RAGE against the Machine: Can Increasing Our Understanding of RAGE Help Us to Battle SARS-CoV-2 Infection in Pregnancy?
Abstract
1. Introduction
2. The Receptor for Advanced Glycation End Products
2.1. RAGE Structure and Forms
2.2. Expression of RAGE Varies across Tissues with Age
2.3. RAGE Signaling in the Fetal Membranes, Placenta, and Uterus
| Clinical Context | Generalized Outcome | Species | Tissue or Cell Target |
|---|---|---|---|
| Preeclampsia | Increased expression/level of 2AGEs, RAGE, and other RAGE ligands | Human | Placenta [43,46,47,48,49,50], Maternal Peripheral Blood [39,47,51], Maternal Serum [14,52,53,54,55], AF [14], Umbilical Blood [47], Myometrium [56], Extravillous Trophoblasts [57], Cord Blood [14], Syncytiotrophoblast [58], Primary Cultured Adipocytes [59] |
| Heparin’s anti-inflammatory effect on HMGB1/RAGE axis in PE | Human | Placenta [35] | |
| Overview of AGE, RAGE, and its signaling molecules in multiple tissues (review) | [60,61] | ||
| Preeclampsia Treatment | Epigallocatechin gallate as a potential treatment to downregulate AGE–RAGE signaling pathway | Genomics [62] | |
| Hypertensive Disorder | Increased expression/level of AGEs, RAGE, and other RAGE and inflammatory ligands | Human | Placenta [63], Primary Cultured Adipocytes [59] |
| Gestational Diabetes | Increased level of AGEs, RAGE, and other RAGE ligands | Human | Placenta [46], Umbilical Cord [64], Plasma [64] |
| In vitro | Umbilical Vein Endothelial Cells [65] | ||
| Association with circular RNAs | Human | Placenta [66] | |
| AGEs, RAGE, and RAGE ligands as both anti- and proinflammatory mediators | Human | FM [67], Omental Adipose Tissue Explants [67], Serum [67] | |
| RAGE gene polymorphisms (review) | Gene Expression [68] | ||
| RAGE clinical opinions for treatment and management (review) | [69] | ||
| Gestational Diabetes Treatment | Ursolic acid and fetal developmental defects | Rat | Placenta [70] |
| sRAGE as a potential protective molecule | Rat | Fetus [71] | |
| Gestational Diabetes Screening | Potential biomarkers | [72] | |
| AGE and RAGE levels remained unchanged, suggesting oral glucose-tolerance tests are safe for pregnant women | Human | Maternal Serum [73] | |
| Diabetes | RAGE knockout mice and diabetic embryopathy | Mouse | Maternal Plasma [74] |
| AGEs, sRAGE, and proinflammatory cytokine pregnancy | Human | Plasma [75] | |
| RAGE and AGE signaling in diabetic pregnancy (review) | Human | Myometrium [76] | |
| Diabetes Treatment | Toxicity of N-Epsilon-(carboxymethyl)lysine and bioaffinity to RAGE | In vitro | Umbilical Vein Endothelial Cells [77] |
| Preterm Birth | Increased expression/levels of AGE, RAGE, and RAGE ligands | Human | AF [40,78,79], Cervix [80], FM [81], Placenta [20] |
| Decreased sRAGE | Human | Maternal Serum [82,83], Plasma [84], Maternal Blood [85] | |
| Germ-free fetal pigs could be a favorable model to study immunocompromised preterm infants | Pig | Fetus [86] | |
| Description of RAGE, TLRs, and NF-kB in inflammatory pathways (review) | [87] | ||
| Preterm Labor | Changes in inflammatory signaling molecules, DAMPs, and RAGE (review) | [88] | |
| Identified multiple AF proteins (including enRAGE) that were associated with women in threatened preterm labor | Human | AF [89] | |
| Preterm Premature Rupture of the Membranes | sRAGE, HMGB1, and AGE levels | Human | Plasma and Serum Extracted Extracellular vesicles [90] |
| Increased HMGB1 and decreased sRAGE levels in clinical chorioamnionitis | Human | AF [91,92] | |
| RAGE increases with cigarette smoke condensate | Human | FM [93] | |
| FM weakening in pPROM and the mechanisms of inflammation in RAGE and NLRP7 inflammasome (review) | Human | FM [25] | |
| Premature Rupture of the Membranes | Increased levels of sRAGE and esRAGE | Human | Plasma [94] |
| Cervical Insufficiency | Identified potential biomarkers for PTB in cervical insufficiency, including enRAGE, S100A8/A9 | Human | AF [95,96] |
| Infection during Pregnancy | Chorioamnionitis–sRAGE expression decreased in airways and circulation | Human | Human Fetal Tracheobronchial Aspirate Fluid [41] |
| Increased expression/level of AGE, RAGE, and RAGE ligands in IAI | Human | AF [97], Placenta [40], FM [45] | |
| Pig | AF [98] | ||
| RAGE inhibition protects against fetal weight loss during secondhand-smoke-induced IUGR | Mouse | Mouse Trophoblast Cells [44] | |
| AGEs and HMGB1 could promote sterile inflammation via monocytes/macrophages | In vitro | Placental Cells [99] | |
| RAGE/NF-KB pathway can increase the risk of placental vascular permeability | In vitro | BeWo Cells [42] | |
| Increased HMGB1 expression/levels correlates with URSA | Human | FM [100,101] | |
| Increased expression in S100 proteins in RAGE receptor binding of patients with HBV | Human | Placenta [102] | |
| Identified genomic instabilities in pregnancy complication, which were potentially due to defective DNA on trophoblast cells and a possible RAGE-mediated mechanism | Human | Placenta [103] | |
| General | RAGE signaling throughout gestation | Human | FM [104] |
| AGE/RAGE and focal adhesion that may contribute to COPD | Computer Model [105] | ||
| Increased levels of sRAGE are associated with recurrent pregnancy loss | Human | Blood [106] | |
| Secondhand smoke exposure increases RAGE | Mouse | Fetal Lung [107] | |
| RAGE upregulation via retinol | [108] | ||
| RAGE and parturition (review) | [109] |
3. SARS-CoV-2
3.1. SARS-CoV-2 and Pregnancy
3.2. Pregnancy Is an Additional Physiological Challenge That Can Exacerbate the Severity of SARS-CoV-2
3.3. SARS-CoV-2 Vaccination
4. The Interaction of RAGE and SARS-CoV-2
4.1. RAGE and Its Role in Comorbidities Associated with SARS-CoV-2
4.1.1. Diabetes and Obesity
4.1.2. Hypertension and Pulmonary Disease
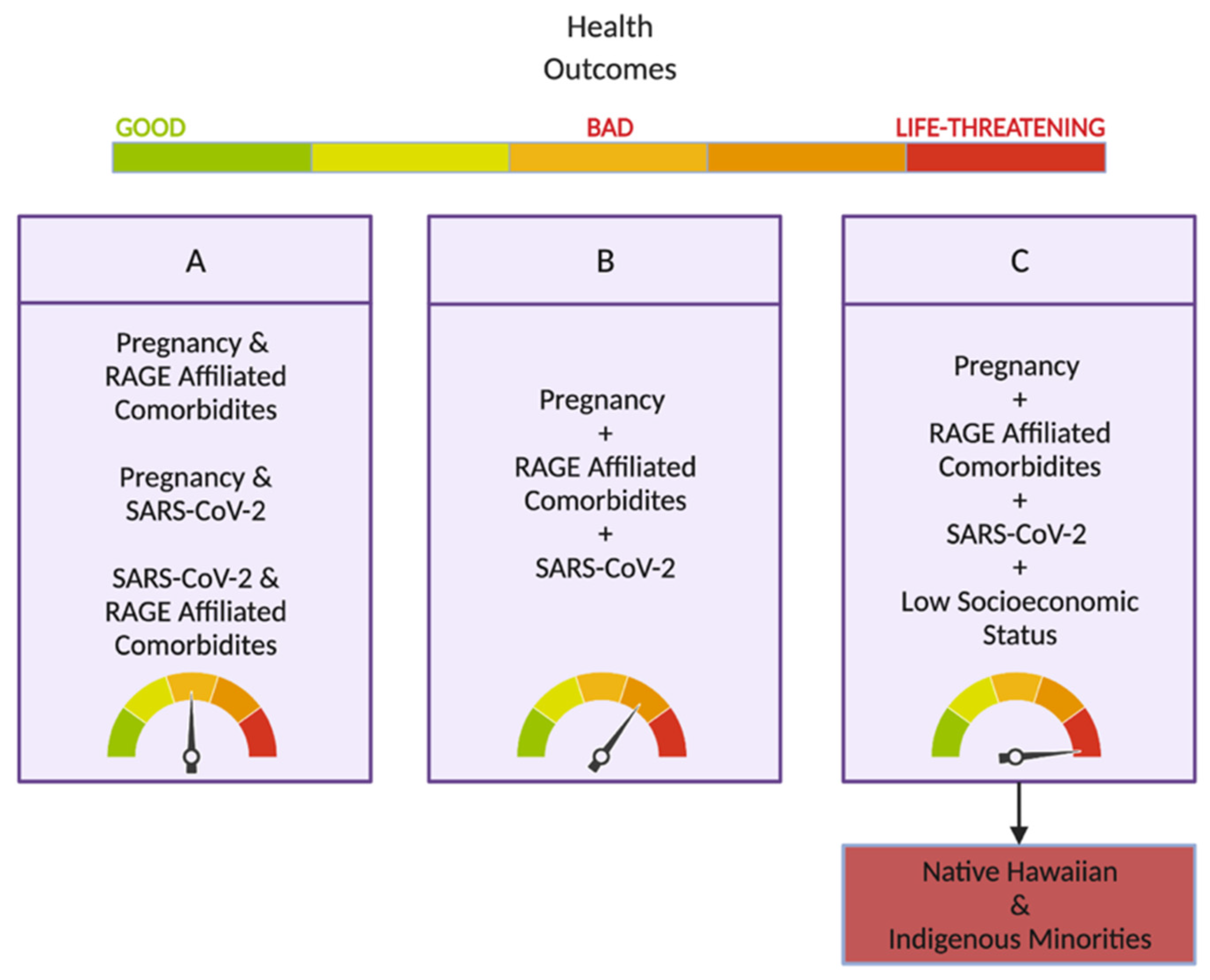
5. SARS-CoV-2 and Its Impact on Hawai’i and Its Vulnerable Populations
Hawai’i and Pregnancy
6. RAGE as a Biomarker or Putative Therapeutic Target
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations List
| ACE-2 | Angiotensin-Converting Enzyme 2 |
| AF | Amniotic Fluid |
| AGE | Advanced Glycation End Product |
| AP-1 | Activator Protein 1 |
| ARDS | Acute Respiratory Distress Syndrome |
| c-RAGE | Cleaved Receptor Advanced Glycation End Products |
| DAMPs | Danger-Associated Molecular Patterns |
| DM | Diabetes Mellitus |
| ECM | Extracellular Matrix |
| EN-RAGE | Extracellular Newly Identified Receptor Advanced Glycation End Products Binding Protein (S100A12) |
| es-RAGE | Endogenous Soluble Receptor Advanced Glycation End Products |
| fl-RAGE | Full-Length Receptor Advanced Glycation End Products |
| FM | Fetal Membrane |
| GM-CSF | Granulocyte Macrophage Colony-Stimulating Factor |
| HMGB1 | High-Mobility Group Box Protein 1 |
| IL-6 | Interleukin 6 |
| MMP | Matrix Metalloproteinase |
| NF-κB | Nuclear Factor Kappa B |
| RAGE | Receptor Advanced Glycation End Products |
| ROS | Reactive Oxidative Species |
| s-RAGE | Secreted Receptor Advanced Glycation End Products |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| TNFα | Tumor Necrosis Factor Alpha |
References
- Zhu, Y.; Hedderson, M.M.; Brown, S.D.; Badon, S.E.; Feng, J.; Quesenberry, C.P.; Ferrara, A. Healthy preconception and early-pregnancy lifestyle and risk of preterm birth: A prospective cohort study. Am. J. Clin. Nutr. 2021, 114, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Mate, A.; Reyes-Goya, C.; Santana-Garrido, Á.; Vázquez, C.M. Lifestyle, Maternal Nutrition and Healthy Pregnancy. Curr. Vasc. Pharmacol. 2021, 19, 132–140. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Cheng, X.; Feng, X.; Wan, H.; Chen, S.; Xiong, M. Clinical Symptom Differences Between Mild and Severe COVID-19 Patients in China: A Meta-Analysis. Front. Public Health 2021, 8, 561264. [Google Scholar] [CrossRef] [PubMed]
- Pregnant People. CDC (Blog). 3 March 2022. Available online: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html (accessed on 29 April 2022).
- Medical Conditions. CDC (Blog). 29 April 2022. Available online: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/pregnant-people.html (accessed on 29 April 2022).
- Wastnedge, E.A.N.; Reynolds, R.M.; van Boeckel, S.R.; Stock, S.J.; Denison, F.C.; Maybin, J.A.; Critchley, H.O.D. Pregnancy and COVID-19. Physiol. Rev. 2021, 101, 303–318. [Google Scholar] [CrossRef]
- Carethers, J.M. Insights into disparities observed with COVID-19. J. Intern. Med. 2021, 289, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Sellegounder, D.; Zafari, P.; Rajabinejad, M.; Taghadosi, M.; Kapahi, P. Advanced glycation end products (AGEs) and its receptor, RAGE, modulate age-dependent COVID-19 morbidity and mortality. A review and hypothesis. Int. Immunopharmacol. 2021, 98, 107806. [Google Scholar] [CrossRef]
- Mor, G.; Cardenas, I.; Abrahams, V.; Guller, S. Inflammation and pregnancy: The role of the immune system at the implantation site. Ann. N. Y. Acad. Sci. 2011, 1221, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Espinoza, J.; Kusanovic, J.P.; Gotsch, F.; Hassan, S.; Erez, O.; Chaiworapongsa, T.; Mazor, M. The preterm parturition syndrome. BJOG 2006, 113, 17–42. [Google Scholar] [CrossRef]
- Padron, J.G.; Saito Reis, C.A.; Kendal-Wright, C.E. The Role of Danger Associated Molecular Patterns in Human Fetal Membrane Weakening. Front. Physiol. 2020, 11, 602. [Google Scholar] [CrossRef] [PubMed]
- Kemp, M.W. Preterm birth, intrauterine infection, and fetal inflammation. Front. Immunol. 2014, 5, 574. [Google Scholar] [CrossRef]
- Agrawal, V.; Hirsch, E. Intrauterine infection and preterm labor. Semin. Fetal Neonatal Med. 2012, 17, 12–19. [Google Scholar] [CrossRef]
- Oliver, E.A.; Buhimschi, C.S.; Dulay, A.T.; Baumbusch, M.A.; Abdel-Razeq, S.S.; Lee, S.Y.; Zhao, G.; Jing, S.; Pettker, C.M.; Buhimschi, I.A. Activation of the receptor for advanced glycation endproducts system in women with severe preeclampsia. J. Clin. Endocrinol. Metab. 2011, 96, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Liu, Y.; Yan, R.; Liu, X.; Duan, L. Assessment of EN-RAGE, sRAGE and EN-RAGE/sRAGE as potential biomarkers in patients with autoimmune hepatitis. J. Transl. Med. 2020, 18, 384. [Google Scholar] [CrossRef]
- Antoniott, G.; Coughlan, M.; Salamonsen, L.; Evans, J. Obesity associated advanced glycation end products within the human uterine cavity adversely impact endometrial function and embryo implantation competence. Hum. Reprod. 2018, 33, 654–665. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, E.M.; Vella, V.; Belfiore, A. COVID-19 and Diabetes: The Importance of Controlling RAGE. Front. Endocrinol. 2020, 11, 526. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Commane, M.; Nie, H.; Hua, X.; Chatterjee-Kishore, M.; Wald, D.; Haag, M.; Stark, G.R. Act1, an NF-kappa B-activating protein. Proc. Natl. Acad. Sci. USA 2000, 97, 10489–10493. [Google Scholar] [CrossRef]
- Koch, M.; Chitayat, S.; Dattilo, B.M.; Schiefner, A.; Diez, J.; Chazin, W.J.; Fritz, G. Structural Basis for Ligand Recognition and Activation of RAGE. Structure 2010, 18, 1342–1352. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Zhu, L.; Zhang, Z.; Li, H.; Li, P.; Wang, Y.; Leng, M. HMGB1-RAGE signaling pathway in pPROM. Taiwan J. Obstet. Gynecol. 2018, 57, 211–216. [Google Scholar] [CrossRef]
- Goldin, C.J.; Vázquez, R.; Polack, F.P.; Alvarez-Paggi, D. Identifying pathophysiological bases of disease in COVID-19. Transl. Med. Commun. 2020, 5, 15. [Google Scholar] [CrossRef]
- Chiappalupi, S.; Salvadori, L.; Donato, R.; Riuzzi, F.; Sorci, G. Hyperactivated RAGE in Comorbidities as a Risk Factor for Severe COVID-19—The Role of RAGE-RAS Crosstalk. Biomolecules 2021, 11, 876. [Google Scholar] [CrossRef] [PubMed]
- What Is Immunoglobulins Superfamily (IGSF). Creative BioMart (Blog). Available online: https://www.creativebiomart.net/gene-family-4-immunoglbulins.htm (accessed on 28 April 2022).
- Wolf, L.; Herr, C.; Niederstraßer, J.; Beisswenger, C.; Bals, R. Receptor for advanced glycation endproducts (RAGE) maintains pulmonary structure and regulates the response to cigarette smoke. PLoS ONE 2017, 12, e0180092. [Google Scholar] [CrossRef]
- Choltus, H.; Lavergne, M.; De Sousa Do Outeiro, C.; Coste, K.; Belville, C.; Blanchon, L.; Sapin, V. Pathophysiological Implication of Pattern Recognition Receptors in Fetal Membranes Rupture: RAGE and NLRP Inflammasome. Biomedicines 2021, 9, 1123. [Google Scholar] [CrossRef]
- Rojas, A.; Morales, M.; Gonzalez, I.; Araya, P. Inhibition of RAGE Axis Signaling: A Pharmacological Challenge. Curr. Drug Targets 2019, 20, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Sparvero, L.J.; Asafu-Adjei, D.; Kang, R.; Tang, D.; Amin, N.; Im, J.; Rutledge, R.; Lin, B.; Amoscato, A.; Zeh, H.J.; et al. RAGE (Receptor for Advanced Glycation Endproducts), RAGE Ligands, and their role in Cancer and Inflammation. J. Transl. Med. 2009, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Pranal, T.; Pereira, B.; Berthelin, P.; Roszyk, L.; Godet, T.; Chabanne, R.; Eisenmann, N.; Lautrette, A.; Belville, C.; Blondonnet, R.; et al. Clinical and Biological Predictors of Plasma Levels of Soluble RAGE in Critically Ill Patients: Secondary Analysis of a Prospective Multicenter Observational Study. Dis. Markers 2018, 2018, 7849675. [Google Scholar] [CrossRef] [PubMed]
- Serveaux-Dancer, M.; Jabaudon, M.; Creveaux, I.; Belville, C.; Blondonnet, R.; Gross, C.; Constantin, J.M.; Blanchon, L.; Sapin, V. Pathological Implications of Receptor for Advanced Glycation End-Product (AGER) Gene Polymorphism. Dis. Markers 2019, 2019, 2067353. [Google Scholar] [CrossRef]
- Lee, E.J.; Park, J.H. Receptor for Advanced Glycation Endproducts (RAGE), Its Ligands, and Soluble RAGE: Potential Biomarkers for Diagnosis and Therapeutic Targets for Human Renal Diseases. Genom. Inform. 2013, 11, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Oczypok, E.A.; Perkins, T.N.; Oury, T.D. All the “RAGE” in lung disease: The receptor for advanced glycation endproducts (RAGE) is a major mediator of pulmonary inflammatory responses. Paediatr. Respir. Rev. 2017, 23, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Son, M.; Chung, W.J.; Oh, S.; Ahn, H.; Choi, C.H.; Hong, S.; Park, K.Y.; Son, K.H.; Byun, K. Age dependent accumulation patterns of advanced glycation end product receptor (RAGE) ligands and binding intensities between RAGE and its ligands differ in the liver, kidney, and skeletal muscle. Immun. Aging 2017, 14, 12. [Google Scholar] [CrossRef]
- Kim, H.J.; Jeong, M.S.; Jang, S.B. Molecular Characteristics of RAGE and Advances in Small-Molecule Inhibitors. Int. J. Mol. Sci. 2021, 22, 6904. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.; Gonzalez, I.; Morales, M.A. SARS-CoV-2-mediated inflammatory response in lungs: Should we look at RAGE? Inflamm. Res. 2020, 69, 641–643. [Google Scholar] [PubMed]
- Zenerino, C.; Nuzzo, A.M.; Giuffrida, D.; Biolcati, M.; Zicari, A.; Todros, T.; Rolfo, A. The HMGB1/RAGE Pro-Inflammatory Axis in the Human Placenta: Modulating Effect of Low Molecular Weight Heparin. Molecules 2017, 22, 1997. [Google Scholar]
- Strauss, J.F. 3rd. Extracellular matrix dynamics and fetal membrane rupture. Reprod. Sci. 2013, 2, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Tian, X.; Huang, H.; Zhong, N. Proteomic Study of Fetal Membrane: Inflammation-Triggered Proteolysis of Extracellular Matrix May Present a Pathogenic Pathway for Spontaneous Preterm Birth. Front. Physiol. 2020, 11, 800. [Google Scholar]
- Klein, L.L.; Gibbs, R.S. Infection and preterm birth. Obstet. Gynecol. Clin. N. Am. 2005, 32, 397–410. [Google Scholar] [CrossRef]
- Zhao, X.; Pan, S.; Liu, C. Effect of S100 calcium binding protein A12 on the pathogenesis of preeclampsia. Zhonghua Fu Chan Ke Za Zhi 2015, 50, 183–187. [Google Scholar] [PubMed]
- Buhimschi, C.S.; Baumbusch, M.A.; Dulay, A.T.; Oliver, E.A.; Lee, S.; Zhao, G.; Bhandari, V.; Ehrenkranz, R.A.; Weiner, C.P.; Madri, J.A.; et al. Characterization of RAGE, HMGB1, and S100beta in inflammation-induced preterm birth and fetal tissue injury. Am. J. Pathol. 2009, 175, 958–975. [Google Scholar] [CrossRef] [PubMed]
- Thomas, W.; Seidenspinner, S.; Kawczyńska-Leda, N.; Wirbelauer, J.; Szymankiewicz, M.; Speer, C.P. Soluble receptor for advanced glycation end products (sRAGE) in tracheobronchial aspirate fluid and cord blood of very low birth weight infants with chorioamnionitis and funisitis. Early Hum. Dev. 2010, 86, 593–598. [Google Scholar] [PubMed]
- Shi, Y.; Qian, J.; Zhang, Q.; Hu, Y.; Sun, D.; Jiang, L. Advanced glycation end products increased placental vascular permeability of human BeWo cells via RAGE/NF-kB signaling pathway. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 250, 93–100. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, Z.; Zhang, L.; Shi, Y.; Qi, J.; Chang, A.; Gao, J.; Feng, Y.; Yang, X. HMGB1-RAGE signaling pathway in severe preeclampsia. Placenta 2015, 36, 1148–1152. [Google Scholar]
- Lewis, J.B.; Mejia, C.; Jordan, C.; Monson, T.D.; Bodine, J.S.; Dunaway, T.M.; Egbert, K.M.; Lewis, A.L.; Wright, T.J.; Ogden, K.C.; et al. Inhibition of the receptor for advanced glycation end-products (RAGE) protects from secondhand smoke (SHS)-induced intrauterine growth restriction IUGR in mice. Cell Tissue Res. 2017, 370, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Plazyo, O.; Romero, R.; Unkel, R.; Balancio, A.; Mial, T.N.; Xu, Y.; Dong, Z.; Hassan, S.S.; Gomez-Lopez, N. HMGB1 Induces an Inflammatory Response in the Chorioamniotic Membranes That Is Partially Mediated by the Inflammasome. Biol. Reprod. 2016, 95, 130. [Google Scholar] [CrossRef] [PubMed]
- Alexander, K.L.; Mejia, C.A.; Jordan, C.; Nelson, M.B.; Howell, B.M.; Jones, C.M.; Reynolds, P.R.; Arroyo, J.A. Differential Receptor for Advanced Glycation End Products Expression in Preeclamptic, Intrauterine Growth Restricted, and Gestational Diabetic Placentas. Am. J. Reprod. Immunol. 2016, 75, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, Y.; Yue, C.; Ye, Y.; Chen, P.; Peng, W.; Wang, Y. Accumulation of Advanced Glycation End Products Involved in Inflammation and Contributing to Severe Preeclampsia, in Maternal Blood, Umbilical Blood and Placental Tissues. Gynecol. Obstet. Investig. 2017, 82, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Liu, W.H.; Luan, N.N.; Feng, C.; Shang, T. Correlation between the expression of high mobility group box 1 and receptor for advanced glycation end products and the onset of pre-eclampsia. Zhonghua Fu Chan Ke Za Zhi 2008, 43, 746–750. [Google Scholar]
- Holmlund, U.; Wähämaa, H.; Bachmayer, N.; Bremme, K.; Sverremark-Ekström, E.; Palmblad, K. The novel inflammatory cytokine high mobility group box protein 1 (HMGB1) is expressed by human term placenta. Immunology 2007, 122, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Xian, N.; Chen, W.; Zhang, Y.; Li, J.; Zhang, N.; Ye, Y. Correlation of the expressions of advanced glycation end products and its receptor in serum and placenta with the pathogenesis of preeclampsia. Zhonghua Fu Chan Ke Za Zhi 2015, 50, 493–499. [Google Scholar] [PubMed]
- Pradervand, P.A.; Waeber, B.; Liaudet, L.; Vial, Y.; Feihl, F. PP125. High mobility group box 1 protein (HMGB1): A pathogenic role in preeclampsia? Pregnancy Hypertens. 2012, 2, 306–307. [Google Scholar] [CrossRef]
- Chekir, C.; Nakatsuka, M.; Noguchi, S.; Konishi, H.; Kamada, Y.; Sasaki, A.; Hao, L.; Hiramatsu, Y. Accumulation of advanced glycation end products in women with preeclampsia: Possible involvement of placental oxidative and nitrative stress. Placenta 2006, 27, 225–233. [Google Scholar] [CrossRef]
- Germanová, A.; Koucký, M.; Hájek, Z.; Parízek, A.; Zima, T.; Kalousová, M. Soluble receptor for advanced glycation end products in physiological and pathological pregnancy. Clin. Biochem. 2010, 43, 442–446. [Google Scholar] [CrossRef]
- Kwon, J.H.; Kim, Y.H.; Kwon, J.Y.; Park, Y.W. Clinical significance of serum sRAGE and esRAGE in women with normal pregnancy and preeclampsia. J. Perinat. Med. 2011, 39, 507–513. [Google Scholar] [CrossRef]
- Naruse, K.; Sado, T.; Noguchi, T.; Tsunemi, T.; Yoshida, S.; Akasaka, J.; Koike, N.; Oi, H.; Kobayashi, H. Peripheral RAGE (receptor for advanced glycation endproducts)-ligands in normal pregnancy and preeclampsia: Novel markers of inflammatory response. J. Reprod. Immunol. 2012, 93, 69–74. [Google Scholar] [CrossRef]
- Cooke, C.L.; Brockelsby, J.C.; Baker, P.N.; Davidge, S.T. The receptor for advanced glycation end products (RAGE) is elevated in women with preeclampsia. Hypertens. Pregnancy 2003, 22, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.T.; Zhang, M.; Zhong, M.; Yu, Y.H.; Liang, W.Z.; Hang, L.L.; Gao, Y.F.; Huang, L.P.; Wang, Z.J. Advanced glycation end products as an upstream molecule triggers ROS-induced sFlt-1 production in extravillous trophoblasts: A novel bridge between oxidative stress and preeclampsia. Placenta 2013, 34, 1177–1182. [Google Scholar] [CrossRef]
- Shao, J.; Zhao, M.; Tong, M.; Wei, J.; Wise, M.R.; Stone, P.; Chamley, L.; Chen, Q. Increased levels of HMGB1 in trophoblastic debris may contribute to preeclampsia. Reproduction 2016, 152, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Akasaka, J.; Naruse, K.; Sado, T.; Uchiyama, T.; Makino, M.; Yamauchi, A.; Ota, H.; Sakuramoto-Tsuchida, S.; Itaya-Hironaka, A.; Takasawa, S.; et al. Involvement of Receptor for Advanced Glycation Endproducts in Hypertensive Disorders of Pregnancy. Int. J. Mol. Sci. 2019, 20, 5462. [Google Scholar] [CrossRef] [PubMed]
- Sado, T.; Naruse, K.; Noguchi, T.; Haruta, S.; Yoshida, S.; Tanase, Y.; Kitanaka, T.; Oi, H.; Kobayashi, H. Inflammatory pattern recognition receptors and their ligands: Factors contributing to the pathogenesis of preeclampsia. Inflamm. Res. 2011, 60, 509–520. [Google Scholar] [CrossRef]
- Guedes-Martins, L.; Matos, L.; Soares, A.; Silva, E.; Almeida, H. AGEs, contributors to placental bed vascular changes leading to preeclampsia. Free Radic. Res. 2013, 47, 70–80. [Google Scholar] [CrossRef]
- Gao, X.; Wang, J.; Shi, J.; Sun, Q.; Jia, N.; Li, H. The Efficacy Mechanism of Epigallocatechin Gallate against Pre-Eclampsia based on Network Pharmacology and Molecular Docking. Reprod. Sci. 2022, 29, 1859–1873. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Tao, Y.; Shang, T.; Yu, M. Calprotectin, RAGE and TNF-α in hypertensive disorders in pregnancy: Expression and significance. Arch. Gynecol. Obstet. 2011, 283, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tang, Z.Z.; Zhang, Y.M.; Kong, L.; Xiao, W.F.; Ma, T.F.; Liu, Y.W. Thrombin/PAR-1 activation induces endothelial damages via NLRP1 inflammasome in gestational diabetes. Biochem. Pharmacol. 2020, 175, 113849. [Google Scholar] [CrossRef] [PubMed]
- Rajaraman, B.; Ramadas, N.; Krishnasamy, S.; Ravi, V.; Pathak, A.; Devasena, C.S.; Swaminathan, K.; Ganeshprasad, A.; Kuppuswamy, A.A.; Vedantham, S. Hyperglycaemia cause vascular inflammation through advanced glycation end products/early growth response-1 axis in gestational diabetes mellitus. Mol. Cell Biochem. 2019, 456, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; She, G.; Zhou, W.; Liu, K.; Miao, J.; Yu, B. Expression profile of circular RNAs in placentas of women with gestational diabetes mellitus. Endocr. J. 2019, 66, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, C.; Filardi, T.; Perrone, G.; Mariani, M.; Mari, E.; Scazzocchio, B.; Masella, R.; Brunelli, R.; Lenzi, A.; Zicari, A.; et al. Cross-talk between fetal membranes and visceral adipose tissue involves HMGB1-RAGE and VIP-VPAC2 pathways in human gestational diabetes mellitus. Acta Diabetol. 2019, 56, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Daher, S.; Torloni, M.R.; Gueuvoghlanian-Silva, B.Y.; Moron, A.F.; Mattar, R. Inflammatory mediator gene polymorphisms and gestational diabetes: A review of the literature. J. Reprod. Immunol. 2011, 90, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, M.; Homko, C.; Reece, E.A. Gestational diabetes mellitus: Controversies and current opinions. Curr. Opin. Obstet. Gynecol. 1999, 11, 11157–11165. [Google Scholar] [CrossRef]
- Dai, S.; Meng, X.; Cai, X.; Yuan, C.; Zhao, Z.; Zhong, L.; Shi, Y.; Yin, F. Therapeutic effect of ursolic acid on fetal development in pregnant rats with gestational diabetes mellitus via AGEs-RAGE signaling pathway. J. Food Biochem. 2021, 45, e13651. [Google Scholar] [CrossRef]
- Tang, X.; Qin, Q.; Xie, X.; He, P. Protective effect of sRAGE on fetal development in pregnant rats with gestational diabetes mellitus. Cell Biochem. Biophys. 2015, 71, 549–556. [Google Scholar] [CrossRef]
- Şimşek Tanin, Ö.; Kara, M.; Engin-Üstün, Y.; Göçmen, A.Y.; Yalvaç, E.S. Comparison of glucose degradation product and receptor levels in diabetic and normal pregnancy. J. Turk. Ger. Gynecol. Assoc. 2021, 22, 127–131. [Google Scholar] [CrossRef]
- Jones, M.L.; Buhimschi, I.A.; Zhao, G.; Bartholomew, A.; Smith-Timms, J.; Rood, K.M.; Buhimschi, C.S. Acute Glucose Load, Inflammation, Oxidative Stress, Nonenzymatic Glycation, and Screening for Gestational Diabetes. Reprod. Sci. 2020, 27, 1587–1594. [Google Scholar] [CrossRef]
- Ejdesjö, A.; Brings, S.; Fleming, T.; Fred, R.G.; Nawroth, P.P.; Eriksson, U.J. Receptor for advanced glycation end products (RAGE) knockout reduces fetal dysmorphogenesis in murine diabetic pregnancy. Reprod. Toxicol. 2016, 62, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Pertyńska-Marczewska, M.; Głowacka, E.; Sobczak, M.; Cypryk, K.; Wilczyński, J. Glycation endproducts, soluble receptor for advanced glycation endproducts and cytokines in diabetic and non-diabetic pregnancies. Am. J. Reprod. Immunol. 2009, 61, 175–182. [Google Scholar] [CrossRef]
- Pertynska-Marczewska, M.; Cypryk, K. The possible impact of advanced glycation end products on pregnancy outcome in women with diabetes mellitus type 1. Minerva Endocrinol. 2017, 42, 271–279. [Google Scholar] [CrossRef]
- Feng, L.; Zhu, M.M.; Zhang, M.H.; Jia, X.B. N-Epsilon-(carboxymethyl)lysine is unable to induce endothelial dysfunction but is able to attenuate Ages-induced endothelium damage in human umbilical vein endothelial cells. Pharmazie 2013, 68, 251–256. [Google Scholar] [PubMed]
- Baumbusch, M.A.; Buhimschi, C.S.; Oliver, E.A.; Zhao, G.; Thung, S.; Rood, K.; Buhimschi, I.A. High Mobility Group-Box 1 (HMGB1) levels are increased in amniotic fluid of women with intra-amniotic inflammation-determined preterm birth, and the source may be the damaged fetal membranes. Cytokine 2016, 81, 82–87. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Park, K.H.; Kim, H.J.; Lee, Y.E.; Kim, Y.M.; Lee, J.E.; Hong, S. Protein microarray analysis of amniotic fluid proteins associated with spontaneous preterm birth in women with preterm labor. Am. J. Reprod. Immunol. 2022, 87, e13517. [Google Scholar] [CrossRef]
- Dubicke, A.; Andersson, P.; Fransson, E.; Andersson, E.; Sioutas, A.; Malmström, A.; Sverremark-Ekström, E.; Ekman-Ordeberg, G. High-mobility group box protein 1 and its signalling receptors in human preterm and term cervix. J. Reprod. Immunol. 2010, 84, 86–94. [Google Scholar] [CrossRef]
- Radnaa, E.; Richardson, L.S.; Sheller-Miller, S.; Baljinnyam, T.; de Castro Silva, M.; Kumar Kammala, A.; Urrabaz-Garza, R.; Kechichian, T.; Kim, S.; Han, A.; et al. Extracellular vesicle mediated feto-maternal HMGB1 signaling induces preterm birth. Lab A Chip 2021, 21, 1956–1973. [Google Scholar] [CrossRef] [PubMed]
- Bastek, J.A.; Brown, A.G.; Foreman, M.N.; McShea, M.A.; Anglim, L.M.; Adamczak, J.E.; Elovitz, M.A. The soluble receptor for advanced glycation end products can prospectively identify patients at greatest risk for preterm birth. J. Matern. Fetal Neonatal Med. 2012, 25, 1762–1768. [Google Scholar] [CrossRef]
- Hájek, Z.; Germanová, A.; Koucký, M.; Zima, T.; Kopecký, P.; Vítkova, M.; Parízek, A.; Kalousová, M. Detection of feto-maternal infection/inflammation by the soluble receptor for advanced glycation end products (sRAGE): Results of a pilot study. J. Perinat. Med. 2008, 36, 399–404. [Google Scholar] [CrossRef]
- Rogers, L.K.; Graf, A.E.; Bhatia, A.; Leonhart, K.L.; Oza-Frank, R. Associations between maternal and infant morbidities and sRAGE within the first week of life in extremely preterm infants. PLoS ONE 2013, 8, e82537. [Google Scholar] [CrossRef]
- Rzepka, R.; Dołęgowska, B.; Rajewska, A.; Sałata, D.; Budkowska, M.; Kwiatkowski, S.; Torbé, A. Diagnostic Potential of Evaluation of SDF-1α and sRAGE Levels in Threatened Premature Labor. Biomed. Res. Int. 2016, 2016, 2719460. [Google Scholar] [CrossRef] [PubMed]
- Splichalova, A.; Slavikova, V.; Splichalova, Z.; Splichal, I. Preterm Life in Sterile Conditions: A Study on Preterm, Germ-Free Piglets. Front. Immunol. 2018, 9, 220. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Sado, T.; Naruse, K.; Shigetomi, H.; Onogi, A.; Haruta, S.; Kawaguchi, R.; Nagai, A.; Tanase, Y.; Yoshida, S.; et al. Evidence for activation of Toll-like receptor and receptor for advanced glycation end products in preterm birth. Mediat. Inflamm. 2010, 2010, 490406. [Google Scholar] [CrossRef]
- Rzepka, R.; Dołęgowska, B.; Rajewska, A.; Kwiatkowski, S. On the significance of new biochemical markers for the diagnosis of premature labour. Mediat. Inflamm. 2014, 2014, 251451. [Google Scholar] [CrossRef]
- Hong, S.; Park, Y.; Kim, Y.M.; Lee, J.E.; Kim, H.J.; Park, K.H. Antibody microarray analysis of amniotic fluid proteins associated with subsequent ruptured membranes in women with threatened preterm labor. Am. J. Reprod. Immunol. 2021, 85, e13371. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, D.; Giguère, Y.; Blanchon, L.; Bujold, E.; Pereira, B.; Bernard, N.; Gallot, D.; Sapin, V.; Forest, J.C. Study of sRAGE, HMGB1, AGE, and S100A8/A9 Concentrations in Plasma and in Serum-Extracted Extracellular Vesicles of Pregnant Women With Preterm Premature Rupture of Membranes. Front. Physiol. 2020, 11, 609. [Google Scholar] [CrossRef]
- Romero, R.; Chaiworapongsa, T.; Savasan, Z.A.; Hussein, Y.; Dong, Z.; Kusanovic, J.P.; Kim, C.J.; Hassan, S.S. Clinical chorioamnionitis is characterized by changes in the expression of the alarmin HMGB1 and one of its receptors, sRAGE. J. Matern. Fetal Neonatal Med. 2012, 25, 558–567. [Google Scholar] [CrossRef]
- Romero, R.; Espinoza, J.; Hassan, S.; Gotsch, F.; Kusanovic, J.P.; Avila, C.; Erez, O.; Edwin, S.; Schmidt, A.M. Soluble receptor for advanced glycation end products (sRAGE) and endogenous secretory RAGE (esRAGE) in amniotic fluid: Modulation by infection and inflammation. J. Perinat. Med. 2008, 36, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Choltus, H.; Minet-Quinard, R.; Belville, C.; Durif, J.; Gallot, D.; Blanchon, L.; Sapin, V. Cigarette Smoke Condensate Exposure Induces Receptor for Advanced Glycation End-Products (RAGE)-Dependent Sterile Inflammation in Amniotic Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 8345. [Google Scholar] [CrossRef]
- Rzepka, R.; Dołegowska, B.; Rajewska, A.; Kwiatkowski, S.; Sałata, D.; Budkowska, M.; Domański, L.; Mikołajek-Bedner, W.; Torbé, A. Soluble and Endogenous Secretory Receptors for Advanced Glycation End Products in Threatened Preterm Labor and Preterm Premature Rupture of Fetal Membranes. Biomed. Res. Int. 2015, 2015, 568042. [Google Scholar] [CrossRef]
- Hong, S.; Park, K.H.; Lee, Y.E.; Lee, J.E.; Kim, Y.M.; Joo, E.; Cho, I. Antibody microarray analysis of amniotic fluid proteomes in women with cervical insufficiency and short cervix, and their association with pregnancy latency length. PLoS ONE 2022, 17, e0263586. [Google Scholar] [CrossRef]
- Lee, K.N.; Park, K.H.; Kim, Y.M.; Cho, I.; Kim, T.E. Prediction of emergency cerclage outcomes in women with cervical insufficiency: The role of inflammatory, angiogenic, and extracellular matrix-related proteins in amniotic fluid. PLoS ONE 2022, 17, e0268291. [Google Scholar] [CrossRef]
- Buhimschi, I.A.; Zhao, G.; Pettker, C.M.; Bahtiyar, M.O.; Magloire, L.K.; Thung, S.; Fairchild, T.; Buhimschi, C.S. The receptor for advanced glycation end products (RAGE) system in women with intraamniotic infection and inflammation. Am. J. Obstet. Gynecol. 2007, 196, 181.e1–181.e13. [Google Scholar] [CrossRef] [PubMed]
- Splichal, I.; Splichalova, A. High Mobility Group Box 1 in Pig Amniotic Membrane Experimentally Infected with E. coli O55. Biomolecules 2021, 11, 1146. [Google Scholar] [CrossRef]
- Shirasuna, K.; Seno, K.; Ohtsu, A.; Shiratsuki, S.; Ohkuchi, A.; Suzuki, H.; Matsubara, S.; Nagayama, S.; Iwata, H.; Kuwayama, T. AGEs and HMGB1 Increase Inflammatory Cytokine Production from Human Placental Cells, Resulting in an Enhancement of Monocyte Migration. Am. J. Reprod. Immunol. 2016, 75, 557–568. [Google Scholar] [CrossRef]
- Zhu, D.; Zou, H.; Liu, J.; Wang, J.; Ma, C.; Yin, J.; Peng, X.; Li, D.; Yang, Y.; Ren, Y.; et al. Inhibition of HMGB1 Ameliorates the Maternal-Fetal Interface Destruction in Unexplained Recurrent Spontaneous Abortion by Suppressing Pyroptosis Activation. Front. Immunol. 2021, 12, 782792. [Google Scholar] [CrossRef]
- Zou, H.; Yin, J.; Zhang, Z.; Xiang, H.; Wang, J.; Zhu, D.; Xu, X.; Cao, Y. Destruction in maternal-fetal interface of URSA patients via the increase of the HMGB1-RAGE/TLR2/TLR4-NF-κB signaling pathway. Life Sci. 2020, 250, 117543. [Google Scholar] [CrossRef]
- Zhao, P.; Wen, J.; Qian, L.; Zhu, X.; Wang, H.; Bai, X. Expression of S100 proteins is associated with HBV intrauterine transmission. Arch. Gynecol. Obstet. 2020, 302, 1389–1399. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.Y.F.; Tullis, B.; Breithaupt, K.L.; Fowers, R.; Jones, N.; Grajeda, S.; Reynolds, P.R.; Arroyo, J.A. A Role for RAGE in DNA Double Strand Breaks (DSBs) Detected in Pathological Placentas and Trophoblast Cells. Cells 2021, 10, 857. [Google Scholar] [CrossRef]
- Choltus, H.; Lavergne, M.; Belville, C.; Gallot, D.; Minet-Quinard, R.; Durif, J.; Blanchon, L.; Sapin, V. Occurrence of a RAGE-Mediated Inflammatory Response in Human Fetal Membranes. Front. Physiol. 2020, 11, 581. [Google Scholar] [CrossRef]
- Röhl, A.; Baek, S.H.; Kachroo, P.; Morrow, J.D.; Tantisira, K.; Silverman, E.K.; Weiss, S.T.; Sharma, A.; Glass, K.; DeMeo, D.L. Protein interaction networks provide insight into fetal origins of chronic obstructive pulmonary disease. Respir. Res. 2022, 23, 69. [Google Scholar] [CrossRef] [PubMed]
- Ota, K.; Yamagishi, S.; Kim, M.; Dambaeva, S.; Gilman-Sachs, A.; Beaman, K.; Kwak-Kim, J. Elevation of soluble form of receptor for advanced glycation end products (sRAGE) in recurrent pregnancy losses (RPL): Possible participation of RAGE in RPL. Fertil. Steril. 2014, 102, 782–789. [Google Scholar] [CrossRef]
- Winden, D.R.; Barton, D.B.; Betteridge, B.C.; Bodine, J.S.; Jones, C.M.; Rogers, G.D.; Chavarria, M.; Wright, A.J.; Jergensen, Z.R.; Jimenez, F.R.; et al. Antenatal exposure of maternal secondhand smoke (SHS) increases fetal lung expression of RAGE and induces RAGE-mediated pulmonary inflammation. Respir. Res. 2014, 15, 129. [Google Scholar] [CrossRef] [PubMed]
- Gelain, D.P.; de Bittencourt Pasquali, M.A.; Caregnato, F.F.; Moreira, J.C. Vitamin A (retinol) up-regulates the receptor for advanced glycation endproducts (RAGE) through p38 and Akt oxidant-dependent activation. Toxicology 2011, 289, 38–44. [Google Scholar] [CrossRef]
- Brockington, I. Parturient rage. Arch. Womens Ment. Health 2006, 9, 223–224. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhou, M.; Dong, X.; Jieming, Q.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- WHO Coronavirus (COVID-19) Dashboard. WHO (Blog). 29 April 2022. Available online: https://covid19.who.int/ (accessed on 29 April 2022).
- Yong, S. Long COVID or post-COVID-19 syndrome: Putative pathophysiology, risk factors, and treatments. Infect. Dis. 2021, 53, 737–754. [Google Scholar] [CrossRef]
- Long COVID: Some COVID-19 Symptoms Last for Months. UC Davis (Blog). 10 February 2022. Available online: https://health.ucdavis.edu/coronavirus/covid-19-information/covid-19-long-haulers (accessed on 28 April 2022).
- Araf, Y.; Akter, F.; Tang, Y.D.; Fatemi, R.; Parvez, M.S.A.; Zheng, C.; Hossain, M.G. Omicron variant of SARS-CoV-2: Genomics, transmissibility, and responses to current COVID-19 vaccines. J. Med. Virol. 2022, 94, 1825–1832. [Google Scholar] [CrossRef]
- SARS-CoV-2 Variant Classifications and Definitions. CDC (Blog). 26 April 2022. Available online: https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-classifications.html (accessed on 28 April 2022).
- Mohamadian, M.; Chiti, H.; Shoghli, A.; Biglari, S.; Parsamanesh, N.; Esmaeilzadeh, A. COVID-19: Virology, biology and novel laboratory diagnosis. J. Gene. Med. 2021, 23, e3303. [Google Scholar] [CrossRef]
- Scudellari, M. How the coronavirus infects cells—And why Delta is so dangerous. Nature 2021, 595, 640–644. [Google Scholar] [CrossRef]
- Mirbeyk, M.; Saghazadeh, A.; Rezaei, N. A systematic review of pregnant women with COVID-19 and their neonates. Arch. Gynecol. Obstet. 2021, 304, 5–38. [Google Scholar] [CrossRef] [PubMed]
- Taglauer, E.; Benarroch, Y.; Rop, K.; Barnett, E.; Sabharwal, V.; Yarrington, C.; Wachman, E.M. Consistent localization of SARS-CoV-2 spike glycoprotein and ACE2 over TMPRSS2 predominance in placental villi of 15 COVID-19 positive maternal-fetal dyads. Placenta 2020, 100, 69–74. [Google Scholar] [CrossRef]
- Rodrigues, C.; Baía, I.; Domingues, R.; Barros, H. Pregnancy and Breastfeeding During COVID-19 Pandemic: A Systematic Review of Published Pregnancy Cases. Front. Public Health 2020, 8, 558144. [Google Scholar] [CrossRef]
- Papageorghiou, A.T.; Deruelle, P.; Gunier, R.B.; Rauch, S.; García-May, P.K.; Mhatre, M.; Usman, M.A.; Abd-Elsalam, S.; Etuk, S.; Simmons, L.E.; et al. Preeclampsia and COVID-19: Results from the INTERCOVID prospective longitudinal study. Am. J. Obstet. Gynecol. 2021, 225, 289.e1–289.e17. [Google Scholar] [CrossRef] [PubMed]
- Villar, J.; Ariff, S.; Gunier, R.B.; Thiruvengadam, R.; Rauch, S.; Kholin, A.; Roggero, P.; Prefumo, F.; Do Vale, M.S.; Cardona-Perez, J.A.; et al. Maternal and Neonatal Morbidity and Mortality among Pregnant Women with and without COVID-19 Infection: The INTERC multinational cohort study. JAMA Pediatri. 2021, 175, 817–826. [Google Scholar] [CrossRef]
- Thompson, J.L.; Nguyen, L.M.; Noble, K.N.; Aronoff, D.M. COVID-19-related disease severity in pregnancy. Am. J. Reprod. Immunol. 2020, 84, e13339. [Google Scholar] [CrossRef]
- Metz, T.D.; Clifton, R.G.; Hughes, B.L.; Sandoval, G.J.; Grobman, W.A.; Saade, G.R.; Manuck, T.A.; Longo, M.; Sowles, A.; Clark, K.; et al. Association of SARS-CoV-2 Infection With Serious Maternal Morbidity and Mortality From Obstetric Complications. JAMA 2022, 327, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Mullins, E.; Evans, D.; Viner, R.M.; O’Brien, P.; Morris, E. Coronavirus in pregnancy and delivery: Rapid review. Ultrasound Obstet. Gynecol. 2020, 55, 586–592. [Google Scholar] [CrossRef]
- Stock, S.J.; Carruthers, J.; Calvert, C.; Denny, C.; Donaghy, J.; Goulding, A.; Hopcroft, L.E.M.; Hopkins, L.; McLaughlin, T.; Pan, J.; et al. SARS-CoV-2 infection and COVID-19 vaccination rates in pregnant women in Scotland. Nat. Med. 2022, 28, 504–512. [Google Scholar] [CrossRef]
- COVID-19 Vaccinations in the United States. CDC (Blog). 28 April 2022. Available online: https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-total-admin-rate-total (accessed on 28 April 2022).
- Goncu Ayhan, S.; Oluklu, D.; Atalay, A.; Menekse Beser, D.; Tanacan, A.; Moraloglu Tekin, O.; Sahin, D. COVID-19 vaccine acceptance in pregnant women. Int. J. Gynaecol. Obstet. 2021, 154, 291–296. [Google Scholar] [CrossRef]
- Heath, P.T.; Le Doare, K.; Khalil, A. Inclusion of pregnant women in COVID-19 vaccine development. Lancet Infect. Dis. 2020, 20, 1007–1008. [Google Scholar] [CrossRef]
- Jorgensen, S.C.J.; Davis, M.R.; Lapinsky, S.E. A review of remdesivir for COVID-19 in pregnancy and lactation. J. Antimicrob. Chemother. 2021, 77, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Garg, I.; Shekhar, R.; Sheikh, A.B.; Pal, S. COVID-19 Vaccine in Pregnant and Lactating Women: A Review of Existing Evidence and Practice Guidelines. Infect. Dis. Rep. 2021, 13, 64. [Google Scholar] [CrossRef] [PubMed]
- Najjar, S.; Najjar, A.; Chong, D.J.; Pramanik, B.K.; Kirsch, C.; Kuzniecky, R.I.; Pacia, S.V.; Azhar, S. Central nervous system complications associated with SARS-CoV-2 infection: Integrative concepts of pathophysiology and case reports. J. Neuroinflamm. 2020, 17, 231. [Google Scholar] [CrossRef]
- Lim, A.; Radujkovic, A.; Weigand, M.A.; Merle, U. Soluble receptor for advanced glycation end products (sRAGE) as a biomarker of COVID-19 disease severity and indicator of the need for mechanical ventilation, ARDS and mortality. Ann. Intensive Care 2021, 11, 50. [Google Scholar] [CrossRef] [PubMed]
- Erusalimsky, J.D. The use of the soluble receptor for advanced glycation-end products (sRAGE) as a potential biomarker of disease risk and adverse outcomes. Redox. Biol. 2021, 42, 101958. [Google Scholar] [CrossRef]
- Feng, Z.; Zhu, L.; Wu, J. RAGE signalling in obesity and diabetes: Focus on the adipose tissue macrophage. Adipocyte 2020, 9, 563–566. [Google Scholar] [CrossRef]
- Roy, D.; Ramasamy, R.; Schmidt, A.M. Journey to a Receptor for Advanced Glycation End Products Connection in Severe Acute Respiratory Syndrome Coronavirus 2 Infection: With Stops Along the Way in the Lung, Heart, Blood Vessels, and Adipose Tissue. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 614–627. [Google Scholar] [CrossRef]
- Birts, C.N.; Wilton, D.C. Age, obesity and hyperglycaemia: Activation of innate immunity initiates a series of molecular interactions involving anionic surfaces leading to COVID-19 morbidity and mortality. Med. Hypotheses 2021, 155, 110646. [Google Scholar] [CrossRef] [PubMed]
- McCartney, S.A.; Kachikis, A.; Huebner, E.M.; Walker, C.L.; Chandrasekaran, S.; Adams Waldorf, K.M. Obesity as a contributor to immunopathology in pregnant and non-pregnant adults with COVID-19. Am. J. Reprod. Immunol. 2020, 84, e13320. [Google Scholar] [CrossRef] [PubMed]
- Stilhano, R.S.; Costa, A.J.; Nishino, M.S.; Shams, S.; Bartolomeo, C.S.; Breithaupt-Faloppa, A.C.; Silva, E.A.; Ramirez, A.L.; Prado, C.M.; Ureshino, R.P. SARS-CoV-2 and the possible connection to ERs, ACE2, and RAGE: Focus on susceptibility factors. FASEB J. 2020, 34, 14103–14119. [Google Scholar] [CrossRef] [PubMed]
- Obesity and Native Hawaiians/Pacific Islanders. OMH (Blog). 2020. Available online: https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=85 (accessed on 28 April 2022).
- Hawai’i Diabetes Prevention & Control Program. State of Hawai’i Department of Health (Blog). 2009. Available online: https://health.hawaii.gov/diabetes/diabetes-prevention-and-control-program/hawaii-data/ (accessed on 28 April 2022).
- Heart Disease and Stroke Program. State of Hawai’i Department of Health (Blog). Available online: https://health.hawaii.gov/heart-disease-stroke/ (accessed on 28 April 2022).
- Trends in Number of COVID-19 Cases and Deaths in the US Reported to CDC, by State/Territory. CDC (Blog). 2022. Available online: https://covid.cdc.gov/covid-data-tracker/#trends_totaldeaths (accessed on 28 April 2022).
- Miller, F.D.; La Croix, S.; Brown, T.; Ramsey, L.T.; Morens, D. Unique pattern of COVID-19 infection in the State of Hawai’i. Int. J. Infect. Dis. 2021, 103, 298–299. [Google Scholar] [CrossRef]
- Penaia, C.S.; Morey, B.N.; Thomas, K.B.; Chang, R.C.; Tran, V.D.; Pierson, N.; Greer, J.; Ponce, N.A. Disparities in Native Hawaiian and Pacific Islander COVID-19 Mortality: A Community-Driven Data Response. Am. J. Public Health 2021, 111, S49–S52. [Google Scholar] [CrossRef] [PubMed]
- McElfish, P.A.; Purvis, R.; Willis, D.E.; Riklon, S. COVID-19 Disparities Among Marshallese Pacific Islanders. Prev. Chronic Dis. 2021, 18, E02. [Google Scholar] [CrossRef]
- Kamaka, M.L.; Watkins-Victorino, L.; Lee, A.; Freitas, S.M.; Ramsey, K.W.; Quint, J.; Ku, T.L.; Nishizaki, K.; Kaholokula, J.K. Addressing Native Hawaiian and Pacific Islander Data Deficiencies Through a Community-based Collaborative Response to the COVID-19 Pandemic. Hawaii J. Health Soc. Welf. 2021, 80, 36–45. [Google Scholar] [PubMed]
- Yamane, D.P.; Oeser, S.G.; Omori, J. Health disparities in the Native Hawaiian homeless. Hawaii Med. J. 2010, 69, 35–41. [Google Scholar]
- Liu, D.M.; Alameda, C.K. Social determinants of health for Native Hawaiian children and adolescents. Hawaii Med. J. 2011, 70, 9–14. [Google Scholar]
- Ju, A.C.; Heymantus, M.B.; Garber, A.K.; Wojcicki, J.M. Maternal Obesity and Risk of Preterm Birth and Low Birthweight in Hawaii PRAMS, 2000–2011. Matern. Child Health J. 2018, 22, 893–902. [Google Scholar] [CrossRef]
- Peristats in Hawai’i. March of Dimes (Blog). 2020. Available online: https://www.marchofdimes.org/peristats/tools/prematurityprofile.aspx?reg=15 (accessed on 28 April 2022).
- Gross, C.; Belville, C.; Lavergne, M.; Choltus, H.; Jabaudon, M.; Blondonnet, R.; Constantin, J.M.; Chiambaretta, F.; Blanchon, L.; Sapin, V. Advanced Glycation End Products and Receptor (RAGE) Promote Wound Healing of Human Corneal Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2020, 61, 14. [Google Scholar] [CrossRef]
- Shen, C.Y.; Lu, C.H.; Wu, C.H.; Li, K.J.; Kuo, Y.M.; Hsieh, S.C.; Yu, C.L. The Development of Maillard Reaction, and Advanced Glycation End Product (AGE)-Receptor for AGE (RAGE) Signaling Inhibitors as Novel Therapeutic Strategies for Patients with AGE-Related Diseases. Molecules 2020, 25, 5591. [Google Scholar] [CrossRef] [PubMed]
- Famous, K.R.; Delucchi, K.; Ware, L.B.; Kangelaris, K.N.; Liu, K.D.; Thompson, B.T.; Calfee, C.S.; ARDS Network. Acute Respiratory Distress Syndrome Subphenotypes Respond Differently to Randomized Fluid Management Strategy. Am. J. Respir. Crit. Care Med. 2017, 195, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Jabaudon, M.; Berthelin, P.; Pranal, T.; Roszyk, L.; Godet, T.; Faure, J.S.; Chabanne, R.; Eisenmann, N.; Lautrette, A.; Belville, C.; et al. Receptor for advanced glycation end-products and ARDS prediction: A multicentre observational study. Sci. Rep. 2018, 8, 2603. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Alkazmi, L.; Habotta, O.A.; Batiha, G.E. High-mobility group box 1 (HMGB1) in COVID-19: Extrapolation of dangerous liaisons. Inflammopharmacology 2022, 30, 811–820, Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurashima, C.K.; Ng, P.K.; Kendal-Wright, C.E. RAGE against the Machine: Can Increasing Our Understanding of RAGE Help Us to Battle SARS-CoV-2 Infection in Pregnancy? Int. J. Mol. Sci. 2022, 23, 6359. https://doi.org/10.3390/ijms23126359
Kurashima CK, Ng PK, Kendal-Wright CE. RAGE against the Machine: Can Increasing Our Understanding of RAGE Help Us to Battle SARS-CoV-2 Infection in Pregnancy? International Journal of Molecular Sciences. 2022; 23(12):6359. https://doi.org/10.3390/ijms23126359
Chicago/Turabian StyleKurashima, Courtney K., Po’okela K. Ng, and Claire E. Kendal-Wright. 2022. "RAGE against the Machine: Can Increasing Our Understanding of RAGE Help Us to Battle SARS-CoV-2 Infection in Pregnancy?" International Journal of Molecular Sciences 23, no. 12: 6359. https://doi.org/10.3390/ijms23126359
APA StyleKurashima, C. K., Ng, P. K., & Kendal-Wright, C. E. (2022). RAGE against the Machine: Can Increasing Our Understanding of RAGE Help Us to Battle SARS-CoV-2 Infection in Pregnancy? International Journal of Molecular Sciences, 23(12), 6359. https://doi.org/10.3390/ijms23126359





