Ginsenoside Rc Promotes Bone Formation in Ovariectomy-Induced Osteoporosis In Vivo and Osteogenic Differentiation In Vitro
Abstract
:1. Introduction
2. Results
2.1. Ginsenoside Rc Promotes Bone Formation in Ovariectomy-Induced Osteoporosis In Vivo
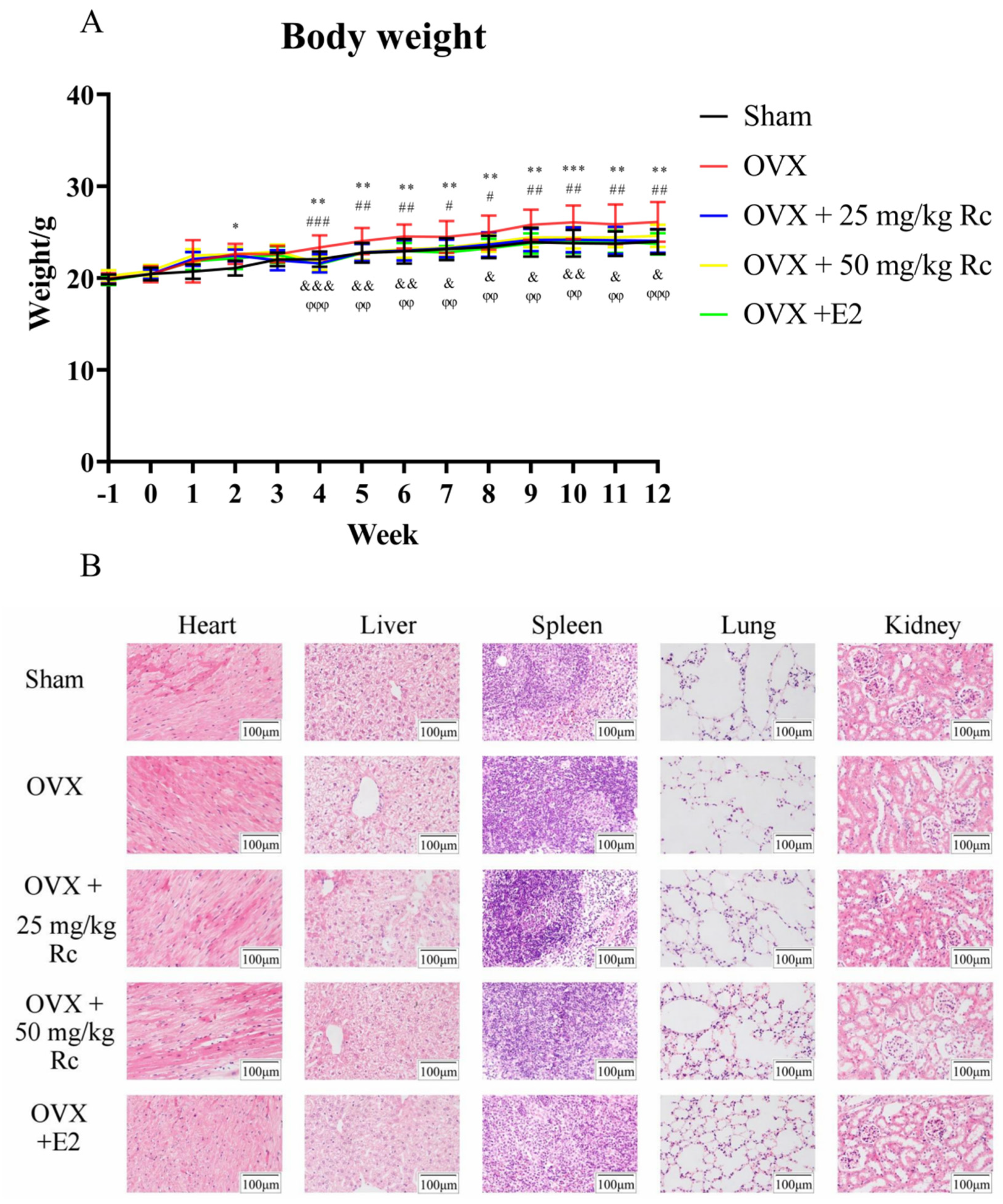
2.1.1. Ovariectomized Mice Have Increased Weight
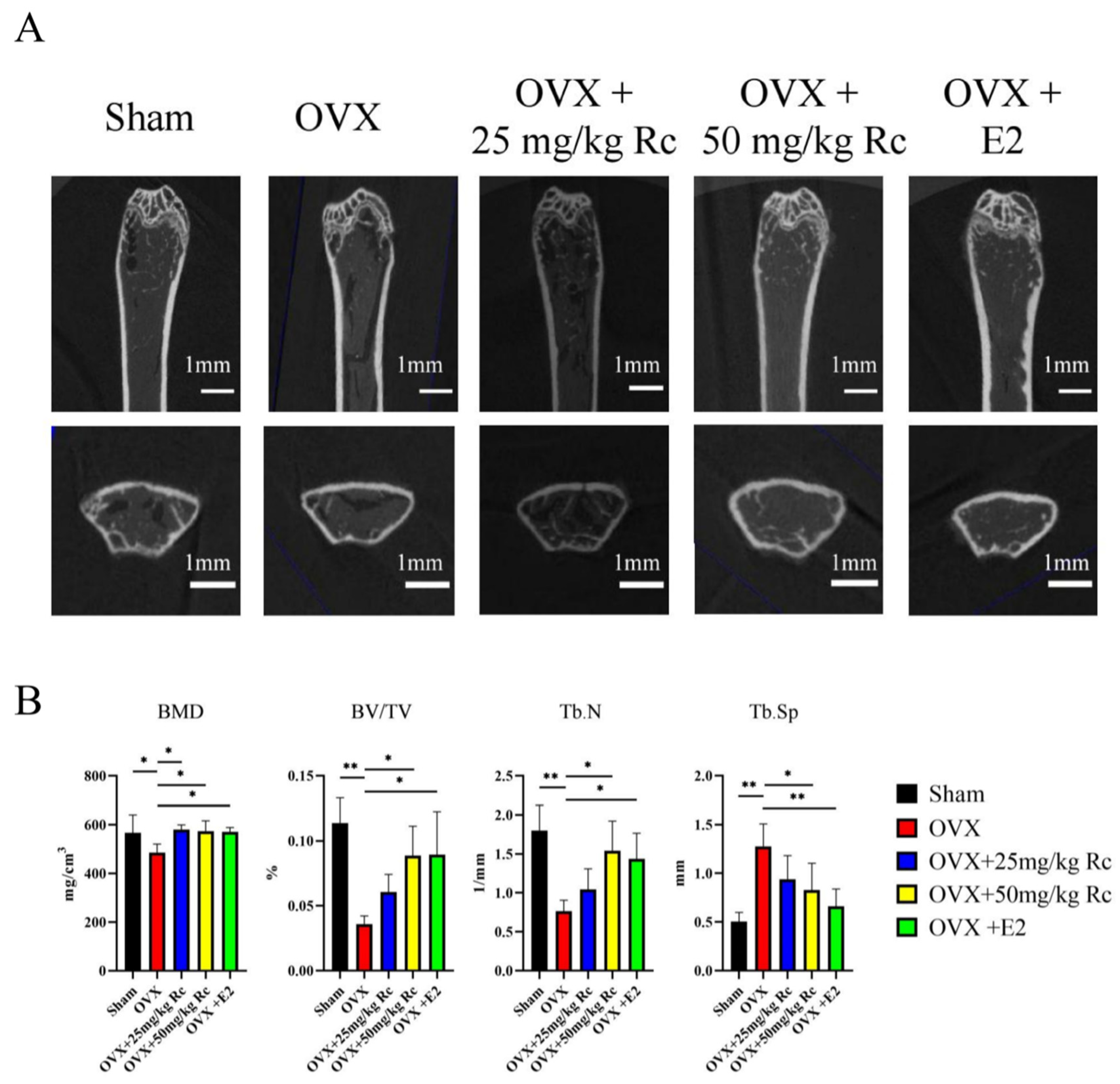
2.1.2. Ginsenoside Rc Can Increase BMD in OVX Mice
2.1.3. Effects of Ginsenoside Rc on Trabecular Bone Tissue of Distal Femur in Mice
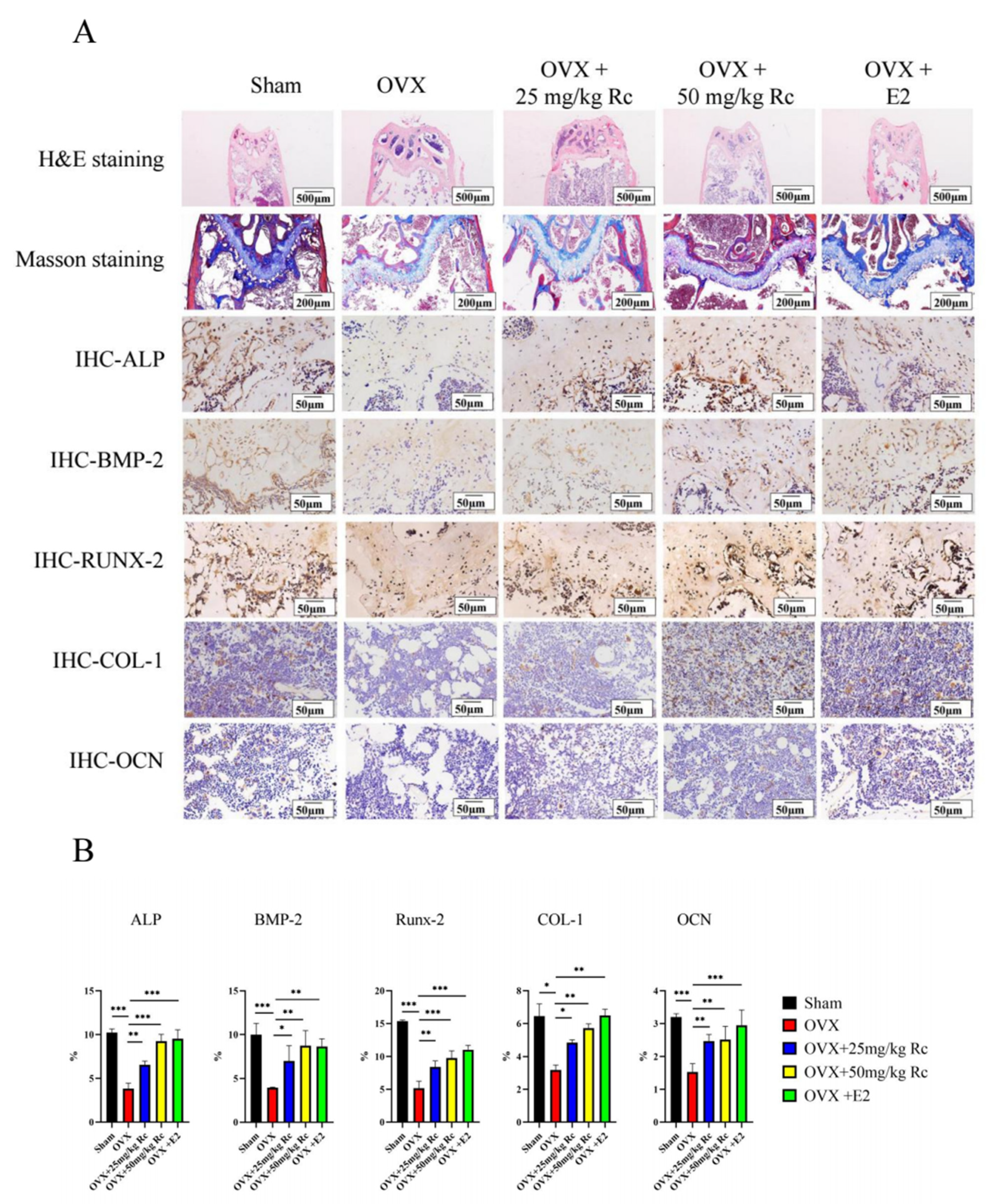
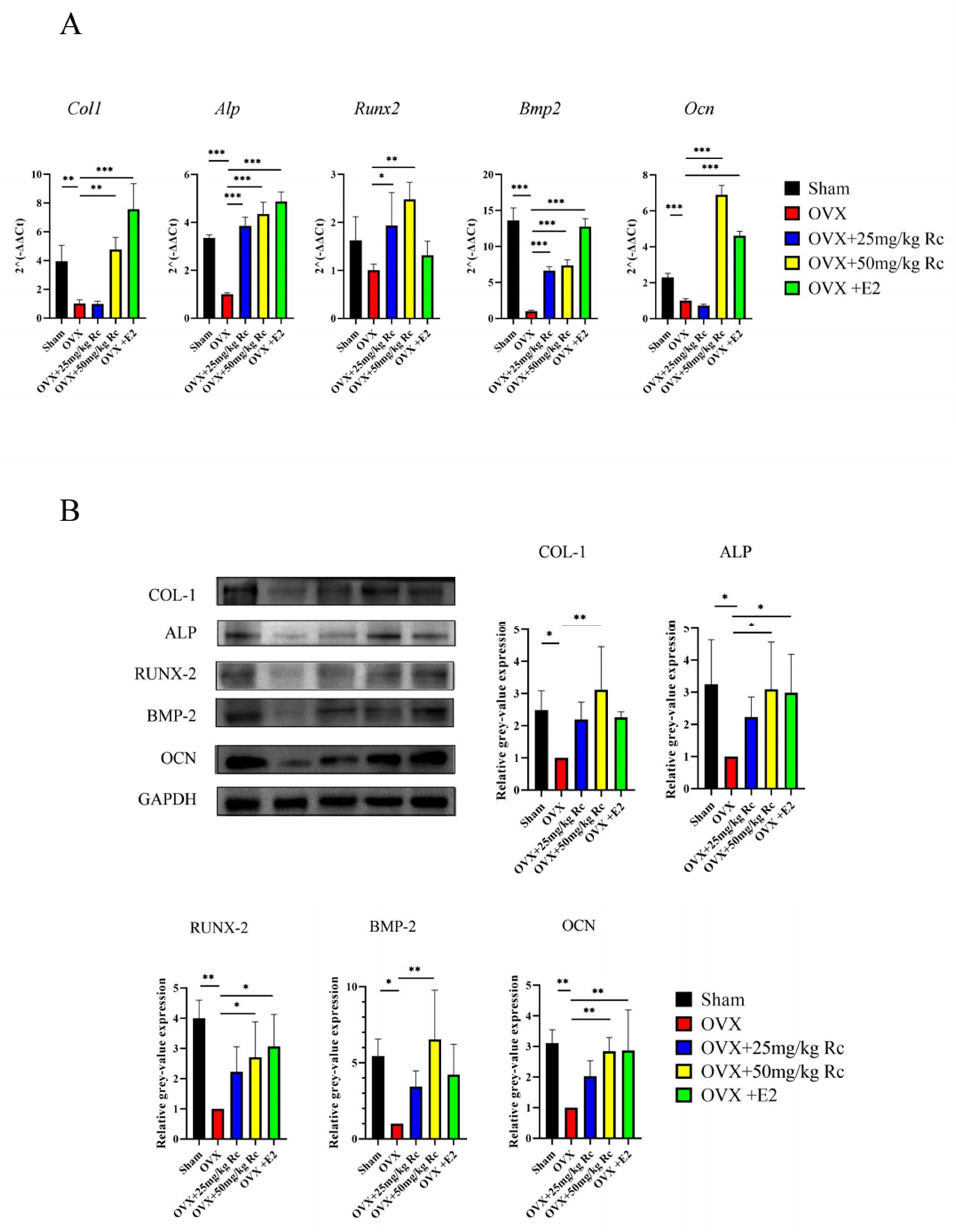
2.1.4. Ginsenoside Rc Prevents OVX-Induced Bone Loss by Promoting the Expression of Bone Formation-Related Genes
2.2. Ginsenoside Rc Promotes Osteogenic Differentiation In Vitro
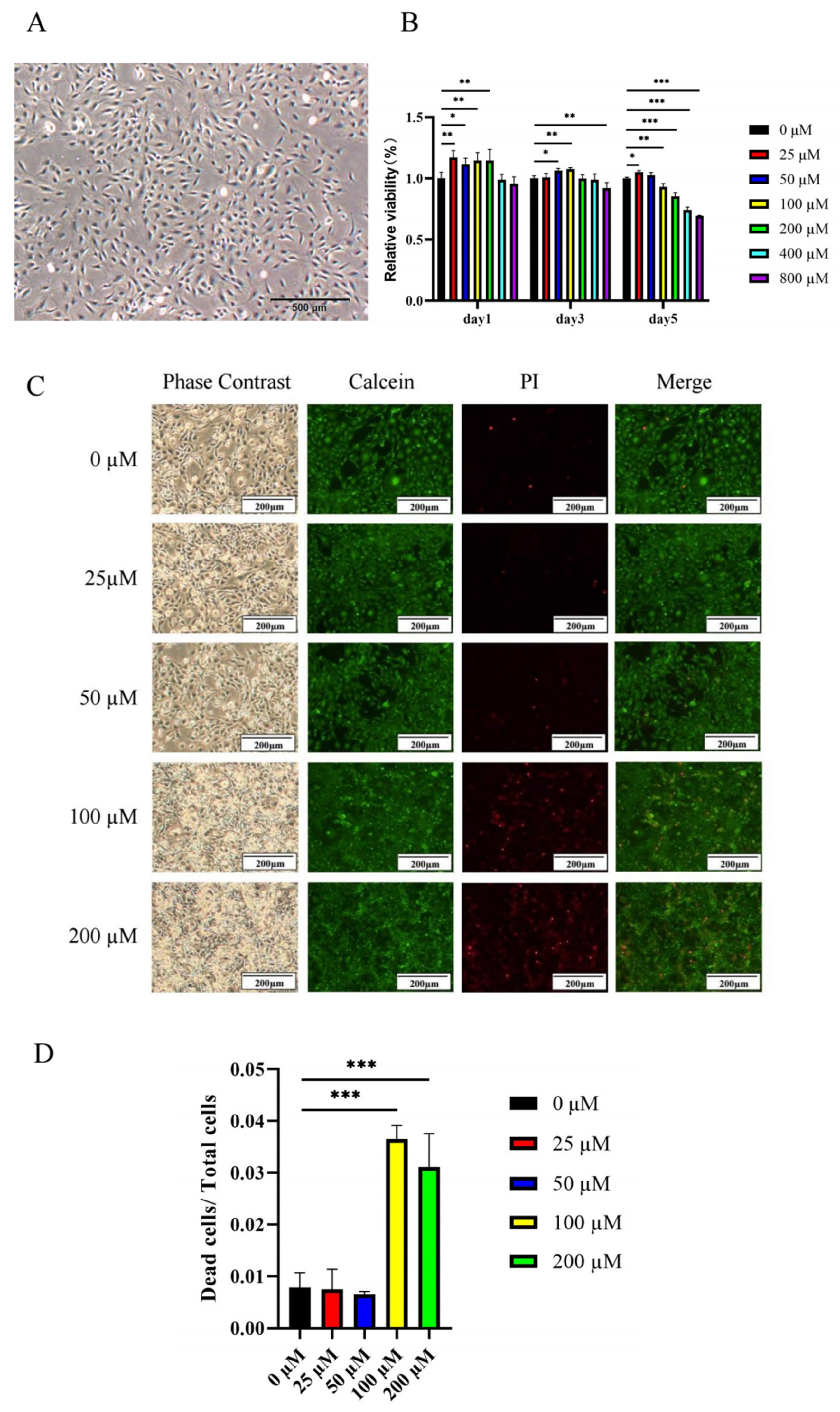
2.2.1. Low Concentrations of Ginsenoside Rc Promote the Viability of MC3T3-E1 Cells
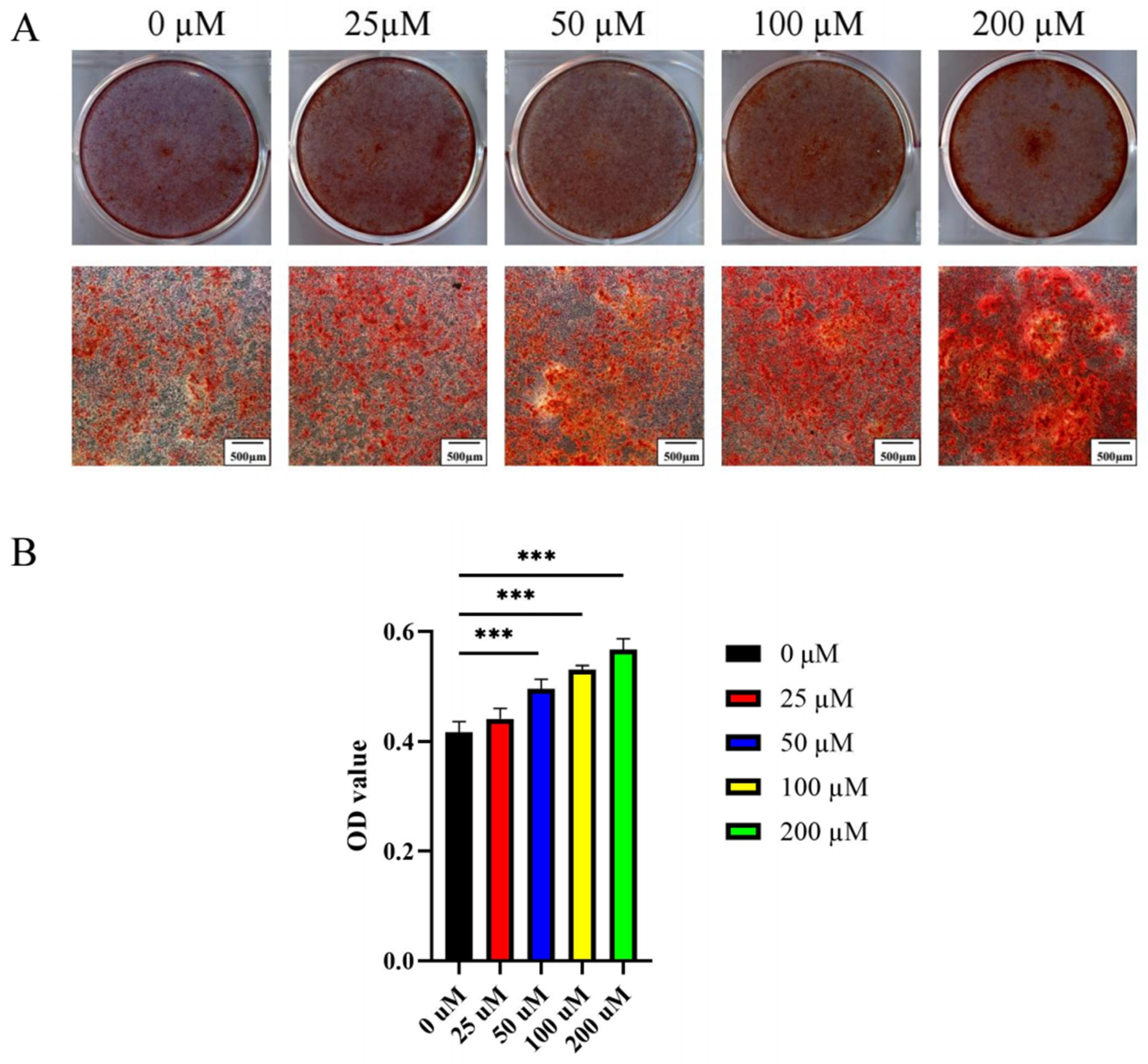
2.2.2. Ginsenoside Rc Dose-Dependently Promotes the Osteogenic Differentiation of MC3T3-E1 Cells
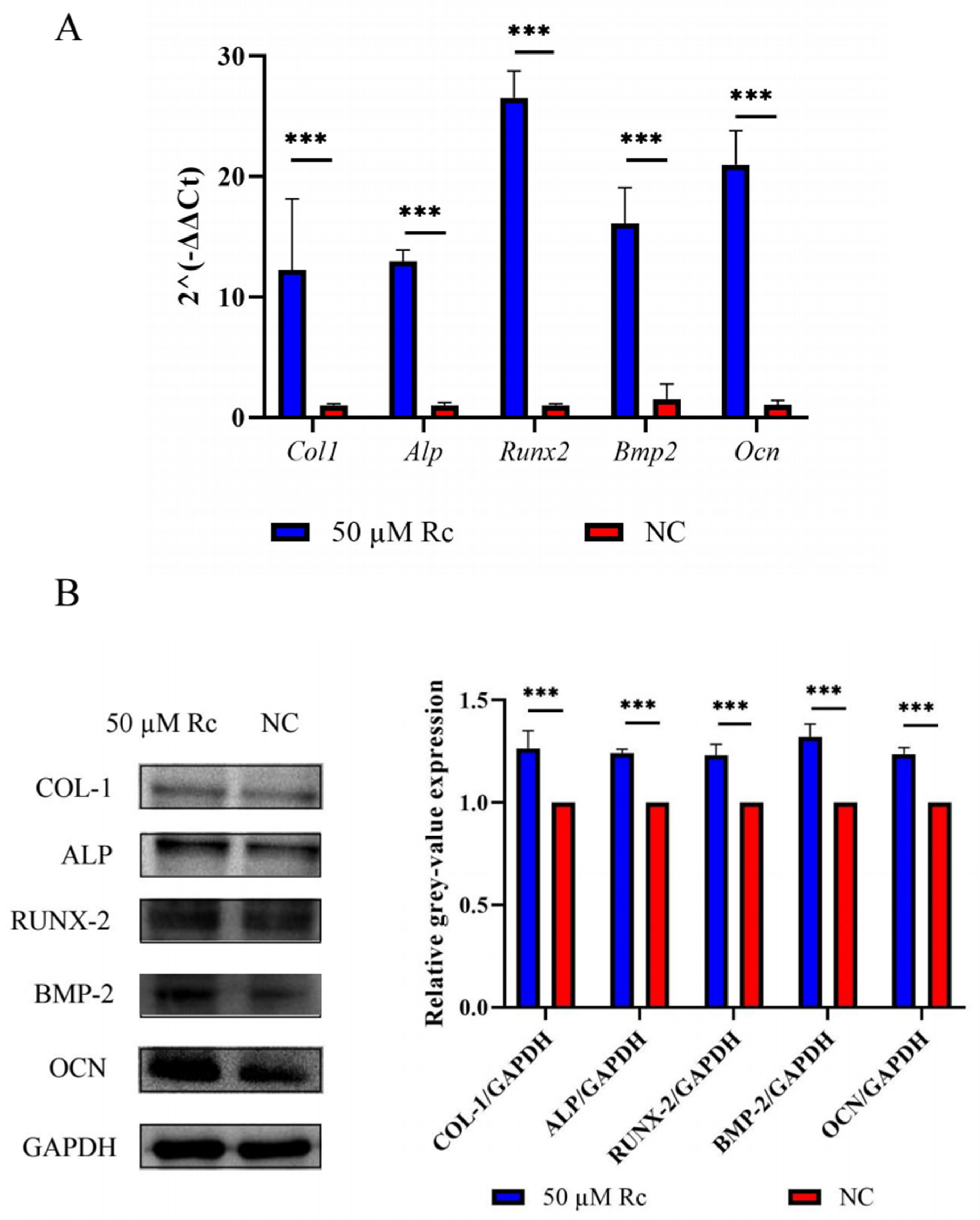
2.2.3. Ginsenoside Rc Promotes Expression of Osteogenesis-Related Genes
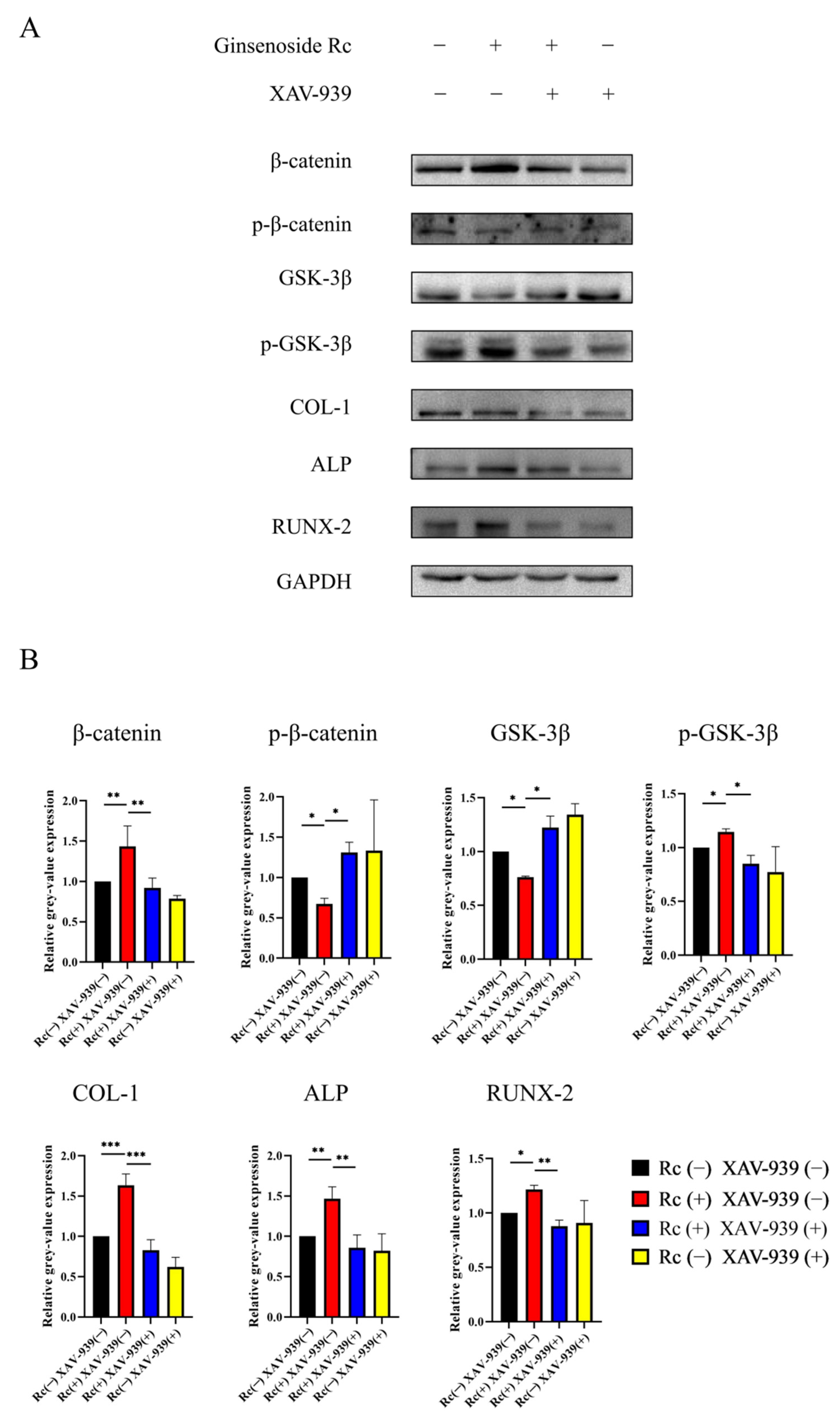
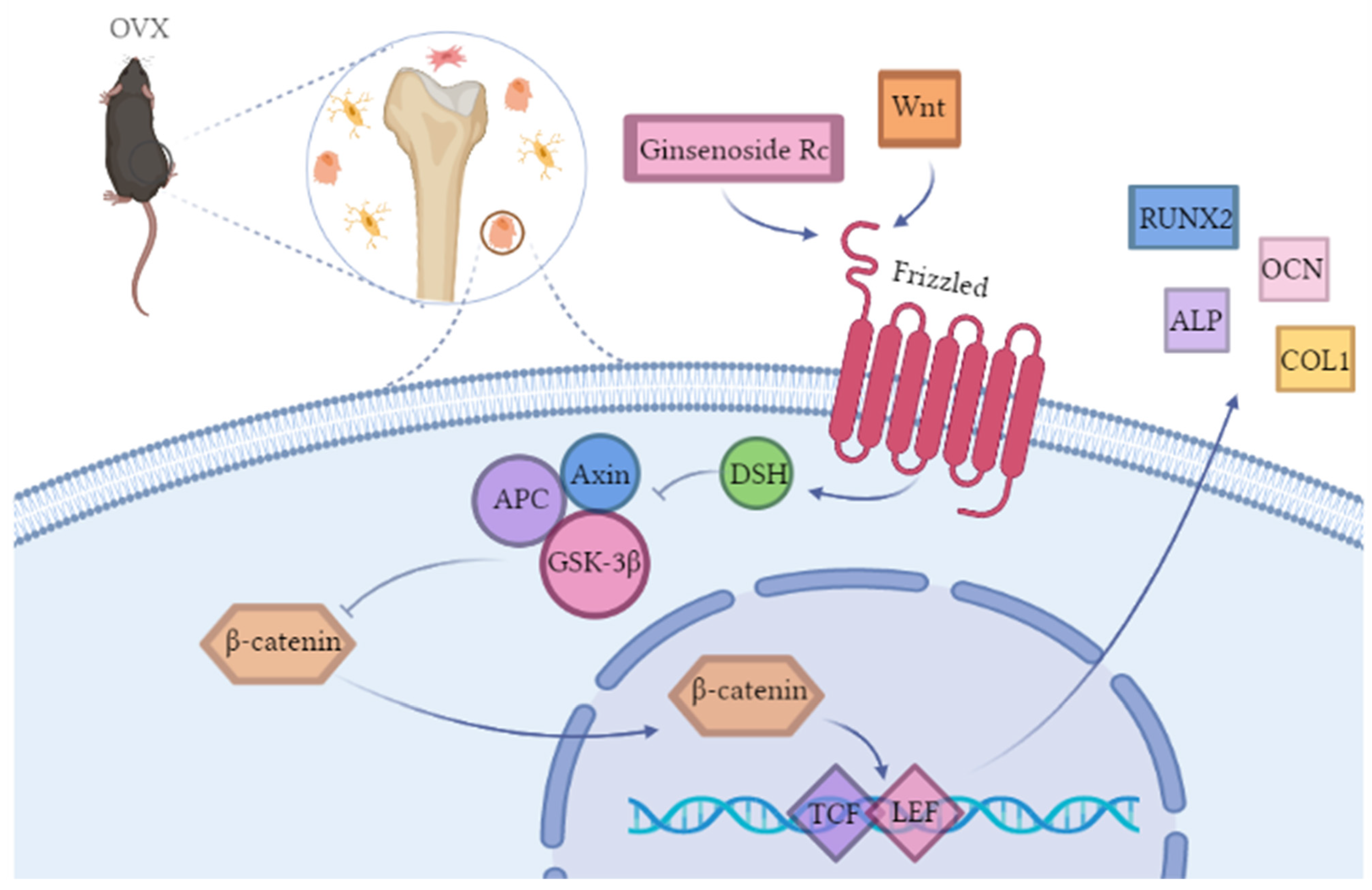
2.2.4. Ginsenoside Rc Promotes Osteogenic Differentiation of MC3T3-E1 Cells via the Wnt/β-Catenin Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Ovariectomy-Induced Osteoporosis Mice Model
4.2. Cell Culture and Differentiation
4.3. Micro-CT
4.4. Histological Analysis of OVX Mice
4.5. qRT-PCR
4.6. Western Blotting
4.7. Cell Viability Assay
4.8. Live-Dead Cell Staining
4.9. ALP Staining and Activity
4.10. Alizarin Red S Staining
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khosla, S.; Hofbauer, L.C. Osteoporosis treatment: Recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 2017, 5, 898–907. [Google Scholar] [CrossRef] [Green Version]
- Teitelbaum, S.L. Bone resorption by osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar] [CrossRef] [PubMed]
- Tomkinson, A.; Reeve, J.; Shaw, R.W.; Noble, B.S. The death of osteocytes via apoptosis accompanies estrogen withdrawal in human bone. J. Clin. Endocrinol. Metab. 1997, 82, 3128–3135. [Google Scholar] [PubMed] [Green Version]
- Lorraine, A.F. Estrogen therapy for postmenopausal osteoporosis. Arq. Bras. Endocrinol. Metab. 2006, 50, 705–719. [Google Scholar]
- Papapoulos, S.; Makras, P. Selection of antiresorptive or anabolic treatments for postmenopausal osteoporosis. Nat. Clin. Pr. Endocrinol. Metab. 2008, 4, 514–523. [Google Scholar] [CrossRef]
- Ettinger, B.; Quesenberry, C.; Schroeder, D.A.; Friedman, G. Long-term postmenopausal estrogen therapy may be associated with increased risk of breast cancer: A cohort study. Menopause 2018, 25, 1191–1194. [Google Scholar] [CrossRef]
- Shin, B.K.; Kwon, S.W.; Park, J.H. Chemical diversity of ginseng saponins from Panax ginseng. J. Ginseng Res. 2015, 39, 287–298. [Google Scholar] [CrossRef] [Green Version]
- Wan, Y.; Wang, J.; Xu, J.F.; Tang, F.; Chen, L.; Tan, Y.-Z.; Rao, C.; Ao, H.; Peng, C. Panax ginseng and its ginsenosides: Potential candidates for the prevention and treatment of chemotherapy-induced side effects. J. Ginseng Res. 2021, 45, 617–630. [Google Scholar] [CrossRef]
- Fu, B.; Wang, N.; Tan, H.Y.; Li, S.; Cheung, F.; Feng, Y. Multi-Component Herbal Products in the Prevention and Treatment of Chemotherapy-Associated Toxicity and Side Effects: A Review on Experimental and Clinical Evidences. Front. Pharm. 2018, 9, 1394. [Google Scholar] [CrossRef] [Green Version]
- Chu, Y.; Zhang, H.C.; Li, S.M.; Wang, J.M.; Wang, X.Y.; Li, W.; Zhang, L.L.; Ma, X.H.; Zhou, S.P.; Zhu, Y.H.; et al. Determination of ginsenoside Rc in rat plasma by LC-MS/MS and its application to a pharmacokinetic study. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 919–920, 75–78. [Google Scholar] [CrossRef]
- Lee, M.S.; Hwang, J.T.; Kim, S.H.; Yoon, S.; Kim, M.S.; Yang, H.J.; Kwon, D.Y. Ginsenoside Rc, an active component of Panax ginseng, stimulates glucose uptake in C2C12 myotubes through an AMPK-dependent mechanism. J. Ethnopharmacol. 2010, 127, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Rhee, M.H.; Lee, J.; Kim, S.H.; Yang, Y.; Kim, H.G.; Kim, Y.; Kim, C.; Kwak, Y.S.; Kim, J.H.; et al. Ginsenoside Rc from Korean Red Ginseng (Panax ginseng C.A. Meyer) Attenuates Inflammatory Symptoms of Gastritis, Hepatitis and Arthritis. Am. J. Chin. Med. 2016, 44, 595–615. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Yang, Y.; Kwak, Y.S.; Song, G.G.; Kim, M.Y.; Rhee, M.H.; Cho, J.Y. Ginsenoside Rc from Panax ginseng exerts anti-inflammatory activity by targeting TANK-binding kinase 1/interferon regulatory factor-3 and p38/ ATF-2. J. Ginseng Res. 2017, 41, 127–133. [Google Scholar] [CrossRef]
- Oh, Y.; Lim, H.W.; Park, K.H.; Huang, Y.H.; Yoon, J.Y.; Kim, K.; Lim, C.J. Ginsenoside Rc protects against UVB-induced photooxidative damage inepidermal keratinocytes. Mol. Med. Rep. 2017, 16, 2907–2914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Z.J.; Li, H.M.; Wang, H.G.; Liu, D.L.; Nan, H. Ginsenoside Rb1 afects the proliferation and osteogenic diferentiation of human adipose-derived stem cells in vitro. Chin. J. Tissue Eng. Res. 2013, 17, 5799–5805. [Google Scholar]
- Huang, Q.; Gao, B.; Jie, Q.; Wei, B.Y.; Fan, J.; Zhang, H.Y.; Zhang, J.K.; Li, X.J.; Shi, J.; Luo, Z.J.; et al. Ginsenoside-Rb2 displays anti-osteoporosis effects through reducing oxidative damage and bone-resorbing cytokines during osteogenesis. Bone 2014, 66, 306–314. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.Y.; Park, Y.G.; Quan, H.Y.; Kim, S.J.; Jung, M.S.; Chung, S.H. Ginsenoside Rd stimulates the diferentiation and mineralization ofosteoblastic MC3T3-E1 cells by activating AMP-activated protein kinase via the BMP-2 signalling pathway. Fitoterapia 2012, 83, 215–222. [Google Scholar] [CrossRef]
- Kim, D.Y.; Jung, M.S.; Park, Y.G.; Yuan, H.D.; Quan, H.Y.; Chung, S.H. Ginsenoside Rh2(S) induces the diferentiation and mineralization of osteoblastic MC3T3-E1 cells through activation of PKD and p38 MAPK pathways. BMB Rep. 2011, 44, 659–664. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.Y.; Park, K.H.; Jung, M.S.; Huang, B.; Yuan, H.D.; Quan, H.Y.; Chung, S.H. Ginsenoside Rh2(S) induces diferentiation and mineralization of MC3T3-E1 cells through activation of the KD/AMPK signalling pathways. Int. J. Mol. Med. 2011, 28, 753–759. [Google Scholar]
- Zhou, W.; Huang, H.; Zhu, H.; Zhou, P.; Shi, X. New metabolites from the biotransformation of ginsenoside Rb1 by Paecilomyces bainier sp.229 and activities in inducing osteogenic differentiation by Wnt/β-catenin signalling activation. J. Ginseng Res. 2018, 42, 199–207. [Google Scholar] [CrossRef]
- Krishnan, V.; Bryant, H.U.; Macdougald, O.A. Regulation of bone mass by Wnt signaling. J. Clin. Investig. 2006, 116, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Lerner, U.H.; Ohlsson, C. The WNT system: Background and its role in bone. J. Intern. Med. 2015, 277, 630–649. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Takahashi, N.; Kobayashi, Y. Roles of Wnt signals in bone resorption during physiological and pathological states. J. Mol. Med. 2013, 91, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.R.; Zhu, G.; Zhang, B.Q.; Luo, Q.; Shi, Q.; Huang, E.; Gao, Y.; Gao, J.-L.; Kim, S.H.; Rastegar, F.; et al. The therapeutic potential of the Wnt signaling pathway in bone disorders. Curr. Mol. Pharm. 2011, 4, 14–25. [Google Scholar] [CrossRef]
- Thompson, D.D.; Simmons, H.A.; Pirie, C.M.; Ke, H.Z. FDA guidelines and animal models forosteoporosis. Bone 1995, 17, 125s–133s. [Google Scholar] [CrossRef]
- Kodama, H.; Amagai, Y.; Sudo, H.; Kasai, S.; Yamamoto, S. Establishment of a clonal osteogenic cell line from newborn mouse calvaria. Jpn. J. Oral Biol. 1981, 23, 899–901. [Google Scholar] [CrossRef] [Green Version]
- Hiura, K.; Sumitani, K.; Kawata, T.; Higashino, K.; Okawa, M.; Sato, T.; Hakeda, Y.; Kumegawa, M. Mouse osteoblastic cells (MC3T3-E1) at different stages of differentiation have opposite effects on osteoclastic cell formation. Endocrinology 1991, 128, 1630–1637. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Takita, H.; Fujisawa, R.; Mizuno, M.; Kuboki, Y. Stimulation by bone sialoprotein of calcification in osteoblast-like MC3T3-E1 cells. Calcif. Tissue Int. 1995, 56, 403–407. [Google Scholar] [CrossRef]
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef]
- Edwards, M.H.; Cooper, C. Bone: The growing cost of fractures in Ireland. Nat. Rev. Endocrinol. 2012, 8, 512–513. [Google Scholar] [CrossRef]
- Banu, J.; Varela, E.; Fernandes, G. Alternative therapies for the prevention and treatment of osteoporosis. Nutr. Rev. 2012, 1, 22–40. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lim, H.; Shehzad, O.; Kim, Y.S.; Kim, H.P. Ginsenosides from Korean red ginseng inhibit matrix metalloproteinase-13 expression in articular chondrocytes and prevent cartilage degradation. Eur. J. Pharm. 2014, 5, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liang, X.; Chen, Y.; Zhao, X. Screening SIRT1 activators from medicinal plants as bioactive compounds against oxidative damage in mitochondrial function. Oxid. Med. Cell. Longev. 2016, 2016, 4206392. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.K.; Choi, Y.G.; Baik, J.Y.; Han, S.Y.; Jeong, D.-W.; Bae, Y.S.; Kim, N.; Lee, S.Y. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood 2005, 106, 852–859. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.Y.; Park, S.H.; Chae, S.W.; Soung, N.K.; Oh, M.J.; Kim, J.S.; Kim, Y.O.; Chae, H.J. Aqueous ginseng extract has a preventive role in RANKL-induced osteoclast diferentiation and estrogen defciency-induced osteoporosis. J. Funct. Food 2015, 13, 192–203. [Google Scholar] [CrossRef]
- Schilling, T.; Ebert, R.; Raaijmakers, N.; Schütze, N.; Jakob, F. Effects of phytoestrogens and other plant-derived compounds on mesenchymal stem cells, bone maintenance and regeneration. J. Steroid Biochem. Mol. Biol. 2014, 139, 252–261. [Google Scholar] [CrossRef]
- Preventing Osteoporosis. J. Midwifery Womens Health 2016, 61, 289–290. [CrossRef] [Green Version]
- Hwang, Y.H.; Kim, K.J.; Kim, S.J.; Mun, S.K.; Hong, S.G.; Son, Y.J.; Yee, S.T. Suppression Effect of Astaxanthin on Osteoclast Formation In Vitro and Bone Loss In Vivo. Int. J. Mol. Sci. 2018, 19, 912. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Zhao, M.; Xiao, G.; Franceschi, R.T. Gene transfer of the Runx2 transcription factor enhances osteogenic activity of bone marrow stromal cells in vitro and in vivo. Mol. Ther. 2005, 12, 247–253. [Google Scholar] [CrossRef]
- Serigano, K.; Sakai, D.; Hiyama, A.; Tamura, F.; Tanaka, M.; Mochida, J. Effect of cell number on mesenchymal stem cell transplantation in a canine disc degeneration model. J. Orthop. Res. 2010, 28, 1267–1275. [Google Scholar] [CrossRef]
- Cosman, F.; Shen, V.; Morgan, D.; Gordon, S.; Parisien, M.; Nieves, J.; Lindsay, R. Biochemical responses of bone metabolism to 1,25-dihydroxyvitamin D administration in black and white women. Osteoporos. Int. 2000, 11, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Pochampally, R.R.; Horwitz, E.M.; DiGirolamo, C.M.; Stokes, D.S.; Prockop, D.J. Correction of a mineralization defect by overexpression of a wild-type cDNA for COL1A1 in marrow stromal cells (MSCs) from a patient with osteogenesis imperfecta: A strategy for rescuing mutations that produce dominant-negative protein defects. Gene Ther. 2005, 12, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Rauch, F.; Glorieux, F.H. Osteogenesis imperfecta. Lancet 2004, 363, 1377–1385. [Google Scholar] [CrossRef]
- Sakkers, R.; Kok, D.; Engelbert, R.; van Dongen, A.; Jansen, M.; Pruijs, H.; Verbout, A.; Schweitzer, D.; Uiterwaal, C. Skeletal efects and functional outcome with olpadronate in children with osteogenesis imperfecta: A 2-year randomised placebo-controlled study. Lancet 2004, 363, 1427–1431. [Google Scholar] [CrossRef]
- Guicheux, J.; Lemonnier, J.; Ghayor, C.; Suzuki, A.; Palmer, G.; Caverzasio, J. Activation of p38 mitogen-activated protein kinase and c-Jun-NH2-terminal kinase by BMP-2 and their implication in the stimulation of osteoblastic cell diferentiation. J. Bone Min. Res. 2003, 18, 2060–2068. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Patel, M.S.; Levasseur, R.; Lobov, I.; Chang, B.H.; Glass, D.A.; Hartmann, C.; Li, L.; Hwang, T.H.; Brayton, C.F.; et al. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice defcient in Lrp5, a Wnt coreceptor. J. Cell Biol. 2002, 157, 303–314. [Google Scholar] [CrossRef]
- Gregory, C.A.; Gunn, W.G.; Reyes, E.; Smolarz, A.J.; Munoz, J.; Spees, J.L.; Prockop, D.J. How Wnt signalling afects bone repair by mesenchymal stem cells from the bone marrow. Ann. N. Y. Acad. Sci. 2005, 1049, 97–106. [Google Scholar] [CrossRef]
- Kestler, H.A.; Kühl, M. From individual Wnt pathways towards a Wnt signaling network. Philos. Trans. R Soc. Lond B Biol. Sci. 2008, 363, 1333–1347. [Google Scholar] [CrossRef] [Green Version]
- Kieslinger, M.; Folberth, S.; Dobreva, G.; Dorn, T.; Croci, L.; Erben, R.; Consalez, G.G.; Grosschedl, R. EBF2 regulates osteoblast-dependent differentiation of osteoclasts. Dev. Cell 2005, 9, 757–767. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.C.; Worton, L.; Estehen, L.; Baldock, P.; Fong, C.; Eisman, J.A.; Gardiner, E.M. Effects of continuous activation of vitamin D and Wnt response pathways on osteoblastic proliferation and differen-tiation. Bone 2007, 41, 87–96. [Google Scholar] [CrossRef]
- Price, M.A. CKI, there’s more than one: Casein kinase I family members in Wnt and Hedgehog signaling. Genes Dev. 2006, 20, 399–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taurin, S.; Sandbo, N.; Qin, Y.; Browning, D.; Dulin, N.O. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase. J. Biol. Chem. 2006, 281, 9971–9976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hua, Y.; Yang, Y.; Li, Q.; He, X.; Zhu, W.; Wang, J.; Gan, X. Oligomerization of frizzled and LRP5/6 protein initiates intracellular signaling for the canonical WNT/β-catenin pathway. J. Biol. Chem. 2018, 293, 19710–19724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, M.D.; Nusse, R. Wnt signaling: Multiple pathways, multiple receptors, and multiple transcription factors. J. Biol. Chem. 2006, 281, 22429–22433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Zhao, X.; Liu, Q.; Li, C.; Graves-Deal, R.; Cao, Z.; Singh, B.; Franklin, J.L.; Wang, J.; Hu, H.; et al. lncRNA MIR100HG-derived miR-100 and miR-125b mediate cetuximab resistance via Wnt/β-catenin signaling. Nat. Med. 2017, 23, 1331–1341. [Google Scholar] [CrossRef]
- Yu, F.; Wu, F.; Li, F.; Liao, X.; Wang, Y.; Li, X.; Wang, C.; Shi, Y.; Ye, L. Wnt7b-induced Sox11 functions enhance self-renewal and osteogenic commitment of bone marrow mesenchymal stem cells. Stem. Cells 2020, 38, 1020–1033. [Google Scholar] [CrossRef]
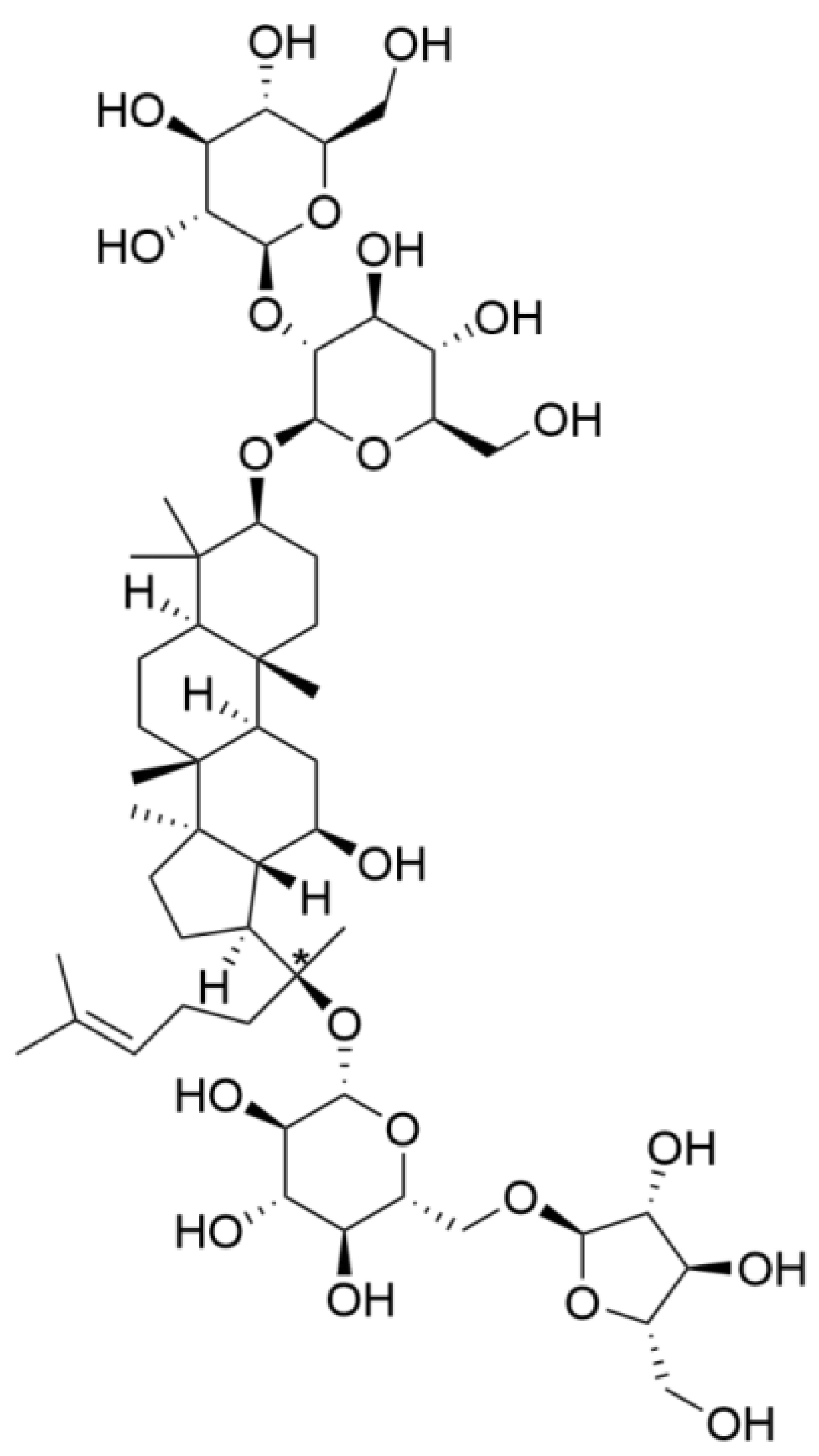

| Genes | Primer Sequence, 5ʹ–3ʹ | |
|---|---|---|
| Forward | Reverse | |
| Runx2 | GATGATGACACTGCCACCTCTGAC | TGAGGGATGAAATGCTTGGGAACTG |
| Alp | CGGCGTCCATGAGCAGAACTAC | CAGGCACAGTGGTCAAGGTTGG |
| Bmp2 | AAGCGTCAAGCCAAACACAAACAG | GAGGTGCCACGATCCAGTCATTC |
| Ocn | CAGAGGAACTGGTTAGCAGGCAAC | ACGCAGGTTCTCAATGGCACAC |
| Col1 | AGGGTCCCGCTGGTCAAGATG | ATGCCTGTTGCTGGTTCTGTAGTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, N.; Zhang, X.; Li, L.; Xu, T.; Li, M.; Zhao, Q.; Yu, J.; Wang, J.; Liu, Z. Ginsenoside Rc Promotes Bone Formation in Ovariectomy-Induced Osteoporosis In Vivo and Osteogenic Differentiation In Vitro. Int. J. Mol. Sci. 2022, 23, 6187. https://doi.org/10.3390/ijms23116187
Yang N, Zhang X, Li L, Xu T, Li M, Zhao Q, Yu J, Wang J, Liu Z. Ginsenoside Rc Promotes Bone Formation in Ovariectomy-Induced Osteoporosis In Vivo and Osteogenic Differentiation In Vitro. International Journal of Molecular Sciences. 2022; 23(11):6187. https://doi.org/10.3390/ijms23116187
Chicago/Turabian StyleYang, Nan, Xiao Zhang, Lingfeng Li, Tongtong Xu, Meihui Li, Qi Zhao, Jinling Yu, Jue Wang, and Zhihui Liu. 2022. "Ginsenoside Rc Promotes Bone Formation in Ovariectomy-Induced Osteoporosis In Vivo and Osteogenic Differentiation In Vitro" International Journal of Molecular Sciences 23, no. 11: 6187. https://doi.org/10.3390/ijms23116187
APA StyleYang, N., Zhang, X., Li, L., Xu, T., Li, M., Zhao, Q., Yu, J., Wang, J., & Liu, Z. (2022). Ginsenoside Rc Promotes Bone Formation in Ovariectomy-Induced Osteoporosis In Vivo and Osteogenic Differentiation In Vitro. International Journal of Molecular Sciences, 23(11), 6187. https://doi.org/10.3390/ijms23116187






