Customized Utilization Strategies of Industrial Lignin to Produce Adsorbents and Flocculants Based on Fractionation and Adequate Structural Interpretation
Abstract
:1. Introduction
2. Results and Discussion
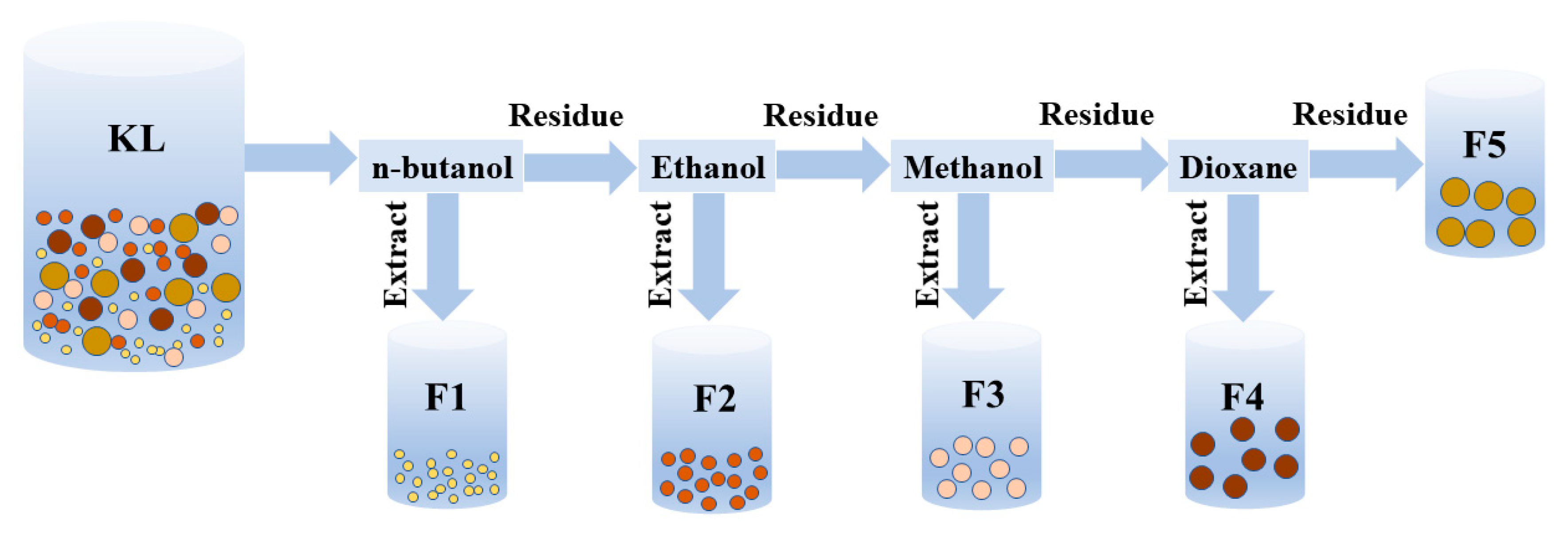
2.1. Classification of Lignin and the Characterization of its Components
2.2. Production of Lignin-Based Flocculant
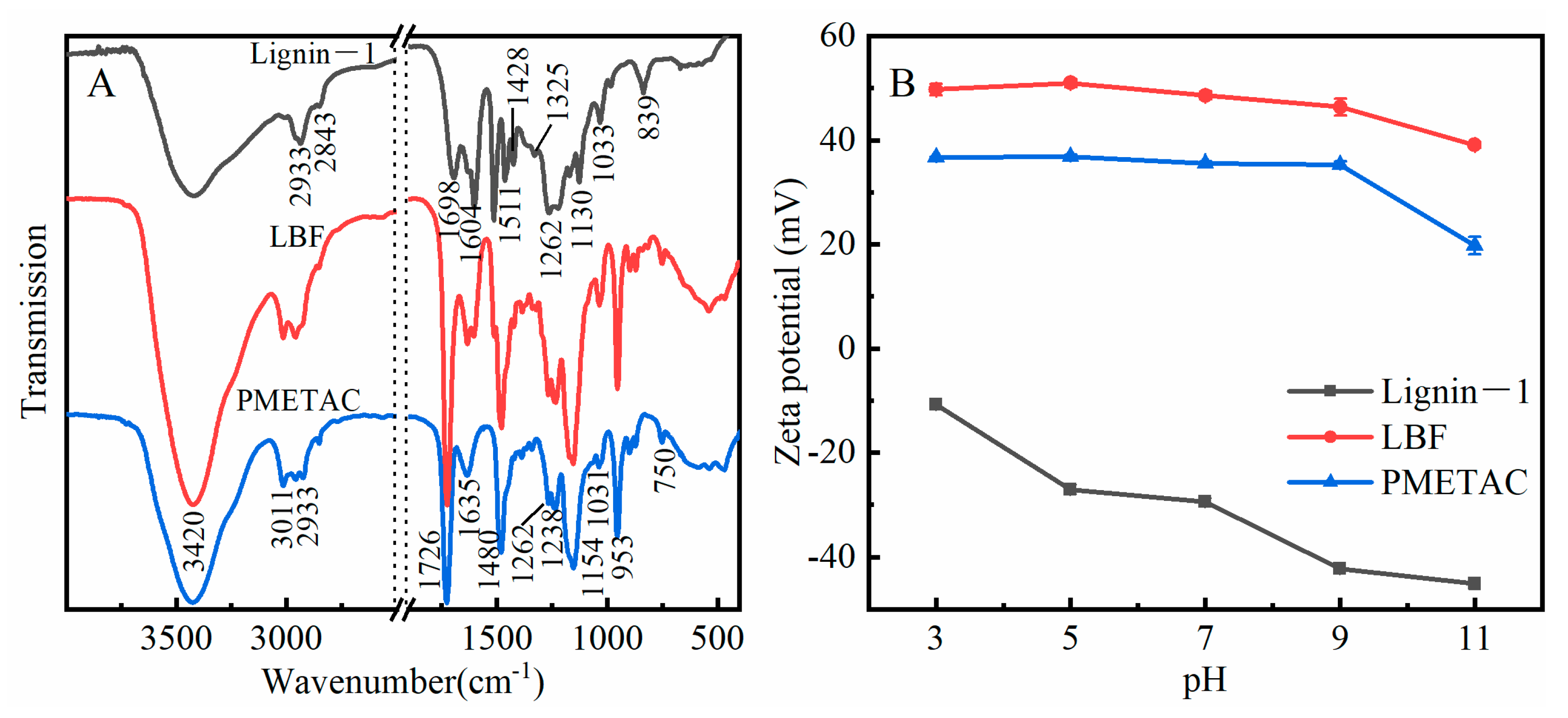
2.3. Characterization of Lignin-Based Flocculant
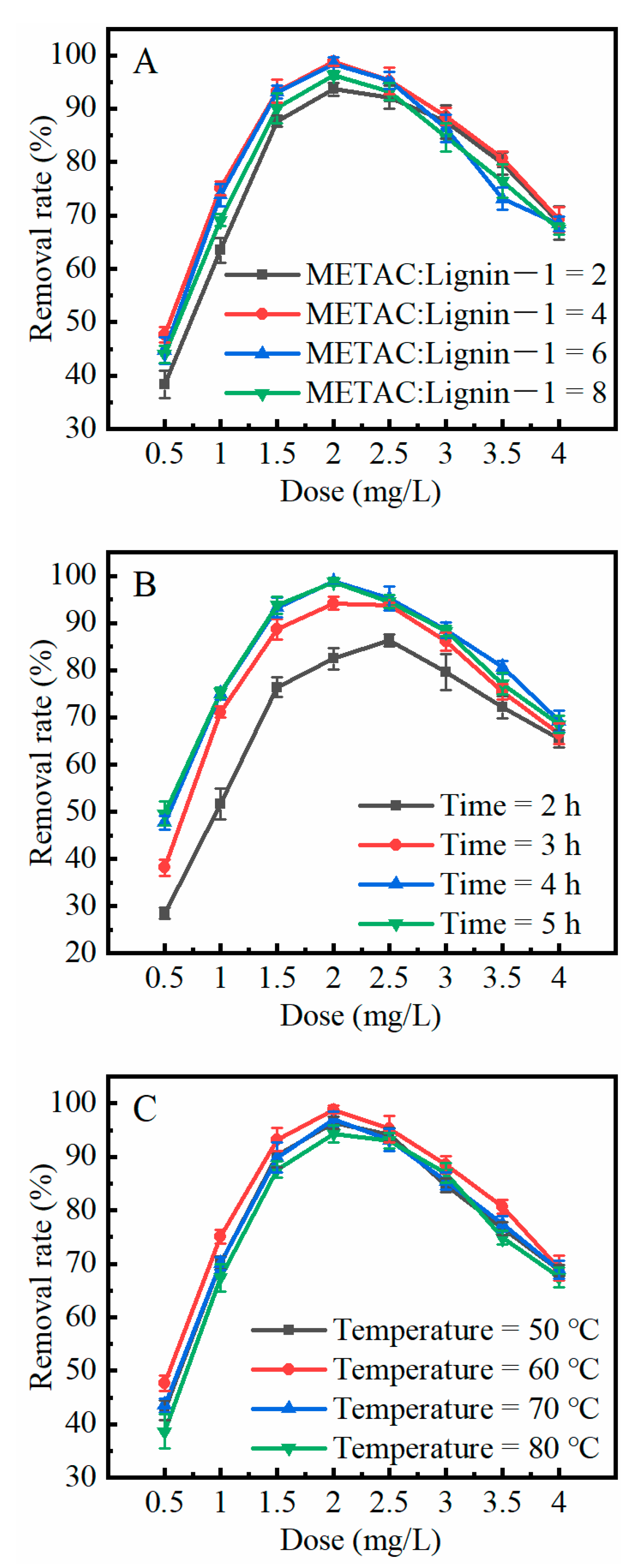
2.4. External Factors Influencing the Flocculation Effect
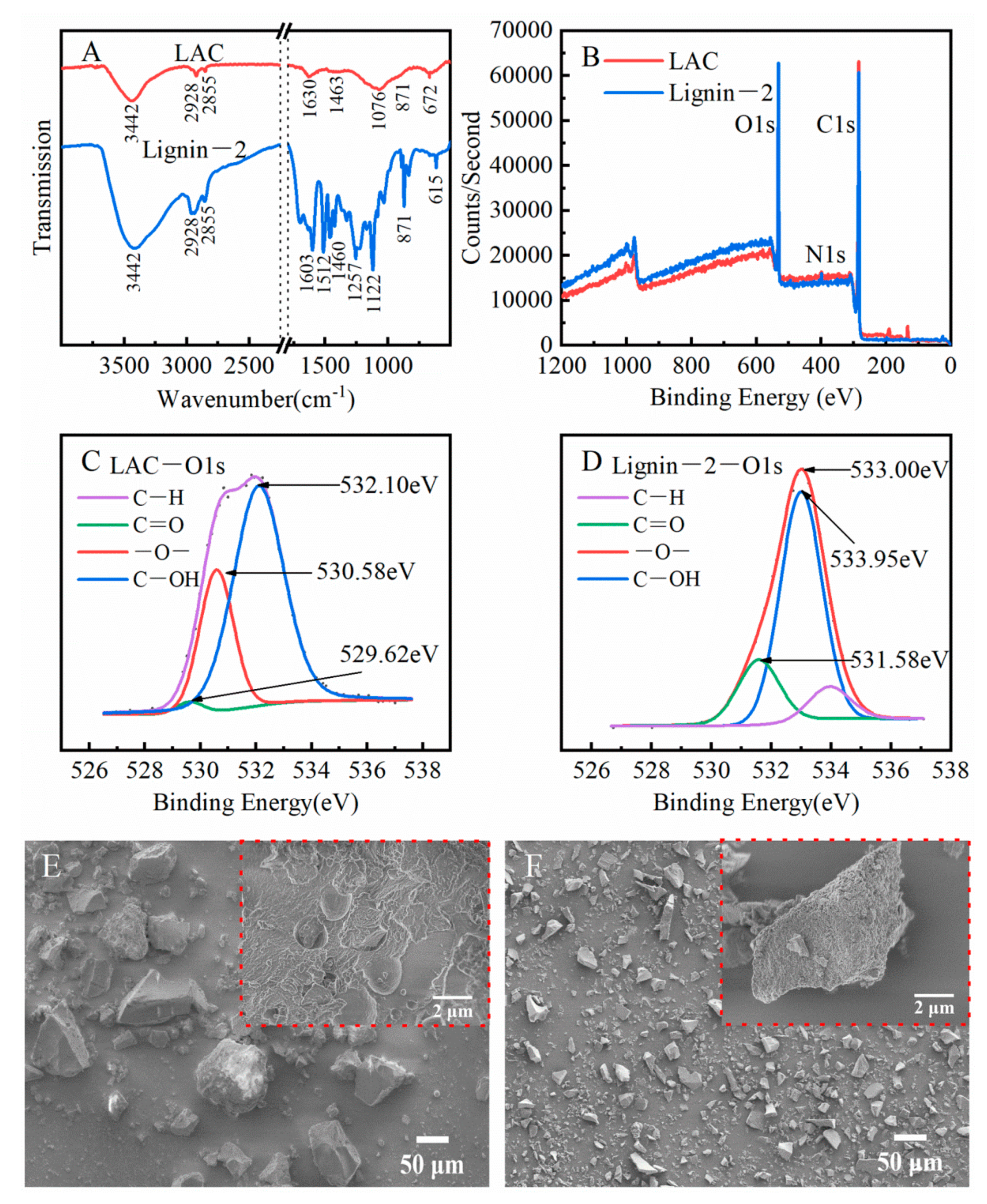
2.5. Production and Characterization of the Lignin-Based Activated Carbon
2.6. Adsorption of LAC to Wastewater Containing Cu (II)
2.6.1. Adsorption Isotherms and Adsorption Kinetics
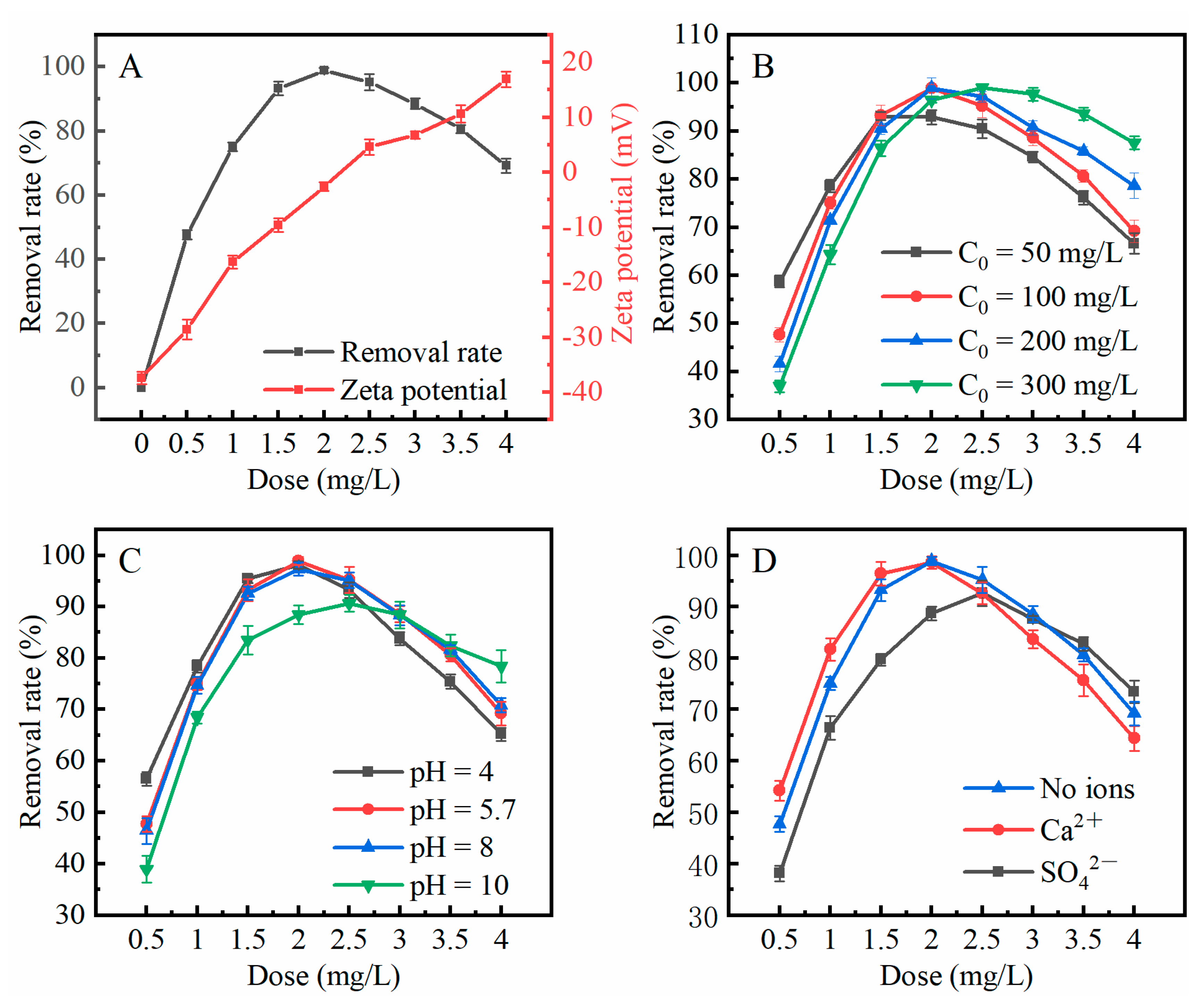
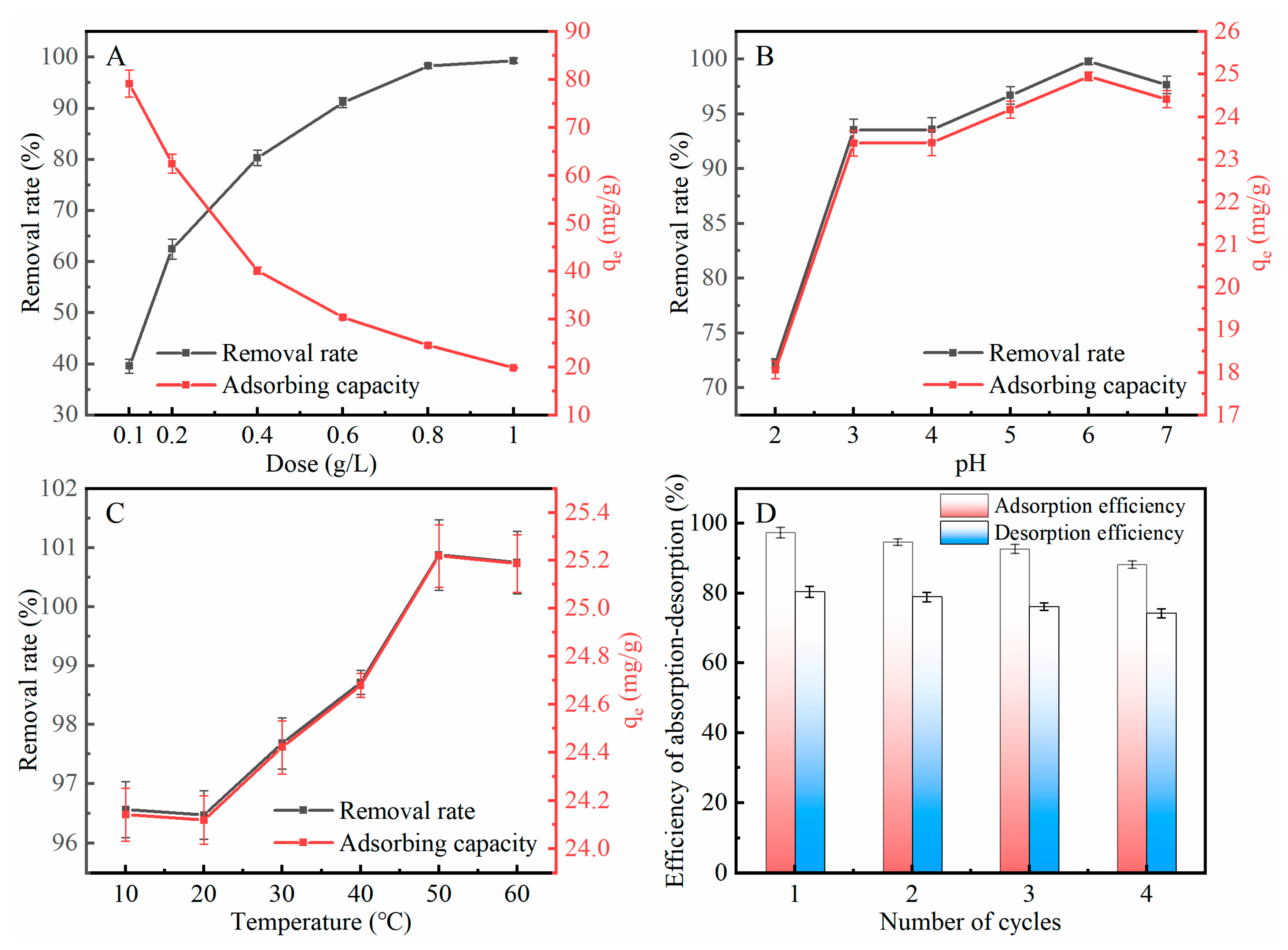
2.6.2. Effect of LAC Dosage
2.6.3. Effect of Wastewater pH
2.6.4. Effect of Temperature
2.6.5. Regeneration of LAC
2.6.6. Prospect of Industrialization
3. Materials and Methods
3.1. Materials
3.2. Sequential Solvent Fractionation
3.3. Production of a Lignin-Based Flocculant
3.4. Production of Lignin-Based Activated Carbon
3.5. Characterization
3.6. Flocculation Experiments
3.7. Adsorption Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sakudo, A.; Yagyu, Y.; Onodera, T. Disinfection and sterilization using plasma technology: Fundamentals and future perspectives for biological applications. Int. J. Mol. Sci. 2019, 20, 5216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teh, C.Y.; Budiman, P.M.; Shak, K.P.Y.; Wu, T.Y. Recent advancement of coagulation–flocculation and its application in wastewater treatment. Ind. Eng. Chem. Res. 2016, 55, 4363–4389. [Google Scholar] [CrossRef]
- Wang, B.; Wang, S.F.; Lam, S.S.; Sonne, C.; Yuan, T.Q.; Song, G.Y.; Sun, R.C. A review on production of lignin-based flocculants: Sustainable feedstock and low carbon footprint applications. Renew. Sustain. Energy Rev. 2020, 134, 110384. [Google Scholar] [CrossRef]
- Kumar, A.; Biswas, B.; Kaur, R.; Krishna, B.B.; Bhaskar, T. Hydrothermal oxidative valorisation of lignin into functional chemicals: A review. Bioresour. Technol. 2021, 342, 126016. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Sun, D.; Yuan, T.; Song, G.; Sun, R. Recent Advances in Lignin Modification and Its Application in Wastewater Treatment. Lignin Util. Strateg. Processing Appl. 2021, 1377, 143–173. [Google Scholar]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin biosynthesis and structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef] [Green Version]
- Beisl, S.; Friedl, A.; Miltner, A. Lignin from micro-to nanosize: Applications. Int. J. Mol. Sci. 2017, 18, 2367. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.; Du, H.S.; Liu, C.; Liu, H.Y.; Wu, M.Y.; Zhang, X.Y.; Si, C.L.; Li, B. An efficient and magnetic adsorbent prepared in a dry process with enzymatic hydrolysis residues for wastewater treatment. J. Clean. Prod. 2021, 313, 127834. [Google Scholar] [CrossRef]
- Yuan, H.; Peng, J.; Ren, T.; Luo, Q.; Luo, Y.; Zhang, N.; Huang, Y.; Guo, X.; Wu, Y. Novel fluorescent lignin-based hydrogel with cellulose nanofibers and carbon dots for highly efficient adsorption and detection of Cr (VI). Sci. Total Environ. 2021, 760, 143395. [Google Scholar] [CrossRef]
- Kwak, H.W.; Shin, M.; Yun, H.; Lee, K.H. Preparation of silk sericin/lignin blend beads for the removal of hexavalent chromium ions. Int. J. Mol. Sci. 2016, 17, 1466. [Google Scholar] [CrossRef] [Green Version]
- Brodin, M.; Vallejos, M.; Opedal, M.T.; Area, M.C.; Chinga-Carrasco, G. Lignocellulosics as sustainable resources for production of bioplastics—A review. J. Clean. Prod. 2017, 162, 646–664. [Google Scholar] [CrossRef]
- Gioia, C.; Colonna, M.; Tagami, A.; Medina, L.; Sevastyanova, O.; Berglund, L.A.; Lawoko, M. Lignin-based epoxy resins: Unravelling the relationship between structure and material properties. Biomacromolecules 2020, 21, 1920–1928. [Google Scholar] [CrossRef] [PubMed]
- Baumberger, S.P.; Lapierre, C.; Monties, B. Utilization of pine kraft lignin in starch composites: Impact of structural heterogeneity. J. Agric. Food Chem. 1998, 46, 2234–2240. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, J.; Lu, X.; Ma, X.; Cao, S.; Pan, X.; Ni, Y. Wearable lignin-based hydrogel electronics: A mini-review. Int. J. Biol. Macromol. 2021, 181, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Gigli, M.; Crestini, C. Fractionation of industrial lignins: Opportunities and challenges. Green Chem. 2020, 22, 4722–4746. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, J.; Erdocia, X.; Hernández-Ramos, F.; Alriols, M.G.; Labidi, J. Lignin separation and fractionation by ultrafiltration. In Separation of Functional Molecules in Food by Membrane Technology; Charis, M., Galanakis, C.M., Eds.; Academic Press: London, UK, 2019; pp. 229–265. [Google Scholar]
- Araújo, L.C.P.; Yamaji, F.M.; Lima, V.H.; Botaro, V.R. Kraft lignin fractionation by organic solvents: Correlation between molar mass and higher heating value. Bioresour. Technol. 2020, 314, 123757. [Google Scholar] [CrossRef]
- Passoni, V.; Scarica, C.; Levi, M.; Turri, S.; Griffini, G. Fractionation of industrial softwood kraft lignin: Solvent selection as a tool for tailored material properties. ACS Sustain. Chem. Eng. 2016, 4, 2232–2242. [Google Scholar] [CrossRef]
- Schuerch, C. The solvent properties of liquids and their relation to the solubility, swelling, isolation and fractionation of lignin. J. Am. Chem. Soc. 1952, 74, 5061–5067. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, S.Y.; Lee, J.H.; Choi, I.G.; Choi, J.W. Sequential solvent fractionation of lignin for selective production of monoaromatics by Ru catalyzed ethanolysis. RSC Adv. 2017, 7, 53117–53125. [Google Scholar] [CrossRef] [Green Version]
- Duarah, P.; Haldar, D.; Purkait, M.K. Technological advancement in the synthesis and applications of lignin-based nanoparticles derived from agro-industrial waste residues: A review. Int. J. Biol. Macromol. 2020, 163, 1828–1843. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lintinen, K.; Hirvonen, J.T.; Kostiainen, M.A.; Santos, H.A. Properties and chemical modifications of lignin: Towards lignin-based nanomaterials for biomedical applications. Prog. Mater. Sci. 2018, 93, 233–269. [Google Scholar] [CrossRef]
- Liu, Z.C.; Wang, Z.W.; Gao, S.; Tong, Y.X.; Le, X.; Hu, N.W.; Yan, Q.S.; Zhou, X.G.; He, Y.R.; Wang, L. Isolation and Fractionation of the Tobacco Stalk Lignin for Customized Value-Added Utilization. Front. Bioeng. Biotechnol. 2021, 9, 811287. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.K.U.; Hadinoto, K. Deep Eutectic Solvent as Green Solvent in Extraction of Biological Macromolecules: A Review. Int. J. Mol. Sci. 2022, 23, 3381. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Lin, W.; Lai, C.; Li, X.; Jin, Y.; Yong, Q. Coupling the post-extraction process to remove residual lignin and alter the recalcitrant structures for improving the enzymatic digestibility of acid-pretreated bamboo residues. Bioresour. Technol. 2019, 285, 121355. [Google Scholar] [CrossRef] [PubMed]
- Faix, O. Classification of lignins from different botanical origins by FT-IR spectroscopy. Holzforschung 1991, 45, 21–28. [Google Scholar] [CrossRef]
- Wang, B.; Sun, D.; Wang, H.M.; Yuan, T.Q.; Sun, R.C. Green and facile preparation of regular lignin nanoparticles with high yield and their natural broad-spectrum sunscreens. ACS Sustain. Chem. Eng. 2018, 7, 2658–2666. [Google Scholar] [CrossRef]
- Yuan, T.Q.; Sun, S.N.; Xu, F.; Sun, R.C. Characterization of lignin structures and lignin-carbohydrate complex (LCC) linkages by quantitative 13C and 2D HSQC NMR spectroscopy. J. Agric. Food Chem. 2011, 59, 10604–10614. [Google Scholar] [CrossRef]
- Heikkinen, S.; Toikka, M.M.; Karhunen, P.T.; Kilpeläinen, I.A. Quantitative 2D HSQC (Q-HSQC) via suppression of J-dependence of polarization transfer in NMR spectroscopy: Application to wood lignin. J. Am. Chem. Soc. 2003, 125, 4362–4367. [Google Scholar] [CrossRef]
- Sette, M.; Wechselberger, R.; Crestini, C. Elucidation of lignin structure by quantitative 2D NMR. Chem.-A Eur. J. 2011, 17, 9529–9535. [Google Scholar] [CrossRef]
- Ikeda, T.; Holtman, K.; Kadla, J.F.; Chang, H.M.; Jameel, H. Studies on the effect of ball milling on lignin structure using a modified DFRC method. J. Agric. Food Chem. 2002, 50, 129–135. [Google Scholar] [CrossRef]
- Wang, B.; Wang, H.M.; Sun, D.; Yuan, T.Q.; Song, G.Y.; Shi, Q.; Zheng, L.; Wang, S.F.; Sun, R.C. Chemosynthesis, characterization and application of lignin-based flocculants with tunable performance prepared by short-wavelength ultraviolet initiation. Ind. Crops Prod. 2020, 157, 112897. [Google Scholar] [CrossRef]
- Chen, R.; Kokta, B.; Valade, J. Study on the graft copolymerization of lignosulfonate and acrylic monomers. J. Appl. Polym. Sci. 1980, 25, 2211–2220. [Google Scholar] [CrossRef]
- Wang, B.; Sun, Z.; Lam, S.S.; Sonne, C.; Yuan, T.Q.; Sun, R.C. A scalable and simple lignin-based polymer for ultra-efficient flocculation and sterilization. Sep. Purif. Technol. 2022, 292, 120960. [Google Scholar] [CrossRef]
- Georges, M.K.; Veregin, R.P.; Kazmaier, P.M.; Hamer, G.K. Narrow molecular weight resins by a free-radical polymerization process. Macromolecules 1993, 26, 2987–2988. [Google Scholar] [CrossRef]
- Binyuan, Z.; Keao, H.; Yongzhong, F.; Rinfang, Z.; Renjie, W. Infrared spectroscopic study on lignosulfonic acid and its derivatives. Chin. J. Anal. Chem. 2000, 120. [Google Scholar] [CrossRef] [Green Version]
- Fang, R.; Cheng, X.; Xu, X. Synthesis of lignin-base cationic flocculant and its application in removing anionic azo-dyes from simulated wastewater. Bioresour. Technol. 2010, 101, 7323–7329. [Google Scholar] [CrossRef]
- Salopek, B.; Krasic, D.; Filipovic, S. Measurement and application of zeta-potential. Rud.-Geol.-Naft. Zb. 1992, 4, 147. [Google Scholar]
- Dickinson, E.; Eriksson, L. Particle flocculation by adsorbing polymers. Adv. Colloid Interface Sci. 1991, 34, 1–29. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, X.; Song, G.; Lou, T. Microwave assisted copolymerization of sodium alginate and dimethyl diallyl ammonium chloride as flocculant for dye removal. Int. J. Biol. Macromol. 2020, 156, 585–590. [Google Scholar] [CrossRef]
- Jia, S.; Yang, Z.; Ren, K.; Tian, Z.; Dong, C.; Ma, R.; Yu, G.; Yang, W. Removal of antibiotics from water in the coexistence of suspended particles and natural organic matters using amino-acid-modified-chitosan flocculants: A combined experimental and theoretical study. J. Hazard. Mater. 2016, 317, 593–601. [Google Scholar] [CrossRef]
- Yu, X.; Somasundaran, P. Role of polymer conformation in interparticle-bridging dominated flocculation. J. Colloid Interface Sci. 1996, 177, 283–287. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Li, P.; Cai, J.; Xiao, S.; Yang, H.; Li, A. Efficient adsorption of both methyl orange and chromium from their aqueous mixtures using a quaternary ammonium salt modified chitosan magnetic composite adsorbent. Chemosphere 2016, 154, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Babeła, K.; Jurewicz, K. KOH activated lignin based nanostructured carbon exhibiting high hydrogen electrosorption. Carbon 2008, 46, 1948–1956. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H. Over the adsorption in solution. J. Phys. Chem 1906, 57, 1100–1107. [Google Scholar]
- Lagergren, S. Zur theorie der sogenannten adsorption geloster stoffe. K. Sven. Vetensk. Handl. 1898, 24, 1–39. [Google Scholar]
- Salam, O.E.A.; Reiad, N.A.; ElShafei, M.M. A study of the removal characteristics of heavy metals from wastewater by low-cost adsorbents. J. Adv. Res. 2011, 2, 297–303. [Google Scholar] [CrossRef] [Green Version]
- Elliott, H.; Huang, C. The adsorption characteristics of Cu (II) in the presence of chelating agents. J. Colloid Interface Sci. 1979, 70, 29–45. [Google Scholar] [CrossRef]
- Dickey, F.H. Specific adsorption. J. Phys. Chem. 1955, 59, 695–707. [Google Scholar] [CrossRef]
- Río, J.C.D.; Rencoret, J.; Prinsen, P.; Martínez, Á.T.; Ralph, J.; Gutiérrez, A. Structural characterization of wheat straw lignin as revealed by analytical pyrolysis, 2D-NMR, and reductive cleavage methods. J. Agric. Food Chem. 2012, 60, 5922–5935. [Google Scholar]
- Wen, J.L.; Sun, S.L.; Yuan, T.Q.; Xu, F.; Sun, R.C. Structural elucidation of lignin polymers of Eucalyptus chips during organosolv pretreatment and extended delignification. J. Agric. Food Chem. 2013, 61, 11067–11075. [Google Scholar] [CrossRef] [PubMed]
- Tolbert, A.; Akinosho, H.; Khunsupat, R.; Naskar, A.K.; Ragauskas, A.J. Characterization and analysis of the molecular weight of lignin for biorefining studies. Biofuels Bioprod. Biorefining 2014, 88, 836–856. [Google Scholar] [CrossRef]
- Fu, K.; Yue, Q.; Gao, B.; Sun, Y.; Zhu, L. Preparation, characterization and application of lignin-based activated carbon from black liquor lignin by steam activation. Chem. Eng. J. 2013, 228, 1074–1082. [Google Scholar] [CrossRef]
- Li, Z.; Chen, J.; Ge, Y. Removal of lead ion and oil droplet from aqueous solution by lignin-grafted carbon nanotubes. Chem. Eng. J. 2017, 308, 809–817. [Google Scholar] [CrossRef]
- Li, Z.; Xiao, D.; Ge, Y.; Koehler, S. Surface-functionalized porous lignin for fast and efficient lead removal from aqueous solution. ACS Appl. Mater. Interfaces 2015, 7, 15000–15009. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Peng, H.; Wang, Z.; Wu, J.; Liu, Z. Dye adsorption from aqueous solution by cellulose/chitosan composite: Equilibrium, kinetics, and thermodynamics. Fibers Polym. 2018, 19, 340–349. [Google Scholar] [CrossRef]
- Lakey, P.S.; Eichler, C.M.; Wang, C.; Little, J.C.; Shiraiwa, M. Kinetic multi-layer model of film formation, growth, and chemistry (KM-FILM): Boundary layer processes, multi-layer adsorption, bulk diffusion, and heterogeneous reactions. Indoor Air 2021, 31, 2070–2083. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Peng, L.; Han, S.; Hao, C.; Jiang, C.; Wang, H.; Fan, X. Effective removal of heavy metals from water using porous lignin-based adsorbents. Chemosphere 2021, 279, 130504. [Google Scholar] [CrossRef]
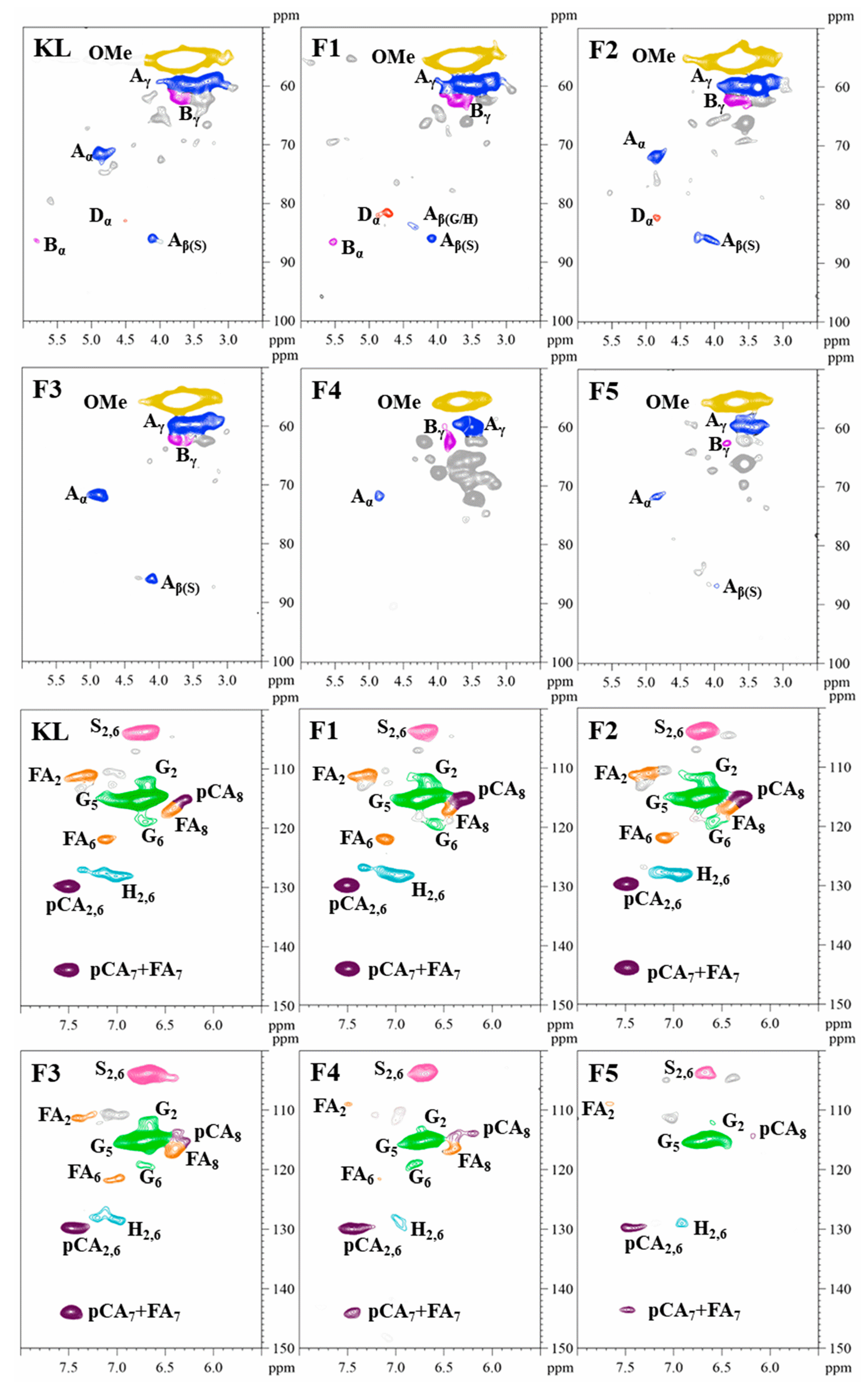

| Lignin Samples | Yield (%) | Mn (g/mol) | Mw (g/mol) | PDI | Total Phenolic−OH (mmol/g) | COOH (mmol/g) |
|---|---|---|---|---|---|---|
| KL | 881 | 2777 | 3.15 | 4.53 | 2.72 | |
| F1 | 15.00 | 781 | 1141 | 1.46 | 4.97 | 3.12 |
| F2 | 34.93 | 1885 | 2696 | 1.43 | 4.55 | 2.74 |
| F3 | 19.10 | 2807 | 3987 | 1.42 | 3.17 | 1.56 |
| F4 | 21.22 | 4355 | 6794 | 1.56 | 2.77 | 1.26 |
| F5 | 9.15 | 4676 | 9820 | 2.10 | 2.30 | 1.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Yang, D.; Li, X.; Zhu, X.; Jiang, J.; Zhang, Y.; Chen, X.; Yu, H. Customized Utilization Strategies of Industrial Lignin to Produce Adsorbents and Flocculants Based on Fractionation and Adequate Structural Interpretation. Int. J. Mol. Sci. 2022, 23, 6617. https://doi.org/10.3390/ijms23126617
Wang L, Yang D, Li X, Zhu X, Jiang J, Zhang Y, Chen X, Yu H. Customized Utilization Strategies of Industrial Lignin to Produce Adsorbents and Flocculants Based on Fractionation and Adequate Structural Interpretation. International Journal of Molecular Sciences. 2022; 23(12):6617. https://doi.org/10.3390/ijms23126617
Chicago/Turabian StyleWang, Lei, Dewei Yang, Xiaohan Li, Xinyi Zhu, Jungang Jiang, Yifan Zhang, Xue Chen, and Hongbo Yu. 2022. "Customized Utilization Strategies of Industrial Lignin to Produce Adsorbents and Flocculants Based on Fractionation and Adequate Structural Interpretation" International Journal of Molecular Sciences 23, no. 12: 6617. https://doi.org/10.3390/ijms23126617
APA StyleWang, L., Yang, D., Li, X., Zhu, X., Jiang, J., Zhang, Y., Chen, X., & Yu, H. (2022). Customized Utilization Strategies of Industrial Lignin to Produce Adsorbents and Flocculants Based on Fractionation and Adequate Structural Interpretation. International Journal of Molecular Sciences, 23(12), 6617. https://doi.org/10.3390/ijms23126617








