Antioxidants Attenuate Heat Shock Induced Premature Senescence of Bovine Mesenchymal Stem Cells
Abstract
:1. Introduction
2. Results
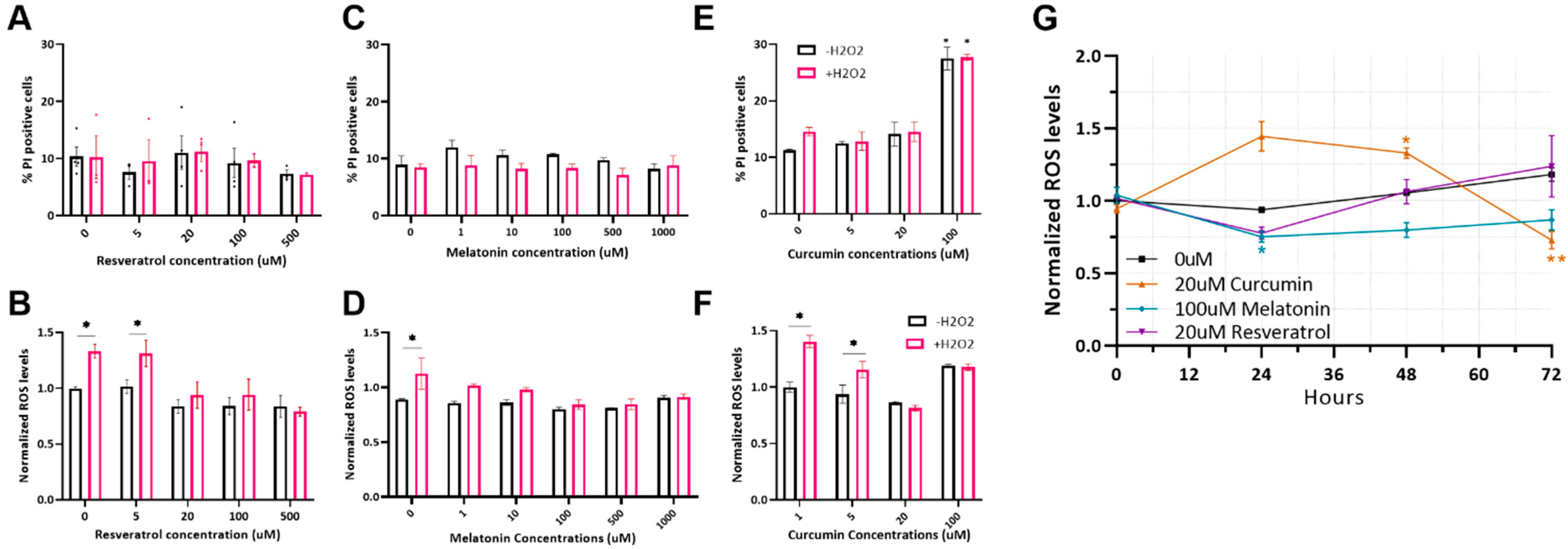
2.1. Optimizing Antioxidant Treatments on MSC
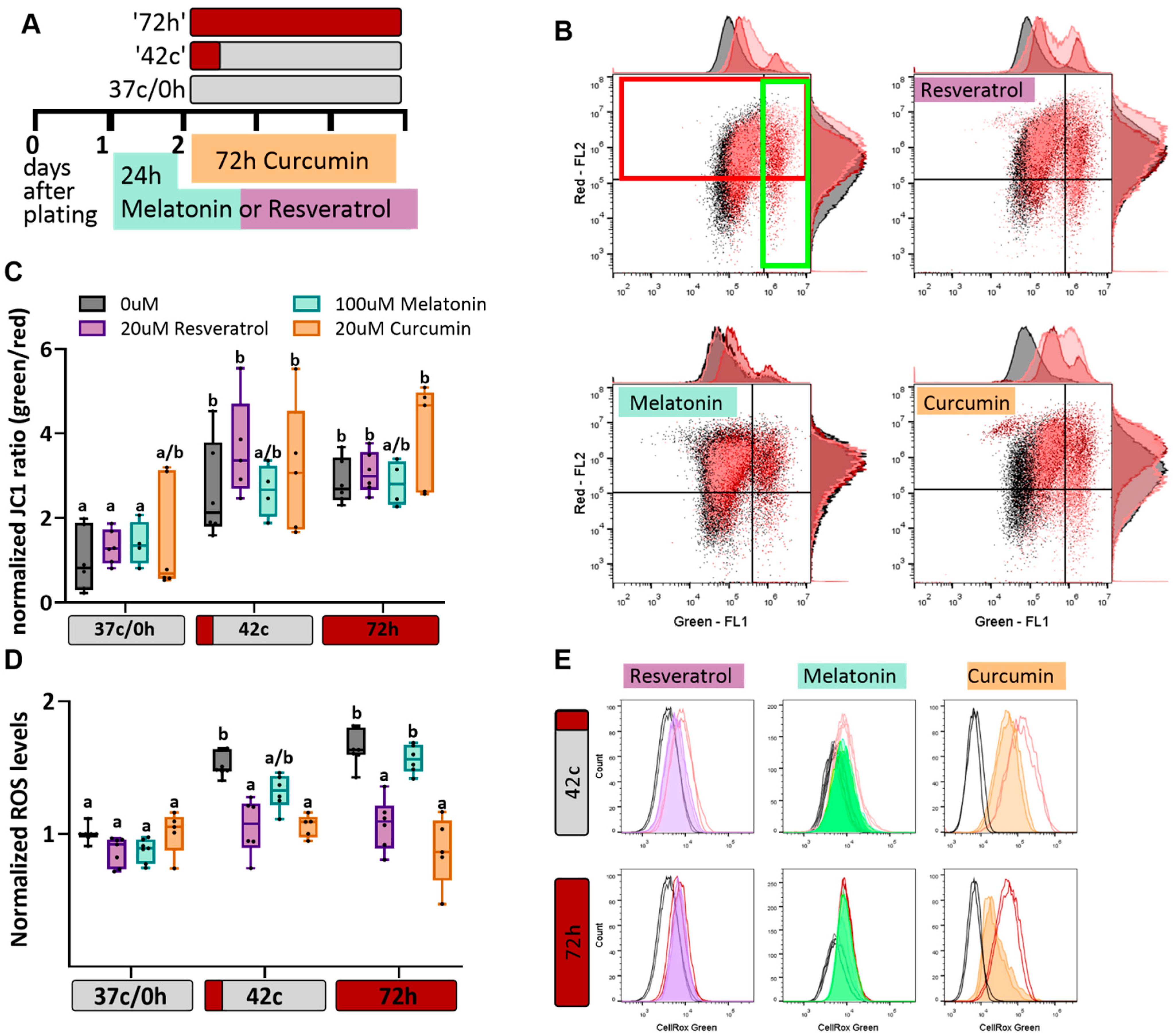
2.2. Antioxidant Protection of MSC from Heat-Shock-Induced Oxidative Stress
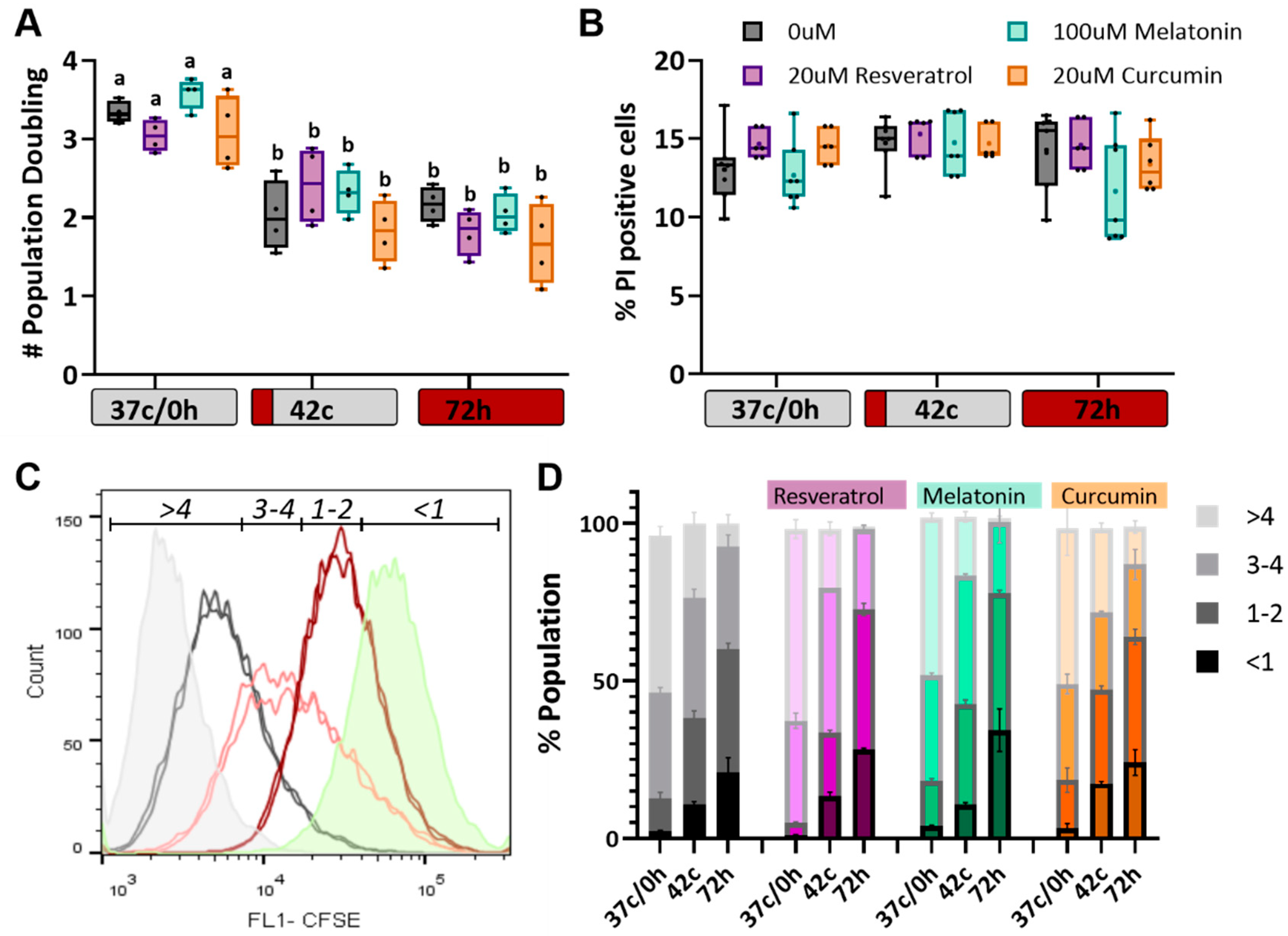
2.3. Antioxidants Have No Effect on Heat Shock-Induced Reduction in Cell Proliferation
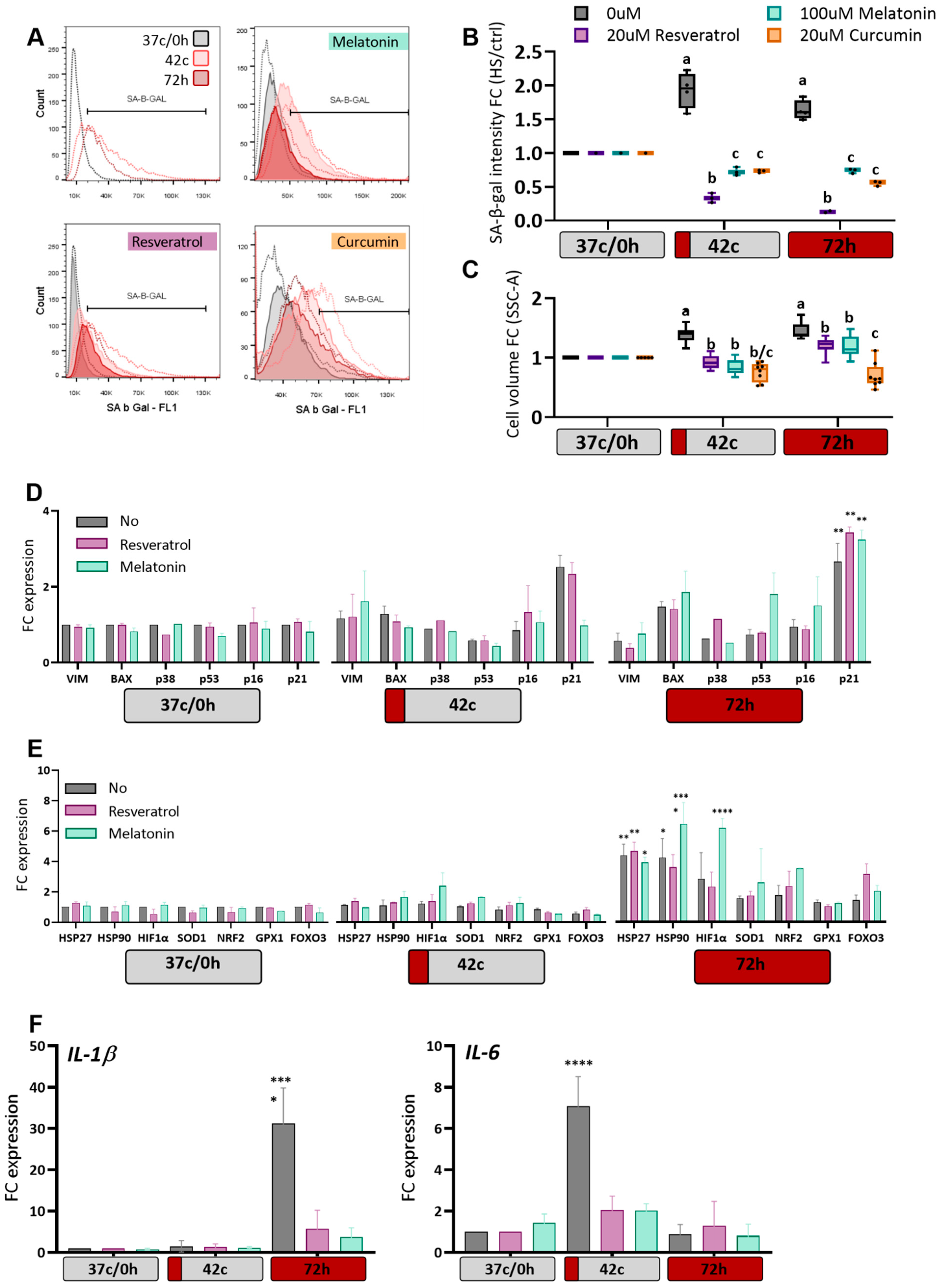
2.4. Antioxidants Protection of MSC from Heat-Shock Induced Premature Senescence
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Induction of Heat-Shock
4.3. Antioxidant Calibration
4.4. Antioxidant Treatments
4.5. Cell Death Quantification Using Propidium Iodide (PI)
4.6. Quantification of Reactive Oxygen Species
4.7. Mitochondrial Membrane Potential Measurement
4.8. Cell Proliferation Assay
4.9. Population Doubling (PD) Calculation
4.10. Senescence-Associated β-Galactosidase Marker Assay
4.11. RNA Extraction, Reverse Transcription (RT), and Quantitative PCR (RT-qPCR)
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olde Riekerink, R.G.; Barkema, H.W.; Kelton, D.F.; Scholl, D.T. Incidence Rate of Clinical Mastitis on Canadian Dairy Farms. J. Dairy Sci. 2008, 91, 1366–1377. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, J.; Peng, D.; Li, G.; Chen, J.; Gu, X. Exposure to heat-stress environment affects the physiology, circulation levels of cytokines, and microbiome in dairy cows. Sci. Rep. 2018, 8, 14606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Key, N.; Sneeringer, S.; Marquardt, D. Economic Research Service Economic Research Report Number 175 United States Department of Agriculture Climate Change, Heat Stress, and U.S. Dairy Production; United States Department of Agriculture: Washington, DC, USA, 2014. [Google Scholar]
- Roth, Z. Influence of heat stress on reproduction in dairy cows—Physiological and practical aspects. J. Anim. Sci. 2020, 98, S80–S87. [Google Scholar] [CrossRef] [PubMed]
- Belhadj Slimen, I.; Najar, T.; Ghram, A.; Dabbebi, H.; Ben Mrad, M.; Abdrabbah, M. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int. J. Hyperth. 2014, 30, 513–523. [Google Scholar] [CrossRef]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef] [Green Version]
- Burgess, R.J.; Agathocleous, M.; Morrison, S.J. Metabolic regulation of stem cell function. J. Intern. Med. 2014, 276, 12–24. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, Y.; Dezawa, M. Mesenchymal Stem Cells and Their Subpopulation, Pluripotent Muse Cells, in Basic Research and Regenerative Medicine. Anat. Rec. 2014, 297, 98–110. [Google Scholar] [CrossRef]
- Gao, F.; Chiu, S.M.; Motan, D.A.L.; Zhang, Z.; Chen, L.; Ji, H.-L.; Tse, H.-F.; Fu, Q.-L.; Lian, Q. Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death Dis. 2016, 7, e2062. [Google Scholar] [CrossRef] [Green Version]
- Deak, E.; Seifried, E.; Henschler, R. Homing Pathways of Mesenchymal Stromal Cells (MSCs) and Their Role in Clinical Applications. Int. Rev. Immunol. 2010, 29, 514–529. [Google Scholar] [CrossRef]
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef]
- Moraes, D.A.; Sibov, T.T.; Pavon, L.F.; Alvim, P.Q.; Bonadio, R.S.; Da Silva, J.R.; Pic-Taylor, A.; Toledo, O.A.; Marti, L.C.; Azevedo, R.B.; et al. A reduction in CD90 (THY-1) expression results in increased differentiation of mesenchymal stromal cells. Stem Cell Res. Ther. 2016, 7, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lara, E.; Acurio, J.; Leon, J.; Penny, J.; Torres-Vergara, P.; Escudero, C. Are the Cognitive Alterations Present in Children Born From Preeclamptic Pregnancies the Result of Impaired Angiogenesis? Focus on the Potential Role of the VEGF Family. Front. Physiol. 2018, 9, 1591. [Google Scholar] [CrossRef] [PubMed]
- Surico, D.; Bordino, V.; Cantaluppi, V.; Mary, D.; Gentilli, S.; Oldani, A.; Farruggio, S.; Melluzza, C.; Raina, G.; Grossini, E. Preeclampsia and intrauterine growth restriction: Role of human umbilical cord mesenchymal stem cells-trophoblast cross-talk. PLoS ONE 2019, 14, e0218437. [Google Scholar] [CrossRef] [PubMed]
- Grompe, M. Tissue Stem Cells: New Tools and Functional Diversity. Cell Stem Cell 2012, 10, 685–689. [Google Scholar] [CrossRef] [Green Version]
- Naaldijk, Y.; Johnson, A.A.; Ishak, S.; Meisel, H.J.; Hohaus, C.; Stolzing, A. Migrational changes of mesenchymal stem cells in response to cytokines, growth factors, hypoxia, and aging. Exp. Cell Res. 2015, 338, 97–104. [Google Scholar] [CrossRef]
- Gugjoo, M.B.; Amarpal; Chandra, V.; Wani, M.Y.; Dhama, K.; Sharma, G.T. Mesenchymal Stem Cell Research in Veterinary Medicine. Curr. Stem Cell Res. Ther. 2018, 13, 645–657. [Google Scholar] [CrossRef]
- Shimoni, C.; Goldstein, M.; Ribarski-Chorev, I.; Schauten, I.; Nir, D.; Strauss, C.; Schlesinger, S. Heat Shock Alters Mesenchymal Stem Cell Identity and Induces Premature Senescence. Front. Cell Dev. Biol. 2020, 8, 956. [Google Scholar] [CrossRef]
- Labora, J.A.F.; O’Loghlen, A. Classical and Nonclassical Intercellular Communication in Senescence and Ageing. Trends Cell Biol. 2020, 30, 628–639. [Google Scholar] [CrossRef]
- Hu, C.; Li, L. The application of resveratrol to mesenchymal stromal cell-based regenerative medicine. Stem Cell Res. Ther. 2019, 10, 307. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Han, Y.-S.; Lee, S.H. Potentiation of biological effects of mesenchymal stem cells in ischemic conditions by melatonin via upregulation of cellular prion protein expression. J. Pineal Res. 2017, 62, e12385. [Google Scholar] [CrossRef]
- Yousefi, F.; Arab, F.L.; Jaafari, M.R.; Rastin, M.; Tabasi, N.; Hatamipour, M.; Nikkhah, K.; Mahmoudi, M. Immunoregulatory, proliferative and anti-oxidant effects of nanocurcuminoids on adipose-derived mesenchymal stem cells. EXCLI J. 2019, 18, 405–421. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Yoon, D.S.; Lee, K.-M.; Choi, S.M.; Lee, M.-H.; Park, K.H.; Han, S.H.; Lee, J.W. Enhancement of Mesenchymal Stem Cell-Driven Bone Regeneration by Resveratrol-Mediated SOX2 Regulation. Aging Dis. 2019, 10, 818–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kornienko, J.S.; Smirnova, I.S.; Pugovkina, N.A.; Ivanova, J.S.; Shilina, M.A.; Grinchuk, T.M.; Shatrova, A.N.; Aksenov, N.D.; Zenin, V.V.; Nikolsky, N.N.; et al. High doses of synthetic antioxidants induce premature senescence in cultivated mesenchymal stem cells. Sci. Rep. 2019, 9, 1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, D.S.; Choi, Y.; Choi, S.M.; Park, K.H.; Lee, J.W. Different effects of resveratrol on early and late passage mesenchymal stem cells through β-catenin regulation. Biochem. Biophys. Res. Commun. 2015, 467, 1026–1032. [Google Scholar] [CrossRef]
- Hu, C.; Li, L. Melatonin plays critical role in mesenchymal stem cell-based regenerative medicine in vitro and in vivo. Stem Cell Res. Ther. 2019, 10, 13. [Google Scholar] [CrossRef] [Green Version]
- Hosseinzadeh, A.; Kamrava, S.K.; Joghataei, M.T.; Darabi, R.; Shakeri-Zadeh, A.; Shahriari, M.; Reiter, R.J.; Ghaznavi, H.; Mehrzadi, S. Apoptosis signaling pathways in osteoarthritis and possible protective role of melatonin. J. Pineal Res. 2016, 61, 411–425. [Google Scholar] [CrossRef]
- Rodriguez, C.; Mayo, J.C.; Sainz, R.M.; Antolin, I.; Herrera, F.; Martin, V.; Reiter, R.J. Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res. 2004, 36, 1–9. [Google Scholar] [CrossRef]
- Song, N.; Kim, A.J.; Kim, H.-J.; Jee, H.J.; Kim, M.; Yoo, Y.H.; Yun, J. Melatonin suppresses doxorubicin-induced premature senescence of A549 lung cancer cells by ameliorating mitochondrial dysfunction. J. Pineal Res. 2012, 53, 335–343. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, D.; Wang, X.; Chen, X.; Long, Y.; Chai, W.; Zhou, X.; Rui, X.; Zhang, Q.; Wang, H.; et al. Melatonin improved rat cardiac mitochondria and survival rate in septic heart injury. J. Pineal Res. 2013, 55, 1–6. [Google Scholar] [CrossRef]
- Lee, J.H.; Yoon, Y.M.; Song, K.H.; Noh, H.; Lee, S.H. Melatonin suppresses senescence-derived mitochondrial dysfunction in mesenchymal stem cells via the HSPA1L–mitophagy pathway. Aging Cell 2020, 19, e13111. [Google Scholar] [CrossRef] [Green Version]
- Yun, S.P.; Han, Y.-S.; Lee, J.H.; Kim, S.M.; Lee, S.H. Melatonin Rescues Mesenchymal Stem Cells from Senescence Induced by the Uremic Toxin p-Cresol via Inhibiting mTOR-Dependent Autophagy. Biomol. Ther. 2018, 26, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Gorabi, A.M.; Kiaie, N.; Hajighasemi, S.; Jamialahmadi, T.; Majeed, M.; Sahebkar, A. The Effect of Curcumin on the Differentiation of Mesenchymal Stem Cells into Mesodermal Lineage. Molecules 2019, 24, 4029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, M.; Huang, W.-Q.; Qiu, W.; Zhu, F.-Q.; Xing, W.; Chen, M.-J.; An, T.-C.; Ao, L.-Q.; Xu, X.; Huang, H. Curcumin protects mesenchymal stem cells against oxidative stress-induced apoptosis via Akt/mTOR/p70S6K pathway. Int. J. Clin. Exp. Pathol. 2017, 10, 6655–6664. [Google Scholar]
- Hsuuw, Y.-D.; Chang, C.-K.; Chan, W.-H.; Yu, J.-S. Curcumin prevents methylglyoxal-induced oxidative stress and apoptosis in mouse embryonic stem cells and blastocysts. J. Cell. Physiol. 2005, 205, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Attari, F.; Zahmatkesh, M.; Aligholi, H.; Mehr, S.E.; Sharifzadeh, M.; Gorji, A.; Mokhtari, T.; Khaksarian, M.; Hassanzadeh, G. Curcumin as a double-edged sword for stem cells: Dose, time and cell type-specific responses to curcumin. DARU J. Pharm. Sci. 2015, 23, 2703–2706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckert, G.P.; Schiborr, C.; Hagl, S.; Abdel-Kader, R.; Müller, W.E.; Rimbach, G.; Frank, J. Curcumin prevents mitochondrial dysfunction in the brain of the senescence-accelerated mouse-prone 8. Neurochem. Int. 2013, 62, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, P.P.; Helson, L. Curcumin and cancer stem cells: Curcumin has asymmetrical effects on cancer and normal stem cells. Anticancer Res. 2015, 35, 599–614. [Google Scholar]
- Watson, J.L.; Hill, R.; Yaffe, P.B.; Greenshields, A.; Walsh, M.; Lee, P.W.; Giacomantonio, C.A.; Hoskin, D.W. Curcumin causes superoxide anion production and p53-independent apoptosis in human colon cancer cells. Cancer Lett. 2010, 297, 1–8. [Google Scholar] [CrossRef]
- Cianfruglia, L.; Minnelli, C.; Laudadio, E.; Scirè, A.; Armeni, T. Side Effects of Curcumin: Epigenetic and Antiproliferative Implications for Normal Dermal Fibroblast and Breast Cancer Cells. Antioxidants 2019, 8, 382. [Google Scholar] [CrossRef] [Green Version]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O.; et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef] [Green Version]
- Alekseenko, L.L.; Zemelko, V.I.; Domnina, A.P.; Lyublinskaya, O.G.; Zenin, V.V.; Pugovkina, N.A.; Kozhukharova, I.V.; Borodkina, A.V.; Grinchuk, T.M.; Fridlyanskaya, I.I.; et al. Sublethal heat shock induces premature senescence rather than apoptosis in human mesenchymal stem cells. Cell Stress Chaperon 2014, 19, 355–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, W.; Yong, D.; El-Jawhari, J.J.; Cuthbert, R.; Mcgonagle, D.; Naing, M.W.; Jones, E. Identification of senescent cells in multipotent mesenchymal stromal cell cultures: Current methods and future directions. Cytotherapy 2019, 21, 803–819. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.M.; Pedersen, B.K. The anti-inflammatory effect of exercise. J. Appl. Physiol. (1985) 2005, 98, 1154–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collier, R.J.; Stiening, C.M.; Pollard, B.C.; VanBaale, M.J.; Baumgard, L.H.; Gentry, P.C.; Coussens, P.M. Use of gene expression microarrays for evaluating environmental stress tolerance at the cellular level in cattle1. J. Anim. Sci. 2006, 84 (Suppl. 13), E1–E13. [Google Scholar] [CrossRef] [PubMed]
- Collier, R.J.; Renquist, B.J.; Xiao, Y. A 100-Year Review: Stress physiology including heat stress. J. Dairy Sci. 2017, 100, 10367–10380. [Google Scholar] [CrossRef]
- Iside, C.; Scafuro, M.; Nebbioso, A.; Altucci, L. SIRT1 Activation by Natural Phytochemicals: An Overview. Front. Pharmacol. 2020, 11, 1225. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Gibellini, L.; Bianchini, E.; De Biasi, S.; Nasi, M.; Cossarizza, A.; Pinti, M. Natural Compounds Modulating Mitochondrial Functions. Evid. Based Complement. Altern. Med. 2015, 2015, 527209. [Google Scholar] [CrossRef] [Green Version]
- Dare, A.J.; Phillips, A.R.; Hickey, A.J.; Mittal, A.; Loveday, B.; Thompson, N.; Windsor, J.A. A systematic review of experimental treatments for mitochondrial dysfunction in sepsis and multiple organ dysfunction syndrome. Free Radic. Biol. Med. 2009, 47, 1517–1525. [Google Scholar] [CrossRef]
- Kra, G.; Daddam, J.R.; Gabay, H.; Yosefi, S.; Zachut, M. Antioxidant Resveratrol Increases Lipolytic and Reduces Lipogenic Gene Expression under In Vitro Heat Stress Conditions in Dedifferentiated Adipocyte-Derived Progeny Cells from Dairy Cows. Antioxidants 2021, 10, 905. [Google Scholar] [CrossRef]
- Alfadda, A.A.; Sallam, R.M. Reactive Oxygen Species in Health and Disease. J. Biomed. Biotechnol. 2012, 2012, 936486. [Google Scholar] [CrossRef] [PubMed]
- Denu, R.A.; Hematti, P. Effects of Oxidative Stress on Mesenchymal Stem Cell Biology. Oxid. Med. Cell. Longev. 2016, 2016, 2989076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Payton, R.R.; Rispoli, L.A.; Nagle, K.A.; Gondro, C.; Saxton, A.M.; Voy, B.H.; Edwards, J.L. Mitochondrial-related consequences of heat stress exposure during bovine oocyte maturation persist in early embryo development. J. Reprod. Dev. 2018, 64, 243–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, P.J. To be or not to be—Determinants of embryonic survival following heat shock. Theriogenology 2007, 68 (Suppl. 1), S40–S48. [Google Scholar] [CrossRef]
- Ko, E.; Lee, K.Y.; Hwang, D.S. Human Umbilical Cord Blood–Derived Mesenchymal Stem Cells Undergo Cellular Senescence in Response to Oxidative Stress. Stem Cells Dev. 2012, 21, 1877–1886. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.K.; Banerjee, S.; Acosta, E.P.; Lillard, J.W.; Singh, R. Resveratrol induces cell cycle arrest and apoptosis with docetaxel in prostate cancer cells via a p53/p21WAF1/CIP1 and p27KIP1 pathway. Oncotarget 2017, 8, 17216. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.-W.; Huang, H.-C. Effect of curcumin on cell cycle progression and apoptosis in vascular smooth muscle cells. Br. J. Pharmacol. 1998, 124, 1029–1040. [Google Scholar] [CrossRef] [Green Version]
- Martín-Renedo, J.; Mauriz, J.L.; Jorquera, F.; Ruiz-Andrés, O.; González, P.; González-Gallego, J. Melatonin induces cell cycle arrest and apoptosis in hepatocarcinoma HepG2 cell line. J. Pineal Res. 2008, 45, 532–540. [Google Scholar] [CrossRef]
- Childs, B.G.; Durik, M.; Baker, D.J.; Van Deursen, J.M. Cellular senescence in aging and age-related disease: From mechanisms to therapy. Nat. Med. 2015, 21, 1424–1435. [Google Scholar] [CrossRef] [Green Version]
- Coppé, J.-P.; Patil, C.K.; Rodier, F.; Krtolica, A.; Beauséjour, C.M.; Parrinello, S.; Hodgson, J.G.; Chin, K.; Desprez, P.-Y.; Campisi, J. A Human-Like Senescence-Associated Secretory Phenotype Is Conserved in Mouse Cells Dependent on Physiological Oxygen. PLoS ONE 2010, 5, e9188. [Google Scholar] [CrossRef]
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular Senescence: Defining a Path Forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, Q.; Yujia, W.; Li, L.; Bu, H.; Bao, J. Senescence of mesenchymal stem cells (Review). Int. J. Mol. Med. 2017, 39, 775–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz-Espín, D.; Serrano, M. Cellular senescence: From physiology to pathology. Nat. Rev. Mol. Cell Biol. 2014, 15, 482–496. [Google Scholar] [CrossRef]
- Kirkland, J.L.; Tchkonia, T. Senolytic drugs: From discovery to translation. J. Intern. Med. 2020, 288, 518–536. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Lam, E.W.; Sun, Y. Senescent cells: A new Achilles’ heel to exploit for cancer medicine? Aging Cell 2019, 18, e12875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Soleimani, M.; Nadri, S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat. Protoc. 2009, 4, 102–106. [Google Scholar] [CrossRef]
- Toupadakis, C.A.; Wong, A.; Genetos, D.C.; Cheung, W.K.; Borjesson, D.L.; Ferraro, G.L.; Galuppo, L.D.; Leach, J.K.; Owens, S.D.; Yellowley, C.E. Comparison of the osteogenic potential of equine mesenchymal stem cells from bone marrow, adipose tissue, umbilical cord blood, and umbilical cord tissue. Am. J. Veter. Res. 2010, 71, 1237–1245. [Google Scholar] [CrossRef]
- Sakai, S.; Hatabu, T.; Yamamoto, Y.; Kimura, K. Alteration of chemokine production in bovine endometrial epithelial and stromal cells under heat stress conditions. Physiol. Rep. 2020, 8, e14640. [Google Scholar] [CrossRef]
- Ido, Y.; Duranton, A.; Lan, F.; Weikel, K.A.; Breton, L.; Ruderman, N.B. Resveratrol Prevents Oxidative Stress-Induced Senescence and Proliferative Dysfunction by Activating the AMPK-FOXO3 Cascade in Cultured Primary Human Keratinocytes. PLoS ONE 2015, 10, e0115341. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
| Name | Target Gene | Sequence | Foreword/ Reverse |
|---|---|---|---|
| RPS9 | Ribosomal protein S9 | GAAGCTGATCGGCGAGTATG | Forward |
| RPS9 | Ribosomal protein S9 | GATCTTGGCCAGGGTGAAT | Reverse |
| PSMB2 | Proteasome subunit beta 2 | GATGCGAAATGGTTATGAACTG | Forward |
| PSMB2 | Proteasome subunit beta 2 | AGGTTTCGGCGAGTGAAAT | Reverse |
| HIF1 | Hypoxia-inducible factor 1 | ATTTTGGCAGCAATGACACA | Forward |
| HIF1 | Hypoxia-inducible factor 1 | CCAAATTTATATTCTGCAATTTCTCA | Reverse |
| NRF2 | Nuclear factor erythroid 2 | CCTCAAAGCACCGTCCTCAG | Forward |
| NRF2 | Nuclear factor erythroid 2 | CAATCAAATCCATGTCCTGCTGG | Reverse |
| FOXO3 | Forkhead box protein O3 | ACAAACGGCTCACTCTGTCC | Forward |
| FOXO3 | Forkhead box protein O3 | GTGCCGGATAGAGTTCTTCCA | Reverse |
| GPX1 | Glutathione Peroxidase 1 | CAACGGTGCGGGACTACA | Forward |
| GPX1 | Glutathione Peroxidase 1 | CCTCGTTCTTGGCGTTTTCC | Reverse |
| SOD1 | Superoxide dismutase 1 | AAGGGAGATACAGTCGTGGT | Forward |
| SOD1 | Superoxide dismutase 1 | CAAACTGATGGACGTGGAATC | Reverse |
| PRDX1 | Peroxiredoxin 1 | GTCACCTGGCATGGATCAAC | Forward |
| PRDX1 | Peroxiredoxin | GACCCCATAGTCCTGAGCAA | Reverse |
| HSP90 | Heat shock protein 90 | GAGCAGTATGCCTGGGAGTC | Forward |
| HSP90 | Heat shock protein 90 | CCATTGGTTCTCCTGTGTCA | Reverse |
| HSP27 | Heat shock protein 27 | CCCTGGACGTCAACCACTT | Forward |
| HSP27 | Heat shock protein 27 | CTCGTGCTTGCCAGTGATCT | Reverse |
| BAX | Bcl-2-associated X | GCTTCAGGGTTTCATCCAGGA | Forward |
| BAX | Bcl-2-associated X | TCAGACACTCGCTCAGCTTC | Reverse |
| P38 | Mitogen-activated protein kinase 14 (MAPK14) | TTCCAAGGGCTACACCAAGT | Forward |
| P38 | Mitogen-activated protein kinase 14 (MAPK14) | TGGTTCAGCTGGTCAAGGTA | Reverse |
| P16 | CDKN2A | CCCTCGTGCTGATGGCTAGT | Forward |
| P16 | CDKN2A | CCCATCATCATCACCTGGTCTA | Reverse |
| P53 | Tumor protein p53 | ACTCTTCAGATCCGTGGGTTTA | Forward |
| P53 | Tumor protein p53 | CCATCCAGAGCATCCTTCAG | Reverse |
| P21 | CDKN1A | CGCCAGCTGAGGTGTGAG | Forward |
| P21 | CDKN1A | ATGGCACCTGTGGCTCTTCT | Reverse |
| VIM | Vimentin | GATGGACAGGTTATCAACGAAACT | Forward |
| VIM | Vimentin | TCCTTCTTGCTGGTAGTATTTTGC | Reverse |
| IL-1β | Interleukin 1 beta | AGCATCCTTTCATTCATCTTTGAAG | Forward |
| IL-1β | Interleukin 1 beta | GGGTGCGTCACACAGAAACTC | Reverse |
| IL-6 | Interleukin 6 | GGGCTCCCATGATTGTGGTA | Forward |
| IL-6 | Interleukin 6 | GTGTGCCCAGTGGACAGGTT | Reverse |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nir, D.; Ribarski-Chorev, I.; Shimoni, C.; Strauss, C.; Frank, J.; Schlesinger, S. Antioxidants Attenuate Heat Shock Induced Premature Senescence of Bovine Mesenchymal Stem Cells. Int. J. Mol. Sci. 2022, 23, 5750. https://doi.org/10.3390/ijms23105750
Nir D, Ribarski-Chorev I, Shimoni C, Strauss C, Frank J, Schlesinger S. Antioxidants Attenuate Heat Shock Induced Premature Senescence of Bovine Mesenchymal Stem Cells. International Journal of Molecular Sciences. 2022; 23(10):5750. https://doi.org/10.3390/ijms23105750
Chicago/Turabian StyleNir, Dana, Ivana Ribarski-Chorev, Chen Shimoni, Carmit Strauss, Jan Frank, and Sharon Schlesinger. 2022. "Antioxidants Attenuate Heat Shock Induced Premature Senescence of Bovine Mesenchymal Stem Cells" International Journal of Molecular Sciences 23, no. 10: 5750. https://doi.org/10.3390/ijms23105750
APA StyleNir, D., Ribarski-Chorev, I., Shimoni, C., Strauss, C., Frank, J., & Schlesinger, S. (2022). Antioxidants Attenuate Heat Shock Induced Premature Senescence of Bovine Mesenchymal Stem Cells. International Journal of Molecular Sciences, 23(10), 5750. https://doi.org/10.3390/ijms23105750






