Copper Modulates Mitochondrial Oxidative Phosphorylation to Enhance Dermal Papilla Cells Proliferation in Rex Rabbits
Abstract
1. Introduction
2. Results
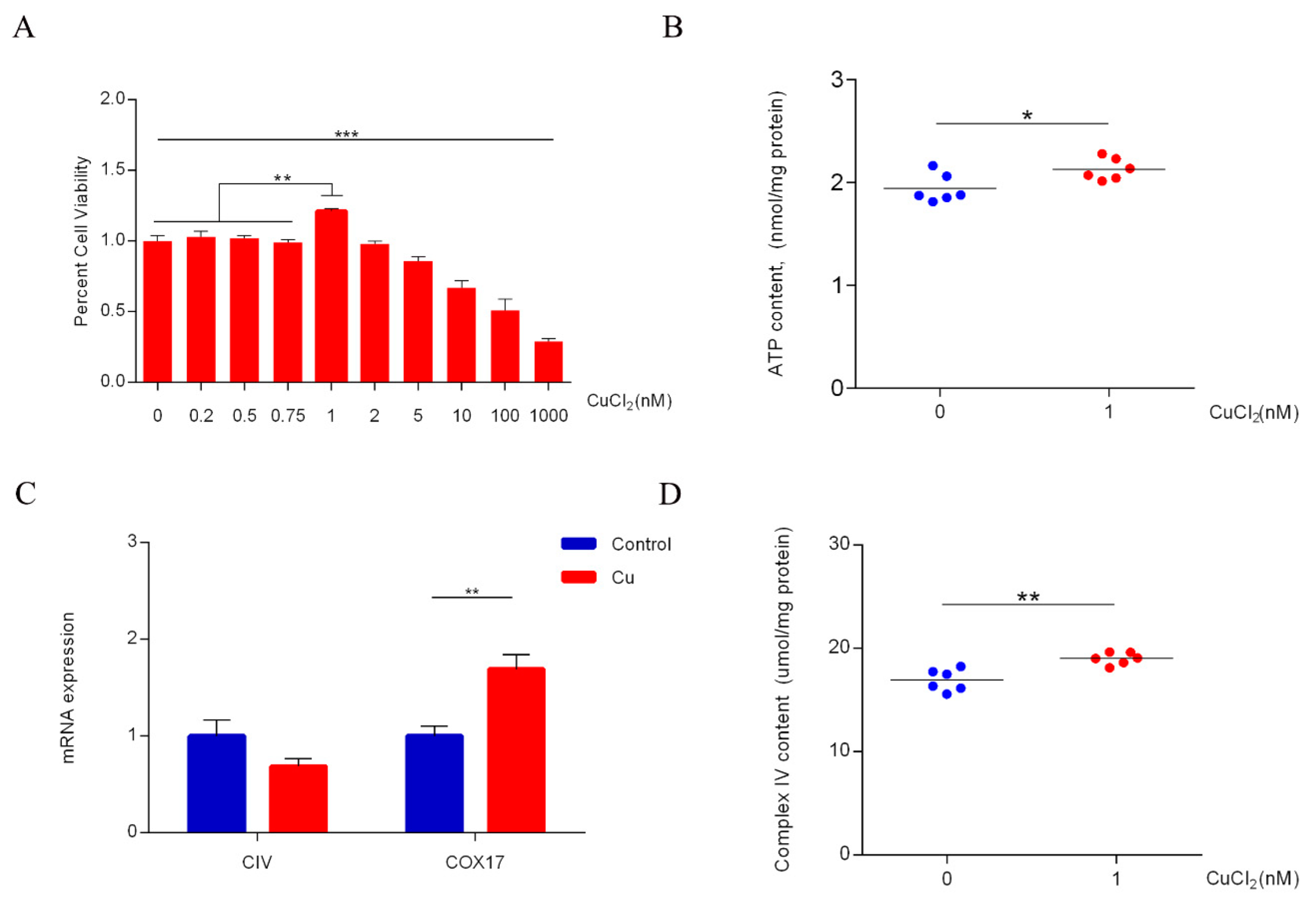
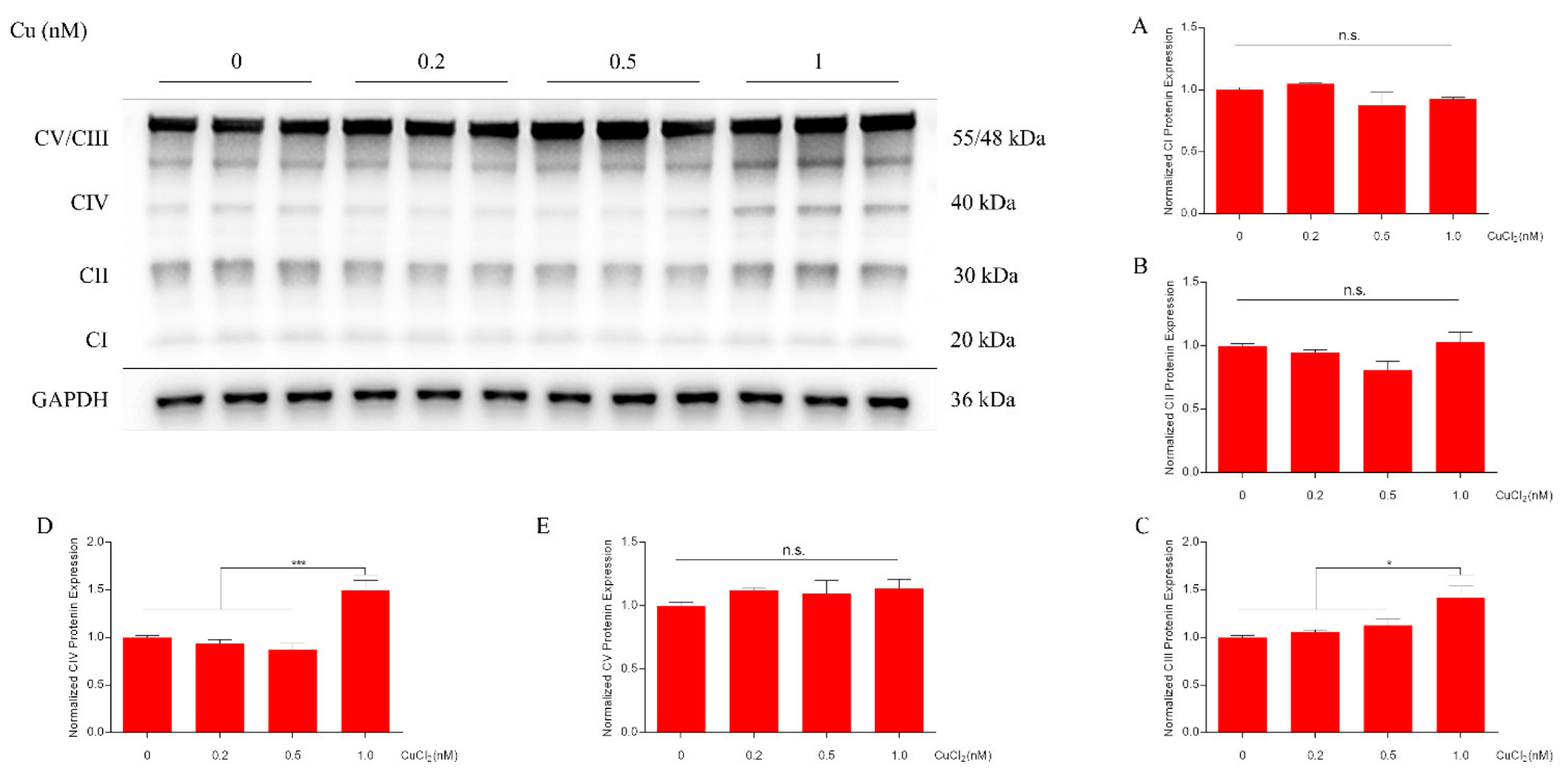
2.1. Copper Increases ATP Levels through Cytochrome c Oxidase
2.2. Metabolites
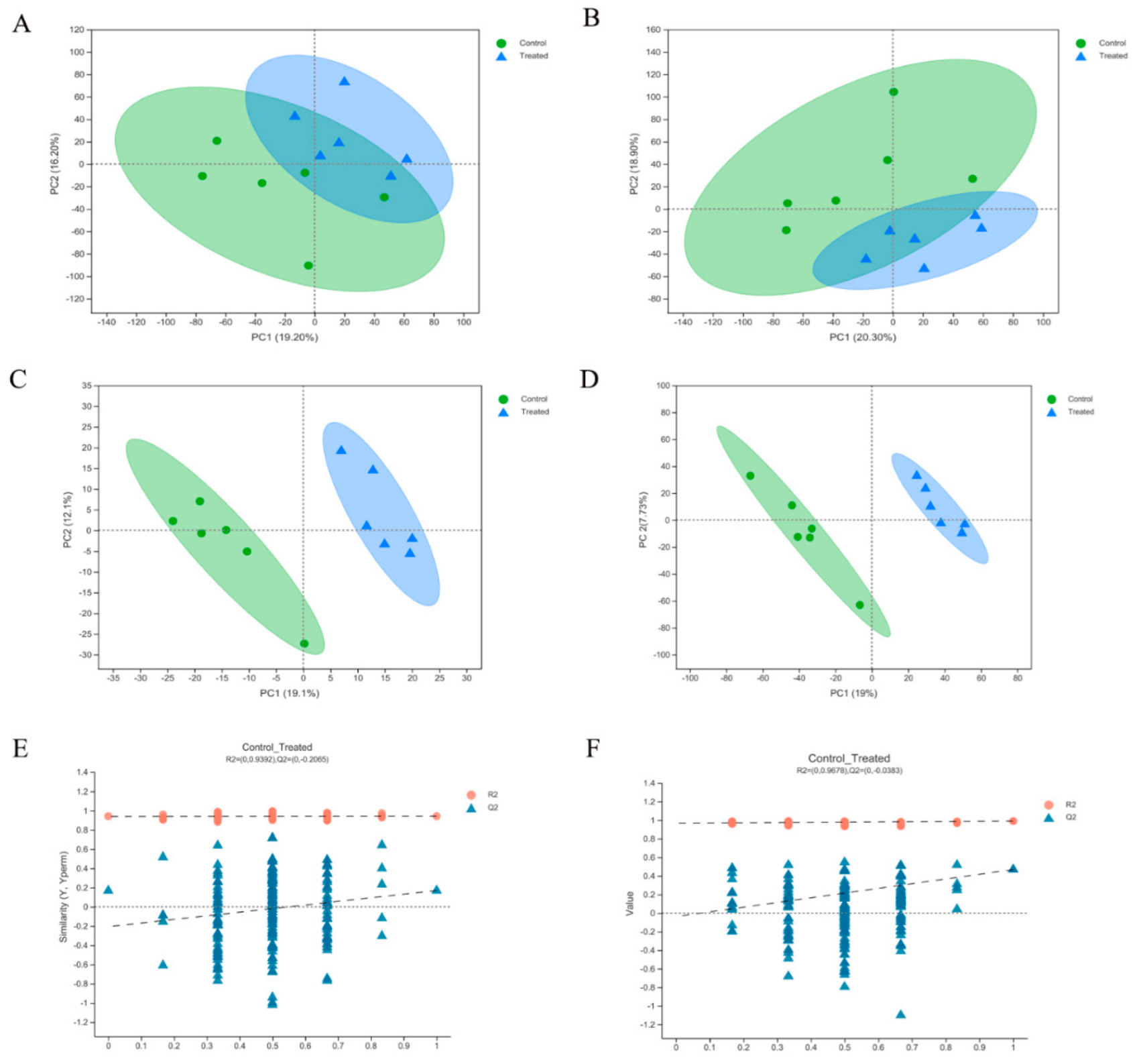
2.3. Multivariate Data Analysis
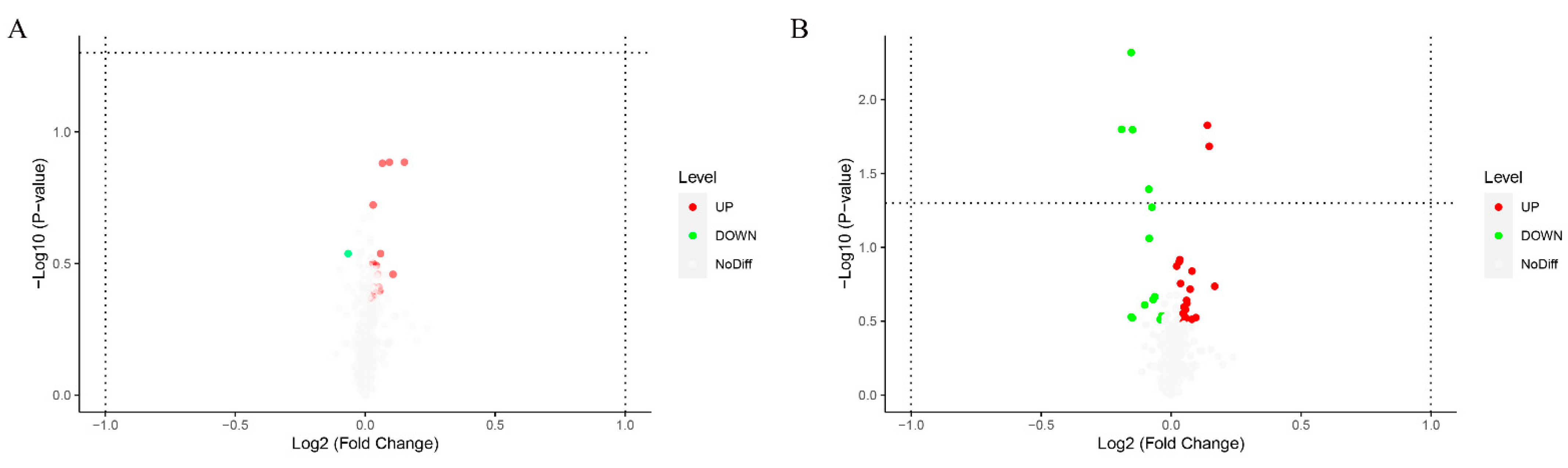
2.4. Significantly Different Metabolites
2.5. Kyoto Encyclopedia of Genes and Genomes’ Functional Pathways
3. Discussion
4. Materials and Methods
4.1. Cells
4.2. Cell Culture
4.3. RNA Isolation and Analysis
4.4. Western Blot
4.5. Metabolite Extraction
4.6. UHPLC–MS Analysis
4.7. Database Search
4.8. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, F.; Liu, L.; Chen, X.; Zhang, B.; Li, F. Dietary Copper Supplementation Increases Growth Performance by Increasing Feed Intake, Digestibility, and Antioxidant Activity in Rex Rabbits. Biol. Trace Elem. Res. 2021, 199, 4614–4623. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wu, X.; Liu, H.; Zhang, B.; Liu, L.; Li, F. Dietary copper supplementation enhances lipolysis in Rex rabbits. J. Trace Elem. Med. Biol. 2021, 68, 126851. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, X.; Zhang, T.; Guo, J.; Gao, X.; Yang, F.; Xing, X. Effects of Dietary Copper and Zinc Supplementation on Growth Performance, Tissue Mineral Retention, Antioxidant Status, and Fur Quality in Growing-Furring Blue Foxes (Alopex lagopus). Biol. Trace Elem. Res. 2015, 168, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, Z.; Guo, J.; Wan, C.; Zhang, T.; Cui, H.; Yang, F.; Gao, X. Influence of dietary zinc and copper on apparent mineral retention and serum biochemical indicators in young male mink (Mustela vison). Biol. Trace Elem. Res. 2015, 165, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.D. Basic and clinical aspects of copper. Crit. Rev. Clin. Lab. Sci. 2003, 40, 547–586. [Google Scholar] [CrossRef]
- Turski, M.L.; Thiele, D.J. New roles for copper metabolism in cell proliferation, signaling, and disease. J. Biol. Chem. 2009, 284, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Lippard, S.J.; Berg, J.M. Principles of Bioinorganic Chemistry; University Science Books: Mill Valley, CA, USA, 1994. [Google Scholar]
- Krishnamoorthy, L.; Cotruvo, J.A.; Chan, J., Jr.; Kaluarachchi, H.; Muchenditsi, A.; Pendyala, V.S.; Jia, S.; Aron, A.T.; Ackerman, C.M.; Wal, M.N.; et al. Copper regulates cyclic-AMP-dependent lipolysis. Nat. Chem. Biol. 2016, 12, 586–592. [Google Scholar] [CrossRef]
- Brady, D.C.; Crowe, M.S.; Turski, M.L.; Hobbs, G.A.; Yao, X.; Chaikuad, A.; Knapp, S.; Xiao, K.; Campbell, S.L.; Thiele, D.J.; et al. Copper is required for oncogenic BRAF signalling and tumorigenesis. Nature 2014, 509, 492–496. [Google Scholar] [CrossRef]
- Horn, D.; Barrientos, A. Mitochondrial copper metabolism and delivery to cytochrome c oxidase. IUBMB Life 2008, 60, 421–429. [Google Scholar] [CrossRef]
- Barrientos, A.; Barros, M.H.; Valnot, I.; Rötig, A.; Rustin, P.; Tzagoloff, A. Cytochrome oxidase in health and disease. Gene 2002, 286, 53–63. [Google Scholar] [CrossRef]
- Stiburek, L.; Hansikova, H.; Tesarova, M.; Cerna, L.; Zeman, J. Biogenesis of eukaryotic cytochrome c oxidase. Physiol. Res. 2006, 55, S27–S41. [Google Scholar] [CrossRef] [PubMed]
- Ramchandani, D.; Berisa, M.; Tavarez, D.A.; Li, Z.; Miele, M.; Bai, Y.; Lee, S.B.; Ban, Y.; Dephoure, N.; Hendrickson, R.C.; et al. Copper depletion modulates mitochondrial oxidative phosphorylation to impair triple negative breast cancer metastasis. Nat. Commun. 2021, 12, 7311. [Google Scholar] [CrossRef]
- Yu, Z.; Jiang, K.; Xu, Z.; Huang, H.; Qian, N.; Lu, Z.; Chen, D.; Di, R.; Yuan, T.; Du, Z.; et al. Hoxc-Dependent Mesenchymal Niche Heterogeneity Drives Regional Hair Follicle Regeneration. Cell Stem Cell 2018, 23, 487–500.e6. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Ding, X.; Allmeroth, K.; Biggs, L.C.; Kolenc, O.I.; L’Hoest, N.; Chacón-Martínez, C.A.; Edlich-Muth, C.; Giavalisco, P.; Quinn, K.P.; et al. Glutamine Metabolism Controls Stem Cell Fate Reversibility and Long-Term Maintenance in the Hair Follicle. Cell Metab. 2020, 32, 629–642.e8. [Google Scholar] [CrossRef] [PubMed]
- Finner, A.M. Nutrition and hair: Deficiencies and supplements. Dermatol. Clin. 2013, 31, 167–172. [Google Scholar] [CrossRef]
- Xiong, Q.Q.; Shen, T.H.; Zhong, L.; Zhu, C.L.; Peng, X.S.; He, X.P.; Fu, J.R.; Ouyang, L.J.; Bian, J.M.; Hu, L.F.; et al. Comprehensive metabolomic, proteomic and physiological analyses of grain yield reduction in rice under abrupt drought-flood alternation stress. Physiol. Plant. 2019, 167, 564–584. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, J.; Wu, R.; Liu, Y.; Hu, X.; Yan, Y.; Ling, X. A novel strategy for rapidly and accurately screening biomarkers based on ultraperformance liquid chromatography-mass spectrometry metabolomics data. Anal. Chim. Acta 2019, 1063, 47–56. [Google Scholar] [CrossRef]
- Zhang, Z.; Dai, Q.; Zhang, Y.; Zhuang, H.; Wang, E.; Xu, Q.; Ma, L.; Wu, C.; Huan, Z.; Guo, F.; et al. Design of a Multifunctional Biomaterial Inspired by Ancient Chinese Medicine for Hair Regeneration in Burned Skin. ACS Appl. Mater. Interfaces 2020, 12, 12489–12499. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.S.; Zhu, X.P.; Wang, R.L.; Jia, Z.H. Effect of different levels of copper and molybdenum supplements on performance, nutrient digestibility, and follicle characteristics in cashmere goats. Biol. Trace Elem. Res. 2011, 143, 1470–1479. [Google Scholar] [CrossRef]
- Martin, S.A.; Brash, A.R.; Murphy, R.C. The discovery and early structural studies of arachidonic acid. J. Lipid Res. 2016, 57, 1126–1132. [Google Scholar] [CrossRef]
- Koundouros, N.; Karali, E.; Tripp, A.; Valle, A.; Inglese, P.; Perry, N.; Magee, D.J.; Anjomani Virmouni, S.; Elder, G.A.; Tyson, A.L.; et al. Metabolic Fingerprinting Links Oncogenic PIK3CA with Enhanced Arachidonic Acid-Derived Eicosanoids. Cell 2020, 181, 1596–1611. [Google Scholar] [CrossRef] [PubMed]
- Bui, P.; Imaizumi, S.; Beedanagari, S.R.; Reddy, S.T.; Hankinson, O. Human CYP2S1 metabolizes cyclooxygenase- and lipoxygenase-derived eicosanoids. Drug Metab. Dispos. Biol. Fate Chem. 2011, 39, 180–190. [Google Scholar] [CrossRef]
- Kanikarla-Marie, P.; Kopetz, S.; Hawk, E.T.; Millward, S.W.; Sood, A.K.; Gresele, P.; Overman, M.; Honn, K.; Menter, D.G. Bioactive lipid metabolism in platelet "first responder" and cancer biology. Cancer Metastasis Rev. 2018, 37, 439–454. [Google Scholar] [CrossRef]
- Yang, C.; Li, C.; Li, M.; Tong, X.; Hu, X.; Yang, X.; Yan, X.; He, L.; Wan, C. CYP2S1 depletion enhances colorectal cell proliferation is associated with PGE2-mediated activation of β-catenin signaling. Exp. Cell Res. 2015, 331, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Dubois, R.N. Prostaglandins and cancer. Gut 2006, 55, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, Y.; Semenov, M.; Han, C.; Baeg, G.; Tan, Y.; Zhang, Z.; Lin, X.; He, X. Control of β-Catenin Phosphorylation/Degradation by a Dual-Kinase Mechanism. Cell 2002, 108, 837–847. [Google Scholar] [CrossRef]
- Wu, G. Dietary requirements of synthesizable amino acids by animals: A paradigm shift in protein nutrition. J. Anim. Sci. Biotechnol. 2014, 5, 34. [Google Scholar] [CrossRef]
- Jewett, M.C.; Miller, M.L.; Chen, Y.; Swartz, J.R. Continued protein synthesis at low [ATP] and [GTP] enables cell adaptation during energy limitation. J. Bacteriol. 2009, 191, 1083–1091. [Google Scholar] [CrossRef]
- Wang, T.; Guo, Z. Copper in medicine: Homeostasis, chelation therapy and antitumor drug design. Curr. Med. Chem. 2006, 13, 525–537. [Google Scholar] [CrossRef]
- Lau, S.J.; Sarkar, B. Ternary coordination complex between human serum albumin, copper (II), and L-histidine. J. Biol. Chem. 1971, 246, 5938–5943. [Google Scholar] [CrossRef]
- Prohaska, J.R. Role of copper transporters in copper homeostasis. Am. J. Clin. Nutr. 2008, 88, 826S–829S. [Google Scholar] [CrossRef] [PubMed]
- Jara, C.P.; de Andrade Berti, B.; Mendes, N.F.; Engel, D.F.; Zanesco, A.M.; Pereira de Souza, G.F.; de Medeiros Bezerra, R.; de Toledo Bagatin, J.; Maria-Engler, S.S.; Morari, J.; et al. Glutamic acid promotes hair growth in mice. Sci. Rep. 2021, 11, 15453. [Google Scholar] [CrossRef]
- Kim, H.S.; Kwon, H.K.; Lee, D.H.; Le, T.N.; Park, H.J.; Kim, M.I. Poly(γ-Glutamic Acid)/Chitosan Hydrogel Nanoparticles For Effective Preservation And Delivery Of Fermented Herbal Extract For Enlarging Hair Bulb And Enhancing Hair Growth. Int. J. Nanomed. 2019, 14, 8409–8419. [Google Scholar] [CrossRef]
- Choi, J.C.; Uyama, H.; Lee, C.H.; Sung, M.H. In vivo hair growth promotion effects of ultra-high molecular weight poly-γ-glutamic acid from Bacillus subtilis (Chungkookjang). J. Microbiol. Biotechnol. 2015, 25, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Rigotti, D.J.; Kirov, I.I.; Djavadi, B.; Perry, N.; Babb, J.S.; Gonen, O. Longitudinal whole-brain N-acetylaspartate concentration in healthy adults. Am. J. Neuroradiol. 2011, 32, 1011–1015. [Google Scholar] [CrossRef] [PubMed]
- Mekala, J.R.; Kurappalli, R.K.; Ramalingam, P.; Moparthi, N.R. N-acetyl l-aspartate and Triacetin modulate tumor suppressor MicroRNA and class I and II HDAC gene expression induce apoptosis in Glioblastoma cancer cells in vitro. Life Sci. 2021, 286, 120024. [Google Scholar] [CrossRef]
- Shi, L.; Tu, B.P. Acetyl-CoA and the regulation of metabolism: Mechanisms and consequences. Curr. Opin. Cell Biol. 2015, 33, 125–131. [Google Scholar] [CrossRef]
- Akram, M. Citric acid cycle and role of its intermediates in metabolism. Cell Biochem. Biophys. 2014, 68, 475–478. [Google Scholar] [CrossRef]
- Baldwin, J.E.; Krebs, H. The evaluation of metabolic cycle. Nature 1981, 292, 291–381. [Google Scholar]
- Ge, Y.D.; Pan, W.; Wang, J.; Zhu, G.P. Advances in molecular biology of citrate synthase. J. Biol. 2010, 27, 59–62. [Google Scholar]
- Bond, D.R.; Mester, T.; Nesbø, C.L.; Izquierdo-Lopez, A.V.; Collart, F.L.; Lovley, D.R. Characterization of citrate synthase from Geobacter sulfurreducens and evidence for a family of citrate synthases similar to those of eukaryotes throughout the Geobacteraceae. Appl. Environ. Microbiol. 2005, 71, 3858–3865. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, J.l.; Liu, M.; Zhou, J.; Chen, Q.R.; Chen, Y.; Chen, S.; Shen, J.Y.; Wu, X. Isolation, Culture and Identification of Rabbit Dermal Papilla Cells. Chin. J. Rabbit. Farm. 2020, 3, 4–7. [Google Scholar]

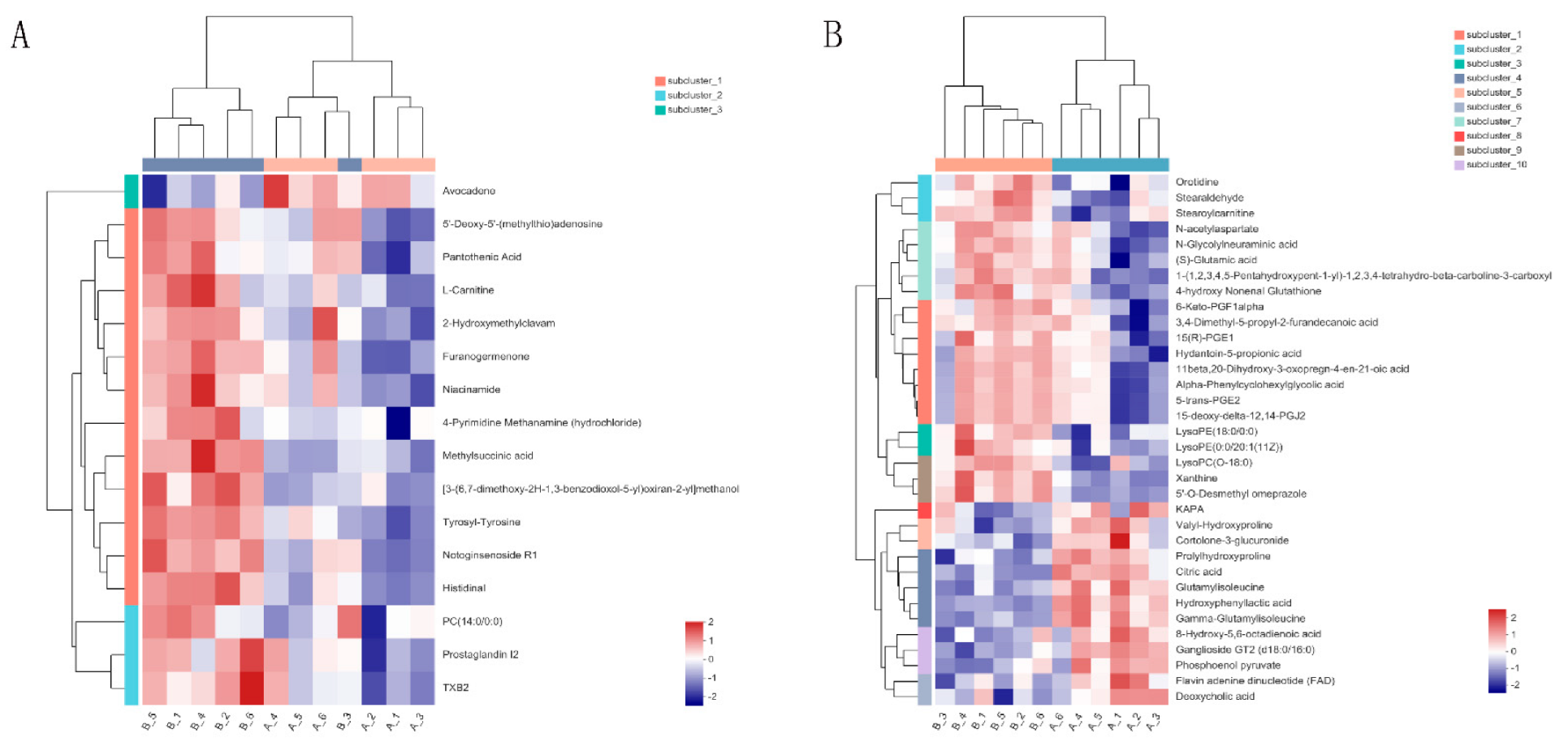
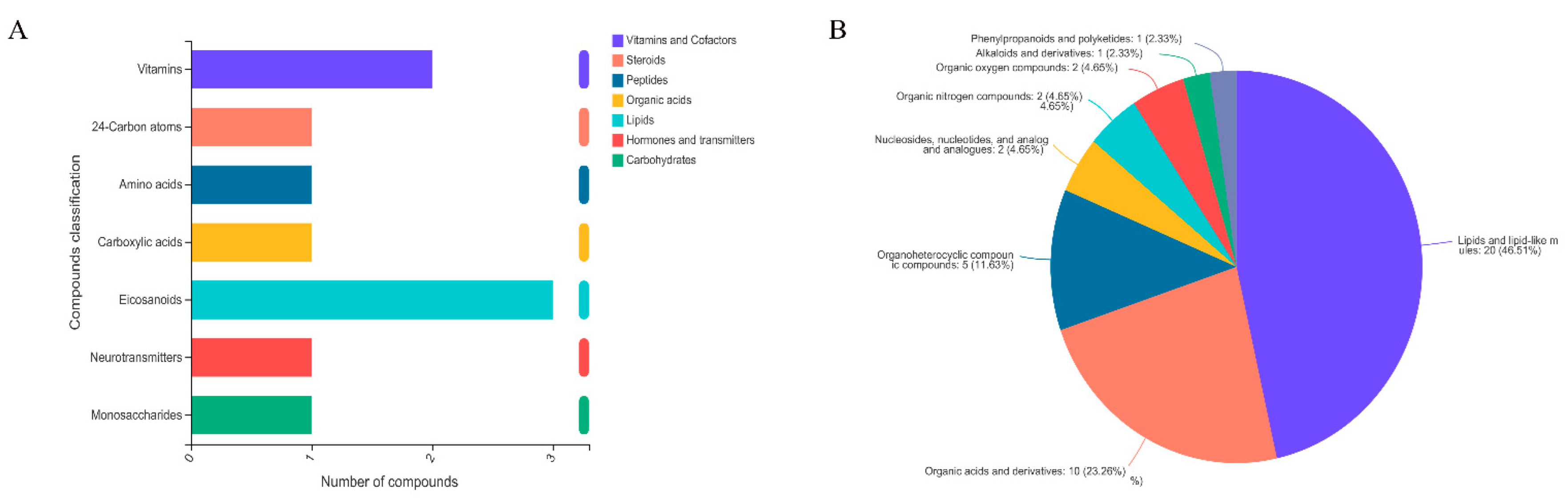
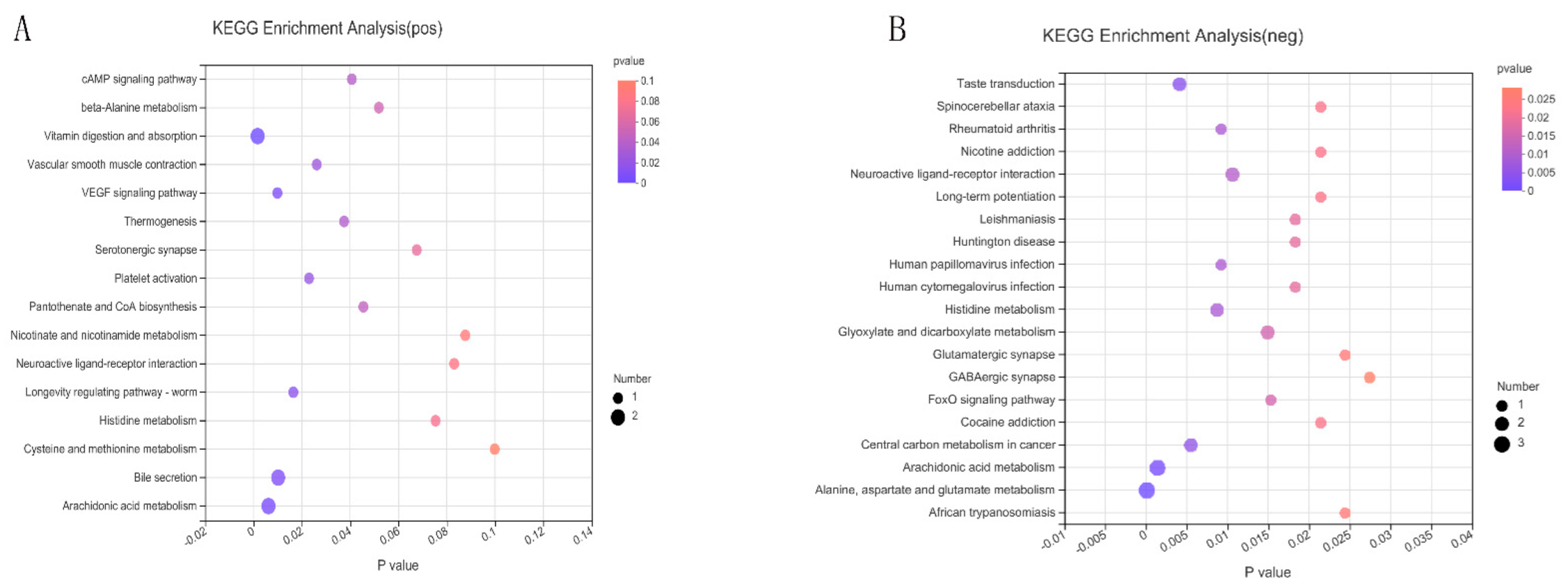
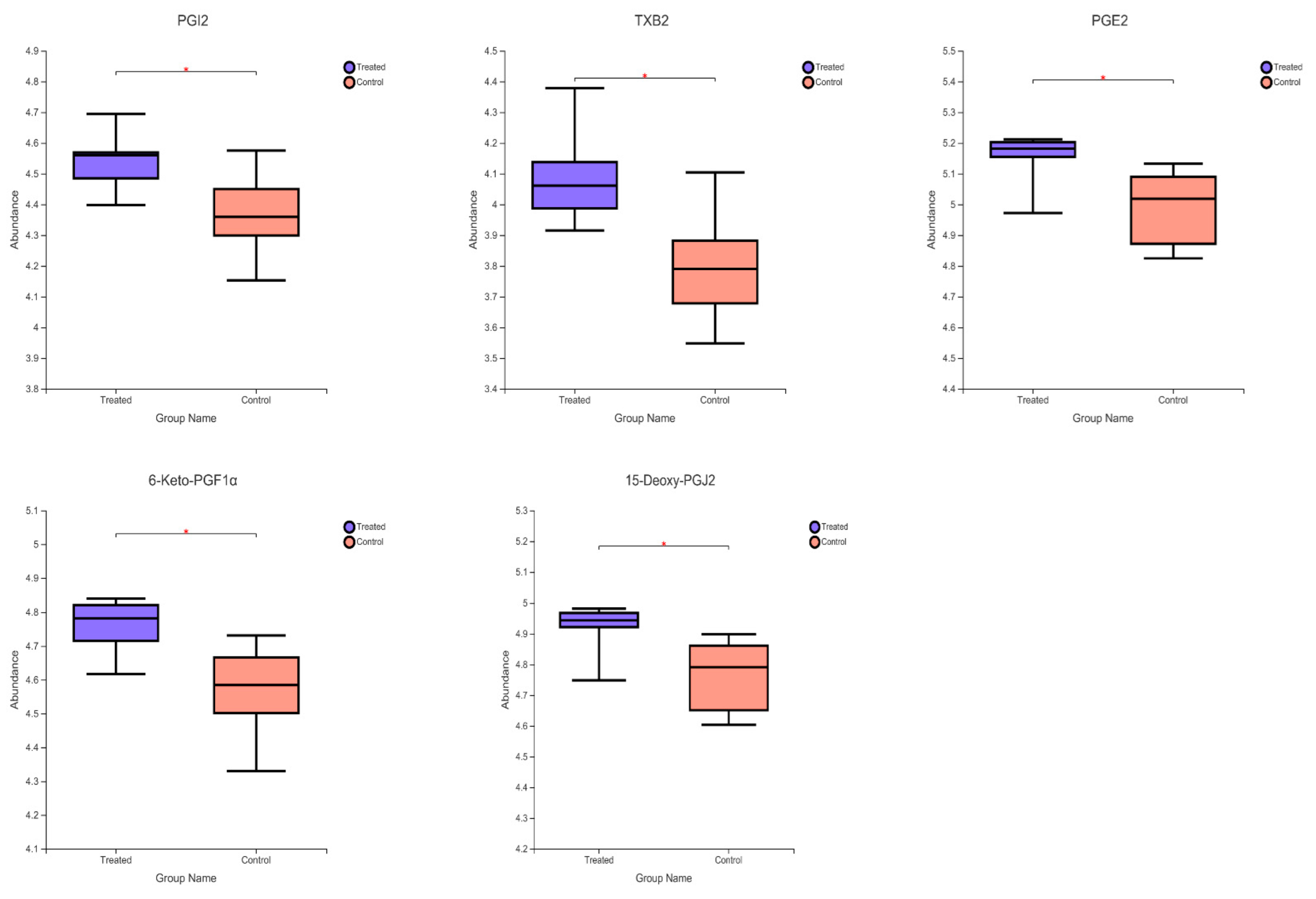
| Metabolite | Formula | M/Z | RT (Min) | FC | p-Value | VIP | Regulate |
|---|---|---|---|---|---|---|---|
| PC (14:0/0:0) | C22H46NO7P | 468.31 | 7.42 | 1.02 | 0.0128 | 1.21 | up |
| Prostaglandin I2 | C20H32O5 | 353.23 | 4.75 | 1.04 | 0.0394 | 1.44 | up |
| Niacinamide | C6H6N2O | 123.06 | 1.32 | 1.02 | 0.044 | 1.18 | up |
| 5’-Deoxy-5’-(methylthio)adenosine | C11H15N5O3S | 298.1 | 1.85 | 1.04 | 0.0093 | 1.75 | up |
| Pantothenic Acid | C9H17NO5 | 220.12 | 1.76 | 1.03 | 0.0197 | 1.38 | up |
| [3-(6,7-dimethoxy-2H-1,3-benzodioxol-5-yl)oxiran-2-yl]methanol | C12H14O6 | 237.08 | 3.24 | 1.02 | 0.0023 | 1.06 | up |
| Methylsuccinic acid | C5H8O4 | 133.05 | 1.65 | 1.11 | 0.0004 | 2.37 | up |
| 4-Pyrimidine Methanamine (hydrochloride) | C5H7N3 | 110.07 | 0.64 | 1.02 | 0.0479 | 0.94 | up |
| Histidinal | C6H9N3O | 122.07 | 0.68 | 1.05 | 0.0006 | 1.74 | up |
| L-Carnitine | C7H15NO3 | 162.11 | 0.68 | 1.02 | 0.0361 | 1.15 | up |
| Tyrosyl-Tyrosine | C18H20N2O5 | 309.13 | 3.48 | 1.03 | 0.0139 | 1.28 | up |
| Furanogermenone | C15H20O2 | 215.14 | 6.06 | 1.04 | 0.032 | 1.35 | up |
| Avocadene | C17H34O3 | 304.28 | 6.3 | 0.96 | 0.0094 | 1.55 | down |
| TXB2 | C20H34O6 | 393.22 | 4.75 | 1.08 | 0.0186 | 1.98 | up |
| Notoginsenoside R1 | C47H80O18 | 478.27 | 2.38 | 1.07 | 0.0004 | 2.07 | up |
| 2-Hydroxymethylclavam | C6H9NO3 | 144.07 | 0.76 | 1.02 | 0.0322 | 1.06 | up |
| Metabolite | Formula | M/Z | RT (Min) | FC | p-Value | VIP | Regulate |
|---|---|---|---|---|---|---|---|
| 5-trans-PGE2 | C20H32O5 | 333.2072 | 5.4926 | 1.032478 | 0.03404 | 1.23838 | up |
| 11beta,20-Dihydroxy-3-oxopregn-4-en-21-oic acid | C22H30O5 | 419.2053 | 5.4926 | 1.039048 | 0.02904 | 1.25489 | up |
| Orotidine | C10H12N2O8 | 333.0592 | 0.65 | 1.034972 | 0.02517 | 1.268658 | up |
| (S)-Glutamic acid | C5H9NO4 | 146.0447 | 0.6788 | 1.035462 | 0.03697 | 1.329292 | up |
| Flavin adenine dinucleotide (FAD) | C27H33N9O15P2 | 784.1511 | 1.9715 | 0.975401 | 0.0371 | 1.047502 | down |
| Hydroxyphenyllactic acid | C9H10O4 | 181.0498 | 2.1269 | 0.898861 | 1.51E-05 | 2.355815 | down |
| KAPA | C9H17NO3 | 186.1127 | 2.9861 | 0.971601 | 0.04326 | 1.199216 | down |
| 1-(1,2,3,4,5-Pentahydroxypent-1-yl)-1,2,3,4-tetrahydro-beta-carboline-3-carboxylate | C17H22N2O7 | 365.1361 | 1.7469 | 1.043125 | 0.02177 | 1.30918 | up |
| 4-hydroxy Nonenal Glutathione | C19H33N3O8S | 462.192 | 3.3535 | 1.057359 | 0.005853 | 1.52711 | up |
| 3,4-Dimethyl-5-propyl-2-furandecanoic acid | C19H32O3 | 307.2279 | 7.5602 | 1.057021 | 0.04261 | 1.368364 | up |
| LysoPC(O-18:0) | C26H56NO6P | 554.383 | 9.0584 | 1.02186 | 0.004398 | 1.2194 | up |
| Citric acid | C6H8O7 | 191.0189 | 1.5058 | 0.876169 | 0.000109 | 2.687449 | down |
| N-Glycolylneuraminic acid | C11H19NO10 | 324.0935 | 0.7009 | 1.05218 | 0.01219 | 1.55297 | up |
| Hydantoin-5-propionic acid | C6H8N2O4 | 343.0911 | 0.7431 | 1.031465 | 0.0479 | 1.059106 | up |
| Xanthine | C5H4N4O2 | 151.0251 | 1.6527 | 1.107071 | 0.000208 | 2.243746 | up |
| 5′-O-Desmethyl omeprazole | C16H17N3O3S | 368.0461 | 1.6527 | 1.101595 | 9.80E-05 | 2.193221 | up |
| Prolylhydroxyproline | C10H16N2O4 | 227.1033 | 2.0353 | 0.943273 | 0.002148 | 1.599332 | down |
| Cortolone-3-glucuronide | C27H42O11 | 587.269 | 2.3248 | 0.953163 | 0.01874 | 1.39429 | down |
| Gamma-Glutamylisoleucine | C11H20N2O5 | 241.1191 | 2.6473 | 0.902102 | 0.000121 | 2.100603 | down |
| 6-Keto-PGF1alpha | C20H34O6 | 369.2282 | 4.7524 | 1.04205 | 0.01969 | 1.421972 | up |
| Deoxycholic acid | C24H40O4 | 391.2858 | 7.4298 | 0.899243 | 0.03853 | 1.777803 | down |
| LysoPE(18:0/0:0) | C23H48NO7P | 480.3097 | 8.8337 | 1.014879 | 0.005051 | 0.995126 | up |
| LysoPE(0:0/20:1(11Z)) | C25H50NO7P | 506.3251 | 8.8789 | 1.025503 | 0.009524 | 1.055846 | up |
| Stearoylcarnitine | C25H49NO4 | 464.3147 | 9.0584 | 1.023518 | 0.003941 | 1.197591 | up |
| Stearaldehyde | C18H36O | 313.2748 | 9.0136 | 1.123257 | 0.01091 | 2.161813 | up |
| 15(R)-PGE1 | C20H34O5 | 353.2333 | 5.5508 | 1.033359 | 0.04541 | 1.076758 | up |
| 15-deoxy-delta-12,14-PGJ2 | C20H28O3 | 315.1964 | 5.4926 | 1.032549 | 0.03407 | 1.213301 | up |
| Alpha-Phenylcyclohexylglycolic acid | C14H18O3 | 233.1178 | 5.4926 | 1.039793 | 0.04039 | 1.189628 | up |
| Glutamylisoleucine | C11H20N2O5 | 241.1191 | 2.8273 | 0.942313 | 0.000573 | 1.700102 | down |
| Ganglioside GT2 (d18:0/16:0) | C88H154N4O42 | 968.4949 | 2.5782 | 0.95 | 0.00089 | 1.774021 | down |
| 8-Hydroxy-5,6-octadienoic acid | C8H12O3 | 467.2263 | 2.1958 | 0.901608 | 0.0406 | 1.763572 | down |
| Valyl-Hydroxyproline | C10H18N2O4 | 251.1036 | 1.7057 | 0.957097 | 0.01674 | 1.273209 | down |
| N-acetylaspartate | C6H9NO5 | 174.0398 | 1.2671 | 1.068341 | 0.03957 | 1.420163 | up |
| Phosphoenol pyruvate | C3H5O6P | 166.974 | 0.7656 | 0.93201 | 0.02386 | 1.694889 | down |
| Genes 1 | GenBank Accession Number | Primer Sequences (5′–3′) | Product Size/bp |
|---|---|---|---|
| CIV | XM_008252337.2 | F: 5′-CTATTTGGAGCTTGAGCTGGGATGG-3′ R:5′-AAGGCATGTGCGGTGACGATTAC-3′ | 128 |
| COX17 | XM_002716664.3 | F: 5′-CAGGAGAAGAAGCCGCTGAAGC-3′ R:5′-GGGCCTCAATTAGATGTCCACAGTG-3′ | 141 |
| GAPDH | NM_001082253.1 | F: 5′-CACCAGGGCTGCTTTTAACTCT-3′ R:5′-CTTCCCGTTCTCAGCCTTGACC-3′ | 163 |
| β-actin | XM_002722894.3 | F: 5′-CGCAGAAACGAGACGAGATT-3′ R:5′-GCAGAACTTTGGGGACTTTG-3′ | 123 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Liu, H.; Wu, X.; Liu, M.; Yue, Z.; Liu, L.; Li, F. Copper Modulates Mitochondrial Oxidative Phosphorylation to Enhance Dermal Papilla Cells Proliferation in Rex Rabbits. Int. J. Mol. Sci. 2022, 23, 6209. https://doi.org/10.3390/ijms23116209
Li F, Liu H, Wu X, Liu M, Yue Z, Liu L, Li F. Copper Modulates Mitochondrial Oxidative Phosphorylation to Enhance Dermal Papilla Cells Proliferation in Rex Rabbits. International Journal of Molecular Sciences. 2022; 23(11):6209. https://doi.org/10.3390/ijms23116209
Chicago/Turabian StyleLi, Fan, Hongli Liu, Xiaojing Wu, Mengqi Liu, Zhengkai Yue, Lei Liu, and Fuchang Li. 2022. "Copper Modulates Mitochondrial Oxidative Phosphorylation to Enhance Dermal Papilla Cells Proliferation in Rex Rabbits" International Journal of Molecular Sciences 23, no. 11: 6209. https://doi.org/10.3390/ijms23116209
APA StyleLi, F., Liu, H., Wu, X., Liu, M., Yue, Z., Liu, L., & Li, F. (2022). Copper Modulates Mitochondrial Oxidative Phosphorylation to Enhance Dermal Papilla Cells Proliferation in Rex Rabbits. International Journal of Molecular Sciences, 23(11), 6209. https://doi.org/10.3390/ijms23116209






