Angiotensin II Inhibits Insulin Receptor Signaling in Adipose Cells
Abstract
1. Introduction
2. Results
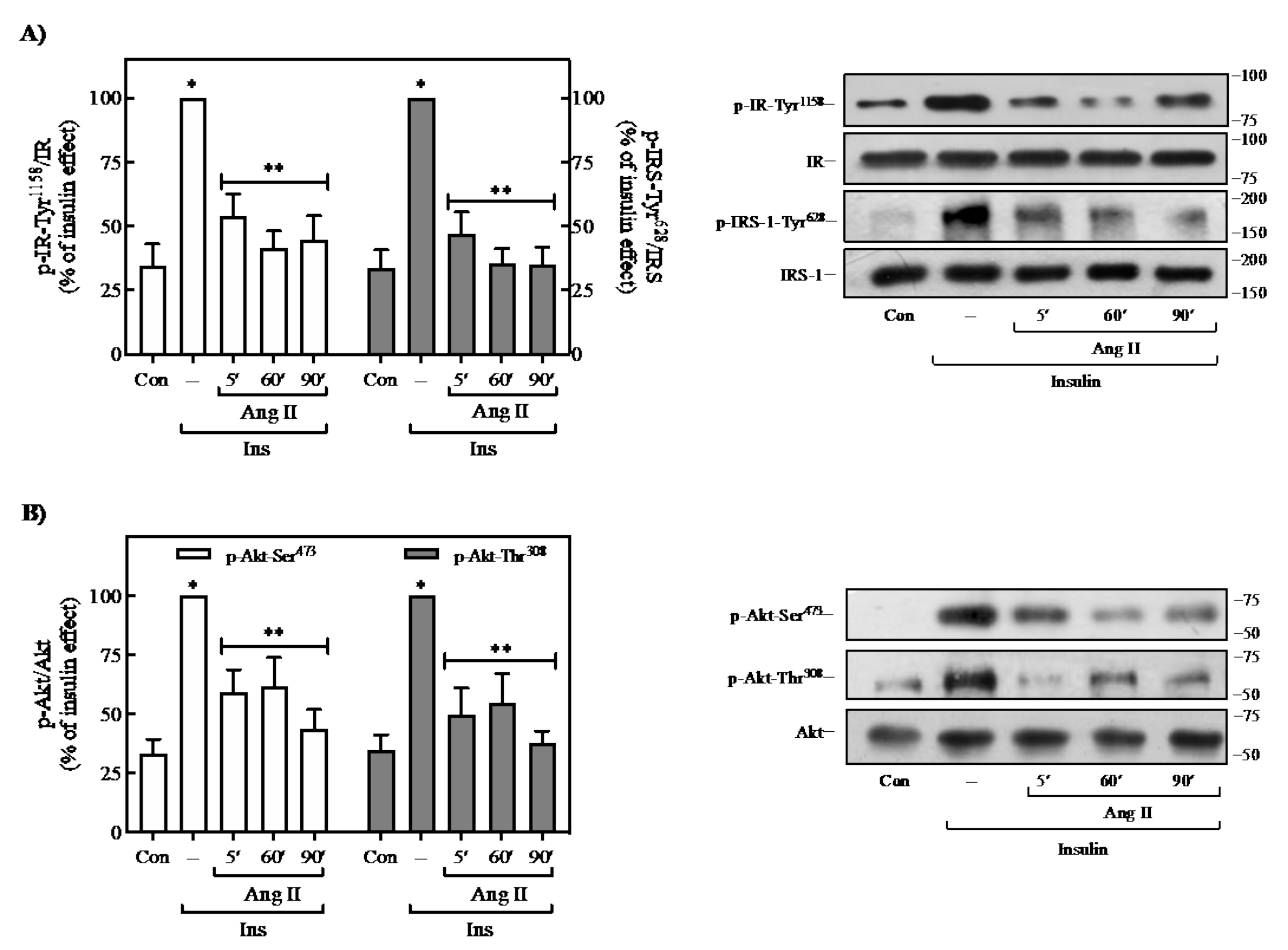
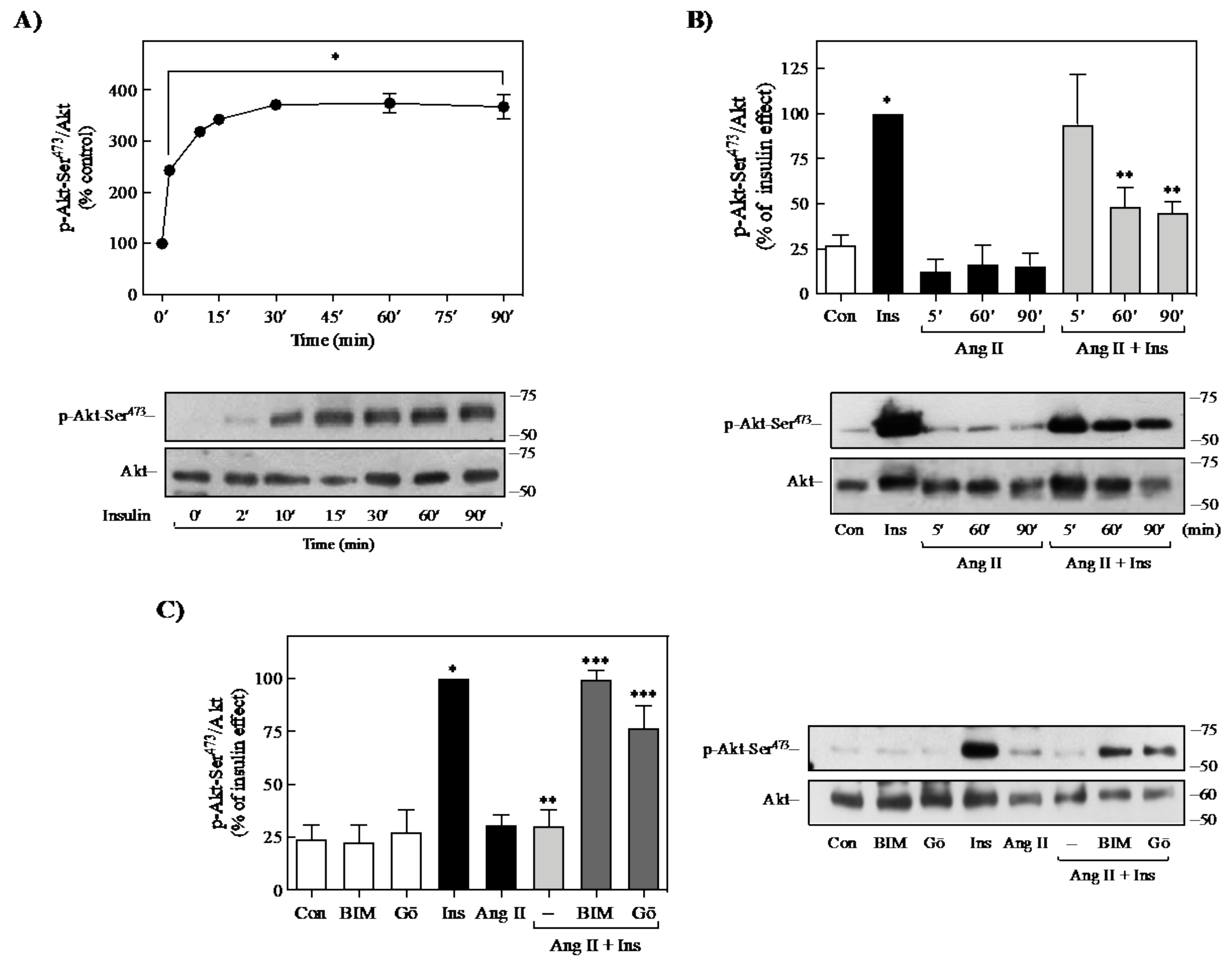
2.1. Effect of Ang II on Insulin-Induced Signaling in 3T3-L1 Adipocytes
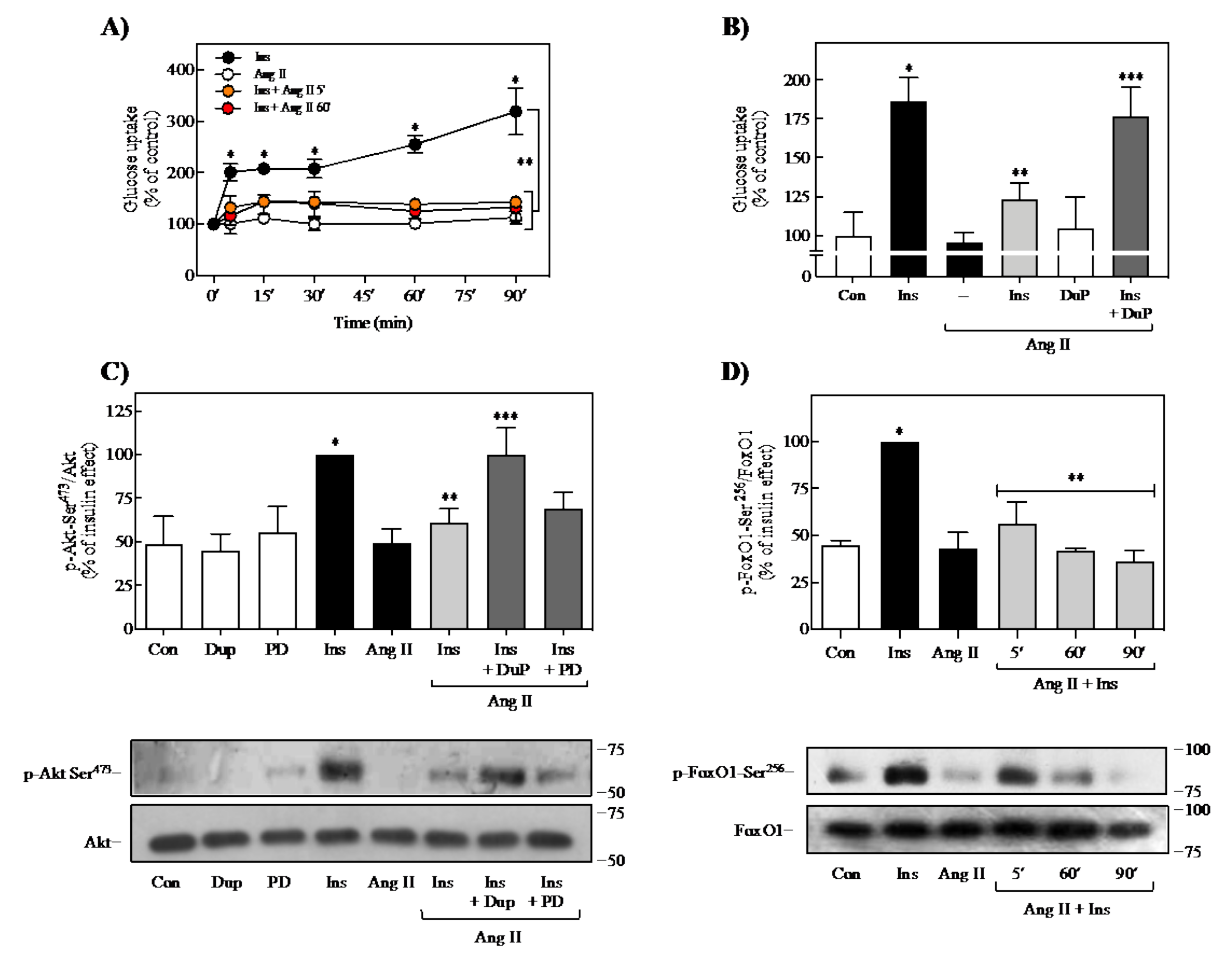
2.2. Effect of Ang II on Insulin-Induced Glucose Uptake and FoxO1 Regulation
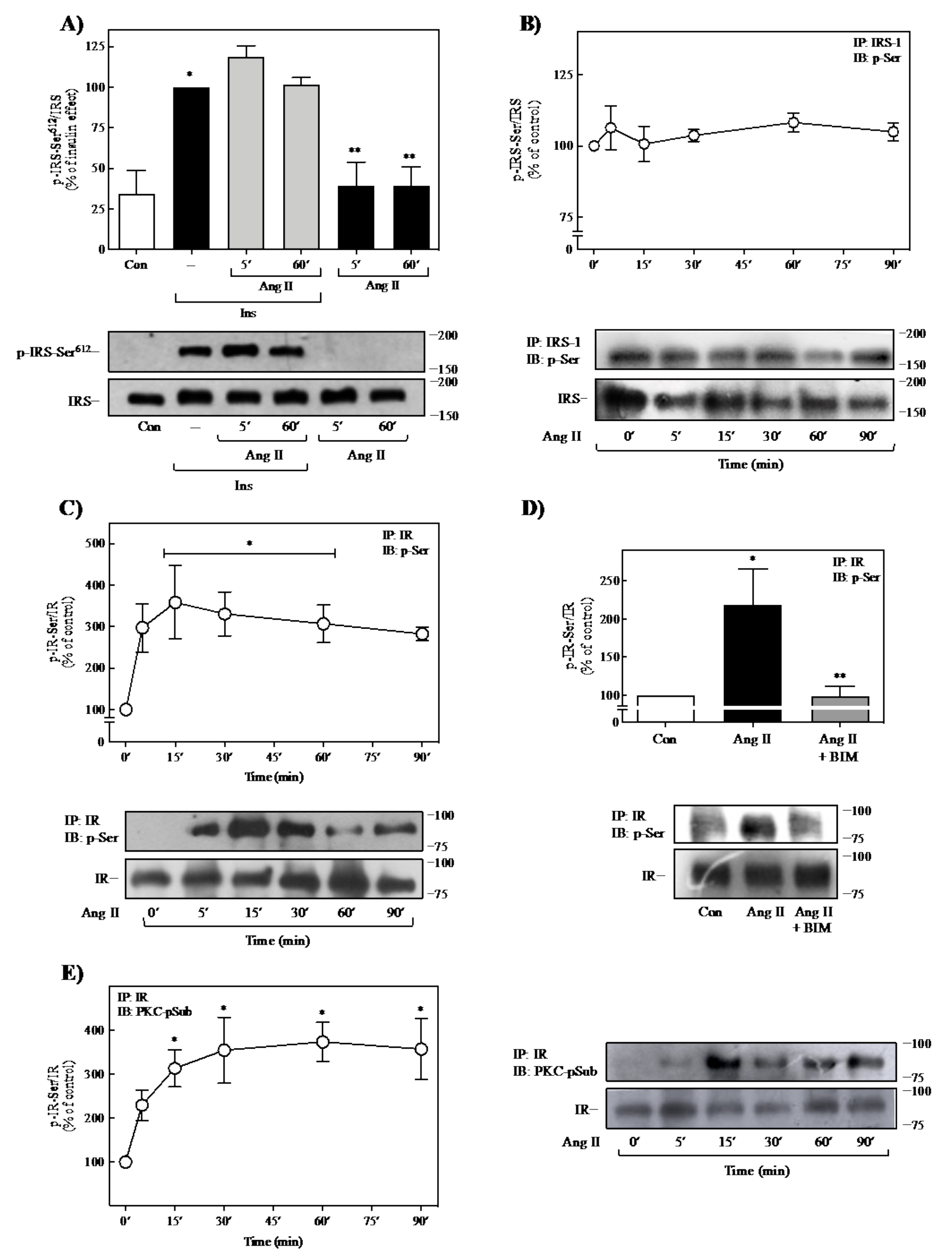
2.3. Effect of Ang II on IR- and IRS-1-Ser-Phosphorylation
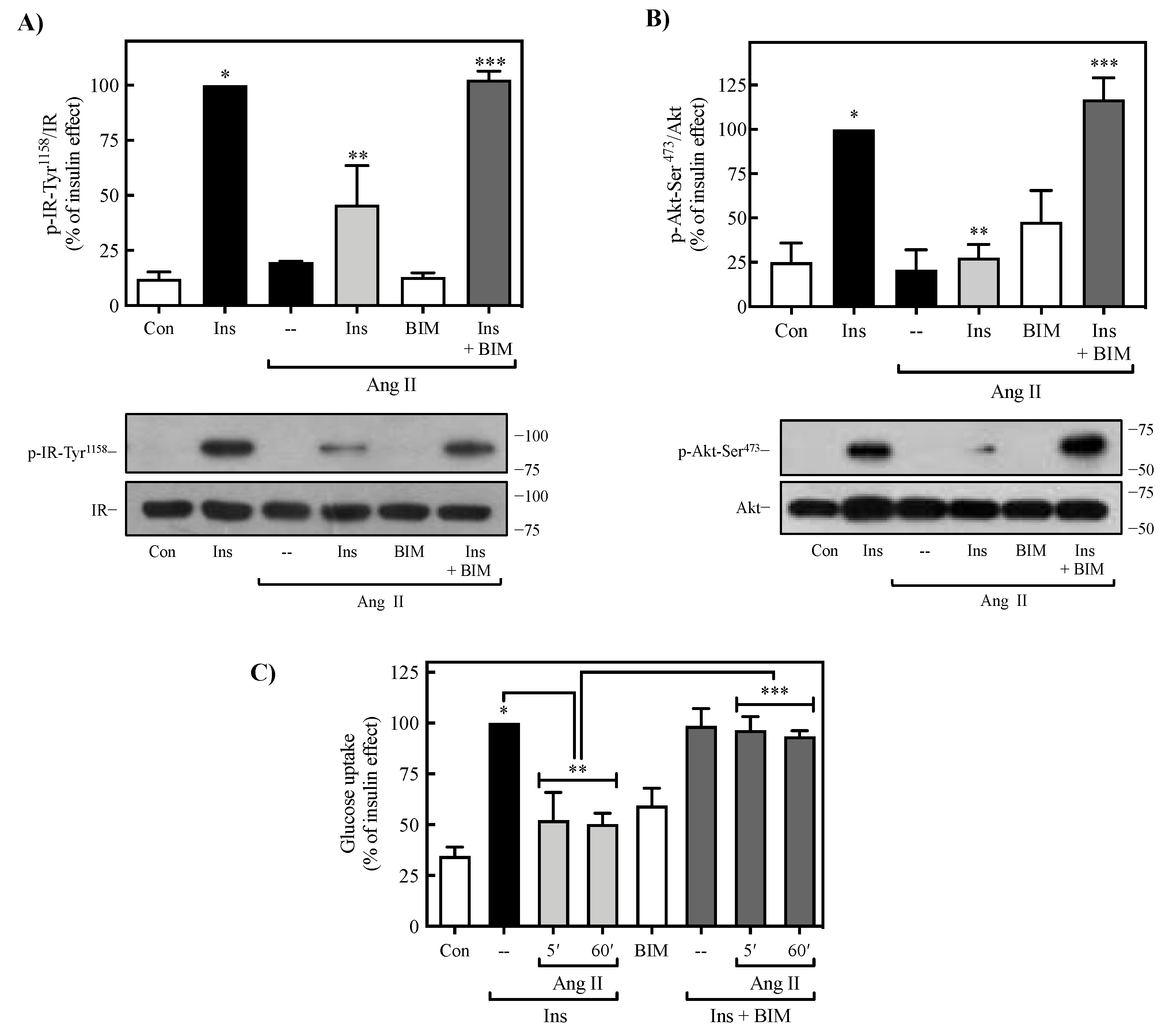
2.4. Role of PKC on Ang II-Mediated Regulation of Insulin Signaling
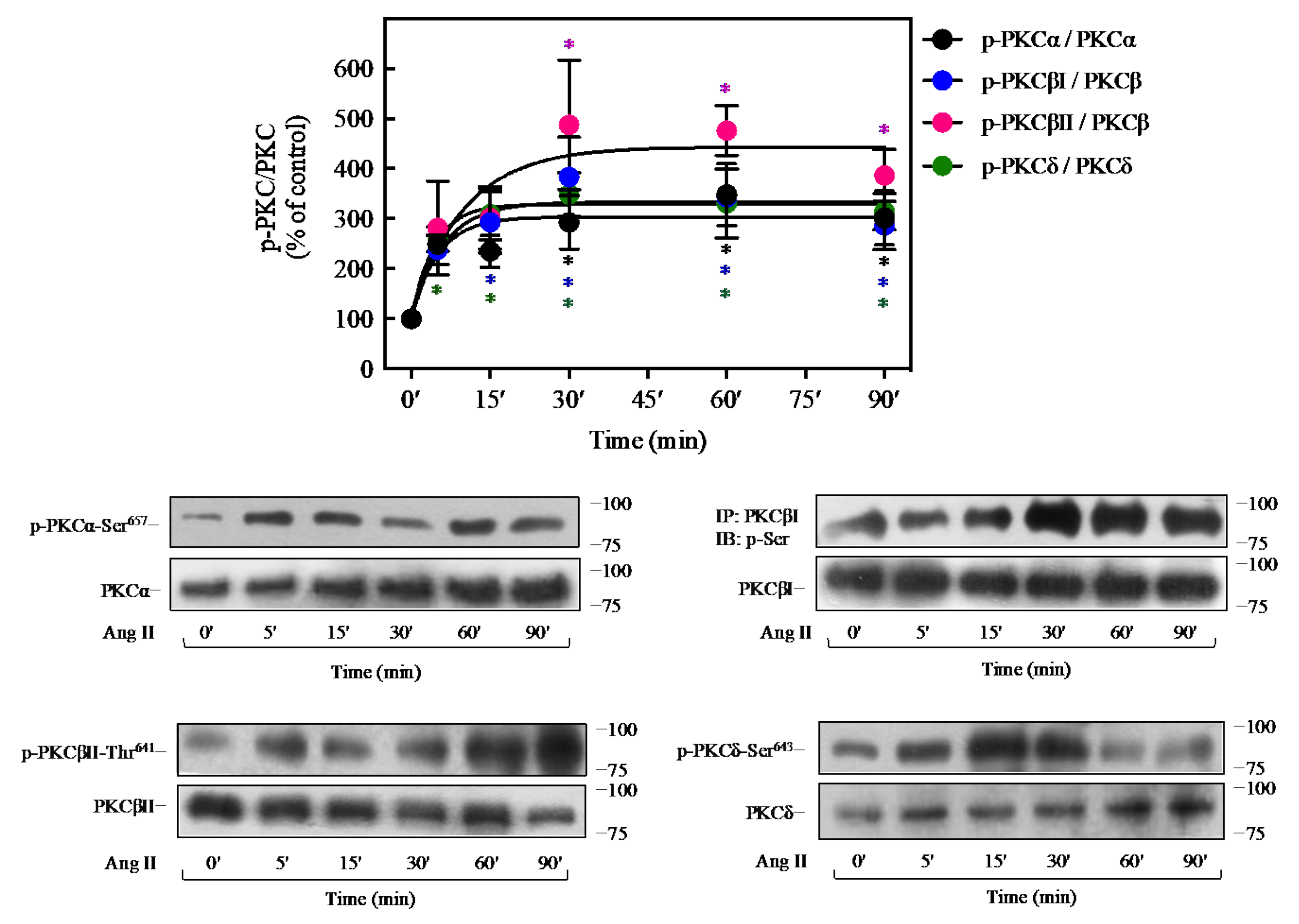
2.5. Ang II Promotes Activation of PKC Isoforms in 3T3-L1 Cells
2.6. Ang II Induces the Interaction of PKC with IR
2.7. Ang II Induces Insulin Resistance in Adipocytes Isolated from BALB/c Mice
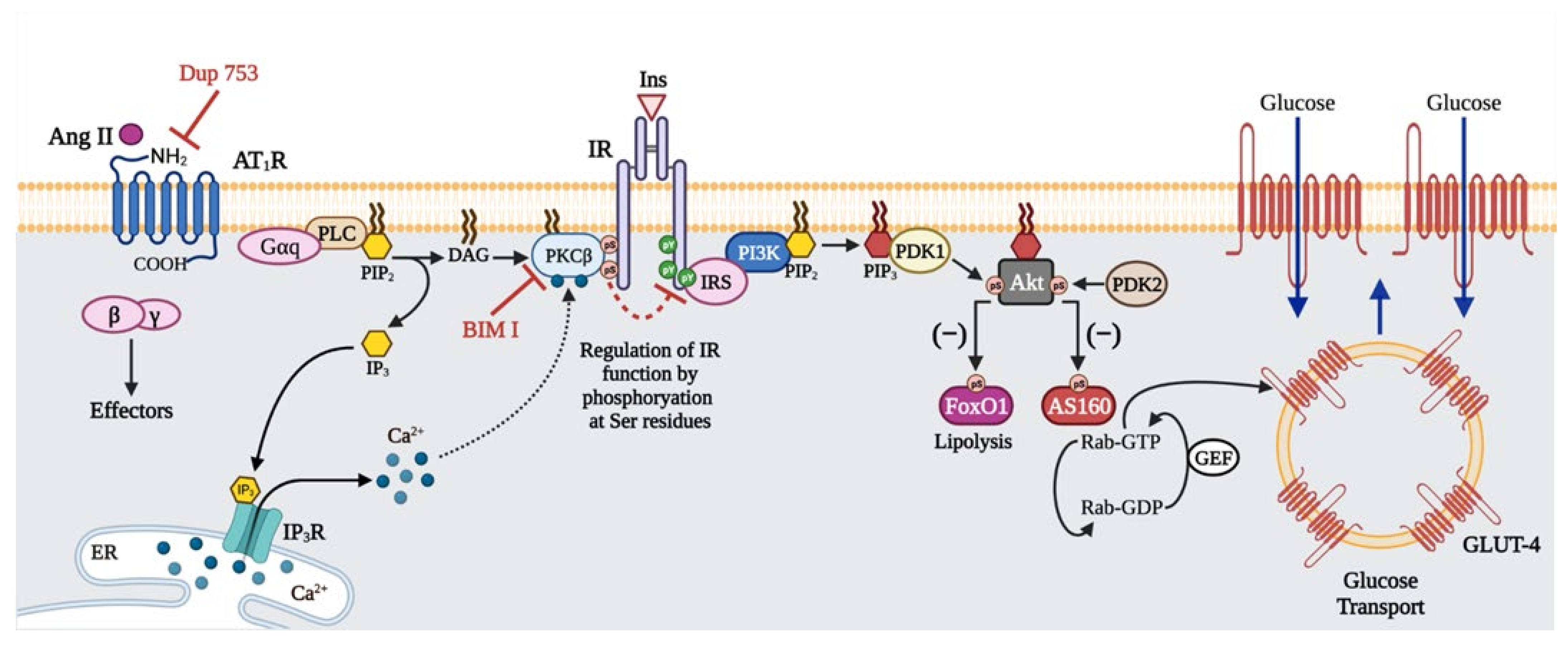
3. Discussion
4. Materials and Methods
4.1. Reagents, Peptides, Inhibitors, and Antibodies
4.2. Animals
4.3. Experiments in Isolated Adipocytes
4.4. Cell Culture and Differentiation of 3T3-L1 Adipocytes
4.5. Western Blotting
4.6. Glucose Uptake Assay
4.7. Immunoprecipitation Assay for Serine Phosphorylated IR and IRS
4.8. Co-Immunoprecipitation Assay
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ang II | Angiotensin II |
| AGT | Angiotensinogen |
| AT1R | Angiotensin receptor type 1 |
| AT2R | Angiotensin receptor type 2 |
| ATGL | Adipose triglyceride lipase |
| BIM I | Bisindolylmaleimide I |
| DM2 | Type 2 diabetes mellitus |
| FoxO1 | Forkhead box protein O1 |
| GLUT4 | Glucose transporter 4 |
| GSK-3 | Glycogen synthase kinase-3 |
| HFD | High-fat diet |
| IR | Insulin receptor |
| IRS | Insulin receptor substrate |
| PI3K | Phosphatidylinositol 3-kinase |
| PKC | Protein kinase C |
| PTPs | Protein tyrosine phosphatases |
| PTP1B | Protein tyrosine phosphatase 1B |
| RAS | Renin-angiotensin system |
| ROS | Reactive oxygen species |
| Ser | Serine |
| SOCS | Suppressor of cytokine signaling |
| Thr | Threonine |
| Tyr | Tyrosine |
| VAT | Visceral adipose tissue |
References
- Boura-Halfon, S.; Zick, Y. Phosphorylation of IRS proteins, insulin action, and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E581–E591. [Google Scholar] [CrossRef]
- Gutierrez-Rodelo, C.; Roura-Guiberna, A.; Olivares-Reyes, J.A. Molecular mechanisms of insulin resistance: An update. Gac. Med. Mex. 2017, 153, 197–209. [Google Scholar]
- Youngren, J. Regulation of insulin receptor function. Cell. Mol. Life Sci. 2007, 64, 873–891. [Google Scholar] [CrossRef]
- Olivares-Reyes, J.A.; Arellano-Plancarte, A.; Castillo-Hernandez, J.R. Angiotensin II and the development of insulin resistance: Implications for diabetes. Mol. Cell. Endocrinol. 2009, 302, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.; Kleinridders, A.; Kahn, C.R. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb. Perspect. Biol. 2014, 6, a009191. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.C.; Madiraju, A.K.; Gassaway, B.M.; Marcel, M.; Nasiri, A.R.; Butrico, G.; Marcucci, M.J.; Zhang, D.; Abulizi, A.; Zhang, X.M.; et al. Insulin receptor Thr1160 phosphorylation mediates lipid-induced hepatic insulin resistance. J. Clin. Investig. 2016, 126, 4361–4371. [Google Scholar] [CrossRef] [PubMed]
- Watarai, T.; Kobayashi, M.; Takata, Y.; Sasaoka, T.; Iwasaki, M.; Shigeta, Y. Alteration of insulin-receptor kinase activity by high-fat feeding. Diabetes 1988, 37, 1397–1404. [Google Scholar] [CrossRef]
- Caro, J.F.; Ittoop, O.; Pories, W.J.; Meelheim, D.; Flickinger, E.G.; Thomas, F.; Jenquin, M.; Silverman, J.F.; Khazanie, P.G.; Sinha, M.K. Studies on the mechanism of insulin resistance in the liver from humans with noninsulin-dependent diabetes. Insulin action and binding in isolated hepatocytes, insulin receptor structure, and kinase activity. J. Clin. Investig. 1986, 78, 249–258. [Google Scholar] [CrossRef]
- Roura-Guiberna, A.; Hernandez-Aranda, J.; Ramirez-Flores, C.J.; Mondragon-Flores, R.; Garibay-Nieto, N.; Queipo-Garcia, G.; Laresgoiti-Servitje, E.; Soh, J.-W.; Olivares-Reyes, J.A. Isomers of conjugated linoleic acid induce insulin resistance through a mechanism involving activation of protein kinase Cε in liver cells. Cell Signal. 2019, 53, 281–293. [Google Scholar] [CrossRef]
- Hunyady, L.; Catt, K.J. Pleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin II. Mol. Endocrinol. 2006, 20, 953–970. [Google Scholar] [CrossRef]
- Henriksen, E.J. Improvement of insulin sensitivity by antagonism of the renin-angiotensin system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R974–R980. [Google Scholar] [CrossRef]
- Carvalheira, J.B.; Calegari, V.C.; Zecchin, H.G.; Nadruz, W., Jr.; Guimaraes, R.B.; Ribeiro, E.B.; Franchini, K.G.; Velloso, L.A.; Saad, M.J. The cross-talk between angiotensin and insulin differentially affects phosphatidylinositol 3-kinase- and mitogen-activated protein kinase-mediated signaling in rat heart: Implications for insulin resistance. Endocrinology 2003, 144, 5604–5614. [Google Scholar] [CrossRef]
- Folli, F.; Kahn, C.R.; Hansen, H.; Bouchie, J.L.; Feener, E.P. Angiotensin II inhibits insulin signaling in aortic smooth muscle cells at multiple levels. A potential role for serine phosphorylation in insulin/angiotensin II crosstalk. J. Clin. Investig. 1997, 100, 2158–2169. [Google Scholar] [CrossRef]
- Arellano-Plancarte, A.; Hernandez-Aranda, J.; Catt, K.J.; Olivares-Reyes, J.A. Angiotensin-induced EGF receptor transactivation inhibits insulin signaling in C9 hepatic cells. Biochem. Pharmacol. 2010, 79, 733–745. [Google Scholar] [CrossRef][Green Version]
- Munoz, M.C.; Argentino, D.P.; Dominici, F.P.; Turyn, D.; Toblli, J.E. Irbesartan restores the in-vivo insulin signaling pathway leading to Akt activation in obese Zucker rats. J. Hypertens. 2006, 24, 1607–1617. [Google Scholar] [CrossRef]
- Wei, Y.; Sowers, J.R.; Clark, S.E.; Li, W.; Ferrario, C.M.; Stump, C.S. Angiotensin II-induced skeletal muscle insulin resistance mediated by NF-kappaB activation via NADPH oxidase. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E345–E351. [Google Scholar] [CrossRef]
- Ketsawatsomkron, P.; Stepp, D.W.; Fulton, D.J.; Marrero, M.B. Molecular mechanism of angiotensin II-induced insulin resistance in aortic vascular smooth muscle cells: Roles of Protein Tyrosine Phosphatase-1B. Vascul. Pharmacol. 2010, 53, 160–168. [Google Scholar] [CrossRef]
- Calegari, V.C.; Alves, M.; Picardi, P.K.; Inoue, R.Y.; Franchini, K.G.; Saad, M.J.; Velloso, L.A. Suppressor of cytokine signaling-3 provides a novel interface in the cross-talk between angiotensin II and insulin signaling systems. Endocrinology 2005, 146, 579–588. [Google Scholar] [CrossRef]
- Jones, B.H.; Standridge, M.K.; Moustaid, N. Angiotensin II increases lipogenesis in 3T3-L1 and human adipose cells. Endocrinology 1997, 138, 1512–1519. [Google Scholar] [CrossRef]
- Goossens, G.H.; Blaak, E.E.; Arner, P.; Saris, W.H.M.; Van Baak, M.A. Angiotensin II: A hormone that affects lipid metabolism in adipose tissue. Int. J. Obes. 2007, 31, 382–384. [Google Scholar] [CrossRef]
- Frantz, E.D.C.; Prodel, E.; Braz, I.D.; Giori, I.G.; Bargut, T.C.L.; Magliano, D.C.; Nobrega, A.C.L. Modulation of the renin–angiotensin system in white adipose tissue and skeletal muscle: Focus on exercise training. Clin. Sci. 2018, 132, 1487–1507. [Google Scholar] [CrossRef]
- Favre, G.A.; Esnault, V.L.M.; Obberghen, E.V. Modulation of glucose metabolism by the renin-angiotensin-aldosterone system. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E435–E449. [Google Scholar] [CrossRef]
- García-Sáinz, J.A.; Macías-Silva, M. Angiotensin II stimulates phosphoinositide turnover and phosphorylase through AII-1 receptors in isolated rat hepatocytes. Biochem. Biophys. Res. Commun. 1990, 172, 780–785. [Google Scholar] [CrossRef]
- Coimbra, C.C.; Garofalo, M.A.R.; Foscolo, D.R.C.; Xavier, A.R.; Migliorini, R.H. Gluconeogenesis activation after intravenous angiotensin II in freely moving rats. Peptides 1999, 20, 823–827. [Google Scholar] [CrossRef]
- Cignarelli, A.; Genchi, V.; Perrini, S.; Natalicchio, A.; Laviola, L.; Giorgino, F. Insulin and Insulin Receptors in Adipose Tissue Development. Int. J. Mol. Sci. 2019, 20, 759. [Google Scholar] [CrossRef]
- Kalupahana, N.S.; Moustaid-Moussa, N. The renin-angiotensin system: A link between obesity, inflammation and insulin resistance. Obes. Rev. 2012, 13, 136–149. [Google Scholar] [CrossRef]
- Valente, V.; Izzo, R.; Manzi, M.V.; De Luca, M.R.; Barbato, E.; Morisco, C. Modulation of insulin resistance by renin angiotensin system inhibitors: Implications for cardiovascular prevention. Monaldi Arch. Chest Dis. 2021, 91, 1602. [Google Scholar] [CrossRef]
- Sarzani, R.; Salvi, F.; Dessi-Fulgheri, P.; Rappelli, A. Renin-angiotensin system, natriuretic peptides, obesity, metabolic syndrome, and hypertension: An integrated view in humans. J. Hypertens. 2008, 26, 831–843. [Google Scholar] [CrossRef]
- Patel, S.; Rauf, A.; Khan, H.; Abu-Izneid, T. Renin-angiotensin-aldosterone (RAAS): The ubiquitous system for homeostasis and pathologies. Biomed. Pharmacother. 2017, 94, 317–325. [Google Scholar] [CrossRef]
- Rüster, C.; Wolf, G. The Role of the Renin-Angiotensin-Aldosterone System in Obesity-Related Renal Diseases. Semin. Nephrol. 2013, 33, 44–53. [Google Scholar] [CrossRef]
- Baba, T.; Drenckhan, M.; Neugebauer, S.; Klein, H. Effect of angiotensin II and bradykinin on insulin-stimulated tyrosine kinase activity of insulin receptor. Diabetologia 1998, 41, 741–742. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sechi, L.A.; Griffin, C.A.; Zingaro, L.; Valentin, J.P.; Bartoli, E.; Schambelan, M. Effects of angiotensin II on insulin receptor binding and mRNA levels in normal and diabetic rats. Diabetologia 1997, 40, 770–777. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Juan, C.C.; Chien, Y.; Wu, L.Y.; Yang, W.M.; Chang, C.L.; Lai, Y.H.; Ho, P.H.; Kwok, C.F.; Ho, L.T. Angiotensin II enhances insulin sensitivity in vitro and in vivo. Endocrinology 2005, 146, 2246–2254. [Google Scholar] [CrossRef] [PubMed]
- Ogihara, T.; Asano, T.; Ando, K.; Chiba, Y.; Sakoda, H.; Anai, M.; Shojima, N.; Ono, H.; Onishi, Y.; Fujishiro, M.; et al. Angiotensin II-induced insulin resistance is associated with enhanced insulin signaling. Hypertension 2002, 40, 872–879. [Google Scholar] [CrossRef]
- Muñoz, M.C.; Giani, J.F.; Burghi, V.; Mayer, M.A.; Carranza, A.; Taira, C.A.; Dominici, F.P. The Mas receptor mediates modulation of insulin signaling by angiotensin-(1–7). Regul. Pept. 2012, 177, 1–11. [Google Scholar] [CrossRef]
- Shi, H.; Tzameli, I.; Bjørbæk, C.; Flier, J.S. Suppressor of cytokine signaling 3 is a physiological regulator of adipocyte insulin signaling. J. Biol. Chem. 2004, 279, 34733–34740. [Google Scholar] [CrossRef]
- Than, A.; Tee, W.T.; Chen, P. Apelin secretion and expression of apelin receptors in 3T3-L1 adipocytes are differentially regulated by angiotensin type 1 and type 2 receptors. Mol. Cell. Endocrinol. 2012, 351, 296–305. [Google Scholar] [CrossRef]
- Thatcher, S.; Yiannikouris, F.; Gupte, M.; Cassis, L. The adipose renin–angiotensin system: Role in cardiovascular disease. Mol. Cell. Endocrinol. 2009, 302, 111–117. [Google Scholar] [CrossRef]
- Weiland, F.; Verspohl, E.J. Variety of angiotensin receptors in 3T3-L1 preadipose cells and differentiated adipocytes. Horm. Metab. Res. 2008, 40, 760–766. [Google Scholar] [CrossRef]
- Iwai, M.; Horiuchi, M. Role of renin–angiotensin system in adipose tissue dysfunction. Hypertens. Res. 2009, 32, 425–427. [Google Scholar] [CrossRef]
- Chakrabarti, P.; Kandror, K.V. FoxO1 controls insulin-dependent adipose triglyceride lipase (ATGL) expression and lipolysis in adipocytes. J. Biol. Chem. 2009, 284, 13296–13300. [Google Scholar] [CrossRef]
- Ugi, S.; Imamura, T.; Maegawa, H.; Egawa, K.; Yoshizaki, T.; Shi, K.; Obata, T.; Ebina, Y.; Kashiwagi, A.; Olefsky, J.M. Protein phosphatase 2A negatively regulates insulin’s metabolic signaling pathway by inhibiting Akt (protein kinase B) activity in 3T3-L1 adipocytes. Mol. Cell. Biol. 2004, 24, 8778–8789. [Google Scholar] [CrossRef]
- Gual, P.; Le Marchand-Brustel, Y.; Tanti, J.F. Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie 2005, 87, 99–109. [Google Scholar] [CrossRef]
- De Fea, K.; Roth, R.A. Protein kinase C modulation of insulin receptor substrate-1 tyrosine phosphorylation requires serine 612. Biochemistry 1997, 36, 12939–12947. [Google Scholar] [CrossRef]
- Andreozzi, F.; Laratta, E.; Sciacqua, A.; Perticone, F.; Sesti, G. Angiotensin II impairs the insulin signaling pathway promoting production of nitric oxide by inducing phosphorylation of insulin receptor substrate-1 on Ser312 and Ser616 in human umbilical vein endothelial cells. Circ. Res. 2004, 94, 1211. [Google Scholar] [CrossRef]
- Hers, I.; Tavare, J.M.; Denton, R.M. The protein kinase C inhibitors bisindolylmaleimide I (GF 109203x) and IX (Ro 31-8220) are potent inhibitors of glycogen synthase kinase-3 activity. FEBS Lett. 1999, 460, 433–436. [Google Scholar] [CrossRef]
- Bandyopadhyay, G.; Standaert, M.L.; Zhao, L.; Yu, B.; Avignon, A.; Galloway, L.; Karnam, P.; Moscat, J.; Farese, R.V. Activation of protein kinase C (alpha, beta, and zeta) by insulin in 3T3/L1 cells. Transfection studies suggest a role for PKC-zeta in glucose transport. J. Biol. Chem. 1997, 272, 2551–2558. [Google Scholar] [CrossRef]
- Tsuru, M.; Katagiri, H.; Asano, T.; Yamada, T.; Ohno, S.; Ogihara, T.; Oka, Y. Role of PKC isoforms in glucose transport in 3T3-L1 adipocytes: Insignificance of atypical PKC. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E338–E345. [Google Scholar] [CrossRef][Green Version]
- Rosenzweig, T.; Braiman, L.; Bak, A.; Alt, A.; Kuroki, T.; Sampson, S.R. Differential effects of tumor necrosis factor-alpha on protein kinase C isoforms alpha and delta mediate inhibition of insulin receptor signaling. Diabetes 2002, 51, 1921–1930. [Google Scholar] [CrossRef]
- Chin, J.E.; Dickens, M.; Tavare, J.M.; Roth, R.A. Overexpression of protein kinase C isoenzymes alpha, beta I, gamma, and epsilon in cells overexpressing the insulin receptor. Effects on receptor phosphorylation and signaling. J. Biol. Chem. 1993, 268, 6338–6347. [Google Scholar] [CrossRef]
- Bossenmaier, B.; Mosthaf, L.; Mischak, H.; Ullrich, A.; Haring, H.U. Protein kinase C isoforms beta 1 and beta 2 inhibit the tyrosine kinase activity of the insulin receptor. Diabetologia 1997, 40, 863–866. [Google Scholar] [CrossRef]
- Martiny-Baron, G.; Kazanietz, M.G.; Mischak, H.; Blumberg, P.M.; Kochs, G.; Hug, H.; Marmé, D.; Schächtele, C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Gö 6976. J. Biol. Chem. 1993, 268, 9194–9197. [Google Scholar] [CrossRef]
- Kalupahana, N.S.; Moustaid-Moussa, N. The adipose tissue renin-angiotensin system and metabolic disorders: A review of molecular mechanisms. Crit. Rev. Biochem. Mol. Biol. 2012, 47, 379–390. [Google Scholar] [CrossRef]
- Karlsson, C.; Lindell, K.; Ottosson, M.; Sjostrom, L.; Carlsson, B.; Carlsson, L.M. Human adipose tissue expresses angiotensinogen and enzymes required for its conversion to angiotensin II. J. Clin. Endocrinol. Metab. 1998, 83, 3925–3929. [Google Scholar] [CrossRef]
- Engeli, S.; Schling, P.; Gorzelniak, K.; Boschmann, M.; Janke, J.; Ailhaud, G.; Teboul, M.; Massiera, F.; Sharma, A.M. The adipose-tissue renin-angiotensin-aldosterone system: Role in the metabolic syndrome? Int. J. Biochem. Cell Biol. 2003, 35, 807–825. [Google Scholar] [CrossRef]
- Mallow, H.; Trindl, A.; Loffler, G. Production of angiotensin II receptors type one (AT1) and type two (AT2) during the differentiation of 3T3-L1 preadipocytes. Horm. Metab. Res. 2000, 32, 500–503. [Google Scholar] [CrossRef]
- Putnam, K.; Batifoulier-Yiannikouris, F.; Bharadwaj, K.G.; Lewis, E.; Karounos, M.; Daugherty, A.; Cassis, L.A. Deficiency of Angiotensin type 1a receptors in adipocytes reduces differentiation and promotes hypertrophy of adipocytes in lean mice. Endocrinology 2012, 153, 4677–4686. [Google Scholar] [CrossRef]
- Luo, C.; Lv, N.; Chang, Z.; Qu, Q.; Huang, J. Adipose angiotensin II type 1 receptor-associated protein ameliorates metabolic disorders via promoting adipose tissue adipogenesis and browning. Eur. J. Cell Biol. 2017, 96, 567–578. [Google Scholar] [CrossRef]
- Jaldin-Fincati, J.R.; Pavarotti, M.; Frendo-Cumbo, S.; Bilan, P.J.; Klip, A. Update on GLUT4 vesicle traffic: A cornerstone of insulin action. Trends Endocrinol. Metab. 2017, 28, 597–611. [Google Scholar] [CrossRef]
- Iwai, M.; Chen, R.; Imura, Y.; Horiuchi, M. TAK-536, a new AT1 receptor blocker, improves glucose intolerance and adipocyte differentiation. Am. J. Hypertens. 2007, 20, 579–586. [Google Scholar] [CrossRef][Green Version]
- Aoki, M.; Jiang, H.; Vogt, P.K. Proteasomal degradation of the FoxO1 transcriptional regulator in cells transformed by the P3k and Akt oncoproteins. Proc. Natl. Acad. Sci. USA 2004, 101, 13613–13617. [Google Scholar] [CrossRef] [PubMed]
- Rena, G.; Guo, S.; Cichy, S.C.; Unterman, T.G.; Cohen, P. Phosphorylation of the transcription factor forkhead family member FKHR by protein kinase B. J. Biol. Chem. 1999, 274, 17179–17183. [Google Scholar] [CrossRef] [PubMed]
- Ioannilli, L.; Ciccarone, F.; Ciriolo, M.R. Adipose tissue and FoxO1: Bridging physiology and mechanisms. Cells 2020, 9, 849. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Rena, G.; Cichy, S.; He, X.; Cohen, P.; Unterman, T. Phosphorylation of serine 256 by protein kinase B disrupts transactivation by FKHR and mediates effects of insulin on insulin-like growth factor-binding protein-1 promoter activity through a conserved insulin response sequence. J. Biol. Chem. 1999, 274, 17184–17192. [Google Scholar] [CrossRef] [PubMed]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef] [PubMed]
- Langlet, F.; Haeusler, R.A.; Lindén, D.; Ericson, E.; Norris, T.; Johansson, A.; Cook, J.R.; Aizawa, K.; Wang, L.; Buettner, C.; et al. Selective inhibition of FOXO1 activator/repressor balance modulates hepatic glucose handling. Cell 2017, 171, 824–835. [Google Scholar] [CrossRef] [PubMed]
- Dunner, N.; Quezada, C.; Berndt, F.A.; Canovas, J.; Rojas, C.V. Angiotensin II signaling in human preadipose cells: Participation of ERK1,2-dependent modulation of Akt. PLoS ONE 2013, 8, e75440. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Farrokhi, F.R.; Butler, A.E.; Sahebkar, A. Insulin resistance: Review of the underlying molecular mechanisms. J. Cell. Physiol. 2019, 234, 8152–8161. [Google Scholar] [CrossRef]
- Lalli, C.A.; Pauli, J.R.; Prada, P.O.; Cintra, D.E.; Ropelle, E.R.; Velloso, L.A.; Saad, M.J.A. Statin modulates insulin signaling and insulin resistance in liver and muscle of rats fed a high-fat diet. Metabolism 2008, 57, 57–65. [Google Scholar] [CrossRef]
- Toullec, D.; Pianetti, P.; Coste, H.; Bellevergue, P.; Grand-Perret, T.; Ajakane, M.; Baudet, V.; Boissin, P.; Boursier, E.; Loriolle, F.; et al. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J. Biol. Chem. 1991, 266, 15771–15781. [Google Scholar] [CrossRef]
- Feener, E.P.; Backer, J.M.; King, G.L.; Wilden, P.A.; Sun, X.J.; Kahn, C.R.; White, M.F. Insulin stimulates serine and tyrosine phosphorylation in the juxtamembrane region of the insulin receptor. J. Biol. Chem. 1993, 268, 11256–11264. [Google Scholar] [CrossRef]
- Coba, M.; Munoz, M.; Dominici, F.; Toblli, J.; Pena, C.; Bartke, A.; Turyn, D. Increased in vivo phosphorylation of insulin receptor at serine 994 in the liver of obese insulin-resistant Zucker rats. J. Endocrinol. 2004, 182, 433–444. [Google Scholar] [CrossRef]
- Liu, F.; Roth, R.A. Identification of serines-1035/1037 in the kinase domain of the insulin receptor as protein kinase Cα mediated phosphorylation sites. FEBS Lett. 1994, 352, 389–392. [Google Scholar] [CrossRef]
- Pillay, T.S.; Xiao, S.; Keranen, L.; Olefsky, J.M. Regulation of the insulin receptor by protein kinase C isoenzymes: Preferential interaction with β isoenzymes and interaction with the catalytic domain of βII. Cell Signal. 2004, 16, 97–104. [Google Scholar] [CrossRef]
- Park, K.; Li, Q.; Rask-Madsen, C.; Mima, A.; Mizutani, K.; Winnay, J.; Maeda, Y.; D’Aquino, K.; White, M.F.; Feener, E.P.; et al. Serine phosphorylation sites on IRS2 activated by angiotensin II and protein kinase C to induce selective insulin resistance in endothelial cells. Mol. Cell. Biol. 2013, 33, 3227–3241. [Google Scholar] [CrossRef]
- Venable, C.L.; Frevert, E.U.; Kim, Y.B.; Fischer, B.M.; Kamatkar, S.; Neel, B.G.; Kahn, B.B. Overexpression of protein-tyrosine phosphatase-1B in adipocytes Inhibits Insulin-stimulated phosphoinositide 3-kinase activity without altering glucose transport or Akt/protein kinase B activation. J. Biol. Chem. 2000, 275, 18318–18326. [Google Scholar] [CrossRef]
- Wronska, A.; Kmiec, Z. Structural and biochemical characteristics of various white adipose tissue depots. Acta Physiol. 2012, 205, 194–208. [Google Scholar] [CrossRef]
- Cassis, L.A.; Police, S.B.; Yiannikouris, F.; Thatcher, S.E. Local adipose tissue renin-angiotensin system. Curr. Hypertens. Rep. 2008, 10, 93–98. [Google Scholar] [CrossRef]
- Fowler, J.D.; Johnson, N.D.; Haroldson, T.A.; Brintnall, J.A.; Herrera, J.E.; Katz, S.A.; Bernlohr, D.A. Regulated renin release from 3T3-L1 adipocytes. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1383–E1391. [Google Scholar] [CrossRef][Green Version]
- Bernlohr, D.A.; Bolanowski, M.A.; Kelly, T.J.; Lane, M.D. Evidence for an increase in transcription of specific mRNAs during differentiation of 3T3-L1 preadipocytes. J. Biol. Chem. 1985, 260, 5563–5567. [Google Scholar] [CrossRef]
- Thomson, M.J.; Williams, M.G.; Frost, S.C. Development of insulin resistance in 3T3-L1 adipocytes. J. Biol. Chem. 1997, 272, 7759–7764. [Google Scholar] [CrossRef]
- Zhang, Y.; Somers, K.R.; Becari, C.; Polonis, K.; Pfeifer, M.A.; Allen, A.M.; Kellogg, T.A.; Covassin, N.; Singh, P. Comparative Expression of Renin-Angiotensin Pathway Proteins in Visceral Versus Subcutaneous Fat. Front. Physiol. 2018, 9, 1370. [Google Scholar] [CrossRef]
- Pinterova, L.; Krizanova, O.; Zorad, S. Rat epididymal fat tissue express all components of the renin-angiotensin system. Gen. Physiol. Biophys. 2000, 19, 329–334. [Google Scholar]
- Schoettl, T.; Fischer, I.P.; Ussar, S. Heterogeneity of adipose tissue in development and metabolic function. J. Exp. Biol. 2018, 221. [Google Scholar] [CrossRef]
- Cases, J.A.; Barzilai, N. The regulation of body fat distribution and the modulation of insulin action. Int. J. Obes. 2000, 24, S63–S66. [Google Scholar] [CrossRef][Green Version]
- Miyazaki, Y.; Defronzo, R.A. Visceral fat dominant distribution in male type 2 diabetic patients is closely related to hepatic insulin resistance, irrespective of body type. Cardiovasc. Diabetol. 2009, 8, 44. [Google Scholar] [CrossRef]
- Ahmed, B.; Sultana, R.; Greene, M.W. Adipose tissue and insulin resistance in obese. Biomed. Pharmacother. 2021, 137, 111315. [Google Scholar] [CrossRef]
- Wang, Z.; Lv, J.; Zhang, R.; Zhu, Y.; Zhu, D.; Sun, Y.; Zhu, J.; Han, X. Co-culture with fat cells induces cellular insulin resistance in primary hepatocytes. Biochem. Biophys. Res. Commun. 2006, 345, 976–983. [Google Scholar] [CrossRef]
- Armani, A.; Mammi, C.; Marzolla, V.; Calanchini, M.; Antelmi, A.; Rosano, G.M.C.; Fabbri, A.; Caprio, M. Cellular models for understanding adipogenesis, adipose dysfunction, and obesity. J. Cell. Biochem. 2010, 110, 564–572. [Google Scholar] [CrossRef]
- Yiannikouris, F.; Gupte, M.; Putnam, K.; Thatcher, S.; Charnigo, R.; Rateri, D.L.; Daugherty, A.; Cassis, L.A. Adipocyte Deficiency of Angiotensinogen Prevents Obesity-Induced Hypertension in Male Mice. Hypertension 2012, 60, 1524–1530. [Google Scholar] [CrossRef]
- Saiki, A.; Ohira, M.; Endo, K.; Koide, N.; Oyama, T.; Murano, T.; Watanabe, H.; Miyashita, Y.; Shirai, K. Circulating angiotensin II is associated with body fat accumulation and insulin resistance in obese subjects with type 2 diabetes mellitus. Metabolism 2009, 58, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Owei, I.; Umekwe, N.; Provo, C.; Wan, J.; Dagogo-Jack, S. Insulin-sensitive and insulin-resistant obese and non-obese phenotypes: Role in prediction of incident pre-diabetes in a longitudinal biracial cohort. BMJ Open Diabetes Res. Care 2017, 5, e000415. [Google Scholar] [CrossRef] [PubMed]
- Reaven, G. All obese individuals are not created equal: Insulin resistance is the major determinant of cardiovascular disease in overweight/obese individuals. Diabetes Vasc. Dis. Res. 2005, 2, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Takemori, K.; Inoue, T.; Ito, H. Effects of Angiotensin II Type 1 receptor blocker and adiponectin on adipocyte dysfunction in stroke-prone spontaneously hypertensive rats. Lipids Health Dis. 2013, 12, 108. [Google Scholar] [CrossRef]
- Bains, R.K.; Wells, S.E.; Flavell, D.M.; Fairhall, K.M.; Strom, M.; Le Tissier, P.; Robinson, I.C.A.F. Visceral Obesity without Insulin Resistance in Late-Onset Obesity Rats. Endocrinology 2004, 145, 2666–2679. [Google Scholar] [CrossRef][Green Version]
- Ahlstrom, P.; Rai, E.; Chakma, S.; Cho, H.H.; Rengasamy, P.; Sweeney, G. Adiponectin improves insulin sensitivity via activation of autophagic flux. J. Mol. Endocrinol. 2017, 59, 339–350. [Google Scholar] [CrossRef]
- Blaslov, K.; Bulum, T.; Zibar, K.; Duvnjak, L. Relationship between Adiponectin Level, Insulin Sensitivity, and Metabolic Syndrome in Type 1 Diabetic Patients. Int. J. Endocrinol. 2013, 2013, 535906. [Google Scholar] [CrossRef]
- Sugihara, H.; Yonemitsu, N.; Miyabara, S.; Yun, K. Primary cultures of unilocular fat cells: Characteristics of growth in vitro and changes in differentiation properties. Differentiation 1986, 31, 42–49. [Google Scholar] [CrossRef]
- Zebisch, K.; Voigt, V.; Wabitsch, M.; Brandsch, M. Protocol for effective differentiation of 3T3-L1 cells to adipocytes. Anal. Biochem. 2012, 425, 88–90. [Google Scholar] [CrossRef]
- Berti, L.; Kellerer, M.; Capp, E.; Haring, H.U. Leptin stimulates glucose transport and glycogen synthesis in C2C12 myotubes: Evidence for a PI3-kinase mediated effect. Diabetologia 1997, 40, 606–609. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutierrez-Rodelo, C.; Arellano-Plancarte, A.; Hernandez-Aranda, J.; Landa-Galvan, H.V.; Parra-Mercado, G.K.; Moreno-Licona, N.J.; Hernandez-Gonzalez, K.D.; Catt, K.J.; Villalobos-Molina, R.; Olivares-Reyes, J.A. Angiotensin II Inhibits Insulin Receptor Signaling in Adipose Cells. Int. J. Mol. Sci. 2022, 23, 6048. https://doi.org/10.3390/ijms23116048
Gutierrez-Rodelo C, Arellano-Plancarte A, Hernandez-Aranda J, Landa-Galvan HV, Parra-Mercado GK, Moreno-Licona NJ, Hernandez-Gonzalez KD, Catt KJ, Villalobos-Molina R, Olivares-Reyes JA. Angiotensin II Inhibits Insulin Receptor Signaling in Adipose Cells. International Journal of Molecular Sciences. 2022; 23(11):6048. https://doi.org/10.3390/ijms23116048
Chicago/Turabian StyleGutierrez-Rodelo, Citlaly, Araceli Arellano-Plancarte, Judith Hernandez-Aranda, Huguet V. Landa-Galvan, G. Karina Parra-Mercado, Nicole J. Moreno-Licona, Karla D. Hernandez-Gonzalez, Kevin J. Catt, Rafael Villalobos-Molina, and J. Alberto Olivares-Reyes. 2022. "Angiotensin II Inhibits Insulin Receptor Signaling in Adipose Cells" International Journal of Molecular Sciences 23, no. 11: 6048. https://doi.org/10.3390/ijms23116048
APA StyleGutierrez-Rodelo, C., Arellano-Plancarte, A., Hernandez-Aranda, J., Landa-Galvan, H. V., Parra-Mercado, G. K., Moreno-Licona, N. J., Hernandez-Gonzalez, K. D., Catt, K. J., Villalobos-Molina, R., & Olivares-Reyes, J. A. (2022). Angiotensin II Inhibits Insulin Receptor Signaling in Adipose Cells. International Journal of Molecular Sciences, 23(11), 6048. https://doi.org/10.3390/ijms23116048







