Mechanisms during Osteogenic Differentiation in Human Dental Follicle Cells
Abstract
1. Introduction
2. Isolation of Cells from Human Dental Follicles
3. Molecular Mechanisms of the Osteogenic Differentiation of DFCs
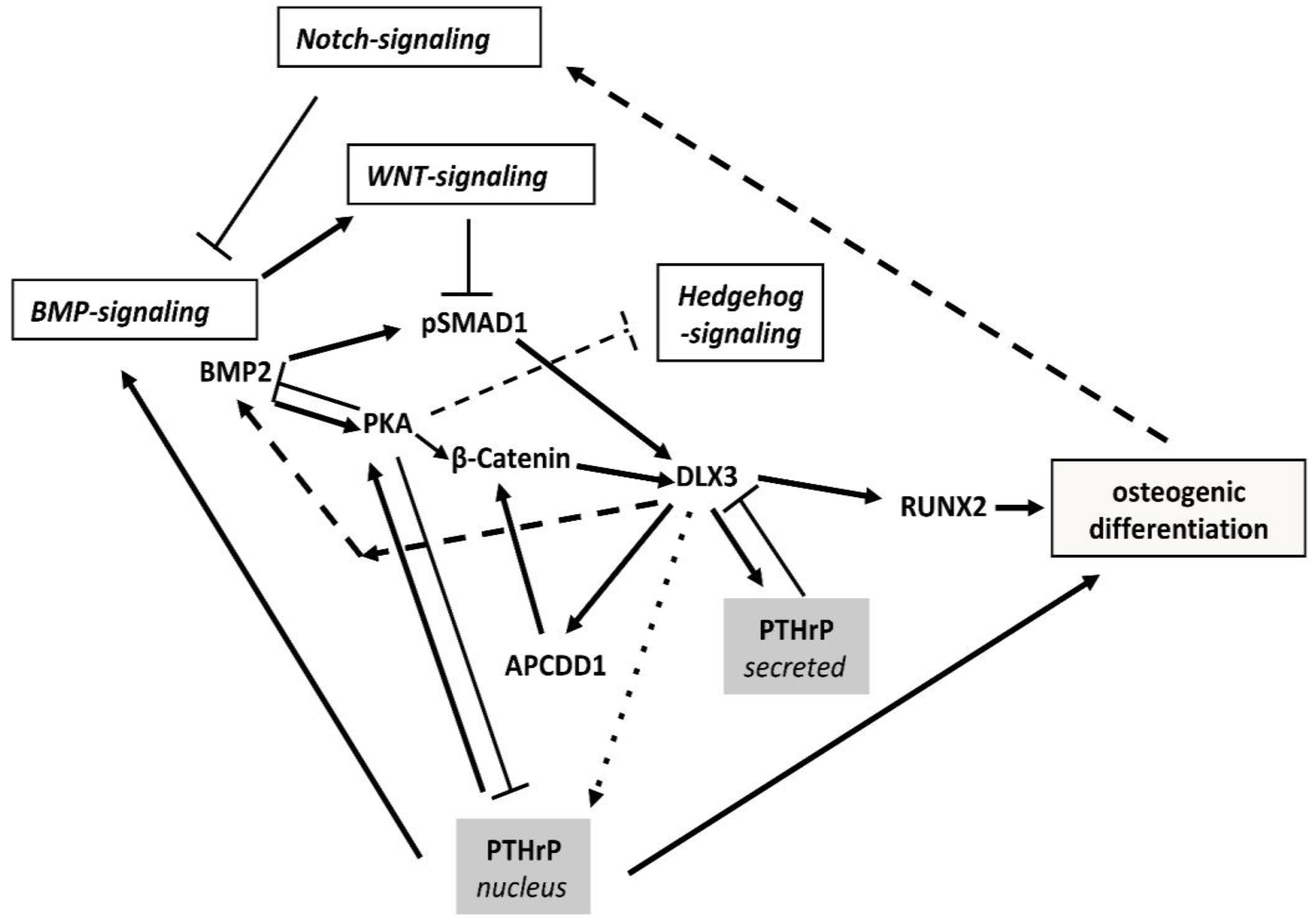
3.1. The BMP2/DLX3 Positive Feedback Loop
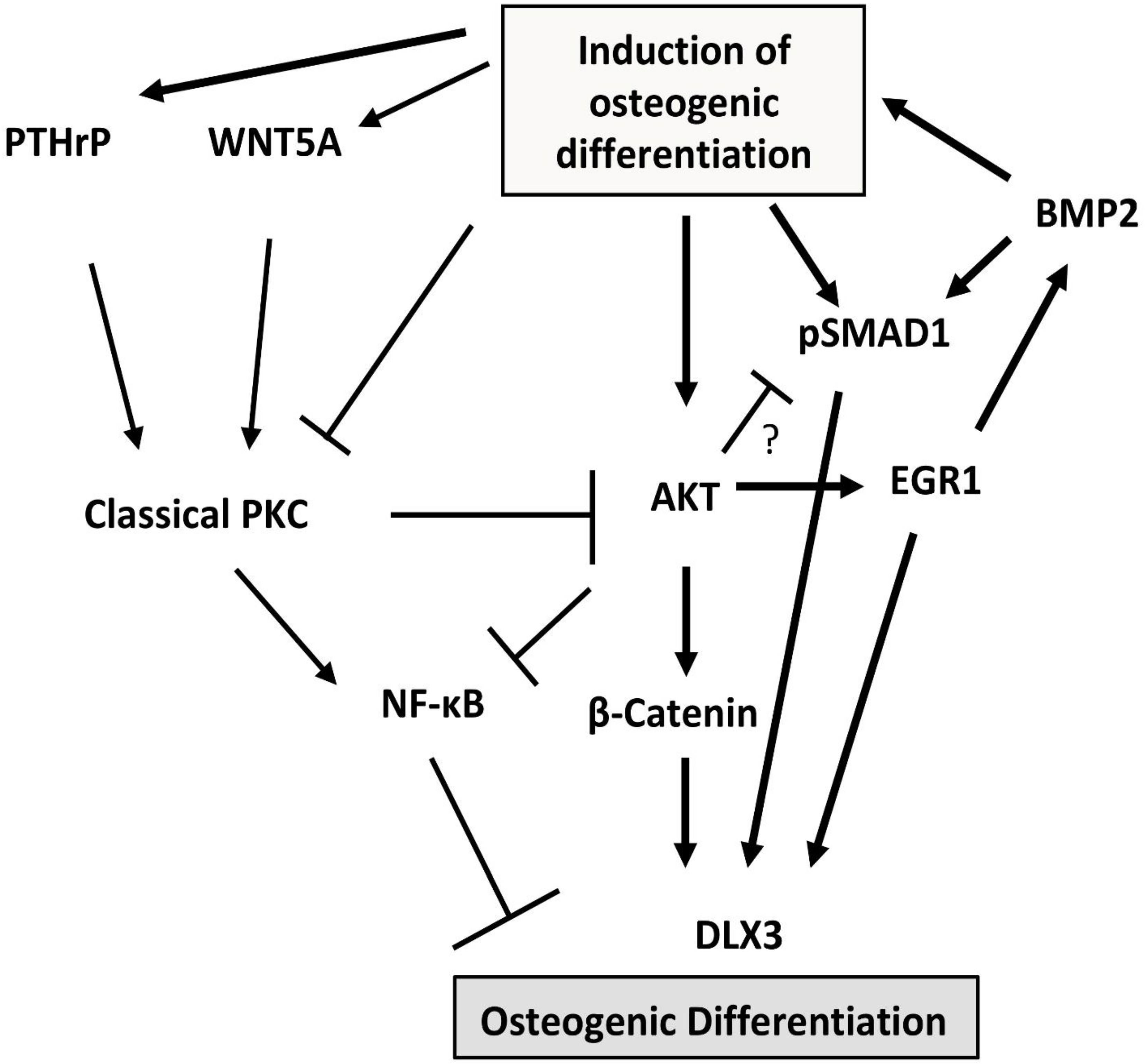
3.2. Protein Kinases C (PKC) and B (AKT)
3.3. Epigenetics and Non-Coding RNAs
3.4. Extracellular Matrix (ECM)
3.5. Influence of Cell Viability and Cellular Senescence
3.6. Pro- and anti-Inflammatory Factors
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Donoghue, P.; Sansom, I.J.; Downs, J.P. Early evolution of vertebrate skeletal tissues and cellular interactions, and the canalization of skeletal development. J. Exp. Zool. Part B Mol. Dev. Evol. 2006, 306, 278–294. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, B.; Lee, A.; Ravi, V.; Maurya, A.K.; Lian, M.M.; Swann, J.B.; Ohta, Y.; Flajnik, M.F.; Sutoh, Y.; Kasahara, M.; et al. Elephant shark genome provides unique insights into gnathostome evolution. Nature 2014, 505, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Diekwisch, T.G. The developmental biology of cementum. Int. J. Dev. Biology. 2001, 45, 695–706. Available online: http://www.ncbi.nlm.nih.gov/pubmed/11669371 (accessed on 4 May 2022).
- Takahashi, A.; Nagata, M.; Gupta, A.; Matsushita, Y.; Yamaguchi, T.; Mizuhashi, K.; Maki, K.; Ruellas, A.C.; Cevidanes, L.S.; Kronenberg, H.M.; et al. Autocrine regulation of mesenchymal progenitor cell fates orchestrates tooth eruption. Proc. Natl. Acad. Sci. USA 2019, 116, 575–580. [Google Scholar] [CrossRef]
- Thesleff, I. Current understanding of the process of tooth formation: Transfer from the laboratory to the clinic. Aust. Dent. J. 2013, 59, 48–54. [Google Scholar] [CrossRef]
- Hermans, F.; Hemeryck, L.; Lambrichts, I.; Bronckaers, A.; Vankelecom, H. Intertwined Signaling Pathways Governing Tooth Development: A Give-and-Take Between Canonical Wnt and Shh. Front. Cell Dev. Biol. 2021, 9, 3043. [Google Scholar] [CrossRef]
- Zhou, T.; Pan, J.; Wu, P.; Huang, R.; Du, W.; Zhou, Y.; Wan, M.; Fan, Y.; Xu, X.; Zhou, X.; et al. Dental Follicle Cells: Roles in Development and Beyond. Stem Cells Int. 2019, 2019, 9159605. [Google Scholar] [CrossRef]
- Lyu, P.; Li, B.; Li, P.; Bi, R.; Cui, C.; Zhao, Z.; Zhou, X.; Fan, Y. Parathyroid Hormone 1 Receptor Signaling in Dental Mesenchymal Stem Cells: Basic and Clinical Implications. Front. Cell Dev. Biol. 2021, 9, 654715. [Google Scholar] [CrossRef]
- Marks, S.; Cahill, D. Experimental study in the dog of the non-active role of the tooth in the eruptive process. Arch. Oral. Biol. 1984, 29, 311–322. [Google Scholar] [CrossRef]
- Zhang, J.W.; Liao, L.J.; Li, Y.Y.; Xu, Y.; Guo, W.H.; Tian, W.D.; Zou, S.J. Parathyroid hormone-related peptide (1–34) promotes tooth eruption and inhibits osteogenesis of dental follicle cells during tooth devel-opment. J. Cell. Physiol. 2019, 234, 11900–11911. [Google Scholar] [CrossRef]
- Izumida, E.; Suzawa, T.; Miyamoto, Y.; Yamada, A.; Otsu, M.; Saito, T.; Yamaguchi, T.; Nishimura, K.; Ohtaka, M.; Nakanishi, M.; et al. Functional Analysis of PTH1R Variants Found in Primary Failure of Eruption. J. Dent. Res. 2020, 99, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Bastos, V.C.; Gomez, R.S.; Gomes, C.C. Revisiting the human dental follicle: From tooth development to its association with unerupted or impacted teeth and pathological changes. Dev. Dyn. 2021, 251, 408–423. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Wu, J.; Huang, L.; Liu, H.; Wang, R.; Karaplis, A.; Goltzman, D.; Miao, D. PTHrP nuclear localization and carboxyl terminus sequences modulate dental and mandibular development in part via the action of p27. Endocrinology 2016, 2016, en20151555. [Google Scholar] [CrossRef] [PubMed]
- Wise, G.E.; Ding, D.; Yao, S. Regulation of secretion of osteoprotegerin in rat dental follicle cells. Eur. J. Oral Sci. 2004, 112, 439–444. [Google Scholar] [CrossRef]
- Wise, G.E. Cellular and molecular basis of tooth eruption. Orthod. Craniofacial Res. 2009, 12, 67–73. [Google Scholar] [CrossRef]
- Sherley, J.L. Asymmetric Cell Kinetics Genes: The Key to Expansion of Adult Stem Cells in Culture. Sci. World J. 2002, 2, 1906–1921. [Google Scholar] [CrossRef][Green Version]
- Mitsiadis, T.A.; Barrandon, O.; Rochat, A.; Barrandon, Y.; De Bari, C. Stem cell niches in mammals. Exp. Cell Res. 2007, 313, 3377–3385. [Google Scholar] [CrossRef]
- Venkei, Z.G.; Yamashita, Y.M. Emerging mechanisms of asymmetric stem cell division. J. Cell Biol. 2018, 217, 3785–3795. [Google Scholar] [CrossRef]
- Sheldrake, A.R. Cellular senescence, rejuvenation and potential immortality. Proc. R. Soc. B Boil. Sci. 2022, 289, 20212434. [Google Scholar] [CrossRef]
- Chiou, S.-H.; Yu, C.-C.; Huang, C.-Y.; Lin, S.-C.; Liu, C.-J.; Tsai, T.-H.; Chou, S.-H.; Chien, C.-S.; Ku, H.-H.; Lo, J.-F. Positive Correlations of Oct-4 and Nanog in Oral Cancer Stem-Like Cells and High-Grade Oral Squamous Cell Carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008, 14, 4085–4095. [Google Scholar] [CrossRef]
- Chen, D.; Wang, C.-Y. Targeting cancer stem cells in squamous cell carcinoma. Precis. Clin. Med. 2019, 2, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Harada, H.; Kettunen, P.; Jung, H.S.; Mustonen, T.; Wang, Y.A.; Thesleff, I. Localization of Putative Stem Cells in Dental Epithelium and Their Association with Notch and Fgf Signaling. J. Cell Biol. 1999, 147, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Arthur, A.; Rychkov, G.; Shi, S.; Koblar, S.A.; Gronthos, S. Adult human dental pulp stem cells dif-ferentiate toward functionally active neurons under appropriate environmental cues. Stem. Cells 2008, 26, 1787–1795. [Google Scholar] [CrossRef] [PubMed]
- Völlner, F.; Ernst, W.; Driemel, O.; Morsczeck, C. A two-step strategy for neuronal differentiation in vitro of human dental follicle cells. Differentiation 2009, 77, 433–441. Available online: http://www.ncbi.nlm.nih.gov/pubmed/19394129 (accessed on 4 May 2022). [CrossRef] [PubMed]
- Morsczeck, C.; Götz, W.; Schierholz, J.; Zeilhofer, F.; Kühn, U.; Möhl, C.; Sippel, C.; Hoffmann, K.H. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005, 24, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.; Kuralesova, A.I. Osteogenic Precursor Cells of Bone Marrow In Radiation Chimeras. Transplantation 1971, 12, 99–108. [Google Scholar] [CrossRef]
- Morsczeck, C.; Huang, G.T.J.; Shi, S. Stem and Progenitor Cells of Dental and Gingival Tissue Origin Morsczeck, Christian; Univ. Hosp. Regensburg, Dept. Cranial and Maxillofacial Surg.: Regensburg, Germany, 2013. [Google Scholar]
- Morsczeck, C. Molecular mechanisms in dental follicle precursor cells during the osteogenic differentiation. Histol. Histopathol. 2015, 30, 1161–1169. [Google Scholar] [CrossRef]
- Oh, J.E.; Yi, J.-K. Isolation and characterization of dental follicle–derived Hertwig’s epithelial root sheath cells. Clin. Oral Investig. 2020, 25, 1787–1796. [Google Scholar] [CrossRef]
- Ono, W.; Sakagami, N.; Nishimori, S.; Ono, N.; Kronenberg, H.M. Parathyroid hormone receptor sig-nalling in osterix-expressing mesenchymal progenitors is essential for tooth root formation. Nat. Commun. 2016, 7, 11277. [Google Scholar] [CrossRef]
- Franceschi, R.T.; Xiao, G.; Jiang, D.; Gopalakrishnan, R.; Yang, S.; Reith, E. Multiple signaling pathways converge on the Cbfa1/Runx2 transcription factor to regulate osteoblast differentiation. Connect. Tissue Res. 2003, 44 (Suppl. S1), 109–116. [Google Scholar] [CrossRef]
- Lin, G.L.; Hankenson, K.D. Integration of BMP, Wnt, and notch signaling pathways in osteoblast dif-ferentiation. J. Cell. Biochem. 2011, 112, 3491–3501. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, R.T.; Ge, C. Control of the Osteoblast Lineage by Mitogen-Activated Protein Kinase Sig-naling. Curr. Mol. Biol. Rep. 2017, 3, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Morsczeck, C. Gene Expression of runx2, Osterix, c-fos, DLX-3, DLX-5, and MSX-2 in Dental Follicle Cells during Osteogenic Differentiation In Vitro. Calcif. Tissue Res. 2006, 78, 98–102. Available online: http://www.ncbi.nlm.nih.gov/pubmed/16467978 (accessed on 4 May 2022). [CrossRef] [PubMed]
- Hassan, M.Q.; Javed, A.; Morasso, M.I.; Karlin, J.; Montecino, M.; van Wijnen, A.J.; Stein, G.S.; Stein, J.L.; Lian, J.B. Dlx3 Transcriptional Regulation of Osteoblast Differentiation: Temporal Recruitment of Msx2, Dlx3, and Dlx5 Homeodomain Proteins to Chromatin of the Osteocalcin Gene. Mol. Cell. Biol. 2004, 24, 9248–9261. Available online: http://www.ncbi.nlm.nih.gov/pubmed/15456894 (accessed on 4 May 2022). [CrossRef]
- Sumiyama, K.; Tanave, A. The regulatory landscape of the Dlx gene system in branchial arches: Shared characteristics among Dlx bigene clusters and evolution. Dev. Growth Differ. 2020, 62, 355–362. [Google Scholar] [CrossRef]
- Zheng, H.; Fu, J.; Chen, Z.; Yang, G.; Yuan, G. Dlx3 Ubiquitination by Nuclear Mdm2 Is Essential for Dentinogenesis in Mice. J. Dent. Res. 2022. [Google Scholar] [CrossRef]
- Choi, S.; Song, I.; Feng, J.; Gao, T.; Haruyama, N.; Gautam, P.; Robey, P.; Hart, T.C. Mutant DLX 3 disrupts odontoblast polarization and dentin formation. Dev. Biol. 2010, 344, 682–692. [Google Scholar] [CrossRef][Green Version]
- Whitehouse, L.L.E.; Smith, C.E.L.; Poulter, J.A.; Brown, C.J.; Patel, A.; Lamb, T.; Brown, L.R.; O’Sullivan, E.A.; Mitchell, R.E.; Berry, I.R.; et al. Novel DLX3 variants in amelogenesis imperfecta with attenuated tricho-dento-osseous syndrome. Oral Dis. 2018, 25, 182–191. [Google Scholar] [CrossRef]
- Zeng, L.; Sun, S.; Dong, L.; Liu, Y.; Liu, H.; Han, D.; Ma, Z.; Wang, Y.; Feng, H. DLX3 epigenetically regulates odontoblastic differentiation of hDPCs through H19/miR-675 axis. Arch. Oral Biol. 2019, 102, 155–163. [Google Scholar] [CrossRef]
- Bonnet, A.L.; Sceosole, K.; Vanderzwalm, A.; Silve, C.; Collignon, A.M.; Gaucher, C. “Isolated” Ame-logenesis Imperfecta Associated with DLX3 Mutation: A Clinical Case. Case Rep. Genet. 2020, 2020, 8217919. [Google Scholar]
- Viale-Bouroncle, S.; Felthaus, O.; Schmalz, G.; Brockhoff, G.; Reichert, E.T.; Morsczeck, C. The Transcription Factor DLX3 Regulates the Osteogenic Differentiation of Human Dental Follicle Precursor Cells. Stem Cells Dev. 2012, 21, 1936–1947. Available online: http://www.ncbi.nlm.nih.gov/pubmed/22107079 (accessed on 4 May 2022). [CrossRef] [PubMed]
- Morsczeck, C.; Schmalz, G. Transcriptomes and Proteomes of Dental Follicle Cells. J. Dent. Res. 2010, 89, 445–456. Available online: http://www.ncbi.nlm.nih.gov/pubmed/20348482 (accessed on 4 May 2022). [CrossRef] [PubMed]
- Hassan, M.Q.; Tare, R.; Lee, S.H.; Mandeville, M.; Morasso, M.I.; Javed, A.; van Wijnen, A.J.; Stein, J.L.; Stein, G.S.; Lian, J.B. BMP2 Commitment to the Osteogenic Lineage Involves Activation of Runx2 by DLX3 and a Homeodomain Transcriptional Network. J. Biol. Chem. 2006, 281, 40515–40526. [Google Scholar] [CrossRef] [PubMed]
- Zhaosong, M.; Na, F.; Shuling, G.; Jiacheng, L.; Ran, W. Heterogeneity affects the differentiation potential of dental follicle stem cells through the TGF-beta signaling pathway. Bioengineered 2021, 12, 12294–12307. [Google Scholar] [CrossRef]
- Li, Z.Z.; Wang, H.T.; Lee, G.Y.; Yang, Y.; Zou, Y.P.; Wang, B.; Gong, C.J.; Cai, Y.; Ren, J.G.; Zhao, J.H. Bleomycin: A novel osteogenesis inhibitor of dental follicle cells via a TGF-beta1/SMAD7/RUNX2 pathway. Br. J. Pharmacol. 2021, 178, 312–327. [Google Scholar] [CrossRef]
- Kemoun, P.; Narayanan, A.S.; Brunel, G.; Salles, J.P.; Laurencin-Dalicieux, S.; Rue, J.; Farges, J.C.; Gennero, I.; Conte-Auriol, F.; Briand-Mesange, F.; et al. Human dental follicle cells acquire cementoblast features under stimulation by BMP-2/-7 and enamel matrix derivatives (EMD) in vitro. Cell Tissue Res. 2007, 329, 283–294. [Google Scholar] [CrossRef]
- Li, C.H.; Yang, X.; He, Y.J.; Ye, G.; Li, X.D.; Zhang, X.N.; Zhou, L.; Deng, F. Bone Morphogenetic Protein-9 Induces Osteogenic Differentiation of Rat Dental Follicle Stem Cells in P38 and ERK1/2 MAPK Dependent Manner. Int. J. Med. Sci. 2012, 9, 862–871. [Google Scholar] [CrossRef]
- Viale-Bouroncle, S.; Klingelhöffer, C.; Ettl, T.; Reichert, T.E.; Morsczeck, C. A protein kinase A (PKA)/β-catenin pathway sustains the BMP2/DLX3-induced osteogenic differentiation in dental follicle cells (DFCs). Cell. Signal. 2015, 27, 598–605. [Google Scholar] [CrossRef]
- Morsczeck, C.; Reck, A.; Beck, H.C. The hedgehog-signaling pathway is repressed during the osteogenic differentiation of dental follicle cells. Mol. Cell. Biochem. 2017, 428, 79–86. [Google Scholar] [CrossRef]
- Nagata, M.; Ono, N.; Ono, W. Mesenchymal Progenitor Regulation of Tooth Eruption: A View from PTHrP. J. Dent. Res. 2020, 99, 133–142. [Google Scholar] [CrossRef]
- Ohba, S. Hedgehog Signaling in Skeletal Development: Roles of Indian Hedgehog and the Mode of Its Action. Int. J. Mol. Sci. 2020, 21, 6665. [Google Scholar] [CrossRef] [PubMed]
- Klingelhöffer, C.; Reck, A.; Ettl, T.; Morsczeck, C. The parathyroid hormone-related protein is secreted during the osteogenic differentiation of human dental follicle cells and inhibits the alkaline phosphatase ac-tivity and the expression of DLX. Tissue Cell 2016, 48, 334–339. Available online: http://www.sciencedirect.com/science/article/pii/S0040816616300593 (accessed on 4 May 2022). [CrossRef] [PubMed]
- Pieles, O.; Reck, A.; Morsczeck, C. High endogenous expression of parathyroid hormone-related pro-tein (PTHrP) supports osteogenic differentiation in human dental follicle cells. Histochem. Cell Biol. 2020, 154, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Miao, D.; Su, H.; He, B.; Gao, J.; Xia, Q.; Zhu, M.; Gu, Z.; Goltzman, D.; Karaplis, A.C. Severe growth retardation and early lethality in mice lacking the nuclear localization sequence and C-terminus of PTH-related protein. Proc. Natl. Acad. Sci. USA 2008, 105, 20309–20314. [Google Scholar] [CrossRef]
- Viale-Bouroncle, S.; Gosau, M.; Morsczeck, C. NOTCH1 signaling regulates the BMP2/DLX-3 directed osteogenic differentiation of dental follicle cells. Biochem. Biophys. Res. Commun. 2014, 443, 500–504. [Google Scholar] [CrossRef]
- Zhou, B.; Lin, W.; Long, Y.; Yang, Y.; Zhang, H.; Wu, K.; Chu, Q. Notch signaling pathway: Architecture, disease, and therapeutics. Signal. Transduct. Target. Ther. 2022, 7, 95. [Google Scholar] [CrossRef]
- Viale-Bouroncle, S.; Klingelhöffer, C.; Ettl, T.; Morsczeck, C. The WNT inhibitor APCDD1 sustains the expression of β-Catenin during the osteogenic differentiation of human dental follicle cells. Biochem. Biophys. Res. Commun. 2015, 457, 314–317. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, J.; Ling, J.; Du, Y.; Hou, Y. Nkd2 promotes the differentiation of dental follicle stem/progenitor cells into osteoblasts. Int. J. Mol. Med. 2018, 42, 2403–2414. [Google Scholar] [CrossRef]
- Li, X.; Chen, D.; Jing, X.; Li, C. DKK1 and TNF-alpha influence osteogenic differentiation of adBMP9-infected-rDFCs. Oral. Dis. 2020, 26, 360–369. [Google Scholar] [CrossRef]
- Pieles, O.; Reichert, T.E.; Morsczeck, C. Protein kinase A is activated during bone morphogenetic pro-tein 2-induced osteogenic differentiation of dental follicle stem cells via endogenous parathyroid hor-mone-related protein. Arch. Oral Biol. 2022, 138, 105409. [Google Scholar] [CrossRef]
- Guerquin, M.-J.; Charvet, B.; Nourissat, G.; Havis, E.; Ronsin, O.; Bonnin, M.-A.; Ruggiu, M.; Olivera-Martinez, I.; Robert, N.; Lu, Y.; et al. Transcription factor EGR1 directs tendon differentiation and promotes tendon repair. J. Clin. Investig. 2013, 123, 3564–3576. [Google Scholar] [CrossRef] [PubMed]
- Press, T.; Vialebouroncle, S.; Felthaus, O.; Gosau, M.; Morsczeck, C. EGR1 supports the osteogenic differentiation of dental stem cells. Int. Endod. J. 2014, 48, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Reumann, M.K.; Strachna, O.; Yagerman, S.; Torrecilla, D.; Kim, J.; Doty, S.B.; Lukashova, L.; Boskey, A.L.; Mayer-Kuckuk, P. Loss of transcription factor early growth response gene 1 results in impaired en-dochondral bone repair. Bone 2011, 49, 743–752. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Viale-Bouroncle, S.; Klingelhöffer, C.; Ettl, T.; Morsczeck, C. The AKT signaling pathway sustains the osteogenic differentiation in human dental follicle cells. Mol. Cell. Biochem. 2015, 406, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Pieles, O.; Reichert, T.E.; Morsczeck, C. Classical isoforms of protein kinase C (PKC) and Akt regulate the osteogenic differentiation of human dental follicle cells via both beta-catenin and NF-kappa B. Stem Cell Res. Ther. 2021, 12, 242. [Google Scholar] [CrossRef]
- Morsczeck, C.; Reck, A.; Reichert, T.E. WNT5A supports viability of senescent human dental follicle cells. Mol. Cell. Biochem. 2018, 455, 21–28. [Google Scholar] [CrossRef]
- Tang, J.; Qing, M.F.; Li, M.; Gao, Z. Dexamethasone inhibits BMP7-induced osteogenic differentiation in rat dental follicle cells via the PI3K/AKT/GSK-3beta/beta-catenin pathway. Int. J. Med. Sci. 2020, 17, 2663–2672. [Google Scholar] [CrossRef]
- Yi, G.; Zhang, S.; Ma, Y.; Yang, X.; Huo, F.; Chen, Y.; Yang, B.; Tian, W. Matrix vesicles from dental follicle cells improve alveolar bone regeneration via activation of the PLC/PKC/MAPK pathway. Stem Cell Res. Ther. 2022, 13, 41. [Google Scholar] [CrossRef]
- Vidoni, C.; Ferraresi, A.; Secomandi, E.; Vallino, L.; Gardin, C.; Zavan, B.; Mortellaro, C.; Isidoro, C. Autophagy drives osteogenic differentiation of human gingival mesenchymal stem cells. Cell Commun. Signal. 2019, 17, 98. [Google Scholar] [CrossRef]
- Pieles, O.; Hartmann, M.; Morsczeck, C. AMP-activated protein kinase and the down-stream activated process of autophagy regulate the osteogenic differentiation of human dental follicle cells. Arch. Oral Biol. 2020, 122, 104951. [Google Scholar] [CrossRef]
- Saha, M.; Kumar, S.; Bukhari, S.; Balaji, S.A.; Kumar, P.; Hindupur, S.K.; Rangarajan, A. AMPK–Akt Double-Negative Feedback Loop in Breast Cancer Cells Regulates Their Adaptation to Matrix Deprivation. Cancer Res. 2018, 78, 1497–1510. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Wu, B.-L.; Fang, F.-C. Overview of noncoding RNAs involved in the osteogenic differentiation of periodontal ligament stem cells. World J. Stem Cells 2020, 12, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Li, C.; Yue, J.; Huang, X.; Chen, M.; Gao, J. miR-21 and miR-101 regulate PLAP-1 expression in periodontal ligament cells. Mol. Med. Rep. 2012, 5, 1340–1346. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Klingelhöffer, C.; Codrin, C.; Ettl, T.; Reichert, T.; Morsczeck, C. miRNA-101 supports the osteogenic differentiation in human dental follicle cells. Arch. Oral Biol. 2016, 72, 47–50. [Google Scholar] [CrossRef]
- Aslani, S.; Rahbarghazi, R.; Rahimzadeh, S.; Rajabi, H.; Abhari, A.; Sakhinia, E. Dynamic of miR-NA-101a-3p and miRNA-200a during Induction of Osteoblast Differentiation in Adipose-derived Mesen-chymal Stem Cells. Int. J. Mol. Cell Med. 2020, 9, 140–146. [Google Scholar]
- Li, Y.; Wang, J.; Ma, Y.; Du, W.; Feng, K.; Wang, S. miR-101-loaded exosomes secreted by bone marrow mesenchymal stem cells requires the FBXW7/HIF1alpha/FOXP3 axis, facilitating osteogenic differ-entiation. J. Cell Physiol. 2021, 236, 4258–4272. [Google Scholar] [CrossRef]
- Ito, K.; Tomoki, R.; Ogura, N.; Takahashi, K.; Eda, T.; Yamazaki, F.; Kato, Y.; Goss, A.; Kondoh, T. MicroRNA-204 regulates osteogenic induction in dental follicle cells. J. Dent. Sci. 2020, 15, 457–465. [Google Scholar] [CrossRef]
- Liu, J.-L.; Liu, Y.-S.; Zheng, M.-J.; He, H.-Y. The management of bone defect using long non-coding RNA as a potential biomarker for regulating the osteogenic differentiation process. Mol. Biol. Rep. 2022, 49, 2443–2453. [Google Scholar] [CrossRef]
- Deng, L.; Hong, H.; Zhang, X.; Chen, D.; Chen, Z.; Ling, J.; Wu, L. Down-regulated lncRNA MEG3 promotes osteogenic differentiation of human dental follicle stem cells by epigenetically regulating Wnt pathway. Biochem. Biophys. Res. Commun. 2018, 503, 2061–2067. [Google Scholar] [CrossRef]
- Wu, L.; Deng, L.; Hong, H.; Peng, C.; Zhang, X.; Chen, Z.; Ling, J. Comparison of long non-coding RNA expression profiles in human dental follicle cells and human periodontal ligament cells. Mol. Med. Rep. 2019, 20, 939–950. [Google Scholar] [CrossRef]
- Chen, Z.; Zheng, J.; Hong, H.; Chen, D.; Deng, L.; Zhang, X.; Ling, J.; Wu, L. lncRNA HOTAIRM1 promotes osteogenesis of hDFSCs by epigenetically regulating HOXA2 via DNMT1 in vitro. J. Cell. Physiol. 2020, 235, 8507–8519. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Pedrosa, E.; Shah, A.; Hrabovsky, A.; Maqbool, S.; Zheng, D.; Lachman, H.M. RNA-Seq of Human Neurons Derived from iPS Cells Reveals Candidate Long Non-Coding RNAs Involved in Neurogenesis and Neuropsychiatric Disorders. PLoS ONE 2011, 6, e23356. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.; Viale-Bouroncle, S.; Morsczeck, C.; Muller, S. The SUMO-Specific Isopeptidase SENP3 Regulates MLL1/MLL2 Methyltransferase Complexes and Controls Osteogenic Differentiation. Mol. Cell 2014, 55, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.; Reck, A.; Morsczeck, C.; Muller, S. Flightless-I governs cell fate by recruiting the SUMO isopeptidase SENP3 to distinct HOX genes. Epigenetics. Chromatin. 2017, 10, 15. [Google Scholar] [CrossRef]
- Liu, C.; Li, Q.; Xiao, Q.; Gong, P.; Kang, N. CHD7 Regulates Osteogenic Differentiation of Human Dental Follicle Cells via PTH1R Signaling. Stem Cells Int. 2020, 2020, 8882857. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhang, Y.; Qi, X.; Chen, Y.; Sheng, R.; Xu, R.; Yuan, Q.; Zhou, C. AFF4 regulates osteogenic differentiation of human dental follicle cells. Int. J. Oral Sci. 2020, 12, 20. [Google Scholar] [CrossRef]
- Li, M.; Fu, T.; Yang, S.; Pan, L.; Tang, J.; Chen, M.; Liang, P.; Gao, Z.; Guo, L. Agarose-based spheroid culture enhanced stemness and promoted odontogenic differentiation potential of human dental follicle cells in vitro. Vitr. Cell. Dev. Biol.-Anim. 2021, 57, 620–630. [Google Scholar] [CrossRef]
- Holle, A.W.; Engler, A.J. More than a feeling: Discovering, understanding, and influencing mechano-sensing pathways. Curr. Opin. Biotechnol. 2011, 22, 648–654. Available online: http://www.ncbi.nlm.nih.gov/pubmed/21536426 (accessed on 4 May 2022). [CrossRef]
- Viale-Bouroncle, S.; Völlner, F.; Möhl, C.; Küpper, K.; Brockhoff, G.; Reichert, T.E.; Schmalz, G.; Morsczeck, C. Soft matrix supports osteogenic differentiation of human dental follicle cells. Biochem. Biophys. Res. Commun. 2011, 410, 587–592. Available online: http://www.ncbi.nlm.nih.gov/pubmed/21684253 (accessed on 4 May 2022). [CrossRef]
- Watt, F.M.; Huck, W.T. Role of the extracellular matrix in regulating stem cell fate. Nat. Rev. Mol. Cell Biol. 2013, 14, 467–473. [Google Scholar] [CrossRef]
- Viale-Bouroncle, S.; Gosau, M.; Morsczeck, C. Collagen I induces the expression of alkaline phosphatase and osteopontin via independent activations of FAK and ERK signalling pathways. Arch. Oral Biol. 2014, 59, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Viale-Bouroncle, S.; Gosau, M.; Morsczeck, C. Laminin regulates the osteogenic differentiation of dental follicle cells via integrin-α2/-β1 and the activation of the FAK/ERK signaling pathway. Cell Tissue Res. 2014, 357, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Jadlowiec, J.A.; Zhang, X.; Li, J.; Campbell, P.G.; Sfeir, C. Extracellular Matrix-mediated Signaling by Dentin Phosphophoryn Involves Activation of the Smad Pathway Independent of Bone Morphogenetic Protein. J. Biol. Chem. 2006, 281, 5341–5347. [Google Scholar] [CrossRef] [PubMed]
- Kundu, A.K.; Putnam, A.J. Vitronectin and collagen I differentially regulate osteogenesis in mesen-chymal stem cells. Biochem. Biophys. Res. Commun. 2006, 347, 347–357. [Google Scholar] [CrossRef]
- Hynes, R.O. Integrins: Bidirectional, Allosteric Signaling Machines. Cell 2002, 110, 673–687. Available online: http://www.ncbi.nlm.nih.gov/pubmed/12297042 (accessed on 4 May 2022). [CrossRef]
- Klees, R.F.; Salasznyk, R.M.; Vandenberg, S.; Bennett, K.; Plopper, G.E. Laminin-5 activates extracel-lular matrix production and osteogenic gene focusing in human mesenchymal stem cells. Matrix Biol. J. Int. Soc. Matrix Biol. 2007, 26, 106–114. [Google Scholar] [CrossRef]
- Morsczeck, C.; Schmalz, G.; Reichert, T.E.; Völlner, F.; Saugspier, M.; Viale-Bouroncle, S.; Driemel, O. Gene expression profiles of dental follicle cells before and after osteogenic differentiation in vitro. Clin. Oral Investig. 2009, 13, 383–391. Available online: http://www.ncbi.nlm.nih.gov/pubmed/19252934 (accessed on 4 May 2022). [CrossRef]
- Mohamed, F.; Ge, C.; Binrayes, A.; Franceschi, R. The Role of Discoidin Domain Receptor 2 in Tooth Development. J. Dent. Res. 2020, 99, 214–222. [Google Scholar] [CrossRef]
- Binrayes, A.; Ge, C.; Mohamed, F.F.; Franceschi, R.T. Role of Discoidin Domain Receptor 2 in Crani-ofacial Bone Regeneration. J. Dent. Res. 2021, 100, 1359–1366. [Google Scholar] [CrossRef]
- Guo, W.; Gong, K.; Shi, H.; Zhu, G.; He, Y.; Ding, B.; Wen, L.; Jin, Y. Dental follicle cells and treated dentin matrix scaffold for tissue engineering the tooth root. Biomaterials 2012, 33, 1291–1302. [Google Scholar] [CrossRef]
- Yang, H.; Li, J.; Hu, Y.; Sun, J.; Guo, W.; Li, H.; Chen, J.; Huo, F.; Tian, W.; Li, S. Treated dentin matrix particles combined with dental follicle cell sheet stimulate periodontal regeneration. Dent. Mater. 2019, 35, 1238–1253. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ma, B.; Yang, H.; Qiao, J.; Tian, W.; Yu, R. Xenogeneic dentin matrix as a scaffold for bio-mineralization and induced odontogenesis. Biomed. Mater. 2021, 16, 045020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lan, T.; Han, X.; Xu, Y.; Liao, L.; Xie, L.; Yang, B.; Tian, W.; Guo, W. Improvement of ECM-based bioroot regeneration via N-acetylcysteine-induced antioxidative effects. Stem Cell Res. Ther. 2021, 12, 202. [Google Scholar] [CrossRef] [PubMed]
- Grotheer, V.; Skrynecki, N.; Oezel, L.; Windolf, J.; Grassmann, J. Osteogenic differentiation of human mesenchymal stromal cells and fibroblasts differs depending on tissue origin and replicative senescence. Sci. Rep. 2021, 11, 11968. [Google Scholar] [CrossRef] [PubMed]
- Kamprom, W.; Tawonsawatruk, T.; Mas-Oodi, S.; Anansilp, K.; Rattanasompattikul, M.; Supokawej, A. P-cresol and Indoxyl Sulfate Impair Osteogenic Differentiation by Triggering Mesenchymal Stem Cell Senescence. Int. J. Med. Sci. 2021, 18, 744–755. [Google Scholar] [CrossRef]
- Choi, Y.J.; Lee, J.Y.; Chung, C.P.; Park, Y.J. Cell-penetrating superoxide dismutase attenuates oxidative stress-induced senescence by regulating the p53-p21Cip1 pathway and restores osteoblastic differentiation in human dental pulp stem cells. Int. J. Nanomed. 2012, 7, 5091–5106. [Google Scholar] [CrossRef][Green Version]
- Alraies, A.; Alaidaroos, N.Y.A.; Waddington, R.J.; Moseley, R.; Sloan, A.J. Variation in human dental pulp stem cell ageing profiles reflect contrasting proliferative and regenerative capabilities. BMC Cell Biol. 2017, 18, 12. [Google Scholar] [CrossRef]
- Mas-Bargues, C.; Vina-Almunia, J.; Ingles, M.; Sanz-Ros, J.; Gambini, J.; Ibanez-Cabellos, J.S.; Garcia-Gimenez, J.L.; Vina, J.; Borras, C. Role of p16(INK4a) and BMI-1 in oxidative stress-induced premature senes-cence in human dental pulp stem cells. Redox. Biol. 2017, 12, 690–698. [Google Scholar] [CrossRef]
- Morsczeck, C.; Gresser, J.; Ettl, T. The induction of cellular senescence in dental follicle cells inhibits the osteogenic differentiation. Mol. Cell. Biochem. 2016, 417, 1–6. [Google Scholar] [CrossRef]
- Morsczeck, C. Effects of Cellular Senescence on Dental Follicle Cells. Pharmacology 2020, 106, 137–142. [Google Scholar] [CrossRef]
- Vicencio, J.M.; Galluzzi, L.; Tajeddine, N.; Ortiz, C.; Criollo, A.; Tasdemir, E.; Morselli, E.; Ben Younes, A.; Maiuri, M.C.; Lavandero, S.; et al. Senescence, apoptosis or autophagy? Gerontology 2008, 54, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Chandeck, C.; Mooi, W.J. Oncogene-induced Cellular Senescence. Adv. Anat. Pathol. 2010, 17, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Morsczeck, C.; Hullmann, M.; Reck, A.; Reichert, T.E. The cell cycle regulator protein P16 and the cellular senescence of dental follicle cells. Mol. Cell. Biochem. 2017, 439, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, D.; Wada, N.; Yoshida, S.; Mitarai, H.; Arima, M.; Tomokiyo, A.; Hamano, S.; Sugii, H.; Maeda, H. Wnt5a suppresses osteoblastic differentiation of human periodontal ligament stem cell-like cells via Ror2/JNK signaling. J. Cell Physiol. 2018, 233, 1752–1762. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Chen, M.; He, L.; Cai, B.; Du, Y.; Zhang, X.; Zhou, C.; Wang, C.; Mao, J.J.; Ling, J. Wnt5a regulates dental follicle stem/progenitor cells of the periodontium. Stem Cell Res. Ther. 2014, 5, 135. [Google Scholar] [CrossRef][Green Version]
- Sakisaka, Y.; Tsuchiya, M.; Nakamura, T.; Tamura, M.; Shimauchi, H.; Nemoto, E. Wnt5a attenuates Wnt3a-induced alkaline phosphatase expression in dental follicle cells. Exp. Cell Res. 2015, 336, 85–93. [Google Scholar] [CrossRef]
- Pieles, O.; Reck, A.; Reichert, T.E.; Morsczeck, C. p53 inhibits the osteogenic differentiation but does not induce senescence in human dental follicle cells. Differentiation 2020, 114, 20–26. [Google Scholar] [CrossRef]
- Xie, Y.; Han, N.; Li, F.; Wang, L.; Liu, G.; Hu, M.; Wang, S.; Wei, X.; Guo, J.; Jiang, H.; et al. Melatonin enhances osteoblastogenesis of senescent bone marrow stromal cells through NSD2-mediated chromatin re-modelling. Clin. Transl. Med. 2022, 12, e746. [Google Scholar] [CrossRef]
- Felthaus, O.; Gosau, M.; Klein, S.; Prantl, L.; Reichert, T.E.; Schmalz, G.; Morsczeck, C. Dexame-thasone-related osteogenic differentiation of dental follicle cells depends on ZBTB16 but not Runx. Cell Tissue Res. 2014, 357, 695–705. [Google Scholar] [CrossRef]
- Bendlová, B.; Vaňková, M.; Hill, M.; Vacínová, G.; Lukášová, P.; Vejražková, D.; Šedová, L.; Šeda, O.; Včelák, J. ZBTB16 Gene Variability Influences Obesity-Related Parameters and Serum Lipid Levels in Czech Adults. Physiol. Res. 2017, 66, S425–S431. [Google Scholar] [CrossRef]
- Liška, F.; Landa, V.; Zídek, V.; Mlejnek, P.; Šilhavý, J.; Šimáková, M.; Strnad, H.; Trnovská, J.; Škop, V.; Kazdová, L.; et al. Downregulation of Plzf Gene Ameliorates Metabolic and Cardiac Traits in the Spontaneously Hypertensive Rat. Hypertension 2017, 69, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.-L.; Liu, W.; Wu, Y.-M.; Sun, W.-L.; Dörfer, C.E.; El-Sayed, K.M.F. Oral Mesenchymal Stem/Progenitor Cells: The Immunomodulatory Masters. Stem Cells Int. 2020, 2020, 1327405. [Google Scholar] [CrossRef] [PubMed]
- Morsczeck, C.O.; Dress, J.; Gosau, M. Lipopolysaccharide from Escherichia coli but not from Porphy-romonas gingivalis induce pro-inflammatory cytokines and alkaline phosphatase in dental follicle cells. Arch. Oral Biol. 2012, 57, 1595–1601. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, B.; Tian, J.; Hong, H.; Du, Y.; Li, K.; Li, X.; Wang, N.; Yu, X.; Wei, X. Dental Follicle Stem Cells Ameliorate Lipopolysaccharide-Induced Inflammation by Secreting TGF-beta3 and TSP-1 to Elicit Macrophage M2 Polarization. Cell Physiol. Biochem. 2018, 51, 2290–2308. [Google Scholar] [CrossRef] [PubMed]
- Genç, D.; Zibandeh, N.; Nain, E.; Gökalp, M.; Özen, A.O.; Göker, M.K.; Akkoç, T. Dental follicle mesenchymal stem cells down-regulate Th2-mediated immune response in asthmatic patients mononuclear cells. Clin. Exp. Allergy 2018, 48, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Genc, D.; Zibandeh, N.; Nain, E.; Arig, U.; Goker, K.; Aydiner, E.K.; Akkoc, T. IFN-gamma stimulation of dental follicle mesenchymal stem cells modulates immune response of CD4(+) T lymphocytes in Der p1(+) asthmatic patients in vitro. Allergol. Immunopathol. (Madr.) 2019, 47, 467–476. [Google Scholar] [CrossRef]
- Sarica, L.T.; Zibandeh, N.; Genç, D.; Gül, F.; Akkoç, T.; Kombak, E.F.; Cinel, L.; Akkoç, T.; Cinel, I. Immunomodulatory and Tissue-preserving Effects of Human Dental Follicle Stem Cells in a Rat Cecal Ligation and Perforation Sepsis Model. Arch. Med. Res. 2020, 51, 397–405. [Google Scholar] [CrossRef]
- Hong, H.; Chen, X.; Li, K.; Wang, N.; Li, M.; Yang, B.; Yu, X.; Wei, X. Dental follicle stem cells rescue the regenerative capacity of inflamed rat dental pulp through a paracrine pathway. Stem. Cell Res. Ther. 2020, 11, 333. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morsczeck, C. Mechanisms during Osteogenic Differentiation in Human Dental Follicle Cells. Int. J. Mol. Sci. 2022, 23, 5945. https://doi.org/10.3390/ijms23115945
Morsczeck C. Mechanisms during Osteogenic Differentiation in Human Dental Follicle Cells. International Journal of Molecular Sciences. 2022; 23(11):5945. https://doi.org/10.3390/ijms23115945
Chicago/Turabian StyleMorsczeck, Christian. 2022. "Mechanisms during Osteogenic Differentiation in Human Dental Follicle Cells" International Journal of Molecular Sciences 23, no. 11: 5945. https://doi.org/10.3390/ijms23115945
APA StyleMorsczeck, C. (2022). Mechanisms during Osteogenic Differentiation in Human Dental Follicle Cells. International Journal of Molecular Sciences, 23(11), 5945. https://doi.org/10.3390/ijms23115945





