Abstract
Crustins are small antimicrobial proteins produced by crustaceans. Of the many reported crustins, very few are from deep sea environments. Crustins are categorized into several types. Recently, the Type I crustin has been further classified into three subtypes, one of which is Type Ib, whose function is unknown. Here, we studied the function of a Type Ib crustin (designated Crus2) identified from a deep-sea crustacean. Crus2 has a whey acidic protein (WAP) domain and a long C-terminal region (named P58). Recombinant Crus2 bound to peptidoglycan (PGN), lipoteichoic acid (LTA), and lipopolysaccharide (LPS), and killed Gram-positive and Gram-negative bacteria by permeabilizing the bacterial cytomembrane. Consistently, Crus2 dramatically attenuated the inflammatory response induced by LPS and LTA. Disruption of the disulfide bonds in the WAP domain abolished the bactericidal ability of Crus2, but had no effect on the bacterial binding ability of Crus2. Deletion of the C-terminal P58 region moderately affected the antimicrobial activity of Crus2 against some bacteria. P58 as a synthesized peptide could bind bacteria and inhibit the bactericidal activity of Crus2. Taken together, these results revealed different roles played by the WAP domain and the P58 region in Type Ib crustin, and provided new insights into the antimicrobial and immunomodulatory functions of crustins.
1. Introduction
Antimicrobial peptides (AMPs) are widely distributed in animals. They are small peptides with various antimicrobial activities against diverse microorganisms [1]. AMPs share certain common features, such as overall positive charge and surface amphiphilicity, that allow them to disrupt the target cells by binding to the cytoplasmic membrane of the cells and/or entering the cells [2,3,4]. With the increase of bacterial antibiotic resistance that has become a global health problem, the search for alternative antimicrobials is an urgent need [5,6]. Compared to antibiotics, AMPs have relatively low resistance development potential. Hence, AMPs have been considered good candidates as antibiotic substitutes [6,7].
Crustins are the largest and a very diverse AMP family in the classified crustacean AMPs [8]. They are usually hydrophobic, cysteine-rich cationic polypeptides containing a whey acid protein (WAP) domain that forms a four-disulfide bond structure [9,10]. Traditionally, crustins were categorized into three main types [11]. Type I crustins exhibit typical crustin characteristics, with four conserved cysteine residues after the signal peptide followed by a C-terminal WAP domain [12,13]. Type II crustins contain two regions rich in glycine and cysteine, respectively, which are replaced by a proline and/or arginine-rich region in Type III crustins [14,15]. All types of crustins possess antibacterial activities. Type II and III crustins can target both Gram-positive and Gram-negative bacteria, while Type I crustins mainly target Gram-positive bacteria [16]. Recent research has expanded the classification of crustins. The Type I crustins are divided into three subtypes, i.e., Ia, Ib, and Ic [17]. Type Ia is the previous Type I; Type Ib has a long random sequence at the C-terminus; Type Ic contains two cysteine-rich regions [17]. The biological functions of the new subtypes, i.e., Ib, and Ic, remain to be investigated.
Deep-sea hydrothermal vents are extreme environments that nurture thriving ecosystems constituted mainly by microorganisms and macro–invertebrates such as shrimp [18]. In particular, the Alvinocarididae family is the dominant shrimp in deep-sea vents, and has been found to encode a variety of antibacterial molecules [19,20,21]. In a previous study, we studied the antibacterial activity of a Type Ia crustin, Crus1, from Alvinocarididae in the deep sea of Manus Basin [22]. In the present work, we characterized another crustin, Crus2, from Rimicaris sp., which differs in structure from Crus1. We investigated the antibacterial activity of Crus2 and its dependence on the different domains, in particular the unique C-terminal region characteristic of Type Ib crustins. In addition, we also examined the immunomodulatory activity of Crus2. Our results add new knowledge to the immune function and working mechanism of deep sea crustins.
2. Results
2.1. Crus2 Is a Type 1b Crustin
Crus2 has 164 amino acid residues, a calculated molecular mass of 18.04 kDa, and a predicted pI of 8.42. Crus2 is predicted to possess an N-terminal signal peptide, a WAP domain with a typical ‘four-disulfide core’ structure, and a C-terminal region of 58 residues (named P58) (Figure 1A). Crus2 shares conserved cysteine residues in the WAP domain and the N-terminus with Type I crustins (Figure 1A). It shows the highest sequence identity (48.60%) with a crustin of Pacifastacus leniusculus (PI- crustin 2) and shares 40.19% identity with Crus1, the previously reported crustin of Rimicaris sp. [22]. Phylogenetic analysis showed that, different from Crus1, Crus2 was clustered together with the Type Ib crustins (Figure 1B). Structural modeling showed that Crus2 mainly contained random coils (Figure 1C). A similar coil-dominant structure was displayed by the Crus2 truncate (named Crus2DC) that lacks the C-terminal P58 region (Figure 1C). Compared to Crus2, Crus2DC exhibited decreased pI (7.8) and a different distribution of surface electrostatic potential (Figure 1D).
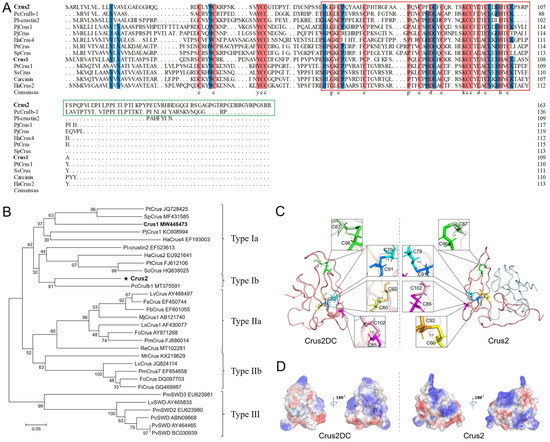
Figure 1.
Crus2 sequence and structure. (A) The Crus2 sequence was aligned with that of Type I crustins. Dots indicate gaps. Red color indicates consensus residues. Blue color indicates ≥75% identity. The WAP domain and the C-terminal region are boxed with red and green lines, respectively. (B) Phylogenetic analysis of Crus2. The numbers indicate bootstrap values based on 1000 replications. The asterisk represents the crustin of this study. In (A,B), the crustin names are followed by their respective GenBank accession numbers. (C) The predicted structures of Crus2 and Crus2DC. The disulfide bonds are shown in yellow (C60–C92), green (C67–C96), blue (C79–C91), and pink (C85–C102). (D) The surface electrostatic potential of Crus2 and Crus2DC.
2.2. Crus2 Binds and Kills Gram-Positive and Gram-Negative Bacteria in a Manner That Is Affected in Degree by the C-Terminal P58 Region
Recombinant Crus2 and Crus2DC were prepared from E. coli (Figure S1), with a yield of 6.2 mg and 5.6 mg per liter of culture, respectively. Both proteins exhibited apparent inhibitory and killing activities against bacteria from different sources, including deep sea (Table 1). In general, the MIC (minimal inhibitory concentration) values of Crus2DC were higher than that of Crus2, except for Micrococcus luteus. Similarly, the MBC (minimal bactericidal concentration) values of Crus2DC were also higher than that of Crus2. Both Crus2 and Crus2DC showed apparent binding to various bacteria (Figure 2A). Consistently, Crus2 and Crus2DC bound well to peptidoglycan (PGN), lipoteichoic acid (LTA) and lipopolysaccharide (LPS) (Figure 2B). When Crus2 and Crus2DC were pre-incubated with PGN or LTA, the bactericidal effects of the proteins on the Gram-positive Bacillus cereus were significantly decreased; similarly, pre-incubation with LPS significantly inhibited the bactericidal activity of Crus2/Crus2DC toward the Gram-negative Vibrio harveyi (Figure 2C,D).

Table 1.
The MIC and MBC of Crus2 and Crus2DC against different bacteria.
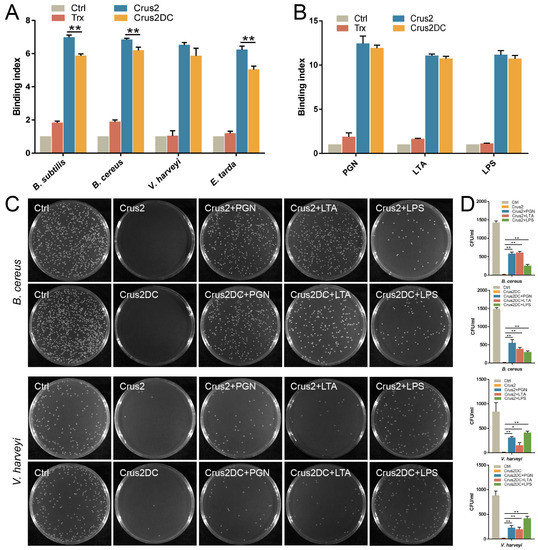
Figure 2.
Binding of Crus2 and Crus2DC to bacteria and bacterial components. (A) Bacteria were incubated with or without (control, Ctrl) Crus2, Crus2DC, or rTrx, and the binding was detected by ELISA. (B) Peptidoglycan (PGN), lipoteichoic acid (LTA), and lipopolysaccharide (LPS) were incubated with or without (Ctrl) Crus2, Crus2DC, or rTrx, and the binding was detected as above. (C,D) Bacillus cereus and Vibrio harveyi were incubated with Crus2 or Crus2DC, or Crus2/Crus2DC that had been pre-treated with PGN, LTA, or LPS. The control bacteria were incubated with PBS. After 1 h of incubation, the bacteria were plated on MH agar plates and observed after 20–24 h incubation (C). The numbers of colony-forming units (CFU) on the plates were counted (D). Values are shown as means ± SD (n = 3). n, the number of replicates. **, p < 0.01; *, p < 0.05 (Student’s t test).
2.3. Crus2 Kills Bacteria by Inducing Damage in the Bacteria Membrane
Propidium iodide (PI) staining showed that following treatment with Crus2/Crus2DC, strong fluorescence was observed in B. cereus and V. harveyi, reflecting disruption of the bacterial membrane (Figure 3A). Cytoplasmic membrane depolarization analysis with DiSC3 (5), a membrane potential-sensitive probe, showed that Crus2 and Crus2DC markedly increased the fluorescent signal in DiSC3 (5)-treated bacteria, indicating changes in the membrane potential (Figure 3B). SEM and TEM revealed that the cells treated with Crus2 exhibited severe structure destruction and release of cellular contents (Figure 3C,D). In contrast, Crus2DC-treated bacteria retained relatively a normal morphology and displayed no apparent structural damage.
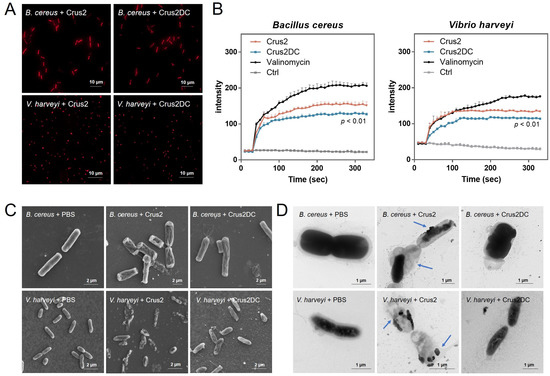
Figure 3.
The effect of Crus2/Crus2DC on bacterial structure. (A) Bacteria (Bacillus cereus or Vibrio harveyi) were treated with Crus2 or Crus2DC. After treatment, PI was used to stain the cells, and the stained cells were observed with a fluorescence microscope. (B) The above bacteria were first treated with DiSC3 (5) and then treated with or without (Ctrl) Crus2, Crus2DC, or valinomycin, and the fluorescence of the cells was subsequently determined. (C,D) B. cereus and V. harveyi were incubated with Crus2, Crus2DC, or PBS. After incubation, scanning (C) or transmission (D) electron microscopy was applied for structural observation. The blue arrows indicate the breakdown of cellular structure and the release of cellular contents.
2.4. The Bactericidal Activity of Crus2 Depends on the Conserved WAP Domain and Is Affected by P58
To explore the essentialness of the conserved disulfide bonds of the Type Ib crustin, the recombinant protein of a Crus2 mutant, Crus2M, was prepared, which bears serine substitutions at C91 and C102. Compared to Crus2, the mutant exhibited no significant difference in the binding to bacteria, LPS, or PGN (Figure 4A,B). However, Crus2M had no apparent effect on the survival of B. cereus or V. harveyi (Figure 4C). To evaluate the functional importance of the C-terminal P58 region of Crus2, P58 was synthesized as a peptide and examined for its anti-bacterial effect. P58 exhibited no killing effect on B. cereus or V. harveyi (Figure 5A), and caused no apparent disruption of bacterial structure (Figure 5C). However, P58 was able to bind to bacteria and bacterial components (Figure 5B). Furthermore, pre-treatment of the bacteria with P58 significantly increased the ability of the bacteria to resist subsequent Crus2 killing (Figure 5D).
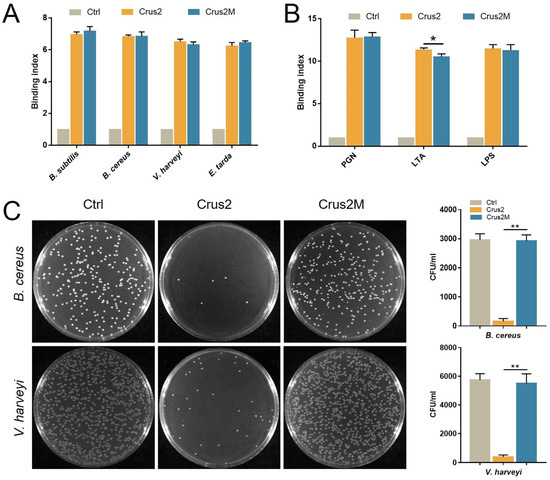
Figure 4.
The bacterial binding and bactericidal activities of Crus2M. (A) Bacillus subtilis, Bacillus cereus, Vibrio harveyi and Edwardsiella tarda were incubated with or without (Ctrl) Crus2 or Crus2M. The binding was subsequently detected by ELISA. (B) Peptidoglycan (PGN), lipoteichoic acid (LTA), and lipopolysaccharide (LPS) were incubated with or without (Ctrl) Crus2 or Crus2M, and the binding was detected as above. (C) B. cereus and V. harveyi were treated with or without (Ctrl) 32 μM Crus2 or Crus2M. After treatment, bacterial growth on MH agar plates was observed. The colony-forming units (CFU) on the plates are shown in the bar graphs. Data are the means triplicate experiments and shown as ±SD. ** and * represent p < 0.01 and p < 0.05, respectively (Student’s t test).
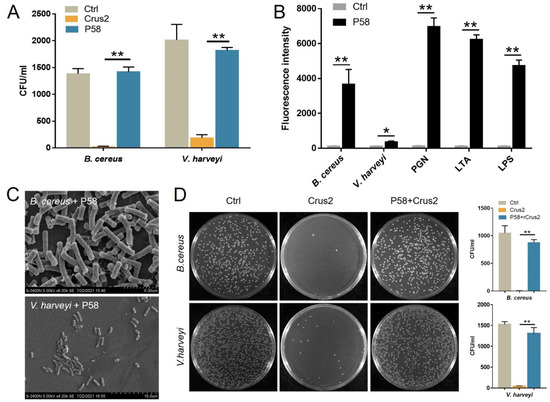
Figure 5.
The bacterial binding and bactericidal activities of P58. (A) Bacillus cereus and Vibrio harveyi were treated with or without (Ctrl) Crus2 or P58 for 1 h. After treatment, bacterial growth on MH agar plates was determined. CFU, colony-forming units. (B) Bacteria, peptidoglycan (PGN), lipoteichoic acid (LTA), and lipopolysaccharide (LPS) were each incubated with or without (Ctrl) FITC-labeled P58, and the binding was determined by measuring fluorescence intensity. (C) B. cereus and V. harveyi were incubated with P58 for 2 h. After incubation, scanning electron microscopy was applied for structural observation. (D) Crus2 was incubated with B. cereus and V. harveyi that had been pre-treated with or without (Ctrl) P58. After incubation, bacterial growth on MH agar plates was determined. The CFU numbers are shown in the graphs. Data are the means triplicate experiments and shown as ± SD. ** and * represent p < 0.01 and p < 0.05, respectively (Student’s t test).
2.5. Crus2 Blocks LPS- and LTA-Induced Inflammatory Response
Since Crus2 and P58 were able to bind LPS and LTA, we examined their potentials to suppress LPS/LTA-induced inflammation. The results showed that pre-incubation with Crus2 significantly impaired the ability of LPS and LTA to induce the release of IL-6, IL-1β and TNF-α from J774.1 cells (Figure 6A–C). Pre-incubation with P58 significantly decreased the release of IL-1β and IL-6, but not TNF-α, caused by LPS (Figure 6D–F). In contrast, pre-incubation with P58 significantly increased LTA-induced releases of IL-1β, IL-6, and TNF-α (Figure 6D–F).
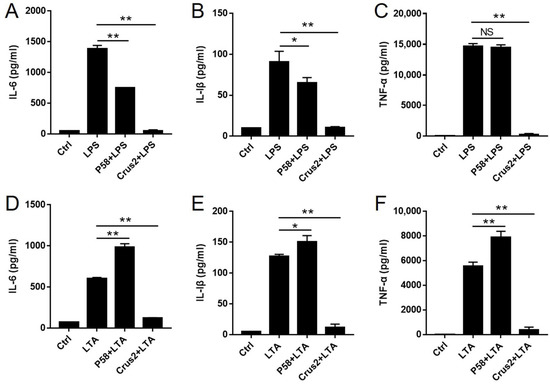
Figure 6.
The effects of Crus2 and P58 on LPS- and LTA-induced inflammatory response. (A–C) J774.1 cells were incubated with or without (Ctrl) lipopolysaccharide (LPS) that had been pre-incubated with or without Crus2 or P58 for 24 h. The release of inflammatory cytokines (IL-6, IL-1β, and TNF-α) was then detected. (D–F) J774.1 cells were incubated with or without (Ctrl) lipoteichoic acid (LTA) that had been pre-incubated with or without Crus2 or P58 for 24 h. The release of inflammatory cytokines (as above) was then detected by ELISA. Data are the means triplicate experiments and shown as ± SD. ** and * represent p < 0.01 and p < 0.05, respectively (Student’s t test).
3. Discussion
Crustins are important innate immune molecules that are widely present in crustaceans [23]. There are accumulating reports on the crustins from coastal or freshwater crustaceans, but studies on crustins from deep-sea environments are limited. Of the documented deep-sea crustins, a type II crustin was extracted from the hydrothermal vent shrimp Rimicaris exoculata, and two other crustins were obtained from the hydrothermal vent shrimp Alvinocaris longirostris [24,25]. Our previous study identified a Crus1 from Rimicaris sp. and classified it as a type I crustin [22]. In the present study, we analyzed another Rimicaris sp. crustin, Crus2. Different from Crus1, Crus2 has a relatively long C-terminal region following the WAP domain, and was therefore classified as a member of the newly identified Type Ib crustin. Interestingly, the eight Cys residues in the WAP domain of Crus2 match that of Type Ia crustins and form four conserved disulfide bonds, suggesting that the extra C-terminal P58 region does not affect the conserved structure of WAP.
Crustins are known to be antimicrobial peptides targeting a broad spectrum of microorganisms. Some reports showed that type I crustins exerted antimicrobial effects only on the bacteria of a Gram-positive nature [22,26,27], while type II crustins are effective antimicrobials against Gram-positive as well as Gram-negative bacteria [28,29]. In this study, we found that unlike the reported classical type I crustins, Crus2 inhibited or killed both Gram-positive and Gram-negative bacteria. In agreement with this observation, Crus2 bound well to the surface molecules of both Gram-positive and Gram-negative bacteria. Furthermore, pre-binding to PGN, LTA, or LPS significantly reduced the bactericidal effect of Crus2, suggesting that these cell wall components are involved in the interaction between Crus2 and its target bacteria.
The ability of AMPs to kill bacteria by breaking down the bacterial cytomembrane has been well demonstrated [30,31]. For some crustins, their antibacterial effects also involve destabilization of the bacterial membrane [13,22]. Similarly, we found that treatment with Crus2 rendered the target bacterial cells permeable by PI and DiSC3 (5), indicating decreased membrane integrity and perturbed membrane potential caused by Crus2. Electron microscopy revealed damaged bacterial structures and leakage of the cytoplasmic content. Together these results indicated that like most AMPs, Crus2 killed the target bacteria by inducing membrane disruption.
The WAP domain of crustin is a key functional structure required for the antimicrobial response, protease inhibition, and potential immunomodulatory activity of crustins [9,32]. A previous study showed that a synthetic WAP peptide displayed bactericidal activity [24]. Another report demonstrated that crustin lost its bactericidal activity when the disulfide bonds were broken or the conserved cysteine in the WAP domain was mutated [22]. In the present study, we found that the C91S-C102S mutant of Crus2 had little bactericidal activity but retained the bacteria binding capacity. These results indicated that, like other types of crustins, the Type Ib crustin also required the disulfide bonds in the WAP domain for effective bacteria killing.
Proteins with entirely disordered sequences are called intrinsically disordered proteins (IDPs) [33]. Most eukaryotic proteins possess structured and disordered regions, and both are required for the proper functioning of the proteins [33,34]. The Type Ib crustins are a recently established new subtype. They all feature a more than 30 aa C-terminal region that adopts a disordered structure similar to that of IDPs [17]. The biological activity of Type Ib crustin has not been reported, and, in particular, the function of the long C-terminal region is poorly understood. In our study, we found that the C-terminal P58 region in the form of a synthesized peptide bound well to bacteria and bacterial cell wall components. Moreover, the peptide P58 significantly reduced the bactericidal effect of Crus2, suggesting that the peptide probably competed with Crus2 for bacterial binding. Considering that Crus2 exhibited stronger bactericidal activity than Crus2DC, it was possible that in Crus2, the C-terminal P58 region functioned to enhance the binding between the protein and the target bacteria, thereby maximizing the bactericidal activity of Crus2.
In addition to their well-known antimicrobial activities, some AMPs are involved in the regulation of inflammatory responses [35]. For example, a Mozambique tilapia AMP (GRN-4) could enhance the innate immune response by up-regulating the expression of inflammatory cytokines [36]. EPI, an orange-spotted grouper AMP, modulated S. aureus LTA-induced inflammatory response [37]. However, to our knowledge, no reports on the immune regulation of crustins have been documented. In our study, we found that Crus2 significantly reduced the inflammatory response elicited by LPS and LTA, suggesting that crustins may not only kill invading microorganisms such as bactericides, but also participate in host immune regulation by neutralizing pathogenic factors.
In conclusion, our study showed that a deep-sea Type Ib crustin, Crus2, killed diverse bacteria, and this killing activity required the stable disulfide bond in the WAP domain. The Type Ib-typical C-terminal region was not essential to the antimicrobial activity of Crus2; however, via its ability to facilitate bacterial interaction, the C-terminal region was required for the optimal bactericidal effect. Furthermore, Crus2 could act as a modulator of inflammatory response by neutralizing LPS and LTA. These results add new insights into the immune function and mechanism of Type Ib crustins.
4. Materials and Methods
4.1. Bacterial Strains and Culture Conditions
The bacteria used in this study are listed in Table 1 and have been reported previously [20,22]. Briefly, Streptococcus iniae was cultured in tryptic soy broth (TSB) (Hopobio, Qingdao, China) at 28 °C; the deep-sea bacteria, i.e., Bacillus subtilis G7, Bacillus cereus H2, Bacillus wiedmannii SR52, Bacillus cereus MB1, and Marinobacter algicola, were grown at 28 °C in marine 2216E medium. Luria-Bertani broth (LB) medium was used for the growth of all other bacterial strains, and the growth was carried out at 37 °C (for Escherichia coli, Micrococcus luteus, and Staphylococcus aureus) or 28 °C (for Edwardsiella tarda, Vibrio harveyi, Vibrio anguillarum, and Pseudomonas fluorescens). For the bacteriostatic assay, S. iniae was cultured in TSB medium, while all other bacteria were cultured in Mueller-Hinton broth (MHB) (Hopobio, Qingdao, China).
4.2. Bioinformatics and Structural Characterization
Crus2 sequence (GenBank accession number MW448474) was analyzed with SignalP and InterPro to predict signal peptide and conserved domains, respectively. A sequence similarity analysis was performed using BLAST (NCBI). Sequence alignment and output were created with ClustalX 2.0 (SFI, Dublin, Ireland) and DNAMAN 6.0 (Lynnon Biosoft, San Ramon, CA, USA), respectively. The phylogenetic analysis was performed with the neighbor-joining (NJ) method using MEGA 6.0 (Mega Limited, Auckland, New Zealand), and the reliability was assessed by 1000 bootstraps. The structure models of Crus2 and Crus2DC (a Crus2 truncate that lacks the C-terminal P58 region) were constructed using the I-TASSER online server, and structural images were generated using the PyMOL Molecular Graphics System (Schrödinger, New York, NY, USA) (ver. 3.7).
4.3. Preparation of Recombinant Proteins
The coding sequence of Crus2 without signal peptide was synthesized by BGI Technology (Beijing, China). The Crus2DC sequence was obtained by PCR with the primer pair F1/R1 (Table S1) using Crus2 as the template. Crus2 and Crus2DC were ligated into pET28a (Sangon Biotech, Shanghai, China) at the Nde I/Xho I sites using ClonExpress II One Step Cloning Kit (Vazyme, Nanjing, China), yielding pETCrus2 and pETCrus2DC. E. coli Transetta (DE3) (TransGen Biotech, Beijing, China) was transformed with the plasmids. After growing in LB medium at 37 °C to OD600 0.6, the transformants were induced to express the recombinant proteins by adding isopropyl-beta-d-thiogalactoside (IPTG) (0.06 mM) into the culture. The bacterial growth was shifted to 16 °C and continued for 12 h. The cells were then collected by centrifugation and disrupted by sonication. Recombinant proteins were purified with Ni-NTA affinity columns (QIAGEN, Germantown, TN, USA) as reported previously [21,22]. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was applied to examine the proteins. A BCA Protein Assay Kit (Beyotime, Shanghai, China) was used to determine protein concentration. To prepare the Crus2 mutant bearing C91S and C102S substitution, reverse PCR was performed with the primers F2/ R2 (Table S1) using pETCrus2 as the template, resulting in the plasmid pETCrus2-C91S. The second PCR was performed with the primers F3/ R3 (Table S1) and pETCrus2-C91S as the template, resulting in the plasmid pETCrus2-C91SC102S. The purification of the Crus2 mutant protein was performed as described above for the purification of Crus2.
4.4. Peptide Synthesis
The peptide P58 was synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). The peptide was 5’-FITC-tagged and purified to >95% using high performance liquid chromatography. The peptides were dissolved in PBS (pH 7.4) prior to use.
4.5. Antibacterial Assay
Determination of the minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC) was performed as reported previously [38]. Briefly, bacteria were grown to OD600 0.5–0.6. The bacterial cells were resuspended in MHB to 105–106 CFU/mL in a 96-well microtiter plate. A two-fold dilution series (2–64 μM) of Crus2 or Crus2DC were each placed into the plate. The plate was incubated at 37 °C or 28 °C for 20–24 h, and the culture in the plate was diluted serially and plated on MH agar plates. The MIC was defined as the lowest concentration of Crus2 or Crus2DC that caused no visible bacterial growth to occur. For MBC, it was defined as the concentration of Crus2 or Crus2DC that killed 99.9% of the tested bacteria within an incubation period of 20–24 h. To examine the effect of bacterial components on the bactericidal activity of Crus2 and Crus2DC, Crus2 and Crus2DC were each pre-incubated with 200 μg/mL of PGN, LTA, or LPS (Sigma, St Louis, MO, USA) for 1 h and then incubated with bacteria for 2 h. The colonies of bacteria on the plate was counted as above. To evaluate the inhibitory effect of P58 on the bactericidal ability of Crus2, bacteria were first pretreated with 32 μM P58 or PBS (control) for 1 h at 28 °C, and then treated with 1 × MBC of Crus2 for 2 h at 28 °C. The growth of the bacteria was determined as described above.
4.6. Binding of Crus2 and Crus2DC to Bacteria
Protein binding to the bacteria and bacterial components was determined by ELISA as described previously [21]. Briefly, bacteria (108 CFU/mL) or bacterial cell wall components (PGN, LTA, or LPS, 200 μg/mL) were each added to a 96-well microtiter plate. The plate was incubated at 4 °C for 12–16 h, followed by washing in PBST (PBS with 0.05% Tween 20) three times. Two hundred microliters of 5% skim milk (Solarbio, Beijing, China) dissolved in PBST was added to the plate. After incubation at 37 °C for 2 h, the plate was washed as above. Two hundred microliters of Crus2, Crus2DC, or PBS (control) was added to the plate. After incubation at 37 °C for 1 h, the plate was washed as above. The bound protein was detected by adding anti-His antibody conjugated with HRP (1/1000 dilution) (ABclonal, Hubei, China) into the plate. After incubation at 37 °C for 1 h, the plate was washed five times with PBST. The TMB substrate solution (Tiangen, Beijing, China) was added to the plate for color development. ELISA stop solution was then added to the plate. The absorbance at 450 nm was measured using a multifunctional microplate reader. The binding index = OD450 of protein/OD450 of PBS.
4.7. PI staining Assay and Electron Microscopy
B. cereus MB1 and V. harveyi were cultured in corresponding media to OD600 of 0.8. The bacteria were resuspended in PBS to 1 × 106 CFU/mL. Crus2 or Crus2DC (1 × MBC) was added to the bacteria, and the mixture was incubated at 28 °C for 2 h. The cells were stained with a PI Staining Kit (BestBio, Shanghai, China) and examined using a fluorescence microscope (TiS/L100, Nikon, Tokyo, Japan). For electron microscopy, the above treated bacterial samples were processed as previously described [22] and observed with scanning electron microscopy (S-3400N, Hitachi, Tokyo, Japan) and transmission microscopy (HT7700, Hitachi, Tokyo, Japan).
4.8. Membrane Depolarization Assay
The cytoplasmic membrane depolarization induced by Crus2/Crus2DC was measured as reported previously [39]. Briefly, B. cereus MB1 and V. harveyi were cultured in corresponding media to OD600 0.6 and placed in a HEPES buffer (5 mM HEPES with 20 mM glucose and 100 mM KCI, pH 7.4) to OD600 0.05. The bacteria were then treated with DiSC3(5) (Macklin, Shanghai, China) (0.4 µM). After incubation in the dark for 30 min, the mixture was quenched at 22–25 °C. The samples were then placed into black 96-well microtiter plates. Crus2 (2 × MBC), Crus2DC (2 × MBC), valinomycin, or PBS (control) was added to the plates. Fluorescence change was recorded using a multifunctional microplate reader (620 nm and 670 nm for excitation and emission, respectively).
4.9. Pro-inflammatory Cytokine Detection
LPS and LTA (5 μg/mL) were each incubated with Crus2 or P58 (32 μM) or PBS (control) for 1 h at room temperature. J774.1 cells were cultured overnight in a 24-well plate (5 × 105 cells/well) and incubated with Crus2/P58-treated LPS/LTA or untreated LPS/LTA for 24 h at 37 °C. The culture supernatant was collected and used to measure IL-1β (EMC001b, Neobioscience, Shenzhen, China), IL-6 (SEKM0007, Solarbio, Beijing, China) and TNF-α (SEKM0034, Solarbio, Beijing, China) by ELISA according to the manufacturer’s instructions.
4.10. Statistical Analysis
Statistical analyses were carried out with GraphPad Prism 7 (GraphPad Software Inc, San Diego, CA, USA) and SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). For two groups, statistical significance was performed with a Student’s t test. All data are presented as mean ± SD. p < 0.05 was considered statistically significant.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms23126444/s1.
Author Contributions
L.S. and Y.-J.W. conceived and designed the research work. Y.-J.W. conducted the experiments and analyzed the data. Y.-J.W. wrote the first draft of the manuscript. L.S. edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the grants from the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA22050403), and the Taishan Scholar Program of Shandong Province.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are included in the article or its Supplementary File.
Acknowledgments
We thank Jian Zhang for assisting sequence analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Izadpanah, A.; Gallo, R.L. Antimicrobial peptides. J. Am. Acad. Dermatol. 2005, 52, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Haney, E.F.; Vogel, H.J. The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol. 2011, 29, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Papo, N.; Shai, Y. Can we predict biological activity of antimicrobial peptides from their interactions with model phospholipid membranes? Peptides 2003, 24, 1693–1703. [Google Scholar] [CrossRef] [PubMed]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Hadley, E.B.; Hancock, R.E. Strategies for the discovery and advancement of novel cationic antimicrobial peptides. Curr. Top. Med. Chem. 2010, 10, 1872–1881. [Google Scholar] [CrossRef]
- Omardien, S.; Brul, S.; Zaat, S.A. Antimicrobial Activity of Cationic Antimicrobial Peptides against Gram-Positives: Current Progress Made in Understanding the Mode of Action and the Response of Bacteria. Front. Cell Dev. Biol. 2016, 4, 111. [Google Scholar] [CrossRef]
- Tennessen, J.A. Molecular evolution of animal antimicrobial peptides: Widespread moderate positive selection. J. Evol. Biol. 2005, 18, 1387–1394. [Google Scholar] [CrossRef]
- Bandeira, P.T.; Vernal, J.; Matos, G.M.; Farias, N.D.; Terenzi, H.; Pinto, A.R.; Barracco, M.A.; Rosa, R.D. A Type IIa crustin from the pink shrimp Farfantepenaeus paulensis (crusFpau) is constitutively synthesized and stored by specific granule-containing hemocyte subpopulations. Fish Shellfish. Immunol. 2020, 97, 294–299. [Google Scholar] [CrossRef]
- Smith, V.J.; Fernandes, J.M.; Kemp, G.D.; Hauton, C. Crustins: Enigmatic WAP domain-containing antibacterial proteins from crustaceans. Dev. Comp. Immunol. 2008, 32, 758–772. [Google Scholar] [CrossRef] [Green Version]
- Ranganathan, S.; Simpson, K.J.; Shaw, D.C.; Nicholas, K.R. The whey acidic protein family: A new signature motif and three-dimensional structure by comparative modeling. J. Mol. Graph. Model. 1999, 17, 106–113. [Google Scholar] [CrossRef]
- Fjell, C.D.; Hiss, J.A.; Hancock, R.E.; Schneider, G. Designing antimicrobial peptides: Form follows function. Nat. Rev. Drug Discov. 2011, 11, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.F.; Wang, J.X. The antimicrobial peptides of the immune response of shrimp. Invertebr. Surviv. J. 2008, 5, 4. [Google Scholar]
- Krusong, K.; Poolpipat, P.; Supungul, P.; Tassanakajon, A. A comparative study of antimicrobial properties of crustinPm1 and crustinPm7 from the black tiger shrimp Penaeus monodon. Dev. Comp. Immunol. 2012, 36, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Amparyup, P.; Kondo, H.; Hirono, I.; Aoki, T.; Tassanakajon, A. Molecular cloning, genomic organization and recombinant expression of a crustin-like antimicrobial peptide from black tiger shrimp Penaeus monodon. Mol. Immunol. 2008, 45, 1085–1093. [Google Scholar] [CrossRef]
- Arockiaraj, J.; Gnanam, A.J.; Muthukrishnan, D.; Gudimella, R.; Milton, J.; Singh, A.; Muthupandian, S.; Kasi, M.; Bhassu, S. Crustin, a WAP domain containing antimicrobial peptide from freshwater prawn Macrobrachium rosenbergii: Immune characterization. Fish Shellfish. Immunol. 2013, 34, 109–118. [Google Scholar] [CrossRef]
- Tassanakajon, A.; Somboonwiwat, K.; Amparyup, P. Sequence diversity and evolution of antimicrobial peptides in invertebrates. Dev. Comp. Immunol. 2015, 48, 324–341. [Google Scholar] [CrossRef]
- Li, S.; Lv, X.; Yu, Y.; Zhang, X.; Li, F. Molecular and Functional Diversity of Crustin-Like Genes in the Shrimp Litopenaeus vannamei. Mar. Drugs 2020, 18, 361. [Google Scholar] [CrossRef]
- Thornburg, C.C.; Zabriskie, T.M.; McPhail, K.L. Deep-sea hydrothermal vents: Potential hot spots for natural products discovery? J. Nat. Prod. 2010, 73, 489–499. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Q.L.; Luan, Z.D.; Lian, C.; Sun, L. Comparative transcriptome analysis of Rimicaris sp. reveals novel molecular features associated with survival in deep-sea hydrothermal vent. Sci. Rep. 2017, 7, 2000. [Google Scholar] [CrossRef]
- Gu, H.J.; Sun, Q.L.; Jiang, S.; Zhang, J.; Sun, L. First characterization of an anti-lipopolysaccharide factor (ALF) from hydrothermal vent shrimp: Insights into the immune function of deep-sea crustacean ALF. Dev. Comp. Immunol. 2018, 84, 382–395. [Google Scholar] [CrossRef]
- Luo, J.C.; Zhang, J.; Sun, L. A g-Type Lysozyme from Deep-Sea Hydrothermal Vent Shrimp Kills Selectively Gram-Negative Bacteria. Molecules 2021, 26, 7624. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, J.; Sun, Y.; Sun, L. A Crustin from Hydrothermal Vent Shrimp: Antimicrobial Activity and Mechanism. Mar. Drugs 2021, 19, 176. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kim, M.; Kim, A.R.; Yi, M.; Choi, J.H.; Park, H.; Park, W.; Kim, H.W. Differences in gene organization between type I and type II crustins in the morotoge shrimp, Pandalopsis japonica. Fish Shellfish. Immunol. 2013, 35, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.L.; Wang, S.L.; Zhu, F.C.; Xue, F.; He, L.S. Characteristics of Two Crustins from Alvinocaris longirostris in Hydrothermal Vents. Mar. Drugs 2021, 19, 600. [Google Scholar] [CrossRef] [PubMed]
- Le Bloa, S.; Boidin-Wichlacz, C.; Cueff-Gauchard, V.; Rosa, R.D.; Cuvillier-Hot, V.; Durand, L.; Methou, P.; Pradillon, F.; Cambon-Bonavita, M.A.; Tasiemski, A. Antimicrobial Peptides and Ectosymbiotic Relationships: Involvement of a Novel Type IIa Crustin in the Life Cycle of a Deep-Sea Vent Shrimp. Front. Immunol. 2020, 11, 1511. [Google Scholar] [CrossRef]
- Donpudsa, S.; Rimphanitchayakit, V.; Tassanakajon, A.; Söderhäll, I.; Söderhäll, K. Characterization of two crustin antimicrobial peptides from the freshwater crayfish Pacifastacus leniusculus. J. Invertebr. Pathol. 2010, 104, 234–238. [Google Scholar] [CrossRef]
- Supungul, P.; Tang, S.; Maneeruttanarungroj, C.; Rimphanitchayakit, V.; Hirono, I.; Aoki, T.; Tassanakajon, A. Cloning, expression and antimicrobial activity of crustinPm1, a major isoform of crustin, from the black tiger shrimp Penaeus monodon. Dev. Comp. Immunol. 2008, 32, 61–70. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Wang, H.; Ma, H.; Huang, Y.Q.; Lu, J.X.; Li, X.C.; Zhang, X.W. Involvement of a newly identified atypical type II crustin (SpCrus5) in the antibacterial immunity of mud crab Scylla paramamosain. Fish Shellfish. Immunol. 2018, 75, 346–356. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.X.; Wang, Y.; Fang, W.H.; Wang, Y.; Zhou, J.F.; Zhao, S.; Li, X.C. Newly identified type II crustin (SpCrus2) in Scylla paramamosain contains a distinct cysteine distribution pattern exhibiting broad antimicrobial activity. Dev. Comp. Immunol. 2018, 84, 1–13. [Google Scholar] [CrossRef]
- Scocchi, M.; Mardirossian, M.; Runti, G.; Benincasa, M. Non-Membrane Permeabilizing Modes of Action of Antimicrobial Peptides on Bacteria. Curr. Top. Med. Chem. 2016, 16, 76–88. [Google Scholar] [CrossRef]
- Malanovic, N.; Lohner, K. Antimicrobial Peptides Targeting Gram-Positive Bacteria. Pharmaceuticals 2016, 9, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, Y.P.; Sun, Y.D.; Wang, Z.H.; Wang, Q.; Wang, X.W.; Zhao, X.F.; Wang, J.X. A single whey acidic protein domain (SWD)-containing peptide from fleshy prawn with antimicrobial and proteinase inhibitory activities. Aquaculture 2008, 284, 246–259. [Google Scholar] [CrossRef]
- van der Lee, R.; Buljan, M.; Lang, B.; Weatheritt, R.J.; Daughdrill, G.W.; Dunker, A.K.; Fuxreiter, M.; Gough, J.; Gsponer, J.; Jones, D.T.; et al. Classification of intrinsically disordered regions and proteins. Chem. Rev. 2014, 114, 6589–6631. [Google Scholar] [CrossRef] [PubMed]
- Dunker, A.K.; Babu, M.M.; Barbar, E.; Blackledge, M.; Bondos, S.E.; Dosztányi, Z.; Dyson, H.J.; Forman-Kay, J.; Fuxreiter, M.; Gsponer, J.; et al. What’s in a name? Why these proteins are intrinsically disordered: Why these proteins are intrinsically disordered. Intrinsically Disord. Proteins 2013, 1, e24157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.J.; Gallo, R.L. Antimicrobial peptides. Curr. Biol. CB 2016, 26, R14–R19. [Google Scholar] [CrossRef]
- Wu, S.H.; Lin, H.J.; Lin, W.F.; Wu, J.L.; Gong, H.Y. A potent tilapia secreted granulin peptide enhances the survival of transgenic zebrafish infected by Vibrio vulnificus via modulation of innate immunity. Fish Shellfish. Immunol. 2018, 75, 74–90. [Google Scholar] [CrossRef]
- Su, B.C.; Chen, J.Y. Epinecidin-1: An orange-spotted grouper antimicrobial peptide that modulates Staphylococcus aureus lipoteichoic acid-induced inflammation in macrophage cells. Fish Shellfish. Immunol. 2020, 99, 362–367. [Google Scholar] [CrossRef]
- Essig, A.; Hofmann, D.; Münch, D.; Gayathri, S.; Künzler, M.; Kallio, P.T.; Sahl, H.G.; Wider, G.; Schneider, T.; Aebi, M. Copsin, a novel peptide-based fungal antibiotic interfering with the peptidoglycan synthesis. J. Biol. Chem. 2014, 289, 34953–34964. [Google Scholar] [CrossRef] [Green Version]
- Bellemare, A.; Vernoux, N.; Morin, S.; Gagné, S.M.; Bourbonnais, Y. Structural and antimicrobial properties of human pre-elafin/trappin-2 and derived peptides against Pseudomonas aeruginosa. BMC Microbiol. 2010, 10, 253. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).