Formation of False Context Fear Memory Is Regulated by Hypothalamic Corticotropin-Releasing Factor in Mice
Abstract
1. Introduction
2. Results
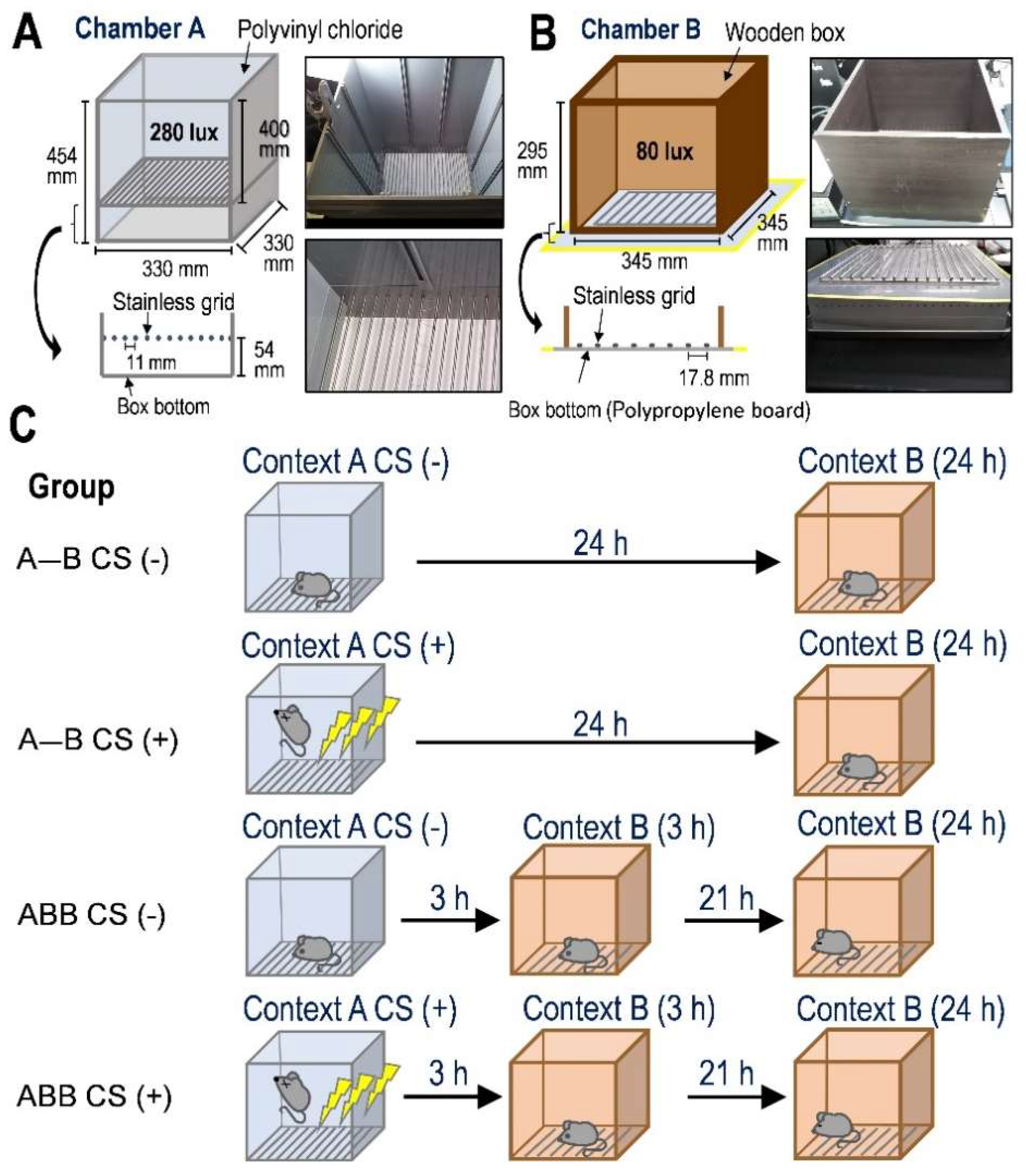
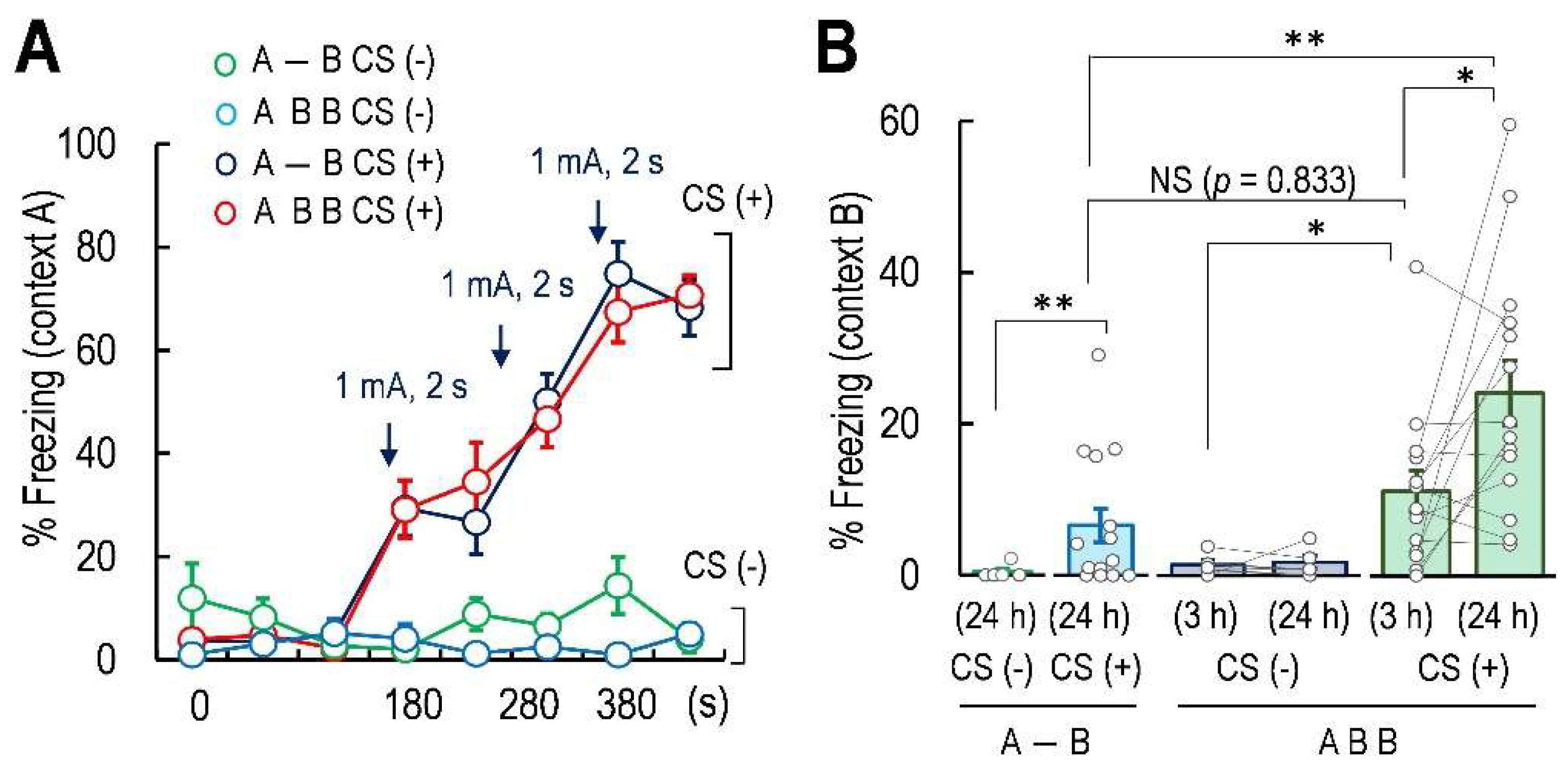
2.1. Exposure of Novel Context in 3 h after Fear Conditioning Formed False Fear Memory That Was Further Enhanced at 24 h after Conditioning
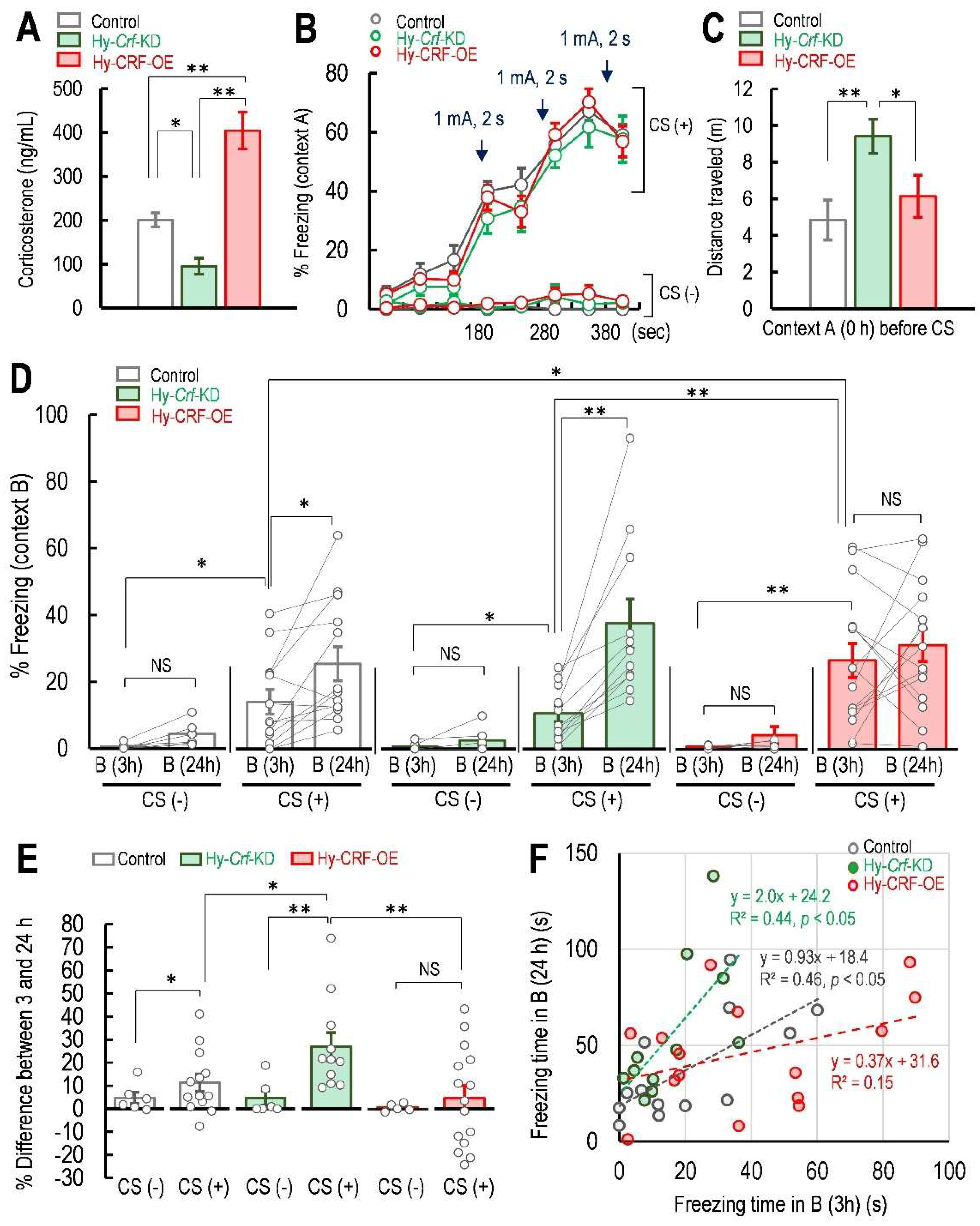
2.2. Hy-Crf-KD Enhanced False Fear Memory Level in 24 h and Hy-CRF-OE Potentiated the False Fear Memory Level within 3 h after Fear Conditioning
3. Discussion
4. Materials and Methods
4.1. Animal Ethics Approval
4.2. Behavioral Tests
4.3. Apparatus for the Contextual Fear Conditioning Test
4.4. Contextual Fear Conditioning Test
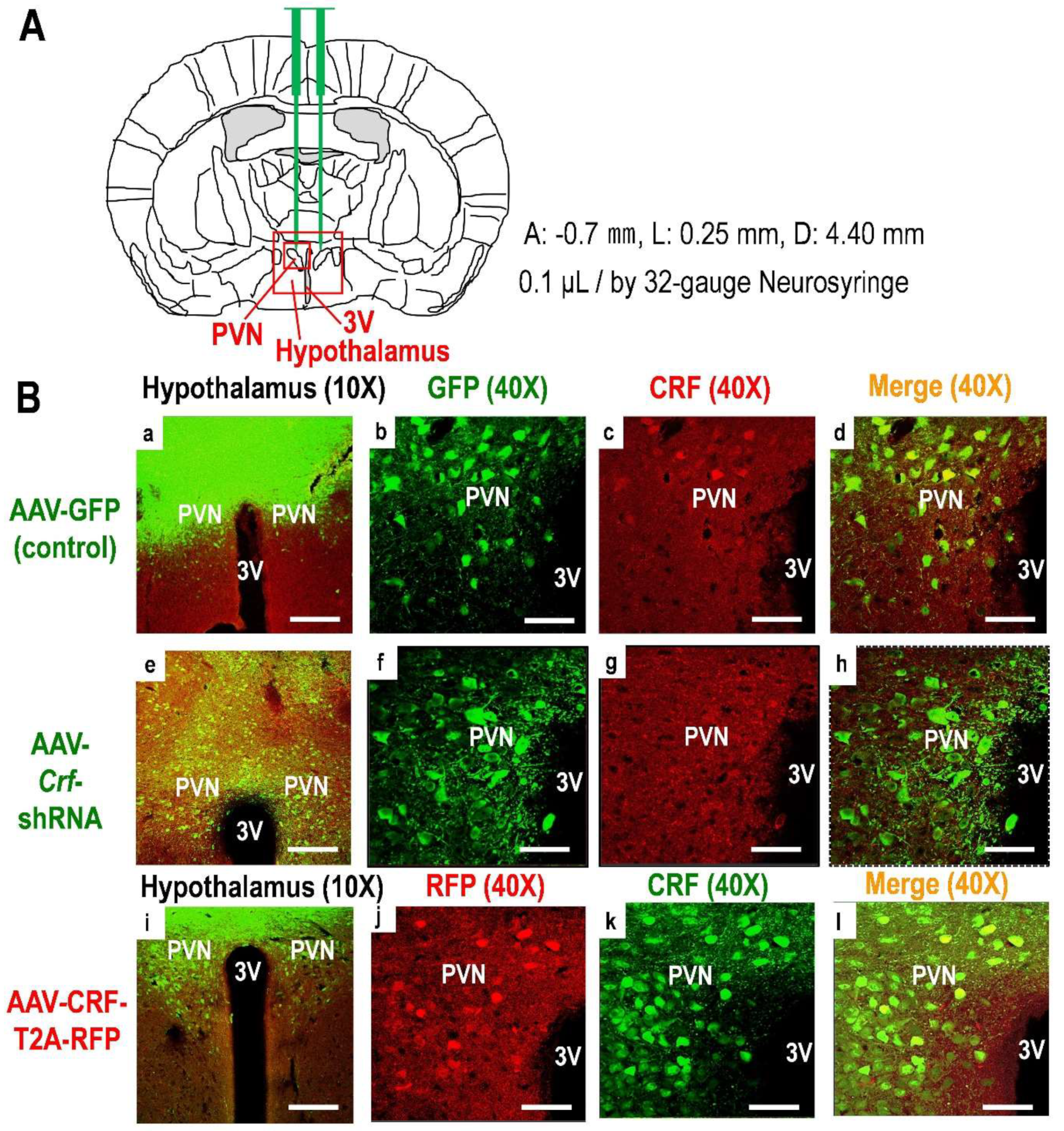
4.5. Producing the AAV for Knockdown and Overexpression of CRF
4.6. AAV Injection into the Hypothalamus
4.7. Verification of AAV Infection and CRF Expression Levels
4.8. Measurement of Plasma Corticosterone
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Quervain, D.J.; Roozendaal, B.; McGaugh, J.L. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature 1998, 394, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Kaouane, N.; Porte, Y.; Vallée, M.; Brayda-Bruno, L.; Mons, N.; Calandreau, L.; Marighetto, A.; Piazza, P.V.; Desmedt, A. Glucocorticoids can induce PTSD-like memory impairments in mice. Science 2012, 335, 1510–1513. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.M.H.; Rashid, A.J.; Jacob, A.D.; Frankland, P.W.; Schacter, D.L.; Josselyn, S.A. The role of neuronal excitability, allocation to an engram and memory linking in the behavioral generation of a false memory in mice. Neurobiol. Learn. Mem. 2020, 174, 107284. [Google Scholar] [CrossRef] [PubMed]
- Nader, K.; Schafe, G.E.; Le Doux, J.E. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 2000, 406, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Millin, p.M.; Riccio, D.C. False memory in nonhuman animals. Learn. Mem. 2019, 26, 381–386. [Google Scholar] [CrossRef]
- Zoellner, L.A.; Foa, E.B.; Brigidi, B.D.; Przeworski, A. Are trauma victims susceptible to “false memories”? J. Abnorm. Psychol. 2000, 109, 517–524. [Google Scholar] [CrossRef]
- Anagnostaras, S.G.; Gale, G.D.; Fanselow, M.S. Hippocampus and contextual fear conditioning: Recent controversies and advances. Hippocampus 2001, 11, 8–17. [Google Scholar] [CrossRef]
- Wiltgen, B.J.; Silva, A.J. Memory for context becomes less specific with time. Learn. Mem. 2007, 14, 313–317. [Google Scholar] [CrossRef]
- Cai, D.J.; Aharoni, D.; Shuman, T.; Shobe, J.; Biane, J.; Song, W.; Wei, B.; Veshkini, M.; La-Vu, M.; Lou, J.; et al. A shared neural ensemble links distinct contextual memories encoded close in time. Nature 2016, 534, 115–118. [Google Scholar] [CrossRef]
- Merz, C.J.; Hermann, A.; Stark, R.; Wolf, O.T. Cortisol modifies extinction learning of recently acquired fear in men. Soc. Cogn. Affect. Neurosci. 2014, 9, 1426–1434. [Google Scholar] [CrossRef]
- Bremner, J.D. Traumatic stress: Effects on the brain. Dialogues Clin. Neurosci. 2006, 8, 445–461. [Google Scholar] [CrossRef] [PubMed]
- Skórzewska, A.; Lehner, M.; Wisłowska-Stanek, A.; Turzyńska, D.; Sobolewska, A.; Krząścik, P.; Szyndler, J.; Maciejak, P.; Chmielewska, N.; Kołosowska, K.; et al. Individual susceptibility or resistance to posttraumatic stress disorder-like behaviours. Behav. Brain Res. 2020, 386, 112591. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, G.; Shi, J.; Xie, X.; Fei, N.; Chen, L.; Liu, N.; Yang, M.; Pan, J.; Huang, W.; et al. trans-Resveratrol ameliorates anxiety-like behaviors and fear memory deficits in a rat model of post-traumatic stress disorder. Neuropharmacology 2018, 133, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Perrine, S.A.; Eagle, A.L.; George, S.A.; Mulo, K.; Kohler, R.J.; Gerard, J.; Harutyunyan, A.; Hool, S.M.; Susick, L.L.; Schneider, B.L.; et al. Severe, multimodal stress exposure induces PTSD-like characteristics in a mouse model of single prolonged stress. Behav. Brain Res. 2016, 303, 228–237. [Google Scholar] [CrossRef]
- Ribeiro, T.O.; Bueno-de-Camargo, L.M.; Waltrick, A.P.F.; de Oliveira, A.R.; Brandão, M.L.; Munhoz, C.D.; Zanoveli, J.M. Activation of mineralocorticoid receptors facilitate the acquisition of fear memory extinction and impair the generalization of fear memory in diabetic animals. Psychopharmacology 2020, 237, 529–542. [Google Scholar] [CrossRef]
- Sarabdjitsingh, R.A.; Zhou, M.; Yau, J.L.; Webster, S.P.; Walker, B.R.; Seckl, J.R.; Joëls, M.; Krugers, H.J. Inhibiting 11β-hydroxysteroid dehydrogenase type 1 prevents stress effects on hippocampal synaptic plasticity and impairs contextual fear conditioning. Neuropharmacology 2014, 81, 231–236. [Google Scholar] [CrossRef]
- Merz, C.J.; Hamacher-Dang, T.C.; Stark, R.; Wolf, O.T.; Hermann, A. Neural Underpinnings of Cortisol Effects on Fear Extinction. Neuropsychopharmacology 2018, 43, 384–392. [Google Scholar] [CrossRef]
- Battaglia, S. Neurobiological advances of learned fear in humans. Adv. Clin. Exp. Med. 2022, 31, 217–221. [Google Scholar] [CrossRef]
- Battaglia, S.; Garofalo, S.; di Pellegrino, G.; Starita, F. Revaluing the Role of vmPFC in the Acquisition of Pavlovian Threat Conditioning in Humans. J. Neurosci. Off. J. Soc. Neurosci. 2020, 40, 8491–8500. [Google Scholar] [CrossRef]
- Fullana, M.A.; Harrison, B.J.; Soriano-Mas, C.; Vervliet, B.; Cardoner, N.; Àvila-Parcet, A.; Radua, J. Neural signatures of human fear conditioning: An updated and extended meta-analysis of fMRI studies. Mol. Psychiatry 2016, 21, 500–508. [Google Scholar] [CrossRef]
- Battaglia, S.; Harrison, B.J.; Fullana, M.A. Does the human ventromedial prefrontal cortex support fear learning, fear extinction or both? A commentary on subregional contributions. Mol. Psychiatry 2022, 27, 784–786. [Google Scholar] [CrossRef] [PubMed]
- Zohar, J.; Juven-Wetzler, A.; Myers, V.; Fostick, L. Post-traumatic stress disorder: Facts and fiction. Curr. Opin. Psychiatry 2008, 21, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Vécsei, L. Editorial of Special Issue “Crosstalk between Depression, Anxiety, and Dementia: Comorbidity in Behavioral Neurology and Neuropsychiatry”. Biomedicines 2021, 9, 517. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Asai, M.; Mahoney, C.E.; Joachim, M.; Shen, Y.; Gunner, G.; Majzoub, J.A. Loss of hypothalamic corticotropin-releasing hormone markedly reduces anxiety behaviors in mice. Mol. Psychiatry 2017, 22, 733–744. [Google Scholar] [CrossRef]
- Malisch, J.L.; Breuner, C.W.; Gomes, F.R.; Chappell, M.A.; Garland, T., Jr. Circadian pattern of total and free corticosterone concentrations, corticosteroid-binding globulin, and physical activity in mice selectively bred for high voluntary wheel-running behavior. Gen. Comp. Endocrinol. 2008, 156, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Fujinaka, A.; Li, R.; Hayashi, M.; Kumar, D.; Changarathil, G.; Naito, K.; Miki, K.; Nishiyama, T.; Lazarus, M.; Sakurai, T.; et al. Effect of context exposure after fear learning on memory generalization in mice. Mol. Brain 2016, 9, 2. [Google Scholar] [CrossRef]
- Xu, W.; Südhof, T.C. A neural circuit for memory specificity and generalization. Science 2013, 339, 1290–1295. [Google Scholar] [CrossRef]
- Mathews, A.; MacLeod, C. Cognitive vulnerability to emotional disorders. Annu. Rev. Clin. Psychol. 2005, 1, 167–195. [Google Scholar] [CrossRef]
- Jovanovic, T.; Norrholm, S.D.; Blanding, N.Q.; Davis, M.; Duncan, E.; Bradley, B.; Ressler, K.J. Impaired fear inhibition is a biomarker of PTSD but not depression. Depress. Anxiety 2010, 27, 244–251. [Google Scholar] [CrossRef]
- Bergstrom, H.C. Assaying Fear Memory Discrimination and Generalization: Methods and Concepts. Curr. Protoc. Neurosci. 2020, 91, e89. [Google Scholar] [CrossRef]
- Kolodziejczyk, M.H.; Fendt, M. Corticosterone Treatment and Incubation Time After Contextual Fear Conditioning Synergistically Induce Fear Memory Generalization in Neuropeptide S Receptor-Deficient Mice. Front. Neurosci. 2020, 14, 128. [Google Scholar] [CrossRef] [PubMed]
- Oishi, N.; Nomoto, M.; Ohkawa, N.; Saitoh, Y.; Sano, Y.; Tsujimura, S.; Nishizono, H.; Matsuo, M.; Muramatsu, S.I.; Inokuchi, K. Artificial association of memory events by optogenetic stimulation of hippocampal CA3 cell ensembles. Mol. Brain 2019, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, S.; Liu, X.; Lin, P.A.; Suh, J.; Pignatelli, M.; Redondo, R.L.; Ryan, T.J.; Tonegawa, S. Creating a false memory in the hippocampus. Science 2013, 341, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ramirez, S.; Tonegawa, S. Inception of a false memory by optogenetic manipulation of a hippocampal memory engram. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130142. [Google Scholar] [CrossRef]
- Eichenbaum, H. On the Integration of Space, Time, and Memory. Neuron 2017, 95, 1007–1018. [Google Scholar] [CrossRef]
- de Voogd, L.D.; Murray, Y.P.J.; Barte, R.M.; van der Heide, A.; Fernández, G.; Doeller, C.F.; Hermans, E.J. The role of hippocampal spatial representations in contextualization and generalization of fear. Neuroimage 2020, 206, 116308. [Google Scholar] [CrossRef]
- Zhang, Y.; Sheng, H.; Qi, J.; Ma, B.; Sun, J.; Li, S.; Ni, X. Glucocorticoid acts on a putative G protein-coupled receptor to rapidly regulate the activity of NMDA receptors in hippocampal neurons. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E747–E758. [Google Scholar] [CrossRef]
- Shavit Stein, E.; Itsekson Hayosh, Z.; Vlachos, A.; Maggio, N. Stress and Corticosteroids Modulate Muscarinic Long Term Potentiation (mLTP) in the Hippocampus. Front. Cell Neurosci. 2017, 11, 299. [Google Scholar] [CrossRef]
- Maggio, N.; Segal, M. Cellular basis of a rapid effect of mineralocorticosteroid receptors activation on LTP in ventral hippocampal slices. Hippocampus 2012, 22, 267–275. [Google Scholar] [CrossRef]
- Qiu, S.; Champagne, D.L.; Peters, M.; Catania, E.H.; Weeber, E.J.; Levitt, P.; Pimenta, A.F. Loss of limbic system-associated membrane protein leads to reduced hippocampal mineralocorticoid receptor expression, impaired synaptic plasticity, and spatial memory deficit. Biol. Psychiatry 2010, 68, 197–204. [Google Scholar] [CrossRef][Green Version]
- Cazakoff, B.N.; Howland, J.G. Acute stress disrupts paired pulse facilitation and long-term potentiation in rat dorsal hippocampus through activation of glucocorticoid receptors. Hippocampus 2010, 20, 1327–1331. [Google Scholar] [CrossRef]
- Dinse, H.R.; Kattenstroth, J.C.; Lenz, M.; Tegenthoff, M.; Wolf, O.T. The stress hormone cortisol blocks perceptual learning in humans. Psychoneuroendocrinology 2017, 77, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Wang, Z.; Wu, X.; Wen, S.W.; Liu, A. Salivary cortisol in post-traumatic stress disorder: A systematic review and meta-analysis. BMC Psychiatry 2018, 18, 324. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Kaminga, A.C.; Wen, S.W.; Wang, Z.; Wu, X.; Liu, A. The 24-hour urinary cortisol in post-traumatic stress disorder: A meta-analysis. PLoS ONE 2020, 15, e0227560. [Google Scholar] [CrossRef] [PubMed]
- Yehuda, R.; Kahana, B.; Binder-Brynes, K.; Southwick, S.M.; Mason, J.W.; Giller, E.L. Low urinary cortisol excretion in Holocaust survivors with posttraumatic stress disorder. Am. J. Psychiatry 1995, 152, 982–986. [Google Scholar] [CrossRef]
- Steudte-Schmiedgen, S.; Stalder, T.; Schönfeld, S.; Wittchen, H.U.; Trautmann, S.; Alexander, N.; Miller, R.; Kirschbaum, C. Hair cortisol concentrations and cortisol stress reactivity predict PTSD symptom increase after trauma exposure during military deployment. Psychoneuroendocrinology 2015, 59, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Hauer, D.; Weis, F.; Papassotiropoulos, A.; Schmoeckel, M.; Beiras-Fernandez, A.; Lieke, J.; Kaufmann, I.; Kirchhoff, F.; Vogeser, M.; Roozendaal, B.; et al. Relationship of a common polymorphism of the glucocorticoid receptor gene to traumatic memories and posttraumatic stress disorder in patients after intensive care therapy. Crit. Care Med. 2011, 39, 643–650. [Google Scholar] [CrossRef]
- Borgomaneri, S.; Battaglia, S.; Sciamanna, G.; Tortora, F.; Laricchiuta, D. Memories are not written in stone: Re-writing fear memories by means of non-invasive brain stimulation and optogenetic manipulations. Neurosci. Biobehav. Rev. 2021, 127, 334–352. [Google Scholar] [CrossRef]
- Stark, R.; Wolf, O.T.; Tabbert, K.; Kagerer, S.; Zimmermann, M.; Kirsch, P.; Schienle, A.; Vaitl, D. Influence of the stress hormone cortisol on fear conditioning in humans: Evidence for sex differences in the response of the prefrontal cortex. NeuroImage 2006, 32, 1290–1298. [Google Scholar] [CrossRef]
- Kan, R.L.D.; Zhang, B.B.B.; Zhang, J.J.Q.; Kranz, G.S. Non-invasive brain stimulation for posttraumatic stress disorder: A systematic review and meta-analysis. Transl. Psychiatry 2020, 10, 168. [Google Scholar] [CrossRef]
- Ruediger, S.; Vittori, C.; Bednarek, E.; Genoud, C.; Strata, P.; Sacchetti, B.; Caroni, P. Learning-related feedforward inhibitory connectivity growth required for memory precision. Nature 2011, 473, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Rohrbaugh, M.; Riccio, D.C. Stimulus generalization of learned fear in infant and adult rats. J. Comp. Physiol. Psychol. 1968, 66, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Huckleberry, K.A.; Ferguson, L.B.; Drew, M.R. Behavioral mechanisms of context fear generalization in mice. Learn. Mem. 2016, 23, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Murawski, N.J.; Asok, A. Understanding the contributions of visual stimuli to contextual fear conditioning: A proof-of-concept study using LCD screens. Neurosci. Lett. 2017, 637, 80–84. [Google Scholar] [CrossRef]
- Elliott, E.; Ezra-Nevo, G.; Regev, L.; Neufeld-Cohen, A.; Chen, A. Resilience to social stress coincides with functional DNA methylation of the Crf gene in adult mice. Nat. Neurosci. 2010, 13, 1351–1353. [Google Scholar] [CrossRef]
- Challis, R.C.; Ravindra Kumar, S.; Chan, K.Y.; Challis, C.; Beadle, K.; Jang, M.J.; Kim, H.M.; Rajendran, P.S.; Tompkins, J.D.; Shivkumar, K.; et al. Systemic AAV vectors for widespread and targeted gene delivery in rodents. Nat. Protoc. 2019, 14, 379–414. [Google Scholar] [CrossRef]
- Williams, R.W. Mapping genes that modulate mouse brain development: A quantitative genetic approach. Results Probl. Cell Differ. 2000, 30, 21–49. [Google Scholar] [CrossRef]
- Franklin, K.; Paxinos, G. The Coronal Plates and Diagrams, Compact. In Mouse Brain Stereotaxic Coordinates, 3rd ed.; Academic Press: Cambridge, MA, USA, 2008; pp. 1–256. [Google Scholar]
- Yang, L.; Shi, L.J.; Yu, J.; Zhang, Y.Q. Activation of protein kinase A in the amygdala modulates anxiety-like behaviors in social defeat exposed mice. Mol. Brain 2016, 9, 3. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Kimura, K.I.; Minami, R.; Yamahama, Y.; Hariyama, T.; Hosoda, N. Framework with cytoskeletal actin filaments forming insect footpad hairs inspires biomimetic adhesive device design. Commun. Biol. 2020, 3, 272. [Google Scholar] [CrossRef]
- Barra de la Tremblaye, P.; Plamondon, H. Alterations in the corticotropin-releasing hormone (CRH) neurocircuitry: Insights into post stroke functional impairments. Front. Neuroendocrinol. 2016, 42, 53–75. [Google Scholar] [CrossRef] [PubMed]
| Two-Way RM ANOVA | Sum Sq | Df | F | p | Significance | |||
| Group | 4040.128 | 5 | 7.0933 | p = 0.0000402 | *** | |||
| Time × CS | 9963.637 | 57 | ||||||
| Group | n | Mean | SEM | t | Df | Cohen’s d | Welch Test | Significance |
| A-B (B-24h) CS− | 5 | 0.440 | 0.440 | 2.566 | 18 | 1.147 | p = 0.0274 | * |
| A-B (B-24h) CS+ | 15 | 6.436 | 2.207 | |||||
| Group | n | Mean | SEM | t | Df | Cohen’s d | Paired t-Test | Significance |
| ABB (B-3h) CS− | 5 | 1.373 | 0.621 | 0.2730 | 4 | 0.2070 | p = 0.798 | N.S. |
| ABB (B-24h) CS− | 5 | 1.680 | 0.843 | |||||
| ABB (B-3h) CS+ | 14 | 23.805 | 4.373 | 2.7170 | 13 | 0.970 | p = 0.0176 | * |
| ABB (B-24h) CS+ | 14 | 11.0095 | 2.767 | |||||
| Group | n | Mean | t | Tukey’s Test | Significance | |||
| A-B in B 24 h (CS+) | 15 | 6.436 | ||||||
| vs. ABB in B 3 h (CS+) | 14 | 11.00952 | 1.153 | p = 0.833 | N.S. | |||
| vs. ABB in B 24 h (CS+) | 14 | 23.805 | 4.379 | p = 0.000688 | *** | |||
| ABB in B 3 h (CS−) | 5 | 1.373 | 2.7330 | p = 0.0478 | * | |||
| ABB in B 3 h (CS+) | 14 | 11.00952 | ||||||
| ABB in B 24 h (CS−) | 5 | 1.680 | 3.9789 | p = 0.00245 | ** | |||
| ABB in B 24 h (CS+) | 14 | 23.805 | ||||||
| Two-Way RM ANOVA | Sum Sq | Df | F Value | p Value | Significance | |||
| Group | 12,587.407 | 5 | 7.602 | p = 0.0000212 | *** | |||
| Time × CS | 40,989.471 | 113 | ||||||
| Group | n | Mean | SEM | t | Df | Cohen’s d Test | Paired t-Test | Significance |
| Control CS (−) B-3h | 6 | 0.567 | 0.394 | 2.260 | 5 | 1.161 | p = 0.733 | N.S. |
| Control CS (−) B-24h | 6 | 3.683 | 1.651 | |||||
| Control CS (+) B-3h | 13 | 13.964 | 3.661 | 3.0222 | 12 | 0.737 | p = 0.0106 | * |
| Control CS (+) B-24h | 13 | 25.185 | 5.019 | |||||
| Hy-Crf-KD CS (−) B-3h | 6 | 0.700 | 0.492 | 1.510 | 5 | 0.951 | p = 0.191 | N.S. |
| Hy-Crf-KD CS (−) B-24h | 6 | 5.367 | 3.064 | |||||
| Hy-Crf-KD CS (+) B-3h | 11 | 10.509 | 2.397 | 4.467 | 10 | 1.557 | p = 0.0012 | ** |
| Hy-Crf-KD CS (+) B-24h | 11 | 37.182 | 7.274 | |||||
| Hy-CRF-OE CS (−) B-3h | 6 | 0.807 | 0.175 | 1.207 | 5 | 0.767 | p = 0.282 | N.S. |
| Hy-CRF-OE CS (−) B-24h | 6 | 4.183 | 2.776 | |||||
| Hy-CRF-OE CS (+) B-3h | 15 | 26.280 | 5.058 | 0.827 | 14 | 0.245 | p = 0.422 | N.S. |
| Hy-CRF-OE CS (+) B-24h | 15 | 30.818 | 4.848 | |||||
| Group | n | Mean | t | Tukey’s Test | Significance | |||
| Control CS (−) B-3h | 6 | 0.189 | 2.536 | p = 0.0316 | * | |||
| Control CS (+) B-3h | 13 | 13.964 | ||||||
| Hy-Crf-KD CS (−) B-3h | 6 | 0.7000 | 1.806 | p = 0.0446 | * | |||
| Hy-Crf-KD CS (+) B-3h | 11 | 10.509 | ||||||
| Hy-CRF-OE CS (−) B-3h | 6 | 0.807 | 4.926 | p = 0.0000565 | *** | |||
| Hy-CRF-OE CS (+) B-3h | 15 | 26.280 | ||||||
| Control CS (−) B-24h | 6 | 4.350 | 3.944 | p = 0.00204 | ** | |||
| Control CS (+) B-24h | 13 | 25.185 | ||||||
| Hy-Crf-KD CS (−) B-24h | 6 | 5.367 | 5.856 | p = 0.00000206 | *** | |||
| Hy-Crf-KD CS (+) B-24h | 11 | 37.182 | ||||||
| Hy-CRF-OE CS (−) B-24h | 6 | 4.183 | 5.151 | p = 0.0000240 | *** | |||
| Hy-CRF-OE CS (+) B-24h | 15 | 30.818 | ||||||
| Control CS (+) B-3h | 13 | 13.964 | 0.788 | p = 0.965 | NS | |||
| Hy-Crf-KD CS (+) B-3h | 11 | 10.509 | ||||||
| Control CS (+) B-3h | 13 | 13.964 | 3.0364 | p = 0.0333 | * | |||
| Hy-CRF-OE CS (+) B-3h | 15 | 26.280 | ||||||
| Hy-Crf-KD CS (+) B-3h | 11 | 10.509 | 3.712 | p = 0.00441 | ** | |||
| Hy-CRF-OE CS (+) B-3h | 15 | 26.280 | ||||||
| Control CS (+) B-24h | 13 | 25.185 | 2.736 | p = 0.0726 | NS | |||
| Hy-Crf-KD CS (+) B-24h | 11 | 37.182 | ||||||
| Control CS (+) B-24h | 13 | 25.185 | 1.389 | p = 0.715 | NS | |||
| Hy-CRF-OE CS (+) B-24h | 15 | 30.818 | ||||||
| Hy-Crf-KD CS (+) B-24h | 11 | 37.182 | 1.498 | p = 0.646 | NS | |||
| Hy-CRF-OE CS (+) B-24h | 15 | 30.818 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasama, E.; Moriya, M.; Kamimura, R.; Matsuki, T.; Seki, K. Formation of False Context Fear Memory Is Regulated by Hypothalamic Corticotropin-Releasing Factor in Mice. Int. J. Mol. Sci. 2022, 23, 6286. https://doi.org/10.3390/ijms23116286
Kasama E, Moriya M, Kamimura R, Matsuki T, Seki K. Formation of False Context Fear Memory Is Regulated by Hypothalamic Corticotropin-Releasing Factor in Mice. International Journal of Molecular Sciences. 2022; 23(11):6286. https://doi.org/10.3390/ijms23116286
Chicago/Turabian StyleKasama, Emi, Miho Moriya, Ryuma Kamimura, Tohru Matsuki, and Kenjiro Seki. 2022. "Formation of False Context Fear Memory Is Regulated by Hypothalamic Corticotropin-Releasing Factor in Mice" International Journal of Molecular Sciences 23, no. 11: 6286. https://doi.org/10.3390/ijms23116286
APA StyleKasama, E., Moriya, M., Kamimura, R., Matsuki, T., & Seki, K. (2022). Formation of False Context Fear Memory Is Regulated by Hypothalamic Corticotropin-Releasing Factor in Mice. International Journal of Molecular Sciences, 23(11), 6286. https://doi.org/10.3390/ijms23116286






