Intratracheal Transplantation of Mesenchymal Stem Cells Attenuates Hyperoxia-Induced Microbial Dysbiosis in the Lungs, Brain, and Gut in Newborn Rats
Abstract
:1. Introduction
2. Results
2.1. Body Weight and Lung Morphometry at P14
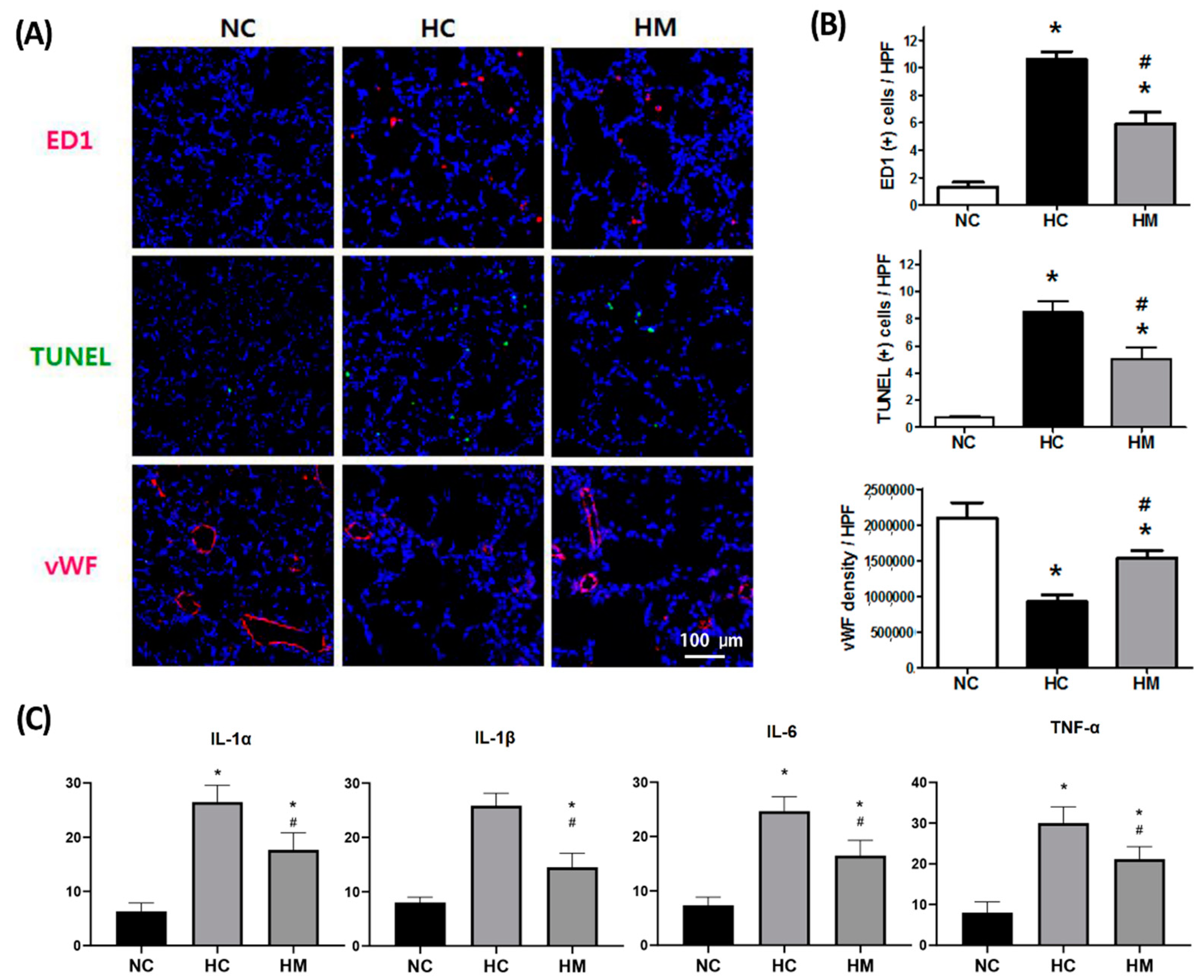
2.2. Changes in the Number of TUNEL- and ED-1-Positive Cells and Levels of Angiogenic and Inflammatory Markers
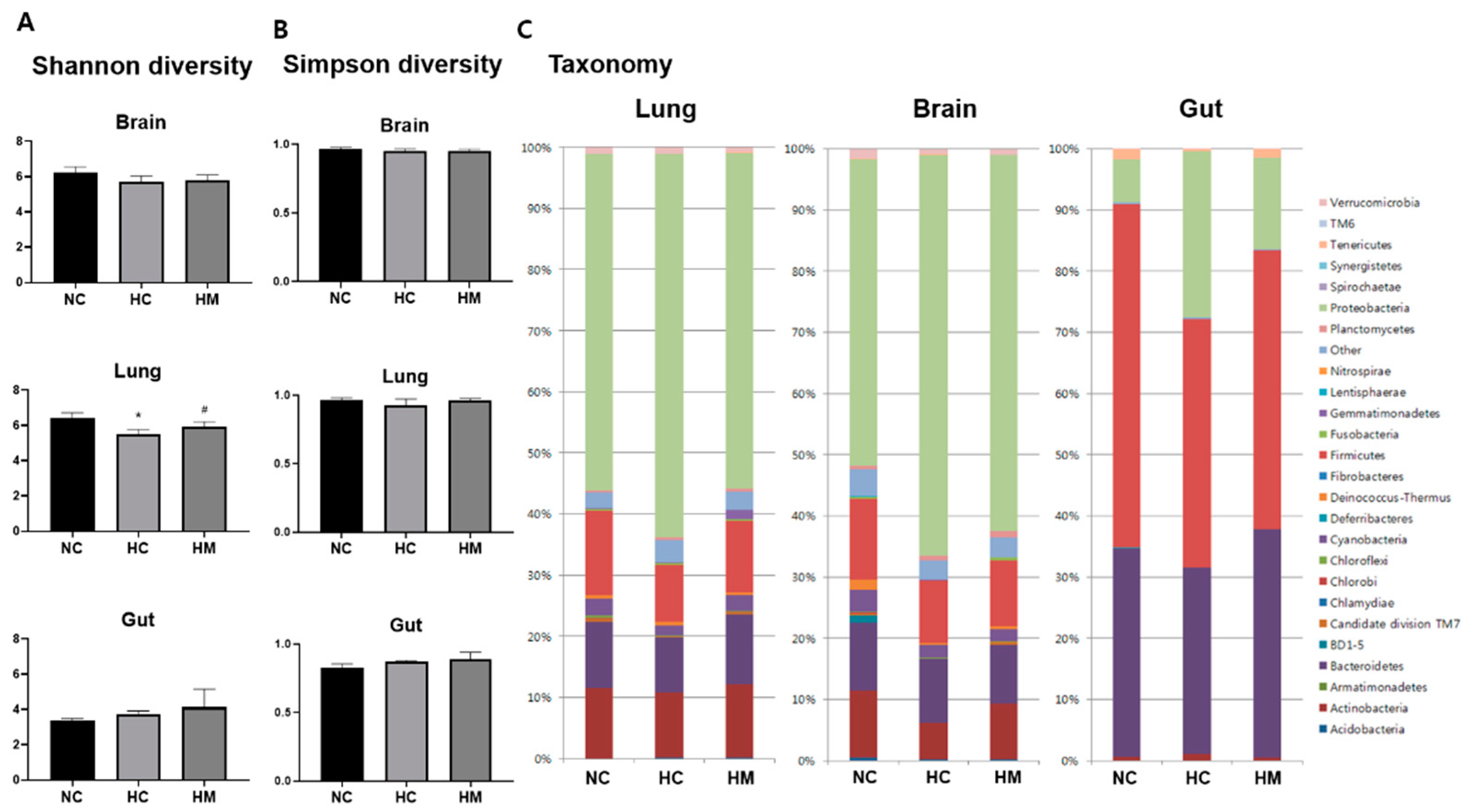
2.3. Relative Abundance of Microbial Taxa
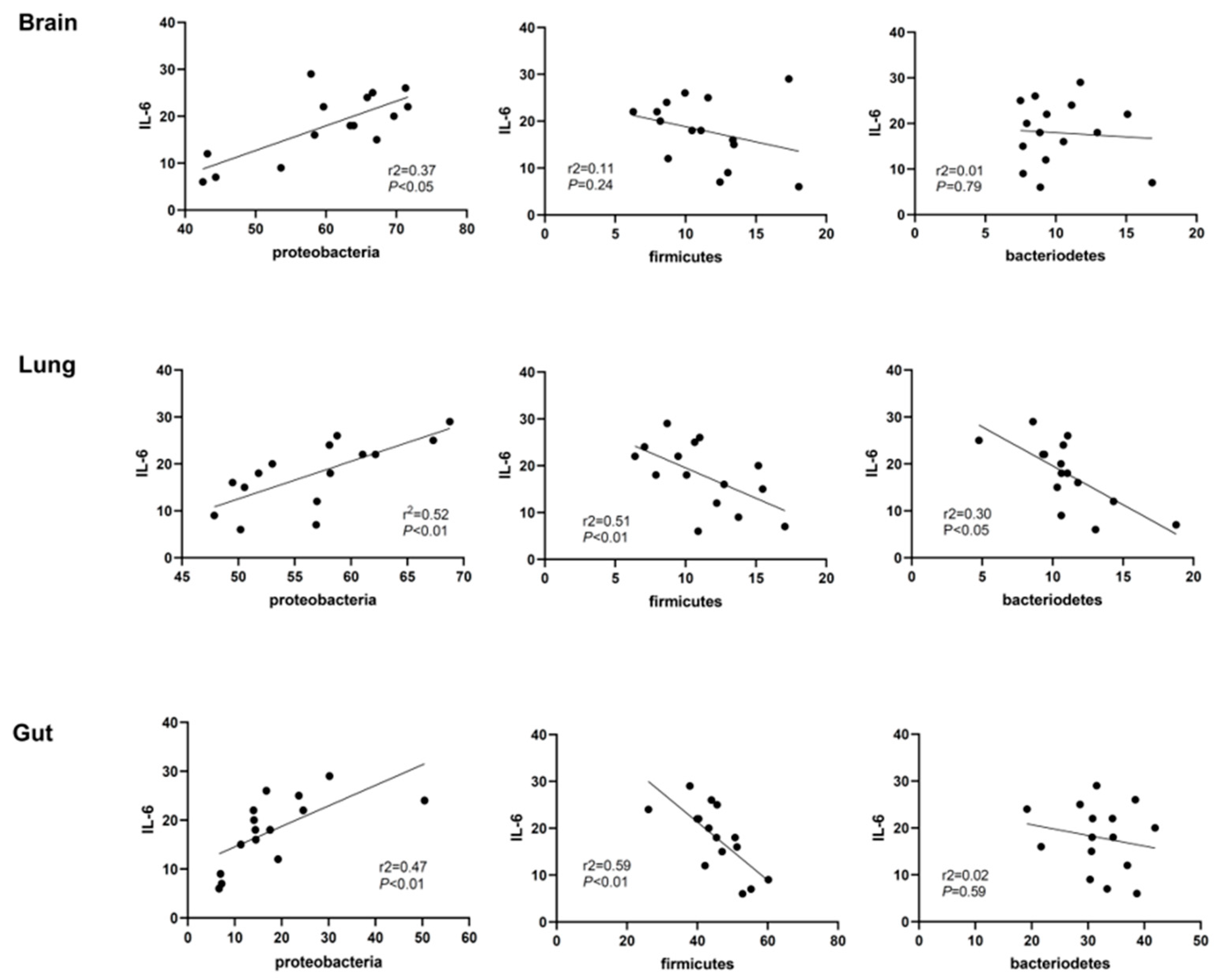
2.4. Correlation Analyses of Lung Inflammation and Lung, Brain, and Gut Microbiota
3. Discussion
4. Materials and Methods
4.1. Animal Model
4.2. Preparation of MSCs and Transplantation
4.3. Morphometric Analysis
4.4. TUNEL Staining
4.5. Quantification of ED-1-Positive Cells
4.6. ELISA Assay
4.7. Oxidative Stress Analysis
4.8. Barcoded Deep 454-Pyrosequencing of 16S rRNA Gene Amplicon
4.9. DNA Sequence Analysis and Taxonomical Identification
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhandari, A.; Panitch, H.B. Pulmonary outcomes in bronchopulmonary dysplasia. Semin. Perinatol. 2006, 30, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.Y.; Chang, Y.S.; Kim, S.Y.; Sung, D.K.; Kim, E.S.; Rime, S.Y.; Yu, W.J.; Choi, S.J.; Oh, W.I.; Park, W.S. Long-term (postnatal day 70) outcome and safety of intratracheal transplantation of human umbilical cord blood-derived mesenchymal stem cells in neonatal hyperoxic lung injury. Yonsei Med. J. 2013, 54, 416–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, S.Y.; Chang, Y.S.; Sung, D.K.; Yoo, H.S.; Sung, S.I.; Choi, S.J.; Park, W.S. Cell type-dependent variation in paracrine potency determines therapeutic efficacy against neonatal hyperoxic lung injury. Cytotherapy 2015, 17, 1025–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.S.; Choi, S.J.; Ahn, S.Y.; Sung, D.K.; Sung, S.I.; Yoo, H.S.; Oh, W.I.; Park, W.S. Timing of umbilical cord blood derived mesenchymal stem cells transplantation determines therapeutic efficacy in the neonatal hyperoxic lung injury. PLoS ONE 2013, 8, e52419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.S.; Choi, S.J.; Sung, D.K.; Kim, S.Y.; Oh, W.; Yang, Y.S.; Park, W.S. Intratracheal transplantation of human umbilical cord blood-derived mesenchymal stem cells dose-dependently attenuates hyperoxia-induced lung injury in neonatal rats. Cell Transplant. 2011, 20, 1843–1854. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.S.; Oh, W.; Choi, S.J.; Sung, D.K.; Kim, S.Y.; Choi, E.Y.; Kang, S.; Jin, H.J.; Yang, Y.S.; Park, W.S. Human umbilical cord blood-derived mesenchymal stem cells attenuate hyperoxia-induced lung injury in neonatal rats. Cell Transplant. 2009, 18, 869–886. [Google Scholar] [CrossRef] [Green Version]
- Ahn, S.Y.; Chang, Y.S.; Kim, J.H.; Sung, S.I.; Park, W.S. Two-Year Follow-Up Outcomes of Premature Infants Enrolled in the Phase I Trial of Mesenchymal Stem Cells Transplantation for Bronchopulmonary Dysplasia. J. Pediatr. 2017, 185, 49–54.e2. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.S.; Ahn, S.Y.; Yoo, H.S.; Sung, S.I.; Choi, S.J.; Oh, W.I.; Park, W.S. Mesenchymal stem cells for bronchopulmonary dysplasia: Phase 1 dose-escalation clinical trial. J. Pediatr. 2014, 164, 966–972.e6. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Chang, Y.S.; Lee, M.H.; Sung, S.I.; Lee, B.S.; Kim, K.S.; Kim, A.R.; Park, W.S. Stem cells for bronchopulmonary dysplasia in preterm infants: A randomized controlled phase II trial. Stem Cells Transl. Med. 2021, 10, 1129–1137. [Google Scholar] [CrossRef]
- Ames, N.J.; Ranucci, A.; Moriyama, B.; Wallen, G.R. The Human Microbiome and Understanding the 16S rRNA Gene in Translational Nursing Science. Nurs. Res. 2017, 66, 184–197. [Google Scholar] [CrossRef] [Green Version]
- Wagner, B.D.; Sontag, M.K.; Harris, J.K.; Miller, J.I.; Morrow, L.; Robertson, C.E.; Stephens, M.; Poindexter, B.B.; Abman, S.H.; Mourani, P.M. Airway Microbial Community Turnover Differs by BPD Severity in Ventilated Preterm Infants. PLoS ONE 2017, 12, e0170120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pammi, M.; Lal, C.V.; Wagner, B.D.; Mourani, P.M.; Lohmann, P.; Luna, R.A.; Sisson, A.; Shivanna, B.; Hollister, E.B.; Abman, S.H.; et al. Airway Microbiome and Development of Bronchopulmonary Dysplasia in Preterm Infants: A Systematic Review. J. Pediatr. 2019, 204, 126–133.e2. [Google Scholar] [CrossRef] [PubMed]
- Tirone, C.; Pezza, L.; Paladini, A.; Tana, M.; Aurilia, C.; Lio, A.; D’Ippolito, S.; Tersigni, C.; Posteraro, B.; Sanguinetti, M.; et al. Gut and Lung Microbiota in Preterm Infants: Immunological Modulation and Implication in Neonatal Outcomes. Front. Immunol. 2019, 10, 2910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, K.; Dong, W. Perspectives on Probiotics and Bronchopulmonary Dysplasia. Front. Pediatr. 2020, 8, 570247. [Google Scholar] [CrossRef] [PubMed]
- Gubert, C.; Kong, G.; Uzungil, V.; Zeleznikow-Johnston, A.M.; Burrows, E.L.; Renoir, T.; Hannan, A.J. Microbiome Profiling Reveals Gut Dysbiosis in the Metabotropic Glutamate Receptor 5 Knockout Mouse Model of Schizophrenia. Front. Cell Dev. Biol. 2020, 8, 582320. [Google Scholar] [CrossRef]
- Yu, G.; Gail, M.H.; Consonni, D.; Carugno, M.; Humphrys, M.; Pesatori, A.C.; Caporaso, N.E.; Goedert, J.J.; Ravel, J.; Landi, M.T. Characterizing human lung tissue microbiota and its relationship to epidemiological and clinical features. Genome Biol. 2016, 17, 163. [Google Scholar] [CrossRef] [Green Version]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Frank, D.N.; St Amand, A.L.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef] [Green Version]
- Henson, M.A.; Phalak, P. Microbiota dysbiosis in inflammatory bowel diseases: In silico investigation of the oxygen hypothesis. BMC Syst. Biol. 2017, 11, 145. [Google Scholar] [CrossRef]
- Rigottier-Gois, L. Dysbiosis in inflammatory bowel diseases: The oxygen hypothesis. ISME J. 2013, 7, 1256–1261. [Google Scholar] [CrossRef]
- Rivera-Chavez, F.; Lopez, C.A.; Baumler, A.J. Oxygen as a driver of gut dysbiosis. Free Radic. Biol. Med. 2017, 105, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Litvak, Y.; Byndloss, M.X.; Tsolis, R.M.; Baumler, A.J. Dysbiotic Proteobacteria expansion: A microbial signature of epithelial dysfunction. Curr. Opin. Microbiol. 2017, 39, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Ahn, S.Y.; Im, G.H.; Sung, D.K.; Park, Y.R.; Choi, S.H.; Choi, S.J.; Chang, Y.S.; Oh, W.; Lee, J.H.; et al. Human umbilical cord blood-derived mesenchymal stem cell transplantation attenuates severe brain injury by permanent middle cerebral artery occlusion in newborn rats. Pediatr. Res. 2012, 72, 277–284. [Google Scholar] [CrossRef] [Green Version]
- Lohmann, P.; Luna, R.A.; Hollister, E.B.; Devaraj, S.; Mistretta, T.A.; Welty, S.E.; Versalovic, J. The airway microbiome of intubated premature infants: Characteristics and changes that predict the development of bronchopulmonary dysplasia. Pediatr. Res. 2014, 76, 294–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pammi, M.; Cope, J.; Tarr, P.I.; Warner, B.B.; Morrow, A.L.; Mai, V.; Gregory, K.E.; Kroll, J.S.; McMurtry, V.; Ferris, M.J.; et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: A systematic review and meta-analysis. Microbiome 2017, 5, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Bafadhel, M.; Haldar, K.; Spivak, A.; Mayhew, D.; Miller, B.E.; Tal-Singer, R.; Johnston, S.L.; Ramsheh, M.Y.; Barer, M.R.; et al. Lung microbiome dynamics in COPD exacerbations. Eur. Respir. J. 2016, 47, 1082–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saugstad, O.D. The oxygen radical disease in neonatology. Indian J. Pediatr. 1989, 56, 585–593. [Google Scholar] [CrossRef]
- Panfoli, I.; Candiano, G.; Malova, M.; De Angelis, L.; Cardiello, V.; Buonocore, G.; Ramenghi, L.A. Oxidative Stress as a Primary Risk Factor for Brain Damage in Preterm Newborns. Front. Pediatr. 2018, 6, 369. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, G.L.V.; Oliveira, C.N.S.; Pinzan, C.F.; de Salis, L.V.V.; Cardoso, C.R.B. Microbiota Modulation of the Gut-Lung Axis in COVID-19. Front. Immunol. 2021, 12, 635471. [Google Scholar] [CrossRef]
- Schirmer, M.; Smeekens, S.P.; Vlamakis, H.; Jaeger, M.; Oosting, M.; Franzosa, E.A.; Ter Horst, R.; Jansen, T.; Jacobs, L.; Bonder, M.J.; et al. Linking the Human Gut Microbiome to Inflammatory Cytokine Production Capacity. Cell 2016, 167, 1125–1136.e1128. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Ramanan, D.; Rozenberg, M.; McGovern, K.; Rastelli, D.; Vijaykumar, B.; Yaghi, O.; Voisin, T.; Mosaheb, M.; Chiu, I.; et al. Interleukin-6 produced by enteric neurons regulates the number and phenotype of microbe-responsive regulatory T cells in the gut. Immunity 2021, 54, 499–513.e5. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.Y.; Sung, D.K.; Kim, Y.E.; Sung, S.; Chang, Y.S.; Park, W.S. Brain-derived neurotropic factor mediates neuroprotection of mesenchymal stem cell-derived extracellular vesicles against severe intraventricular hemorrhage in newborn rats. Stem Cells Transl. Med. 2021, 10, 374–384. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Chang, Y.S.; Sung, D.K.; Sung, S.I.; Ahn, J.Y.; Park, W.S. Pivotal Role of Brain Derived Neurotrophic Factor Secreted by Mesenchymal Stem Cells in Severe Intraventricular Hemorrhage in the Newborn Rats. Cell Transplant. 2016, 26, 145–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.K.; Lee, M.K.; Jin, H.J.; Kim, D.S.; Yang, Y.S.; Oh, W.; Yang, S.E.; Park, T.S.; Lee, S.Y.; Kim, B.S.; et al. Efficient intracytoplasmic labeling of human umbilical cord blood mesenchymal stromal cells with ferumoxides. Cell Transplant. 2007, 16, 849–857. [Google Scholar] [CrossRef] [Green Version]
- Jang, Y.K.; Jung, D.H.; Jung, M.H.; Kim, D.H.; Yoo, K.H.; Sung, K.W.; Koo, H.H.; Oh, W.; Yang, Y.S.; Yang, S.E. Mesenchymal stem cells feeder layer from human umbilical cord blood for ex vivo expanded growth and proliferation of hematopoietic progenitor cells. Ann. Hematol. 2006, 85, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Muhling, M.; Woolven-Allen, J.; Murrell, J.C.; Joint, I. Improved group-specific PCR primers for denaturing gradient gel electrophoresis analysis of the genetic diversity of complex microbial communities. ISME J. 2008, 2, 379–392. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Ondov, B.D.; Bergman, N.H.; Phillippy, A.M. Interactive metagenomic visualization in a Web browser. BMC Bioinform. 2011, 12, 385. [Google Scholar] [CrossRef] [Green Version]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]

| Lung Phylum | NC | HC | HM |
| Proteobacteria | 51.66 ± 4.69 | 62.69 ± 4.42 * | 52.69 ± 3.63 # |
| Firmicutes | 13.9 ± 3.08 | 8.89 ± 1.87 * | 11.78 ± 2.97 |
| Bacteroidetes | 14.15 ± 4.19 | 8.99 ± 2.27 | 11.81 ± 1.67 |
| Actinobacteria | 11.5 ± 2.75 | 10.54 ± 3.74 | 11.66 ± 3.01 |
| Cyanobacteria | 2.7 ± 1.64 | 1.69 ± 0.87 | 2.02 ± 0.99 |
| Brain Phylum | NC | HC | HM |
| Proteobacteria | 48.16 ± 8.24 | 65.49 ± 5.74 * | 61.53 ± 8.82 |
| Firmicutes | 14.5 ± 3.09 | 10.30 ± 3.89 | 10.75 ± 2.06 |
| Bacteroidetes | 11.15 ± 4.97 | 10.56 ± 2.73 | 9.63 ± 1.8 |
| Actinobacteria | 13.01 ± 4.26 | 5.94 ± 2.66 * | 9.12 ± 8.08 |
| Cyanobacteria | 3.23 ± 0.5 | 2.07 ± 0.27 * | 1.86 ± 0.32 |
| Gut Phylum | NC | HC | HM |
| Firmicutes | 56.11 ± 0.36 | 39.0 ± 6.91 * | 46.59 ± 3.45 *,# |
| Bacteroidetes | 34.16 ± 4.2 | 30.48 ± 6.48 | 32.73 ± 6.27 |
| Proteobacteria | 6.96 ± 0.27 | 26.63 ± 13.06 * | 15.20 ± 2.8 |
| Tenericutes | 1.66 ± 1.5 | 1.08 ± 1.7 | 0.95 ± 1.75 |
| Actinobacteria | 0.57 ± 0.3 | 0.87 ± 1.15 | 1.61 ± 0.72 |
| Lung Phylum | Lung Genus | NC | HC | HM |
| Proteobacteria | Methylophaga | 10.51 ± 4.65 | 12.85 ± 2.79 | 7.07 ± 3.03 # |
| Proteobacteria | Sphingomonas | 8.44 ± 0.89 | 8.80 ± 1.56 | 6.71 ± 2.29 |
| Actinomycetota | Priopionibacterium | 6.11 ± 0.88 | 5.30 ± 3.21 | 7.05 ± 4.11 |
| Proteobacteria | Aeromonas | 5.21 ± 1.47 | 3.41 ± 0.72 | 3.83 ± 1.76 |
| Bacillota | Streoptococcus | 4.33 ± 0.41 | 3.86 ± 1.33 | 3.89 ± 1.97 |
| Brain Phylum | Brain Genus | NC | HC | HM |
| Proteobacteria | Methylophaga | 7.99 ± 0.70 | 15.14 ± 5.72 * | 15.54 ± 5.21 |
| Proteobacteria | Sphingomonas | 6.76 ± 0.74 | 9.67 ± 2.15 | 8.45 ± 1.52 |
| Bacillota | Streoptococcus | 4.81 ± 2.35 | 5.29 ± 2.72 | 5.55 ± 1.50 |
| Proteobacteria | Aeromonas | 3.16 ± 0.13 | 4.90 ± 1.12 * | 4.04 ± 1.67 |
| Actinomycetota | Priopionibacterium | 5.44 ± 1.45 | 2.89 ± 1.43 * | 4.72 ± 4.70 |
| Gut Phylum | Gut Genus | NC | HC | HM |
| Firmicutes | Lactobacillus | 43.78 ± 8.29 | 22.27 ± 9.58 * | 34.51 ± 4.61 # |
| Bacteroidetes | Bacteroides | 21.52 ± 4.49 | 14.22 ± 6.39 | 17.50 ± 10.03 |
| Bacteroidetes | Parabacteroides | 12.61 ± 4.02 | 15.78 ± 2.02 | 10.57 ± 6.97 |
| Proteobacteria | Escherichia-Shigella | 4.00 ± 0.82 | 19.59 ± 6.18 * | 8.32 ± 6.50 # |
| Unknown | Unknown | 4.90 ± 4.24 | 7.75 ± 4.59 | 2.73 ± 2.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, S.Y.; Sung, D.K.; Chang, Y.S.; Park, W.S. Intratracheal Transplantation of Mesenchymal Stem Cells Attenuates Hyperoxia-Induced Microbial Dysbiosis in the Lungs, Brain, and Gut in Newborn Rats. Int. J. Mol. Sci. 2022, 23, 6601. https://doi.org/10.3390/ijms23126601
Ahn SY, Sung DK, Chang YS, Park WS. Intratracheal Transplantation of Mesenchymal Stem Cells Attenuates Hyperoxia-Induced Microbial Dysbiosis in the Lungs, Brain, and Gut in Newborn Rats. International Journal of Molecular Sciences. 2022; 23(12):6601. https://doi.org/10.3390/ijms23126601
Chicago/Turabian StyleAhn, So Yoon, Dong Kyung Sung, Yun Sil Chang, and Won Soon Park. 2022. "Intratracheal Transplantation of Mesenchymal Stem Cells Attenuates Hyperoxia-Induced Microbial Dysbiosis in the Lungs, Brain, and Gut in Newborn Rats" International Journal of Molecular Sciences 23, no. 12: 6601. https://doi.org/10.3390/ijms23126601
APA StyleAhn, S. Y., Sung, D. K., Chang, Y. S., & Park, W. S. (2022). Intratracheal Transplantation of Mesenchymal Stem Cells Attenuates Hyperoxia-Induced Microbial Dysbiosis in the Lungs, Brain, and Gut in Newborn Rats. International Journal of Molecular Sciences, 23(12), 6601. https://doi.org/10.3390/ijms23126601






