Biochemical, Sensory, and Molecular Evaluation of Flavour and Consumer Acceptability in Australian Papaya (Carica papaya L.) Varieties
Abstract
:1. Introduction
2. Results
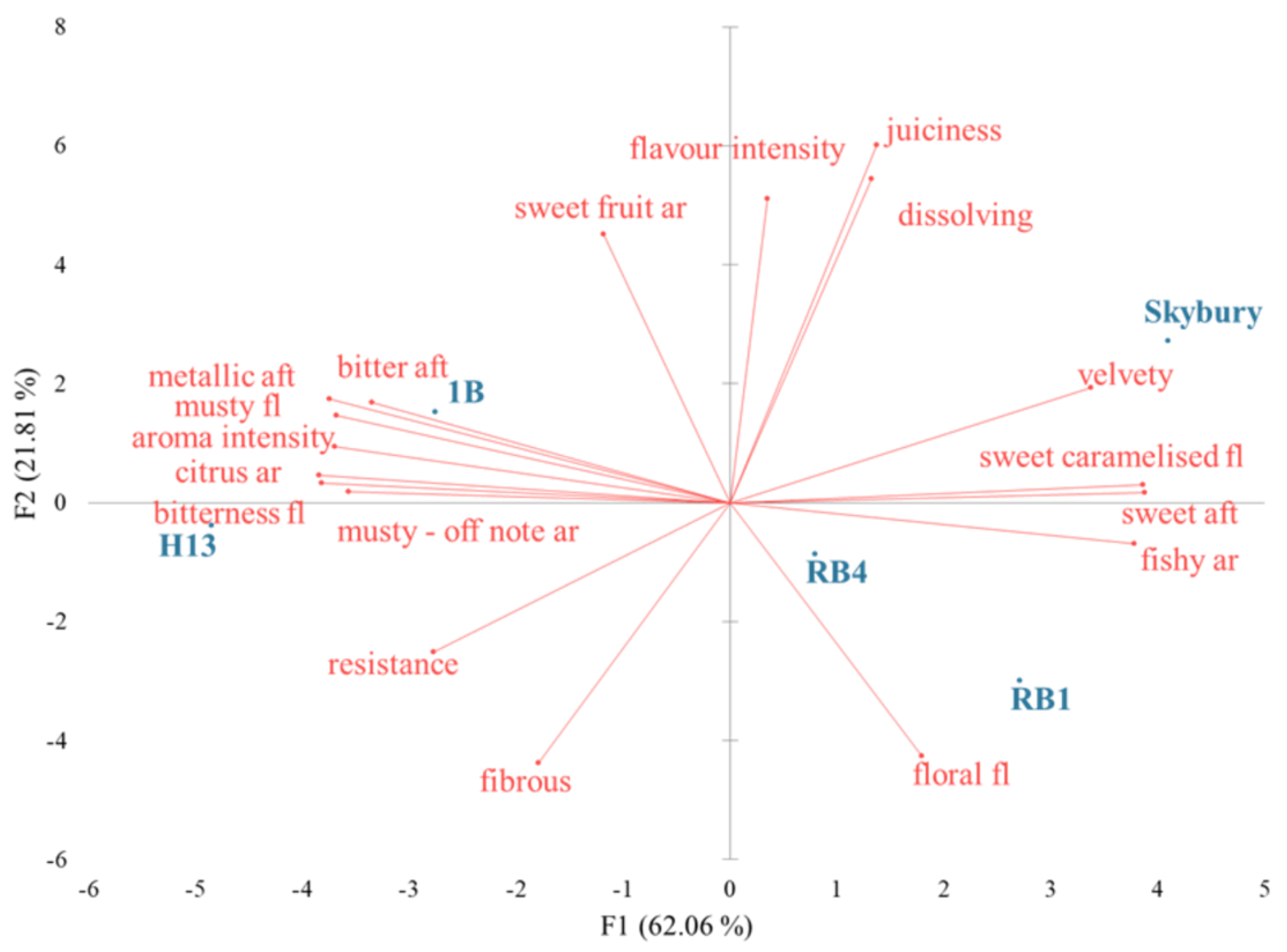
2.1. Sensory Descriptive Profiling
2.2. Sugar Content Determination
2.3. Volatile Compound Analysis
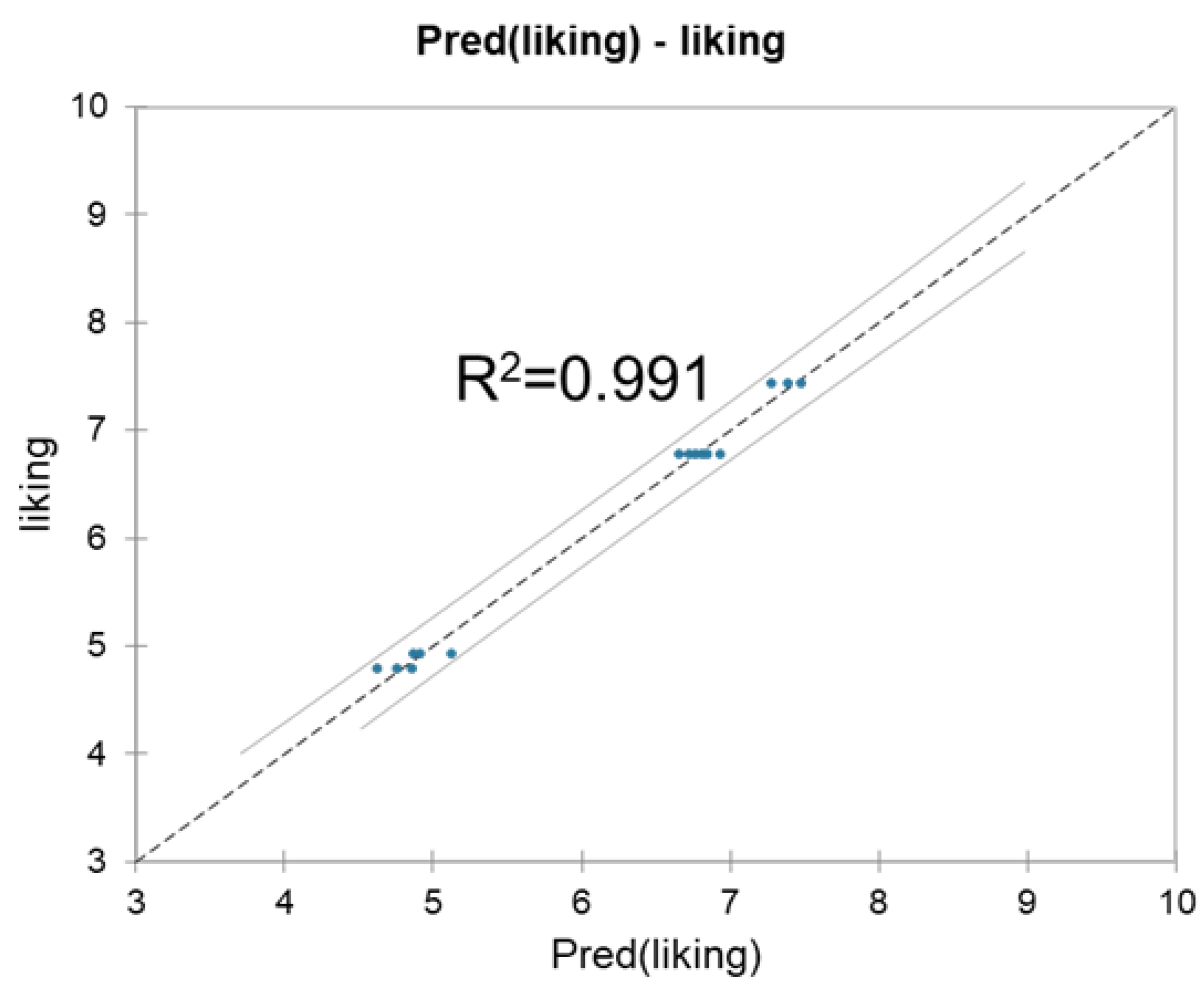
2.4. Correlation and Multiple Linear Regression Analyses
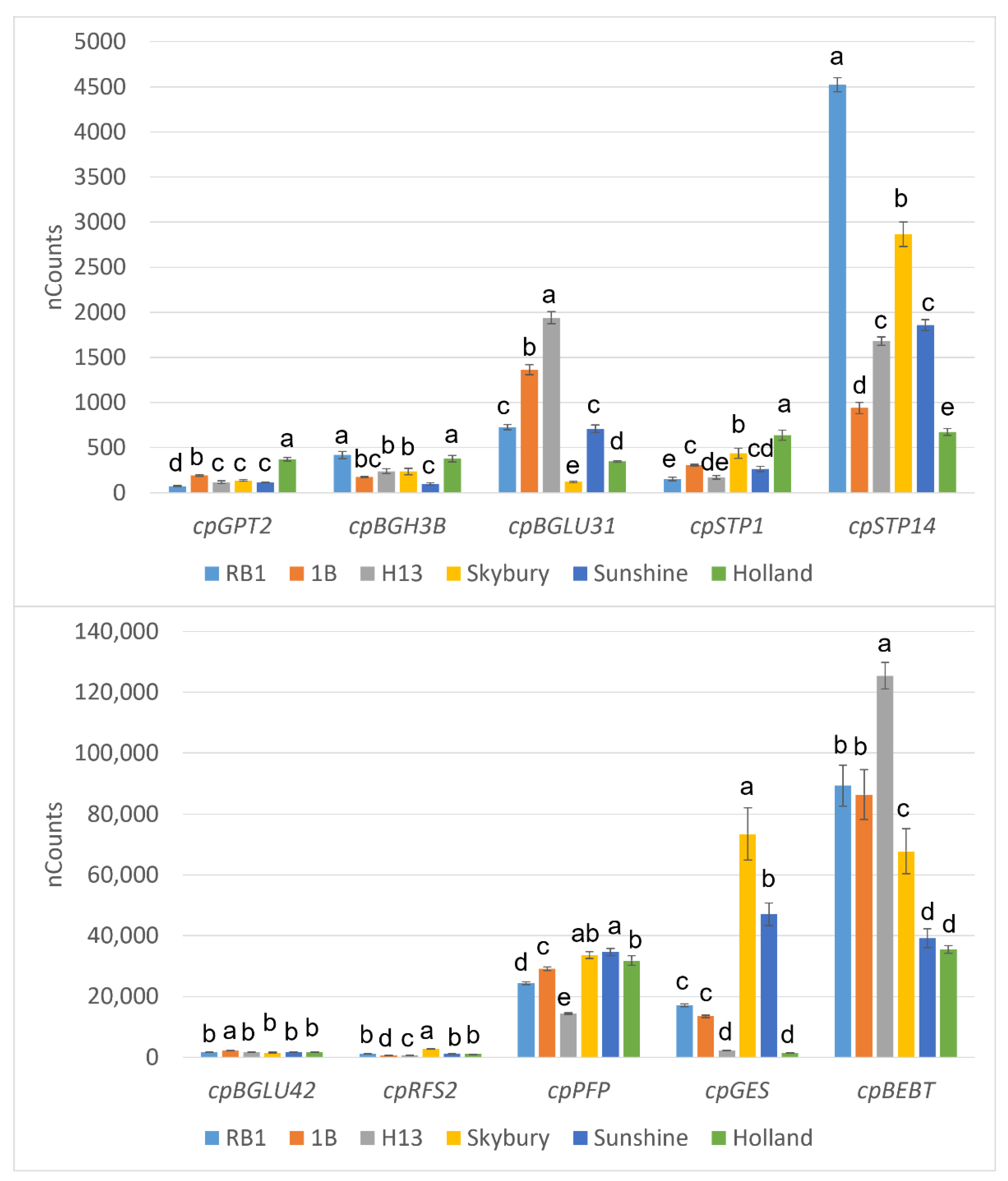
2.5. Differential Gene Expression Analysis of Flavour-Related Genes
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Sensory Descriptive Profiling
4.3. Sugar Content Determination
4.4. Volatile Compound Analysis
4.5. Consumer Acceptability Study
4.6. Data Analysis
4.7. Differential Gene Expression Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kanchana-udomkan, C.; Nantawan, U.; Ford, R. New Genetic Targets to Improve Quality in Papaya (PP15000); Hort Innovation: Sydney, NSW, Australia, 2018; p. 53. [Google Scholar]
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 1 June 2021).
- Kamil, A.; Wijaya, C.H.; Sobir; Adawiyah, D.R. Correlation between off-flavor and morphology of papaya (Carica papaya L.) fruits. J. Exp. Agric. Int. 2020, 42, 121–132. [Google Scholar] [CrossRef]
- Zhou, Z.; Ford, R.; Bar, I.; Kanchana-udomkan, C. Papaya (Carica papaya L.) Flavour Profiling. Genes 2021, 12, 1416. [Google Scholar] [CrossRef] [PubMed]
- Wijaya, C.H.; Chen, F. Flavour of papaya (L.) Fruit Carica papaya. Biotropia 2013, 20, 50–71. [Google Scholar]
- Ulrich, D.; Wijaya, C. Volatile patterns of different papaya (Carica papaya L.) varieties. J. Appl. Bot. Food Qual. 2010, 83, 128–132. [Google Scholar]
- Del Carmen, D.R.; Esguerra, E.B.; Absulio, W.L.; Maunahan, M.V.; Masilungan, G.P. Understanding consumers’ preference for fresh table-ripe papaya. Philipp. J. Crop Sci. 2011, 36, 38–39. [Google Scholar]
- Lieb, V.M.; Esquivel, P.; Cubero Castillo, E.; Carle, R.; Steingass, C.B. GC–MS profiling, descriptive sensory analysis, and consumer acceptance of Costa Rican papaya (Carica papaya L.) fruit purees. Food Chem. 2018, 248, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Flath, R.A.; Forrey, R.R. Volatile components of papaya (Carica papaya L., Solo variety). J. Agric. Food Chem. 1977, 25, 103–109. [Google Scholar] [CrossRef]
- Flath, R.A.; Light, D.M.; Jang, E.B.; Mon, T.R.; John, J.O. Headspace examination of volatile emissions from ripening papaya (Carica papaya L., Solo variety). J. Agric. Food Chem. 1990, 38, 1060–1063. [Google Scholar] [CrossRef]
- Jing, G.; Li, T.; Qu, H.; Yun, Z.; Jia, Y.; Zheng, X.; Jiang, Y. Carotenoids and volatile profiles of yellow- and red-fleshed papaya fruit in relation to the expression of carotenoid cleavage dioxygenase genes. Postharvest Biol. Technol. 2015, 109, 114–119. [Google Scholar] [CrossRef]
- Pino, J.A.; Almora, K.; Marbot, R. Volatile components of papaya (Carica papaya L., Maradol variety) 555 fruit. Flavour Fragr. J. 2003, 18, 492–496. [Google Scholar] [CrossRef]
- Chan, H.T.; Flath, R.A.; Forrey, R.R.; Cavaletto, C.G.; Nakayama, T.O.M.; Brekke, J.E. Development of off-odors and off-flavors in papaya puree. J. Agric. Food Chem. 1973, 21, 566–570. [Google Scholar] [CrossRef]
- Fabi, J.P.; Peroni, F.H.G. Papaya, Mango and Guava Fruit Metabolism during Ripening: Postharvest Changes Affecting Tropical Fruit Nutritional Content and Quality. Fresh Prod. 2010, 4, 56–66. [Google Scholar]
- Gomez, M.; Lajolo, F.; Cordenunsi, B. Evolution of Soluble Sugars During Ripening of Papaya Fruit and its Relation to Sweet Taste. J. Food Sci. 2002, 67, 442–447. [Google Scholar] [CrossRef]
- Ferrão, L.F.V.; Johnson, T.S.; Benevenuto, J.; Edger, P.P.; Colquhoun, T.A.; Munoz, P.R. Genome-wide association of volatiles reveals candidate loci for blueberry flavor. New Phytol. 2020, 226, 1725–1737. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Scott, J.W.; Einstein, M.A.; Malundo, T.M.M.; Carr, B.T.; Shewfelt, R.L.; Tandon, K.S. Relationship between Sensory and Instrumental Analysis for Tomato Flavor. J. Am. Soc. Hortic. Sci. 1998, 123, 906–915. [Google Scholar] [CrossRef] [Green Version]
- Yamaki, S. Metabolism and Accumulation of Sugars Translocated to Fruit and Their Regulation. J. Jpn. Soc. Hortic. Sci. 2010, 79, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Nantawan, U.; Kanchana-udomkan, C.; Drew, R.; Ford, R. Identification of genes related to sugar content in Carica papaya L.: Differential expression and candidate marker development. Acta Hortic. 2018, 1203, 129–136. [Google Scholar] [CrossRef]
- Fabi, J.P.; Seymour, G.B.; Graham, N.S.; Broadley, M.R.; May, S.T.; Lajolo, F.M.; Cordenunsi, B.R.; Oliveira do Nascimento, J.R. Analysis of ripening-related gene expression in papaya using an Arabidopsis-based microarray. BMC Plant Biol. 2012, 12, 242. [Google Scholar] [CrossRef] [Green Version]
- Nantawan, U.; Kanchana-udomkan, C.; Bar, I.; Ford, R. Linkage mapping and quantitative trait loci analysis of sweetness and other fruit quality traits in papaya. BMC Plant Biol. 2019, 19, 449. [Google Scholar] [CrossRef] [PubMed]
- Klee, H.J. Improving the flavor of fresh fruits: Genomics, biochemistry, and biotechnology. New Phytol. 2010, 187, 44–56. [Google Scholar] [CrossRef]
- Rongtong, B.; Suwonsichon, T.; Ritthiruangdej, P.; Kasemsumran, S. Determination of water activity, total soluble solids and moisture, sucrose, glucose and fructose contents in osmotically dehydrated papaya using near-infrared spectroscopy. Agric. Nat. Resour. 2018, 52, 557–564. [Google Scholar] [CrossRef]
- Schweiggert, R.M.; Steingass, C.B.; Esquivel, P.; Carle, R. Chemical and Morphological Characterization of Costa Rican Papaya (Carica papaya L.) Hybrids and Lines with Particular Focus on Their Genuine Carotenoid Profiles. J. Agric. Food Chem. 2012, 60, 2577–2585. [Google Scholar] [CrossRef] [PubMed]
- Pangborn, R.M. Relative Taste Intensities of Selected Sugars and Organic Acidsa. J. Food Sci. 1963, 28, 726–733. [Google Scholar] [CrossRef]
- Leonardou, V.K.; Doudoumis, E.; Tsormpatsidis, E.; Vysini, E.; Papanikolopoulos, T.; Papasotiropoulos, V.; Lamari, F.N. Quality Traits, Volatile Organic Compounds, and Expression of Key Flavor Genes in Strawberry Genotypes over Harvest Period. Int. J. Mol. Sci. 2021, 22, 13499. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.; Pereira, J.; Câmara, J.S. Effectiveness of different solid-phase microextraction fibres for differentiation of selected Madeira island fruits based on their volatile metabolite profile—Identification of novel compounds. Talanta 2011, 83, 899–906. [Google Scholar] [CrossRef]
- Mostafa, S.; Wang, Y.; Zeng, W.; Jin, B. Floral Scents and Fruit Aromas: Functions, Compositions, Biosynthesis, and Regulation. Front. Plant Sci. 2022, 13, 860157. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Kate, A.; Mohapatra, D.; Tripathi, M.K.; Ray, H.; Akuli, A.; Ghosh, A.; Modhera, B. Volatile organic compounds (VOCs): Biomarkers for quality management of horticultural commodities during storage through e-sensing. Trends Food Sci. Technol. 2020, 106, 417–433. [Google Scholar] [CrossRef]
- Kupska, M.; Wasilewski, T.; Jędrkiewicz, R.; Gromadzka, J.; Namieśnik, J. Determination of Terpene Profiles in Potential Superfruits. Int. J. Food Prop. 2016, 19, 2726–2738. [Google Scholar] [CrossRef]
- San, A.T.; Joyce, D.C.; Hofman, P.J.; Macnish, A.J.; Webb, R.I.; Matovic, N.J.; Williams, C.M.; De Voss, J.J.; Wong, S.H.; Smyth, H.E. Stable isotope dilution assay (SIDA) and HS-SPME-GCMS quantification of key aroma volatiles for fruit and sap of Australian mango cultivars. Food Chem. 2017, 221, 613–619. [Google Scholar] [CrossRef] [Green Version]
- Andrade, E.H.A.; Maia, J.G.S.; Zoghbi, M.d.G.B. Aroma Volatile Constituents of Brazilian Varieties of Mango Fruit. J. Food Compos. Anal. 2000, 13, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Castro-Vázquez, L.; Díaz-Maroto, M.C.; Pérez-Coello, M.S. Aroma composition and new chemical markers of Spanish citrus honeys. Food Chem. 2007, 103, 601–606. [Google Scholar] [CrossRef]
- Kulkarni, M.M. Digital Multiplexed Gene Expression Analysis Using the NanoString nCounter System. Curr. Protoc. Mol. Biol. 2011, 94, 25B.10.1–25B.10.17. [Google Scholar] [CrossRef]
- Veldman-Jones, M.H.; Brant, R.; Rooney, C.; Geh, C.; Emery, H.; Harbron, C.G.; Wappett, M.; Sharpe, A.; Dymond, M.; Barrett, J.C.; et al. Evaluating Robustness and Sensitivity of the NanoString Technologies nCounter Platform to Enable Multiplexed Gene Expression Analysis of Clinical Samples. Cancer Res. 2015, 75, 2587–2593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunz, H.H.; Häusler, R.E.; Fettke, J.; Herbst, K.; Niewiadomski, P.; Gierth, M.; Bell, K.; Steup, M.; Flügge, U.-I.; Schneider, A. The role of plastidial glucose-6-phosphate/phosphate translocators in vegetative tissues of Arabidopsis thaliana mutants impaired in starch biosynthesis. Plant Biol. Stuttg. Ger. 2010, 12 (Suppl. S1), 115–128. [Google Scholar] [CrossRef]
- Weise, S.E.; Liu, T.; Childs, K.L.; Preiser, A.L.; Katulski, H.M.; Perrin-Porzondek, C.; Sharkey, T.D. Transcriptional Regulation of the Glucose-6-Phosphate/Phosphate Translocator 2 Is Related to Carbon Exchange Across the Chloroplast Envelope. Front. Plant Sci. 2019, 10, 827. [Google Scholar] [CrossRef]
- Yeoman, C.J.; Han, Y.; Dodd, D.; Schroeder, C.M.; Mackie, R.I.; Cann, I.K.O. Chapter 1—Thermostable Enzymes as Biocatalysts in the Biofuel Industry. In Advances in Applied Microbiology; Academic Press: Cambridge, MA, USA, 2010; Volume 70, pp. 1–55. [Google Scholar]
- Larsbrink, J.; Rogers, T.E.; Hemsworth, G.R.; McKee, L.S.; Tauzin, A.S.; Spadiut, O.; Klinter, S.; Pudlo, N.A.; Urs, K.; Koropatkin, N.M.; et al. A discrete genetic locus confers xyloglucan metabolism in select human gut Bacteroidetes. Nature 2014, 506, 498–502. [Google Scholar] [CrossRef]
- Nuñez-Lillo, G.; Balladares, C.; Pavez, C.; Urra, C.; Sanhueza, D.; Vendramin, E.; Dettori, M.T.; Arús, P.; Verde, I.; Blanco-Herrera, F.; et al. High-density genetic map and QTL analysis of soluble solid content, maturity date, and mealiness in peach using genotyping by sequencing. Sci. Hortic. 2019, 257, 108734. [Google Scholar] [CrossRef]
- Lehle, L.; Tanner, W. The Function of myo-Inositol in the Biosynthesis of Raffinose. Eur. J. Biochem. 1973, 38, 103–110. [Google Scholar] [CrossRef]
- Duan, E.; Wang, Y.; Liu, L.; Zhu, J.; Zhong, M.; Zhang, H.; Li, S.; Ding, B.; Zhang, X.; Guo, X.; et al. Pyrophosphate: Fructose-6-phosphate 1-phosphotransferase (PFP) regulates carbon metabolism during grain filling in rice. Plant Cell Rep. 2016, 35, 1321–1331. [Google Scholar] [CrossRef] [Green Version]
- Groenewald, J.-H.; Botha, F.C.; Groenewald, J.-H.; Botha, F.C. Molecular and kinetic characterisation of sugarcane pyrophosphate: Fructose-6-phosphate 1-phosphotransferase and its possible role in the sucrose accumulation phenotype. Funct. Plant Biol. 2007, 34, 517–525. [Google Scholar] [CrossRef]
- Kong, W.; An, B.; Zhang, Y.; Yang, J.; Li, S.; Sun, T.; Li, Y. Sugar Transporter Proteins (STPs) in Gramineae Crops: Comparative Analysis, Phylogeny, Evolution, and Expression Profiling. Cells 2019, 8, 560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherson, S.M.; Hemmann, G.; Wallace, G.; Forbes, S.; Germain, V.; Stadler, R.; Bechtold, N.; Sauer, N.; Smith, S.M. Monosaccharide/proton symporter AtSTP1 plays a major role in uptake and response of Arabidopsis seeds and seedlings to sugars. Plant J. 2000, 24, 849–857. [Google Scholar] [CrossRef]
- Poschet, G.; Hannich, B.; Büttner, M. Identification and characterization of AtSTP14, a novel galactose transporter from Arabidopsis. Plant Cell Physiol. 2010, 51, 1571–1580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adair, W.L. Carbohydrates. In xPharm: The Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D.B., Eds.; Elsevier: New York, NY, USA, 2007; pp. 1–12. ISBN 978-0-08-055232-3. [Google Scholar]
- Tholl, D.; Sohrabi, R.; Huh, J.-H.; Lee, S. The biochemistry of homoterpenes--common constituents of floral and herbivore-induced plant volatile bouquets. Phytochemistry 2011, 72, 1635–1646. [Google Scholar] [CrossRef]
- Herde, M.; Gärtner, K.; Köllner, T.G.; Fode, B.; Boland, W.; Gershenzon, J.; Gatz, C.; Tholl, D. Identification and regulation of TPS04/GES, an Arabidopsis geranyllinalool synthase catalyzing the first step in the formation of the insect-induced volatile C16-homoterpene TMTT. Plant Cell 2008, 20, 1152–1168. [Google Scholar] [CrossRef] [Green Version]
- D’Auria, J.C.; Chen, F.; Pichersky, E. Characterization of an acyltransferase capable of synthesizing benzylbenzoate and other volatile esters in flowers and damaged leaves of Clarkia breweri. Plant Physiol. 2002, 130, 466–476. [Google Scholar] [CrossRef] [Green Version]
- Hirvi, T.; Honkanen, E.; Pyysalo, T. The aroma of cranberries. Z. Für Lebensm. Unters. Forsch. 1981, 172, 365–367. [Google Scholar] [CrossRef]
- Kelebek, H.; Selli, S.; Gubbuk, H.; Gunes, E. Comparative evaluation of volatiles, phenolics, sugars, organic acids and antioxidant properties of Sel-42 and Tainung papaya varieties. Food Chem. 2015, 173, 912–919. [Google Scholar] [CrossRef]
- Velterop, J.S.; Vos, F. A rapid and inexpensive microplate assay for the enzymatic determination of glucose, fructose, sucrose, L-malate and citrate in tomato (Lycopersicon esculentum) extracts and in orange juice. Phytochem. Anal. 2001, 12, 299–304. [Google Scholar] [CrossRef]
- Schlotter, Y.M.; Veenhof, E.Z.; Brinkhof, B.; Rutten, V.P.M.G.; Spee, B.; Willemse, T.; Penning, L.C. A GeNorm algorithm-based selection of reference genes for quantitative real-time PCR in skin biopsies of healthy dogs and dogs with atopic dermatitis. Vet. Immunol. Immunopathol. 2009, 129, 115–118. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Horbinski, C.; Wu, H.; Liu, Y.; Sheng, S.; Liu, J.; Wang, C. NanoStringDiff: A novel statistical method 669 for differential expression analysis based on NanoString nCounter data. Nucleic Acids Res. 2016, 44, e151–670. [Google Scholar] [CrossRef] [Green Version]
| Variety | Glucose (mg/g FW) | Fructose (mg/g FW) | Sucrose (mg/g FW) | Glucose (%, w/w) * | Fructose (%, w/w) | Sucrose (%, w/w) | TSS (◦Brix) * |
|---|---|---|---|---|---|---|---|
| RB1 | 18.1 ± 6.8 | 32.4 ± 3.4 | 37.1 ± 2.5 | 20.6 ± 0.02 ab | 36.8 ± 0.02 | 42.5 ± 0.02 | 11.1 ± 0.7 b |
| RB4 | 17.8 ± 6.8 | 31.5 ± 3.4 | 36.6 ± 2.4 | 20.7 ± 0.01 ab | 36.6 ± 0.02 | 42.7 ± 0.03 | 10.6 ± 0.7 bc |
| Skybury | 15.0 ± 6.5 | 29.0 ± 3.3 | 37.4 ± 2.4 | 18.4 ± 0.02 b | 35.6 ± 0.03 | 46.0 ± 0.02 | 12.8 ± 1.3 a |
| 1B | 20.7 ± 9.5 | 28.1 ± 7.4 | 33.3 ± 9.3 | 24.1 ± 0.05 a | 34.0 ± 0.01 | 42.0 ± 0.05 | 9.5 ± 0.7 c |
| H13 | 22.6 ± 4.9 | 32.1 ± 3.9 | 36.5 ± 2.7 | 24.4 ± 0.03 a | 35.2 ± 0.01 | 40.4 ± 0.03 | 9.2 ± 0.9 c |
| p-Value | 0.148 | 0.519 | 0.245 | 0.008 | 0.116 | 0.144 | <0.0001 |
| Volatile Identity | Classification | Concentration (mg/kg) * | |||||
|---|---|---|---|---|---|---|---|
| RB1 | RB4 | Skybury | 1B | H13 | p-Value | ||
| Linalool oxide | Monoterpene | 0.087 b | 0.108 ab | 0.031 b | 0.289 a | 0.193 ab | 0.013 |
| Linalool | Monoterpene | 0.016 | 0.029 | 0.004 | 0.098 | 0.024 | 0.078 |
| Terpinolene | Monoterpene | 0.017 bc | 0.016 abc | 0.002 c | 0.071 a | 0.051 ab | 0.006 |
| α-Pinene | Monoterpene | 0.003 | - | 0.040 | - | 0.008 | 0.441 |
| β-Pinene | Monoterpene | 0.004 | 0.001 | 0.014 | 0.002 | 0.003 | 0.397 |
| β-Myrcene | Monoterpene | 0.009 | 0.002 | 0.004 | - | 0.002 | 0.719 |
| 3-Carene | Monoterpene | <0.001 | - | 0.016 | - | 0.002 | 0.413 |
| p-Cymene | Monoterpene | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 | 0.312 |
| D-Limonene | Monoterpene | 0.002 | 0.003 | 0.017 | 0.003 | 0.003 | 0.297 |
| Eucalyptol | Monoterpene | 0.063 | 0.030 | 0.015 | 0.052 | 0.014 | 0.065 |
| Citronellol | Monoterpene | 0.025 | 0.019 | 0.038 | 0.026 | 0.027 | 0.137 |
| Citral | Monoterpene | 0.079 ab | 0.038 ab | 0.059 ab | 0.116 a | 0.046 b | 0.035 |
| Benzyl isothiocyanate | Benzene | 0.201 | 0.269 | 0.046 | 0.198 | 0.121 | 0.267 |
| Naphthalene | Benzene | 0.343 | 0.329 | 0.354 | 0.382 | 0.335 | 0.447 |
| Sensory Descriptor | Consumer Liking |
|---|---|
| sweet caramelised flavour | 0.593 |
| sweet aftertaste | 0.542 |
| velvety | 0.302 |
| fishy aroma | 0.286 |
| floral flavour | 0.223 |
| flavour intensity | 0.119 |
| juiciness | 0.083 |
| resistance | 0.044 |
| dissolving | −0.060 |
| metallic aftertaste | −0.121 |
| sweet fruit aroma | −0.166 |
| musty off-note aroma | −0.205 |
| fibrous | −0.261 |
| bitter aftertaste | −0.298 |
| bitterness flavour | −0.321 |
| musty flavour | −0.325 |
| aroma intensity | −0.360 |
| citrus aroma | −0.441 |
| (a) Correlation between target sensory descriptors and sweetness-related variables | ||||||
|---|---|---|---|---|---|---|
| Variables | Sweet Aftertaste | Sweet Caramelised Flavour | Floral Flavour | Bitter Aftertaste | Bitterness Flavour | Musty Flavour |
| TSS | 0.797 | 0.782 | 0.325 | −0.474 | −0.436 | −0.565 |
| Glucose | −0.298 | −0.323 | −0.156 | 0.372 | 0.431 | 0.452 |
| Fructose | 0.087 | 0.060 | 0.071 | 0.075 | 0.143 | 0.179 |
| Sucrose | 0.263 | 0.222 | 0.178 | −0.107 | −0.160 | −0.225 |
| %Sucrose | 0.258 | 0.265 | 0.118 | −0.319 | −0.417 | −0.504 |
| %Glucose | −0.453 | −0.463 | −0.226 | 0.444 | 0.504 | 0.540 |
| (b) Correlation between target sensory descriptors and volatile-related variables | ||||||
| Variables | citrus aroma | fishy aroma | aroma intensity | musty off-note aroma | sweet fruit aroma | |
| Naphthalene | 0.083 | 0.217 | 0.025 | −0.058 | −0.275 | |
| Citral | 0.209 | 0.151 | 0.215 | 0.186 | −0.016 | |
| Eucalyptol | −0.023 | 0.167 | 0.014 | −0.121 | 0.198 | |
| p-Cymene | −0.475 | 0.432 | −0.594 | −0.462 | −0.185 | |
| D-Limonene | −0.375 | 0.297 | −0.395 | −0.286 | −0.164 | |
| α-Pinene | −0.313 | 0.181 | −0.369 | −0.274 | −0.102 | |
| 3-Carene | −0.343 | 0.245 | −0.395 | −0.286 | −0.125 | |
| β-Pinene | −0.346 | 0.209 | −0.377 | −0.279 | −0.113 | |
| Citronellol | −0.468 | 0.365 | −0.278 | −0.329 | 0.017 | |
| Benzyl isothiocyanate | 0.285 | −0.177 | 0.052 | 0.074 | 0.038 | |
| Linalool | 0.722 | −0.614 | 0.649 | 0.400 | 0.507 | |
| Terpinolene | 0.946 | −0.828 | 0.850 | 0.662 | 0.275 | |
| Linalool oxide | 0.916 | −0.768 | 0.799 | 0.631 | 0.265 | |
| β-Myrcene | −0.439 | 0.252 | −0.348 | −0.411 | 0.127 | |
| Attribute | Definition |
|---|---|
| Aroma | |
| overall aroma intensity | The overall intensity of the sample aroma |
| sweet fruit | An aroma of fresh sweet fruit such as honeydew melon or mango |
| musty off-note | An aroma of ripe rock melon, over-ripe fruit, eggy, sulphurous |
| fishy | An aroma of tuna, fishy, or seaweed |
| citrus | An aroma of citrus peel or juice |
| Texture | |
| resistance | The degree to which the sample resists initial bite, firmness |
| juiciness | The degree to which liquid is released upon mastication |
| dissolving | The degree to which the sample dissolves/disintegrates in the mouth |
| velvety | The smoothness of the sample (lack of particles/grit) |
| fibrous | The presence of fibrous pieces, debris in the sample |
| Flavour | |
| flavour intensity | The overall flavour intensity of the sample |
| sweet caramelised | A flavour associated with cooked sweet potato/carrot, sweet melon with caramel notes |
| bitterness | A bitter flavour |
| musty | A flavour of over-ripe rockmelon with skin, stale |
| floral | A flavour of floral notes (jasmine flower) |
| Aftertaste | |
| bitter | A bitter aftertaste |
| sweet | A sweet aftertaste |
| metallic | A metallic aftertaste |
| Gene ID | Accession ID | Description |
|---|---|---|
| SAND | JQ678783 | Carica papaya SAND family protein-like (SAND) mRNA |
| TBP2 | JQ678779.1 | TATA-binding protein 2 |
| CYP | JQ678769.1 | Cyclophilin |
| cpGPT2 | XM_022031675.1 | PREDICTED: Carica papaya glucose-6-phosphate/phosphate translocator 2, chloroplastic (LOC110806751), mRNA |
| cpBGH3B | XM_022036929.1 | PREDICTED: Carica papaya beta-glucosidase BoGH3B-like (LOC110810687), mRNA |
| cpBGLU42 | XM_022038006.1 | PREDICTED: Carica papaya beta-glucosidase 42-like (LOC110811486), mRNA |
| cpBGLU31 | XM_022053230.1 | PREDICTED: Carica papaya beta-glucosidase 31-like (LOC110822982), mRNA |
| cpRFS2 | XM_022037673.1 | PREDICTED: Carica papaya probable galactinol--sucrose galactosyltransferase 2 (LOC110811248), transcript variant X3, mRNA |
| cpPFP | XM_022039277.1 | PREDICTED: Carica papaya pyrophosphate-fructose 6-phosphate 1-phosphotransferase subunit beta (LOC110812486), mRNA |
| cpSTP14 | XM_022044378.1 | PREDICTED: Carica papaya sugar transport protein 14 (LOC110816261), mRNA |
| cpSTP1 | XM_022055661.1 | PREDICTED: Carica papaya sugar transport protein 1-like (LOC110825204), mRNA |
| cpGES | XM_022046578.1 | PREDICTED: Carica papaya (E, E)-geranyllinalool synthase-like (LOC110817861), mRNA |
| cpBEBT | XM_022055614.1 | PREDICTED: Carica papaya benzyl alcohol O-benzoyltransferase-like (LOC110825152), mRNA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Bar, I.; Ford, R.; Smyth, H.; Kanchana-udomkan, C. Biochemical, Sensory, and Molecular Evaluation of Flavour and Consumer Acceptability in Australian Papaya (Carica papaya L.) Varieties. Int. J. Mol. Sci. 2022, 23, 6313. https://doi.org/10.3390/ijms23116313
Zhou Z, Bar I, Ford R, Smyth H, Kanchana-udomkan C. Biochemical, Sensory, and Molecular Evaluation of Flavour and Consumer Acceptability in Australian Papaya (Carica papaya L.) Varieties. International Journal of Molecular Sciences. 2022; 23(11):6313. https://doi.org/10.3390/ijms23116313
Chicago/Turabian StyleZhou, Ziwei, Ido Bar, Rebecca Ford, Heather Smyth, and Chutchamas Kanchana-udomkan. 2022. "Biochemical, Sensory, and Molecular Evaluation of Flavour and Consumer Acceptability in Australian Papaya (Carica papaya L.) Varieties" International Journal of Molecular Sciences 23, no. 11: 6313. https://doi.org/10.3390/ijms23116313
APA StyleZhou, Z., Bar, I., Ford, R., Smyth, H., & Kanchana-udomkan, C. (2022). Biochemical, Sensory, and Molecular Evaluation of Flavour and Consumer Acceptability in Australian Papaya (Carica papaya L.) Varieties. International Journal of Molecular Sciences, 23(11), 6313. https://doi.org/10.3390/ijms23116313










