Platelets and the Role of P2X Receptors in Nociception, Pain, Neuronal Toxicity and Thromboinflammation
Abstract
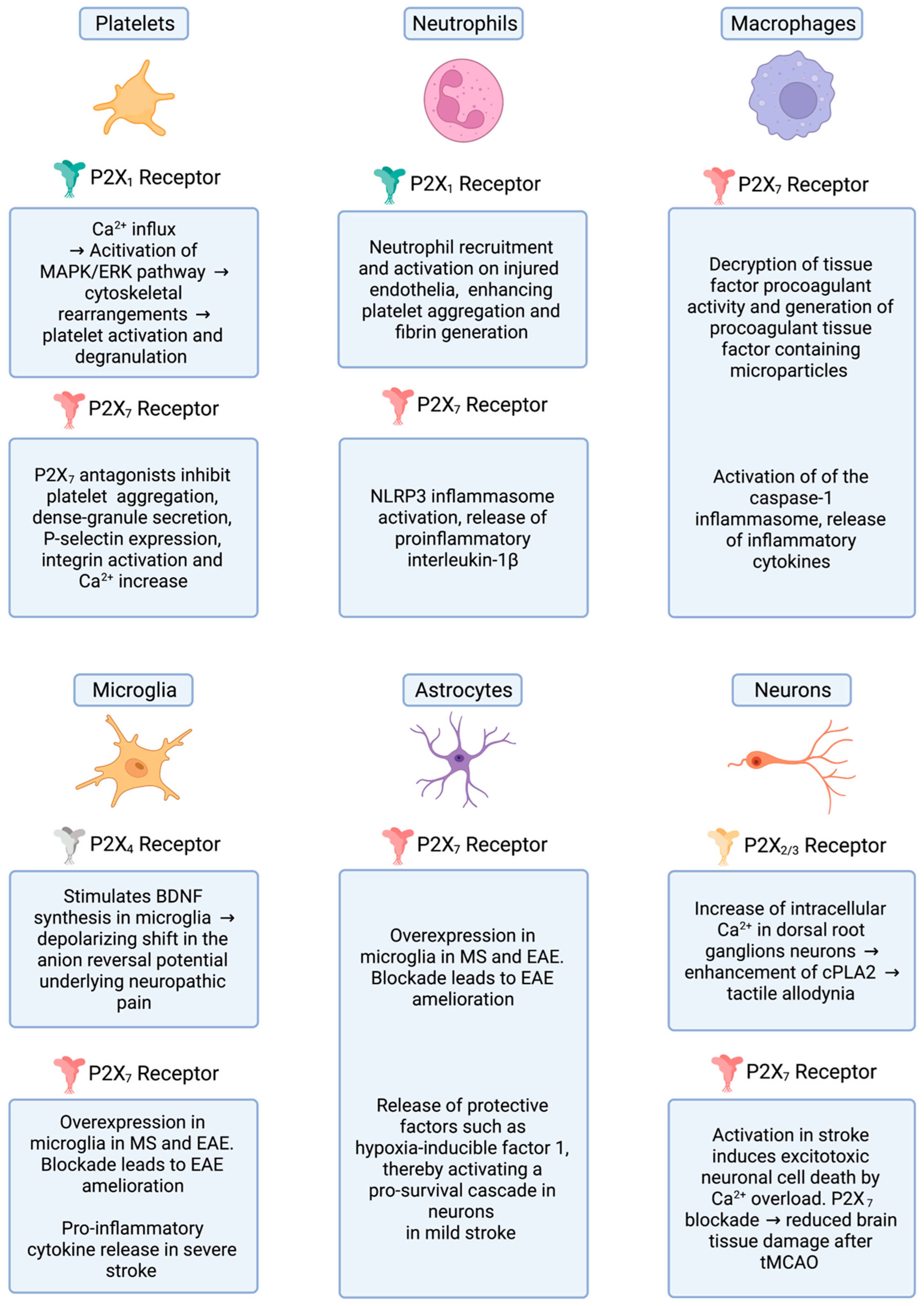
:1. The P2X Receptor Family
2. Regulation of Platelet Function by Purinergic Receptors
3. Platelet-Mediated Inflammation in MS/EAE
P2X Receptors in Familial Multiple Sclerosis
4. Platelet Activation in Alzheimer’s Disease
5. Purinergic Receptors in Parkinson’s Disease
6. P2X Receptors, Thromboinflammation and Brain Ischemia
7. P2X Receptors, Neuropathic Pain and Nociception
8. Potential Translational and Clinical Applications
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- North, R.A. P2X receptors. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150427. [Google Scholar] [CrossRef] [PubMed]
- Illes, P.; Müller, C.E.; Jacobson, K.A.; Grutter, T.; Nicke, A.; Fountain, S.J.; Kennedy, C.; Schmalzing, G.; Jarvis, M.F.; Stojilkovic, S.S.; et al. Update of P2X receptor properties and their pharmacology: IUPHAR Review 30. Br. J. Pharmacol. 2021, 178, 489–514. [Google Scholar] [CrossRef] [PubMed]
- Gever, J.R.; Cockayne, D.A.; Dillon, M.P.; Burnstock, G.; Ford, A.P. Pharmacology of P2X channels. Pharmacol. P2X Channels 2006, 452, 513–537. [Google Scholar] [CrossRef] [PubMed]
- Oury, C.; Lecut, C.; Hego, A.; Wéra, O.; Delierneux, C. Purinergic control of inflammation and thrombosis: Role of P2X1 receptors. Comput. Struct. Biotechnol. J. 2014, 13, 106–110. [Google Scholar] [CrossRef] [Green Version]
- Dosch, M.; Gerber, J.; Jebbawi, F.; Beldi, G. Mechanisms of ATP Release by Inflammatory Cells. Int. J. Mol. Sci. 2018, 19, 1222. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Karlsson, L.; Moses, S.; Hultgårdh-Nilsson, A.; Andersson, M.; Borna, C.; Gudbjartsson, T.; Jern, S.; Erlinge, D. P2 Receptor Expression Profiles in Human Vascular Smooth Muscle and Endothelial Cells. J. Cardiovasc. Pharmacol. 2002, 40, 841–853. [Google Scholar] [CrossRef]
- Mulryan, K.; Gitterman, D.P.; Lewis, C.J.; Vial, C.; Leckie, B.J.; Cobb, A.L.; Brown, J.E.; Conley, E.C.; Buell, G.; Pritchard, C.A.; et al. Reduced vas deferens contraction and male infertility in mice lacking P2X1 receptors. Nature 2000, 403, 86–89. [Google Scholar] [CrossRef]
- Wewers, M.D.; Sarkar, A. P2X7 receptor and macrophage function. Purinergic Signal. 2009, 5, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Kawano, A.; Tsukimoto, M.; Noguchi, T.; Hotta, N.; Harada, H.; Takenouchi, T.; Kitani, H.; Kojima, S. Involvement of P2X4 receptor in P2X7 receptor-dependent cell death of mouse macrophages. Biochem. Biophys. Res. Commun. 2012, 419, 374–380. [Google Scholar] [CrossRef]
- Gachet, C. P2 receptors, platelet function and pharmacological implications. Thromb. Haemost. 2008, 99, 466–472. [Google Scholar] [CrossRef]
- Khakh, B.S.; North, R.A. Neuromodulation by Extracellular ATP and P2X Receptors in the CNS. Neuron 2012, 76, 51–69. [Google Scholar] [CrossRef] [Green Version]
- Kuan, Y.-H.; Shyu, B.-C. Nociceptive transmission and modulation via P2X receptors in central pain syndrome. Mol. Brain 2016, 9, 58. [Google Scholar] [CrossRef] [Green Version]
- Golebiewska, E.M.; Poole, A.W. Platelet secretion: From haemostasis to wound healing and beyond. Blood Rev. 2015, 29, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Savage, B.; Saldívar, E.; Ruggeri, Z.M. Initiation of Platelet Adhesion by Arrest onto Fibrinogen or Translocation on von Willebrand Factor. Cell 1996, 84, 289–297. [Google Scholar] [CrossRef] [Green Version]
- Schneider, S.W.; Nuschele, S.; Wixforth, A.; Gorzelanny, C.; Alexander-Katz, A.; Netz, R.R.; Schneider, M.F. Shear-induced unfolding triggers adhesion of von Willebrand factor fibers. Proc. Natl. Acad. Sci. USA 2007, 104, 7899–7903. [Google Scholar] [CrossRef] [Green Version]
- Furie, B.; Furie, B.C. Thrombus formation in vivo. J. Clin. Investig. 2005, 115, 3355–3362. [Google Scholar] [CrossRef]
- Harrison, P.; Cramer, E.M. Platelet alpha-granules. Blood Rev. 1993, 7, 52–62. [Google Scholar] [CrossRef]
- Blair, P.; Flaumenhaft, R. Platelet α-granules: Basic biology and clinical correlates. Blood Rev. 2009, 23, 177–189. [Google Scholar] [CrossRef] [Green Version]
- Storey, R.; Thomas, M.R. The role of platelets in inflammation. Thromb. Haemost. 2015, 114, 449–458. [Google Scholar] [CrossRef]
- Hechler, B.; Lenain, N.; Marchese, P.; Vial, C.; Heim, V.; Freund, M.; Cazenave, J.-P.; Cattaneo, M.; Ruggeri, Z.M.; Evans, R.; et al. A Role of the Fast ATP-gated P2X1 Cation Channel in Thrombosis of Small Arteries In Vivo. J. Exp. Med. 2003, 198, 661–667. [Google Scholar] [CrossRef] [Green Version]
- Oury, C.; Sticker, E.; Cornelissen, H.; de Vos, R.; Vermylen, J.; Hoylaerts, M.F. ATP augments von Willebrand factor-dependent shear-induced platelet aggregation through Ca2+-calmodulin and myosin light chain kinase activation. J. Biol. Chem. 2004, 279, 26266–26273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hechler, B.; Cattaneo, M.; Gachet, C. The P2 Receptors in Platelet Function. Semin. Thromb. Hemost. 2005, 31, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Oury, C.C.; Kuijpers, M.J.E.; Toth-Zsamboki, E.; Bonnefoy, A.; Danloy, S.; Vreys, I.; Feijge, M.A.H.; de Vos, R.; Vermylen, J.; Heemskerk, J.W.M.; et al. Overexpression of the platelet P2X1 ion channel in transgenic mice generates a novel prothrombotic phenotype. Blood 2003, 101, 3969–3976. [Google Scholar] [CrossRef] [PubMed]
- Hechler, B.; Gachet, C. Purinergic Receptors in Thrombosis and Inflammation. Arter. Thromb. Vasc. Biol. 2015, 35, 2307–2315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grenegård, M.; Vretenbrant-Öberg, K.; Nylander, M.; Désilets, S.; Lindström, E.G.; Larsson, A.; Ramström, I.; Ramström, S.; Lindahl, T.L. The ATP-gated P2X1 Receptor Plays a Pivotal Role in Activation of Aspirin-treated Platelets by Thrombin and Epinephrine. J. Biol. Chem. 2008, 283, 18493–18504. [Google Scholar] [CrossRef] [Green Version]
- Furlan-Freguia, C.; Marchese, P.; Gruber, A.; Ruggeri, Z.M.; Ruf, W. P2X7 receptor signaling contributes to tissue factor-dependent thrombosis in mice. J. Clin. Investig. 2011, 121, 2932–2944. [Google Scholar] [CrossRef] [Green Version]
- Ming, Y.; Xin, G.; Ji, B.; Ji, C.; Wei, Z.; Zhang, B.; Zhang, J.; Yu, K.; Zhang, X.; Li, S.; et al. Entecavir as a P2X7R antagonist ameliorates platelet activation and thrombus formation. J. Pharmacol. Sci. 2020, 144, 43–51. [Google Scholar] [CrossRef]
- Kim, S.; Kunapuli, S.P. P2Y12 receptor in platelet activation. Platelets 2011, 22, 56–60. [Google Scholar] [CrossRef]
- Mezger, M.; Nording, H.; Sauter, R.; Graf, T.; Heim, C.; Von Bubnoff, N.; Ensminger, S.M.; Langer, H.F. Platelets and Immune Responses During Thromboinflammation. Front. Immunol. 2019, 10, 1731. [Google Scholar] [CrossRef]
- Rawish, E.; Sauter, M.; Sauter, R.; Nording, H.; Langer, H.F. Complement, inflammation and thrombosis. Br. J. Pharmacol. 2021, 178, 2892–2904. [Google Scholar] [CrossRef]
- Rawish, E.; Nording, H.; Münte, T.; Langer, H.F. Platelets as Mediators of Neuroinflammation and Thrombosis. Front. Immunol. 2020, 11, 548631. [Google Scholar] [CrossRef]
- Gilhus, N.E.; Deuschl, G. Neuroinflammation—A common thread in neurological disorders. Nat. Rev. Neurol. 2019, 15, 429–430. [Google Scholar] [CrossRef]
- Rawish, E.; Nickel, L.; Schuster, F.; Stölting, I.; Frydrychowicz, A.; Saar, K.; Hübner, N.; Othman, A.; Kuerschner, L.; Raasch, W.; et al. Telmisartan prevents development of obesity and normalizes hypothalamic lipid droplets. J. Endocrinol. 2020, 244, 95–110. [Google Scholar] [CrossRef]
- Leiter, O.; Walker, T.L. Platelets in Neurodegenerative Conditions-Friend or Foe? Front. Immunol. 2020, 11, 747. [Google Scholar] [CrossRef]
- Filippi, M.; Bar-Or, A.; Piehl, F.; Preziosa, P.; Solari, A.; Vukusic, S.; Rocca, M.A. Multiple sclerosis. Nat. Rev. Dis. Primers 2018, 4, 43. [Google Scholar] [CrossRef]
- Lock, C.; Hermans, G.; Pedotti, R.; Brendolan, A.; Schadt, E.; Garren, H.; Langer-Gould, A.; Strober, S.; Cannella, B.; Allard, J.; et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat. Med. 2002, 8, 500–508. [Google Scholar] [CrossRef]
- Langer, H.F.; Choi, E.Y.; Zhou, H.; Schleicher, R.; Chung, K.J.; Tang, Z.; Gobel, K.; Bdeir, K.; Chatzigeorgiou, A.; Wong, C.; et al. Platelets contribute to the pathogenesis of experimental autoimmune encephalomyelitis. Circ. Res. 2012, 110, 1202–1210. [Google Scholar] [CrossRef] [Green Version]
- Callea, L.; Arese, M.; Orlandini, A.; Bargnani, C.; Priori, A.; Bussolino, F. Platelet activating factor is elevated in cerebral spinal fluid and plasma of patients with relapsing–remitting multiple sclerosis. J. Neuroimmunol. 1999, 94, 212–221. [Google Scholar] [CrossRef]
- Kihara, Y.; Ishii, S.; Kita, Y.; Toda, A.; Shimada, A.; Shimizu, T. Dual phase regulation of experimental allergic encephalomyelitis by platelet-activating factor. J. Exp. Med. 2005, 202, 853–863. [Google Scholar] [CrossRef]
- D’Souza, C.S.; Li, Z.; Maxwell, D.L.; Trusler, O.; Murphy, M.; Crewther, S.; Peter, K.; Orian, J.M. Platelets Drive Inflammation and Target Gray Matter and the Retina in Autoimmune-Mediated Encephalomyelitis. J. Neuropathol. Exp. Neurol. 2018, 77, 567–576. [Google Scholar] [CrossRef]
- Qin, C.; Zhou, J.; Gao, Y.; Lai, W.; Yang, C.; Cai, Y.; Chen, S.; Du, C. Critical Role of P2Y12 Receptor in Regulation of Th17 Differentiation and Experimental Autoimmune Encephalomyelitis Pathogenesis. J. Immunol. 2017, 199, 72–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grygorowicz, T.; Welniak-Kaminska, M.; Strużyńska, L. Early P2X7R-related astrogliosis in autoimmune encephalomyelitis. Mol. Cell. Neurosci. 2016, 74, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yiangou, Y.; Facer, P.; Durrenberger, P.; Chessell, I.P.; Naylor, A.; Bountra, C.; Banati, R.R.; Anand, P. COX-2, CB2 and P2X7-immunoreactivities are increased in activated microglial cells/macrophages of multiple sclerosis and amyotrophic lateral sclerosis spinal cord. BMC Neurol. 2006, 6, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narcisse, L.; Scemes, E.; Zhao, Y.; Lee, S.C.; Brosnan, C.F. The cytokine IL-1beta transiently enhances P2X7 receptor expression and function in human astrocytes. Glia 2005, 49, 245–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matute, C.; Torre, I.; Pérez-Cerdá, F.; Pérez-Samartín, A.; Alberdi, E.; Etxebarria, E.; Arranz, A.M.; Ravid, R.; Rodríguez-Antigüedad, A.; Sánchez-Gómez, M.; et al. P2X(7) receptor blockade prevents ATP excitotoxicity in oligodendrocytes and ameliorates experimental autoimmune encephalomyelitis. J. Neurosci. 2007, 27, 9525–9533. [Google Scholar] [CrossRef]
- Zabala, A.; Vazquez-Villoldo, N.; Rissiek, B.; Gejo, J.; Martin, A.; Palomino, A.; Perez-Samartín, A.; Pulagam, K.R.; Lukowiak, M.; Capetillo-Zarate, E.; et al. P2X4 receptor controls microglia activation and favors remyelination in autoimmune encephalitis. EMBO Mol. Med. 2018, 10, e8743. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef]
- Li, Q.X.; Whyte, S.; Tanner, J.E.; Evin, G.; Beyreuther, K.; Masters, C.L. Secretion of Alzheimer’s disease Abeta amyloid peptide by activated human platelets. Lab. Investig. J. Tech. Methods Pathol. 1998, 78, 461–469. [Google Scholar]
- Bush, A.I.; Martins, R.N.; Rumble, B.; Moir, R.; Fuller, S.; Milward, E.; Currie, J.; Ames, D.; Weidemann, A.; Fischer, P.; et al. The amyloid precursor protein of Alzheimer’s disease is released by human platelets. J. Biol. Chem. 1990, 265, 15977–15983. [Google Scholar] [CrossRef]
- Chen, M.; Inestrosa, N.C.; Ross, G.S.; Fernandez, H.L. Platelets are the primary source of amyloid beta-peptide in human blood. Biochem. Biophys. Res. Commun. 1995, 213, 96–103. [Google Scholar] [CrossRef]
- Li, Q.X.; Evin, G.; Small, D.H.; Multhaup, G.; Beyreuther, K.; Masters, C.L. Proteolytic processing of Alzheimer’s disease beta A4 amyloid precursor protein in human platelets. J. Biol. Chem. 1995, 270, 14140–14147. [Google Scholar] [CrossRef] [Green Version]
- Kowalska, M.A.; Badellino, K. Beta-Amyloid protein induces platelet aggregation and supports platelet adhesion. Biochem. Biophys. Res. Commun. 1994, 205, 1829–1835. [Google Scholar] [CrossRef]
- Visconte, C.; Canino, J.; Guidetti, G.F.; Zarà, M.; Seppi, C.; Abubaker, A.A.; Pula, G.; Torti, M.; Canobbio, I. Amyloid precursor protein is required for in vitro platelet adhesion to amyloid peptides and potentiation of thrombus formation. Cell. Signal. 2018, 52, 95–102. [Google Scholar] [CrossRef]
- Sevush, S.; Jy, W.; Horstman, L.L.; Mao, W.-W.; Kolodny, L.; Ahn, Y.S. Platelet Activation in Alzheimer Disease. Arch. Neurol. 1998, 55, 530–536. [Google Scholar] [CrossRef]
- Ciabattoni, G.; Porreca, E.; di Febbo, C.; di Iorio, A.; Paganelli, R.; Bucciarelli, T.; Pescara, L.; del Re, L.; Giusti, C.; Falco, A.; et al. Determinants of platelet activation in Alzheimer’s disease. Neurobiol. Aging 2007, 28, 336–342. [Google Scholar] [CrossRef]
- Jarre, A.; Gowert, N.S.; Donner, L.; Munzer, P.; Klier, M.; Borst, O.; Schaller, M.; Lang, F.; Korth, C.; Elvers, M. Pre-activated blood platelets and a pro-thrombotic phenotype in APP23 mice modeling Alzheimer’s disease. Cell. Signal. 2014, 26, 2040–2050. [Google Scholar] [CrossRef]
- Canobbio, I.; Visconte, C.; Oliviero, B.; Guidetti, G.; Zara, M.; Pula, G.; Torti, M. Increased platelet adhesion and thrombus formation in a mouse model of Alzheimer’s disease. Cell. Signal. 2016, 28, 1863–1871. [Google Scholar] [CrossRef] [Green Version]
- Johnston, J.A.; Liu, W.W.; Coulson, D.T.; Todd, S.; Murphy, S.; Brennan, S.; Foy, C.J.; Craig, D.; Irvine, G.B.; Passmore, A.P. Platelet beta-secretase activity is increased in Alzheimer’s disease. Neurobiol. Aging 2008, 29, 661–668. [Google Scholar] [CrossRef]
- Kniewallner, K.M.; Wenzel, D.; Humpel, C. Thiazine Red+ platelet inclusions in Cerebral Blood Vessels are first signs in an Alzheimer’s Disease mouse model. Sci. Rep. 2016, 6, 28447. [Google Scholar] [CrossRef] [Green Version]
- Donner, L.; Falker, K.; Gremer, L.; Klinker, S.; Pagani, G.; Ljungberg, L.U.; Lothmann, K.; Rizzi, F.; Schaller, M.; Gohlke, H.; et al. Platelets contribute to amyloid-beta aggregation in cerebral vessels through integrin alphaIIbbeta3-induced outside-in signaling and clusterin release. Sci. Signal. 2016, 9, ra52. [Google Scholar] [CrossRef]
- Kniewallner, K.M.; Foidl, B.M.; Humpel, C. Platelets isolated from an Alzheimer mouse damage healthy cortical vessels and cause inflammation in an organotypic ex vivo brain slice model. Sci. Rep. 2018, 8, 15483. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Orellana, F.; Fuentes-Fuentes, M.C.; Godoy, P.A.; Silva-Grecchi, T.; Panes, J.D.; Guzmán, L.; Yévenes, G.E.; Gavilán, J.; Egan, T.M.; Aguayo, L.G.; et al. P2X receptor overexpression induced by soluble oligomers of amyloid beta peptide potentiates synaptic failure and neuronal dyshomeostasis in cellular models of Alzheimer’s disease. Neuropharmacology 2018, 128, 366–378. [Google Scholar] [CrossRef]
- Godoy, P.A.; Molina, O.R.; Fuentealba, J. Exploring the Role of P2X Receptors in Alzheimer’s Disease. Front. Pharmacol. 2019, 10, 1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parvathenani, L.K.; Tertyshnikova, S.; Greco, C.R.; Roberts, S.B.; Robertson, B.; Posmantur, R. P2X7 mediates superoxide production in primary microglia and is up-regulated in a transgenic mouse model of Alzheimer’s disease. J. Biol. Chem. 2003, 278, 13309–13317. [Google Scholar] [CrossRef] [Green Version]
- McLarnon, J.G.; Ryu, J.K.; Walker, D.G.; Choi, H.B. Upregulated expression of purinergic P2X(7) receptor in Alzheimer disease and amyloid-beta peptide-treated microglia and in peptide-injected rat hippocampus. J. Neuropathol. Exp. Neurol. 2006, 65, 1090–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, J.K.; McLarnon, J.G. Block of purinergic P2X(7) receptor is neuroprotective in an animal model of Alzheimer’s disease. Neuroreport 2008, 19, 1715–1719. [Google Scholar] [CrossRef] [PubMed]
- Delarasse, C.; Auger, R.; Gonnord, P.; Fontaine, B.; Kanellopoulos, J.M. The purinergic receptor P2X7 triggers alpha-secretase-dependent processing of the amyloid precursor protein. J. Biol. Chem. 2011, 286, 2596–2606. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Frailes, C.; di Lauro, C.; Bianchi, C.; de Diego-García, L.; Sebastián-Serrano, Á.; Boscá, L.; Díaz-Hernández, M. Amyloid Peptide Induced Neuroinflammation Increases the P2X7 Receptor Expression in Microglial Cells, Impacting on Its Functionality. Front. Cell. Neurosci. 2019, 13, 143. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Hu, J.; Jiang, L.; Xu, S.; Zheng, B.; Wang, C.; Zhang, J.; Wei, X.; Chang, L.; Wang, Q. Brilliant Blue G improves cognition in an animal model of Alzheimer’s disease and inhibits amyloid-β-induced loss of filopodia and dendrite spines in hippocampal neurons. Neuroscience 2014, 279, 94–101. [Google Scholar] [CrossRef]
- Diaz-Hernandez, J.I.; Gomez-Villafuertes, R.; León-Otegui, M.; Hontecillas-Prieto, L.; del Puerto, A.; Trejo, J.L.; Lucas, J.J.; Garrido, J.J.; Gualix, J.; Miras-Portugal, M.T.; et al. In vivo P2X7 inhibition reduces amyloid plaques in Alzheimer’s disease through GSK3β and secretases. Neurobiol. Aging 2012, 33, 1816–1828. [Google Scholar] [CrossRef] [Green Version]
- Martin, E.; Amar, M.; Dalle, C.; Youssef, I.; Boucher, C.; Le Duigou, C.; Brückner, M.; Prigent, A.; Sazdovitch, V.; Halle, A.; et al. New role of P2X7 receptor in an Alzheimer’s disease mouse model. Mol. Psychiatry 2018, 24, 108–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Lau, L.M.; Breteler, M.M. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006, 5, 525–535. [Google Scholar] [CrossRef]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.-E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef] [PubMed]
- Salat, D.; Tolosa, E. Levodopa in the treatment of Parkinson’s disease: Current status and new developments. J. Parkinsons Dis. 2013, 3, 255–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcellino, D.; Suárez-Boomgaard, D.; Sánchez-Reina, M.D.; Aguirre, J.A.; Yoshitake, T.; Yoshitake, S.; Hagman, B.; Kehr, J.; Agnati, L.F.; Fuxe, K.; et al. On the role of P2X7 receptors in dopamine nerve cell degeneration in a rat model of Parkinson’s disease: Studies with the P2X7 receptor antagonist A-438079. J. Neural Transm. 2010, 117, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Carmo, M.; Menezes, A.P.F.; Nunes, A.C.L.; Pliássova, A.; Rolo, A.; Palmeira, C.; Cunha, R.A.; Canas, P.; Andrade, G. The P2X7 receptor antagonist Brilliant Blue G attenuates contralateral rotations in a rat model of Parkinsonism through a combined control of synaptotoxicity, neurotoxicity and gliosis. Neuropharmacology 2014, 81, 142–152. [Google Scholar] [CrossRef]
- Ferrazoli, E.G.; de Souza, H.D.N.; Nascimento, I.C.; Oliveira-Giacomelli, Á.; Schwindt, T.T.; Britto, L.R.; Ulrich, H. Brilliant Blue G, but not Fenofibrate, Treatment Reverts Hemiparkinsonian Behavior and Restores Dopamine Levels in an Animal Model of Parkinson’s Disease. Cell Transplant. 2017, 26, 669–677. [Google Scholar] [CrossRef] [Green Version]
- Gan, M.; Moussaud, S.; Jiang, P.; McLean, P.J. Extracellular ATP induces intracellular alpha-synuclein accumulation via P2X1 receptor-mediated lysosomal dysfunction. Neurobiol. Aging 2015, 36, 1209–1220. [Google Scholar] [CrossRef] [Green Version]
- Oliveira-Giacomelli, Á.; Naaldijk, Y.; Sardá-Arroyo, L.; Gonçalves, M.C.B.; Corrêa-Velloso, J.; Pillat, M.M.; de Souza, H.D.N.; Ulrich, H. Purinergic Receptors in Neurological Diseases With Motor Symptoms: Targets for Therapy. Front. Pharmacol. 2018, 9, 325. [Google Scholar] [CrossRef]
- Wilkaniec, A.; Gąssowska, M.; Czapski, G.; Cieślik, M.; Sulkowski, G.; Adamczyk, A. P2X7 receptor-pannexin 1 interaction mediates extracellular alpha-synuclein-induced ATP release in neuroblastoma SH-SY5Y cells. Purinergic Signal. 2017, 13, 347–361. [Google Scholar] [CrossRef] [Green Version]
- Jiang, T.; Hoekstra, J.; Heng, X.; Kang, W.; Ding, J.; Liu, J.; Chen, S.; Zhang, J. P2X7 receptor is critical in α-synuclein–mediated microglial NADPH oxidase activation. Neurobiol. Aging 2015, 36, 2304–2318. [Google Scholar] [CrossRef]
- Espinosa-Parrilla, Y.; Gonzalez-Billault, C.; Fuentes, E.; Palomo, I.; Alarcón, M. Decoding the Role of Platelets and Related MicroRNAs in Aging and Neurodegenerative Disorders. Front. Aging Neurosci. 2019, 11, 151. [Google Scholar] [CrossRef]
- Haas, R.H.; Nasirian, F.; Nakano, K.; Ward, D.; Pay, M.; Hill, R.; Shults, C.W. Low platelet mitochondrial complex I and complex II/III activity in early untreated parkinson’s disease. Ann. Neurol. 1995, 37, 714–722. [Google Scholar] [CrossRef]
- Bronstein, J.M.; Paul, K.; Yang, L.; Haas, R.H.; Shults, C.W.; Le, T.; Ritz, B. Platelet mitochondrial activity and pesticide exposure in early Parkinson’s disease. Mov. Disord. 2015, 30, 862–866. [Google Scholar] [CrossRef] [Green Version]
- Koçer, A.; Yaman, A.; Niftaliyev, E.; Dürüyen, H.; Eryılmaz, M.; Koçer, E. Assessment of Platelet Indices in Patients with Neurodegenerative Diseases: Mean Platelet Volume Was Increased in Patients with Parkinson’s Disease. Curr. Gerontol. Geriatr. Res. 2013, 2013, 986254. [Google Scholar] [CrossRef]
- Johnson, C.O. Global, regional, and national burden of stroke, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 439–458. [Google Scholar] [CrossRef] [Green Version]
- Campbell, B.C.V.; de Silva, D.A.; Macleod, M.R.; Coutts, S.B.; Schwamm, L.H.; Davis, S.M.; Donnan, G.A. Ischaemic stroke. Nat. Rev. Dis. Primers 2019, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [PubMed]
- Nieswandt, B.; Kleinschnitz, C.; Stoll, G. Ischaemic stroke: A thrombo-inflammatory disease? J. Physiol. 2011, 589, 4115–4123. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, G.; Arumugam, T.V.; Stokes, K.Y.; Granger, D.N. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation 2006, 113, 2105–2112. [Google Scholar] [CrossRef] [Green Version]
- Kleinschnitz, C.; Schwab, N.; Kraft, P.; Hagedorn, I.; Dreykluft, A.; Schwarz, T.; Austinat, M.; Nieswandt, B.; Wiendl, H.; Stoll, G. Early detrimental T-cell effects in experimental cerebral ischemia are neither related to adaptive immunity nor thrombus formation. Blood 2010, 115, 3835–3842. [Google Scholar] [CrossRef] [PubMed]
- Kleinschnitz, C.; Kraft, P.; Dreykluft, A.; Hagedorn, I.; Goebel, K.; Schuhmann, M.K.; Langhauser, F.; Helluy, X.; Schwarz, T.; Bittner, S.; et al. Regulatory T cells are strong promoters of acute ischemic stroke in mice by inducing dysfunction of the cerebral microvasculature. Blood 2013, 121, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Lecut, C.; Frederix, K.; Johnson, D.M.; Deroanne, C.; Thiry, M.; Faccinetto, C.; Marée, R.; Evans, R.J.; Volders, P.G.A.; Bours, V.; et al. P2X1 Ion Channels Promote Neutrophil Chemotaxis through Rho Kinase Activation. J. Immunol. 2009, 183, 2801. [Google Scholar] [PubMed] [Green Version]
- Chou, W.-H.; Choi, D.-S.; Zhang, H.; Mu, D.; McMahon, T.; Kharazia, V.N.; Lowell, C.A.; Ferriero, D.M.; Messing, R. Neutrophil protein kinase Cδ as a mediator of stroke-reperfusion injury. J. Clin. Investig. 2004, 114, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Zhang, X.; Gu, L.; Zhu, H.; Zhong, Y.; Ye, Y.; Xiong, X.; Jian, Z. New Insight Into Neutrophils: A Potential Therapeutic Target for Cerebral Ischemia. Front. Immunol. 2021, 12, 2618. [Google Scholar] [CrossRef]
- Darbousset, R.; Delierneux, C.; Mezouar, S.; Hego, A.; Lecut, C.; Guillaumat, I.; Riederer, M.A.; Evans, R.J.; Dignat-George, F.; Panicot-Dubois, L.; et al. P2X1 expressed on polymorphonuclear neutrophils and platelets is required for thrombosis in mice. Blood 2014, 124, 2575–2585. [Google Scholar] [CrossRef] [Green Version]
- Melani, A.; Turchi, D.; Vannucchi, M.G.; Cipriani, S.; Gianfriddo, M.; Pedata, F. ATP extracellular concentrations are increased in the rat striatum during in vivo ischemia. Neurochem. Int. 2005, 47, 442–448. [Google Scholar] [CrossRef]
- Rossi, D.J.; Brady, J.D.; Mohr, C. Astrocyte metabolism and signaling during brain ischemia. Nat. Neurosci. 2007, 10, 1377–1386. [Google Scholar] [CrossRef]
- Braun, N.; Zhu, Y.; Krieglstein, J.; Culmsee, C.; Zimmermann, H. Upregulation of the Enzyme Chain Hydrolyzing Extracellular ATP after Transient Forebrain Ischemia in the Rat. J. Neurosci. 1998, 18, 4891. [Google Scholar] [CrossRef]
- Arbeloa, J.; Pérez-Samartín, A.; Gottlieb, M.; Matute, C. P2X7 receptor blockade prevents ATP excitotoxicity in neurons and reduces brain damage after ischemia. Neurobiol. Dis. 2012, 45, 954–961. [Google Scholar] [CrossRef]
- Chu, K.; Yin, B.; Wang, J.; Peng, G.; Liang, H.; Xu, Z.; Du, Y.; Fang, M.; Xia, Q.; Luo, B. Inhibition of P2X7 receptor ameliorates transient global cerebral ischemia/reperfusion injury via modulating inflammatory responses in the rat hippocampus. J. Neuroinflammation 2012, 9, 69. [Google Scholar] [CrossRef] [Green Version]
- Andrejew, R.; Oliveira-Giacomelli, Á.; Ribeiro, D.E.; Glaser, T.; Arnaud-Sampaio, V.F.; Lameu, C.; Ulrich, H. The P2X7 Receptor: Central Hub of Brain Diseases. Front. Mol. Neurosci. 2020, 13, 124. [Google Scholar] [CrossRef]
- Karmakar, M.; Katsnelson, M.A.; Dubyak, G.R.; Pearlman, E. Neutrophil P2X7 receptors mediate NLRP3 inflammasome-dependent IL-1β secretion in response to ATP. Nat. Commun. 2016, 7, 10555. [Google Scholar] [CrossRef] [Green Version]
- Hirayama, Y.; Ikeda-Matsuo, Y.; Notomi, S.; Enaida, H.; Kinouchi, H.; Koizumi, S. Astrocyte-Mediated Ischemic Tolerance. J. Neurosci. 2015, 35, 3794. [Google Scholar] [CrossRef]
- Cisneros-Mejorado, A.J.; Pérez-Samartín, A.; Domercq, M.; Arellano, R.O.; Gottlieb, M.; Koch-Nolte, F.; Matute, C. P2X7 Receptors as a Therapeutic Target in Cerebrovascular Diseases. Front. Mol. Neurosci. 2020, 13, 92. [Google Scholar] [CrossRef] [PubMed]
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.B.; et al. Neuropathic pain. Nat. Rev. Dis. Primers 2017, 3, 17002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernier, L.-P.; Ase, A.R.; Séguéla, P. P2X receptor channels in chronic pain pathways. J. Cereb. Blood Flow Metab. 2018, 175, 2219–2230. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K. P2 receptors and chronic pain. Purinergic Signal 2007, 3, 135–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rae, M.G.; Rowan, E.G.; Kennedy, C. Pharmacological properties of P2X3-receptors present in neurones of the rat dorsal root ganglia. Br. J. Pharmacol. 1998, 124, 176–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueno, S.; Tsuda, M.; Iwanaga, T.; Inoue, K. Cell type-specific ATP-activated responses in rat dorsal root ganglion neurons. J. Cereb. Blood Flow Metab. 1999, 126, 429–436. [Google Scholar] [CrossRef] [Green Version]
- Brederson, J.D.; Jarvis, M.F. Homomeric and heteromeric P2X3 receptors in peripheral sensory neurons. Curr. Opin. Investig. Drugs 2008, 9, 716–725. [Google Scholar]
- Inoue, K.; Tsuda, M.; Koizumi, S. ATP receptors in pain sensation: Involvement of spinal microglia and P2X4 receptors. Purinergic Signal. 2005, 1, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, S.; Kohro, Y.; Tsuda, M.; Inoue, K. Activation of cytosolic phospholipase A2 in dorsal root ganglion neurons by Ca2+/calmodulin-dependent protein kinase II after peripheral nerve injury. Mol. Pain 2009, 5, 22. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, S.; Kohro, Y.; Shiratori, M.; Ishii, S.; Shimizu, T.; Tsuda, M.; Inoue, K. Role of PAF Receptor in Proinflammatory Cytokine Expression in the Dorsal Root Ganglion and Tactile Allodynia in a Rodent Model of Neuropathic Pain. PLoS ONE 2010, 5, e10467. [Google Scholar] [CrossRef] [Green Version]
- Schäfers, M.; Svensson, C.; Sommer, C.; Sorkin, L.S. Tumor Necrosis Factor-α Induces Mechanical Allodynia after Spinal Nerve Ligation by Activation of p38 MAPK in Primary Sensory Neurons. J. Neurosci. 2003, 23, 2517–2521. [Google Scholar] [CrossRef] [Green Version]
- Bekő, K.; Koványi, B.; Gölöncsér, F.; Horváth, G.; Dénes, Á.; Környei, Z.; Botz, B.; Helyes, Z.; Müller, C.E.; Sperlágh, B. Contribution of platelet P2Y(12) receptors to chronic Complete Freund’s adjuvant-induced inflammatory pain. J. Thromb. Haemost. 2017, 15, 1223–1235. [Google Scholar] [CrossRef] [Green Version]
- Tsuda, M.; Shigemoto-Mogami, Y.; Koizumi, S.; Mizokoshi, A.; Kohsaka, S.; Salter, M.W.; Inoue, K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 2003, 424, 778–783. [Google Scholar] [CrossRef]
- Ulmann, L.; Hatcher, J.P.; Hughes, J.P.; Chaumont, S.; Green, P.J.; Conquet, F.; Buell, G.N.; Reeve, A.J.; Chessell, I.P.; Rassendren, F. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J. Neurosci. 2008, 28, 11263–11268. [Google Scholar] [CrossRef]
- Coull, J.A.M.; Beggs, S.; Boudreau, D.; Boivin, D.; Tsuda, M.; Inoue, K.; Gravel, C.; Salter, M.W.; De Koninck, Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 2005, 438, 1017–1021. [Google Scholar] [CrossRef]
- Masuda, T.; Ozono, Y.; Mikuriya, S.; Kohro, Y.; Tozaki-Saitoh, H.; Iwatsuki, K.; Uneyama, H.; Ichikawa, K.I.H.U.R.; Salter, M.W.; Tsuda, T.M.Y.K.H.T.-S.M.; et al. Dorsal horn neurons release extracellular ATP in a VNUT-dependent manner that underlies neuropathic pain. Nat. Commun. 2016, 7, 12529. [Google Scholar] [CrossRef] [Green Version]
- Schüpke, S.; Neumann, F.-J.; Menichelli, M.; Mayer, K.; Bernlochner, I.; Wöhrle, J.; Richardt, G.; Liebetrau, C.; Witzenbichler, B.; Antoniucci, D.; et al. Ticagrelor or prasugrel in patients with acute coronary syndromes. N. Engl. J. Med. 2019, 381, 1524–1534. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Wang, F.; Sundararajan, S. Use of Dual Antiplatelet Therapy Following Ischemic Stroke. Stroke 2020, 51, e78–e80. [Google Scholar] [CrossRef] [PubMed]
- Stock, T.C.; Bloom, B.J.; Wei, N.; Ishaq, S.; Park, W.; Wang, X.; Gupta, P.; Mebus, C.A. Efficacy and Safety of CE-224,535, an Antagonist of P2X7 Receptor, in Treatment of Patients with Rheumatoid Arthritis Inadequately Controlled by Methotrexate. J. Rheumatol. 2012, 39, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Keystone, E.C.; Wang, M.M.; Layton, M.; Hollis, S.; McInnes, I.B. Clinical evaluation of the efficacy of the P2X7 purinergic receptor antagonist AZD9056 on the signs and symptoms of rheumatoid arthritis in patients with active disease despite treatment with methotrexate or sulphasalazine. Ann. Rheum. Dis. 2012, 71, 1630–1635. [Google Scholar] [CrossRef]
- Eser, A.; Colombel, J.F.; Rutgeerts, P.; Vermeire, S.; Vogelsang, H.; Braddock, M.; Persson, T.; Reinisch, W. Safety and Efficacy of an Oral Inhibitor of the Purinergic Receptor P2X7 in Adult Patients with Moderately to Severely Active Crohn’s Disease: A Randomized Placebo-controlled, Double-blind, Phase IIa Study. Inflamm. Bowel Dis. 2015, 21, 2247–2253. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Wang, Q.; Ao, H.; Shoblock, J.R.; Lord, B.; Aluisio, L.; Fraser, I.; Nepomuceno, D.; Neff, R.A.; Welty, N.; et al. Pharmacological characterization of a novel centrally permeable P2X7 receptor antagonist: JNJ-47965567. Br. J. Pharmacol. 2013, 170, 624–640. [Google Scholar] [CrossRef] [Green Version]
- Letavic, M.A.; Lord, B.; Bischoff, F.; Hawryluk, N.A.; Pieters, S.; Rech, J.C.; Sales, Z.; Velter, A.I.; Ao, H.; Bonaventure, P.; et al. Synthesis and Pharmacological Characterization of Two Novel, Brain Penetrating P2X7 Antagonists. ACS Med. Chem. Lett. 2013, 4, 419–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharya, A.; Jones, D.N. Emerging role of the P2X7-NLRP3-IL1β pathway in mood disorders. Psychoneuroendocrinology 2018, 98, 95–100. [Google Scholar] [CrossRef]
- Matsumura, Y.; Yamashita, T.; Sasaki, A.; Nakata, E.; Kohno, K.; Masuda, T.; Tozaki-Saitoh, H.; Imai, T.; Kuraishi, Y.; Tsuda, M.; et al. A novel P2X4 receptor-selective antagonist produces anti-allodynic effect in a mouse model of herpetic pain. Sci. Rep. 2016, 6, 32461. [Google Scholar] [CrossRef] [Green Version]
- Richards, D.; Gever, J.R.; Ford, A.P.; Fountain, S.J. Action of MK-7264 (gefapixant) at human P2X3 and P2X2/3 receptors and in vivo efficacy in models of sensitisation. Br. J. Pharmacol. 2019, 176, 2279–2291. [Google Scholar] [CrossRef] [Green Version]
- McGarvey, L.; Birring, S.; Morice, A.; Dicpinigaitis, P.; Pavord, I.; Schelfhout, J.; Nguyen, A.M.; Li, Q.; Tzontcheva, A.; Iskold, B.; et al. Late Breaking Abstract-Two Phase 3 Randomized Clinical Trials of Gefapixant, a P2X3 Receptor Antagonist, in Refractory or Unexplained Chronic Cough (COUGH-1 and COUGH-2). Eur. Respir. J. 2020, 56, 3800. [Google Scholar]
- Abu-Zaid, A.; Aljaili, A.; Althaqib, A.; Adem, F.; Alhalal, D.; Almubarak, A.; Aldughaither, S.; Alghabban, S.; Alfaraj, G.; Masoud, A.; et al. Safety and efficacy of gefapixant, a novel drug for the treatment of chronic cough: A systematic review and meta-analysis of randomized controlled trials. Ann. Thorac. Med. 2021, 16, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Morice, A.; Smith, J.A.; McGarvey, L.; Birring, S.S.; Parker, S.M.; Turner, A.; Hummel, T.; Gashaw, I.; Fels, L.; Klein, S.; et al. Eliapixant (BAY 1817080), a P2X3 receptor antagonist, in refractory chronic cough: A randomised, placebo-controlled, crossover phase 2a study. Eur. Respir. J. 2021, 58, 2004240. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rawish, E.; Langer, H.F. Platelets and the Role of P2X Receptors in Nociception, Pain, Neuronal Toxicity and Thromboinflammation. Int. J. Mol. Sci. 2022, 23, 6585. https://doi.org/10.3390/ijms23126585
Rawish E, Langer HF. Platelets and the Role of P2X Receptors in Nociception, Pain, Neuronal Toxicity and Thromboinflammation. International Journal of Molecular Sciences. 2022; 23(12):6585. https://doi.org/10.3390/ijms23126585
Chicago/Turabian StyleRawish, Elias, and Harald F. Langer. 2022. "Platelets and the Role of P2X Receptors in Nociception, Pain, Neuronal Toxicity and Thromboinflammation" International Journal of Molecular Sciences 23, no. 12: 6585. https://doi.org/10.3390/ijms23126585
APA StyleRawish, E., & Langer, H. F. (2022). Platelets and the Role of P2X Receptors in Nociception, Pain, Neuronal Toxicity and Thromboinflammation. International Journal of Molecular Sciences, 23(12), 6585. https://doi.org/10.3390/ijms23126585





