Biomarkers of Dementia with Lewy Bodies: Differential Diagnostic with Alzheimer’s Disease
Abstract
:1. Introduction
1.1. Epidemiology of DLB
1.2. Diagnostic Criteria
1.3. Differential Diagnosis with AD and PD
1.4. Therapeutic Management
1.5. Diagnostic Tools Available at the Present Time
2. Methodology
3. Brain Imaging Diagnostic Biomarkers
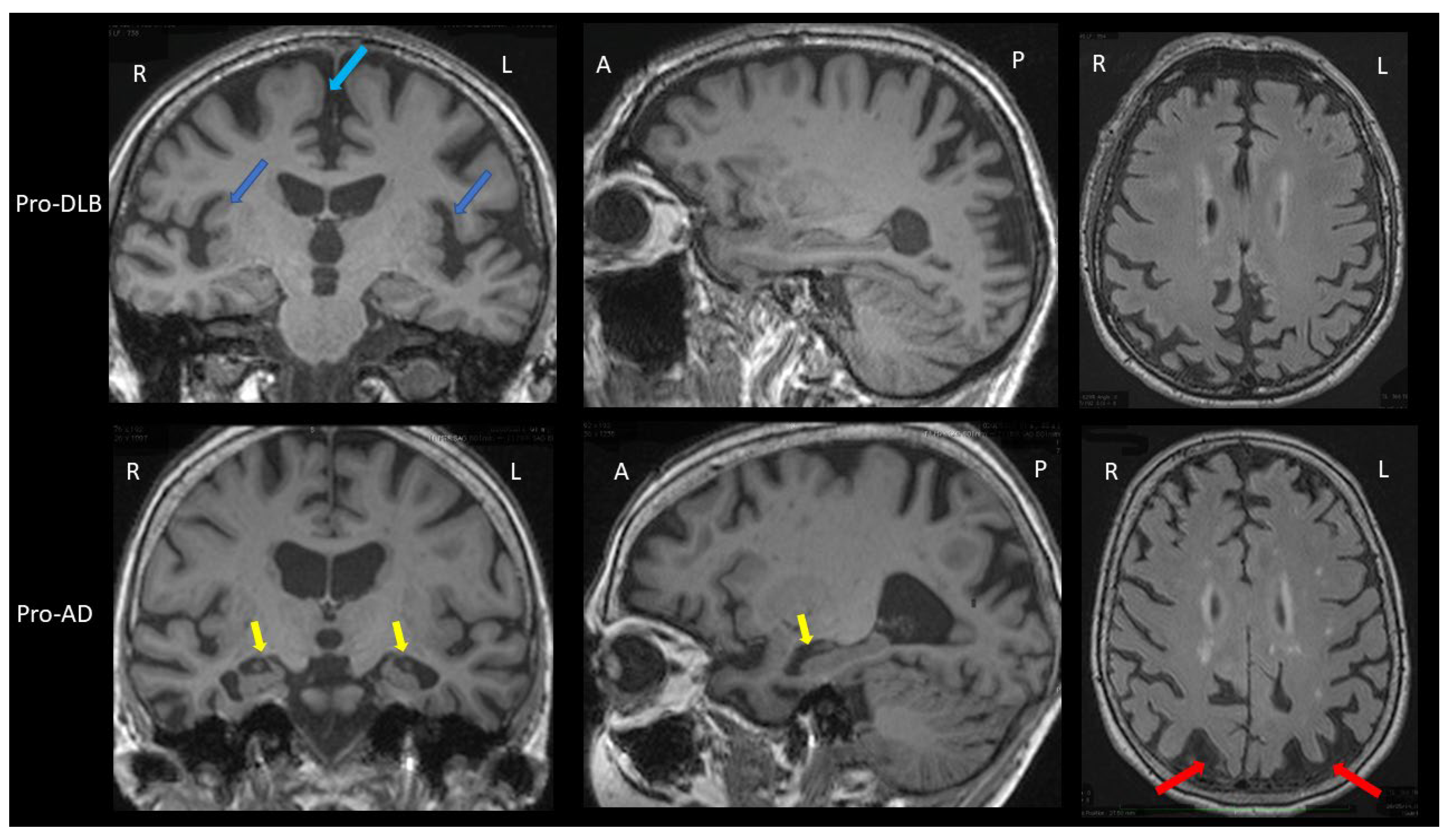
3.1. Brain MRI
3.2. Scintigraphy
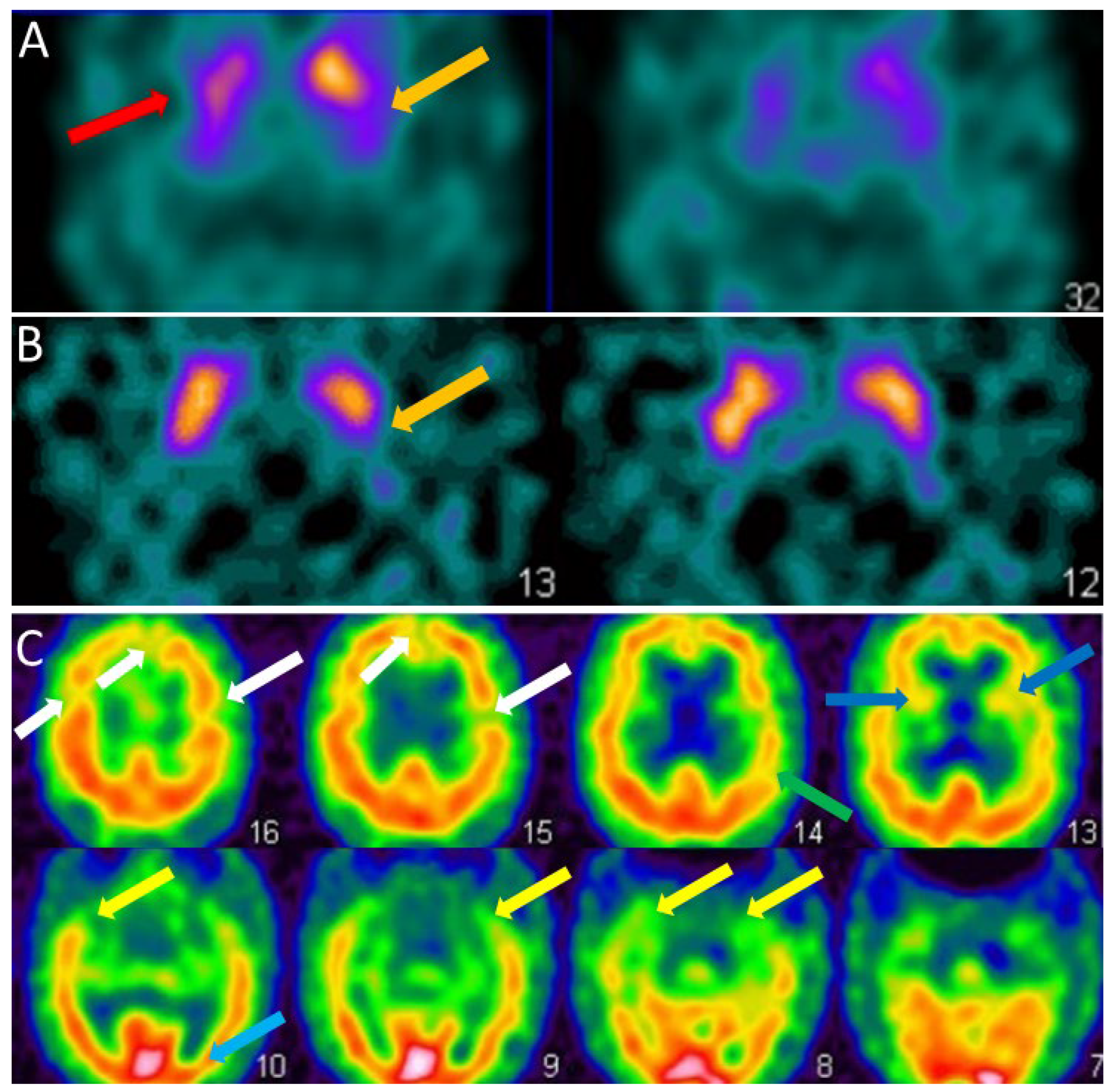
3.2.1. FP-CIT SPECT
3.2.2. Perfusion SPECT
3.2.3. FDG-PET
3.2.4. Synuclein PET
3.2.5. 123 I-Metaiodobenzylguanidine (MIBG) Myocardial Scintigraphy
4. CSF Biomarkers
4.1. RT-QuIC Technique
4.2. Potentially Interesting Biomarkers in the Differential Diagnosis between AD and DLB
4.2.1. Alpha-Synuclein Protease
4.2.2. Neuroinflammation
4.2.3. Neurotransmitter Metabolites
4.2.4. Amino Acids and Neuropeptides
4.2.5. Minerals and Metals
4.2.6. Synaptic Proteins
4.2.7. Others
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zaccai, J.; McCracken, C.; Brayne, C. A systematic review of prevalence and incidence studies of dementia with Lewy bodies. Age Ageing 2005, 34, 561–566. [Google Scholar] [CrossRef] [Green Version]
- McKeith, I.; Mintzer, J.; Aarsland, D.; Burn, D.; Chiu, H.; Cohen-Mansfield, J.; Dickson, D.; Dubois, B.; Duda, J.E.; Feldman, H.; et al. Dementia with Lewy bodies. Lancet Neurol. 2004, 3, 19–28. [Google Scholar] [CrossRef]
- Okazaki, H.; Lipkin, L.E.; Aronson, S.M. Diffuse intracytoplasmic ganglionic inclusions (Lewy type) associated with progressive dementia and quadriparesis in flexion. J. Neuropathol. Exp. Neurol. 1961, 20, 237–244. [Google Scholar] [CrossRef]
- McKeith, I.G.; Galasko, D.; Kosaka, K.; Perry, E.K.; Dickson, D.W.; Hansen, L.A.; Salmon, D.P.; Lowe, J.; Byrne, E.J.; Lennox, G.; et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): Report of the consortium on DLB international workshop. Neurology 1996, 47, 1113–1124. [Google Scholar] [CrossRef]
- McKeith, I.G.; Dickson, D.W.; Lowe, J.; Emre, M.; O’Brien, J.T.; Feldman, H.; Cummings, J.; Duda, J.E.; Lippa, C.; Perry, E.K.; et al. Diagnosis and management of dementia with Lewy bodies: Third report of the DLB Consortium. Neurology 2005, 65, 1863–1872. [Google Scholar] [CrossRef] [Green Version]
- McKeith, I.G.; Boeve, B.F.; Dickson, D.W.; Halliday, G.; Taylor, J.P.; Weintraub, D.; Aarsland, D.; Galvin, J.; Attems, J.; Ballard, C.G.; et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 2017, 89, 88–100. [Google Scholar] [CrossRef] [Green Version]
- McKeith, I.G.; Ferman, T.J.; Thomas, A.J.; Blanc, F.; Boeve, B.F.; Fujishiro, H.; Kantarci, K.; Muscio, C.; O’Brien, J.T.; Postuma, R.B.; et al. Research criteria for the diagnosis of prodromal dementia with Lewy bodies. Neurology 2020, 94, 743–755. [Google Scholar] [CrossRef]
- Donaghy, P.C.; McKeith, I.G. The clinical characteristics of dementia with Lewy bodies and a consideration of prodromal diagnosis. Alzheimer’s Res. Ther. 2014, 6, 46. [Google Scholar] [CrossRef] [Green Version]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. Alpha-synuclein in Lewy bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef] [PubMed]
- Van der Perren, A.; Gelders, G.; Fenyi, A.; Bousset, L.; Brito, F.; Peelaerts, W.; Van den Haute, C.; Gentleman, S.; Melki, R.; Baekelandt, V. The structural differences between patient-derived alpha-synuclein strains dictate characteristics of Parkinson’s disease, multiple system atrophy and dementia with Lewy bodies. Acta Neuropathol. 2020, 139, 977–1000. [Google Scholar] [CrossRef]
- Kemp, J.; Philippi, N.; Phillipps, C.; Demuynck, C.; Albasser, T.; Martin-Hunyadi, C.; Schmidt-Mutter, C.; Cretin, B.; Blanc, F. Cognitive profile in prodromal dementia with Lewy bodies. Alzheimer’s Res. Ther. 2017, 9, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballard, C.; Ziabreva, I.; Perry, R.; Larsen, J.P.; O’Brien, J.; McKeith, I.; Perry, E.; Aarsland, D. Differences in neuropathologic characteristics across the Lewy body dementia spectrum. Neurology 2006, 67, 1931–1934. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, G.; Arcuti, S.; Copetti, M.; Alessandria, M.; Savica, R.; Fontana, A.; Liguori, R.; Logroscino, G. Accuracy of clinical diagnosis of dementia with Lewy bodies: A systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2018, 89, 358–366. [Google Scholar] [CrossRef]
- Nelson, P.T.; Jicha, G.A.; Kryscio, R.J.; Abner, E.L.; Schmitt, F.A.; Cooper, G.; Xu, L.O.; Smith, C.D.; Markesbery, W.R. Low sensitivity in clinical diagnoses of dementia with Lewy bodies. J. Neurol. 2010, 257, 359–366. [Google Scholar] [CrossRef] [Green Version]
- Verny, M.; Blanc, F. Lewy body dementia: Therapeutic propositions according to evidence based medicine and practice. Geriatr. Psychol. Neuropsychiatr. Vieil. 2019, 17, 189–197. [Google Scholar] [PubMed]
- McKeith, I.; Fairbairn, A.; Perry, R.; Thompson, P.; Perry, E. Neuroleptic sensitivity in patients with senile dementia of Lewy body type. BMJ 1992, 305, 673–678. [Google Scholar] [CrossRef] [Green Version]
- Bousiges, O.; Blanc, F. Diagnostic value of cerebro-spinal fluid biomarkers in dementia with lewy bodies. Clin. Chim. Acta 2019, 490, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Bousiges, O.; Bombois, S.; Schraen, S.; Wallon, D.; Quillard, M.M.; Gabelle, A.; Lehmann, S.; Paquet, C.; Amar-Bouaziz, E.; Magnin, E.; et al. Cerebrospinal fluid Alzheimer biomarkers can be useful for discriminating dementia with Lewy bodies from Alzheimer’s disease at the prodromal stage. J. Neurol. Neurosurg. Psychiatry 2018, 89, 467–475. [Google Scholar] [CrossRef]
- Hall, S.; Ohrfelt, A.; Constantinescu, R.; Andreasson, U.; Surova, Y.; Bostrom, F.; Nilsson, C.; Håkan, W.; Decraemer, H.; Någga, K.; et al. Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. Arch. Neurol. 2012, 69, 1445–1452. [Google Scholar] [CrossRef]
- Wennstrom, M.; Surova, Y.; Hall, S.; Nilsson, C.; Minthon, L.; Bostrom, F.; Hansson, O.; Nielsen, H.M. Low CSF levels of both alpha-synuclein and the alpha-synuclein cleaving enzyme neurosin in patients with synucleinopathy. PLoS ONE 2013, 8, e53250. [Google Scholar] [CrossRef] [Green Version]
- Chiasserini, D.; Biscetti, L.; Eusebi, P.; Salvadori, N.; Frattini, G.; Simoni, S.; De Roeck, N.; Tambasco, N.; Stoops, E.; Vanderstichele, H.; et al. Differential role of CSF fatty acid binding protein 3, alpha-synuclein, and Alzheimer’s disease core biomarkers in Lewy body disorders and Alzheimer’s dementia. Alzheimer’s Res. Ther. 2017, 9, 52. [Google Scholar] [CrossRef]
- Law, Z.K.; Todd, C.; Mehraram, R.; Schumacher, J.; Baker, M.R.; LeBeau, F.E.N.; Yarnall, A.; Onofrj, M.; Bonanni, L.; Thomas, A.; et al. The Role of EEG in the Diagnosis, Prognosis and Clinical Correlations of Dementia with Lewy Bodies—A Systematic Review. Diagnostics 2020, 10, 616. [Google Scholar] [CrossRef] [PubMed]
- Blanc, F.; Colloby, S.J.; Philippi, N.; de Petigny, X.; Jung, B.; Demuynck, C.; Phillipps, C.; Anthony, P.; Thomas, A.; Bing, F.; et al. Cortical Thickness in Dementia with Lewy Bodies and Alzheimer’s Disease: A Comparison of Prodromal and Dementia Stages. PLoS ONE 2015, 10, e0127396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roquet, D.; Noblet, V.; Anthony, P.; Philippi, N.; Demuynck, C.; Cretin, B.; Martin-Hunyadi, C.; Loureiro de Sousa, P.; Blanc, F. Insular atrophy at the prodromal stage of dementia with Lewy bodies: A VBM DARTEL study. Sci. Rep. 2017, 7, 9437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanc, F.; Colloby, S.J.; Cretin, B.; de Sousa, P.L.; Demuynck, C.; O’Brien, J.T.; Martin-Hunyadi, C.; McKeith, I.; Philippi, N.; Taylor, J.P. Grey matter atrophy in prodromal stage of dementia with Lewy bodies and Alzheimer’s disease. Alzheimer’s Res. Ther. 2016, 8, 31. [Google Scholar] [CrossRef] [Green Version]
- Firbank, M.J.; Durcan, R.; O’Brien, J.T.; Allan, L.M.; Barker, S.; Ciafone, J.; Donaghy, P.C.; Hamilton, C.A.; Lawley, S.; Roberts, G.; et al. Hippocampal and insula volume in mild cognitive impairment with Lewy bodies. Parkinsonism Relat. Disord. 2021, 86, 27–33. [Google Scholar] [CrossRef]
- Kantarci, K.; Lesnick, T.; Ferman, T.J.; Przybelski, S.A.; Boeve, B.F.; Smith, G.E.; Kremers, W.K.; Knopman, D.S.; Jack, C.R., Jr.; Petersen, R.C. Hippocampal volumes predict risk of dementia with Lewy bodies in mild cognitive impairment. Neurology 2016, 87, 2317–2323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanc, F.; Schulz, I.; Mondino, M.; Gobin, M.S.I.; Gudewiez, R.; Loureiro De Sousa, P.; Philippi, N.; Baloglu, S.; Cretin, B.; Demuynck, C.; et al. Atrophy of insulae and hippocampi in prodromal dementia with Lewy bodies and Alzheimer’s Disease: Visual assessment. ADPD 2019. [Google Scholar]
- Burton, E.J.; Barber, R.; Mukaetova-Ladinska, E.B.; Robson, J.; Perry, R.H.; Jaros, E.; Kalaria, R.N.; O’Brien, J.T. Medial temporal lobe atrophy on MRI differentiates Alzheimer’s disease from dementia with Lewy bodies and vascular cognitive impairment: A prospective study with pathological verification of diagnosis. Brain A J. Neurol. 2009, 132 Pt 1, 195–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harper, L.; Fumagalli, G.G.; Barkhof, F.; Scheltens, P.; O’Brien, J.T.; Bouwman, F.; Burton, E.J.; Rohrer, J.D.; Fox, N.C.; Ridgway, G.R.; et al. MRI visual rating scales in the diagnosis of dementia: Evaluation in 184 post-mortem confirmed cases. Brain A J. Neurol. 2016, 139 Pt 4, 1211–1225. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, G.; De Blasi, R.; Capozzo, R.; Tortelli, R.; Barulli, M.R.; Liguori, R.; Grasso, D.; Logroscino, G. Loss of Swallow Tail Sign on Susceptibility-Weighted Imaging in Dementia with Lewy Bodies. J. Alzheimer’s Dis. JAD 2019, 67, 61–65. [Google Scholar] [CrossRef]
- Shams, S.; Fallmar, D.; Schwarz, S.; Wahlund, L.O.; van Westen, D.; Hansson, O.; Larsson, E.M.; Haller, S. MRI of the Swallow Tail Sign: A Useful Marker in the Diagnosis of Lewy Body Dementia? AJNR Am. J. Neuroradiol. 2017, 38, 1737–1741. [Google Scholar] [CrossRef] [Green Version]
- Blanc, F.; Verny, M. Prodromal stage of disease (dementia) with Lewy bodies, how to diagnose in practice? Geriatr. Psychol. Neuropsychiatr. Vieil. 2017, 15, 196–204. [Google Scholar] [CrossRef]
- Colloby, S.J.; McParland, S.; O’Brien, J.T.; Attems, J. Neuropathological correlates of dopaminergic imaging in Alzheimer’s disease and Lewy body dementias. Brain A J. Neurol. 2012, 135 Pt 9, 2798–2808. [Google Scholar] [CrossRef] [Green Version]
- Walker, Z.; Jaros, E.; Walker, R.W.; Lee, L.; Costa, D.C.; Livingston, G.; Ince, P.G.; Perry, R.; McKeith, I.; Katona, C.L. Dementia with Lewy bodies: A comparison of clinical diagnosis, FP-CIT single photon emission computed tomography imaging and autopsy. J. Neurol. Neurosurg. Psychiatry 2007, 78, 1176–1181. [Google Scholar] [CrossRef]
- McKeith, I.; O’Brien, J.; Walker, Z.; Tatsch, K.; Booij, J.; Darcourt, J.; Padovani, A.; Giubbini, R.; Bonuccelli, U.; Volterrani, D.; et al. Sensitivity and specificity of dopamine transporter imaging with 123I-FP-CIT SPECT in dementia with Lewy bodies: A phase III, multicentre study. Lancet Neurol. 2007, 6, 305–313. [Google Scholar] [CrossRef]
- Morgan, S.; Kemp, P.; Booij, J.; Costa, D.C.; Padayachee, S.; Lee, L.; Barber, C.; Carter, J.; Walker, Z. Differentiation of frontotemporal dementia from dementia with Lewy bodies using FP-CIT SPECT. J. Neurol. Neurosurg. Psychiatry 2012, 83, 1063–1070. [Google Scholar] [CrossRef] [Green Version]
- Nihashi, T.; Ito, K.; Terasawa, T. Diagnostic accuracy of DAT-SPECT and MIBG scintigraphy for dementia with Lewy bodies: An updated systematic review and Bayesian latent class model meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1984–1997. [Google Scholar] [CrossRef]
- Thomas, A.J.; Donaghy, P.; Roberts, G.; Colloby, S.J.; Barnett, N.A.; Petrides, G.; Lloyd, J.; Olsen, K.; Taylor, J.P.; McKeith, I.; et al. Diagnostic accuracy of dopaminergic imaging in prodromal dementia with Lewy bodies. Psychol. Med. 2019, 49, 396–402. [Google Scholar] [CrossRef] [Green Version]
- Lobotesis, K.; Fenwick, J.D.; Phipps, A.; Ryman, A.; Swann, A.; Ballard, C.; McKeith, I.G.; O’Brien, J.T. Occipital hypoperfusion on SPECT in dementia with Lewy bodies but not AD. Neurology 2001, 56, 643–649. [Google Scholar] [CrossRef]
- Pasquier, J.; Michel, B.F.; Brenot-Rossi, I.; Hassan-Sebbag, N.; Sauvan, R.; Gastaut, J.L. Value of (99m)Tc-ECD SPET for the diagnosis of dementia with Lewy bodies. Eur. J. Nucl. Med. Mol. Imaging 2002, 29, 1342–1348. [Google Scholar] [CrossRef] [PubMed]
- Hanyu, H.; Shimizu, S.; Hirao, K.; Kanetaka, H.; Iwamoto, T.; Chikamori, T.; Usui, Y.; Yamashina, A.; Koizumi, K.; Abe, K. Comparative value of brain perfusion SPECT and [(123)I]MIBG myocardial scintigraphy in distinguishing between dementia with Lewy bodies and Alzheimer’s disease. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Imabayashi, E.; Soma, T.; Sone, D.; Tsukamoto, T.; Kimura, Y.; Sato, N.; Murata, M.; Matsuda, H. Validation of the cingulate island sign with optimized ratios for discriminating dementia with Lewy bodies from Alzheimer’s disease using brain perfusion SPECT. Ann. Nucl. Med. 2017, 31, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Kanetaka, H.; Shimizu, S.; Inagawa, Y.; Hirose, D.; Takenoshita, N.; Sakurai, H.; Hanyu, H. Differentiating Mild Cognitive Impairment, Alzheimer’s Disease, and Dementia with Lewy Bodies Using Cingulate Island Sign on Perfusion IMP-SPECT. Front. Neurol. 2020, 11, 568438. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.T.; Firbank, M.J.; Davison, C.; Barnett, N.; Bamford, C.; Donaldson, C.; Olsen, K.; Herholz, K.; Williams, D.; Lloyd, J. 18F-FDG PET and perfusion SPECT in the diagnosis of Alzheimer and Lewy body dementias. J. Nucl. Med. 2014, 55, 1959–1965. [Google Scholar] [CrossRef] [Green Version]
- Imamura, T.; Ishii, K.; Sasaki, M.; Kitagaki, H.; Yamaji, S.; Hirono, N.; Shimomura, T.; Hashimoto, M.; Tanimukai, S.; Kazui, H.; et al. Regional cerebral glucose metabolism in dementia with Lewy bodies and Alzheimer’s disease: A comparative study using positron emission tomography. Neurosci. Lett. 1997, 235, 49–52. [Google Scholar] [CrossRef]
- Lim, S.M.; Katsifis, A.; Villemagne, V.L.; Best, R.; Jones, G.; Saling, M.; Bradshaw, J.; Merory, J.; Woodward, M.; Hopwood, M.; et al. The 18F-FDG PET Cingulate Island Sign and Comparison to 123I-β-CIT SPECT for Diagnosis of Dementia with Lewy Bodies. J. Nucl. Med. 2009, 50, 1638–1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iizuka, T.; Kameyama, M. Cingulate island sign on FDG-PET is associated with medial temporal lobe atrophy in dementia with Lewy bodies. Ann. Nucl. Med. 2016, 30, 421–429. [Google Scholar] [CrossRef]
- Whitwell, J.L.; Graff-Radford, J.; Singh, T.D.; Drubach, D.A.; Senjem, M.L.; Spychalla, A.J.; Tosakulwong, N.; Lowe, V.J.; Josephs, K.A. (18)F-FDG PET in Posterior Cortical Atrophy and Dementia with Lewy Bodies. J. Nucl. Med. 2017, 58, 632–638. [Google Scholar] [CrossRef] [Green Version]
- Korat, Š.; Bidesi, N.S.R.; Bonanno, F.; Di Nanni, A.; Hoàng, A.N.N.; Herfert, K.; Maurer, A.; Battisti, U.M.; Bowden, G.D.; Thonon, D.; et al. Alpha-Synuclein PET Tracer Development—An Overview about Current Efforts. Pharmaceuticals 2021, 14, 847. [Google Scholar] [CrossRef]
- Kuebler, L.; Buss, S.; Leonov, A.; Ryazanov, S.; Schmidt, F.; Maurer, A.; Weckbecker, D.; Landau, A.M.; Lillethorup, T.P.; Bleher, D.; et al. [(11)C]MODAG-001-towards a PET tracer targeting alpha-synuclein aggregates. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1759–1772. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, A.; Takeda, A.; Okamura, N.; Tashiro, M.; Hasegawa, T.; Furumoto, S.; Kobayashi, M.; Sugeno, N.; Baba, T.; Miki, Y.; et al. In vivo visualization of alpha-synuclein deposition by carbon-11-labelled 2-[2-(2-dimethylaminothiazol-5-yl)ethenyl]-6-[2-(fluoro)ethoxy]benzoxazole positron emission tomography in multiple system atrophy. Brain A J. Neurol. 2010, 133 Pt 6, 1772–1778. [Google Scholar] [CrossRef] [Green Version]
- Yoshita, M.; Arai, H.; Arai, H.; Arai, T.; Asada, T.; Fujishiro, H.; Hanyu, H.; Iizuka, O.; Iseki, E.; Kashihara, K.; et al. Diagnostic accuracy of 123I-meta-iodobenzylguanidine myocardial scintigraphy in dementia with Lewy bodies: A multicenter study. PLoS ONE 2015, 10, e0120540. [Google Scholar] [CrossRef]
- Sonni, I.; Ratib, O.; Boccardi, M.; Picco, A.; Herholz, K.; Nobili, F.; Varrone, A. Clinical validity of presynaptic dopaminergic imaging with (123)I-ioflupane and noradrenergic imaging with (123)I-MIBG in the differential diagnosis between Alzheimer’s disease and dementia with Lewy bodies in the context of a structured 5-phase development framework. Neurobiol. Aging 2017, 52, 228–242. [Google Scholar] [PubMed]
- Roberts, G.; Durcan, R.; Donaghy, P.C.; Lawley, S.; Ciafone, J.; Hamilton, C.A.; Colloby, S.J.; Firbank, M.J.; Allan, L.; Barnett, N.; et al. Accuracy of Cardiac Innervation Scintigraphy for Mild Cognitive Impairment with Lewy Bodies. Neurology 2021, 96, e2801–e2811. [Google Scholar] [CrossRef]
- Fairfoul, G.; McGuire, L.I.; Pal, S.; Ironside, J.W.; Neumann, J.; Christie, S.; Joachim, C.; Esiri, M.; Evetts, S.G.; Rolinski, M.; et al. Alpha-synuclein RT-QuIC in the CSF of patients with alpha-synucleinopathies. Ann. Clin. Transl. Neurol. 2016, 3, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Bongianni, M.; Ladogana, A.; Capaldi, S.; Klotz, S.; Baiardi, S.; Cagnin, A.; Perra, D.; Fiorini, M.; Poleggi, A.; Legname, G.; et al. alpha-Synuclein RT-QuIC assay in cerebrospinal fluid of patients with dementia with Lewy bodies. Ann. Clin. Transl. Neurol. 2019, 6, 2120–2126. [Google Scholar] [CrossRef]
- Bargar, C.; Wang, W.; Gunzler, S.A.; LeFevre, A.; Wang, Z.; Lerner, A.J.; Singh, N.; Tatsuoka, C.; Appleby, B.; Zhu, X.; et al. Streamlined alpha-synuclein RT-QuIC assay for various biospecimens in Parkinson’s disease and dementia with Lewy bodies. Acta Neuropathol. Commun. 2021, 9, 62. [Google Scholar] [CrossRef]
- Groveman, B.R.; Orru, C.D.; Hughson, A.G.; Raymond, L.D.; Zanusso, G.; Ghetti, B.; Campbell, K.J.; Safar, J.; Galasko, D.; Caughey, B. Rapid and ultra-sensitive quantitation of disease-associated alpha-synuclein seeds in brain and cerebrospinal fluid by alphaSyn RT-QuIC. Acta Neuropathol. Commun. 2018, 6, 7. [Google Scholar] [CrossRef]
- Rossi, M.; Candelise, N.; Baiardi, S.; Capellari, S.; Giannini, G.; Orru, C.D.; Antelmi, E.; Mammana, A.; Hughson, A.G.; Calandra-Buonaura, G.; et al. Ultrasensitive RT-QuIC assay with high sensitivity and specificity for Lewy body-associated synucleinopathies. Acta Neuropathol. 2020, 140, 49–62. [Google Scholar] [CrossRef]
- McGrowder, D.A.; Miller, F.; Vaz, K.; Nwokocha, C.; Wilson-Clarke, C.; Anderson-Cross, M.; Brown, J.; Anderson-Jackson, L.; Williams, L.; Latore, L.; et al. Cerebrospinal Fluid Biomarkers of Alzheimer’s Disease: Current Evidence and Future Perspectives. Brain Sci. 2021, 11, 215. [Google Scholar] [CrossRef]
- Morenas-Rodriguez, E.; Alcolea, D.; Suarez-Calvet, M.; Munoz-Llahuna, L.; Vilaplana, E.; Sala, I.; Subirana, A.; Querol-Vilaseca, M.; Carmona-Iragui, M.; Illán-Gala, I.; et al. Different pattern of CSF glial markers between dementia with Lewy bodies and Alzheimer’s disease. Sci. Rep. 2019, 9, 7803. [Google Scholar] [CrossRef]
- Wennstrom, M.; Hall, S.; Nagga, K.; Londos, E.; Minthon, L.; Hansson, O. Cerebrospinal fluid levels of IL-6 are decreased and correlate with cognitive status in DLB patients. Alzheimer’s Res. Ther. 2015, 7, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lourenco, M.V.; Ribeiro, F.C.; Santos, L.E.; Beckman, D.; Melo, H.M.; Sudo, F.K.; Drummond, C.; Assunção, N.; Vanderborght, B.; Tovar-Moll, F.; et al. Cerebrospinal Fluid Neurotransmitters, Cytokines, and Chemokines in Alzheimer’s and Lewy Body Diseases. J. Alzheimer’s Dis. JAD 2021, 82, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Mukaetova-Ladinska, E.B.; Monteith, R.; Perry, E.K. Cerebrospinal fluid biomarkers for dementia with lewy bodies. Int. J. Alzheimer’s Dis. 2010, 2010, 536538. [Google Scholar] [CrossRef] [Green Version]
- Gmitterova, K.; Varges, D.; Schmitz, M.; Zafar, S.; Maass, F.; Lingor, P.; Zerr, I. Chromogranin A Analysis in the Differential Diagnosis Across Lewy Body Disorders. J. Alzheimer’s Dis. JAD 2020, 73, 1355–1361. [Google Scholar] [CrossRef]
- Duits, F.H.; Brinkmalm, G.; Teunissen, C.E.; Brinkmalm, A.; Scheltens, P.; Van der Flier, W.M.; Zetterberg, H.; Blennow, K. Synaptic proteins in CSF as potential novel biomarkers for prognosis in prodromal Alzheimer’s disease. Alzheimer’s Res. Ther. 2018, 10, 5. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Hou, L.; Shi, H.; Zhong, X.; Zhang, Y.; Zheng, D.; Tan, Y.; Hu, G.; Mu, N.; Chan, J.; et al. CSF levels of the neuronal injury biomarker visinin-like protein-1 in Alzheimer’s disease and dementia with Lewy bodies. J. Neurochem. 2013, 127, 681–690. [Google Scholar] [CrossRef]
- Zou, K.; Abdullah, M.; Michikawa, M. Current Biomarkers for Alzheimer’s Disease: From CSF to Blood. J. Pers. Med. 2020, 10, 85. [Google Scholar] [CrossRef]
- Van Steenoven, I.; Koel-Simmelink, M.J.A.; Vergouw, L.J.M.; Tijms, B.M.; Piersma, S.R.; Pham, T.V.; Bridel, C.; Ferri, G.L.; Cocco, C.; Noli, B.; et al. Identification of novel cerebrospinal fluid biomarker candidates for dementia with Lewy bodies: A proteomic approach. Mol. Neurodegener. 2020, 15, 36. [Google Scholar] [CrossRef]
- Van Steenoven, I.; Noli, B.; Cocco, C.; Ferri, G.L.; Oeckl, P.; Otto, M.; Koel-Simmelink, M.J.A.; Bridel, C.; van der Flier, W.M.; Lemstra, A.W.; et al. VGF Peptides in Cerebrospinal Fluid of Patients with Dementia with Lewy Bodies. Int. J. Mol. Sci. 2019, 20, 4674. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, J.; Gobom, J.; Sjodin, S.; Brinkmalm, G.; Ashton, N.J.; Svensson, J.; Johansson, P.; Portelius, E.; Zetterberg, H.; Blennow, K.; et al. Cerebrospinal fluid biomarker panel for synaptic dysfunction in Alzheimer’s disease. Alzheimers Dement. 2021, 13, e12179. [Google Scholar] [CrossRef] [PubMed]
- Libiger, O.; Shaw, L.M.; Watson, M.H.; Nairn, A.C.; Umana, K.L.; Biarnes, M.C.; Canet-Avilés, R.M.; Jack, C.R., Jr.; Breton, Y.A.; Cortes, L.; et al. Longitudinal CSF proteomics identifies NPTX2 as a prognostic biomarker of Alzheimer’s disease. Alzheimers Dement. 2021, 17, 1976–1987. [Google Scholar] [CrossRef] [PubMed]
- Boiten, W.A.; van Steenoven, I.; Xiao, M.; Worley, P.F.; Lemstra, A.W.; Teunissen, C.E. Pathologically Decreased CSF Levels of Synaptic Marker NPTX2 in DLB Are Correlated with Levels of Alpha-Synuclein and VGF. Cells 2020, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Brinkmalm, G.; Sjodin, S.; Simonsen, A.H.; Hasselbalch, S.G.; Zetterberg, H.; Brinkmalm, A.; Blennow, K. A Parallel Reaction Monitoring Mass Spectrometric Method for Analysis of Potential CSF Biomarkers for Alzheimer’s Disease. PROTEOMICS Clin. Appl. 2018, 12, 1700131. [Google Scholar] [CrossRef] [PubMed]
- Llano, D.A.; Devanarayan, P.; Devanarayan, V.; Alzheimer’s Disease Neuroimaging Initiative (ADNI). VGF in Cerebrospinal Fluid Combined With Conventional Biomarkers Enhances Prediction of Conversion from MCI to AD. Alzheimer Dis. Assoc. Disord. 2019, 33, 307–314. [Google Scholar]
- Bartolomucci, A.; Possenti, R.; Mahata, S.K.; Fischer-Colbrie, R.; Loh, Y.P.; Salton, S.R. The extended granin family: Structure, function, and biomedical implications. Endocr. Rev. 2011, 32, 755–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferri, G.L.; Noli, B.; Brancia, C.; D’Amato, F.; Cocco, C. VGF: An inducible gene product, precursor of a diverse array of neuro-endocrine peptides and tissue-specific disease biomarkers. J. Chem. Neuroanat. 2011, 42, 249–261. [Google Scholar] [CrossRef]
- Xu, D.; Hopf, C.; Reddy, R.; Cho, R.W.; Guo, L.; Lanahan, A.; Petralia, R.S.; Wenthold, R.J.; O’Brien, R.J.; Worley, P. Narp and NP1 form heterocomplexes that function in developmental and activity-dependent synaptic plasticity. Neuron 2003, 39, 513–528. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, R.J.; Xu, D.; Petralia, R.S.; Steward, O.; Huganir, R.L.; Worley, P. Synaptic clustering of AMPA receptors by the extracellular immediate-early gene product Narp. Neuron 1999, 23, 309–323. [Google Scholar] [CrossRef] [Green Version]
- Schwarzer, C. 30 years of dynorphins—New insights on their functions in neuropsychiatric diseases. Pharmacol. Ther. 2009, 123, 353–370. [Google Scholar] [CrossRef] [Green Version]
- Tejeda, H.A.; Shippenberg, T.S.; Henriksson, R. The dynorphin/kappa-opioid receptor system and its role in psychiatric disorders. Cell. Mol. Life Sci. 2012, 69, 857–896. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Irwin, D.J.; Leverenz, J.B.; Gamez, N.; Taylor, A.; Galvin, J.E. Biomarker Use for Dementia With Lewy Body Diagnosis: Survey of US Experts. Alzheimer Dis. Assoc. Disord. 2021, 35, 55–61. [Google Scholar] [CrossRef]
- Boot, B.P.; Orr, C.F.; Ahlskog, J.E.; Ferman, T.J.; Roberts, R.; Pankratz, V.S.; Dickson, D.W.; Parisi, J.; Aakre, J.A.; Geda, Y.E.; et al. Risk factors for dementia with Lewy bodies: A case-control study. Neurology 2013, 81, 833–840. [Google Scholar] [CrossRef] [Green Version]
- Chouliaras, L.; Thomas, A.; Malpetti, M.; Donaghy, P.; Kane, J.; Mak, E.; Savulich, G.; Prats-Sedano, M.A.; Heslegrave, A.J.; Zetterberg, H.; et al. Differential levels of plasma biomarkers of neurodegeneration in Lewy body dementia, Alzheimer’s disease, frontotemporal dementia and progressive supranuclear palsy. J. Neurol. Neurosurg. Psychiatry 2022, 93, 651–658. [Google Scholar] [CrossRef]
| Clinical Characteristics | |
|---|---|
| Essential Criterion | |
| Cognitive decline of sufficient severity to interfere with activities of daily living. The deficits frequently concern attentional, executive and visuospatial abilities. | |
| Core Criteria | Supportive Criteria |
|
|
| Biomarkers | |
| Indicative | Supportive |
|
|
| Prodromal DLB | Validity | DLB dementia | Validity | References | |
|---|---|---|---|---|---|
| Brain MRI T1 | Insular atrophy | Not demonstrated | No or mild hippocampal atrophy | Sensitivity = 64% Specificity = 68% (compared to AD) | [30] |
| Brain MRI SWI | Loss of the swallow tail sign | Not demonstrated | Loss of the swallow tail sign | Sensitivity = 63% Specificity = 75% (compared to AD) | [32] |
| FP-CIT SPECT (DAT-scan) | Presynaptic striatal dopaminergic decrease | Sensitivity = 54.2% Specificity = 89.0% (compared to prodromal AD) | Presynaptic striatal dopaminergic decrease | Sensitivity = 77.7% Specificity = 90.4% (compared to AD) | [36,39] |
| Perfusion SPECT | Occipital hypoperfusion | Not demonstrated | Occipital hypoperfusion | Sensitivity = 74.0% Specificity = 82.0% (compared to AD) | [42] |
| FDG-PET | Occipital hypometabolism and Cingulate Island Sign | Not demonstrated | Occipital hypometabolism and Cingulate Island Sign | Sensitivity = 77.0% Specificity = 80.0% (compared to AD) | [47] |
| Synuclein-PET | Cortical and basal ganglia accumulation? | Not existing | Cortical and basal ganglia accumulation? | Not existing | |
| MIBG scintigraphy | Decrease cardiac sympathetic activity | Sensitivity = 46.2% Specificity = 88.0% (compared to prodromal AD) | Decrease cardiac sympathetic activity | Sensitivity = 68.9% Specificity = 87.0% (compared to AD) | [53,55] |
| Biomarkers | AD | DLB | References |
|---|---|---|---|
| YKL-40 | ↗ | - | [61,62] |
| neurogranin | ↗ | - | [61] |
| VILIP-1 | ↗ | - | [68,69] |
| Magnesium, calcium, copper | - | ↗ | [65] |
| Neurosin | - | ↘ | [20] |
| Il-6 | - | ↘ | [63] |
| CART | - | ↘ | [65] |
| Chromogranin A | ↗ | ↗ | [66,67] |
| Asparagine, glycine | ? | ↗ | [65] |
| HVA, 5-HIAA et MHPG | ↘ compared to AD | [64] | |
| NPTX2, VGF, SCG2, | ↘ | ↘(↘) | [70] |
| PDYN | ↘ | [70] | |
| RT-QuIC | - | ↗ | [56,57,58,59,60] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bousiges, O.; Blanc, F. Biomarkers of Dementia with Lewy Bodies: Differential Diagnostic with Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 6371. https://doi.org/10.3390/ijms23126371
Bousiges O, Blanc F. Biomarkers of Dementia with Lewy Bodies: Differential Diagnostic with Alzheimer’s Disease. International Journal of Molecular Sciences. 2022; 23(12):6371. https://doi.org/10.3390/ijms23126371
Chicago/Turabian StyleBousiges, Olivier, and Frédéric Blanc. 2022. "Biomarkers of Dementia with Lewy Bodies: Differential Diagnostic with Alzheimer’s Disease" International Journal of Molecular Sciences 23, no. 12: 6371. https://doi.org/10.3390/ijms23126371
APA StyleBousiges, O., & Blanc, F. (2022). Biomarkers of Dementia with Lewy Bodies: Differential Diagnostic with Alzheimer’s Disease. International Journal of Molecular Sciences, 23(12), 6371. https://doi.org/10.3390/ijms23126371






