Application Progress of Modified Chitosan and Its Composite Biomaterials for Bone Tissue Engineering
Abstract
:1. Introduction
2. Post-Implantation Complications and the Safety of Chitosan
3. Fabrication Strategies
4. Modification Methods of Chitosan for BTE
5. Applications of Chitosan Cross-Linking Modification for BTE
5.1. Physically Cross-Linked Chitosan for BTE
5.2. Chemically Cross-Linked Chitosan for BTE
5.2.1. Aldehyde
5.2.2. Genipin
5.2.3. Tripolyphosphate (TPP)
5.2.4. Other Cross-linkers
5.2.5. Photo-Cross-Linked Chitosan
5.3. Enzymatic Cross-Linked Chitosan for BTE
6. Application of Structure-Modified Chitosan for BTE
6.1. Carboxymethyl Chitosan, CMCS
6.2. Hydroxypropyltrimethyl Ammonium Chloride Chitosan, HACC
6.3. Sulfated Chitosan, SCS
6.4. Glycol Chitosan, GCS
6.5. Guanidinylated Chitosan, GC
7. Application of Chitosan Grafted with Biodegradable Polymers for BTE
| Modification | Fabrication | Materials | Effect | Cell/Model | Ref. |
|---|---|---|---|---|---|
| Physically cross-linked | ice template-assisted freeze-drying | EO-loaded CS/Dex | Exhibit antioxidant, antifungal properties and the inhibition of Candida parapsilosis fungi | - | [129] |
| freeze-drying | nanoporous chitin hydrogels | Enhance the strength and Young’s modulus of hydrogel, mBMSC adhesion, and proliferation | mBMSC | [133] | |
| direct ink writing (DWI) | CS/PVA | Promote toughness performance | - | [134] | |
| double network | CS/PVA/HAp | Increase cell adhesion, proliferation, OCN, ALP, COL I, and osteochondral repair efficacy | Rat bone marrow mesenchymal stem cells (rBMSCs) and L929 cells (Mouse fibroblast cell line)/New Zealand white rabbits with a bone defect (5 mm in diameter and 8 mm deep) in the lateral femoral condyle | [147] | |
| Aldehyde-crosslinking | freeze-drying | CS/HA/β-TCP | Promote biological performance, metabolic activity, ALP expression, cell morphology, cell/scaffold interaction, and gene expression | MG63 human osteoblastic-like cells | [153] |
| freeze-drying | CS/vanillin hydrogel | Achieve a good balance between self-healing capability and mechanical strength | - | [156] | |
| emulsion method | CS/vanillin hydrogel | Provide favorable cell attachment and biocompatibility | MG63 cell/ muscular incision 20 mm long on the backs of SD rats | [157] | |
| freeze-drying | vanillin-CS/CS | Exhibit suitable viscosity values and shear thinning behavior for 3D printing applications | - | [158] | |
| freeze-drying | Cinnamaldehyde/CS | Show thermal characteristics and stability and synergistic antibacterial activity against Staphylococcus aureus and Escherichia coli bacteria | Staphylococcus aureus or S. aureus (ATCC 25923) and Escherichia coli or E. coli (ATCC 35218) bacteria | [161] | |
| Genipin cross-linking | CS and hyaluronic acid solutions PEC+BMP-2 | Control the swelling ratio and degradation of PEC and achieve quite a high loading efficacy, prolonged, and sustained BMP-2 release profile | MC3T3-E1 cells | [165] | |
| mixing | gentamycin sulfate (GS)-loaded CMCS hydrogel | Achieve superb inhibition of bacterial growth and biofilm formation of Staphylococcus aureus, enhance the adhesion, proliferation, and differentiation of MC3T3-E1 cells | MC3T3-E1 cells | [168] | |
| electrospinning | CS/HA nanofibers | Increase in Young’s modulus and osteoinductive bioactivity | Murine 7F2 osteoblast-like cells | [172] | |
| mixing | CS/methylcellulose | Enhance fibroblast, endothelial, and osteoblast proliferation and adhesion | Osteoblasts, fibroblasts, and HUVECs | [173] | |
| DWI and freeze-drying | HA/CS composite scaffolds | Friendly environment, increase cell population, levels of viability, and attachment | MG63 human osteoblast-like cells | [274] | |
| self-assembly | HA/GO/CS composite hydrogel | Improve the microstructure and mechanical strength. Balance the rigidity and toughness of the composite hydrogel | Rat bone marrow mesenchymal stem cells (rBMSCs) | [275] | |
| Tripolyphosphate (TPP) cross-linking | coacervation and lyophilization | nHA/CS/TPP Scaffolds | Exhibit highest ultimate compressive strength and show good osteoblast adhesion and proliferation | OB-6 line cell | [188] |
| freeze-drying | CS/Gel/β-TCP scaffolds | Show mechanical improvements, bioactivity, high proliferation rate, high extracellular calcium deposition, excellent cell adhesion, and characteristic osteoblast cell morphology | Human osteoblast cells (CRL-11372) (hOB) | [179] | |
| freeze-drying | HA/β-TCP/CS composites | Show good swelling properties, and higher levels of cell proliferation and growth | Human osteoblast-like cells (Saos-2) and mouse fibroblastic-like cells (L929) | [276] | |
| Glycerylphytate (G1Phy) | 3d-printing and photopolymerization | GelMA/CS scaffold | Exhibit excellent shape fidelity, resolution, swelling behavior, and mechanical and biological properties; enhance cell adhesion and proliferation | L929 fibroblasts | [191] |
| Carbodiimide and citric acid | extruded in a coagulant bath using viscose-type stainless steel spinneret | citrate–CS fibers | Improve the mechanical property, higher stability against enzymatic degradation and hydrophobicity, and superior bio-mineralization | MSCs New Zealand white male rabbits | [193] |
| Photo-crosslinking | UV light | MCS/TPVA (Darocur 2959) | Exhibit rapid gelation behavior, improved stiff and compressive strength. Promote L929 cell attachment and proliferation | L929 cell | [200] |
| visible blue light with riboflavin | CS-MTT hydrogel | Recruit native cells and promote calvarial healing without the delivery of additional therapeutic agents or stem cells | male CD-1 nude mice | [277] | |
| blue light (420–460 nm) | ChI-MA/GO | Showe intermediate platelet aggregation hemolytic tendencies, enhance tissue regeneration | NHOst cells Reconstruction of the distal epiphysis of the femur | [199] | |
| Enzymatic-crosslinking | Standard carbodiimide coupling method | HPP-GC + BMP(HRP + H2O2) | Localize osteoprogenitor recruitment and osteogenesis | Col3.6 rat critical sized bilateral calvarial defect model | [278] |
| Carboxymethyl chitosan, CMCS | electrospinning | CMCS/HA | Increase the ALP activity and Runx2 expression, promote new bone formation and maturation | mBMSCs circular critical-size Calvarial bone defects (diameter of 5 mm) on both parietal bones of Sprague–Dawley rats | [211] |
| electrospinning | PCL/CMCS nanofibrous scaffolds | Adjust the viscosity and charge density and exhibited excellent initial cell attachment and proliferation | human osteoblast cells (MG63) | [214] | |
| freeze-drying | NOCC/FD composite hydrogel | Enhance the proliferation, ALP activity, and mineralization of osteoblast cells | L929 mouse fibroblasts and 7F2 osteoblast cell | [279] | |
| freeze-drying | SF/CMCS/CNCs/Sr-HAp | Maintain high porosity with a lower swelling ratio, enhanced protein adsorption and ALP activity | bone mesenchymal stem cell (BMSC) | [217] | |
| Hydroxypropyltrimethyl ammonium chloride chitosan (HACC) | 3D-printing | PLGA/HA/HACC composite scaffold | Favor cell attachment, proliferation, spreading, and osteogenic differentiation and exhibit good neovascularization and tissue integration | human bone marrow-derived mesenchymal stem cells (hBMSCs) | [219] |
| solvent casting-particulate leaching method | silica/HACC/zein scaffold | Exhibit long-lasting antibacterial activity against Escherichia coli and Staphylococcus aureus, and significant early osteogenic differentiation | Rabbit model of critical-sized radius bone defect | [221] | |
| PTFE mould | HACC-PMMA | Improve properties, stem cell proliferation, osteogenic differentiation, and osteogenesis-associated gene expression | human mesenchymal stem cells (hMSCs) | [224] | |
| Sulfated chitosan (SCS) | - | 2-N,6-O- SCS + BMP-2 | Exhibit a higher cell viability and sprouting ability, secrete more VEGF and NO, and improve the angiogenic potential | Rat bone marrow stromal cells (BMSCs) | [231] |
| solution casting | SCS coated on poly(d,l-lactide) (PDLLA) | Increase osteogenic- and angiogenic-related gene and protein expression | Mouse preosteoblast cells (MC3T3-E1s) and human umbilical vein endothelial cells (HUVECs) | [231] | |
| - | 2-N,6-O- SCS + BMP-2 | Enhance BMP-2 bioactivity to induce osteoblastic differentiation in vitro and in vivo by promoting the BMP-2 signaling pathway | C2C12 cells | [232] | |
| Glycol chitosan (GCS) | solvent cast and evaporation | nHA/GCS composites | No cytotoxicity and promotion of cell ingrowth and osteoconduction | osteoblastic-like (SAOS) and embryonic cell lines (HEK293T) | [238] |
| solvent cast and evaporation | CHA/ SF/GCS/DF-PEG self-healing hydrogel + BMP-2 | Promote osteogenic differentiation of mOPCs and promote the proliferation and migration of HUVECs | C57BL/6 suckling rat A 4-mm-deep hole in the femoral condyle of SD rat | [280] | |
| electrostatic interaction | GCS-HA NPs + PEGDA+ SrRNPs-H | Increase the level of bone regeneration | Dorsal incision around the lumbar and sacral pine area of male Wistar rats | [240] | |
| Guanidinylated chitosan (GC) | sol–gel chemistry and freeze-drying | Sulfonate and carboxylate-containing chitosan/silica hybrid composites | Showed a substantial effect on the mineralization of calcium phosphate and was more efficient to induce heterogeneous nucleation and growth of hydroxyapatite | - | [281] |
| - | GC/PANI-containing self-healing semi-conductive waterborne scaffolds | Exhibit excellent shape memory properties and shape recovery ratio, enhanced cell attachment, COL-1, ALP, RUNX2, and OCN expression | Human adipose-derived mesenchymal stem cells (hADSCs) | [282] | |
| mixing | LNSs/GC | Show inherent osteogenic properties, a versatile moldable vehicle, facilitating handling and osteogenic potential | Mouse bone marrow stromal cell line (BMSCs, D1 ORL UVA [D1], D1 cell, CRL-12424) | [242] | |
| Grafted with PLA | electrospun | CS/PLA/HA | Enhance proliferation of MC3T3-E1 cells used in applications as coating materials on medical devices | MC3T3-E1 cells | [268] |
| Grafted with HPMC | coupling reagent-mediated approach | CS/HPMC | Highly water-soluble across a wide pH range, high pH buffering capacity, and a high drug encapsulation efficiency | Metronidazole, methylene blue, tetracycline hydrochloride, and mometasone furoate as drug models | [270] |
| Grafted with CMC | Freeze-drying | CS/HPMC/ mesoporous wollastonite | Cyto-friendly nature to human osteoblastic cells, confirmed by calcium deposition and expression of an osteoblast-specific microRNA | MG-63 | [271] |
| Grafted with cycle RGD peptide | noncovalent method | CS/cRGD/GO | Provide a multifunctional drug delivery system and can be efficiently loaded with a number of therapeutic agents for biomedical applications | hepatoma cells (Bel-7402, SMMC-7721, HepG2) | [273] |
| Grafted with HVP-aldehyde peptide | mixing | CS/HVP | Support the adhesion of osteoblasts, the formation of elongated cell shapes, and increased osteoblast differentiation. | Human (h) osteoblast cells | [283] |
8. Future Directions for Modified CS-Based Bone Scaffolds
9. Challenges and Future Prospects
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yair, R.; Shahar, R.; Uni, Z. Prenatal nutritional manipulation by in ovo enrichment influences bone structure, composition, and mechanical properties. J. Anim. Sci. 2013, 91, 2784–2793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayliss, L.; Mahoney, D.J.; Monk, P. Normal bone physiology, remodelling and its hormonal regulation. Surg. (Oxf. ) 2012, 30, 47–53. [Google Scholar] [CrossRef]
- Aguilar, A.; Zein, N.; Harmouch, E.; Hafdi, B.; Bornert, F.; Offner, D.; Clauss, F.; Fioretti, F.; Huck, O.; Benkirane-Jessel, N.; et al. Application of Chitosan in Bone and Dental Engineering. Molecules 2019, 24, 3009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cameron, J.A.; Milner, D.J.; Lee, J.S.; Cheng, J.; Fang, N.X.; Jasiuk, I.M. Employing the biology of successful fracture repair to heal critical size bone defects. Curr. Top. Microbiol. Immunol. 2013, 367, 113–132. [Google Scholar] [CrossRef]
- Jin, M.; Kim, B.S.; Seo, S.H.; Kim, M.; Kang, Y.G.; Shin, J.W.; Cho, K.H.; Shin, M.C.; Yoon, C.; Min, K.A. Synergistic Effect of Growth Factor Releasing Polymeric Nanoparticles and Ultrasound Stimulation on Osteogenic Differentiation. Pharmaceutics 2021, 13, 457. [Google Scholar] [CrossRef]
- Wang, B.; Guo, Y.; Chen, X.; Zeng, C.; Hu, Q.; Yin, W.; Li, W.; Xie, H.; Zhang, B.; Huang, X.; et al. Nanoparticle-modified chitosan-agarose-gelatin scaffold for sustained release of SDF-1 and BMP-2. Int. J. Nanomed. 2018, 13, 7395–7408. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Ma, K.; Zhao, H.; Zhang, Q.; Liu, Y.; Bai, N. Bioactive effects of nonthermal argon-oxygen plasma on inorganic bovine bone surface. Sci. Rep. 2020, 10, 17973. [Google Scholar] [CrossRef]
- Urbani, L.; Maghsoudlou, P.; Milan, A.; Menikou, M.; Hagen, C.K.; Totonelli, G.; Camilli, C.; Eaton, S.; Burns, A.; Olivo, A.; et al. Long-term cryopreservation of decellularised oesophagi for tissue engineering clinical application. PLoS ONE 2017, 12, e0179341. [Google Scholar] [CrossRef] [Green Version]
- Bharadwaz, A.; Jayasuriya, A.C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110698. [Google Scholar] [CrossRef]
- Balagangadharan, K.; Dhivya, S.; Selvamurugan, N. Chitosan based nanofibers in bone tissue engineering. Int. J. Biol. Macromol. 2017, 104, 1372–1382. [Google Scholar] [CrossRef]
- Bastami, F.; Paknejad, Z.; Jafari, M.; Salehi, M.; Rezai Rad, M.; Khojasteh, A. Fabrication of a three-dimensional β-tricalcium-phosphate/gelatin containing chitosan-based nanoparticles for sustained release of bone morphogenetic protein-2: Implication for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 72, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Alidadi, S.; Bigham-Sadegh, A.; Meimandi-Parizi, A. Chitosan/gelatin/platelet gel enriched by a combination of hydroxyapatite and beta-tricalcium phosphate in healing of a radial bone defect model in rat. Int. J. Biol. Macromol. 2017, 101, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Su, K.; Su, L.; Liang, P.; Ji, P.; Wang, C. Comparison of 3D-printed porous tantalum and titanium scaffolds on osteointegration and osteogenesis. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109908. [Google Scholar] [CrossRef] [PubMed]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Yuan, Y.; Yang, Q.; Zhu, P.; Lian, H. Versatile acid base sustainable solvent for fast extraction of various molecular weight chitin from lobster shell. Carbohydr. Polym. 2018, 201, 211–217. [Google Scholar] [CrossRef]
- Azuma, K.; Izumi, R.; Osaki, T.; Ifuku, S.; Morimoto, M.; Saimoto, H.; Minami, S.; Okamoto, Y. Chitin, chitosan, and its derivatives for wound healing: Old and new materials. J. Funct. Biomater. 2015, 6, 104–142, Retraction published on 7 June 2018, see J. Funct. Biomater. 2018, 9, 38. [Google Scholar] [CrossRef] [Green Version]
- Bedian, L.; Villalba-Rodríguez, A.M.; Hernández-Vargas, G.; Parra-Saldivar, R.; Iqbal, H.M. Bio-based materials with novel characteristics for tissue engineering applications—A review. Int. J. Biol. Macromol. 2017, 98, 837–846. [Google Scholar] [CrossRef]
- Fakhri, E.; Eslami, H.; Maroufi, P.; Pakdel, F.; Taghizadeh, S.; Ganbarov, K.; Yousefi, M.; Tanomand, A.; Yousefi, B.; Mahmoudi, S.; et al. Chitosan biomaterials application in dentistry. Int. J. Biol. Macromol. 2020, 162, 956–974. [Google Scholar] [CrossRef]
- Satitsri, S.; Muanprasat, C. Chitin and Chitosan Derivatives as Biomaterial Resources for Biological and Biomedical Applications. Molecules 2020, 25, 5961. [Google Scholar] [CrossRef]
- Lizardi-Mendoza, J.; Argüelles Monal, W.M.; Goycoolea Valencia, F.M. Chemical characteristics and functional properties of chitosan. In Chitosan in the Preservation of Agricultural Commodities; Elsevier: Amsterdam, The Netherlands, 2016; pp. 3–31. [Google Scholar] [CrossRef]
- Kadouche, S.; Farhat, M.; Lounici, H.; Fiallo, M.; Sharrock, P.; Mecherri, M.; Hadioui, M. Low Cost Chitosan Biopolymer for Environmental Use Made from Abundant Shrimp Wastes. Waste Biomass Valorization 2017, 8, 401–406. [Google Scholar] [CrossRef]
- Arbia, W.; Arbia, L.; Adour, L.; Amrane, A. Chitin Extraction from Crustacean Shells Using Biological Methods—A Review. Food Technol. Biotechnol. 2013, 51, 12–25. [Google Scholar]
- Vázquez, J.A.; Rodríguez-Amado, I.; Montemayor, M.I.; Fraguas, J.; González Mdel, P.; Murado, M.A. Chondroitin sulfate, hyaluronic acid and chitin/chitosan production using marine waste sources: Characteristics, applications and eco-friendly processes: A review. Mar. Drugs 2013, 11, 747–774. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Kumar, A.; Kumar, R.; Rana, N.K.; Koch, B. Development of a novel chitosan based biocompatible and self-healing hydrogel for controlled release of hydrophilic drug. Int. J. Biol. Macromol. 2018, 116, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas-Triviño, G.; Elgueta, C.; Vergara, L.; Ojeda, J.; Valenzuela, A.; Cruzat, C. Chitosan doped with nanoparticles of copper, nickel and cobalt. Int. J. Biol. Macromol. 2017, 104, 498–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.; Ramos, D.; Lee, P.; Liang, D.; Yu, X.; Kumbar, S.G. Collagen functionalized bioactive nanofiber matrices for osteogenic differentiation of mesenchymal stem cells: Bone tissue engineering. J. Biomed. Nanotechnol. 2014, 10, 287–298. [Google Scholar] [CrossRef]
- Lou, T.; Wang, X.; Yan, X.; Miao, Y.; Long, Y.Z.; Yin, H.L.; Sun, B.; Song, G. Fabrication and biocompatibility of poly(l-lactic acid) and chitosan composite scaffolds with hierarchical microstructures. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 64, 341–345. [Google Scholar] [CrossRef]
- Amidi, M.; Mastrobattista, E.; Jiskoot, W.; Hennink, W.E. Chitosan-based delivery systems for protein therapeutics and antigens. Adv. Drug Deliv. Rev. 2010, 62, 59–82. [Google Scholar] [CrossRef]
- Zhong, Z.; Qin, J.; Ma, J. Cellulose acetate/hydroxyapatite/chitosan coatings for improved corrosion resistance and bioactivity. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 49, 251–255. [Google Scholar] [CrossRef]
- Olanipekun, E.O.; Ayodele, O.; Olatunde, O.C.; Olusegun, S.J. Comparative studies of chitosan and carboxymethyl chitosan doped with nickel and copper: Characterization and antibacterial potential. Int. J. Biol. Macromol. 2021, 183, 1971–1977. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Sun, T.; Jiang, C. Biomacromolecules as carriers in drug delivery and tissue engineering. Acta Pharm. Sin. B 2018, 8, 34–50. [Google Scholar] [CrossRef] [PubMed]
- Lall, A.; Kamdem Tamo, A.; Doench, I.; David, L.; Nunes de Oliveira, P.; Gorzelanny, C.; Osorio-Madrazo, A. Nanoparticles and Colloidal Hydrogels of Chitosan-Caseinate Polyelectrolyte Complexes for Drug-Controlled Release Applications. Int. J. Mol. Sci. 2020, 21, 5602. [Google Scholar] [CrossRef]
- Niranjan, R.; Koushik, C.; Saravanan, S.; Moorthi, A.; Vairamani, M.; Selvamurugan, N. A novel injectable temperature-sensitive zinc doped chitosan/β-glycerophosphate hydrogel for bone tissue engineering. Int. J. Biol. Macromol. 2013, 54, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Zaveri, T.D.; Dolgova, N.V.; Lewis, J.S.; Hamaker, K.; Clare-Salzler, M.J.; Keselowsky, B.G. Macrophage integrins modulate response to ultra-high molecular weight polyethylene particles and direct particle-induced osteolysis. Biomaterials 2017, 115, 128–140. [Google Scholar] [CrossRef] [Green Version]
- Palomo, J.; Dietrich, D.; Martin, P.; Palmer, G.; Gabay, C. The interleukin (IL)-1 cytokine family--Balance between agonists and antagonists in inflammatory diseases. Cytokine 2015, 76, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Sinder, B.P.; Pettit, A.R.; McCauley, L.K. Macrophages: Their Emerging Roles in Bone. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2015, 30, 2140–2149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazimierczak, P.; Koziol, M.; Przekora, A. The Chitosan/Agarose/NanoHA Bone Scaffold-Induced M2 Macrophage Polarization and Its Effect on Osteogenic Differentiation In Vitro. Int. J. Mol. Sci. 2021, 22, 1109. [Google Scholar] [CrossRef] [PubMed]
- Chumnanvej, S.; Luangwattanawilai, T.; Rawiwet, V.; Suwanprateeb, J.; Rattanapinyopituk, K.; Huaijantug, S.; Yinharnmingmongkol, C.; Hemstapat, R. In vivo evaluation of bilayer ORC/PCL composites in a rabbit model for using as a dural substitute. Neurol. Res. 2020, 42, 879–889. [Google Scholar] [CrossRef]
- Przekora, A. The summary of the most important cell-biomaterial interactions that need to be considered during in vitro biocompatibility testing of bone scaffolds for tissue engineering applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 97, 1036–1051. [Google Scholar] [CrossRef]
- Wang, M.; Yu, Y.; Dai, K.; Ma, Z.; Liu, Y.; Wang, J.; Liu, C. Improved osteogenesis and angiogenesis of magnesium-doped calcium phosphate cement via macrophage immunomodulation. Biomater. Sci. 2016, 4, 1574–1583. [Google Scholar] [CrossRef]
- Brown, B.N.; Ratner, B.D.; Goodman, S.B.; Amar, S.; Badylak, S.F. Macrophage polarization: An opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials 2012, 33, 3792–3802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruber, R. Osteoimmunology: Inflammatory osteolysis and regeneration of the alveolar bone. J. Clin. Periodontol. 2019, 46 (Suppl. S21), 52–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajishengallis, G.; Chavakis, T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat. Rev. Immunol. 2021, 21, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Haynes, D.R.; Crotti, T.N.; Zreiqat, H. Regulation of osteoclast activity in peri-implant tissues. Biomaterials 2004, 25, 4877–4885. [Google Scholar] [CrossRef] [PubMed]
- Kapasa, E.R.; Giannoudis, P.V.; Jia, X.; Hatton, P.V.; Yang, X.B. The Effect of RANKL/OPG Balance on Reducing Implant Complications. J. Funct. Biomater. 2017, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Cerqueni, G.; Scalzone, A.; Licini, C.; Gentile, P.; Mattioli-Belmonte, M. Insights into oxidative stress in bone tissue and novel challenges for biomaterials. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 130, 112433. [Google Scholar] [CrossRef]
- Ozmen, I.; Naziroglu, M.; Okutan, R. Comparative study of antioxidant enzymes in tissues surrounding implant in rabbits. Cell Biochem. Funct. 2006, 24, 275–281. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Abd El-Ghany, N.A.; Abdel-Aziz, M.M. Synthesis, characterization, anti-inflammatory and anti-Helicobacter pylori activities of novel benzophenone tetracarboxylimide benzoyl thiourea cross-linked chitosan hydrogels. Int. J. Biol. Macromol. 2021, 181, 956–965. [Google Scholar] [CrossRef]
- Fasolino, I.; Raucci, M.G.; Soriente, A.; Demitri, C.; Madaghiele, M.; Sannino, A.; Ambrosio, L. Osteoinductive and anti-inflammatory properties of chitosan-based scaffolds for bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110046. [Google Scholar] [CrossRef]
- Soriente, A.; Fasolino, I.; Gomez-Sánchez, A.; Prokhorov, E.; Buonocore, G.G.; Luna-Barcenas, G.; Ambrosio, L.; Raucci, M.G. Chitosan/hydroxyapatite nanocomposite scaffolds to modulate osteogenic and inflammatory response. J. Biomed. Mater. Res. Part A 2022, 110, 266–272. [Google Scholar] [CrossRef]
- Ribeiro, J.C.V.; Forte, T.C.M.; Tavares, S.J.S.; Andrade, F.K.; Vieira, R.S.; Lima, V. The effects of the molecular weight of chitosan on the tissue inflammatory response. J. Biomed. Mater. Research. Part A 2021, 109, 2556–2569. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Mori, T.; Fujinaga, T. Topical formulations and wound healing applications of chitosan. Adv. Drug Deliv. Rev. 2001, 52, 105–115. [Google Scholar] [CrossRef]
- Penha, E.S.D.; Lacerda-Santos, R.; de Medeiros, L.; Araújo Rosendo, R.; Dos Santos, A.; Fook, M.V.L.; de Sousa, W.J.B.; de Oliveira Firmino, M.; Montagna, E. Effect of chitosan and Dysphania ambrosioides on the bone regeneration process: A randomized controlled trial in an animal model. Microsc. Res. Tech. 2020, 83, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Le, B.Q.; Nurcombe, V.; Cool, S.M.; van Blitterswijk, C.A.; de Boer, J.; LaPointe, V.L.S. The Components of Bone and What They Can Teach Us about Regeneration. Materials 2017, 11, 14. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Li, H.Y.; Wang, Z.; Zhang, H.N.; Wang, Y.Z.; Xu, H. Carboxymethyl chitosan reduces inflammation and promotes osteogenesis in a rabbit knee replacement model. BMC Musculoskelet. Disord. 2020, 21, 775. [Google Scholar] [CrossRef]
- Wang, C.H.; Cherng, J.H.; Liu, C.C.; Fang, T.J.; Hong, Z.J.; Chang, S.J.; Fan, G.Y.; Hsu, S.D. Procoagulant and Antimicrobial Effects of Chitosan in Wound Healing. Int. J. Mol. Sci. 2021, 22, 7067. [Google Scholar] [CrossRef]
- Moutzouri, A.G.; Athanassiou, G.M. Insights into the alteration of osteoblast mechanical properties upon adhesion on chitosan. BioMed Res. Int. 2014, 2014, 740726. [Google Scholar] [CrossRef]
- Dejob, L.; Toury, B.; Tadier, S.; Grémillard, L.; Gaillard, C.; Salles, V. Electrospinning of in situ synthesized silica-based and calcium phosphate bioceramics for applications in bone tissue engineering: A review. Acta Biomater. 2021, 123, 123–153. [Google Scholar] [CrossRef]
- Wasupalli, G.K.; Verma, D. Injectable and thermosensitive nanofibrous hydrogel for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110343. [Google Scholar] [CrossRef]
- Chen, T.; Zou, Q.; Du, C.; Wang, C.; Li, Y.; Fu, B. Biodegradable 3D printed HA/CMCS/PDA scaffold for repairing lacunar bone defect. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 116, 111148. [Google Scholar] [CrossRef]
- Yao, M.; Zou, Q.; Zou, W.; Xie, Z.; Li, Z.; Zhao, X.; Du, C. Bifunctional scaffolds of hydroxyapatite/poly(dopamine)/carboxymethyl chitosan with osteogenesis and anti-osteosarcoma effect. Biomater. Sci. 2021, 9, 3319–3333. [Google Scholar] [CrossRef] [PubMed]
- Qasim, S.B.; Zafar, M.S.; Najeeb, S.; Khurshid, Z.; Shah, A.H.; Husain, S.; Rehman, I.U. Electrospinning of Chitosan-Based Solutions for Tissue Engineering and Regenerative Medicine. Int. J. Mol. Sci. 2018, 19, 407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taskin, M.B.; Xu, R.; Gregersen, H.; Nygaard, J.V.; Besenbacher, F.; Chen, M. Three-Dimensional Polydopamine Functionalized Coiled Microfibrous Scaffolds Enhance Human Mesenchymal Stem Cells Colonization and Mild Myofibroblastic Differentiation. ACS Appl. Mater. Interfaces 2016, 8, 15864–15873. [Google Scholar] [CrossRef] [PubMed]
- Formica, F.A.; Öztürk, E.; Hess, S.C.; Stark, W.J.; Maniura-Weber, K.; Rottmar, M.; Zenobi-Wong, M. A Bioinspired Ultraporous Nanofiber-Hydrogel Mimic of the Cartilage Extracellular Matrix. Adv. Healthc. Mater. 2016, 5, 3129–3138. [Google Scholar] [CrossRef]
- Ramadurai, K.W. 3-Dimensional Printing and Bio-Based Materials in Global Health: An Interventional Approach to Addressing Healthcare Disparities in Low and Middle-Income Countries. Master’s thesis, Harvard Extension School, Cambridge, MA, USA, 2017. [Google Scholar]
- Hwang, P.T.; Murdock, K.; Alexander, G.C.; Salaam, A.D.; Ng, J.I.; Lim, D.J.; Dean, D.; Jun, H.W. Poly(ε-caprolactone)/gelatin composite electrospun scaffolds with porous crater-like structures for tissue engineering. J. Biomed. Mater. Research. Part A 2016, 104, 1017–1029. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Kim, G. Three-Dimensional Hierarchical Nanofibrous Collagen Scaffold Fabricated Using Fibrillated Collagen and Pluronic F-127 for Regenerating Bone Tissue. ACS Appl. Mater. Interfaces 2018, 10, 35801–35811. [Google Scholar] [CrossRef]
- Saito, M.; Tsuji, T. Extracellular matrix administration as a potential therapeutic strategy for periodontal ligament regeneration. Expert Opin. Biol. Ther. 2012, 12, 299–309. [Google Scholar] [CrossRef]
- Qasim, S.B.; Najeeb, S.; Delaine-Smith, R.M.; Rawlinson, A.; Ur Rehman, I. Potential of electrospun chitosan fibers as a surface layer in functionally graded GTR membrane for periodontal regeneration. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2017, 33, 71–83. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.; Chen, M.; Qu, T.; Li, J.; Man, Y. Three-dimensional electrospun nanofibrous scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 1311–1321. [Google Scholar] [CrossRef]
- Zhou, Y.; Liang, K.; Zhao, S.; Zhang, C.; Li, J.; Yang, H.; Liu, X.; Yin, X.; Chen, D.; Xu, W.; et al. Photopolymerized maleilated chitosan/methacrylated silk fibroin micro/nanocomposite hydrogels as potential scaffolds for cartilage tissue engineering. Int. J. Biol. Macromol. 2018, 108, 383–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Reddy, V.J.; Wong, S.Y.; Li, X.; Su, B.; Ramakrishna, S.; Lim, C.T. Enhanced biomineralization in osteoblasts on a novel electrospun biocomposite nanofibrous substrate of hydroxyapatite/collagen/chitosan. Tissue Eng. Part A 2010, 16, 1949–1960. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.H.; Liao, M.H.; Lin, Y.L.; Lai, C.H.; Lin, P.I.; Chen, R.M. Improving effects of chitosan nanofiber scaffolds on osteoblast proliferation and maturation. Int. J. Nanomed. 2014, 9, 4293–4304. [Google Scholar] [CrossRef] [Green Version]
- Jing, X.; Mi, H.Y.; Wang, X.C.; Peng, X.F.; Turng, L.S. Shish-kebab-structured poly(ε-caprolactone) nanofibers hierarchically decorated with chitosan-poly(ε-caprolactone) copolymers for bone tissue engineering. ACS Appl. Mater. Interfaces 2015, 7, 6955–6965. [Google Scholar] [CrossRef] [PubMed]
- Jhala, D.; Rather, H.; Vasita, R. Polycaprolactone-chitosan nanofibers influence cell morphology to induce early osteogenic differentiation. Biomater. Sci. 2016, 4, 1584–1595. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Kim, D.; Kang, D.; Yang, G.H.; Jung, B.; Yeo, M.; Park, M.J.; An, S.; Lee, K.; Kim, J.S.; et al. 3D-printed gelatin methacrylate (GelMA)/silanated silica scaffold assisted by two-stage cooling system for hard tissue regeneration. Regen. Biomater. 2021, 8, rbab001. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, T.; Tenorio, A.J.; Campbell, K.T.; Silva, E.A.; Leach, J.K. Alginate-Based Bioinks for 3D Bioprinting and Fabrication of Anatomically Accurate Bone Grafts. Tissue Eng. Part A 2021, 27, 1168–1181. [Google Scholar] [CrossRef]
- Ozbolat, I.T. Bioprinting scale-up tissue and organ constructs for transplantation. Trends Biotechnol. 2015, 33, 395–400. [Google Scholar] [CrossRef]
- Dey, M.; Ozbolat, I.T. 3D bioprinting of cells, tissues and organs. Sci. Rep. 2020, 10, 14023. [Google Scholar] [CrossRef]
- Wu, C.S. Modulation, functionality, and cytocompatibility of three-dimensional printing materials made from chitosan-based polysaccharide composites. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 27–36. [Google Scholar] [CrossRef]
- Morris, V.B.; Nimbalkar, S.; Younesi, M.; McClellan, P.; Akkus, O. Mechanical Properties, Cytocompatibility and Manufacturability of Chitosan:PEGDA Hybrid-Gel Scaffolds by Stereolithography. Ann. Biomed. Eng. 2017, 45, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.Q.; Liu, W.G.; Xu, Z.Y.; Li, J.G.; Huang, T.T.; Lu, Y.J.; Huang, H.G.; Lin, J.X. Chitosan ducts fabricated by extrusion-based 3D printing for soft-tissue engineering. Carbohydr. Polym. 2020, 236, 116058. [Google Scholar] [CrossRef] [PubMed]
- Sadeghianmaryan, A.; Naghieh, S.; Alizadeh Sardroud, H.; Yazdanpanah, Z.; Afzal Soltani, Y.; Sernaglia, J.; Chen, X. Extrusion-based printing of chitosan scaffolds and their in vitro characterization for cartilage tissue engineering. Int. J. Biol. Macromol. 2020, 164, 3179–3192. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.F.; Lu, T.Y.; Li, Y.E.; Teng, K.C.; Chen, Y.C.; Wei, Y.; Lin, T.E.; Cheng, N.C.; Yu, J. Design and Synthesis of Stem Cell-Laden Keratin/Glycol Chitosan Methacrylate Bioinks for 3D Bioprinting. Biomacromolecules 2022. [Google Scholar] [CrossRef]
- Wu, C.; Yu, Z.; Li, Y.; Zhou, K.; Cao, C.; Zhang, P.; Li, W. Cryogenically printed flexible chitosan/bioglass scaffolds with stable and hierarchical porous structures for wound healing. Biomed. Mater. 2020, 16, 015004. [Google Scholar] [CrossRef]
- Suo, H.; Zhang, J.; Xu, M.; Wang, L. Low-temperature 3D printing of collagen and chitosan composite for tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 123, 111963. [Google Scholar] [CrossRef]
- Ozbolat, I.T.; Peng, W.; Ozbolat, V. Application areas of 3D bioprinting. Drug Discov. Today 2016, 21, 1257–1271. [Google Scholar] [CrossRef] [Green Version]
- Kelder, C.; Bakker, A.D.; Klein-Nulend, J.; Wismeijer, D. The 3D Printing of Calcium Phosphate with K-Carrageenan under Conditions Permitting the Incorporation of Biological Components-A Method. J. Funct. Biomater. 2018, 9, 57. [Google Scholar] [CrossRef] [Green Version]
- Atala, A.; Yoo, J.J. Essentials of 3D Biofabrication and Translation; Academic Press: Cambridge, MA, USA, 2015; pp. 1–422. [Google Scholar]
- Kurian, M.; Stevens, R.; McGrath, K.M. Towards the Development of Artificial Bone Grafts: Combining Synthetic Biomineralisation with 3D Printing. J. Funct. Biomater. 2019, 10, 12. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Maire, M.; Lerouge, S.; Daniel, T.; Heuzey, M.-C. 3D Printing of Microstructured and Stretchable Chitosan Hydrogel for Guided Cell Growth. Adv. Biosyst. 2017, 1, 1700058. [Google Scholar] [CrossRef]
- Rajabi, M.; Ali, A.; McConnell, M.; Cabral, J. Keratinous materials: Structures and functions in biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110612. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liao, J.; Wu, F.; Shi, J. Mechanical strength improvement of chitosan/hydroxyapatite scaffolds by coating and cross-linking. J. Mech. Behav. Biomed. Mater. 2021, 114, 104169. [Google Scholar] [CrossRef] [PubMed]
- Michailidou, G.; Terzopoulou, Z.; Kehagia, A.; Michopoulou, A.; Bikiaris, D.N. Preliminary Evaluation of 3D Printed Chitosan/Pectin Constructs for Biomedical Applications. Mar. Drugs 2021, 19, 36. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Q.; Jiang, B.J.; Guo, W.J.; Zhao, Y.M. Indirect 3D printing technology for the fabrication of customised β-TCP/chitosan scaffold with the shape of rabbit radial head-an in vitro study. J. Orthop. Surg. Res. 2019, 14, 102. [Google Scholar] [CrossRef]
- Ahmed, J.; Mulla, M.; Maniruzzaman, M. Rheological and Dielectric Behavior of 3D-Printable Chitosan/Graphene Oxide Hydrogels. ACS Biomater. Sci. Eng. 2020, 6, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Kazemi-Aghdam, F.; Jahed, V.; Dehghan-Niri, M.; Ganji, F.; Vasheghani-Farahani, E. Injectable chitosan hydrogel embedding modified halloysite nanotubes for bone tissue engineering. Carbohydr. Polym. 2021, 269, 118311. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Ren, W.; Hou, R.; Liu, H.; Li, R.; Du, S.; Wang, L.; Liu, J. PEI-modified diatomite/chitosan composites as bone tissue engineering scaffold for sustained release of BMP-2. J. Biomater. Science. Polym. Ed. 2021, 32, 1337–1355. [Google Scholar] [CrossRef] [PubMed]
- Koç Demir, A.; Elçin, A.E.; Elçin, Y.M. Strontium-modified chitosan/montmorillonite composites as bone tissue engineering scaffold. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 89, 8–14. [Google Scholar] [CrossRef]
- Daemi, H.; Barikani, M. Synthesis and characterization of calcium alginate nanoparticles, sodium homopolymannuronate salt and its calcium nanoparticles. Sci. Iran. 2012, 19, 2023–2028. [Google Scholar] [CrossRef] [Green Version]
- Speer, D.P.; Chvapil, M.; Eskelson, C.D.; Ulreich, J. Biological effects of residual glutaraldehyde in glutaraldehyde-tanned collagen biomaterials. J. Biomed. Mater. Res. 1980, 14, 753–764. [Google Scholar] [CrossRef]
- Zheng, Z.; Wu, J.; Liu, M.; Wang, H.; Li, C.; Rodriguez, M.J.; Li, G.; Wang, X.; Kaplan, D.L. 3D Bioprinting of Self-Standing Silk-Based Bioink. Adv. Healthc. Mater. 2018, 7, e1701026. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Ma, Y.; Hou, C.; Gao, F.; Zhang, Y.; Ruan, C.; Pan, H.; Lu, W.W.; Liu, W. 3D-Printed High Strength Bioactive Supramolecular Polymer/Clay Nanocomposite Hydrogel Scaffold for Bone Regeneration. ACS Biomater. Sci. Eng. 2017, 3, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Ventura, I.; Bianco-Peled, H. Small-angle X-ray scattering study on pectin-chitosan mixed solutions and thermoreversible gels. Carbohydr. Polym. 2015, 123, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tan, Y.J.; Liu, S.; Li, L. Three-Dimensional Bioprinting of Oppositely Charged Hydrogels with Super Strong Interface Bonding. ACS Appl. Mater. Interfaces 2018, 10, 11164–11174. [Google Scholar] [CrossRef]
- Gierszewska, M.; Ostrowska-Czubenko, J. Chitosan-based membranes with different ionic crosslinking density for pharmaceutical and industrial applications. Carbohydr. Polym. 2016, 153, 501–511. [Google Scholar] [CrossRef]
- Censi, R.; Fieten, P.J.; Di Martino, P.; Hennink, W.E.; Vermonden, T. In-situ forming hydrogels by simultaneous thermal gelling and Michael addition reaction between methacrylate bearing thermosensitive triblock copolymers and thiolated hyaluronan. J. Control. Release Off. J. Control. Release Soc. 2010, 148, e28–e29. [Google Scholar] [CrossRef]
- Cai, Z.; Tang, Y.; Wei, Y.; Wang, P.; Zhang, H. Physically Cross-Linked Hyaluronan-Based Ultrasoft Cryogel Prepared by Freeze-Thaw Technique as a Barrier for Prevention of Postoperative Adhesions. Biomacromolecules 2021, 22, 4967–4979. [Google Scholar] [CrossRef]
- Parhi, R. Cross-Linked Hydrogel for Pharmaceutical Applications: A Review. Adv. Pharm. Bull. 2017, 7, 515–530. [Google Scholar] [CrossRef]
- Li, X.; Sun, Q.; Li, Q.; Kawazoe, N.; Chen, G. Functional Hydrogels With Tunable Structures and Properties for Tissue Engineering Applications. Front. Chem. 2018, 6, 499. [Google Scholar] [CrossRef] [Green Version]
- Pan, W.; Dong, J.; Gui, T.; Liu, R.; Liu, X.; Luo, J. Fabrication of dual anti-corrosive polyaniline microcapsules via Pickering emulsion for active corrosion protection of steel. Soft Matter. 2022, 18, 2829–2841. [Google Scholar] [CrossRef]
- Gyarmati, B.; Mészár, E.Z.; Kiss, L.; Deli, M.A.; László, K.; Szilágyi, A. Supermacroporous chemically cross-linked poly(aspartic acid) hydrogels. Acta Biomater. 2015, 22, 32–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellá, M.C.G.; Lima-Tenório, M.K.; Tenório-Neto, E.T.; Guilherme, M.R.; Muniz, E.C.; Rubira, A.F. Chitosan-based hydrogels: From preparation to biomedical applications. Carbohydr. Polym. 2018, 196, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhang, X.; Tan, G.; Tian, L.; Liu, D.; Liu, Y.; Yang, X.; Pan, W. A novel pH-induced thermosensitive hydrogel composed of carboxymethyl chitosan and poloxamer cross-linked by glutaraldehyde for ophthalmic drug delivery. Carbohydr. Polym. 2017, 155, 208–217. [Google Scholar] [CrossRef]
- Wei, L.; Mi, Y.; Zhang, J.; Li, Q.; Dong, F.; Guo, Z. Evaluation of quaternary ammonium chitosan derivatives differing in the length of alkyl side-chain: Synthesis and antifungal activity. Int. J. Biol. Macromol. 2019, 129, 1127–1132. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Yuan, Z.; Han, H.; Li, T.; Li, L.; Guo, X. Chitosan cross-linked poly(acrylic acid) hydrogels: Drug release control and mechanism. Colloids Surf. B Biointerfaces 2017, 152, 252–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, X.; Zhang, W.; Liu, Y.; Zhao, Y.; Zhang, J.; Hou, M. Hygroscopicity modulation of hydrogels based on carboxymethyl chitosan/Alginate polyelectrolyte complexes and its application as pH-sensitive delivery system. Carbohydr. Polym. 2018, 198, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Braz, E.M.A.; Silva, S.C.C.C.; Sousa Brito, C.A.R.; Brito, L.M.; Barreto, H.M.; Carvalho, F.A.A.; Santos, L.S.; Lobo, A.O.; Osajima, J.A.; Sousa, K.S.; et al. Spectroscopic, thermal characterizations and bacteria inhibition of chemically modified chitosan with phthalic anhydride. Mater. Chem. Phys. 2020, 240, 122053. [Google Scholar] [CrossRef]
- Medeiros Borsagli, F.G.L.; Carvalho, I.C.; Mansur, H.S. Amino acid-grafted and N-acylated chitosan thiomers: Construction of 3D bio-scaffolds for potential cartilage repair applications. Int. J. Biol. Macromol. 2018, 114, 270–282. [Google Scholar] [CrossRef]
- Bhavsar, C.; Momin, M.; Gharat, S.; Omri, A. Functionalized and graft copolymers of chitosan and its pharmaceutical applications. Expert Opin. Drug Deliv. 2017, 14, 1189–1204. [Google Scholar] [CrossRef]
- Alves, N.M.; Mano, J.F. Chitosan derivatives obtained by chemical modifications for biomedical and environmental applications. Int. J. Biol. Macromol. 2008, 43, 401–414. [Google Scholar] [CrossRef] [Green Version]
- Ninan, N.; Mohan Bhagyaraj, S.; Francis, E. Natural Polymers, Biopolymers, Biomaterials, and Their Composites, Blends, and IPNs; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Campos, E.V.R.; Oliveira, J.L.; Fraceto, L.F. Poly(ethylene glycol) and Cyclodextrin-Grafted Chitosan: From Methodologies to Preparation and Potential Biotechnological Applications. Front. Chem. 2017, 5, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, D.; Kumar, S. Grafting of Acrylic Acid on to Plantago psyllium Mucilage. IOSR J. Appl. Chem. 2014, 7, 76–82. [Google Scholar] [CrossRef]
- Kumar, D.; Khan, N.; Kumar, P.; Panday, J. Improve the native characteristics of polysaccharides by grafting through the gamma radiation: A review. Green Chem. Technol. Lett. 2016, 2, 151–159. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, P.; Pandey, J. Binary grafted chitosan film: Synthesis, characterization, antibacterial activity and prospects for food packaging. Int. J. Biol. Macromol. 2018, 115, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Tripodo, G.; Trapani, A.; Rosato, A.; Di Franco, C.; Tamma, R.; Trapani, G.; Ribatti, D.; Mandracchia, D. Hydrogels for biomedical applications from glycol chitosan and PEG diglycidyl ether exhibit pro-angiogenic and antibacterial activity. Carbohydr. Polym. 2018, 198, 124–130. [Google Scholar] [CrossRef]
- Dinu, M.V.; Gradinaru, A.C.; Lazar, M.M.; Dinu, I.A.; Raschip, I.E.; Ciocarlan, N.; Aprotosoaie, A.C. Physically cross-linked chitosan/dextrin cryogels entrapping Thymus vulgaris essential oil with enhanced mechanical, antioxidant and antifungal properties. Int. J. Biol. Macromol. 2021, 184, 898–908. [Google Scholar] [CrossRef]
- Li, P.; Feng, Z.; Yu, Z.; Chen, Y.; Li, P.; Yang, Z.; Li, S.; Jin, S. Preparation of chitosan-Cu(2+)/NH(3) physical hydrogel and its properties. Int. J. Biol. Macromol. 2019, 133, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Takei, T.; Danjo, S.; Sakoguchi, S.; Tanaka, S.; Yoshinaga, T.; Nishimata, H.; Yoshida, M. Autoclavable physically-crosslinked chitosan cryogel as a wound dressing. J. Biosci. Bioeng. 2018, 125, 490–495. [Google Scholar] [CrossRef]
- Neufeld, L.; Bianco-Peled, H. Pectin-chitosan physical hydrogels as potential drug delivery vehicles. Int. J. Biol. Macromol. 2017, 101, 852–861. [Google Scholar] [CrossRef]
- Ding, B.; Gao, H.; Song, J.; Li, Y.; Zhang, L.; Cao, X.; Xu, M.; Cai, J. Tough and Cell-Compatible Chitosan Physical Hydrogels for Mouse Bone Mesenchymal Stem Cells in Vitro. ACS Appl. Mater. Interfaces 2016, 8, 19739–19746. [Google Scholar] [CrossRef]
- Jiang, P.; Lin, P.; Yang, C.; Qin, H.; Wang, X.; Zhou, F. 3D Printing of Dual-Physical Cross-linking Hydrogel with Ultrahigh Strength and Toughness. Chem. Mater. 2020, 32, 9983–9995. [Google Scholar] [CrossRef]
- Singh, Y.P.; Dasgupta, S.; Bhaskar, R. Preparation, characterization and bioactivities of nano anhydrous calcium phosphate added gelatin-chitosan scaffolds for bone tissue engineering. J. Biomater. Sci. Polym. Ed. 2019, 30, 1756–1778. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, C.; Cui, Y.; Li, A.; Qiao, Y.; Qiu, D. Conjoined-network rendered stiff and tough hydrogels from biogenic molecules. Sci. Adv. 2019, 5, eaau3442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, C.; Shao, C.; Meng, L.; Yang, J. High-Strength, Self-Adhesive, and Strain-Sensitive Chitosan/Poly(acrylic acid) Double-Network Nanocomposite Hydrogels Fabricated by Salt-Soaking Strategy for Flexible Sensors. ACS Appl. Mater. Interfaces 2019, 11, 39228–39237. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.A.; Kurokawa, T.; Gong, J.P. Super tough double network hydrogels and their application as biomaterials. Polymer 2012, 53, 1805–1822. [Google Scholar] [CrossRef]
- Nonoyama, T.; Gong, J.P. Tough Double Network Hydrogel and Its Biomedical Applications. Annu. Rev. Chem. Biomol. Eng. 2021, 12, 393–410. [Google Scholar] [CrossRef]
- Shaw, S.; White, J.D. Asymmetric Catalysis Using Chiral Salen-Metal Complexes: Recent Advances. Chem. Rev. 2019, 119, 9381–9426. [Google Scholar] [CrossRef]
- Shi, A.; Dai, X.; Jing, Z. Tough and Self-Healing Chitosan/Poly(acrylamide-co-acrylic acid) Double Network Hydrogels. Polym. Sci. Ser. A 2020, 62, 228–239. [Google Scholar] [CrossRef]
- Peak, C.; Wilker, J.; Schmidt, G. A Review on Tough and Sticky Hydrogels. Colloid Polym. Sci. 2013, 291, 2031–2049. [Google Scholar] [CrossRef]
- Matricardi, P.; Di Meo, C.; Coviello, T.; Hennink, W.E.; Alhaique, F. Interpenetrating Polymer Networks polysaccharide hydrogels for drug delivery and tissue engineering. Adv. Drug Deliv. Rev. 2013, 65, 1172–1187. [Google Scholar] [CrossRef]
- Fukao, K.; Tanaka, K.; Kiyama, R.; Nonoyama, T.; Gong, J.P. Hydrogels toughened by biominerals providing energy-dissipative sacrificial bonds. J. Mater. Chem. B 2020, 8, 5184–5188. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.; Sun, T.L.; Saruwatari, Y.; Kurokawa, T.; King, D.R.; Gong, J.P. Creating Stiff, Tough, and Functional Hydrogel Composites with Low-Melting-Point Alloys. Adv. Mater. 2018, 30, e1706885. [Google Scholar] [CrossRef] [PubMed]
- Lieou, C.K.; Elbanna, A.E.; Carlson, J.M. Sacrificial bonds and hidden length in biomaterials: A kinetic constitutive description of strength and toughness in bone. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2013, 88, 012703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bi, S.; Wang, P.; Hu, S.; Li, S.; Pang, J.; Zhou, Z.; Sun, G.; Huang, L.; Cheng, X.; Xing, S.; et al. Construction of physical-crosslink chitosan/PVA double-network hydrogel with surface mineralization for bone repair. Carbohydr. Polym. 2019, 224, 115176. [Google Scholar] [CrossRef]
- Duan, J.; Liang, X.; Guo, J.; Zhu, K.; Zhang, L. Ultra-Stretchable and Force-Sensitive Hydrogels Reinforced with Chitosan Microspheres Embedded in Polymer Networks. Adv. Mater. 2016, 28, 8037–8044. [Google Scholar] [CrossRef]
- Bi, S.; Pang, J.; Huang, L.; Sun, M.; Cheng, X.; Chen, X. The toughness chitosan-PVA double network hydrogel based on alkali solution system and hydrogen bonding for tissue engineering applications. Int. J. Biol. Macromol. 2020, 146, 99–109. [Google Scholar] [CrossRef]
- Cong, J.; Fan, Z.; Pan, S.; Tian, J.; Lian, W.; Li, S.; Wang, S.; Zheng, D.; Miao, C.; Ding, W.; et al. Polyacrylamide/Chitosan-Based Conductive Double Network Hydrogels with Outstanding Electrical and Mechanical Performance at Low Temperatures. ACS Appl. Mater. Interfaces 2021, 13, 34942–34953. [Google Scholar] [CrossRef]
- Gadhave, R.V.; Mahanwar, P.A.; Gadekar, P.T. Effect of glutaraldehyde on thermal and mechanical properties of starch and polyvinyl alcohol blends. Des. Monomers Polym. 2019, 22, 164–170. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Venugopal, J.; Huang, Z.M.; Lim, C.T. Crosslinking of the electrospun gelatin nanofibers. Polymer 2006, 47, 2911–2917. [Google Scholar] [CrossRef]
- Pinto, R.V.; Gomes, P.S.; Fernandes, M.H.; Costa, M.E.V.; Almeida, M.M. Glutaraldehyde-crosslinking chitosan scaffolds reinforced with calcium phosphate spray-dried granules for bone tissue applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 109, 110557. [Google Scholar] [CrossRef]
- Jalaja, K.; Naskar, D.; Kundu, S.C.; James, N.R. Potential of electrospun core-shell structured gelatin-chitosan nanofibers for biomedical applications. Carbohydr. Polym. 2016, 136, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Augustin, M.A.; Hemar, Y. Nano- and micro-structured assemblies for encapsulation of food ingredients. Chem. Soc. Rev. 2009, 38, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhan, W.; Tang, X.; Mo, F.; Fu, L.; Lin, B. Self-healing chitosan/vanillin hydrogels based on Schiff-base bond/hydrogen bond hybrid linkages. Polym. Test. 2018, 66, 155–163. [Google Scholar] [CrossRef]
- Zou, Q.; Li, J.; Li, Y. Preparation and characterization of vanillin-crosslinked chitosan therapeutic bioactive microcarriers. Int. J. Biol. Macromol. 2015, 79, 736–747. [Google Scholar] [CrossRef]
- Michailidou, G.; Koukaras, E.N.; Bikiaris, D.N. Vanillin chitosan miscible hydrogel blends and their prospects for 3D printing biomedical applications. Int. J. Biol. Macromol. 2021, 192, 1266–1275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Han, Z.; Zeng, X.A.; Xiong, X.Y.; Liu, Y.J. Enhancing mechanical properties of chitosan films via modification with vanillin. Int. J. Biol. Macromol. 2015, 81, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yu, M.; Li, Y.; Li, Q.; Yang, H.; Zheng, M.; Han, Y.; Lu, D.; Lu, S.; Gui, L. Synergistic anti-inflammatory and osteogenic n-HA/resveratrol/chitosan composite microspheres for osteoporotic bone regeneration. Bioact. Mater. 2021, 6, 1255–1266. [Google Scholar] [CrossRef]
- Gadkari, R.R.; Suwalka, S.; Yogi, M.R.; Ali, W.; Das, A.; Alagirusamy, R. Green synthesis of chitosan-cinnamaldehyde cross-linked nanoparticles: Characterization and antibacterial activity. Carbohydr. Polym. 2019, 226, 115298. [Google Scholar] [CrossRef]
- Wu, X.; Miao, L.; Yao, Y.; Wu, W.; Liu, Y.; Chen, X.; Sun, W. Electrospun fibrous scaffolds combined with nanoscale hydroxyapatite induce osteogenic differentiation of human periodontal ligament cells. Int. J. Nanomed. 2014, 9, 4135–4143. [Google Scholar] [CrossRef] [Green Version]
- Muzzarelli, R.A.; El Mehtedi, M.; Bottegoni, C.; Aquili, A.; Gigante, A. Genipin-Crosslinked Chitosan Gels and Scaffolds for Tissue Engineering and Regeneration of Cartilage and Bone. Mar. Drugs 2015, 13, 7314–7338. [Google Scholar] [CrossRef] [Green Version]
- Oryan, A.; Kamali, A.; Moshiri, A.; Baharvand, H.; Daemi, H. Chemical crosslinking of biopolymeric scaffolds: Current knowledge and future directions of crosslinked engineered bone scaffolds. Int. J. Biol. Macromol. 2018, 107, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.D.; Abueva, C.; Kim, B.; Lee, B.T. Chitosan-hyaluronic acid polyelectrolyte complex scaffold crosslinked with genipin for immobilization and controlled release of BMP-2. Carbohydr. Polym. 2015, 115, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, Q.; Zhu, J.; Xiao, J.; Wan, P.; Zhou, C.; Huang, Z.; Qiang, N.; Zhang, W.; Wu, Z.; et al. Using genipin-crosslinked acellular porcine corneal stroma for cosmetic corneal lens implants. Biomaterials 2012, 33, 7336–7346. [Google Scholar] [CrossRef] [PubMed]
- Su, W.-T.; Wang, Y.-T.; Chou, C.-M. Optimal fluid flow enhanced mineralization of MG-63 cells in porous chitosan scaffold. J. Taiwan Inst. Chem. Eng. 2014, 45, 1111–1118. [Google Scholar] [CrossRef]
- Wu, F.; Meng, G.; He, J.; Wu, Y.; Wu, F.; Gu, Z. Antibiotic-loaded chitosan hydrogel with superior dual functions: Antibacterial efficacy and osteoblastic cell responses. ACS Appl. Mater. Interfaces 2014, 6, 10005–10013. [Google Scholar] [CrossRef]
- Yan, L.P.; Wang, Y.J.; Ren, L.; Wu, G.; Caridade, S.G.; Fan, J.B.; Wang, L.Y.; Ji, P.H.; Oliveira, J.M.; Oliveira, J.T.; et al. Genipin-cross-linked collagen/chitosan biomimetic scaffolds for articular cartilage tissue engineering applications. J. Biomed. Mater. Res. Part A 2010, 95, 465–475. [Google Scholar] [CrossRef] [Green Version]
- Garnica-Palafox, I.M.; Sánchez-Arévalo, F.M. Influence of natural and synthetic crosslinking reagents on the structural and mechanical properties of chitosan-based hybrid hydrogels. Carbohydr. Polym. 2016, 151, 1073–1081. [Google Scholar] [CrossRef]
- Wahba, M.I. Enhancement of the mechanical properties of chitosan. J. Biomater. Sci. Polym. Ed. 2020, 31, 350–375. [Google Scholar] [CrossRef]
- Frohbergh, M.E.; Katsman, A.; Botta, G.P.; Lazarovici, P.; Schauer, C.L.; Wegst, U.G.; Lelkes, P.I. Electrospun hydroxyapatite-containing chitosan nanofibers crosslinked with genipin for bone tissue engineering. Biomaterials 2012, 33, 9167–9178. [Google Scholar] [CrossRef] [Green Version]
- Pandit, V.; Zuidema, J.M.; Venuto, K.N.; Macione, J.; Dai, G.; Gilbert, R.J.; Kotha, S.P. Evaluation of multifunctional polysaccharide hydrogels with varying stiffness for bone tissue engineering. Tissue Eng. Part A 2013, 19, 2452–2463. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [Green Version]
- Dimida, S.; Santin, M.; Verri, T.; Barca, A.; Demitri, C. Assessment of Cytocompatibility and Anti-Inflammatory (Inter)Actions of Genipin-Crosslinked Chitosan Powders. Biology 2020, 9, 159. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.S.; Lee, S.E.; Ng, C.L.; Kim, J.K.; Park, J.S. Exploring the Preparation of Albendazole-Loaded Chitosan-Tripolyphosphate Nanoparticles. Materials 2015, 8, 486–498. [Google Scholar] [CrossRef] [PubMed]
- Bahreini, E.; Aghaiypour, K.; Abbasalipourkabir, R.; Mokarram, A.R.; Goodarzi, M.T.; Saidijam, M. Preparation and nanoencapsulation of l-asparaginase II in chitosan-tripolyphosphate nanoparticles and in vitro release study. Nanoscale Res. Lett. 2014, 9, 340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jing, Z.W.; Jia, Y.Y.; Wan, N.; Luo, M.; Huan, M.L.; Kang, T.B.; Zhou, S.Y.; Zhang, B.L. Design and evaluation of novel pH-sensitive ureido-conjugated chitosan/TPP nanoparticles targeted to Helicobacter pylori. Biomaterials 2016, 84, 276–285. [Google Scholar] [CrossRef]
- Rázga, F.; Vnuková, D.; Némethová, V.; Mazancová, P.; Lacík, I. Preparation of chitosan-TPP sub-micron particles: Critical evaluation and derived recommendations. Carbohydr. Polym. 2016, 151, 488–499. [Google Scholar] [CrossRef]
- Liao, C.T.; Ho, M.H. The Fabrication of Biomimetic Chitosan Scaffolds by Using SBF Treatment with Different Crosslinking Agents. Membranes 2010, 1, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Rho, J.Y.; Tsui, T.Y.; Pharr, G.M. Elastic properties of human cortical and trabecular lamellar bone measured by nanoindentation. Biomaterials 1997, 18, 1325–1330. [Google Scholar] [CrossRef]
- Aryaei, A.; Jayatissa, A.H.; Jayasuriya, A.C. Nano and micro mechanical properties of uncross-linked and cross-linked chitosan films. J. Mech. Behav. Biomed. Mater. 2012, 5, 82–89. [Google Scholar] [CrossRef] [Green Version]
- Aryaei, A.; Liu, J.; Jayatissa, A.H.; Jayasuriya, A.C. Cross-linked chitosan improves the mechanical properties of calcium phosphate-chitosan cement. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 54, 14–19. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, L.B.; Avalos, A.; Chiaia, N.; Nadarajah, A. Effect of Formulation and Process Parameters on Chitosan Microparticles Prepared by an Emulsion Crosslinking Technique. AAPS PharmSciTech 2017, 18, 1084–1094. [Google Scholar] [CrossRef]
- Mantripragada, V.P.; Jayasuriya, A.C. IGF-1 release kinetics from chitosan microparticles fabricated using environmentally benign conditions. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 42, 506–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medellín-Castillo, N.A.; Isaacs-Páez, E.D.; Rodríguez-Méndez, I.; González-García, R.; Labrada-Delgado, G.J.; Aragón-Piña, A.; García-Arreola, M.E. Formaldehyde and tripolyphosphate crosslinked chitosan hydrogels: Synthesis, characterization and modeling. Int. J. Biol. Macromol. 2021, 183, 2293–2304. [Google Scholar] [CrossRef] [PubMed]
- Silvestro, I.; Francolini, I.; Di Lisio, V.; Martinelli, A.; Pietrelli, L.; Scotto d’Abusco, A.; Scoppio, A.; Piozzi, A. Preparation and Characterization of TPP-Chitosan Crosslinked Scaffolds for Tissue Engineering. Materials 2020, 13, 3577. [Google Scholar] [CrossRef]
- Uswatta, S.P.; Okeke, I.U.; Jayasuriya, A.C. Injectable porous nano-hydroxyapatite/chitosan/tripolyphosphate scaffolds with improved compressive strength for bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 505–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sacco, P.; Borgogna, M.; Travan, A.; Marsich, E.; Paoletti, S.; Asaro, F.; Grassi, M.; Donati, I. Polysaccharide-based networks from homogeneous chitosan-tripolyphosphate hydrogels: Synthesis and characterization. Biomacromolecules 2014, 15, 3396–3405. [Google Scholar] [CrossRef]
- Izzo, D.; Palazzo, B.; Scalera, F.; Gullotta, F.; Lapesa, V.; Scialla, S.; Sannino, A.; Gervaso, F. Chitosan scaffolds for cartilage regeneration: Influence of different ionic crosslinkers on biomaterial properties. Int. J. Polym. Mater. Polym. Biomater. 2018, 68, 936–945. [Google Scholar] [CrossRef]
- Mora-Boza, A.; Włodarczyk-Biegun, M.K.; Del Campo, A.; Vázquez-Lasa, B.; Román, J.S. Glycerylphytate as an ionic crosslinker for 3D printing of multi-layered scaffolds with improved shape fidelity and biological features. Biomater. Sci. 2019, 8, 506–516. [Google Scholar] [CrossRef] [Green Version]
- Diaz Blanco, C.; Ortner, A.; Dimitrov, R.; Navarro, A.; Mendoza, E.; Tzanov, T. Building an antifouling zwitterionic coating on urinary catheters using an enzymatically triggered bottom-up approach. ACS Appl. Mater. Interfaces 2014, 6, 11385–11393. [Google Scholar] [CrossRef]
- Ghosh, P.; Rameshbabu, A.P.; Das, D.; Francis, N.K.; Pawar, H.S.; Subramanian, B.; Pal, S.; Dhara, S. Covalent cross-links in polyampholytic chitosan fibers enhances bone regeneration in a rabbit model. Colloids Surf. B Biointerfaces 2015, 125, 160–169. [Google Scholar] [CrossRef]
- Lim, K.S.; Levato, R.; Costa, P.F.; Castilho, M.D.; Alcala-Orozco, C.R.; van Dorenmalen, K.M.A.; Melchels, F.P.W.; Gawlitta, D.; Hooper, G.J.; Malda, J.; et al. Bio-resin for high resolution lithography-based biofabrication of complex cell-laden constructs. Biofabrication 2018, 10, 034101. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Ma, X.; Gou, M.; Mei, D.; Zhang, K.; Chen, S. 3D printing of functional biomaterials for tissue engineering. Curr. Opin. Biotechnol. 2016, 40, 103–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krkobabić, M.; Medarević, D.; Pešić, N.; Vasiljević, D.; Ivković, B.; Ibrić, S. Digital Light Processing (DLP) 3D Printing of Atomoxetine Hydrochloride Tablets Using Photoreactive Suspensions. Pharmaceutics 2020, 12, 833. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wei, D.; Yang, K.; Yang, Y.; Liu, X.; Fan, H.; Zhang, X. The development of cell-initiated degradable hydrogel based on methacrylated alginate applicable to multiple microfabrication technologies. J. Mater. Chem. B 2017, 5, 8060–8069. [Google Scholar] [CrossRef]
- Shen, Y.; Tang, H.; Huang, X.; Hang, R.; Zhang, X.; Wang, Y.; Yao, X. DLP printing photocurable chitosan to build bio-constructs for tissue engineering. Carbohydr. Polym. 2020, 235, 115970. [Google Scholar] [CrossRef]
- Céspedes-Valenzuela, D.N.; Sánchez-Rentería, S.; Cifuentes, J.; Gantiva-Diaz, M.; Serna, J.A.; Reyes, L.H.; Ostos, C.; Cifuentes-De la Portilla, C.; Muñoz-Camargo, C.; Cruz, J.C. Preparation and Characterization of an Injectable and Photo-Responsive Chitosan Methacrylate/Graphene Oxide Hydrogel: Potential Applications in Bone Tissue Adhesion and Repair. Polymers 2021, 14, 126. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, S.; Zhang, C.; Liang, K.; Li, J.; Yang, H.; Gu, S.; Bai, Z.; Ye, D.; Xu, W. Photopolymerized maleilated chitosan/thiol-terminated poly (vinyl alcohol) hydrogels as potential tissue engineering scaffolds. Carbohydr. Polym. 2018, 184, 383–389. [Google Scholar] [CrossRef]
- Nair; Lakshmi, S. Injectable Hydrogels by Enzymatic Crosslinking; Imperial College Press: London, UK, 2016; pp. 201–238. [Google Scholar]
- Wang, L.S.; Du, C.; Chung, J.E.; Kurisawa, M. Enzymatically cross-linked gelatin-phenol hydrogels with a broader stiffness range for osteogenic differentiation of human mesenchymal stem cells. Acta Biomater. 2012, 8, 1826–1837. [Google Scholar] [CrossRef]
- Wei, Q.; Duan, J.; Ma, G.; Zhang, W.; Wang, Q.; Hu, Z. Enzymatic crosslinking to fabricate antioxidant peptide-based supramolecular hydrogel for improving cutaneous wound healing. J. Mater. Chem. B 2019, 7, 2220–2225. [Google Scholar] [CrossRef]
- Maddock, R.M.A.; Pollard, G.J.; Moreau, N.G.; Perry, J.J.; Race, P.R. Enzyme-catalysed polymer cross-linking: Biocatalytic tools for chemical biology, materials science and beyond. Biopolymers 2020, 111, e23390. [Google Scholar] [CrossRef]
- El-Hag Ali, A.; AlArifi, A. Characterization and in vitro evaluation of starch based hydrogels as carriers for colon specific drug delivery systems. Carbohydr. Polym. 2009, 78, 725–730. [Google Scholar] [CrossRef]
- Del Valle, L.J.; Díaz, A.; Puiggalí, J. Hydrogels for Biomedical Applications: Cellulose, Chitosan, and Protein/Peptide Derivatives. Gels 2017, 3, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Zhou, L.; Sun, Q.; Cai, H.; Tan, W.S. Porous chitosan derivative scaffolds affect proliferation and osteogenesis of mesenchymal stem cell via reducing intracellular ROS. Carbohydr. Polym. 2020, 237, 116108. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Tolba, E.; Schröder, H.C.; Neufurth, M.; Wang, S.; Link, T.; Al-Nawas, B.; Wang, X. A new printable and durable N,O-carboxymethyl chitosan-Ca(2+)-polyphosphate complex with morphogenetic activity. J. Mater. Chem. B 2015, 3, 1722–1730. [Google Scholar] [CrossRef] [PubMed]
- Hirata, I.; Akamatsu, M.; Fujii, E.; Poolthong, S.; Okazaki, M. Chemical analyses of hydroxyapatite formation on SAM surfaces modified with COOH, NH(2), CH(3), and OH functions. Dent. Mater. J. 2010, 29, 438–445. [Google Scholar] [CrossRef] [Green Version]
- Dumont, V.C.; Mansur, A.A.P.; Carvalho, S.M.; Medeiros Borsagli, F.G.L.; Pereira, M.M.; Mansur, H.S. Chitosan and carboxymethyl-chitosan capping ligands: Effects on the nucleation and growth of hydroxyapatite nanoparticles for producing biocomposite membranes. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 265–277. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, L.; Li, Q.; Zou, Q.; Du, C. Biomimetic mineralization of carboxymethyl chitosan nanofibers with improved osteogenic activity in vitro and in vivo. Carbohydr. Polym. 2018, 195, 225–234. [Google Scholar] [CrossRef]
- LogithKumar, R.; KeshavNarayan, A.; Dhivya, S.; Chawla, A.; Saravanan, S.; Selvamurugan, N. A review of chitosan and its derivatives in bone tissue engineering. Carbohydr. Polym. 2016, 151, 172–188. [Google Scholar] [CrossRef]
- Upadhyaya, L.; Singh, J.; Agarwal, V.; Tewari, R.P. The implications of recent advances in carboxymethyl chitosan based targeted drug delivery and tissue engineering applications. J. Control. Release Off. J. Control. Release Soc. 2014, 186, 54–87. [Google Scholar] [CrossRef]
- Sharifi, F.; Atyabi, S.M.; Norouzian, D.; Zandi, M.; Irani, S.; Bakhshi, H. Polycaprolactone/carboxymethyl chitosan nanofibrous scaffolds for bone tissue engineering application. Int. J. Biol. Macromol. 2018, 115, 243–248. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Chen, Y.P.; Han, J.; Mo, J.; Dong, P.F.; Zhuo, Y.H.; Feng, Y. Biocompatiable silk fibroin/carboxymethyl chitosan/strontium substituted hydroxyapatite/cellulose nanocrystal composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2019, 136, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Adnan, S.; Ranjha, N.M.; Hanif, M.; Asghar, S. O-Carboxymethylated chitosan; A promising tool with in-vivo anti-inflammatory and analgesic properties in albino rats. Int. J. Biol. Macromol. 2020, 156, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Santos, B.; Chorilli, M. An overview of carboxymethyl derivatives of chitosan: Their use as biomaterials and drug delivery systems. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 1349–1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, H.; Ma, R.; Lin, C.; Liu, Z.; Tang, T. Quaternized chitosan as an antimicrobial agent: Antimicrobial activity, mechanism of action and biomedical applications in orthopedics. Int. J. Mol. Sci. 2013, 14, 1854–1869. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, S.; Wang, Y.; Yu, Z.; Ao, H.; Zhang, H.; Qin, L.; Guillaume, O.; Eglin, D.; Richards, R.G.; et al. Anti-infective efficacy, cytocompatibility and biocompatibility of a 3D-printed osteoconductive composite scaffold functionalized with quaternized chitosan. Acta Biomater. 2016, 46, 112–128. [Google Scholar] [CrossRef]
- Crismaru, M.; Asri, L.A.; Loontjens, T.J.; Krom, B.P.; de Vries, J.; van der Mei, H.C.; Busscher, H.J. Survival of adhering staphylococci during exposure to a quaternary ammonium compound evaluated by using atomic force microscopy imaging. Antimicrob. Agents Chemother. 2011, 55, 5010–5017. [Google Scholar] [CrossRef] [Green Version]
- Zhou, P.; Xia, Y.; Cheng, X.; Wang, P.; Xie, Y.; Xu, S. Enhanced bone tissue regeneration by antibacterial and osteoinductive silica-HACC-zein composite scaffolds loaded with rhBMP-2. Biomaterials 2014, 35, 10033–10045. [Google Scholar] [CrossRef]
- Hanssen, A.D. Local antibiotic delivery vehicles in the treatment of musculoskeletal infection. Clin. Orthop. Relat. Res. 2005, 437, 91–96. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Speziale, P.; Arciola, C.R. Antibiotic-loaded biomaterials and the risks for the spread of antibiotic resistance following their prophylactic and therapeutic clinical use. Biomaterials 2010, 31, 6363–6377. [Google Scholar] [CrossRef]
- Tan, H.; Guo, S.; Yang, S.; Xu, X.; Tang, T. Physical characterization and osteogenic activity of the quaternized chitosan-loaded PMMA bone cement. Acta Biomater. 2012, 8, 2166–2174. [Google Scholar] [CrossRef]
- Yang, J.; Xie, Q.; Zhu, J.; Zou, C.; Chen, L.; Du, Y.; Li, D. Preparation and in vitro antioxidant activities of 6-amino-6-deoxychitosan and its sulfonated derivatives. Biopolymers 2015, 103, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Krichen, F.; Ghlissi, Z.; Amor, I.B.; Sayari, N.; Kallel, R.; Gargouri, J.; Sahnoun, Z.; Boudawara, T.; Bougatef, A. In vitro and in vivo anti-coagulant activity and toxicological studies of marine sulfated glycosaminoglycans. Exp. Toxicol. Pathol. Off. J. Ges. Fur Toxikol. Pathol. 2017, 69, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Valcarcel, J.; Novoa-Carballal, R.; Pérez-Martín, R.I.; Reis, R.L.; Vázquez, J.A. Glycosaminoglycans from marine sources as therapeutic agents. Biotechnol. Adv. 2017, 35, 711–725. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-H.; Huang, J.-J. Biodegradability and anticoagulant properties of chitosan and sulfonated chitosan films coated on TiNi alloys. Surf. Coat. Technol. 2012, 206, 4959–4963. [Google Scholar] [CrossRef]
- Campelo, C.S.; Lima, L.D.; Rebêlo, L.M.; Mantovani, D.; Beppu, M.M.; Vieira, R.S. In vitro evaluation of anti-calcification and anti-coagulation on sulfonated chitosan and carrageenan surfaces. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 241–248. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, J.; Yu, Y.; Dai, K.; Wang, J.; Liu, C. Enhancement of BMP-2-mediated angiogenesis and osteogenesis by 2-N,6-O-sulfated chitosan in bone regeneration. Biomater. Sci. 2018, 6, 431–439. [Google Scholar] [CrossRef]
- Han, G.; Zheng, Z.; Pan, Z.; Lin, Y.; Gan, S.; Jiao, Y.; Li, H.; Zhou, C.; Ding, S.; Li, L. Sulfated chitosan coated polylactide membrane enhanced osteogenic and vascularization differentiation in MC3T3-E1s and HUVECs co-cultures system. Carbohydr. Polym. 2020, 245, 116522. [Google Scholar] [CrossRef]
- Zhou, H.; Qian, J.; Wang, J.; Yao, W.; Liu, C.; Chen, J.; Cao, X. Enhanced bioactivity of bone morphogenetic protein-2 with low dose of 2-N, 6-O-sulfated chitosan in vitro and in vivo. Biomaterials 2009, 30, 1715–1724. [Google Scholar] [CrossRef]
- Giri, T.; Thakur, A.; Alexander, A.; Ajaz, A.; Badwaik, H.; Tripathi, D. Modified chitosan hydrogels as drug delivery and tissue engineering systems: Present status and applications. Acta Pharm. Sin. B 2012, 2, 439–449. [Google Scholar] [CrossRef] [Green Version]
- Sun, I.C.; Na, J.H.; Jeong, S.Y.; Kim, D.E.; Kwon, I.C.; Choi, K.; Ahn, C.H.; Kim, K. Biocompatible glycol chitosan-coated gold nanoparticles for tumor-targeting CT imaging. Pharm. Res. 2014, 31, 1418–1425. [Google Scholar] [CrossRef]
- Ko, E.S.; Kim, C.; Choi, Y.; Lee, K.Y. 3D printing of self-healing ferrogel prepared from glycol chitosan, oxidized hyaluronate, and iron oxide nanoparticles. Carbohydr. Polym. 2020, 245, 116496. [Google Scholar] [CrossRef] [PubMed]
- Mansur, A.A.P.; de Almeida, C.G.; de Carvalho, S.M.; de Faria, L.V.; de Almeida, M.V.; Mansur, H.S. Cytocompatible Fluorescent Quantum Dot/PEG-Chitosan Bioconjugates for Nanomedicine Applications. Eur. J. Inorg. Chem. 2015, 27, 4555–4564. [Google Scholar] [CrossRef]
- Costa, H.S.; Mansur, A.A.P.; Barbosa-Stancioli, E.F.; Pereira, M.M.; Mansur, H.S. Morphological, mechanical, and biocompatibility characterization of macroporous alumina scaffolds coated with calcium phosphate/PVA. J. Mater. Sci. 2008, 43, 510–524. [Google Scholar] [CrossRef]
- Dumont, V.C.; Mansur, H.S.; Mansur, A.A.; Carvalho, S.M.; Capanema, N.S.; Barrioni, B.R. Glycol chitosan/nanohydroxyapatite biocomposites for potential bone tissue engineering and regenerative medicine. Int. J. Biol. Macromol. 2016, 93, 1465–1478. [Google Scholar] [CrossRef] [PubMed]
- Deepthi, S.; Abdul Gafoor, A.A.; Sivashanmugam, A.; Nair, S.V.; Jayakumar, R. Nanostrontium ranelate incorporated injectable hydrogel enhanced matrix production supporting chondrogenesis in vitro. J. Mater. Chem. B 2016, 4, 4092–4103. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.W.; Chen, C.H.; Manga, Y.B.; Huang, S.C.; Chao, K.M.; Jheng, P.R.; Wong, P.C.; Nyambat, B.; Satapathy, M.K.; Chuang, E.Y. Facilitated and Controlled Strontium Ranelate Delivery Using GCS-HA Nanocarriers Embedded into PEGDA Coupled with Decortication Driven Spinal Regeneration. Int. J. Nanomed. 2021, 16, 4209–4224. [Google Scholar] [CrossRef]
- Sahariah, P.; Óskarsson, B.M.; Hjálmarsdóttir, M.; Másson, M. Synthesis of guanidinylated chitosan with the aid of multiple protecting groups and investigation of antibacterial activity. Carbohydr. Polym. 2015, 127, 407–417. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, J.; Lee, C.S.; Kim, S.; Chen, C.; Lee, M. Supramolecular Hydrogels Based on Nanoclay and Guanidine-Rich Chitosan: Injectable and Moldable Osteoinductive Carriers. ACS Appl. Mater. Interfaces 2020, 12, 16088–16096. [Google Scholar] [CrossRef]
- He, T.; Wang, W.; Chen, B.; Wang, J.; Liang, Q.; Chen, B. 5-Fluorouracil monodispersed chitosan microspheres: Microfluidic chip fabrication with crosslinking, characterization, drug release and anticancer activity. Carbohydr. Polym. 2020, 236, 116094. [Google Scholar] [CrossRef]
- Abdullayev, E.; Lvov, Y. Halloysite clay nanotubes as a ceramic “skeleton” for functional biopolymer composites with sustained drug release. J. Mater. Chem. B 2013, 1, 2894–2903. [Google Scholar] [CrossRef]
- De Silva, R.T.; Pasbakhsh, P.; Goh, K.L.; Chai, S.-P.; Ismail, H. Physico-chemical characterisation of chitosan/halloysite composite membranes. Polym. Test. 2013, 32, 265–271. [Google Scholar] [CrossRef]
- Naumenko, E.A.; Guryanov, I.D.; Yendluri, R.; Lvov, Y.M.; Fakhrullin, R.F. Clay nanotube-biopolymer composite scaffolds for tissue engineering. Nanoscale 2016, 8, 7257–7271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousa, M.; Evans, N.D.; Oreffo, R.O.C.; Dawson, J.I. Clay nanoparticles for regenerative medicine and biomaterial design: A review of clay bioactivity. Biomaterials 2018, 159, 204–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satish, S.; Tharmavaram, M.; Rawtani, D. Halloysite nanotubes as a nature’s boon for biomedical applications. Nanobiomedicine 2019, 6, 1849543519863625. [Google Scholar] [CrossRef] [Green Version]
- Huang, B.; Liu, M.; Zhou, C. Chitosan composite hydrogels reinforced with natural clay nanotubes. Carbohydr. Polym. 2017, 175, 689–698. [Google Scholar] [CrossRef]
- Liu, M.; Wu, C.; Jiao, Y.; Xiong, S.; Zhou, C. Chitosan-halloysite nanotubes nanocomposite scaffolds for tissue engineering. J. Mater. Chem. B 2013, 1, 2078–2089. [Google Scholar] [CrossRef] [PubMed]
- Kadam, A.A.; Shinde, S.K.; Ghodake, G.S.; Saratale, G.D.; Saratale, R.G.; Sharma, B.; Hyun, S.; Sung, J.S. Chitosan-Grafted Halloysite Nanotubes-Fe(3)O(4) Composite for Laccase-Immobilization and Sulfamethoxazole-Degradation. Polymers 2020, 12, 2221. [Google Scholar] [CrossRef]
- Naumenko, E.; Zakirova, E.; Guryanov, I.; Akhatova, F.; Sergeev, M.; Valeeva, A.; Fakhrullin, R. Composite biodegradable polymeric matrix doped with halloysite nanotubes for the repair of bone defects in dogs. Clays Clay Miner. 2021, 69, 522–532. [Google Scholar] [CrossRef]
- Sanchez-Salvador, J.L.; Balea, A.; Monte, M.C.; Negro, C.; Blanco, A. Chitosan grafted/cross-linked with biodegradable polymers: A review. Int. J. Biol. Macromol. 2021, 178, 325–343. [Google Scholar] [CrossRef]
- Qu, B.; Luo, Y. Chitosan-based hydrogel beads: Preparations, modifications and applications in food and agriculture sectors—A review. Int. J. Biol. Macromol. 2020, 152, 437–448. [Google Scholar] [CrossRef]
- Baek, P.; Voorhaar, L.; Barker, D.; Travas-Sejdic, J. Molecular Approach to Conjugated Polymers with Biomimetic Properties. Acc. Chem. Res. 2018, 51, 1581–1589. [Google Scholar] [CrossRef]
- D’Souza, A.A.; Shegokar, R. Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Pu, H.; Liu, S.; Kan, J.; Jin, C. Synthesis, characterization, bioactivity and potential application of phenolic acid grafted chitosan: A review. Carbohydr. Polym. 2017, 174, 999–1017. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.; Coimbra, M.A.; Ferreira, P. Tailoring Functional Chitosan-Based Composites for Food Applications. Chem. Rec. 2018, 18, 1138–1149. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Gihar, S.; Shrivash, M.K.; Kumar, P.; Kundu, P.P. A review on the synthesis of graft copolymers of chitosan and their potential applications. Int. J. Biol. Macromol. 2020, 163, 2097–2112. [Google Scholar] [CrossRef]
- Amini, Z.; Rudsary, S.S.; Shahraeini, S.S.; Dizaji, B.F.; Goleij, P.; Bakhtiari, A.; Irani, M.; Sharifianjazi, F. Magnetic bioactive glasses/Cisplatin loaded-chitosan (CS)-grafted- poly (ε-caprolactone) nanofibers against bone cancer treatment. Carbohydr. Polym. 2021, 258, 117680. [Google Scholar] [CrossRef]
- Acevedo, C.A.; Olguín, Y.; Briceño, M.; Forero, J.C.; Osses, N.; Díaz-Calderón, P.; Jaques, A.; Ortiz, R. Design of a biodegradable UV-irradiated gelatin-chitosan/nanocomposed membrane with osteogenic ability for application in bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 875–886. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Z.; Cao, Z.; Zhuang, L.; Xu, Y.; Liu, X.; Xu, Y.; Gong, Y. Enhancing proliferation and osteogenic differentiation of HMSCs on casein/chitosan multilayer films. Colloids Surf. B Biointerfaces 2016, 141, 397–407. [Google Scholar] [CrossRef]
- Fan, L.; Zou, S.; Ge, H.; Xiao, Y.; Wen, H.; Feng, H.; Liu, M.; Nie, M. Preparation and characterization of hydroxypropyl chitosan modified with collagen peptide. Int. J. Biol. Macromol. 2016, 93, 636–643. [Google Scholar] [CrossRef]
- Piaia, L.; Silva, S.S.; Gomes, J.M.; Franco, A.R.; Fernandes, E.M.; Lobo, F.C.M.; Rodrigues, L.C.; Leonor, I.B.; Fredel, M.C.; Salmoria, G.V.; et al. Chitosan/β-TCP composites scaffolds coated with silk fibroin: A bone tissue engineering approach. Biomed. Mater. 2021, 17, 015003. [Google Scholar] [CrossRef]
- Li, D.W.; Lei, X.; He, F.L.; He, J.; Liu, Y.L.; Ye, Y.J.; Deng, X.; Duan, E.; Yin, D.C. Silk fibroin/chitosan scaffold with tunable properties and low inflammatory response assists the differentiation of bone marrow mesenchymal stem cells. Int. J. Biol. Macromol. 2017, 105, 584–597. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.R.; Murray, E.; McAdam, C.J.; McConnell, M.A.; Cabral, J.D. Peptide Chitosan/Dextran Core/Shell Vascularized 3D Constructs for Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 32328–32339. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, X.; Liu, R.; Gong, Y.; Wang, M.; Huang, Q.; Feng, Q.; Yu, B. Zero-order controlled release of BMP2-derived peptide P24 from the chitosan scaffold by chemical grafting modification technique for promotion of osteogenesis in vitro and enhancement of bone repair in vivo. Theranostics 2017, 7, 1072–1087. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.G.; Li, X.H.; Xie, C.Y.; Chen, J.G.; Zou, J.E.; Zhou, S.B.; Weng, J.E. Controllable growth of hydroxyapatite on electrospun poly(DL-lactide) fibers grafted with chitosan as potential tissue engineering scaffolds. Polymer 2010, 51, 2320–2328. [Google Scholar] [CrossRef]
- Yao, F.L.; Chen, W.; Wang, H.; Liu, H.F.; Yao, K.D.; Sun, P.C.; Lin, H. A study on cytocompatible poly (chitosan-g-L-lactic acid). Polymer 2003, 44, 6435–6441. [Google Scholar] [CrossRef]
- Lai, W.F.; Shum, H.C. Hypromellose-graft-chitosan and Its Polyelectrolyte Complex as Novel Systems for Sustained Drug Delivery. ACS Appl. Mater. Interfaces 2015, 7, 10501–10510. [Google Scholar] [CrossRef]
- Sainitya, R.; Sriram, M.; Kayanaraman, V.; Dhivya, S.; Saravanan, S.; Vairamani, M.; Sastry, T.P.; Selvamurugan, N. Scaffolds containing chitosan/carboxymethyl cellulose/mesoporous wollastonite for bone tissue engineering. Int. J. Biol. Macromol. 2015, 80, 481–488. [Google Scholar] [CrossRef]
- Kulkarni, N.; Shinde, S.D.; Jadhav, G.S.; Adsare, D.R.; Rao, K.; Kachhia, M.; Maingle, M.; Patil, S.P.; Arya, N.; Sahu, B. Peptide-Chitosan Engineered Scaffolds for Biomedical Applications. Bioconjugate Chem. 2021, 32, 448–465. [Google Scholar] [CrossRef]
- Wang, C.; Chen, B.B.; Zou, M.J.; Cheng, G. Cyclic RGD-modified chitosan/graphene oxide polymers for drug delivery and cellular imaging. Colloids Surf. B-Biointerfaces 2014, 122, 332–340. [Google Scholar] [CrossRef]
- Zafeiris, K.; Brasinika, D.; Karatza, A.; Koumoulos, E.; Karoussis, I.K.; Kyriakidou, K.; Charitidis, C.A. Additive manufacturing of hydroxyapatite-chitosan-genipin composite scaffolds for bone tissue engineering applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111639. [Google Scholar] [CrossRef]
- Yu, P.; Bao, R.Y.; Shi, X.J.; Yang, W.; Yang, M.B. Self-assembled high-strength hydroxyapatite/graphene oxide/chitosan composite hydrogel for bone tissue engineering. Carbohydr. Polym. 2017, 155, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Shavandi, A.; Bekhit Ael, D.; Ali, M.A.; Sun, Z.; Gould, M. Development and characterization of hydroxyapatite/β-TCP/chitosan composites for tissue engineering applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 56, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.K.; Kim, S.; Baljon, J.J.; Wu, B.M.; Aghaloo, T.; Lee, M. Microporous methacrylated glycol chitosan-montmorillonite nanocomposite hydrogel for bone tissue engineering. Nat. Commun. 2019, 10, 3523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gohil, S.V.; Wang, L.; Rowe, D.W.; Nair, L.S. Spatially controlled rhBMP-2 mediated calvarial bone formation in a transgenic mouse model. Int. J. Biol. Macromol. 2018, 106, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.T.; Lu, T.W.; Chen, C.H.; Lu, K.Y.; Mi, F.L. Development of nanocomposite scaffolds based on biomineralization of N,O-carboxymethyl chitosan/fucoidan conjugates for bone tissue engineering. Int. J. Biol. Macromol. 2018, 120, 2335–2345. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, J.; Zhou, X.; Sun, J.; Zhu, B.; Duan, C.; Chen, P.; Guo, X.; Zhang, T.; Guo, H. A New Self-Healing Hydrogel Containing hucMSC-Derived Exosomes Promotes Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 564731. [Google Scholar] [CrossRef]
- Salama, A.; Hesemann, P. Synthesis and characterization of N-guanidinium chitosan/silica ionic hybrids as templates for calcium phosphate mineralization. Int. J. Biol. Macromol. 2020, 147, 276–283. [Google Scholar] [CrossRef]
- Shaabani, A.; Sedghi, R. Preparation of chitosan biguanidine/PANI-containing self-healing semi-conductive waterborne scaffolds for bone tissue engineering. Carbohydr. Polym. 2021, 264, 118045. [Google Scholar] [CrossRef]
- Brun, P.; Zamuner, A.; Battocchio, C.; Cassari, L.; Todesco, M.; Graziani, V.; Iucci, G.; Marsotto, M.; Tortora, L.; Secchi, V.; et al. Bio-Functionalized Chitosan for Bone Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 5916. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Fahmy, A.; Taha, T.H.; El-Fakharany, E.M.; Makram, M.; Soliman, H.M.A.; Shehata, H. Thermo-and pH-sensitive hydrogel membranes composed of poly(N-isopropylacrylamide)-hyaluronan for biomedical applications: Influence of hyaluronan incorporation on the membrane properties. Int. J. Biol. Macromol. 2018, 106, 158–167. [Google Scholar] [CrossRef]
- Ahmad, U.; Sohail, M.; Ahmad, M.; Minhas, M.U.; Khan, S.; Hussain, Z.; Kousar, M.; Mohsin, S.; Abbasi, M.; Shah, S.A.; et al. Chitosan based thermosensitive injectable hydrogels for controlled delivery of loxoprofen: Development, characterization and in-vivo evaluation. Int. J. Biol. Macromol. 2019, 129, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, C.; Zhang, X.; Chen, G.; Hu, Q.; Li, H.; Wang, J.; Wen, D.; Zhang, Y.; Lu, Y.; et al. In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment. Nat. Nanotechnol. 2019, 14, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhao, X.; Ma, P.X.; Guo, B. pH-responsive self-healing injectable hydrogel based on N-carboxyethyl chitosan for hepatocellular carcinoma therapy. Acta Biomater. 2017, 58, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wang, X.; Xu, Q.; Lu, Y.; Zhang, Y.; Xia, H.; Lu, A.; Zhang, L. Rubbery Chitosan/Carrageenan Hydrogels Constructed through an Electroneutrality System and Their Potential Application as Cartilage Scaffolds. Biomacromolecules 2018, 19, 340–352. [Google Scholar] [CrossRef]
- Wang, L.; Li, B.; Xu, F.; Xu, Z.; Wei, D.; Feng, Y.; Wang, Y.; Jia, D.; Zhou, Y. UV-crosslinkable and thermo-responsive chitosan hybrid hydrogel for NIR-triggered localized on-demand drug delivery. Carbohydr. Polym. 2017, 174, 904–914. [Google Scholar] [CrossRef]
- Xu, Y.; Cui, M.; Patsis, P.A.; Günther, M.; Yang, X.; Eckert, K.; Zhang, Y. Reversibly Assembled Electroconductive Hydrogel via a Host-Guest Interaction for 3D Cell Culture. ACS Appl. Mater. Interfaces 2019, 11, 7715–7724. [Google Scholar] [CrossRef]
- Das, D.; Pal, S. Dextrin/poly (HEMA): pH responsive porous hydrogel for controlled release of ciprofloxacin. Int. J. Biol. Macromol. 2015, 72, 171–178. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Han, H.; Liu, J.; Zhao, H.; Shen, M.; Xu, Y.; Xu, J.; Li, L.; Guo, X. Self-assembled micelles of N-phthaloylchitosan-g-poly (N-vinylcaprolactam) for temperature-triggered non-steroidal anti-inflammatory drug delivery. J. Mater. Sci. 2016, 51, 1591–1599. [Google Scholar] [CrossRef]
- Hu, L.M.; Sun, Y.; Wu, Y. Advances in chitosan-based drug delivery vehicles. Nanoscale 2013, 5, 3103–3111. [Google Scholar] [CrossRef]
- Lin, Y.J.; Hsu, F.C.; Chou, C.W.; Wu, T.H.; Lin, H.R. Poly(acrylic acid)-chitosan-silica hydrogels carrying platelet gels for bone defect repair. J. Mater. Chem. B 2014, 2, 8329–8337. [Google Scholar] [CrossRef]
- Cok, M.; Viola, M.; Vecchies, F.; Sacco, P.; Furlani, F.; Marsich, E.; Donati, I. N-isopropyl chitosan. A pH- and thermo-responsive polysaccharide for gel formation. Carbohydr. Polym. 2020, 230, 115641. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Qazvini, N.T.; Sadati, M.; Zeng, Z.; Huang, S.; De La Lastra, A.L.; Zhang, L.; Feng, Y.; Liu, W.; Huang, B.; et al. A pH-Triggered, Self-Assembled, and Bioprintable Hybrid Hydrogel Scaffold for Mesenchymal Stem Cell Based Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2019, 11, 8749–8762. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.S.J.; Rosencrantz, S.; Tepper, L.; Chea, S.; Klöpzig, S.; Krüger-Genge, A.; Storsberg, J.; Rosencrantz, R.R. Functional Glyco-Nanogels for Multivalent Interaction with Lectins. Molecules 2019, 24, 1865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, S.; Bremner, D.H.; Wu, J.; Wu, J.; Wang, H.; Li, H.; Qian, Q.; Zheng, H.; Zhu, L. l-Peptide functionalized dual-responsive nanoparticles for controlled paclitaxel release and enhanced apoptosis in breast cancer cells. Drug Deliv. 2018, 25, 1275–1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motamedian, S.R.; Hosseinpour, S.; Ahsaie, M.G.; Khojasteh, A. Smart scaffolds in bone tissue engineering: A systematic review of literature. World J. Stem Cells 2015, 7, 657–668. [Google Scholar] [CrossRef]
- Chocholata, P.; Kulda, V.; Babuska, V. Fabrication of Scaffolds for Bone-Tissue Regeneration. Materials 2019, 12, 568. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.N.; Pramanik, K. Generation of bioactive nano-composite scaffold of nanobioglass/silk fibroin/carboxymethyl cellulose for bone tissue engineering. J. Biomater. Sci. Polym. Ed. 2018, 29, 2011–2034. [Google Scholar] [CrossRef]
- Dhivya, S.; Saravanan, S.; Sastry, T.P.; Selvamurugan, N. Nanohydroxyapatite-reinforced chitosan composite hydrogel for bone tissue repair in vitro and in vivo. J. Nanobiotechnology 2015, 13, 40. [Google Scholar] [CrossRef] [Green Version]
- Saravanan, S.; Sameera, D.K.; Moorthi, A.; Selvamurugan, N. Chitosan scaffolds containing chicken feather keratin nanoparticles for bone tissue engineering. Int. J. Biol. Macromol. 2013, 62, 481–486. [Google Scholar] [CrossRef]
- Riaz Rajoka, M.S.; Zhao, L.; Mehwish, H.M.; Wu, Y.; Mahmood, S. Chitosan and its derivatives: Synthesis, biotechnological applications, and future challenges. Appl. Microbiol. Biotechnol. 2019, 103, 1557–1571. [Google Scholar] [CrossRef]
- Abinaya, B.; Prasith, T.P.; Ashwin, B.; Viji Chandran, S.; Selvamurugan, N. Chitosan in Surface Modification for Bone Tissue Engineering Applications. Biotechnol. J. 2019, 14, e1900171. [Google Scholar] [CrossRef] [PubMed]
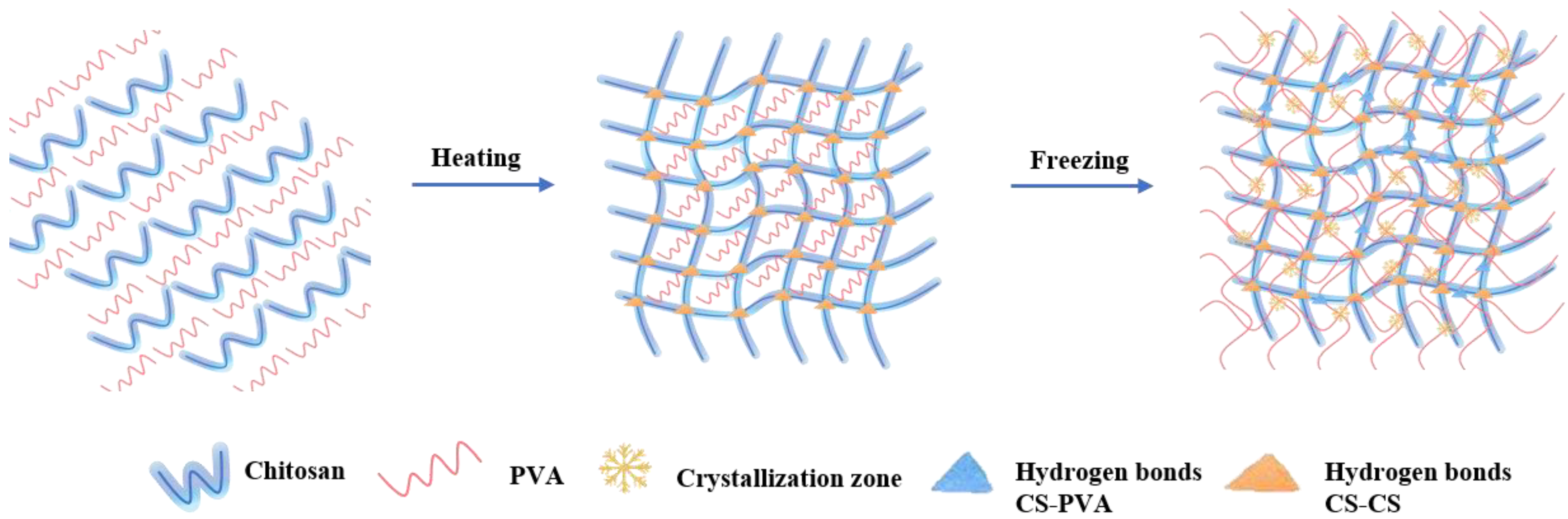
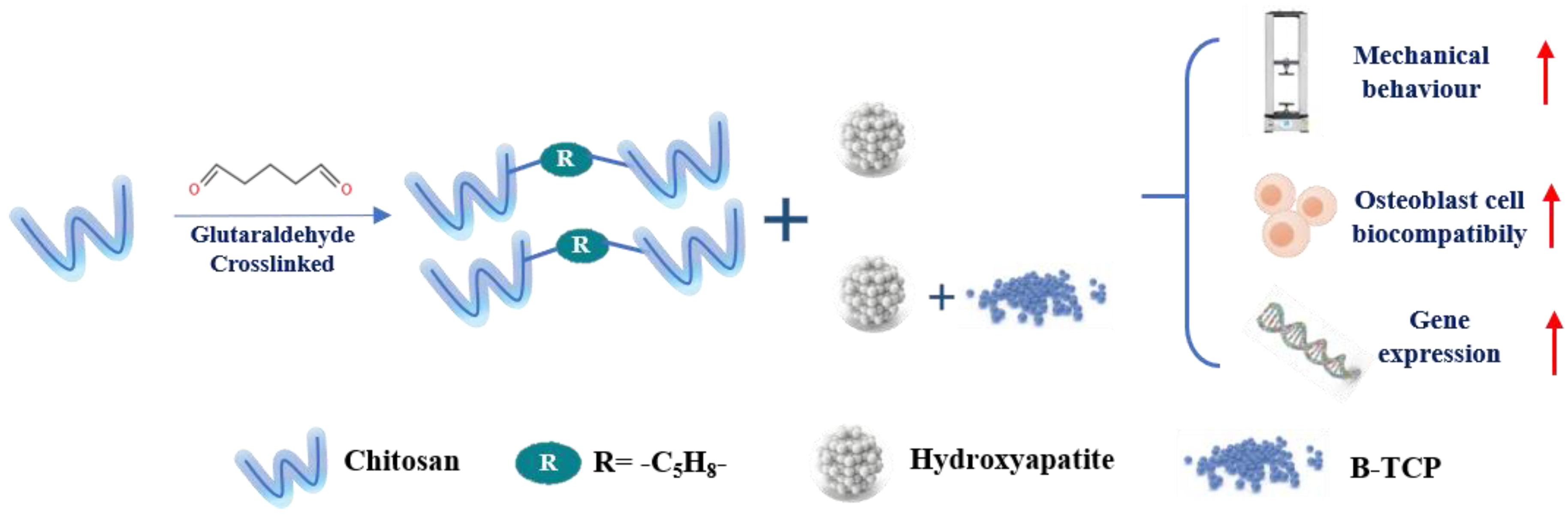
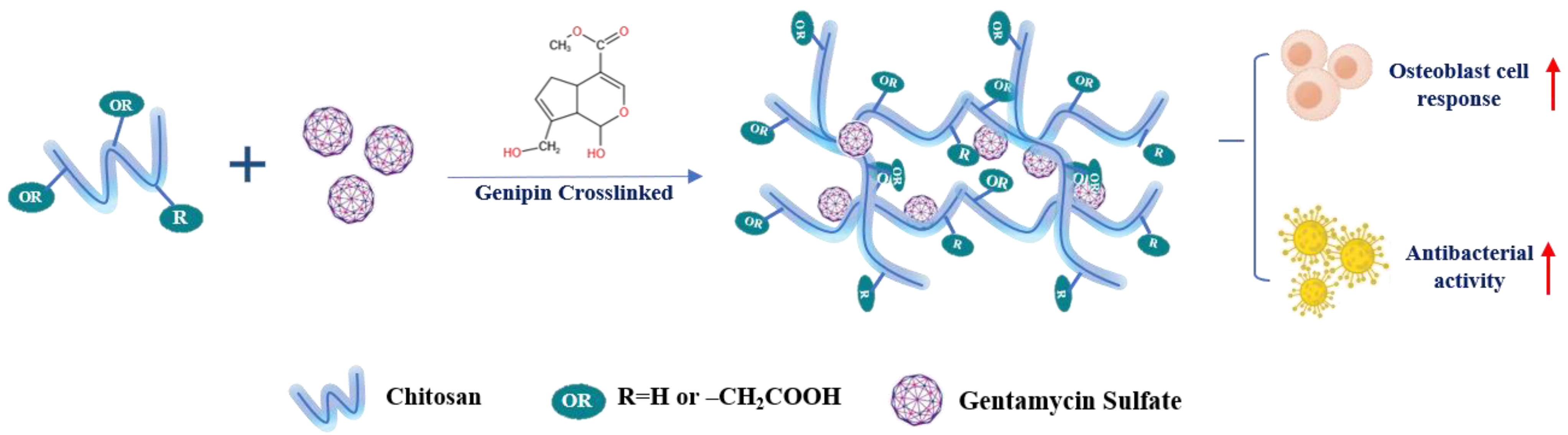
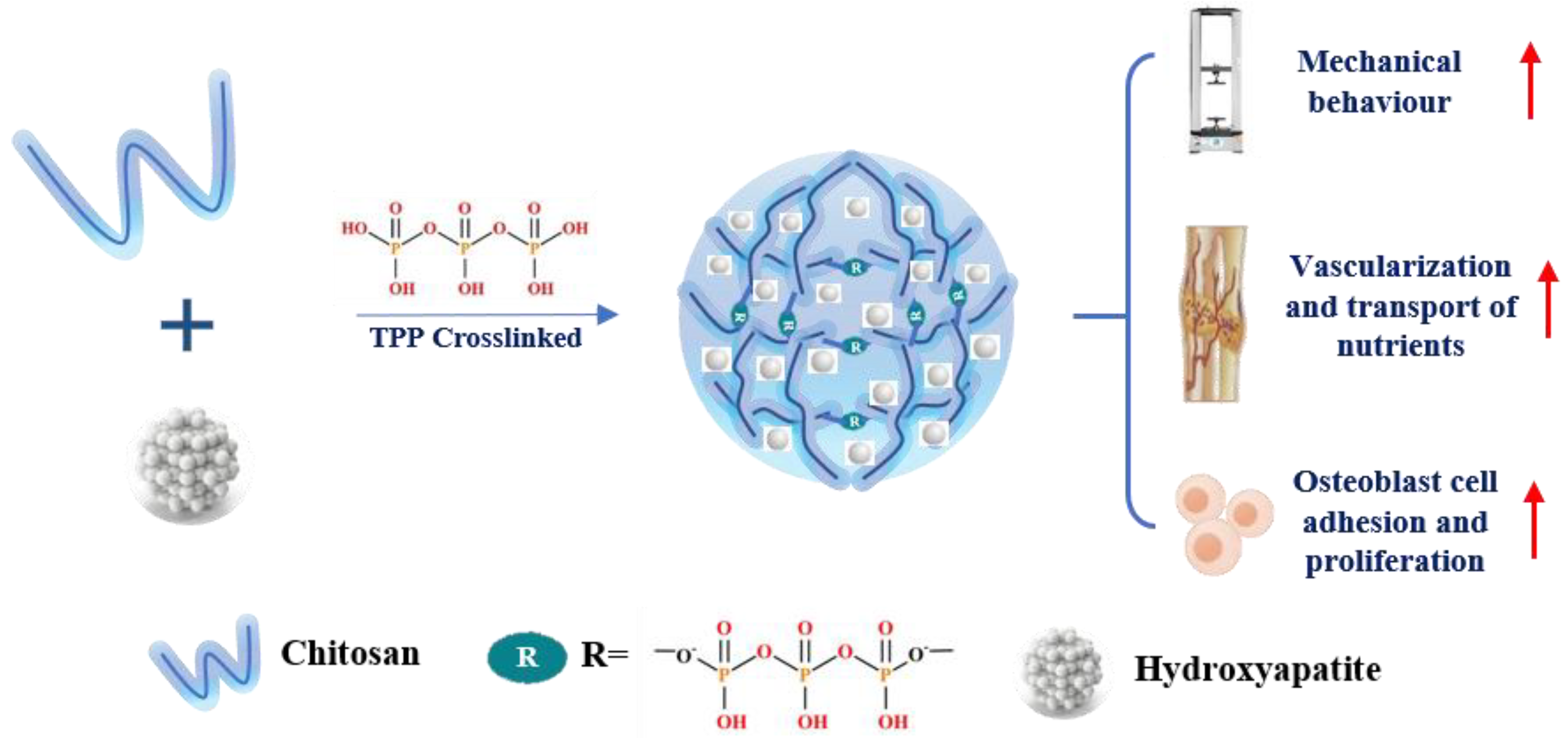

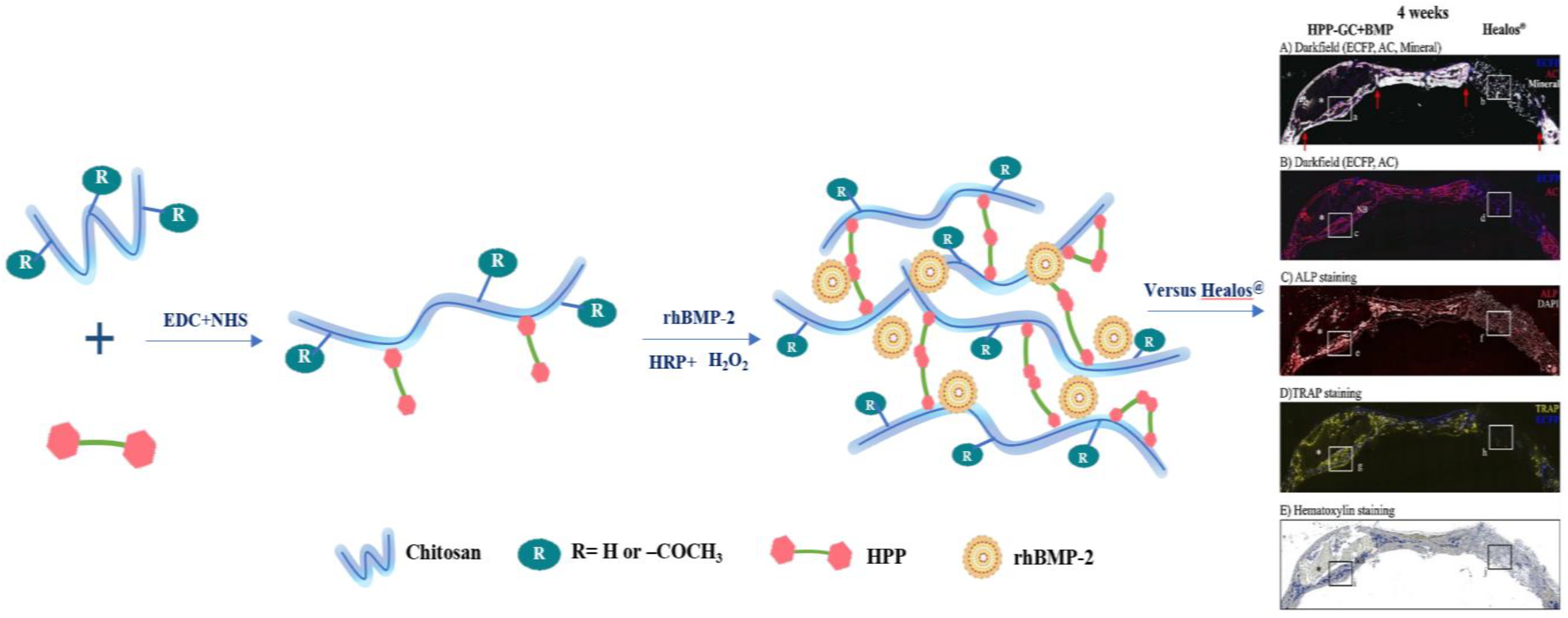
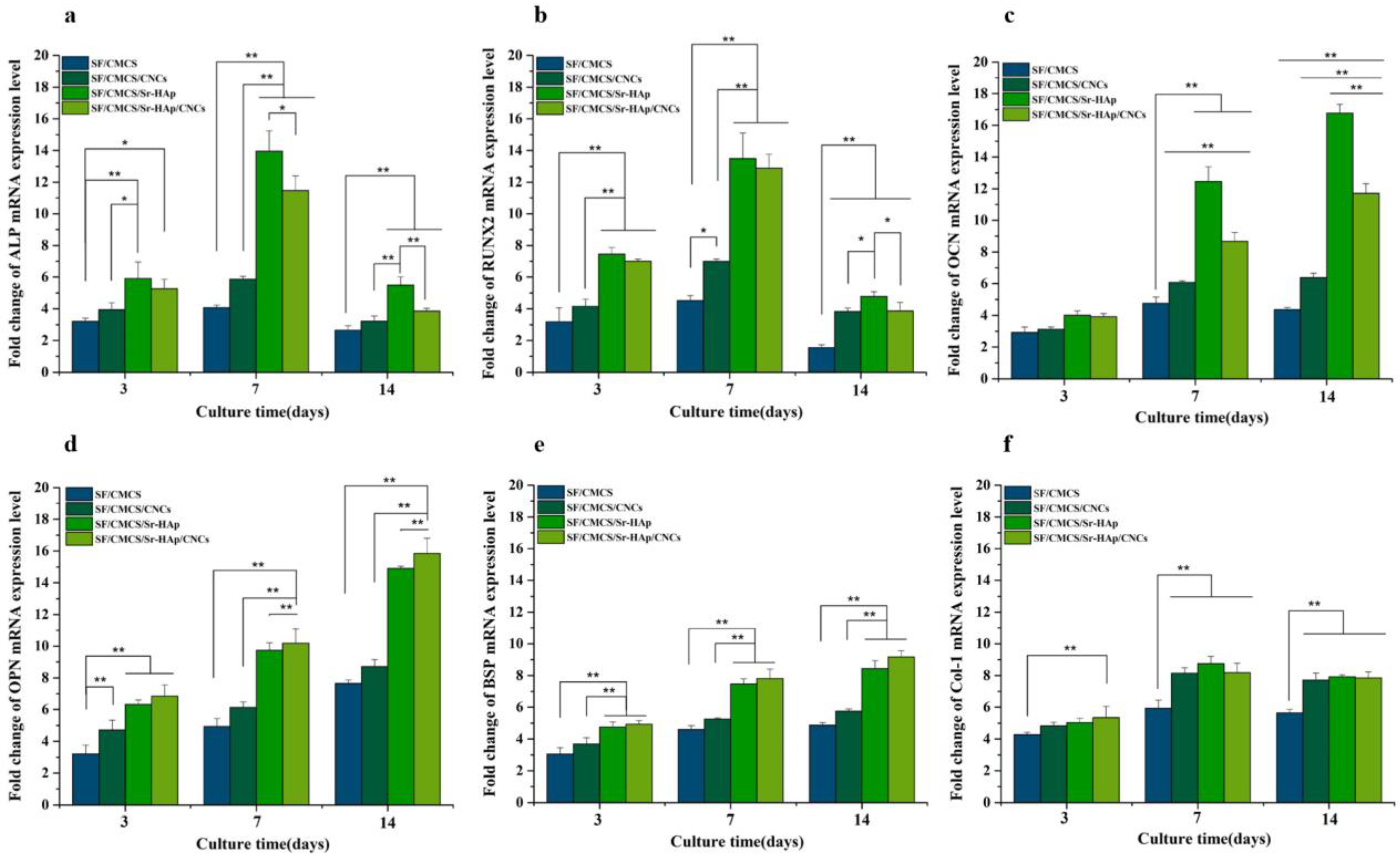

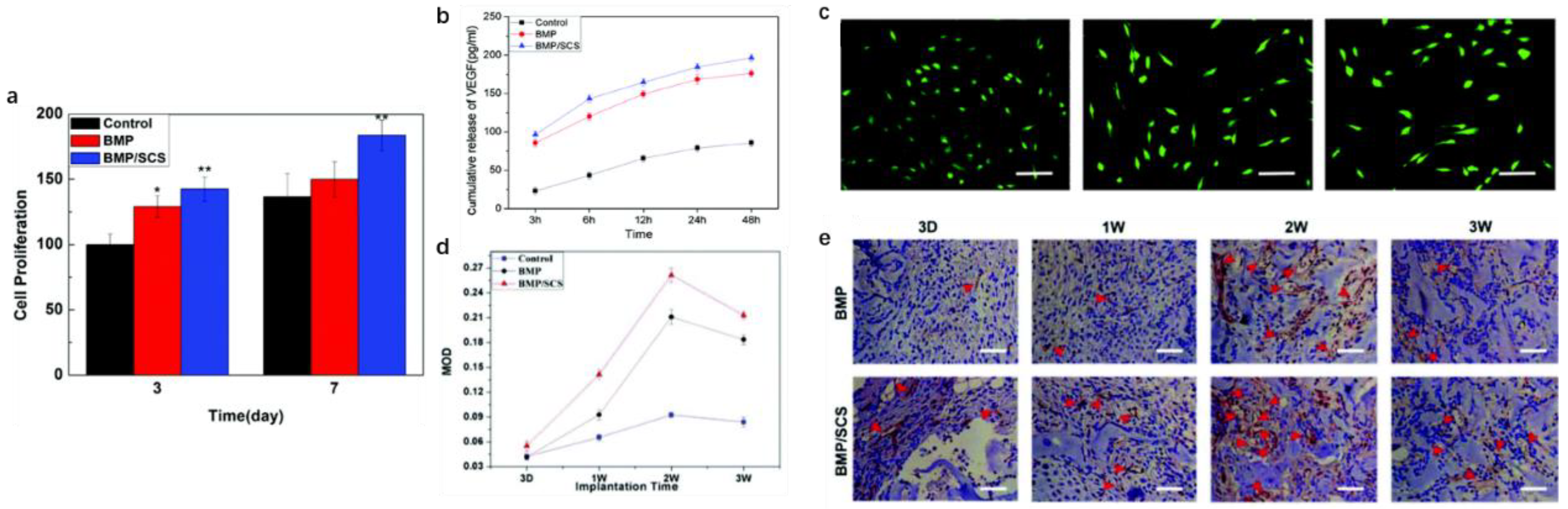

| Animals/Volunteers (Total) | Incisions/Defects/Cells | Chitosan-Based Form | Effects | Ref. | |
|---|---|---|---|---|---|
| Preclinical trials | Beagles (n/d) | Open skin wounds on the dorsal side | 20 mg/wound (2 × 2 cm) | Activate immunocytes and inflammatory cells | [53] |
| Mail Wistar rats (60) | Bone defects measuring 2 mm in diameter in both tibias | CS/D. ambrosioides spheres | Faster bone regeneration and a controlled release of the extract | [54] | |
| New Zealand white rabbits (20) | Undergoing TKA surgery and implanted with titanium rod prostheses | CMCS hydrogel | Reduce the inflammatory response around rabbit knee prostheses, affect the OPG/RANKL/RANK signaling pathway, and promote osteogenesis. | [56] | |
| Clinical trials | Patients undergoing abdominal surgery (30) | Wound incisions | CS membrane | An effective antimicrobial and procoagulant and promote wound repair by providing a suitable environment for beneficial microbiota | [57] |
| Patients aged 50–70 years old undergoing total or elective hip replacement (n/d) | Human bone marrow stromal cells | CS immobilized glasses | Stimulate fast osteoblast response, displaying rapid cell spreading and cytoskeleton reorganization | [58] |
| Cross-linking Method | Strength | Limit |
|---|---|---|
| Physical cross-linking |
|
|
| Chemical cross-linking |
|
|
| Enzymatic cross-linking |
|
|
| Cross-Linking Agents | Cross-Linking Mechanism |
|---|---|
| Glutaraldehyde(GA) | 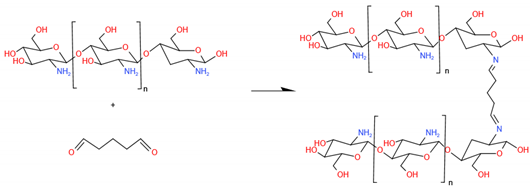 |
| Vanillin |  |
| Genipin |  |
| Tripolyphosphate, TPP | 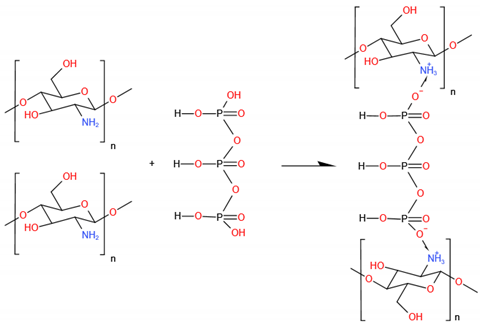 |
| Carbodiimide(NHS/EDC) | 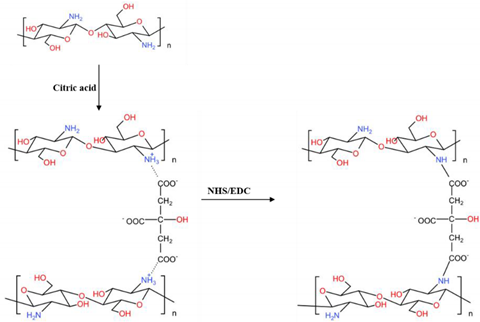 |
| Enzymatic-cross-linking (HRP) | 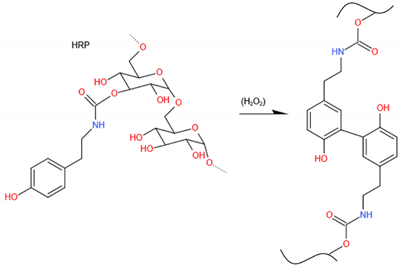 |
| Photoinitiators | 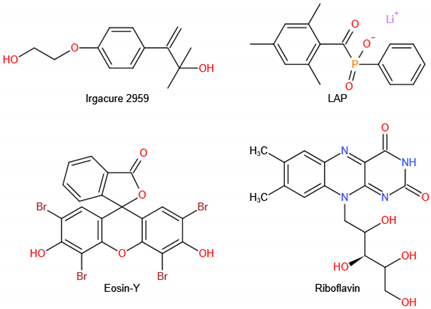 |
| Methylmethacrylate chitosan, ChMA | 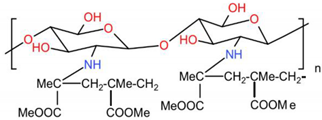 |
| CS derivates | Chemical formula |
|---|---|
| Carboxymethyl chitosan, CMC | 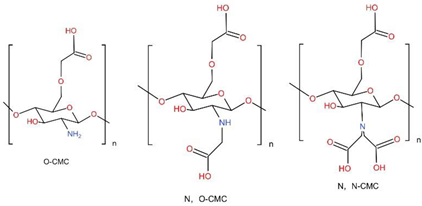 |
| Hydroxypropyltrimethyl ammonium chloride chitosan, HACC | 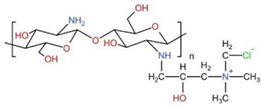 |
| Sulfated chitosan, SCS | 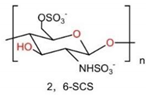 |
| Glycol chitosan, GCS | 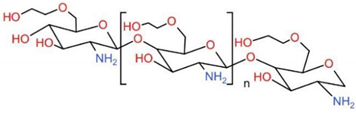 |
| Guanidinylated chitosan, GC | 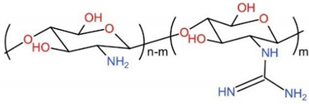 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Zhang, Y.; Zhou, Y. Application Progress of Modified Chitosan and Its Composite Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2022, 23, 6574. https://doi.org/10.3390/ijms23126574
Zhu Y, Zhang Y, Zhou Y. Application Progress of Modified Chitosan and Its Composite Biomaterials for Bone Tissue Engineering. International Journal of Molecular Sciences. 2022; 23(12):6574. https://doi.org/10.3390/ijms23126574
Chicago/Turabian StyleZhu, Yuemeng, Yidi Zhang, and Yanmin Zhou. 2022. "Application Progress of Modified Chitosan and Its Composite Biomaterials for Bone Tissue Engineering" International Journal of Molecular Sciences 23, no. 12: 6574. https://doi.org/10.3390/ijms23126574
APA StyleZhu, Y., Zhang, Y., & Zhou, Y. (2022). Application Progress of Modified Chitosan and Its Composite Biomaterials for Bone Tissue Engineering. International Journal of Molecular Sciences, 23(12), 6574. https://doi.org/10.3390/ijms23126574





