Expression of a Chlorophyll b Reductase Gene from Zoysia japonica Causes Changes in Leaf Color and Chlorophyll Morphology in Agrostis stolonifera
Abstract
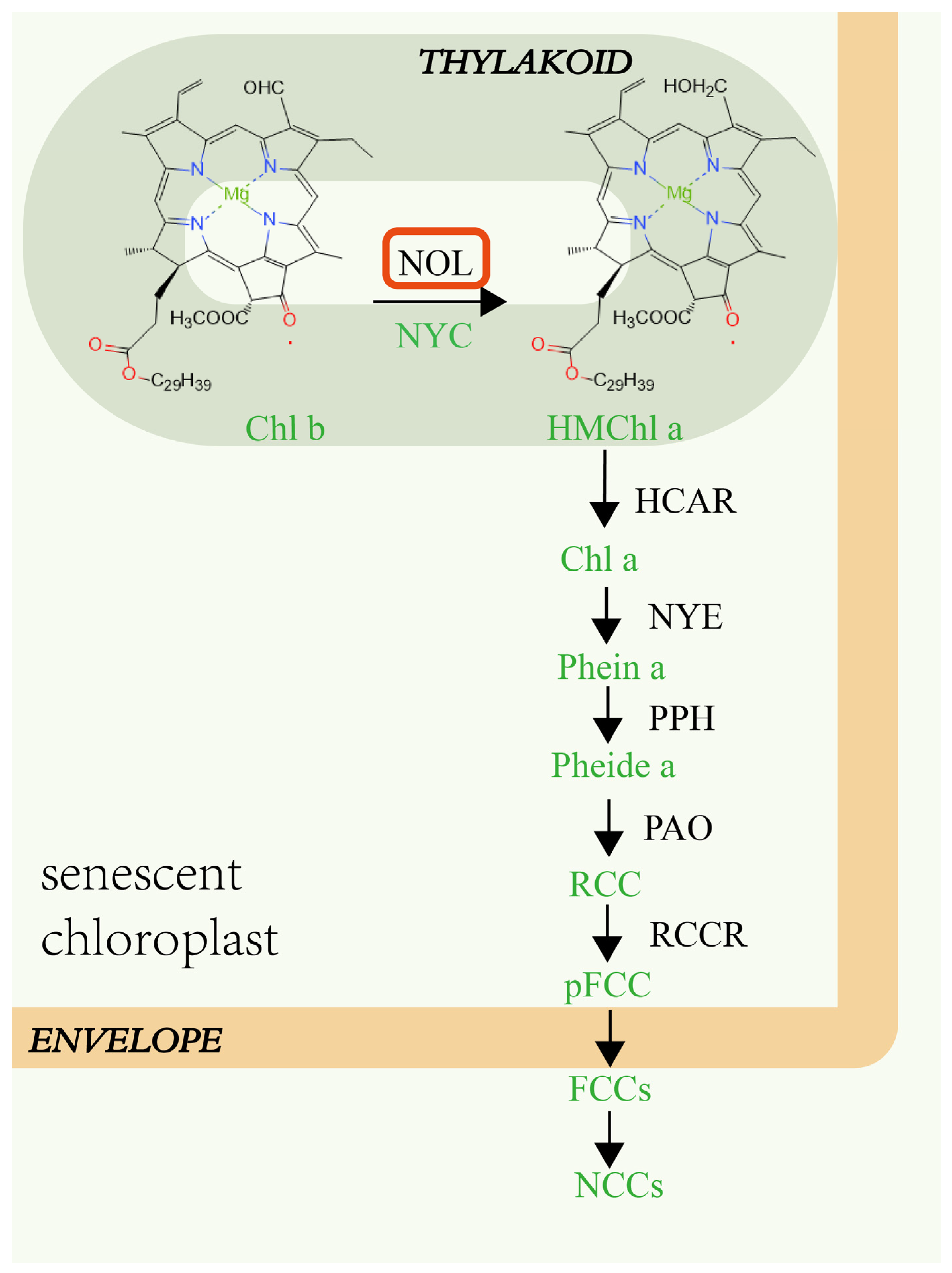
1. Introduction
2. Results
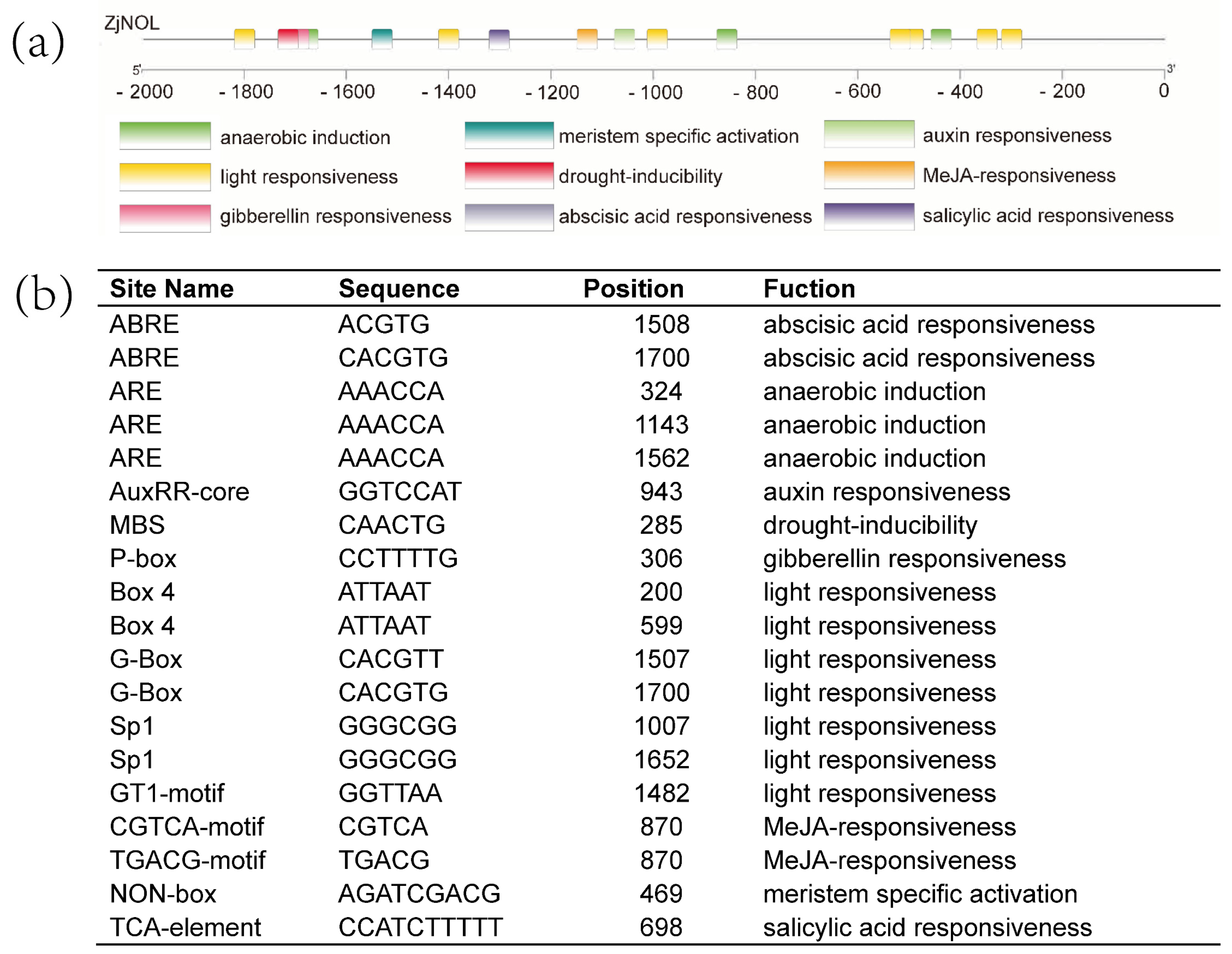
2.1. The Isolation and Analysis of the Gene ZjNOL
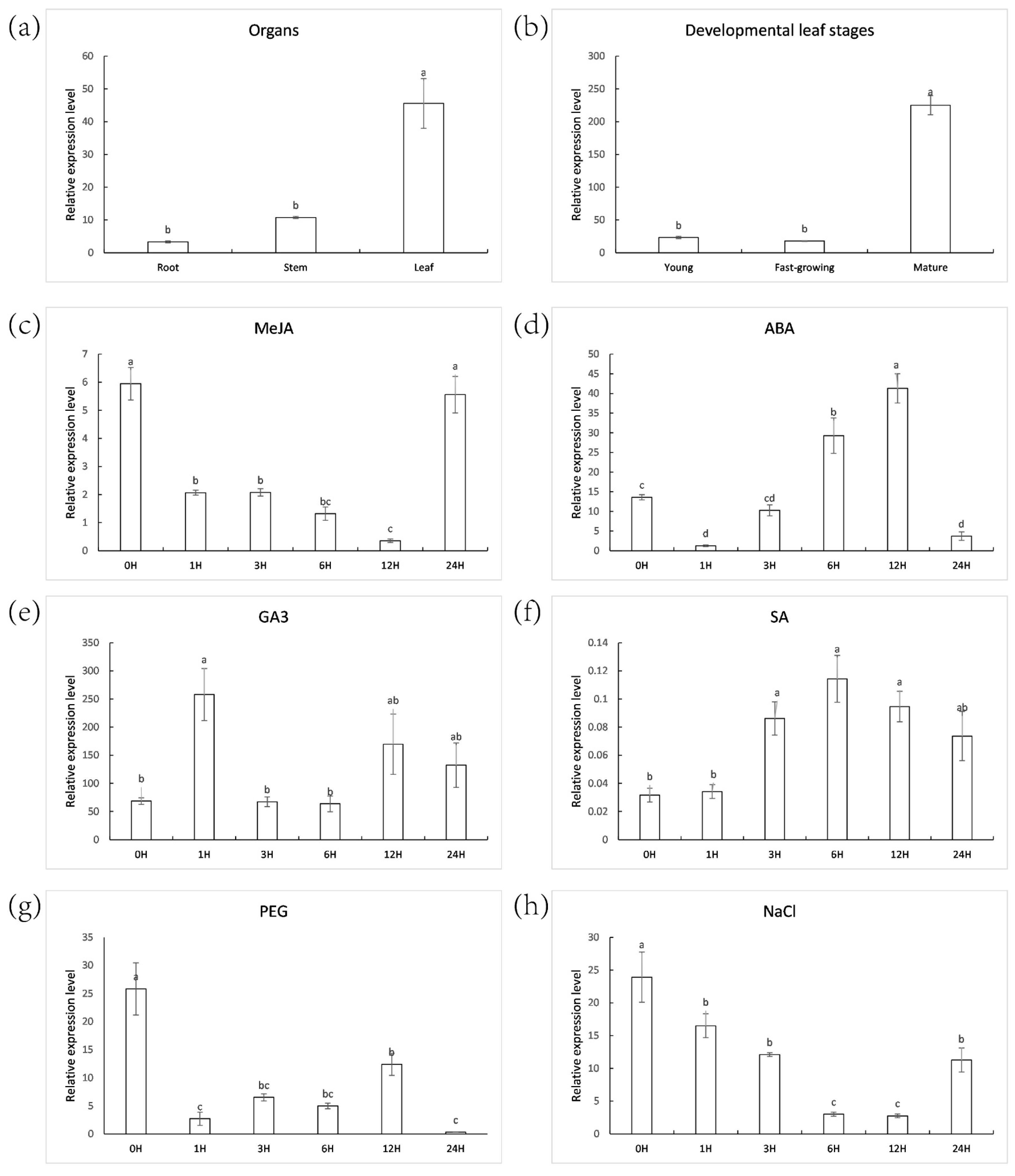
2.2. ZjNOL Expression Profiles and Expression Analysis of AsZDS, AsNCED, AsCLH1 and AsNYC Induced by Dark
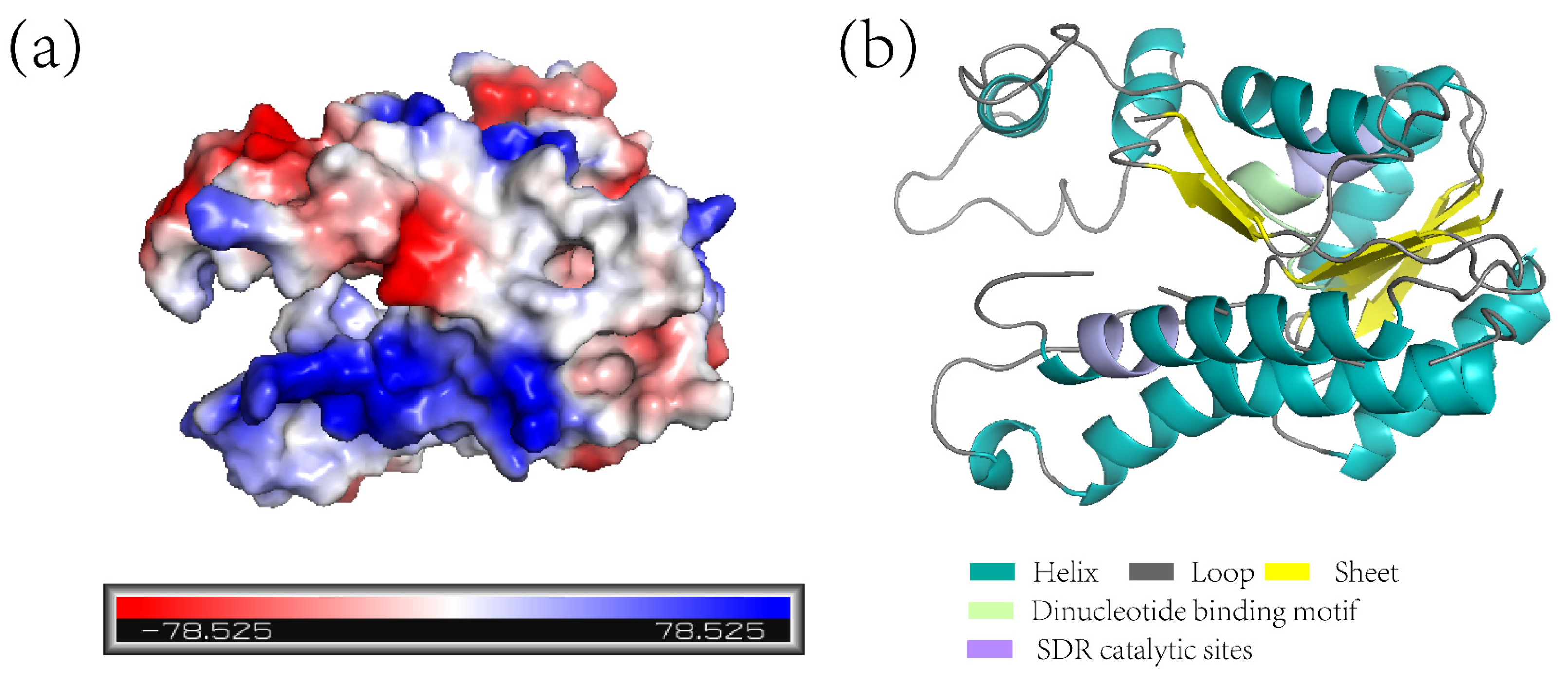
2.3. Analysis of the Protein ZjNOL
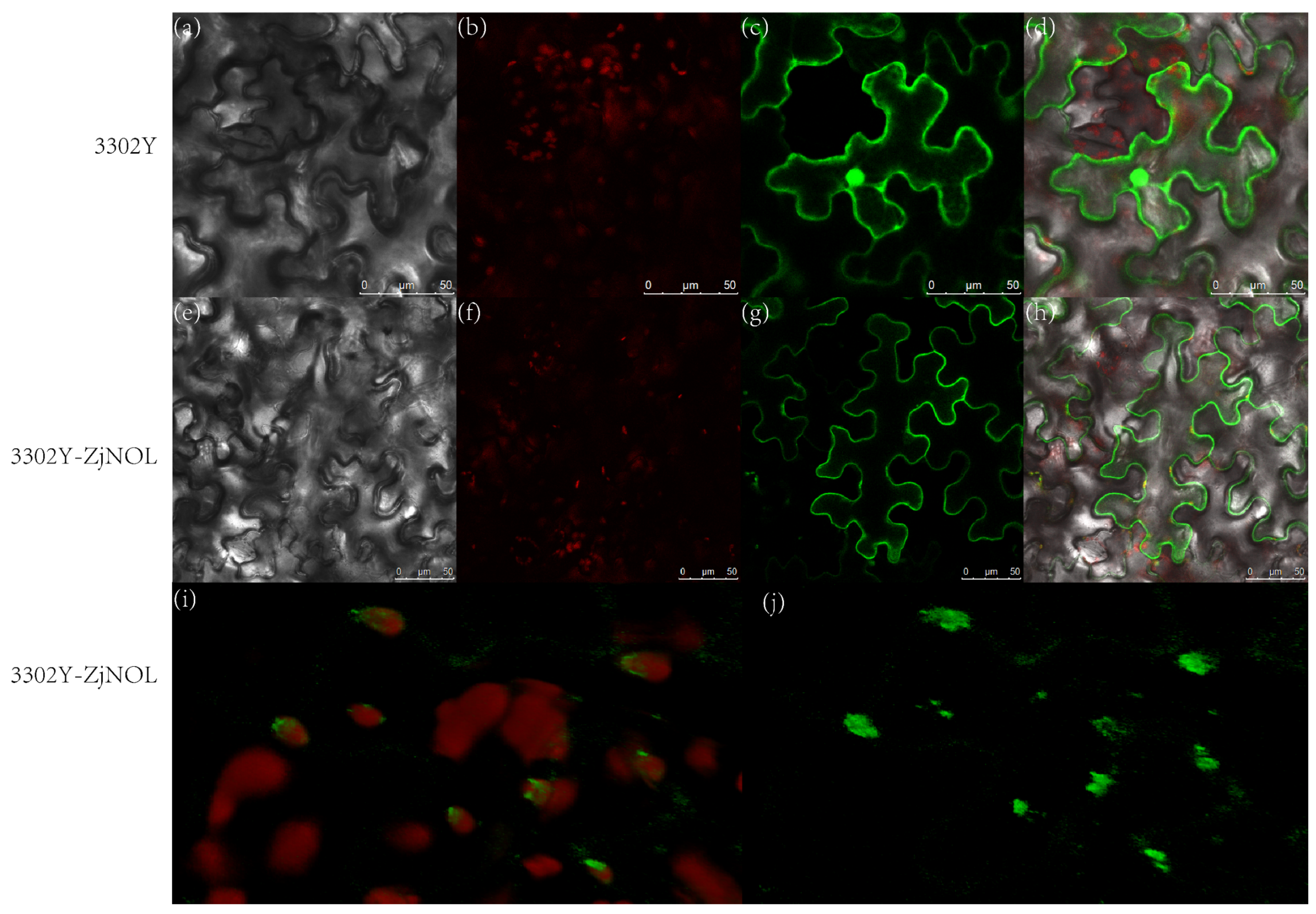
2.4. Subcellular Localization of ZjNOL
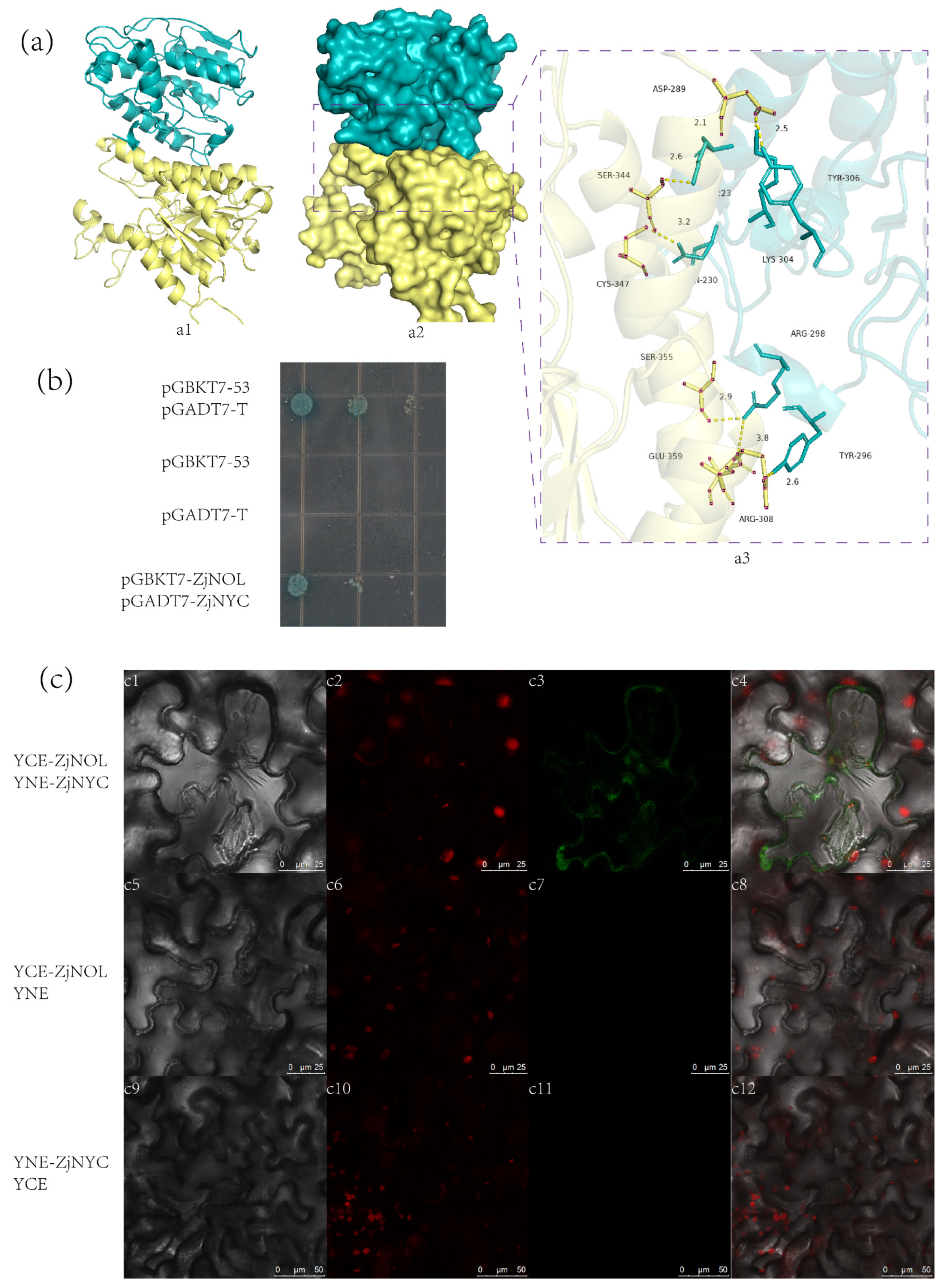
2.5. ZjNOL Interacts with ZjNYC
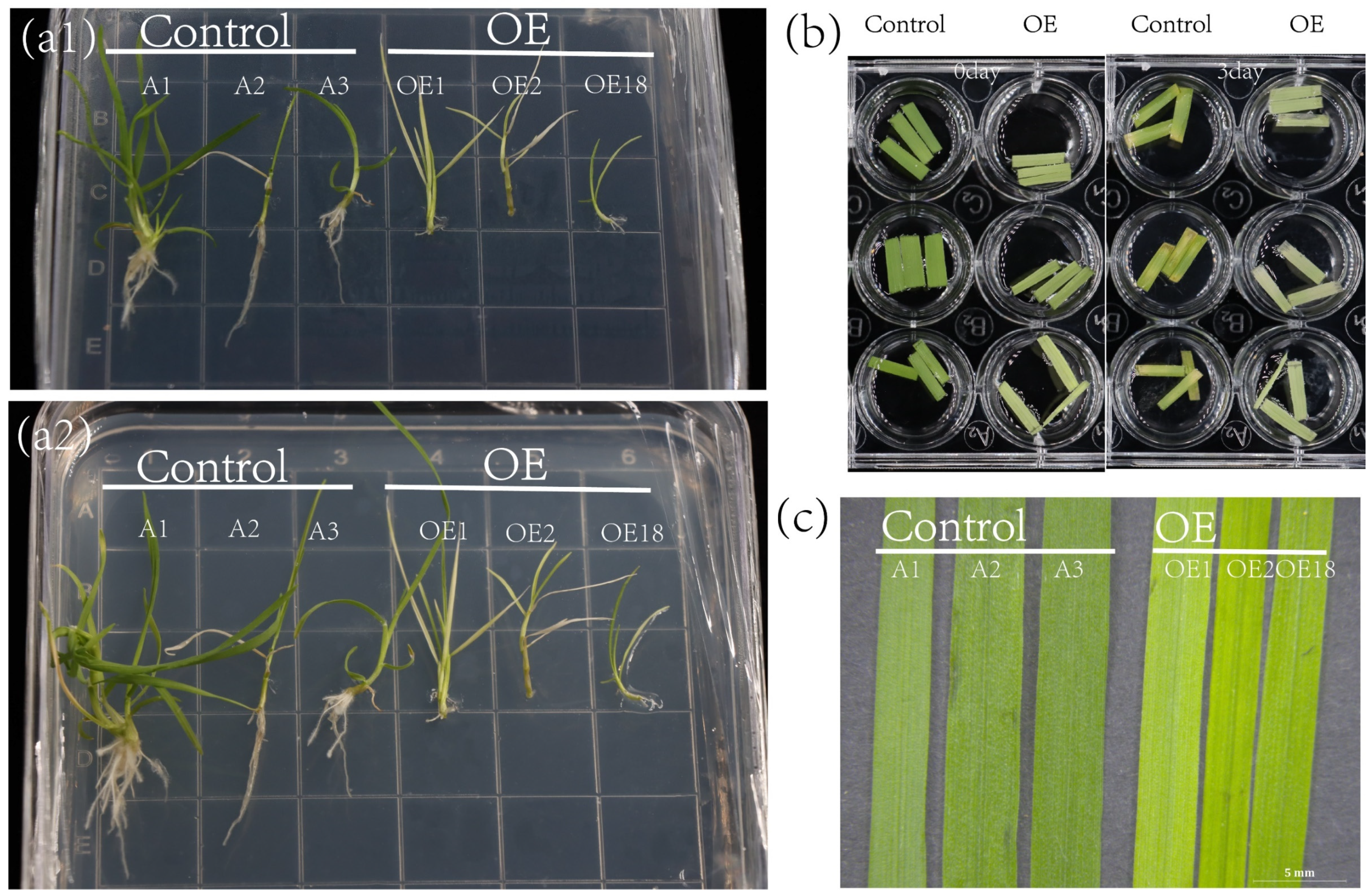
2.6. Ectopic Expression of ZjNOL Resulted in Leaf Yellowing and Sluggish Development
2.7. Morphological Changes of Transgenic Plants
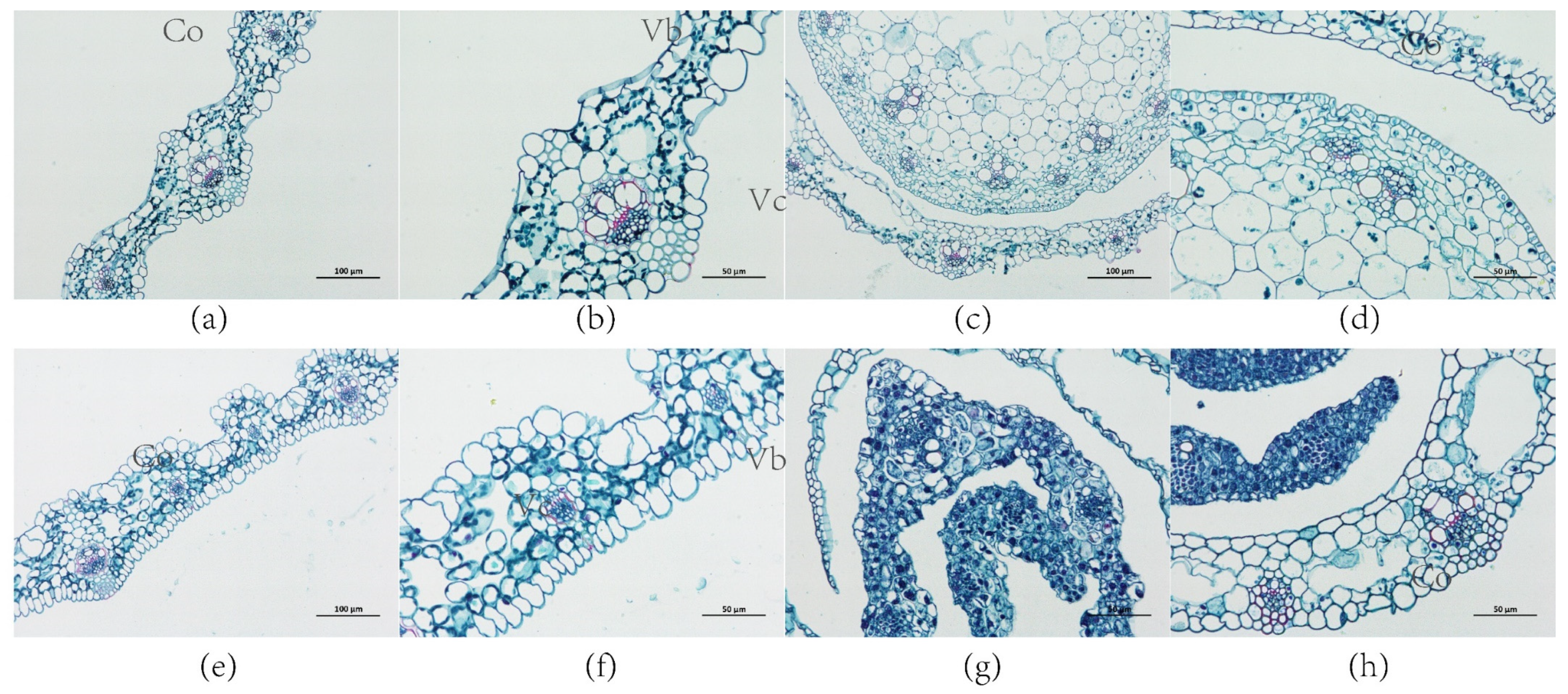
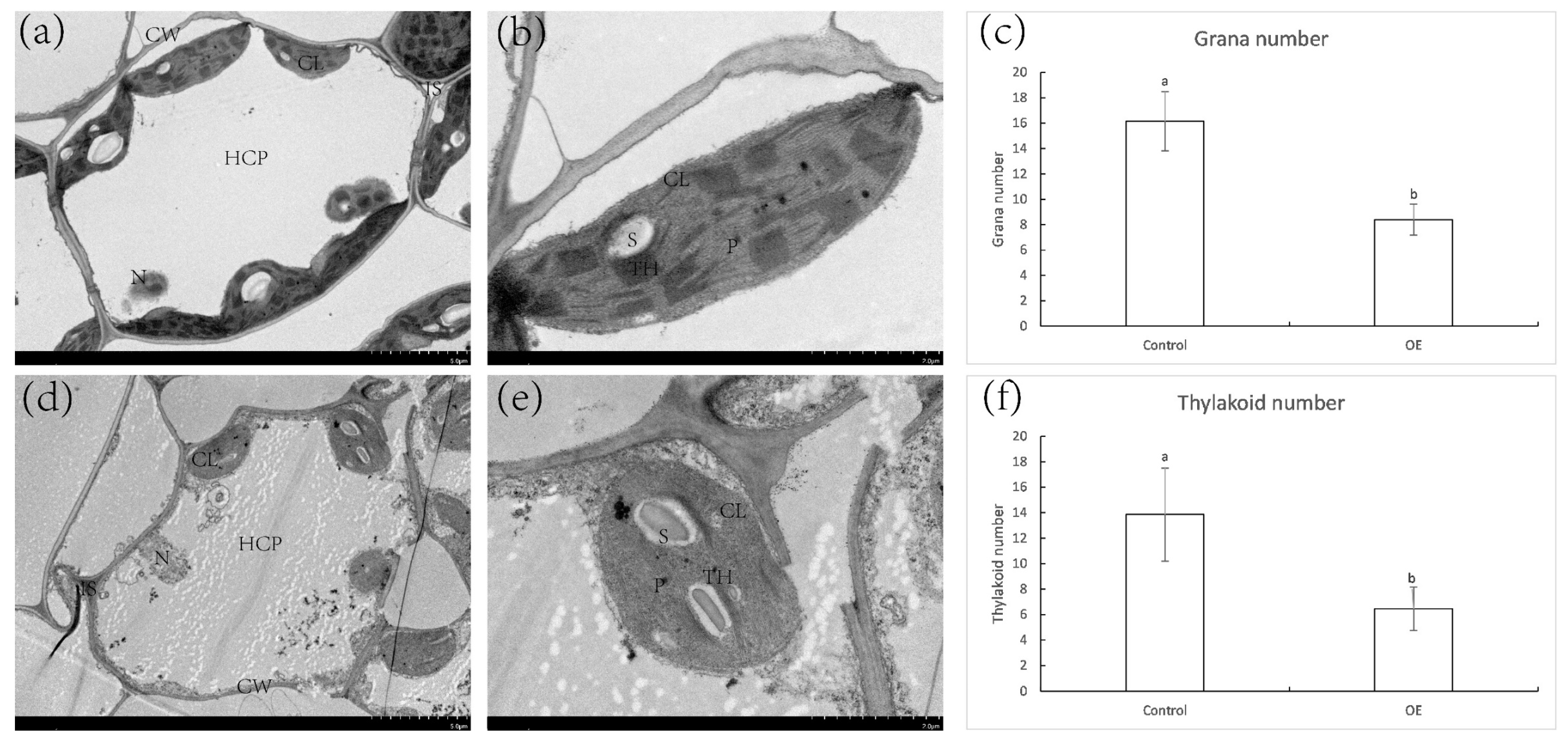
2.8. Expression of ZjNOL Gene Results in Chloroplast Morphological Changes
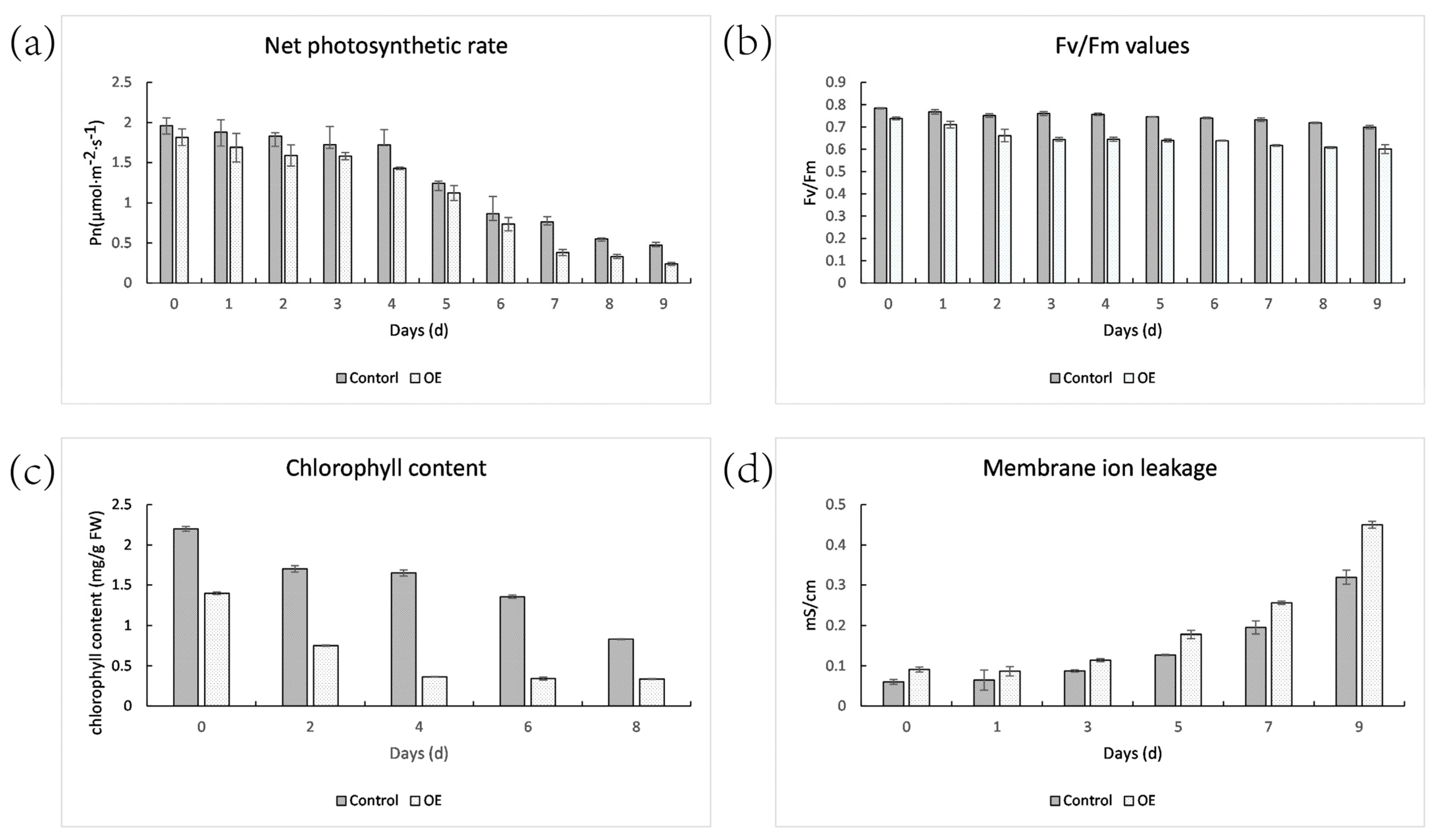
2.9. Net Photosynthetic Rate, Fv/Fm, Chlorophyll Content, Membrane Ion Leakage of Control and OE
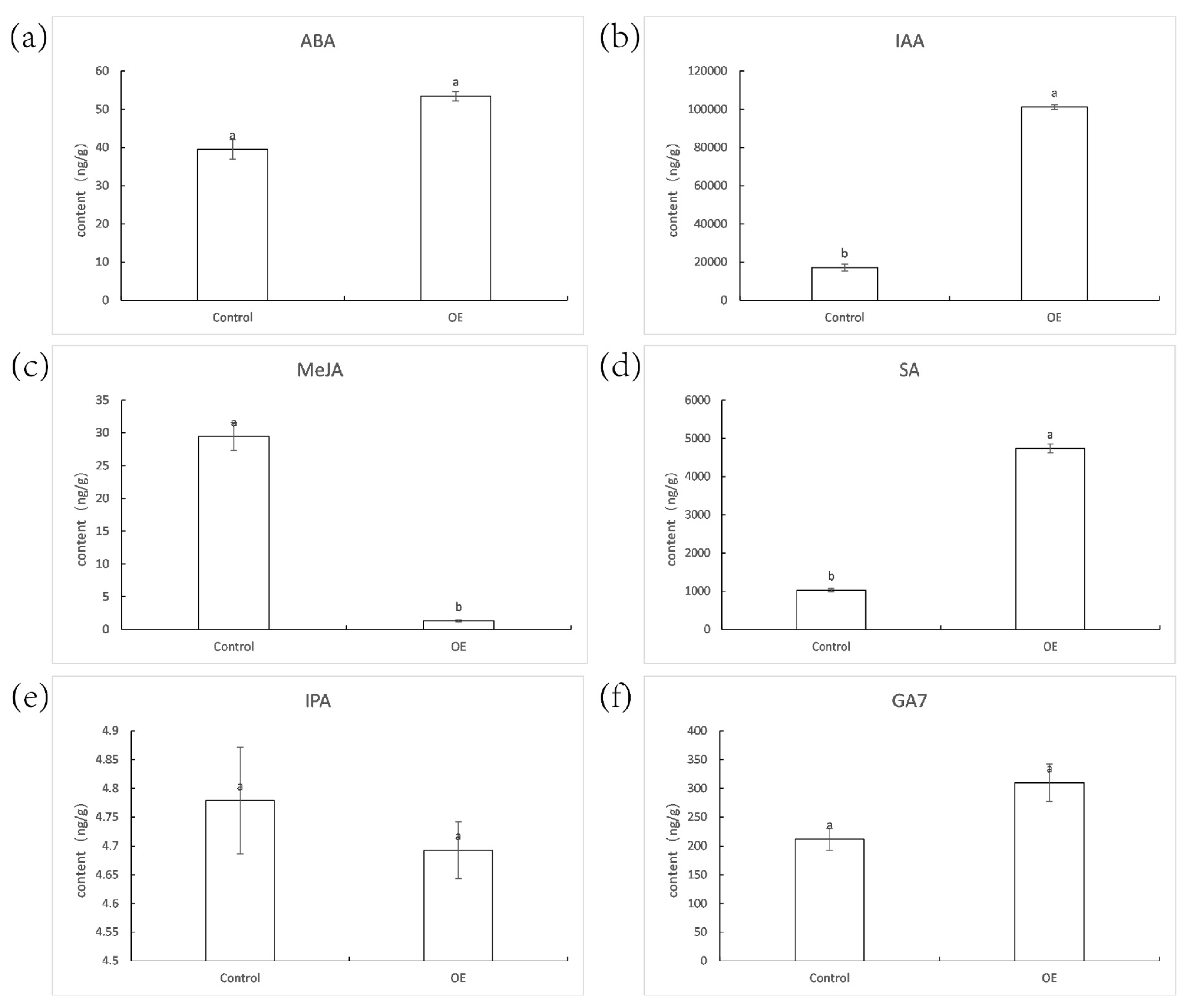
2.10. Hormone Content
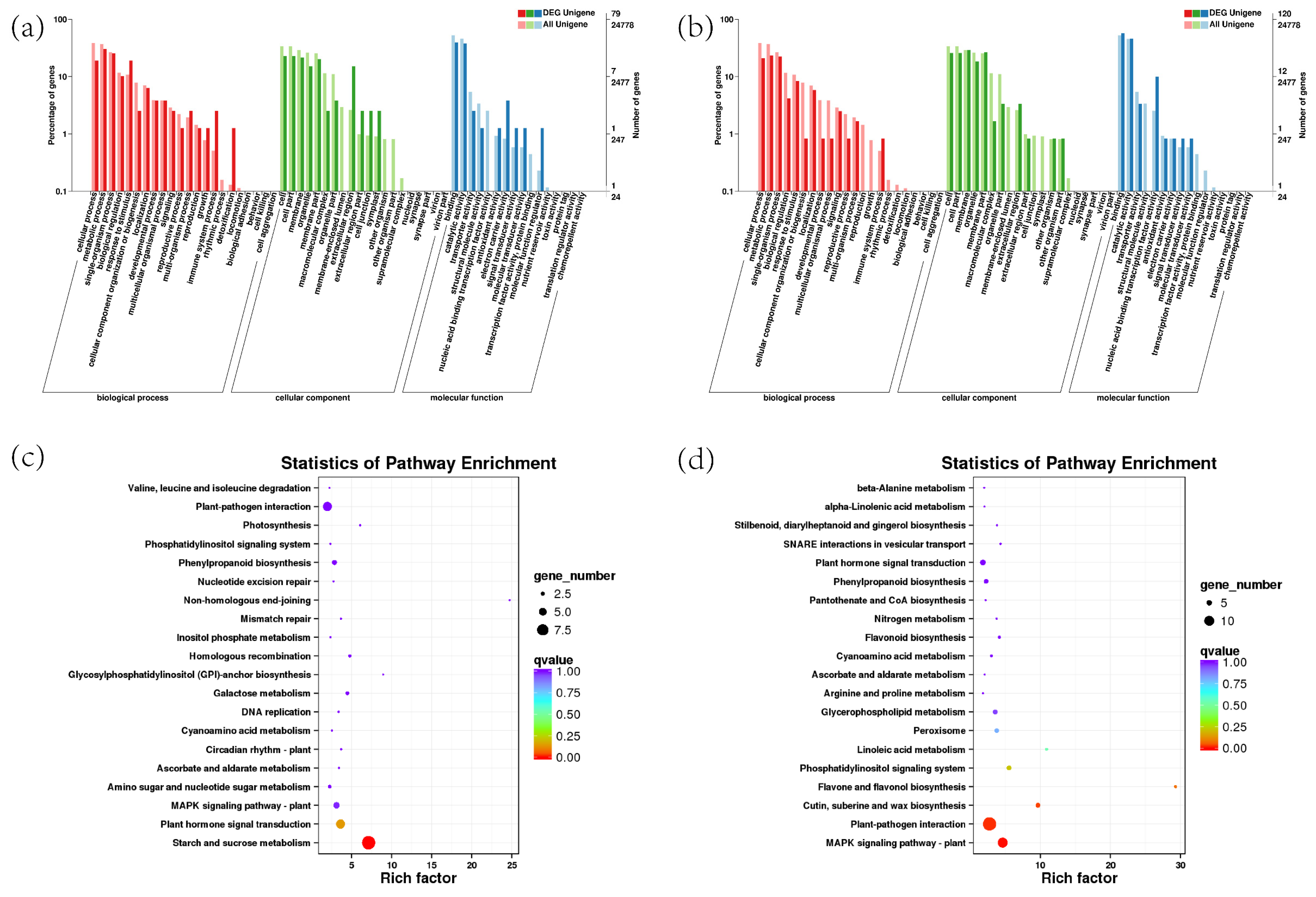
2.11. The Transcriptome Analysis
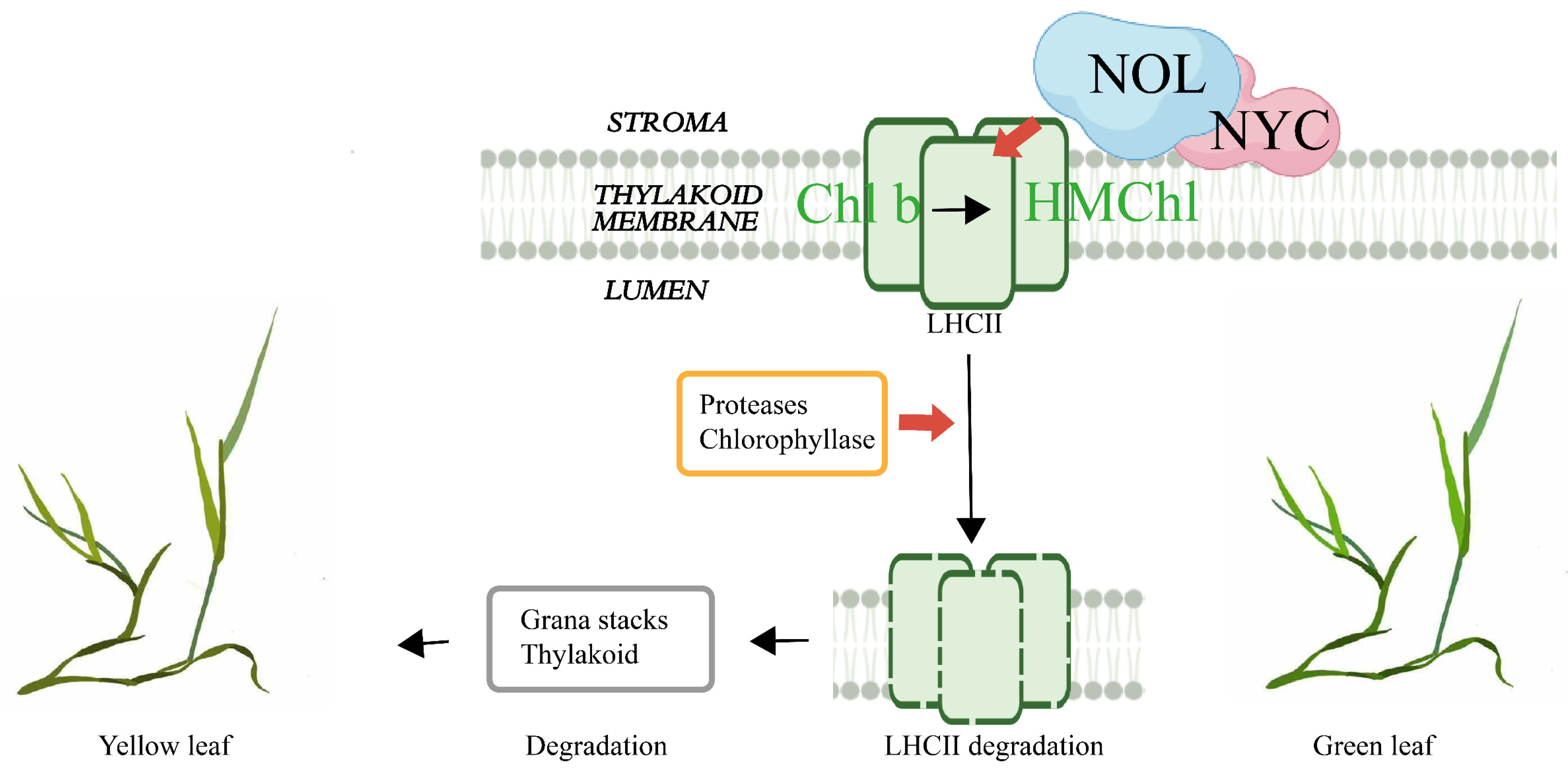
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Gene Cloning and Vector Construction
4.3. Generation of Transgenic Plants
4.4. Subcellular Localization and BiFC Analysis
4.5. Yeast Two-Hybrid Assay
4.6. Measurement of Net Photosynthesis of Leaves
4.7. Photosynthetic Pigment Analysis and Electrolyte Leakage (EL) Measurement
4.8. Transmission Electron Microscopy
4.9. Morphological Observation
4.10. Bioinformatics Analysis
4.11. Expression Levels of ZjNOL Gene and Pigment-Related Genes
4.12. Hormone Contents Assay
4.13. Transcriptomic Analysis
4.14. Experimental Validation of DEGs by RT-PCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hörtensteiner, S. The Loss of Green Color during Chlorophyll Degradation—A Prerequisite to Prevent Cell Death? Planta 2004, 219, 191–194. [Google Scholar] [CrossRef]
- KiJhlbrandt, W. Structure and Function of the Plant Light-Harvesting Complex, LHC-II. Curr. Opin. Struct. Biol. 1994, 4, 519–528. [Google Scholar] [CrossRef]
- Allen, J.F.; Forsberg, J. Molecular Recognition in Thylakoid Structure and Function. Trends Plant Sci. 2001, 6, 317–326. [Google Scholar] [CrossRef]
- Sakuraba, Y.; Kim, Y.S.; Yoo, S.C.; H?Rtensteiner, S.; Paek, N.C. 7-Hydroxymethyl Chlorophyll a Reductase Functions in Metabolic Channeling of Chlorophyll Breakdown Intermediates during Leaf Senescence. Biochem. Biophys. Res. Commun. 2013, 430, 32–37. [Google Scholar] [CrossRef]
- Sakuraba, Y.; Schelbert, S.; Park, S.Y.; Han, S.H.; Lee, B.D.; Andres, C.B.; Kessler, F.; Hortensteiner, S.; Paek, N.C. STAY-GREEN and Chlorophyll Catabolic Enzymes Interact at Light-Harvesting Complex II for Chlorophyll Detoxification during Leaf Senescence in Arabidopsis. Plant Cell 2012, 24, 507–518. [Google Scholar] [CrossRef]
- Rubina, J.; Sullivan, K.L.; Ross, C.; Erridge, Z.A.; David, C.; Mclachlan, A.R.G.; Brummell, D.A.; Dijkwel, P.P.; Hunter, D.A. Staying Green Postharvest: How Three Mutations in the Arabidopsis Chlorophyll b Reductase Gene NYC1 Delay Degreening by Distinct Mechanisms. J. Exp. Bot. 2015, 66, 6849–6862. [Google Scholar]
- Luo, F.; Cheng, S.C.; Cai, J.H.; Wei, B.D.; Zhou, X.; Zhou, Q.; Zhao, Y.B.; Ji, S.J. Chlorophyll Degradation and Carotenoid Biosynthetic Pathways: Gene Expression and Pigment Content in Broccoli during Yellowing. Food Chem. 2019, 297, 124964. [Google Scholar] [CrossRef]
- HRtensteiner, S.; Vicentini, F.; Matile, P. Chlorophyll Breakdown in Senescent Leaves: Enzymatic Cleavage of Pheophorbide a In Vitro. New Phytol. 1995, 129, 237–246. [Google Scholar] [CrossRef]
- Jia, T.; Ito, H.; Tanaka, A. The Chlorophyll b Reductase NOL Participates in Regulating the Antenna Size of Photosystem II in Arabidopsis thaliana. Procedia Chem. 2015, 14, 422–427. [Google Scholar] [CrossRef]
- Sato, Y.; Morita, R.; Katsuma, S.; Nishimura, M.; Kusaba, M. Two Short-Chain Dehydrogenase/Reductases, NON-YELLOW COLORING 1 and NYC1-LIKE, Are Required for Chlorophyll b and Light-Harvesting Complex II Degradation during Senescence in Rice. Plant J. 2010, 57, 120–131. [Google Scholar] [CrossRef]
- Yu, G.; Xie, Z.; Zhang, J.; Lei, S.; Lin, W.; Xu, B.; Huang, B. NOL-mediated Functional Stay-green Traits in Perennial Ryegrass (Lolium Perenne L.) Involving Multifaceted Molecular Factors and Metabolic Pathways Regulating Leaf Senescence. Plant J. 2021, 106, 1219–1232. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, B. Comparative Transcriptomic Analysis Reveals Common Molecular Factors Responsive to Heat and Drought Stress in Agrostis stolonifera. Sci. Rep. 2018, 8, 15181. [Google Scholar] [CrossRef]
- Tanaka, H.; Hirakawa, H.; Kosugi, S.; Nakayama, S.; Ono, A.; Watanabe, A.; Hashiguchi, M.; Gondo, T.; Ishigaki, G.; Muguerza, M. Sequencing and Comparative Analyses of the Genomes of Zoysiagrasses. DNA Res. 2016, 2, 171–180. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Ohta, H.; Okawa, K.; Iwamatsu, A.; Shimada, H.; Masuda, T.; Takamiya, K.I. Cloning of Chlorophyllase, the Key Enzyme in Chlorophyll Degradation: Finding of a Lipase Motif and the Induction by Methyl Jasmonate. Proc. Natl. Acad. Sci. USA 1999, 96, 15362–15367. [Google Scholar] [CrossRef]
- Araya-Garay, J.M.; Feijoo-Siota, L.; Veiga-Crespo, P.; Sánchez-Pérez, A.; Villa, T.G. Cloning and Functional Expression of Z-Carotene Desaturase, A Novel Carotenoid Biosynthesis Gene from Ficus carica. Int. J. Microbiol. Adv. Immunol. 2014, 2, 1016–1033. [Google Scholar]
- Leng, P.; Zhang, G.L.; Li, X.X.; Wang, L.H.; Zheng, Z.M. Cloning of 9-Cis-Epoxycarotenoid Dioxygenase (NCED) Gene Encoding a Key Enzyme during Abscisic Acid (ABA) Biosynthesis and ABA-Regulated Ethylene Production in Detached Young Persimmon Calyx. Sci. Bull. 2009, 54, 2830–2838. [Google Scholar] [CrossRef]
- Moummou, H.; Kallberg, Y.; Tonfack, L.B.; Persson, B.; Rest, B.V.D. The Plant Short-Chain Dehydrogenase (SDR) Superfamily: Genome-Wide Inventory and Diversification Patterns. BMC Plant Biol. 2012, 12, 219. [Google Scholar] [CrossRef]
- Filling, C. Critical Residues for Structure and Catalysis in Short-Chain Dehydrogenases/Reductases. J. Biol. Chem. 2002, 277, 25677–25684. [Google Scholar] [CrossRef]
- Kavanagh, K.L.; Jörnvall, H.; Persson, B.; Oppermann, U. Medium- and short-chain dehydrogenase/reductase gene and protein families: The SDR superfamily: Functional and structural diversity within a family of metabolic and regulatory enzymes. Cell. Mol. Life Sci. 2008, 65, 3895–3906. [Google Scholar] [CrossRef]
- Jörnvall, H.; Persson, B.; Krook, M.; Atrian, S.; Gonzalez-Duarte, R.; Jeffery, J.; Ghosh, D. Short-Chain Dehydrogenases/Reductases (SDRs). Eur. J. Biochem. 1995, 34, 6003–6013. [Google Scholar] [CrossRef]
- Shimada, Y.; Wu, G.-J.; Watanabe, A. A Protein Encoded by Dinl, a Dark-Inducible and Senescence-Associated Gene of Radish, Can Be Imported by Isolated Chloroplasts and Has Sequence Similarity to Sulfide Dehydrogenase and Other Small Stress Proteins. Plant Cell Physiol. 1998, 39, 139–143. [Google Scholar] [CrossRef][Green Version]
- Chen, Y.; Fu, X.; Mei, X.; Zhou, Y.; Cheng, S.; Zeng, L.; Dong, F.; Yang, Z. Proteolysis of Chloroplast Proteins Is Responsible for Accumulation of Free Amino Acids in Dark-Treated Tea (Camellia Sinensis) Leaves. J. Proteom. 2017, 157, 10–17. [Google Scholar] [CrossRef]
- Liu, H. The Rice Aspartyl-TRNA Synthetase YLC3 Regulates Amino Acid Homeostasis and Chloroplast Development under Low Temperature. Front. Plant Sci. 2022, 13, 847364. [Google Scholar] [CrossRef]
- Soto, D.; Córdoba, J.P.; Villarreal, F.; Bartoli, C.; Schmitz, J.; Maurino, V.G.; Braun, H.P.; Pagnussat, G.C.; Zabaleta, E. Functional Characterization of Mutants Affected in the Carbonic Anhydrase Domain of the Respiratory ComplexI in Arabidopsis Thaliana. Plant J. 2015, 83, 831–844. [Google Scholar] [CrossRef]
- Guyer, L.; Hofstetter, S.S.; Christ, B.; Lira, B.S.; Rossi, M.; Hörtensteiner, S. Different Mechanisms Are Responsible for Chlorophyll Dephytylation during Fruit Ripening and Leaf Senescence in Tomato. Plant Physiol. 2014, 166, 44–56. [Google Scholar] [CrossRef]
- Schelbert, S.; Aubry, S.; Burla, B.; Agne, B.; Kessler, F.; Krupinska, K.; Hortensteiner, S. Pheophytin Pheophorbide Hydrolase (Pheophytinase) Is Involved in Chlorophyll Breakdown during Leaf Senescence in Arabidopsis. Plant Cell 2009, 21, 767–785. [Google Scholar] [CrossRef]
- Guyer, L.; Salinger, K.; Krügel, U.; Hrtensteiner, S. Catalytic and Structural Properties of Pheophytinase, the Phytol Esterase Involved in Chlorophyll Breakdown. J. Exp. Bot. 2017, 69, 879–889. [Google Scholar] [CrossRef]
- Sakuraba, Y.; Kim, D.; Kim, Y.S.; Hörtensteiner, S.; Paek, N.C. Arabidopsis STAYGREEN-LIKE (SGRL) Promotes Abiotic Stress-Induced Leaf Yellowing during Vegetative Growth. FEBS Lett. 2014, 588, 3830–3837. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral Dip: A Simplified Method for Agrobacterium-Mediated Transformation of Arabidopsis thaliana. Plant J. 2010, 16, 735–743. [Google Scholar] [CrossRef]
- Luo, H.; Hu, Q.; Nelson, K.; Longo, C.; Kausch, A.P.; Chandlee, J.M.; Wip, J.K.; Fricker, C.R. Agrobacterium Tumefaciens-Mediated Creeping Bentgrass (Agrostis stolonifera L.) Transformation Using Phosphinothricin Selection Results in a High Frequency of Single-Copy Transgene Integration. Plant Cell Rep. 2004, 22, 645–652. [Google Scholar] [CrossRef]
- Kokkirala, V.R.; Peng, Y.; Abbagani, S.; Zhu, Z.; Umate, P. Subcellular Localization of Proteins of Oryza sativa L. in the Model Tobacco and Tomato Plants. Plant Signal. Behav. 2010, 1, 1336–1341. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.-Q.; Chen, J.-Y.; Kuang, J.-F.; Lu, W.-J.; Shan, W. The Banana Fruit SINA Ubiquitin Ligase MaSINA1 Regulates the Stability of MaICE1 to Be Negatively Involved in Cold Stress Response. Front. Plant Sci. 2017, 8, 995. [Google Scholar]
- Ding, F.; Wang, R. Amelioration of Postharvest Chilling Stress by Trehalose in Pepper. Sci. Hortic. 2018, 232, 52–56. [Google Scholar] [CrossRef]
- Bajji, M.; Kinet, J.M.; Lutts, S. The Use of the Electrolyte Leakage Method for Assessing Cell Membrane Stability as a Water Stress Tolerance Test in Durum Wheat. Plant Growth Regul. 2002, 36, 61–70. [Google Scholar] [CrossRef]
- Winey, M.; Meehl, J.B.; O’Toole, E.T.; Jr, G.T. Conventional Transmission Electron Microscopy. Ultramicroscopy 1978, 3, 169–174. [Google Scholar] [CrossRef]
- Wang, G.L.; Xiong, F.; Que, F.; Xu, Z.S.; Wang, F.; Xiong, A.S. Morphological characteristics, anatomical structure, and gene expression: Novel insights into gibberellin biosynthesis and perception during carrot growth and development. Hortic. Res. 2015, 2, 15028. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.-Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An Upgraded Gene Feature Visualization Server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Lescot, M. PlantCARE, a Database of Plant Cis-Acting Regulatory Elements and a Portal to Tools for In Silico Analysis of Promoter Sequences. Nucleic. Acids. Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Sudhir, K.; Glen, S.; Li, M.; Christina, K.; Koichiro, T. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar]
- Bailey, T.L.; Elkan, C. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc. Int. Conf. Intell. Syst. Mol. Biol. 1994, 2, 28–36. [Google Scholar]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Yumeng, Y.; Di, Z.; Pei, Z.; Botong, L.; Sheng-You, H. HDOCK: A Web Server for Protein-Protein and Protein-DNA/RNA Docking Based on a Hybrid Strategy. Nucleic. Acids. Res. 2017, 45, W365–W373. [Google Scholar]
- Dong, D.; Zhao, Y.; Teng, K.; Tan, P.; Liu, Z.; Yang, Z.; Han, L.; Chao, Y. Expression of ZjPSY, a Phytoene Synthase Gene from Zoysia japonica Affects Plant Height and Photosynthetic Pigment Contents. Plants 2022, 11, 395. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, J.; Burgess, P.; Rossi, S.; Huang, B. Interactive Effects of Melatonin and Cytokinin on Alleviating Drought-Induced Leaf Senescence in Creeping Bentgrass (Agrostis stolonifera). Environ. Exp. Bot. 2018, 145, 1–11. [Google Scholar] [CrossRef]
- Schmittgen, T.D. Analyzing Real-Time PCR Data by the Comparative CT Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, C.; Yan, X.; Zhang, J.; Xu, J. Simultaneous Analysis of Ten Phytohormones in Sargassum Horneri by High-performance Liquid Chromatography with Electrospray Ionization Tandem Mass Spectrometry. J. Sep. Sci. 2016, 39, 1804–1813. [Google Scholar] [CrossRef] [PubMed]
- Imura, J.; Antoniadi, I.; Iroká, J.; Tarkowská, D.; Strnad, M.; Ljung, K.; Novák, O. Plant Hormonomics: Multiple Phytohormone Profiling by Targeted Metabolomics 1. Plant Physiol. 2018, 177, 476–489. [Google Scholar]
- Dong, D.; Wang, M.; Li, Y.; Liu, Z.; Han, L. Melatonin Influences the Early Growth Stage in Zoysia japonica Steud. by Regulating Plant Oxidation and Genes of Hormones. Sci. Rep. 2021, 11, 12381. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, D.; Yang, Z.; Ma, Y.; Li, S.; Wang, M.; Li, Y.; Liu, Z.; Han, L.; Chao, Y. Expression of a Chlorophyll b Reductase Gene from Zoysia japonica Causes Changes in Leaf Color and Chlorophyll Morphology in Agrostis stolonifera. Int. J. Mol. Sci. 2022, 23, 6032. https://doi.org/10.3390/ijms23116032
Dong D, Yang Z, Ma Y, Li S, Wang M, Li Y, Liu Z, Han L, Chao Y. Expression of a Chlorophyll b Reductase Gene from Zoysia japonica Causes Changes in Leaf Color and Chlorophyll Morphology in Agrostis stolonifera. International Journal of Molecular Sciences. 2022; 23(11):6032. https://doi.org/10.3390/ijms23116032
Chicago/Turabian StyleDong, Di, Zhuoxiong Yang, Yuan Ma, Shuwen Li, Mengdi Wang, Yinruizhi Li, Zhuocheng Liu, Liebao Han, and Yuehui Chao. 2022. "Expression of a Chlorophyll b Reductase Gene from Zoysia japonica Causes Changes in Leaf Color and Chlorophyll Morphology in Agrostis stolonifera" International Journal of Molecular Sciences 23, no. 11: 6032. https://doi.org/10.3390/ijms23116032
APA StyleDong, D., Yang, Z., Ma, Y., Li, S., Wang, M., Li, Y., Liu, Z., Han, L., & Chao, Y. (2022). Expression of a Chlorophyll b Reductase Gene from Zoysia japonica Causes Changes in Leaf Color and Chlorophyll Morphology in Agrostis stolonifera. International Journal of Molecular Sciences, 23(11), 6032. https://doi.org/10.3390/ijms23116032





