Structural and Molecular Kinetic Features of Activities of DNA Polymerases
Abstract
1. Introduction
2. Classification of DNA Polymerases
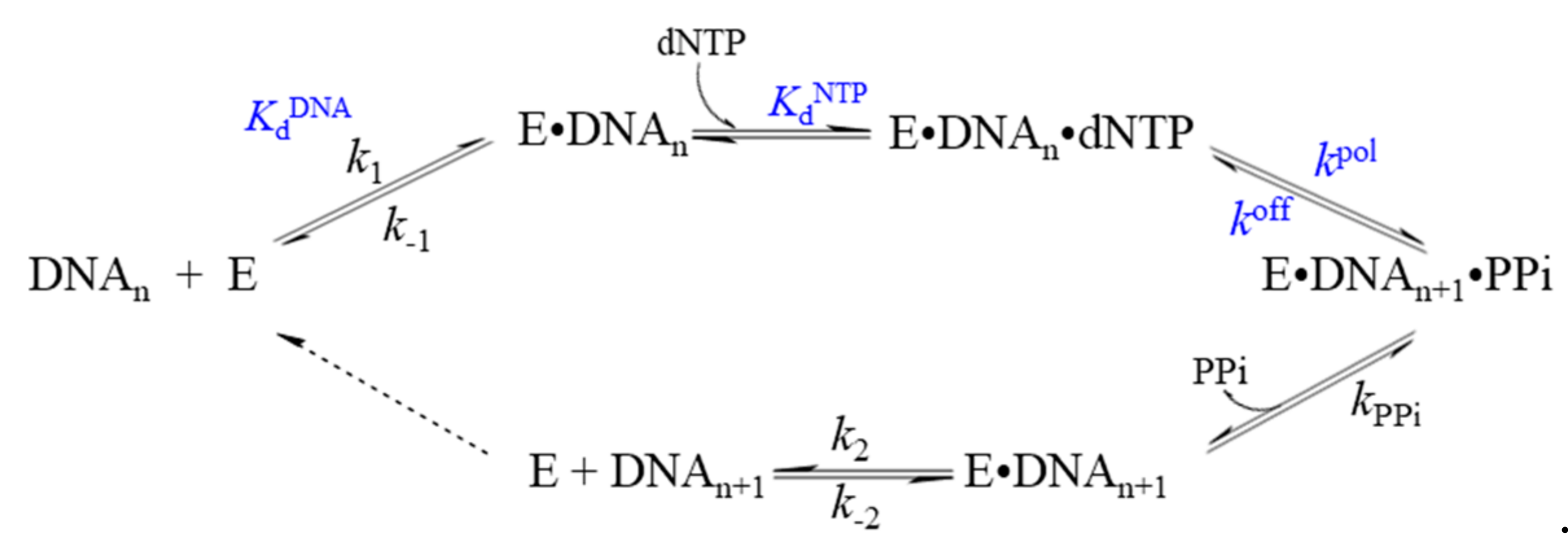
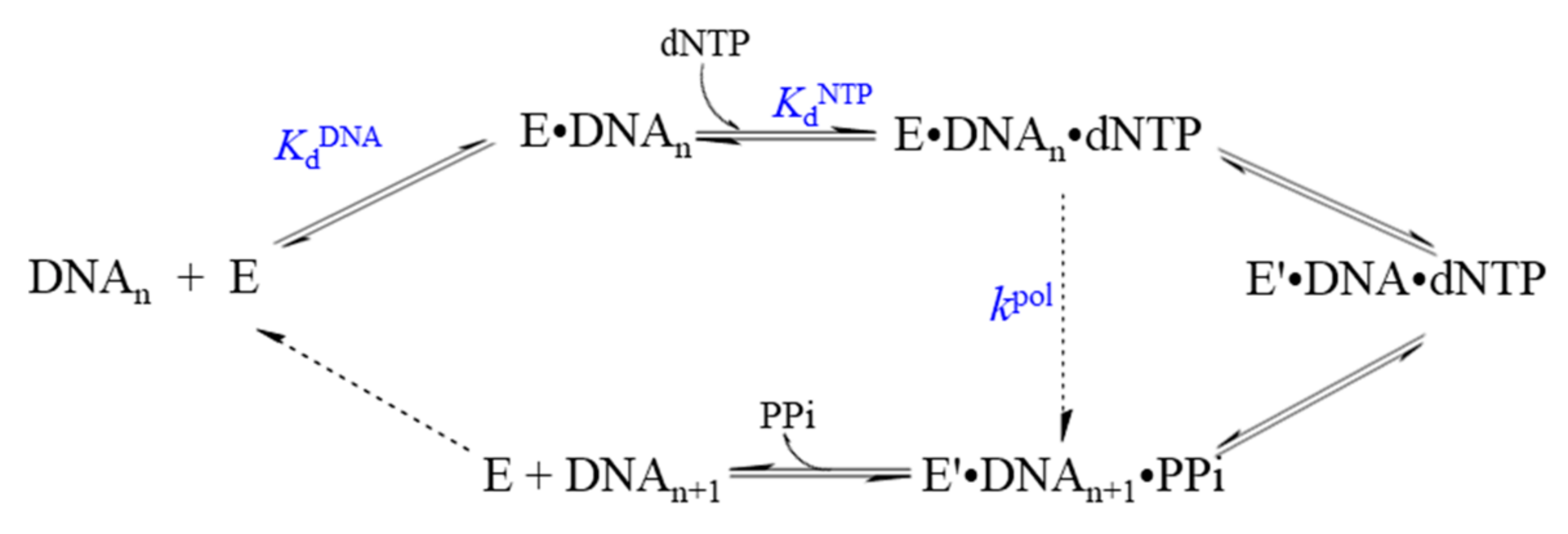
3. Methods for Studying the Mechanism of Action of DNA Polymerases
4. Family A DNA Polymerases
5. Family B DNA Polymerases
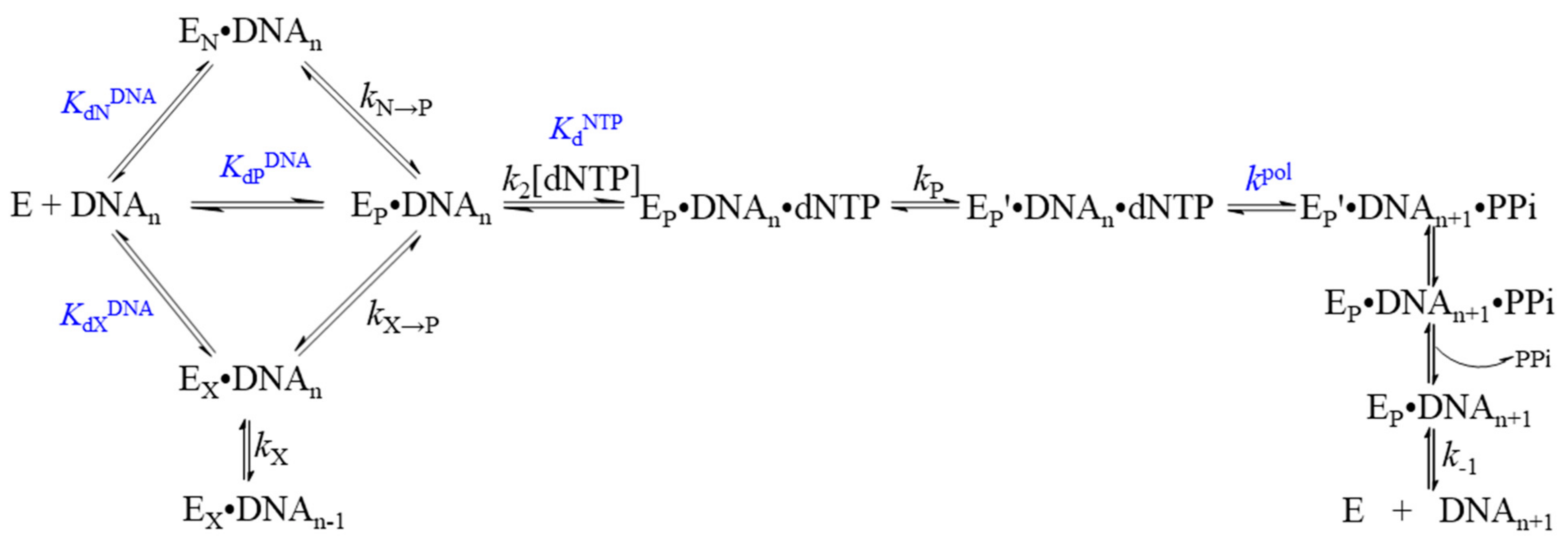
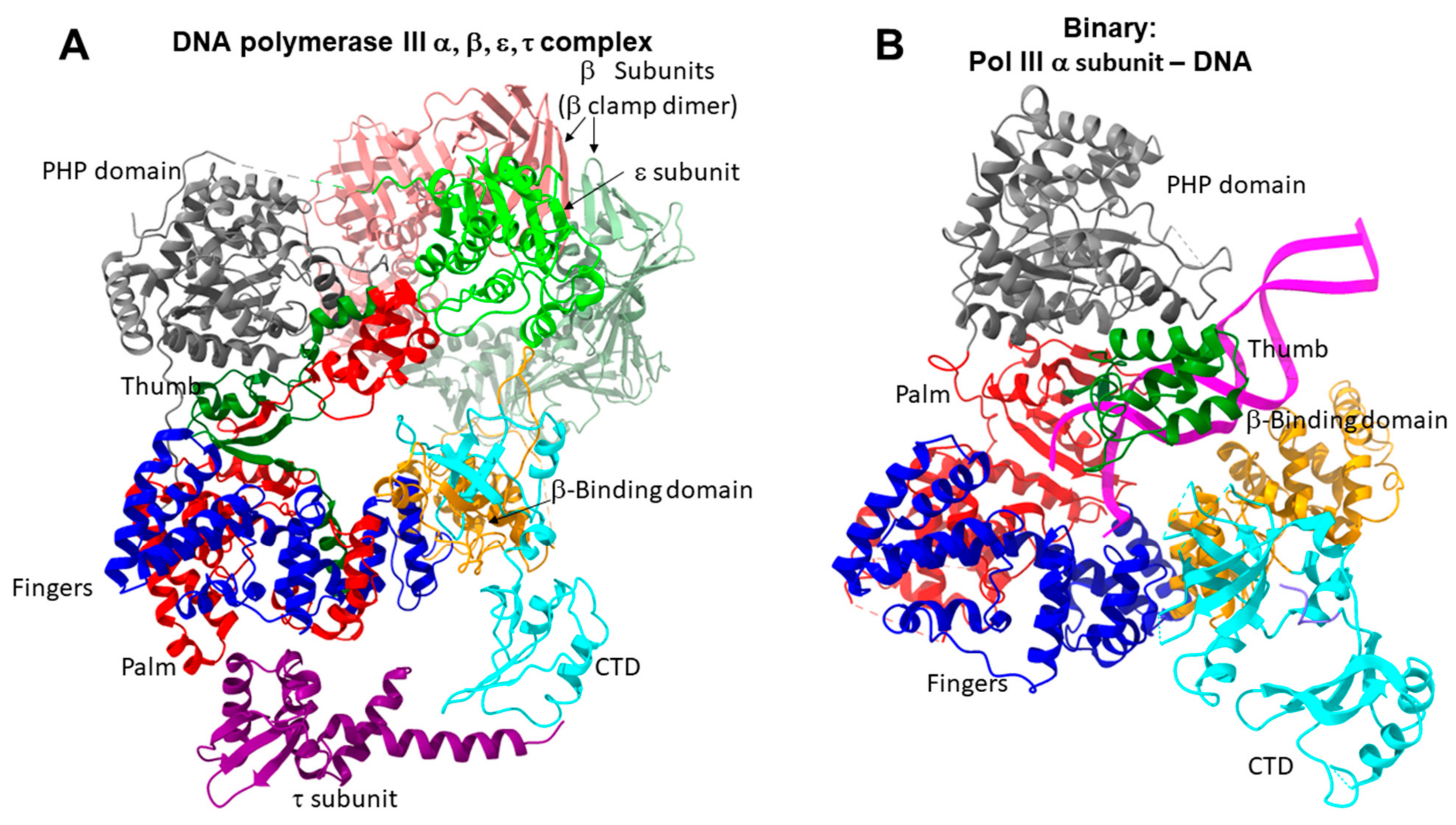
6. Family C DNA Polymerases
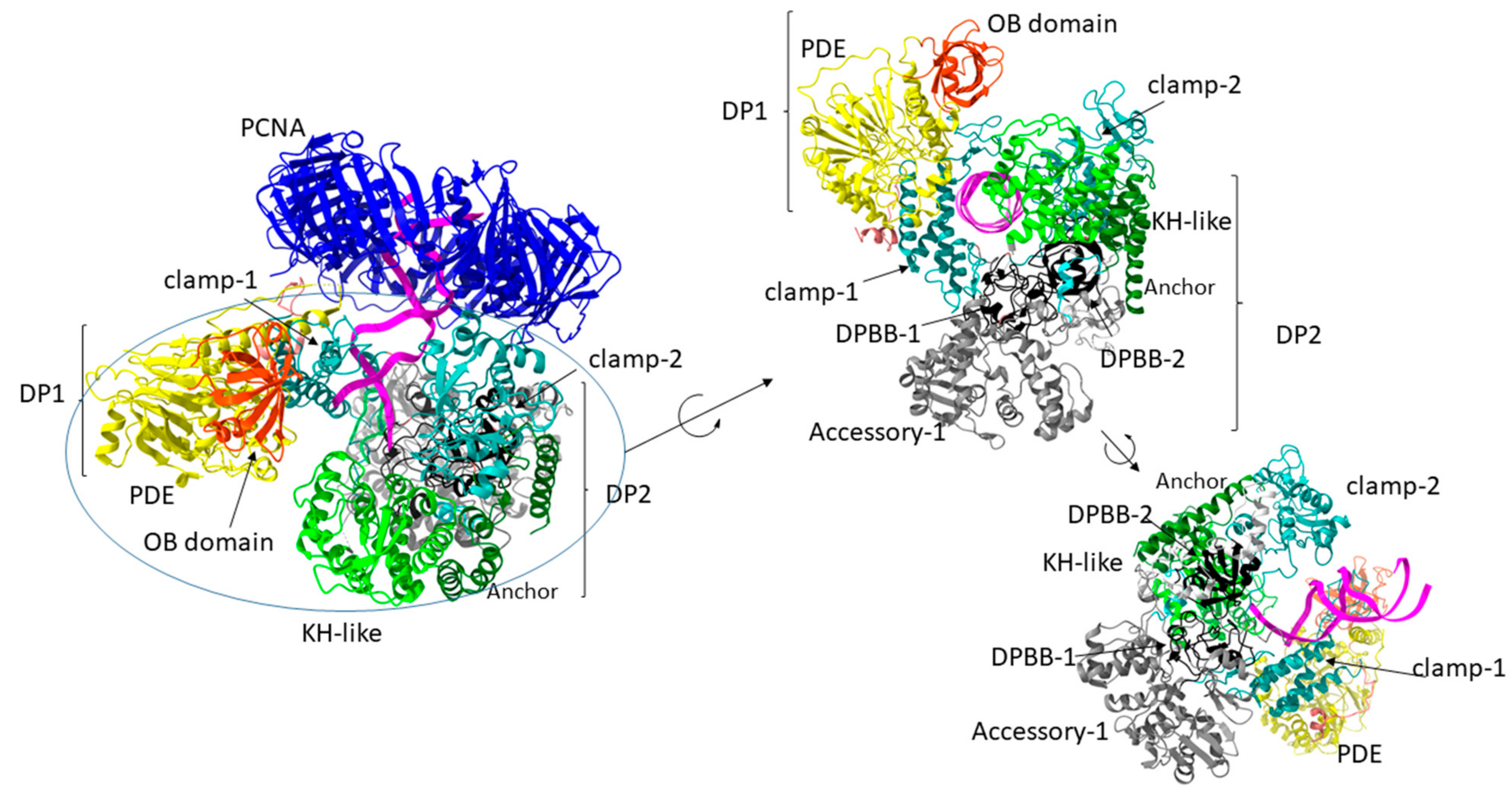
7. Family D DNA Polymerases
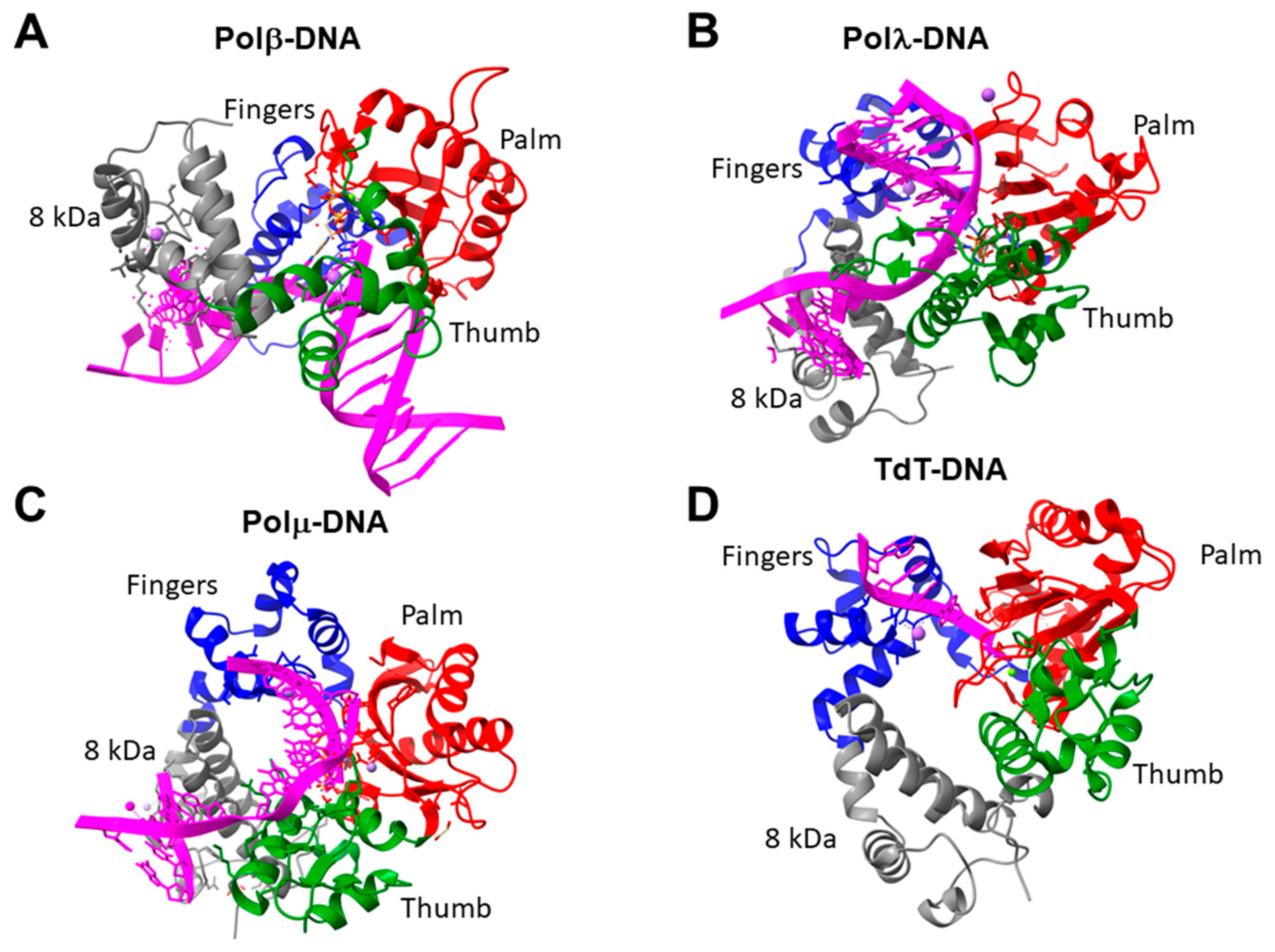
8. Family X DNA Polymerases
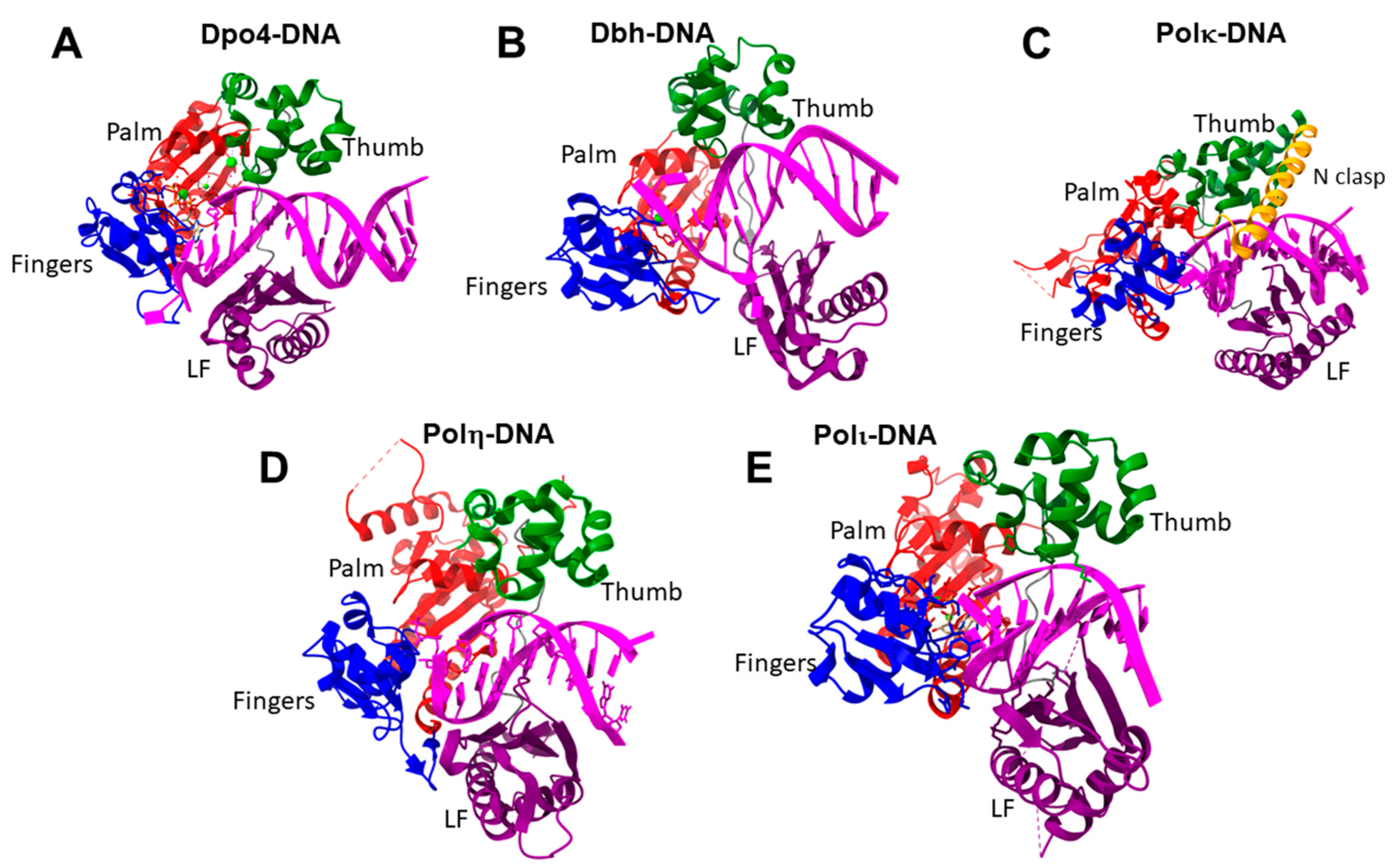
9. Family Y DNA Polymerases
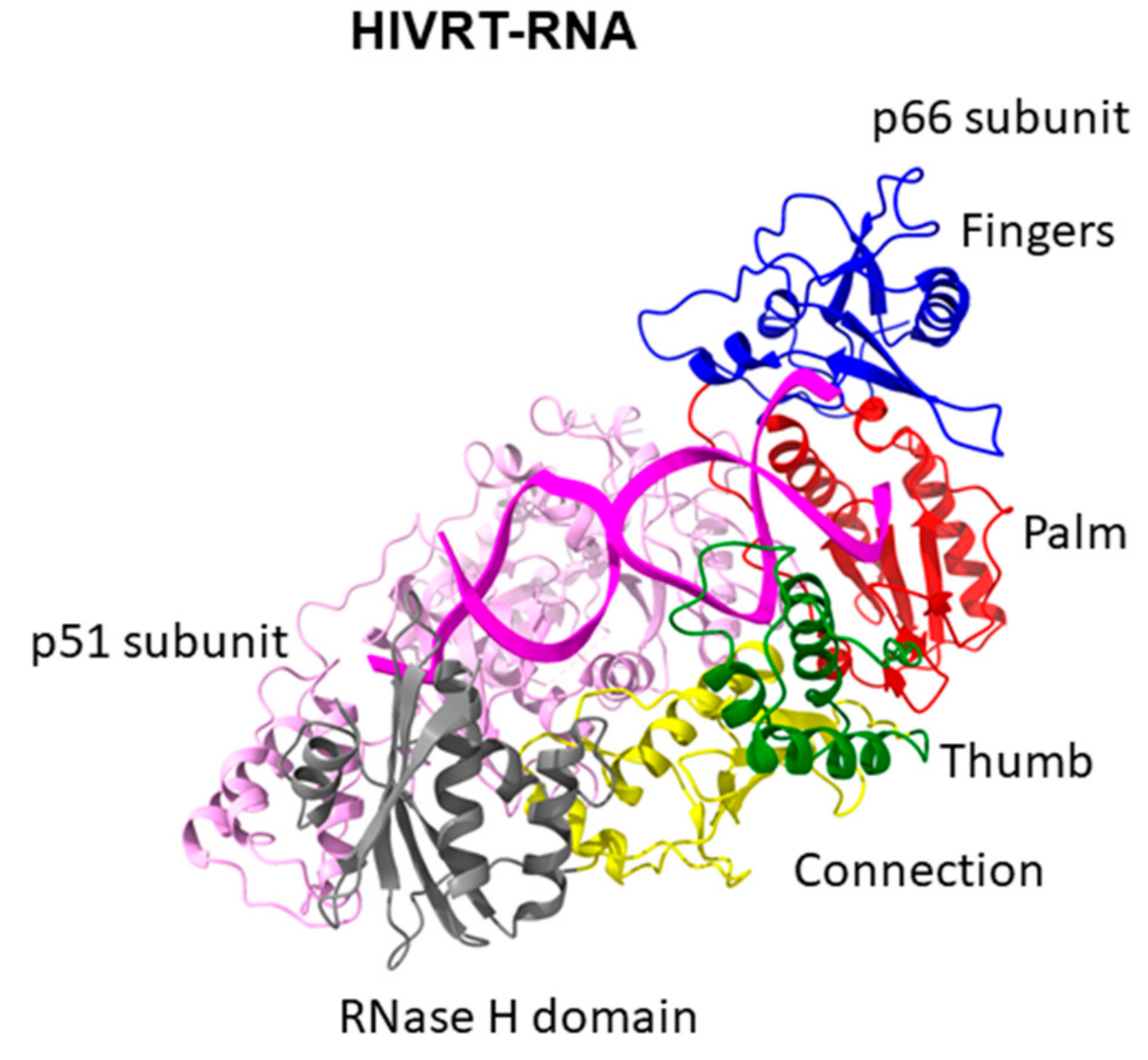
10. DNA Polymerases of RT Family
11. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Steitz, T.A. DNA Polymerases: Structural Diversity and Common Mechanisms. J. Biol. Chem. 1999, 274, 17395–17398. [Google Scholar] [CrossRef]
- Raper, A.T.; Reed, A.J.; Suo, Z. Kinetic Mechanism of DNA Polymerases: Contributions of Conformational Dynamics and a Third Divalent Metal Ion. Chem. Rev. 2018, 118, 6000–6025. [Google Scholar] [CrossRef]
- Berdis, A.J. Mechanisms of DNA Polymerases. Chem. Rev. 2009, 109, 2862–2879. [Google Scholar] [CrossRef]
- Rothwell, P.J.; Waksman, G. Structure and Mechanism of DNA Polymerases. Adv. Protein Chem. 2005, 71, 401–440. [Google Scholar] [CrossRef]
- Steitz, T.A. DNA- and RNA-Dependent DNA Polymerases. Curr. Opin. Struct. Biol. 1993, 3, 31–38. [Google Scholar] [CrossRef]
- Steitz, T.A. A Mechanism for All Polymerases. Nature 1998, 391, 231–232. [Google Scholar] [CrossRef]
- Palermo, G.; Cavalli, A.; Klein, M.L.; Alfonso-Prieto, M.; Dal Peraro, M.; De Vivo, M. Catalytic Metal Ions and Enzymatic Processing of DNA and RNA. Acc. Chem. Res. 2015, 48, 220–228. [Google Scholar] [CrossRef]
- Nakamura, T.; Zhao, Y.; Yamagata, Y.; Hua, Y.J.; Yang, W. Watching DNA Polymerase η Make a Phosphodiester Bond. Nature 2012, 487, 196–201. [Google Scholar] [CrossRef]
- Wang, J.; Konigsberg, W.H. Two-Metal-Ion Catalysis: Inhibition of DNA Polymerase Activity by a Third Divalent Metal Ion. Front. Mol. Biosci. 2022, 9, 824794. [Google Scholar] [CrossRef]
- Tsai, M.D. Catalytic Mechanism of DNA Polymerases—Two Metal Ions or Three? Protein Sci. 2019, 28, 288–291. [Google Scholar] [CrossRef]
- Wu, W.J.; Yang, W.; Tsai, M.D. How DNA Polymerases Catalyse Replication and Repair with Contrasting Fidelity. Nat. Rev. Chem. 2017, 1, 0068. [Google Scholar] [CrossRef]
- Joyce, C.M.; Steitz, T.A. Relationships in DNA Polymerases. Annu. Rev. Biochem. 1994, 63, 777–822. [Google Scholar] [CrossRef] [PubMed]
- Hubscher, U.; Maga, G.; Villani, G.; Spadari, S. DNA Polymerases Discovery, Characterization and Functions in Cellular DNA Transactions; Brenner’s; Illustrated Edition; World Scientific Publishing Company: Singapore, 2013; ISBN 9814299162. [Google Scholar]
- Garcia-Diaz, M.; Bebenek, K. Multiple Functions of DNA Polymerases. CRC Crit. Rev. Plant Sci. 2007, 26, 105–122. [Google Scholar] [CrossRef]
- Alba, M.M. Replicative DNA Polymerases. Genome Biol. 2001, 2, reviews3002.1. [Google Scholar] [CrossRef]
- Miller, E.S.; Kutter, E.; Mosig, G.; Arisaka, F.; Kunisawa, T.; Rüger, W. Bacteriophage T4 Genome. Microbiol. Mol. Biol. Rev. 2003, 67, 86–156. [Google Scholar] [CrossRef]
- Wang, J.; Sattar, A.K.M.A.; Wang, C.C.; Karam, J.D.; Konigsberg, W.H.; Steitz, T.A. Crystal Structure of a Pol α Family Replication DNA Polymerase from Bacteriophage RB69. Cell 1997, 89, 1087–1099. [Google Scholar] [CrossRef]
- Hashimoto, H.; Nishioka, M.; Fujiwara, S.; Takagi, M.; Imanaka, T.; Inoue, T.; Kai, Y. Crystal Structure of DNA Polymerase from Hyperthermophilic Archaeon Pyrococcus kodakaraensis KOD1. J. Mol. Biol. 2001, 306, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.P.; Ito, J. DNA Polymerase C of the Thermophilic Bacterium Thermus Aquaticus: Classification and Phylogenetic Analysis of the Family C DNA Polymerases. J. Mol. Evol. 1999, 48, 756–769. [Google Scholar] [CrossRef] [PubMed]
- Uemori, T.; Sato, Y.; Kato, I.; Doi, H.; Ishino, Y. A Novel DNA Polymerase in the Hyperthermophilic Archaeon, Pyrococcus furiosus: Gene Cloning, Expression, and Characterization. Genes Cells 1997, 2, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Cann, I.K.O.; Komori, K.; Toh, H.; Kanai, S.; Ishino, Y. A Heterodimeric DNA Polymerase: Evidence That Members of Euryarchaeota Possess a Distinct DNA Polymerase. Proc. Natl. Acad. Sci. USA 1998, 95, 14250–14255. [Google Scholar] [CrossRef]
- Yamtich, J.; Sweasy, J.B. DNA Polymerase Family X: Function, Structure, and Cellular Roles. Biochim. Biophys. Acta-Proteins Proteom. 2010, 1804, 1136–1150. [Google Scholar] [CrossRef]
- Yang, W. An Overview of Y-Family DNA Polymerases and a Case Study of Human DNA Polymerase π. Biochemistry 2014, 53, 2793–2803. [Google Scholar] [CrossRef] [PubMed]
- Götte, M.; Li, X.; Wainberg, M.A. HIV-1 Reverse Transcription: A Brief Overview Focused on Structure-Function Relationships among Molecules Involved in Initiation of the Reaction. Arch. Biochem. Biophys. 1999, 365, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.W.; Wright, W.E. Telomeres and Telomerase: Three Decades of Progress. Nat. Rev. Genet. 2019, 20, 299–309. [Google Scholar] [CrossRef]
- Kirby, T.W.; Derose, E.F.; Cavanaugh, N.A.; Beard, W.A.; Shock, D.D.; Mueller, G.A.; Wilson, S.H.; London, R.E. Metal-Induced DNA Translocation Leads to DNA Polymerase Conformational Activation. Nucleic Acids Res. 2012, 40, 2974–2983. [Google Scholar] [CrossRef] [PubMed]
- Pustovalova, Y.; MacIejewski, M.W.; Korzhnev, D.M. NMR Mapping of PCNA Interaction with Translesion Synthesis DNA Polymerase Rev1 Mediated by Rev1-BRCT Domain. J. Mol. Biol. 2013, 425, 3091–3105. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sušac, L.; Feigon, J. Structural Biology of Telomerase. Cold Spring Harb. Perspect. Biol. 2019, 11, a032383. [Google Scholar] [CrossRef]
- Broyde, S.; Wang, L.; Zhang, L.; Rechkoblit, O.; Geacintov, N.E.; Patel, D.J. DNA Adduct Structure-Function Relationships: Comparing Solution with Polymerase Structures. Chem. Res. Toxicol. 2008, 21, 45–52. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cao, D.; Liang, B. Cryo-Electron Microscopy Structures of the Pneumoviridae Polymerases. Viral Immunol. 2021, 34, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Wolf, M. Cryo-Electron Microscopy for Structural Analysis of Dynamic Biological Macromolecules. Biochim. Biophys. Acta-Gen. Subj. 2018, 1862, 324–334. [Google Scholar] [CrossRef]
- Bhella, D. Cryo-Electron Microscopy: An Introduction to the Technique, and Considerations When Working to Establish a National Facility. Biophys. Rev. 2019, 11, 515–519. [Google Scholar] [CrossRef]
- Joyce, C.M. Techniques Used to Study the DNA Polymerase Reaction Pathway. Biochim. Biophys. Acta 2010, 1804, 1032–1040. [Google Scholar] [CrossRef]
- Lavrik, O.; Belousova, E.; Crespan, E.; Lebedeva, N.; Rechkunova, N.; Hubscher, U.; Maga, G. Photoreactive DNA Probes as a Tool for Studying the Translesion Synthesis System in Mammalian Cell Extracts. Med. Chem. 2008, 4, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Ruoho, A.E.; Kiefer, H.; Roeder, P.E.; Singer, S.J. The Mechanism of Photoaffinity Labeling. Proc. Natl. Acad. Sci. USA 1973, 70, 2567–2571. [Google Scholar] [CrossRef] [PubMed]
- Murale, D.P.; Hong, S.C.; Haque, M.M.; Lee, J.S. Photo-Affinity Labeling (PAL) in Chemical Proteomics: A Handy Tool to Investigate Protein-Protein Interactions (PPIs). Proteome Sci. 2017, 15, 14. [Google Scholar] [CrossRef]
- Knorre, D.G.; Godovikova, T.S. Photoaffinity Labeling as an Approach to Study Supramolecular Nucleoprotein Complexes. FEBS Lett. 1998, 433, 9–14. [Google Scholar] [CrossRef]
- Grachev, M.A.; Mustaev, A.A. Cyclic Adenosine-5′-Trimetaphosphate Phosphorylates a Histidine Residue Nearby the Initiating Substrate Binding Site of Escherichia coli DNA-Dependent RNA-Polymerase. FEBS Lett. 1982, 137, 89–94. [Google Scholar] [CrossRef]
- Lavrik, O.I.; Prasad, R.; Beard, W.A.; Safronov, I.V.; Dobrikov, M.I.; Srivastava, D.K.; Shishkin, G.V.; Wood, T.G.; Wilson, S.H. DNTP Binding to HIV-1 Reverse Transcriptase and Mammalian DNA Polymerase β as Revealed by Affinity Labeling with a Photoreactive DNTP Analog. J. Biol. Chem. 1996, 271, 21891–21897. [Google Scholar] [CrossRef]
- Doronin, S.V.; Dobrikov, M.I.; Buckle, M.; Roux, P.; Buc, H.; Lavrik, O.I. Affinity Modification of Human Immunodeficiency Virus Reverse Transcriptase and DNA Template by Photoreactive DCTP Analogs. FEBS Lett. 1994, 354, 200–202. [Google Scholar] [CrossRef]
- Doronin, S.V.; Dobrikov, M.I.; Lavrik, O.I. Photoaffinity Labeling of DNA Polymerase α DNA Primase Complex Based on the Catalytic Competence of a DNTP Reactive Analog. FEBS Lett. 1992, 313, 31–33. [Google Scholar] [CrossRef]
- Mitina, R.L.; Mustaev, A.A.; Zaychikov, E.F.; Khomov, V.V.; Lavrik, O.I. Highly Selective Affinity Labeling of the Primer-Binding Site of E. coli DNA Polymerase I. FEBS Lett. 1990, 272, 181–183. [Google Scholar] [CrossRef]
- Zakharova, O.D.; Podust, V.N.; Mustaev, A.A.; Anarbaev, R.O.; Lavrik, O.I. Highly Selective Affinity Labeling of DNA Polymerase α-Primase from Human Placenta by Reactive Analogs of ATP. Biochimie 1995, 77, 699–702. [Google Scholar] [CrossRef]
- Kuchta, R.D.; Mizrahi, V.; Benkovic, P.A.; Johnson, K.A.; Benkovic, S.J. Kinetic Mechanism of DNA Polymerase I (Klenow). Biochemistry 1987, 26, 8410–8417. [Google Scholar] [CrossRef]
- Dahlberg, M.E.; Benkovic, S.J. Kinetic Mechanism of DNA Polymerase I (Klenow Fragment): Identification of a Second Conformational Change and Evaluation of the Internal Equilibrium Constant. Biochemistry 1991, 30, 4835–4843. [Google Scholar] [CrossRef] [PubMed]
- Datta, K.; LiCata, V.J. Thermodynamics of the Binding of Thermus Aquaticus DNA Polymerase to Primed-Template DNA. Nucleic Acids Res. 2003, 31, 5590–5597. [Google Scholar] [CrossRef][Green Version]
- Datta, K.; Wowor, A.J.; Richard, A.J.; LiCata, V.J. Temperature Dependence and Thermodynamics of Klenow Polymerase Binding to Primed-Template DNA. Biophys. J. 2006, 90, 1739–1751. [Google Scholar] [CrossRef] [PubMed]
- Datta, K.; LiCata, V.J. Salt Dependence of DNA Binding by Thermus Aquaticus and Escherichia coli DNA Polymerases. J. Biol. Chem. 2003, 278, 5694–5701. [Google Scholar] [CrossRef] [PubMed]
- Purohit, V.; Grindley, N.D.F.; Joyce, C.M. Use of 2-Aminopurine Fluorescence to Examine Conformational Changes during Nucleotide Incorporation by DNA Polymerase I (Klenow Fragment). Biochemistry 2003, 42, 10200–10211. [Google Scholar] [CrossRef]
- Sandin, P.; Stengel, G.; Ljungdahl, T.; Borjesson, K.; Macao, B.; Wilhelmsson, L.M. Highly Efficient Incorporation of the Fluorescent Nucleotide Analogs TC and TCO by Klenow Fragment. Nucleic Acids Res. 2009, 37, 3924–3933. [Google Scholar] [CrossRef] [PubMed]
- Stengel, G.; Knoll, W. Surface Plasmon Field-Enhanced Fluorescence Spectroscopy Studies of Primer Extension Reactions. Nucleic Acids Res. 2005, 33, e69. [Google Scholar] [CrossRef][Green Version]
- Driscoll, M.D.; Rentergent, J.; Hay, S. A Quantitative Fluorescence-Based Steady-State Assay of DNA Polymerase. FEBS J. 2014, 281, 2042–2050. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, J.L.; Rejali, N.; Wittwer, C.T. Stopped-Flow DNA Polymerase Assay by Continuous Monitoring of DNTP Incorporation by Fluorescence. Anal. Biochem. 2013, 441, 133–139. [Google Scholar] [CrossRef]
- Schwartz, J.J.; Quake, S.R. Single Molecule Measurement of the “Speed Limit” of DNA Polymerase. Proc. Natl. Acad. Sci. USA 2009, 106, 20294–20299. [Google Scholar] [CrossRef] [PubMed]
- Santoso, Y.; Joyce, C.M.; Potapova, O.; Le Reste, L.; Hohlbein, J.; Torella, J.P.; Grindley, N.D.F.; Kapanidis, A.N. Conformational Transitions in DNA Polymerase I Revealed by Single-Molecule FRET. Proc. Natl. Acad. Sci. USA 2010, 107, 715–720. [Google Scholar] [CrossRef]
- Markiewicz, R.P.; Vrtis, K.B.; Rueda, D.; Romano, L.J. Single-Molecule Microscopy Reveals New Insights into Nucleotide Selection by DNA Polymerase I. Nucleic Acids Res. 2012, 40, 7975–7984. [Google Scholar] [CrossRef] [PubMed]
- Pauszek, R.F.; Lamichhane, R.; Singh, A.R.; Millar, D.P. Single-Molecule View of Coordination in a Multi-Functional DNA Polymerase. eLife 2021, 10, e62046. [Google Scholar] [CrossRef]
- Geertsema, H.J.; Kulczyk, A.W.; Richardson, C.C.; Van Oijen, A.M. Single-Molecule Studies of Polymerase Dynamics and Stoichiometry at the Bacteriophage T7 Replication Machinery. Proc. Natl. Acad. Sci. USA 2014, 111, 4073–4078. [Google Scholar] [CrossRef] [PubMed]
- Turvey, M.W.; Gabriel, K.N.; Lee, W.; Taulbee, J.J.; Kim, J.K.; Chen, S.; Lau, C.J.; Kattan, R.E.; Pham, J.T.; Majumdar, S.; et al. Single-Molecule Taq DNA Polymerase Dynamics. Sci. Adv. 2022, 8, eabl3522. [Google Scholar] [CrossRef]
- Mazumder, A.; Wang, A.; Uhm, H.; Ebright, R.H.; Kapanidis, A.N. RNA Polymerase Clamp Conformational Dynamics: Long-Lived States and Modulation by Crowding, Cations, and Nonspecific DNA Binding. Nucleic Acids Res. 2021, 49, 2790–2802. [Google Scholar] [CrossRef]
- Sun, B.; Wang, M.D. Single-Molecule Optical-Trapping Techniques to Study Molecular Mechanisms of a Replisome, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; Volume 582. [Google Scholar]
- Morin, J.A.; Cao, F.J.; Lázaro, J.M.; Arias-Gonzalez, J.R.; Valpuesta, J.M.; Carrascosa, J.L.; Salas, M.; Ibarra, B. Mechano-Chemical Kinetics of DNA Replication: Identification of the Translocation Step of a Replicative DNA Polymerase. Nucleic Acids Res. 2015, 43, 3643–3652. [Google Scholar] [CrossRef]
- Stoloff, D.H.; Wanunu, M. Recent Trends in Nanopores for Biotechnology. Curr. Opin. Biotechnol. 2013, 24, 699–704. [Google Scholar] [CrossRef]
- Hurt, N.; Wang, H.; Akeson, M.; Lieberman, K.R. Specific Nucleotide Binding and Rebinding to Individual DNA Polymerase Complexes Captured on a Nanopore. J. Am. Chem. Soc. 2009, 131, 3772–3778. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, P.Y.; Yang, M. Kinetic Study of Various Binding Modes between Human DNA Polymerase β and Different DNA Substrates by Surface-Plasmon-Resonance Biosensor. Biochem. J. 2002, 361, 317–325. [Google Scholar] [CrossRef]
- Tsoi, P.Y.; Zhang, X.; Sui, S.F.; Yang, M. Effects of DNA Mismatches on Binding Affinity and Kinetics of Polymerase-DNA Complexes as Revealed by Surface Plasmon Resonance Biosensor. Analyst 2003, 128, 1169–1174. [Google Scholar] [CrossRef]
- Johnson, K.A. Kinetic Analysis for the New Enzymology: Using Computer Simulation to Learn Kinetics and Solve Mechanisms; Kintek Corporation: Austin, TX, USA, 2019; ISBN 9781733998208, 1733998209. [Google Scholar]
- Patel, S.S.; Wong, I.; Johnson, K.A. Pre-Steady-State Kinetic Analysis of Processive DNA Replication Including Complete Characterization of an Exonuclease-Deficient Mutant. Biochemistry 1991, 30, 511–525. [Google Scholar] [CrossRef]
- Wu, P.; Nossal, N.; Benkovic, S.J. Kinetic Characterization of a Bacteriophage T4 Antimutator DNA Polymerase. Biochemistry 1998, 37, 14748–14755. [Google Scholar] [CrossRef] [PubMed]
- Baranovskiy, A.G.; Duong, V.N.; Babayeva, N.D.; Zhang, Y.; Pavlov, Y.I.; Anderson, K.S.; Tahirov, T.H. Activity and Fidelity of Human DNA Polymerase Depend on Primer Structure. J. Biol. Chem. 2018, 293, 6824–6843. [Google Scholar] [CrossRef]
- Zahurancik, W.J.; Suo, Z. Kinetic Investigation of the Polymerase and Exonuclease Activities of Human DNA Polymerase e Holoenzyme. J. Biol. Chem. 2020, 295, 17251–17264. [Google Scholar] [CrossRef] [PubMed]
- Lowe, L.G.; Guengerich, F.P. Steady-State and Pre-Steady-State Kinetic Analysis of DNTP Insertion Opposite 8-Oxo-7,8-Dihydroguanine by Escherichia coli Polymerases I Exo- and II Exo-. Biochemistry 1996, 35, 9840–9849. [Google Scholar] [CrossRef]
- Einolf, H.J.; Guengerich, F.P. Fidelity of Nucleotide Insertion at 8-Oxo-7,8-Dihydroguanine by Mammalian DNA Polymerase Δ: Steady-State and Pre-Steady-State Kinetic Analysis. J. Biol. Chem. 2001, 276, 3764–3771. [Google Scholar] [CrossRef] [PubMed]
- Dieckman, L.M.; Johnson, R.E.; Prakash, S.; Washington, M.T. Pre-Steady State Kinetic Studies of the Fidelity of Nucleotide Incorporation by Yeast DNA Polymerase δ. Biochemistry 2010, 49, 7344–7350. [Google Scholar] [CrossRef] [PubMed]
- Graves, S.W.; Johnson, A.A.; Johnson, K.A. Expression, Purification, and Initial Kinetic Characterization of the Large Subunit of the Human Mitochondrial DNA Polymerase. Biochemistry 1998, 37, 6050–6058. [Google Scholar] [CrossRef]
- Johnson, A.A.; Tsai, Y.C.; Graves, S.W.; Johnson, K.A. Human Mitochondrial DNA Polymerase Holoenzyme: Reconstitution and Characterization. Biochemistry 2000, 39, 1702–1708. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.F.; Joyce, C.M.; Jack, W.E. Comparative Kinetics of Nucleotide Analog Incorporation by Vent DNA Polymerase. J. Biol. Chem. 2004, 279, 11834–11842. [Google Scholar] [CrossRef]
- Yang, G.; Franklin, M.; Li, J.; Lin, T.C.; Konigsberg, W. Correlation of the Kinetics of Finger Domain Mutants in RB69 DNA Polymerase with Its Structure. Biochemistry 2002, 41, 2526–2534. [Google Scholar] [CrossRef]
- Lahiri, I.; Mukherjee, P.; Pata, J.D. Kinetic Characterization of Exonuclease-Deficient Staphylococcus Aureus PolC, a C-Family Replicative DNA Polymerase. PLoS ONE 2013, 8, e63489. [Google Scholar] [CrossRef] [PubMed]
- Schermerhorn, K.M.; Gardner, A.F. Pre-Steady-State Kinetic Analysis of a Family D DNA Polymerase from Thermococcus sp. 9°N Reveals Mechanisms for Archaeal Genomic Replication and Maintenance. J. Biol. Chem. 2015, 290, 21800–21810. [Google Scholar] [CrossRef] [PubMed]
- Werneburg, B.G.; Ahn, J.; Zhong, X.; Hondal, R.J.; Kraynov, V.S.; Tsai, M.D. DNA Polymerase β: Pre-Steady-State Kinetic Analysis and Roles of Arginine-283 in Catalysis and Fidelity. Biochemistry 1996, 35, 7041–7050. [Google Scholar] [CrossRef]
- García-Díaz, M.; Bebenek, K.; Sabariegos, R.; Domínguez, O.; Rodríguez, J.; Kirchhoff, T.; García-Palomero, E.; Picher, A.J.; Juárez, R.; Ruiz, J.F.; et al. DNA Polymerase λ, a Novel DNA Repair Enzyme in Human Cells. J. Biol. Chem. 2002, 277, 13184–13191. [Google Scholar] [CrossRef]
- Fiala, K.A.; Abdel-Gawad, W.; Suo, Z. Pre-Steady-State Kinetic Studies of the Fidelity and Mechanism of Polymerization Catalyzed by Truncated Human DNA Polymerase λ. Biochemistry 2004, 43, 6751–6762. [Google Scholar] [CrossRef]
- Roettger, M.P.; Fiala, K.A.; Sompalli, S.; Dong, Y.; Suo, Z. Pre-Steady-State Kinetic Studies of the Fidelity of Human DNA Polymerase. Biochemistry 2004, 43, 13827–13838. [Google Scholar] [CrossRef]
- Bertram, J.G.; Bloom, L.B.; O’Donnell, M.; Goodman, M.F. Increased DNTP Binding Affinity Reveals a Nonprocessive Role for Escherichia coli β Clamp with DNA Polymerase IV. J. Biol. Chem. 2004, 279, 33047–33050. [Google Scholar] [CrossRef]
- Cramer, J.; Restle, T. Pre-Steady-State Kinetic Characterization of the DinB Homologue DNA Polymerase of Sulfolobus solfataricus. J. Biol. Chem. 2005, 280, 40552–40558. [Google Scholar] [CrossRef]
- Fiala, K.A.; Suo, Z. Pre-Steady-State Kinetic Studies of the Fidelity of Sulfolobus solfataricus P2 DNA Polymerase IV. Biochemistry 2004, 43, 2106–2115. [Google Scholar] [CrossRef] [PubMed]
- Fiala, K.A.; Sherrer, S.M.; Brown, J.A.; Suo, Z. Mechanistic Consequences of Temperature on DNA Polymerization Catalyzed by a Y-Family DNA Polymerase. Nucleic Acids Res. 2008, 36, 1990–2001. [Google Scholar] [CrossRef]
- Brown, J.A.; Zhang, L.; Sherrer, S.M.; Taylor, J.S.; Burgers, P.M.J.; Suo, Z. Pre-Steady-State Kinetic Analysis of Truncated and Full-Length Saccharomyces Cerevisiae DNA Polymerase Eta. J. Nucleic Acids 2010, 2010, 871939. [Google Scholar] [CrossRef]
- Kati, W.M.; Johnson, K.A.; Jerva, L.F.; Anderson, K.S. Mechanism and Fidelity of HIV Reverse Transcriptase. J. Biol. Chem. 1992, 267, 25988–25997. [Google Scholar] [CrossRef]
- Brautigam, C.A.; Steitz, T.A. Structural and Functional Insights Provided by Crystal Structures of DNA Polymerases and Their Substrate Complexes. Curr. Biol. 1998, 8, 54–63. [Google Scholar] [CrossRef]
- Joyce, C.M. DNA Polymerase I, Bacterial, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; ISBN 9780123786319. [Google Scholar]
- Wood, R.D.; Doublié, S. DNA Polymerase θ (POLQ), Double-Strand Break Repair, and Cancer. DNA Repair 2016, 44, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Arana, M.E.; Seki, M.; Wood, R.D.; Rogozin, I.B.; Kunkel, T.A. Low-Fidelity DNA Synthesis by Human DNA Polymerase Theta. Nucleic Acids Res. 2008, 36, 3847–3856. [Google Scholar] [CrossRef]
- Doublié, S.; Zahn, K.E. Structural Insights into Eukaryotic DNA Replication. Front. Microbiol. 2014, 5, 444. [Google Scholar] [CrossRef]
- Pellegrini, L. The Pol α -Primase Complex. Subcell. Biochem. 2012, 62, 157–169. [Google Scholar] [CrossRef]
- Zahurancik, W.J.; Klein, S.J.; Suo, Z. Significant Contribution of the 3′→5′ Exonuclease Activity to the High Fidelity of Nucleotide Incorporation Catalyzed by Human DNA Polymerase ∈. Nucleic Acids Res. 2014, 42, 13853–13860. [Google Scholar] [CrossRef]
- Prindle, M.J.; Loeb, L.A. DNA Polymerase Delta in DNA Replication and Genome Maintenance. Environ. Mol. Mutagen. 2012, 53, 666–682. [Google Scholar] [CrossRef]
- Foley, M.C.; Couto, L.; Rauf, S.; Boyke, A. Insights into DNA Polymerase δ’s Mechanism for Accurate DNA Replication. J. Mol. Model. 2019, 25, 80. [Google Scholar] [CrossRef]
- Khandagale, P.; Peroumal, D.; Manohar, K.; Acharya, N. Human DNA Polymerase Delta Is a Pentameric Holoenzyme with a Dimeric P12 Subunit. Life Sci. Alliance 2019, 2, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pursell, Z.F.; Linn, S. Identification and Cloning of Two Histone Fold Motif-Containing Subunits of HeLa DNA Polymerase ε. J. Biol. Chem. 2000, 275, 23247–23252. [Google Scholar] [CrossRef] [PubMed]
- Muzi-Falconi, M.; Giannattasio, M.; Foiani, M.; Plevani, P. The DNA Polymerase Alpha-Primase Complex: Multiple Functions and Interactions. Sci. World J. 2003, 3, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Ramírez, R.; Klinge, S.; Sauguet, L.; Melero, R.; Recuero-Checa, M.A.; Kilkenny, M.; Perera, R.L.; García-Alvarez, B.; Hall, R.J.; Nogales, E.; et al. Flexible Tethering of Primase and DNA Pol α in the Eukaryotic Primosome. Nucleic Acids Res. 2011, 39, 8187–8199. [Google Scholar] [CrossRef]
- Jain, R.; Hammel, M.; Johnson, R.E.; Prakash, L.; Prakash, S.; Aggarwal, A.K. Structural Insights into Yeast DNA Polymerase δ by Small Angle X-ray Scattering. J. Mol. Biol. 2009, 394, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Rice, W.J.; Malik, R.; Johnson, R.E.; Prakash, L.; Prakash, S.; Ubarretxena-Belandia, I.; Aggarwal, A.K. Cryo-EM Structure and Dynamics of Eukaryotic DNA Polymerase δ Holoenzyme. Nat. Struct. Mol. Biol. 2019, 26, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Asturias, F.J.; Cheung, I.K.; Sabouri, N.; Chilkova, O.; Wepplo, D.; Johansson, E. Structure of Saccharomyces Cerevisiae DNA Polymerase Epsilon by Cryo-Electron Microscopy. Nat. Struct. Mol. Biol. 2006, 13, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Lancey, C.; Tehseen, M.; Raducanu, V.S.; Rashid, F.; Merino, N.; Ragan, T.J.; Savva, C.G.; Zaher, M.S.; Shirbini, A.; Blanco, F.J.; et al. Structure of the Processive Human Pol δ Holoenzyme. Nat. Commun. 2020, 11, 1109. [Google Scholar] [CrossRef] [PubMed]
- Baranovskiy, A.G.; Babayeva, N.D.; Zhang, Y.; Gu, J.; Suwa, Y.; Pavlov, Y.I.; Tahirov, T.H. Mechanism of Concerted RNA-DNA Primer Synthesis by the Human Primosome. J. Biol. Chem. 2016, 291, 10006–10020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Baranovskiy, A.G.; Tahirov, T.H.; Pavlov, Y.I. The C-Terminal Domain of the DNA Polymerase Catalytic Subunit Regulates the Primase and Polymerase Activities of the Human DNA Polymerase α-Primase Complex. J. Biol. Chem. 2014, 289, 22021–22034. [Google Scholar] [CrossRef] [PubMed]
- Ganai, R.A.; Osterman, P.; Johansson, E. Yeast DNA Polymerase ε Catalytic Core and Holoenzyme Have Comparable Catalytic Rates. J. Biol. Chem. 2015, 290, 3825–3835. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhou, Y.; Lee, E.Y.C.; Lee, M.Y.W.T.; Frick, D.N. The P12 Subunit of Human Polymerase Delta Modulates the Rate and Fidelity of DNA Synthesis. Biochemistry 2010, 49, 3545–3554. [Google Scholar] [CrossRef]
- Zahurancik, W.J.; Klein, S.J.; Suo, Z. Kinetic Mechanism of DNA Polymerization Catalyzed by Human DNA Polymerase ε. Biochemistry 2013, 52, 7041–7049. [Google Scholar] [CrossRef]
- Lamers, M.H.; O’Donnell, M. A Consensus View of DNA Binding by the C Family of Replicative DNA Polymerases. Proc. Natl. Acad. Sci. USA 2008, 105, 20565–20566. [Google Scholar] [CrossRef]
- Bailey, S.; Wing, R.A.; Steitz, T.A. The Structure of T. Aquaticus DNA Polymerase III Is Distinct from Eukaryotic Replicative DNA Polymerases. Cell 2006, 126, 893–904. [Google Scholar] [CrossRef]
- Evans, R.J.; Davies, D.R.; Bullard, J.M.; Christensen, J.; Green, L.S.; Guiles, J.W.; Pata, J.D.; Ribble, W.K.; Janjic, N.; Jarvis, T.C. Structure of PolC Reveals Unique DNA Binding and Fidelity Determinants. Proc. Natl. Acad. Sci. USA 2008, 105, 20695–20700. [Google Scholar] [CrossRef]
- Wing, R.A.; Bailey, S.; Steitz, T.A. Insights into the Replisome from the Structure of a Ternary Complex of the DNA Polymerase III Alpha-Subunit. J. Mol. Biol. 2008, 382, 859–869. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lamers, M.H.; Georgescu, R.E.; Lee, S.G.; O’Donnell, M.; Kuriyan, J. Crystal Structure of the Catalytic α Subunit of E. coli Replicative DNA Polymerase III. Cell 2006, 126, 881–892. [Google Scholar] [CrossRef]
- Filée, J.; Forterre, P.; Sen-Lin, T.; Laurent, J. Evolution of DNA Polymerase Families: Evidences for Multiple Gene Exchange between Cellular and Viral Proteins. J. Mol. Evol. 2002, 54, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Takeshi, N. NII-Electronic Library Service. Chem. Pharm. Bull. 1977, 57, 364–370. [Google Scholar]
- Ishino, Y.; Komori, K.; Cann, I.K.O.; Koga, Y. A Novel DNA Polymerase Family Found in Archaea. J. Bacteriol. 1998, 180, 2232–2236. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Tang, X.F.; Matsui, I. Subunit Interaction and Regulation of Activity through Terminal Domains of the Family D DNA Polymerase from Pyrococcus Horikoshii. J. Biol. Chem. 2003, 278, 21247–21257. [Google Scholar] [CrossRef]
- Shen, Y.; Tang, X.F.; Matsui, E.; Matsui, I. Subunit Interaction and Regulation of Activity through Terminal Domains of the Family D DNA Polymerase from Pyrococcus Horikoshii. Biochem. Soc. Trans. 2004, 32, 245–249. [Google Scholar] [CrossRef]
- Sauguet, L.; Raia, P.; Henneke, G.; Delarue, M. Shared Active Site Architecture between Archaeal PolD and Multi-Subunit RNA Polymerases Revealed by X-Ray Crystallography. Nat. Commun. 2016, 7, 12227. [Google Scholar] [CrossRef]
- Ruprich-Robert, G.; Thuriaux, P. Non-Canonical DNA Transcription Enzymes and the Conservation of Two-Barrel RNA Polymerases. Nucleic Acids Res. 2010, 38, 4559–4569. [Google Scholar] [CrossRef] [PubMed]
- Raia, P.; Carroni, M.; Henry, E.; Pehau-Arnaudet, G.; Brûlé, S.; Béguin, P.; Henneke, G.; Lindahl, E.; Delarue, M.; Sauguet, L. Structure of the DP1-DP2 PolD Complex Bound with DNA and Its Implications for the Evolutionary History of DNA and RNA Polymerases. PLoS Biol. 2019, 17, e3000122. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, S.; Carr, P.D.; Brown, S.E.; Ollis, D.L.; Dixon, N.E. Structural Basis for Proofreading during Replication of the Escherichia coli Chromosome. Structure 2002, 10, 535–546. [Google Scholar] [CrossRef]
- Belousova, E.A.; Lavrik, O.I. DNA Polymerases β and λ and Their Roles in Cell. DNA Repair 2015, 29, 112–126. [Google Scholar] [CrossRef]
- Sawaya, M.R.; Prasad, R.; Wilson, S.H.; Kraut, J.; Pelletier, H. Crystal Structures of Human DNA Polymerase β Complexed with Gapped and Nicked DNA: Evidence for an Induced Fit Mechanism. Biochemistry 1997, 36, 11205–11215. [Google Scholar] [CrossRef]
- Beard, W.A.; Wilson, S.H. Structure and Mechanism of DNA Polymerase β. Chem Rev. 2006, 106, 361–382. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, B.; Hübscher, U.; Maga, G. Living on the Edge: DNA Polymerase Lambda between Genome Stability and Mutagenesis. Chem. Res. Toxicol. 2017, 30, 1936–1941. [Google Scholar] [CrossRef]
- Garcia-Diaz, M.; Bebenek, K.; Krahn, J.M.; Kunkel, T.A.; Pedersen, L.C. A Closed Conformation for the Pol λ Catalytic Cycle. Nat. Struct. Mol. Biol. 2005, 12, 97–98. [Google Scholar] [CrossRef] [PubMed]
- Moon, A.F.; Garcia-Diaz, M.; Batra, V.K.; Beard, W.A.; Bebenek, K.; Kunkel, T.A.; Wilson, S.H.; Pedersen, L.C. The X Family Portrait: Structural Insights into Biological Functions of X Family Polymerases. DNA Repair 2007, 6, 1709–1725. [Google Scholar] [CrossRef] [PubMed]
- Bebenek, K.; Pedersen, L.C.; Kunkel, T.A. Structure-Function Studies of DNA Polymerase λ. Biochemistry 2014, 53, 2781–2792. [Google Scholar] [CrossRef]
- Moon, A.F.; Garcia-Diaz, M.; Bebenek, K.; Davis, B.J.; Zhong, X.; Ramsden, D.A.; Kunkel, T.A.; Pedersen, L.C. Structural Insight into the Substrate Specificity of DNA Polymerase μ. Nat. Struct. Mol. Biol. 2007, 14, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Juárez, R.; Ruiz, J.F.; McElhinny, S.A.N.; Ramsden, D.; Blanco, L. A Specific Loop in Human DNA Polymerase Mu Allows Switching between Creative and DNA-Instructed Synthesis. Nucleic Acids Res. 2006, 34, 4572–4582. [Google Scholar] [CrossRef]
- Moon, A.F.; Pryor, J.M.; Ramsden, D.A.; Kunkel, T.A.; Bebenek, K.; Pedersen, L.C. Sustained Active Site Rigidity during Synthesis by Human DNA Polymerase μ. Nat. Struct. Mol. Biol. 2014, 21, 253–260. [Google Scholar] [CrossRef]
- Dominguez, O. DNA Polymerase Mu (Pol Micro), Homologous to TdT, Could Act as a DNA Mutator in Eukaryotic Cells. EMBO J. 2000, 19, 1731–1742. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, X.; Yuan, F.; Xie, Z.; Wang, Z. Highly Frequent Frameshift DNA Synthesis by Human DNA Polymerase μ. Mol. Cell. Biol. 2001, 21, 7995–8006. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Freudenthal, B.D.; Beard, W.A.; Shock, D.D.; Wilson, S.H. XObserving a DNA Polymerase Choose Right from Wrong. Cell 2013, 154, 157. [Google Scholar] [CrossRef]
- Freudenthal, B.D.; Beard, W.A.; Perera, L.; Shock, D.D.; Kim, T.; Schlick, T.; Wilson, S.H. Uncovering the Polymerase-Induced Cytotoxicity of an Oxidized Nucleotide. Nature 2015, 517, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Vyas, R.; Reed, A.J.; Tokarsky, E.J.; Suo, Z. Viewing Human DNA Polymerase β Faithfully and Unfaithfully Bypass an Oxidative Lesion by Time-Dependent Crystallography. J. Am. Chem. Soc. 2015, 137, 5225–5230. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, A.M.; Smith, M.R.; Schaich, M.A.; Freudenthal, B.D. Capturing a Mammalian DNA Polymerase Extending from an Oxidized Nucleotide. Nucleic Acids Res. 2017, 45, 6934–6944. [Google Scholar] [CrossRef]
- Reed, A.J.; Suo, Z. Time-Dependent Extension from an 8-Oxoguanine Lesion by Human DNA Polymerase Beta. J. Am. Chem. Soc. 2017, 139, 9684–9690. [Google Scholar] [CrossRef]
- Jamsen, J.A.; Beard, W.A.; Pedersen, L.C.; Shock, D.D.; Moon, A.F.; Krahn, J.M.; Bebenek, K.; Kunkel, T.A.; Wilson, S.H. Time-Lapse Crystallography Snapshots of a Double-Strand Break Repair Polymerase in Action. Nat. Commun. 2017, 8, 253. [Google Scholar] [CrossRef]
- Loc’h, J.; Delarue, M. Terminal Deoxynucleotidyltransferase: The Story of an Untemplated DNA Polymerase Capable of DNA Bridging and Templated Synthesis across Strands. Curr. Opin. Struct. Biol. 2018, 53, 22–31. [Google Scholar] [CrossRef]
- Delarue, M.; Boulé, J.B.; Lescar, J.; Expert-Bezançon, N.; Jourdan, N.; Sukumar, N.; Rougeon, F.; Papanicolaou, C. Crystal Structures of a Template-Independent DNA Polymerase: Murine Terminal Deoxynucleotidyltransferase. EMBO J. 2002, 21, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Gouge, J.; Rosario, S.; Romain, F.; Beguin, P.; Delarue, M. Structures of Intermediates along the Catalytic Cycle of Terminal Deoxynucleotidyltransferase: Dynamical Aspects of the Two-Metal Ion Mechanism. J. Mol. Biol. 2013, 425, 4334–4352. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.S.; Murakami, K.S. Watching the Bacteriophage N4 RNA Polymerase Transcription by Time-Dependent Soak-Trigger-Freeze X-ray Crystallography. J. Biol. Chem. 2013, 288, 3305–3311. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Woodgate, R. What a Difference a Decade Makes: Insights into Translesion DNA Synthesis. Proc. Natl. Acad. Sci. USA 2007, 104, 15591–15598. [Google Scholar] [CrossRef]
- Waters, L.S.; Minesinger, B.K.; Wiltrout, M.E.; D’Souza, S.; Woodruff, R.V.; Walker, G.C. Eukaryotic Translesion Polymerases and Their Roles and Regulation in DNA Damage Tolerance. Microbiol. Mol. Biol. Rev. 2009, 73, 134–154. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.C.; Jackson, M.A.; Pata, J.D. Y-Family Polymerase Conformation Is a Major Determinant of Fidelity and Translesion Specificity. Structure 2013, 21, 20–31. [Google Scholar] [CrossRef]
- Alt, A.; Lammens, K.; Chiocchini, C.; Lammens, A.; Pieck, J.C.; Kuch, D.; Hopfner, K.-P.; Carell, T. Bypass of DNA Lesions Generated During Anticancer Treatment with Cisplatin by DNA Polymerase Eta. Science 2007, 318, 967–970. [Google Scholar] [CrossRef]
- Zhao, Y.; Gregory, M.T.; Biertümpfel, C.; Hua, Y.J.; Hanaoka, F.; Yang, W. Mechanism of Somatic Hypermutation at the WA Motif by Human DNA Polymerase N. Proc. Natl. Acad. Sci. USA 2013, 110, 8146–8151. [Google Scholar] [CrossRef]
- Wong, J.H.; Fiala, K.A.; Suo, Z.; Ling, H. Snapshots of a Y-Family DNA Polymerase in Replication: Substrate-Induced Conformational Transitions and Implications for Fidelity of Dpo4. J. Mol. Biol. 2008, 379, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Uljon, S.N.; Johnson, R.E.; Edwards, T.A.; Prakash, S.; Prakash, L.; Aggarwal, A.K. Crystal Structure of the Catalytic Core of Human DNA Polymerase Kappa. Structure 2004, 12, 1395–1404. [Google Scholar] [CrossRef]
- Bauer, J.; Xing, G.; Yagi, H.; Sayer, J.M.; Jerina, D.M.; Ling, H. A Structural Gap in Dpo4 Supports Mutagenic Bypass of a Major Benzo [a]Pyrene DG Adduct in DNA through Template Misalignment. Proc. Natl. Acad. Sci. USA 2007, 104, 14905–14910. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Tang, T.S.; Zhang, H.; Wang, Z.; Friedberg, E.; Yang, W.; Guo, C. Variants of Mouse DNA Polymerase κ Reveal a Mechanism of Efficient and Accurate Translesion Synthesis Past a Benzo[a]Pyrene DG Adduct. Proc. Natl. Acad. Sci. USA 2014, 111, 1789–1794. [Google Scholar] [CrossRef]
- Wu, Y.; Wilson, R.C.; Pata, J.D. The Y-Family DNA Polymerase Dpo4 Uses a Template Slippage Mechanism to Create Single-Base Deletions. J. Bacteriol. 2011, 193, 2630–2636. [Google Scholar] [CrossRef][Green Version]
- Gao, Y.; Yang, W. Capture of a Third Mg2+ Is Essential for Catalyzing DNA Synthesis. Science 2016, 352, 1334–1337. [Google Scholar] [CrossRef]
- Kohlstaed, L.A.; Wang, J.; Friedman, J.M.; Rice, P.A.; Steitz, T.A. Crystal Structure at 3.5 Å Resolution of h Iv -1 Reverse Transcriptase Complexed with an Inhibitor. Science 2020, 256, 254–261. [Google Scholar] [CrossRef]
- Jacobo-Molina, A.; Ding, J.; Nanni, R.G.; Clark, A.D.; Lu, X.; Tantillo, C.; Williams, R.L.; Kamer, G.; Ferris, A.L.; Clark, P.; et al. Crystal Structure of Human Immunodeficiency Virus Type 1 Reverse Transcriptase Complexed with Double-Stranded DNA at 3.0 Å Resolution Shows Bent DNA. Proc. Natl. Acad. Sci. USA 1993, 90, 6320–6324. [Google Scholar] [CrossRef]
- Huang, H.; Chopra, R.; Verdine, G.L.; Harrison, S.C. Structure of a Covalently Trapped Catalytic Complex of HIV-1 Reverse Transcriptase: Implications for Drug Resistance. Science 1998, 282, 1669–1675. [Google Scholar] [CrossRef]
- Kirmizialtin, S.; Nguyen, V.; Johnson, K.A.; Elber, R. How Conformational Dynamics of DNA Polymerase Select Correct Substrates: Experiments and Simulations. Structure 2012, 20, 618–627. [Google Scholar] [CrossRef]
- Kellinger, M.W.; Johnson, K.A. Nucleotide-Dependent Conformational Change Governs Specificity and Analog Discrimination by HIV Reverse Transcriptase. Proc. Natl. Acad. Sci. USA 2010, 107, 7734–7739. [Google Scholar] [CrossRef]
- Kellinger, M.W.; Johnson, K.A. Role of Induced-Fit in Limiting Discrimination Against AZT by HIV Reverse Transcriptase. Biochemistry 2011, 50, 5008–5015. [Google Scholar] [CrossRef] [PubMed]
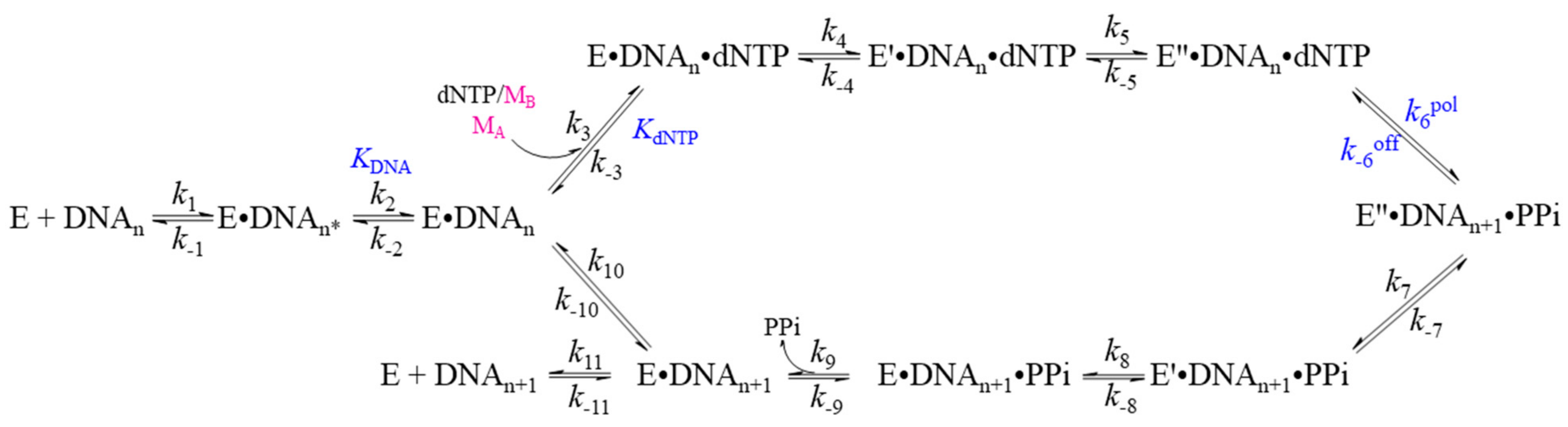
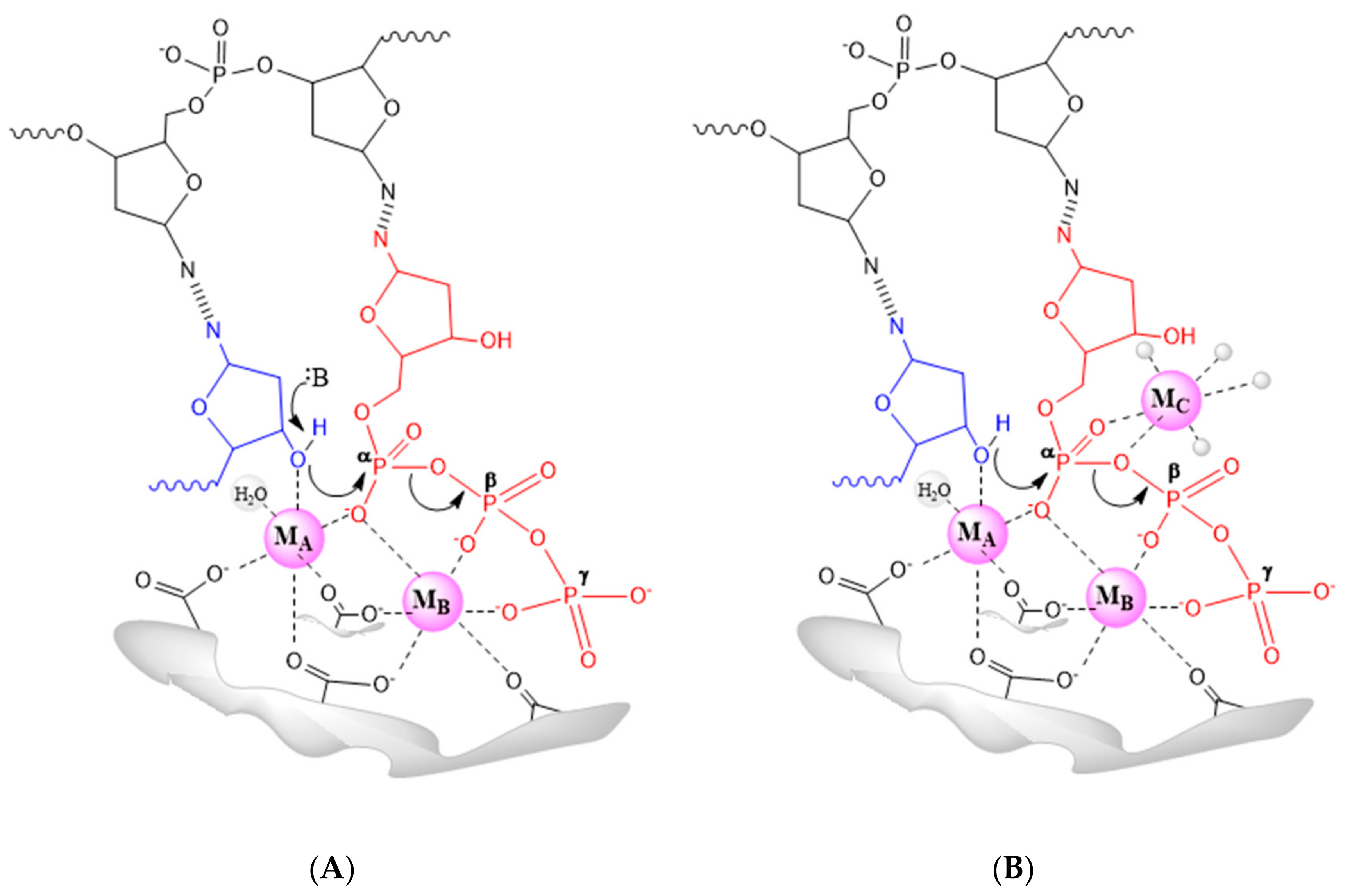
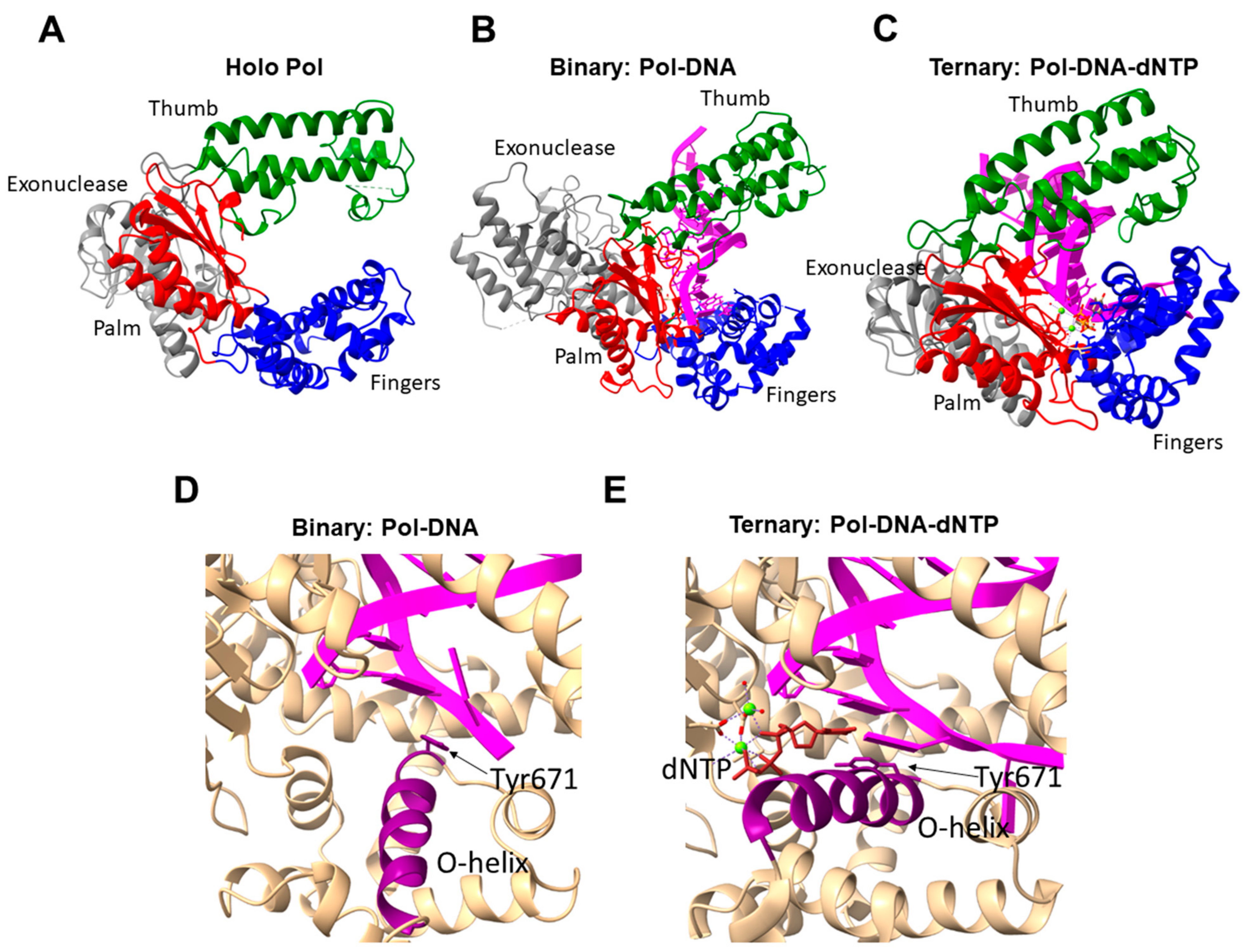
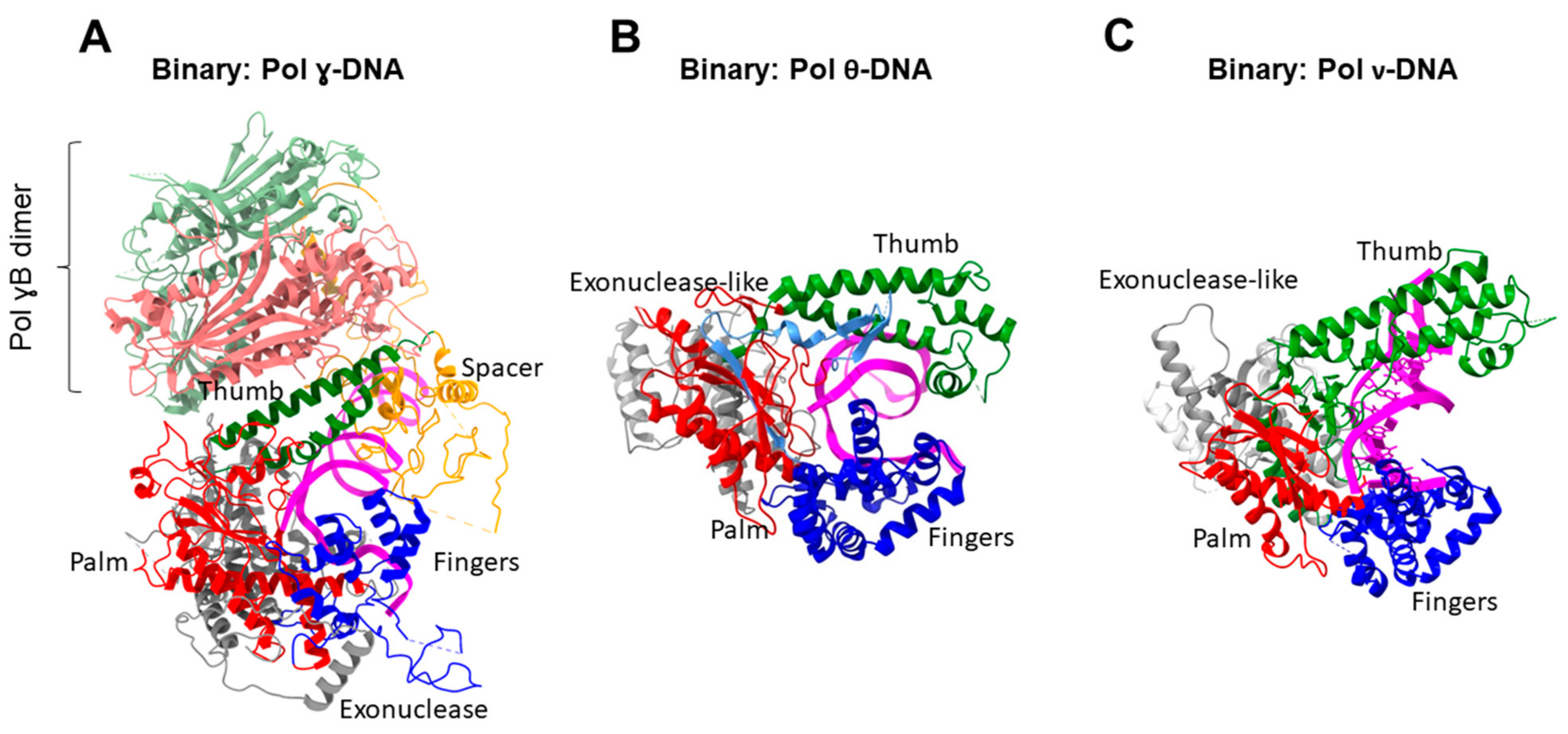

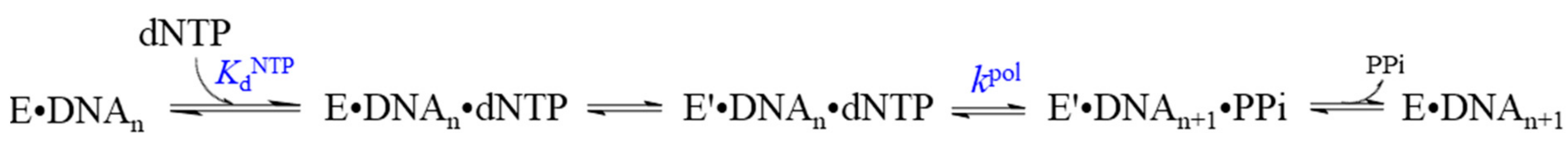
| Family | Taxon | DNA Polymerases | Functions |
|---|---|---|---|
| A | Eukaryota Bacteria Viruses | Pol γ, Pol θ, and Pol ν Pol I T7 DNA Pol | Replication, repair |
| B | Eukaryota Bacteria Archea Viruses | Pol ζ, Pol α, Pol δ, Pol ε Pol II DNA pol B T4 DNA Pol | Replication, repair |
| C | Bacteria | Pol III | Replication |
| D | Archea | Pol D | Replication |
| X | Eukaryota Bacteria Archea Viruses | Pol β, Pol σ, Pol λ, Pol µ, TdT Pol X Pol X ASFV DNA Pol | Repair |
| Y | Eukaryota Bacteria Archea | Rev1, Pol ι, Pol κ, and Pol η Dbh, Pol IV and Pol V Dpo4 DNA Pol | Translesion synthesis |
| RT | Eukaryota Viruses | Telomerase Reverse transcriptase | RNA-dependent DNA synthesis |
| Family | Polymerase Domain | Special Domains | Other Activities Present in DNA Polymerase |
|---|---|---|---|
| A | The catalytic domain includes palm, fingers, thumb subdomains | Exonuclease domain | 3′ → 5′ exonuclease corrective activity (for most members); 5′ → 3′ exonuclease activity |
| B | Multi-subunit complex, catalytic core includes palm, fingers, thumb subdomains | CTD (responsible for connection of the catalytic domain with B-subunit and primase) | 3′ → 5′ exonuclease corrective activity (devoid for most members from eukaryotes); primase activity (DNA synthesis de novo) |
| C | Large multidomain proteins, catalytic core includes palm, fingers, thumb subdomains | PHP domain; β-sliding clamp-binding domain; CTD containing an oligonucleotide-binding fold | 3′ → 5′ exonuclease corrective activity |
| D | The heterodimeric polymerase consisting of DP1 and DP2 subunits | PDE domain; clamp-1 and clamp-2 domains; DPBB-1 and DPBB-2 domains; KH-like domain | 3′ → 5′ exonuclease corrective activity |
| X | Small proteins, catalytic core includes palm, fingers, thumb subdomains | 8 kDa domain; BRCT domain (important for protein–protein interactions) | dRP-lyase activity; single-strand DNA extension (for Pol µ, TdT) |
| Y | The catalytic core includes palm, fingers, thumb subdomains | regulatory region; little fingers domain | translesion DNA synthesis |
| RT | Heterodimeric polymerase consisting of two subunits, catalytic core includes palm, fingers, thumb subdomains | RNase H domain; connection domain | RNA template-dependent DNA polymerase activity; ribonuclease H activity |
| Polymerase | Family | KdDNA, (nM) | KddNTP, (µM) Correct N | kpol, (s−1) | koff, (s−1) | Ref. |
|---|---|---|---|---|---|---|
| Pol I (Klenow) | A | 5 | 5.5 | 50 | 0.2 | [44] |
| DNA polymerase T7 | A | 23 | 18 | 287 | 0.2 | [68] |
| Pol T4 | A | 70 | 20 | 400 | 6 | [69] |
| Human Pol α | B | 58 | 9.2 | 26.8 | 7.0 | [70] |
| Human Pol ε | B | 22 | 11 | 411 | ND | [71] |
| E. coli Pol II | B | 21 | 4.4 | 13.1 | 0.05 | [72] |
| Mammalian Pol δ | B | 300 | 0.93 | 13 | ND | [73] |
| Yeast Pol δ | B | 30 | 24 | 0.93 | 0.03 | [74] |
| Human mitochondrial large subunit Pol γ Holo Pol γ | B | 39 9.9 | 14 0.78 | 3.5 45 | 0.03 0.02 | [75] [76] |
| Vent Pol B | B | 70 | 66 | 1.1 | [77] | |
| RB69 | B | 69 | 200 | 0.35 | [78] | |
| Sau-PolC-∆N∆Exo | C | 390 | 4 | 180 | 150 | [79] |
| Pol D | D | 0.9–2.5 | 1.8–3.1 | 0.4 | [80] | |
| Pol β | X | 49 | 110 | 10 | 0.3 | [81] |
| Pol λ | X | 0.15 | 1.1–2.4 | 3.0–6.0 | ND | [82,83] |
| Pol µ | X | 0.35–1.8 | 0.006–0.076 | ND | [84] | |
| E. coli Pol IV | Y | 50 | 441 | 12 | 0.18 | [85] |
| Sulfolobus solfataricus Dbh | Y | 60 | 600 | 0.64–5.6 | ND | [86] |
| S. solfataricus Dpo4 | Y | 10–40 | 70–230 | 7.6–16.1 | ND | [87,88] |
| Yeast Pol η | Y | 16 | 6.8–15 | 3.9–15.6 | ND | [89] |
| RT | RT | 4.7 | 4–14 | 33–74 | 0.16 | [90] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuznetsova, A.A.; Fedorova, O.S.; Kuznetsov, N.A. Structural and Molecular Kinetic Features of Activities of DNA Polymerases. Int. J. Mol. Sci. 2022, 23, 6373. https://doi.org/10.3390/ijms23126373
Kuznetsova AA, Fedorova OS, Kuznetsov NA. Structural and Molecular Kinetic Features of Activities of DNA Polymerases. International Journal of Molecular Sciences. 2022; 23(12):6373. https://doi.org/10.3390/ijms23126373
Chicago/Turabian StyleKuznetsova, Aleksandra A., Olga S. Fedorova, and Nikita A. Kuznetsov. 2022. "Structural and Molecular Kinetic Features of Activities of DNA Polymerases" International Journal of Molecular Sciences 23, no. 12: 6373. https://doi.org/10.3390/ijms23126373
APA StyleKuznetsova, A. A., Fedorova, O. S., & Kuznetsov, N. A. (2022). Structural and Molecular Kinetic Features of Activities of DNA Polymerases. International Journal of Molecular Sciences, 23(12), 6373. https://doi.org/10.3390/ijms23126373







