Non-Aziridination Approaches to 3-Arylaziridine-2-carboxylic Acid Derivatives and 3-Aryl-(aziridin-2-yl)ketones
Abstract
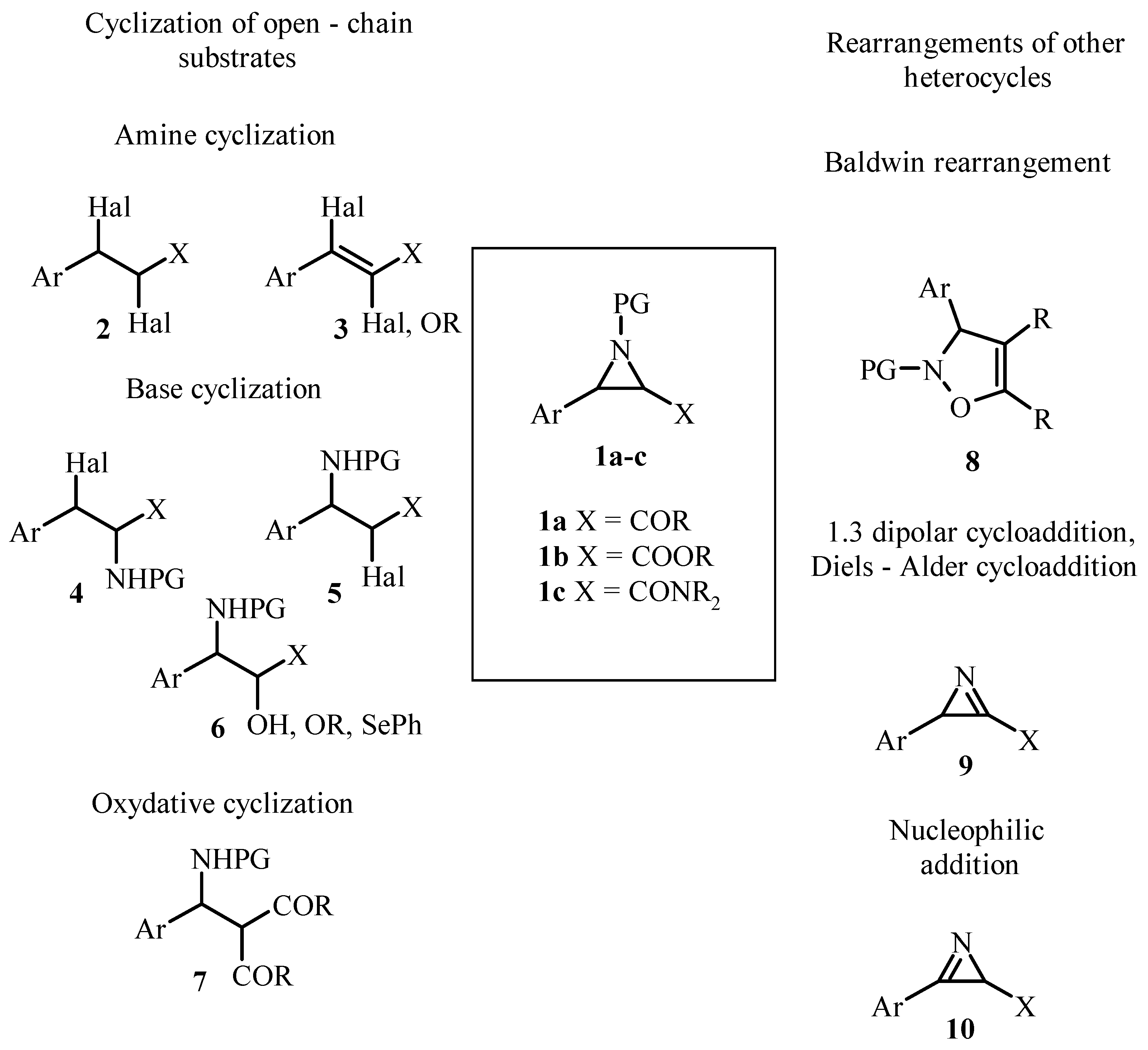
1. Introduction
- Cyclization of open-chain substrates;
- Rearrangement of other heterocycles.
2. Cyclization of Open-Chain Substrates
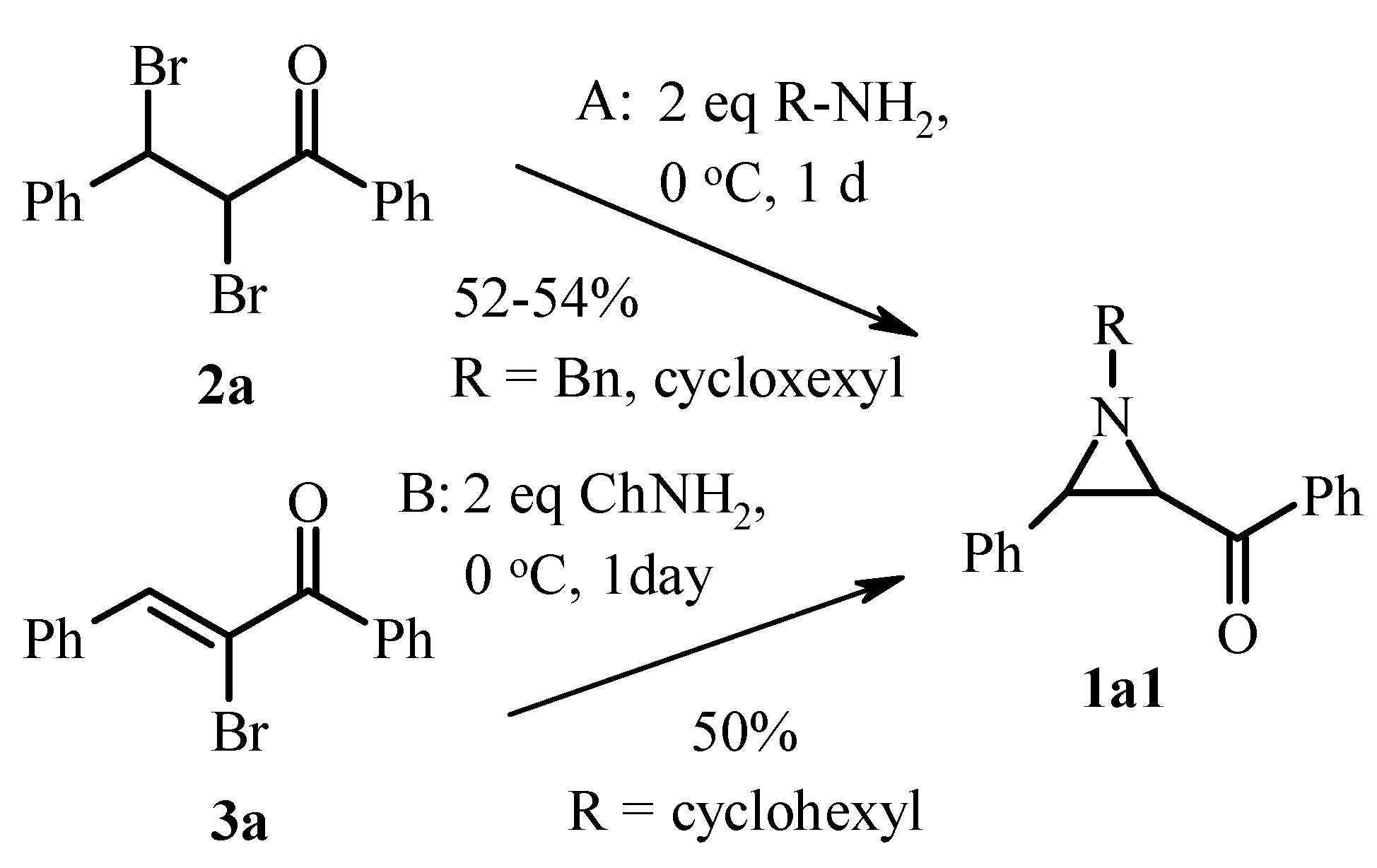
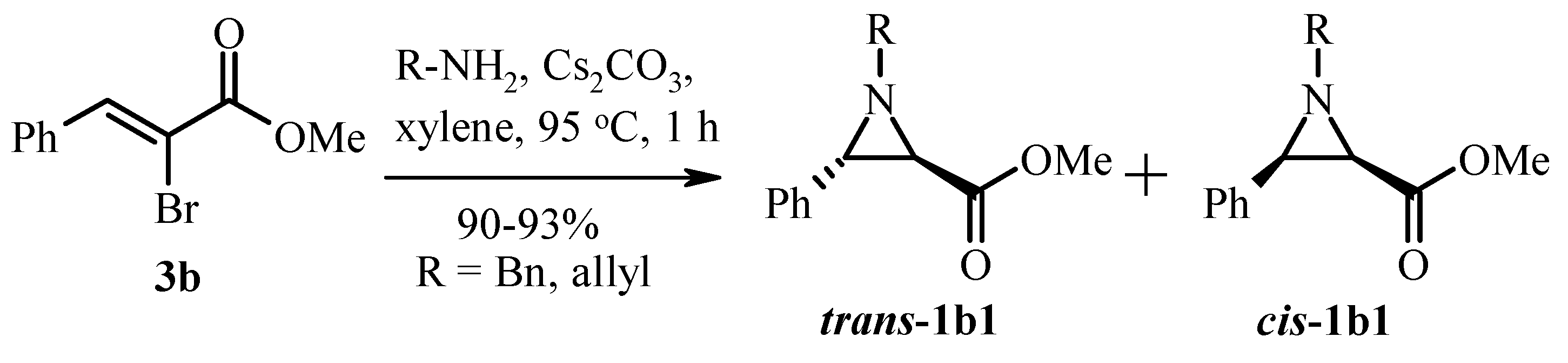
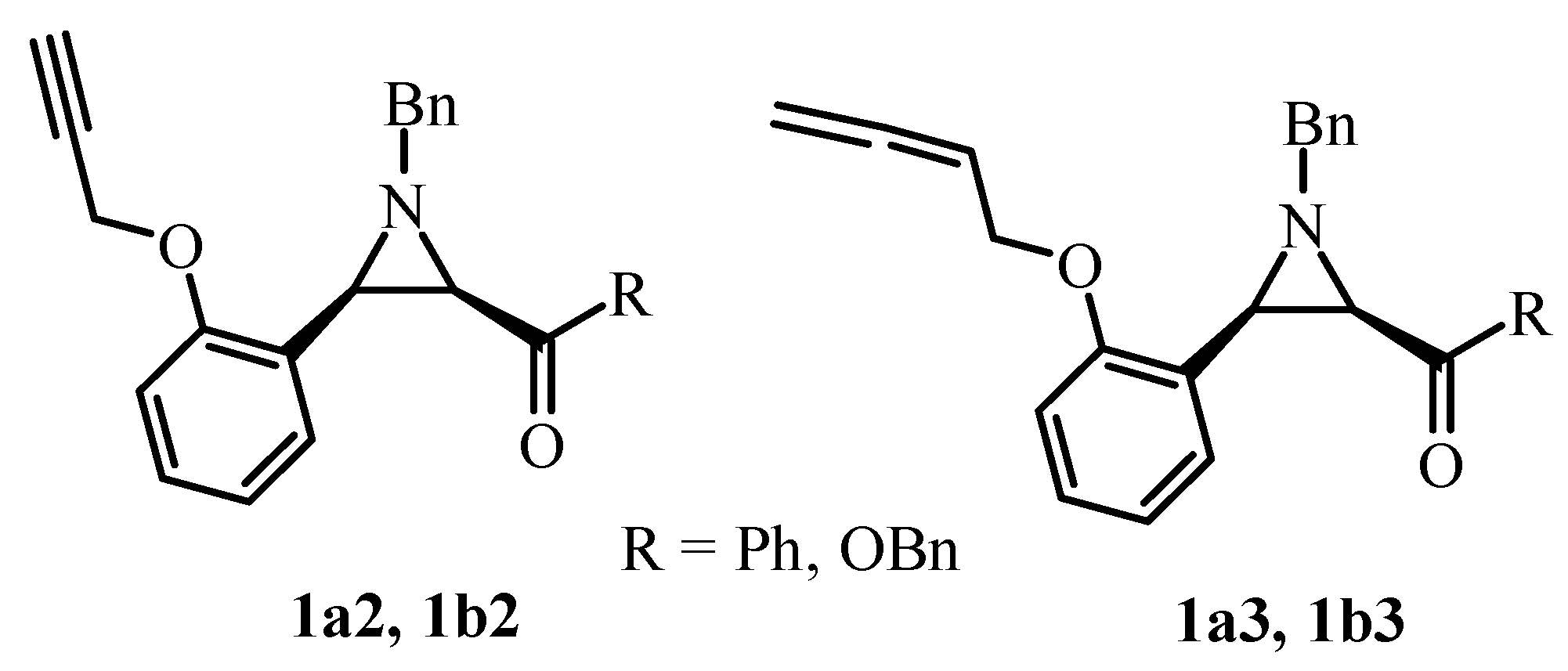
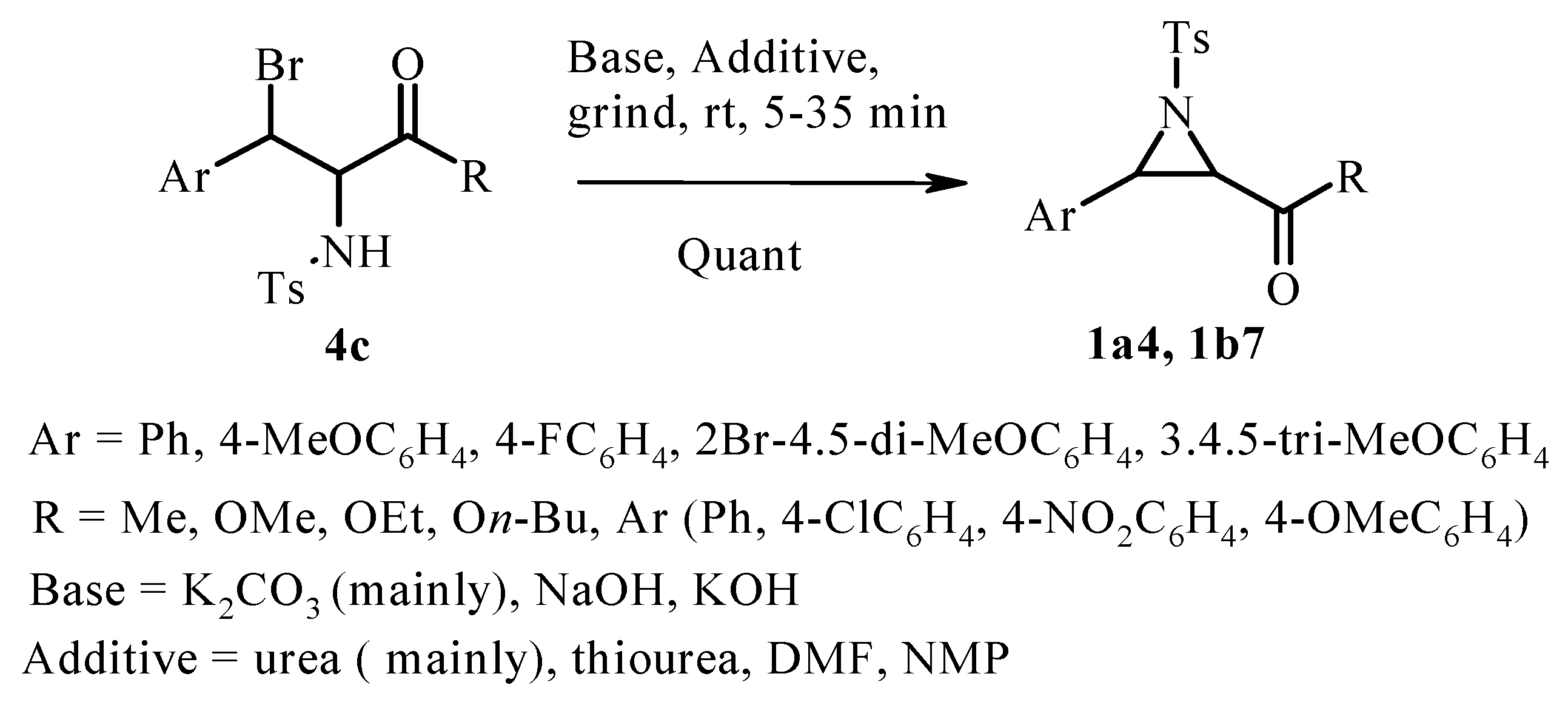
2.1. Classic Gabriel-Cromwell Type Cyclization of Halogenated Substrates
2.2. Base-Promoted Cyclization of β-Substituted Amino Substrates
2.3. Oxidative Cyclization
3. Aziridines from Other Heterocycles
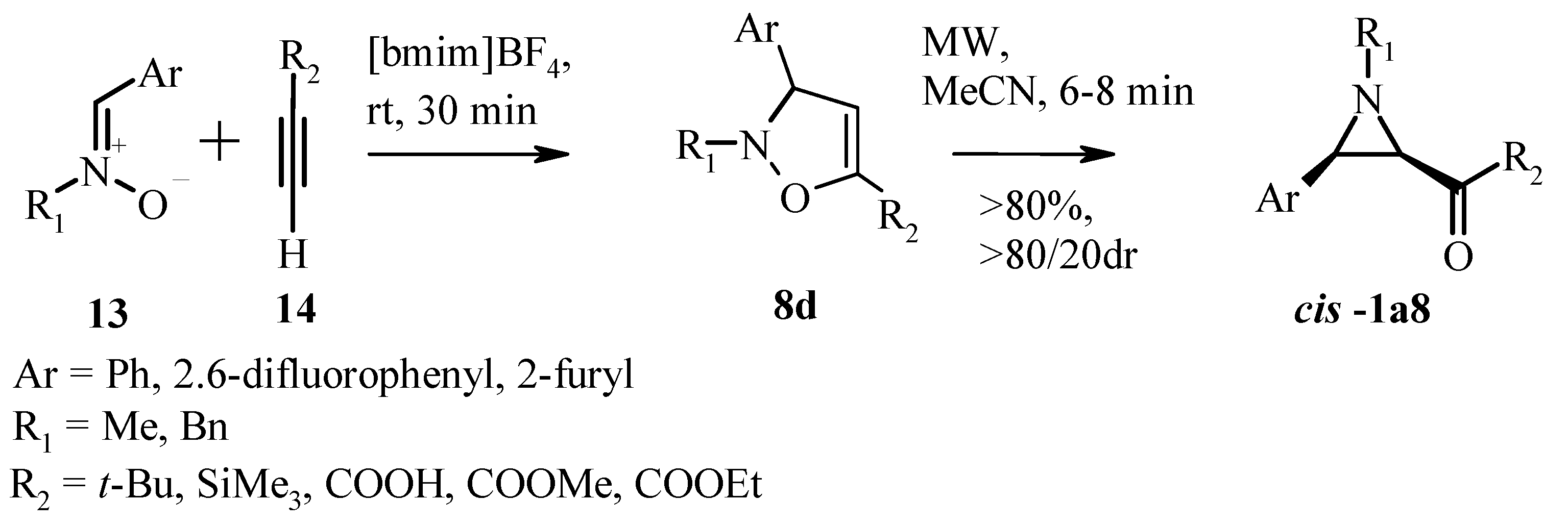
3.1. Aziridines from Isoxazolines. Baldwin Rearrangement
3.2. Aziridines from Azirines
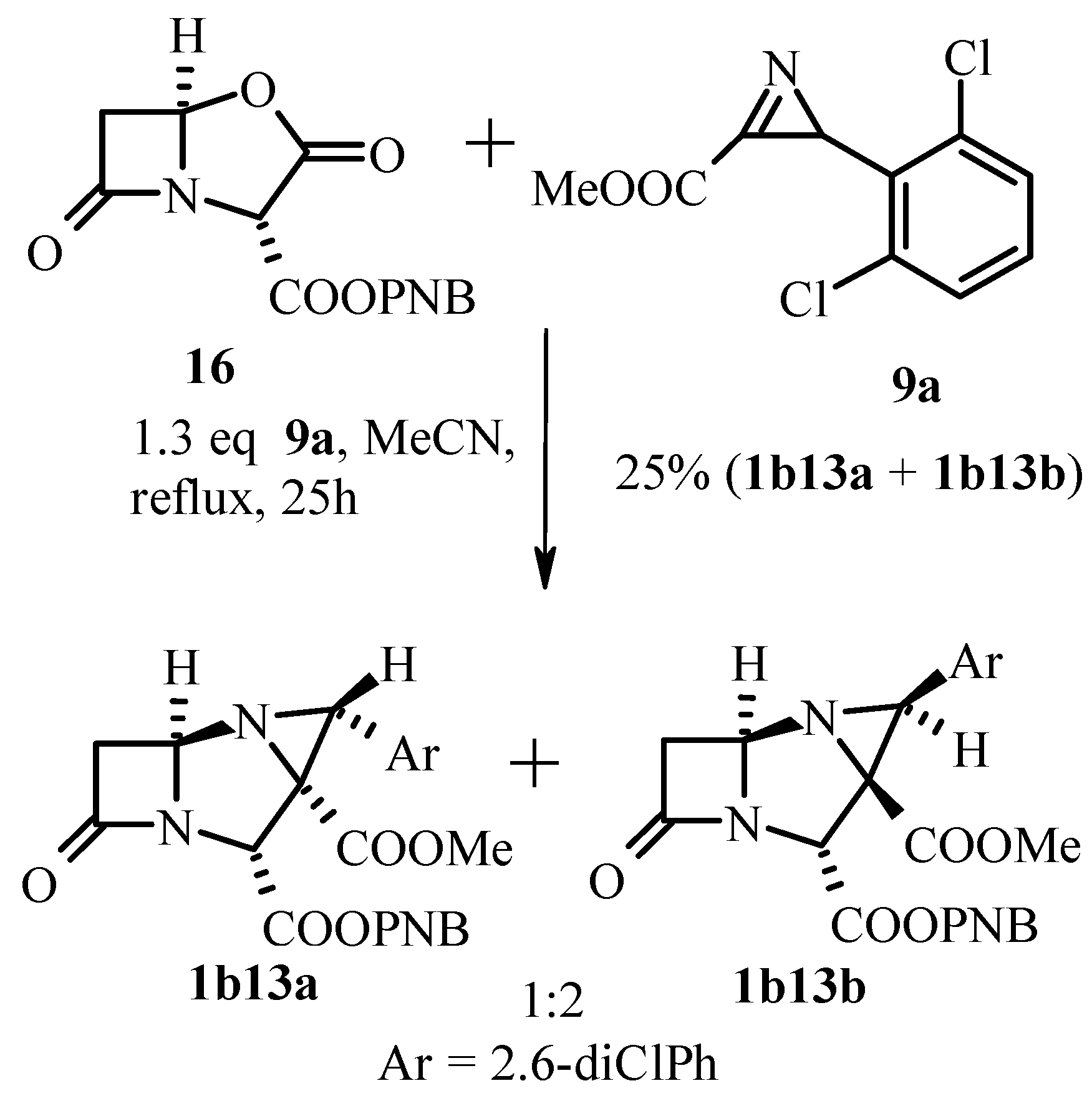
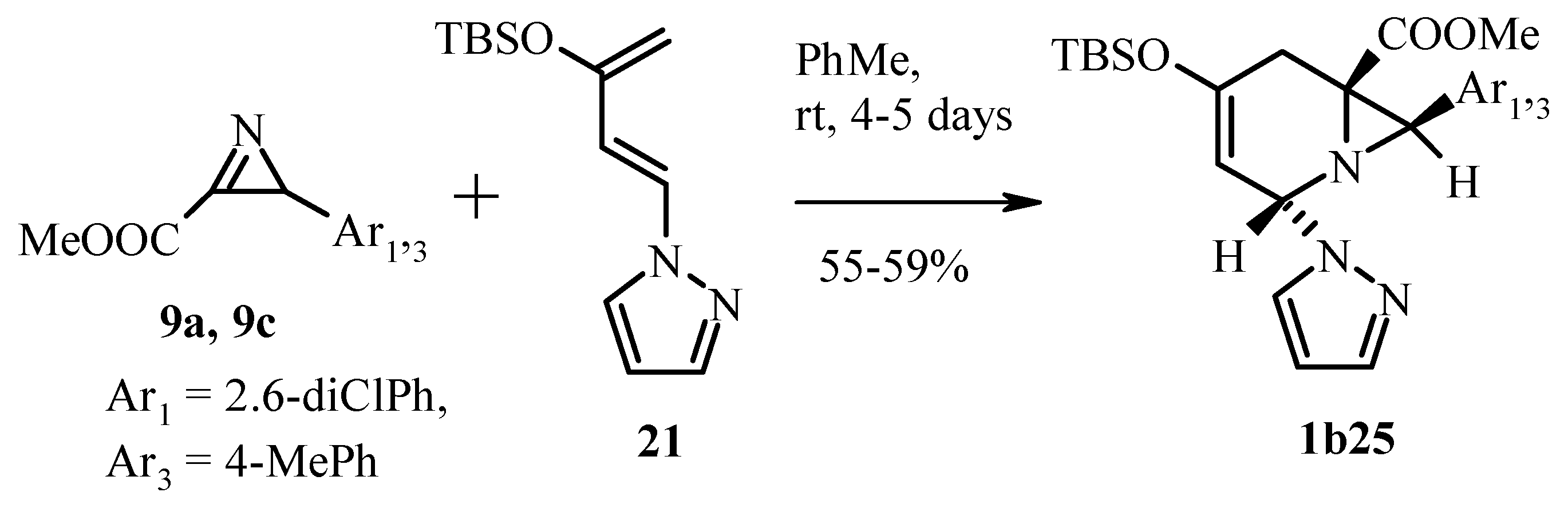
3.2.1. 2H-Azirine as 1.3-Dipolarophile
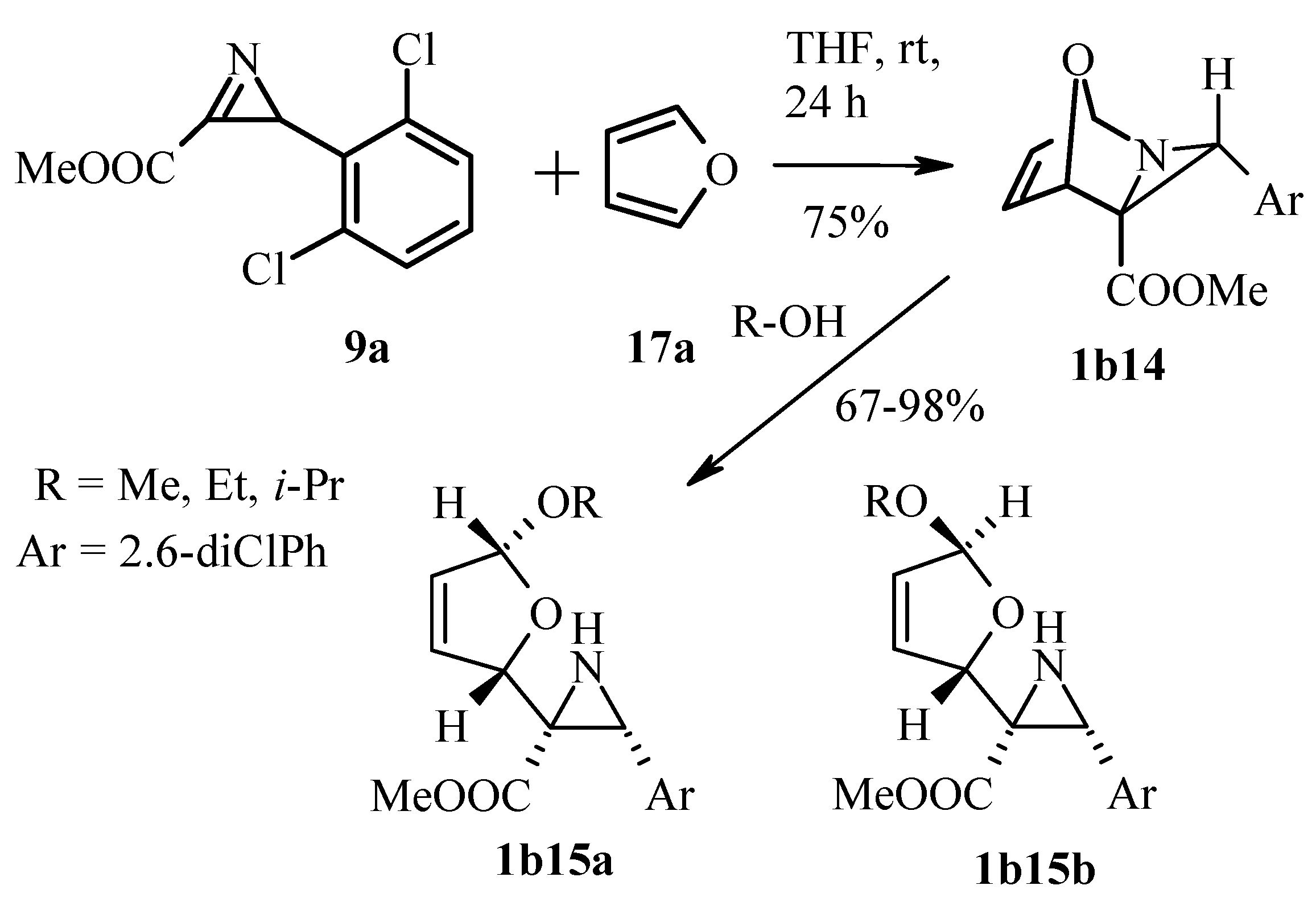
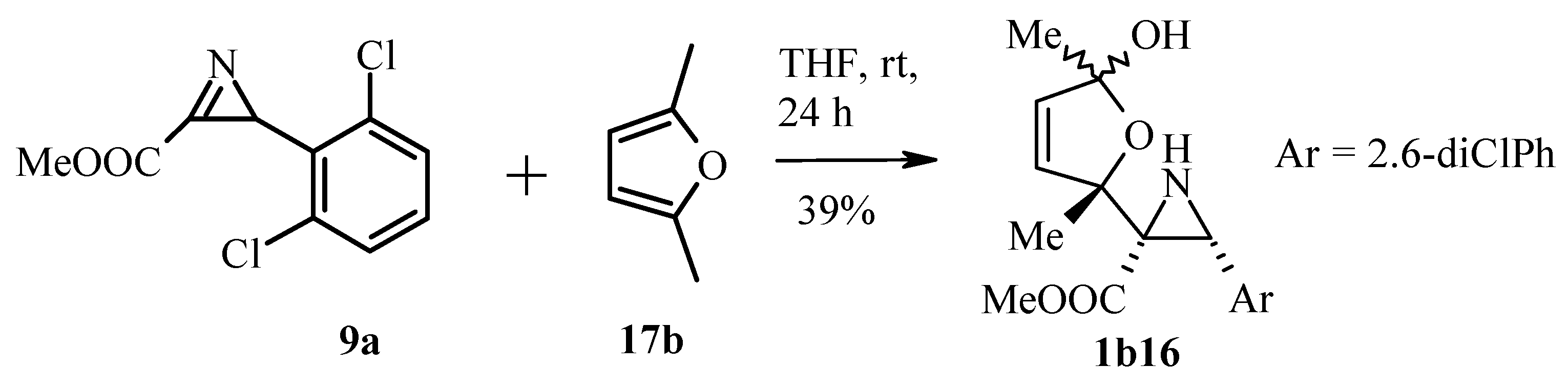
3.2.2. Azirines as Dienophiles
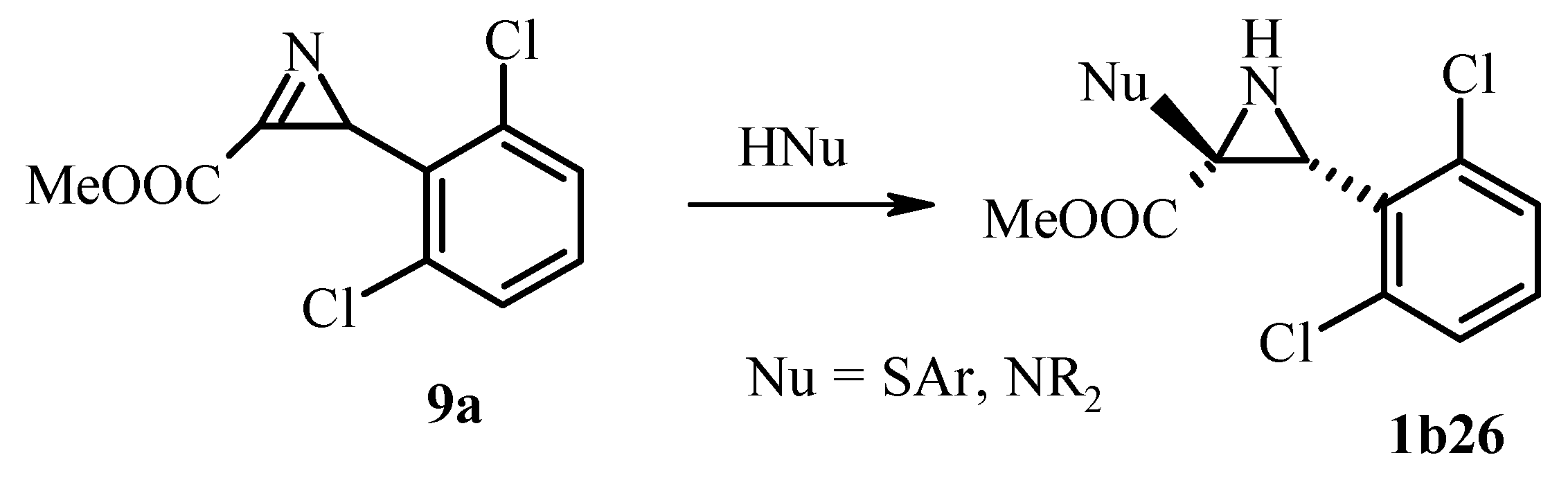
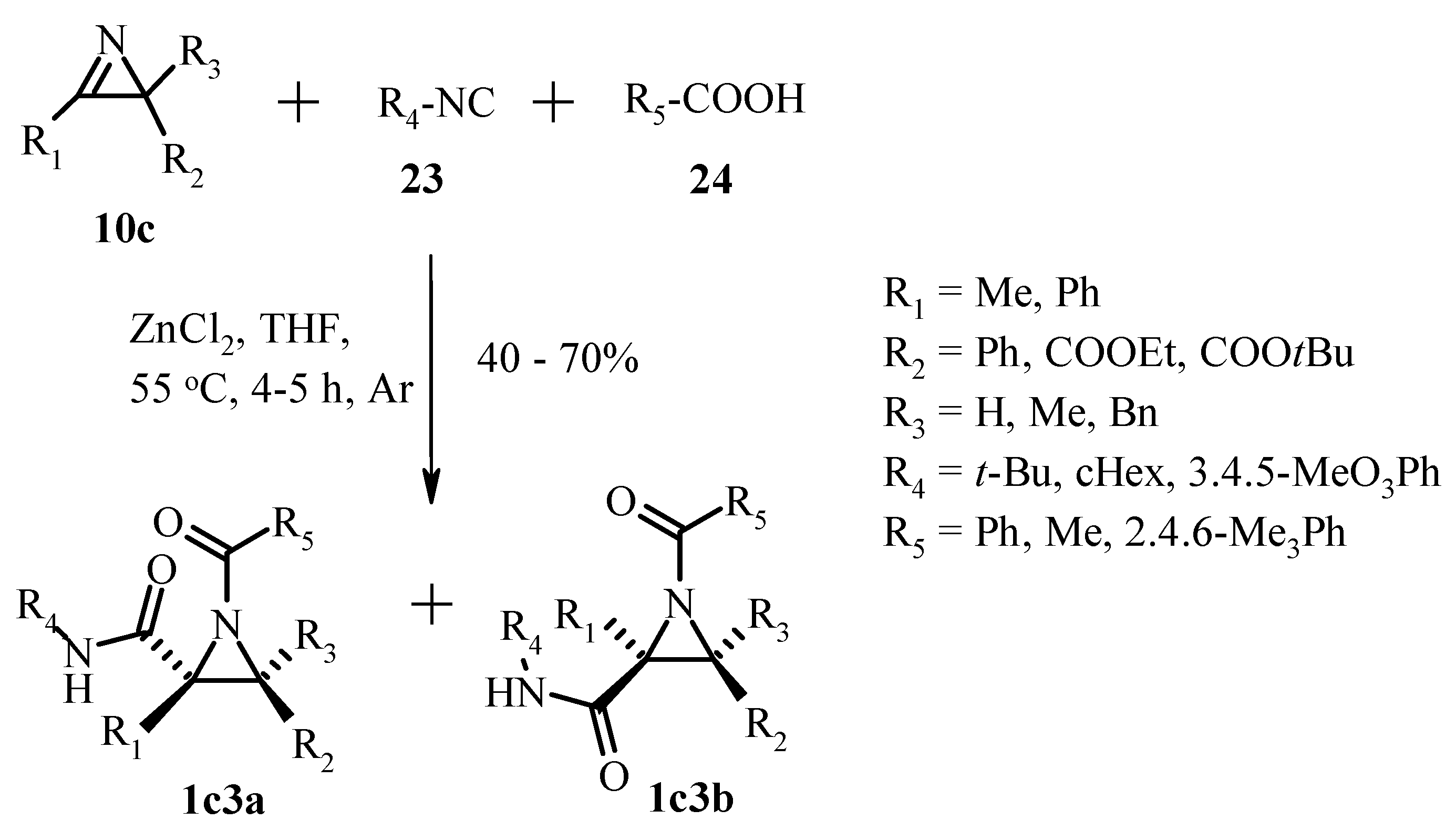
3.2.3. Nucleophilic Addition to the Azirine Double Bond
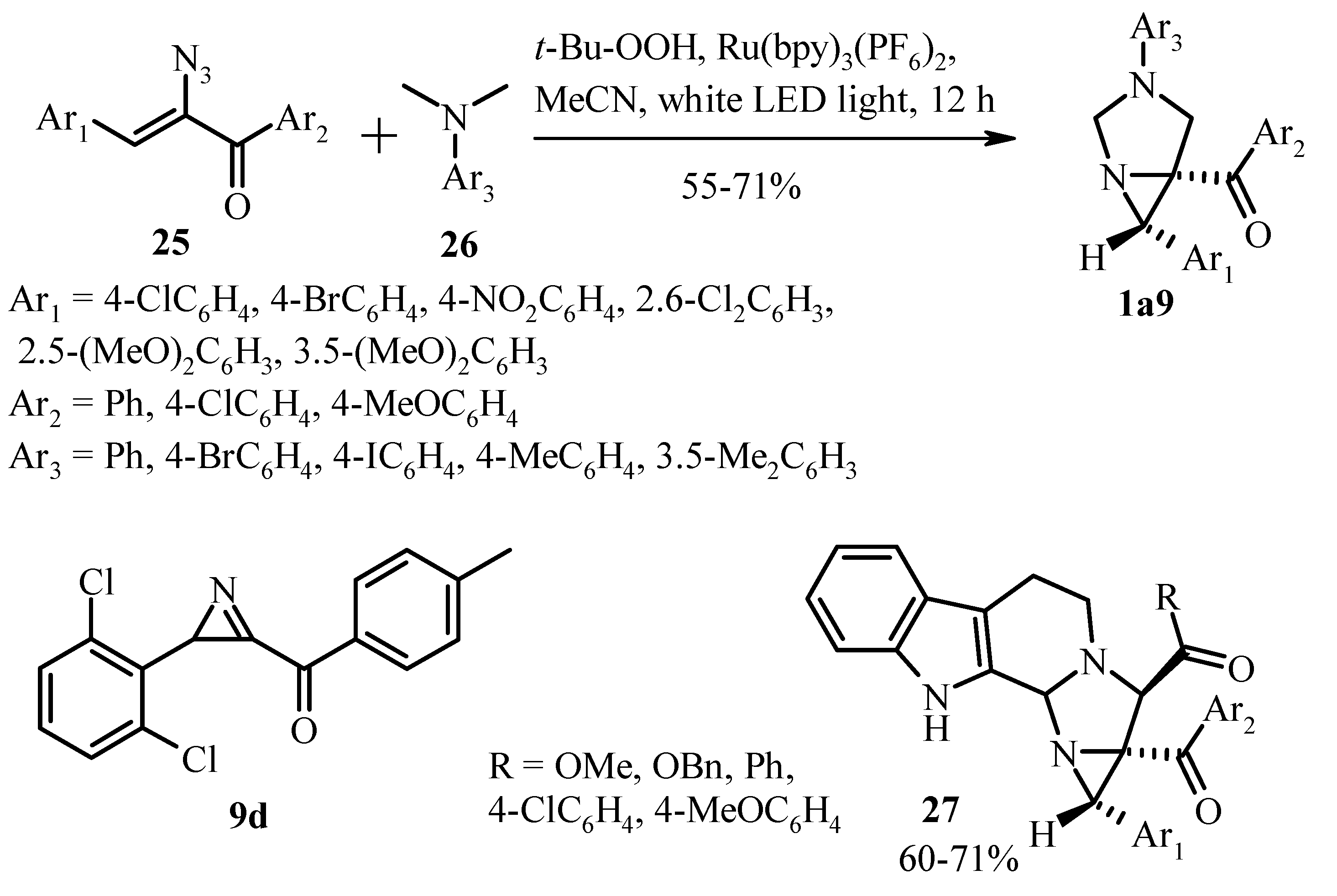
4. Perspective Photo- and Electrochemical Methods in the Synthesis of 3-Arylated Aziridines
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tanner, D. Chiral Aziridines—Their Synthesis and Use in Stereoselective Transformations. Angew. Chem. Int. Ed. 1994, 33, 599–619. [Google Scholar] [CrossRef]
- Iynegar, B.S.; Dorr, R.T.; Remers, W.A. Chemical basis for the biological activity of imexon and related cyanoaziridines. J. Med. Chem. 2004, 47, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Kalvinsh, I.Y.; Astapenok, E.B. Pharmaceutical Composition and Method for Treating Tumors Susceptible to 2-Carbamoylaziridine. U.S. Patent US4686215, appl. US 06/693,171, 11 August 1987. [Google Scholar]
- Rajendra Prasad, N.; Karthikeyan, A.; Karthikeyan, S.; Reddy, B.V. Inhibitory effect of caffeic acid on cancer cell proliferation by oxidative mechanism in human HT-1080 fibrosarcoma cell line. Mol. Cell. Biochem. 2011, 349, 11–19. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Kuo, Y.-H.; Yang, N.-C.; Liu, C.-W.; Chang, W.-T.; Hsu, C.-L.J. Cytotoxic and apoptotic effects of caffeate derivatives on A549 human lung carcinoma cells. Chin. Med. Assoc. 2014, 77, 535–543. [Google Scholar] [CrossRef][Green Version]
- Kong, C.-S.; Jeong, C.-H.; Choi, J.-S.; Kim, K.-J.; Jeong, J.-W. Antiangiogenic effects of p-coumaric acid in human endothelial cells. Phytother. Res. 2013, 27, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, J.; Žalubovskis, R. Derivatives of 2-Aziridinyl Ketones and Aziridinyl-2-Carboxylates. Chem. Heterocycl. Compd. 2016, 52, 535–537. [Google Scholar] [CrossRef]
- Singh, G.S. Advances in synthesis and chemistry of aziridines. Adv. Heterocycl. Chem. 2019, 129, 246–335. [Google Scholar] [CrossRef]
- Degennaro, L.; Trinchera, P.; Luisi, R. Recent Advances in the Stereoselective Synthesis of Aziridines. Chem. Rev. 2014, 114, 7881–7929. [Google Scholar] [CrossRef]
- Strumfs, B.; Uljanovs, R.; Velikijs, K.; Trapencieris, P.; Strumfa, I. 3-Arylaziridine-2-carboxylic Acid derivatives and (3-Arylaziridin-2-yl)ketones: The Aziridination Approaches. Int. J. Mol. Sci. 2021, 22, 9861. [Google Scholar] [CrossRef]
- Cromwell, N.H.; Babson, R.D.; Harris, C.E. α, β-Unsaturated Aminoketones. VIII. Reaction of Primary Amines with 1.3-Diketones and Bromine Derivatives of Benzalacetophenone. Ethylene Imines. J. Am. Che. Soc. 1943, 65, 312–315. [Google Scholar] [CrossRef]
- Madkour, H.M.F.; Salem, M.A.I.; Soliman, E.A.; Mahmoud, N.F.H. A facile One-Pot Synthesis and Antibacterial Activity og Aziridines and Thiazines from 1.3-diarylprop-2-enones. Phosphorus Sulfur Silicon Relat. Elem. 2001, 170, 15–27. [Google Scholar] [CrossRef]
- Barros, M.T.; Maycock, C.D.; Ventura, M.R. A synthesis of aziridines from α-iodoenones. Tet. Lett. 2002, 43, 4329–4331. [Google Scholar] [CrossRef]
- Sharma, D.D.; Kanwar, S.; Rajpoot, S. Aziridines as Templates: A General Strategy for the Stereospecific Synthesis of 2-Azetidinones. J. Heterocycl. Chem. 2006, 43, 11–19. [Google Scholar] [CrossRef]
- Taylor, A.M.; Schreiber, S.L. Aziridines as intermediates in diversity-oriented syntheses of alkaloids. Tet. Lett. 2009, 50, 3230–3233. [Google Scholar] [CrossRef]
- Laia, F.M.R.; e Melo, T.M.P. Synthesis and Reactivity of aziridines with Internal Dipolarophiles: An Approach to 1.4-Dihydrochromeno [4.3-b]pyrroles and 3-Methylenechromano[4.3-b] pyrroles. Synthesis 2015, 47, 2781–2790. [Google Scholar] [CrossRef]
- Shulz, F.; Gelhaus, C.; Degel, B.; Vicik, R.; Heppner, S.; Breuning, A.; Leippe, M.; Gut, J.; Rosenthal, P.J.; Shirmeister, T. Screening of Protease Inhibitors as Antiplasmodial Agents. Part I: Aziridines and Epocides. ChemMedChem 2007, 2, 1214–1224. [Google Scholar] [CrossRef]
- Degel, B.; Staib, P.; Rohrer, S.; Scheiber, J.; Martina, E.; Buchold, C.; Baumann, K.; Morschhauser, J.; Shirmeister, T. Cis-Configured Aziridines Are new Pseudo-Irreversible Dual-Mode Inhibitors of Candida Albicans Secreted Aspartic Protease 2. ChemMedChem 2008, 3, 302–315. [Google Scholar] [CrossRef]
- Buchold, C.; Hemberger, Y.; Heindl, C.; Welker, A.; Degel, B.; Pfeuffer, T.; Staib, P.; Schneider, S.; Rosenthal, P.J.; Gut, J.; et al. New cis-Configured Aziridine-2-carboxylates as Aspartic Acid Protease Inhibitors. ChemMedChem 2011, 6, 141–152. [Google Scholar] [CrossRef]
- Tranchant, M.-J.; Dalla, V.; Jabin, I.; Decroix, B. Reaction of vinyl triflates of α-keto esters with primary amines: Efficient synthesis of aziridine carboxylates. Tetrahedron 2002, 58, 8425–8432. [Google Scholar] [CrossRef]
- Chen, F.; Kim, S.H.; Hodges, B.; Li, G. The cinnamate –based aminohalogenation provides an easy access to anti methyl 3-aryl-N-p-tosyl and N-o-nosyl-aziridine-2-carboxylates. ARKIVOC 2003, 12, 56–63. [Google Scholar] [CrossRef]
- Colpaert, F.; Mangelinckx, S.; De Brabandere, S.; De Kimpe, N. Asymmetric Synthesis of α-chloro-β-amino-N-sulfinyl Imidates as Chiral Building Blocks. J. Org. Chem. 2011, 76, 2204–2213. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Timmone, C.; Guo, L.; Xu, X.; Li, G. One-Pot Stereoselective Synthesis of anti 3-Alkyl and 3-Aryl-N-p-tosyl-aziridine-2-ketones and 3-Aryl-N-p-tosyl-aziridine-2-carboxylates. Synthesis 2004, 15, 2479–2484. [Google Scholar] [CrossRef]
- Boukhris, S.; Souizi, A. Easy access to β-halo amino esters and aziridine-2-carboxylic esters from halohydrins. Tet. Lett. 2004, 44, 3259–3261. [Google Scholar] [CrossRef]
- Wang, I.-N.; Sun, G.-X.; Qi, G. β-Amino functionalisation of cinnamic Weinereb amides in ionic liquid. Beilstein J. Org. Chem. 2016, 12, 2372–2377. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Chen, Z.; Gao, Y.; Zhang, P.; Wang, C.; Zhao, P.; Wang, Y.; Shi, X. A Rapid and simple Method for Quantitative Aziridination from Aminobrominated Derivatives of Olefins under Solvent-free and Mild Conditions. Chin. J. Chem. 2012, 30, 391–399. [Google Scholar] [CrossRef]
- Kaabi, A.; Elemine, B.O.; Besbes, R. A One-Pot synthesis of trans-N-Alkylaziridine-2-carboxylates from Amino Alcohol Esters. Synth. Comm. 2011, 41, 1472–1480. [Google Scholar] [CrossRef]
- Davies, S.G.; Fletcher, A.M.; Frost, A.B.; Lee, J.A.; Roberts, P.M.; Thomson, J.E. Trading N and O: Asymmetric syntheses of β-hydroxy-α-amino acids via α-hydroxy-β-amino esters. Tetrahedron 2013, 69, 8885–8898. [Google Scholar] [CrossRef]
- Yan, Z.; Guan, C.; Yu, Z.; Tian, W. Fluoroalkanosulfonyl fluorides-mediated cyclodehydration of β-hydroxy sulfonamides and β-hydroxy thioamides to the corresponding aziridines and thiazolines. Tet. Lett. 2013, 54, 5788–5790. [Google Scholar] [CrossRef]
- Miniejew, C.; Outurquin, F.; Pannecoucke, X. Diastereoselective synthesis of aziridine esters via amino selanyl esters. Tetrahedron 2006, 62, 2657–2670. [Google Scholar] [CrossRef]
- Fan, R.; Wang, H.; Ye, Y.; Gan, J. PhIO/Bu4NI mediated oxidative cyclization of amidoalkylation adducts for the synthesis of N-benzoyl aziridines and oxazolines. Tet. Lett. 2010, 51, 453–456. [Google Scholar] [CrossRef]
- Baldwin, J.E.; Pudussery, R.G.; Qreshi, A.K.; Sklarz, B. Valence rarrangement of hetero systems. The 4-isoxazolines. J. Am. Chem. Soc. 1968, 90, 5325–5326. [Google Scholar] [CrossRef]
- Ishikawa, T.; Kudeh, T.; Yoshida, J.; Yasuhara, A.; Manabe, S.; Saito, S. Dicobalt Octacaronyl Promoted Rerrangement of 4-Isoxazolines to Acylaziridines: Dramatic Rate Acceleration with Very High Substrate tolerance. Org. Lett. 2002, 4, 1907–1910. [Google Scholar] [CrossRef] [PubMed]
- Gayon, E.; Debleds, O.; Nicouleau, M.; Lamaty, F.; van der Lee, A.; Vrancken, E.; Campagne, J.-M. Highly Diastereoselective Baldwin Rearrangement of Isoxazolines into cis—Acylaziridines. J. Org. Chem. 2010, 75, 6050–6053. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, Y.; Wada, N.; Soeta, T.; Fujinami, S.; Inomata, K.; Ukaji, Y. One-Pot Stereoselective Synthesis of 1-Acylaziridines and 2-Acylpyrrolidines from N-(proprargylic)hydroxylamnes. Chem. Asian J. 2013, 8, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, B.; Chhetri, M.S.; Chhetri, E. Highly Stereoselective Synthesis of new aziridines via Baldwin Rearrangement and their Potential Biological Activities. J. Het. Chem. 2017, 54, 110–120. [Google Scholar] [CrossRef][Green Version]
- Chakraborty, B.; Chhetri, M.S.; Luite, G.P. Synthesis of Some Novel Class of Bis (isoxazoline) and Bis (aziridine) Derivatives. J. Het. Chem. 2017, 54, 1611–1618. [Google Scholar] [CrossRef]
- Brown, D.; Brown, G.A.; Andrews, M.; Large, J.M.; Urban, D.; Butts, C.P.; Hales, N.J.; Gallagher, T. The azomethine ylide strategy for β-lactam synthesis. Azaphenams and 1-azacephams. J. Chem. Soc. Perkin Trans. 2002, 1, 2014–2021. [Google Scholar] [CrossRef]
- Alves, M.J.; Gilchrist, T.L. Methyl 2-aryl-2H-azirine-3-carboxylates as dienophiles. J. Chem. Soc. Perkin Trans. 1998, 1, 299–304. [Google Scholar] [CrossRef]
- Alves, M.J.; Azoia, N.G.; Bickley, J.F.; Gil Fortes, A.; Gilchrist, T.L.; Mendonca, R. Diels-Alder reactions of alkyl 2H-aziridine-3-carboxylates with furans. J. Chem. Soc. Perkin Trans. 2001, 1, 2969–2976. [Google Scholar] [CrossRef][Green Version]
- Alves, M.J.; Duraes, M.M.; Fortes, A.G. Cycloaddition of methyl 2-(2.6-dichlorophenyl)-2H-azirine-3-carboxylate to electron-rich 2-azadienes. Tet. Lett. 2003, 44, 5079–5082. [Google Scholar] [CrossRef][Green Version]
- Alves, M.J.; Duraes, M.M.; Fortes, A.G. Diels—Alder cycloaddition of 2-azadienes to methyl 2-(2.6-dichlorophenyl)-2H-azirine-3-carboxylate in the synthesis if 4-oxo-1.3-diazabicyclo[4.1.10] heptane-6-carboxylates. Tetrahedron 2004, 60, 6541–6553. [Google Scholar] [CrossRef]
- Alves, M.J.; Costa, C.; Duraes, M.M. Diastereoselective Diels-Alder cycloaddition of [(1R)-10-(N,N-diethylsulfamoyl)isobornyl]2H-azirine to nucleophilic 1.4-disubstituted 1.3-dienes. Tetrahedron Asymmetry 2009, 20, 1378–1382. [Google Scholar] [CrossRef]
- Alves, M.J.; Fortes, A.G.; Costa, F.T. Diels—Alder cycloaddition of electrophilic 2H-azirines with 3-(3-(tert-butyldimethylsiloxy)buta-1.3-dienyl)oxazolidin-2-ones. Treatment of the cycloadducts under acidic conditions. Tetrahedron 2006, 62, 3095–3102. [Google Scholar] [CrossRef]
- Alves, M.J.; Fortes, A.G.; Costa, F.T.; Duarte, V.C.M. Formation of pyridine-4(1H)-one versus 1H-azepin-4(7H)-one by treatment of 4-tert-butyldimethylsilyslocy-2-amino-1-azabicyclo[4.1.10]hept-3-enes with tetrabutylammonium fluoride. Tetrahedron 2007, 63, 11167–11173. [Google Scholar] [CrossRef]
- Alves, M.J.; Ferreira, P.M.; Maina, H.L.S.; Monteiro, L.S.; Gilchrist, T.L. Novel aziridine esters by the addition of aromatic nitrogen heterocycles to a 2H-azirine-3-carboxylic ester. Tet. Lett. 2000, 41, 4991–4995. [Google Scholar] [CrossRef]
- Davis, F.A.; Liang, C.-H.; Liu, H. Asymmetric Synthesis of β-Substituted α-Amino Acids using 2H Azirine-2-carboxylate Esters. Synthesis of 3.3-Disubstituted Aziridine-2-carboxylate Esters. J. Org. Chem. 1997, 62, 3796–3797. [Google Scholar] [CrossRef]
- Davis, F.A.; Zhang, Y.; Rao, A.; Zhang, Z. Aziridine mediated asymmetric synthesis of α-benzylserine and α-n-butylserine. Tetrahedron 2001, 57, 6345–6352. [Google Scholar] [CrossRef]
- Alves, M.J.; Fortes, A.G.; Goncalves, L.F. Optically active aziridine esters by nucleophilic addition of nitrogen heterocycles to a chiral 2H-azirine-2-carboxylic ester. Tet. Lett. 2003, 44, 6277–6279. [Google Scholar] [CrossRef]
- Angyal, A.; Demjen, A.; Weber, E.; Kovacs, A.K.; Wolfling, J.; Puskas, L.G.; Kanizsai, I. Lewis Acid-Catalyzed Diastereoselective Synthesis of Multisubstituted N-Acylaziridine-2-carboxamides from 2H-Azirines via Joullie-Ugi Three-Component Reaction. J. Org. Chem. 2018, 83, 3570–3581. [Google Scholar] [CrossRef]
- Chandarsekhar, D.; Borra, S.; Nanubolu, J.B.; Maurya, R.A. Visible Light Driven Photocascade Catalysis: Ru(bpy)3(PF6)2/THBP-Mediated Synthesis of Fused β-Carbolines ub Batch and Flow Microreactors. Org. Lett. 2016, 18, 2974–2977. [Google Scholar] [CrossRef]
- Borra, S.; Chandarsekhar, D.; Adhikary, S.; Rasala, S.; Gokulnath, S.; Maurya, R.A. Visible-Light Driven Photocascade Catalysis: Union of N,N-Dimethylanilines and α-azidochalcones in Flow Microreactors. J. Org. Chem. 2017, 82, 2249–2256. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shi, J.; Tan, J.; Xu, W.; Zhang, S.; Xu, K. Electrochemical Synthesis of trans-2.3-Disubstituted Aziridines via Oxidative Dehydrogenative Intramolecular C(sp3)-H Amination. Org. Lett. 2019, 21, 9430–9433. [Google Scholar] [CrossRef] [PubMed]
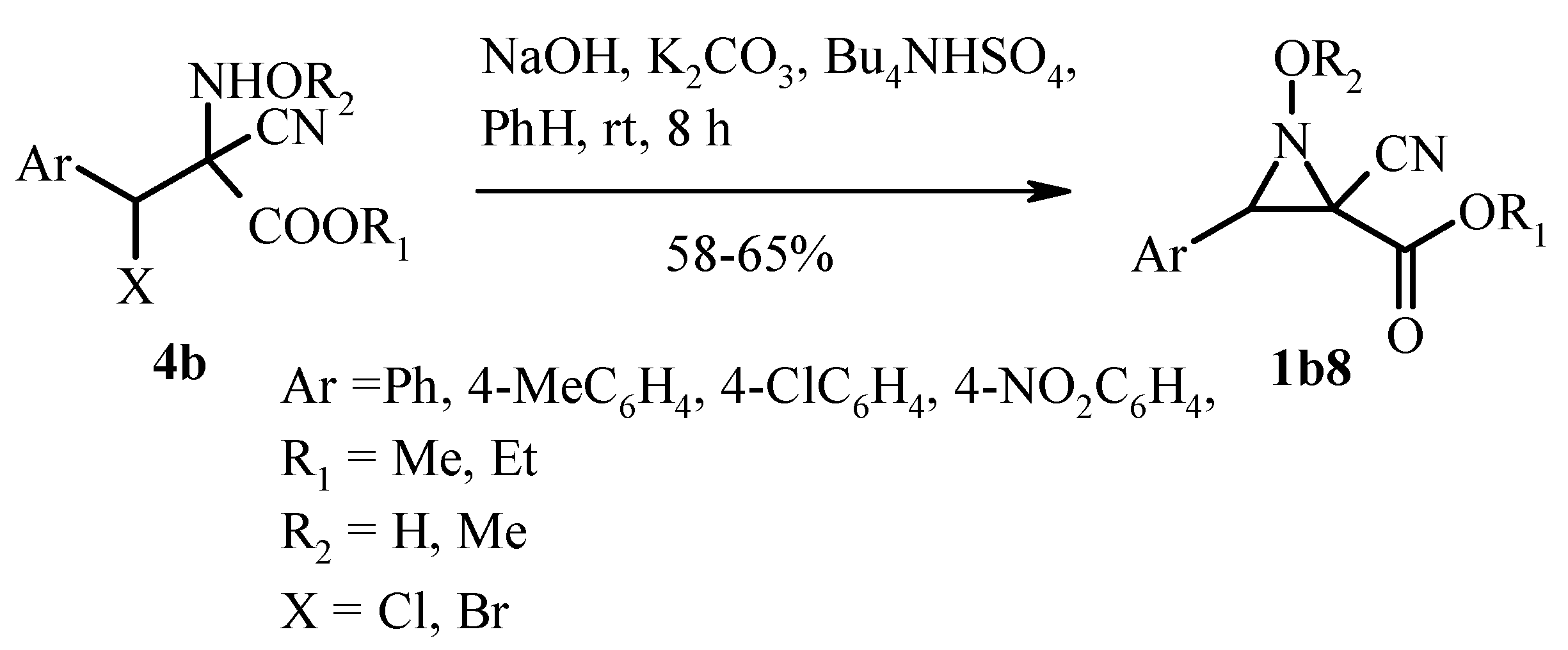
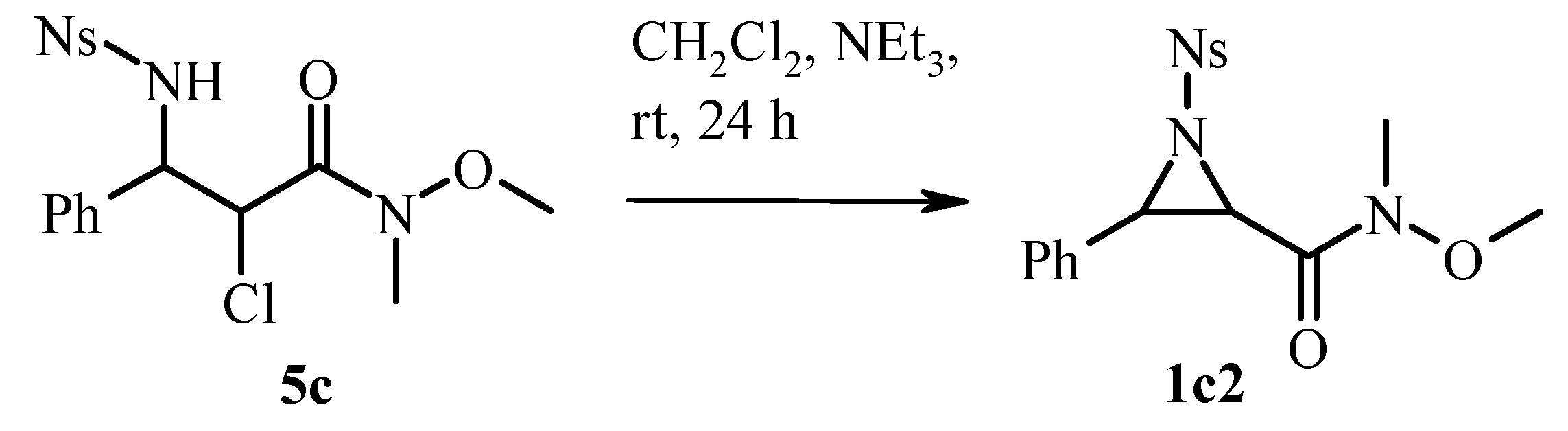

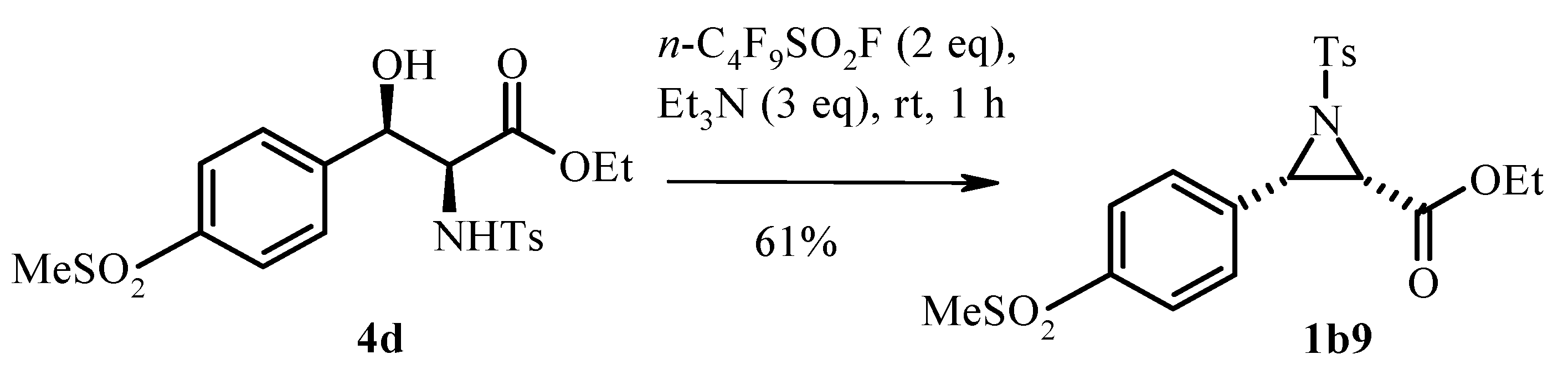
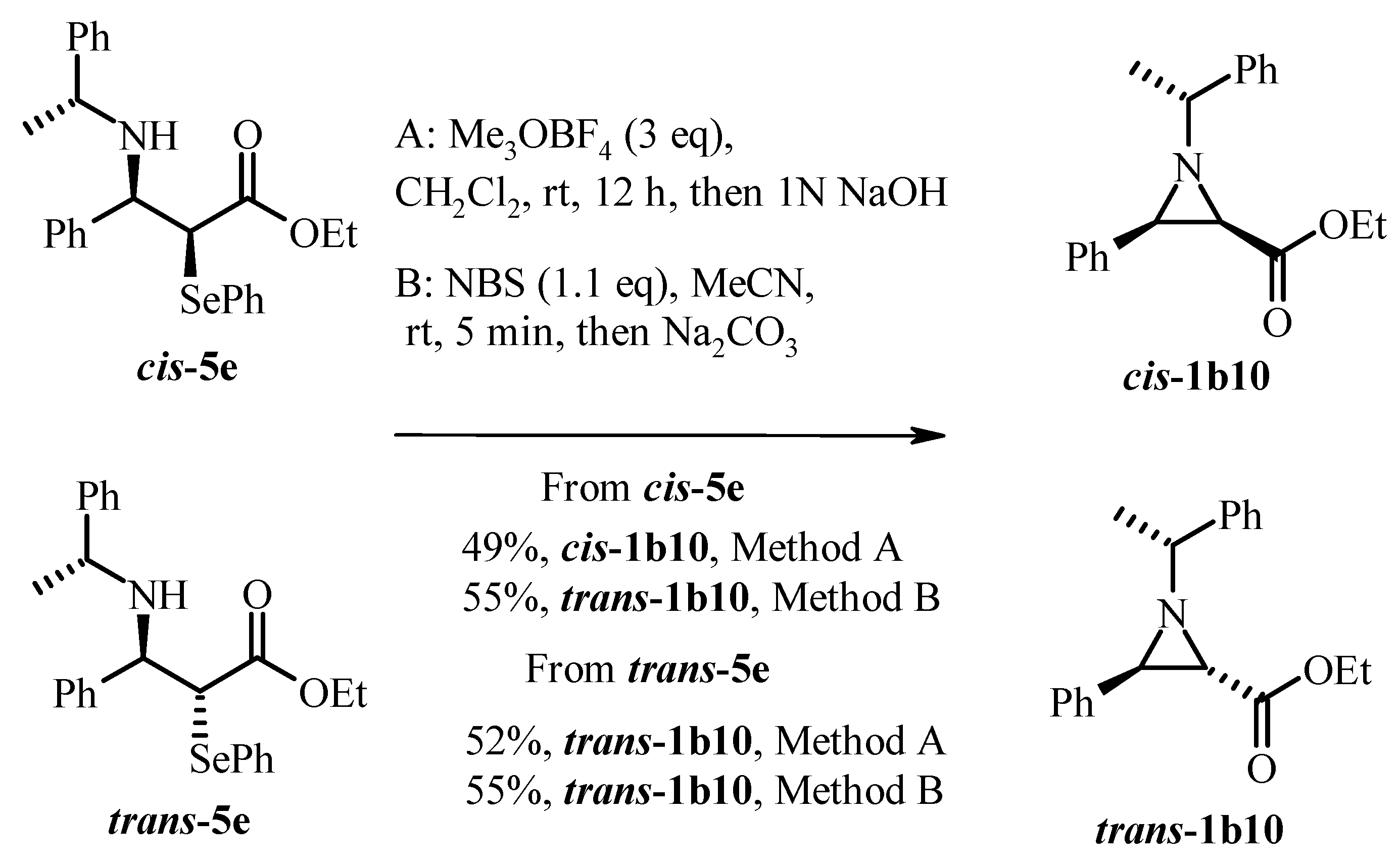
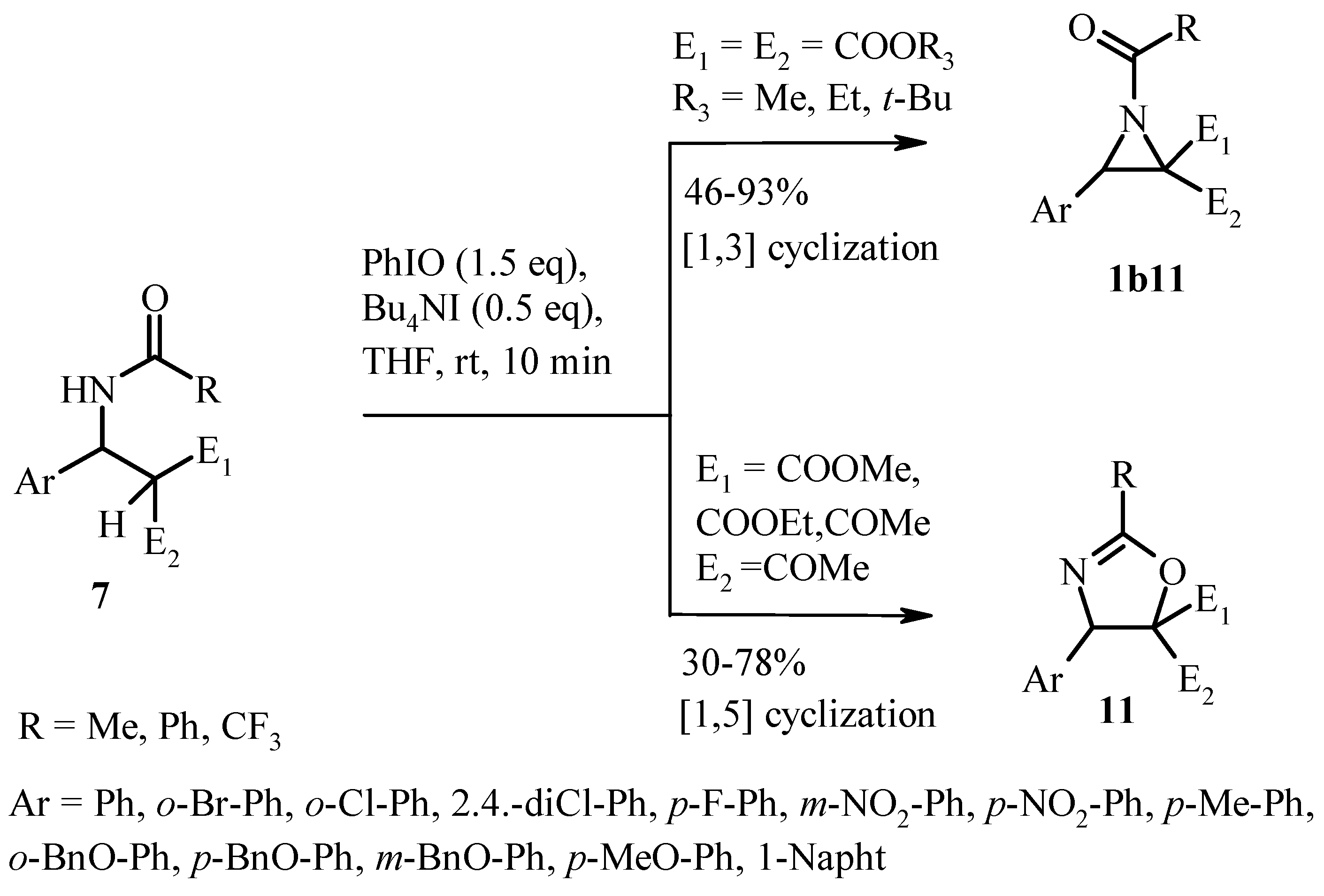
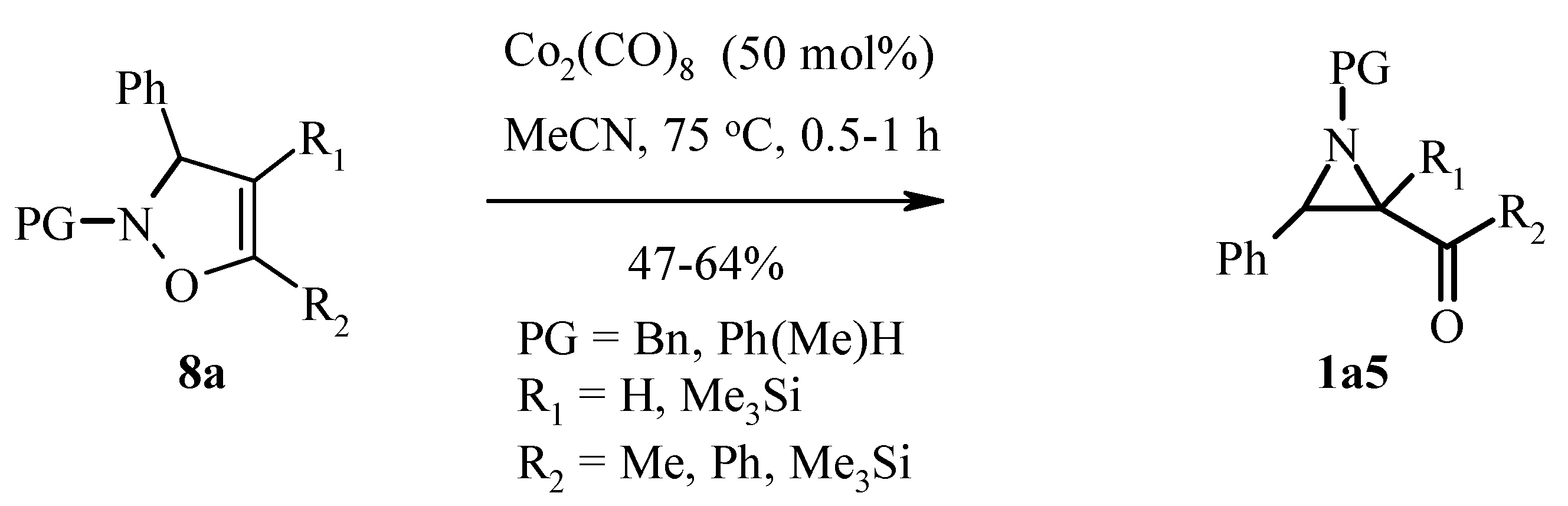
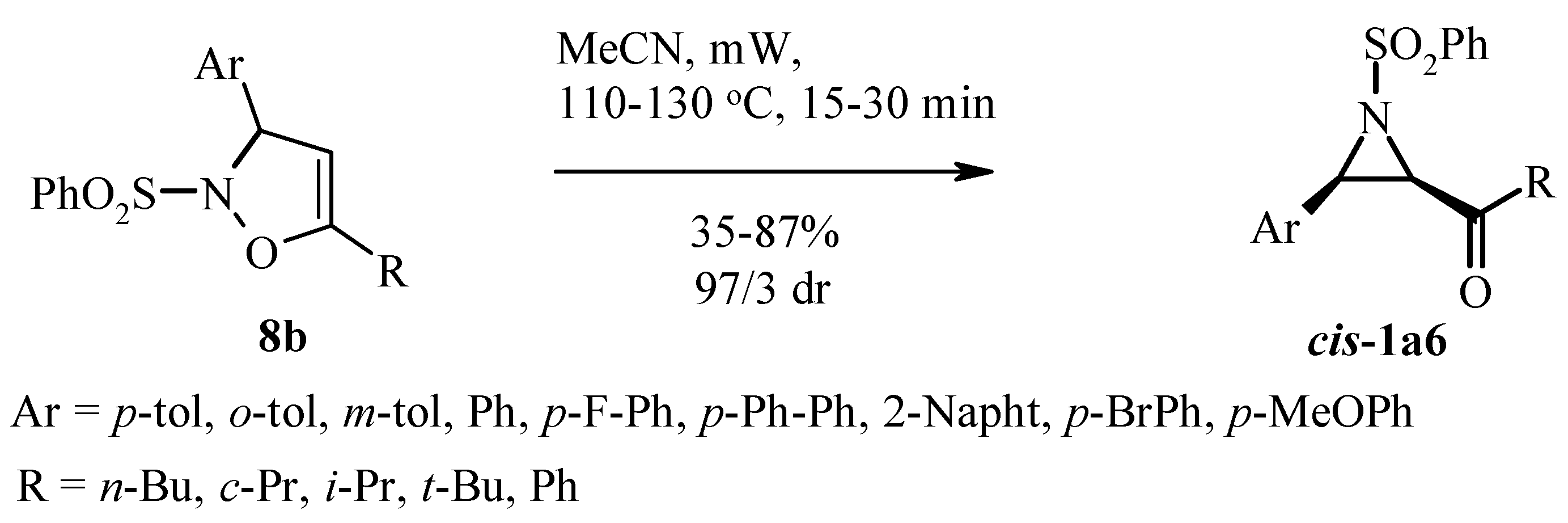


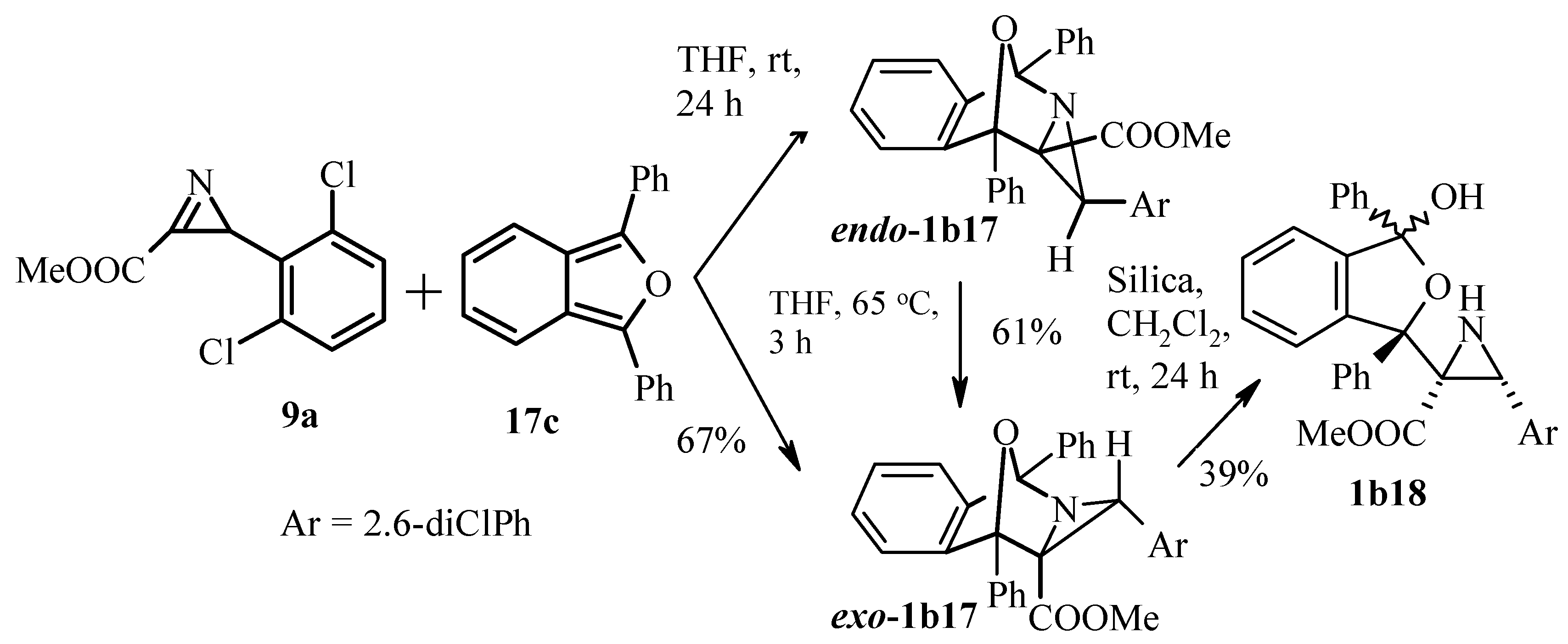
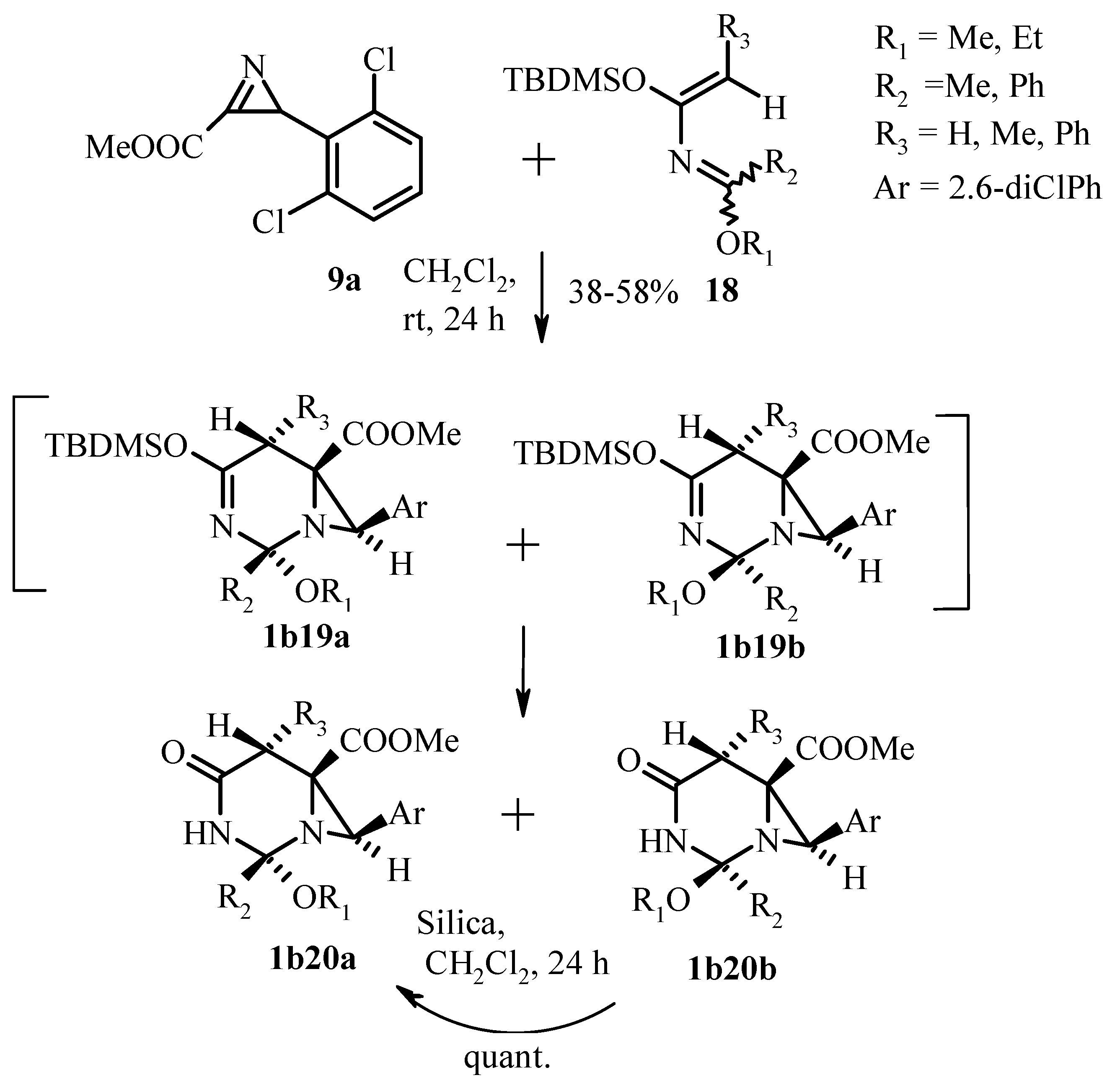
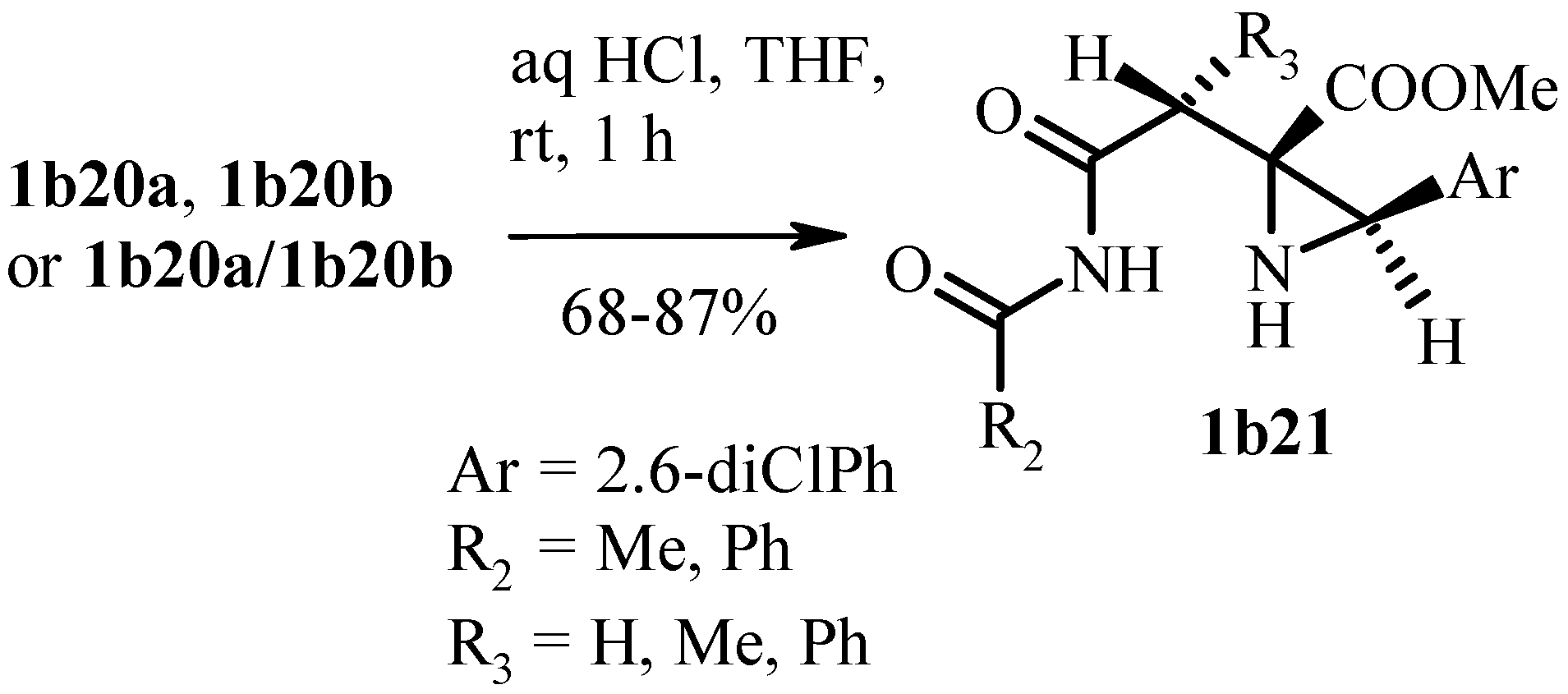
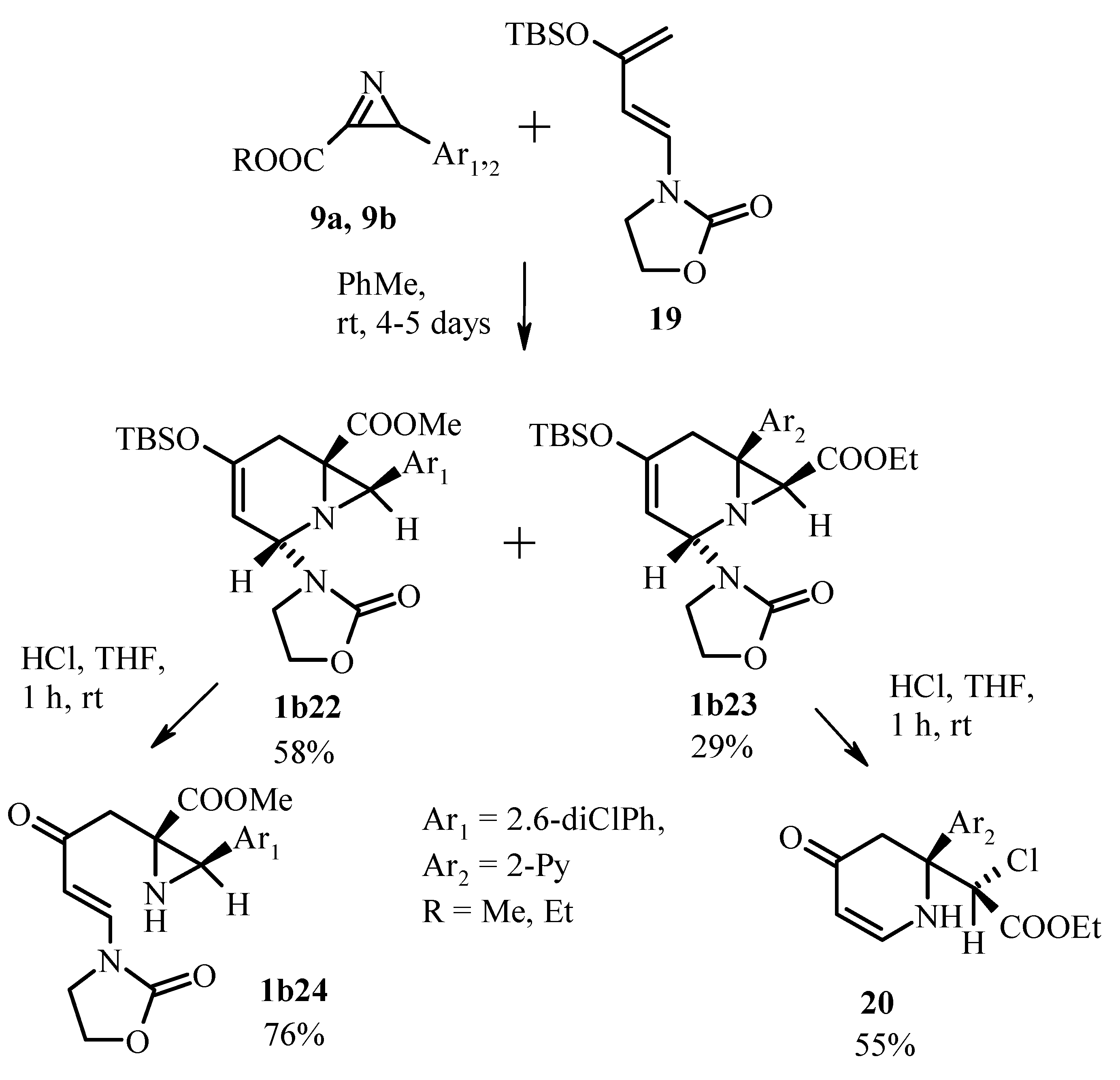
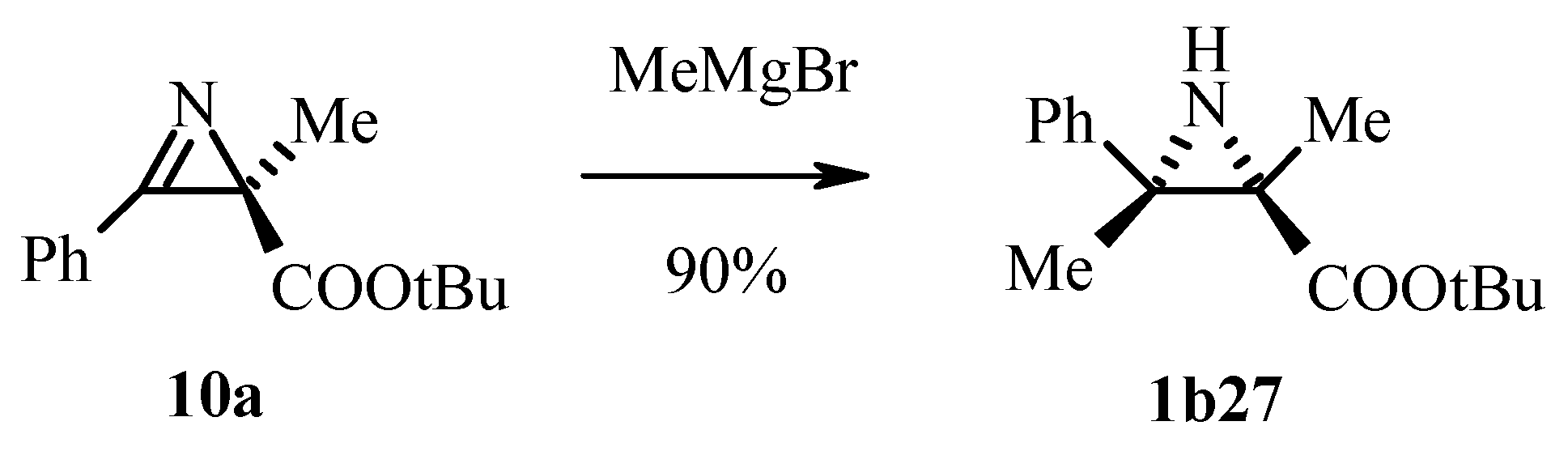

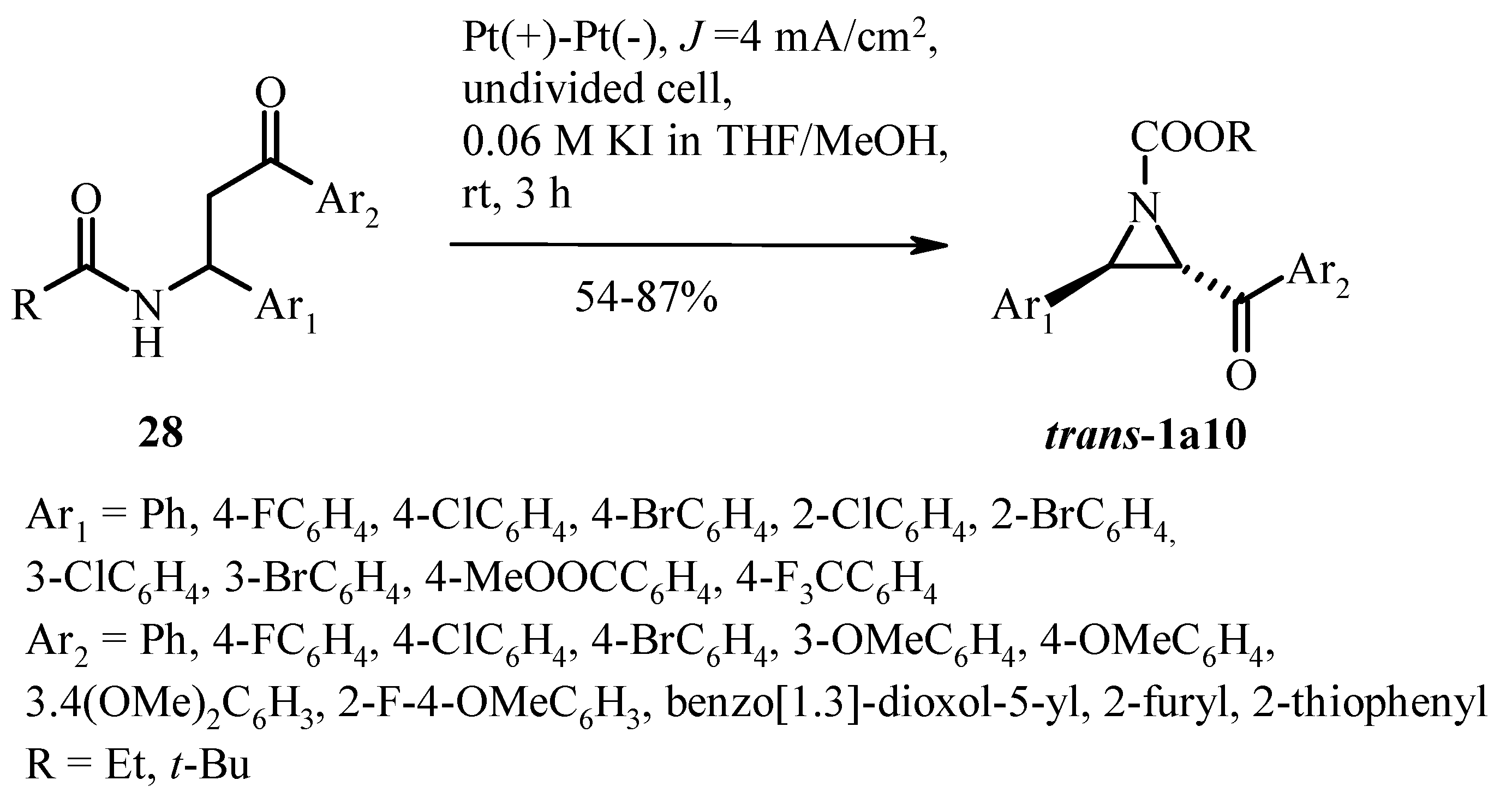
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strumfs, B.; Velikijs, K.; Uljanovs, R.; Sinkarevs, S.; Strumfa, I. Non-Aziridination Approaches to 3-Arylaziridine-2-carboxylic Acid Derivatives and 3-Aryl-(aziridin-2-yl)ketones. Int. J. Mol. Sci. 2022, 23, 5919. https://doi.org/10.3390/ijms23115919
Strumfs B, Velikijs K, Uljanovs R, Sinkarevs S, Strumfa I. Non-Aziridination Approaches to 3-Arylaziridine-2-carboxylic Acid Derivatives and 3-Aryl-(aziridin-2-yl)ketones. International Journal of Molecular Sciences. 2022; 23(11):5919. https://doi.org/10.3390/ijms23115919
Chicago/Turabian StyleStrumfs, Boriss, Kirils Velikijs, Romans Uljanovs, Stanislavs Sinkarevs, and Ilze Strumfa. 2022. "Non-Aziridination Approaches to 3-Arylaziridine-2-carboxylic Acid Derivatives and 3-Aryl-(aziridin-2-yl)ketones" International Journal of Molecular Sciences 23, no. 11: 5919. https://doi.org/10.3390/ijms23115919
APA StyleStrumfs, B., Velikijs, K., Uljanovs, R., Sinkarevs, S., & Strumfa, I. (2022). Non-Aziridination Approaches to 3-Arylaziridine-2-carboxylic Acid Derivatives and 3-Aryl-(aziridin-2-yl)ketones. International Journal of Molecular Sciences, 23(11), 5919. https://doi.org/10.3390/ijms23115919





