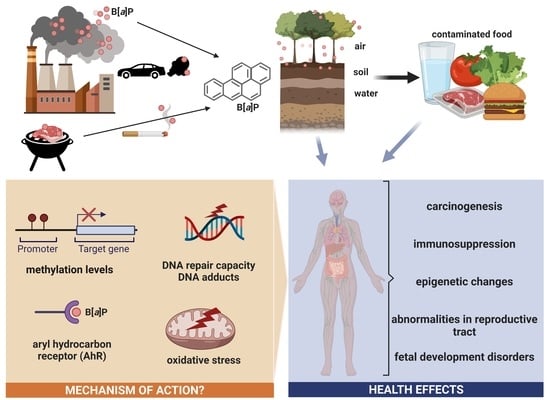
Benzo[a]pyrene—Environmental Occurrence, Human Exposure, and Mechanisms of Toxicity
Abstract
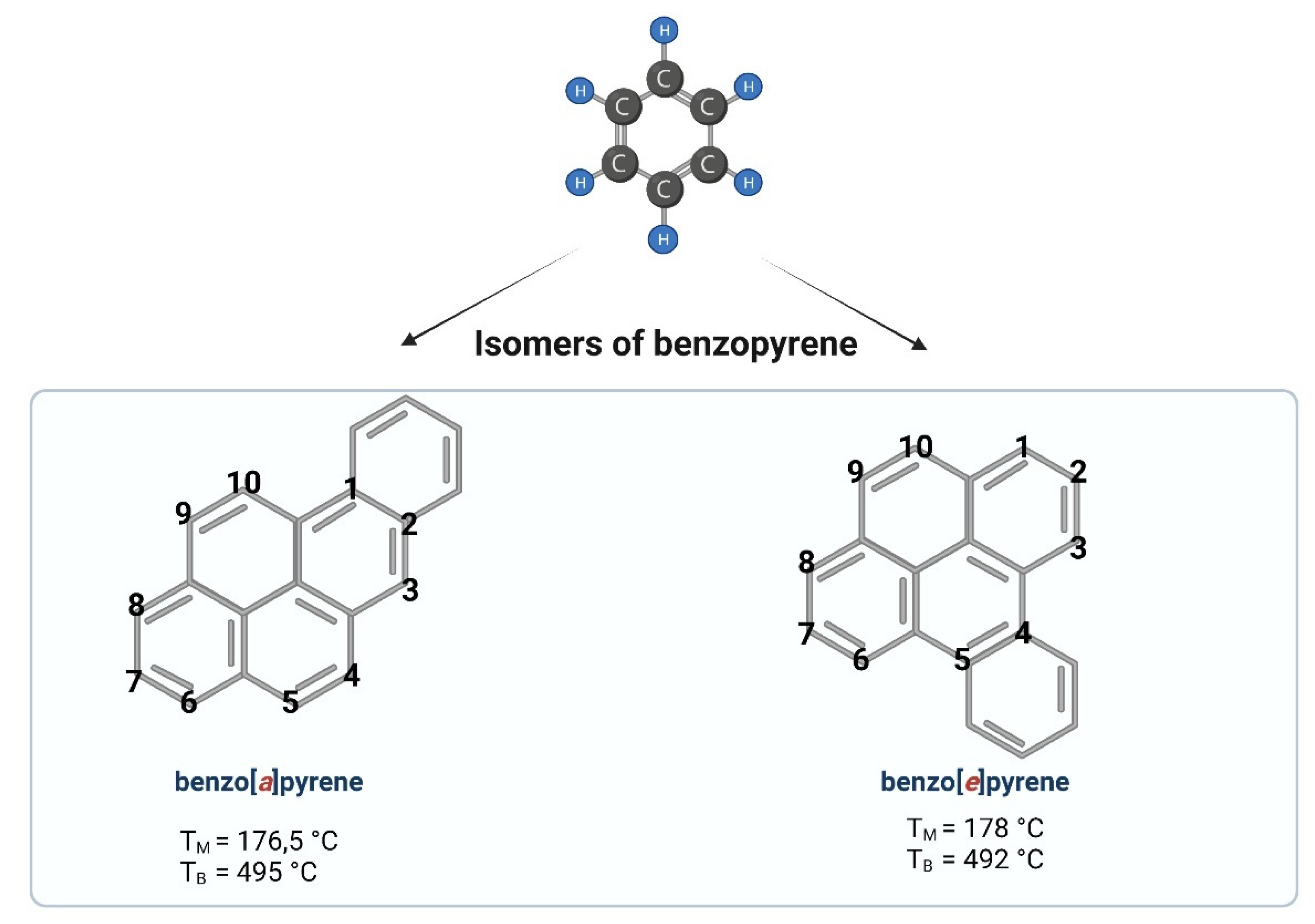
1. General Information
2. Occupational Exposure to PAHs, Including B[a]P
3. Sources of Human Exposure to B[a]P
3.1. Air
3.1.1. Outdoor Air
3.1.2. Indoor Air
3.2. Surface Water
3.3. Soil
Biodegradation B[a]P by Microorganisms
3.4. Food Contamination
Drinking Water
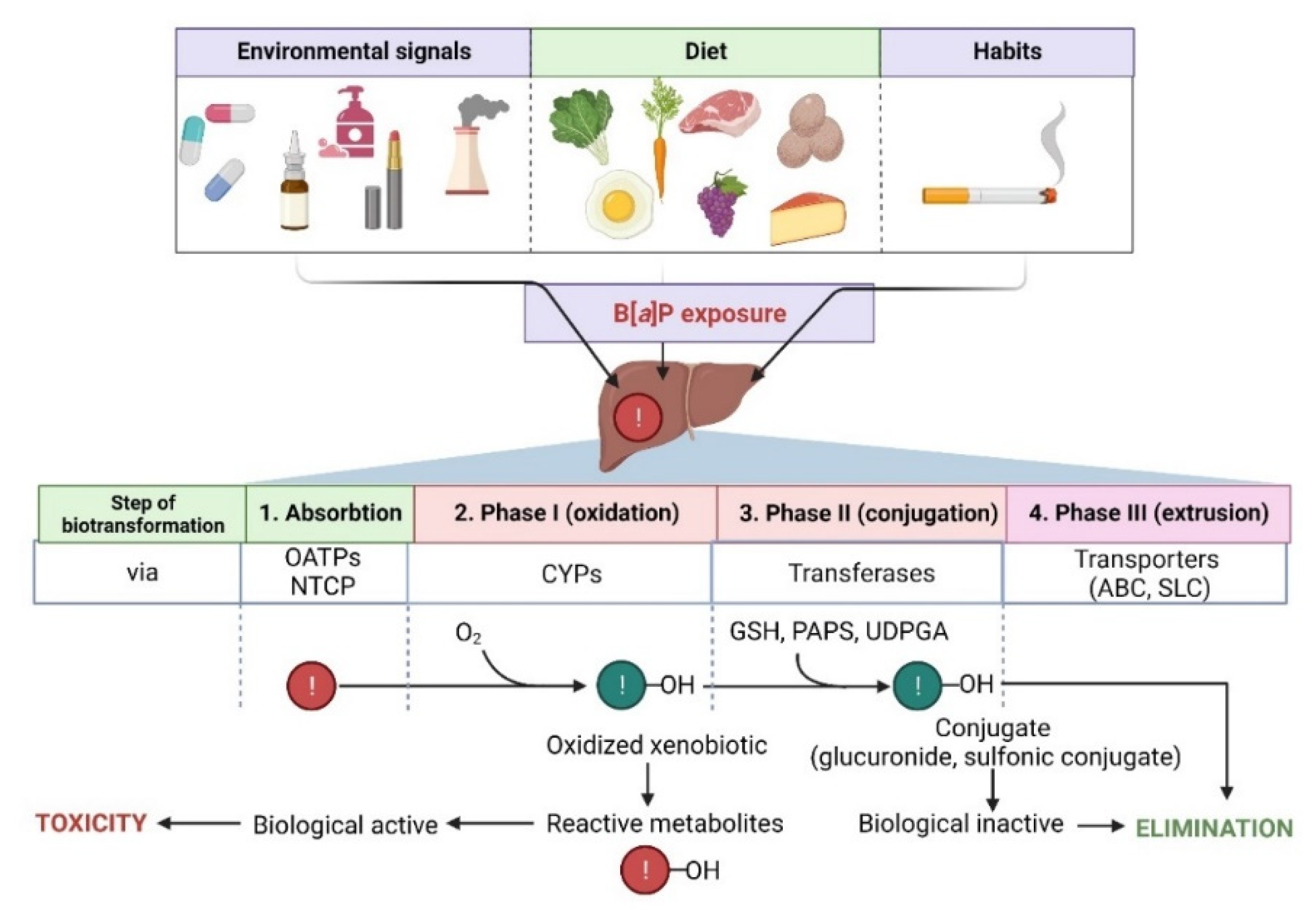
4. Metabolism of B[a]P
4.1. Polymorphism of Genes Involved in B[a]P Metabolism and DNA Repair, Influencing Its Toxicity
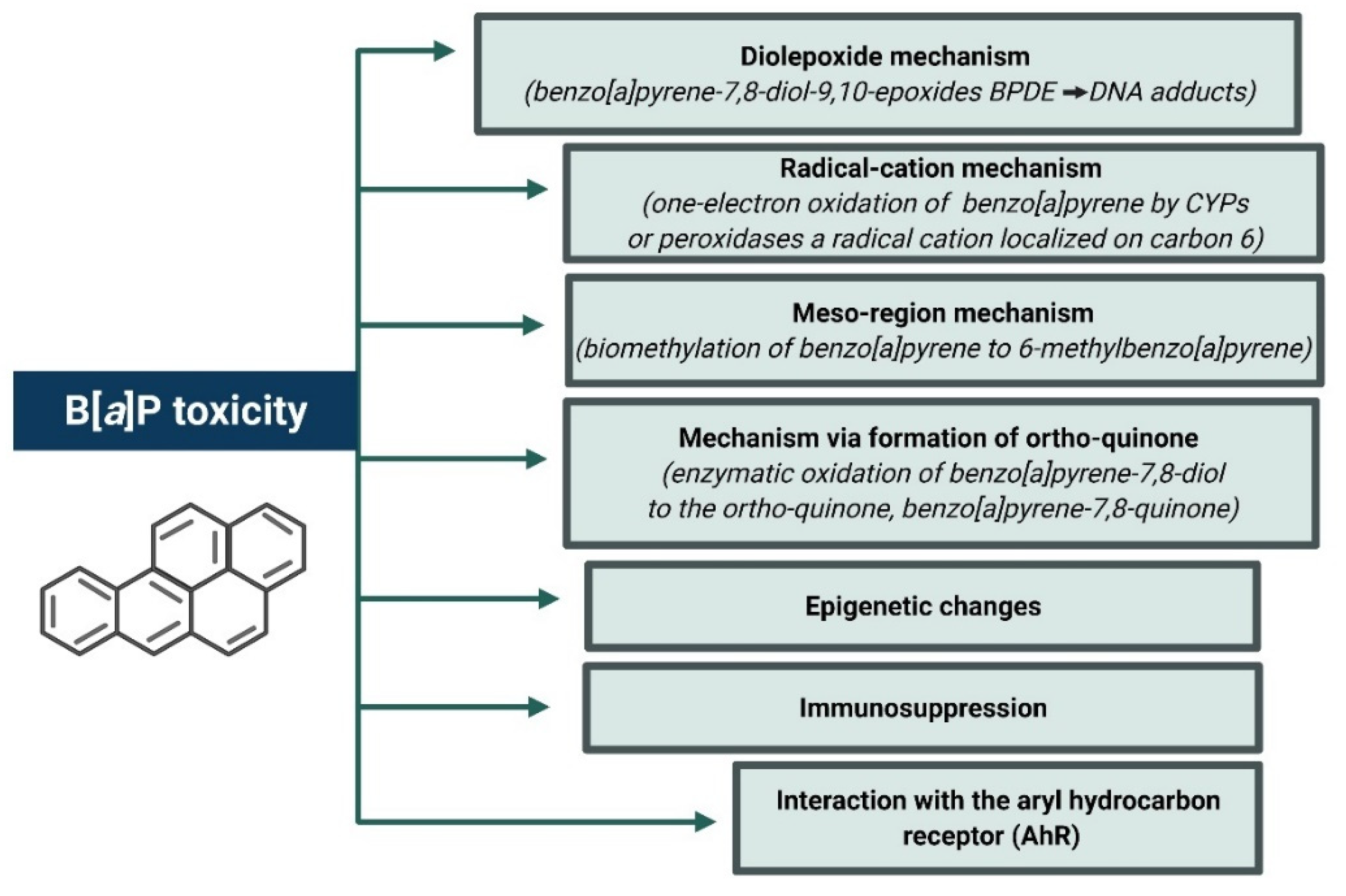
4.2. B[a]P Carcinogenesis Mechanisms Associated with Its Metabolism
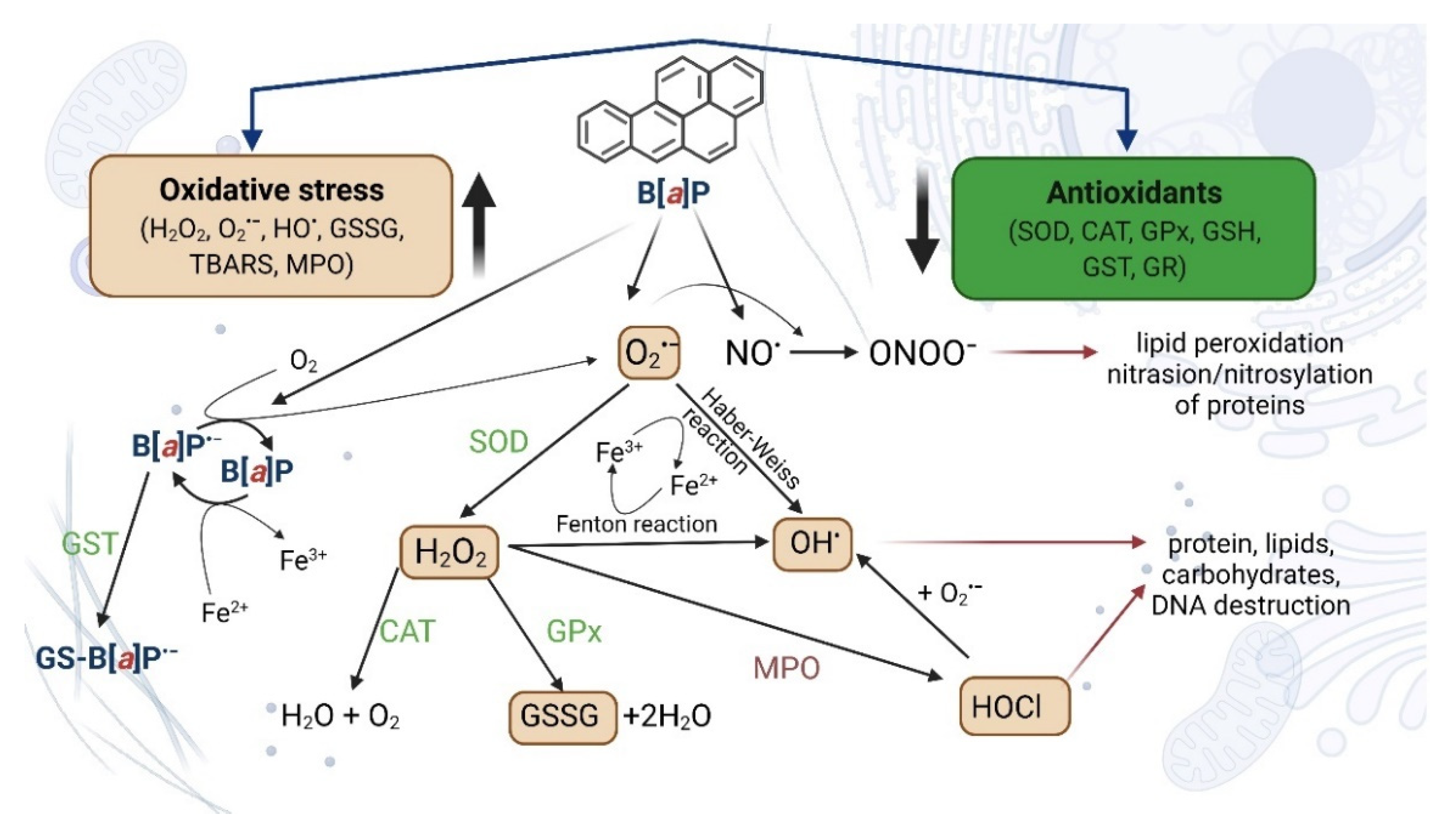
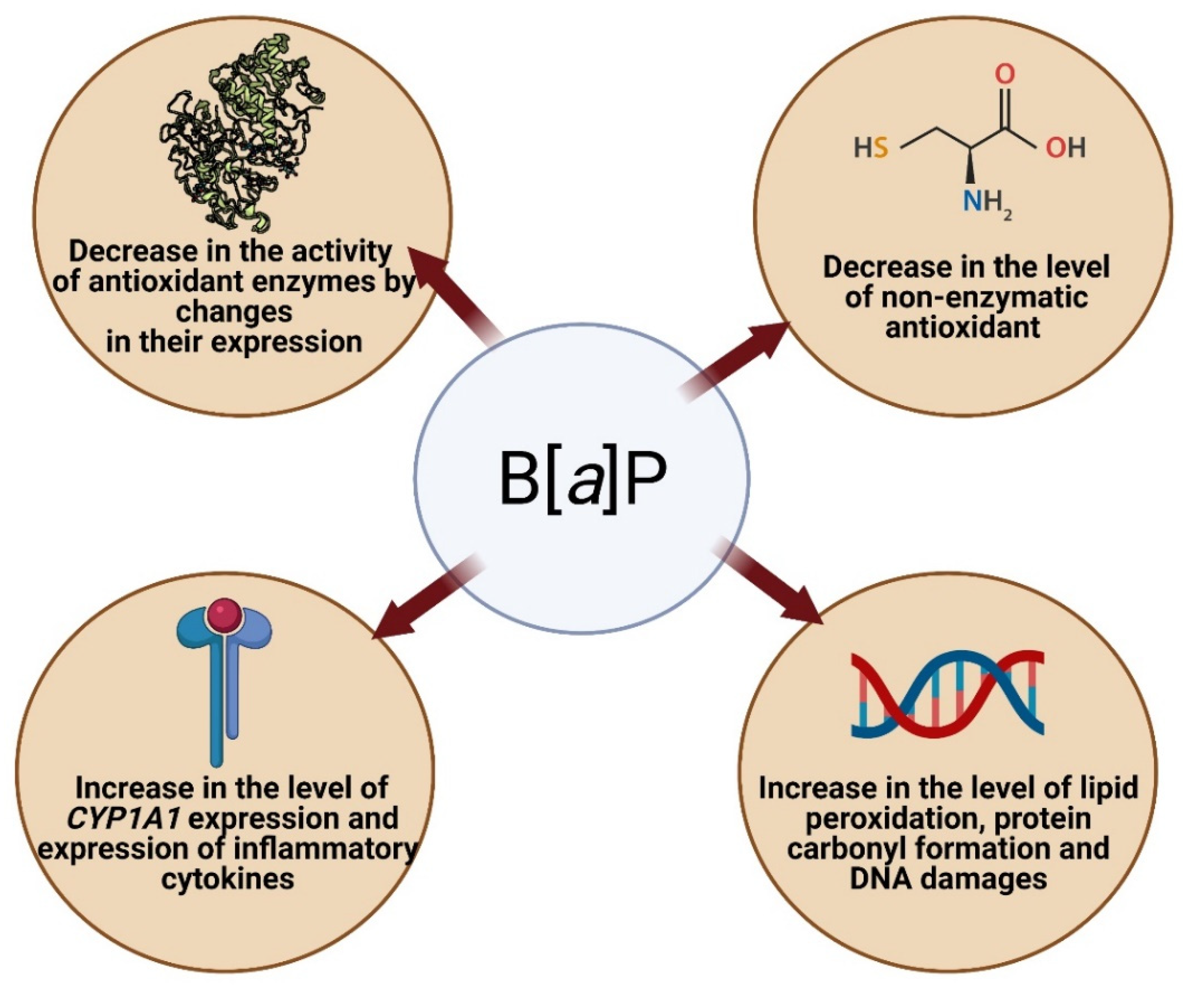
4.2.1. Oxidative Stress and Apoptosis as a Result of Increased Expression of Selected Genes and Increased CYP Activity
4.2.2. Oxidative Stress and Neurotoxicity
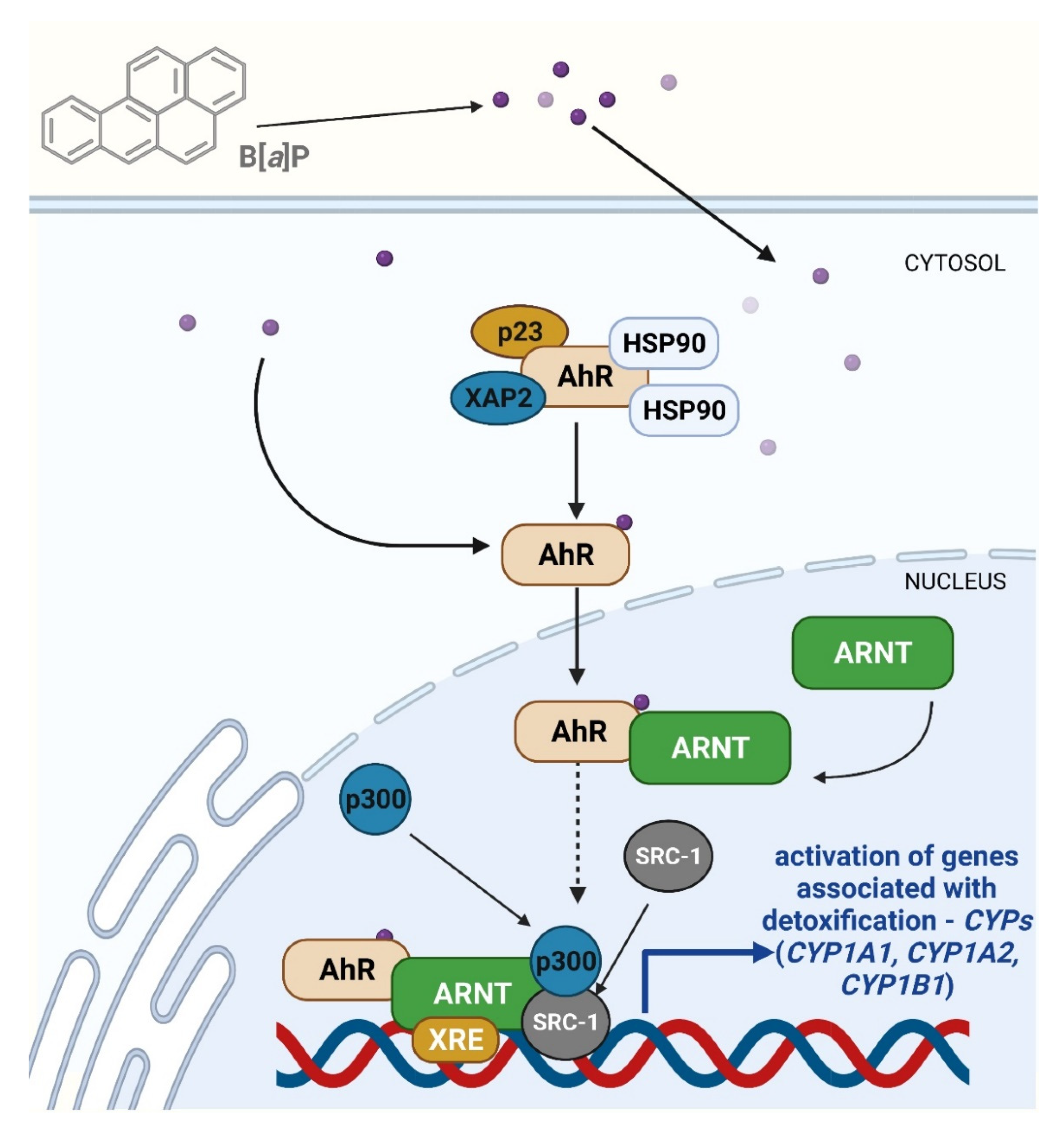
4.2.3. The Role of AhR Receptor in Toxicity and Carcinogenicity of B[a]P
5. Adverse Effects Observed in In Vitro and In Vivo Studies
5.1. Genotoxicity and Carcinogenicity
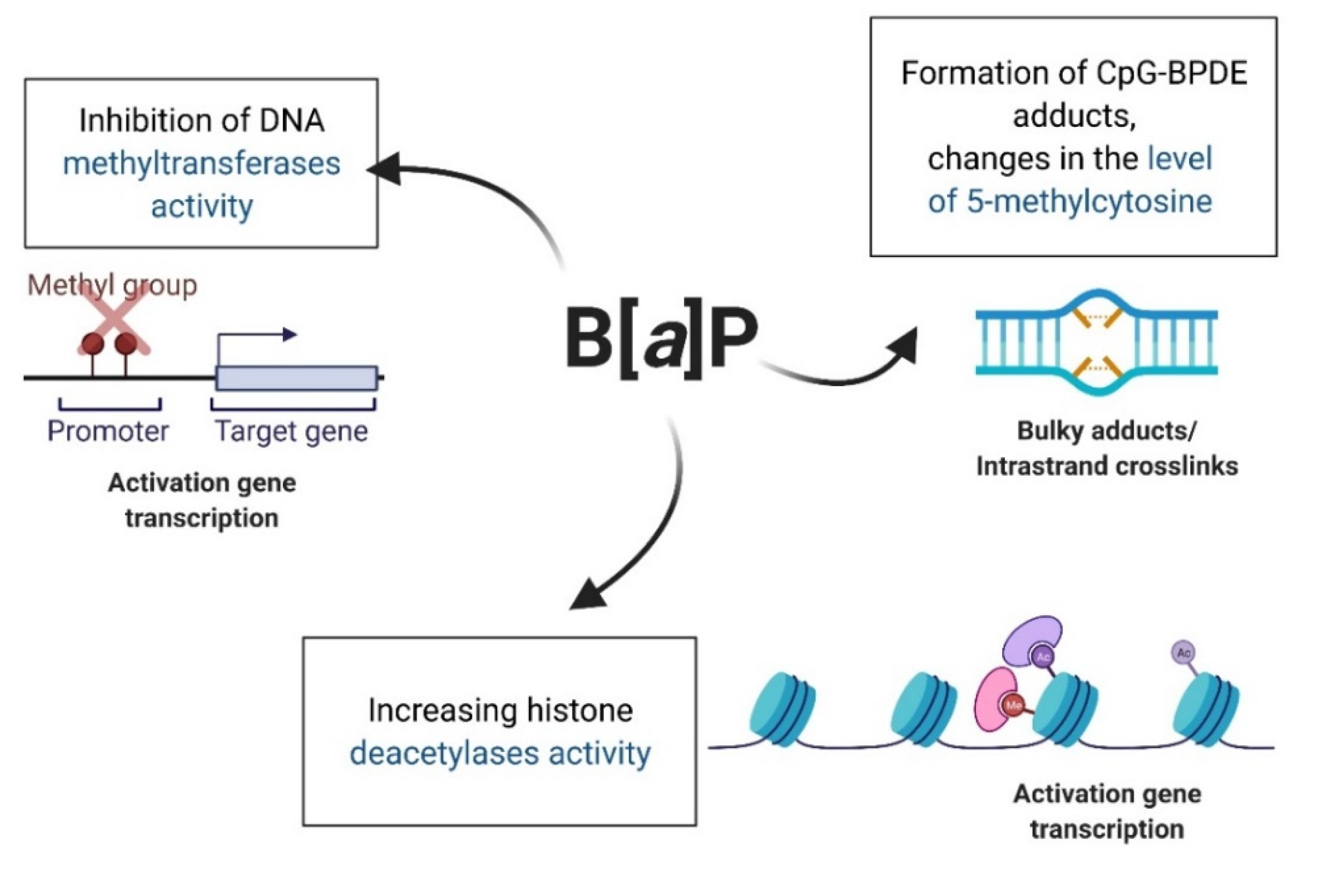
5.2. Epigenetic Effect
5.3. Epigenetic and Carcinogenic Effect of Benzo[a]pyrene in Epidemiological Studies—Exposure to B[a]P Present in PAH Mixtures
5.4. Effects on Reproduction
5.4.1. B[a]P Effect on Males
5.4.2. Effect on Females
5.4.3. Effect on Fetal Development
6. Impact on Virus Development
7. Other Effects
8. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bezza, F.A.; Chirwa, E.M.N. The Role of Lipopeptide Biosurfactant on Microbial Remediation of Aged Polycyclic Aromatic Hydrocarbons (PAHs)-Contaminated Soil. Chem. Eng. J. 2017, 309, 563–576. [Google Scholar] [CrossRef]
- Boström, C.E.; Gerde, P.; Hanberg, A.; Jernström, B.; Johansson, C.; Kyrklund, T.; Rannug, A.; Törnqvist, M.; Victorin, K.; Westerholm, R. Cancer Risk Assessment, Indicators, and Guidelines for Polycyclic Aromatic Hydrocarbons in the Ambient Air. Environ. Health Perspect. 2002, 110, 451–488. [Google Scholar] [PubMed]
- Saravanakumar, K.; Sivasantosh, S.; Sathiyaseelan, A.; Sankaranarayanan, A.; Naveen, K.V.; Zhang, X.; Jamla, M.; Vijayasarathy, S.; Vishnu Priya, V.; MubarakAli, D.; et al. Impact of Benzo[a]Pyrene with Other Pollutants Induce the Molecular Alternation in the Biological System: Existence, Detection and Remediation Methods. Environ. Pollut. 2022, 304, 119207. [Google Scholar] [CrossRef]
- Knafla, A.; Phillipps, K.A.; Brecher, R.W.; Petrovic, S.; Richardson, M. Development of a Dermal Cancer Slope Factor for Benzo[a]Pyrene. Regul. Toxicol. Pharmacol. 2006, 45, 159–168. [Google Scholar] [CrossRef]
- Bukowska, B.; Sicińska, P. Influence of Benzo(a)Pyrene on Different Epigenetic Processes. Int. J. Mol. Sci. 2021, 22, 13453. [Google Scholar] [CrossRef] [PubMed]
- IARC Working Group Benzo[a]Pyrene. Iarc Monographs on the Identification of Carcinogenic Hazards to Humans. 2010. Available online: https://monographs.iarc.who.int/wp-content/uploads/2018/06/mono100F-14.pdf (accessed on 12 May 2022).
- Susanto, A.; Yusril, N.; Zaini, J.; Nuwidya, F. Comparison of Serum Benzo(a)Pyrene Diol Epoxide—Protein Adducts Level between Kretek Cigarette Smokers and Nonsmokers and the Related Factors. J. Nat. Sci. Biol. Med. 2021, 12, 52. [Google Scholar] [CrossRef]
- Motejlek, K.; Palluch, F.; Neulen, J.; Grümmer, R. Smoking Impairs Angiogenesis during Maturation of Human Oocytes. Fertil. Steril. 2006, 86, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Younglai, E.V.; Holloway, A.C.; Foster, W.G. Environmental and Occupational Factors Affecting Fertility and IVF Success. Hum. Reprod. Update 2005, 11, 43–57. [Google Scholar] [CrossRef]
- Saleh, S.A.K.; Adly, H.M.; Aljahdali, I.A.; Khafagy, A.A. Correlation of Occupational Exposure to Carcinogenic Polycyclic Aromatic Hydrocarbons (CPAHs) and Blood Levels of P53 and P21 Proteins. Biomolecules 2022, 12, 260. [Google Scholar] [CrossRef]
- Petit, P.; Maître, A.; Persoons, R.; Bicout, D.J. Lung Cancer Risk Assessment for Workers Exposed to Polycyclic Aromatic Hydrocarbons in Various Industries. Environ. Int. 2019, 124, 109–120. [Google Scholar] [CrossRef]
- Guerreiro, C.B.B.; Horálek, J.; de Leeuw, F.; Couvidat, F. Benzo(a)Pyrene in Europe: Ambient Air Concentrations, Population Exposure and Health Effects. Environ. Pollut. 2016, 214, 657–667. [Google Scholar] [CrossRef]
- Amadou, A.; Praud, D.; Coudon, T.; Deygas, F.; Grassot, L.; Faure, E.; Couvidat, F.; Caudeville, J.; Bessagnet, B.; Salizzoni, P.; et al. Risk of Breast Cancer Associated with Long-Term Exposure to Benzo[a]Pyrene (BaP) Air Pollution: Evidence from the French E3N Cohort Study. Environ. Int. 2021, 149, 106399. [Google Scholar] [CrossRef]
- Mahasakpan, N.; Chaisongkaew, P.; Inerb, M.; Nim, N.; Phairuang, W.; Tekasakul, S.; Furuuchi, M.; Hata, M.; Kaosol, T.; Tekasakul, P.; et al. Fine and Ultrafine Particle- and Gas-Polycyclic Aromatic Hydrocarbons Affecting Southern Thailand Air Quality during Transboundary Haze and Potential Health Effects. J. Environ. Sci. 2023, 124, 253–267. [Google Scholar] [CrossRef]
- San José, R.; Pérez, J.L.; Callén, M.S.; López, J.M.; Mastral, A. BaP (PAH) Air Quality Modelling Exercise over Zaragoza (Spain) Using an Adapted Version of WRF-CMAQ Model. Environ. Pollut. 2013, 183, 151–158. [Google Scholar] [CrossRef]
- Valerio, F.; Pala, M.; Lazzarotto, A.; Stella, A.; Ciccarelli, F.; Balducci, D.; Brescianini, C. Air Quality Standard for Benzo(a)Pyrene (BaP) in Genoa (1994–1995). Polycycl. Aromat. Compd. 1996, 9, 61–66. [Google Scholar] [CrossRef]
- Flaga-Maryańczyk, A.; Baran-Gurgul, K. The Impact of Local Anti-Smog Resolution in Cracow (Poland) on the Concentrations of PM10 and BaP Based on the Results of Measurements of the State Environmental Monitoring. Energies 2021, 15, 56. [Google Scholar] [CrossRef]
- Deziel, N.C.; Wei, W.-Q.; Abnet, C.C.; Qiao, Y.-L.; Sunderland, D.; Ren, J.-S.; Schantz, M.M.; Zhang, Y.; Strickland, P.T.; Abubaker, S.; et al. A Multi-Day Environmental Study of Polycyclic Aromatic Hydrocarbon Exposure in a High-Risk Region for Esophageal Cancer in China. J. Expo. Sci. Environ. Epidemiol. 2013, 23, 52–59. [Google Scholar] [CrossRef]
- Fismes, J.; Perrin-Ganier, C.; Empereur-Bissonnet, P.; Morel, J.L. Soil-to-Root Transfer and Translocation of Polycyclic Aromatic Hydrocarbons by Vegetables Grown on Industrial Contaminated Soils. J. Environ. Qual. 2002, 31, 1649–1656. [Google Scholar] [CrossRef]
- Sushkova, S.; Deryabkina, I.; Antonenko, E.; Kizilkaya, R.; Rajput, V.; Vasilyeva, G. Benzo[a]Pyrene Degradation and Bioaccumulation in Soil-Plant System under Artificial Contamination. Sci. Total Environ. 2018, 633, 1386–1391. [Google Scholar] [CrossRef]
- Research Committee Report, 1984-Epa Nepis. Available online: https://nepis.epa.gov (accessed on 12 May 2022).
- Dejchanchaiwong, R.; Tekasakul, P.; Tekasakul, S.; Phairuang, W.; Nim, N.; Sresawasd, C.; Thongboon, K.; Thongyen, T.; Suwattiga, P. Impact of Transport of Fine and Ultrafine Particles from Open Biomass Burning on Air Quality during 2019 Bangkok Haze Episode. J. Environ. Sci. 2020, 97, 149–161. [Google Scholar] [CrossRef]
- Jin, T.; Han, M.; Han, K.; Fu, X.; Xu, L.; Xu, X. Health Risk of Ambient PM10-Bound PAHs at Bus Stops in Spring and Autumn in Tianjin, China. Aerosol Air Qual. Res. 2018, 18, 1828–1838. [Google Scholar] [CrossRef]
- Air Quality in Europe. 2020. Available online: https://www.eea.europa.eu/publications/air-quality-in-europe-2020-report (accessed on 12 May 2022).
- Schreiberová, M.; Vlasáková, L.; Vlček, O.; Šmejdířová, J.; Horálek, J.; Bieser, J. Benzo[a]Pyrene in the Ambient Air in the Czech Republic: Emission Sources, Current and Long-Term Monitoring Analysis and Human Exposure. Atmosphere 2020, 11, 955. [Google Scholar] [CrossRef]
- Eionet Report—ETC/ATNI Benzo(a)Pyrene (B[a]P) Annual Mapping. Benzo(a)pyrene (BaP) annual mapping—Eionet. 2021. Available online: https://www.eionet.europa.eu (accessed on 12 May 2022).
- Gordon, S.B.; Bruce, N.G.; Grigg, J.; Hibberd, P.L.; Kurmi, O.P.; Lam, K.H.; Mortimer, K.; Asante, K.P.; Balakrishnan, K.; Balmes, J.; et al. Respiratory Risks from Household Air Pollution in Low and Middle Income Countries. Lancet Respir. Med. 2014, 2, 823–860. [Google Scholar] [CrossRef]
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-Year Trends of the Global Burden of Disease Attributable to Ambient Air Pollution: An Analysis of Data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef]
- Feng, S.; Shen, X.; Hao, X.; Cao, X.; Li, X.; Yao, X.; Shi, Y.; Lv, T.; Yao, Z. Polycyclic and Nitro-Polycyclic Aromatic Hydrocarbon Pollution Characteristics and Carcinogenic Risk Assessment of Indoor Kitchen Air during Cooking Periods in Rural Households in North China. Environ. Sci. Pollut. Res. 2021, 28, 11498–11508. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Qiu, J.Q.; Shu, M.; Ho, S.S.H.; Cao, J.J.; Wang, G.H.; Wang, X.X.; Zhao, X.Q. Characteristics of Polycyclic Aromatic Hydrocarbons in PM2.5 Emitted from Different Cooking Activities in China. Environ. Sci. Pollut. Res. 2018, 25, 4750–4760. [Google Scholar] [CrossRef]
- Cui, L.; Duo, B.; Zhang, F.; Li, C.; Fu, H.; Chen, J. Physiochemical Characteristics of Aerosol Particles Collected from the Jokhang Temple Indoors and the Implication to Human Exposure. Environ. Pollut. 2018, 236, 992–1003. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, N.; Liang, W.; Chen, X.; Hou, R.; Kang, Y.; Guo, Q.; Cao, S.; Duan, X. Polycycl. Aromatic Hydrocarbon Exposure of Children in Typical Household Coal Combustion Environments: Seasonal Variations, Sources, and Carcinogenic Risks. Int. J. Environ. Res. Public Health 2020, 17, 6520. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, M.A.; Hassan, S.K.; Alzahrani, N.A.; Al Sharif, M.Y.; Khoder, M.I. Classroom Dust-Bound Polycyclic Aromatic Hydrocarbons in Jeddah Primary Schools, Saudi Arabia: Level, Characteristics and Health Risk Assessment. Int. J. Environ. Res. Public Health 2020, 17, 2779. [Google Scholar] [CrossRef] [PubMed]
- Błaszczyk, E.; Rogula-Kozłowska, W.; Klejnowski, K.; Kubiesa, P.; Fulara, I.; Mielżyńska-Švach, D. Indoor Air Quality in Urban and Rural Kindergartens: Short-Term Studies in Silesia, Poland. Air Qual. Atmos. Health 2017, 10, 1207–1220. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Narayana, J.; Ravichandran, B.; Dhananjayan, V. Polyaromatic Hydrocarbons Depositions and Their Carcinogenic Risk Assessment in the Foundry Workers. Aerosol Sci. Eng. 2018, 2, 173–181. [Google Scholar] [CrossRef]
- Ny, E.T.; Heederik, D.; Kromhout, H.; Jongeneelen, F. The Relationship Between Polycyclic Aromatic Hydrocarbons in Air and in Urine of Workers in Söderberg Potroom. Am. Ind. Hyg. Assoc. J. 1993, 54, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Brandt, H.C.; Watson, W.P. Monitoring Human Occupational and Environmental Exposures to Polycyclic Aromatic Compounds. Ann. Occup. Hyg. 2003, 47, 349–378. [Google Scholar]
- He, Y.; Yang, C.; He, W.; Xu, F. Nationwide Health Risk Assessment of Juvenile Exposure to Polycyclic Aromatic Hydrocarbons (PAHs) in the Water Body of Chinese Lakes. Sci. Total Environ. 2020, 723, 138099. [Google Scholar] [CrossRef]
- Singare, P.U. Carcinogenic and Endocrine-Disrupting PAHs in the Aquatic Ecosystem of India. Environ. Monit. Assess. 2016, 188, 599. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Peng, B.; Huang, H.; Kuang, Y.; Qian, Z.; Zhu, W.; Liu, W.; Zhang, Y.; Liao, Y.; Zhao, X.; et al. Distribution and Potential Sources of OCPs and PAHs in Waters from the Danshui River Basin in Yichang, China. Int. J. Environ. Res. Public Health 2021, 19, 263. [Google Scholar] [CrossRef]
- Hayakawa, K.; Makino, F.; Yasuma, M.; Yoshida, S.; Chondo, Y.; Toriba, A.; Kameda, T.; Tang, N.; Kunugi, M.; Nakase, H.; et al. Polycyclic Aromatic Hydrocarbons in Surface Water of the Southeastern Japan Sea. Chem. Pharm. Bull. 2016, 64, 625–631. [Google Scholar] [CrossRef]
- Li, R.; Cheng, M.; Cui, Y.; He, Q.; Guo, X.; Chen, L.; Wang, X. Distribution of the Soil PAHs and Health Risk Influenced by Coal Usage Processes in Taiyuan City, Northern China. Int. J. Environ. Res. Public Health 2020, 17, 6319. [Google Scholar] [CrossRef] [PubMed]
- Twardowska, I.; Kolodziejczyk, A.M. Benzo[a]Pyrene in Soils and Ground Water: Occurrence, Sources, Distribution, Interrelation. Toxicol. Environ. Chem. 1998, 66, 127–144. [Google Scholar] [CrossRef]
- Wydro, U.; Wołejko, E.; Łoboda, T.; Kowczyk-Sadowy, M. Changes the Concentration of Selected PAHs in Urban Soils Fertilized with Municipal Sewage Sludge. J. Ecol. Eng. 2015, 16, 62–67. [Google Scholar] [CrossRef]
- Obrycki, J.F.; Basta, N.T.; Culman, S.W. Management Options for Contaminated Urban Soils to Reduce Public Exposure and Maintain Soil Health. J. Environ. Qual. 2017, 46, 420–430. [Google Scholar] [CrossRef]
- Olgun, B.; Doğan, G. Polycyclic Aromatic Hydrocarbon Concentrations in Soils of Greenhouses Located in Aksu Antalya, Turkey. Water Sci. Technol. 2020, 81, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Wilcke, W. SYNOPSIS Polycyclic Aromatic Hydrocarbons (PAHs) in Soil—A Review. J. Plant Nutr. Soil Sci. 2000, 163, 229–248. [Google Scholar] [CrossRef]
- Mielke, H.W.; Wang, G.; Gonzales, C.R.; Le, B.; Quach, V.N.; Mielke, P.W. PAH and Metal Mixtures in New Orleans Soils and Sediments. Sci. Total Environ. 2001, 281, 217–227. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, J.; Yang, P.; Qiao, X.; Tian, F. Polycyclic Aromatic Hydrocarbons in Dalian Soils: Distribution and Toxicity Assessment. J. Environ. Monit. 2007, 9, 199–204. [Google Scholar] [CrossRef]
- Nam, J.J.; Thomas, G.O.; Jaward, F.M.; Steinnes, E.; Gustafsson, O.; Jones, K.C. PAHs in Background Soils from Western Europe: Influence of Atmospheric Deposition and Soil Organic Matter. Chemosphere 2008, 70, 1596–1602. [Google Scholar] [CrossRef]
- Nadal, M.; Schuhmacher, M.; Domingo, J.L. Levels of PAHs in Soil and Vegetation Samples from Tarragona County, Spain. Environ. Pollut. 2004, 132, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Maliszewska-Kordybach, B.; Smreczak, B.; Klimkowicz-Pawlas, A. Concentrations, Sources and Spatial Distribution of Individual Polycyclic Aromatic Hydrocarbons (PAHs) in Agricultural Soils in the Eastern Part of the EU: Poland as a Case Study. Sci. Total Environ. 2009, 407, 3746–3753. [Google Scholar] [CrossRef]
- Shamilishvily, G.; Abakumov, E.; Gabov, D. Polycyclic Aromatic Hydrocarbon in Urban Soils of an Eastern European Megalopolis: Distribution, Source Identification and Cancer Risk Evaluation. Solid Earth 2018, 9, 669–682. [Google Scholar] [CrossRef]
- Abakumov, E.; Nizamutdinov, T.; Yaneva, R.; Zhiyanski, M. Polycyclic Aromatic Hydrocarbons and Potentially Toxic Elements in Soils of the Vicinity of the Bulgarian Antarctic Station “St. Kliment Ohridski” (Antarctic Peninsula). Front. Environ. Sci. 2021, 9, 656271. [Google Scholar] [CrossRef]
- Bezberdaya, L.; Kosheleva, N.; Chernitsova, O.; Lychagin, M.; Kasimov, N. Pollution Level, Partition and Spatial Distribution of Benzo(a)Pyrene in Urban Soils, Road Dust and Their PM10 Fraction of Health-Resorts (Alushta, Yalta) and Industrial (Sebastopol) Cities of Crimea. Water 2022, 14, 561. [Google Scholar] [CrossRef]
- Lee, C.C.; Chen, C.S.; Wang, Z.X.; Tien, C.J. Polycyclic Aromatic Hydrocarbons in 30 River Ecosystems, Taiwan: Sources and Ecological and Human Health Risks. Sci. Total Environ. 2021, 795, 148867. [Google Scholar] [CrossRef]
- Punetha, A.; Saraswat, S.; Rai, J.P.N. An Insight on Microbial Degradation of Benzo[a]Pyrene: Current Status and Advances in Research. World J. Microbiol. Biotechnol. 2022, 38, 61. [Google Scholar] [CrossRef]
- Nzila, A.; Musa, M.M.; Sankara, S.; Al-Momani, M.; Xiang, L.; Li, Q.X. Degradation of Benzo[a]Pyrene by Halophilic Bacterial Strain Staphylococcus haemoliticus Strain 10SBZ1A. PLoS ONE 2021, 16, e0247723. [Google Scholar] [CrossRef]
- Delgado-Saborit, J.M.; Alam, M.S.; Godri Pollitt, K.J.; Stark, C.; Harrison, R.M. Analysis of Atmospheric Concentrations of Quinones and Polycyclic Aromatic Hydrocarbons in Vapour and Particulate Phases. Atmos. Environ. 2013, 77, 974–982. [Google Scholar] [CrossRef]
- Sampaio, G.R.; Guizellini, G.M.; da Silva, S.A.; de Almeida, A.P.; Pinaffi-Langley, A.C.C.; Rogero, M.M.; de Camargo, A.C.; Torres, E.A.F.S. Polycyclic Aromatic Hydrocarbons in Foods: Biological Effects, Legislation, Occurrence, Analytical Methods, and Strategies to Reduce Their Formation. Int. J. Mol. Sci. 2021, 22, 6010. [Google Scholar] [CrossRef]
- Gharby, S.; Harhar, H.; Farssi, M.; Ait Taleb, A.; Guillaume, D.; Laknifli, A. Influence of Roasting Olive Fruit on the Chemical Composition and Polycyclic Aromatic Hydrocarbon Content of Olive Oil. OCL 2018, 25, A303. [Google Scholar] [CrossRef]
- Santonicola, S.; Albrizio, S.; Murru, N.; Ferrante, M.C.; Mercogliano, R. Study on the Occurrence of Polycyclic Aromatic Hydrocarbons in Milk and Meat/Fish Based Baby Food Available in Italy. Chemosphere 2017, 184, 467–472. [Google Scholar] [CrossRef]
- Migdał, W.; Walczycka, M.; Migdał, Ł. The Levels of Polycyclic Aromatic Hydrocarbons in Traditionally Smoked Cheeses in Poland. Polycycl. Aromat. Compd. 2020, 42, 1–13. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C.; Li, C.; Xu, X.; Zhou, G. Effects of Phenolic Acid Marinades on the Formation of Polycyclic Aromatic Hydrocarbons in Charcoal-Grilled Chicken Wings. J. Food Prot. 2019, 82, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Bogdanović, T.; Pleadin, J.; Petričević, S.; Listeš, E.; Sokolić, D.; Marković, K.; Ozogul, F.; Šimat, V. The Occurrence of Polycyclic Aromatic Hydrocarbons in Fish and Meat Products of Croatia and Dietary Exposure. J. Food Compos. Anal. 2019, 75, 49–60. [Google Scholar] [CrossRef]
- Kamalabadi, M.; Kamankesh, M.; Mohammadi, A.; Hadian, Z.; Ferdowsi, R. Contamination and Daily Intake of Polycyclic Aromatic Hydrocarbons in Iranian Bread Samples. Polycycl. Aromat. Compd. 2020, 40, 1187–1195. [Google Scholar] [CrossRef]
- Żyżelewicz, D.; Oracz, J.; Krysiak, W.; Budryn, G.; Nebesny, E. Effects of Various Roasting Conditions on Acrylamide, Acrolein and Polycyclic Aromatic Hydrocarbons Content in Cocoa Bean and the Derived Chocolates. Dry. Technol. 2017, 35, 363–374. [Google Scholar] [CrossRef]
- Kazerouni, N.; Sinha, R.; Hsu, C.H.; Greenberg, A.; Rothman, N. Analysis of 200 Food Items for Benzo[a]Pyrene and Estimation of Its Intake in an Epidemiologic Study. Food Chem. Toxicol. 2001, 39, 423–4367. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Abdel-Shafy, H.I.; Mansour, M.S.M. Removal of Pyrene and Benzo(a)Pyrene Micropollutant from Water via Adsorption by Green Synthesized Iron Oxide Nanoparticles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 015006. [Google Scholar] [CrossRef]
- European Union. 5AD European Union Council Directive 98/83/EC of 3 November 1998 on the Quality of Water Intended for Human Consumption (Council Directive). Off. J. Eur. Communities 1998, 330, 32–54. [Google Scholar]
- Kabziński, A.K.M.; Cyran, J.; Juszczak, R. Determination of Polycyclic Aromatic Hydrocarbons in Water (Including Drinking Water) of Łódź. Pol. J. Environ. Stud. 2002, 11, 695–706. [Google Scholar]
- Aygun, S.F.; Bagcevan, B. Determination of Polycyclic Aromatic Hydrocarbons (PAHs) in Drinking Water of Samsun and Its Surrounding Areas, Turkey. J. Environ. Health Sci. Eng. 2019, 17, 1205–1212. [Google Scholar] [CrossRef]
- Vermillion Maier, M.L.; Siddens, L.K.; Pennington, J.M.; Uesugi, S.L.; Anderson, K.A.; Tidwell, L.G.; Tilton, S.C.; Ognibene, T.J.; Turteltaub, K.W.; Smith, J.N.; et al. Benzo[a]Pyrene (BaP) Metabolites Predominant in Human Plasma Following Escalating Oral Micro-Dosing with [14C]-BaP. Environ. Int. 2022, 159, 107045. [Google Scholar] [CrossRef]
- Nebert, D.W.; Shi, Z.; Gálvez-Peralta, M.; Uno, S.; Dragin, N. Oral Benzo[a]Pyrene: Understanding Pharmacokinetics, Detoxication and Consequences—Cyp1 Knockout Mouse Lines as a Paradigm. Mol. Pharmacol. 2013, 84, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, W.; Ma, L.; Zhang, S.; Zhang, K.; Ye, P.; Xing, G.; Zhang, X.; Cao, Y.; Xi, J.; et al. Detoxification of Benzo[a]Pyrene Primarily Depends on Cytochrome P450, While Bioactivation Involves Additional Oxidoreductases Including 5-Lipoxygenase, Cyclooxygenase and Aldo-Keto Reductase in the Liver. J. Biochem. Mol. Toxicol. 2017, 31, e21902. [Google Scholar] [CrossRef]
- Barnes, J.L.; Zubair, M.; John, K.; Poirier, M.C.; Martin, F.L. Carcinogens and DNA Damage. Biochem. Soc. Trans. 2018, 46, 1213–1224. [Google Scholar] [CrossRef]
- Chung, J.Y.; Kim, J.Y.; Kim, Y.J.; Jung, S.J.; Park, J.E.; Lee, S.G.; Kim, J.T.; Oh, S.; Lee, C.J.; Yoon, Y.D.; et al. Cellular Defense Mechanisms against Benzo[a]Pyrene in Testicular Leydig Cells: Implications of P53, Aryl-Hydrocarbon Receptor and Cytochrome P450 1A1 Status. Endocrinology 2007, 148, 6134–6144. [Google Scholar] [CrossRef]
- Bukowska, B. Hemoglobin Adducts as Biomarkers of Human Exposure to Selected Xenobiotics. Postepy Hig. Med. Dosw. 2015, 12, 668–680. [Google Scholar] [CrossRef]
- Shiizaki, K.; Kawanishi, M.; Yagi, T. Modulation of Benzo[a]Pyrene–DNA Adduct Formation by CYP1 Inducer and Inhibitor. Genes Environ. 2017, 39, 14. [Google Scholar] [CrossRef]
- Gelboin, H.V. Benzo[Alpha]Pyrene Metabolism, Activation and Carcinogenesis: Role and Regulation of Mixed-Function Oxidases and Related Enzymes. Physiol. Rev. 1980, 60, 1107–1166. [Google Scholar] [CrossRef] [PubMed]
- Koreeda, M.; Moore, P.D.; Wislocki, P.G.; Levin, W.; Conney, A.H.; Yagi, H.; Jerina, D.M. Binding of Nenzo[a]Pyrene 7,8-Diol-9,10-Epoxides to DNA, RNA, and Protein of Mouse Skin Occurs with High Stereoselectivity. Science 1978, 199, 778–781. [Google Scholar] [CrossRef] [PubMed]
- Fantel, A.G. Reactive Oxygen Species in Developmental Toxicity: Review and Hypothesis. Teratology 1996, 53, 196–217. [Google Scholar] [CrossRef]
- McNeill, J.M.; Wills, E.D. The Formation of Mutagenic Derivatives of Benzo[a]Pyrene by Peroxidising Fatty Acids. Chem.-Biol. Interact. 1985, 53, 197–207. [Google Scholar] [CrossRef]
- Marinković, N.; Pašalić, D.; Potočki, S. Polymorphisms of Genes Involved in Polycyclic Aromatic Hydrocarbons’ Biotransformation and Atherosclerosis. Biochem. Med. 2013, 23, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Esteves, F.; Rueff, J.; Kranendonk, M. The Central Role of Cytochrome P450 in Xenobiotic Metabolism—A Brief Review on a Fascinating Enzyme Family. JoX 2021, 11, 94–114. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H. Xenobiotic Metabolic Enzymes: Bioactivation and Antioxidant Defense; Springer International Publishing: Cham, Switzerland, 2020; ISBN 9783030416782. [Google Scholar]
- Monostory, K.; Dvorak, Z. Steroid Regulation of Drug-Metabolizing Cytochromes P450. Curr. Drug Metab. 2011, 12, 154–172. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T. Xenobiotic-Metabolizing Enzymes Involved in Activation and Detoxification of Carcinogenic Polycyclic Aromatic Hydrocarbons. Drug Metab. Pharmacokinet. 2006, 21, 257–276. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Altaf, N.; Ejaz, M.; Altaf, A.; Amin, A.; Janjua, K.; Khan, A.U.; Imran, I.; Khan, S. Variations in the Frequencies of Polymorphisms in the CYP2C9 Gene in Six Major Ethnicities of Pakistan. Sci. Rep. 2020, 10, 19370. [Google Scholar] [CrossRef] [PubMed]
- Hannah, R.; Ramani, P.; Ramanathan, A.; Merlin, J.; Gheena, S.; Ramasubramanian, A.; Monika, K. CYP2 C9 Polymorphism among Patients with Oral Squamous Cell Carcinoma and Its Role in Altering the Metabolism of Benzo[a]Pyrene. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 130, 306–312. [Google Scholar]
- Bukowski, K.; Woźniak, K. Polymorphism of Genes Encoding Proteins of DNA Repair vs. Occupational and Environmental Exposure to Lead, Arsenic and Pesticides. Med. Pr. 2017, 69, 225–236. [Google Scholar]
- Xiao, S.; Cui, S.; Lu, X.; Guan, Y.; Li, D.; Liu, Q.; Cai, Y.; Jin, C.; Yang, J.; Wu, S.; et al. The ERCC2/XPD Lys751Gln Polymorphism Affects DNA Repair of Benzo[a]Pyrene Induced Damage, Tested in an in Vitro Model. Toxicol. Vitr. 2016, 34, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Warshawsky, D. Metabolic Activation of Polycyclic and Heterocyclic Aromatic Hydrocarbons and DNA Damage: A Review. Toxicol. Appl. Pharmacol. 2005, 206, 73–93. [Google Scholar] [CrossRef] [PubMed]
- Chakravarti, D.; Venugopal, D.; Mailander, P.C.; Meza, J.L.; Higginbotham, S.; Cavalieri, E.L.; Rogan, E.G. The Role of Polycyclic Aromatic Hydrocarbon–DNA Adducts in Inducing Mutations in Mouse Skin. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2008, 649, 161–178. [Google Scholar] [CrossRef]
- Mass, M.J.; Ross, J.A.; Nesnow, S.; Jeffers, A.J.; Nelson, G.; Galati, A.J.; Stoner, G.D. Ki-Ras Oncogene Mutations in Tumors and DNA Adducts Formed by Benz[j]Aceanthrylene and Benzo[a]Pyrene in the Lungs of Strain A/J Mice. Mol. Carcinog. 1993, 8, 186–192. [Google Scholar] [CrossRef]
- Surh, Y.-J.; Liem, A.; Miller, E.C.; Miller, J.A. Metabolic Activation of the Carcinogen 6-Hydroxymethylbenzo[a]Pyrene: Formation of an Electrophilic Sulftiric Acid Ester and Benzylic DNA Adducts in Rat Liver In Vivo and in Reactions In Vitro. Carcinogenesis 1989, 10, 1519–1528. [Google Scholar] [CrossRef]
- Mangal, D.; Vudathala, D.; Park, J.-H.; Lee, S.H.; Penning, T.M.; Blair, I.A. Analysis of 7,8-Dihydro-8-Oxo-2′-Deoxyguanosine in Cellular DNA during Oxidative Stress. Chem. Res. Toxicol. 2009, 22, 788–797. [Google Scholar] [CrossRef]
- Shimizu, Y.; Nakatsuru, Y.; Ichinose, M.; Takahashi, Y.; Kume, H.; Mimura, J.; Fujii-Kuriyama, Y.; Ishikawa, T. Benzo[a]Pyrene Carcinogenicity Is Lost in Mice Lacking the Aryl Hydrocarbon Receptor. Proc. Natl. Acad. Sci. USA 2000, 97, 779–782. [Google Scholar] [CrossRef]
- Bartosz, G. The Second Face of Oxygen: Free Radical in Nature, 2nd ed.; PWN: Warszawa, Poland, 2009. [Google Scholar]
- Gao, M.; Zheng, A.; Chen, L.; Dang, F.; Liu, X.; Gao, J. Benzo(a)Pyrene Affects Proliferation with Reference to Metabolic Genes and ROS/HIF-1α/HO-1 Signaling in A549 and MCF-7 Cancer Cells. Drug Chem. Toxicol. 2022, 45, 741–749. [Google Scholar] [CrossRef]
- Elfawy, H.A.; Anupriya, S.; Mohanty, S.; Patel, P.; Ghosal, S.; Panda, P.K.; Das, B.; Verma, S.K.; Patnaik, S. Molecular Toxicity of Benzo(a)Pyrene Mediated by Elicited Oxidative Stress Infer Skeletal Deformities and Apoptosis in Embryonic Zebrafish. Sci. Total Environ. 2021, 789, 147989. [Google Scholar] [CrossRef]
- Aparna, S.; Patri, M. Benzo[a]Pyrene Exposure and Overcrowding Stress Impacts Anxiety-like Behavior and Impairs Learning and Memory in Adult Zebrafish, Danio rerio. Environ. Toxicol. 2021, 36, 352–361. [Google Scholar] [CrossRef]
- Bukowska, B.; Duchnowicz, P. Molecular Mechanisms of Action of Selected Substances Involved in the Reduction of Benzo[a]Pyrene-Induced Oxidative Stress. Molecules 2022, 27, 1379. [Google Scholar] [CrossRef]
- González, A.; Espinoza, D.; Vidal, C.; Moenne, A. Benzopyrene Induces Oxidative Stress and Increases Expression and Activities of Antioxidant Enzymes, and CYP450 and GST Metabolizing Enzymes in Ulva lactuca (Chlorophyta). Planta 2020, 252, 107. [Google Scholar] [CrossRef]
- Lin, S.; Ren, A.; Wang, L.; Huang, Y.; Wang, Y.; Wang, C.; Greene, N.D. Oxidative Stress and Apoptosis in Benzo[a]Pyrene-Induced Neural Tube Defects. Free Radic. Biol. Med. 2018, 116, 149–158. [Google Scholar] [CrossRef]
- Lin, Y.C.; Wu, C.Y.; Hu, C.H.; Pai, T.W.; Chen, Y.R.; Wang, W.D. Integrated Hypoxia Signaling and Oxidative Stress in Developmental Neurotoxicity of Benzo[a]Pyrene in Zebrafish Embryos. Antioxidants 2020, 9, 731. [Google Scholar] [CrossRef]
- Dröge, W. Free Radicals in the Physiological Control of Cell Function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Feng, D.; Xu, Z.; Qi, P.; Yan, X. Acute Benzo[a]Pyrene Exposure Induced Oxidative Stress, Neurotoxicity and Epigenetic Change in Blood Clam Tegillarca granosa. Sci. Rep. 2021, 11, 18744. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, P.; Rahman, I. Oxidative Stress in Asthma and COPD: Antioxidants as a Therapeutic Strategy. Pharmacol. Ther. 2006, 111, 476–494. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, P.D. Free Radicals of Benzo(a)Pyrene and Derivatives. Environ. Health Perspect. 1985, 64, 288–295. [Google Scholar] [CrossRef]
- Allmann, S.; Mayer, L.; Olma, J.; Kaina, B.; Hofmann, T.G.; Tomicic, M.T.; Christmann, M. Benzo[a]Pyrene Represses DNA Repair through Altered E2F1/E2F4 Function Marking an Early Event in DNA Damage-Induced Cellular Senescence. Nucleic Acids Res. 2020, 48, 12085–12101. [Google Scholar] [CrossRef] [PubMed]
- Das, L.; Patel, B.; Patri, M. Adolescence Benzo[a]Pyrene Treatment Induces Learning and Memory Impairment and Anxiolytic like Behavioral Response Altering Neuronal Morphology of Hippocampus in Adult Male Wistar Rats. Toxicol. Rep. 2019, 6, 1104–1113. [Google Scholar] [CrossRef] [PubMed]
- Grova, N.; Salquèbre, G.; Schroeder, H.; Appenzeller, B.M.R. Determination of PAHs and OH-PAHs in Rat Brain by Gas Chromatography Tandem (Triple Quadrupole) Mass Spectrometry. Chem. Res. Toxicol. 2011, 24, 1653–1667. [Google Scholar] [CrossRef] [PubMed]
- Chahin, A.; Peiffer, J.; Olry, J.-C.; Crepeaux, G.; Schroeder, H.; Rychen, G.; Guiavarc’h, Y. EROD Activity Induction in Peripheral Blood Lymphocytes, Liver and Brain Tissues of Rats Orally Exposed to Polycyclic Aromatic Hydrocarbons. Food Chem. Toxicol. 2013, 56, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhao, Y.; Qi, Y.; Gao, Y.; Tu, D.; Wang, Y.; Gao, H.M.; Zhou, H. Benzo(a)Pyrene Exposure Induced Neuronal Loss, Plaque Deposition, and Cognitive Decline in APP/PS1 Mice. J. Neuroinflamm. 2020, 17, 258. [Google Scholar] [CrossRef] [PubMed]
- Grova, N.; Valley, A.; Turner, J.D.; Morel, A.; Muller, C.P.; Schroeder, H. Modulation of Behavior and NMDA-R1 Gene MRNA Expression in Adult Female Mice after Sub-Acute Administration of Benzo(a)Pyrene. Neurotoxicology 2007, 28, 630–636. [Google Scholar] [CrossRef]
- Grova, N.; Schroeder, H.; Farinelle, S.; Prodhomme, E.; Valley, A.; Muller, C.P. Sub-Acute Administration of Benzo[a]Pyrene (B[a]P) Reduces Anxiety-Related Behaviour in Adult Mice and Modulates Regional Expression of N-Methyl-d-Aspartate (NMDA) Receptors Genes in Relevant Brain Regions. Chemosphere 2008, 73, S295–S302. [Google Scholar] [CrossRef] [PubMed]
- Cherif, L.S.; Cao-Lei, L.; Farinelle, S.; Muller, C.P.; Turner, J.D.; Schroeder, H.; Grova, N. Assessment of 9-OH- and 7,8-Diol-Benzo[a]Pyrene in Blood as Potent Markers of Cognitive Impairment Related to Benzo[a]Pyrene Exposure: An Animal Model Study. Toxics 2021, 9, 50. [Google Scholar] [CrossRef]
- Tsuji, N.; Fukuda, K.; Nagata, Y.; Okada, H.; Haga, A.; Hatakeyama, S.; Yoshida, S.; Okamoto, T.; Hosaka, M.; Sekine, K.; et al. The Activation Mechanism of the Aryl Hydrocarbon Receptor (AhR) by Molecular Chaperone HSP90. FEBS Open Bio 2014, 4, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, T.; Fujimura, T.; Aiba, S. Aryl Hydrocarbon Receptor Modulates Carcinogenesis and Maintenance of Skin Cancers. Front. Med. 2019, 6, 194. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.; Yin, Q.; Qi, J.; Wang, M.; Fan, L.; Han, R. The Benzo(a)Pyrene-Induced MRNA Expression of Aromatic Hydrocarbon Receptor and Cytochrome P4501A1 Genes in Rat Liver. J. Pharm. Anal. 2010, 22, 30–33. [Google Scholar]
- Zhu, K.; Meng, Q.; Zhang, Z.; Yi, T.; He, Y.; Zheng, J.; Lei, W. Aryl Hydrocarbon Receptor Pathway: Role, Regulation and Intervention in Atherosclerosis Therapy (Review). Mol. Med. Rep. 2019, 20, 4763–4773. [Google Scholar] [CrossRef] [PubMed]
- Tsay, J.J.; Tchou-Wong, K.M.; Greenberg, A.K.; Pass, H.; Rom, W.N. Aryl Hydrocarbon Receptor and Lung Cancer. Anticancer. Res. 2013, 33, 1247–1256. [Google Scholar]
- Safe, S.; Cheng, Y.; Jin, U.-H. The Aryl Hydrocarbon Receptor (AhR) as a Drug Target for Cancer Chemotherapy. Curr. Opin. Toxicol. 2017, 2, 24–29. [Google Scholar] [CrossRef]
- Chahal, H.S.; Lin, Y.; Ransohoff, K.J.; Hinds, D.A.; Wu, W.; Dai, H.J.; Qureshi, A.A.; Li, W.Q.; Kraft, P.; Tang, J.Y.; et al. Genome-Wide Association Study Identifies Novel Susceptibility Loci for Cutaneous Squamous Cell Carcinoma. Nat. Commun. 2016, 7, 12048. [Google Scholar] [CrossRef]
- Ye, G.; Gao, H.; Zhang, X.; Liu, X.; Chen, J.; Liao, X.; Zhang, H.; Huang, Q. Aryl Hydrocarbon Receptor Mediates Benzo[a]Pyrene-Induced Metabolic Reprogramming in Human Lung Epithelial BEAS-2B Cells. Sci. Total Environ. 2021, 756, 144130. [Google Scholar] [CrossRef]
- Lou, W.; Zhang, M.; Chen, Q.; Bai, T.Y.; Hu, Y.X.; Gao, F.; Li, J.; Lv, X.L.; Zhang, Q.; Chang, F.H. Molecular Mechanism of Benzo[a]Pyrene Regulating Lipid Metabolism via Aryl Hydrocarbon Receptor. Lipids Health Dis. 2022, 21, 13. [Google Scholar] [CrossRef] [PubMed]
- Hardonnière, K.; Saunier, E.; Lemarié, A.; Fernier, M.; Gallais, I.; Héliès-Toussaint, C.; Mograbi, B.; Antonio, S.; Bénit, P.; Rustin, P.; et al. The Environmental Carcinogen Benzo[a]Pyrene Induces a Warburg-like Metabolic Reprogramming Dependent on NHE1 and Associated with Cell Survival. Sci. Rep. 2016, 6, 30776. [Google Scholar] [CrossRef] [PubMed]
- Słowikowski, B.K.; Jankowski, M.; Jagodziński, P.P. The Smoking Estrogens—A Potential Synergy between Estradiol and Benzo(a)Pyrene. Biomed. Pharmacother. 2021, 139, 111658. [Google Scholar] [CrossRef]
- Tseng, Y.H.; Chen, Y.C.; Yu, A.L.; Yu, J. Benzo[a]Pyrene Induces Fibrotic Changes and Impairs Differentiation in Lung Stem Cells. Ecotoxicol. Environ. Saf. 2021, 210, 111892. [Google Scholar] [CrossRef]
- Fanali, L.Z.; De Oliveira, C.; Sturve, J. Enzymatic, Morphological, and Genotoxic Effects of Benzo[a]Pyrene in Rainbow Trout (Oncorhynchus mykiss). Environ. Sci. Pollut. Res. 2021, 28, 53926–53935. [Google Scholar] [CrossRef]
- Bollati, V.; Baccarelli, A. Environmental Epigenetics. Heredity 2010, 105, 105–112. [Google Scholar] [CrossRef]
- Herbstman, J.B.; Tang, D.; Zhu, D.; Qu, L.; Sjödin, A.; Li, Z.; Camann, D.; Perera, F.P. Prenatal Exposure to Polycyclic Aromatic Hydrocarbons, Benzo[a]Pyrene–DNA Adducts and Genomic DNA Methylation in Cord Blood. Environ. Health Perspect. 2012, 120, 733–738. [Google Scholar] [CrossRef]
- Joubert, B.R.; Håberg, S.E.; Nilsen, R.M.; Wang, X.; Vollset, S.E.; Murphy, S.K.; Huang, Z.; Hoyo, C.; Midttun, Ø.; Cupul-Uicab, L.A.; et al. 450K Epigenome-Wide Scan Identifies Differential DNA Methylation in Newborns Related to Maternal Smoking during Pregnancy. Environ. Health Perspect. 2012, 120, 1425–1431. [Google Scholar] [CrossRef]
- Suter, M.; Ma, J.; Harris, A.S.; Patterson, L.; Brown, K.A.; Shope, C.; Showalter, L.; Abramovici, A.; Aagaard-Tillery, K.M. Maternal Tobacco Use Modestly Alters Correlated Epigenome-Wide Placental DNA Methylation and Gene Expression. Epigenetics 2011, 6, 1284–1294. [Google Scholar] [CrossRef]
- Tang, W.; Levin, L.; Talaska, G.; Cheung, Y.Y.; Herbstman, J.; Tang, D.; Miller, R.L.; Perera, F.; Ho, S.M. Maternal Exposure to Polycyclic Aromatic Hydrocarbons and 5’-CpG Methylation of Interferon-γ in Cord White Blood Cells. Environ. Health Perspect. 2012, 120, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Yang, L.Q.; Huang, H.Y.; Pang, L.; Yang, X.F.; Yi, Y.J.; Ren, X.H.; Li, J.; Zhuang, Z.X.; Liu, J.J. Repression of Biotin-Related Proteins by Benzo[a]Pyrene-Induced Epigenetic Modifications in Human Bronchial Epithelial Cells. Int. J. Toxicol. 2016, 35, 336–343. [Google Scholar] [CrossRef]
- Sadikovic, B.; Andrews, J.; Carter, D.; Robinson, J.; Rodenhiser, D.I. Genome-Wide H3K9 Histone Acetylation Profiles Are Altered in Benzopyrene-Treated MCF7 Breast Cancer Cells. J. Biol. Chem. 2008, 283, 4051–4060. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, Q.; Liu, C.; Duan, H.; Zeng, X.; Zhang, B.; Li, X.; Zhao, J.; Tang, S.; Li, Z.; et al. Aberrant Expression of MiR-638 Contributes to Benzo(a)Pyrene-Induced Human Cell Transformation. Toxicol. Sci. 2012, 125, 382–391. [Google Scholar] [CrossRef]
- Subach, O.M.; Baskunov, V.B.; Darii, M.V.; Maltseva, D.V.; Alexandrov, D.A.; Kirsanova, O.V.; Kolbanovskiy, A.; Kolbanovskiy, M.; Johnson, F.; Bonala, R.; et al. Impact of Benzo[a]Pyrene-2‘-Deoxyguanosine Lesions on Methylation of DNA by SssI and HhaI DNA Methyltransferases. Biochemistry 2006, 45, 6142–6159. [Google Scholar] [CrossRef] [PubMed]
- Yauk, C.L.; Polyzos, A.; Rowan-Carroll, A.; Kortubash, I.; Williams, A.; Kovalchuk, O. Tandem Repeat Mutation, Global DNA Methylation, and Regulation of DNA Methyltransferases in Cultured Mouse Embryonic Fibroblast Cells Chronically Exposed to Chemicals with Different Modes of Action. Environ. Mol. Mutagen. 2008, 49, 26–35. [Google Scholar] [CrossRef]
- Mo, J.; Au, D.W.T.; Guo, J.; Winkler, C.; Kong, R.Y.C.; Seemann, F. Benzo[a]Pyrene Osteotoxicity and the Regulatory Roles of Genetic and Epigenetic Factors: A Review. Crit. Rev. Environ. Sci. Technol. 2021, 1–39. [Google Scholar] [CrossRef]
- Yin, X.; Liu, Y.; Zeb, R.; Chen, F.; Chen, H.; Wang, K.J. The Intergenerational Toxic Effects on Offspring of Medaka Fish Oryzias melastigma from Parental Benzo[a]Pyrene Exposure via Interference of the Circadian Rhythm. Environ. Pollut. 2020, 267, 115437. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, C.; Boffetta, P.; La Vecchia, C. Occupational Exposures to Polycyclic Aromatic Hydrocarbons, and Respiratory and Urinary Tract Cancers: A Quantitative Review to 2005. Ann. Oncol. 2007, 18, 431–446. [Google Scholar] [CrossRef] [PubMed]
- Ifegwu, O.C.; Anyakora, C. Polycyclic Aromatic Hydrocarbons. In Advances in Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 2015; Volume 72, pp. 277–304. [Google Scholar]
- Kim, K.E.; Cho, D.; Park, H.J. Air Pollution and Skin Diseases: Adverse Effects of Airborne Particulate Matter on Various Skin Diseases. Life Sci. 2016, 152, 126–134. [Google Scholar] [CrossRef]
- Rota, M.; Bosetti, C.; Boccia, S.; Boffetta, P.; La Vecchia, C. Occupational Exposures to Polycyclic Aromatic Hydrocarbons and Respiratory and Urinary Tract Cancers: An Updated Systematic Review and a Meta-Analysis to 2014. Arch. Toxicol. 2014, 88, 1479–1490. [Google Scholar] [CrossRef]
- Hamidi, E.N.; Hajeb, P.; Selamat, J.; Razis, A.F.A. Polycyclic Aromatic Hydrocarbons (PAHs) and Their Bioaccessibility in Meat: A Tool for Assessing Human Cancer Risk. Asian Pac. J. Cancer Prev. 2016, 17, 15–23. [Google Scholar] [CrossRef]
- Korsh, J.; Shen, A.; Aliano, K.; Davenport, T. Polycyclic Aromatic Hydrocarbons and Breast Cancer: A Review of the Literature. Breast Care 2015, 10, 316–318. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, G.P.; Denissenko, M.F.; Olivier, M.; Tretyakova, N.; Hecht, S.S.; Hainaut, P. Tobacco Smoke Carcinogens, DNA Damage and P53 Mutations in Smoking-Associated Cancers. Oncogene 2002, 21, 7435–7451. [Google Scholar] [CrossRef] [PubMed]
- DeMarini, D.; Landi, S.; Tian, D. Lung Tumor KRAS and TP53 Mutations in Nonsmokers Reflect Exposure to PAH-Rich Coal Combustion Emissions. Cancer Res. 2001, 61, 6679–6681. [Google Scholar] [CrossRef]
- Widziewicz, K.; Rogula-Kozłowska, W.; Majewski, G. Lung Cancer Risk Associated with Exposure to Benzo(a)Pyrene in Polish Agglomerations, Cities, and Other Areas. Int. J. Environ. Res. 2017, 11, 685–693. [Google Scholar] [CrossRef]
- Vilariño-Güell, C.; Smith, A.G.; Dubrova, Y.E. Germline Mutation Induction at Mouse Repeat DNA Loci by Chemical Mutagens. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2003, 526, 63–73. [Google Scholar] [CrossRef]
- Verhofstad, N.; van Oostrom, C.T.M.; van Benthem, J.; van Schooten, F.J.; van Steeg, H.; Godschalk, R.W.L. DNA Adduct Kinetics in Reproductive Tissues of DNA Repair Proficient and Deficient Male Mice after Oral Exposure to Benzo(a)Pyrene. Environ. Mol. Mutagen. 2010, 51, 123–129. [Google Scholar] [CrossRef]
- National Toxicology Program. Toxicology and Carcinogenesis Studies of Naphthalene (CAS No. 91-20-3) in F344/N Rats (Inhalation Studies). Natl. Toxicol. Program Tech. Rep. Ser. 2000, 500, 1–173. [Google Scholar]
- Zenzes, M.T. Smoking and Reproduction: Gene Damage to Human Gametes and Embryos. Hum. Reprod. Update 2000, 6, 122–131. [Google Scholar] [CrossRef]
- Reddy, K.P.; Girish, B.P.; Reddy, P.S. Reproductive and Paternal Mediated Developmental Toxicity of Benzo(a)Pyrene in Adult Male Wistar Rats. Toxicol. Res. 2015, 4, 223–232. [Google Scholar] [CrossRef]
- Jorge, B.C.; Reis, A.C.C.; Stein, J.; da Silva Balin, P.; Sterde, É.T.; Barbosa, M.G.; de Aquino, A.M.; Kassuya, C.A.L.; Arena, A.C. Parental Exposure to Benzo(a)Pyrene in the Peripubertal Period Impacts Reproductive Aspects of the F1 Generation in Rats. Reprod. Toxicol. 2021, 100, 126–136. [Google Scholar] [CrossRef]
- Hombach-Klonisch, S.; Pocar, P.; Kietz, S.; Klonisch, T. Molecular Actions of Polyhalogenated Arylhydrocarbons (PAHs) in Female Reproduction. Curr. Med. Chem. 2005, 12, 599–616. [Google Scholar]
- El-Nemr, A.; Al-Shawaf, T.; Sabatini, L.; Wilson, C.; Lower, A.M.; Grudzinskas, J.G. Effect of Smoking on Ovarian Reserve and Ovarian Stimulation in In Vitro Fertilization and Embryo Transfer. Hum. Reprod. 1998, 13, 2192–2198. [Google Scholar] [CrossRef] [PubMed]
- Shiverick, K.T.; Salafia, C. Cigarette Smoking and Pregnancy I: Ovarian, Uterine and Placental Effects. Placenta 1999, 20, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Ramesh, A.; Shioda, T.; Leon Parada, K.; Luderer, U. Sex Differences in Embryonic Gonad Transcriptomes and Benzo[a]Pyrene Metabolite Levels After Transplacental Exposure. Endocrinology 2022, 163, bqab228. [Google Scholar] [CrossRef]
- Wright, K.P.; Trimarchi, J.R.; Allsworth, J.; Keefe, D. The Effect of Female Tobacco Smoking on IVF Outcomes. Hum. Reprod. 2006, 21, 2930–2934. [Google Scholar] [CrossRef]
- Karttunen, V.; Myllynen, P.; Prochazka, G.; Pelkonen, O.; Segerbäck, D.; Vähäkangas, K. Placental Transfer and DNA Binding of Benzo(a)Pyrene in Human Placental Perfusion. Toxicol. Lett. 2010, 197, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ramesh, A.; Nayyar, T.; Hood, D.B. Assessment of Metabolites and AhR and CYP1A1 MRNA Expression Subsequent to Prenatal Exposure to Inhaled Benzo(a)Pyrene. Int. J. Dev. Neurosci. 2003, 21, 333–346. [Google Scholar] [CrossRef]
- Bui, Q.; Tran, M.; West, W. A Comparative Study of the Reproductive Effects of Methadone and Benzo [a] Pyrene in the Pregnant and Pseudopregnant Rat. Toxicology 1986, 42, 195–204. [Google Scholar] [CrossRef]
- Perera, F.P.; Rauh, V.; Whyatt, R.M.; Tsai, W.Y.; Bernert, J.T.; Tu, Y.H.; Andrews, H.; Ramirez, J.; Qu, L.; Tang, D. Molecular Evidence of an Interaction between Prenatal Environmental Exposures and Birth Outcomes in a Multiethnic Population. Environ. Health Perspect. 2004, 112, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhou, Y.; Pan, L.; Xu, R.; Li, D. Benzo[a]Pyrene Exposure Induced Reproductive Endocrine-Disrupting Effects via the Steroidogenic Pathway and Estrogen Signaling Pathway in Female Scallop Chlamys farreri. Sci. Total Environ. 2020, 726, 138585. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Uchida, M.; Ishibashi, H.; Hirano, M.; Ichikawa, N.; Arizono, K.; Koyama, J.; Tominaga, N. Potential Mechanisms Underlying Embryonic Developmental Toxicity Caused by Benzo[a]Pyrene in Japanese Medaka (Oryzias latipes). Chemosphere 2020, 242, 125243. [Google Scholar] [CrossRef]
- Perera, F.P.; Tang, D.; Rauh, V.; Lester, K.; Tsai, W.Y.; Tu, Y.H.; Weiss, L.; Hoepner, L.; King, J.; Del Priore, G.; et al. Relationships among Polycyclic Aromatic Hydrocarbon–DNA Adducts, Proximity to the World Trade Center and Effects on Fetal Growth. Environ. Health Perspect. 2005, 113, 1062–1067. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ye, Y.; Jiang, S.; Zhang, C.; Cheng, Y.; Zhong, H.; Du, T.; Xu, W.; Azziz, R.; Zhang, H.; Zhao, X. Environmental Pollutant Benzo[a]Pyrene Induces Recurrent Pregnancy Loss through Promoting Apoptosis and Suppressing Migration of Extravillous trophoblast. BioMed Res. Int. 2020, 2020, 8983494. [Google Scholar] [CrossRef]
- Legraverend, C.; Guenthner, T.M.; Nebert, D.W. Importance of the Route of Administration for Genetic Differences in Benzo[a]Pyrene-Induced in Utero Toxicity and Teratogenicity. Teratology 1984, 29, 35–47. [Google Scholar] [CrossRef]
- Ranjit, S.; Sinha, N.; Kodidela, S.; Kumar, S. Benzo(a)Pyrene in Cigarette Smoke Enhances HIV-1 Replication through NF-ΚB Activation via CYP-Mediated Oxidative Stress Pathway. Sci. Rep. 2018, 8, 10394. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Luo, Y.; Zhong, R.; Law, P.T.Y.; Boon, S.S.; Chen, Z.; Wong, C.H.; Chan, P.K.S. Role of Polycyclic Aromatic Hydrocarbons as a Co-Factor in Human Papillomavirus-Mediated Carcinogenesis. BMC Cancer 2019, 19, 138. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, Z.; Lin, Y.; Zhang, M.; Liu, J.; Zhu, L.; Chen, Q.; Bi, J.; Li, S.; Ni, Z.; et al. Benzo(a)Pyrene Induces MUC5AC Expression through the AhR/Mitochondrial ROS/ERK Pathway in Airway Epithelial Cells. Ecotoxicol. Environ. Saf. 2021, 210, 111857. [Google Scholar] [CrossRef] [PubMed]
- Tajima, H.; Tajiki-Nishino, R.; Watanabe, Y.; Kurata, K.; Fukuyama, T. Activation of Aryl Hydrocarbon Receptor by Benzo[a]Pyrene Increases Interleukin 33 Expression and Eosinophil Infiltration in a Mouse Model of Allergic Airway Inflammation. J. Appl. Toxicol. 2020, 40, 1545–1553. [Google Scholar] [CrossRef]
- Choi, H.; Dostal, M.; Pastorkova, A.; Rossner, P.; Sram, R.J. Airborne Benzo[a]Pyrene May Contribute to Divergent Pheno-Endotypes in Children. Environ. Health 2021, 20, 40. [Google Scholar] [CrossRef]

| Location | Concentration [ng/m3] | Reference |
|---|---|---|
| The European Union | 7% of EU citizens live in areas with a tolerable risk level of 0.12 ng/m3 | [12] |
| France | 1 ng to 2.49 ng/m3 | [13] |
| Thailand | 0.052 and 0.095 ng/m3 in PM 2.5 fraction | [14] |
| Iberian Peninsula | exceeded target value of 1 ng/m3 | [15] |
| Italy—Genoa | 2 ng/m3 (along heavy traffic streets) | [16] |
| 14 ng/m3 (300 m from a coke oven) | ||
| Poland—Cracow | 4–10 ng/m3, | [17] |
| Tarnow | 4–6 ng/m3 | |
| Nowy Sacz | 10–11 ng/m3 | |
| China—Linzhou | 5.1–20.2 ng/m3 | [18] |
| Saudi Arabia—Makkah | 0.082 ± 0.032 ng/m3 (occupationally exposed workers) | [10] |
| 0.044 ± 0.006 ng/m3 (unexposed group) |
| Location | Concentration | Reference |
|---|---|---|
| North China kitchens, indoor air | 14.3 ± 23.0 ng/m3 (gaseous phase) | [29] |
| 6.7 ± 17.4 ng/m3 (particulate phase) | ||
| China Yucheng City kitchens, indoor air | 25.8 ± 10.6 ng/m3 (oil-based cooking) | [30] |
| 7.3 ± 4.6 ng/m3 (water-based cooking) | ||
| Tibet Jokhang Temple, indoor air | 18.5 ± 4.3 ng/m3 | [31] |
| China Shanxi Provence schools, indoor air | 0.05 ng/m3 (nonheating season) | [32] |
| 10.3 ng/m3 (heating season) | ||
| Saudi Arabia, Jeddah’s schools, indoor air | 163.87 ± 68.53 ng/m3 | [33] |
| Poland, Silesia kindergartens, indoor air | 3.7 ± 0.8 ng/m3 | [34] |
| India, Shimoga, iron foundry | 7.20 ± 1.11 μg/m3 (melting section) 45.37 µg/m3 (molding section) | [35] |
| Sweden, Aluminum manufacturing factories | 14 μg/m3 | [36] |
| United Kingdom, Coke oven facilities | 3.3 μg/m3 | [37] |
| Location | Land Type | Concentration (µg/kg) | Reference |
|---|---|---|---|
| Bangkok | Urban–tropical | 5.5 | [47] |
| Brazil | Forest–tropical | 0.3 | |
| New Orleans | Urban | 276 | [48] |
| Dalian, China | Traffic | 388 | [49] |
| Park | 71 | ||
| Suburban | 27 | ||
| Rural | 9 | ||
| United Kingdom | Rural | 46 | [50] |
| Norway | Rural | 5.3 | |
| Spain | Industrial–chemical | 100 | [51] |
| Industrial–petrochemical | 18 | ||
| Residential | 56 | ||
| Rural | 22 | ||
| Poland | Agricultural | 30 | [52] |
| Poland, Bialystok | Urban | 300–900 | [44] |
| USA, Cleveland | Municipal plots | 280–5500 | [45] |
| Russia, St. Petersburg | Parkland | 220 | [53] |
| Residential | 430 | ||
| Industrial | 340 | ||
| Turkey, Antalya Aksu region | greenhouse crops | 2.31 | [46] |
| Taiyuan | Urban | 94.03 | [42] |
| Agricultural | 65.57 | ||
| Montane | 16.60 | ||
| Antarctic Peninsula | Antarctic station territory | 1.5 | [54] |
| United Kingdom, Cities of Crimea | Alushta | 60 | [55] |
| Yalta | 139 | ||
| Sebastopol | 260 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bukowska, B.; Mokra, K.; Michałowicz, J. Benzo[a]pyrene—Environmental Occurrence, Human Exposure, and Mechanisms of Toxicity. Int. J. Mol. Sci. 2022, 23, 6348. https://doi.org/10.3390/ijms23116348
Bukowska B, Mokra K, Michałowicz J. Benzo[a]pyrene—Environmental Occurrence, Human Exposure, and Mechanisms of Toxicity. International Journal of Molecular Sciences. 2022; 23(11):6348. https://doi.org/10.3390/ijms23116348
Chicago/Turabian StyleBukowska, Bożena, Katarzyna Mokra, and Jaromir Michałowicz. 2022. "Benzo[a]pyrene—Environmental Occurrence, Human Exposure, and Mechanisms of Toxicity" International Journal of Molecular Sciences 23, no. 11: 6348. https://doi.org/10.3390/ijms23116348
APA StyleBukowska, B., Mokra, K., & Michałowicz, J. (2022). Benzo[a]pyrene—Environmental Occurrence, Human Exposure, and Mechanisms of Toxicity. International Journal of Molecular Sciences, 23(11), 6348. https://doi.org/10.3390/ijms23116348