Liver Graft Hypothermic Static and Oxygenated Perfusion (HOPE) Strategies: A Mitochondrial Crossroads
Abstract
1. Introduction
2. Cold Ischemic Insult and Liver Graft Cold Storage
3. Mitochondrial Protection and Preservation Solutions in Static Cold Storage
4. New Additives for Improving Cold Static Preservation: Oxygen Carriers, Ozone, and AMPK Inducers
4.1. Oxygen Carriers: M-101
4.2. Ozone
4.3. Other Antioxidants
4.4. Adenosine Monophosphate Protein Kinase (AMPK) Inducers
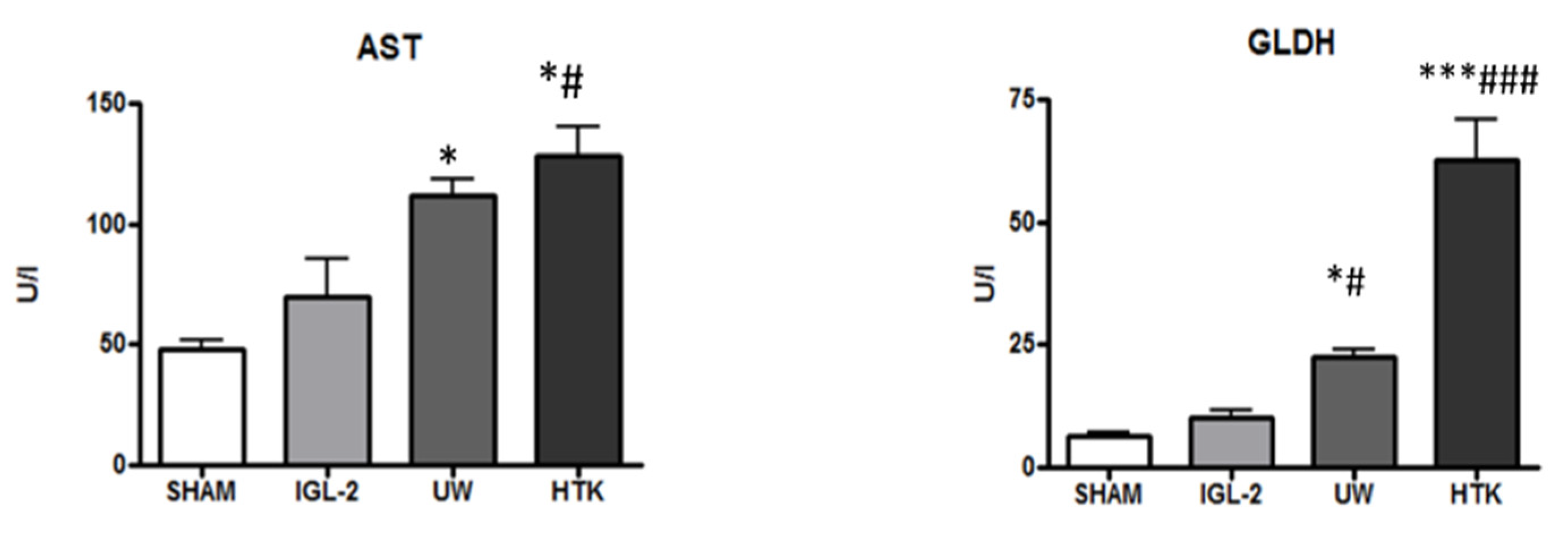
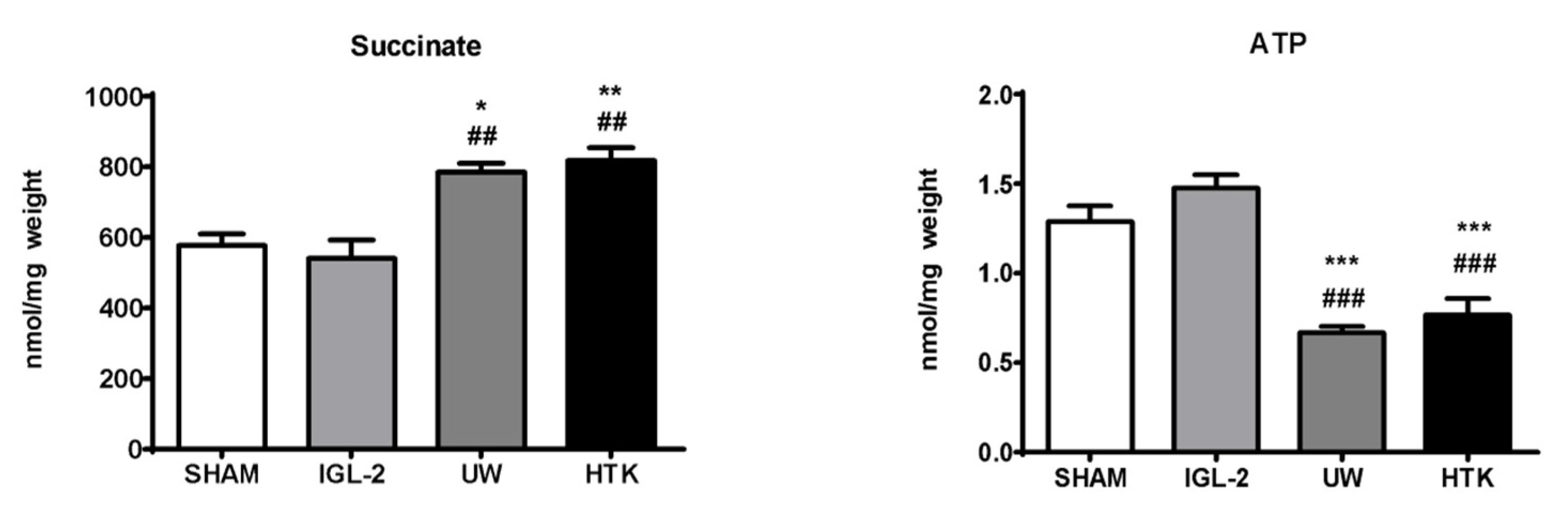
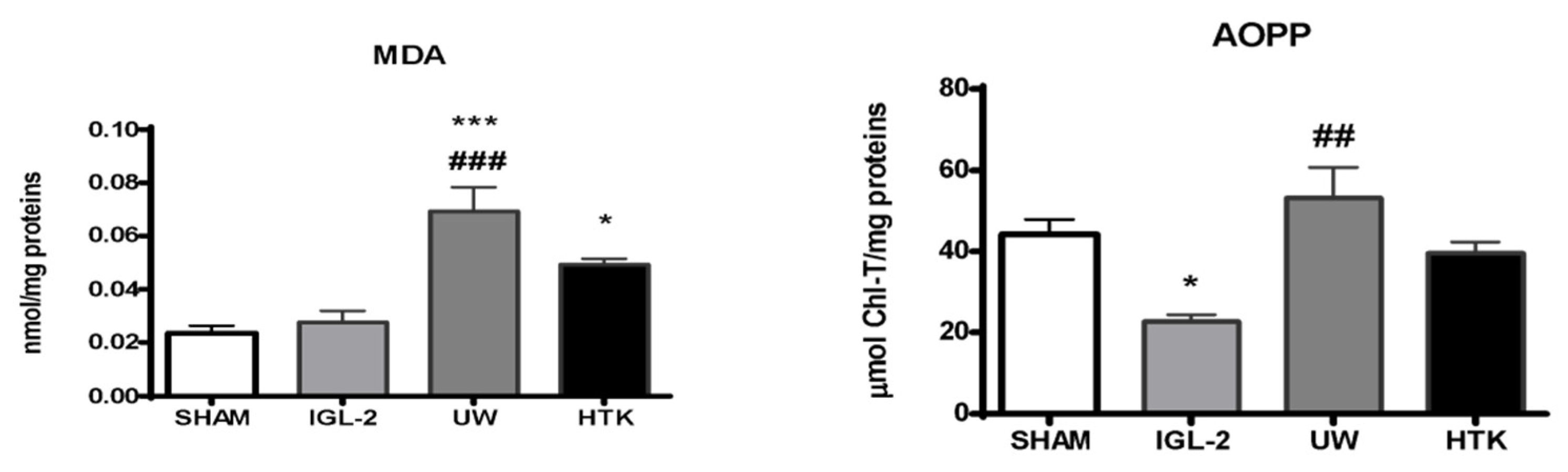
5. HOPE, Mitochondrial and Glycocalyx Protection, and PEG35 Effluents (IGL-2)
6. Some Considerations and Concluding Remarks
Summary
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 4H NE | 4-Hydroxynonenal |
| ALDH2 | Aldehyde dehydrogenase-2 |
| ALT | Alanine aminotransferase |
| AMPK | AMP-activated protein kinase |
| AST | Aspartate aminotransferase |
| Belzer MPS | Belzer Machine Perfusion Solution |
| DCD | Donor after cardiac death |
| eNOS | Endothelial nitric oxide synthase |
| GCX | Glycocalyx |
| GLDH | Glutamate dehydrogenase |
| PEG35 | Polyethylene glycol 35 |
| HES | Hydroxyethyl starch |
| HOPE | Hypothermic oxygenated perfusion |
| HTK | Histidine-tryptophan-ketoglutarate |
| IGL-1/IGL-2 | Institut Georges Lopez 1/Institut Georges Lopez 2 |
| IRI | Ischemia–reperfusion injury |
| MP | Machine perfusion |
| NO | Nitric oxide |
| ROS | Radical oxygen species |
| SCS | Static cold storage |
| TX | Liver transplantation |
| UCP2 | Uncoupling protein-2 |
| UW | University of Wisconsin |
References
- Belzer, F.O. Organ Preservation: Organ Preservation: A Personal Perspective. Early Experience in Kidney Transplantation. Available online: https://web.stanford.edu/dept/HPST/transplant/html/belzer.html (accessed on 15 March 2022).
- Southard, J.H. UW organ preservation solution. Transplantation 2020, 104, 1764–1766. [Google Scholar] [CrossRef] [PubMed]
- Belzer, F.O.; Southard, J.H. Principles of solid organ preservation by cold storage. Transplantation 1988, 45, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Kalayoglu, M.; Sollinger, H.W.; Stratta, R.J.; D’Alessandro, A.M.; Hoffmann, R.M.; Pirsch, J.D.; Belzer, F.O. Extended preservation of the liver for clinical preservation. Lancet 1988, 1, 617–619. [Google Scholar] [PubMed]
- D’Alessandro, A.M.; Stratta, R.J.; Sollinger, H.W.; Kalayoglu, M.; Pirsch, J.D.; Belzer, F.O. Use of UW solution in pancreas transplantation. Diabetes 1989, 38 (Suppl. S1), 7–9. [Google Scholar] [CrossRef]
- Southard, J.H.; van Gulik, T.M.; Ametani, M.S.; Vreugdenhil, P.K.; Lindell, S.L.; Pienaar, B.L.; Belzer, F.O. Important components of the UW Wisconsin solution. Transplantation 1990, 49, 251–257. [Google Scholar] [CrossRef]
- Belzer, F.O. Organ preservation. Annu. Rev. Med. 1995, 46, 235–247. [Google Scholar]
- Zaouali, M.A.; Ben Abdennebi, H.; Padrissa-Altés, S.; Mahfoudh-Boussaid, A.; Rosello-Catafau, J. Pharmacological strategies against cold ischemia reperfusion injury. Expert Opin. Pharm. 2010, 11, 537–555. [Google Scholar] [CrossRef]
- Guibert, E.E.; Petrenko, A.Y.; Balaban, C.L.; Somov, A.Y.; Rodriguez, J.V.; Fuller, B.J. Organ preservation: Current concepts and new strategies for the next decade. Transfus. Med. Hemother. 2011, 38, 125–142. [Google Scholar] [CrossRef]
- Fuller, B.; Froghi, F.; Davidson, B. Organ preservation solutions: Linking pharmacology to survival for the donor organ pathway. Curr. Opin. Organ Transplant. 2018, 23, 361–368. [Google Scholar] [CrossRef]
- Peralta, C.; Roselló-Catafau, J. The future of fatty livers. J. Hepatol. 2004, 41, 14–15. [Google Scholar] [CrossRef]
- Busutil, R.W.; Tanaka, K. The utility of marginal donors in liver transplantation. Liver Transpl. 2003, 9, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Selzner, M.; Clavien, P.A. Fatty liver transplantation and surgery. Semin. Liver Dis. 2001, 21, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Linares, I.; Hamar, M.; Selzner, N.; Selzner, M. Steatosis in Liver Transplantation: Current Limitations and Future Strategies. Transplantation 2019, 103, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.J.; Hickey, A.J.; Phillips, A.R.; Bartlett, A.S. The impact of hepatic steatosis on hepatic ischemia-reperfusion injury in experimental studies: A systematic review. BioMed Res. Int. 2013, 2013, 192029. [Google Scholar]
- Ijaz, S.; Yang, W.; Winslet, M.C.; Seifalian, A.M. Impairment of hepatic microcirculation in fatty liver. Microcirculation 2003, 10, 447–456. [Google Scholar] [CrossRef]
- Deschenes, M.; Belle, S.H.; Krom, R.A.; Zetterman, R.K.; Lake, J.R. Early allograft dysfunction after liver transplantation: A definition and predictors of outcome. National Institute of Diabetes and Digestive and Kidney Diseases Liver Transplantation Database. Transplantation 1998, 66, 302–310. [Google Scholar] [CrossRef]
- Tashiro, H.; Kuroda, S.; Mikuriya, Y.; Ohdan, H. Ischemia reperfusion injury in patients with fatty liver and the clinical impact of steatotic liver on hepatic surgery. Surg. Today 2014, 44, 1611–1625. [Google Scholar] [CrossRef]
- Said, A. Non alcoholic fatty liver diseases and trasplantation: Outcomes and advances. World J. Gastroenterol. 2013, 28, 9126–9155. [Google Scholar]
- Panisello-Roselló, A.; Alva, N.; Flores, M.; Lopez, A.; Castro Benítez, C.; Folch-Puy, E.; Rolo, A.; Palmeira, C.; Adam, R.; Carbonell, T.; et al. Aldehyde Dehydrogenase 2 (ALDH2) in rat fatty liver cold ischemia injury. Int. J. Mol. Sci. 2018, 19, 2479. [Google Scholar]
- Zaoualí, M.A.; Reiter, R.J.; Padrissa-Altés, S.; Boncompagni, E.; García, J.J.; Ben Abnennebi, H.; Freitas, I.; García-Gil, F.A.; Rosello-Catafau, J. Melatonin protects steatotic and non steatotic liver grafts against old ischemia and reperfusion injury. J. Pineal Res. 2011, 50, 13–21. [Google Scholar]
- Adam, R.; Cailliez, V.; Majno, P.; Karam, V.; McMaster, P.; Caine, R.Y.; O’Grady, J.; Pichlmayr, R.; Neuhaus, P.; Otte, J.B.; et al. 416 Normalised intrinsic mortality risk in liver transplantation: European Liver Transplant Registry study. Lancet 2000, 56, 621–627. [Google Scholar] [CrossRef]
- Horváth, T.; Jász, D.K.; Baraáth, B.; Poles, M.Z.; Boros, M.; Hartmann, P. Mitochondrial consequences of organ preservation techniques during liver transplantation. Int. J. Mol. Sci. 2021, 22, 2816. [Google Scholar] [CrossRef] [PubMed]
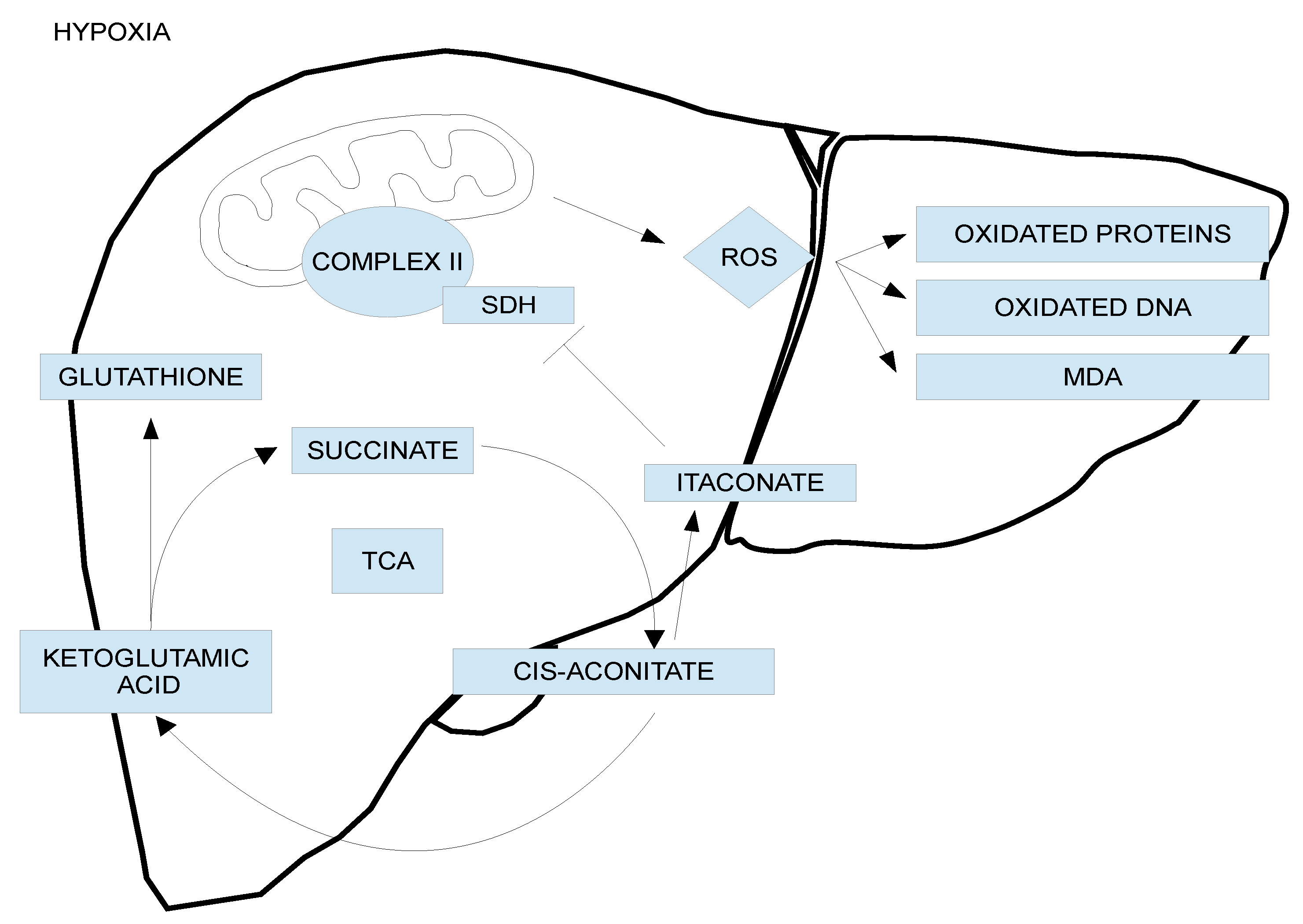
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijevic, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.; Muller, X.; Mueller, M.; Stepanova, A.; Kron, P.; de Rougemont, O.; Muiesan, P.; Clavien, P.A.; Galkin, A.; Meierhofer, D.; et al. Hypothermic oxygenated perfusion protects from mitochondrial injury before liver transplantation. eBioMedicine 2020, 60, 103014. [Google Scholar] [CrossRef]
- Martins, P.N.; Schlegel, A.; Ghinolfi, D. Cold or Not So Cold?—Static Organ Preservation at 10 °C May Prolong Organ Preservation and Facilitate Transplant Logistics. Transplantation 2022, 106, 427–429. [Google Scholar] [CrossRef]
- Cordes, T.; Lucas, A.; Divakaruni, A.S.; Murphy, A.N.; Cabrales, P.; Metallo, C.M. Itaconate modulates tricarboxylic acid and redox metabolim to mitigate reperfusion injury. Mol. Metab. 2020, 32, 122–135. [Google Scholar] [CrossRef]
- Lampropoulou, V.; Sergushichev, A.; Bambouskova, M.; Nair, S.; Vincent, E.E.; Loginicheva, E.; Cervantes-Barragan, L.; Ma, X.; Huang, S.C.-C.; Griss, T.; et al. Itaconate links inhibition of succinate dehydrogenase with macrophage metabolic remodeling and regulation of inflammation. Cell Metab. 2016, 24, 158–166. [Google Scholar] [CrossRef]
- Bejaoui, M.; Pantazi, E.; Folch-Puy, E.; Baptista, P.M.; Garcia-Gil, A.; Adam, R.; Rosello-Catafau, J. Emerging concepts in liver graft preservation. World J. Gastroenterol. 2015, 21, 396–407. [Google Scholar] [CrossRef]
- Guarrera, J.V.; Henry, S.D.; Samstein, B.; Odeh-Ramadan, R.; Kinkhabwala, M.; Goldstein, M.J.; Ratner, L.E.; Renz, J.F.; Lee, H.T.; Brown, R.S., Jr.; et al. Hypothermic machine preservation in human liver transplantation: The first inical series. Am. J. Transplant. 2010, 10, 372–381. [Google Scholar] [CrossRef]
- Kron, P.; Schlegel, A.; Mancina, L.; Clavien, P.A.; Dutkowski, P. Hypothermic oxigenated perfusion (HOPE) for fatty livers in rats and humans. J. Hepatol. 2018, 68, 82–91. [Google Scholar] [CrossRef]
- Adam, R.; Delvart, V.; Karam, V.; Ducerf, C.; Navarro, F.; Letoublon, C.; Belghiti, J.; Pezet, D.; Castaing, D.; Le Treut, Y.P.; et al. ELTR contributing centers, the European Liver, Intestine Transplant Association (ELITA). Compared eficacy of preservation solutions in liver transplantation: A long-term graft outcome study from the European Liver Transplant Registry. Am. J. Transplant. 2015, 15, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Ben Mosbah, I.; Roselló-Catafau, J.; Franco-Gou, R.; Abdennebi, H.B.; Saidane, D.; Ramella-Virieux, S.; Boillot, O.; Peralta, C. Preservation of steatotic livers in IGL-1 solution. Liver Transplant. 2006, 12, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Zaouali, M.A.; Bejaoui, M.; Calvo, M.; Folch-Puy, E.; Pantazi, E.; Pasut, G.; Rimola, A.; Abdennebi, H.B.; Adam, R.; Roselló-Catafau, J. Polyethylene glycol rinse solution: An efective way to prevent ischemia-reperfusion injury. World J. Gastroenterol. 2015, 20, 16203–16214. [Google Scholar] [CrossRef] [PubMed]
- Pasut, G.; Panisello, A.; Folch-Puy, E.; Lopez, A.; Castro-Benítez, C.; Calvo, M.; Carbonell, T.; García-Gil, A.; Adam, R.; Roselló-Catafau, J. Polyethylene glycols: An effective strategy for limiting liver ischemia reperfusion injury. World J. Gastroenterol. 2016, 22, 6501–6508. [Google Scholar] [CrossRef] [PubMed]
- Padrissa-Altes, S.; Zaouali, M.A.; Rosello-Catafau, J. AMP-activated protein kinase as a target for preconditioning in transplantation medicine. Transplantation 2010, 90, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Ben Abdennebi, H.; Zaoualí, M.A.; Alfany-Fernandez, I.; Donia Tabka, D.; Roselló-Catafau, J. How to protect liver graft with nitric oxide. World J. Gastroenterol. 2011, 17, 879–2889. [Google Scholar] [CrossRef] [PubMed]
- Bardallo, R.G.; da Silva, R.T.; Carbonell, T.; Folch-Puy, E.; Carlos Palmeira, C.; Roselló-Catafau, J.; Pirenne, J.; Adam, R.; Panisello-Roselló, A. Mitochondrial ALDH2, and Glutathione in Cold Fatty Liver Graft Preservation: An IGL-2 Approach. Int. J. Mol. Sci. 2021, 22, 5332. [Google Scholar] [CrossRef] [PubMed]
- Evans, Z.P.; Ellett, J.D.; Schmidt, M.G.; Schnellmann, R.G.; Chavin, K.D. Mitochondrial Uncoupling Protein-2 Mediates Steatotic Liver Injury following Ischemia/Reperfusion. J. Biol.Chem. 2008, 283, 8573–8579. [Google Scholar] [CrossRef]
- Chen, G.G.; Yan, J.B.; Wang, X.M.; Zheng, M.Z.; Jiang, J.P.; Zhou, X.M.; Cai, B.; Shen, Y.L. Mechanism of uncoupling protein 2 mediated myocardial injury in hypothermic preserved rat hearts. Mol. Med. Rep. 2016, 14, 1857–1864. [Google Scholar] [CrossRef][Green Version]
- Petrenko, A.Y.; Cherkashina, D.V.; Somov, A.Y.; Tkacheva, E.N.; Semenchenko, O.A.; Lebedinsky, A.S.; Fuller, B.J. Reversible mitochondrial uncoupling in the cold phase during liver preservation/reperfusion reduces oxidative injury in the rat model. Cryobiology 2010, 60, 293–300. [Google Scholar] [CrossRef]
- Rial, E.; Rodríguez-Sánchez, L.; Aller, P.; Guisado, A.; González-Barroso, M.M.; Gallardo-Vara, E.; Redondo-Horcajo, M.; Castellanos, E.; de la Pradilla, R.F.; Viso, A. Development of chromanes as novel inhibitors of the uncoupling proteins. Chem. Biol. 2011, 18, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Panisello-Roselló, A.; Lopez, A.; Folch-Puy, E.; Carbonell, T.; Rolo, A.; Palmeira, C.; Adam, R.; Net, M.; Roselló-Catafau, J. Role of aldehyde dehydrogenase 2 in ischemia reperfusion injury: An update. World J. Gastroenterol. 2018, 24, 2984–2994. [Google Scholar] [CrossRef] [PubMed]
- Bardallo, R.G.; Company-Marin, I.; Folch-Puy, E.; Roselló-Catafau, J.; Panisello-Roselló, A.; Carbonell, T. PEG35 and Glutathione Improve Mitochondrial Function and Reduce Oxidative Stress in Cold Fatty Liver Graft Preservation. Antioxidants 2022, 11, 158. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, F.S.; Fernandez Monteiro, I.; Rosello-Catafau, J.; Peralta, C. Hepatic microcirculatory failure. Acta Cir. Bras. 2006, 21 (Suppl. S1), 48–53. [Google Scholar] [CrossRef] [PubMed]
- Alix, P.; Val-Laillet, D.; Turlin, B.; Ben Mosbah, I.; Burel, A.; Bobillier, E.; Bendavid, C.; Delpy, E.; Zal, F.; Corlu, A.; et al. Adding the oxygen carrier M101 to a cold-storage solution could be an alternative to HOPE for liver graft preservation. JHEP Rep. 2020, 2, 100119. [Google Scholar] [CrossRef]
- Asong-Fontem, N.; Panisello-Rosello, A.; Lopez, A.; Katsunor, I.; Zal, F.; Delpy, E.; Rosello-Catafau, J.; Adam, R. A Novel Oxygen Carrier (M101) Attenuates Ischemia-Reperfusion Injuries during Static Cold Storage in Steatotic Livers. Int. J. Mol. Sci. 2021, 22, 8542. [Google Scholar] [CrossRef]
- Hernandez, F.; Menendez, S.; Wong, R. Decrease of blood cholesterol and stimulation of antioxidative response in cardiopathy patients treated with endovenous ozone therapy. Free Radic. Biol. Med. 1995, 19, 115–119. [Google Scholar] [CrossRef]
- Peralta, C.; Leon, O.S.; Xaus, C.; Prats, N.; Jalil, E.C.; Planell, E.S.; Puig-Parellada, P.; Gelpí, E.; Roselló-Catafau, J. Protective effect of ozone treatment on the injury associated with hepatic ischemia reperfusion: Antioxidant-prooxidant balance. Free Radic. Res. 1999, 31, 191–196. [Google Scholar] [CrossRef]
- Aydın, H.O.; Ayvazoğlu, E.H.; Soy, T.T.; Avc, T.; Erken, M.; Yıldırım, S.; Haberal, M. Effect of Ozone Added to University of Wisconsin Solution on Preservation Damage in Perfused Liver. Exp. Clin. Transplant. 2022; Online ahead of print. [Google Scholar] [CrossRef]
- Peralta, C.; Xaus, C.; Bartrons, R.; Leon, O.S.; Gelpi, E.; Rosello-Catafau, J. Effect of Ozone Treatment on Reactive Oxygen Species and Adenosine Production During Hepatic Ischemia-Reperfusion. Free Radic. Res. 2000, 33, 595–605. [Google Scholar] [CrossRef]
- Boudjema, K.; Van Gulik, T.M.; Lindell, S.L.; Vreugdenhil, P.S.; Southard, J.H.; Belzer, F.O. Effect of oxidized and reduced glutathione in liver preservation. Transplantation 1990, 50, 948–951. [Google Scholar] [CrossRef] [PubMed]
- van Breussegem, A.; van Pelt, J.; Wylin, T.; Heedfeld, V.; Zeegers, M.; Monbaliu, D.; Pirenne, J.; Vekemans, K. Presumed and actual concentrations of reduced glutathione in preservation solutions. Transplant. Proc. 2011, 43, 3451–3454. [Google Scholar] [CrossRef] [PubMed]
- Ostrózka-Cieslik, A. The Effect of Antioxidant Added to Preservation Solution on the Protection of Kidneys before Transplantation. Int. J. Mol. Sci. 2022, 23, 3141. [Google Scholar] [CrossRef] [PubMed]
- Haberal, M.; Kirnap, M.; Erdem, S.R.; Ozdemir, B.H.; Lux, K.M.; Bacanli, D. Evaluation of New Baskent University Preservation Solution for Kidney Graft During Cold Ischemia: Preliminary Experimental Animal Study. Exp. Clin. Transplant. 2019, 17, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Aliakbarian, M.; Nikeghbalian, S.; Ghaffaripour, S.; Bahreini, A.; Shafiee, M.; Rashidi, M.; Rajabnejad, Y. Effects of N-Acetylcysteine Addition to University Wisconsin solution on the Rate of Ischemia-Reperfusion Injury in Adult Orthotopic Liver Transplant. Exp. Clin. Transplant. 2017, 15, 432–436. [Google Scholar] [PubMed]
- Baker, C.J.; Longoria, J.; Gade, P.V.; Starnes, V.A.; Barr, M.L. Addition of a water-soluble alpha-tocopherol analogue to University of Wisconsin solution improves endothelial viability and decreases lung reperfusion injury. J. Surg. Res. 1999, 86, 145–149. [Google Scholar] [CrossRef]
- Carling, D. AMPK signalling in health and disease. Curr. Opin. Cell Biol. 2017, 45, 31–37. [Google Scholar] [CrossRef]
- Hardie, D.G. The AMP-activated protein kinase cascade: The key sensor of cellular energy status. Endocrinology 2003, 144, 5179–5183. [Google Scholar] [CrossRef]
- Hardie, D.G.; Hawley, S.A.; Scott, J.W. AMP-activated protein kinase–development of the energy sensor concept. J. Physiol. 2006, 574, 7–15. [Google Scholar] [CrossRef]
- Ben Mosbah, I.; Massip-Salcedo, M.; Fernández-Monteiro, I.; Xaus, C.; Bartrons, R.; Boillot, O.; Rosello-Catafau, J.; Peralta, C. Addition of adenosine monophosphate activated protein kinase activators to University of Wisconsin solution: A way of protecting rat steatotic livers. Liver Transpl. 2007, 13, 410–425. [Google Scholar] [CrossRef]
- Chai, Y.C.; Dang, G.X.; He, H.Q.; Shi, J.H.; Zhang, H.K.; Zhang, R.T.; Wang, B.; Hu, L.S.; Lv, Y. Hypothermic machine perfusion with metformin-University of Wisconsin solution for ex vivo preservation of standard and marginal liver grafts in a rat model. World J. Gastroenterol. 2017, 23, 7221–7231. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Mohammadi, P.; Hozeififi, S.; Hoseinzadeh-Chahkandak, F.; Xu, S.; Farzaei, M.H. Natural AMPK Activators in Cardiovascular Disease Prevention. Front. Pharmacol. 2022, 12, 738420. [Google Scholar]
- Zaouali, M.A.; Boncompagni, E.; Reiter, R.J.; Bejaoui, M.; Freitas, I.; Pantazi, E.; Folch-Puy, E.; Abdennebi, H.B.; Garcia-Gil, F.A.; Rosello-Catafau, J. AMPK involvement in endoplasmic reticulum stress and autophagy modulation after fatty liver graft preservation: A role for melatonin and trimetazidine cocktail. J. Pineal Res. 2013, 55, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Bejaoui, M.; Pantazi, E.; De Luca, V.; Arnau Panisello, A.; Folch-Puy, E.; Hotter, G.; Clemente Capasso, C.; Supuran, C.; Roselló-Catafau, J. Carbonic Anhydrase Protects Fatty Liver Grafts against Ischemic Reperfusion Damage. PLoS ONE 2015, 10, e0134499. [Google Scholar] [CrossRef]
- Panisello-Roselló, A.; Verde, E.; Amine Zaouali, M.; Flores, M.; Alva, N.; Lopez, A.; Folch-Puy, E.; Carbonell, T.; Hotter, G.; Adam, R.; et al. The Relevance of the UPS in Fatty Liver Graft Preservation: A New Approach for IGL-1 and HTK Solutions. Int. J. Mol. Sci. 2017, 18, 2287. [Google Scholar] [CrossRef]
- Panisello Rosello, A.; da Silva, R.T.; Castro, C.; Bardallo, R.G.; Calvo, M.; Folch-Puy, E.; Carbonell, T.; Palmeira, C.; Roselló Catafau, J.; René Adam, R. Polyethylene Glycol 35 as a Perfusate Additive for Mitochondrial and Glycocalyx Protection in HOPE Liver Preservation. Int. J. Mol. Sci. 2020, 21, 5703. [Google Scholar] [CrossRef]
- Muller, X.; Mohkam, K.; Mueller, M.; Schlegel, A.; Dondero, F.; Sepulveda, A.; Savier, E.; Scatton, O.; Bucur, P.; Salame, E.; et al. Hypothermic Oxygenated Perfusion Versus Normothermic Regional Perfusion in Liver Transplantation From Controlled Donation After Circulatory Death: First International Comparative Study. Ann. Surg. 2020, 272, 751–758. [Google Scholar] [CrossRef]
- van Rijn, R.; Schurink, I.J.; de Vries, Y.; van den Berg, A.P.; Cortes Cerisuelo, M.; Darwish Murad, S.; Erdmann, J.I.; Gilbo, N.; de Haas, R.J.; Heaton, N.; et al. Hypothermic Machine Perfusion in Liver Transplantation—A Randomized Trial. N. Engl. J. Med. 2021, 384, 1391–1401. [Google Scholar] [CrossRef]
- Czigany, Z.; Pratschke, J.; Froněk, J.; Guba, M.; Schöning, W.; Raptis, D.A.; Andrassy, J.; Kramer, M.; Strnad, P.; Tolba, R.H.; et al. Hypothermic Oxygenated Machine Perfusion Reduces Early Allograft Injury and Improves Post-transplant Outcomes in Extended Criteria Donation Liver transplantation From Donation After Brain Death: Results From a Multicenter Randomized Controlled Trial (HOPE ECD-DBD). Ann. Surg. 2021, 274, 705–712. [Google Scholar]
- Thorne, A.M.; Lantinga, V.; Bodewes, S.; de Kleine, R.H.J.; Nijkamp, M.W.; Sprakel, J.; Hartog, H.; Polak, W.G.; Porte, R.J.; de Meijer, V.E. Ex Situ Dual Hypothermic Oxigenated Machine Perfusion for Human split liver Transplantation. Transplant. Direct 2021, 7, e666. [Google Scholar] [CrossRef]
- Wang, S.; Zeng, X.; Yang, Y.; Li, S.; Wang, Y.; Ye, Q.; Fan, X. Hypothermic oxigenated perfusion ameliorates ischemia-reperfusion injury of fatty liver in mice via Brg1/Nrf2/HO-1 axis. Artif. Organs 2022, 46, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Morariu, A.M.; Van der Plaats, A.; Oeveren, W.V.; Hart, N.A.T.; Leuvenik, H.G.D.; Graaff, R.; Ploegh, R.J.; Rakhorst, G. Hyperaggregating effect of hydroxyethyl starch components and University of Wisconsin solution on human red blood cells:a risk of impaired graft perfusion in organ procurement? Transplantation 2003, 76, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Ben Mosbah, I.; Franco-Gou, R.; Ben Abdennebi, H.; Hernandez, R.; Escolar, G.; Saidane, D.; Rosello-Catafau, J.; Peralta, C. Effects of polyethylene glycol and hydroxyethyl starch in University of Wisconsin preservation on human red blood cell aggregation and viscosity. Transplant. Proc. 2006, 38, 1229–1235. [Google Scholar] [CrossRef]
- Van Golen, R.F.; van Gulik, T.M.; Heger, M. Mechanistic overview of reactive species-induced degradation of the endothelial glycocalyx during hepatic ischemia/reperfusion injury. Free Radic. Biol. Med. 2012, 52, 1382–1402. [Google Scholar] [CrossRef]
- van Golen, R.; Reiniers, M.J.; Vrisekoop, N.; Zuurbier, C.J.; Olthof, P.B.; van Rheenen, J.; van Gulik, T.M.; Parsons, B.J.; Heger, M. The mechanisms and physiological relevance of glycocalyx degradation in hepatic is chemia/reperfusion injury. Antioxid. Redox Signal. 2014, 21, 1098–1118. [Google Scholar] [CrossRef] [PubMed]
- Panisello-Rosello, A.; Roselló-Catafau, J. HOPE (hypothermic oxygenated perfusion) strategies in the era of dynamic liver graft preservation. eBioMedicine 2020, 61, 103071. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Xiang, T.; Qiu, Q.; Leung, J.; Xu, J.; Zhou, W.; Hu, Q.; Lan, J.; Liu, Z.; Zhong, Z.; et al. Aldehyde dehydrogenase 2 regulates autophagy via the Akt-mTOR pathway to mitigate renal ischemia-reperfusion injury in hypothermic machine perfusion. Life Sci. 2020, 253, 117705. [Google Scholar] [CrossRef]
- Panisello-Roselló, A.; Verde, E.; Lopez, A.; Flores, M.; Folch-Puy, E.; Rolo, A.; Palmeira, C.; Hotter, G.; Carbonell, T.; Adam, R.; et al. Cytoprotective Mechanisms in Fatty Liver Preservation against Cold Ischemia Injury: A Comparison between IGL-1 and HTK. Int. J. Mol. Sci. 2018, 19, 348. [Google Scholar] [CrossRef]
- Lopez, A.; Panisello-Rosello, A.; Castro-Benitez, C.; Adam, R. Glycocalyx Preservation and NO Production in Fatty Livers-The Protective Role of High Molecular Polyethylene Glycol in Cold Ischemia Injury. Int. J. Mol. Sci. 2018, 19, 2375. [Google Scholar] [CrossRef]
- Da Silva, R.T.; Bardallo, R.G.; Folch-Puy, E.; Carbonell, T.; Palmeira, C.M.; Fondevila, C.; Adam, R.; Rosello-Catafau, J.; Panisello-Roselló, A. IGL-2 as a Unique Solution for Cold Static Preservation and Machine Perfusion in Liver and Mitochondrial Protection. Transplant. Proc. 2022, 54, 73–76. [Google Scholar] [CrossRef]
- Mabrut, J.Y.; Lesurtel, M.; Muller, X.; Dubois, R.; Ducerf, C.; Rossignol, G.; Mohkam, K. Ex Vivo Liver Splitting and Hypothermic Oxygenated Machine Perfusion: Technical Refinements of a Promising Preservation Strategy in Split Liver Transplantation. Transplantation 2021, 105, e89–e90. [Google Scholar] [CrossRef] [PubMed]
- Panisello-Roselló, A.; da Silva, R.T.; Folch-Puy, E.; Carbonell, T.; Palmeira, C.M.; Fondevila, C.; Roselló-Catafau, J.; Adam, R. The Use of a Single, Novel Preservation Solution in Split Liver Transplantation and Hypothermic Oxygenated Machine Perfusion. Transplantation 2022, 106, e187–e188. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A. The Long Road to Identify a Reliable Viability Test in Liver Transplantation. Transplantation 2022, 106, 702–704. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhang, X.F.; Fu, B.M.; Tarbell, J.M. The Role of Endothelial Surface Glycocalyx in Mechanosensing and Transduction. Adv. Exp. Med. Biol. 2018, 1097, 1–27. [Google Scholar] [PubMed]
- Passov, A.; Schramko, A.; Mäkisalo, H.; Nordin, A.; Andersson, S.; Pesonen, E.; Ilmakunnas, M. Graft glycocalyx degradation in human liver transplantation. PLoS ONE 2019, 14, e0221010. [Google Scholar] [CrossRef]
- Schiefer, J.; Faybik, P.; Koch, S.; Tudor, B.; Kollmann, D.; Kuessel, L.; Krenn, C.G.; Berlakovich, G.; Baron, D.M.; Baron-Stefaniak, J. Glycocalyx Damage Within Human Liver Grafts Correlates With Graft Injury and Postoperative Graft Function After Orthotopic Liver Transplantation. Transplantation 2020, 104, 72–78. [Google Scholar] [CrossRef]
- Panisello-Roselló, A.; Castro Benitez, C.; Lopez, A.; da Silva, R.T.; Roselló-Catafau, J.; Adam, R. Glycocalyx as a Useful Marker of Endothelial Injury in Liver Transplantation. The Role of Preservation Solution. Transplantation 2020, 104, e356–e357. [Google Scholar] [CrossRef]
- Marsh, D.S.; Lindell, S.L.; Fox, L.E.; Belzer, O.F.; Southard, J.H. Hypothermic preservation of hepatocytes. Role of cell swelling. Criobiology 1989, 26, 524–534. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bardallo, R.G.; Da Silva, R.T.; Carbonell, T.; Palmeira, C.; Folch-Puy, E.; Roselló-Catafau, J.; Adam, R.; Panisello-Rosello, A. Liver Graft Hypothermic Static and Oxygenated Perfusion (HOPE) Strategies: A Mitochondrial Crossroads. Int. J. Mol. Sci. 2022, 23, 5742. https://doi.org/10.3390/ijms23105742
Bardallo RG, Da Silva RT, Carbonell T, Palmeira C, Folch-Puy E, Roselló-Catafau J, Adam R, Panisello-Rosello A. Liver Graft Hypothermic Static and Oxygenated Perfusion (HOPE) Strategies: A Mitochondrial Crossroads. International Journal of Molecular Sciences. 2022; 23(10):5742. https://doi.org/10.3390/ijms23105742
Chicago/Turabian StyleBardallo, Raquel G., Rui T. Da Silva, Teresa Carbonell, Carlos Palmeira, Emma Folch-Puy, Joan Roselló-Catafau, René Adam, and Arnau Panisello-Rosello. 2022. "Liver Graft Hypothermic Static and Oxygenated Perfusion (HOPE) Strategies: A Mitochondrial Crossroads" International Journal of Molecular Sciences 23, no. 10: 5742. https://doi.org/10.3390/ijms23105742
APA StyleBardallo, R. G., Da Silva, R. T., Carbonell, T., Palmeira, C., Folch-Puy, E., Roselló-Catafau, J., Adam, R., & Panisello-Rosello, A. (2022). Liver Graft Hypothermic Static and Oxygenated Perfusion (HOPE) Strategies: A Mitochondrial Crossroads. International Journal of Molecular Sciences, 23(10), 5742. https://doi.org/10.3390/ijms23105742










