Effect of Acrylamide Treatment on Cyp2e1 Expression and Redox Status in Rat Hepatocytes
Abstract
:1. Introduction
2. Material and Methods
2.1. Animals and Treatment
2.2. Organ Processing and Immunohistochemical (IHC) Staining Procedures
2.3. Quantitative Analyses of Digital Immunohistochemistry Images
2.4. Cell Culture and Treatment
2.5. Cell Viability Assays
2.6. Evaluation of Redox Status and Glutathione-S-Transferase (GST) Activity
2.7. RNA Isolation and Real-Time RT-PCR (RT-qPCR)
2.8. Statistical Analysis
3. Results
3.1. Cell Viability Analysis
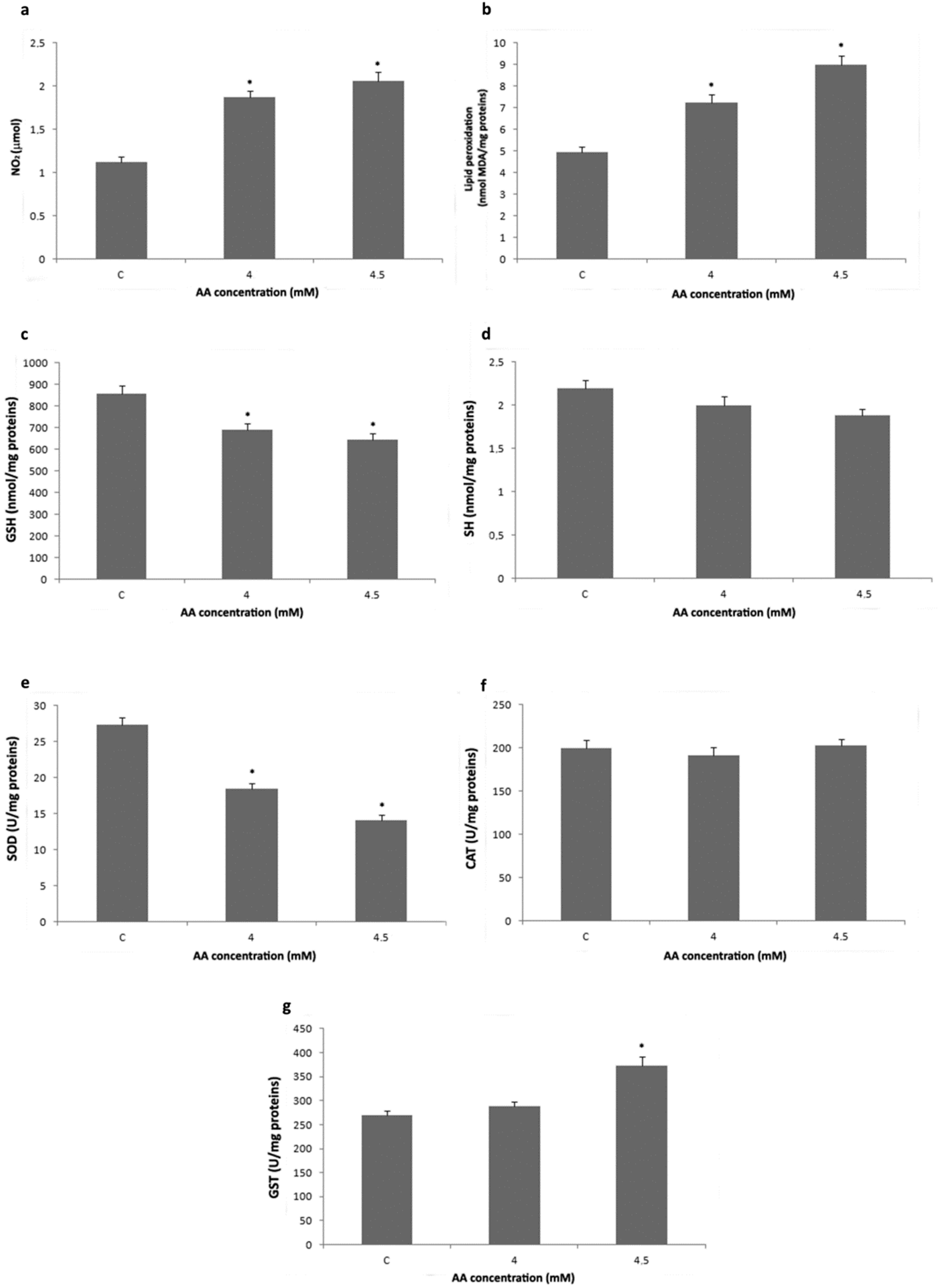
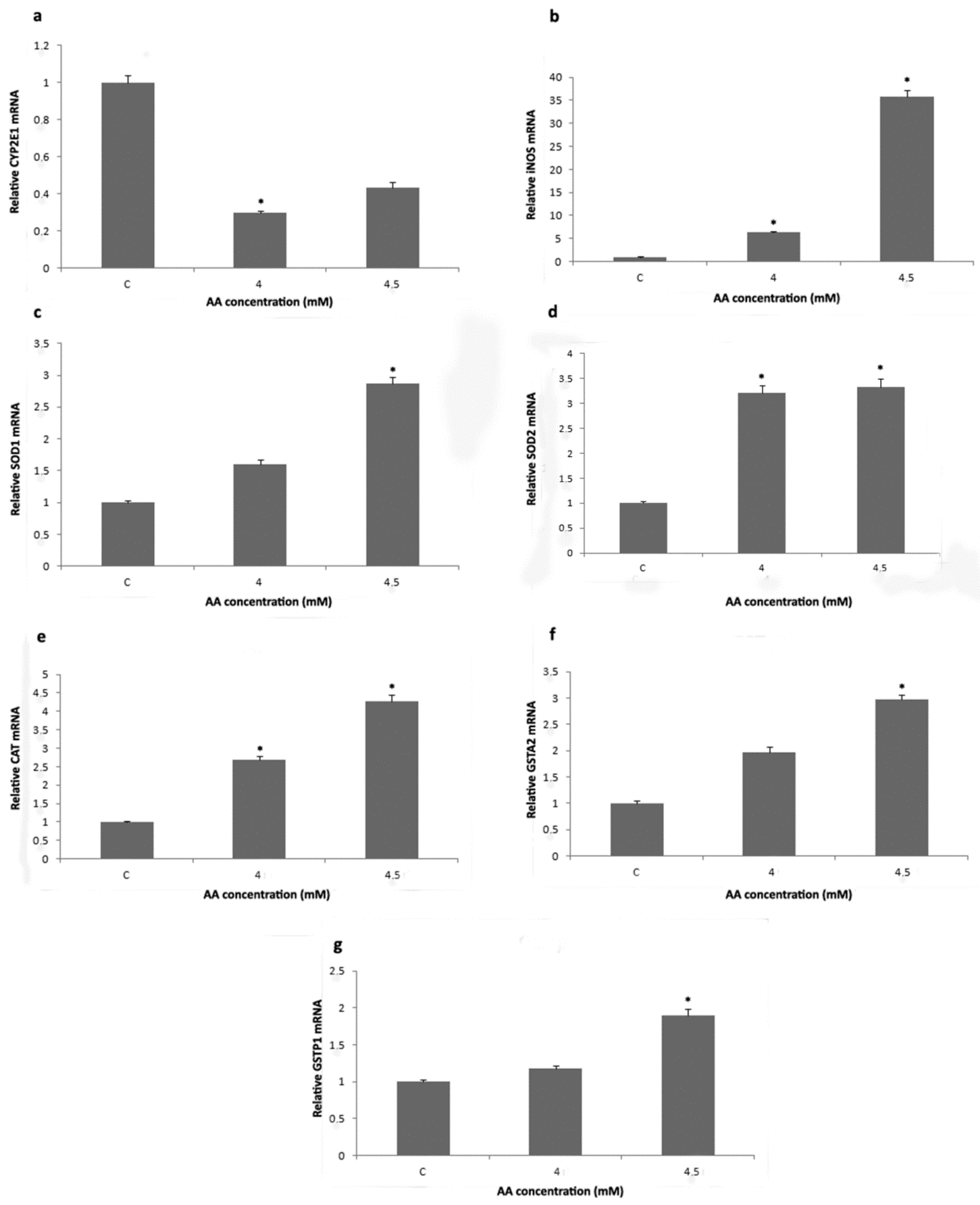
3.2. Analysis of Redox Status and GST Activity
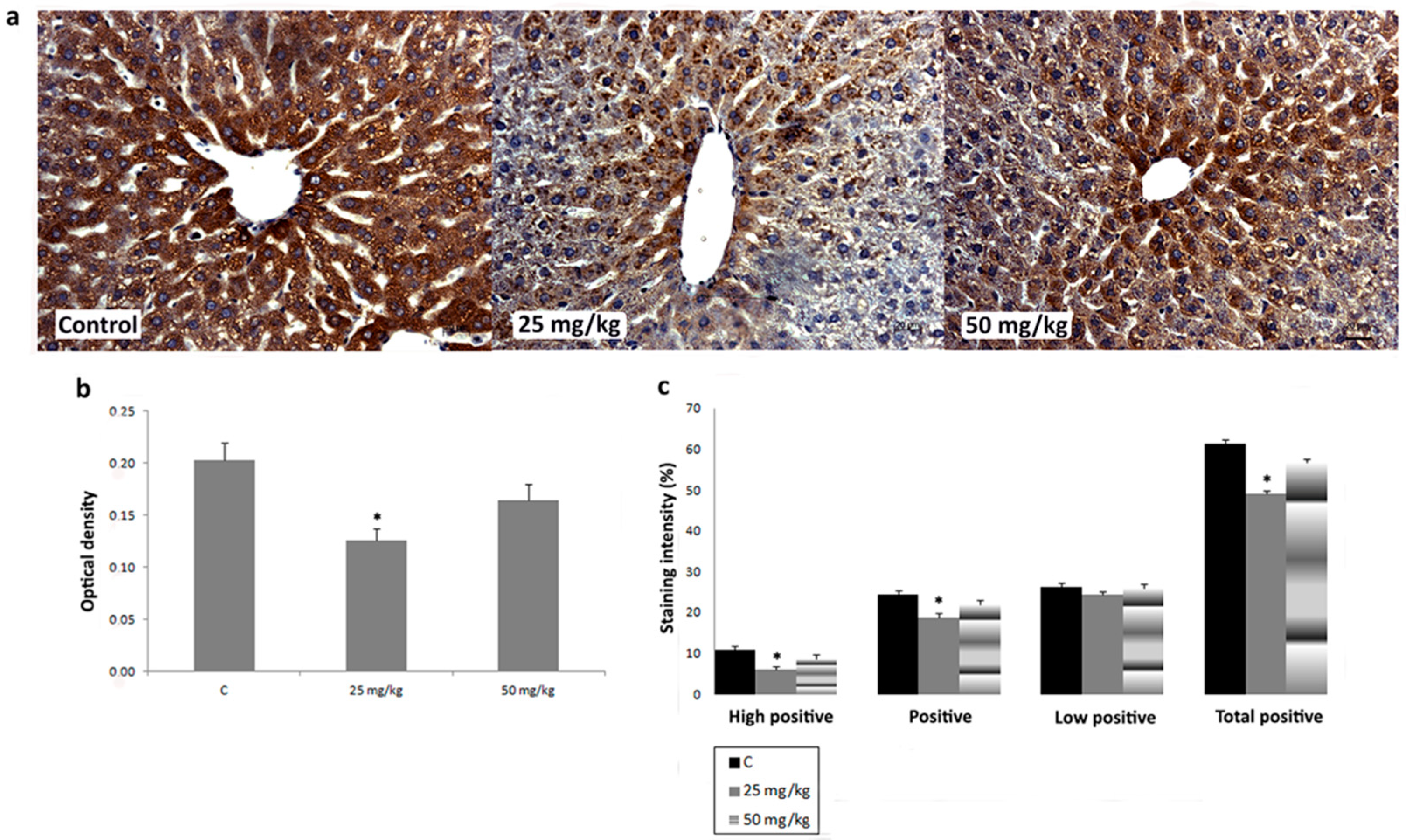
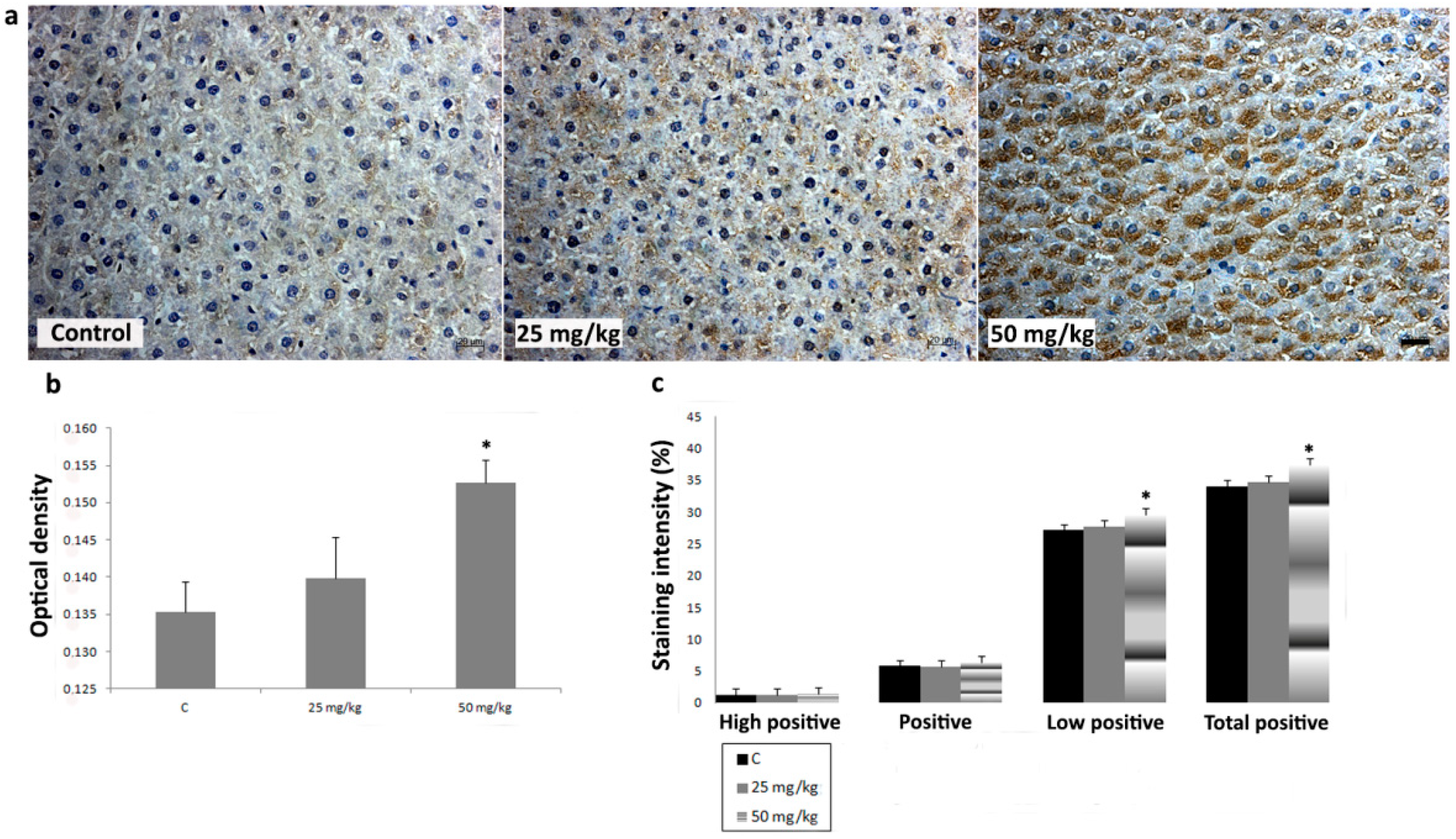
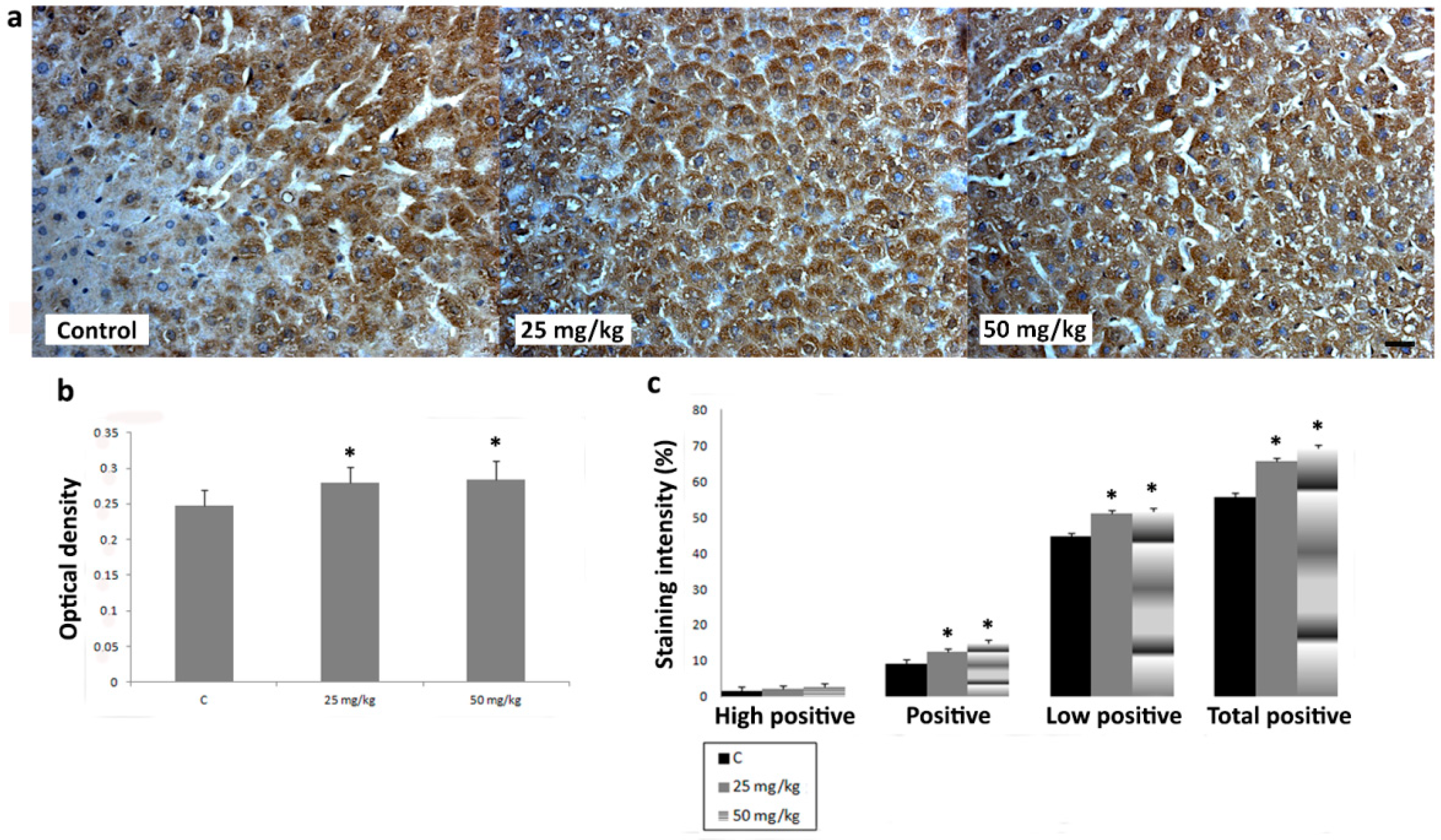
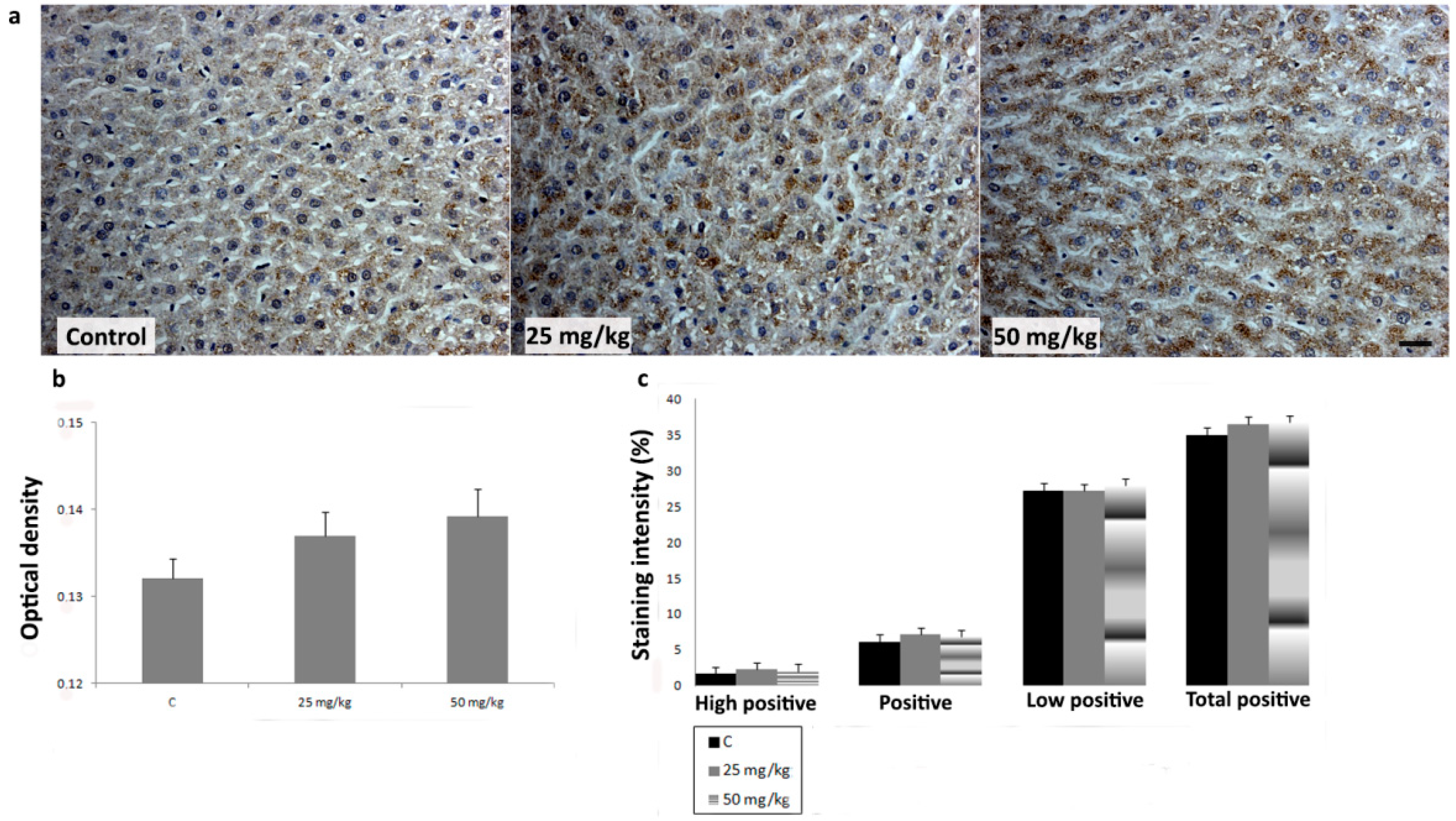
3.3. Immunihistochemical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rice, J.M. The carcinogenicity of acrylamide. Mutat. Res. 2005, 580, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Stošić, M.; Matavulj, M.; Marković, J. Subchronic exposure to acrylamide leads to pancreatic islet remodeling determined by alpha cell expansion and beta cell mass reduction in adult rats. Acta Histochem. 2018, 120, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Patel, D. Gel electrophoresis: Essential Data, 1st ed.; John Wiley and Son: New York, NY, USA, 1994. [Google Scholar]
- Woodrow, J.E.; Seiber, J.N.; Miller, G.C. Acrylamide release resulting from sunlight irradiation of aqueous polyacrylamide/iron mixtures. J. Agr. Food Chem. 2008, 56, 2773–2779. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Liu, K.; Yang, J.; Hong, L.; Xie, X.; Wang, S. Review of Research into the Determination of Acrylamide in Foods. Foods 2020, 9, 524. [Google Scholar] [CrossRef]
- Rifai, L.; Saleh, F.A. A review on acrylamide in food: Occurrence, toxicity, and mitigation strategies. Int. J. Toxicol. 2020, 39, 93–102. [Google Scholar] [CrossRef]
- Törnqvist, M. Acrylamide in food: The discovery and its implications. In Chemistry and Safety of Acrylamide in Food; Friedman, M., Mottram, D., Eds.; Springer Science and Business Media Inc.: New York, NY, USA, 2005; Volume 561, pp. 1–19. [Google Scholar]
- Keramat, J.; LeBail, A.; Prost, C.; Soltanizadeh, N. Acrylamide in foods: Chemistry and analysis. A review. Food Bioprocess. Technol. 2011, 4, 340–363. [Google Scholar] [CrossRef]
- Tareke, E.; Rydberg, P.; Karlsson, P.; Eriksson, S.; Törnqvist, M. Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J. Agr. Food Chem. 2002, 50, 4998–5006. [Google Scholar] [CrossRef]
- Gonzalez, V.; Navarro, C.; Ronco, A.M. Acrylamide in foods: Reference values, recommendations and mitigation actions. Rev. Chil. Nutr. 2021, 48, 109–117. [Google Scholar]
- Codex Alimentarius. Code of Practice for the Reduction of Acrylamide in Foods. CAC/RCP 67-2009. 2009. Available online: http://www.codexalimentarius.org/input/download/standards/11258/CXP_067e.pdf (accessed on 20 December 2019).
- Agencia Española de Seguridad Alimentaria y Nutrición. Acrylamide in Food, New Standards and Recommendations for Your Health. AECOSAN. 2018. Available online: http://www.aecosan.msssi.gob.es/AECOSAN/docs/documentos/noticias/2018/CUADRIPTICO_ACRILAMIDA_AECOSAN.PDF (accessed on 8 December 2018).
- Food and Drug Administration. Guidance for Industry Acrylamide in Foods; FDA: Rockville, MA, USA, 2016. Available online: https://www.fda.gov/media/87150/download (accessed on 18 March 2016).
- Diario Oficial de la Unión Europea. Commission Regulation (EU) 2017/2158. Available online: https://www.boe.es/doue/2017/304/L00024-00044.pdf (accessed on 20 November 2017).
- Fooddrink Europe. Acrylamide. Toolbox 2019. Brussels. Available online: https://www.fooddrinkeurope.eu/uploads/publications_documents/FoodDrinkEurope_Acrylamide_Toolbox_2019.pdf (accessed on 25 March 2019).
- Tardiff, R.G.; Gargas, M.L.; Kirman, C.R.; Carson, M.L.; Sweeney, L.M. Estimation of safe dietary intake levels of acrylamide for humans. Food Chem. Toxicol. 2010, 48, 658–667. [Google Scholar] [CrossRef]
- Wang, H.; Huang, P.; Lie, T.; Li, J.; Hutz, R.J.; Li, K.; Shi, F. Reproductive toxicity of acrylamide-treated male rats. Reprod. Toxicol. 2010, 29, 225–230. [Google Scholar] [CrossRef]
- Chen, W.; Hongming, S.; Yang, X.; Chao, J. In vitro gastrointestinal digestion promotes the protective effect of blackberry extract against acrylamide-induced oxidative stress. Sci. Rep. 2017, 7, 40514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Agency for Research on Cancer (IARC). Monographs on the Evaluation of Carcinogenic Risks to Humans: Some Industrial Chemicals No. 60; IARC: Lyon, France, 1994. [Google Scholar]
- Katen, A.L.; Shaun, D.; Roman, S.A. The genetic consequences of paternal acrylamide exposure and potential for amelioration. Mutat. Res. 2015, 777, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.N. Evidence of acrylamide induced oxidative stress and neurotoxicity in Drosophila melanogaster–Its amelioration with spice active enrichment: Relevance to neuropathy. Neurotoxicology 2012, 33, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Werner, M.; Driftmann, S.; Kleinehr, K.; Kaiser, G.M.; Mathé, Z.; Treckmann, J.W.; Broering, R. All-In-One: Advanced preparation of human parenchymal and non-parenchymal liver cells. PLoS ONE 2015, 10, e0138655. [Google Scholar] [CrossRef] [Green Version]
- Tillitt, D.E.; Giesy, J.P.; Ankley, G.T. Characterization of the H4IIE rat hepatoma cell bioassay as a tool for assessing toxic potency of planar halogenated hydrocarbons in environmental samples. Environ. Sci. Technol. 1991, 25, 87–92. [Google Scholar] [CrossRef]
- Fujimura, H.; Murakami, N.; Miwa, S.; Aruga, C.; Toriumi, W. The suitability of rat hepatoma cell line H4IIE for evaluating the potentials of compounds to induce CYP3A23 expression. Exp. Toxicol. Pathol. 2012, 64, 527–533. [Google Scholar] [CrossRef]
- Exon, J.H. A review of the toxicology of acrylamide. J. Toxicol. Environ. Health B Crit. Rev. 2006, 9, 397–412. [Google Scholar] [CrossRef]
- Zhao, T.; Guo, Y.; Ji, H.; Mao, G.; Feng, W.; Chen, Y.; Yang, L. Short-term exposure to acrylamide exacerbated metabolic disorders and increased metabolic toxicity susceptibility on adult male mice with diabetes. Toxicol. Lett. 2021, 356, 41–53. [Google Scholar] [CrossRef]
- OECD. OECD Guidelines for the Testing of Chemicals. Organization for Economic. Protocol for a Repeated Dose (28 Days) Toxicity (Oral) Study, Based on OECD 407. 2008. Available online: https://www.oecd-ilibrary.org/docserver/9789264070684-en.pdf?expires=1653323855&id=id&accname=guest&checksum=DDA1DA6F4F0E5B4C8132A185A731277B. (accessed on 3 October 2008).
- El-Bohi, K.M.; Moustafa, G.G.; El Sharkawi, N.I.; Sabik, L.M.E. Acrylamide-induced genotoxic, biochemical and pathological perturbations in male rats liver. J. Am. Sc. 2011, 7, 1092–1096. [Google Scholar]
- Marković, J.; Stošić, M.; Kojić, D.; Matavulj, M. Effects of acrylamide on oxidant/antioxidant parameters and CYP2E1 expression in rat pancreatic endocrine cells. Acta. Histochem. 2018, 120, 73–83. [Google Scholar] [CrossRef]
- Ruifrok, A.C.; Johnston, D.A. Quantification of histochemical staining by color deconvolution. Anal. Quant. Cytol. Histol. 2001, 23, 291–299. [Google Scholar] [PubMed]
- Varghese, F.; Bukhari, A.B.; Malhotra, R.; De, A. IHC Profiler: An open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS ONE 2014, 9, e96801. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ghanayem, B.I.; McDaniel, L.P.; Churchwell, M.I.; Twaddle, N.C.; Snyder, R.; Fennell, T.R.; Doerge, D.R. Role of CYP2E1 in the epoxidation of acrylamide to glycidamide and formation of DNA and hemoglobin adducts. Toxicol. Sci. 2005, 88, 311–318. [Google Scholar] [CrossRef] [Green Version]
- Prenzler, P.D.; Omar, S.H.; Ismael, R.; Servili, M.; Esposto, S.; Taticchi, A.; Selvaggini, R.; Urbani, S. Pharmacology of olive biophenols. In Advances in Molecular Toxicology, 1st ed.; Fishbein, J.C., Ed.; Elsevier: Oxford, UK, 2012; Volume 6, pp. 195–242. [Google Scholar]
- Ingelman-Sundberg, M.; Johansson, I.; Penttilä, K.E.; Glaumann, H.; Lindros, K.O. Centrilobular expression of ethanol-inducible cytochrome P-450 (IIE1) in rat liver. Biochem. Biophys. Res. Commun. 1988, 157, 55–60. [Google Scholar] [CrossRef]
- Tsutsumi, M.; Lasker, J.M.; Shimizu, M.; Rosman, A.S.; Lieber, C.S. The intralobular distribution of ethanol-inducible P450IIE1 in rat and human liver. Hepatology 1989, 10, 437–446. [Google Scholar] [CrossRef]
- Singh, M.P.; Jakhar, R.; Kang, S.C. Morin hydrate attenuates the acrylamide-induced imbalance in antioxidant enzymes in a murine model. Int. J. Mol. Med. 2015, 36, 992–1000. [Google Scholar] [CrossRef] [Green Version]
- Denucci, S.M.; Tong, M.; Longato, L.; Lawton, M.; Setshedi, M.; Carlson, R.I.; Wands, J.R.; de la Monte, S.M. Rat strain differences in susceptibility to alcohol-induced chronic liver injury and hepatic insulin resistance. Gastroent. Res. Pract. 2010, 2010, 312790. [Google Scholar] [CrossRef] [Green Version]
- González-Jasso, E.; López, T.; Lucas, D.; Berthou, F.; Manno, M.; Ortega, A.; Albores, A. CYP2E1 regulation by benzene and other small organic chemicals in rat liver and peripheral lymphocytes. Toxicol. Lett. 2003, 144, 55–67. [Google Scholar] [CrossRef]
- Ma, X.-L.; Li, Y.-H.; Gao, J.-X.; Li, J.; Guo, L.; Wu, C.-Z. Expression of inducible nitric oxide synthase in the liver is under the control of nuclear factor kappa B in concanavalin A-induced hepatitis. J. Gastroenterol. Hepatol. 2007, 23, e231–e235. [Google Scholar] [CrossRef]
- Tache, D.-T.; Stănciulescu, C.E.; Baniţă, I.M.; Purcaru, S.O.; Andrei, A.M.; Comănescu, V.; Pisoschi, C.G. Inducible nitric oxide synthase expression (iNOS) in chronic viral hepatitis and its correlation with liver fibrosis. Rom. J. Morphol. Embryol. 2014, 55, 539–543. [Google Scholar] [PubMed]
- Iwakiri, Y. Nitric oxide in liver fibrosis: The role of inducible nitric oxide synthase. Clin. Mol. Hepatol. 2015, 21, 319–325. [Google Scholar] [CrossRef]
- Lyn-Cook, L.E., Jr.; Tareke, E.; Word, B.; Starlard-Davenport, A.; Lyn-Cook, B.D.; Hammons, G.J. Food contaminant acrylamide increases expression of Cox-2 and nitric oxide synthase in breast epithelial cells. Toxicol. Ind. Health. 2011, 27, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, P.; Larsson, R.; Krupitza, G.; Muehlematter, D.; Crawford, D.; Amstad, P. Pathophysiological mechanisms of oxidants. In Oxy-Radicals in Molecular Biology and Pathology, 1st ed.; Cerutti, P., Ed.; Alan R. Liss: New York, NY, USA, 1988; Volume 214, pp. 81–88. [Google Scholar]
- Hussain, S.P.; Amstad, P.; He, P.; Robles, A.; Lupold, S.; Kaneko, I.; Ichimiya, M.; Sengupta, S.; Mechanic, L.; Okamura, S.; et al. p53-induced up-regulation of MnSOD and GPx but not catalase increases oxidative stress and apoptosis. Cancer Res. 2004, 64, 2350–2356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Huangyou, L.; Lingzhu, L.; Haiyang, Y.; Yuan, Y. Antioxidant activity of blueberry anthocyanin extracts and their protective effects against acrylamide-induced toxicity in HepG2 cells. Int. J. Food. Sci. Technol. 2018, 53, 147–155. [Google Scholar] [CrossRef]
- Chu, Q.; Chen, W.; Ruoyi, J.; Xiang, Y.; Yonglu, L.; Yangyang, L.; Yong, J.; Xiaodong, Z. Tetrastigma hemsleyanum leaves extract against acrylamide-induced toxicity in HepG2 cells and Caenorhabditis elegans. J. Hazard. Mater. 2020, 393, 122364. [Google Scholar] [CrossRef]
- Sadek, K.M. Antioxidant and immunostimulant effect of Carica papaya Linn. Aqueous extract in acrylamide intoxicated rats. Acta Inform. Med. 2012, 20, 180–185. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Wang, P.; Zhu, Y.; Liu, X.; Hu, X.; Chen, F. The chemoprotection of a blueberry anthocyanin extract against the acrylamide-induced oxidative stress in mitochondria: Unequivocal evidence in mice liver. Food Funct. 2015, 6, 3006–3012. [Google Scholar] [CrossRef]
- Omar, R.; Chyan, Y.-J.; Andorn, A.C.; Poeggeler, B.; Robakis, N.K.; Pappolla, M.A. Increased expression but reduced activity of antioxidant enzymes in Alzheimer’s disease. J. Alzheimers Dis. 1999, 1, 139–145. [Google Scholar] [CrossRef]
- Parzefall, W. Minireview on the toxicity of dietary acrylamide. Food Chem. Toxicol. 2008, 46, 1360–1364. [Google Scholar] [CrossRef]
- Awad, M.E.; Abdel-Rahman, M.S.; Hassan, S.A. Acrylamide toxicity in isolated rat hepatocytes. Toxicol. In Vitro 1998, 12, 699–704. [Google Scholar] [CrossRef]
- Azari, A.; Shokrzadeh, M.; Zamani, E.; Amani, N.; Shaki, F. Cerium oxide nanoparticles protects against acrylamide induced toxicity in HepG2 cells through modulation of oxidative stress. Drug Chem. Toxicol. 2019, 42, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Alturfan, E.I.; Beceren, A.; Şehirli, A.Ö.; Demiral, Z.E.; Şener, G.; Omurtag, G.Z. Protective effect of N-acetyl-L-cysteine against acrylamide-induced oxidative stress in rats. Turk. J. Vet. Anim. Sci. 2012, 36, 438–445. [Google Scholar]
- El-Beltagi, H.S.; Ahmed, M.M. Assessment the protective role of quercetin on acrylamide induced oxidative stress in rats. J. Food Biochem. 2016, 40, 715–723. [Google Scholar] [CrossRef]
- Yousef, M.I.; El-Demerdash, F.M. Acrylamide induced oxidative stress and biochemical perturbations in rats. Toxicology 2006, 219, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Arnér, E.S.; Holmgren, A. Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem. 2000, 267, 6102–6109. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marković Filipović, J.; Miler, M.; Kojić, D.; Karan, J.; Ivelja, I.; Čukuranović Kokoris, J.; Matavulj, M. Effect of Acrylamide Treatment on Cyp2e1 Expression and Redox Status in Rat Hepatocytes. Int. J. Mol. Sci. 2022, 23, 6062. https://doi.org/10.3390/ijms23116062
Marković Filipović J, Miler M, Kojić D, Karan J, Ivelja I, Čukuranović Kokoris J, Matavulj M. Effect of Acrylamide Treatment on Cyp2e1 Expression and Redox Status in Rat Hepatocytes. International Journal of Molecular Sciences. 2022; 23(11):6062. https://doi.org/10.3390/ijms23116062
Chicago/Turabian StyleMarković Filipović, Jelena, Marko Miler, Danijela Kojić, Jelena Karan, Ivana Ivelja, Jovana Čukuranović Kokoris, and Milica Matavulj. 2022. "Effect of Acrylamide Treatment on Cyp2e1 Expression and Redox Status in Rat Hepatocytes" International Journal of Molecular Sciences 23, no. 11: 6062. https://doi.org/10.3390/ijms23116062
APA StyleMarković Filipović, J., Miler, M., Kojić, D., Karan, J., Ivelja, I., Čukuranović Kokoris, J., & Matavulj, M. (2022). Effect of Acrylamide Treatment on Cyp2e1 Expression and Redox Status in Rat Hepatocytes. International Journal of Molecular Sciences, 23(11), 6062. https://doi.org/10.3390/ijms23116062





