Abstract
Lipids are not only constituents of cellular membranes, but they are also key signaling mediators, thus acting as “bioactive lipids”. Among the prominent roles exerted by bioactive lipids are immune regulation, inflammation, and maintenance of homeostasis. Accumulated evidence indicates the existence of a bidirectional relationship between the immune and nervous systems, and lipids can interact particularly with the aggregation and propagation of many pathogenic proteins that are well-renowned hallmarks of several neurodegenerative disorders, including Alzheimer’s (AD) and Parkinson’s (PD) diseases. In this review, we summarize the current knowledge about the presence and quantification of the main classes of endogenous bioactive lipids, namely glycerophospholipids/sphingolipids, classical eicosanoids, pro-resolving lipid mediators, and endocannabinoids, in AD and PD patients, as well as their most-used animal models, by means of lipidomic analyses, advocating for these lipid mediators as powerful biomarkers of pathology, diagnosis, and progression, as well as predictors of response or activity to different current therapies for these neurodegenerative diseases.
1. Bioactive Lipids: Main Families and Their Members
Although lipids are the major constituents of the cell membranes of all living organisms and the most efficient source of energy [1], they also act as intercellular and intracellular signaling mediators that bear many cell functions upon binding to specific G protein-coupled receptors (GPCRs) and, for this reason, are termed “bioactive lipids” [2]. Importantly, the formation and functions of these molecules strictly rely on the prevalence of omega-6 or omega-3 polyunsaturated fatty acid (PUFA) precursors and, as such, can depend on diet or be modified by supplementation. The main families of bioactive lipids are classical eicosanoids, glycerophospholipids, and sphingolipids, specialized pro-resolving mediators (SPMs), and endocannabinoids (eCBs) [3,4], as displayed in Figure 1.
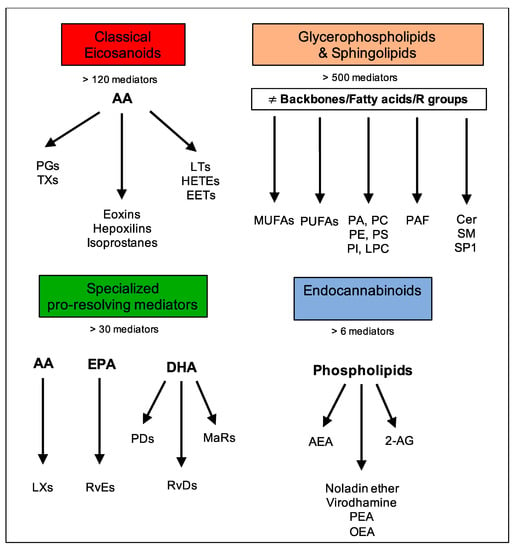
Figure 1.
Main families of bioactive lipids and their mediators.
Classical eicosanoids, mostly derived from the omega-6 polyunsaturated fatty acid (PUFA) arachidonic acid (AA), are the most renowned superfamily of bioactive lipids, comprising more than 120 known mediators. These include leukotrienes (LTs), prostaglandins (PGs), thromboxanes (TXs), hydroxyeicosatetraenoic acids (HETEs), epoxides (EETs), eoxins, and hepoxilins. These are involved in immune and inflammatory processes, with the aims of amplifying inflammation, coordinating leukocyte recruitment, producing cytokines and chemokines as well as presenting antigens and forming antibodies, inducing cell proliferation and migration [3].
Glycerophospholipids and sphingolipids comprise several compounds with great molecular diversity (e.g., phosphoinositides, phosphatidic acids, phospholipids, sphingosines, and ceramides) and with glycerol or sphingosine as respective backbones to which two fatty acids and a phosphoric acid are attached as esters [5]. The presence of an additional group attached to the phosphate allows for many different phosphoglycerides. Among the fatty acids esterified to carbon-1 and -2 (respectively denoted as the sn-1 and sn-2 positions), both saturated and unsaturated ones can occur. Typically, saturated fatty acids or monounsaturated fatty acids (MUFAs) are present at the sn-1 position, and PUFAs, such as 20:4 (AA), 22:6 (docosahexaenoic acid, DHA), and 20:5 (eicosapentaenoic acid, EPA), are present at the sn-2 position of phospholipids. This superfamily exerts pleiotropic effects, including inflammation, vesicular trafficking, endocytosis, cell cycle, cell migration and survival, as well as apoptosis and senescence [6].
The third superfamily is a relatively new class of bioactive lipids called SPMs which were identified in the laboratory of Prof. Charles Serhan. SPMs comprise over 30 different mediators that are actively synthesized during acute inflammation, either from omega-6 AA or omega-3 PUFAs, such as EPA and DHA, and they include AA-derived lipoxins (LXs); EPA-derived resolvins (RvEs); and DHA-derived resolvins (RvDs), protectins (PDs), and maresins (MaRs). They all act as immunoresolvents; that is, they stimulate the cardinal signs of the resolution of inflammation: removal, relief, restoration, regeneration, and remission [3,7,8].
The family of eCBs is a group of bioactive lipids that are able to activate type-1 and type-2 cannabinoid receptors (CB1 and CB2) and include a few lipid mediators such as N-arachidonoylethanolamine (commonly known as anandamide, AEA) and 2-arachidonoylglycerol (2-AG), as well as eCB-like 2-AG-ether (noladin ether), O-arachidonoylethanolamine (virodhamine), N-palmitoylethanolamine (PEA), and N-oleoylethanolamine (OEA). These molecules are produced ubiquitously by all tissues, and they serve as a homeostatic system to control several pathophysiological states and maintain human health [9].
It has been established that dysregulation of the bioactive lipid network can foster neuroinflammation and contribute to the etiopathogenesis, severity, and outcomes of many neurodegenerative and neuroinflammatory disorders. In this review, we will discuss the current literature on the quantification of the different superfamilies of bioactive lipids in the tissues and body fluids of patients affected by Alzheimer’s disease (AD) and Parkinson’s disease (PD), or their respective animal models, by the golden standard methodology of liquid chromatography or gas chromatography–mass spectrometry or mass spectrometry imaging.
2. Lipids and Alzheimer’s Disease
It is now evident that alterations in the brain lipid content and the composition of lipids and cerebral lipid peroxidation by genetic and environmental factors, such as apolipoprotein and lipid transporter carrying status, are key determinants for AD pathology. As a matter of fact, many studies using cell culture and transgenic animals have investigated the potential mechanisms by which ApoE4 is implicated in the pathogenesis of Alzheimer’s disease, including studies that investigated alterations in lipid metabolism, causing the inhibition of neurite extension [10]. Moreover, amyloidogenesis, an important pathogenetic factor for AD, is strictly associated with lipid composition within membrane lipid rafts, which are characterized by a combination of sphingolipids, cholesterol, saturated FAs, and a reduced content of PUFAs that serve as platforms for β-amyloid (Aβ) interactions with ApoE and tau to promote the aggregation of Aβ oligomers and hyperphosphorylation [11]. In addition, cortical and free unsaturated FAs induce the assembly of amyloid and tau filaments in vitro [12]. Over the last 30 years, all of the families of bioactive lipids have been analyzed in AD by lipidomics and will be described here and summarized in Table 1.

Table 1.
Bioactive lipid evaluation in Alzheimer disease by lipidomics.
2.1. Glycerophospholipids and Sphingolipids
Abnormal phospholipid composition characterizes the brain of AD patients and mainly affects the cortex area. In the brain of AD patients, phosphatidylcholine (PC) and phosphatidylethanolamine (PE), the two most predominant phospholipids, were significantly decreased, and phospholipid diacylation products glycerophosphocholine were increased in the frontal, primary auditory, and parietal cortices [13]. Three PCs were found significantly diminished in AD: PC (16:0/20:5), PC (16:0/22:6), and PC (18:0/22:6) [14]. Mapstone et al. conducted a 5-year, observational study in healthy, elderly patients and identified ten metabolites, comprising seven PCs, one lysophosphatidylcholine, and two acylcarnitines, that were depleted in the plasma of patients with mild cognitive impairment (MCI) or AD, and the depletion could identify (with accuracy above 90%) cognitively normal individuals who, on average, will convert to MCI or AD within 2–3 years [15]. Although most studies reported a reduction of PC levels in AD, contradictory findings have also been reported. Proitsi et al. found that the lipids most strongly associated with AD were PC 40:4 and PC 36:3, both of which were increased in AD [16]. An increase in CSF PC was observed in AD brains, as compared to control brains. During normal aging, the plasma levels of lysophosphatidylcholine, choline plasmalogen, and lyso-PAF increase significantly; similar but more pronounced changes in these choline-containing phospholipids were observed in AD patients [58].
Cardiolipin is another glycerophospholipid, mainly found in the inner mitochondrial membrane, and responsible for the maintenance of the fluidity and activity of mitochondrial electron transport chain enzymes. A reduction of cardiolipin in the synaptic mitochondrial membranes was reported in the brains of AD patients [59].
Concerning sphingolipids, several studies report variations of these subtype of lipids although there is some inconsistency among them. Levels of sphingomyelins (SMs) and ceramides were studied in two different post-mortem brain regions of patients with AD. The region with extensive Aβ has increased ceramide and decreased sphingomyelin, while in the region with only diffuse Aβ deposits, the ceramide/sphingomyelin ratio was reversed [17]. In the white matter, no differences in sphingomyelin between AD and healthy subjects were found, but significant decreases in ceramide C16:0, C22:0, and C24:1 were observed [18]. Additionally, metabolomic assays reported increased SM levels in the brain tissue of AD subjects, and these were associated with the severity of AD pathology and an increased risk of abnormal cognition, including three SMs with acyl residue (SM C16:0, SM C16:1, and SM C18:1) and one hydroxysphingomyelin with acyl residue (SM (OH) C14:1) [19]. These findings came as support of former evidence in a mouse model of AD, where the intracerebral injection of Aβ promoted the catalysis of sphingomyelin to ceramide by hydrolysis and thus an increase in ceramide levels [60].
Lipidomic studies found increased ceramide levels in AD brains, in particular, ceramides Cer16, Cer18, Cer20, and Cer24 [20]. Senile plaques were abundant in saturated ceramides Cer(d18:1/18:0) and Cer(d18:1/20:0) [61]. Increased levels of ceramides were also found in the CSF [21]. Han et al. observed an enhancement of ceramide in the early AD stages in brain tissue lipid extracts by electrospray ionization mass spectrometry, while its concentration reduced with disease severity [22]. So far, few studies have measured glycerophospholipids and sphingolipids in the serum of AD patients. In particular, only three specific ceramides (containing C16:0, C20:0, and stearoyl as fatty acids) were increased, indicating that they are important predictors of cell damage and memory impairment [23]. The major metabolic product derived from ceramide is Sphingosine 1-Phosphate, and its level was decreased in the AD brain [24,62].
Over the past 15 years, it has been useful to analyze the concentration of lipids in CSF to better understand levels of brain impairment. A research team in Pasadena, California has described the presence of lipid-rich nanoparticles (NPs) in the cerebrospinal fluid of older adults [25]. During LC/MS analysis of the CSF fraction, they revealed that the NPs had a higher glycerophospholipid (PC, PE, PS, and LPS) levels than the supernatant fluid in AD patients [25]. Specifically, a subsequent study revealed that the NPs fraction composition showed increased levels of two odd-numbered MUFAs (C15:1 and C19:1) [63]. The CSF levels of sphingomyelins and phosphatidylcholine-containing MUFAs significantly correlated with p-tau levels, suggesting that this family of bioactive lipids might be associated with disease severity [64].
In search of biomarkers for the non-clinical recognition of AD stages, a method was suggested to distinguish cognitively healthy individuals with normal (CH-NAT) from pathological Aβ42/tau (CH-PAT) and AD. This study revealed that phosphatidylcholine molecular species from the supernatant fraction of CH-PAT were higher than in the CH-NAT AD participants. Furthermore, sphingomyelin levels in the supernatant fraction were lower in the CH-PAT and AD than in the CH-NAT group. The decrease in sphingomyelin corresponded with an increase in ceramide and dihydroceramide and an increase in the ceramide to sphingomyelin ratio in AD. In contrast with the supernatant fraction, sphingomyelin was higher in the nanoparticle fraction from the CH-PAT group, accompanied by lower ceramide and dihydroceramide and a decrease in the ratio of ceramide to sphingomyelin in CH-PAT, as compared with CH-NAT [26]. Other types of sphingolipids, such as sulfatides and gangliosides, although being reported to significantly vary in AD, were not the focus of the present review since they are more constituent of myelin or lipid rafts than actual bioactive lipids.
Since both glycerophospholipids and sphingolipids respectively have two or one fatty acid chains attached to the molecule of glycerol or sphingosine, a great part of literature has focused on measuring levels of FAs. The brain is highly enriched in the PUFAs DHA (22:6n-3) and AA (20:4n-6), and linoleic acid, EPA and DHA account for ~10% of brain lipids.
Patients with MCI and AD had elevated levels of AA, but they had reduced levels of its precursor, linoleic acid (LA), as compared with healthy controls, with the latter progressively decreasing in cognitive performance [27]. Oleic acid (OA), an omega-9 FA and the most abundant dietary FA, was decreased in the frontal cortex and hippocampus of AD brains [28]. Furthermore, a case-control study performed on 148 AD patients reported significantly lower serum levels of DHA, and this reduction was associated with the severity of clinical dementia [65]. A more complete study analyzed the level of FFAs in the serum of AD patients and found that several of them significantly decreased when compared to the control; the study included 3 saturated fatty acids (C14:0, C16:0, and C18:0) and 6 unsaturated fatty acids (C16:1, C18:1, C18:2, γ-C18:3, C20:2, and C22:6). The serum level of C18:3 was significantly higher in AD patients [30]. In an elegant study by Martin et al., lipid composition was analyzed in lipid rafts of human frontal brain cortex obtained between 3- and 18-h post-mortem, revealing that lipid rafts from AD brains exhibit aberrant lipid profiles compared to healthy brains. In particular, lower levels of omega-3 long-chain PUFAs (mainly DHA) and oleic acid were observed [31].
Studies testing six unsaturated FAs, including linoleic acid (LA), AA, α-linolenic acid (ALA), DHA, EPA, and OA, showed that all these unsaturated FAs were positively associated with neuritic plaques and neurofibrillary tangle burdens, and they were negatively correlated with cognitive performance. In brain regions vulnerable to AD pathology—the middle frontal and inferior temporal gyri, there were decrements in LA, ALA, and AA, and increases in DHA [32]. All these unsaturated FAs can directly interact with Aβ40 and Aβ42 peptides, and they display excellent anti-aggregation properties by preventing amyloid fibril formation, especially OA and DHA [66]. However, when investigating the role of different unsaturated FAs in modulation of neuroprotective α-secretase-cleaved soluble APP (sAPPα) secretion and cell membrane fluidity, only AA, EPA, and DHA with four or more double bonds are capable of increasing membranous fluidity and sAPPα secretion, whereas stearic acid (SA, 18:0), LA, ALA, and OA cannot [67].
Among all PUFAs, DHA is assuredly the most investigated in AD, and since the end of the 1990s, many studies have concurred that a reduction of this lipid in several tissues [10,28] suggests a key role in AD pathology. AD patients have decreased DHA levels throughout their brains, including the disease-resistant regions, but the most prominent reduction occurs in the hippocampus, and DHA content has been found to show a positive correlation with dementia [33]. Livers from AD patients also contain lower levels of DHA but higher levels of short-chain n-3 precursors, including tetracosahexaenoic acid, suggesting a defect in its bioconversion into DHA [34]. However, some studies reported no significant changes in DHA levels between erythrocytes or the brain tissue of AD and control subjects [68,69].
In contrast, a very recent study with 2 years of follow-up reported that DHA is a strong protective factor for cognitive decline, inasmuch as AD patients who had stable cognitive impairment for 2 years resulted in higher baseline serum DHA levels than patients with declining AD [70].
2.2. Classical Eicosanoids
Most of the eicosanoids derived from arachidonic acid, i.e., PGs, LTs, HETEs and EETs, have been detected and associated with AD, as elegantly reviewed by Biringer [40] and reported by other studies that analyzed their oxidation products [71] or revealed specific increases in 12-HETE in plasma and brain and decreases in 15-HETE and PGD2 during the early phase [35]. An eicosanoid known to be a specific marker of in vivo lipid peroxidation is isoprostane-F2a, which is present in elevated concentrations in the CSF of AD [36] and in plasma, too [37,72]. Although plasma levels of F2a-isoprostanes do not qualify as robust biomarkers for AD diagnosis as in the CSF, even if plasma measurements may still have value in clinical trials. A hypothetical classification system for AD diagnosis is based on CSF, Aβ42, and tau levels, which allowed the improvement of the diagnostic accuracy for AD. For discriminating AD from non-AD, the level of CSF F2-IsoP needs to be greater than 25 pg/mL [38]. However, in another study, it was found that peripheral F2A and F4-Neuroprostatene levels in urine and plasma are not increased in AD [73,74].
The alteration of neurotransmitters, lipids, and sterol metabolism are the pathways impaired in the CSF of AD patients. The major metabolites altered are prostaglandins (PGG2 and PGJ2), hydrocortisone, and tetrahydrocortisone [39]. In a recent work on the CSF of AD patients, the levels of both pro-inflammatory and pro-resolving LMs seem to be associated with cognitive dysfunction [50].
Although analyses of leukotrienes in CSF have been performed since the 1980s, no significant alteration in the context of AD is known. The levels of PGs in post-mortem brains revealed regional differences in the patterns of PG profiles between the parieto-occipital cortex and the other cortical areas; only TBX2 and PGD2 were greater than in controls [41]; specifically, PGD2 was significantly increased in the frontal cortex [40], and PGE2 and PGF2 were reduced [29].
Regarding those fluid samples more easily accessible from the patient, such as CSF, plasma, and urine, the levels of eicosanoids were significantly different from those of control subjects. Many studies observed alterations in the levels of diacylglycerols, prostaglandins, and phospholipids, which can be related to oxidative stress and membrane breakdown.
Elevations in serum prostaglandin levels are important markers of oxidative stress [75]. PGE2 concentrations in CSF were approximately 5 times higher, and 6-keto-PGF1a levels were 4-fold less than those of control subjects [42]. In contrast, the detection of PGF2a, PGD2, and TXB2 did not reveal differences between AD and the control. Subsequently, a study investigated the correlation between PGE2 and cognitive scores, and they found that PGE2 levels were higher when learning scores were just below the normal range (early Alzheimer’s disease), but declined with progressive learning impairment [39,43,76]. These findings support the hypothesis that inflammatory processes predominate in early AD.
In urinary samples, the concentrations of F2-isoprostanes, PGF2 and 8-isoPGF2, significantly increased in AD patients when compared to those of the healthy subjects [44]. Thromboxane (TX), the last class of eicosanoids, did not seem to change in plasma or CS; instead, urinary samples with higher concentrations of 11-dehydro-TXB2 were reported in AD patients, as compared to healthy controls [77]. Concerning this lipid class, an Alzheimer’s Disease Anti-inflammatory Prevention Trial (ADAPT) analyzed the urine and plasma of participants to determine whether treatments for disease produced serious, adverse cardiovascular (CV) events, and an association between high urine Tx-M/PGI-M ratios and CV events was observed [78].
2.3. Specialized Pro-Resolving Mediators
Despite the relatively recent discovery of this superfamily of bioactive lipids, which is constantly flourishing and up-to-date counts include more than 30 members, measurement and quantification of SPMs in AD started as early as 2005 with the work by the group of Prof. Nicolas Bazan, when the levels in post-mortem AD brain tissues of the DHA-derived neuroprotectin D1 (NPD1) were found to be reduced in the post-mortem hippocampal CA1 region, but not in the thalamus or occipital lobes of AD patients, as compared to age-matched controls [45]. After this study, the reduction of NPD1 levels during the AD course have been corroborated in the hippocampus of the 3xTg mouse model of AD [46]. Although only two studies used the less-standardized enzyme immune assay (EIA) developed for a few SPMs by Cayman, whereby LXA4, RvD2, and RvE1 were reduced in the brain or hippocampus of 3xTg mice and 5xFAD mice, respectively [79,80], the first complete lipidomic profiling was undertaken in 2015 by the group of Prof. Serhan. This study found a reduction of RvD1 in CSF but not in the hippocampus, and it found decreased levels of MaR1 in the hippocampus in post-mortem human AD brains [47]. The same group conducted randomized, double-blind, and placebo-controlled clinical trial on AD patients—the OmegAD study—in which a placebo or a supplement of 1.7 g DHA and 0.6 g EPA was taken daily for 6 months. After the treatment, RvD1 and LXA4 levels were measured by EIA on the peripheral blood mononuclear cells (PBMCs), and their levels remained unchanged, suggesting that omega-3 supplementation prevented a reduction in SPMs [81]. A similar result was obtained in another clinical trial in human patients affected by mild cognitive impairment (MCI) and pre-MCI conditions, whereby the supplementation with omega-3 fatty acids and antioxidants led to increased levels of RvD1 in macrophage cultures isolated from PBMCs, as quantified by EIA [82]. The search for SPM measurements as potential biomarkers of AD pathology was then performed on a brain area different from the previously analyzed hippocampus, namely the entorhinal cortex, an anatomical region of importance for memory which is affected early in AD pathogenesis. In this study, several SPMs were significantly reduced as compared to healthy controls, such as PD1, MaR1, and RvD5, while LXA4, LXB4, RvD1, RvD2, RvE1, and RvE2 did not show relevant changes [48]. Subsequently, SPMs were analyzed in 3 different mouse models of AD: Fat-1 mice, Tg2576 mice, and APP/PS1/SphK1 mice. As expected, these studies led to contrasting results, and several SPMs were undetectable in the brain [83], increased in the plasma [84]—in both cases following a DHA-enriched diet, or reduced in neuronal cells [49].
Recently, the first lipidomic profiling of cerebrospinal fluid in AD was undertaken to simultaneously analyze both SPMs and classical eicosanoids in patients with cognitive impairment, ranging from subjective impairment to a diagnosis of AD, and correlated to cognition, CSF tau, and β-amyloid. In this study, RvD4, RvD1, PD1, MaR1, and RvE4 were lower in AD and/or MCI, as compared to patients with spinal cord injuries. Furthermore, levels of RvD1 showed a negative correlation with p-tau levels, while RvD4 negatively correlated with AD tangle biomarkers, and positive correlations with cognitive test scores were observed for both SPMs and their precursor fatty acids [50].
2.4. Endocannabinoids
Despite eCBs having been discovered in the early 1990s, very few reports have measured their levels in AD patients. The first report was in 2006, but that was in a rat model of AD injected with Aβ that reported an increase in the hippocampal levels of 2-AG, but not AEA [51]. This was confirmed 10 years later in the more-commonly used APP/PS1 transgenic mouse model of AD, in which 2-AG levels were increased in the cortex although that occurred upon inhibition of its degrading enzyme, monoacylglycerol lipase (MAGL) [52]. The first LC-MS/MS study on eCBs levels on AD patients was not performed until 2009. Here, no differences in the plasma levels of 2-AG and AEA were found between AD patients and healthy controls. However, only 2-AG—but not AEA—was detected in the CSF of these patients, and its levels, although they did not correlate with cognitive performance in healthy controls at risk for AD, showed an inverse correlation with TNF-alpha [85]. Moreover, 2-AG levels were found to be increased in blood samples of AD patients, which nonetheless showed no differences in other eCBs, such as AEA, PEA, or OEA [53].
LC-MS/MS analyses of the brain areas of post-mortem AD patients revealed no variations in 2-AG or PEA, but there was a significant reduction in AEA and its precursor 1-stearoyl,2-docosahexaenoyl-sn-glycerophosphoethanolamine-N-arachidonoyl (NArPE) in midfrontal and temporal cortex tissue, but not in the cerebellum [54]. Over the following 5 years, levels of eCBs were only investigated in murine models of AD. A metabolomic brain profiling of PS1/APP AD mice was performed and revealed an increase in both AEA and 2-AG, as compared to their wild-type littermates, especially for 2-AG in mice that also carried a genetic deficiency of MAGL [55]. A similar result was obtained in another genetic model of AD, where 2-AG increased in the brain of 5XFAD upon administration of a potent MAGL inhibitor [56]. Another study on the AβPPswe/PS1ΔE9 mouse model quantified eCB levels and assessed lipidomic profiles in the frontal cortex, hippocampus, and striatum tissues to determine whether regional variations would be observed with age and disease progression. This study showed age-related increased levels of AEA, PEA, and OEA in the hippocampal and frontal cortex tissues of both AD and control mice, but the hippocampi of the former had higher concentrations of AEA than in those of the latter, while in the striatum, lower levels of 2-AG have been reported [57]. More recently, a new study quantified the AEA and 2-AG in the human plasma of AD patients by means of a novel, selective column-switching ultra-high performance liquid chromatography–tandem mass spectrometry (UHPLC–MS/MS) method, allowing for a faster approach with fewer steps [86]. Although the values for both AEA and 2-AG agreed with data previously reported in the literature, their concentration ranges were smaller.
3. Lipids and Parkinson’s Disease
One of the neuropathophysiological hallmarks of PD is characterized by the accumulation of α-synuclein (α-syn) and the formation of filamentous aggregates called “Lewy bodies” in the brainstem, limbic system, and cortical areas, leading to a progressive loss of dopaminergic neurons in the substantia nigra and striatum, and thus motor dysfunction [87]. Although the etiology of PD is generally unknown, the formation of α-syn aggregates seems to be closely associated to an altered lipid metabolism. Indeed, despite the fact that the physiological role of α-syn is yet not fully understood, it appears that its accumulation and aggregation are either enhanced by membrane lipid composition or they further cause alterations in lipid homeostasis [88]. Furthermore, genome-wide screening studies in yeast support the involvement of lipid metabolism in α-syn toxicity, with many genes associated with lipid metabolism, modifying toxicity, and vesicle-mediated transport [89]. Additionally, many known PD-risk genes, such as glucocerebrosidase (GBA), leucine-rich repeat kinase 2 (LRRK2), and parkin (PARK2), show a clear association with lipid metabolism, and particularly lipid accumulation [90,91,92]. Modern lipidomic approaches further underline the significant roles of lipids, and particularly dysfunctions in lipid metabolism, in the pathogenesis of PD, which are discussed in this review and summarized in Table 2.

Table 2.
Bioactive lipids evaluation in Parkinson’s disease by lipidomics.
3.1. Glycerophospholipids and Sphingolipids
Similar to AD, PD changes in the lipid membrane composition are strictly associated with its pathology, and superfamilies of several bioactive lipids seem to be involved in the pathogenesis of PD [133,134].
One prominent gene involved in the pathogenesis of PD is the GBA gene, which encodes for the β-glucocerebrosidase enzyme, which hydrolyzes glucosylceramide. Mutations in this gene are found in up to 10% of patients with PD, thus implicating aberrant sphingolipid metabolism [135]. Indeed, the CSF lipidome signatures of Parkinson’s patients at early stages, de novo PD patients with abnormal dopamine transporters, and healthy controls show distinct lipidome changes, with a significant increase in glucosylceramide (GlcCer), while sphingomyelin (SM) was significantly reduced in GBA-PD patients [93]. Furthermore, applying the ratio of GlcCer to SM for stratifying cases of idiopathic PD revealed that patients with a high GlcCer/SM ratio present increased cognitive deterioration compared to those in the lowest quartiles. However, other studies did not find any significant accumulation of GluCer in the CSF of PD subjects [136]. Of note, PD patients with neuropathic and dyskinetic pain had higher plasma levels of GlcCer compared to PD patients with no sensory pain [116].
Interestingly, plasma ceramide lipidomics linked cognitive impairments among PD patients with higher levels of the plasma ceramides C16:0, C18:0, C20:0, C22:0, and C24:1, and the monohexosylceramide species C16:0, C20:0, and C24:0 [94]. Thus, plasma ceramide and monohexosylceramide metabolism seem to be altered in PD and also in non-GBA mutation carriers, with higher levels being accompanied by worse cognition. These findings were confirmed by shotgun lipidomics of L444PGBA mutation fibroblasts, an ex vivo PD system that presents a mutation of the GBA gene, showing distinct, increased proportions of ceramides and hexosylceramide, while total phospholipids were significantly decreased [95]. Intriguingly, isolated membrane lipids from PD-GBA cultures proved to be more potent triggers of recombinant human α-syn in vitro fibrillation compared to lipids from healthy controls [95]. LRRK2 is another important gene for the development of PD, and it has been implicated in a variety of pathways, including mitochondrial dynamics. LRRK2 is mutated in families with autosomal-dominantly inherited PD, and it has been acknowledged as a susceptibility factor for PD [137]. Although in vivo models of LRRK−/− show an intact dopaminergic system [138], targeted lipidomics approaches identified altered sphingolipid composition in LRRK2−/− mouse brains, with significantly increased levels of Cer in LRRK2−/− mice and direct alterations in GBA1 enzymatic activity [96].
Furthermore, new machine learning analysis has been introduced to link lipidome data of PD patients to the course of disease severity by using whole blood lipidomics and an established lipid panel containing dihydrosphingomyelin (dhSM), GlcCer, dihydro globotriaosylceramide (dhGB3), and dihydro GM3 ganglioside (dhGM3). [139]. In addition, untargeted lipidome assessments were utilized to detect incipient dementia in PD. Lipidome analysis by isotope-standard-assisted liquid chromatography of PD patients’ serum revealed that an association with patients transitioning to the development of dementia could be characterized by a 5-lipid biomarker panel [97].
A multi-omic integration analysis on 30 drug-naive, de novo PD patients and 30 matched controls revealed that a longitudinal trajectory with 3 long-chain fatty acids (FA) (FA C14:0, FA C17:1, and FA C20:1) and dopaminergic medication exerted a strong change in lipidomic signature, with a prevalence of phospholipid species and breakdown products of phospholipids [98].
The substantia nigra forms the major spot of interest in PD research, as this brain area is characterized by the loss of dopaminergic neurons, resulting in the clinical manifestation of PD. However, information regarding the lipid alterations in established PD animal models is still scarce. HPLC-ESI-MS/MS measurements of the substantia nigra from rats 21 days after an infusion of 20 µg of 6-OHDA, or a saline vehicle into the anterior dorsal striatum, displayed a relative down-regulated abundance of several PC species, while known neuroinflammatory signaling lipids, namely lysophosphotidylcholine (16:0 and 18:1), were up-regulated [99].
In post-mortem human substantia nigra samples, 5 lipid species have shown different abundances between PD patients and controls, namely Bis (Monoacylglycerol) Phosphate (BMP) 42:8, PC 36:3, PE A36:2, PI 42:10, and PS 36:3, with BMP and PI showing a significant upregulation in PD samples [100]. The same study also showed a saturated sphingomyelin species depletion in the putamen of PD patients. Post-mortem-acquired sphingolipidome data of the caudate, putamen, and globus pallidus revealed altered levels of sphingolipid species, including ceramides (Cer), dihydroceramides (DHC), hydoxyceramides (OH-Cer), phytoceramides (Phyto-Cer), and phosphoethanolamine ceramides (PE-Cer) in the PD subjects compared to healthy controls. The putamen showed the strongest effect of depletion of Cer and Cer-OH, while sphingomyelin levels remained largely unchanged across the basal ganglia of both PD and control samples [101]. These results are in line with prior studies reporting unchanged total glucosylceramide levels in the putamen and cerebellum in both GBA-PD and sporadic PD brain samples [140]. However, other studies have reported decreased levels of SM in the anterior cingulate cortex [102] and increased levels in the substantia nigra [103], while lipidome analysis of the visual cortex, a brain area that does not show any formation of Lewy bodies in PD, showed increased overall sphingolipid levels [104]. Applied lipidomics on the Lewy bodies in the post-mortem mesencephalon and corpus callosum of PD patients with dementia confirmed a high cell-membrane-related lipid content within the Lewy bodies, with the mass spectra showing strong peaks corresponding to SM and PC for positive immunostained α-syn inclusions isolated from both the SN and hippocampal CA2 sector [141]. However, the profile of SM/PC does not seem to be specific for LB.
It is important to note that, while post-mortem data acquired from PD human brain samples show distinct changes in lipid profiles, post-mortem-acquired parkinsonian CSF seems to present increased lipid levels of almost the complete profile of PC and the majority of CM and SM [105]. This effect might result from the breakdown of the blood–brain–barrier and the subsequent influx of non-CSF lipids into the CSF, thus potentially leading to an impaired lipid homeostasis.
Although most lipidomics approaches in PD are focused on the CSF and blood, lipidomics have also been introduced for the examination of other biological substances and tissues, such as the sebum or skin fibroblasts. Sebum is an oil-like substance produced by the sebaceous glands and is predominantly composed of triglycerides, fatty acids, wax esters, squalene, and cholesterol. LC-MS analyzation of sebum samples, including drug-naïve PD, medicated PD, and healthy controls, revealed an enrichment of the sphingolipid metabolism in both drug-naïve and medicated PD [106]. Lipid dysregulation in the sphingolipid metabolism in the sebum of PD patients reflected an overall lipid dysregulation. Additionally, lipid profiling of parkin-mutant, human skin fibroblasts also found these lysophophatidylcholine species to be upregulated [107]. Also, while a majority of the presented lipidomic data were acquired by mass spectrometry, different lipidomic approaches involving lipoprotein profiling by the implementation of NMR spectroscopy, bundled with multivariate data pre-processing, show promising results in discriminating between early-stage PD and PD-related dementia [142].
3.2. Classical Eicosanoids
Higher PUFA levels were detected in the supernatants and high-speed membrane fractions of neuronal cells over-expressing wild-type or PD-causing mutant α-syn. This increased PUFA content in the membrane fraction was accompanied by increased membrane fluidity in the α-syn overexpressing neurons. Membrane fluidity and the levels of certain PUFAs were lower in the brains of mice that have had α-syn genetically deleted [108]. Furthermore, lipid rafts were purified from human frontal cortex from the normal, early motor stages of PD and incidental PD subjects, and the lipid composition was analyzed. Lipid classes were separated from a fraction of total lipid by one-dimensional, double-development, high-performance, thin-layer chromatography. The results show that lipid rafts from both cohorts of patients exhibited dramatic reductions in their contents of omega-3 and omega-6 long-chain PUFAs, especially DHA and AA [109].
Metabolomic approaches using UPLS-MS measured the plasma levels of 158 fatty-acid metabolites in a cohort including 42 PD patients and 54 healthy volunteers. Results showed an increase in 2 eicosanoids (arachidonic acid and 13-hydroxy-octadecatrienoic acid) and a decrease in 11 eicosanoids (DHA, lyso-platelet-activating factor, 12-hydroxy-eicosatetraenoic acid, dihydroxy-eicosatrienoic acids, dihidroxy-octadecenoic acids, 17,18-dihydroxy-eicosatetraenoic acid, and hydroperoxy-octadecadienoic acids) in PD patients compared to healthy controls [110]. A similar study identified LTB3 as a potential biomarker for PD [111].
Among PGs, PGE2 is the most associated with PD, and several lipidomic studies identified this eicosanoid in PD patients. Indeed, PGE2 levels were increased in the substantia nigra [143] and the CSF of PD patients with mild cognitive impairment and dementia although, in the first study, it was measured by enzyme immunoassay, whereas in the second study, its levels were not associated with elevated total-tau, phosphorylated-tau, or other inflammatory mediators [144]. In an α-syn model of PD, among all prostaglandins assayed in the brain (PGE2, PGD2, and PGF2α), PGD2 showed the greatest increase (two-fold) compared to wild-type animals [112]. Furthermore, analysis of brain extracts by electrospray ionization–Fourier transform resonance mass spectrometry (ESI–FT-ICR-MS) revealed an increase in two different prostaglandins (PGB1 and PGH2) and in 15(S)-HETE in brain extracts of a Parkinson-like, rat model chronically exposed to manganese [113].
Other eicosanoid members, such as isoprostanes and HETEs, were also identified in PD. Indeed, the levels of plasma F2-isoprostanes (F2-IsoPs), neuroprostanes (F4-NPs), and HETEs were found to be higher in PD patients compared to controls, especially in the early stages of PD. In the same study, a significant negative correlation between the cumulative intake of L-DOPA and plasma total HETEs was also observed [114]. Changes in PGE2 and PGD2 levels were corroborated in other models of PD. Indeed, while mice carrying the mutated disease gene DJ-1 showed reduced PGE2 and PGD2 levels [145,146], LPS-injected mice displayed increased PGE2 levels [147,148], and MPTP-injected mice presented enhanced striatal and nigral levels of LTB4 [149]. However, 6-OHDA-treated rats showed lower PGE2 levels 4 weeks after treatment compared to controls [150]. Of note, in these studies, eicosanoids were always measured with the commercially available enzyme immunoassay kits from Cayman, and not by lipidomics.
Additionally, LC/MS analysis in rotenone-treated rats showed a significant reduction of oxidizable PUFA-containing phospholipid cardiolipin species and increased contents of mono-oxygenated CL species in later stages of exposure in the substantia nigra. Notably, linoleic acid in the sn-1 position was the major oxidation substrate yielding its mono-hydroxy- and epoxy-derivatives, whereas more readily “oxidizable” fatty-acid residues, AA and DHA, remained non-oxidized in the substantia nigra. On the other hand, elevated levels of PUFA CLs were detected in plasma [115].
3.3. Specialized Pro-Resolving Mediators
As for this superfamily of bioactive lipids, so far, only 1 study from our group measured 2 specific SPMs, i.e., RvD1 and RvD2, in both the plasma and CSF of the transgenic and over-expressing α-syn (Syn) rat model of PD, as well as in early-PD patients. In particular, although no changes were observed in RvD2 levels, the content of RvD1 was higher in 2-month-old Syn rats and progressively decreased in 4-month-old and 18-month-old rats compared to their wild-type littermates, suggesting an impairment in the production of this SPM along disease progression, starting at an early phase when neuroinflammatory, synaptic, and motor-behavioral dysfunctions started to appear. Early-onset PD patients also showed strong reductions in RvD1 levels in both plasma and CSF [128]. However, the measurement of RvD1 and RvD2 were performed by commercially available EIA kits from Cayman, and so far, no study has ever attempted to measure all of the other SPMs through targeted-lipidomics analysis.
3.4. Endocannabinoids
Studies on the quantification of eCBs in PD are numerous. The very first report of eCB analysis in PD came from the group of Prof. di Marzo, in which AEA and 2-AG were measured in two regions of the basal ganglia in the reserpine-treated rat model of PD, namely the globus pallidus and the substantia nigra. Although the levels of AEA in these anatomical regions were three-fold higher than those previously reported in any other brain region, only 2-AG showed a strong increase in the globus pallidus compared to control animals [117]. In another rat model of PD, induced by a unilateral nigral lesion with 6-hydroxydopamine (6-OHDA), the levels of AEA were increased in the striatum 6-OHDA-lesioned rats compared to control animals, whereas endogenous 2-AG was unaffected. The authors suggested that this was attributable to decreased AEA degradation, rather than an increased synthesis [118]. This was supported by similar findings observed by the group of Prof. Maccarrone, in which, once again, only AEA striatal levels were increased in 6-OHDA, and these were reversed upon chronic L-DOPA treatment [119]. In the same year, another study investigated the effect of L-DOPA on eCB levels in 6-OHDA rats and found that systemic administration of L-DOPA increased anandamide concentrations in the basal ganglia via activation of dopamine D1/D2 receptors, whereas its levels were significantly reduced in the caudate–putamen ipsilateral to the lesion. These results indicate that a deficiency in eCB transmission may contribute to levodopa-induced dyskinesias [120]. However, a similar study reported years later that both striatal AEA and 2-AG were reduced in these 6-OHDA rats upon L-DOPA administration [121], suggesting that further studies on other LID models and human samples are still required to validate the mechanism of endocannabinoid in LID.
A more detailed L-DOPA treatment was undertaken in the MPTP-lesioned, non-human primate model of PD, where cynomolgus monkeys were either acutely treated (non-dyskinetic) or long-term treated with L-DOPA (levodopa-induced dyskinesia, LID). In the untreated, MPTP-lesioned primates, parkinsonism was associated with increases in both 2-AG and AEA in the striatum, and in only 2-AG in the substantia nigra. Increased levels of AEA in the external globus pallidus of MPTP-lesioned animals were normalized by L-DOPA treatment and may contribute to the generation of parkinsonian symptoms. However, no evident alterations in eCB levels were associated with the expression of LID [122]. Levels of 2-AG were also found to be increased in MPTP-lesioned mice [123]. Following MPTP administration, levels of 2-AG in the ventral midbrain started to increase 2 days, and up to 4 days, after MPTP injection and returned close to control levels after 7 days, whereas in the striatum, 2-AG levels were reduced after 7 days [124]. Thus, changes in 2-AG were time- and region-specific following MPTP administration, indicating that eCBs represent a natural defense mechanism against inflammation. Subsequently in neurotoxic and inflammation-driven rat models of PD with striatal injections of 4 different toxins/inflammatory stimuli (6-OHDA, LPS, rotenone, and Poly I:C), not only AEA and 2-AG, but also other eCB-like members, such as PEA and OEA, were measured for the first time. Interestingly, while LPS caused significant increases in all 4 eCBs at specific time-points, 6-OHDA was only able to induce PEA and OEA, but not AEA or 2-AG, in the striatum [125]. On the other hand, rotenone injection led to an increase in the striatal levels of AEA, PEA, and OEA, while viral stimulus Poly I:C induced PEA and OEA, but not AEA or 2-AG, albeit at specific time-points [126]. These findings suggest that changes in eCB levels may be functionally important in inflammation-driven neurodegeneration, rather than in direct neurotoxicity per se. Despite the fact that changes in eCB levels were indeed observed in several neurotoxic models of PD, no variation in AEA or 2-AG was observed in the striatum of PINK-deficient mice, a gene involved in early-onset PD [129]. However, in yet another PD animal model overexpressing α-syn, eCBs not only were significantly reduced (especially 2-AG and OEA) but such reduction was manifested not at an early stage, but at an advanced stage, suggesting that α-syn overexpression triggers a dysregulation of eCB biosynthesis [127].
The very first report on eCB measurement in PD patients was performed in 2005 by the group of Maccarrone in the CSF of 16 patients at different stages of disease, from de novo PD diagnosis to early-mild or advanced, and all had undergone a complete drug washout before CSF examination. AEA levels showed a 2-fold increase in PD patients compared to control subjects, and this increase was independent of the stage of disease [151]. The same group extended the study in PD patients to include subjects undergoing drug withdrawal and those under pharmacological therapy. In untreated patients AEA levels were more than doubled as compared to controls, and the levels were restored following chronic dopaminergic replacement [152,153]. Based on these studies, the authors suggested that the increase in AEA might reflect a compensatory mechanism occurring in the striatum of PD patients, aimed at normalizing chronic dopamine depletion. Plasma and CSF levels of eCBs were also measured in PD patients, with and without LID, and LC-MS/MS analysis showed elevated CSF levels of AEA and decreased CSF and plasma levels of 2-AG, as compared to healthy controls, and these changes were curiously observed only in patients without LID [130]. Of note, AEA levels were reduced in PD patients with ongoing pain and showed a linear relationship with pain intensity and sensory losses [116].
More recently, several groups focused on developing novel mass-spectrometry approaches to measure eCB in PD patients or animal tissues. For instance, a study described the development and validation of an ultra-high performance liquid chromatography–tandem mass spectrometry method that uses disposable pipette extraction (DPX-UHPLC–MS/MS), and this novel approach was successfully applied to determine AEA in CSF samples of PD patients, providing a simpler, faster, and highly sensitive procedure that requires smaller CSF sample and organic solvent volumes [131]. The same group developed 2 additional simple and reliable methods to determine AEA and 2-AG levels in the brain hemispheres of 6-OHDA rats: (i) by micro salting-out assisted liquid–liquid extraction combined with ultra-high performance liquid chromatography–tandem mass spectrometry (SALLE/UHPLC-MS/MS) [154] with no significant matrix effect, and inter- and intra-assay precision and accuracy with CV and RSE values lower than 15%, respectively; and (ii) by in-tube solid-phase microextraction (in-tube SPME) directly coupled to a tandem mass spectrometry (MS/MS). This showed similar advantages to the first approach, but the selectivity of the in-tube SPME and MS/MS (MRM mode) techniques allowed them to be directly coupled online, which dismissed the need for the chromatographic separation step [132]. Due to space limitations, studies measuring eCB levels in different models of PD upon the pharmacological or genetic modulation of their respective degrading enzymes are not discussed here.
4. Concluding Remarks
Changes in the lipidomic profiles of the different superfamilies of bioactive lipids are apparent in biological fluids, such as plasma and CSF, as well in specific anatomical brain regions that are associated with the pathological hallmarks of both AD and PD. With the exception of SPMs in Parkinson disease, where no targeted-based lipidomic has ever been performed, it is now clear that dysregulated lipid homeostasis in several members of glycerophospholipids/sphingolipids, classical eicosanoids, SPMs and endocannabinoids (only for AD) contributes greatly to the pathogenesis and even progression of these neurodegenerative diseases. Since each superfamily of these bioactive lipids has a distinct role in either sustaining the neuroinflammatory and degenerative processes or in reducing them, it is important that future studies apply integrated multi-omic approaches that will be able to simultaneously measure all of them in the same sample in order to have a clear metabolic fingerprint and profile of the inflammatory status of each patient. This is undermined by the limited availability of human samples, and novel mass-spectrometry implementations, which can reduce the amount of a sample needed to few microliters to detect all lipid mediators, will be crucial.
In most cases, the detection of certain lipid mediators in plasma or CSF could be potentially useful for monitoring the diagnosis, progression, and prognosis of the disease. However, confounding factors form a major issue in lipidomics analysis with gender-, gut-microbiota-, and diet-induced alterations in lipid composition affecting the identification of disease- and therapy-specific lipidome signatures. Most importantly, modern mass spectrometry technologies provide quantitative readouts, but many studies do not report absolute lipid concentrations and differ vastly in methodology, even in regard to lipid extraction, workflow, and data presentation. Hence, a network of lipidomic standards is highly encouraged in order to minimize interlaboratory differences and to prove its clinical use [155].
Author Contributions
V.C. designed the manuscript. V.C., M.T., A.M., F.F. and H.S. wrote the manuscript. S.S., N.B.M. and G.S. edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Italian Ministry of Health grant (RF-2018-12365509) to N.B.M. and V.C.
Conflicts of Interest
The authors declare no conflict of interest.
List of Abbreviations
| 2-AG | 2-Arachidonoylglycerol |
| 6-OHDA | 6-Hydroxydopamine |
| AA | Arachidonic Acid |
| Aβ | β-Amyloid |
| AD | Alzheimer’s Disease |
| ADAPT | Alzheimer’s Disease Anti-Inflammatory Prevention Trial |
| AEA | Anandamide |
| ALA | α-Linolenic Acid |
| APCI | Atmospheric pressure chemical ionization |
| APPα | Neuroprotective α-Secretase-Cleaved Soluble APP |
| ApoE | Apolipoprotein E |
| α-syn | α-synuclein |
| BMP | Bis (Monoacylglycero) Phosphate |
| eCBs | Endocannabinoids |
| CB | Cannabinoid Receptor |
| CSF | Cerebrospinal Fluid |
| CH-NAT | Cognitively Healthy Individuals With Normal Aβ42/Tau |
| CH-PAT | Cognitively Healthy Individuals With Pathological Aβ42/Tau |
| DHA | Docosahexaenoic Acid |
| DHC | Dihydroceramides |
| DPX | Disposable Pipette Extraction |
| EETs | Epoxides |
| EI | Electron impact |
| EIA | Enzyme Immune Assay |
| EPA | Eicosapentaenoic Acid |
| ESI | Electrospray Ionization |
| FAs | Saturated Fatty Acid |
| FT | Fourier Transform |
| F2-IsoPs | F2-Isoprostanes |
| F4-NPs | Neuroprostanes |
| GBA | Glucocerebrosidase |
| GC | Gas Chromatography |
| Β-GBA | Β-Glucocerebrosidase Enzyme |
| dhGB3 | Dihydro Globotriaosylceramide |
| dhGM3 | Dihydro GM3 Ganglioside |
| dhSM | Dihydrosphingomyelin |
| GlcCer | Glucosylceramide |
| GPCRs | G Protein-Coupled Receptors |
| HETEs | Hydroxyeicosatetraenoic Acids |
| HILIC | Hydrophilic Liquid Interaction Chromatography |
| HPLC | High-Performance Liquid Chromatography |
| HR | High Resolution |
| ICR | Ion Cyclotron Resonance |
| LA | Linoleic Acid |
| LC | Liquid Chromatography |
| L-DOPA | Levodopa |
| LC | Liquid Chromatography |
| LID | Levodopa-Induced Dyskinesia |
| LIF | Laser-Induced Fluorescence |
| LPS | Lipopolysaccharides |
| LRRK2 | Leucine-Rich Repeat Kinase 2 |
| LTs | Leukotrienes |
| LXA4 | Lipoxin A4 |
| LXB4 | Lipoxin B4 |
| LXs | AA-Derived Lipoxins |
| MAGL | Monoacylglycerol Lipase |
| MALDI | Matrix-Assisted Laser Desorption Ionization |
| MaRs | Maresins |
| MCI | Mild Cognitive Impairment |
| MPTP | 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine |
| MRM | Multiple Reaction Monitoring |
| MS/MS | Tandem Mass Spectrometry |
| MUFAs | Monounsaturated Fatty Acids |
| NArPE | 1-Stearoyl,2-Docosahexaenoyl-Sn-Glycerophosphoethanolamine-N-Arachidonoyl |
| NMR | Nuclear Magnetic Resonance |
| NPs | Lipid-Rich Nanoparticles |
| NPD1 | Derived Neuroprotectin D1 |
| OA | Oleic Acid |
| OEA | N-Oleoylethanolamine |
| OH-Cer | Hydoxyceramides |
| PBMCs | Peripheral Blood Mononuclear Cells |
| PC | Phosphatidylcholine |
| PD | Parkinson’s Disease |
| PDA | Photodiode Array |
| PDs | Protectins |
| PE | Phosphatidylethanolamine |
| PEA | N-Palmitoylethanolamine |
| PE-Cer | Phosphoethanolamine Ceramides |
| PUFA | Polyunsaturated Fatty Acid |
| PGs | Prostaglandins |
| Phyto-Cer | Phytoceramides |
| RP | Reverse-Phase |
| RSE | Relative Standard Error |
| RvDs | D-Series Resolvins |
| RvEs | E-Series Resolvins |
| SMs | Sphingomyelins |
| SM (OH) | Hydroxysphingomyelin |
| SPMs | Specialized Pro-Resolving Mediators |
| SPME | Solid-Phase Microextraction |
| TLC | Thin-Layer Chromatography |
| TNF-alpha | Tumor Necrosis Factor alpha |
| TOF-MS | Time-of-Flight Mass Spectrometry |
| TXs | Thromboxanes |
| UHPLC | Ultra-High Performance Liquid Chromatography |
| UPLC | Ultra-High-Pressure Liquid |
References
- Vigh, L.; Escribá, P.V.; Sonnleitner, A.; Sonnleitner, M.; Piotto, S.; Maresca, B.; Horváth, I.; Harwood, J.L. The significance of lipid composition for membrane activity: New concepts and ways of assessing function. Prog. Lipid Res. 2005, 44, 303–344. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T. Lipid mediators in health and disease: Enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 123–150. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiù, V.; Maccarrone, M. Bioactive lipids as modulators of immunity, inflammation and emotions. Curr. Opin. Pharmacol. 2016, 29, 54–62. [Google Scholar] [CrossRef]
- Chiurchiù, V.; Leuti, A.; Maccarrone, M. Bioactive Lipids and Chronic Inflammation: Managing the Fire within. Front. Immunol. 2018, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A.; Obeid, L.M. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef] [PubMed]
- El Alwani, M.; Wu, B.X.; Obeid, L.M.; Hannun, Y.A. Bioactive sphingolipids in the modulation of the inflammatory response. Pharmacol. Ther. 2006, 112, 171–183. [Google Scholar] [CrossRef]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef]
- Basil, M.C.; Levy, B.D. Specialized pro-resolving mediators: Endogenous regulators of infection and inflammation. Nat. Rev. Immunol. 2016, 16, 51–67. [Google Scholar] [CrossRef]
- Chiurchiù, V. Endocannabinoids and Immunity. Cannabis. Cannabinoid. Res. 2016, 1, 59–66. [Google Scholar] [CrossRef]
- Kao, Y.C.; Ho, P.C.; Tu, Y.K.; Jou, I.M.; Tsai, K.J. Lipids and Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 1505. [Google Scholar] [CrossRef]
- Kawarabayashi, T.; Shoji, M.; Younkin, L.H.; Wen-Lang, L.; Dickson, D.W.; Murakami, T.; Matsubara, E.; Abe, K.; Ashe, K.H.; Younkin, S.G. Dimeric amyloid beta protein rapidly accumulates in lipid rafts followed by apolipoprotein E and phosphorylated tau accumulation in the Tg2576 mouse model of Alzheimer’s disease. J. Neurosci. 2004, 24, 3801–3809. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.M.; Binder, L.I. Free fatty acids stimulate the polymerization of tau and amyloid beta peptides. In vitro evidence for a common effector of pathogenesis in Alzheimer’s disease. Am. J. Pathol. 1997, 150, 2181–2195. [Google Scholar] [PubMed]
- Nitsch, R.M.; Blusztajn, J.K.; Pittas, A.G.; Slack, B.E.; Growdon, J.H.; Wurtman, R.J. Evidence for a membrane defect in Alzheimer disease brain. Proc. Natl. Acad. Sci. USA 1992, 89, 1671–1675. [Google Scholar] [CrossRef] [PubMed]
- Whiley, L.; Sen, A.; Heaton, J.; Proitsi, P.; García-Gómez, D.; Leung, R.; Smith, N.; Thambisetty, M.; Kloszewska, I.; Mecocci, P.; et al. Evidence of altered phosphatidylcholine metabolism in Alzheimer’s disease. Neurobiol. Aging 2014, 35, 271–278. [Google Scholar] [CrossRef]
- Mapstone, M.; Cheema, A.K.; Fiandaca, M.S.; Zhong, X.; Mhyre, T.R.; MacArthur, L.H.; Hall, W.J.; Fisher, S.G.; Peterson, D.R.; Haley, J.M.; et al. Plasma phospholipids identify antecedent memory impairment in older adults. Nat. Med. 2014, 20, 415. [Google Scholar] [CrossRef]
- Proitsi, P.; Kim, M.; Whiley, L.; Simmons, A.; Sattlecker, M.; Velayudhan, L.; Lupton, M.K.; Soininen, H.; Kloszewska, I.; Mecocci, P.; et al. Association of blood lipids with Alzheimer’s disease: A comprehensive lipidomics analysis. Alzheimer’s Dement. 2017, 13, 140–151. [Google Scholar] [CrossRef]
- Cutler, R.G.; Kelly, J.; Storie, K.; Pedersen, W.A.; Tammara, A.; Hatanpaa, K.; Troncoso, J.C.; Mattson, M.P. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2004, 101, 2070–2075. [Google Scholar] [CrossRef]
- Barbash, S.; Garfinkel, B.P.; Maoz, R.; Simchovitz, A.; Nadorp, B.; Guffanti, A.; Bennett, E.R.; Nadeau, C.; Türk, A.; Paul, L.; et al. Alzheimer’s brains show inter-related changes in RNA and lipid metabolism. Neurobiol. Dis. 2017, 106, 1–13. [Google Scholar] [CrossRef]
- Varma, V.R.; Oommen, A.M.; Varma, S.; Casanova, R.; An, Y.; Andrews, R.M.; O’Brien, R.; Pletnikova, O.; Troncoso, J.C.; Toledo, J.; et al. Brain and blood metabolite signatures of pathology and progression in Alzheimer disease: A targeted metabolomics study. PLoS Med. 2018, 15, e1002482. [Google Scholar] [CrossRef]
- Filippov, V.; Song, M.A.; Zhang, K.; Vinters, H.V.; Tung, S.; Kirsch, W.M.; Yang, J.; Duerksen-Hughes, P.J. Increased ceramide in brains with Alzheimer’s and other neurodegenerative diseases. J. Alzheimer’s Dis. 2012, 29, 537–547. [Google Scholar] [CrossRef]
- Satoi, H.; Tomimoto, H.; Ohtani, R.; Kitano, T.; Kondo, T.; Watanabe, M.; Oka, N.; Akiguchi, I.; Furuya, S.; Hirabayashi, Y.; et al. Astroglial expression of ceramide in Alzheimer’s disease brains: A role during neuronal apoptosis. Neuroscience 2005, 130, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Han, X.D.M.H.; McKeel, D.W., Jr.; Kelley, J.; Morris, J.C. Substantial sulfatide deficiency and ceramide elevation in very early Alzheimer’s disease: Potential role in disease pathogenesis. J. Neurochem. 2002, 82, 809–818. [Google Scholar] [CrossRef]
- Mielke, M.M.; Bandaru, V.V.; Haughey, N.J.; Rabins, P.V.; Lyketsos, C.G.; Carlson, M.C. Serum sphingomyelins and ceramides are early predictors of memory impairment. Neurobiol. Aging 2010, 31, 17–24. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Huang, Y.; Li, B.; Gong, C.X.; Schuchman, E.H. Deregulation of sphingolipid metabolism in Alzheimer’s disease. Neurobiol. Aging 2010, 31, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Harrington, M.G.; Fonteh, A.N.; Oborina, E.; Liao, P.; Cowan, R.P.; McComb, G.; Chavez, J.N.; Rush, J.; Biringer, R.G.; Hühmer, A.F. The morphology and biochemistry of nanostructures provide evidence for synthesis and signaling functions in human cerebrospinal fluid. Cerebrospinal. Fluid Res. 2009, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Fonteh, A.N.; Chiang, A.J.; Arakaki, X.; Edminster, S.P.; Harrington, M.G. Accumulation of Cerebrospinal Fluid Glycerophospholipids and Sphingolipids in Cognitively Healthy Participants With Alzheimer’s Biomarkers Precedes Lipolysis in the Dementia Stage. Front. Neurosci. 2020, 14, 611393. [Google Scholar] [CrossRef] [PubMed]
- Iuliano, L.; Pacelli, A.; Ciacciarelli, M.; Zerbinati, C.; Fagioli, S.; Piras, F.; Orfei, M.D.; Bossu, P.; Pazzelli, F.; Serviddio, G.; et al. Plasma fatty acid lipidomics in amnestic mild cognitive impairment and Alzheimer’s disease. J. Alzheimer’s Dis. 2013, 36, 545–553. [Google Scholar] [CrossRef]
- Prasad, M.R.; Lovell, M.A.; Yatin, M.; Dhillon, H.; Markesbery, W.R. Regional membrane phospholipid alterations in Alzheimer’s disease. Neurochem. Res. 1998, 23, 81–88. [Google Scholar] [CrossRef]
- Wong, P.T.; McGeer, P.L.; McGeer, E.G. Decreased prostaglandin synthesis in postmortem cerebral cortex from patients with Alzheimer’s disease. Neurochem. Int. 1992, 21, 197–202. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, X.; Liu, L.; Xie, W.; Wu, Q.; Wang, D. Gas chromatography-mass spectrometry analysis of the free fatty acids in serum obtained from patients with Alzheimer’s disease. Biomed. Mater. Eng. 2015, 26 (Suppl. 1), 2165–2177. [Google Scholar]
- Martín, V.; Fabelo, N.; Santpere, G.; Puig, B.; Marín, R.; Ferrer, I.; Díaz, M. Lipid alterations in lipid rafts from Alzheimer’s disease human brain cortex. J. Alzheimer’s Dis. 2010, 19, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Snowden, S.G.; Ebshiana, A.A.; Hye, A.; An, Y.; Pletnikova, O.; O’Brien, R.; Troncoso, J.; Legido-Quigley, C.; Thambisetty, M. Association between fatty acid metabolism in the brain and Alzheimer disease neuropathology and cognitive performance: Anontargeted metabolomic study. PLoS Med. 2017, 14, e1002266. [Google Scholar] [CrossRef] [PubMed]
- Astarita, G.; Jung, K.M.; Berchtold, N.C.; Nguyen, V.Q.; Gillen, D.L.; Head, E.; Cotman, C.W.; Piomelli, D. Deficient liver biosynthesis of docosahexaenoic acid correlates with cognitive impairment in Alzheimer’s disease. PLoS ONE 2010, 5, e12538. [Google Scholar] [CrossRef] [PubMed]
- Astarita, G.; Piomelli, D. Towards a whole-body systems [multi-organ] lipidomics in Alzheimer’s disease. Leukot. Essent. Fat. Acids 2011, 85, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Tajima, Y.; Ishikawa, M.; Maekawa, K.; Murayama, M.; Senoo, Y.; Nishimaki-Mogami, T.; Nakanishi, H.; Ikeda, K.; Arita, M.; Taguchi, R.; et al. Lipidomic analysis of brain tissues and plasma in a mouse model expressing mutated human amyloid precursor protein/tau for Alzheimer’s disease. Lipids Health Dis. 2013, 12, 68. [Google Scholar] [CrossRef] [PubMed]
- Praticò, D.; Yao, Y.; Rokach, J.; Mayo, M.; Silverberg, G.G.; McGuire, D. Reduction of brain lipid peroxidation by CSF drainage in Alzheimer’s disease patients. J. Alzheimer’s Dis. 2004, 6, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Peña-Bautista, C.; López-Cuevas, R.; Cuevas, A.; Baquero, M.; Cháfer-Pericás, C. Lipid peroxidation biomarkers correlation with medial temporal atrophy in early Alzheimer Disease. Neurochem. Int. 2019, 129, 104519. [Google Scholar] [CrossRef]
- Montine, T.J.; Kaye, J.A.; Montine, K.S.; McFarland, L.; Morrow, J.D.; Quinn, J.F. Cerebrospinal fluid abeta42, tau, and f2-isoprostane concentrations in patients with Alzheimer disease, other dementias, and in age-matched controls. Arch. Pathol. Lab. Med. 2001, 125, 510–512. [Google Scholar] [CrossRef]
- Trushina, E.; Dutta, T.; Persson, X.M.; Mielke, M.M.; Petersen, R.C. Identification of altered metabolic pathways in plasma and CSF in mild cognitive impairment and Alzheimer’s disease using metabolomics. PLoS ONE 2013, 8, e63644. [Google Scholar] [CrossRef]
- Biringer, R.G. The Role of Eicosanoids in Alzheimer’s Disease. Int. J. Environ. Res. Public Health 2019, 16, 2560. [Google Scholar] [CrossRef]
- Iwamoto, N.; Kobayashi, K.; Kosaka, K. The formation of prostaglandins in the postmortem cerebral cortex of Alzheimer-type dementia patients. J. Neurol. 1989, 236, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Montine, T.J.; Sidell, K.R.; Crews, B.C.; Markesbery, W.R.; Marnett, L.J.; Roberts, L.J., 2nd; Morrow, J.D. Elevated CSF prostaglandin E2 levels in patients with probable AD. Neurology 1999, 53, 1495–1498. [Google Scholar] [CrossRef] [PubMed]
- Ferré-González, L.; Peña-Bautista, C.; Baquero, M.; Cháfer-Pericás, C. Assessment of Lipid Peroxidation in Alzheimer’s Disease Differential Diagnosis and Prognosis. Antioxidants 2022, 11, 551. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Jung, B.H.; Paeng, K.J.; Kim, I.; Chung, B.C. Increased urinary F(2)-isoprostanes levels in the patients with Alzheimer’s disease. Brain Res. Bull. 2004, 64, 47–51. [Google Scholar] [CrossRef]
- Lukiw, W.J. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J. Clin. Investig. 2005, 115, 2774–2783. [Google Scholar] [CrossRef]
- Zhao, Y.; Calon, F.; Julien, C.; Winkler, J.W.; Petasis, N.A.; Lukiw, W.J.; Bazan, N.G. Docosahexaenoic acid-derived neuroprotectin D1 induces neuronal survival via secretase- and PPARγ-mediated mechanisms in Alzheimer’s disease models. PLoS ONE 2011, 6, e15816. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, M.; Hjorth, E.; Cortés-Toro, V.; Eyjolfsdottir, H.; Graff, C.; Nennesmo, I.; Palmblad, J.; Eriksdotter, M.; Sambamurti, K.; et al. Resolution of inflammation is altered in Alzheimer’s disease. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2015, 11, 40–50. [Google Scholar]
- Zhu, M.; Wang, X.; Hjorth, E.; Colas, R.A.; Schroeder, L.; Granholm, A.C.; Serhan, C.N.; Schultzberg, M. Pro-Resolving Lipid Mediators Improve Neuronal Survival and Increase Aβ42 Phagocytosis. Mol. Neurobiol. 2016, 53, 2733–2749. [Google Scholar] [CrossRef]
- Lee, J.Y.; Han, S.H.; Park, M.H.; Baek, B.; Song, I.-S.; Choi, M.-K.; Takuwa, Y.; Ryu, H.; Kim, S.H.; He, X.; et al. Neuronal SphK1 acetylates COX2 and contributes to pathogenesis in a model of Alzheimer’s Disease. Nat. Commun. 2018, 9, 1479. [Google Scholar] [CrossRef]
- Do, K.V.; Hjorth, E.; Wang, Y.; Jun, B.; Kautzmann, M.I.; Ohshima, M.; Eriksdotter, M.; Schultzberg, M.; Bazan, N.G. Cerebrospinal Fluid Profile of Lipid Mediators in Alzheimer’s Disease. Cell Mol. Neurobiol. 2022. [Google Scholar] [CrossRef]
- van der Stelt, M.; Mazzola, C.; Esposito, G.; Matias, I.; Petrosino, S.; De Filippis, D.; Micale, V.; Steardo, L.; Drago, F.; Iuvone, T.; et al. Endocannabinoids and beta-amyloid-induced neurotoxicity in vivo: Effect of pharmacological elevation of endocannabinoid levels. Cell Mol. Life Sci. 2006, 63, 1410–1424. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Yun, Y.; Ku, T.; Li, G.; Sang, N. NO2 inhalation promotes Alzheimer’s disease-like progression: Cyclooxygenase-2-derived prostaglandin E2 modulation and monoacylglycerol lipase inhibition-targeted medication. Sci. Rep. 2016, 6, 22429. [Google Scholar] [CrossRef] [PubMed]
- Altamura, C.; Ventriglia, M.; Martini, M.G.; Montesano, D.; Errante, Y.; Piscitelli, F.; Scrascia, F.; Quattrocchi, C.; Palazzo, P.; Seccia, S.; et al. Elevation of Plasma 2-Arachidonoylglycerol Levels in Alzheimer’s Disease Patients as a Potential Protective Mechanism against Neurodegenerative Decline. J. Alzheimer’s Dis. 2015, 46, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.-M.; Astarita, G.; Yasar, S.; Vasilevko, V.; Cribbs, D.H.; Head, E.; Cotman, C.W.; Piomelli, D. An amyloid β42-dependent deficit in anandamide mobilization is associated with cognitive dysfunction in Alzheimer’s disease. Neurobiol. Aging 2012, 33, 1522–1532. [Google Scholar] [CrossRef] [PubMed]
- Piro, J.R.; Benjamin, D.I.; Duerr, J.M.; Pi, Y.; Gonzales, C.; Wood, K.M.; Schwartz, J.W.; Nomura, D.K.; Samad, T.A. A Dysregulated Endocannabinoid-Eicosanoid Network Supports Pathogenesis in a Mouse Model of Alzheimer’s Disease. Cell Rep. 2012, 1, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhang, J.; Wu, Y.; Wang, D.; Feng, G.; Tang, Y.-P.; Teng, Z.; Chen, C. Monoacylglycerol lipase is a therapeutic target for Alzheimer’s disease. Cell Rep. 2012, 2, 1329–1339. [Google Scholar] [CrossRef]
- Maroof, N.; Ravipati, S.; Pardon, M.C.; Barrett, D.A.; Kendall, D.A. Reductions in endocannabinoid levels and enhanced coupling of cannabinoid receptors in the striatum are accompanied by cognitive impairments in the AβPPswe/PS1ΔE9 mouse model of Alzheimer’s disease. J. Alzheimer’s Dis. JAD 2014, 42, 227–245. [Google Scholar] [CrossRef]
- Dorninger, F.; Moser, A.B.; Kou, J.; Wiesinger, C.; Forss-Petter, S.; Gleiss, A.; Hinterberger, M.; Jungwirth, S.; Fischer, P.; Berger, J.; et al. Alterations in the plasma levels of specific choline phospholipids in Alzheimer’s disease mimic accelerated aging. J. Alzheimer’s Dis. 2018, 62, 841–854. [Google Scholar] [CrossRef]
- Pettegrew, J.W.; Panchalingam, K.; Hamilton, R.L.; McClure, R.J. Brain membrane phospholipid alterations in Alzheimer’s disease. Neurochem. Res. 2001, 26, 771–782. [Google Scholar] [CrossRef]
- Alessenko, A.V.; Bugrova, A.E.; Dudnik, L.B. Connection of lipid peroxide oxidation with the sphingomyelin pathway in the development of Alzheimer’s disease. Biochem. Soc. Trans. 2004, 32 Pt 1, 144–146. [Google Scholar] [CrossRef]
- Panchal, M.; Gaudin, M.; Lazar, A.N.; Salvati, E.; Rivals, I.; Ayciriex, S.; Dauphinot, L.; Dargere, D.; Auzeil, N.; Masserini, M.; et al. Ceramides and sphingomyelinases in senile plaques. Neurobiol. Dis. 2014, 65, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, G.; Maddelein, M.L.; Pucelle, M.; Nicaise, Y.; Maurage, C.A.; Duyckaerts, C.; Cuvillier, O.; Delisle, M.B. Neuronal sphingosine kinase 2 subcellular localization is altered in Alzheimer’s disease brain. Acta Neuropathol. Commun. 2018, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Fonteh, A.N.; Cipolla, M.; Chiang, J.; Arakaki, X.; Harrington, M.G. Human cerebrospinal fluid fatty acid levels differ between supernatant fluid and brain-derived nanoparticle fractions, and are altered in Alzheimer’s disease. PLoS ONE 2014, 9, e100519. [Google Scholar] [CrossRef] [PubMed]
- Solomon, V.; Hafez, M.; Xian, H.; Harrington, M.; Fonteh, A.; Yassine, H. An Association Between Saturated Fatty Acid-Containing Phosphatidylcholine in Cerebrospinal Fluid with Tau Phosphorylation. J. Alzheimer’s Dis. 2022. Epub ahead of print. [Google Scholar] [CrossRef]
- Tully, A.M.; Roche, H.M.; Doyle, R.; Fallon, C.; Bruce, I.; Lawlor, B.; Coakley, D.; Gibney, M.J. Low serum cholesteryl ester-docosahexaenoic acid levels in Alzheimer’s disease: A case-control study. Br. J. Nutr. 2003, 89, 483–489. [Google Scholar] [CrossRef]
- El Shatshat, A.; Pham, A.T.; Rao, P.P.N. Interactions of polyunsaturated fatty acids with amyloid peptides Abeta40 and AbetaArch. Biochem. Biophys. 2019, 663, 34–43. [Google Scholar] [CrossRef]
- Yang, X.; Sheng, W.; Sun, G.Y.; Lee, J.C. Effects of fatty acid unsaturation numbers on membrane fluidity and alpha-secretase-dependent amyloid precursor protein processing. Neurochem. Int. 2011, 58, 321–329. [Google Scholar] [CrossRef]
- Kroger, E.; Verreault, R.; Carmichael, P.H.; Lindsay, J.; Julien, P.; Dewailly, E.; Ayotte, P.; Laurin, D. Omega-3 fatty acids and risk of dementia: The Canadian Study of Health and Aging. Am. J. Clin. Nutr. 2009, 90, 184–192. [Google Scholar] [CrossRef]
- Fraser, T.; Tayler, H.; Love, S. Fatty acid composition of frontal, temporal and parietal neocortex in the normal human brain and in Alzheimer’s disease. Neurochem. Res. 2010, 35, 503–513. [Google Scholar] [CrossRef]
- Chu, C.S.; Hung, C.F.; Ponnusamy, V.K.; Chen, K.C.; Chen, N.C. Higher Serum DHA and Slower Cognitive Decline in Patients with Alzheimer’s Disease: Two-Year Follow-Up. Nutrients 2022, 14, 1159. [Google Scholar] [CrossRef]
- Furman, R.; Lee, J.V.; Axelsen, P.H. Analysis of eicosanoid oxidation products in Alzheimer brain by LC-MS with uniformly 13C-labeled internal standards. Free Radic. Biol. Med. 2018, 118, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Irizarry, M.C.; Yao, Y.; Hyman, B.T.; Growdon, J.H.; Praticò, D. Plasma F2A isoprostane levels in Alzheimer’s and Parkinson’s disease. Neurodegener. Dis. 2007, 4, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Montine, T.J.; Quinn, J.F.; Milatovic, D.; Silbert, L.C.; Dang, T.; Sanchez, S.; Terry, E.; Roberts, L.J., 2nd; Kaye, J.A.; Morrow, J.D. Peripheral F2-isoprostanes and F4-neuroprostanes are not increased in Alzheimer’s disease. Ann. Neurol. 2002, 52, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.C.; Yen, H.C.; Huang, C.C.; Hsu, W.C.; Wei, H.J.; Lin, C.L. Cerebrospinal fluid biomarkers for neuropsychological symptoms in early stage of late-onset Alzheimer’s disease. Int. J. Neurosci. 2015, 125, 747–754. [Google Scholar] [CrossRef] [PubMed]
- González-Domínguez, R.; García-Barrera, T.; Gómez-Ariza, J.L. Metabolomic study of lipids in serum for biomarker discovery in Alzheimer’s disease using direct infusion mass spectrometry. J. Pharm. Biomed. Anal. 2014, 98, 321–326. [Google Scholar] [CrossRef]
- Combrinck, M.; Williams, J.; De Berardinis, M.; Warden, D.; Puopolo, M.; Smith, A.D.; Minghetti, L. Levels of CSF prostaglandin E2, cognitive decline, and survival in Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2006, 77, 85–88. [Google Scholar] [CrossRef]
- Ciabattoni, G.; Porreca, E.; Di Febbo, C.; Di Iorio, A.; Paganelli, R.; Bucciarelli, T.; Pescara, L.; Del Re, L.; Giusti, C.; Falco, A.; et al. Determinants of platelet activation in Alzheimer’s disease. Neurobiol. Aging 2007, 28, 336–342. [Google Scholar] [CrossRef]
- Montine, T.J.; Sonnen, J.A.; Milne, G.; Baker, L.D.; Breitner, J.C. Elevated ratio of urinary metabolites of thromboxane and prostacyclin is associated with adverse cardiovascular events in ADAPT. PLoS ONE 2010, 5, e9340. [Google Scholar] [CrossRef][Green Version]
- Dunn, H.C.; Ager, R.R.; Baglietto-Vargas, D.; Cheng, D.; Kitazawa, M.; Cribbs, D.H.; Medeiros, R. Restoration of Lipoxin A4 Signaling Reduces Alzheimer’s Disease-Like Pathology in the 3xTg-AD Mouse Model. J. Alzheimer’s Dis. 2014, 43, 893–903. [Google Scholar] [CrossRef]
- Kantarci, A.; Aytan, N.; Palaska, I.; Stephens, D.; Crabtree, L.; Benincasa, C.; Jenkins, B.G.; Carreras, I.; and Dedeoglu, A. Combined administration of resolvin E1 and lipoxin A4 resolves inflammation in a murine model of Alzheimer’s disease. Exp. Neurol. 2018, 300, 111–120. [Google Scholar] [CrossRef]
- Wang, X.; Hjorth, E.; Vedin, I.; Eriksdotter, M.; Freund-Levi, Y.; Wahlund, L.-O.; Cederholm, T.; Palmblad, J.; Schultzberg, M. Effects of n-3 FA supplementation on the release of proresolving lipid mediators by blood mononuclear cells: The OmegAD study. J. Lipid Res. 2015, 56, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Fiala, M.; Halder, R.C.; Sagong, B.; Ross, O.; Sayre, J.; Porter, V.; and Bredesen, D.E. ω-3 Supplementation increases amyloid-β phagocytosis and resolvin D1 in patients with minor cognitive impairment. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2015, 29, 2681–2689. [Google Scholar] [CrossRef] [PubMed]
- Hopperton, K.E.; Trépanier, M.-O.; James, N.C.E.; Chouinard-Watkins, R.; Bazinet, R.P. Fish oil feeding attenuates neuroinflammatory gene expression without concomitant changes in brain eicosanoids and docosanoids in a mouse model of Alzheimer’s disease. Brain Behav. Immun. 2018, 69, 74–90. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.; Mateo, V.; Amalric, N.; Babiak, M.; Béréziat, G.; Kanony-Truc, C.; Clerc, T.; Blaise, R.; Limon, I. Cerebrovascular β-amyloid deposition and associated microhemorrhages in a Tg2576 Alzheimer mouse model are reduced with a DHA-enriched diet. FASEB J. 2018, 32, 4972–4983. [Google Scholar] [CrossRef] [PubMed]
- Koppel, J.; Bradshaw, H.; Goldberg, T.E.; Khalili, H.; Marambaud, P.; Walker, M.J.; Pazos, M.; Gordon, M.L.; Christen, E.; Davies, P. Endocannabinoids in Alzheimer’s disease and their impact on normative cognitive performance: A case-control and cohort study. Lipids Health Dis. 2009, 8, 2. [Google Scholar] [CrossRef]
- Marchioni, C.; de Souza, I.D.; Grecco, C.F.; Crippa, J.A.; Tumas, V.; Queiroz, M.E.C. A column switching ultrahigh-performance liquid chromatography-tandem mass spectrometry method to determine anandamide and 2-arachidonoylglycerol in plasma samples. Anal. Bioanal. Chem. 2017, 409, 3587–3596. [Google Scholar] [CrossRef]
- Obeso, J.A.; Stamelou, M.; Goetz, C.G.; Poewe, W.; Lang, A.E.; Weintraub, D.; Burn, D.; Halliday, G.M.; Bezard, E.; Przedborski, S.; et al. Past, present, and future of Parkinson’s disease: A special essay on the 200th Anniversary of the Shaking Palsy. Mov. Disord. 2017, 32, 1264–1310. [Google Scholar] [CrossRef]
- Sarchione, A.; Marchand, A.; Taymans, J.-M.; Chartier-Harlin, M.-C. Alpha-Synuclein and Lipids: The Elephant in the Room? Cells 2021, 10, 2452. [Google Scholar] [CrossRef]
- Willingham, S.; Outeiro, T.F.; DeVit, M.J.; Lindquist, S.L.; Muchowski, P.J. Yeast genes that enhance the toxicity of a mutant huntingtin fragment or alpha-synuclein. Science 2003, 302, 1769–1772. [Google Scholar] [CrossRef]
- Aufschnaiter, A.; Kohler, V.; Diessl, J.; Peselj, C.; Carmona-Gutierrez, D.; Keller, W.; Büttner, S. Mitochondrial lipids in neurodegeneration. Cell Tissue Res. 2017, 367, 125–140. [Google Scholar] [CrossRef]
- Riboldi, G.M.; Di Fonzo, A.B. GBA, Gaucher Disease, and Parkinson’s Disease: From Genetic to Clinic to New Therapeutic Approaches. Cells 2019, 8, 364. [Google Scholar] [CrossRef] [PubMed]
- Fais, M.; Dore, A.; Galioto, M.; Galleri, G.; Crosio, C.; Iaccarino, C. Parkinson’s Disease-Related Genes and Lipid Alteration. Int. J. Mol. Sci. 2021, 22, 7630. [Google Scholar] [CrossRef] [PubMed]
- Huh, Y.E.; Park, H.; Chiang, M.S.R.; Tuncali, I.; Liu, G.; Locascio, J.J.; Shirvan, J.; Hutten, S.J.; Rotunno, M.; Scherzer, C.R.; et al. Glucosylceramide in cerebrospinal fluid of patients with GBA-associated and idiopathic Parkinson’s disease en-rolled in PPMI. NPJ Park. Dis. 2021, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- Mielke, M.M.; Maetzler, W.; Haughey, N.J.; Bandaru, V.R.; Savica, R.; Deutschle, C.; Gasser, T.; Hauser, A.H.; Grä-ber-Sultan, S.; Liepelt-Scarfone, I.; et al. Plasma Ceramide and Glucosylceramide Metabolism Is Altered in Sporadic Parkinson’s Disease and Associated with Cognitive Impairment: A Pilot Study. PLoS ONE 2013, 8, e73094. [Google Scholar] [CrossRef]
- Galvagnion, C.; Marlet, F.R.; Cerrie, S.; Schapira, A.H.V.; Blandini, F.; Di Monte, D.A. Sphingolipid changes in Parkinson L444P GBA mutation fibroblasts promote α-synuclein aggregation. Brain Awab. 2022, 371, 1038–1051. [Google Scholar] [CrossRef]
- Ferrazza, R.; Cogo, S.; Melrose, H.; Bubacco, L.; Greggio, E.; Guella, G.; Civiero, L.; Plotegher, N. LRRK2 deficiency im-pacts ceramide metabolism in brain. Biochem. Biophys. Res. Commun. 2016, 478, 1141–1146. [Google Scholar] [CrossRef]
- Zardini Buzatto, A.; Tatlay, J.; Bajwa, B.; Mung, D.; Camicioli, R.; Dixon, R.A.; Li, L. Comprehensive Serum Lipidomics for Detecting Incipient Dementia in Parkinson’s Disease. J. Proteome Res. 2021, 20, 4053–4067. [Google Scholar] [CrossRef]
- Hertel, J.; Harms, A.C.; Heinken, A.; Baldini, F.; Thinnes, C.C.; Glaab, E.; Vasco, D.A.; Pietzner, M.; Stewart, I.D.; Thiele, I.; et al. Integrated Analyses of Microbiome and Longitudinal Metabolome Data Reveal Microbial-Host Interactions on Sulfur Metabolism in Parkinson’s Disease. Cell Rep. 2019, 29, 1767–1777.e8. [Google Scholar] [CrossRef]
- Farmer, K.; Smith, C.A.; Hayley, S.; Smith, J. Major Alterations of Phosphatidylcholine and Lysophosphotidylcholine Lipids in the Substantia Nigra Using an Early Stage Model of Parkinson’s Disease. Int. J. Mol. Sci. 2015, 16, 18865–18877. [Google Scholar] [CrossRef]
- Xicoy, H.; Brouwers, J.F.; Wieringa, B.; Martens, G.J.M. Explorative Combined Lipid and Transcriptomic Profiling of Substantia Nigra and Putamen in Parkinson’s Disease. Cells 2020, 9, 1966. [Google Scholar] [CrossRef]
- Beger, A.W.; Dudzik, B.; Woltjer, R.L.; Wood, P.L. Human Brain Lipidomics: Pilot Analysis of the Basal Ganglia Sphingolipidome in Parkinson’s Disease and Lewy Body Disease. Metabolites 2022, 12, 187. [Google Scholar] [CrossRef] [PubMed]
- Abbott, S.K.; Li, H.; Sanz Munoz, S.; Knoch, B.; Batterham, M.; Murphy, K.E.; Halliday, G.M.; Garner, B. Altered ceramide acyl chain length and ceramide synthase gene expression in Parkinson’s disease. Mov. Disord. 2014, 29, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Seyfried, T.N.; Choi, H.; Chevalier, A.; Hogan, D.; Akgoc, Z.; Schneider, J.S. Sex-Related Abnormalities in Substantia Nigra Lipids in Parkinson’s Disease. ASN Neuro. 2018, 10, 1759091418781889. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Jenner, A.M.; Shui, G.; Cheong, W.F.; Mitchell, T.W.; Nealon, J.R.; Kim, W.S.; McCann, H.; Wenk, M.R.; Garner, B.; et al. Lipid pathway alterations in Parkinson’s disease primary visual cortex. PLoS ONE 2011, 6, e17299. [Google Scholar] [CrossRef]
- Fernández-Irigoyen, J.; Cartas-Cejudo, P.; Iruarrizaga-Lejarreta, M.; Santamaría, E. Alteration in the Cerebrospinal Fluid Lipidome in Parkinson’s Disease: A Post-Mortem Pilot Study. Biomedicines 2021, 9, 491. [Google Scholar] [CrossRef]
- Sinclair, E.; Trivedi, D.K.; Sarkar, D.; Walton-Doyle, C.; Milne, J.; Kunath, T.; Rijs, A.M.; de Bie, R.M.A.; Goodacre, R.; Barran, P.; et al. Metabolomics of sebum reveals lipid dysregulation in Parkinson’s disease. Nat. Commun. 2021, 12, 1592. [Google Scholar] [CrossRef]
- Lobasso, S.; Tanzarella, P.; Vergara, D.; Maffia, M.; Cocco, T.; Corcelli, A. Lipid profiling of parkin-mutant human skin fibroblasts. J. Cell. Physiol. 2017, 232, 3540–3551. [Google Scholar] [CrossRef]
- Sharon, R.; Bar-Joseph, I.; Mirick, G.E.; Serhan, C.N.; Selkoe, D.J. Altered Fatty Acid Composition of Dopaminergic Neurons Expressing alpha-synuclein and human brains with α-Synucleinopathies. J. Biol. Chem. 2003, 278, 49874–49881. [Google Scholar] [CrossRef]
- Fabelo, N.; Martín, V.; Santpere, G.; Marín, R.; Torrent, L.; Ferrer, I.; Díaz, M. Severe alterations in lipid composition of frontal cortex lipid rafts from Parkinson’s disease and incidental Parkinson’s disease. Mol. Med. 2011, 17, 1107–1118. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Zhang, L.; Chen, S.; Chen, Y.; Cai, C. Targeted fatty acid metabolomics to discover Parkinson’s disease associated metabolic alteration. J. Mass Spectrom. 2021, 56, e4781. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, C.; Zhao, N.; Li, W.; Yang, Z.; Liu, X.; Le, W.; Zhang, X. Potential biomarkers of Parkinson’s disease revealed by plasma metabolic profiling. J. Chromatogr. B 2018, 1081–1082, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Golovko, M.Y.; Murphy, E.J. Brain prostaglandin formation is increased by alpha-synuclein gene-ablation during global ischemia. Neurosci. Lett. 2008, 432, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Fernsebner, K.; Zorn, J.; Kanawati, B.; Walker, A.; Michalke, B. Manganese leads to an increase in markers of oxidative stress as well as to a shift in the ratio of Fe(ii)/(iii) in rat brain tissue. Metallomics 2014, 6, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Seet, R.C.S.; Lee, C.Y.J.; Lim, E.C.H.; Tan, J.J.H.; Quek, A.M.L.; Chong, W.L.; Looi, W.F.; Huang, S.H.; Chan, Y.H.; Halliwell, B. Oxidative damage in Parkinson disease: Measurement using accurate biomarkers. Free Radic. Biol. Med. 2010, 48, 560–566. [Google Scholar] [CrossRef]
- Tyurina, Y.Y.; Polimova, A.M.; Maciel, E.; Tyurin, V.A.; Kapralova, V.I.; Winnica, D.E.; Vikulina, A.S.; Domingues, M.R.M.; McCoy, J.; Kagan, V.E.; et al. LC/MS analysis of cardiolipins in substantia nigra and plasma of rotenone-treated rats: Implication for mitochondrial dysfunction in Parkinson’s disease. Free Radic. Res. 2015, 49, 681–691. [Google Scholar] [CrossRef]
- Klatt-Schreiner, K.; Valek, L.; Kang, J.S.; Khlebtosky, A.; Trautmann, S.; Hahnfeld, L.; Schreiber, Y.; Gurke, R.; Thomas, D.; Tegeder, I.; et al. High Glucosylceramides and Low Anandamide Contribute to Sensory Loss and Pain in Parkinson’s Disease. Mov. Disord. 2020, 35, 1822–1833. [Google Scholar] [CrossRef]
- Di Marzo, V.; Hill, M.P.; Bisogno, T.; Crossman, A.R.; Brotchie, J.M. Enhanced levels of endogenous cannabinoids in the globus pallidus are associated with a reduction in movement in an animal model of Parkinson’s disease. FASEB J. 2000, 14, 1432–1438. [Google Scholar] [CrossRef]
- Gubellini, P.; Picconi, B.; Bari, M.; Battista, N.; Calabresi, P.; Centonze, D.; Bernardi, G.; Finazzi-Agrò, A.; Maccarrone, M. Experimental parkinsonism alters endocannabinoid degradation: Implications for striatal glutamatergic transmission. J. Neurosci. 2002, 22, 6900–6907. [Google Scholar] [CrossRef]
- Maccarrone, M.; Gubellini, P.; Bari, M.; Picconi, B.; Battista, N.; Centonze, D.; Bernardi, G.; Finazzi-Agrò, A.; Calabresi, P. Levodopa treatment reverses endocannabinoid system abnormalities in experimental parkinsonism. J. Neurochem. 2003, 4, 1018–1025. [Google Scholar] [CrossRef]
- Ferrer, B.; Asbrock, N.; Kathuria, S.; Piomelli, D.; Giuffrida, A. Effects of levodopa on endocannabinoid levels in rat basal ganglia: Implications for the treatment of levodopa-induced dyskinesias. Eur. J. Neurosci. 2003, 6, 1607–1614. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, G.J.; Sun, Y.N.; Yao, L.; Wang, H.S.; Du, C.X.; Zhang, L.; Liu, J. Identification of metabolite biomarkers for L-DOPA-induced dyskinesia in a rat model of Parkinson’s disease by metabolomic technology. Behav. Brain Res. 2018, 347, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Van der Stelt, M.; Fox, S.H.; Hill, M.; Crossman, A.R.; Petrosino, S.; Di Marzo, V.; Brotchie, J.M. A role for endocannabinoids in the generation of parkinsonism and levodopa-induced dyskinesia in MPTP-lesioned non-human primate models of Parkinson’s disease. FASEB J. 2005, 9, 1140–1142. [Google Scholar] [CrossRef] [PubMed]
- Nomura, D.K.; Morrison, B.E.; Blankman, J.L.; Long, J.Z.; Kinsey, S.G.; Marcondes, M.C.; Ward, A.M.; Hahn, Y.K.; Lichtman, A.H.; Conti, B.; et al. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science 2011, 6057, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Mounsey, R.B.; Mustafa, S.; Robinson, L.; Ross, R.A.; Riedel, G.; Pertwee, R.G.; Teismann, P. Increasing levels of the endocannabinoid 2-AG is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Exp. Neurol. 2015, 273, 36–44. [Google Scholar] [CrossRef]
- Concannon, R.M.; Okine, B.N.; Finn, D.P.; Dowd, E. Differential upregulation of the cannabinoid CB2 receptor in neurotoxic and inflammation-driven rat models of Parkinson’s disease. Exp. Neurol. 2015, 269, 133–141. [Google Scholar] [CrossRef]
- Concannon, R.M.; Okine, B.N.; Finn, D.P.; Dowd, E. Upregulation of the cannabinoid CB2 receptor in environmental and viral inflammation-driven rat models of Parkinson’s disease. Exp. Neurol. 2016, 283, 204–212. [Google Scholar] [CrossRef]
- Kelly, R.; Bemelmans, A.P.; Joséphine, C.; Brouillet, E.; McKernan, D.P.; Dowd, E. Time-Course of Alterations in the Endocannabinoid System after Viral-Mediated Overexpression of α-Synuclein in the Rat Brain. Molecules 2022, 507, 14. [Google Scholar] [CrossRef]
- Krashia, P.; Cordella, A.; Nobili, A.; La Barbera, L.; Federici, M.; Leuti, A.; Campanelli, F.; Natale, G.; Marino, G.; Calabrese, V.; et al. Blunting neuroinflammation with resolvin D1 prevents early pathology in a rat model of Parkinson’s disease. Nat. Commun. 2019, 10, 3945. [Google Scholar] [CrossRef]
- Madeo, G.; Schirinzi, T.; Maltese, M.; Martella, G.; Rapino, C.; Fezza, F.; Mastrangelo, N.; Bonsi, P.; Maccarrone, M.; Pisani, A. Dopamine-dependent CB1 receptor dysfunction at corticostriatal synapses in homozygous PINK1 knockout mice. Neuropharmacology 2016, 101, 460–470. [Google Scholar] [CrossRef]
- Marchioni, C.; Santos-Lobato, B.L.; Queiroz, M.E.C.; Crippa, J.A.S.; Tumas, V. Endocannabinoid levels in patients with Parkinson’s disease with and without levodopa-induced dyskinesias. J. Neural Transm. 2020, 10, 1359–1367. [Google Scholar] [CrossRef]
- Oliveira, I.G.C.; Marchioni, C.; Queiroz, M.E.C. Determination of anandamide in cerebrospinal fluid samples by disposable pipette extraction and ultra-high performance liquid chromatography tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1130, 121809. [Google Scholar] [CrossRef]
- Oliveira, I.G.C.; Souza, I.D.; Nascimento, G.C.D.; Del Bel, E.; Queiroz, M.E.C. In-tube solid-phase microextraction directly coupled to tandem mass spectrometry for anandamide and 2-arachidonoylglycerol determination in rat brain samples from an animal model of Parkinson’s disease. J. Chromatogr. A 2021, 1636, 461766. [Google Scholar] [CrossRef]
- Lin, G.; Wang, L.; Marcogliese, P.C.; Bellen, H.J. Sphingolipids in the Pathogenesis of Parkinson’s Disease and Parkinsonism. Trends Endocrinol. Metab. 2019, 30, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Vendruscolo, M. Lipid Homeostasis and its Links with Protein Misfolding Diseases. Front. Mol. Neurosci. 2022, 15, 829291. [Google Scholar] [CrossRef] [PubMed]
- Nalls, A.M.; Duran, R.; Lopez, G.; Kurzawa-Akabni, M.; McKeith, I.G.; Chinnery, P.; Morris, C.M.; Theus, J.; Crosiers, D.; Cras, P.; et al. Multicenter Analysis of Glucocerebrosidase Mutations in Parkinson’s Disease. N. Engl. J. Med. 2009, 361, 1651–1661. [Google Scholar]
- Niimi, Y.; Mizutani, Y.; Akiyama, H.; Watanabe, H.; Shiroki, R.; Hirabayashi, Y.; Hoshinaga, K.; Mutoh, T. Cerebrospinal Fluid Profiles in Parkinson’s Disease: No Accumulation of Glucosylceramide, but Significant Downregulation of Active Complement C5 Fragment. J. Parkinsons. Dis. 2021, 11, 221–232. [Google Scholar] [CrossRef]
- Mata, I.F.; Wedemeyer, W.J.; Farrer, M.J.; Taylor, J.P.; Gallo, K.A. LRRK2 in Parkinson’s disease: Protein domains and functional insights. Trends Neurosci. 2006, 29, 286–293. [Google Scholar] [CrossRef]
- Hinkle, K.M.; Yue, M.; Behrouz, B.; Dächsel, J.C.; Lincoln, S.J.; Bowles, E.E.; Beevers, J.E.; Dugger, B.; Winner, B.; Melrose, H.L.; et al. LRRK2 knockout mice have an intact dopaminergic system but display alterations in exploratory and motor co-ordination behaviors. Mol. Neurodegener. 2012, 7, 25. [Google Scholar] [CrossRef]
- Avisar, H.; Guardia-Laguarta, C.; Area-Gomez, E.; Surface, M.; Chan, A.K.; Alcalay, R.N.; Lerner, B. Lipidomics Predic-tion of Parkinson’s Disease Severity: A Machine-Learning Analysis. J. Parkinsons. Dis. 2021, 11, 1141–1155. [Google Scholar] [CrossRef]
- Gegg, M.E.; Sweet, L.; Wang, B.H.; Shihabuddin, L.; Sardi, S.P.; Schapira, A.H.V. No evidence for substrate accumulation in Parkinson brains with GBA mutations. Mov. Disord. 2015, 30, 1085–1089. [Google Scholar] [CrossRef]
- Shahmoradian, S.H.; Lewis, A.J.; Genoud, C.; Hench, J.; Moors, T.E.; Navarro, P.P.; Castano-Diaz, D.; Schweighauser, G.; Graff-Meyer, A.; Lauer, M.E.; et al. Lewy pathology in Parkinson’s disease consists of crowded organelles and lipid membranes. Nat. Neurosci. 2019, 22, 1099–1109. [Google Scholar] [CrossRef]
- Pizarro, C.; Esteban-Díez, I.; Espinosa, M.; Rodríguez-Royo, F.; González-Sáiz, J.-M. An NMR-based lipidomic approach to identify Parkinson’s disease-stage specific lipoprotein-lipid signatures in plasma. Analyst 2019, 144, 1334–1344. [Google Scholar] [CrossRef]
- Mattammal, M.B.; Strong, R.; Lakshmi, V.M.; Chung, H.D.; Stephenson, A.H. Prostaglandin H Synthetase-Mediated Metabolism of Dopamine: Implication for Parkinson’s Disease. J. Neurochem. 1995, 64, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zou, L.; Wang, F.; Chen, Z.; Hu, Y.; Wang, Y.; Wang, X.; Zhang, W. Potential biomarkers relating pathological proteins, neuroinflammatory factors and free radicals in PD patients with cognitive impairment: A cross-sectional study. BMC Neurol. 2014, 14, 113. [Google Scholar] [CrossRef] [PubMed]
- Ashley, A.K.; Hinds, A.I.; Hanneman, W.H.; Tjalkens, R.B.; Legare, M.E. DJ-1 mutation decreases astroglial release of inflammatory mediators. Neurotoxicology 2016, 52, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.J.; An, J.; Jou, I.; Park, S.M.; Joe, E.-H. A Parkinson’s disease gene, DJ-1, regulates anti-inflammatory roles of astrocytes through prostaglandin D2 synthase expression. Neurobiol. Dis. 2019, 127, 482–491. [Google Scholar] [CrossRef]
- Bai, L.; Zhang, X.; Li, X.; Liu, N.; Lou, F.; Ma, H.M.; Luo, X.; Ren, Y. Somatostatin prevents lipopolysaccharide-induced neurodegeneration in the rat substantia nigra by inhibiting the activation of microglia. Mol. Med. Rep. 2015, 12, 1002–1008. [Google Scholar] [CrossRef]
- McLaughlin, P.; Zhou, Y.; Ma, T.; Liu, J.; Zhang, W.; Hong, J.S.; Kovacs, M.; Zhang, J. Proteomic analysis of microglial contribution to mouse strain-dependent dopaminergic neurotoxicity. Glia 2006, 53, 567–582. [Google Scholar] [CrossRef]
- Kang, K.H.; Liou, H.-H.; Hour, M.-J.; Liou, H.-C.; Fu, W.-M. Protection of dopaminergic neurons by 5-lipoxygenase inhibitor. Neuropharmacology 2013, 73, 380–387. [Google Scholar] [CrossRef]
- Branchi, I.; D’Andrea, I.; Armida, M.; Carnevale, D.; Ajomne-Cat, M.A.; Pèzzola, A.; Potenza, R.L.; Morgese, M.G.; Cassano, T.; Alleva, E.; et al. Striatal 6-OHDA lesion in mice: Investigating early neurochemical changes underlying Parkinson’s disease. Behav. Brain Res. 2010, 208, 137–143. [Google Scholar] [CrossRef]
- Pisani, A.; Fezza, F.; Galati, S.; Battista, N.; Napolitano, S.; Finazzi-Agrò, A.; Bernardi, G.; Brusa, L.; Pierantozzi, M.; Stanzione, P.; et al. High endogenous cannabinoid levels in the cerebrospinal fluid of untreated Parkinson’s disease patients. Ann. Neurol. 2005, 57, 777–779. [Google Scholar] [CrossRef] [PubMed]
- Pisani, V.; Moschella, V.; Bari, M.; Fezza, F.; Galati, S.; Bernardi, G.; Stanzione, P.; Pisani, A.; Maccarrone, M. Dynamic changes of anandamide in the cerebrospinal fluid of Parkinson’s disease patients. Mov. Disord. 2010, 7, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Pisani, V.; Madeo, G.; Tassone, A.; Sciamanna, G.; Maccarrone, M.; Stanzione, P.; Pisani, A. Homeostatic changes of the endocannabinoid system in Parkinson’s disease. Mov. Disord. 2011, 2, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, I.G.; Queiroz, M.E. A micro salting-out assisted liquid-liquid extraction combined with ultra-high performance liquid chromatography tandem mass spectrometry to determine anandamide and 2-arachidonoylglycerol in rat brain samples. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1158, 122351. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Gupta, S.K.; Perretti, M.; Godson, C.; Brennan, E.; Li, Y.; Soehnlein, O.; Shimizu, T.; Werz, O.; Chiurchiù, V.; et al. The Atlas of Inflammation Resolution (AIR). Mol Aspects Med. 2020, 74, 100894. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).