Epoxyeicosatrienoic Acid and Prostanoid Crosstalk at the Receptor and Intracellular Signaling Levels to Maintain Vascular Tone
Abstract
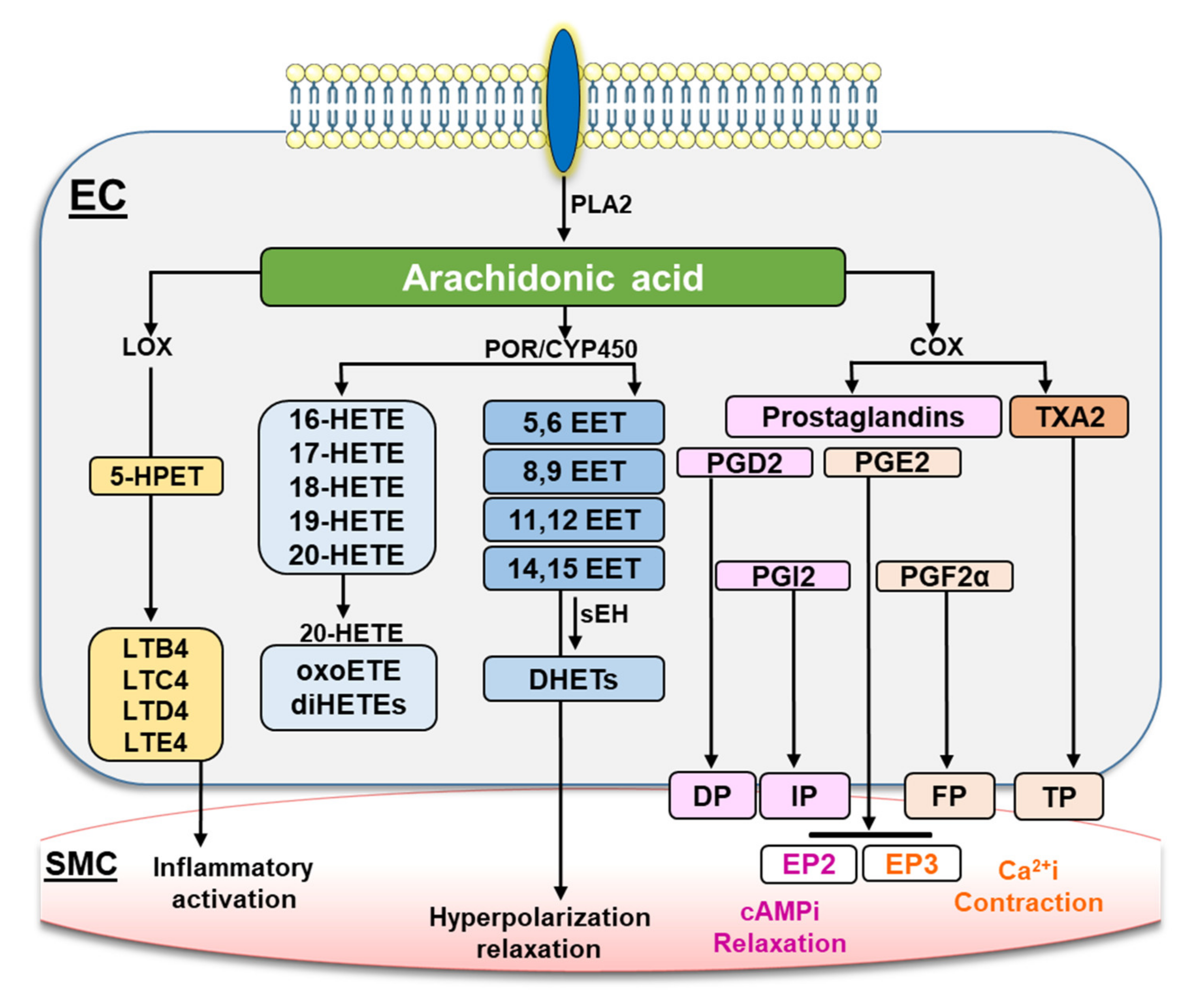
1. Introduction
2. Results
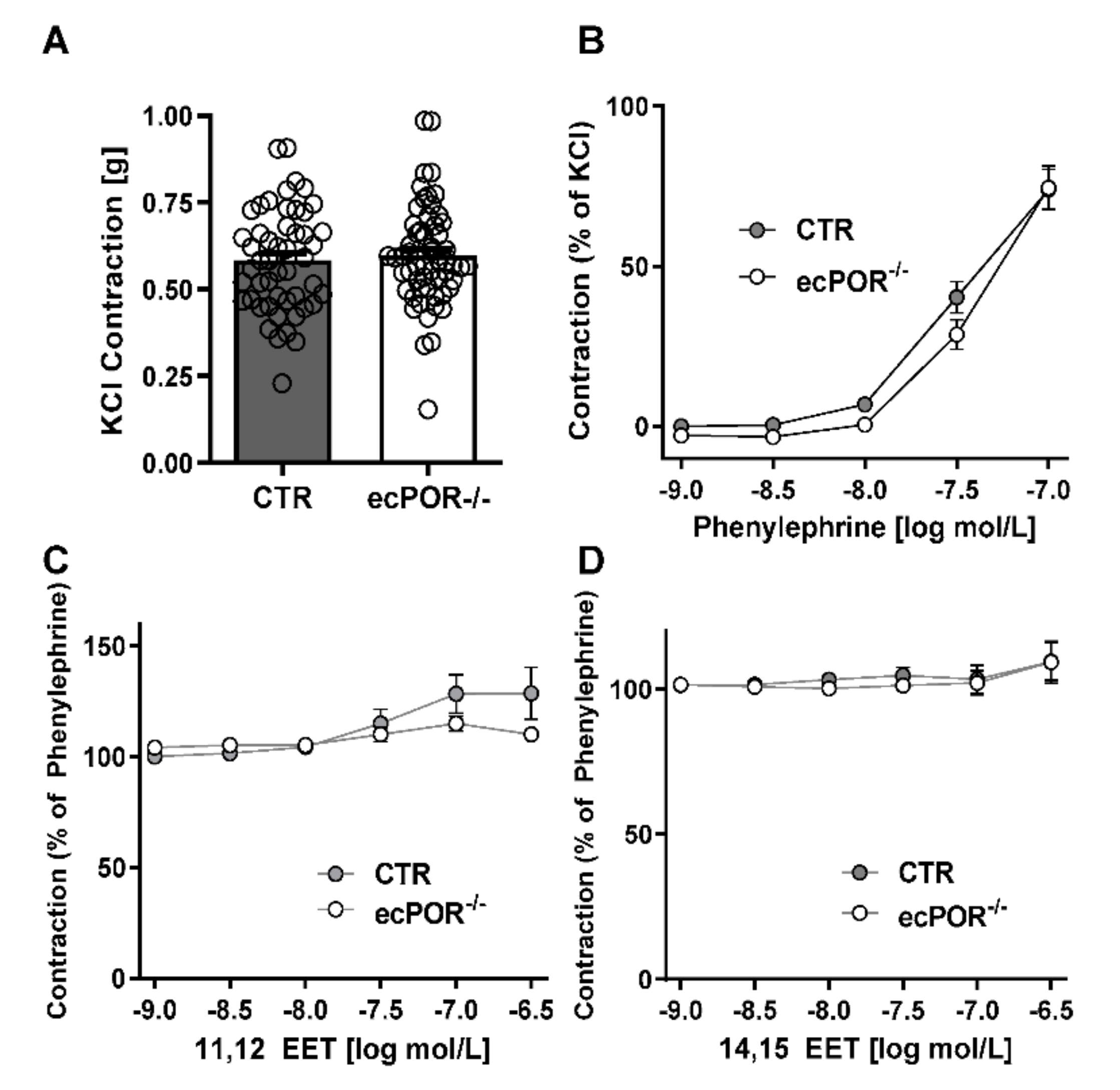
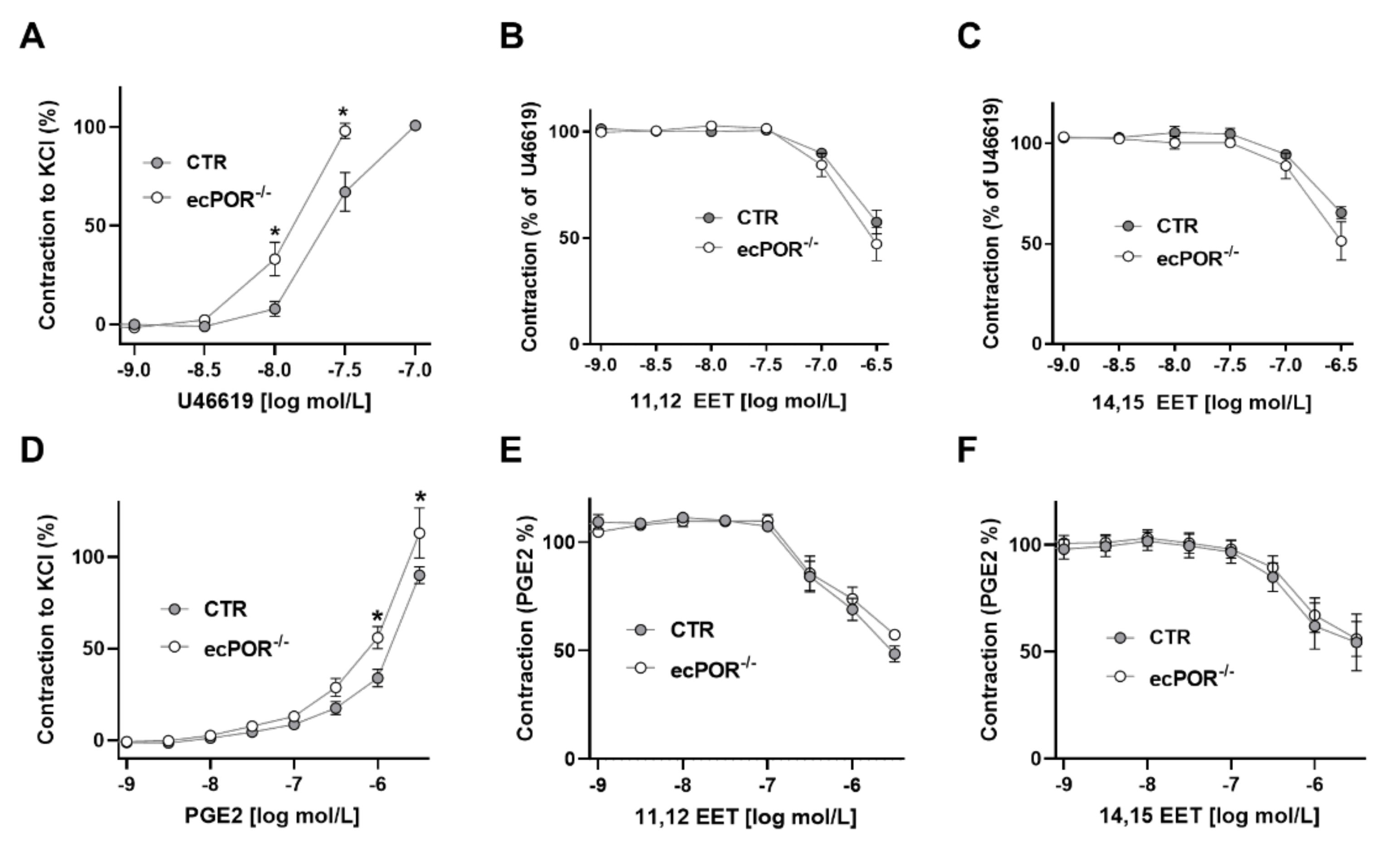
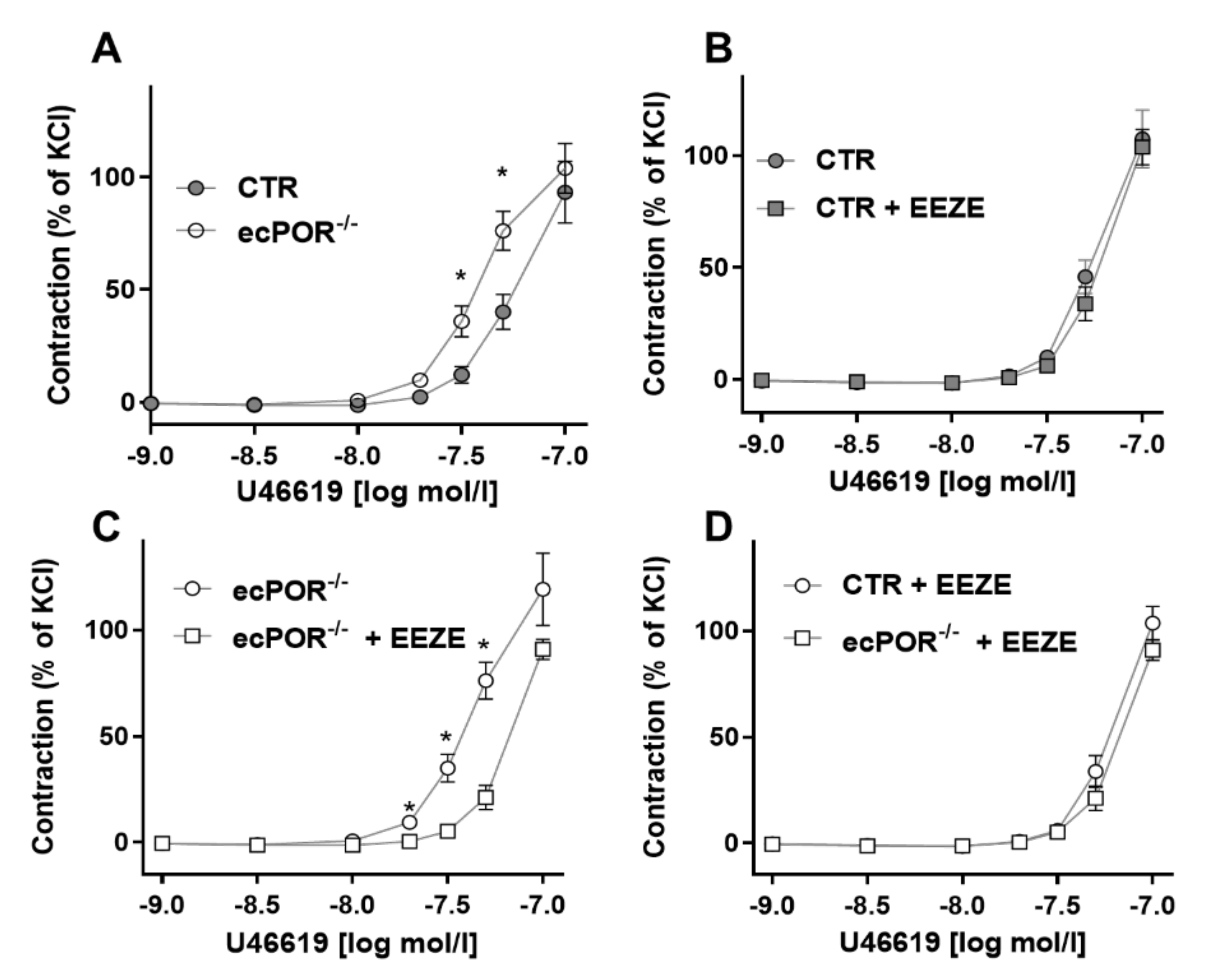
2.1. Knockout of Endothelial POR Increases Vessel Constriction to the Thromboxane Receptor Agonist U46619 or PGE2
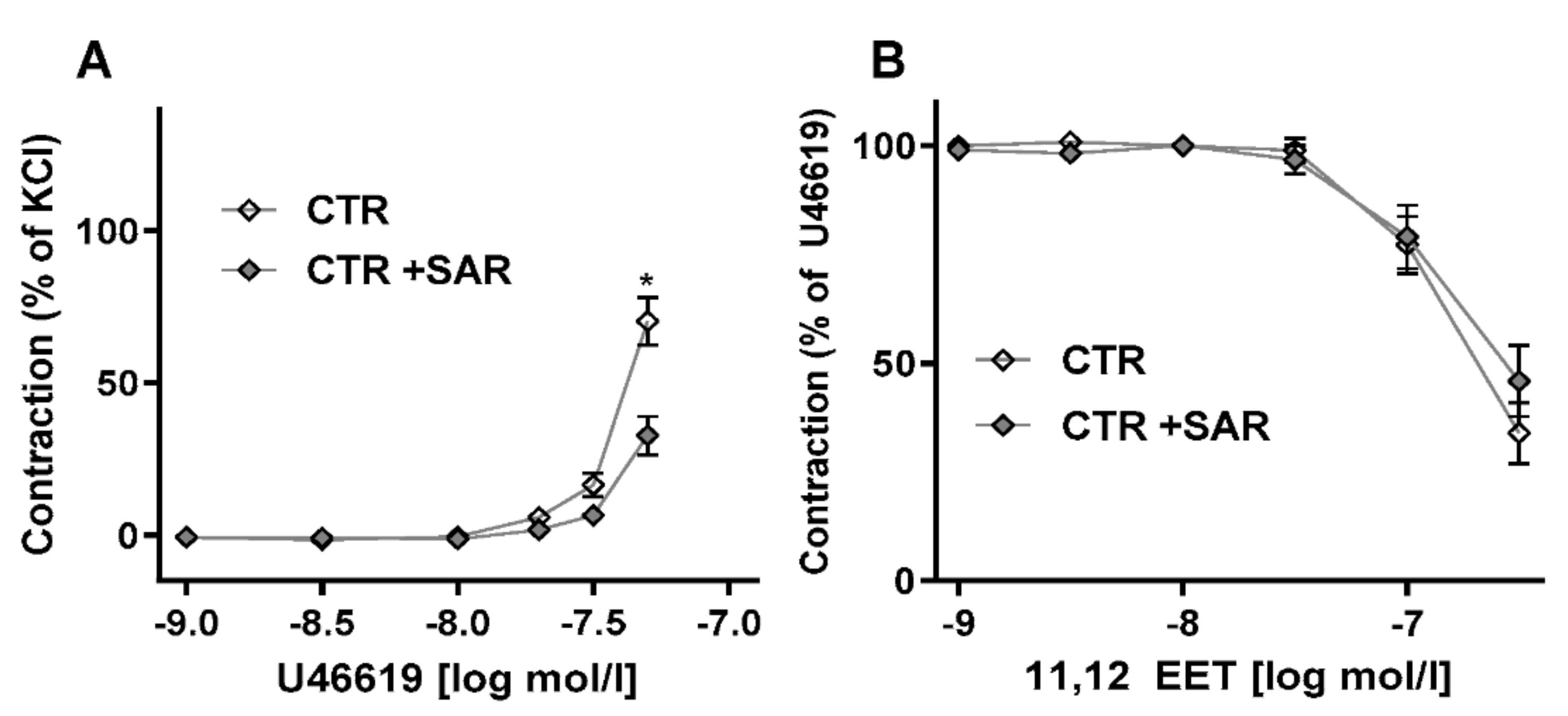
2.2. Restoring EET Pools in Aortic Rings of ecPOR−/− Mice Normalizes Contraction to U46619
2.3. Knockout of POR in Endothelial Cells (HUVEC) Increases Expression of Genes Related to Rho Signaling
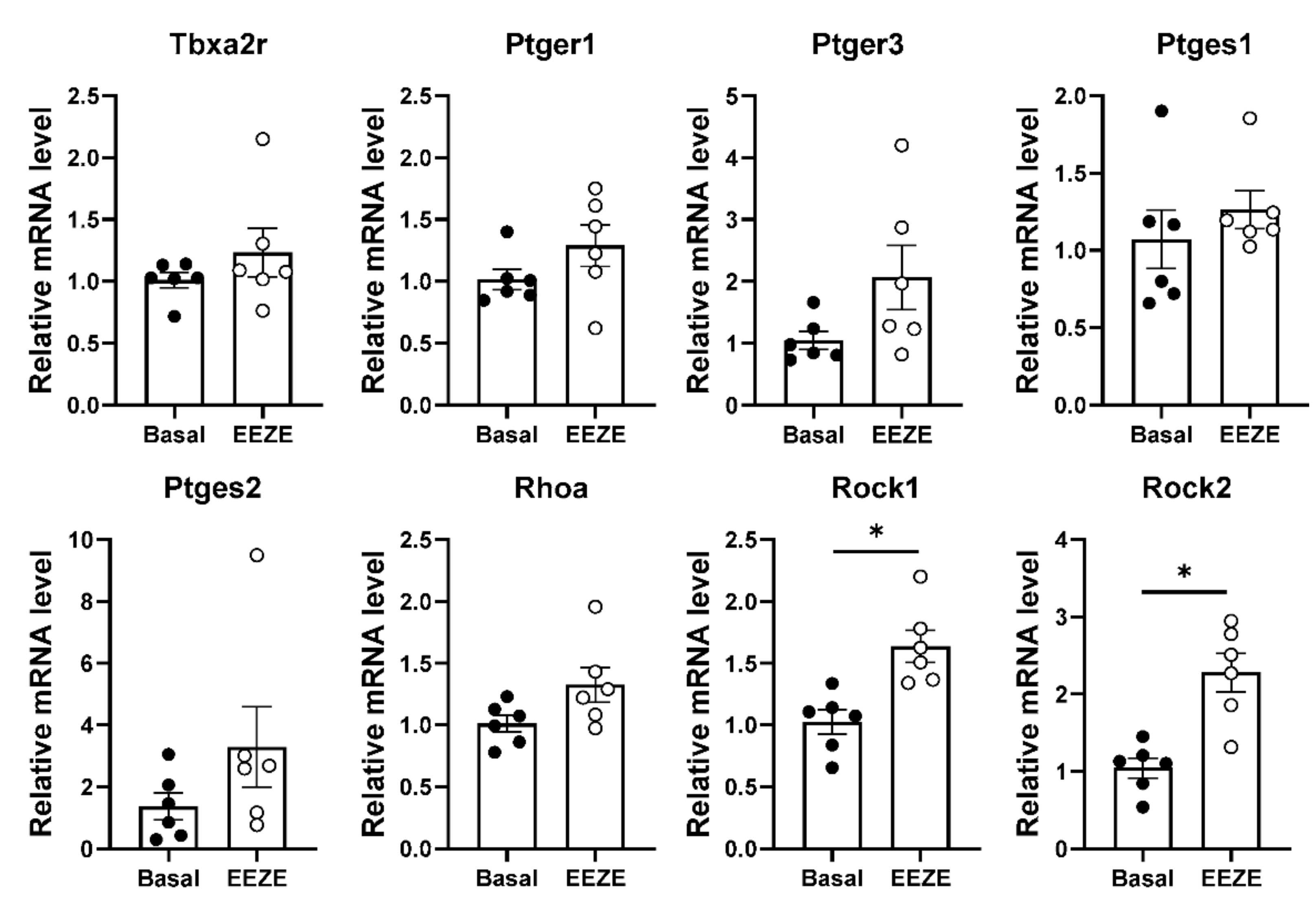
2.4. Addition of EET to Aortic Tissue Increases Expression of Rho Kinases
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animal Procedure
4.3. Vascular Reactivity Measurements
4.4. EET Supplementation
4.5. RNA Isolation and RT-qPCR
4.6. CRISPR/Cas9 for Cytochrome P450 Reductase (POR)
4.7. RNA Seq from HUVEC
4.8. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, L.; Mäki-Petäjä, K.; Cheriyan, J.; McEniery, C.; Wilkinson, I.B. The role of epoxyeicosatrienoic acids in the cardiovascular system. Br. J. Clin. Pharmacol. 2015, 80, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Fleming, I. The factor in EDHF: Cytochrome P450 derived lipid mediators and vascular signaling. Vasc. Pharmacol. 2016, 86, 3140. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Signal Transduct. Target. Ther. 2021, 6, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.S.-K.; Vanhoutte, P.M. COX-mediated endothelium-dependent contractions: From the past to recent discoveries. Acta Pharmacol. Sin. 2010, 31, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Wise, H.; Wong, Y.H.; Jones, R. Prostanoid Signal Integration and Cross Talk. Neurosignals 2002, 11, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.J.; Roche, A.M.; Kostetskaia, E.; Smyth, E.M. Dimerization of the Human Receptors for Prostacyclin and Thromboxane Facilitates Thromboxane Receptor-mediated cAMP Generation. J. Biol. Chem. 2004, 279, 53036–53047. [Google Scholar] [CrossRef] [PubMed]
- Momotani, K.; Artamonov, M.V.; Utepbergenov, D.; Derewenda, U.; Derewenda, Z.S.; Somlyo, A.V. p63RhoGEF Couples Gαq/11-Mediated Signaling to Ca2+ Sensitization of Vascular Smooth Muscle Contractility. Circ. Res. 2011, 109, 993–1002. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Jiang, M.J. Rho-kinase-mediated regulation of receptor-agonist-stimulated smooth muscle contraction. Pflug. Arch. 2006, 453, 223–232. [Google Scholar] [CrossRef]
- Campbell, W.B.; Gebremedhin, D.; Pratt, P.F.; Harder, D.R. Identification of Epoxyeicosatrienoic Acids as Endothelium-Derived Hyperpolarizing Factors. Circ. Res. 1996, 78, 415–423. [Google Scholar] [CrossRef]
- Imig, J.D.; Zhao, X.; Capdevila, J.H.; Morisseau, C.; Hammock, B.D. Soluble epoxide hydrolase inhibition lowers arterial blood pressure in angiotensin II hypertension. Hypertension 2002, 39, 690–694. [Google Scholar] [CrossRef]
- Sinal, C.J.; Miyata, M.; Tohkin, M.; Nagata, K.; Bend, J.R.; Gonzalez, F.J. Targeted Disruption of Soluble Epoxide Hydrolase Reveals a Role in Blood Pressure Regulation. J. Biol. Chem. 2000, 275, 40504–40510. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Xu, F.; Huse, L.M.; Morisseau, C.; Draper, A.J.; Newman, J.; Parker, C.; Graham, L.; Engler, M.M.; Hammock, B.D.; et al. Soluble Epoxide Hydrolase Regulates Hydrolysis of Vasoactive Epoxyeicosatrienoic Acids. Circ. Res. 2000, 87, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.R.; Imig, J.D.; Edin, M.L.; Foley, J.; Degraff, L.M.; Bradbury, J.A.; Graves, J.P.; Lih, F.B.; Clark, J.; Myers, P.; et al. Endothelial expression of human cytochrome P450 epoxygenases lowers blood pressure and attenuates hypertension-induced renal injury in mice. FASEB J. 2010, 24, 3770–3781. [Google Scholar] [CrossRef] [PubMed]
- Malacarne, P.F.; Ratiu, C.; Gajos-Draus, A.; Müller, N.; Lopez, M.; Pflüger-Müller, B.; Ding, X.; Warwick, T.; Oo, J.; Siragusa, M.; et al. Loss of Endothelial Cytochrome P450 Reductase Induces Vascular Dysfunction in Mice. Hypertension 2022, 79, 1216–1226. [Google Scholar] [CrossRef]
- Behm, D.J.; Ogbonna, A.; Wu, C.; Burns-Kurtis, C.L.; Douglas, S.A. Epoxyeicosatrienoic Acids Function as Selective, Endogenous Antagonists of Native Thromboxane Receptors: Identification of a Novel Mechanism of Vasodilation. J. Pharmacol. Exp. Ther. 2008, 328, 231–239. [Google Scholar] [CrossRef]
- Weintraub, N.L.; Fang, X.; Kaduce, T.L.; VanRollins, M.; Chatterjee, P.; Spector, A.A. Potentiation of Endothelium-Dependent Relaxation by Epoxyeicosatrienoic Acids. Circ. Res. 1997, 81, 258–267. [Google Scholar] [CrossRef]
- Pang, H.; Guo, Z.; Su, W.; Xie, Z.; Eto, M.; Gong, M.C. RhoA-Rho kinase pathway mediates thrombin- and U-46619-induced phosphorylation of a myosin phosphatase inhibitor, CPI-17, in vascular smooth muscle cells. Am. J. Physiol. Physiol. 2005, 289, C352–C360. [Google Scholar] [CrossRef][Green Version]
- He, Z.; Yang, Y.; Wen, Z.; Chen, C.; Xu, X.; Zhu, Y.; Wang, Y.; Wang, D.W. CYP2J2 metabolites, epoxyeicosatrienoic acids, attenuate Ang II-induced cardiac fibrotic response by targeting Gα12/13. J. Lipid Res. 2017, 58, 1338–1353. [Google Scholar] [CrossRef]
- Capdevila, J.; Chacos, N.; Werringloer, J.; Prough, R.A.; Estabrook, R.W. Liver microsomal cytochrome P-450 and the oxidative metabolism of arachidonic acid. Proc. Natl. Acad. Sci. USA 1981, 78, 5362–5366. [Google Scholar] [CrossRef]
- Zou, A.P.; Fleming, J.T.; Falck, J.R.; Jacobs, E.R.; Gebremedhin, D.; Harder, D.R.; Roman, R.J. Stereospecific effects of epoxyeicosatrienoic acids on renal vascular tone and K(+)-channel activity. Am. J. Physiol. Physiol. 1996, 270, F822–F832. [Google Scholar] [CrossRef]
- Earley, S.; Heppner, T.J.; Nelson, M.T.; Brayden, J.E. TRPV4 Forms a Novel Ca2+ Signaling Complex With Ryanodine Receptors and BKCa Channels. Circ. Res. 2005, 97, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Tuniki, V.R.; Anjaiah, S.; Falck, J.R.; Hillard, C.J.; Campbell, W.B. Characterization of Epoxyeicosatrienoic Acid Binding Site in U937 Membranes Using a Novel Radiolabeled Agonist, 20-125i-14,15-Epoxyeicosa-8(Z)-Enoic Acid. J. Pharmacol. Exp. Ther. 2008, 324, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Holmes, B.B.; Gopal, V.R.; Kishore, R.V.K.; Sangras, B.; Yi, X.-Y.; Falck, J.R.; Campbell, W.B. Characterization of 14,15-Epoxyeicosatrienoyl-Sulfonamides as 14,15-Epoxyeicosatrienoic Acid Agonists: Use for Studies of Metabolism and Ligand Binding. J. Pharmacol. Exp. Ther. 2007, 321, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Du, Y. Distinct Roles of Central and Peripheral Prostaglandin E2 and EP Subtypes in Blood Pressure Regulation. Am. J. Hypertens. 2012, 25, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- Campbell, W.B.; Fleming, I. Epoxyeicosatrienoic acids and endothelium-dependent responses. Pflug. Arch. 2010, 459, 881–895. [Google Scholar] [CrossRef]
- Rezende, F.; Prior, K.-K.; Löwe, O.; Wittig, I.; Strecker, V.; Moll, F.; Helfinger, V.; Schnütgen, F.; Kurrle, N.; Wempe, F.; et al. Cytochrome P450 enzymes but not NADPH oxidases are the source of the NADPH-dependent lucigenin chemiluminescence in membrane assays. Free Radic. Biol. Med. 2017, 102, 57–66. [Google Scholar] [CrossRef] [PubMed]

| Name | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| Eef2 | GACATCACCAAGGGTGTGCAG | GCGGTCAGCACACTGGCATA |
| Tbxa2r | TGGTTCAGCTCGTGGGCATCAT | ACACGCAGGTAGATGAGCAGCT |
| Ptger1 | TCATGGTGGTGTCGTGCATCTG | GTCCAGGATCTGGTTCCACGAT |
| Ptger3 | GCTTCGCTGAACCAGATCTTGG | CAGGTACTGCAATGAAAGTCCAC |
| Ptges1 | GAATGCCACCTTCATCCGAGAAG | GCTCACATTGGAGAAGGACTCC |
| Ptges2 | GCGACATACTCAAGCAGGAGCA | AGTGGTAACCGCTCAGGTGTTG |
| Rock1 | CACGCCTAACTGACAAGCACCA | CAGGTCAACATCTAGCATGGAAC |
| Rock2 | GTGACCTCAAACAGTCTCAGCAG | GACAACGCTTCTGAGTTTCCTGC |
| Rhoa | CTTCAGCAAGGACCAGTTCCCA | GGCGGTCATAATCTTCCTGTCC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malacarne, P.F.; Bezzenberger, J.; Lopez, M.; Warwick, T.; Müller, N.; Brandes, R.P.; Rezende, F. Epoxyeicosatrienoic Acid and Prostanoid Crosstalk at the Receptor and Intracellular Signaling Levels to Maintain Vascular Tone. Int. J. Mol. Sci. 2022, 23, 5939. https://doi.org/10.3390/ijms23115939
Malacarne PF, Bezzenberger J, Lopez M, Warwick T, Müller N, Brandes RP, Rezende F. Epoxyeicosatrienoic Acid and Prostanoid Crosstalk at the Receptor and Intracellular Signaling Levels to Maintain Vascular Tone. International Journal of Molecular Sciences. 2022; 23(11):5939. https://doi.org/10.3390/ijms23115939
Chicago/Turabian StyleMalacarne, Pedro Felipe, Justus Bezzenberger, Melina Lopez, Timothy Warwick, Niklas Müller, Ralf P. Brandes, and Flávia Rezende. 2022. "Epoxyeicosatrienoic Acid and Prostanoid Crosstalk at the Receptor and Intracellular Signaling Levels to Maintain Vascular Tone" International Journal of Molecular Sciences 23, no. 11: 5939. https://doi.org/10.3390/ijms23115939
APA StyleMalacarne, P. F., Bezzenberger, J., Lopez, M., Warwick, T., Müller, N., Brandes, R. P., & Rezende, F. (2022). Epoxyeicosatrienoic Acid and Prostanoid Crosstalk at the Receptor and Intracellular Signaling Levels to Maintain Vascular Tone. International Journal of Molecular Sciences, 23(11), 5939. https://doi.org/10.3390/ijms23115939







