Retrograde Axonal Transport of Liposomes from Peripheral Tissue to Spinal Cord and DRGs by Optimized Phospholipid and CTB Modification
Abstract
1. Introduction
2. Results
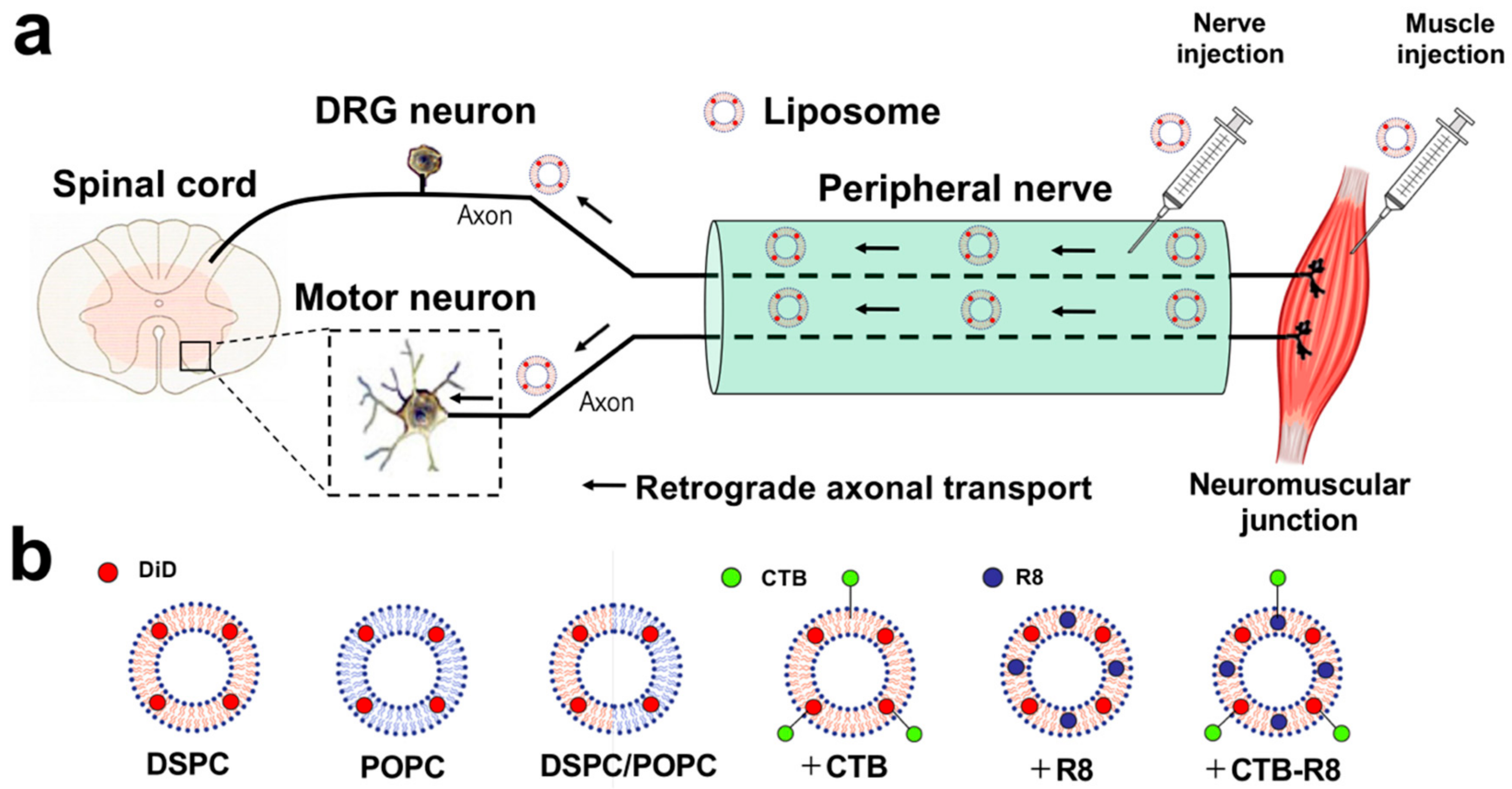
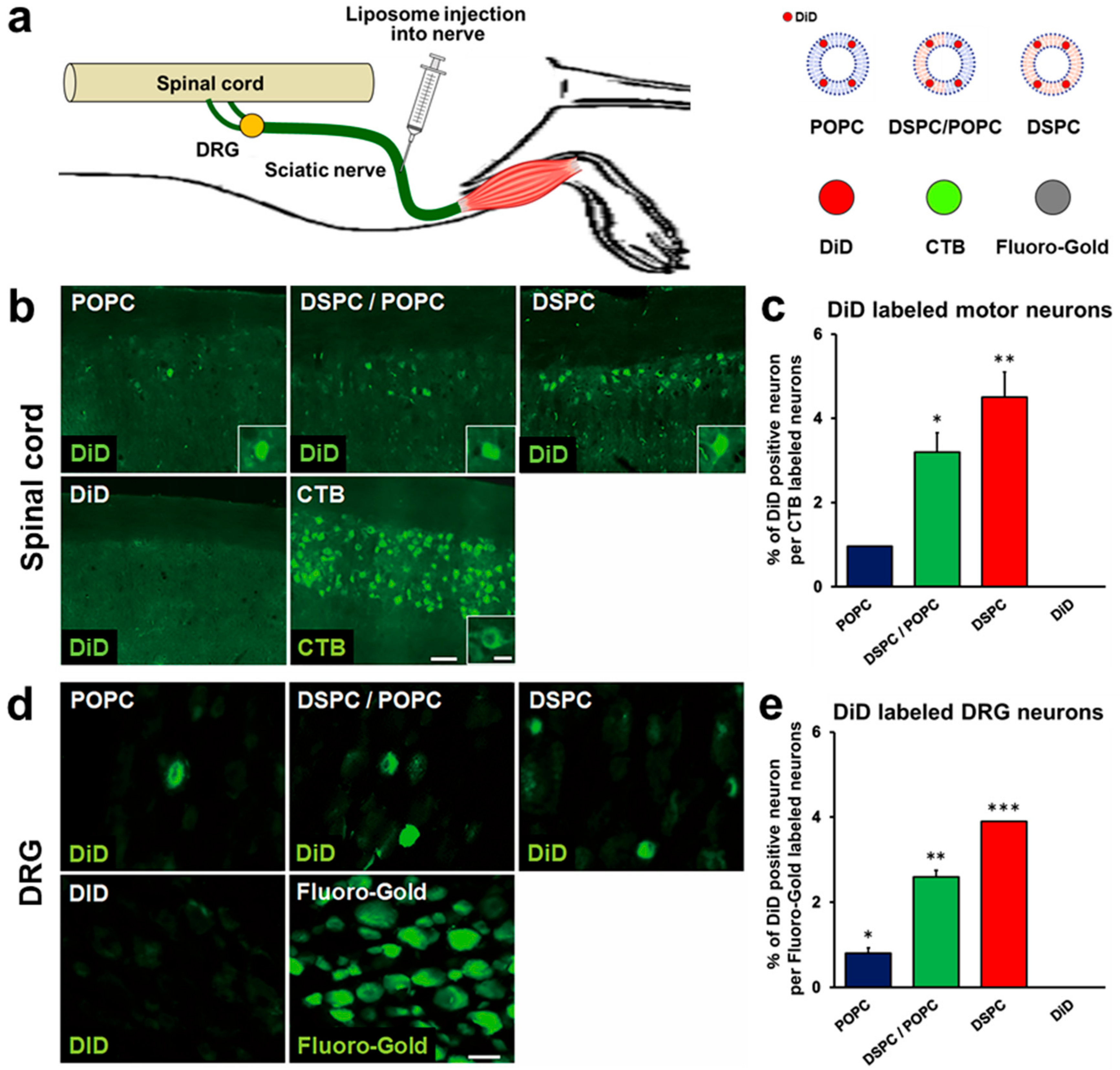
2.1. Liposomes Consisting of DSPC, Chol, and PEG Lipid Are Retrogradely Transported from Peripheral Nerves to the Spinal Cord and DRG
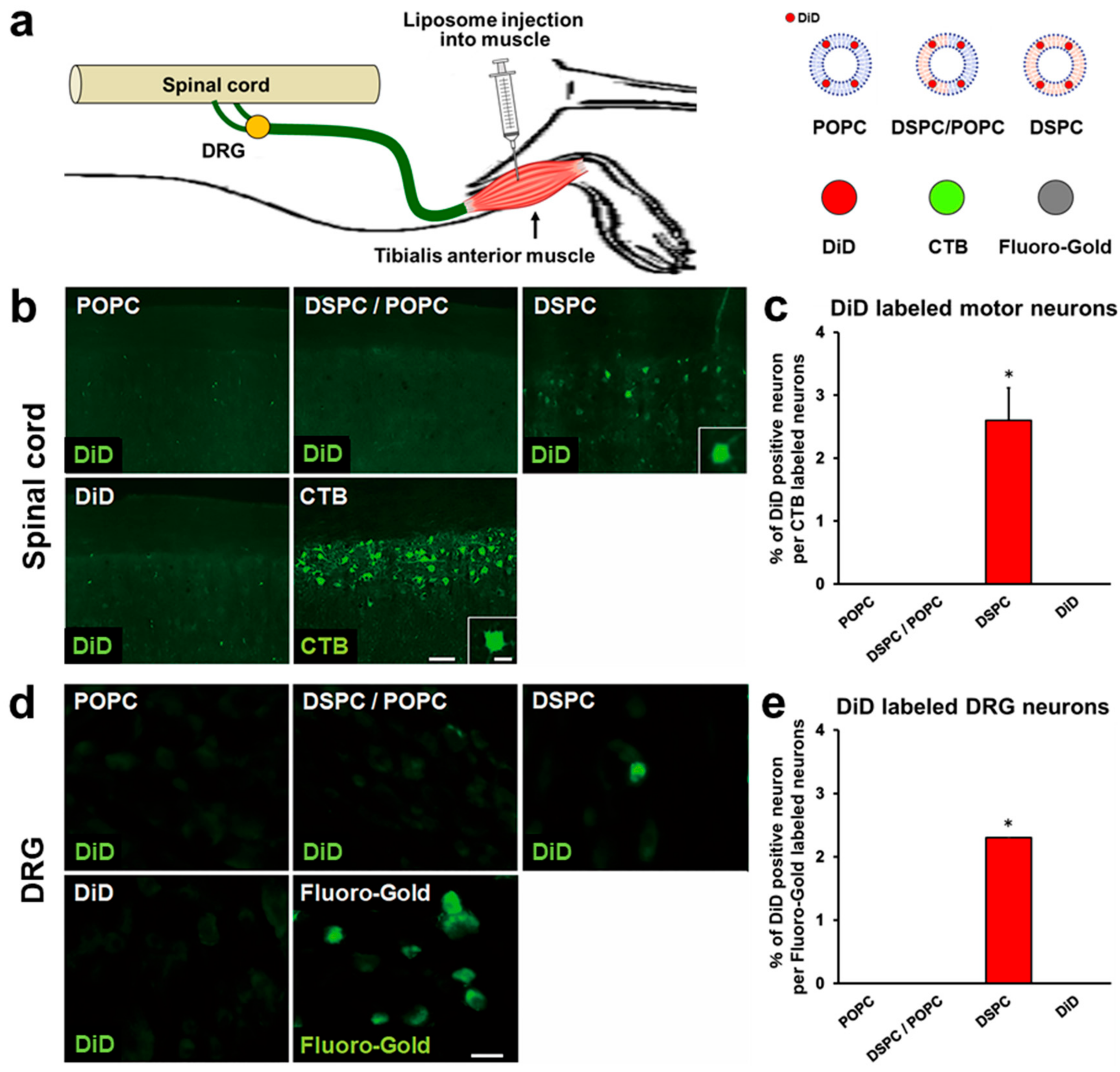
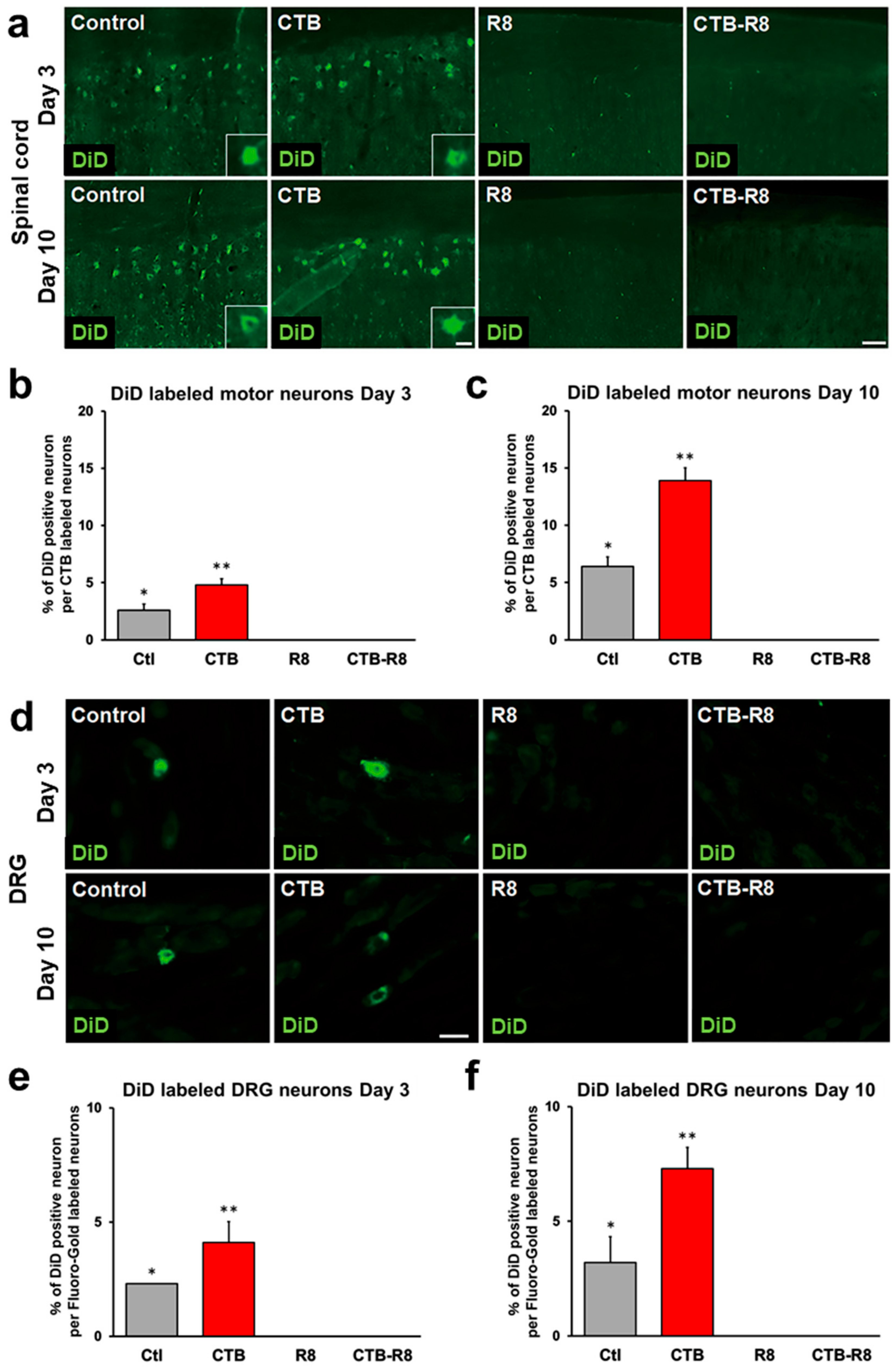
2.2. Liposomes Consisting of DSPC, Chol, and PEG Lipid Are Retrogradely Transported from Muscle to Spinal Cord and DRGs
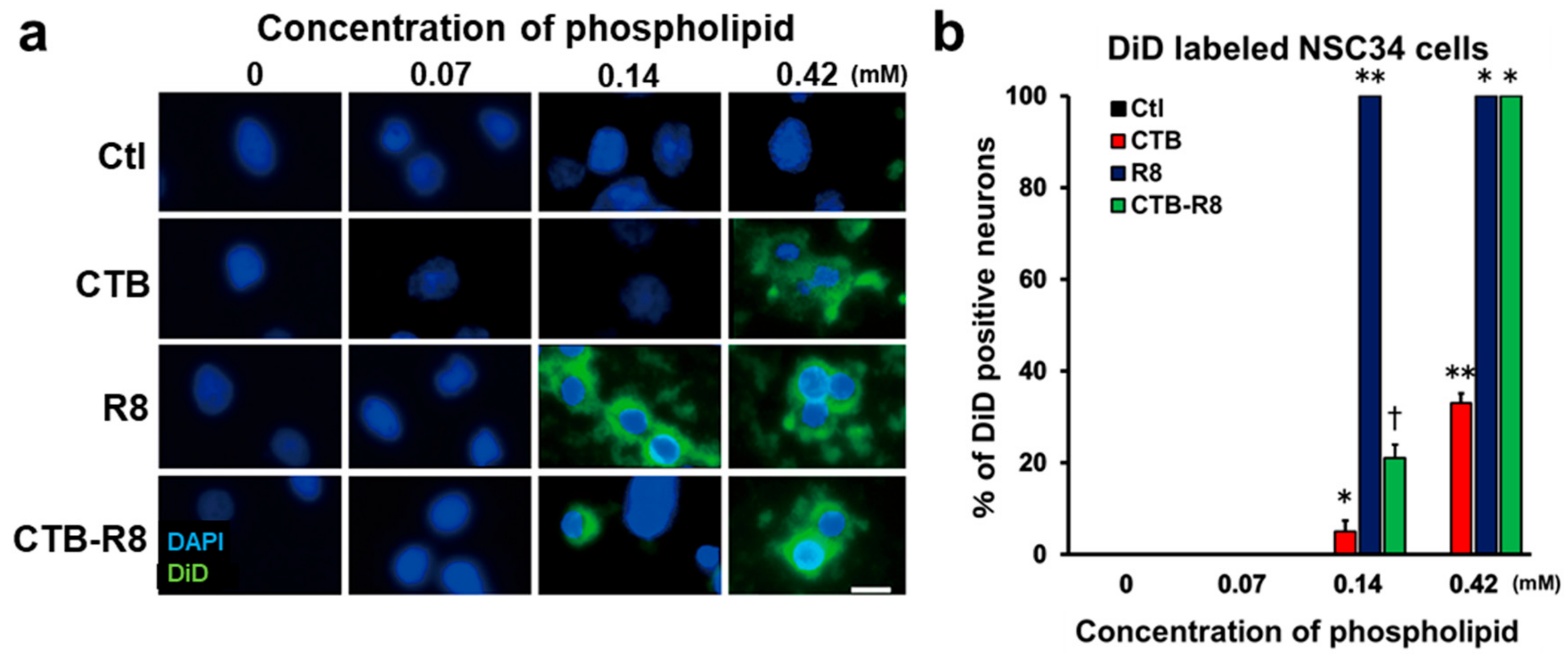
2.3. Modification with R8 and CTB Improves Uptake of Liposomes by Cultured Spinal Motor Neurons
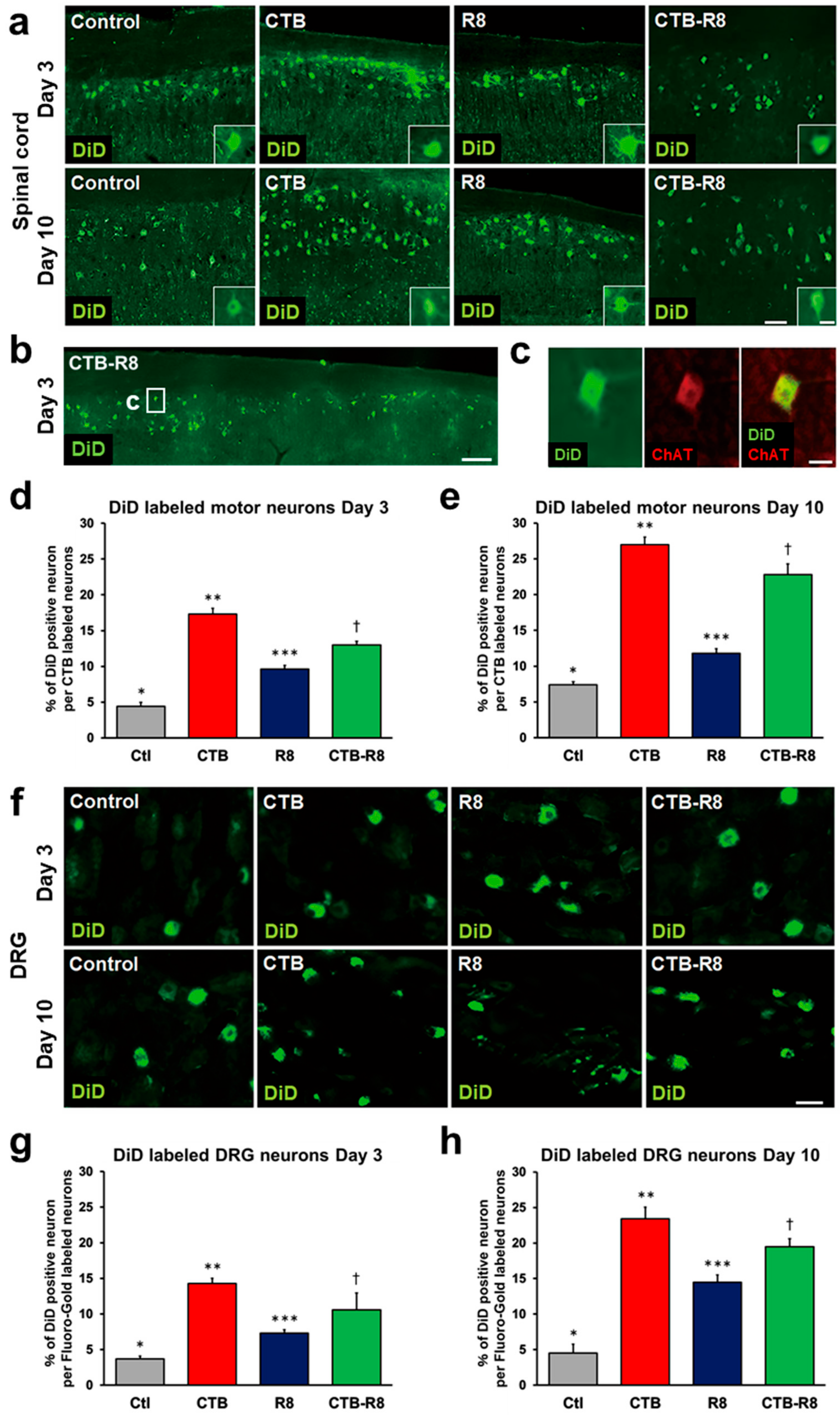
2.4. Modification with CTB Improves Retrograde Axonal Transport of Liposomes from Peripheral Nerves to the Spinal Cord and DRGs
2.5. Modification with CTB Improves Retrograde Axonal Transport of Liposomes from Muscles to the Spinal Cord and DRGs
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Preparation of Liposomes
4.3. Surgical Procedures
4.4. Histology
4.5. Immunolabeling
4.6. Quantification
4.7. Cell Culture
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Federici, T.; Boulis, N. Gene therapy for peripheral nervous system diseases. Curr. Gene Ther. 2007, 7, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Kheder, A.; Nair, K.P. Spasticity: Pathophysiology, evaluation and management. Pract. Neurol. 2012, 12, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Mòdol, L.; Cobianchi, S.; Navarro, X. Prevention of NKCC1 phosphorylation avoids downregulation of KCC2 in central sensory pathways and reduces neuropathic pain after peripheral nerve injury. Pain 2014, 155, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- Nabizadeh, F.; Balabandian, M.; Sharafi, A.M.; Ghaderi, A.; Rostami, M.R.; Naser Moghadasi, A. Statins and risk of amyotrophic lateral sclerosis: A systematic review and meta-analysis. Acta Neurol. Belg. 2021. [Google Scholar] [CrossRef]
- Houdebine, L.; D’Amico, D.; Bastin, J.; Chali, F.; Desseille, C.; Rumeau, V.; Soukkari, J.; Oudot, C.; Rouquet, T.; Bariohay, B.; et al. Low-Intensity Running and High-Intensity Swimming Exercises Differentially Improve Energy Metabolism in Mice with Mild Spinal Muscular Atrophy. Front. Physiol. 2019, 10, 1258. [Google Scholar] [CrossRef]
- Lee-Hotta, S.; Uchiyama, Y.; Kametaka, S. Role of the BDNF-TrkB pathway in KCC2 regulation and rehabilitation following neuronal injury: A mini review. Neurochem. Int. 2019, 128, 32–38. [Google Scholar] [CrossRef]
- Banks, W.A. From blood-brain barrier to blood-brain interface: New opportunities for CNS drug delivery. Nat. Rev. Drug Discov. 2016, 15, 275–292. [Google Scholar] [CrossRef]
- Montero, A.S.; Bielle, F.; Goldwirt, L.; Lalot, A.; Bouchoux, G.; Canney, M.; Belin, F.; Beccaria, K.; Pradat, P.F.; Salachas, F.; et al. Ultrasound-Induced Blood-Spinal Cord Barrier Opening in Rabbits. Ultrasound Med. Biol. 2019, 45, 2417–2426. [Google Scholar] [CrossRef]
- Reinhold, A.K.; Rittner, H.L. Characteristics of the nerve barrier and the blood dorsal root ganglion barrier in health and disease. Exp. Neurol. 2020, 327, 113244. [Google Scholar] [CrossRef]
- Fowler, M.J.; Cotter, J.D.; Knight, B.E.; Sevick-Muraca, E.M.; Sandberg, D.I.; Sirianni, R.W. Intrathecal drug delivery in the era of nanomedicine. Adv. Drug Deliv. Rev. 2020, 165–166, 77–95. [Google Scholar] [CrossRef]
- Papisov, M.I.; Belov, V.V.; Gannon, K.S. Physiology of the intrathecal bolus: The leptomeningeal route for macromolecule and particle delivery to CNS. Mol. Pharm. 2013, 10, 1522–1532. [Google Scholar] [CrossRef]
- Lu, C.T.; Zhao, Y.Z.; Wong, H.L.; Cai, J.; Peng, L.; Tian, X.Q. Current approaches to enhance CNS delivery of drugs across the brain barriers. Int. J. Nanomed. 2014, 9, 2241–2257. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L. Retrograde Axonal Transport Property of Adeno-Associated Virus and Its Possible Application in Future. Microbes Infect. 2021, 23, 104829. [Google Scholar] [CrossRef]
- Jan, A.; Richner, M.; Vægter, C.B.; Nyengaard, J.R.; Jensen, P.H. Gene Transfer in Rodent Nervous Tissue following Hindlimb Intramuscular Delivery of Recombinant Adeno-Associated Virus Serotypes AAV2/6, AAV2/8, and AAV2/9. Neurosci. Insights 2019, 14, 1179069519889022. [Google Scholar] [CrossRef]
- Wang, L.J.; Lu, Y.Y.; Muramatsu, S.; Ikeguchi, K.; Fujimoto, K.; Okada, T.; Mizukami, H.; Matsushita, T.; Hanazono, Y.; Kume, A.; et al. Neuroprotective effects of glial cell line-derived neurotrophic factor mediated by an adeno-associated virus vector in a transgenic animal model of amyotrophic lateral sclerosis. J. Neurosci. Off. J. Soc. Neurosci. 2002, 22, 6920–6928. [Google Scholar] [CrossRef]
- Chen, Z.; Fan, G.; Li, A.; Yuan, J.; Xu, T. rAAV2-Retro Enables Extensive and High-Efficient Transduction of Lower Motor Neurons following Intramuscular Injection. Mol. Ther. Methods Clin. Dev. 2020, 17, 21–33. [Google Scholar] [CrossRef]
- Benkhelifa-Ziyyat, S.; Besse, A.; Roda, M.; Duque, S.; Astord, S.; Carcenac, R.; Marais, T.; Barkats, M. Intramuscular scAAV9-SMN injection mediates widespread gene delivery to the spinal cord and decreases disease severity in SMA mice. Mol. Ther. 2013, 21, 282–290. [Google Scholar] [CrossRef]
- Jaiswal, P.B.; Tung, J.K.; Gross, R.E.; English, A.W. Motoneuron activity is required for enhancements in functional recovery after peripheral nerve injury in exercised female mice. J. Neurosci. Res. 2020, 98, 448–457. [Google Scholar] [CrossRef]
- Foust, K.D.; Poirier, A.; Pacak, C.A.; Mandel, R.J.; Flotte, T.R. Neonatal intraperitoneal or intravenous injections of recombinant adeno-associated virus type 8 transduce dorsal root ganglia and lower motor neurons. Hum. Gene Ther. 2008, 19, 61–70. [Google Scholar] [CrossRef]
- Hollis Ii, E.R.; Kadoya, K.; Hirsch, M.; Samulski, R.J.; Tuszynski, M.H. Efficient Retrograde Neuronal Transduction Utilizing Self-complementary AAV1. Mol. Ther. 2008, 16, 296–301. [Google Scholar] [CrossRef]
- Zheng, H.; Qiao, C.; Wang, C.H.; Li, J.; Li, J.; Yuan, Z.; Zhang, C.; Xiao, X. Efficient retrograde transport of adeno-associated virus type 8 to spinal cord and dorsal root ganglion after vector delivery in muscle. Hum. Gene Ther. 2010, 21, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Bloom, D.C.; Watson, Z.L.; Neumann, D.M. Peripheral AAV Injection for Retrograde Transduction of Dorsal Root and Trigeminal Ganglia. Methods Mol. Biol. 2019, 1950, 237–247. [Google Scholar] [PubMed]
- Ross, C.; Taylor, M.; Fullwood, N.; Allsop, D. Liposome delivery systems for the treatment of Alzheimer’s disease. Int. J. Nanomed. 2018, 13, 8507–8522. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Yamada, Y.; Sato, Y.; Khalil, I.A.; Harashima, H. Innovative nanotechnologies for enhancing nucleic acids/gene therapy: Controlling intracellular trafficking to targeted biodistribution. Biomaterials 2019, 218, 119329. [Google Scholar] [CrossRef]
- Abu Lila, A.S.; Ishida, T. Liposomal Delivery Systems: Design Optimization and Current Applications. Biol. Pharm. Bull. 2017, 40, 1–10. [Google Scholar] [CrossRef]
- Raoufi, E.; Bahramimeimandi, B.; Salehi-Shadkami, M.; Chaosri, P.; Mozafari, M.R. Methodical Design of Viral Vaccines Based on Avant-Garde Nanocarriers: A Multi-Domain Narrative Review. Biomedicines 2021, 9, 520. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Lauritano, D.; Ronconi, G.; Calvisi, V.; Conti, P. Antibodies for COVID-19—Which, when and how long? J. Biol. Regul. Homeost. Agents 2021, 35, 417–422. [Google Scholar]
- Nguyen, T.X.; Huang, L.; Gauthier, M.; Yang, G.; Wang, Q. Recent advances in liposome surface modification for oral drug delivery. Nanomed. Lond. Engl. 2016, 11, 1169–1185. [Google Scholar] [CrossRef]
- Gonzalez Porras, M.A.; Durfee, P.N.; Gregory, A.M.; Sieck, G.C.; Brinker, C.J.; Mantilla, C.B. A novel approach for targeted delivery to motoneurons using cholera toxin-B modified protocells. J. Neurosci. Methods 2016, 273, 160–174. [Google Scholar] [CrossRef]
- Walker, W.A.; Tarannum, M.; Vivero-Escoto, J.L. Cellular Endocytosis and Trafficking of Cholera Toxin B-Modified Mesoporous Silica Nanoparticles. J. Mater. Chem. B 2016, 4, 1254–1262. [Google Scholar] [CrossRef]
- Futaki, S.; Suzuki, T.; Ohashi, W.; Yagami, T.; Tanaka, S.; Ueda, K.; Sugiura, Y. Arginine-rich peptides. An abundant source of membrane-permeable peptides having potential as carriers for intracellular protein delivery. J. Biol. Chem. 2001, 276, 5836–5840. [Google Scholar] [CrossRef]
- Gonzalez Porras, M.A.; Durfee, P.; Giambini, S.; Sieck, G.C.; Brinker, C.J.; Mantilla, C.B. Uptake and intracellular fate of cholera toxin subunit b-modified mesoporous silica nanoparticle-supported lipid bilayers (aka protocells) in motoneurons. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 661–672. [Google Scholar] [CrossRef]
- Schmued, L.C.; Fallon, J.H. Fluoro-Gold: A new fluorescent retrograde axonal tracer with numerous unique properties. Brain Res. 1986, 377, 147–154. [Google Scholar] [CrossRef]
- Lai, B.Q.; Qiu, X.C.; Zhang, K.; Zhang, R.Y.; Jin, H.; Li, G.; Shen, H.Y.; Wu, J.L.; Ling, E.A.; Zeng, Y.S. Cholera Toxin B Subunit Shows Transneuronal Tracing after Injection in an Injured Sciatic Nerve. PLoS ONE 2015, 10, e0144030. [Google Scholar] [CrossRef]
- Hayakawa, T.; Takanaga, A.; Tanaka, K.; Maeda, S.; Seki, M. Cells of origin of vagal motor neurons projecting to different parts of the stomach in the rat: Confocal laser scanning and electron microscopic study. Anat. Embryol. 2003, 207, 289–297. [Google Scholar] [CrossRef]
- Chien, J.Y.; Sheu, J.H.; Wen, Z.H.; Tsai, R.K.; Huang, S.P. Neuroprotective effect of 4-(Phenylsulfanyl)butan-2-one on optic nerve crush model in rats. Exp. Eye Res. 2016, 143, 148–157. [Google Scholar] [CrossRef]
- Abbott, C.J.; Choe, T.E.; Lusardi, T.A.; Burgoyne, C.F.; Wang, L.; Fortune, B. Evaluation of retinal nerve fiber layer thickness and axonal transport 1 and 2 weeks after 8 hours of acute intraocular pressure elevation in rats. Investig. Ophthalmol. Vis. Sci. 2014, 55, 674–687. [Google Scholar] [CrossRef][Green Version]
- Zhao, Y.; Maharjan, S.; Sun, Y.; Yang, Z.; Yang, E.; Zhou, N.; Lu, L.; Whittaker, A.K.; Yang, B.; Lin, Q. Red fluorescent AuNDs with conjugation of cholera toxin subunit B (CTB) for extended-distance retro-nerve transporting and long-time neural tracing. Acta Biomater. 2020, 102, 394–402. [Google Scholar] [CrossRef]
- Rivero-Melián, C.; Rosario, C.; Grant, G. Demonstration of transganglionically transported choleragenoid in rat spinal cord by immunofluorescence cytochemistry. Neurosci. Lett. 1992, 145, 114–117. [Google Scholar] [CrossRef]
- Prodanov, D.; Feirabend, H.K. Automated characterization of nerve fibers labeled fluorescently: Determination of size, class and spatial distribution. Brain Res. 2008, 1233, 35–50. [Google Scholar] [CrossRef]
- Inoh, Y.; Hirose, T.; Yokoi, A.; Yokawa, S.; Furuno, T. Effects of lipid composition in cationic liposomes on suppression of mast cell activation. Chem. Phys. Lipids 2020, 231, 104948. [Google Scholar] [CrossRef]
- Inoh, Y.; Nagai, M.; Matsushita, K.; Nakanishi, M.; Furuno, T. Gene transfection efficiency into dendritic cells is influenced by the size of cationic liposomes/DNA complexes. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2017, 102, 230–236. [Google Scholar] [CrossRef]
- Sakai-Kato, K.; Yoshida, K.; Takechi-Haraya, Y.; Izutsu, K.I. Physicochemical Characterization of Liposomes That Mimic the Lipid Composition of Exosomes for Effective Intracellular Trafficking. Langmuir ACS J. Surf. Colloids 2020, 36, 12735–12744. [Google Scholar] [CrossRef]
- Suesca, E.; Alejo, J.L.; Bolaños, N.I.; Ocampo, J.; Leidy, C.; González, J.M. Sulfocerebrosides upregulate liposome uptake in human astrocytes without inducing a proinflammatory response. Cytometry A 2013, 83, 627–635. [Google Scholar] [CrossRef]
- Nakamura, T.; Kuroi, M.; Harashima, H. Influence of Endosomal Escape and Degradation of α-Galactosylceramide Loaded Liposomes on CD1d Antigen Presentation. Mol. Pharm. 2015, 12, 2791–2799. [Google Scholar] [CrossRef]
- Yamada, Y.; Furukawa, R.; Harashima, H. A Dual-Ligand Liposomal System Composed of a Cell-Penetrating Peptide and a Mitochondrial RNA Aptamer Synergistically Facilitates Cellular Uptake and Mitochondrial Targeting. J. Pharm. Sci. 2016, 105, 1705–1713. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, G.; Li, J.; Fu, Y.; Mavlyutov, T.A.; Yao, A.; Nickells, R.W.; Gong, S.; Guo, L.W. An intraocular drug delivery system using targeted nanocarriers attenuates retinal ganglion cell degeneration. J. Control. Release 2017, 247, 153–166. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, Q.; Jiang, Q.; Huang, Y.; Liu, H.; Zhao, Y.; Cao, W.; Ma, G.; Dai, F.; Liang, X.; et al. Enhanced endosomal/lysosomal escape by distearoyl phosphoethanolamine-polycarboxybetaine lipid for systemic delivery of siRNA. J. Control. Release 2014, 176, 104–114. [Google Scholar] [CrossRef]
- Khalil, I.A.; Kogure, K.; Futaki, S.; Harashima, H. Octaarginine-modified liposomes: Enhanced cellular uptake and controlled intracellular trafficking. Int. J. Pharm. 2008, 354, 39–48. [Google Scholar] [CrossRef]
- Zuilhof, H. Fighting Cholera One-on-One: The Development and Efficacy of Multivalent Cholera-Toxin-Binding Molecules. Acc. Chem. Res. 2016, 49, 274–285. [Google Scholar] [CrossRef]
- Masuda, H.; Nakamura, T.; Harashima, H. Distribution of BCG-CWS-Loaded Nanoparticles in the Spleen After Intravenous Injection Affects Cytotoxic T Lymphocyte Activity. J. Pharm. Sci. 2020, 109, 1943–1950. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Somiya, K.; Miyauchi, A.; Osaka, H.; Harashima, H. Validation of a mitochondrial RNA therapeutic strategy using fibroblasts from a Leigh syndrome patient with a mutation in the mitochondrial ND3 gene. Sci. Rep. 2020, 10, 7511. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Moriguchi, R.; Kogure, K.; Shastri, N.; Harashima, H. Efficient MHC class I presentation by controlled intracellular trafficking of antigens in octaarginine-modified liposomes. Mol. Ther. 2008, 16, 1507–1514. [Google Scholar] [CrossRef] [PubMed]
- Rusmini, P.; Cortese, K.; Crippa, V.; Cristofani, R.; Cicardi, M.E.; Ferrari, V.; Vezzoli, G.; Tedesco, B.; Meroni, M.; Messi, E.; et al. Trehalose induces autophagy via lysosomal-mediated TFEB activation in models of motoneuron degeneration. Autophagy 2019, 15, 631–651. [Google Scholar] [CrossRef]
- Abraham, Z.; Hawley, E.; Hayosh, D.; Webster-Wood, V.A.; Akkus, O. Kinesin and Dynein Mechanics: Measurement Methods and Research Applications. J. Biomech. Eng. 2018, 140, 0208051–02080511. [Google Scholar] [CrossRef]
- Godement, P.; Vanselow, J.; Thanos, S.; Bonhoeffer, F. A study in developing visual systems with a new method of staining neurones and their processes in fixed tissue. Development 1987, 101, 697–713. [Google Scholar] [CrossRef]
- Balice-Gordon, R.J.; Chua, C.K.; Nelson, C.C.; Lichtman, J.W. Gradual loss of synaptic cartels precedes axon withdrawal at developing neuromuscular junctions. Neuron 1993, 11, 801–815. [Google Scholar] [CrossRef]
- Muzio, M.R.; Cascella, M. Histology, Axon. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- Katsuki, T.; Joshi, R.; Ailani, D.; Hiromi, Y. Compartmentalization within neurites: Its mechanisms and implications. Dev. Neurobiol. 2011, 71, 458–473. [Google Scholar] [CrossRef]
- Aravamudhan, P.; Raghunathan, K.; Konopka-Anstadt, J.; Pathak, A.; Sutherland, D.M.; Carter, B.D.; Dermody, T.S. Reovirus uses macropinocytosis-mediated entry and fast axonal transport to infect neurons. PLoS Pathog. 2020, 16, e1008380. [Google Scholar] [CrossRef]
- Morrison, B.M. Neuromuscular Diseases. Semin. Neurol. 2016, 36, 409–418. [Google Scholar] [CrossRef]
- Banks, R.W.; Hulliger, M.; Saed, H.H.; Stacey, M.J. A comparative analysis of the encapsulated end-organs of mammalian skeletal muscles and of their sensory nerve endings. J. Anat. 2009, 214, 859–887. [Google Scholar] [CrossRef]
- Sudhof, T.C. The synaptic vesicle cycle. Annu. Rev. Neurosci. 2004, 27, 509–547. [Google Scholar] [CrossRef]
- Ribeiro, F.M.; Black, S.A.; Prado, V.F.; Rylett, R.J.; Ferguson, S.S.; Prado, M.A. The “ins” and “outs” of the high-affinity choline transporter CHT1. J. Neurochem. 2006, 97, 1–12. [Google Scholar] [CrossRef]
- Royle, S.J.; Lagnado, L. Endocytosis at the synaptic terminal. J. Physiol. 2003, 553, 345–355. [Google Scholar] [CrossRef]
- Hirokawa, N.; Niwa, S.; Tanaka, Y. Molecular motors in neurons: Transport mechanisms and roles in brain function, development, and disease. Neuron 2010, 68, 610–638. [Google Scholar] [CrossRef]
- Walsby, A.E. Gas vesicles. Microbiol. Rev. 1994, 58, 94–144. [Google Scholar] [CrossRef]
- Boggs, J.M. Lipid intermolecular hydrogen bonding: Influence on structural organization and membrane function. Biochim. Et Biophys. Acta 1987, 906, 353–404. [Google Scholar] [CrossRef]
- Melkoumov, A.; St-Jean, I.; Banquy, X.; Leclair, G.; Leblond Chain, J. GM1-Binding Conjugates To Improve Intestinal Permeability. Mol. Pharm. 2019, 16, 60–70. [Google Scholar] [CrossRef]
- Jobling, M.G.; Yang, Z.; Kam, W.R.; Lencer, W.I.; Holmes, R.K. A single native ganglioside GM1-binding site is sufficient for cholera toxin to bind to cells and complete the intoxication pathway. mBio 2012, 3, e00401-12. [Google Scholar] [CrossRef]
- Fujiwara, T.; Akita, H.; Harashima, H. Intracellular fate of octaarginine-modified liposomes in polarized MDCK cells. Int. J. Pharm. 2010, 386, 122–130. [Google Scholar] [CrossRef]
- Khalil, I.A.; Kogure, K.; Futaki, S.; Harashima, H. High density of octaarginine stimulates macropinocytosis leading to efficient intracellular trafficking for gene expression. J. Biol. Chem. 2006, 281, 3544–3551. [Google Scholar] [CrossRef] [PubMed]
- Sellers, D.L.; Bergen, J.M.; Johnson, R.N.; Back, H.; Ravits, J.M.; Horner, P.J.; Pun, S.H. Targeted axonal import (TAxI) peptide delivers functional proteins into spinal cord motor neurons after peripheral administration. Proc. Natl. Acad. Sci. USA 2016, 113, 2514–2519. [Google Scholar] [CrossRef] [PubMed]
- Futaki, S. Membrane translocation of arginine-rich cell-penetrating peptides. Seikagaku. J. Jpn. Biochem. Soc. 2017, 89, 8–14. [Google Scholar]
- Boulenguez, P.; Liabeuf, S.; Bos, R.; Bras, H.; Jean-Xavier, C.; Brocard, C.; Stil, A.; Darbon, P.; Cattaert, D.; Delpire, E.; et al. Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat. Med. 2010, 16, 302–307. [Google Scholar] [CrossRef]
- Gagnon, M.; Bergeron, M.J.; Lavertu, G.; Castonguay, A.; Tripathy, S.; Bonin, R.P.; Perez-Sanchez, J.; Boudreau, D.; Wang, B.; Dumas, L.; et al. Chloride extrusion enhancers as novel therapeutics for neurological diseases. Nat. Med. 2013, 19, 1524–1528. [Google Scholar] [CrossRef]
- Cappella, M.; Ciotti, C.; Cohen-Tannoudji, M.; Biferi, M.G. Gene Therapy for ALS-A Perspective. Int. J. Mol. Sci. 2019, 20, 4388. [Google Scholar] [CrossRef]
- Mathew, B.; Ruiz, P.; Pathak, V.; Suto, M.J. Development of novel small molecules for the treatment of ALS. Bioorg. Med. Chem. Lett. 2020, 30, 126950. [Google Scholar] [CrossRef]
- Amporndanai, K.; Rogers, M.; Watanabe, S.; Yamanaka, K.; O’Neill, P.M.; Hasnain, S.S. Novel Selenium-based compounds with therapeutic potential for SOD1-linked amyotrophic lateral sclerosis. EBioMedicine 2020, 59, 102980. [Google Scholar] [CrossRef]
- Barkats, M. SMA: From gene discovery to gene therapy. Med. Sci. M/S 2020, 36, 137–140. [Google Scholar]
- Peyronnard, J.M.; Charron, L.F.; Lavoie, J.; Messier, J.P. Motor, sympathetic and sensory innervation of rat skeletal muscles. Brain Res. 1986, 373, 288–302. [Google Scholar] [CrossRef]
- Brink, E.E.; Morrell, J.I.; Pfaff, D.W. Localization of lumbar epaxial motoneurons in the rat. Brain Res. 1979, 170, 23–41. [Google Scholar] [CrossRef]
| Liposome | Composition | Diameter (nm) | PDI | Zeta-Potential (mV) | Lipid Concentration (mM) |
|---|---|---|---|---|---|
| DSPC | DSPC/Chol/DSPE-PEG2000 = 70/30/5 | 136.00 ± 9.27 | 0.26 ± 0.01 | −15.77 ± 0.31 | 4.73 ± 0.77 |
| POPC | POPC/Chol/DSPE-PEG2000 = 70/30/5 | 146.67 ± 13.07 | 0.13 ± 0.01 | −16.10 ± 2.62 | 5.91 ± 0.25 |
| DSPC/POPC | DSPC/POPC/Chol/DSPE-PEG2000 = 30/40/30/5 | 154.33 ± 14.52 | 0.15 ± 0.08 | −16.57 ± 3.69 | 5.27 ± 0.17 |
| CTB-DSPC | DSPC/Chol/DSPE-PEG2000 = 70/30/5 CTB modification | 173.00 ± 20.61 | 0.23 ± 0.03 | −11.6 ± 2.36 | 3.68 ± 0.26 |
| R8-DSPC | DSPC/Chol/DSPE-PEG2000 = 70/30/5 R8 modification | 116.67 ± 13.07 | 0.20 ± 0.03 | 17.9 ± 1.85 | 4.49 ± 0.23 |
| CTB-R8-DSPC | DSPC/Chol/DSPE-PEG2000 = 70/30/5 CTB + R8 modification | 125.00 ± 6.16 | 0.23 ± 0.03 | 16.3 ± 1.44 | 4.2 ± 0.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukui, T.; Tateno, H.; Nakamura, T.; Yamada, Y.; Sato, Y.; Iwasaki, N.; Harashima, H.; Kadoya, K. Retrograde Axonal Transport of Liposomes from Peripheral Tissue to Spinal Cord and DRGs by Optimized Phospholipid and CTB Modification. Int. J. Mol. Sci. 2022, 23, 6661. https://doi.org/10.3390/ijms23126661
Fukui T, Tateno H, Nakamura T, Yamada Y, Sato Y, Iwasaki N, Harashima H, Kadoya K. Retrograde Axonal Transport of Liposomes from Peripheral Tissue to Spinal Cord and DRGs by Optimized Phospholipid and CTB Modification. International Journal of Molecular Sciences. 2022; 23(12):6661. https://doi.org/10.3390/ijms23126661
Chicago/Turabian StyleFukui, Takafumi, Hironao Tateno, Takashi Nakamura, Yuma Yamada, Yusuke Sato, Norimasa Iwasaki, Hideyoshi Harashima, and Ken Kadoya. 2022. "Retrograde Axonal Transport of Liposomes from Peripheral Tissue to Spinal Cord and DRGs by Optimized Phospholipid and CTB Modification" International Journal of Molecular Sciences 23, no. 12: 6661. https://doi.org/10.3390/ijms23126661
APA StyleFukui, T., Tateno, H., Nakamura, T., Yamada, Y., Sato, Y., Iwasaki, N., Harashima, H., & Kadoya, K. (2022). Retrograde Axonal Transport of Liposomes from Peripheral Tissue to Spinal Cord and DRGs by Optimized Phospholipid and CTB Modification. International Journal of Molecular Sciences, 23(12), 6661. https://doi.org/10.3390/ijms23126661







