Targeting of Glycosaminoglycans in Genetic and Inflammatory Airway Disease
Abstract
1. Introduction
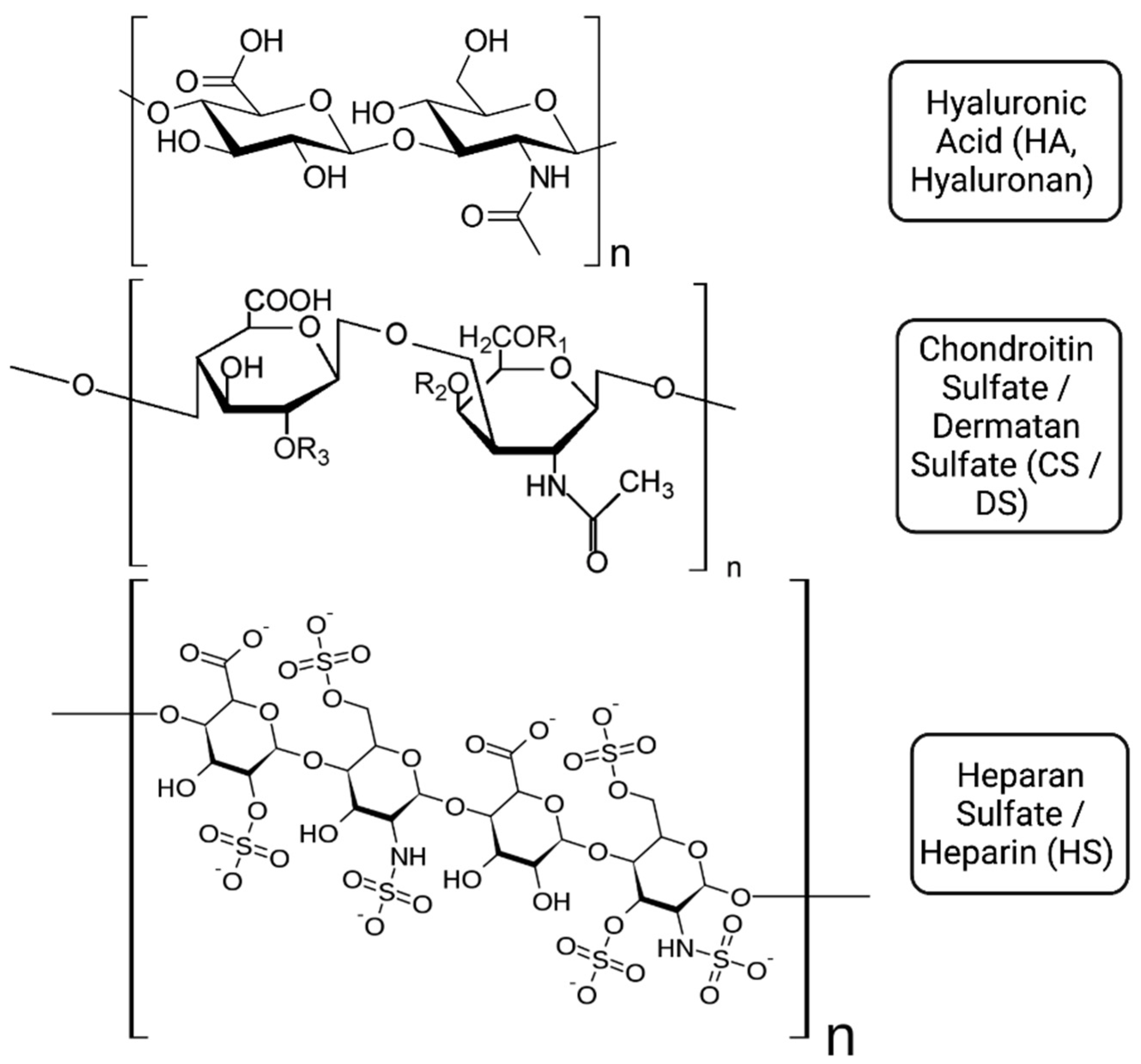
2. Glycosaminoglycans in the Airways: Physical Properties
3. Biological Roles and Protein Interactions of Glycosaminoglycans
3.1. GAGs and Pro-Inflammatory Protein Interactions
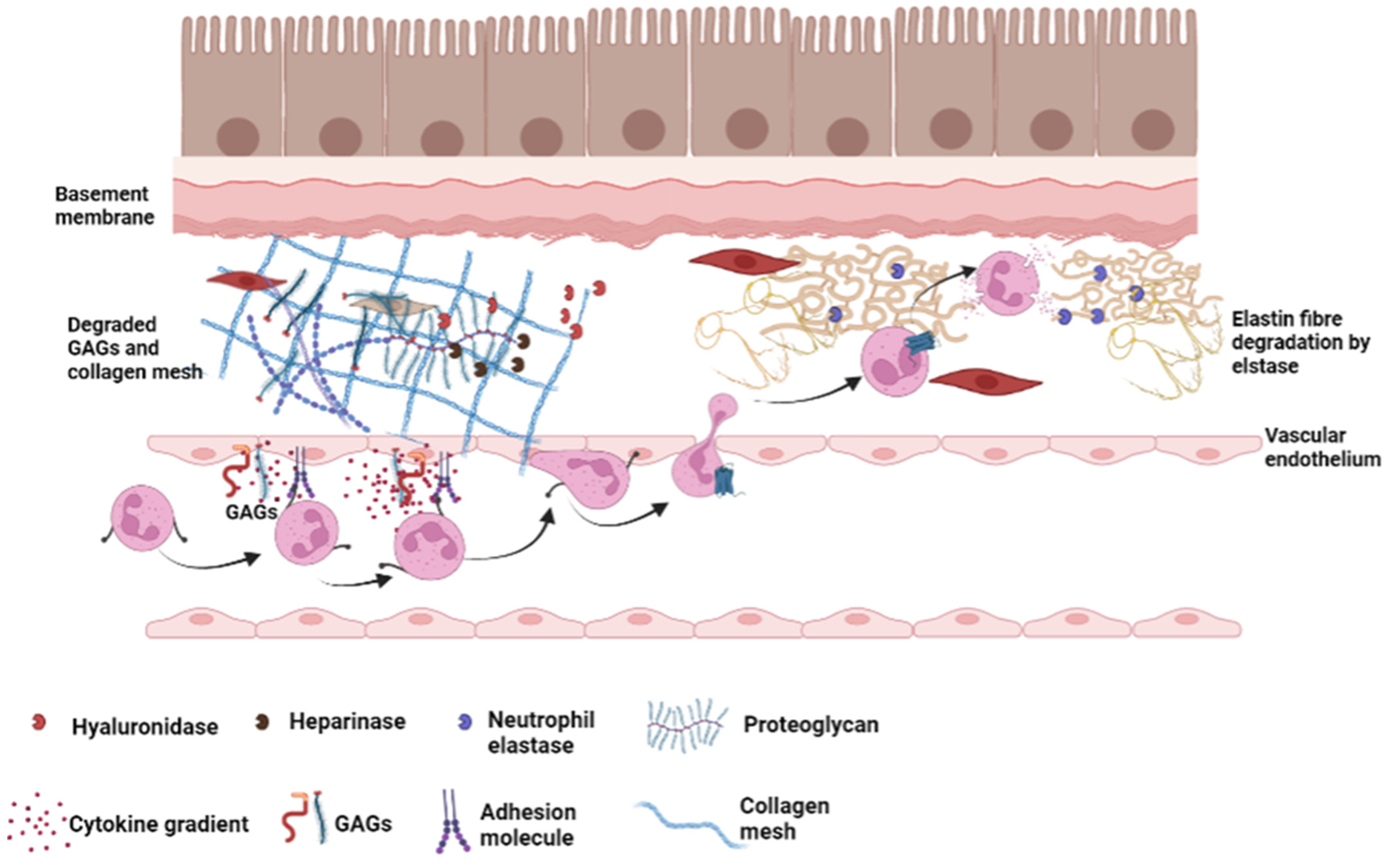
3.2. GAG Proteases Interactions
4. The Role of Glycosaminoglycans in Airways Disease
4.1. An Introduction to Cystic Fibrosis
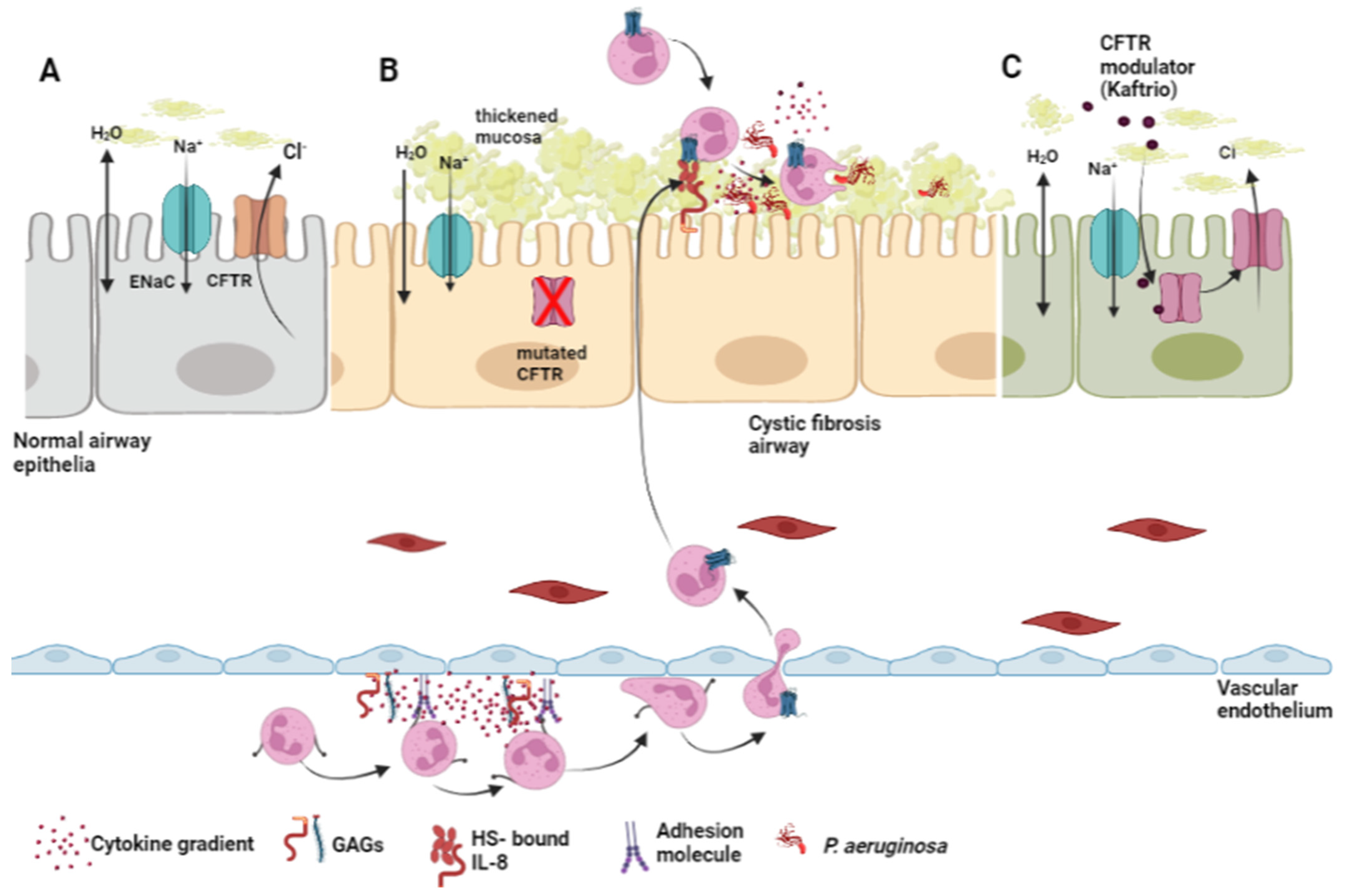
4.1.1. The Role of Glycosaminoglycans in the Pathology of Cystic Fibrosis
4.1.2. Glycosaminoglycans and the Management of Cystic Fibrosis
4.2. Glycosaminoglycans in Chronic Obstructive Pulmonary Disease
4.3. Glycosaminoglycans in Asthma
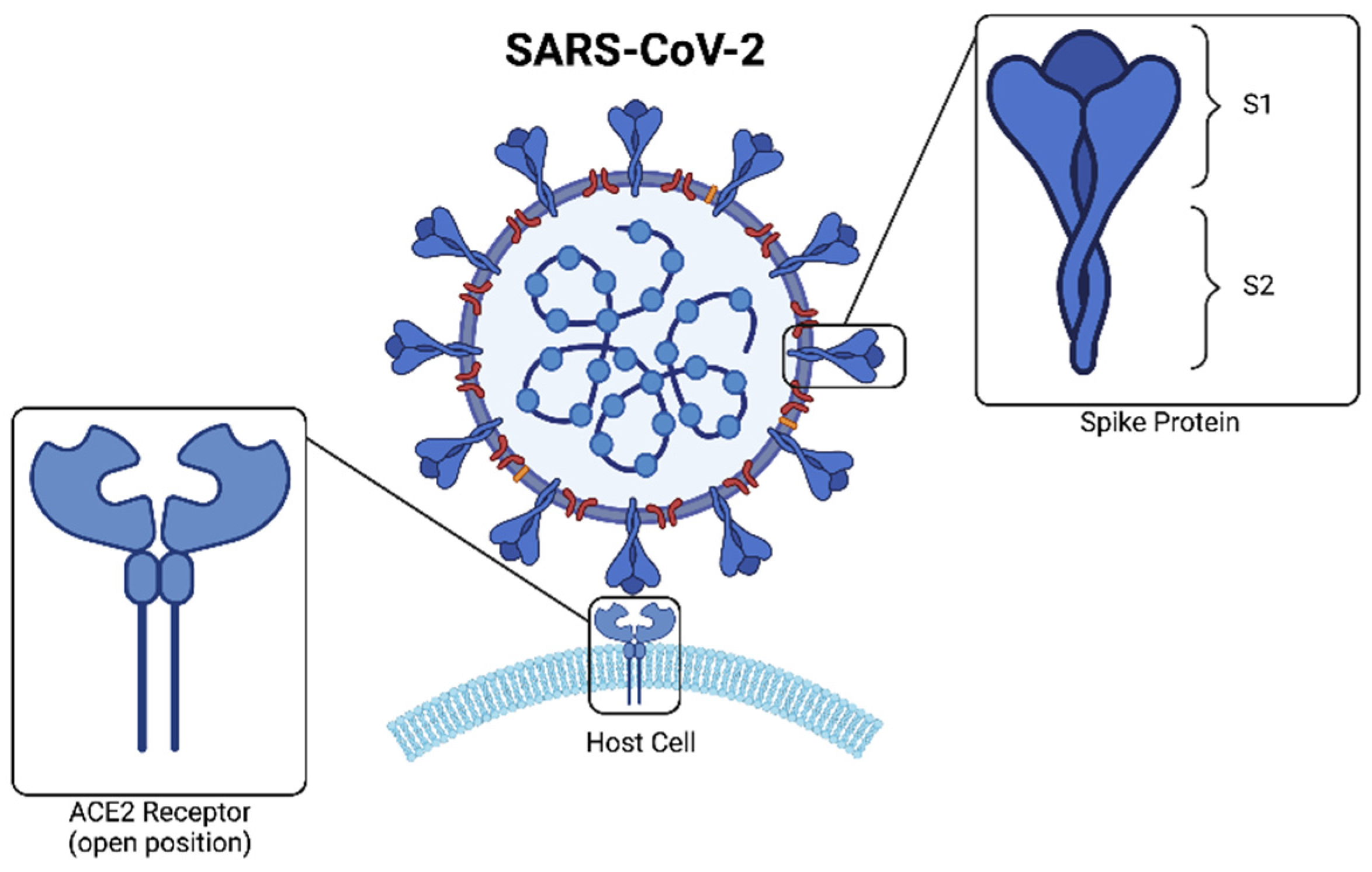
4.4. Glycosaminoglycans in COVID-19
Glycosaminoglycans and the Potential Management of COVID
4.5. Glycosaminoglycans and Non-Infectious Lung Injury
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmed, A.U. An overview of inflammation: Mechanism and consequences. Front. Biol. 2011, 6, 274–281. [Google Scholar] [CrossRef]
- Aghasafari, P.; George, U.; Pidaparti, R. A review of inflammatory mechanism in airway diseases. Inflamm. Res. 2019, 68, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cao, X.; Guo, M.; Xie, M.; Liu, X. Trends and risk factors of mortality and disability adjusted life years for chronic respiratory diseases from 1990 to 2017: Systematic analysis for the Global Burden of Disease Study 2017. BMJ 2020, 368, m234. [Google Scholar] [CrossRef] [PubMed]
- Garth, J.; Barnes, J.W.; Krick, S. Targeting Cytokines as Evolving Treatment Strategies in Chronic Inflammatory Airway Diseases. Int. J. Mol. Sci. 2018, 19, 3402. [Google Scholar] [CrossRef]
- Walma, D.A.C.; Yamada, K.M. The extracellular matrix in development. Development 2020, 147, dev175596. [Google Scholar] [CrossRef]
- Kamhi, E.; Joo, E.J.; Dordick, J.S.; Linhardt, R.J. Glycosaminoglycans in infectious disease. Biol. Rev. Camb. Philos. Soc. 2013, 88, 928–943. [Google Scholar] [CrossRef]
- Zimmermann, P.; Zhang, Z.; Degeest, G.; Mortier, E.; Leenaerts, I.; Coomans, C.; Schulz, J.; N’Kuli, F.; Courtoy, P.J.; David, G.J.D.c. Syndecan recyling is controlled by syntenin-PIP2 interaction and Arf6. Dev. Cell 2005, 9, 377–388. [Google Scholar] [CrossRef]
- Thompson, S.; Martinez-Burgo, B.; Sepuru, K.M.; Rajarathnam, K.; Kirby, J.A.; Sheerin, N.S.; Ali, S. Regulation of Chemokine Function: The Roles of GAG-Binding and Post-Translational Nitration. Int. J. Mol. Sci. 2017, 18, 1692. [Google Scholar] [CrossRef]
- Roberts, M.B.V. Biology: A Functional Approach; Nelson: Cheltenham, UK, 1986. [Google Scholar]
- Lin, C.Q.; Bissell, M.J. Multi-faceted regulation of cell differentiation by extracellular matrix. FASEB J. 1993, 7, 737–743. [Google Scholar] [CrossRef]
- Sasisekharan, R.; Raman, R.; Prabhakar, V. Glycomics approach to structure-function relationships of glycosaminoglycans. Annu. Rev. Biomed. Eng. 2006, 8, 181–231. [Google Scholar] [CrossRef]
- Morla, S. Glycosaminoglycans and Glycosaminoglycan Mimetics in Cancer and Inflammation. Int. J. Mol. Sci. 2019, 20, 1963. [Google Scholar] [CrossRef] [PubMed]
- Wight, T.N. A role for proteoglycans in vascular disease. Matrix Biol. 2018, 71–72, 396–420. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Zhang, F.; Linhardt, R.J. Glycosaminoglycans in Neurodegenerative Diseases. Adv. Exp. Med. Biol. 2021, 1325, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Voynow, J.A.; Zheng, S.; Kummarapurugu, A.B. Glycosaminoglycans as Multifunctional Anti-Elastase and Anti-Inflammatory Drugs in Cystic Fibrosis Lung Disease. Front. Pharmacol. 2020, 11, 1011. [Google Scholar] [CrossRef] [PubMed]
- Hallgren, O.; Nybom, A.; Zhou, X.-H.; Dellgren, G.; Bjermer, L.; Westergren-Thorsson, G. Disease-Specific Alterations in Glycosaminoglycans in IPF and COPD. Am. J. Respir. Crit. Care Med. 2018, 197, A5756. [Google Scholar]
- Martin, C.; Lozano-Iturbe, V.; Giron, R.M.; Vazquez-Espinosa, E.; Rodriguez, D.; Merayo-Lloves, J.; Vazquez, F.; Quiros, L.M.; Garcia, B. Glycosaminoglycans are differentially involved in bacterial binding to healthy and cystic fibrosis lung cells. J. Cyst. Fibros 2019, 18, e19–e25. [Google Scholar] [CrossRef] [PubMed]
- Sampson, P.M.; Boyd, R.B.; Pietra, G.G.; Fishman, A.P. Glycosaminoglycan biosynthesis in the isolated perfused rat lung. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1984, 57, 1648–1654. [Google Scholar] [CrossRef]
- Mulloy, B.; Rider, C.C. Cytokines and proteoglycans: An introductory overview. Biochemical. Soc. Trans. 2006, 34, 409–413. [Google Scholar] [CrossRef]
- Whitelock, J.M.; Iozzo, R.V. Heparan sulfate: A complex polymer charged with biological activity. Chem. Rev. 2005, 105, 2745–2764. [Google Scholar] [CrossRef]
- Weiss, R.J.; Esko, J.D.; Tor, Y. Targeting heparin and heparan sulfate protein interactions. Org. Biomol. Chem. 2017, 15, 5656–5668. [Google Scholar] [CrossRef]
- Fraser, J.R.; Laurent, T.C.; Laurent, U.B. Hyaluronan: Its nature, distribution, functions and turnover. J. Intern. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Souza-Fernandes, A.B.; Pelosi, P.; Rocco, P.R. Bench-to-bedside review: The role of glycosaminoglycans in respiratory disease. Crit. Care 2006, 10, 237. [Google Scholar] [CrossRef] [PubMed]
- Handel, T.M.; Johnson, Z.; Crown, S.E.; Lau, E.K.; Proudfoot, A.E. Regulation of protein function by glycosaminoglycans--as exemplified by chemokines. Annu. Rev. Biochem. 2005, 74, 385–410. [Google Scholar] [CrossRef] [PubMed]
- Gerdin, B.; Hallgren, R. Dynamic role of hyaluronan (HYA) in connective tissue activation and inflammation. J. Intern. Med. 1997, 242, 49–55. [Google Scholar] [CrossRef]
- Cantor, J.O.; Cerreta, J.M.; Armand, G.; Turino, G.M. Aerosolized hyaluronic acid decreases alveolar injury induced by human neutrophil elastase. Proc. Soc. Exp. Biol. Med. 1998, 217, 471–475. [Google Scholar] [CrossRef]
- Skold, C.M.; Blaschke, E.; Eklund, A. Transient increases in albumin and hyaluronan in bronchoalveolar lavage fluid after quitting smoking: Possible signs of reparative mechanisms. Respir. Med. 1996, 90, 523–529. [Google Scholar] [CrossRef][Green Version]
- Lu, K.W.; Goerke, J.; Clements, J.A.; Taeusch, H.W. Hyaluronan reduces surfactant inhibition and improves rat lung function after meconium injury. Pediatr. Res. 2005, 58, 206–210. [Google Scholar] [CrossRef]
- Sawant, K.V.; Sepuru, K.M.; Lowry, E.; Penaranda, B.; Frevert, C.W.; Garofalo, R.P.; Rajarathnam, K. Neutrophil recruitment by chemokines Cxcl1/KC and Cxcl2/MIP2: Role of Cxcr2 activation and glycosaminoglycan interactions. J. Leukoc. Biol. 2021, 109, 777–791. [Google Scholar] [CrossRef]
- Hillyer, P.; Male, D. Expression of chemokines on the surface of different human endothelia. Immunol. Cell Biol. 2005, 83, 375–382. [Google Scholar] [CrossRef]
- Rot, A. Endothelial cell binding of NAP-1/IL-8: Role in neutrophil emigration. Immunol. Today 1992, 13, 291–294. [Google Scholar] [CrossRef]
- Middleton, J.; Neil, S.; Wintle, J.; Clark-Lewis, I.; Moore, H.; Lam, C.; Auer, M.; Hub, E.; Rot, A. Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell 1997, 91, 385–395. [Google Scholar] [CrossRef]
- Proudfoot, A.E. The biological relevance of chemokine-proteoglycan interactions. Biochem. Soc. Trans. 2006, 34, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Proudfoot, A.E.; Handel, T.M.; Johnson, Z.; Lau, E.K.; LiWang, P.; Clark-Lewis, I.; Borlat, F.; Wells, T.N.; Kosco-Vilbois, M.H. Glycosaminoglycan binding and oligomerization are essential for the in vivo activity of certain chemokines. Proc. Natl. Acad. Sci. USA 2003, 100, 1885–1890. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.R.; Hyduk, S.J.; Cybulsky, M.I. Chemoattractants induce a rapid and transient upregulation of monocyte alpha4 integrin affinity for vascular cell adhesion molecule 1 which mediates arrest: An early step in the process of emigration. J. Exp. Med. 2001, 193, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Constantin, G.; Majeed, M.; Giagulli, C.; Piccio, L.; Kim, J.Y.; Butcher, E.C.; Laudanna, C. Chemokines trigger immediate beta2 integrin affinity and mobility changes: Differential regulation and roles in lymphocyte arrest under flow. Immunity 2000, 13, 759–769. [Google Scholar] [CrossRef]
- Cinamon, G.; Shinder, V.; Alon, R. Shear forces promote lymphocyte migration across vascular endothelium bearing apical chemokines. Nat. Immunol. 2001, 2, 515–522. [Google Scholar] [CrossRef]
- Smits, N.C.; Robbesom, A.A.; Versteeg, E.M.; van de Westerlo, E.M.; Dekhuijzen, P.N.; van Kuppevelt, T.H. Heterogeneity of heparan sulfates in human lung. Am. J. Respir. Cell Mol. Biol. 2004, 30, 166–173. [Google Scholar] [CrossRef]
- Bao, X.; Moseman, E.A.; Saito, H.; Petryniak, B.; Thiriot, A.; Hatakeyama, S.; Ito, Y.; Kawashima, H.; Yamaguchi, Y.; Lowe, J.B.; et al. Endothelial heparan sulfate controls chemokine presentation in recruitment of lymphocytes and dendritic cells to lymph nodes. Immunity 2010, 33, 817–829. [Google Scholar] [CrossRef]
- Webb, L.M.; Ehrengruber, M.U.; Clark-Lewis, I.; Baggiolini, M.; Rot, A. Binding to heparan sulfate or heparin enhances neutrophil responses to interleukin 8. Proc. Natl. Acad. Sci. USA 1993, 90, 7158–7162. [Google Scholar] [CrossRef]
- Frevert, C.W.; Kinsella, M.G.; Vathanaprida, C.; Goodman, R.B.; Baskin, D.G.; Proudfoot, A.; Wells, T.N.; Wight, T.N.; Martin, T.R. Binding of interleukin-8 to heparan sulfate and chondroitin sulfate in lung tissue. Am. J. Respir. Cell Mol. Biol. 2003, 28, 464–472. [Google Scholar] [CrossRef]
- Hoogewerf, A.J.; Kuschert, G.S.; Proudfoot, A.E.; Borlat, F.; Clark-Lewis, I.; Power, C.A.; Wells, T.N. Glycosaminoglycans mediate cell surface oligomerization of chemokines.s. Biochemistry 1997, 36, 13570–13578. [Google Scholar] [CrossRef] [PubMed]
- Breuer, O.; Caudri, D.; Stick, S.; Turkovic, L. Predicting disease progression in cystic fibrosis. Expert Rev. Respir. Med. 2018, 12, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Kummarapurugu, A.B.; Afosah, D.K.; Sankaranarayanan, N.V.; Gangji, R.N.; Zheng, S.; Kennedy, T.; Rubin, B.K.; Voynow, J.A.; Umesh, R.D. Molecular principles for heparin oligosaccharide-based inhibition of neutrophil elastase in cystic fibrosis. J. Biol. Chem. 2018, 10, 12480–12490. [Google Scholar] [CrossRef] [PubMed]
- Clancy, D.M.; Sullivan, G.P.; Moran, H.B.T.; Henry, C.M.; Reeves, E.P.; McElvaney, N.G.; Lavelle, E.C.; Martin, S.J. Extracellular Neutrophil Proteases Are Efficient Regulators of IL-1, IL-33, and IL-36 Cytokine Activity but Poor Effectors of Microbial Killing. Cell Rep. 2018, 22, 2937–2950. [Google Scholar] [CrossRef]
- Cosgrove, S.; Chotirmall, S.H.; Greene, C.M.; McElvaney, N.G. Pulmonary proteases in the cystic fibrosis lung induce interleukin 8 expression from bronchial epithelial cells via a heme/meprin/epidermal growth factor receptor/Toll-like receptor pathway. J. Biol. Chem. 2011, 286, 7692–7704. [Google Scholar] [CrossRef]
- Kuschert, G.S.; Coulin, F.; Power, C.A.; Proudfoot, A.E.; Hubbard, R.E.; Hoogewerf, A.J.; Wells, T.N. Glycosaminoglycans interact selectively with chemokines and modulate receptor binding and cellular responses. Biochemistry 1999, 38, 12959–12968. [Google Scholar] [CrossRef]
- Metzemaekers, M.; Mortier, A.; Janssens, R.; Boff, D.; Vanbrabant, L.; Lamoen, N.; Van Damme, J.; Teixeira, M.M.; De Meester, I.; Amaral, F.A.; et al. Glycosaminoglycans Regulate CXCR3 Ligands at Distinct Levels: Protection against Processing by Dipeptidyl Peptidase IV/CD26 and Interference with Receptor Signaling. Int. J. Mol. Sci. 2017, 18, 1513. [Google Scholar] [CrossRef]
- Tissue destruction by neutrophils. N. Engl. J. Med. 1989, 321, 327–329. [CrossRef]
- Janoff, A. Elastase in tissue injury. Annu. Rev. Med. 1985, 36, 207–216. [Google Scholar] [CrossRef]
- Walsh, R.L.; Dillon, T.J.; Scicchitano, R.; McLennan, G. Heparin and heparan sulphate are inhibitors of human leucocyte elastase. Clin. Sci. 1991, 81, 341–346. [Google Scholar] [CrossRef]
- Buczek-Thomas, J.A.; Nugent, M.A. Elastase-mediated release of heparan sulfate proteoglycans from pulmonary fibroblast cultures. A mechanism for basic fibroblast growth factor (bFGF) release and attenuation of bfgf binding following elastase-induced injury. J. Biol. Chem. 1999, 274, 25167–25172. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.L.; Stone, P.J.; Nugent, M.A. New insights into the inhibition of human neutrophil elastase by heparin. Biochemistry 2006, 45, 9104–9120. [Google Scholar] [CrossRef] [PubMed]
- Shastri, M.D.; Peterson, G.M.; Stewart, N.; Sohal, S.S.; Patel, R.P. Non-anticoagulant derivatives of heparin for the management of asthma: Distant dream or close reality? Expert Opin. Investig. Drugs 2014, 23, 357–373. [Google Scholar] [CrossRef] [PubMed]
- Sissi, C.; Lucatello, L.; Naggi, A.; Torri, G.; Palumbo, M. Interactions of low-molecular-weight semi-synthetic sulfated heparins with human leukocyte elastase and human Cathepsin G. Biochem. Pharmacol. 2006, 71, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Ying, Q.L.; Kemme, M.; Saunders, D.; Simon, S.R. Glycosaminoglycans regulate elastase inhibition by oxidized secretory leukoprotease inhibitor. Am. J. Physiol. 1997, 272, L533–L541. [Google Scholar] [CrossRef] [PubMed]
- Cutting, G.R. Cystic fibrosis genetics: From molecular understanding to clinical application. Nat. Rev. Genet. 2015, 16, 45–56. [Google Scholar] [CrossRef]
- Laube, B.L.; Carson, K.A.; Evans, C.M.; Richardson, V.L.; Sharpless, G.; Zeitlin, P.L.; Mogayzel, P.J., Jr. Changes in mucociliary clearance over time in children with cystic fibrosis. Pediatr. Pulmonol. 2020, 55, 2307–2314. [Google Scholar] [CrossRef]
- Stallings, V.A.; Stark, L.J.; Robinson, K.A.; Feranchak, A.P.; Quinton, H.; Clinical Practice Guidelines on Growth and Nutrition Subcommittee; Ad Hoc Working Group. Evidence-based practice recommendations for nutrition-related management of children and adults with cystic fibrosis and pancreatic insufficiency: Results of a systematic review. J. Am. Diet Assoc. 2008, 108, 832–839. [Google Scholar] [CrossRef]
- McKone, E.F.; Borowitz, D.; Drevinek, P.; Griese, M.; Konstan, M.W.; Wainwright, C.; Ratjen, F.; Sermet-Gaudelus, I.; Plant, B.; Munck, A.; et al. Long-term safety and efficacy of ivacaftor in patients with cystic fibrosis who have the Gly551Asp-CFTR mutation: A phase 3, open-label extension study (PERSIST). Lancet Respir. Med. 2014, 2, 902–910. [Google Scholar] [CrossRef]
- Ramsey, B.W.; Davies, J.; McElvaney, N.G.; Tullis, E.; Bell, S.C.; Drevinek, P.; Griese, M.; McKone, E.F.; Wainwright, C.E.; Konstan, M.W.; et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N. Engl. J. Med. 2011, 365, 1663–1672. [Google Scholar] [CrossRef]
- Rosenfeld, M.; Wainwright, C.E.; Higgins, M.; Wang, L.T.; McKee, C.; Campbell, D.; Tian, S.; Schneider, J.; Cunningham, S.; Davies, J.C.; et al. Ivacaftor treatment of cystic fibrosis in children aged 12 to <24 months and with a CFTR gating mutation (ARRIVAL): A phase 3 single-arm study. Lancet Respir. Med. 2018, 6, 545–553. [Google Scholar] [CrossRef]
- Middleton, P.G.; Mall, M.A.; Drevinek, P.; Lands, L.C.; McKone, E.F.; Polineni, D.; Ramsey, B.W.; Taylor-Cousar, J.L.; Tullis, E.; Vermeulen, F.; et al. Elexacaftor-Tezacaftor-Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele. N. Engl. J. Med. 2019, 381, 1809–1819. [Google Scholar] [CrossRef] [PubMed]
- Reeves, E.P.; Williamson, M.; O’Neill, S.J.; Greally, P.; McElvaney, N.G. Nebulized hypertonic saline decreases IL-8 in sputum of patients with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2011, 183, 1517–1523. [Google Scholar] [CrossRef] [PubMed]
- Solic, N.; Wilson, J.; Wilson, S.J.; Shute, J.K. Endothelial activation and increased heparan sulfate expression in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2005, 172, 892–898. [Google Scholar] [CrossRef]
- Wyatt, H.A.; Dhawan, A.; Cheeseman, P.; Mieli-Vergani, G.; Price, J.F. Serum hyaluronic acid concentrations are increased in cystic fibrosis patients with liver disease. Arch. Dis. Child. 2002, 86, 190–193. [Google Scholar] [CrossRef][Green Version]
- Boat, T.F.; Cheng, P.W.; Iyer, R.N.; Carlson, D.M.; Polony, I. Human respiratory tract secretion. Mucous glycoproteins of nonpurulent tracheobronchial secretions, and sputum of patients with bronchitis and cystic fibrosis. Arch. Biochem. Biophys. 1976, 177, 95–104. [Google Scholar] [CrossRef]
- Cheng, P.W.; Boat, T.F.; Cranfill, K.; Yankaskas, J.R.; Boucher, R.C. Increased sulfation of glycoconjugates by cultured nasal epithelial cells from patients with cystic fibrosis. J. Clin. Investig. 1989, 84, 68–72. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Look, D.; Tobacman, J.K. Increased arylsulfatase B activity in cystic fibrosis cells following correction of CFTR. Clin. Chim. Acta 2007, 380, 122–127. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Solakyildirim, K.; Zhang, Z.; Chen, M.L.; Linhardt, R.J.; Tobacman, J.K. Cell-bound IL-8 increases in bronchial epithelial cells after arylsulfatase B silencing due to sequestration with chondroitin-4-sulfate. Am. J. Respir. Cell Mol. Biol. 2010, 42, 51–61. [Google Scholar] [CrossRef]
- Reeves, E.P.; Bergin, D.A.; Murray, M.A.; McElvaney, N.G. The involvement of glycosaminoglycans in airway disease associated with cystic fibrosis. Sci. World J. 2011, 11, 959–971. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Feferman, L.; Tobacman, J.K. Effect of CFTR modifiers on arylsulfatase B activity in cystic fibrosis and normal human bronchial epithelial cells. Pulm. Pharmacol. Ther. 2016, 36, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Johnson, Z.; Proudfoot, A.E.; Handel, T.M. Interaction of chemokines and glycosaminoglycans: A new twist in the regulation of chemokine function with opportunities for therapeutic intervention. Cytokine Growth Factor Rev. 2005, 16, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Spillmann, D.L.; Lindahl, U. Glycosamioglycan-protein interactions, a question of specificity. Curr. Opin. Struct. Biol. 1994, 4, 677–682. [Google Scholar] [CrossRef]
- Jin, L.; Abrahams, J.P.; Skinner, R.; Petitou, M.; Pike, R.N.; Carrell, R.W. The anticoagulant activation of antithrombin by heparin. Proc. Natl. Acad. Sci. USA 1997, 94, 14683–14688. [Google Scholar] [CrossRef]
- Lyon, M.; Gallagher, J.T. Bio-specific sequences and domains in heparan sulphate and the regulation of cell growth and adhesion. Matrix Biol. 1998, 17, 485–493. [Google Scholar] [CrossRef]
- Jouy, F.; Lohmann, N.; Wandel, E.; Ruiz-Gomez, G.; Pisabarro, M.T.; Beck-Sickinger, A.G.; Schnabelrauch, M.; Moller, S.; Simon, J.C.; Kalkhof, S.; et al. Sulfated hyaluronan attenuates inflammatory signaling pathways in macrophages involving induction of antioxidants. Proteomics 2017, 17, e1700082. [Google Scholar] [CrossRef]
- Pulsipher, A.; Qin, X.; Thomas, A.J.; Prestwich, G.D.; Oottamasathien, S.; Alt, J.A. Prevention of sinonasal inflammation by a synthetic glycosaminoglycan. Int. Forum Allergy Rhinol. 2017, 7, 177–184. [Google Scholar] [CrossRef]
- Gavina, M.; Luciani, A.; Villella, V.R.; Esposito, S.; Ferrari, E.; Bressani, I.; Casale, A.; Bruscia, E.M.; Maiuri, L.; Raia, V. Nebulized hyaluronan ameliorates lung inflammation in cystic fibrosis mice. Pediatr. Pulmonol. 2013, 48, 761–771. [Google Scholar] [CrossRef]
- Buonpensiero, P.; De Gregorio, F.; Sepe, A.; Di Pasqua, A.; Ferri, P.; Siano, M.; Terlizzi, V.; Raia, V. Hyaluronic acid improves “pleasantness” and tolerability of nebulized hypertonic saline in a cohort of patients with cystic fibrosis. Adv. Ther. 2010, 27, 870–878. [Google Scholar] [CrossRef]
- Furnari, M.L.; Termini, L.; Traverso, G.; Barrale, S.; Bonaccorso, M.R.; Damiani, G.; Piparo, C.L.; Collura, M. Nebulized hypertonic saline containing hyaluronic acid improves tolerability in patients with cystic fibrosis and lung disease compared with nebulized hypertonic saline alone: A prospective, randomized, double-blind, controlled study. Ther. Adv. Respir. Dis. 2012, 6, 315–322. [Google Scholar] [CrossRef]
- Carro, L.M.; Ferreiro, A.L.; de Valbuena Maiz, M.R.; Struwing, C.W.; Alvarez, G.G.; Cortina, L.S. Tolerance of two inhaled hypertonic saline solutions in patients with cystic fibrosis. Med. Clin. 2012, 138, 57–59. [Google Scholar] [CrossRef]
- Ros, M.; Casciaro, R.; Lucca, F.; Troiani, P.; Salonini, E.; Favilli, F.; Quattrucci, S.; Sher, D.; Assael, B.M. Hyaluronic acid improves the tolerability of hypertonic saline in the chronic treatment of cystic fibrosis patients: A multicenter, randomized, controlled clinical trial. J. Aerosol. Med. Pulm. Drug Deliv. 2014, 27, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Greene, C.M.; Marciniak, S.J.; Teckman, J.; Ferrarotti, I.; Brantly, M.L.; Lomas, D.A.; Stoller, J.K.; McElvaney, N.G. alpha1-Antitrypsin deficiency. Nat. Rev. Dis. Primers 2016, 2, 16051. [Google Scholar] [CrossRef]
- Disease, G.B.D.; Injury, I.; Prevalence, C. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- World Healthy Organisation. Chronic Respiratory Diseases. Available online: https://www.who.int/health-topics/chronic-respiratory-diseases#tab=tab_1 (accessed on 1 January 2021).
- Irish Thoracic Society. Lung Disease in Ireland-Prevalence and Trends: Implications for Work Force Planning. Available online: http://www.irishthoracicsociety.com/images/uploads/Lung%20Disease%20in%20Ireland%20December%202013.pdf (accessed on 1 September 2020).
- O’Farrell, A.; De La Harpe, D.; Johnson, H.; Bennett, K. Trends in COPD mortality and in-patient admissions in men & women: Evidence of convergence. Ir. Med. J. 2011, 104, 245–248. [Google Scholar] [PubMed]
- Alpha-1 Foundation Ireland Annual Report 2017. Available online: https://www.alpha1.ie/info-centre/annual-reports (accessed on 1 January 2020).
- Thoracic, S.A.; Respiratory, S.E. American Thoracic Society/European Respiratory Society statement: Standards for the diagnosis and management of individuals with alpha-1 antitrypsin deficiency. Am. J. Respir. Crit. Care Med. 2003, 168, 818–900. [Google Scholar] [CrossRef]
- Karakioulaki, M.; Papakonstantinou, E.; Stolz, D. Extracellular matrix remodelling in COPD. Eur. Respir. Rev. 2020, 29, 190124. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Klagas, I.; Roth, M.; Tamm, M.; Stolz, D. Acute Exacerbations of COPD Are Associated With Increased Expression of Heparan Sulfate and Chondroitin Sulfate in BAL. Chest 2016, 149, 685–695. [Google Scholar] [CrossRef]
- Bihlet, A.R.; Karsdal, M.A.; Sand, J.M.; Leeming, D.J.; Roberts, M.; White, W.; Bowler, R. Biomarkers of extracellular matrix turnover are associated with emphysema and eosinophilic-bronchitis in COPD. Respir. Res. 2017, 18, 22. [Google Scholar] [CrossRef]
- Stolz, D.; Leeming, D.J.; Kristensen, J.H.E.; Karsdal, M.A.; Boersma, W.; Louis, R.; Milenkovic, B.; Kostikas, K.; Blasi, F.; Aerts, J.; et al. Systemic Biomarkers of Collagen and Elastin Turnover Are Associated With Clinically Relevant Outcomes in COPD. Chest 2017, 151, 47–59. [Google Scholar] [CrossRef]
- Churg, A.; Zhou, S.; Wright, J.L. Series “matrix metalloproteinases in lung health and disease”: Matrix metalloproteinases in COPD. Eur. Respir. J. 2012, 39, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Waeijen-Smit, K.; Reynaert, N.L.; Beijers, R.; Houben-Wilke, S.; Simons, S.O.; Spruit, M.A.; Franssen, F.M.E. Alterations in plasma hyaluronic acid in patients with clinically stable COPD versus (non)smoking controls. Sci. Rep. 2021, 11, 15883. [Google Scholar] [CrossRef] [PubMed]
- Garantziotis, S.; Brezina, M.; Castelnuovo, P.; Drago, L. The role of hyaluronan in the pathobiology and treatment of respiratory disease. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 310, L785–L795. [Google Scholar] [CrossRef]
- Turino, G.M.; Ma, S.; Lin, Y.Y.; Cantor, J.O. The Therapeutic Potential of Hyaluronan in COPD. Chest 2018, 153, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Maiz Carro, L.; Martinez-Garcia, M.A. Use of Hyaluronic Acid (HA) in Chronic Airway Diseases. Cells 2020, 9, 2210. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Roth, M.; Klagas, I.; Karakiulakis, G.; Tamm, M.; Stolz, D. COPD Exacerbations Are Associated With Proinflammatory Degradation of Hyaluronic Acid. Chest 2015, 148, 1497–1507. [Google Scholar] [CrossRef]
- Tsai, K.Y.F.; Hirschi Budge, K.M.; Llavina, S.; Davis, T.; Long, M.; Bennett, A.; Sitton, B.; Arroyo, J.A.; Reynolds, P.R. RAGE and AXL expression following secondhand smoke (SHS) exposure in mice. Exp. Lung Res. 2019, 45, 297–309. [Google Scholar] [CrossRef]
- Galdi, F.; Pedone, C.; McGee, C.A.; George, M.; Rice, A.B.; Hussain, S.S.; Vijaykumar, K.; Boitet, E.R.; Tearney, G.J.; McGrath, J.A.; et al. Inhaled high molecular weight hyaluronan ameliorates respiratory failure in acute COPD exacerbation: A pilot study. Respir. Res. 2021, 22, 30. [Google Scholar] [CrossRef]
- Shute, J.K.; Calzetta, L.; Cardaci, V.; di Toro, S.; Page, C.P.; Cazzola, M. Inhaled nebulised unfractionated heparin improves lung function in moderate to very severe COPD: A pilot study. Pulm. Pharmacol. Ther. 2018, 48, 88–96. [Google Scholar] [CrossRef]
- Schmidt, E.P.; Li, G.; Li, L.; Fu, L.; Yang, Y.; Overdier, K.H.; Douglas, I.S.; Linhardt, R.J. The circulating glycosaminoglycan signature of respiratory failure in critically ill adults. J. Biol. Chem. 2014, 289, 8194–8202. [Google Scholar] [CrossRef]
- Pihtili, A.; Bingol, Z.; Kiyan, E. Serum endocan levels in patients with stable COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 3367–3372. [Google Scholar] [CrossRef] [PubMed]
- In, E.; Kuluozturk, M.; Turgut, T.; Altintop Geckil, A.; Ilhan, N. Endocan as a potential biomarker of disease severity and exacerbations in COPD. Clin. Respir. J. 2021, 15, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, E.; Baty, F.; Roth, M.; Tamm, M.; Stolz, D. Predictors for significant changes in serum sulfated glycosaminoglycans at COPD exacerbations. Eur. Respir. J. 2019, 54 (Suppl. 63), PA3850. [Google Scholar]
- Svitich, O.A.; Sobolev, V.V.; Gankovskaya, L.V.; Zhigalkina, P.V.; Zverev, V.V. The role of regulatory RNAs (miRNAs) in asthma. Allergol. Immunopathol. 2018, 46, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Maciag, M.C.; Phipatanakul, W. Prevention of Asthma: Targets for Intervention. Chest 2020, 158, 913–922. [Google Scholar] [CrossRef]
- Liang, J.; Jiang, D.; Jung, Y.; Xie, T.; Ingram, J.; Church, T.; Degan, S.; Leonard, M.; Kraft, M.; Noble, P.W. Role of hyaluronan and hyaluronan-binding proteins in human asthma. J. Allergy Clin. Immunol. 2011, 128, 403–411.e403. [Google Scholar] [CrossRef]
- Misra, S.; Hascall, V.C.; Markwald, R.R.; Ghatak, S. Interactions between Hyaluronan and Its Receptors (CD44, RHAMM) Regulate the Activities of Inflammation and Cancer. Front. Immunol. 2015, 6, 201. [Google Scholar] [CrossRef]
- Petrigni, G.; Allegra, L. Aerosolised hyaluronic acid prevents exercise-induced bronchoconstriction, suggesting novel hypotheses on the correction of matrix defects in asthma. Pulm. Pharmacol. Ther. 2006, 19, 166–171. [Google Scholar] [CrossRef]
- Ghonim, M.A.; Wang, J.; Ibba, S.V.; Luu, H.H.; Pyakurel, K.; Benslimane, I.; Mousa, S.; Boulares, A.H. Sulfated non-anticoagulant heparin blocks Th2-induced asthma by modulating the IL-4/signal transducer and activator of transcription 6/Janus kinase 1 pathway. J. Transl. Med. 2018, 16, 243. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Ontong, P.; Prachayasittikul, V. Unraveled roles of hyaluronan in severe COVID-19. EXCLI J. 2021, 20, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Magnani, H.N. Rationale for the Role of Heparin and Related GAG Antithrombotics in COVID-19 Infection. Clin. Appl. Thromb. Hemost. 2021, 27, 1076029620977702. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, C.; Xu, X.F.; Xu, W.; Liu, S.W. Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020, 41, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Zhang, T.; Zhang, W.; Sun, Q.; Li, H.; Li, J.P. Elucidating the Interactions Between Heparin/Heparan Sulfate and SARS-CoV-2-Related Proteins-An Important Strategy for Developing Novel Therapeutics for the COVID-19 Pandemic. Front. Mol. Biosci. 2020, 7, 628551. [Google Scholar] [CrossRef]
- Shi, C.; Wang, C.; Wang, H.; Yang, C.; Cai, F.; Zeng, F.; Cheng, F.; Liu, Y.; Zhou, T.; Deng, B.; et al. The Potential of Low Molecular Weight Heparin to Mitigate Cytokine Storm in Severe COVID-19 Patients: A Retrospective Cohort Study. Clin. Transl. Sci. 2020, 13, 1087–1095. [Google Scholar] [CrossRef]
- McElvaney, O.J.; McEvoy, N.L.; Boland, F.; McElvaney, O.F.; Hogan, G.; Donnelly, K.; Friel, O.; Browne, E.; Fraughen, D.D.; Murphy, M.P.; et al. A randomized, double-blind, placebo-controlled trial of intravenous alpha-1 antitrypsin for acute respiratory distress syndrome secondary to COVID-19. Med 2022, 3, 233–248.e6. [Google Scholar] [CrossRef]
- McElvaney, O.J.; McEvoy, N.L.; McElvaney, O.F.; Carroll, T.P.; Murphy, M.P.; Dunlea, D.M.; Ni Choileain, O.; Clarke, J.; O’Connor, E.; Hogan, G.; et al. Characterization of the Inflammatory Response to Severe COVID-19 Illness. Am. J. Respir. Crit. Care Med. 2020, 202, 812–821. [Google Scholar] [CrossRef]
- McElvaney, O.F.; Asakura, T.; Meinig, S.L.; Torres-Castillo, J.L.; Hagan, R.S.; Gabillard-Lefort, C.; Murphy, M.P.; Thorne, L.B.; Borczuk, A.; Reeves, E.P.; et al. Protease-anti-protease compartmentalization in SARS-CoV-2 ARDS: Therapeutic implications. EBioMedicine 2022, 77, 103894. [Google Scholar] [CrossRef]
- Schuurs, Z.P.; Hammond, E.; Elli, S.; Rudd, T.R.; Mycroft-West, C.J.; Lima, M.A.; Skidmore, M.A.; Karlsson, R.; Chen, Y.H.; Bagdonaite, I.; et al. Evidence of a putative glycosaminoglycan binding site on the glycosylated SARS-CoV-2 spike protein N-terminal domain. Comput. Struct. Biotechnol. J. 2021, 19, 2806–2818. [Google Scholar] [CrossRef]
- Clausen, T.M.; Sandoval, D.R.; Spliid, C.B.; Pihl, J.; Perrett, H.R.; Painter, C.D.; Narayanan, A.; Majowicz, S.A.; Kwong, E.M.; McVicar, R.N.; et al. SARS-CoV-2 Infection Depends on Cellular Heparan Sulfate and ACE2. Cell 2020, 183, 1043–1057.e1015. [Google Scholar] [CrossRef]
- Queisser, K.A.; Mellema, R.A.; Middleton, E.A.; Portier, I.; Manne, B.K.; Denorme, F.; Beswick, E.J.; Rondina, M.T.; Campbell, R.A.; Petrey, A.C. COVID-19 generates hyaluronan fragments that directly induce endothelial barrier dysfunction. JCI Insight 2021, 6, e147472. [Google Scholar] [CrossRef] [PubMed]
- Potje, S.R.; Costa, T.J.; Fraga-Silva, T.F.C.; Martins, R.B.; Benatti, M.N.; Almado, C.E.L.; de Sa, K.S.G.; Bonato, V.L.D.; Arruda, E.; Louzada-Junior, P.; et al. Heparin prevents in vitro glycocalyx shedding induced by plasma from COVID-19 patients. Life Sci. 2021, 276, 119376. [Google Scholar] [CrossRef] [PubMed]
- Broekhuizen, L.N.; Lemkes, B.A.; Mooij, H.L.; Meuwese, M.C.; Verberne, H.; Holleman, F.; Schlingemann, R.O.; Nieuwdorp, M.; Stroes, E.S.; Vink, H. Effect of sulodexide on endothelial glycocalyx and vascular permeability in patients with type 2 diabetes mellitus. Diabetologia 2010, 53, 2646–2655. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka-Tojo, M. Vascular endothelial glycocalyx as a mechanism of vascular endothelial dysfunction and atherosclerosis. World J. Cardiovasc. Dis. 2020, 10, 731. [Google Scholar] [CrossRef]
- Cagno, V.; Tseligka, E.D.; Jones, S.T.; Tapparel, C. Heparan Sulfate Proteoglycans and Viral Attachment: True Receptors or Adaptation Bias? Viruses 2019, 11, 596. [Google Scholar] [CrossRef] [PubMed]
- Mycroft-West, C.J.; Su, D.; Pagani, I.; Rudd, T.R.; Elli, S.; Gandhi, N.S.; Guimond, S.E.; Miller, G.J.; Meneghetti, M.C.Z.; Nader, H.B.; et al. Heparin Inhibits Cellular Invasion by SARS-CoV-2: Structural Dependence of the Interaction of the Spike S1 Receptor-Binding Domain with Heparin. Thromb. Haemost. 2020, 120, 1700–1715. [Google Scholar] [CrossRef]
- Kim, S.Y.; Jin, W.; Sood, A.; Montgomery, D.W.; Grant, O.C.; Fuster, M.M.; Fu, L.; Dordick, J.S.; Woods, R.J.; Zhang, F.; et al. Characterization of heparin and severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) spike glycoprotein binding interactions. Antivir. Res. 2020, 181, 104873. [Google Scholar] [CrossRef]
- Tandon, R.; Sharp, J.S.; Zhang, F.; Pomin, V.H.; Ashpole, N.M.; Mitra, D.; McCandless, M.G.; Jin, W.; Liu, H.; Sharma, P.; et al. Effective Inhibition of SARS-CoV-2 Entry by Heparin and Enoxaparin Derivatives. J. Virol. 2021, 13, e01987-20. [Google Scholar] [CrossRef]
- Shi, C.; Tingting, W.; Li, J.P.; Sullivan, M.A.; Wang, C.; Wang, H.; Deng, B.; Zhang, Y. Comprehensive Landscape of Heparin Therapy for COVID-19. Carbohydr. Polym. 2021, 254, 117232. [Google Scholar] [CrossRef]
- Di Castelnuovo, A.; Costanzo, S.; Antinori, A.; Berselli, N.; Blandi, L.; Bonaccio, M.; Cauda, R.; Guaraldi, G.; Menicanti, L.; Mennuni, M.; et al. Heparin in COVID-19 Patients Is Associated with Reduced In-Hospital Mortality: The Multicenter Italian CORIST Study. Thromb. Haemost. 2021, 121, 1054–1065. [Google Scholar] [CrossRef]
- Lin, L.; Lu, L.; Cao, W.; Li, T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection-a review of immune changes in patients with viral pneumonia. Emerg. Microbes. Infect. 2020, 9, 727–732. [Google Scholar] [CrossRef]
- Russo, V.; Cardillo, G.; Viggiano, G.V.; Mangiacapra, S.; Cavalli, A.; Fontanella, A.; Agrusta, F.; Bellizzi, A.; Amitrano, M.; Iannuzzo, M.; et al. Fondaparinux Use in Patients With COVID-19: A Preliminary Multicenter Real-World Experience. J. Cardiovasc. Pharmacol. 2020, 76, 369–371. [Google Scholar] [CrossRef]
- Möller, S.; Theiß, J.; Deinert, T.I.L.; Golat, K.; Heinze, J.; Niemeyer, D.; Wyrwa, R.; Schnabelrauch, M.; Bogner, E. High-Sulfated Glycosaminoglycans Prevent Coronavirus Replication. Viruses 2022, 14, 413. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, Y.; Chen, T.; Tang, J.; Ma, X. Unfractionated heparin inhibits lipopolysaccharide-induced expression of chemokines in human endothelial cells through nuclear factor-KappaB signaling pathway. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2016, 28, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Yazeji, T.; Moulari, B.; Beduneau, A.; Stein, V.; Dietrich, D.; Pellequer, Y.; Lamprecht, A. Nanoparticle-based delivery enhances anti-inflammatory effect of low molecular weight heparin in experimental ulcerative colitis. Drug Deliv. 2017, 24, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, A.; Ferrara, F. Low Molecular Weight Heparin, Anti-inflammatory/Immunoregulatory and Antiviral Effects, a Short Update. Cardiovasc. Drugs. Ther. 2021, 30, 1–5. [Google Scholar] [CrossRef]
- Billett, H.H.; Reyes-Gil, M.; Szymanski, J.; Ikemura, K.; Stahl, L.R.; Lo, Y.; Rahman, S.; Gonzalez-Lugo, J.D.; Kushnir, M.; Barouqa, M.; et al. Anticoagulation in COVID-19: Effect of Enoxaparin, Heparin, and Apixaban on Mortality. Thromb. Haemost. 2020, 120, 1691–1699. [Google Scholar] [CrossRef]
- Han, H.; Ma, Q.; Li, C.; Liu, R.; Zhao, L.; Wang, W.; Zhang, P.; Liu, X.; Gao, G.; Liu, F.; et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg. Microbes. Infect. 2020, 9, 1123–1130. [Google Scholar] [CrossRef]
- Itano, N.; Kimata, K. Mammalian hyaluronan synthases. IUBMB Life 2002, 54, 195–199. [Google Scholar] [CrossRef]
- Itano, N.; Sawai, T.; Yoshida, M.; Lenas, P.; Yamada, Y.; Imagawa, M.; Shinomura, T.; Hamaguchi, M.; Yoshida, Y.; Ohnuki, Y.; et al. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J. Biol. Chem. 1999, 274, 25085–25092. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Bonovolias, I.; Roth, M.; Tamm, M.; Schumann, D.; Baty, F.; Louis, R.; Milenkovic, B.; Boersma, W.; Stieltjes, B.; et al. Serum levels of hyaluronic acid are associated with COPD severity and predict survival. Eur. Respir. J. 2019, 53, 1801183. [Google Scholar] [CrossRef] [PubMed]
- Hodge-Dufour, J.; Noble, P.W.; Horton, M.R.; Bao, C.; Wysoka, M.; Burdick, M.D.; Strieter, R.M.; Trinchieri, G.; Pure, E. Induction of IL-12 and chemokines by hyaluronan requires adhesion-dependent priming of resident but not elicited macrophages. J. Immunol. 1997, 159, 2492–2500. [Google Scholar] [PubMed]
- Horton, M.R.; Olman, M.A.; Bao, C.; White, K.E.; Choi, A.M.; Chin, B.Y.; Noble, P.W.; Lowenstein, C.J. Regulation of plasminogen activator inhibitor-1 and urokinase by hyaluronan fragments in mouse macrophages. Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 279, L707–L715. [Google Scholar] [CrossRef] [PubMed]
- Horton, M.R.; Shapiro, S.; Bao, C.; Lowenstein, C.J.; Noble, P.W. Induction and regulation of macrophage metalloelastase by hyaluronan fragments in mouse macrophages. J. Immunol. 1999, 162, 4171–4176. [Google Scholar] [PubMed]
- McKee, C.M.; Penno, M.B.; Cowman, M.; Burdick, M.D.; Strieter, R.M.; Bao, C.; Noble, P.W. Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages. The role of HA size and CD44. J. Clin. Investig. 1996, 98, 2403–2413. [Google Scholar] [CrossRef] [PubMed]
- Haslinger, B.; Mandl-Weber, S.; Sellmayer, A.; Sitter, T. Hyaluronan fragments induce the synthesis of MCP-1 and IL-8 in cultured human peritoneal mesothelial cells. Cell Tissue Res. 2001, 305, 79–86. [Google Scholar] [CrossRef]
- Eberlein, M.; Scheibner, K.A.; Black, K.E.; Collins, S.L.; Chan-Li, Y.; Powell, J.D.; Horton, M.R. Anti-oxidant inhibition of hyaluronan fragment-induced inflammatory gene expression. J. Inflamm. 2008, 5, 20. [Google Scholar] [CrossRef]
- Lesley, J.; Hascall, V.C.; Tammi, M.; Hyman, R. Hyaluronan binding by cell surface CD44. J. Biol. Chem. 2000, 275, 26967–26975. [Google Scholar] [CrossRef]
- Teder, P.; Vandivier, R.W.; Jiang, D.; Liang, J.; Cohn, L.; Pure, E.; Henson, P.M.; Noble, P.W. Resolution of lung inflammation by CD44. Science 2002, 296, 155–158. [Google Scholar] [CrossRef]
- Haeger, S.M.; Yang, Y.; Schmidt, E.P. Heparan Sulfate in the Developing, Healthy, and Injured Lung. Am. J. Respir. Cell Mol. Biol. 2016, 55, 5–11. [Google Scholar] [CrossRef]
- Douglas, M.S.; Rix, D.A.; Dark, J.H.; Talbot, D.; Kirby, J.A. Examination of the mechanism by which heparin antagonizes activation of a model endothelium by interferon-gamma (IFN-gamma). Clin. Exp. Immunol. 1997, 107, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Baltus, T.; Weber, K.S.; Johnson, Z.; Proudfoot, A.E.; Weber, C. Oligomerization of RANTES is required for CCR1-mediated arrest but not CCR5-mediated transmigration of leukocytes on inflamed endothelium. Blood 2003, 102, 1985–1988. [Google Scholar] [CrossRef] [PubMed]
- Cripps, J.G.; Crespo, F.A.; Romanovskis, P.; Spatola, A.F.; Fernandez-Botran, R. Modulation of acute inflammation by targeting glycosaminoglycan-cytokine interactions. Int. Immunopharmacol. 2005, 5, 1622–1632. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caird, R.; Williamson, M.; Yusuf, A.; Gogoi, D.; Casey, M.; McElvaney, N.G.; Reeves, E.P. Targeting of Glycosaminoglycans in Genetic and Inflammatory Airway Disease. Int. J. Mol. Sci. 2022, 23, 6400. https://doi.org/10.3390/ijms23126400
Caird R, Williamson M, Yusuf A, Gogoi D, Casey M, McElvaney NG, Reeves EP. Targeting of Glycosaminoglycans in Genetic and Inflammatory Airway Disease. International Journal of Molecular Sciences. 2022; 23(12):6400. https://doi.org/10.3390/ijms23126400
Chicago/Turabian StyleCaird, Robin, Michael Williamson, Azeez Yusuf, Debananda Gogoi, Michelle Casey, Noel G. McElvaney, and Emer P. Reeves. 2022. "Targeting of Glycosaminoglycans in Genetic and Inflammatory Airway Disease" International Journal of Molecular Sciences 23, no. 12: 6400. https://doi.org/10.3390/ijms23126400
APA StyleCaird, R., Williamson, M., Yusuf, A., Gogoi, D., Casey, M., McElvaney, N. G., & Reeves, E. P. (2022). Targeting of Glycosaminoglycans in Genetic and Inflammatory Airway Disease. International Journal of Molecular Sciences, 23(12), 6400. https://doi.org/10.3390/ijms23126400





