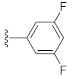
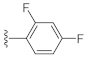
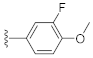
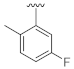
Abstract
Millions of people worldwide suffer from acute or chronic liver inflammation caused by the hepatitis C virus (HCV). Metal ion chelators have achieved widespread success in the development of antiviral drugs. Some inhibitors with metal ion chelating structures have been proven to have good inhibitory activities on non-structural protein 5B (NS5B) polymerase. However, most of the reported metal ion chelators showed poor anti-HCV potency at the cellular level. Hence, we designed and synthesized a series of 3-hydroxyquinazoline-2,4(1H,3H)-dione derivatives with novel metal ion chelating structures. Typical compounds such as 21h, 21k, and 21t showed better anti-HCV activities than ribavirin with EC50 values less than 10 μM. 21t is currently known as one of the metal ion chelators with the best anti-HCV potency (EC50 = 2.0 μM) at the cellular level and has a better therapeutic index (TI > 25) as compared to ribavirin and the reported compound 6. In the thermal shift assay, the representative compounds 21e and 21k increased the melting temperature (Tm) of NS5B protein solution by 1.6 °C and 2.1 °C, respectively, at the test concentration, indicating that these compounds may exert an anti-HCV effect by targeting NS5B. This speculation was also supported by our molecular docking studies and ultraviolet-visible (UV-Vis) spectrophotometry assay, in which the possibility of binding of 3-hydroxyquinazoline-2,4(1H,3H)-diones with Mg2+ in the NS5B catalytic center was observed.
1. Introduction
Hepatitis C is an acute or chronic liver inflammation caused by the hepatitis C virus (HCV), which can lead to cirrhosis or liver cancer [1]. According to statistics from the World Health Organization (WHO), about 58 million people worldwide are infected with the hepatitis C virus, with about 1.5 million new infections occurring every year [2]. In 2019, approximately 290,000 people died of hepatitis C, mainly due to primary liver cancer and cirrhosis [2]. HCV is a small positive sense, single-stranded RNA virus of the Flaviviridae family. Due to the high error rate when synthesizing RNA, HCV has a variety of subtypes. Based on the genetic differences between HCV isolates, 8 genotypes (1–8) and 93 subtypes of hepatitis C virus have been confirmed [3], in which subtypes 1a and 1b were found to be predominant [4,5]. Unlike hepatitis A and hepatitis B viruses, there is currently no effective vaccine against HCV [6], which makes the overall prevention and control of HCV very difficult.
At present, direct-acting antivirals (DAAs) mainly target non-structural proteins NS3/4A, NS5A, and NS5B in the treatment of HCV infection. Non-structural protein 5B (NS5B) is a key enzyme in the synthesis of HCV RNA strands. As an RNA-dependent RNA polymerase (RdRp), NS5B takes the original RNA chain as a template and catalyzes the polymerization of ribonucleoside triphosphates (rNTP) to synthesize the new RNA chains [7,8].
The NS5B protein consists of three domains: fingers, thumb, and palm regions [8]. The thumb and palm regions contain four allosteric sites which can regulate the conformation of the NS5B protein, thereby affecting the RNA synthesis [9,10]. Inhibitors of various structural types, such as benzothiadiazines [11] and benzofurans [12], have been identified to be capable of binding to the allosteric sites and show potent HCV inhibitory activities [10,11,12,13,14,15]. Due to the mutability and high mutation rates of the allosteric sites, NS5B allosteric inhibitors are mostly effective against only a small range of virus subtypes and are prone to drug resistance [16,17].
The active center of NS5B in the palm region is responsible for catalyzing the nucleophilic attack of the 3′-terminal hydroxyl group of the RNA extension chain to the rNTP substrates [18]. Since the NS5B polymerase active site is highly conserved, inhibitors targeting the NS5B active site have a higher genetic barrier to drug resistance and more pan-genotypic activities as compared to other HCV DAAs [17,19,20]. Nucleoside or nucleotide inhibitors target the active center of NS5B in their active form, which could be accepted as substrates for NS5B polymerase and ultimately incorporated into growing RNA strands, terminating the HCV replication cycle [8]. A variety of nucleoside or nucleotide inhibitors have been shown to have good anti-HCV activities [21,22]. However, the development of many nucleoside or nucleotide analogs was halted in clinical trials due to the widespread mitochondrial toxicity [23]. In addition, nucleoside inhibitors represented by ribavirin can also exert toxicity through the disruption of natural nucleoside triphosphate (NTP) pools [24]. These features make the development of nucleoside or nucleotide-based anti-HCV drugs more risky. Sofosbuvir (trade name Sovaldi) is currently the only nucleotide prodrug approved by the U.S. Food and Drug Administration (FDA) for the treatment of HCV infection [25]. Although the combination regimens of sofosbuvir with other DAAs have achieved high HCV clearance rates, 5–10% of patients still do not respond well to the current therapies [26]. More importantly, a growing list of HCV mutations associated with DAAs resistance has been found in clinical practice, including sofosbuvir [27,28,29,30,31]. Therefore, the development of novel HCV inhibitors is still of great importance.
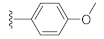
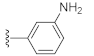
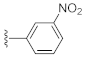
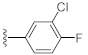
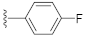
Two Mg2+ ions in the active center of NS5B form a chelating complex with the conserved amino acid residues D220, D318, and D319, stabilizing the central structure of the active site [8]. In addition to nucleoside or nucleotide inhibitors, some metal ion chelators can also target the conserved active center of NS5B by chelating with the Mg2+, which are essential for the polymerase active center [32]. Metal ion chelators can be treated as pyrophosphate (PPi) mimetics that block viral RNA replication by competing with the phosphate group of NTP for binding to the catalytic center of polymerases [33]. As shown in Figure 1, a variety of structural types of metal ion chelators, such as α,γ-diketo acids (compound 1 and 2) [34], meconic acids (compound 3) [35], 5,6-dihydroxypyrimidine-4-carboxylic acids (compound 4 and 5) [36,37], and 2-hydroxyisoquinoline-1,3-diones (compound 6) [38] have been identified to have potent inhibitory activities against NS5B. Molecular simulations also suggested that these compounds might bind to the two Mg2+ ions in the active center of NS5B through a “tridentate” chelation mode [37,38]. However, the anti-HCV activities of most reported metal ion chelators at the cellular level did not reach expectations, probably due to the low membrane permeability of the compounds caused by the carboxyl-containing metal-chelating functional groups. Interestingly, the representative compound 6 of the 2-hydroxyisoquinoline-1,3-diones with no carboxyl group possesses a good cellular-level anti-HCV activity (EC50 = 1.9 μM). Nevertheless, the therapeutic index of such compounds still needs optimization [38].
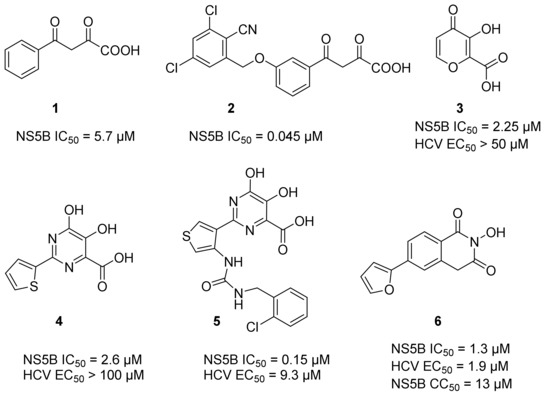
Figure 1.
A series of representative non-structural protein 5B (NS5B) metal ion chelators.
Magnesium ions play a central role as metal cofactors in a variety of enzymes, especially those involved in nucleic acid biochemistry [39,40]. In addition to HCV NS5B polymerase, the representative ones are HIV-1 integrase, HIV-1 ribonuclease H (RNase H), and influenza virus endonuclease. Targeting these metal cofactor-containing viral proteins to design and develop a series of metal ion chelators has been shown to be a practical and effective antiviral strategy [41,42,43,44,45,46,47]. To date, five HIV-1 integrase inhibitors (raltegravir, elvitegravir, dolutegravir, bictegravir, and cabotegravir) containing metal ion chelating structures have been approved by the FDA [48]. These encouraging results confirm that the metal ion chelation strategy has a broad prospect in the development of HCV DAAs.
Herein, we designed and synthesized a series of 3-hydroxyquinazoline-2,4(1H,3H)-dione derivatives acting as metal ion chelators. The cellular-level anti-HCV activities of the compounds were evaluated based on the HCV replicon model. In addition, the binding of representative compounds to the NS5B protein was determined using the thermal shift assay (TSA) to validate the targeted protein of the compounds. The in vitro binding properties of the preferred compound with Mg2+ were studied by ultraviolet-visible (UV-Vis) spectrophotometry. Molecular modeling was conducted to explore the binding mode of this series of metal ion chelators to the active center of NS5B.
2. Results and Discussion
2.1. Chemistry
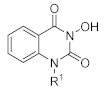
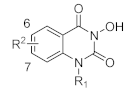
In this study, we synthesized a series of novel metal ion chelators with 3-hydroxyquinazoline-2,4(1H,3H)-dione parent nucleus. Based on the strategy of partition structure modification, we first tried different substituents on the 1-nitrogen of hydroxyquinazolinedione core to explore the preliminary structure-activity relationship (SAR) (Scheme 1). Further, structural modifications at the phenyl ring region of the parent nucleus were implemented (Scheme 2). As shown in Scheme 1, the starting material, methyl anthranilate 7, underwent a one-pot two-step reaction to give the key intermediate 3-(benzyloxy)quinazoline-2,4(1H,3H)-dione (8). Initially, methyl anthranilate 7 was condensed with 1,1′-carbonyldiimidazole (CDI) and benzyloxyamine successively to introduce the carbonyl fragment. Then the intermediate 8 was obtained after the intramolecular cyclization reaction under a strongly alkaline environment. In step b, the intermediate 8 and the corresponding halide underwent an alkylation reaction with various substituents introduced on the N-1 position to yield compounds 9a–o. Finally, to obtain the target compounds N-1 substituted 3-hydroxyquinazoline-2,4(1H,3H)-diones (10a–p), the benzyl groups in 8 and 9a–o were removed using different conditions of deprotection, i.e., heating in hydrobromic acid/acetic acid mixture under reflux, or palladium-carbon catalyzed hydrogenation.
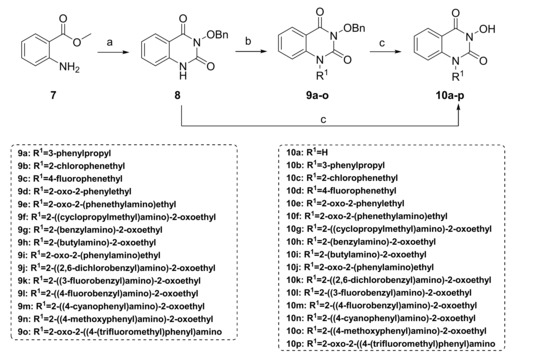
Scheme 1.
Synthesis of N-1 substituted 3-hydroxy-quinazoline-2,4(1H,3H)-diones. Reagents and conditions: (a) (i) CDI/toluene, reflux, 2 h, then NH2OBn, reflux, 4 h (ii) NaOH/H2O/EtOH, reflux, 2 h; (b) R1X, K2CO3, DMF, 80 °C, 2 h; (c) 48% HBr, AcOH, reflux, 2 h; or H2, 10% Pd/C, THF/MeOH, room temperature (r.t.), 4–12 h.
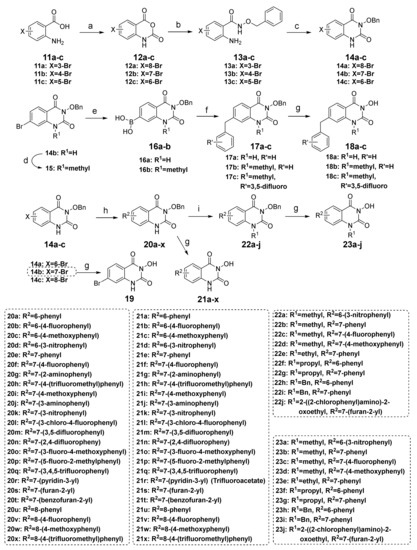
Scheme 2.
Synthesis of C-6/C-7/C-8 substituted 3-hydroxyquinazoline-2,4(1H,3H)-diones and the key intermediates. Reagents and conditions: (a) triphosgene, 1,4-dioxane, reflux, 6 h; (b) NH2OBn, triethylamine, EtOH, reflux, 3 h; (c) triphosgene, triethylamine, THF, r.t., 2 h; (d) CH3I, K2CO3, DMF, r.t., 2 h; (e) bis(pinacolato)diboron, Pd(dppf)Cl2, KOAc, 1,4-dioxane, 100 °C, 8 h; (f) BnBr or substituted BnBr, Pd(dppf)Cl2, KOAc, dioxane/H2O, 100 °C, 3 h; (g) 48% HBr, AcOH, reflux, 2 h; or H2, 10% Pd/C, THF/MeOH, 4–12 h; or TFA, reflux, 12–16 h; or TiCl4/potassium sodium tartrate, DCM, 1.5 h; (h) R2B(OH)2, K2CO3, Pd(PPh3)4, dioxane/H2O, 100 °C, 12 h; (i) R1Br, K2CO3, DMF, 80 °C, 2 h; or R1I, K2CO3, DMF, r.t., 2 h.
The synthetic route depicted in Scheme 2 produced the C-6/C-7/C-8 substituted 3-hydroxyquinazoline-2,4(1H,3H)-diones. Bromoanthranilic acid (11a–c) and triphosgene were refluxed in dioxane to afford the oxazinedione intermediates 12a–c, which were treated with benzyloxyamine to produce 13a–c through the nucleophilic reaction. Then, the aniline groups of 13a–c were amidated by reacting with triphosgene, and subsequently, the products underwent an intramolecular cyclization reaction to yield the key intermediates 14a–c possessing the quinazolinedione parent nucleus. Next, we tried to introduce benzyl substitution on the phenyl ring of the parent nucleus. The C-7 brominated compound 14b and its N-1 methylated product 15 were treated with bis(pinacolato)diboron to achieve the boron intermediates 16a and 16b, respectively, which were then subjected to the Pd(dppf)Cl2 catalyzed Suzuki coupling reaction with benzyl bromide or substituted benzyl bromide to obtain compounds 17a–c. Finally, under the catalysis of palladium-carbon, the benzyl groups of 17a–c were removed by hydrogenation reaction to yield three target compounds 18a–c substituted by various benzyl groups at the C-7 position.
In order to fully study the SAR on the phenyl ring of the parent nucleus, we also prepared a series of C-6/C-7/C-8 substituted 3-hydroxyquinazoline-2,4(1H,3H)-diones according to the synthetic route in Scheme 2. The key intermediates 14a–c were subjected to Suzuki coupling reaction with different arylboronic acids under the catalysis of Pd(PPh3)4 to obtain intermediates 20a–x, which were then debenzylated by different conditions to yield the target compounds 21a–x. The C-7 brominated compound 14b was directly debenzylated in the mixture of hydrobromic acid/acetic acid to give the target compound 19. On the other hand, alkylation of the N-1 position of 20a–x by different halides under the condition of inorganic base afforded intermediates 22a–j, which were then debenzylated to produce the target compounds 23a–j using the conditions as described in converting 20a–x to 21a–x. In the selection of debenzylation conditions, the more environmentally friendly palladium-carbon catalyzed hydrogenation is preferred. Strong acids such as trifluoroacetic acid, hydrobromic acid, and acetic acid are used for debenzylation unless the hydrogenation conditions are not applicable. As for compound 20s, the debenzylation reaction was performed using titanium tetrachloride (TiCl4) in dichloromethane (DCM) to obtain the target compound 21s in high purity. The structures of various target compounds were confirmed by ESI-MS, 1H NMR, and 13C NMR spectral data, which are shown in the Materials and Methods section.
2.2. Anti-HCV Assay
The HCV replicons were validated as convenient and effective models for testing the anti-HCV activity [49]. Since HCV subtype 1b is one of the major subtypes worldwide, especially in China [4,5], the replicon model from the Huh-7.5.1 cell line integrating HCV 1b genome encoding the nonstructural proteins was chosen to evaluate the anti-HCV activities of 3-hydroxyquinazoline-2,4(1H,3H)-diones. The inhibitory rates of the compounds on HCV replicon cells were determined at concentrations of 25 μM and 10 μM, respectively. The broad-spectrum antiviral drug ribavirin (RBV) and the NS5B nucleoside inhibitor 2′-C-methyladenosine (2CMA) were selected as positive controls. The cytotoxicity of these compounds on replicon cells was determined using the cell counting kit-8 (CCK-8) assay.
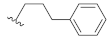
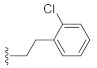
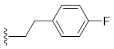
As shown in Table 1, the EC50 values of ribavirin and 2CMA measured under the experimental conditions were 20.0 μM and 0.36 μM, respectively, which were close to the reported 14 μM [38] and 0.3 μM [50], indicating the reliability of the test method in this study. As compared to 3-hydroxyquinazoline-2,4(1H,3H)-dione (10a), the introduction of phenylpropyl group (10b) and substituted phenethyl groups (10c, 10d) at the N-1 position can increase the HCV inhibitory rate at 25 μM and 10 μM. The EC50 value of 10d reaches 13.3 μM, while compounds 10c and 10d both show some cytotoxicity. The ketone carbonyl group or different types of amide fragments were further introduced into the N-1 position nitrogen of the parent nucleus. The results indicated that when there is no aromatic group in the N-1 substituents (10g, 10i), the compounds inhibited HCV by less than 30% at the tested concentrations. Compared with 10j, the compounds with longer N-1 substituted chain (10f, 10h) bearing aryl groups had lower HCV inhibitory activities. The inhibitory rate of 10f and 10h did not exceed 50% at 25 μM; however, 10j had the corresponding value of 62.7%. When halogen (10k, 10l, 10m), methoxy (10o), cyano (10n), and trifluoromethyl (10p) groups were introduced into the phenyl ring of the substituent at the N-1 position, the activity was not significantly improved. In general, 10n with cyano-substituted on the phenyl ring of the amide fragment had the best anti-HCV activity, with an EC50 value of 6.4 μM. Similar to ribavirin, these compounds have certain cytotoxicity, with therapeutic indexes (TI) of about 1.7–1.9, which is comparable to the tested TI of ribavirin (TI = 2.3). Among the synthesized N-1 substituted 3-hydroxyquinazoline-2,4(1H,3H)-diones, compounds 10n and 10p showed anti-HCV EC50 values less than 10 μM, which were more potent than ribavirin (EC50 = 20.0 μM). These results preliminarily validated the anti-HCV potential of 3-hydroxyquinazoline-2,4(1H,3H)-dione derivatives.

Table 1.
Anti-HCV assay of N-1 substituted 3-hydroxy-quinazoline-2,4(1H,3H)-diones.
Further optimization of the anti-HCV activity and therapeutic index was conducted by exploring the SAR on the phenyl ring of the quinazolinedione parent nucleus. As listed in Table 2, C-6/C-7/C-8 aryl or benzyl groups substituted 3-hydroxyquinazoline-2,4(1H,3H)-diones were subjected to an anti-HCV assay based on the HCV 1b replicon model. The C-7 benzyl substituted compound 18a (inhibitory rate = 59.8% at 25 μM) had a higher inhibitory rate of HCV than that of the unsubstituted 10a (inhibitory rate = 36.0% at 25 μM). However, the introduction of a methyl group (18b) at the N-1 position of 18a failed to effectively improve the anti-HCV potency. Compared with the C-7 brominated compound 19, the inhibitory rates of 18a–c did not exceed 40% at 10 μM, indicating that the benzyl substituents at the phenyl ring of the hydroxyquinazolinedione core may not be dominant in enhancing the inhibitory activity of HCV.

Table 2.
Anti-HCV assay of C-6/C-7/C-8 substituted 3-hydroxy-quinazoline-2,4(1H,3H)-diones.
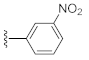
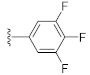
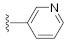
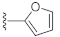
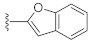
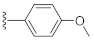
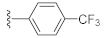
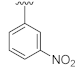
Unlike the benzyl substituents, when the C-7 position of the parent nucleus was substituted with phenyl (21e), the anti-HCV activity of the compound was significantly improved (78.1% inhibitory rate at 10 μM). Based on these results, various aryl substituents at the C-6, C-7, or 8-position of the hydroxyquinazolinedione core were introduced to observe their effects on inhibiting HCV replication. It should be noted that compounds 21a–d with C-6 aryl groups showed significant cytotoxicity. For instance, the therapeutic index of 21d with m-nitrophenyl at the C-6 position was only 1.4. Interestingly, as compared to 21d, the C-7 regioisomer 21k had better HCV inhibitory activity (EC50 = 3.5 μM) and significantly improved therapeutic index (TI = 11.7). These encouraging results led to the further exploration of different C-7 aryl groups on the parent nucleus. Most of the compounds 21e–p substituted with phenyl groups at the C-7 position showed good antiviral activities with EC50 values below 10 μM. Among the C-7 substituents, disubstituted phenyl groups had no advantage over monosubstituted ones for anti-HCV potency, while the C-7 trisubstituted phenyl led to the near loss of activity (21q). It is remarkable that, in comparison with an electron-donating group, when the C-7 aromatic substituent contains an electron-withdrawing group, the compound is less toxic to the tested cells and has a better therapeutic index. For example, no cytotoxicity was observed in the tested concentrations of 21h with trifluoromethyl at the C-7 position (TI > 5.2). In the cases of 21k bearing nitro group and 21l bearing chlorine group, the therapeutic indexes were 11.7 and 5.2, respectively. The cytotoxicity profiles of compounds 21h, 21k, and 21l were distinctly better than those of 21g, 21i, 21j, and 21o, in which C-7 phenyl substituents contain electron-donating groups such as amino and methoxy (TI = 1.1–1.8). Compared with ribavirin (TI = 2.3, EC50 = 20.0 μM) tested under the same condition, 21h, 21k, 21l, and 21n all have better therapeutic windows and HCV inhibitory activities. At the C-7 position of the parent nucleus, we also tried some heteroaryl groups, such as the pyridine group of 21r, the furan group of 21s, and the benzofuran group of 21t. The HCV inhibitory rate of 21r at 10 μM turned out to be 26.9%, which showed no advantage over other compounds possessing C-7 aryl substituents. The reason could be that the introduction of the pyridine group increased the hydrophilicity of the compound and thus reduced the cell membrane permeability. The activity of C-7 furyl substituted 21s (EC50 = 4.0 μM) is comparable to that of C-7 phenyl substituted 21e (EC50 = 3.2 μM). Intriguingly, 21s has a therapeutic index of 4.1, which is better than 21e (TI = 1.7), suggesting that the furyl group is beneficial in reducing the cytotoxicity. This result was also confirmed by compound 21t, which contains a benzofuranyl group at the C-7 position. No cytotoxic effect was observed for 21t at the tested concentrations. On the other hand, the HCV inhibitory activity of 21t (EC50 = 2.0 μM) is also superior to other synthesized compounds, rendering the therapeutic index of 21t greater than 25. It is worth mentioning that 21t showed a comparable EC50 value to compound 6 (EC50 = 1.9 μM), which is one of the reported metal ion chelators with the best anti-HCV activity at the cellular level. More importantly, no obvious cytotoxic effect was observed at the tested concentration of 21t (TI > 25), leading to a better therapeutic index (TI > 25) than that of compound 6 (TI = 6.8) [38]. The EC50 fitting curves of representative compounds 21k and 21t measured using HCV 1b replicon cells are depicted in Figure 2. The fitting graph validated that the HCV inhibitory rate of the compounds has a gradient increasing relationship with the concentration.
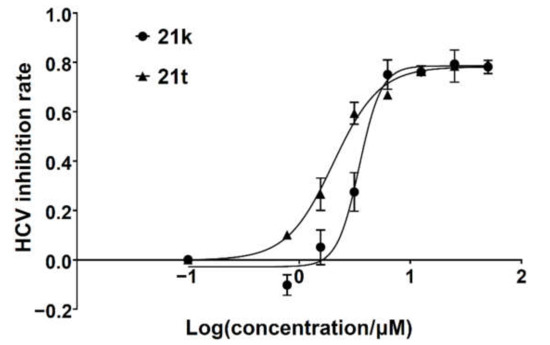
Figure 2.
HCV inhibition fitting curves of compounds 21k and 21t.
Compared with the unsubstituted 10a, C-8 aryl-substituted compounds exhibited no significant improvement in the HCV inhibitory activity. For example, the HCV inhibitory rates of compounds 21u–x at 25 μM did not exceed 50%, whether the introduced C-8 substituents had an electron-donating group (21w) or an electron-withdrawing group (21x). Methylation of 21d–f and 21i at the N-1 position gave the compounds 23a–d, while the EC50 values did not change significantly. However, the cytotoxicity profiles of the methylated products 23a–d were slightly better. The introduction of the ethyl group at the N-1 position of 21e (EC50 = 3.2 μM) greatly reduced the anti-HCV potency, which is exemplified by compound 23e (EC50 = 26.8 μM). The same situation occurred in 23f–i, in which the N-1 position is substituted by a propyl or benzyl group. These results indicated that when the phenyl ring of the hydroxyquinazolinedione core is substituted by aryl groups, sterically large substituents may not be preferred in improving the anti-HCV activity. However, compound 23j is an exception to this rule. One possible reason is that 23j adopts a different binding mode with the target. Overall, the SAR study elucidated the characteristics of the HCV inhibitory profile of 3-hydroxyquinazoline-2,4(1H,3H)-diones. These findings also verified the feasibility of the metal ion chelation strategy in the development of novel anti-HCV drugs.
2.3. Thermal Shift Assay
The stability of a protein system is enhanced after binding to its specific ligand, leading to an increase in the melting temperature (Tm). Taking advantage of this feature, the thermal shift assay was extensively used to assess the binding of small molecules to the protein targets [51,52,53]. The metal ion chelators were thought to exert an anti-HCV effect by chelating with Mg2+ in the catalytic center of NS5B [34,36,38]. Herein, to evaluate the binding of 3-hydroxyquinazoline-2,4(1H,3H)-dione derivatives with NS5B protein, representative compounds 21e and 21k with superior anti-HCV activities or good therapeutic indexes were selected and subjected to the thermal shift assay. C-terminal His-tagged NS5BΔ21 from HCV 1b subtype was used as the protein in the experiment. Compared with the blank control (DMSO), the fluorescence–temperature curves of the NS5B system in the presence of 21e (Figure 3A) and 21k (Figure 3B) at 50 μM, 100 μM, and 200 μM all shifted to the right of the axis. The Tm values of 21e and 21k increased in a concentration-dependent manner, presenting higher values than that of the blank control (Tm = 63.5 °C) at all tested concentrations. The addition of 200 μM 21e or 21k to the NS5B protein solution resulted in a shift in the melting temperature (ΔTm) with a value of 1.5 °C or 2.1 °C, respectively (Table 3), validating the binding abilities of 21e and 21k to NS5B. The co-crystal structure of NS5B complexed with ADP (PDB code: 4WTD) [8] proved that ADP could bind to the catalytic center of NS5B. Hence, ADP was chosen as the positive control in the thermal shift assay. As listed in Table 3, under the same experimental conditions, the ΔTm values of protein–ligand complexes containing ADP at the concentrations of 50 μM, 100 μM, and 200 μM were 0.44 °C, 0.62 °C, and 0.87 °C, respectively, which were significantly lower than the corresponding concentration groups of compounds 21e and 21k. These data indicated that the affinities of 21e and 21k to NS5B protein might be better than that of ADP. The thermal shift assay suggested that the metal ion chelators with 3-hydroxyquinazoline-2,4(1H,3H)-dione parent nucleus could inhibit the replication of HCV by binding to NS5B protein.
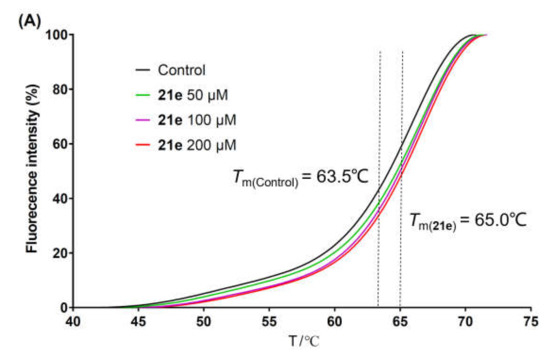
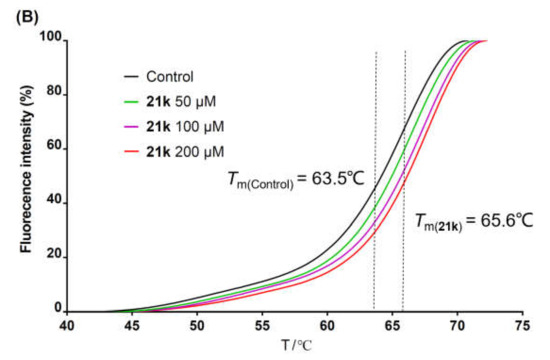
Figure 3.
Fluorescence intensity change versus temperature of NS5B complexed with different concentrations of 21e (A) or 21k (B).

Table 3.
Thermal shifts of the melting temperatures of NS5B complexed with tested compounds.
2.4. Molecular Docking
In order to provide detailed insights into the possible binding mode of 3-hydroxyquinazoline-2,4(1H,3H)-diones with HCV NS5B polymerase and to interpret the potential reasons for the structure–activity relationship of the compounds, the 7-phenyl substituted compound 21e was docked into the crystal structure of NS5B 1b subtype (PDB code: 1GX6) [9] using the Schrödinger software package [54]. Before the docking studies, the original ligand UTP in 1GX6 was removed. In addition, the structures of NS5B protein and 21e were optimized by Schrödinger. The best scoring docking model was subjected to subsequent binding analysis.
From the docking model (Figure 4), it can be seen that the two Mg2+ ions in the NS5B protein maintain the core conformation of the catalytic center in a “hexadentate coordination” mode. The side chain of amino acid residues D318, D319, and D220, the main chain of T221, and two water molecules together form a chelation complex with the central metal ions. The oxygen at the N-3 position and the diketone carbonyl group of compound 21e occupy the remaining coordination space of Mg2+. The distance between Mg2+ and the oxygen atoms involved in chelation in the simulation model is 2.2–2.4 Å, which is very close to the corresponding distance (2.3 Å) between the phosphate group of UTP and the metal ions in the co-crystal structure (1GX6). In addition, one of the carbonyl oxygen atoms of 21e chelated with Mg2+ also forms a hydrogen bond with the main chain of residue F224, maintaining the chelation stability of the compound in the active center of NS5B. It is worth noting that the positive charge center of the side chain of R158 is in the vertical direction of the hydroxyquinazolinedione core of 21e, contributing a cation–π interaction with the nuclear parent of 21e. Interestingly, the cation–π interaction could also be observed between the positively charged amino in the side chain of K141 and the 7-phenyl ring of compound 21e. In fact, such interactions were widely observed among structures in the Protein Data Bank (PDB) [55]. In the aforementioned anti-HCV SAR studies of 3-hydroxyquinazoline-2,4(1H,3H)-diones, we found that the C-6 or C-7 aryl substituents are beneficial to the improvement of HCV inhibitory activity. This finding could be attributed to the cation–π interactions observed in the docking model. Meanwhile, the docking results showed that the C-7 phenyl group of the compound also had a van der Waals interaction with the hydrophobic residue I160. As shown in Table 2, compound 21t with benzofuranyl at the C-7 position had the best HCV inhibitory activity (EC50 = 2.0 μM) and therapeutic index (TI > 25) among the tested compounds. According to the docking model, the superior performance of 21t might be because the substitution of a larger aromatic ring at the C-7 position is advantageous to improving the cation–π interaction with K141 and the van der Waals interaction with I160. The docking conformation also suggested that the C-8 position of the hydroxyquinazolinedione core is spatially farther from the pocket formed by K141 and I160 as compared to the C-7 position, which might be the reason why C-8 aromatic substituents have no apparent contribution to the HCV inhibitory activities of 3-hydroxyquinazoline-2,4(1H,3H)-dione derivatives. The molecular simulation results are in accordance with the SAR studies from multiple perspectives, indicating the mechanism by which the compounds exert their anti-HCV efficacy.
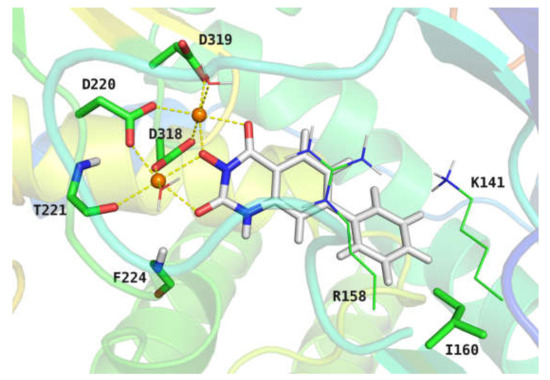
Figure 4.
Binding model of compound 21e with NS5B protein from molecular docking study. The central two Mg2+ ions are shown in orange spheres. The ligand molecule is shown in sticks with carbon atoms and hydrogen atoms colored grey. The key amino acid residues and two water molecules in the catalytic center are presented in sticks or lines with non-polar hydrogen atoms hidden for clarity. The chelation interactions of Mg2+ with the ligand, water molecules, and central amino acid residues are shown in golden dash lines.
2.5. Metal Ion Chelation Assay Using UV-Vis Spectrophotometry
The chelation rules of the 3-hydroxyquinazoline-2,4(1H,3H)-diones with magnesium ions were explored by UV-Vis spectrophotometry, which was widely used to study the binding properties of compounds with metal ions in vitro [56,57,58]. For the sake of eliminating the interference of heteroaryl groups on the determination of metal ion chelation, the representative compound 21e with phenyl group substituted at the C-7 position was selected to study its chelation ability with Mg2+ under different conditions. As illustrated in Figure 5A, the increase in absorbance of 21e methanol solution at 254 nm after the addition of ascending concentrations of MgCl2 was analyzed. The results revealed that with the increase in Mg2+ concentration, the value of absorbance difference at 254 nm between the control group and sample group was always no more than 0.1 even if the concentration of MgCl2 was up to 640 μM, and no concentration dependence was observed. This is probably because compound 21e mainly exists in the free molecular state with the N-hydroxyl group protonated in methanol, which is difficult to chelate with Mg2+. Intriguingly, after the introduction of 5 mm NaOAc to the methanol solution containing 21e, the effect of MgCl2 on the absorbance of the system totally changed (Figure 5B). Compared to the control group containing 50 μM 21e and 5 mm NaOAc, the sample group, which had additional MgCl2, showed significantly higher absorbance. Remarkably, merely 20 μM MgCl2 was able to increase the absorbance by more than 0.5. In the presence of NaOAc, higher concentrations of MgCl2 resulted in larger changes in absorbance at 254 nm, with equilibrium reached at about 400 μM Mg2+. A reasonable logic is that the addition of NaOAc raised the pH of the methanol solution, resulting in partial ionization of compound 21e at the N-hydroxyl group. Subsequently, 21e in the ionic state formed a chelation complex with Mg2+, which increased the absorbance of the solution.
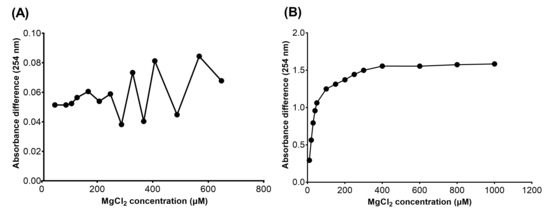
Figure 5.
Absorbance increasement at 254 nm of the methanol solution containing 50 μM 21e in the presence (A) or absence (B) of NaOAc caused by the addition of ascending concentrations of MgCl2. (A) Absorbance difference at 254 nm obtained by subtracting the absorbance of the control group (50 μM 21e) from that of the sample group (50 μM 21e, 40–640 μM MgCl2) containing MgCl2. (B) Absorbance difference at 254 nm obtained by subtracting the absorbance of the control group (50 μM 21e, 5 mm NaOAc) from that of the sample group (50 μM 21e, 5 mm NaOAc, 10–1000 μM MgCl2) containing MgCl2.
With the aim of verifying whether 21e has the ability to chelate with Mg2+ in the ionic state, we further investigated the influence of various concentrations of NaOAc on the differential UV-Vis spectra caused by the addition of MgCl2. As shown in Figure 6, when the system contained no NaOAc, 80 μM MgCl2 could not effectively change the UV-Vis spectrum of the solution containing 21e. Nevertheless, in the presence of ascending concentrations of NaOAc (from 200 μM to 5 mm), compared with the control group (50 μM 21e, 200 μM to 5 mm NaOAc), the UV spectra of the sample group (50 μM 21e, the same concentration of NaOAc as in the control group, 80 μM MgCl2) containing MgCl2 changed significantly, with the absorbance at 254 nm increased by 0.8–1.2. Moreover, the magnitude of the variation in the spectrum is proportional to the concentration of NaOAc. These findings demonstrated that with the increase of NaOAc concentration in methanol solution, the concentration of N-hydroxyl deprotonated 21e increased synchronously, leading to a higher amount of chelation complex with Mg2+. The comparison between different UV-Vis spectra also verified that compound 21e could chelate with Mg2+ in the N-hydroxyl deprotonated form rather than the free molecular state. The UV-Vis spectra studies illustrated the metal ion chelating properties of 3-hydroxyquinazoline-2,4(1H,3H)-diones represented by compound 21e, suggesting that these compounds in the ionic state may have the ability to chelate the metal ions in the catalytic center of NS5B.
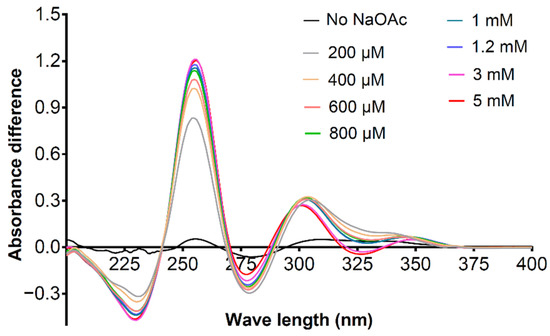
Figure 6.
The differential ultraviolet-visible (UV-Vis) spectra caused by the addition of 80 μM MgCl2 in the presence of 0–5 mm NaOAc. The differential spectra were obtained by subtracting the absorbance of the control group (50 μM 21e, 0–5 mm NaOAc) from that of the sample group (50 μM 21e, the same concentration of NaOAc as in the control group, 80 μM MgCl2) at the corresponding wavelength (200–400 nm). Methanol was used as the solvent.
3. Materials and Methods
3.1. Chemistry
The reagents used in the chemical experiments were purchased from qualified chemical sellers, with purity greater than 95%. Most of the solvents used in the synthesis experiments were obtained from the China National Pharmaceutical Group Corporation. Unless otherwise specified, all solvents used in the reactions are of analytical grade and have not been further processed. Anhydrous solvents such as DMF, DCM, THF, etc., were purchased from the Innovative Technology Solvent Purification System (Innovative Technology Ltd., Hong Kong, China). Some reaction products were purified by flash column chromatography (CombiFlash® EZ Prep, Teledyne ISCO, Lincoln, NE, USA) with 200–300 mesh silica gel (Qingdao Haiyang Chemical Co., Ltd., Qingdao, China).
The nuclear magnetic resonance spectra (1H NMR, 13C NMR) of the synthesized compounds were recorded by Varian Mercury Plus 400 MHz and Bruker AscendTM 600 MHz spectrometers with TMS as an internal standard to calibrate the chemical shifts (δ). Deuterated DMSO or Deuterated trifluoroacetic acid purchased from J&K Scientific was used as the solvent for NMR spectroscopy. The purities and molecular weights of the compounds were determined using an Agilent 1100s mass spectrometer and an Agilent 1260 LC-Agilent 6120 MS liquid chromatography-mass spectrometer with an ESI ion source. The mobile phase was a chromatographically pure water/methanol mixture.
3.1.1. Synthesis of Compounds 8 and 10a
3-(Benzyloxy)quinazoline-2,4(1H,3H)-dione (8). To O-benzylhydroxylamine hydrochloride (6.25 g, 39.1 mmol, 1.5 equiv.) was added 5% NaOH aqueous solution (80 mL) and ether (230 mL), and the mixture was stirred at room temperature for 2 h. The layers were left to stand, and the ether layer was washed three times with saturated brine. The ether layer was dried by Na2SO4 and concentrated to give O-benzylhydroxylamine (4.8 g, 100%), which was stored at 4 °C for later use. A suspension of methyl anthranilate (3.95 g, 26.1 mmol, 1.0 equiv.) and CDI (5.3 g, 32.6 mmol, 1.25 equiv.) in toluene (240 mL) was heated under reflux for 2 h. After the reaction mixture was cooled, the above prepared O-benzylhydroxylamine (4.8 g, 39.1 mmol, 1.5 equiv.) was added, and the suspension was heated under reflux for 4 h and then evaporated. Subsequently, ethanol (70 mL) and 2 mol/L NaOH aqueous solution (18 mL) were added to the flask, and the mixture was refluxed for 2 h. After cooling, 15% volume fraction of acetic acid aqueous solution (240 mL) was slowly added to the reaction mixture. The white precipitate was filtrated and recrystallized with methanol (40 mL) to obtain 8 as a white solid (4.2 g, 60% in all steps). MS (ESI) m/z: 269.1 [M + H]+. 1H NMR (400 MHz, DMSO-d6) δ 8.08 (dd, J = 8.7, 1.5 Hz, 1H), 7.54 (td, J = 7.9, 1.5 Hz, 1H), 7.38–7.31 (m, 4H), 7.35–7.17 (m, 4H), 5.01 (s, 2H).
3-Hydroxyquinazoline-2,4(1H,3H)-dione (10a). To compound 8 (100 mg, 0.373 mmol, 1.0 equiv.) was added 10 wt.% loading palladium–carbon catalyst (10% of the mass of 8), THF (4 mL), and methanol (1 mL). The solution was stirred under hydrogen (1 atm) at room temperature for 8 h until the raw material 8 was completely converted. The palladium–carbon catalyst was removed by filtration. The filtrate was evaporated to dryness, recrystallized with methanol to give 10a as a white solid (38 mg, 57%). MS (ESI) m/z: 177.1 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 11.51 (s, 1H), 10.56 (s, 1H), 7.95 (d, J = 8.0 Hz, 1H), 7.66 (t, J = 7.8 Hz, 1H), 7.27–7.12 (m, 2H). 13C NMR (151 MHz, DMSO-d6) δ 159.23, 148.58, 138.18, 134.53, 126.90, 122.36, 115.10, 113.97.
The ESI-MS, 1H NMR, and 13C NMR spectra of 10a are shown in Supplementary Materials (Figures S1–S4).
3.1.2. Synthesis of Compounds 10b–p
A suspension of compound 8 (300 mg, 1.12 mmol, 1.0 equiv.), K2CO3 (309.6 mg, 2.24 mmol, 2.0 equiv.), the corresponding halide (1.34 mmol, 1.2 equiv.) was stirred in DMF (3 mL) at 80 °C for 2 h. After cooling, the reaction mixture was poured into water. The precipitate was washed with water and ether and dried to give the crude product 9a–o, respectively. Debenzylation reactions of 9a–o were subsequently conducted using two different conditions to obtain 10b–p, respectively.
For 10b–e: to compound 9a–d (0.388 mmol, 1.0 equiv.) was added 48% hydrobromic acid (1.5 mL) and glacial acetic acid (1.5 mL). The solution was refluxed for 2 h until the complete conversion of 9a–d. The reaction mixture was cooled to 0 °C under an ice bath and neutralized with 1 mol/L sodium hydroxide aqueous solution. The precipitate was collected by filtration, washed with water and diethyl ether, dried, and re-slurried or recrystallized with a mixture of ethyl acetate/methanol to obtain 10b–e as a solid, respectively.
For 10f–p: A suspension of compound 9e–o (0.233 mmol, 1.0 equiv.), 10 wt.% loading palladium–carbon catalyst (10% of the mass of 9e–o), THF (4 mL), and methanol (1 mL) was stirred under hydrogen (1 atm) at room temperature for 4–12 h until the raw material 9e–o was completely consumed. The catalyst was removed by filtration. The filtrate was evaporated to dryness, recrystallized or re-slurried with methanol, and dried to give 10f–p as a solid, respectively.
3-Hydroxy-1-(3-phenylpropyl)quinazoline-2,4(1H,3H)-dione (10b). According to the above general procedure with 1-bromo-3-phenylpropane as the halide in the reactants, the crude 9a was obtained as a white solid. MS (ESI) m/z: 387.0 [M + H]+. Subsequent debenzylation in the mixture of hydrobromic acid/glacial acetic acid gave 10b as a pale yellow solid (82.2 mg, 55% over two steps). MS (ESI) m/z: 294.9 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 8.00 (d, J = 7.8 Hz, 1H), 7.66 (d, J = 8.0 Hz, 1H), 7.35 (d, J = 8.4 Hz, 1H), 7.31–7.07 (m, 6H), 4.09 (t, J = 7.7 Hz, 2H), 2.67 (d, J = 8.2 Hz, 2H), 1.89 (t, J = 8.6 Hz, 2H). 13C NMR (151 MHz, DMSO-d6) δ 158.71, 149.74, 141.08, 137.98, 134.21, 128.51, 128.25, 128.17, 128.09, 127.36, 125.74, 122.30, 115.13, 114.14, 42.74, 31.98, 28.40.
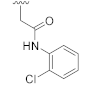
1-(2-Chlorophenethyl)-3-hydroxyquinazoline-2,4(1H,3H)-dione (10c). According to the above general procedure with 1-(2-bromoethyl)-2-chlorobenzene as the halide in the reactants, the crude 9b was obtained as a white solid. MS (ESI) m/z: 406.9 [M + H]+. Subsequent debenzylation in the mixture of hydrobromic acid/glacial acetic acid gave 10c as a pale yellow solid (43 mg, 18% over two steps). MS (ESI) m/z: 315.1 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 10.76 (s, 1H), 8.07 (d, J = 7.5 Hz, 1H), 7.74 (t, J = 7.6 Hz, 1H), 7.46 (d, J = 8.2 Hz, 1H), 7.40 (d, J = 7.2 Hz, 2H), 7.29 (dt, J = 14.7, 6.9 Hz, 3H), 4.37 (t, J = 7.5 Hz, 2H), 3.09 (t, J = 7.3 Hz, 2H). 13C NMR (151 MHz, DMSO-d6) δ 158.28, 148.91, 138.39, 135.50, 134.87, 133.10, 131.35, 129.13, 128.55, 127.67, 127.36, 122.73, 114.89, 114.25, 42.87, 30.58.
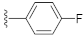
1-(4-Fluorophenethyl)-3-hydroxyquinazoline-2,4(1H,3H)-dione (10d). According to the above general procedure with 1-(2-bromoethyl)-4-fluorobenzene as the halide in the reactants, the crude 9c was obtained as a white solid. MS (ESI) m/z: 391.1 [M + H]+. Subsequent debenzylation in the mixture of hydrobromic acid/glacial acetic acid gave 10d as a pale yellow solid (60 mg, 65% over two steps). MS (ESI) m/z: 299.0 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 10.76 (s, 1H), 8.08 (d, J = 8.0 Hz, 1H), 7.92–7.71 (m, 1H), 7.56 (d, J = 8.7 Hz, 1H), 7.49–7.27 (m, 3H), 7.23–7.07 (m, 2H), 4.33 (d, J = 7.7 Hz, 2H), 2.95 (d, J = 7.4 Hz, 2H). 13C NMR (151 MHz, DMSO-d6) δ 161.74, 160.14, 158.29, 148.90, 138.32, 134.98, 134.03, 130.66, 130.61, 127.64, 122.78, 115.12, 114.98, 114.86, 114.67, 44.34, 31.94.
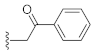
3-Hydroxy-1-(2-oxo-2-phenylethyl)quinazoline-2,4(1H,3H)-dione (10e). According to the above general procedure with 2-bromo-1-phenylethan-1-one as the halide in the reactants, the crude 9d was obtained as a white solid. MS (ESI) m/z: 386.9 [M + H]+. Subsequent debenzylation in the mixture of hydrobromic acid/glacial acetic acid gave 10e as a pale yellow solid (30 mg, 27% over two steps). MS (ESI) m/z: 295.0 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 10.90 (s, 1H), 8.14 (d, J = 7.2 Hz, 3H), 7.85–7.57 (m, 4H), 7.43–7.26 (m, 2H), 5.82 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 192.91, 158.44, 149.36, 139.06, 135.01, 134.15, 128.85 (2C), 128.17 (2C), 127.58, 123.02, 114.75, 114.61, 50.12.
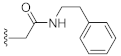
2-(3-Hydroxy-2,4-dioxo-3,4-dihydroquinazolin-1(2H)-yl)-N-phenethylacetamide (10f). According to the above general procedure with 2-bromo-N-phenethylacetamide as the halide in the reactants, the crude 9e was obtained as a white solid. MS (ESI) m/z: 430.2 [M + H]+. Subsequent debenzylation by catalytic hydrogenation gave 10f as a white solid (36.3 mg, 35% over two steps). MS (ESI) m/z: 338.1 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 10.89 (s, 1H), 8.36 (t, J = 5.9 Hz, 1H), 8.05 (d, J = 7.5 Hz, 1H), 7.70 (t, J = 7.7 Hz, 1H), 7.28 (t, J = 8.0 Hz, 3H), 7.20 (t, J = 8.6 Hz, 3H), 7.11 (d, J = 7.9 Hz, 1H), 4.72 (d, J = 4.9 Hz, 2H), 3.32 (d, J = 7.3 Hz, 2H), 2.71 (dd, J = 10.0, 4.6 Hz, 2H). 13C NMR (151 MHz, DMSO-d6) δ 166.20, 158.55, 149.50, 139.12, 138.95, 134.68, 128.55 (2C), 128.20 (2C), 127.38, 125.99, 122.79, 114.92, 114.30, 45.89, 40.21, 34.89.
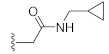
N-(Cyclopropylmethyl)-2-(3-hydroxy-2,4-dioxo-3,4-dihydroquinazolin-1(2H)-yl)acetamide (10g). According to the above general procedure with 2-bromo-N-(cyclopropylmethyl)acetamide as the halide in the reactants, the crude 9f was obtained as a white solid. MS (ESI) m/z: 380.2 [M + H]+. Subsequent debenzylation by catalytic hydrogenation gave 10g as a white solid (85 mg, 79% over two steps). MS (ESI) m/z: 288.1 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 8.33 (s, 1H), 8.00 (d, J = 6.8 Hz, 1H), 7.69 (s, 1H), 7.26 (d, J = 7.5 Hz, 1H), 7.17 (d, J = 8.1 Hz, 1H), 4.73 (s, 2H), 2.97 (s, 2H), 2.50 (s, 2H), 0.88 (s, 1H), 0.38 (d, J = 5.7 Hz, 2H), 0.13 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 166.23, 158.80, 149.91, 138.78, 134.23, 127.21, 122.53, 115.06, 114.16, 45.84, 42.81, 10.58, 3.06 (2C).
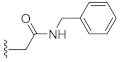
N-Benzyl-2-(3-hydroxy-2,4-dioxo-3,4-dihydroquinazolin-1(2H)-yl)acetamide (10h). According to the above general procedure with N-benzyl-2-bromoacetamide as the halide in the reactants, the crude 9g was obtained as a white solid. MS (ESI) m/z: 416.1 [M + H]+. Subsequent debenzylation by catalytic hydrogenation gave 10h as a pale yellow solid (90 mg, 81% over two steps). MS (ESI) m/z: 324.1 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 10.95 (s, 1H), 8.78 (s, 1H), 8.06 (d, J = 7.6 Hz, 1H), 7.73 (s, 1H), 7.31 (t, J = 8.0 Hz, 3H), 7.27–7.19 (m, 4H), 4.84 (s, 2H), 4.30 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 166.49, 158.61, 149.57, 138.90, 134.63, 128.14, 127.38 (2C), 126.99 (2C), 126.71, 122.84, 114.98, 114.41, 46.03, 42.02.
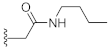
N-Butyl-2-(3-hydroxy-2,4-dioxo-3,4-dihydroquinazolin-1(2H)-yl)acetamide (10i). According to the above general procedure with 2-bromo-N-butylacetamide as the halide in the reactants, the crude 9h was obtained as a white solid. MS (ESI) m/z: 382.2 [M + H]+. Subsequent debenzylation by catalytic hydrogenation gave 10i as a white solid (19 mg, 11% over two steps). MS (ESI) m/z: 290.2 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 10.82 (s, 1H), 8.20 (d, J = 5.7 Hz, 1H), 8.07 (d, J = 7.9 Hz, 1H), 7.73 (t, J = 7.8 Hz, 1H), 7.31 (t, J = 7.7 Hz, 1H), 7.19 (d, J = 7.8 Hz, 1H), 4.73 (s, 2H), 3.06 (p, J = 5.6, 5.0 Hz, 2H), 1.37 (t, J = 8.0 Hz, 2H), 1.24 (q, J = 7.5 Hz, 2H), 0.90–0.78 (m, 3H). 13C NMR (151 MHz, DMSO-d6) δ 166.03, 158.51, 149.41, 139.05, 134.74, 127.43, 122.84, 114.90, 114.37, 45.92, 38.18, 30.97, 19.33, 13.52.
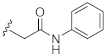
2-(3-Hydroxy-2,4-dioxo-3,4-dihydroquinazolin-1(2H)-yl)-N-phenylacetamide (10j). According to the above general procedure with 2-bromo-N-phenylacetamide as the halide in the reactants, the crude 9i was obtained as a white solid. MS (ESI) m/z: 402.1 [M + H]+. Subsequent debenzylation by catalytic hydrogenation gave 10j as a white solid (13 mg, 7% over two steps). MS (ESI) m/z: 310.1 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 10.89 (s, 1H), 10.38 (s, 1H), 8.10 (d, J = 7.0 Hz, 1H), 7.76 (t, J = 8.0 Hz, 1H), 7.57 (d, J = 6.8 Hz, 2H), 7.40 (d, J = 7.9 Hz, 1H), 7.32 (d, J = 6.7 Hz, 3H), 7.08 (d, J = 7.1 Hz, 1H), 5.00 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 165.13, 158.41, 149.42, 139.15, 138.39, 134.89, 128.67 (2C), 127.45, 123.42, 122.90, 119.01 (2C), 114.62, 114.57, 46.30.
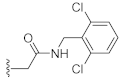
N-(2,6-Dichlorobenzyl)-2-(3-hydroxy-2,4-dioxo-3,4-dihydroquinazolin-1(2H)-yl)acetamide (10k). According to the above general procedure with 2-bromo-N-(2,6-dichlorobenzyl)acetamide as the halide in the reactants, the crude 9j was obtained as a white solid. MS (ESI) m/z: 484.1 [M + H]+. Subsequent debenzylation by catalytic hydrogenation gave 10k as a grey solid (28.4 mg, 25% over two steps). MS (ESI) m/z: 393.0 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 10.83 (s, 1H), 8.56 (s, 1H), 8.07 (d, J = 8.5 Hz, 1H), 7.71 (t, J = 8.3 Hz, 1H), 7.50 (d, J = 8.2 Hz, 2H), 7.38 (d, J = 6.8 Hz, 1H), 7.34–7.24 (m, 1H), 7.18 (d, J = 8.8 Hz, 1H), 4.79 (s, 2H), 4.54 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 166.11, 158.44, 149.40, 139.02, 135.46 (2C), 134.74, 132.83, 130.27, 128.50 (2C), 127.44, 122.88, 114.78, 114.41, 45.55, 38.80.
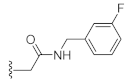
N-(3-Fluorobenzyl)-2-(3-hydroxy-2,4-dioxo-3,4-dihydroquinazolin-1(2H)-yl)acetamide (10l). According to the above general procedure with 2-bromo-N-(3-fluorobenzyl)acetamide as the halide in the reactants, the crude 9k was obtained as a white solid. MS (ESI) m/z: 434.1 [M + H]+. Subsequent debenzylation by catalytic hydrogenation gave 10l as a white solid (40 mg, 30% over two steps). MS (ESI) m/z: 342.2 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 10.84 (s, 1H), 8.80 (d, J = 6.7 Hz, 1H), 8.08 (d, J = 7.8 Hz, 1H), 7.73 (t, J = 7.8 Hz, 1H), 7.39–7.24 (m, 3H), 7.05 (dd, J = 17.2, 9.0 Hz, 3H), 4.85 (d, J = 2.9 Hz, 2H), 4.32 (d, J = 6.0 Hz, 2H). 13C NMR (151 MHz, DMSO-d6) δ 166.70, 162.11 (d, J = 243.3 Hz), 158.55, 149.49, 142.02 (d, J = 7.2 Hz), 139.03, 134.76, 130.10 (d, J = 8.4 Hz), 127.49, 122.94, 115.01, 114.46, 113.52.
N-(4-Fluorobenzyl)-2-(3-hydroxy-2,4-dioxo-3,4-dihydroquinazolin-1(2H)-yl)acetamide (10m). According to the above general procedure with 2-bromo-N-(4-fluorobenzyl)acetamide as the halide in the reactants, the crude 9l was obtained as a white solid. MS (ESI) m/z: 434.1 [M + H]+. Subsequent debenzylation by catalytic hydrogenation gave 10m as a white solid (49.4 mg, 36% over two steps). MS (ESI) m/z: 342.1 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 10.84 (s, 1H), 8.79 (d, J = 6.0 Hz, 1H), 8.08 (d, J = 7.7 Hz, 1H), 7.74 (t, J = 7.9 Hz, 1H), 7.32 (t, J = 8.0 Hz, 1H), 7.26 (d, J = 7.7 Hz, 3H), 7.19–7.09 (m, 2H), 4.83 (s, 2H), 4.28 (d, J = 5.7 Hz, 2H). 13C NMR (151 MHz, DMSO-d6) δ 166.53, 161.08 (d, J = 242.2 Hz), 158.54, 149.47, 139.03, 135.15, 134.79, 128.99 (d, J = 8.3 Hz), 127.47, 122.93, 114.97 (d, J = 4.4 Hz), 114.81, 114.44, 46.05, 41.33.
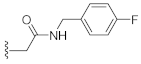
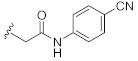
N-(4-Cyanophenyl)-2-(3-hydroxy-2,4-dioxo-3,4-dihydroquinazolin-1(2H)-yl)acetamide (10n). According to the above general procedure with 2-bromo-N-(4-cyanophenyl)acetamide as the halide in the reactants, the crude 9m was obtained as a white solid. MS (ESI) m/z: 427.1 [M + H]+. Subsequent debenzylation by catalytic hydrogenation gave 10n as a white solid (20 mg, 19% over two steps). MS (ESI) m/z: 335.1 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 10.90 (s, 1H), 10.85 (s, 1H), 8.11 (dd, J = 7.5, 3.6 Hz, 1H), 7.78 (d, J = 11.4 Hz, 5H), 7.47–7.40 (m, 1H), 7.34 (td, J = 7.4, 3.6 Hz, 1H), 5.05 (d, J = 3.7 Hz, 2H). 13C NMR (151 MHz, DMSO-d6) δ 166.14, 158.40, 149.41, 142.57, 139.10, 134.96, 133.27, 123.00, 119.07, 118.79, 114.61, 105.23, 46.50.
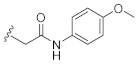
2-(3-Hydroxy-2,4-dioxo-3,4-dihydroquinazolin-1(2H)-yl)-N-(4-methoxyphenyl)acetamide (10o). According to the above general procedure with 2-bromo-N-(4-methoxyphenyl)acetamide as the halide in the reactants, the crude 9n was obtained as a white solid. MS (ESI) m/z: 432.1 [M + H]+. Subsequent debenzylation by catalytic hydrogenation gave 10o as a white solid (23.8 mg, 18% over two steps). MS (ESI) m/z: 340.1 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 10.87 (s, 1H), 10.22 (s, 1H), 8.09 (d, J = 7.9 Hz, 1H), 7.74 (t, J = 8.2 Hz, 1H), 7.47 (d, J = 8.6 Hz, 2H), 7.35 (dd, J = 20.2, 8.4 Hz, 2H), 6.88 (d, J = 8.4 Hz, 2H), 4.96 (s, 2H), 3.71 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 164.60, 158.41, 155.22, 149.41, 139.14, 134.85, 131.47, 127.41, 122.85, 120.59, 114.63, 114.54, 113.73, 54.96, 46.17.
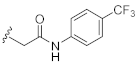
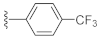
2-(3-Hydroxy-2,4-dioxo-3,4-dihydroquinazolin-1(2H)-yl)-N-(4-(trifluoromethyl)phenyl)acetamide (10p). According to the above general procedure with 2-bromo-N-(4-(trifluoromethyl)phenyl)acetamide as the halide in the reactants, the crude 9o was obtained as a white solid. MS (ESI) m/z: 470.1 [M + H]+. Subsequent debenzylation by catalytic hydrogenation gave 10p as a white solid (30 mg, 18% over two steps). MS (ESI) m/z: 378.1 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 10.91 (s, 1H), 10.77 (s, 1H), 8.10 (d, J = 7.8 Hz, 1H), 7.78 (d, J = 9.0 Hz, 2H), 7.74 (d, J = 7.3 Hz, 1H), 7.69 (d, J = 8.4 Hz, 2H), 7.43 (d, J = 7.9 Hz, 1H), 7.33 (d, J = 7.3 Hz, 1H), 5.05 (s, 2H). 13C NMR (151 MHz, Chloroform-d) δ 165.95, 158.42, 149.44, 141.95, 139.12, 134.92, 127.48, 126.04, 125.04, 123.54, 123.28 (d, J = 12.2 Hz), 122.96, 118.96, 114.61, 46.44.
3.1.3. Synthesis of Compounds 14a–c
To compound 11a–c (37.0 mmol, 1.0 equiv.) was added triphosgene (3.84 g, 12.9 mmol, 0.35 equiv.), and 1,4-dioxane (40 mL). The suspension was refluxed at 110 °C for 6 h and then evaporated to 1/3 of the volume. The precipitate was collected by filtration, washed with petroleum ether (50 mL) and ethyl acetate (10 mL) successively, and dried to give the crude product 12a–c. A suspension of O-benzylhydroxylamine hydrochloride (3.72 g, 23.3 mmol, 1.02 equiv.) and triethylamine (2.36 g, 23.3 mmol, 1.02 equiv.) in ethanol (100 mL) was stirred at room temperature for 1 h, and then crude 12a–c (22.9 mmol, 1.0 equiv.) was added. The reaction mixture was refluxed for 3 h, then poured into water (350 mL). The precipitate was collected by filtration, washed with water, and dried to obtain crude 13a–c. Without further purification, crude 13a–c (16.7 mmol, 1.0 equiv.) was stirred with triphosgene (1.98 g, 6.63 mmol, 0.4 equiv.) and triethylamine (4.05 g, 40.0 mmol, 2.4 equiv.) in THF (200 mL) at room temperature for 2 h, then quenched with water (600 mL). The precipitate was filtrated, washed with water, dried, and re-slurried with petroleum ether/ethyl acetate to give compound 14a–c, respectively.
3-(Benzyloxy)-8-bromoquinazoline-2,4(1H,3H)-dione (14a). According to the above general procedure, cyclization of 11a with triphosgene afforded crude 12a. MS (ESI) m/z: 242.1 [M + H]+. Reaction of 12a with O-benzylhydroxylamine gave crude 13a. MS (ESI) m/z: 321.0 [M + H]+. Compound 14a was finally obtained from crude 13a as a white solid (4.52 g, 36% over three steps). MS (ESI) m/z: 347.1 [M + H]+.
3-(Benzyloxy)-7-bromoquinazoline-2,4(1H,3H)-dione (14b). According to the above general procedure, cyclization of 11b with triphosgene afforded crude 12b. MS (ESI) m/z: 242.1 [M + H]+. Reaction of 12b with O-benzylhydroxylamine gave crude 13b. MS (ESI) m/z: 321.0 [M + H]+. Compound 14b was finally obtained from crude 13b as a white solid (5.28 g, 42% over three steps). MS (ESI) m/z: 347.1 [M + H]+. 1H NMR (400 MHz, DMSO-d6) δ 11.78 (s, 1H), 7.88 (d, J = 8.5 Hz, 1H), 7.62–7.54 (m, 2H), 7.42 (d, J = 7.3 Hz, 4H), 7.37 (d, J = 1.8 Hz, 1H), 5.09 (s, 2H).
3-(Benzyloxy)-6-bromoquinazoline-2,4(1H,3H)-dione (14c). According to the above general procedure, cyclization of 11c with triphosgene afforded crude 12c. MS (ESI) m/z: 242.1 [M + H]+. Reaction of 12c with O-benzylhydroxylamine gave crude 13c. MS (ESI) m/z: 321.0 [M + H]+. Compound 14c was finally obtained from crude 13c as a white solid (1.1 g, 18% over three steps). MS (ESI) m/z: 347.1 [M + H]+.
The 1H NMR, and 13C NMR spectra of 10b–p are shown in Supplementary Materials (Figures S5–S49).
3.1.4. Synthesis of Compounds 18a–c and 19
A mixture of 14b or 15 (2.88 mmol, 1.0 equiv.), bis(pinacolato)diboron (3.47 mmol, 1.2 equiv.), Pd(dppf)Cl2 (0.145 mmol, 0.05 equiv.), and KOAc (8.66 mmol, 3.0 equiv.) were heated at 100 °C under nitrogen atmosphere for 8 h. The suspension was filtered while hot to remove the catalyst and KOAc, and the filtrate was poured into water (175 mL). The precipitate was collected by filtration, washed with water and petroleum ether, and dried to give crude boron 16a or 16b. A portion of crude 16a or 16b (0.769 mmol, 1.0 equiv.) was added to benzyl bromide or substituted benzyl bromide (0.641 mmol, 1.2 equiv.), Pd(dppf)Cl2 (0.032 mmol, 0.05 equiv.), KOAc (1.28 mmol, 2.0 equiv.), and water (2 mL) in 1,4-dioxane (20 mL). The suspension was heated at 80 °C for 3 h and then filtered while hot. The filtrate was concentrated, and the residue was purified by chromatography on silica gel with 20–30% ethyl acetate in petroleum ether to afford 17a–c, which was subsequently subjected to catalytic hydrogenation following the same procedure as described in the preparation of 10a to obtain 18a–c.
7-Benzyl-3-hydroxyquinazoline-2,4(1H,3H)-dione (18a). According to the above general procedure, 14b was first converted to the boron intermediate 16a. MS (ESI) m/z: 310.8 [M−H]−. The crude 16a was then subjected to the Suzuki coupling reaction with benzyl bromide to afford crude 17a. MS (ESI) m/z: 359.1 [M + H]+. Subsequent debenzylation of 17a by catalytic hydrogenation gave 18a as a white solid (11 mg, 10% over three steps). MS (ESI) m/z: 266.9 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 11.43 (s, 1H), 10.50 (s, 1H), 7.86 (dd, J = 8.1, 2.3 Hz, 1H), 7.32 (dd, J = 10.9, 4.6 Hz, 2H), 7.23 (d, J = 6.3 Hz, 3H), 7.09 (d, J = 8.1 Hz, 1H), 7.00 (s, 1H), 4.01 (d, J = 4.1 Hz, 2H). 13C NMR (151 MHz, DMSO-d6) δ 159.09, 148.64, 148.55, 139.79, 138.36, 128.73 (2C), 128.45 (2C), 127.16, 126.18, 123.33, 114.66, 112.13, 40.83.
7-Benzyl-3-hydroxy-1-methylquinazoline-2,4(1H,3H)-dione (18b). According to the above general procedure, 15 was first converted to the boron intermediate 16b. MS (ESI) m/z: 324.8 [M−H]−. The crude 16b was then subjected to the Suzuki coupling reaction with benzyl bromide to afford crude 17b. MS (ESI) m/z: 373.1 [M + H]+. Subsequent debenzylation of 17b by catalytic hydrogenation gave 18b as a white solid (30 mg, 18% over three steps). MS (ESI) m/z: 280.9 [M−H]−. 1H NMR (400 MHz, DMSO-d6) 1H NMR (400 MHz, DMSO-d6) δ 10.67 (s, 1H), 7.95 (d, J = 8.1 Hz, 1H), 7.41 (s, 1H), 7.30 (d, J = 3.5 Hz, 4H), 7.21 (p, J = 4.2 Hz, 1H), 7.15 (d, J = 7.9 Hz, 1H), 4.09 (s, 2H), 3.52 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 158.21, 149.24, 148.95, 140.01, 139.42, 128.66 (2C), 128.40 (2C), 127.57, 126.14, 123.38, 114.51, 112.77, 41.11, 30.59.
7-(3,5-Difluorobenzyl)-3-hydroxy-1-methylquinazoline-2,4(1H,3H)-dione (18c). According to the above general procedure, 14b was first converted to the boron intermediate 16a. MS (ESI) m/z: 310.8 [M−H]−. The crude 16a was then subjected to the Suzuki coupling reaction with 1-(bromomethyl)-3,5-difluorobenzene to afford crude 17c. MS (ESI) m/z: 373.1 [M + H]+. Subsequent debenzylation of 17c by catalytic hydrogenation gave 18c as a white solid (30 mg, 18% over three steps). MS (ESI) m/z: 316.8 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 10.69 (s, 1H), 7.98 (d, J = 8.0 Hz, 1H), 7.46 (s, 1H), 7.21 (d, J = 7.9 Hz, 1H), 7.14–7.00 (m, 3H), 4.11 (s, 2H), 3.54 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 162.22 (dd, J = 246.0, 13.3 Hz, 2C), 158.18, 149.23, 147.52, 144.63, 139.51, 127.71, 123.32, 114.75, 113.07, 111.88 (d, J = 5.0 Hz), 111.75 (d, J = 4.9 Hz), 101.69 (t, J = 25.7 Hz), 40.41, 30.65.
7-Bromo-3-hydroxyquinazoline-2,4(1H,3H)-dione (19). To compound 14b (200 mg, 0.576 mmol, 1.0 equiv.) was added 48% hydrobromic acid (1.5 mL) and glacial acetic acid (1.5 mL). The solution was refluxed for 2 h. The reaction mixture was cooled to 0 °C under an ice bath and neutralized with 1 mol/L sodium hydroxide aqueous solution. The precipitate was collected by filtration, washed with water, dried, and recrystallized with methanol to obtain 19 as a pale yellow solid (120 mg, 81%). MS (ESI) m/z: 255.1 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 11.63 (s, 1H), 10.65 (s, 1H), 7.86 (s, 1H), 7.39 (d, J = 13.7 Hz, 2H). 13C NMR (151 MHz, DMSO-d6) δ 158.78, 148.45, 139.33, 129.04, 127.84, 125.40, 117.51, 113.36.
The 1H NMR, 13C NMR, and representative ESI-MS spectra of 18a–c and 19 are shown in Supplementary Materials (Figures S50–S63).
3.1.5. Synthesis of Compounds 21a–x
The brominated compound 14a, 14b, or 14c (0.576 mmol, 1.0 equiv.), the corresponding arylboronic acid (0.864 mmol, 1.5 equiv.), Pd(PPh3)4 (0.029 mmol, 0.05 equiv.), K2CO3 (2.88 mmol, 5.0 equiv.), and water (1.5 mL) were suspended in 1,4-dioxane (15 mL). The mixture was heated at 100 °C under nitrogen for 12 h until the complete conversion of 14a–c and then filtrated while hot. The filtrate was poured into water (15 mL), and the precipitate was collected by filtration, washed with water, dried, and re-slurried with methanol or ethyl acetate (5 mL) to give the crude solid 20a–x. Debenzylation reactions of 20a–x were subsequently performed using four different conditions to obtain 21a–x, respectively. For 20a–i, 20m–q, and 20t–x, the same method of catalytic hydrogenation as described in the preparation of 10f–q was used to give 21a–i, 21m–q, and 20t–x, respectively. For 20j–l, the same procedure of debenzylation in the mixture of hydrobromic acid/glacial acetic acid as described in the preparation of 10b–e was conducted to afford 21j–l, respectively. 21r or 21s was obtained from the debenzylation of 20r or 20s using trifluoroacetate (TFA) or TiCl4, respectively.
3-Hydroxy-6-phenylquinazoline-2,4(1H,3H)-dione (21a). According to the above general procedure, 14a was first subjected to the Suzuki coupling reaction with phenylboronic acid to afford crude 20a. MS (ESI) m/z: 343.3 [M−H]−. Subsequent debenzylation of 20a by catalytic hydrogenation gave 21a as a white solid (28 mg, 30% over two steps). MS (ESI) m/z: 253.2 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 11.64 (s, 1H), 10.63 (s, 1H), 8.15 (s, 1H), 8.00 (d, J = 6.4 Hz, 1H), 7.69 (d, J = 5.4 Hz, 2H), 7.48 (d, J = 6.0 Hz, 2H), 7.38 (t, J = 6.8 Hz, 1H), 7.30 (d, J = 6.9 Hz, 1H). 13C NMR (151 MHz, DMSO-d6) δ 159.24, 148.51, 138.47, 137.53, 134.31, 133.04, 128.95 (2C), 127.44, 126.25 (2C), 124.23, 115.97, 114.43.
6-(4-Fluorophenyl)-3-hydroxyquinazoline-2,4(1H,3H)-dione (21b). According to the above general procedure, 14a was first subjected to the Suzuki coupling reaction with (4-fluorophenyl)boronic acid to afford crude 20b. MS (ESI) m/z: 361.1 [M−H]−. Subsequent debenzylation of 20b by catalytic hydrogenation gave 21b as a white solid (38.9 mg, 29% over two steps). MS (ESI) m/z: 271.1 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 11.64 (s, 1H), 10.63 (s, 1H), 8.13 (s, 1H), 8.01–7.90 (m, 1H), 7.74 (dd, J = 7.7, 4.3 Hz, 2H), 7.30 (t, J = 6.8 Hz, 3H). 13C NMR (151 MHz, DMSO-d6) δ 161.75 (d, J = 244.8 Hz), 159.19, 148.49, 137.49, 134.98, 133.32, 132.98, 128.35, 128.30, 124.22, 115.97, 115.79, 115.65, 114.42.
3-Hydroxy-6-(4-methoxyphenyl)quinazoline-2,4(1H,3H)-dione (21c). According to the above general procedure, 14a was first subjected to the Suzuki coupling reaction with (4-methoxyphenyl)boronic acid to afford crude 20c. MS (ESI) m/z: 373.1 [M−H]−. Subsequent debenzylation of 20c by catalytic hydrogenation gave 21c as a white solid (25 mg, 18% over two steps). MS (ESI) m/z: 283.2 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 11.58 (s, 1H), 10.61 (s, 1H), 8.10 (s, 1H), 7.95 (d, J = 8.6 Hz, 1H), 7.64 (d, J = 8.4 Hz, 2H), 7.27 (d, J = 8.6 Hz, 1H), 7.04 (d, J = 8.3 Hz, 2H), 3.81 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 159.27, 158.83, 148.49, 136.96, 134.10, 132.62, 130.83, 127.39 (2C), 123.47, 115.88, 114.39, 114.35 (2C), 55.03.
3-Hydroxy-6-(3-nitrophenyl)quinazoline-2,4(1H,3H)-dione (21d). According to the above general procedure, 14a was first subjected to the Suzuki coupling reaction with (3-nitrophenyl)boronic acid to afford crude 20d. MS (ESI) m/z: 388.1 [M−H]−. Subsequent debenzylation of 20d by catalytic hydrogenation gave 21d as a white solid (25 mg, 29% over two steps). MS (ESI) m/z: 298.2 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 11.72 (s, 1H), 10.68 (s, 1H), 8.49–8.42 (m, 1H), 8.30–8.16 (m, 3H), 8.12 (dd, J = 8.7, 2.3 Hz, 1H), 7.77 (t, J = 8.0 Hz, 1H), 7.33 (d, J = 8.5 Hz, 1H). 13C NMR (151 MHz, DMSO-d6) δ 159.15, 148.54, 148.38, 140.12, 138.39, 133.26, 132.91, 131.92, 130.53, 125.05, 122.13, 120.76, 116.26, 114.64.
3-Hydroxy-7-phenylquinazoline-2,4(1H,3H)-dione (21e). According to the above general procedure, 14b was first subjected to the Suzuki coupling reaction with phenylboronic acid to afford crude 20e. MS (ESI) m/z: 343.1 [M−H]−. Subsequent debenzylation of 20e by catalytic hydrogenation gave 21e as an off-white solid (42 mg, 42% over two steps). MS (ESI) m/z: 253.2 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 11.58 (s, 1H), 10.63 (s, 1H), 8.02 (d, J = 8.2 Hz, 1H), 7.68 (d, J = 7.6 Hz, 2H), 7.53 (d, J = 7.6 Hz, 3H), 7.48 (d, J = 6.7 Hz, 1H), 7.41 (d, J = 6.2 Hz, 1H). 13C NMR (151 MHz, DMSO-d6) δ 159.14, 148.77, 146.17, 138.75, 138.55, 129.14, 128.69, 127.76, 126.90, 121.23, 113.07, 112.83.
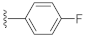
7-(4-Fluorophenyl)-3-hydroxyquinazoline-2,4(1H,3H)-dione (21f). According to the above general procedure, 14b was first subjected to the Suzuki coupling reaction with (4-fluorophenyl)boronic acid to afford crude 20f. MS (ESI) m/z: 361.1 [M−H]−. Subsequent debenzylation of 20f by catalytic hydrogenation gave 21f as a white solid (20 mg, 15% over two steps). MS (ESI) m/z: 271.1 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 11.61 (s, 1H), 10.61 (s, 1H), 8.01 (d, J = 7.6 Hz, 1H), 7.73 (dd, J = 8.6, 5.0 Hz, 2H), 7.50 (d, J = 8.3 Hz, 1H), 7.36 (d, J = 10.6 Hz, 3H). 13C NMR (151 MHz, DMSO-d6) δ 162.36 (d, J = 246.2 Hz), 158.99, 148.63, 144.96, 138.62, 134.91, 128.97, 128.92, 127.67, 121.06, 115.98, 115.84, 112.92, 112.65.
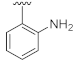
7-(2-Aminophenyl)-3-hydroxyquinazoline-2,4(1H,3H)-dione (21g). According to the above general procedure, 14b was first subjected to the Suzuki coupling reaction with (2-aminophenyl)boronic acid to afford crude 20g. MS (ESI) m/z: 360.1 [M + H]+. Subsequent debenzylation of 20g by catalytic hydrogenation gave 21g as a white solid (34.5 mg, 37% over two steps). MS (ESI) m/z: 268.1 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 11.50 (s, 1H), 10.65 (s, 1H), 7.98 (d, J = 8.6 Hz, 1H), 7.26 (d, J = 5.8 Hz, 2H), 7.09 (t, J = 7.0 Hz, 1H), 7.01 (d, J = 7.7 Hz, 1H), 6.77 (d, J = 8.2 Hz, 1H), 6.65 (t, J = 7.3 Hz, 1H), 4.97 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 159.18, 148.77, 146.12, 145.13, 138.57, 129.66, 129.03, 127.41, 123.82, 123.22, 116.56, 115.41, 114.99, 112.44.
3-Hydroxy-7-(4-(trifluoromethyl)phenyl)quinazoline-2,4(1H,3H)-dione (21h). According to the above general procedure, 14b was first subjected to the Suzuki coupling reaction with (4-(trifluoromethyl)phenyl)boronic acid to afford crude 20h. MS (ESI) m/z: 413.1 [M + H]+. Subsequent debenzylation of 20h by catalytic hydrogenation gave 21h as a white solid (21 mg, 12% over two steps). MS (ESI) m/z: 321.1 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 11.62 (s, 1H), 10.67 (s, 1H), 8.05 (dt, J = 10.8, 5.2 Hz, 1H), 7.88 (d, J = 8.3 Hz, 4H), 7.57 (d, J = 8.7 Hz, 1H), 7.44 (d, J = 10.0 Hz, 1H). 13C NMR (151 MHz, DMSO-d6) δ 158.97, 148.65, 144.41, 142.52, 138.72, 128.78 (d, J = 31.9 Hz), 127.90, 127.76, 125.94 (d, J = 3.9 Hz), 124.03 (d, J = 271.8 Hz), 121.34, 113.75, 113.33.
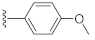
3-Hydroxy-7-(4-methoxyphenyl)quinazoline-2,4(1H,3H)-dione (21i). According to the above general procedure, 14b was first subjected to the Suzuki coupling reaction with (4-methoxyphenyl)boronic acid to afford crude 20i. MS (ESI) m/z: 373.1 [M−H]−. Subsequent debenzylation of 20i by catalytic hydrogenation gave 21i as a white solid (14.2 mg, 17% over two steps). MS (ESI) m/z: 283.2 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 11.56 (s, 1H), 10.57 (s, 1H), 8.07–7.89 (m, 1H), 7.69–7.60 (m, 2H), 7.54–7.45 (m, 1H), 7.36 (d, J = 2.1 Hz, 1H), 7.14–7.05 (m, 2H), 3.82 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 159.82, 159.16, 148.78, 145.80, 138.77, 130.66, 128.11 (2C), 127.68, 120.74, 114.57 (2C), 112.43, 111.96, 55.19.
7-(3-Aminophenyl)-3-hydroxyquinazoline-2,4(1H,3H)-dione (21j). According to the above general procedure, 14b was first subjected to the Suzuki coupling reaction with (3-aminophenyl)boronic acid to afford crude 20j. MS (ESI) m/z: 358.1 [M−H]−. Subsequent debenzylation of 20j in the mixture of hydrobromic acid/glacial acetic acid gave 21j as a white solid (32.4 mg, 42% over two steps). MS (ESI) m/z: 268.2 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 11.59 (s, 1H), 10.58 (s, 1H), 7.98 (d, J = 7.5 Hz, 1H), 7.41 (d, J = 8.2 Hz, 1H), 7.33 (s, 1H), 7.16 (t, J = 7.0 Hz, 1H), 6.86 (s, 1H), 6.79 (d, J = 7.4 Hz, 1H), 6.66 (d, J = 7.8 Hz, 1H), 5.42 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 159.11, 148.85, 148.73, 147.08, 139.23, 138.59, 129.56, 127.53, 121.00, 114.50, 114.34, 112.76, 112.43, 112.08.
3-Hydroxy-7-(3-nitrophenyl)quinazoline-2,4(1H,3H)-dione (21k). According to the above general procedure, 14b was first subjected to the Suzuki coupling reaction with (3-nitrophenyl)boronic acid to afford crude 20k. MS (ESI) m/z: 388.1 [M−H]−. Subsequent debenzylation of 20k in the mixture of hydrobromic acid/glacial acetic acid gave 21k as a white solid (16 mg, 13% over two steps). MS (ESI) m/z: 298.1 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 11.63 (s, 1H), 10.65 (s, 1H), 8.45 (s, 1H), 8.31 (s, 1H), 8.16 (s, 1H), 8.07 (d, J = 8.1 Hz, 1H), 7.84 (s, 1H), 7.64 (d, J = 7.6 Hz, 1H), 7.50 (s, 1H). 13C NMR (151 MHz, DMSO-d6) δ 159.00, 148.67, 148.33, 143.60, 140.06, 138.81, 133.44, 130.78, 128.07, 123.31, 121.41, 121.35, 113.90, 113.37.
7-(3-Chloro-4-fluorophenyl)-3-hydroxyquinazoline-2,4(1H,3H)-dione (21l). According to the above general procedure, 14b was first subjected to the Suzuki coupling reaction with (3-chloro-4-fluorophenyl)boronic acid to afford crude 20l. MS (ESI) m/z: 395.0 [M−H]−. Subsequent debenzylation of 20l in the mixture of hydrobromic acid/glacial acetic acid gave 21l as a white solid (34 mg, 42% over two steps). MS (ESI) m/z: 306.8 [M + H]+. 1H NMR (400 MHz, DMSO-d6) δ 8.00 (s, 1H), 7.90 (s, 1H), 7.69 (s, 1H), 7.56 (d, J = 18.8 Hz, 2H), 7.37 (s, 1H). 13C NMR (151 MHz, DMSO-d6) δ 158.97, 157.37 (d, J = 248.7 Hz), 148.68, 143.61, 138.68, 136.40, 128.97, 128.07–126.56 (m), 121.21, 120.19 (d, J = 17.7 Hz), 117.59, 117.45, 113.40, 113.10.
7-(3,5-Difluorophenyl)-3-hydroxyquinazoline-2,4(1H,3H)-dione (21m). According to the above general procedure, 14b was first subjected to the Suzuki coupling reaction with (3,5-difluorophenyl)boronic acid to afford crude 20m. MS (ESI) m/z: 379.1 [M−H]−. Subsequent debenzylation of 20m by catalytic hydrogenation gave 21m as a white solid (30 mg, 48% over two steps). MS (ESI) m/z: 290.8 [M + H]+. 1H NMR (400 MHz, DMSO-d6) δ 11.58 (s, 1H), 10.67 (s, 1H), 8.02 (d, J = 8.3 Hz, 1H), 7.61–7.52 (m, 1H), 7.48–7.31 (m, 4H). 13C NMR (151 MHz, DMSO-d6) δ 162.67 (dd, J = 246.4, 13.4 Hz, 2C), 158.91, 148.62, 143.43, 142.17, 138.62, 127.78, 121.28, 113.88, 113.34, 110.30 (d, J = 5.8 Hz), 110.17 (d, J = 5.7 Hz), 103.91 (t, J = 25.8 Hz).
7-(2,4-Difluorophenyl)-3-hydroxyquinazoline-2,4(1H,3H)-dione (21n). According to the above general procedure, 14b was first subjected to the Suzuki coupling reaction with (2,4-difluorophenyl)boronic acid to afford crude 20n. MS (ESI) m/z: 379.1 [M−H]−. Subsequent debenzylation of 20n by catalytic hydrogenation gave 21n as a white solid (39.5 mg, 62% over two steps). MS (ESI) m/z: 290.8 [M + H]+. 1H NMR (400 MHz, DMSO-d6) δ 11.62 (s, 1H), 10.63 (s, 1H), 8.03 (d, J = 7.8 Hz, 1H), 7.64 (q, J = 8.2 Hz, 1H), 7.44 (t, J = 10.3 Hz, 1H), 7.38 (d, J = 8.3 Hz, 1H), 7.33 (s, 1H), 7.25 (t, J = 8.8 Hz, 1H). 13C NMR (151 MHz, DMSO-d6) δ 163.18–161.23 (m), 158.99 (dd, J = 249.7, 12.7 Hz), 158.96, 148.64, 140.04, 138.33, 134.90–130.51 (m), 127.40, 123.26 (d, J = 12.9 Hz), 122.90, 115.08, 113.37, 112.31 (d, J = 21.2 Hz), 104.69 (t, J = 26.4 Hz).
7-(3-Fluoro-4-methoxyphenyl)-3-hydroxyquinazoline-2,4(1H,3H)-dione (21o). According to the above general procedure, 14b was first subjected to the Suzuki coupling reaction with (3-fluoro-4-methoxyphenyl)boronic acid to afford crude 20o. MS (ESI) m/z: 391.1 [M−H]−. Subsequent debenzylation of 20o by catalytic hydrogenation gave 21o as a white solid (20.6 mg, 32% over two steps). MS (ESI) m/z: 302.8 [M + H]+. 1H NMR (400 MHz, DMSO-d6) δ 11.56 (s, 1H), 10.60 (s, 1H), 7.98 (d, J = 8.4 Hz, 1H), 7.63–7.54 (m, 1H), 7.54–7.46 (m, 2H), 7.40–7.35 (m, 1H), 7.33 (t, J = 8.4 Hz, 1H), 3.91 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 159.02, 151.56 (d, J = 244.3 Hz), 148.68, 147.54 (d, J = 10.5 Hz), 144.51, 138.67, 131.16 (d, J = 6.2 Hz), 127.64, 123.19, 120.79, 114.26 (d, J = 6.6 Hz), 114.15, 112.79, 112.25, 55.97.
7-(5-Fluoro-2-methylphenyl)-3-hydroxyquinazoline-2,4(1H,3H)-dione (21p). According to the above general procedure, 14b was first subjected to the Suzuki coupling reaction with (5-fluoro-2-methylphenyl)boronic acid to afford crude 20p. MS (ESI) m/z: 375.1 [M−H]−. Subsequent debenzylation of 20p by catalytic hydrogenation gave 21p as a white solid (22.6 mg, 39% over two steps). MS (ESI) m/z: 286.8 [M + H]+. 1H NMR (400 MHz, DMSO-d6) δ 11.60 (s, 1H), 10.62 (s, 1H), 8.00 (dd, J = 8.1, 2.4 Hz, 1H), 7.38 (t, J = 7.0 Hz, 1H), 7.26–7.14 (m, 2H), 7.10 (d, J = 12.7 Hz, 2H), 2.19 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 160.20 (d, J = 242.5 Hz), 159.03, 148.65, 146.07, 141.31 (d, J = 7.7 Hz), 138.15, 132.21 (d, J = 7.9 Hz), 130.66, 127.04, 123.28, 115.51 (d, J = 21.7 Hz), 115.24, 114.68 (d, J = 20.3 Hz), 113.03, 19.05.
3-Hydroxy-7-(3,4,5-trifluorophenyl)quinazoline-2,4(1H,3H)-dione (21q). According to the above general procedure, 14b was first subjected to the Suzuki coupling reaction with (3,4,5-trifluorophenyl)boronic acid to afford crude 20q. MS (ESI) m/z: 397.1 [M−H]−. Subsequent debenzylation of 20q by catalytic hydrogenation gave 21q as a white solid (30.5 mg, 37% over two steps). MS (ESI) m/z: 307.0 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 11.64 (s, 1H), 10.64 (s, 1H), 8.06–7.97 (m, 1H), 7.68 (td, J = 6.9, 2.8 Hz, 2H), 7.54 (dd, J = 8.4, 1.9 Hz, 1H), 7.36 (d, J = 1.9 Hz, 1H). 13C NMR (151 MHz, DMSO-d6) δ 158.89, 150.47 (d, J = 246.6 Hz), 148.61, 142.81, 138.90 (d, J = 251.3 Hz), 138.58, 135.37, 127.76, 121.25, 113.77, 113.31, 111.86 (d, J = 4.6 Hz), 111.75 (d, J = 4.4 Hz).
3-Hydroxy-7-(pyridin-3-yl)quinazoline-2,4(1H,3H)-dione (21r). According to the above general procedure, 14b was first subjected to the Suzuki coupling reaction with pyridin-3-ylboronic acid to afford crude 20r. MS (ESI) m/z: 344.1 [M−H]−. To 20r (90 mg, 0.261 mmol, 1.0 equiv.) was added TFA (3 mL) and the suspension was refluxed at 80 °C for 12 h. The reaction mixture was evaporated to dryness and diethyl ether (5 mL) was added. The suspension was well stirred for 15 min and then filtrated. The filtrate was discarded and the residue was washed with diethyl ether (20 mL), dried, and recrystallized with methanol to give the trifluoroacetate 21r as a white solid (20 mg, 10% over two steps). MS (ESI) m/z: 254.1 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 11.70 (s, 1H), 8.98 (d, J = 7.9 Hz, 1H), 8.74 (d, J = 6.0 Hz, 1H), 8.28 (d, J = 9.2 Hz, 1H), 8.07 (d, J = 8.6 Hz, 1H), 7.70 (d, J = 6.7 Hz, 1H), 7.60 (d, J = 8.6 Hz, 1H), 7.45 (d, J = 7.9 Hz, 1H). 13C NMR (151 MHz, DMSO-d6) δ 163.70, 153.37, 152.43, 150.72 (d, J = 33.6 Hz), 146.95, 143.48, 141.35, 139.63, 132.70, 129.49, 126.06, 118.55, 118.06 (d, J = 4.7 Hz).
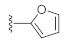
7-(Furan-2-yl)-3-hydroxyquinazoline-2,4(1H,3H)-dione (21s). According to the above general procedure, 14b was first subjected to the Suzuki coupling reaction with furan-2-ylboronic acid to afford crude 20s. MS (ESI) m/z: 333.1 [M−H]−. To a suspension of 20s (100 mg, 0.299 mmol, 1.0 equiv.) in anhydrous DCM (5 mL) was added a solution of TiCl4 (75 μL) in anhydrous DCM (750 μL) dropwise at 0 °C, and the mixture was stirred at 0 °C for 1 h. 1 mol/L potassium sodium tartrate aqueous solution (3 mL) was subsequently added, and the mixture was stirred for 30 min at room temperature. After completion of the reaction, DCM was evaporated, and water (10 mL) was added. The resulting suspension was extracted with ethyl acetate (4 × 10 mL). The combined organic layer was dried by Na2SO4, filtrated, concentrated, and recrystallized in petroleum ether/ethyl acetate to obtain 21s as a pale yellow solid (9.7 mg, 10% over two steps). MS (ESI) m/z: 243.1 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 11.60 (s, 1H), 10.57 (s, 1H), 7.95 (d, J = 8.0 Hz, 1H), 7.88 (s, 1H), 7.57 (d, J = 8.2 Hz, 1H), 7.46 (s, 1H), 7.16 (d, J = 3.8 Hz, 1H), 6.68 (d, J = 3.8 Hz, 1H). 13C NMR (151 MHz, DMSO-d6) δ 158.98, 151.32, 148.73, 144.57, 138.92, 135.39, 127.79, 117.95, 112.62, 112.51, 109.12, 108.63.
7-(Benzofuran-2-yl)-3-hydroxyquinazoline-2,4(1H,3H)-dione (21t). According to the above general procedure, 14b was first subjected to the Suzuki coupling reaction with benzofuran-2-ylboronic acid to afford crude 20t. MS (ESI) m/z: 383.1 [M−H]−. Subsequent debenzylation of 20t by catalytic hydrogenation gave 21t as a white solid (21 mg, 21% over two steps). MS (ESI) m/z: 293.1 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 11.67 (s, 1H), 10.64 (s, 1H), 8.03 (d, J = 8.4 Hz, 1H), 7.79 (d, J = 8.1 Hz, 1H), 7.73 (d, J = 7.3 Hz, 1H), 7.69 (t, J = 3.6 Hz, 2H), 7.66–7.62 (m, 1H), 7.40 (t, J = 7.3 Hz, 1H), 7.32 (t, J = 7.3 Hz, 1H). 13C NMR (151 MHz, DMSO-d6) δ 158.93, 154.48, 153.28, 148.71, 138.91, 134.94, 128.34, 127.87, 125.62, 123.51, 121.72, 118.96, 113.72, 111.25, 110.17, 105.10.
3-Hydroxy-8-phenylquinazoline-2,4(1H,3H)-dione (21u). According to the above general procedure, 14c was first subjected to the Suzuki coupling reaction with phenylboronic acid to afford crude 20u. MS (ESI) m/z: 342.8 [M−H]−. Subsequent debenzylation of 20u by catalytic hydrogenation gave 21u as a white solid (17.6 mg, 23% over two steps). MS (ESI) m/z: 253.0 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 10.64 (s, 1H), 10.16 (s, 1H), 8.01 (dd, J = 7.4, 2.2 Hz, 1H), 7.57–7.47 (m, 3H), 7.47–7.38 (m, 3H), 7.31 (t, J = 7.8 Hz, 1H). 13C NMR (151 MHz, DMSO-d6) δ 159.08, 148.36, 136.10, 135.72, 135.28, 129.07 (2C), 128.89, 128.81 (2C), 127.93, 126.41, 122.59, 114.93.
8-(4-Fluorophenyl)-3-hydroxyquinazoline-2,4(1H,3H)-dione (21v). According to the above general procedure, 14c was first subjected to the Suzuki coupling reaction with (4-fluorophenyl)boronic acid to afford crude 20v. MS (ESI) m/z: 361.1 [M−H]−. Subsequent debenzylation of 20v by catalytic hydrogenation gave 21v as a white solid (18 mg, 32% over two steps). MS (ESI) m/z: 270.9 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 10.62 (d, J = 8.1 Hz, 1H), 10.34 (d, J = 6.9 Hz, 1H), 8.00 (d, J = 8.1 Hz, 1H), 7.55–7.48 (m, 1H), 7.45 (ddd, J = 8.4, 5.7, 2.3 Hz, 2H), 7.36–7.21 (m, 3H). 13C NMR (151 MHz, DMSO-d6) δ 162.06 (d, J = 244.0 Hz), 159.04, 148.45, 135.73, 135.57, 132.52, 131.36, 131.31, 128.01, 126.52, 122.48, 115.69, 115.55, 114.92.
3-Hydroxy-8-(4-methoxyphenyl)quinazoline-2,4(1H,3H)-dione (21w). According to the above general procedure, 14c was first subjected to the Suzuki coupling reaction with (4-methoxyphenyl)boronic acid to afford crude 20w. MS (ESI) m/z: 373.1 [M−H]−. Subsequent debenzylation of 20w by catalytic hydrogenation gave 21w as a white solid (45.1 mg, 61% over two steps). MS (ESI) m/z: 282.9 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 10.63 (s, 1H), 10.07 (s, 1H), 8.07–7.92 (m, 1H), 7.57–7.47 (m, 1H), 7.35 (d, J = 8.8 Hz, 2H), 7.29 (t, J = 7.8 Hz, 1H), 7.13–7.03 (m, 2H), 3.82 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 159.12, 159.06, 148.33, 135.60, 135.41, 130.30 (2C), 128.70, 128.23, 126.00, 122.55, 114.84, 114.30 (2C), 55.03.
3-Hydroxy-8-(4-(trifluoromethyl)phenyl)quinazoline-2,4(1H,3H)-dione (21x). According to the above general procedure, 14c was first subjected to the Suzuki coupling reaction with (4-(trifluoromethyl)phenyl)boronic acid to afford crude 20x. MS (ESI) m/z: 411.1 [M−H]−. Subsequent debenzylation of 20x by catalytic hydrogenation gave 21x as a white solid (28.5 mg, 20% over two steps). MS (ESI) m/z: 320.8 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 10.65 (s, 1H), 10.58 (s, 1H), 8.13–7.98 (m, 1H), 7.84 (d, J = 7.9 Hz, 2H), 7.64 (d, J = 8.1 Hz, 2H), 7.60–7.51 (m, 1H), 7.34 (dd, J = 9.4, 6.4 Hz, 1H). 13C NMR (151 MHz, DMSO-d6) δ 158.97, 148.53, 140.51, 135.67, 135.50, 130.16, 128.33 (d, J = 31.5 Hz), 127.57, 127.10, 125.59 (d, J = 4.1 Hz), 124.26 (d, J = 272.0 Hz), 122.54, 115.07.
The 1H NMR, 13C NMR, and representative ESI-MS spectra of 21a–x are shown in Supplementary Materials (Figures S64–S151).
3.1.6. Synthesis of Compounds 23a–j
To compound 20a, 20d–f, 20i or 20s (0.436 mmol, 1.0 equiv.) was added K2CO3 (0.872 mmol, 2.0 equiv.), the corresponding halide (0.872 mmol, 2.0 equiv.), and DMF (4 mL). The suspension was heated at 80 °C for 2 h or stirred at room temperature for 2 h when the halide was iodomethane. After cooling, the reaction was quenched with water (10 mL), and the precipitate was collected by filtration, washed with water, and dried to give the crude product 22a–j. Debenzylation reactions of 22a–j were subsequently conducted using three different conditions to obtain 23a–j, respectively. For 22a, the same method of debenzylation using TFA as described in the preparation of 21r was performed to give 23a. For 22b or 22c, the same procedure of debenzylation in the mixture of hydrobromic acid/glacial acetic acid as described in the preparation of 10b–e was conducted to afford 23b or 23c, respectively. For 22d–j, the method of catalytic hydrogenation described in the preparation of 10f–q was used to give 23d–j, respectively.
3-Hydroxy-1-methyl-6-(3-nitrophenyl)quinazoline-2,4(1H,3H)-dione (23a). According to the above general procedure, 20d was subjected to the alkylation reaction with iodomethane to afford crude 22a. MS (ESI) m/z: 404.1 [M + H]+. Subsequent debenzylation of 22a in TFA gave 23a as a grey solid (53.4 mg, 56% over two steps). MS (ESI) m/z: 312.1 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 10.80 (s, 1H), 8.61 (s, 1H), 8.31 (d, J = 8.2 Hz, 2H), 8.16 (d, J = 8.1 Hz, 1H), 7.90–7.80 (m, 1H), 7.76 (s, 1H), 7.71 (d, J = 8.0 Hz, 1H), 3.66 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 158.06, 149.24, 148.30, 143.98, 140.26, 139.81, 133.95, 130.45, 128.24, 123.21, 121.87, 121.60, 114.47, 113.30, 30.80.
3-Hydroxy-1-methyl-7-phenylquinazoline-2,4(1H,3H)-dione (23b). According to the above general procedure, 20e was subjected to the alkylation reaction with iodomethane to afford crude 22b. MS (ESI) m/z: 359.1 [M + H]+. Subsequent debenzylation of 22b in the mixture of hydrobromic acid/glacial acetic acid gave 23b as a grey solid (209 mg, 73% over two steps). MS (ESI) m/z: 267.1 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 10.74 (s, 1H), 8.12 (d, J = 8.1 Hz, 1H), 7.83 (d, J = 7.4 Hz, 2H), 7.61 (d, J = 7.6 Hz, 2H), 7.54 (t, J = 7.4 Hz, 2H), 7.47 (t, J = 7.2 Hz, 1H), 3.64 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 158.19, 149.30, 146.46, 139.73, 138.65, 128.93 (2C), 128.63, 128.01, 127.27 (2C), 121.36, 113.65, 112.61, 30.65.
7-(4-Fluorophenyl)-3-hydroxy-1-methylquinazoline-2,4(1H,3H)-dione (23c). According to the above general procedure, 20f was subjected to the alkylation reaction with iodomethane to afford crude 22c. MS (ESI) m/z: 377.1 [M + H]+. Subsequent debenzylation of 22c in the mixture of hydrobromic acid/glacial acetic acid gave 23c as a white solid (30.8 mg, 45% over two steps). MS (ESI) m/z: 285.2 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 10.75 (s, 1H), 8.11 (d, J = 8.0 Hz, 1H), 7.98–7.85 (m, 2H), 7.60 (d, J = 9.4 Hz, 2H), 7.37 (t, J = 8.4 Hz, 2H), 3.64 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 163.36, 161.73, 158.21, 149.35, 145.39, 139.79, 135.15, 129.56, 129.50, 128.09, 121.34, 115.91, 115.76, 113.68, 112.64, 30.73.
3-Hydroxy-7-(4-methoxyphenyl)-1-methylquinazoline-2,4(1H,3H)-dione (23d). According to the above general procedure, 20i was subjected to the alkylation reaction with iodomethane to afford crude 22d. MS (ESI) m/z: 389.2 [M + H]+. Subsequent debenzylation of 22d by catalytic hydrogenation gave 23d as a white solid (20 mg, 19% over two steps). MS (ESI) m/z: 297.2 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 10.72 (s, 1H), 8.08 (s, 1H), 7.81 (s, 2H), 7.58 (s, 2H), 7.09 (s, 2H), 3.83 (s, 3H), 3.64 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 159.86, 158.27, 149.39, 146.15, 139.80, 130.86, 128.58 (2C), 128.00, 120.89, 114.40 (2C), 113.06, 111.80, 55.21, 39.94, 39.81, 39.68, 39.54, 39.40, 39.26, 39.12, 38.98, 30.68.
1-Ethyl-3-hydroxy-7-phenylquinazoline-2,4(1H,3H)-dione (23e). According to the above general procedure, 20e was subjected to the alkylation reaction with bromoethane to afford crude 22e. MS (ESI) m/z: 373.2 [M + H]+. Subsequent debenzylation of 22e by catalytic hydrogenation gave 23e as a white solid (6.8 mg, 8% over two steps). MS (ESI) m/z: 281.2 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 10.76 (s, 1H), 8.14 (d, J = 8.7 Hz, 1H), 7.82 (d, J = 7.2 Hz, 2H), 7.56 (td, J = 30.5, 26.6, 9.5 Hz, 5H), 4.40–4.18 (m, 2H), 1.28 (dt, J = 11.9, 5.8 Hz, 3H). 13C NMR (151 MHz, DMSO-d6) δ 158.11, 148.86, 146.71, 138.68, 138.62, 128.93 (2C), 128.62, 128.40, 127.36 (2C), 121.43, 113.92, 112.13, 38.24, 12.40.
3-Hydroxy-6-phenyl-1-propylquinazoline-2,4(1H,3H)-dione (23f). According to the above general procedure, 20a was subjected to the alkylation reaction with 1-bromopropane to afford crude 22f. MS (ESI) m/z: 387.0 [M + H]+. Subsequent debenzylation of 22f by catalytic hydrogenation gave 23f as a white solid (22.3 mg, 41% over two steps). MS (ESI) m/z: 295.1 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 10.80 (s, 1H), 8.28 (s, 1H), 8.08 (d, J = 8.0 Hz, 1H), 7.73 (d, J = 8.1 Hz, 2H), 7.62 (d, J = 7.2 Hz, 1H), 7.51 (t, J = 7.3 Hz, 2H), 7.41 (d, J = 7.1 Hz, 1H), 4.12 (t, J = 7.5 Hz, 2H), 1.69 (q, J = 7.6 Hz, 2H), 0.97 (t, J = 6.8 Hz, 3H). 13C NMR (151 MHz, DMSO-d6) δ 158.26, 148.91, 138.08, 137.74, 134.42, 133.08, 128.99 (2C), 127.59, 126.30 (2C), 124.87, 115.50, 115.36, 44.60, 20.14, 10.70.
3-Hydroxy-7-phenyl-1-propylquinazoline-2,4(1H,3H)-dione (23g). According to the above general procedure, 20e was subjected to the alkylation reaction with 1-bromopropane to afford crude 22g. MS (ESI) m/z: 387.2 [M + H]+. Subsequent debenzylation of 22g by catalytic hydrogenation gave 23g as a white solid (25 mg, 25% over two steps). MS (ESI) m/z: 295.1 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 10.76 (s, 1H), 8.19–8.10 (m, 1H), 7.80 (d, J = 4.7 Hz, 2H), 7.66–7.57 (m, 2H), 7.57–7.51 (m, 2H), 7.52–7.43 (m, 1H), 4.23 (t, J = 8.0 Hz, 2H), 1.78–1.61 (m, 2H), 0.96 (t, J = 6.3 Hz, 3H). 13C NMR (151 MHz, DMSO-d6) δ 158.08, 149.18, 146.65, 138.90, 138.71, 128.96 (2C), 128.60, 128.35, 127.33 (2C), 121.48, 113.85, 112.35, 44.32, 20.16, 10.82.
1-Benzyl-3-hydroxy-6-phenylquinazoline-2,4(1H,3H)-dione (23h). According to the above general procedure, 20a was subjected to the alkylation reaction with benzyl bromide to afford crude 22h. MS (ESI) m/z: 435.0 [M + H]+. Subsequent debenzylation of 22h by catalytic hydrogenation gave 23h as a white solid (20 mg, 33% over two steps). MS (ESI) m/z: 343.1 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 10.92 (s, 1H), 8.31 (d, J = 8.6 Hz, 1H), 7.99 (d, J = 9.1 Hz, 1H), 7.70 (d, J = 8.0 Hz, 2H), 7.48 (t, J = 7.6 Hz, 2H), 7.45–7.22 (m, 7H), 5.44 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 158.41, 149.61, 138.05, 137.87, 136.00, 134.78, 133.09, 129.04 (2C), 128.66 (2C), 127.69, 127.25, 126.38 (2C), 126.32 (2C), 124.93, 115.90, 115.62, 46.40.
1-Benzyl-3-hydroxy-7-phenylquinazoline-2,4(1H,3H)-dione (23i). According to the above general procedure, 20e was subjected to the alkylation reaction with benzyl bromide to afford crude 22i. MS (ESI) m/z: 435.1 [M + H]+. Subsequent debenzylation of 22i by catalytic hydrogenation gave 23i as a white solid (26 mg, 29% over two steps). MS (ESI) m/z: 345.2 [M + H]+. 1H NMR (400 MHz, DMSO-d6) δ 10.91 (d, J = 20.9 Hz, 1H), 8.24–8.10 (m, 1H), 7.74–7.20 (m, 12H), 5.57 (d, J = 21.3 Hz, 2H). 13C NMR (151 MHz, DMSO-d6) δ 158.23, 149.87, 146.26, 138.95, 138.49, 136.27, 129.03 (2C), 128.72 (2C), 128.67, 128.39, 127.25, 127.07 (2C), 126.42 (2C), 121.61, 114.09, 112.96, 46.18.
N-(2-Chlorophenyl)-2-(7-(furan-2-yl)-3-hydroxy-2,4-dioxo-3,4-dihydroquinazolin-1(2H)-yl)acetamide (23j). According to the above general procedure, 20s was subjected to the alkylation reaction with 2-chloro-N-(2-chlorophenyl)acetamide to afford crude 22j. MS (ESI) m/z: 502.1 [M + H]+. Subsequent debenzylation of 22j by catalytic hydrogenation gave 23j as a white solid (26 mg, 13% over two steps). MS (ESI) m/z: 410.0 [M−H]−. 1H NMR (400 MHz, DMSO-d6) δ 10.89 (s, 1H), 10.10 (s, 1H), 8.11 (d, J = 9.0 Hz, 1H), 7.88 (s, 1H), 7.67 (d, J = 8.2 Hz, 1H), 7.65–7.56 (m, 2H), 7.51 (d, J = 7.7 Hz, 1H), 7.32 (t, J = 6.5 Hz, 1H), 7.27 (s, 1H), 7.24 (d, J = 10.8 Hz, 1H), 6.70 (s, 1H), 5.17 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 165.98, 158.16, 151.37, 149.59, 139.66, 135.80, 134.22, 129.52, 128.45, 127.47, 127.22, 126.89, 126.46, 118.21, 113.40, 112.63, 112.54, 109.56, 108.37, 46.22.
The 1H NMR, 13C NMR, and representative ESI-MS spectra of 23a–j are shown in Supplementary Materials (Figures S152–S185).
3.2. Anti-HCV Assay
HCV 1b replicon cells (Huh-7.5.1) containing luciferase reporter gene were kindly provided by Prof. Zhenghong Yuan (Key Laboratory of Medical Molecular Virology, Fudan University, Shanghai, China). The replicon cells were plated at 1.5 × 104 cells per well in a 48-well plate (Corning Inc., New York, NY, USA) at 5% CO2/37 °C. The culture medium was Dulbecco’s Modified Eagle Medium (DMEM, pH 7.3, Shanghai XP Biomed Ltd., Shanghai, China) containing 10% fetal bovine serum (Shanghai XP Biomed Ltd., Shanghai, China), 105 U/L penicillin, 0.1 g/L streptomycin, and 0.5 μg/mL blasticidin. After 12 h, the culture medium in each well was replaced with 300 μL of pre-prepared fresh culture medium containing test compound (dissolved in DMSO with specific concentration) or blank DMSO. The final concentration of DMSO in the culture medium in the plate was 0.1%. Each group was tested at least twice in parallel. Ribavirin and 2CMA were used as the positive controls. After incubating the drug-added plate at 5% CO2/37 °C for 72 h, the medium was removed, and each well was washed with 300 μL of phosphate buffer solution (PBS, Shanghai XP Biomed Ltd., Shanghai, China). The cells in each well were then lysed with 50 μL of 1× lysis buffer (E2820, Promega, Madison, WI, USA). Subsequently, 50 μL of 1× luciferase assay substrate (E2820, Promega, Madison, WI, USA) was mixed with 20 μL of the harvested cell lysates. The sample was immediately placed in an ultra-weak luminescence analyzer (BPCL-GP21Q, Guangzhou Weiguang Technology Co., Ltd., Guangzhou, China), and the sum of emitted photons in the first 10 s was measured, which was represented by L. The inhibitory rate of a compound in a given concentration on HCV replicon cells was calculated using the following equation, in which Lcompound or Lblank represents the measured signal of the compound group or the blank control (DMSO), respectively:
inhibitory rate = (1−Lcompound/Lblank) × 100%
Compounds that showed good inhibitory rates at 10 μM or 25 μM were subjected to further anti-HCV EC50 (50% effective concentration) determination. The inhibitory rates under at least six different concentration gradients were imported into GraphPad Prism 7.0 software to calculate the anti-HCV EC50 values of the compounds using the nonlinear fitting method.
3.3. Cytotoxicity Assay
The HCV 1b replicon cells were incubated and treated with test compounds or blank DMSO in a 48-well plate using the same materials and methods as described in the experimental procedure of anti-HCV assay. The final concentration of DMSO in each well was 0.1%. After 72 h of drug treatment, the culture medium in the well plate was removed, and cells in each well were washed with 300 μL of PBS. A total of 300 μL of pre-prepared fresh culture medium containing 30 μL CCK-8 reagent (Donjindo, Kumamoto, Japan) was added to each well, and the plate was incubated at 5% CO2/37 °C for 2 h. The OD (optical density) value of each well at 450 nm was measured using a multi-function microplate reader (SpectraMax M5, Molecular Devices, San Jose, CA, USA). The death ratio of each well was calculated using the following equation, in which ODcompound or ODblank represents the measured OD value of the compound group or the blank control (DMSO), respectively:
death ratio = (1−ODcompound/ODblank) × 100%
The cell death data under at least six different concentration gradients were imported into GraphPad Prism 7.0.0 software (GraphPad Software Inc., San Diego, CA, USA), and the CC50 (50% cytotoxic concentration) values were calculated using the nonlinear fitting method.
3.4. Thermal Shift Assay
C-terminal His-tagged NS5BΔ21 from HCV 1b subtype was expressed from pET28a HCR6 plasmid in our lab and dissolved in a Tris-HCl buffer (20 mm, pH 7.2) containing 150 mm NaCl. Then, 1 μL of corresponding concentration (1 mm, 2 mm, or 4 mm) of the test compound in DMSO or 1 μL DMSO alone was added to each well of a 96-well PCR plate (Shanghai Sangon Biotech Co., Ltd., Shanghai, China). Subsequently, 19 μL of pre-prepared PBS solution containing 10 mm MgCl2, 10× SYPRO® orange protein gel stain (Sigma-Aldrich, St. Louis, MO, USA), and 5 μM NS5B protein was added to each well and mixed. The final concentration of DMSO in each well was 5%. The PCR plate was covered with a high-permeable film, incubated at room temperature for 10 min, and then placed on the Applied BiosystemsTM 7500 Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA, USA). The temperature was increased to 90 °C gradually, and the change curve of fluorescence intensity versus system temperature was recorded.
3.5. UV-Vis spectrophotometry Assay
2 mL of methanol solution containing the components in the sample or control group (Table 4) was added to a quartz cuvette (BQ-114-2, Chongqing Xinweier Glass Co., Ltd., Chongqing, China). The absorbance at 200–400 nm was scanned by a UV-Vis spectrophotometer (Agilent Cary 60, Agilent Technologies, Santa Clara, CA, USA), and the blank methanol was used for baseline calibration. The absorbance of the control group was subtracted from that of the sample group at the corresponding wavelength to obtain the differential UV-Vis spectra, which were used to analyze the chelating property of the compound with Mg2+.

Table 4.
Sample and control groups with different components in methanol.
3.6. Molecular Docking
Molecular docking simulations were performed using the Schrödinger software package [54]. The protein structure was obtained from the co-crystal structure of NS5B 1b subtype in complex with UTP (PDB code: 1GX6) [9]. Except for the water molecules interacting with the metal ions in the catalytic center, all other water molecules in the co-crystal structure were deleted. The protein structure was automatically hydrogenated and optimized by limiting the heavy atoms within the range of RMSD = 0.3 Å using the OPLS3 force field. The structure of the ligand was built in the 3D Builder module with an ionization state generated by Epik and optimized under an OPLS3 force field in the LigPrep program. Finally, the ligand was docked into the catalytic center of NS5B protein using the Glide module at standard precision (SP). Among the five docking models in the output, the conformation of the ligand–protein complex with the best Glide score (the lowest value) was selected for subsequent binding mode analysis.
4. Conclusions
In this study, we designed and synthesized a series of 3-hydroxyquinazoline-2,4(1H,3H)-dione derivatives with metal ion chelating structures. The anti-HCV assay using the HCV replicon model showed that most compounds possessed potent HCV inhibitory activities at the cellular level. Some compounds, represented by 21h, 21k, and 21t, exhibited superior activities and therapeutic indexes to ribavirin. Among them, 21t is currently known as one of the metal ion chelators with the best anti-HCV activity (EC50 = 2.0 μM) at the cellular level, and no obvious cytotoxic effect at the tested concentrations (TI > 25) was observed. Thermal shift assay and molecular docking studies suggested that 3-hydroxyquinazoline-2,4(1H,3H)-diones may exert an anti-HCV effect by binding to NS5B. The UV-Vis spectrophotometry assay demonstrated that compound 21e with the 3-OH-quinazolinedione core could chelate Mg2+ in the N-hydroxyl deprotonated form, supporting the design idea of inhibiting HCV by targeting the metal ions in the catalytic center of NS5B. These findings verified the feasibility of developing novel anti-HCV inhibitors based on metal ion chelating structures and provided a structural reference for further research on metal ion chelators in the field of anti-HCV.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms23115930/s1: 1H NMR, 13C NMR, and representative ESI-MS spectra of the target compounds.
Author Contributions
Conceptualization, Y.C., D.Y. and L.Z.; methodology, Y.C., D.Y. and L.Z.; validation, Y.C.; formal analysis, Y.C. and A.A.; investigation, Y.C., A.A. and Z.Z.; writing—original draft preparation, Y.C.; writing—review and editing, Y.C., D.Y. and L.Z.; visualization, Y.C.; supervision, Y.C., D.Y. and L.Z.; project administration, Y.C., D.Y. and L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Chinese National Natural Science Foundation (Grant No. 81373330) and Specialized Research Fund for the Doctoral Program of Higher Education, Chinese Ministry of Education (Grant No. 20130071110068).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The research group of Zhenghong Yuan (Key Laboratory of Medical Molecular Virology, Fudan University, China) is gratefully acknowledged for providing the HCV 1b replicon cells.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
2CMA: 2′-C-methyladenosine; 3D: three dimension; ADP: adenosine diphosphate; CC50: 50% cytotoxic concentration; CCK-8: cell counting kit-8; CDI: 1,1′-carbonyldiimidazole; Comp.: compound; DAA: direct-acting antiviral; DCM: dichloromethane; DMEM: Dulbecco’s Modified Eagle Medium; DMF: N,N-dimethylformamide; DMSO: dimethyl sulfoxide; EC50: 50% effective concentration; equiv.: equivalent; ESI: electrospray ionization; FDA: U.S. Food and Drug Administration; HCV: hepatitis C virus; HIV: human immunodeficiency virus; IC50: 50% inhibitory concentration; MS: mass spectrometry; NMR: nuclear magnetic resonance; NS3/4A: nonstructural protein 3/4A; NS5A: nonstructural protein 5A; NS5B: non-structural protein 5B; NTP: nucleoside triphosphate; OD: optical density; PBS: phosphate buffered saline; PCR: polymerase chain reaction; PDB: Protein Data Bank; Pos.: position; PPi: pyrophosphate; r.t.: room temperature; RBV: ribavirin; RdRp: RNA-dependent RNA polymerase; RMSD: root-mean-square deviation; RNA: ribonucleic acid; RNase H: ribonuclease H; rNTP: ribonucleoside triphosphate; SAR: structure-activity relationship; SP: standard precision; TFA: trifluoroacetate; THF: tetrahydrofuran; TI: therapeutic index; Tm: melting temperature; TMS: tetramethylsilane; Tris-HCl: tris(hydroxymethyl)aminomethane hydrochloride; TSA: thermal shift assay; UTP: uridine triphosphate; UV-Vis: ultraviolet-visible; WHO: World Health Organization.
References
- Perz, J.F.; Armstrong, G.L.; Farrington, L.A.; Hutin, Y.J.; Bell, B.P. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J. Hepatol. 2006, 45, 529–538. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Recommendations and guidance on hepatitis C virus self-testing. In Recommendations and Guidance on Hepatitis C Virus Self-Testing; World Health Organization: Geneva, Switzerland, 2021; p. 32. [Google Scholar]
- Smith, D.; Kuiken, C.; Muerhoff, A.; Rice, C.; Stapleton, J.; Simmonds, P. A Web Resource To Manage the Classification and Genotype and Subtype Assignments of Hepatitis C Virus. Available online: https://talk.ictvonline.org/ictv_wikis/flaviviridae/w/sg_flavi/56/hcv-classification (accessed on 5 May 2022).
- Du, G.; Li, X.; Musa, T.H.; Ji, Y.; Wu, B.; He, Y.; Ni, Q.; Su, L.; Li, W.; Ge, Y. The nationwide distribution and trends of hepatitis C virus genotypes in mainland China. J. Med. Virol. 2019, 91, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Hedskog, C.; Parhy, B.; Chang, S.; Zeuzem, S.; Moreno, C.; Shafran, S.D.; Borgia, S.M.; Asselah, T.; Alric, L.; Abergel, A.; et al. Identification of 19 novel hepatitis C virus subtypes—further expanding HCV classification. Open Forum Infect. Dis. 2019, 6, ofz076. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.I.; Chiang, B.-L. A new insight into hepatitis C vaccine development. J. Biomed. Biotechnol. 2010, 2010, 548280. [Google Scholar] [CrossRef] [PubMed]
- Moradpour, D.; Penin, F.; Rice, C.M. Replication of hepatitis C virus. Nat. Rev. Microbiol. 2007, 5, 453–463. [Google Scholar] [CrossRef]
- Appleby, T.C.; Perry, J.K.; Murakami, E.; Barauskas, O.; Feng, J.; Cho, A.; Fox, D., III; Wetmore, D.R.; McGrath, M.E.; Ray, A.S. Structural basis for RNA replication by the hepatitis C virus polymerase. Science 2015, 347, 771–775. [Google Scholar] [CrossRef]
- Bressanelli, S.; Tomei, L.; Rey, F.A.; De Francesco, R. Structural analysis of the hepatitis C virus RNA polymerase in complex with ribonucleotides. J. Virol. 2002, 76, 3482–3492. [Google Scholar] [CrossRef] [Green Version]
- Khalid, H.; Landry, K.B.; Ijaz, B.; Ashfaq, U.A.; Ahmed, M.; Kanwal, A.; Froeyen, M.; Mirza, M.U. Discovery of novel Hepatitis C virus inhibitor targeting multiple allosteric sites of NS5B polymerase. Infect. Genet. Evol. 2020, 84, 104371. [Google Scholar] [CrossRef]
- Dhanak, D.; Duffy, K.J.; Johnston, V.K.; Lin-Goerke, J.; Darcy, M.; Shaw, A.N.; Gu, B.; Silverman, C.; Gates, A.T.; Nonnemacher, M.R. Identification and biological characterization of heterocyclic inhibitors of the hepatitis C virus RNA-dependent RNA polymerase. J. Biol. Chem. 2002, 277, 38322–38327. [Google Scholar] [CrossRef] [Green Version]
- Howe, A.Y.; Cheng, H.; Johann, S.; Mullen, S.; Chunduru, S.K.; Young, D.C.; Bard, J.; Chopra, R.; Krishnamurthy, G.; Mansour, T. Molecular mechanism of hepatitis C virus replicon variants with reduced susceptibility to a benzofuran inhibitor, HCV-796. Antimicrob. Agents Chemother. 2008, 52, 3327–3338. [Google Scholar] [CrossRef] [Green Version]
- Kirkovsky, L.; Zhou, Y.; Norris, D.; Okamoto, E.; Nolan, T.G.; Bartkowski, D.; Khandurina, J.; Sergeeva, M.; Murphy, D.; Ayida, B. ANA598, a novel non-nucleoside inhibitor of HCVNS5B polymerase, exhibits favorable pharmacokinetic properties in multiple preclinical species. Hepatology. 2007, 46, 858A. [Google Scholar]
- Hang, J.Q.; Yang, Y.; Harris, S.F.; Leveque, V.; Whittington, H.J.; Rajyaguru, S.; Ao-Ieong, G.; McCown, M.F.; Wong, A.; Giannetti, A.M. Slow binding inhibition and mechanism of resistance of non-nucleoside polymerase inhibitors of hepatitis C virus. J. Biol. Chem. 2009, 284, 15517–15529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandra, P.; Raible, D.; Harper, D.; Speth, J.; Villano, S.; Bichier, G. Antiviral activity of the non-nucleoside polymerase inhibitor, HCV-796, in patients with chronic hepatitis C virus: Preliminary results from a randomized, double-blind, placebo-controlled, ascending multiple dose study. Gastroenterology 2006, 130, A748. [Google Scholar]
- Liu, M.; Tuttle, M.; Gao, M.; Lemm, J.A. Potency and resistance analysis of hepatitis C virus NS5B polymerase inhibitor BMS-791325 on all major genotypes. Antimicrob. Agents Chemother. 2014, 58, 7416–7423. [Google Scholar] [CrossRef] [Green Version]
- Sorbo, M.C.; Cento, V.; Di Maio, V.C.; Howe, A.Y.; Garcia, F.; Perno, C.F.; Ceccherini-Silberstein, F. Hepatitis C virus drug resistance associated substitutions and their clinical relevance: Update 2018. Drug Resist. Updates 2018, 37, 17–39. [Google Scholar] [CrossRef]
- Joyce, C.M.; Steitz, T.A. Polymerase structures and function: Variations on a theme? J. Bacteriol. 1995, 177, 6321–6329. [Google Scholar] [CrossRef] [Green Version]
- Le Pogam, S.; Seshaadri, A.; Kosaka, A.; Chiu, S.; Kang, H.; Hu, S.; Rajyaguru, S.; Symons, J.; Cammack, N.; Najera, I. Existence of hepatitis C virus NS5B variants naturally resistant to non-nucleoside, but not to nucleoside, polymerase inhibitors among untreated patients. J. Antimicrob. Chemother. 2008, 61, 1205–1216. [Google Scholar] [CrossRef]
- Sofia, M.J. Nucleotide prodrugs for the treatment of HCV infection. Adv. Pharmacol. 2013, 67, 39–73. [Google Scholar]
- Sofia, M.J.; Chang, W.; Furman, P.A.; Mosley, R.T.; Ross, B.S. Nucleoside, nucleotide, and non-nucleoside inhibitors of hepatitis C virus NS5B RNA-dependent RNA-polymerase. J. Med. Chem. 2012, 55, 2481–2531. [Google Scholar] [CrossRef]
- Cho, A. Evolution of HCV NS5B Nucleoside and Nucleotide Inhibitors. In HCV: The Journey from Discovery to a Cure; Springer: Berlin/Heidelberg, Germany, 2019; pp. 117–139. [Google Scholar]
- Feng, J.Y. Addressing the selectivity and toxicity of antiviral nucleosides. Antiviral Chem. Chemother. 2018, 26, 2040206618758524. [Google Scholar] [CrossRef]
- Graci, J.D.; Cameron, C.E. Mechanisms of action of ribavirin against distinct viruses. Rev. Med. Virol. 2006, 16, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, H.K.; Singh, H.; Grewal, N.; Natt, N.K. Sofosbuvir: A novel treatment option for chronic hepatitis C infection. J. Pharmacol. Pharmacother. 2014, 5, 278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soria, M.E.; Gregori, J.; Chen, Q.; García-Cehic, D.; Llorens, M.; de Ávila, A.I.; Beach, N.M.; Domingo, E.; Rodríguez-Frías, F.; Buti, M. Pipeline for specific subtype amplification and drug resistance detection in hepatitis C virus. BMC Infect. Dis. 2018, 18, 446. [Google Scholar] [CrossRef] [PubMed]
- Sarrazin, C. The importance of resistance to direct antiviral drugs in HCV infection in clinical practice. J. Hepatol. 2016, 64, 486–504. [Google Scholar] [CrossRef] [PubMed]
- Dietz, J.; Susser, S.; Vermehren, J.; Peiffer, K.-H.; Grammatikos, G.; Berger, A.; Ferenci, P.; Buti, M.; Müllhaupt, B.; Hunyady, B. Patterns of resistance-associated substitutions in patients with chronic HCV infection following treatment with direct-acting antivirals. Gastroenterology 2018, 154, 976–988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svarovskaia, E.S.; Gane, E.; Dvory-Sobol, H.; Martin, R.; Doehle, B.; Hedskog, C.; Jacobson, I.M.; Nelson, D.R.; Lawitz, E.; Brainard, D.M. L159F and V321A sofosbuvir-associated hepatitis C virus NS5B substitutions. J. Infect. Dis. 2016, 213, 1240–1247. [Google Scholar] [CrossRef] [Green Version]
- Hedskog, C.; Dvory-Sobol, H.; Gontcharova, V.; Martin, R.; Ouyang, W.; Han, B.; Gane, E.; Brainard, D.; Hyland, R.; Miller, M. Evolution of the HCV viral population from a patient with S282T detected at relapse after sofosbuvir monotherapy. J. Viral Hepat. 2015, 22, 871–881. [Google Scholar] [CrossRef]
- Raj, V.S.; Hundie, G.B.; Schürch, A.C.; Smits, S.L.; Pas, S.D.; Le Pogam, S.; Janssen, H.L.; de Knegt, R.J.; Osterhaus, A.D.; Najera, I. Identification of HCV resistant variants against direct acting antivirals in plasma and liver of treatment naïve patients. Sci. Rep. 2017, 7, 4688. [Google Scholar] [CrossRef]
- Rogolino, D.; Carcelli, M.; Sechi, M.; Neamati, N. Viral enzymes containing magnesium: Metal binding as a successful strategy in drug design. Coord. Chem. Rev. 2012, 256, 3063–3086. [Google Scholar] [CrossRef]
- Hutchinson, D. Metal chelators as potential antiviral agents. Antiviral Res. 1985, 5, 193–205. [Google Scholar] [CrossRef]
- Summa, V.; Petrocchi, A.; Pace, P.; Matassa, V.G.; De Francesco, R.; Altamura, S.; Tomei, L.; Koch, U.; Neuner, P. Discovery of α, γ-diketo acids as potent selective and reversible inhibitors of hepatitis C virus NS5b RNA-dependent RNA polymerase. J. Med. Chem. 2004, 47, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Pace, P.; Nizi, E.; Pacini, B.; Pesci, S.; Matassa, V.; De Francesco, R.; Altamura, S.; Summa, V. The monoethyl ester of meconic acid is an active site inhibitor of HCV NS5B RNA-dependent RNA polymerase. Bioorg. Med. Chem. Lett. 2004, 14, 3257–3261. [Google Scholar] [CrossRef] [PubMed]
- Koch, U.; Attenni, B.; Malancona, S.; Colarusso, S.; Conte, I.; Di Filippo, M.; Harper, S.; Pacini, B.; Giomini, C.; Thomas, S. 2-(2-Thienyl)-5, 6-dihydroxy-4-carboxypyrimidines as inhibitors of the hepatitis C virus NS5B polymerase: Discovery, SAR, modeling, and mutagenesis. J. Med. Chem. 2006, 49, 1693–1705. [Google Scholar] [CrossRef] [PubMed]
- Pacini, B.; Avolio, S.; Ercolani, C.; Koch, U.; Migliaccio, G.; Narjes, F.; Pacini, L.; Tomei, L.; Harper, S. 2-(3-Thienyl)-5, 6-dihydroxypyrimidine-4-carboxylic acids as inhibitors of HCV NS5B RdRp. Bioorg. Med. Chem. Lett. 2009, 19, 6245–6249. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Tang, J.; Kesler, M.J.; Sham, Y.Y.; Vince, R.; Geraghty, R.J.; Wang, Z. The design, synthesis and biological evaluations of C-6 or C-7 substituted 2-hydroxyisoquinoline-1, 3-diones as inhibitors of hepatitis C virus. Biorg. Med. Chem. 2012, 20, 467–479. [Google Scholar] [CrossRef]
- Yang, W.; Lee, J.Y.; Nowotny, M. Making and breaking nucleic acids: Two-Mg2+-ion catalysis and substrate specificity. Mol. Cell 2006, 22, 5–13. [Google Scholar] [CrossRef]
- Kirschberg, T.; Parrish, J. Metal chelators as antiviral agents. Curr. Opin. Drug discov. Dev. 2007, 10, 460–472. [Google Scholar]
- Choi, E.; Mallareddy, J.R.; Lu, D.; Kolluru, S. Recent advances in the discovery of small-molecule inhibitors of HIV-1 integrase. Future Sci. OA 2018, 4, FSO338. [Google Scholar] [CrossRef] [Green Version]
- Shaw-Reid, C.A.; Munshi, V.; Graham, P.; Wolfe, A.; Witmer, M.; Danzeisen, R.; Olsen, D.B.; Carroll, S.S.; Embrey, M.; Wai, J.S. Inhibition of HIV-1 ribonuclease H by a novel diketo acid, 4-[5-(benzoylamino) thien-2-yl]-2, 4-dioxobutanoic acid. J. Biol. Chem. 2003, 278, 2777–2780. [Google Scholar] [CrossRef] [Green Version]
- Didierjean, J.; Isel, C.; Querré, F.; Mouscadet, J.-F.; Aubertin, A.-M.; Valnot, J.-Y.; Piettre, S.R.; Marquet, R. Inhibition of human immunodeficiency virus type 1 reverse transcriptase, RNase H, and integrase activities by hydroxytropolones. Antimicrob. Agents Chemother. 2005, 49, 4884–4894. [Google Scholar] [CrossRef] [Green Version]
- Kirschberg, T.A.; Balakrishnan, M.; Squires, N.H.; Barnes, T.; Brendza, K.M.; Chen, X.; Eisenberg, E.J.; Jin, W.; Kutty, N.; Leavitt, S. RNase H active site inhibitors of human immunodeficiency virus type 1 reverse transcriptase: Design, biochemical activity, and structural information. J. Med. Chem. 2009, 52, 5781–5784. [Google Scholar] [CrossRef] [PubMed]
- Credille, C.V.; Chen, Y.; Cohen, S.M. Fragment-based identification of influenza endonuclease inhibitors. J. Med. Chem. 2016, 59, 6444–6454. [Google Scholar] [CrossRef] [PubMed]
- Credille, C.V.; Dick, B.L.; Morrison, C.N.; Stokes, R.W.; Adamek, R.N.; Wu, N.C.; Wilson, I.A.; Cohen, S.M. Structure–activity relationships in metal-binding pharmacophores for influenza endonuclease. J. Med. Chem. 2018, 61, 10206–10217. [Google Scholar] [CrossRef] [PubMed]
- O’Hanlon, R.; Shaw, M.L. Baloxavir marboxil: The new influenza drug on the market. Curr. Opin. Virol. 2019, 35, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Ha, C.H.X.; Lee, N.K.; Rahman, T.; Hwang, S.S.; Yam, W.K.; Chee, X.W. Repurposing FDA-approved drugs as HIV-1 integrase inhibitors: An in silico investigation. J. Biomol. Struct. Dyn. 2022, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bartenschlager, R. Hepatitis C virus replicons: Potential role for drug development. Nat. Rev. Drug Discov. 2002, 1, 911–916. [Google Scholar] [CrossRef]
- Carroll, S.S.; Tomassini, J.E.; Bosserman, M.; Getty, K.; Stahlhut, M.W.; Eldrup, A.B.; Bhat, B.; Hall, D.; Simcoe, A.L.; LaFemina, R. Inhibition of hepatitis C virus RNA replication by 2′-modified nucleoside analogs. J. Biol. Chem. 2003, 278, 11979–11984. [Google Scholar] [CrossRef] [Green Version]
- Huynh, K.; Partch, C.L. Analysis of protein stability and ligand interactions by thermal shift assay. Curr. Protoc. Protein Sci. 2015, 79, 28–29. [Google Scholar] [CrossRef]
- Andreotti, G.; Monticelli, M.; Cubellis, M.V. Looking for protein stabilizing drugs with thermal shift assay. Drug Test. Anal. 2015, 7, 831–834. [Google Scholar] [CrossRef]
- Pang, L.; Weeks, S.D.; Juhás, M.; Strelkov, S.V.; Zitko, J.; Van Aerschot, A. Towards Novel 3-Aminopyrazinamide-Based Prolyl-tRNA Synthetase Inhibitors: In Silico Modelling, Thermal Shift Assay and Structural Studies. Int. J. Mol. Sci. 2021, 22, 7793. [Google Scholar] [CrossRef]
- Schrodinger, L. Schrodinger Software Suite; Schrödinger LLC.: New York, NY, USA, 2011; p. 670. [Google Scholar]
- Gallivan, J.P.; Dougherty, D.A. Cation–π interactions in structural biology. Proc. Natl. Acad. Sci. USA 1999, 96, 9459–9464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Billamboz, M.; Bailly, F.; Lion, C.; Touati, N.; Vezin, H.; Calmels, C.; Andréola, M.-L.; Christ, F.; Debyser, Z.; Cotelle, P. Magnesium chelating 2-hydroxyisoquinoline-1, 3 (2 H, 4 H)-diones, as inhibitors of HIV-1 integrase and/or the HIV-1 reverse transcriptase ribonuclease H domain: Discovery of a novel selective inhibitor of the ribonuclease H function. J. Med. Chem. 2011, 54, 1812–1824. [Google Scholar] [CrossRef] [PubMed]
- Holtomo, O.; Nsangou, M.; Fifen, J.; Motapon, O. Antioxidative Potency and UV–Vis spectra features of the compounds resulting from the chelation of Fe2+ by Caffeic Acid Phenethyl Ester and two of its derivatives. Comput. Theor. Chem. 2015, 1067, 135–147. [Google Scholar] [CrossRef]
- Li, S.-Y.; Wang, X.-B.; Xie, S.-S.; Jiang, N.; Wang, K.D.; Yao, H.-Q.; Sun, H.-B.; Kong, L.-Y. Multifunctional tacrine–flavonoid hybrids with cholinergic, β-amyloid-reducing, and metal chelating properties for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2013, 69, 632–646. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).