular Sciences. 2022; 23(12):6724. https://doi.org/10.3390/ijms23126724
Chicago/Turabian StylePei, Xingyao, Haiyang Jiang, Gang Xu, Cun Li, Daowen Li, and Shusheng Tang. 2022. "Lethality of Zinc Oxide Nanoparticles Surpasses Conventional Zinc Oxide via Oxidative Stress, Mitochondrial Damage and Calcium Overload: A Comparative Hepatotoxicity Study" International Journal of Molecular Sciences 23, no. 12: 6724. https://doi.org/10.3390/ijms23126724
APA StylePei, X., Jiang, H., Xu, G., Li, C., Li, D., & Tang, S. (2022). Lethality of Zinc Oxide Nanoparticles Surpasses Conventional Zinc Oxide via Oxidative Stress, Mitochondrial Damage and Calcium Overload: A Comparative Hepatotoxicity Study. International Journal of Molecular Sciences, 23(12), 6724. https://doi.org/10.3390/ijms23126724







