Brexpiprazole Reduces 5-HT7 Receptor Function on Astroglial Transmission Systems
Abstract
:1. Introduction
2. Results
2.1. Effects of Brex on Astroglial Signalling
2.1.1. Effects of Subchronic Administration of a Therapeutically Relevant Concentration of Brex on the Protein Expression of 5-HT1A and 5-HT7R in the Plasma Membrane Fraction of Astrocytes
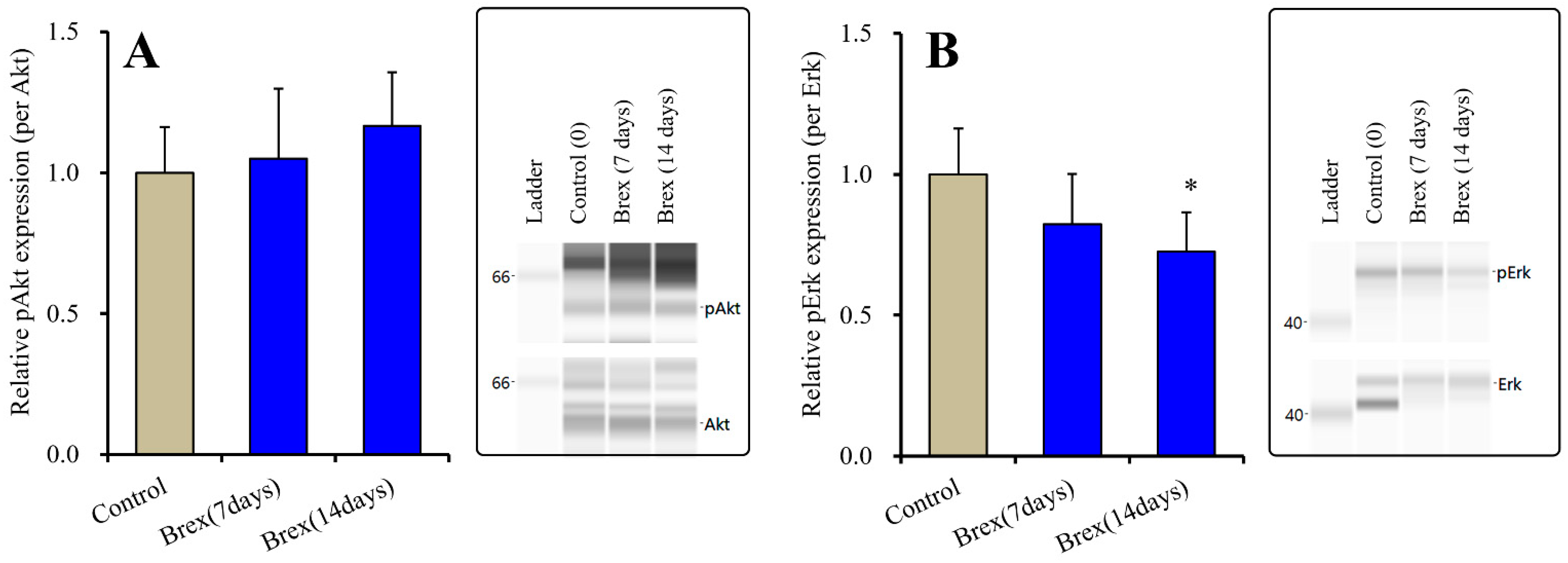
2.1.2. Effects of Brex on Intracellular Signal Transduction Protein in the Plasma Membrane Fraction of Astrocytes
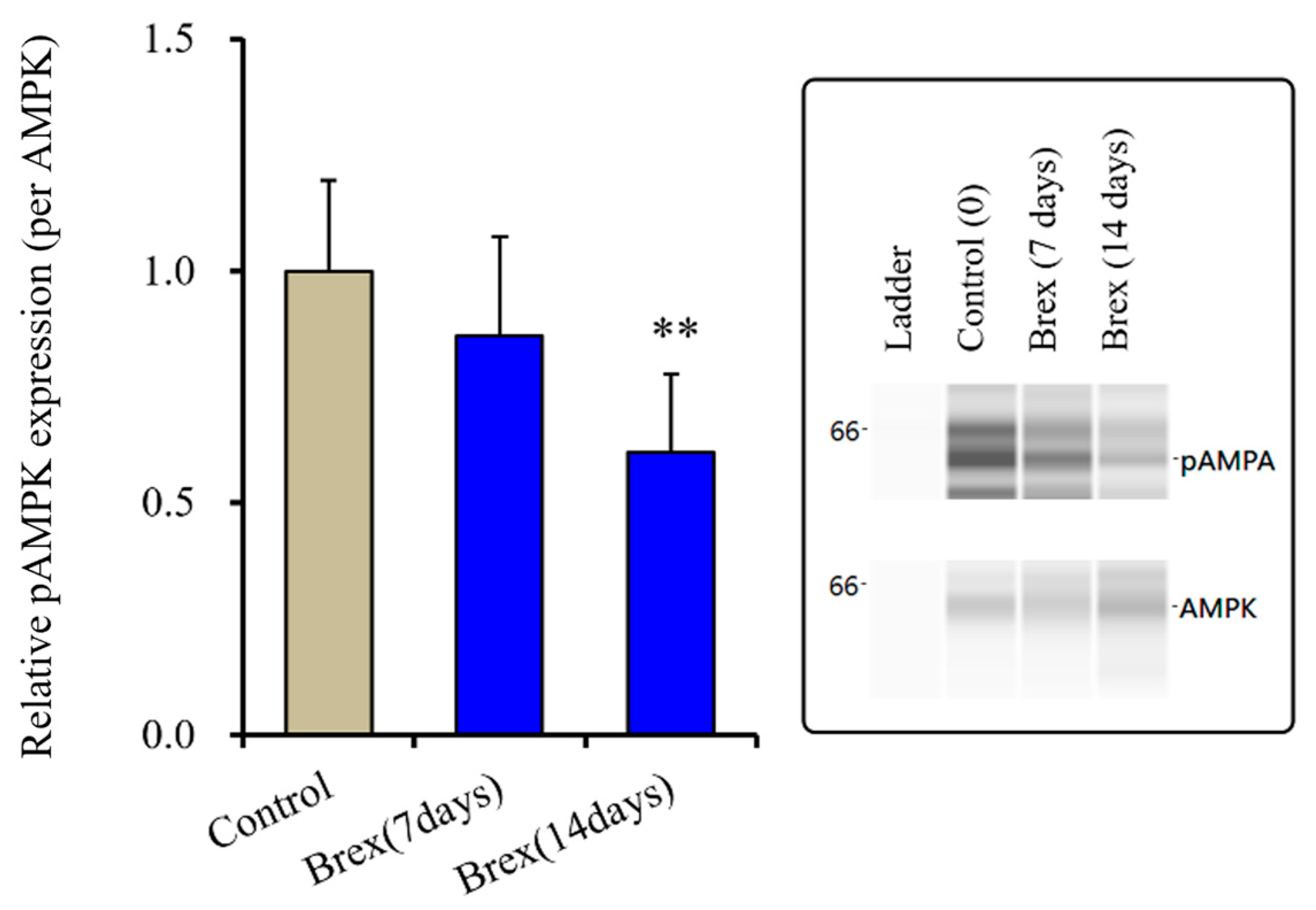
2.1.3. Effects of Brex on pAMPK in the Astroglial Cytosol Fraction
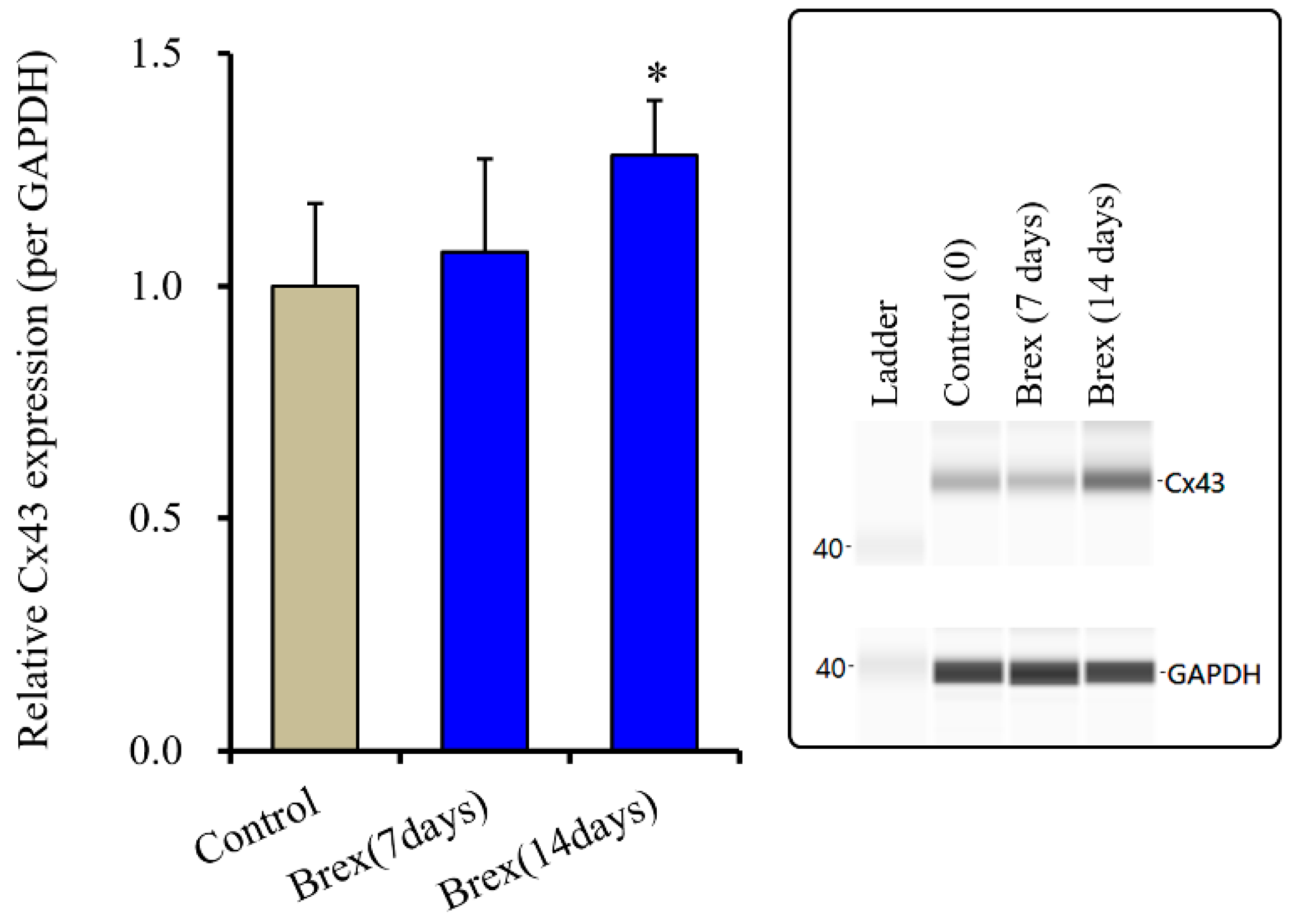
2.2. Effects of Subchronic Administration of a Therapeutically Relevant Concentration of Brex on Connexin43 Protein Expression in Astrocytes
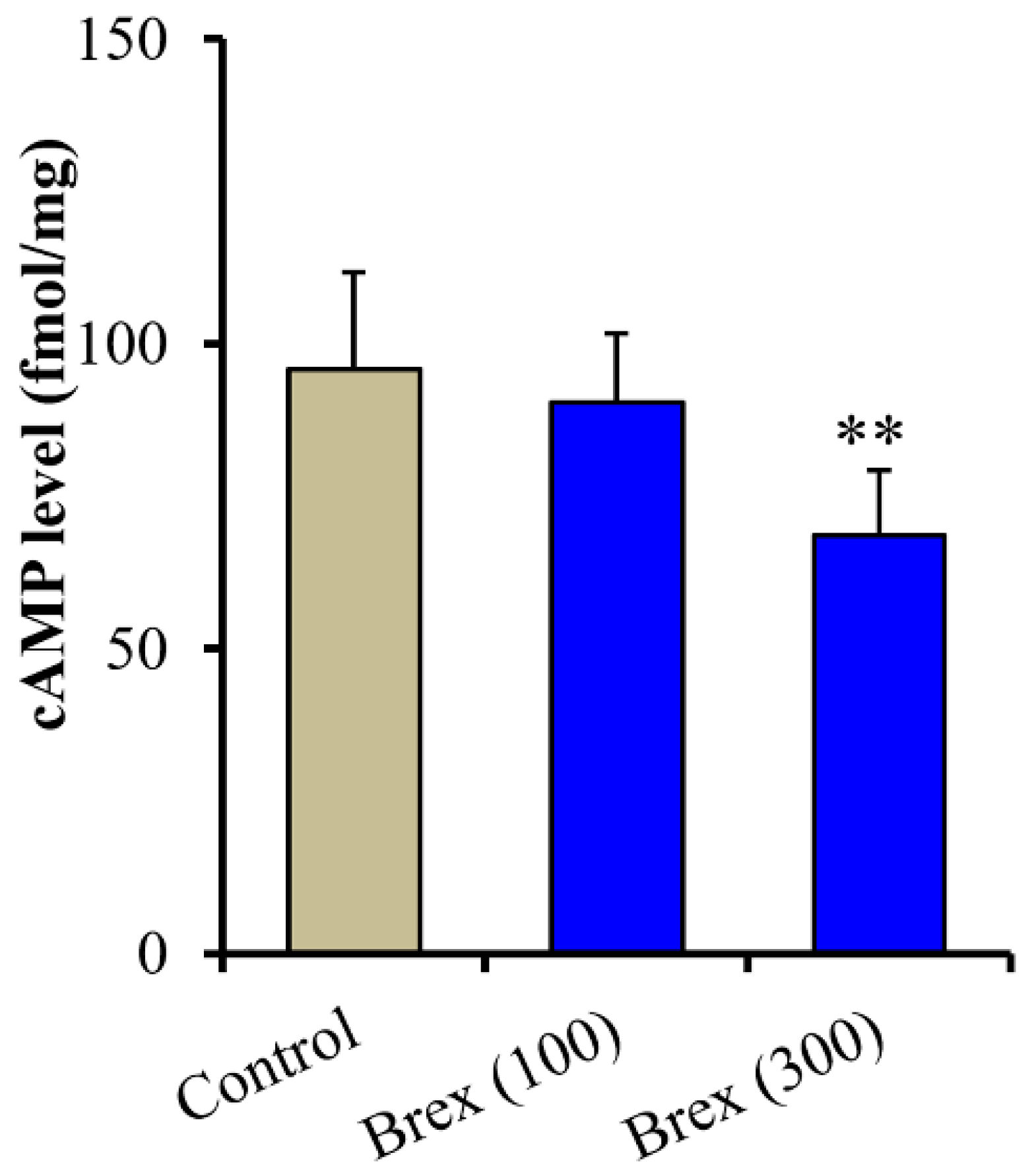
2.3. Effects of Brex on the Intracellular cAMP Level in Astrocytes
2.4. Effects of Subchronic Systemic Administration of Brex on the cAMP Level and AMPK Signalling in the Hypothalamus In Vivo
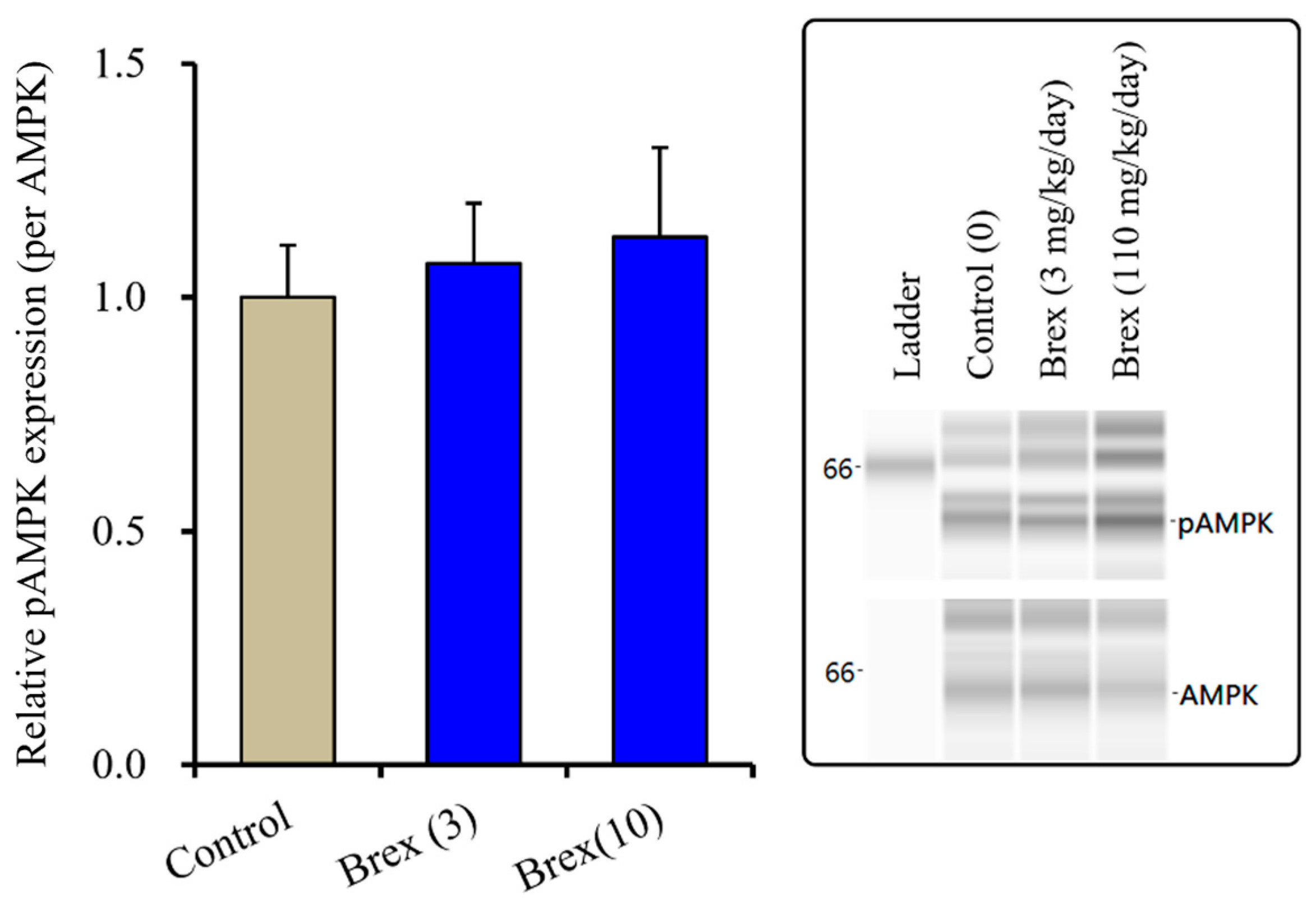
2.4.1. Effects of Subchronic Systemic Administration of Brex on pAMPK in the Hypothalamus
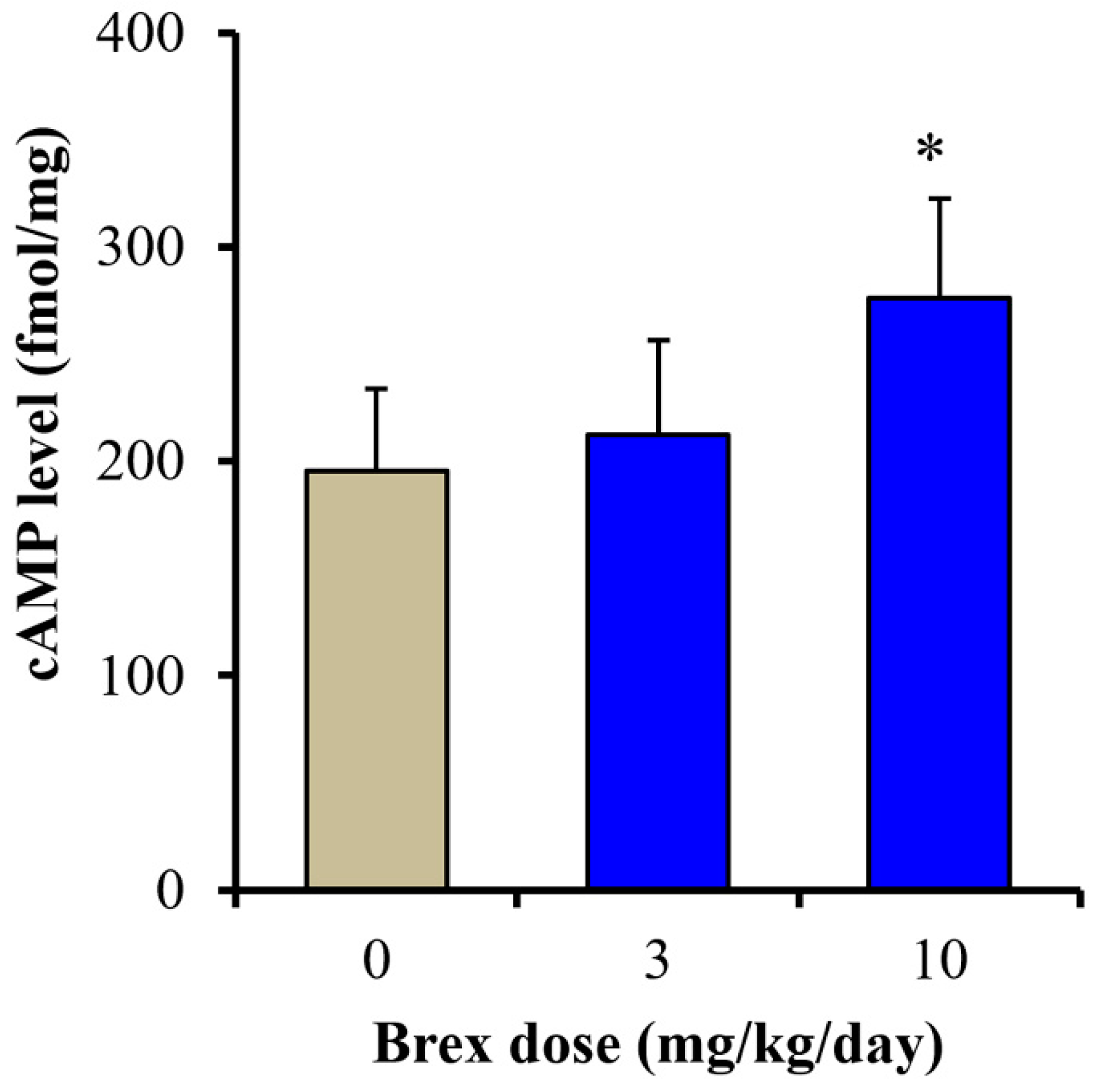
2.4.2. Effects of Subchronic Systemic Administration of Brex on cAMP Levels in the Hypothalamus
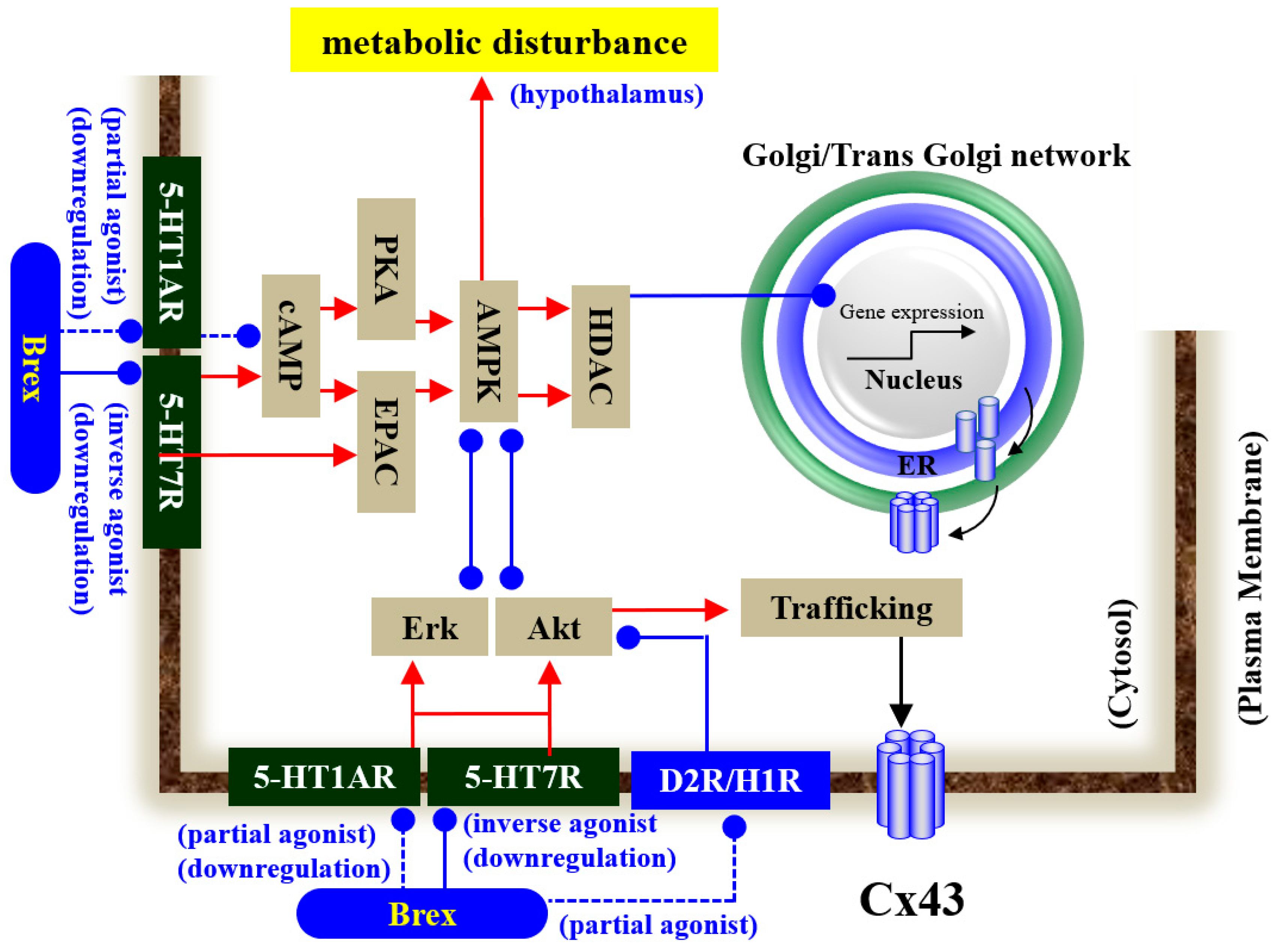
3. Discussion
3.1. Effects of Subchronic Administration of Brex on the Expression of 5-HT1AR and 5-HT7R in Astrocytes
3.2. Effects of Subchronic Administration of Brex on Intracellular Signalling in Astrocytes
3.3. Impacts of the Suppression of AMPK Signalling on Tripartite Synaptic Transmission
3.4. Clinical Implication and Low Risk of Metabolic Syndrome
4. Materials and Methods
4.1. Chemical Agents and Drug Administration
4.2. Preparation of Primary Astrocyte Culture
4.3. Extraction of Cultured Astrocytes and Rat Hypothalamus
4.4. Capillary Immunoblotting Analysis
4.5. Determination of Intracellular cAMP Levels in Cultured Astrocytes and Rat Hypothalamus
4.6. Data Analysis
4.7. Nomenclature of Targets and Ligands
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Otsuka Pharmaceutical Co., Ltd. Rexulti® Tablets, a New Antipsychotic Drug, Launched in Japan. Available online: https://www.otsuka.co.jp/en/company/newsreleases/2018/20180418_1.html (accessed on 31 March 2022).
- Huhn, M.; Nikolakopoulou, A.; Schneider-Thoma, J.; Krause, M.; Samara, M.; Peter, N.; Arndt, T.; Backers, L.; Rothe, P.; Cipriani, A.; et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: A systematic review and network meta-analysis. Lancet 2019, 394, 939–951. [Google Scholar] [CrossRef]
- Watson, P.; Zhang, J.P.; Rizvi, A.; Tamale, J.; Birnbaum, M.L.; Kane, J. A meta-analysis of factors associated with quality of life in first episode psychosis. Schizophr. Res. 2018, 202, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Nunez, N.A.; Joseph, B.; Pahwa, M.; Kumar, R.; Resendez, M.G.; Prokop, L.J.; Veldic, M.; Seshadri, A.; Biernacka, J.M.; Frye, M.A.; et al. Augmentation strategies for treatment resistant major depression: A systematic review and network meta-analysis. J. Affect. Disord. 2022, 302, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Fava, M.; Menard, F.; Davidsen, C.K.; Baker, R.A. Adjunctive brexpiprazole in patients with major depressive disorder and irritability: An exploratory study. J. Clin. Psychiatry 2016, 77, 1695–1701. [Google Scholar] [CrossRef]
- Thase, M.E.; Youakim, J.M.; Skuban, A.; Hobart, M.; Augustine, C.; Zhang, P.; McQuade, R.D.; Carson, W.H.; Nyilas, M.; Sanchez, R.; et al. Efficacy and safety of adjunctive brexpiprazole 2 mg in major depressive disorder: A phase 3, randomized, placebo-controlled study in patients with inadequate response to antidepressants. J. Clin. Psychiatry 2015, 76, 1224–1231. [Google Scholar] [CrossRef]
- Thase, M.E.; Youakim, J.M.; Skuban, A.; Hobart, M.; Zhang, P.; McQuade, R.D.; Nyilas, M.; Carson, W.H.; Sanchez, R.; Eriksson, H. Adjunctive brexpiprazole 1 and 3 mg for patients with major depressive disorder following inadequate response to antidepressants: A phase 3, randomized, double-blind study. J. Clin. Psychiatry 2015, 76, 1232–1240. [Google Scholar] [CrossRef]
- Pillinger, T.; McCutcheon, R.A.; Vano, L.; Mizuno, Y.; Arumuham, A.; Hindley, G.; Beck, K.; Natesan, S.; Efthimiou, O.; Cipriani, A.; et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: A systematic review and network meta-analysis. Lancet Psychiatry 2020, 7, 64–77. [Google Scholar] [CrossRef]
- Girgis, R.R.; Forbes, A.; Abi-Dargham, A.; Slifstein, M. A positron emission tomography occupancy study of brexpiprazole at dopamine d2 and d3 and serotonin 5-ht1a and 5-ht2a receptors, and serotonin reuptake transporters in subjects with schizophrenia. Neuropsychopharmacology 2020, 45, 786–792. [Google Scholar] [CrossRef]
- Maeda, K.; Sugino, H.; Akazawa, H.; Amada, N.; Shimada, J.; Futamura, T.; Yamashita, H.; Ito, N.; McQuade, R.D.; Mork, A.; et al. Brexpiprazole i: In vitro and in vivo characterization of a novel serotonin-dopamine activity modulator. J. Pharm. Exp. 2014, 350, 589–604. [Google Scholar] [CrossRef]
- Citrome, L.; Stensbol, T.B.; Maeda, K. The preclinical profile of brexpiprazole: What is its clinical relevance for the treatment of psychiatric disorders? Expert Rev. Neurother. 2015, 15, 1219–1229. [Google Scholar] [CrossRef]
- Fukuyama, K.; Okada, M. Effects of atypical antipsychotics, clozapine, quetiapine and brexpiprazole on astroglial transmission associated with connexin43. Int. J. Mol. Sci. 2021, 22, 5623. [Google Scholar] [CrossRef] [PubMed]
- Okubo, R.; Hasegawa, T.; Fukuyama, K.; Shiroyama, T.; Okada, M. Current limitations and candidate potential of 5-ht7 receptor antagonism in psychiatric pharmacotherapy. Front. Psychiatry 2021, 12, 623684. [Google Scholar] [CrossRef] [PubMed]
- Okada, M. Can rodent models elucidate pathomechanisms of genetic epilepsy? Br. J. Pharmacol. 2022, 179, 1620–1639. [Google Scholar] [CrossRef]
- Okada, M.; Oka, T.; Nakamoto, M.; Fukuyama, K.; Shiroyama, T. Astroglial connexin43 as a potential target for a mood stabiliser. Int. J. Mol. Sci. 2020, 22, 339. [Google Scholar] [CrossRef]
- Maxishima, M.; Shiga, T.; Shutoh, F.; Hamada, S.; Maeshima, T.; Okado, N. Serotonin 2a receptor-like immunoreactivity is detected in astrocytes but not in oligodendrocytes of rat spinal cord. Brain Res. 2001, 889, 270–273. [Google Scholar] [CrossRef]
- Harris, K.L.; Mason, S.L.; Vallin, B.; Barker, R.A. Reduced expression of dopamine d2 receptors on astrocytes in r6/1 hd mice and hd post-mortem tissue. Neurosci. Lett. 2021, 767, 136289. [Google Scholar] [CrossRef] [PubMed]
- Shiroyama, T.; Fukuyama, K.; Okada, M. Distinct effects of escitalopram and vortioxetine on astroglial l-glutamate release associated with connexin43. Int. J. Mol. Sci. 2021, 22, 10013. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Fukuyama, K.; Shiroyama, T.; Murata, M. A working hypothesis regarding identical pathomechanisms between clinical efficacy and adverse reaction of clozapine via the activation of connexin43. Int. J. Mol. Sci. 2020, 21, 7019. [Google Scholar] [CrossRef]
- Shapiro, D.A.; Renock, S.; Arrington, E.; Chiodo, L.A.; Liu, L.X.; Sibley, D.R.; Roth, B.L.; Mailman, R. Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology 2003, 28, 1400–1411. [Google Scholar] [CrossRef]
- Ghanizadeh, A.; Sahraeizadeh, A.; Berk, M. A head-to-head comparison of aripiprazole and risperidone for safety and treating autistic disorders, a randomized double blind clinical trial. Child Psychiatry Hum. Dev. 2014, 45, 185–192. [Google Scholar] [CrossRef]
- Su, T.P.; Malhotra, A.K.; Hadd, K.; Breier, A.; Pickar, D. D2 dopamine receptor occupancy: A crossover comparison of risperidone with clozapine therapy in schizophrenic patients. Arch. Gen. Psychiatry 1997, 54, 972–973. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, H.Y. The mechanism of action of novel antipsychotic drugs. Schizophr. Bull. 1991, 17, 263–287. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, T.; Horisawa, T.; Tokuda, K.; Ishiyama, T.; Ogasa, M.; Tagashira, R.; Matsumoto, K.; Nishikawa, H.; Ueda, Y.; Toma, S.; et al. Pharmacological profile of lurasidone, a novel antipsychotic agent with potent 5-hydroxytryptamine 7 (5-ht7) and 5-ht1a receptor activity. J. Pharm. Exp. 2010, 334, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Munoz, F.; Alamo, C. Active metabolites as antidepressant drugs: The role of norquetiapine in the mechanism of action of quetiapine in the treatment of mood disorders. Front. Psychiatry 2013, 4, 102. [Google Scholar] [CrossRef]
- Schmidt, A.W.; Lebel, L.A.; Howard, H.R., Jr.; Zorn, S.H. Ziprasidone: A novel antipsychotic agent with a unique human receptor binding profile. Eur. J. Pharmacol. 2001, 425, 197–201. [Google Scholar] [CrossRef]
- Schotte, A.; Janssen, P.F.; Gommeren, W.; Luyten, W.H.; Van Gompel, P.; Lesage, A.S.; De Loore, K.; Leysen, J.E. Risperidone compared with new and reference antipsychotic drugs: In vitro and in vivo receptor binding. Psychopharmacology 1996, 124, 57–73. [Google Scholar] [CrossRef]
- Fukuyama, K.; Okubo, R.; Murata, M.; Shiroyama, T.; Okada, M. Activation of astroglial connexin is involved in concentration-dependent double-edged sword clinical action of clozapine. Cells 2020, 9, 414. [Google Scholar] [CrossRef]
- Fukuyama, K.; Kato, R.; Murata, M.; Shiroyama, T.; Okada, M. Clozapine normalizes a glutamatergic transmission abnormality induced by an impaired nmda receptor in the thalamocortical pathway via the activation of a group iii metabotropic glutamate receptor. Biomolecules 2019, 9, 234. [Google Scholar] [CrossRef]
- Fatemi, S.H.; Folsom, T.D.; Reutiman, T.J.; Pandian, T.; Braun, N.N.; Haug, K. Chronic psychotropic drug treatment causes differential expression of connexin 43 and gfap in frontal cortex of rats. Schizophr. Res. 2008, 104, 127–134. [Google Scholar] [CrossRef]
- Sun, J.-D.; Liu, Y.; Yuan, Y.-H.; Li, J.; Chen, N.-H. Gap junction dysfunction in the prefrontal cortex induces depressive-like behaviors in rats. Neuropsychopharmacology 2012, 37, 1305–1320. [Google Scholar] [CrossRef]
- Jeanson, T.; Pondaven, A.; Ezan, P.; Mouthon, F.; Charveriat, M.; Giaume, C. Antidepressants impact connexin 43 channel functions in astrocytes. Front. Cell. Neurosci. 2015, 9, 495. [Google Scholar] [CrossRef]
- Quesseveur, G.; Portal, B.; Basile, J.A.; Ezan, P.; Mathou, A.; Halley, H.; Leloup, C.; Fioramonti, X.; Deglon, N.; Giaume, C.; et al. Attenuated levels of hippocampal connexin 43 and its phosphorylation correlate with antidepressant- and anxiolytic-like activities in mice. Front. Cell. Neurosci. 2015, 9, 490. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, K.; Motomura, E.; Shiroyama, T.; Okada, M. Impact of 5-ht7 receptor inverse agonism of lurasidone on monoaminergic tripartite synaptic transmission and pathophysiology of lower risk of weight gain. Biomed. Pharmacother. 2022, 148, 112750. [Google Scholar] [CrossRef]
- Schoretsanitis, G.; Paulzen, M.; Unterecker, S.; Schwarz, M.; Conca, A.; Zernig, G.; Grunder, G.; Haen, E.; Baumann, P.; Bergemann, N.; et al. Tdm in psychiatry and neurology: A comprehensive summary of the consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology, update 2017; a tool for clinicians. World J. Biol. Psychiatry 2018, 19, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Hiemke, C.; Bergemann, N.; Clement, H.W.; Conca, A.; Deckert, J.; Domschke, K.; Eckermann, G.; Egberts, K.; Gerlach, M.; Greiner, C.; et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: Update 2017. Pharmacopsychiatry 2018, 51, 9–62. [Google Scholar] [PubMed]
- Tanahashi, S.; Yamamura, S.; Nakagawa, M.; Motomura, E.; Okada, M. Clozapine, but not haloperidol, enhances glial d-serine and l-glutamate release in rat frontal cortex and primary cultured astrocytes. Br. J. Pharmacol. 2012, 165, 1543–1555. [Google Scholar] [CrossRef]
- Yamamura, S.; Hoshikawa, M.; Dai, K.; Saito, H.; Suzuki, N.; Niwa, O.; Okada, M. Ono-2506 inhibits spike-wave discharges in a genetic animal model without affecting traditional convulsive tests via gliotransmission regulation. Br. J. Pharmacol. 2013, 168, 1088–1100. [Google Scholar] [CrossRef]
- Fukuyama, K.; Okada, M. Effects of levetiracetam on astroglial release of kynurenine-pathway metabolites. Br. J. Pharmacol. 2018, 175, 4253–4265. [Google Scholar] [CrossRef]
- Fukuyama, K.; Ueda, Y.; Okada, M. Effects of carbamazepine, lacosamide and zonisamide on gliotransmitter release associated with activated astroglial hemichannels. Pharmaceuticals 2020, 13, 117. [Google Scholar] [CrossRef]
- Fukuyama, K.; Okada, M. Effects of an atypical antipsychotic, zotepine, on astroglial l-glutamate release through hemichannels: Exploring the mechanism of mood-stabilising antipsychotic actions and antipsychotic-induced convulsion. Pharmaceuticals 2021, 14, 1116. [Google Scholar] [CrossRef]
- Okada, M.; Fukuyama, K.; Shiroyama, T.; Ueda, Y. Brivaracetam prevents astroglial l-glutamate release associated with hemichannel through modulation of synaptic vesicle protein. Biomed. Pharmacother. 2021, 138, 111462. [Google Scholar] [CrossRef]
- Okada, M.; Matsumoto, R.; Yamamoto, Y.; Fukuyama, K. Effects of subchronic administrations of vortioxetine, lurasidone, and escitalopram on thalamocortical glutamatergic transmission associated with serotonin 5-ht7 receptor. Int. J. Mol. Sci. 2021, 22, 1351. [Google Scholar] [CrossRef]
- Okada, M.; Kawano, Y.; Fukuyama, K.; Motomura, E.; Shiroyama, T. Candidate strategies for development of a rapid-acting antidepressant class that does not result in neuropsychiatric adverse effects: Prevention of ketamine-induced neuropsychiatric adverse reactions. Int. J. Mol. Sci. 2020, 21, 7951. [Google Scholar] [CrossRef]
- Jeon, S.M. Regulation and function of ampk in physiology and diseases. Exp. Mol. Med. 2016, 48, e245. [Google Scholar] [CrossRef]
- Zmudzka, E.; Salaciak, K.; Sapa, J.; Pytka, K. Serotonin receptors in depression and anxiety: Insights from animal studies. Life Sci. 2018, 210, 106–124. [Google Scholar] [CrossRef]
- Andressen, K.W.; Manfra, O.; Brevik, C.H.; Ulsund, A.H.; Vanhoenacker, P.; Levy, F.O.; Krobert, K.A. The atypical antipsychotics clozapine and olanzapine promote down-regulation and display functional selectivity at human 5-ht7 receptors. Br. J. Pharmacol. 2015, 172, 3846–3860. [Google Scholar] [CrossRef]
- Albert, P.R.; Vahid-Ansari, F. The 5-ht1a receptor: Signaling to behavior. Biochimie 2019, 161, 34–45. [Google Scholar] [CrossRef]
- Yoon, D.H.; Yoon, S.; Kim, D.; Kim, H.; Baik, J.H. Regulation of dopamine d2 receptor-mediated extracellular signal-regulated kinase signaling and spine formation by gabaa receptors in hippocampal neurons. Neurosci. Lett. 2015, 586, 24–30. [Google Scholar] [CrossRef]
- Aringhieri, S.; Kolachalam, S.; Gerace, C.; Carli, M.; Verdesca, V.; Brunacci, M.G.; Rossi, C.; Ippolito, C.; Solini, A.; Corsini, G.U.; et al. Clozapine as the most efficacious antipsychotic for activating erk 1/2 kinases: Role of 5-ht2a receptor agonism. Eur. Neuropsychopharmacol. 2017, 27, 383–398. [Google Scholar] [CrossRef]
- Yang, B.H.; Son, H.; Kim, S.H.; Nam, J.H.; Choi, J.H.; Lee, J.S. Phosphorylation of erk and creb in cultured hippocampal neurons after haloperidol and risperidone administration. Psychiatry Clin. Neurosci. 2004, 58, 262–267. [Google Scholar] [CrossRef]
- Beaulieu, J.M.; Sotnikova, T.D.; Marion, S.; Lefkowitz, R.J.; Gainetdinov, R.R.; Caron, M.G. An akt/beta-arrestin 2/pp2a signaling complex mediates dopaminergic neurotransmission and behavior. Cell 2005, 122, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Daniele, S.; Zappelli, E.; Martini, C. Trazodone regulates neurotrophic/growth factors, mitogen-activated protein kinases and lactate release in human primary astrocytes. J. Neuroinflamm. 2015, 12, 225. [Google Scholar] [CrossRef]
- Emamian, E.S.; Hall, D.; Birnbaum, M.J.; Karayiorgou, M.; Gogos, J.A. Convergent evidence for impaired akt1-gsk3beta signaling in schizophrenia. Nat. Genet. 2004, 36, 131–137. [Google Scholar] [CrossRef]
- Wang, Y.J.; Ren, Q.G.; Gong, W.G.; Wu, D.; Tang, X.; Li, X.L.; Wu, F.F.; Bai, F.; Xu, L.; Zhang, Z.J. Escitalopram attenuates beta-amyloid-induced tau hyperphosphorylation in primary hippocampal neurons through the 5-ht1a receptor mediated akt/gsk-3beta pathway. Oncotarget 2016, 7, 13328–13339. [Google Scholar] [CrossRef]
- Petrunich-Rutherford, M.L.; Garcia, F.; Battaglia, G. 5-ht1a receptor-mediated activation of neuroendocrine responses and multiple protein kinase pathways in the peripubertal rat hypothalamus. Neuropharmacology 2018, 139, 173–181. [Google Scholar] [CrossRef]
- Okada, M.; Okubo, R.; Fukuyama, K. Vortioxetine subchronically activates serotonergic transmission via desensitization of serotonin 5-ht1a receptor with 5-ht3 receptor inhibition in rats. Int. J. Mol. Sci. 2019, 20, 6235. [Google Scholar] [CrossRef]
- Okada, M.; Fukuyama, K.; Shiroyama, T.; Ueda, Y. Lurasidone inhibits nmda antagonist-induced functional abnormality of thalamocortical glutamatergic transmission via 5-ht7 receptor blockade. Br. J. Pharmacol. 2019, 176, 4002–4018. [Google Scholar] [CrossRef]
- Okada, M.; Fukuyama, K.; Okubo, R.; Shiroyama, T.; Ueda, Y. Lurasidone sub-chronically activates serotonergic transmission via desensitization of 5-ht1a and 5-ht7 receptors in dorsal raphe nucleus. Pharmaceuticals 2019, 12, 149. [Google Scholar] [CrossRef]
- Svejda, B.; Kidd, M.; Timberlake, A.; Harry, K.; Kazberouk, A.; Schimmack, S.; Lawrence, B.; Pfragner, R.; Modlin, I.M. Serotonin and the 5-ht7 receptor: The link between hepatocytes, igf-1 and small intestinal neuroendocrine tumors. Cancer Sci. 2013, 104, 844–855. [Google Scholar] [CrossRef]
- Hedlund, P.B.; Huitron-Resendiz, S.; Henriksen, S.J.; Sutcliffe, J.G. 5-ht7 receptor inhibition and inactivation induce antidepressantlike behavior and sleep pattern. Biol. Psychiatry 2005, 58, 831–837. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, J.; Gao, K.; Chi, Y.; Mitsui, T.; Ihara, T.; Sawada, N.; Kamiyama, M.; Fan, J.; Takeda, M. Ampk suppresses connexin43 expression in the bladder and ameliorates voiding dysfunction in cyclophosphamide-induced mouse cystitis. Sci. Rep. 2016, 6, 19708. [Google Scholar] [CrossRef]
- Rebello, C.J.; Greenway, F.L. Obesity medications in development. Expert Opin. Investig. Drugs 2020, 29, 63–71. [Google Scholar] [CrossRef]
- Steinberg, G.R.; Kemp, B.E. Ampk in health and disease. Physiol. Rev. 2009, 89, 1025–1078. [Google Scholar] [CrossRef]
- Carli, M.; Kolachalam, S.; Longoni, B.; Pintaudi, A.; Baldini, M.; Aringhieri, S.; Fasciani, I.; Annibale, P.; Maggio, R.; Scarselli, M. Atypical antipsychotics and metabolic syndrome: From molecular mechanisms to clinical differences. Pharmaceuticals 2021, 14, 238. [Google Scholar] [CrossRef]
- Kim, S.F.; Huang, A.S.; Snowman, A.M.; Teuscher, C.; Snyder, S.H. From the cover: Antipsychotic drug-induced weight gain mediated by histamine h1 receptor-linked activation of hypothalamic amp-kinase. Proc. Natl. Acad. Sci. USA 2007, 104, 3456–3459. [Google Scholar] [CrossRef]
- Stahl, S.M. Mechanism of action of brexpiprazole: Comparison with aripiprazole. CNS Spectr. 2016, 21, 1–6. [Google Scholar] [CrossRef]
- Samy, D.M.; Mostafa, D.K.; Abdelmonsif, D.A.; Ismail, C.A.; Hassaan, P.S. Crosstalk of hypothalamic chemerin, histamine, and ampk in diet-and olanzapine-induced obesity in rats. Life Sci. 2021, 284, 119897. [Google Scholar] [CrossRef]
- Wojtys, E.M. Perspective. Sports Health 2019, 11, 115. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, S.; Yu, H.S.; Ko, K.H.; Park, H.G.; Kim, Y.S. The antipsychotic agent clozapine induces autophagy via the ampk-ulk1-beclin1 signaling pathway in the rat frontal cortex. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 81, 96–104. [Google Scholar] [CrossRef]
- Mombereau, C.; Arnt, J.; Mork, A. Involvement of presynaptic 5-ht1a receptors in the low propensity of brexpiprazole to induce extrapyramidal side effects in rats. Pharm. Biochem. Behav. 2017, 153, 141–146. [Google Scholar] [CrossRef]
- Harvima, I.T.; Levi-Schaffer, F.; Draber, P.; Friedman, S.; Polakovicova, I.; Gibbs, B.F.; Blank, U.; Nilsson, G.; Maurer, M. Molecular targets on mast cells and basophils for novel therapies. J. Allergy Clin. Immunol. 2014, 134, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Maruko, T.; Nakahara, T.; Sakamoto, K.; Saito, M.; Sugimoto, N.; Takuwa, Y.; Ishii, K. Involvement of the betagamma subunits of g proteins in the camp response induced by stimulation of the histamine h1 receptor. Naunyn Schmiedebergs Arch. Pharm. 2005, 372, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Guan, T.; Zhang, H.; Xia, Y.; Liu, F.; Zhang, Y. Inhibitory crosstalk between erk and ampk in the growth and proliferation of cardiac fibroblasts. Biochem. Biophys. Res. Commun. 2008, 368, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hu, X.; Liu, Y.; Dong, S.; Wen, Z.; He, W.; Zhang, S.; Huang, Q.; Shi, M. Ros signaling under metabolic stress: Cross-talk between ampk and akt pathway. Mol. Cancer 2017, 16, 79. [Google Scholar] [CrossRef]
- Soengas, J.L. Integration of nutrient sensing in fish hypothalamus. Front. Neurosci. 2021, 15, 653928. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.P.; Christopoulos, A.; Davenport, A.P.; Kelly, E.; Mathie, A.; Peters, J.A.; Veale, E.L.; Armstrong, J.F.; Faccenda, E.; Harding, S.D.; et al. The concise guide to pharmacology 2021/22: G protein-coupled receptors. Br. J. Pharmacol. 2021, 178, S27–S156. [Google Scholar] [CrossRef] [PubMed]
- Lilley, E.; Stanford, S.C.; Kendall, D.E.; Alexander, S.P.H.; Cirino, G.; Docherty, J.R.; George, C.H.; Insel, P.A.; Izzo, A.A.; Ji, Y.; et al. Arrive 2.0 and the british journal of pharmacology: Updated guidance for 2020. Br. J. Pharmacol. 2020, 177, 3611–3616. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, K.; Okada, M. Age-dependent and sleep/seizure-induced pathomechanisms of autosomal dominant sleep-related hypermotor epilepsy. Int. J. Mol. Sci. 2020, 21, 8142. [Google Scholar] [CrossRef]
- Iversen, L.L.; Glowinski, J. Regional studies of catecholamines in the rat brain. Ii. Rate of turnover of catecholamines in various brain regions. J. Neurochem. 1966, 13, 671–682. [Google Scholar] [CrossRef]
- Curtis, M.J.; Alexander, S.; Cirino, G.; Docherty, J.R.; George, C.H.; Giembycz, M.A.; Hoyer, D.; Insel, P.A.; Izzo, A.A.; Ji, Y.; et al. Experimental design and analysis and their reporting ii: Updated and simplified guidance for authors and peer reviewers. Br. J. Pharmacol. 2018, 175, 987–993. [Google Scholar] [CrossRef]



| Receptor | Brex | APZ | CLZ | LUR | QTP | ZIP | ZTP |
|---|---|---|---|---|---|---|---|
| 5-HT1AR | 0.12 | 5.6 | 124 | 6.8 | 432 | 2.5 | 471 |
| 5-HT2AR | 0.47 | 8.7 | 5.4 | 2.0 | 100 | 0.08 | 2.7 |
| 5-HT7R | 3.7 | 10.3 | 18.0 | 0.5 | 307 | 6 | 12.0 |
| H1R | 19 | 27.9 | 1.13 | >1000 | 2.2–11 | 15 | 3.21 |
| D1R | 160 | >1000 | 266 | 262 | 712 | 30 | 71.0 |
| D2R | 0.3 | 3.3 | 157 | 1.7 | 245 | 4.6 | 25.0 |
| Reference | [10] | [20,21] | [22,23] | [24] | [25] | [26] | [27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukuyama, K.; Motomura, E.; Okada, M. Brexpiprazole Reduces 5-HT7 Receptor Function on Astroglial Transmission Systems. Int. J. Mol. Sci. 2022, 23, 6571. https://doi.org/10.3390/ijms23126571
Fukuyama K, Motomura E, Okada M. Brexpiprazole Reduces 5-HT7 Receptor Function on Astroglial Transmission Systems. International Journal of Molecular Sciences. 2022; 23(12):6571. https://doi.org/10.3390/ijms23126571
Chicago/Turabian StyleFukuyama, Kouji, Eishi Motomura, and Motohiro Okada. 2022. "Brexpiprazole Reduces 5-HT7 Receptor Function on Astroglial Transmission Systems" International Journal of Molecular Sciences 23, no. 12: 6571. https://doi.org/10.3390/ijms23126571
APA StyleFukuyama, K., Motomura, E., & Okada, M. (2022). Brexpiprazole Reduces 5-HT7 Receptor Function on Astroglial Transmission Systems. International Journal of Molecular Sciences, 23(12), 6571. https://doi.org/10.3390/ijms23126571






