TGFβ-Treated Placenta-Derived Mesenchymal Stem Cells Selectively Promote Anti-Adipogenesis in Thyroid-Associated Ophthalmopathy
Abstract
:1. Introduction
2. Results
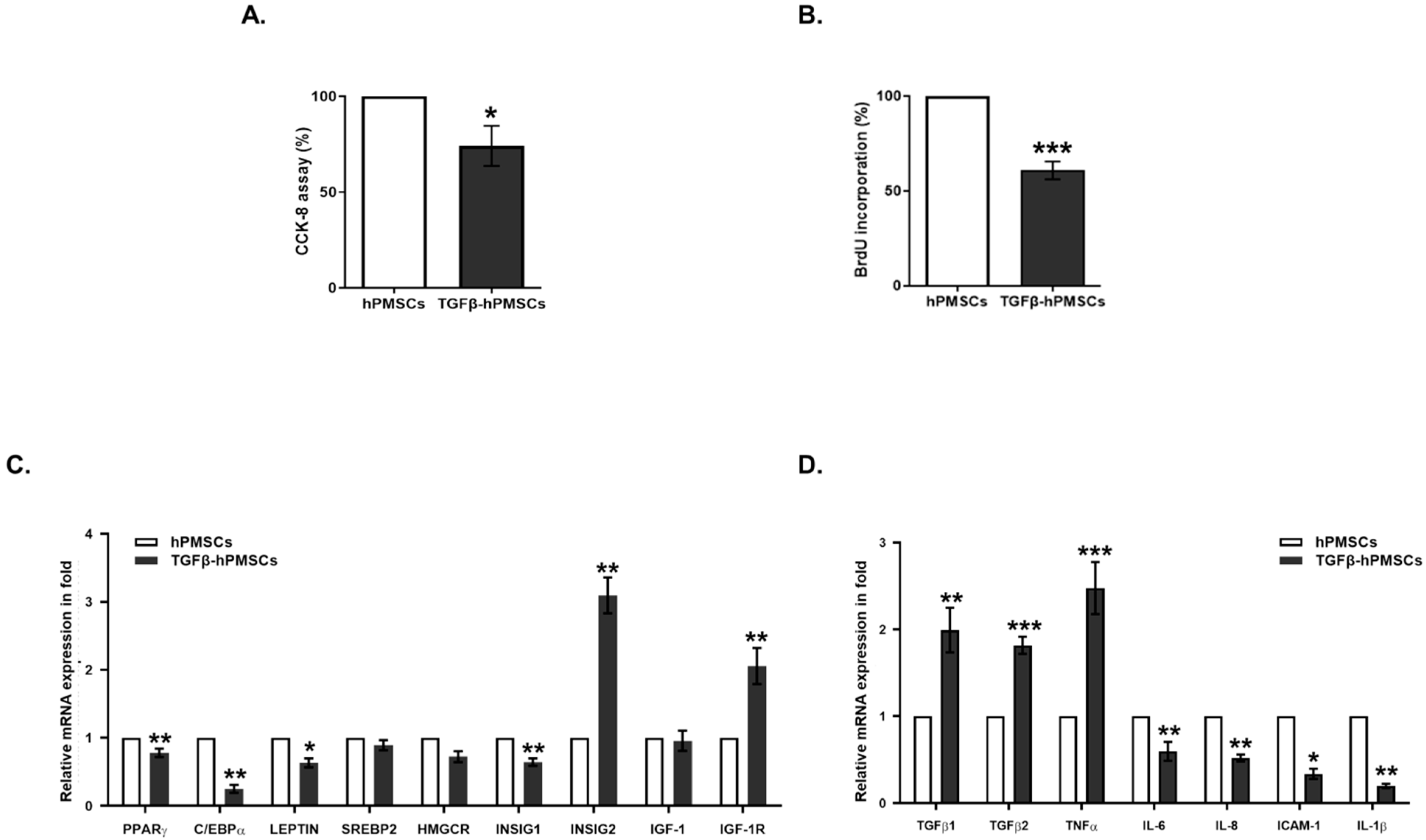
2.1. Characterization of hPMSCs Treated with TGFβ1
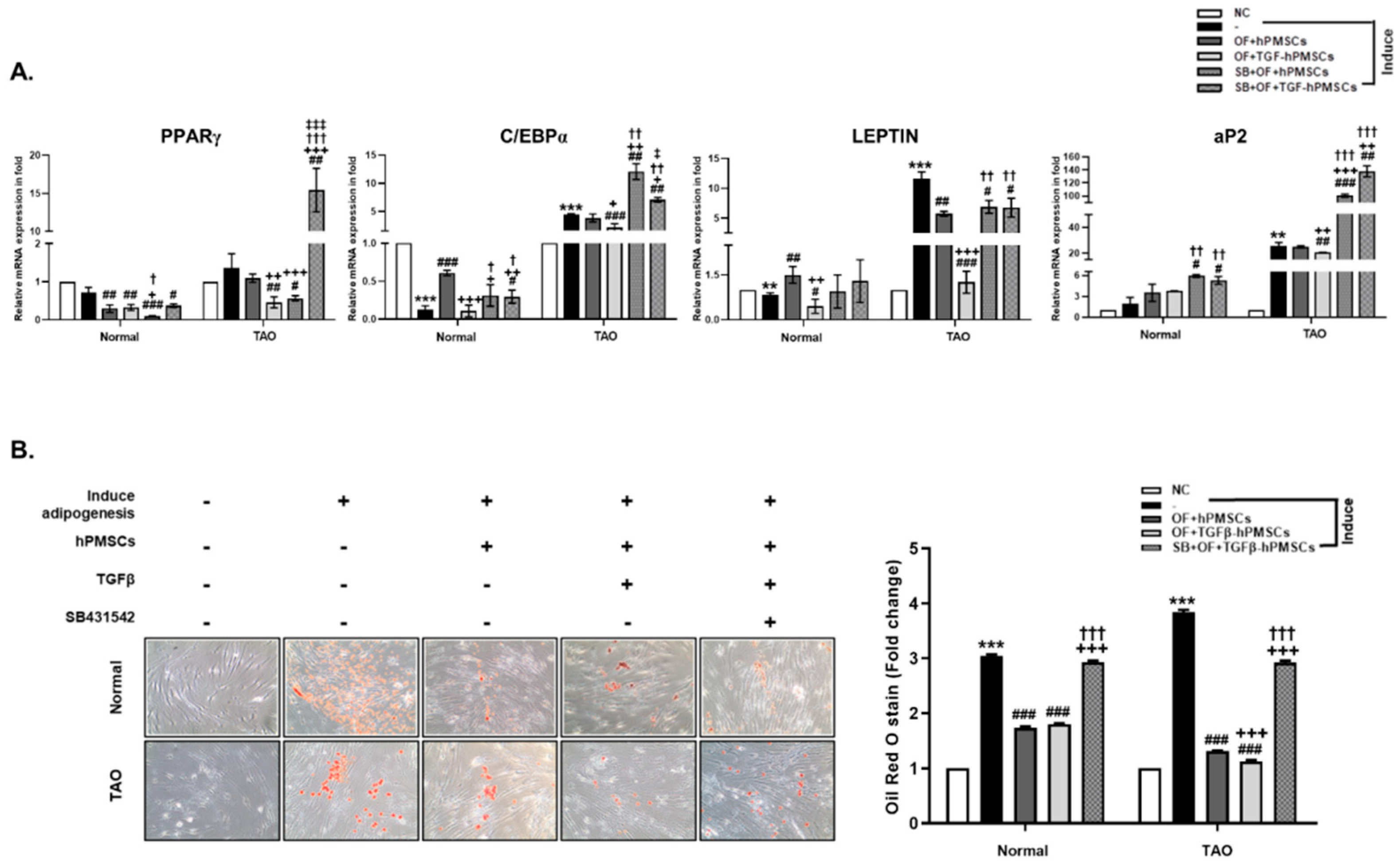
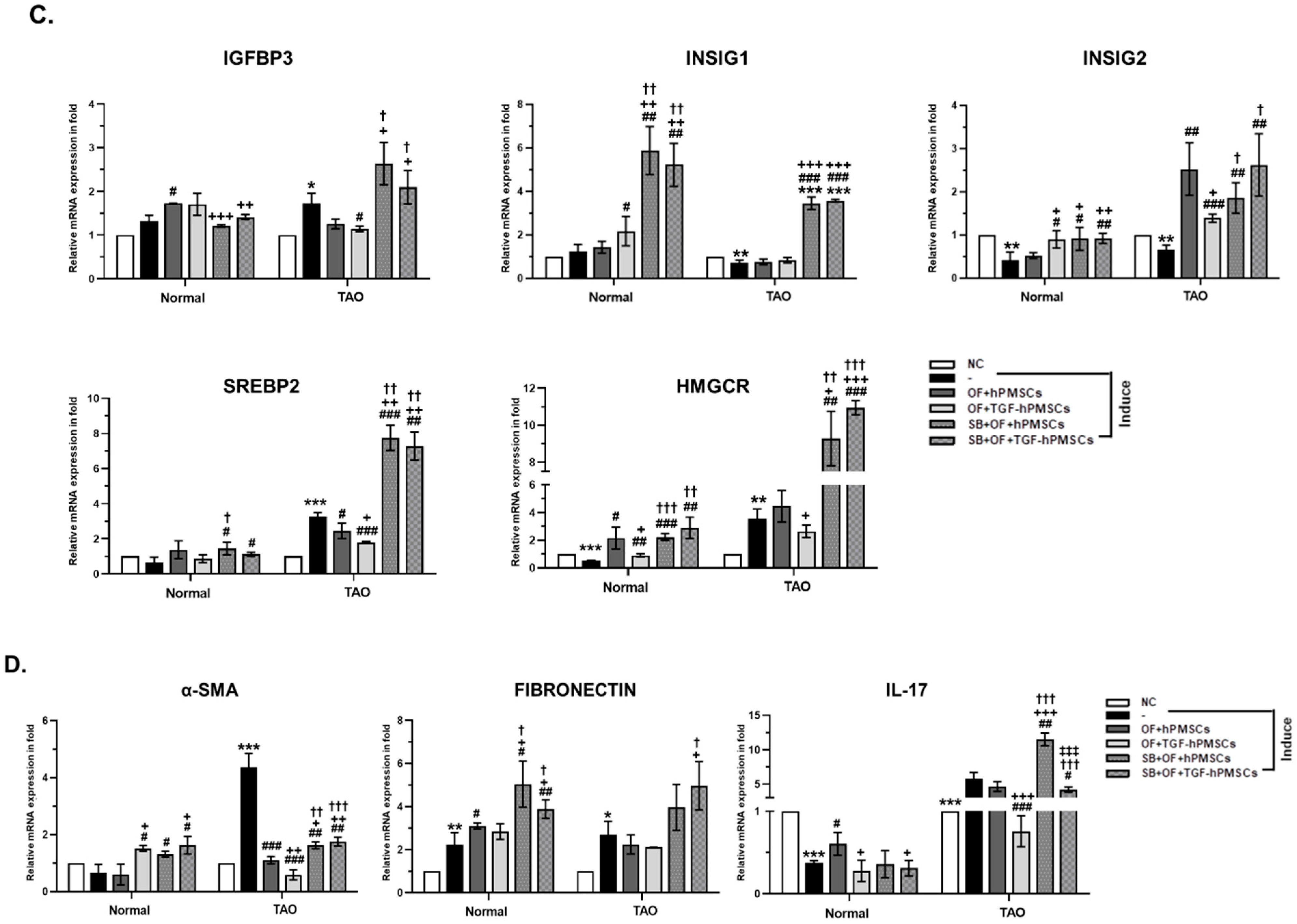
2.2. Effect of TGFβ-hPMSCs on TAO-Derived OFs
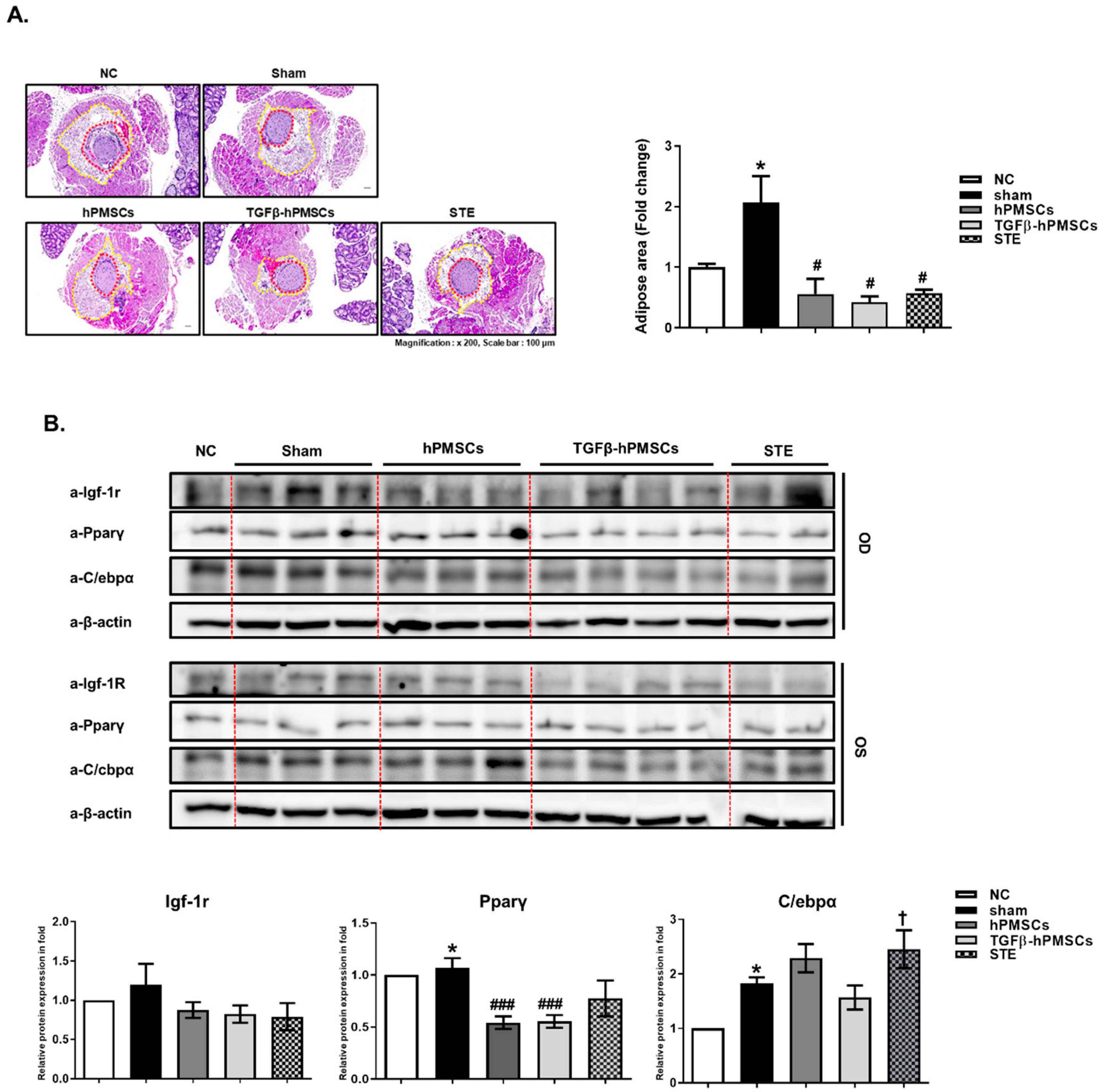
2.3. Pathology Assessment
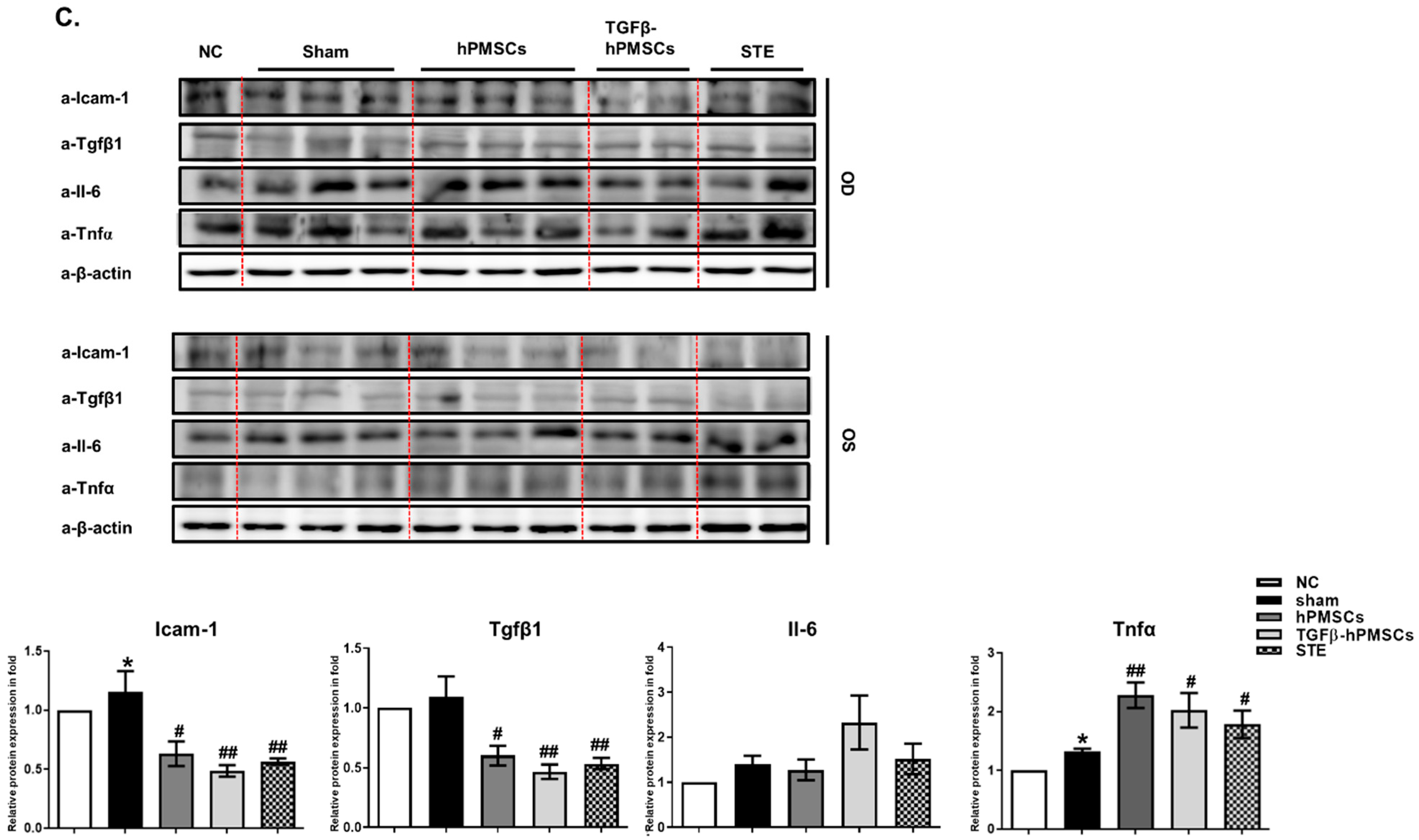
2.4. TGFβ-hPMSCs Inhibit Adipogenesis and Inflammation in TAO Animals
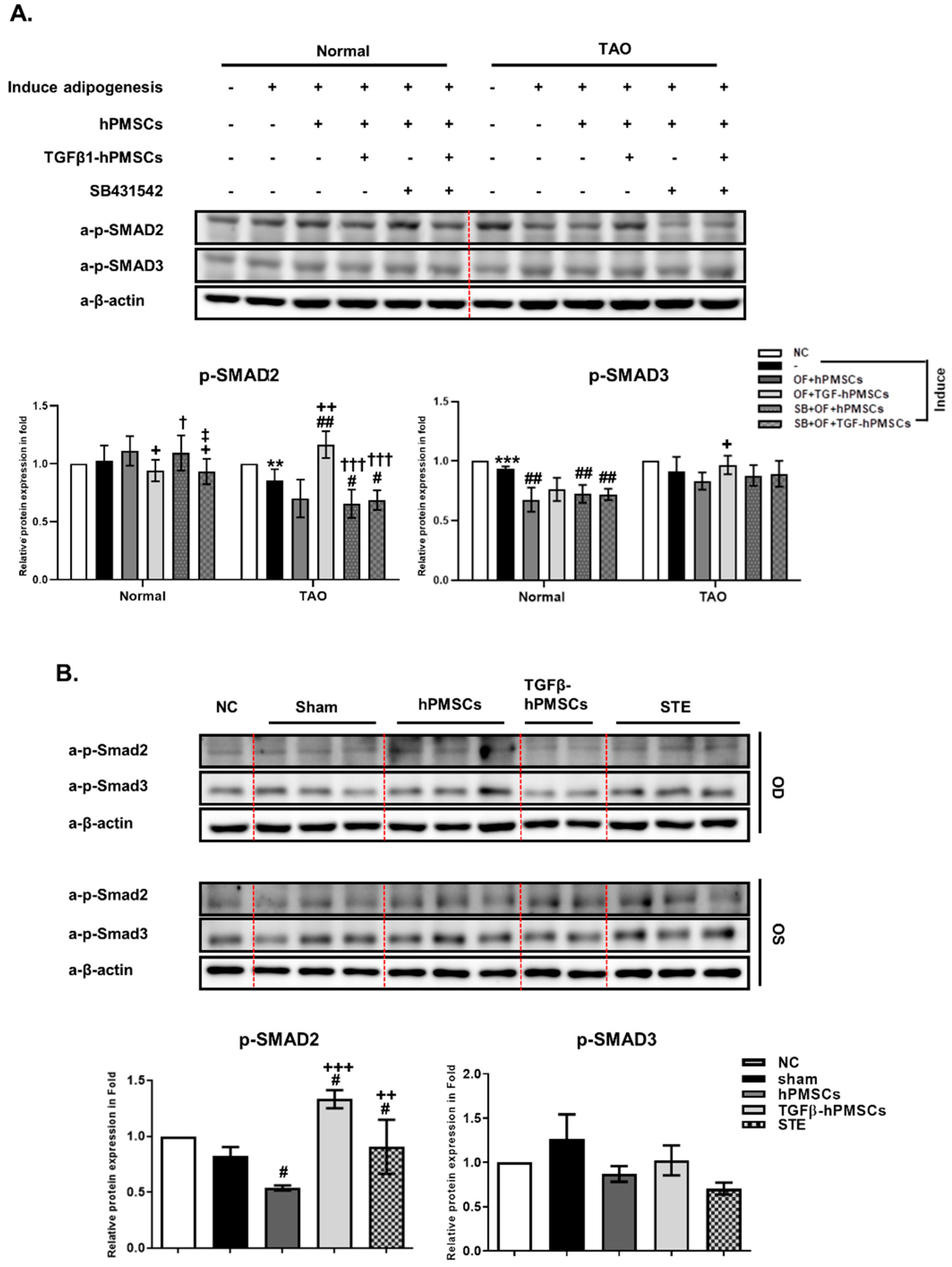
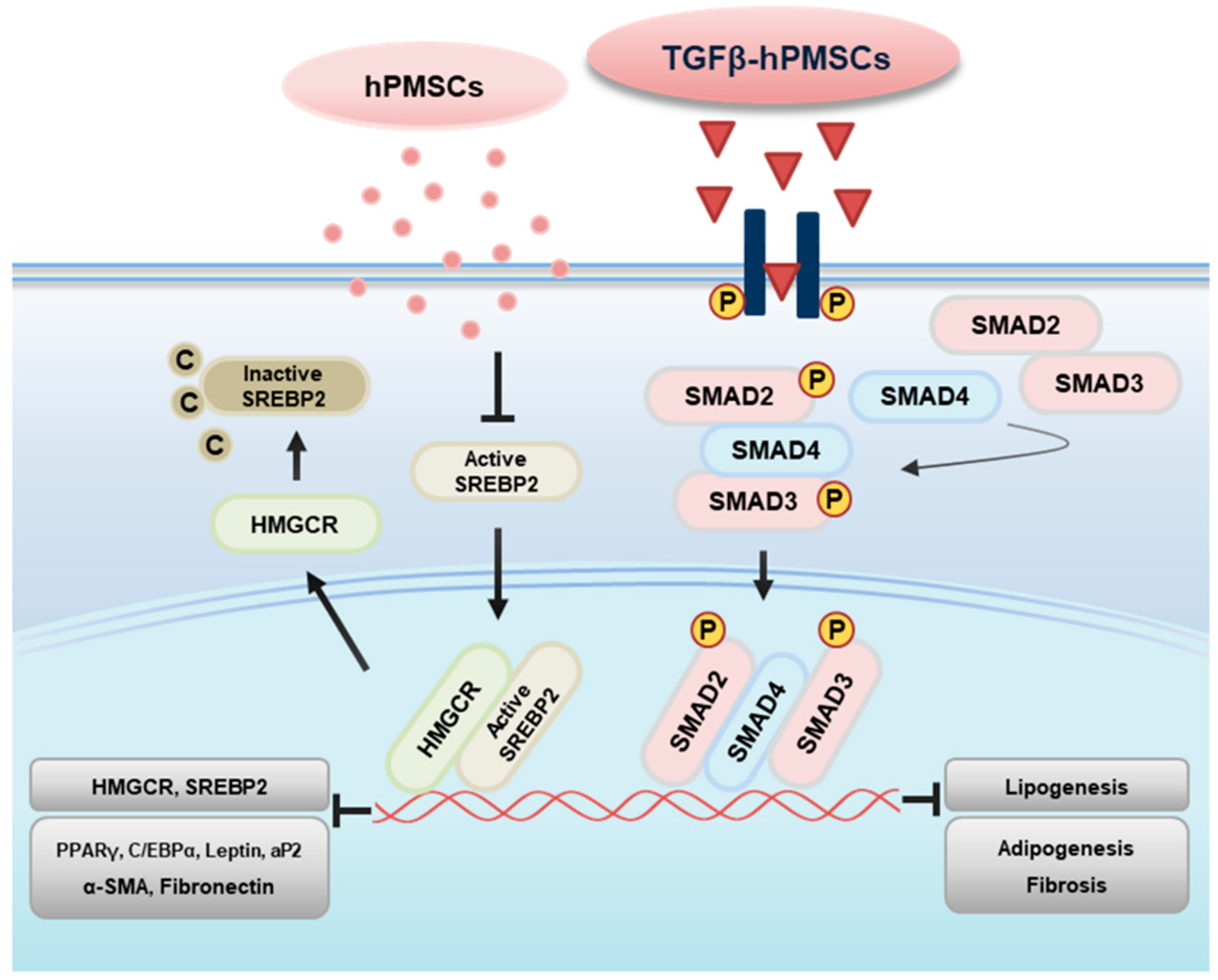
2.5. TGFβ-hPMSCs Regulate TAO OFs via the SMAD Pathway
3. Discussion
4. Material and Methods
4.1. Cell Preparation of hPMSCs and Orbital Fibroblast
4.2. Adipocyte Differentiation
4.3. Co-Culture Experiments
4.4. CCK-8 Assay
4.5. BrdU Incorporation Assay
4.6. Oil Red O Staining
4.7. Quantitative Real-Time Polymerase Chain Reaction
4.8. Development of an Experimental Mouse Model of TAO Using Female BALB/c Mice
4.9. Orbital Tissue Histopathology
4.10. Western Blot Analysis
4.11. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sahli, E.; Gunduz, K. Thyroid-associated Ophthalmopathy. Turk. J. Ophthalmol. 2017, 47, 94–105. [Google Scholar] [CrossRef]
- Bahn, R.S. Clinical review 157: Pathophysiology of Graves’ ophthalmopathy: The cycle of disease. J. Clin. Endocrinol. Metab. 2003, 88, 1939–1946. [Google Scholar] [CrossRef] [Green Version]
- Smith, T.J.; Koumas, L.; Gagnon, A.; Bell, A.; Sempowski, G.D.; Phipps, R.P.; Sorisky, A. Orbital fibroblast heterogeneity may determine the clinical presentation of thyroid-associated ophthalmopathy. J. Clin. Endocrinol. Metab. 2002, 87, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Sarugaser, R.; Hanoun, L.; Keating, A.; Stanford, W.L.; Davies, J.E. Human mesenchymal stem cells self-renew and differentiate according to a deterministic hierarchy. PLoS ONE 2009, 4, e6498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, N.; Wang, Y.; Lu, X.; Liu, R.; Zhang, L.; Zhao, W.; Yuan, W.; Luo, Q.; Wu, H.; Luan, X.; et al. hPMSC transplantation restoring ovarian function in premature ovarian failure mice is associated with change of Th17/Tc17 and Th17/Treg cell ratios through the PI3K/Akt signal pathway. Stem Cell Res. Ther. 2018, 9, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, W.; Abu-El-Rub, E.; Saravanan, S.; Kirshenbaum, L.A.; Arora, R.C.; Dhingra, S. Inflammation in myocardial injury: Mesenchymal stem cells as potential immunomodulators. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H213–H225. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Kim, J.Y.; Kang, J.M.; Lee, H.J.; Banga, J.P.; Kim, G.J.; Lew, H. PRL-1 overexpressed placenta-derived mesenchymal stem cells suppress adipogenesis in Graves’ ophthalmopathy through SREBP2/HMGCR pathway. Stem Cell Res. Ther. 2021, 12, 304. [Google Scholar] [CrossRef]
- Morikawa, M.; Derynck, R.; Miyazono, K. TGF-beta and the TGF-beta Family: Context-Dependent Roles in Cell and Tissue Physiology. Cold Spring Harb. Perspect. Biol. 2016, 8, 5. [Google Scholar] [CrossRef] [Green Version]
- Li, S.N.; Wu, J.F. TGF-beta/SMAD signaling regulation of mesenchymal stem cells in adipocyte commitment. Stem Cell Res. Ther. 2020, 11, 41. [Google Scholar] [CrossRef]
- Li, C.; Wang, M.; Zhang, T.; He, Q.; Shi, H.; Luo, J.; Loor, J.J. Insulin-induced gene 1 and 2 isoforms synergistically regulate triacylglycerol accumulation, lipid droplet formation, and lipogenic gene expression in goat mammary epithelial cells. J. Dairy Sci. 2019, 102, 1736–1746. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef] [Green Version]
- Pittenger, M.F.; Discher, D.E.; Peault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Markov, A.; Thangavelu, L.; Aravindhan, S.; Zekiy, A.O.; Jarahian, M.; Chartrand, M.S.; Pathak, Y.; Marofi, F.; Shamlou, S.; Hassanzadeh, A. Mesenchymal stem/stromal cells as a valuable source for the treatment of immune-mediated disorders. Stem Cell Res. Ther. 2021, 12, 192. [Google Scholar] [CrossRef] [PubMed]
- Moseti, D.; Regassa, A.; Kim, W.K. Molecular Regulation of Adipogenesis and Potential Anti-Adipogenic Bioactive Molecules. Int. J. Mol. Sci. 2016, 17, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Khan, D.; Delling, J.; Tobiasch, E. Mechanisms underlying the osteo- and adipo-differentiation of human mesenchymal stem cells. Sci. World J. 2012, 2012, 793823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grafe, I.; Alexander, S.; Peterson, J.R.; Snider, T.N.; Levi, B.; Lee, B.; Mishina, Y. TGF-beta Family Signaling in Mesenchymal Differentiation. Cold Spring Harb. Perspect. Biol. 2018, 10, a022202. [Google Scholar] [CrossRef] [PubMed]
- Furutani, Y.; Umemoto, T.; Murakami, M.; Matsui, T.; Funaba, M. Role of endogenous TGF-beta family in myogenic differentiation of C2C12 cells. J. Cell. Biochem. 2011, 112, 614–624. [Google Scholar] [CrossRef] [Green Version]
- Ahdjoudj, S.; Kaabeche, K.; Holy, X.; Fromigue, O.; Modrowski, D.; Zerath, E.; Marie, P.J. Transforming growth factor-beta inhibits CCAAT/enhancer-binding protein expression and PPARgamma activity in unloaded bone marrow stromal cells. Exp. Cell Res. 2005, 303, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Choy, L.; Derynck, R. Transforming growth factor-beta inhibits adipocyte differentiation by Smad3 interacting with CCAAT/enhancer-binding protein (C/EBP) and repressing C/EBP transactivation function. J. Biol. Chem. 2003, 278, 9609–9619. [Google Scholar] [CrossRef] [Green Version]
- Massague, J.; Wotton, D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 2000, 19, 1745–1754. [Google Scholar] [CrossRef] [Green Version]
- Aykul, S.; Maust, J.; Thamilselvan, V.; Floer, M.; Martinez-Hackert, E. Smad2/3 Activation Regulates Smad1/5/8 Signaling via a Negative Feedback Loop to Inhibit 3T3-L1 Adipogenesis. Int. J. Mol. Sci. 2021, 22, 8472. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Huang, X.R.; Li, A.G.; Liu, F.; Li, J.H.; Truong, L.D.; Wang, X.J.; Lan, H.Y. Signaling mechanism of TGF-beta1 in prevention of renal inflammation: Role of Smad7. J. Am. Soc. Nephrol. 2005, 16, 1371–1383. [Google Scholar] [CrossRef]
- Luo, H.; Guo, Y.; Liu, Y.; Wang, Y.; Zheng, R.; Ban, Y.; Peng, L.; Yuan, Q.; Liu, W. Growth differentiation factor 11 inhibits adipogenic differentiation by activating TGF-beta/Smad signalling pathway. Cell Prolif. 2019, 52, e12631. [Google Scholar] [CrossRef]
- Budi, E.H.; Hoffman, S.; Gao, S.; Zhang, Y.E.; Derynck, R. Integration of TGF-beta-induced Smad signaling in the insulin-induced transcriptional response in endothelial cells. Sci. Rep. 2019, 9, 16992. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.Y. Diverse roles of TGF-beta/Smads in renal fibrosis and inflammation. Int. J. Biol. Sci. 2011, 7, 1056–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ihara, S.; Hirata, Y.; Koike, K. TGF-beta in inflammatory bowel disease: A key regulator of immune cells, epithelium, and the intestinal microbiota. J. Gastroenterol. 2017, 52, 777–787. [Google Scholar] [CrossRef] [Green Version]
- Syed, S.; Dinallo, V.; Iqbal, N.T.; Di Iorio, L.; Di Fusco, D.; Guleria, S.; Amadi, B.C.; Sadiq, K.; Moskaluk, C.; Ali, S.A.; et al. High SMAD7 and p-SMAD2,3 expression is associated with environmental enteropathy in children. PLoS Negl. Trop. Dis. 2018, 12, e0006224. [Google Scholar] [CrossRef] [Green Version]
- Sedda, S.; Marafini, I.; Dinallo, V.; Di Fusco, D.; Monteleone, G. The TGF-beta/Smad System in IBD Pathogenesis. Inflamm. Bowel Dis. 2015, 21, 2921–2925. [Google Scholar] [CrossRef] [Green Version]
- Park, M.; Banga, J.P.; Kim, G.J.; Kim, M.; Lew, H. Human placenta-derived mesenchymal stem cells ameliorate orbital adipogenesis in female mice models of Graves’ ophthalmopathy. Stem Cell Res. Ther. 2019, 10, 246. [Google Scholar] [CrossRef]
- Frangogiannis, N. Transforming growth factor-beta in tissue fibrosis. J. Exp. Med. 2020, 217, e20190103. [Google Scholar] [CrossRef]
- Gyorfi, A.H.; Matei, A.E.; Distler, J.H.W. Targeting TGF-beta signaling for the treatment of fibrosis. Matrix Biol. 2018, 68–69, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.T.; Han, Y.P.; Yan, C.; Shaw, M.C.; Garner, W.L. TNF-alpha suppresses alpha-smooth muscle actin expression in human dermal fibroblasts: An implication for abnormal wound healing. J. Investig. Dermatol. 2007, 127, 2645–2655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biernacka, A.; Dobaczewski, M.; Frangogiannis, N.G. TGF-beta signaling in fibrosis. Growth Factors 2011, 29, 196–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.; Han, C.; Xian, S.; Zhuang, F.; Ding, F.; Zhang, W.; Liu, Y. Placental Mesenchymal Stromal Cells (PMSCs) and PMSC-Derived Extracellular Vesicles (PMSC-EVs) Attenuated Renal Fibrosis in Rats with Unilateral Ureteral Obstruction (UUO) by Regulating CD4(+) T Cell Polarization. Stem Cells Int. 2020, 2020, 2685820. [Google Scholar] [CrossRef]
- Park, M.; Kim, H.C.; Kim, O.; Lew, H. Human placenta mesenchymal stem cells promote axon survival following optic nerve compression through activation of NF-kappaB pathway. J. Tissue Eng. Regen. Med. 2018, 12, e1441–e1449. [Google Scholar] [CrossRef]
- Zhao, S.X.; Tsui, S.; Cheung, A.; Douglas, R.S.; Smith, T.J.; Banga, J.P. Orbital fibrosis in a mouse model of Graves’ disease induced by genetic immunization of thyrotropin receptor cDNA. J. Endocrinol. 2011, 210, 369–377. [Google Scholar] [CrossRef]
| Genes | Primer Sequences | Tm | |
|---|---|---|---|
| PPARγ | Forward | 5’-TTGACCCAGAAAGCGATTCC-3’ | 50 |
| Reverse | 5’-AAAGTTGGTGGGCCAGAATG-3’ | ||
| C/EBPα | Forward | 5’-TGTATACCCCTGGTGGGAGA -3’ | 50 |
| Reverse | 5’-TCATAACTCCGGTCCCTCTG-3’ | ||
| LEPTIN | Forward | 5’-GGTTGCAAGGCCCAAGAA-3’ | 50 |
| Reverse | 5’-ACATAGAAAAGATAGGGCCAGC-3’ | ||
| AP2 | Forward | 5’-ATGGGGGTGTCCTGGTACAT-3’ | 60 |
| Reverse | 5’-ACGTCCCTTGGCTTATGCTC-3’ | ||
| SREBP2 | Forward | 5’-AACGGTCATTCACCCAGGTC-3’ | 60 |
| Reverse | 5’-GGCTGAAGAATAGGAGTTGCC-3’ | ||
| HMGCR | Forward | 5’-AAGGAGGCATTTGACAGCAC-3’ | 60 |
| Reverse | 5’-CTGACCTGGACTGGAAACG-3’ | ||
| IGFBP3 | Forward | 5’-AAGTTGACTACGAGTCTCAG-3 | 60 |
| Reverse | 5’-ACGGCAGGGACCATATTC-3 | ||
| α-SMA | Forward | 5’-CTCCCAGGGCTGTTTTCCCA-3’ | 60 |
| Reverse | 5’-CCATGTCGTCCCAGTTGGTG-3’ | ||
| FIBRONECTIN | Forward | 5’-CCAAGAAGGGCTCGTGTG-3’ | 60 |
| Reverse | 5’-TGGCTGGAACGGCATCA-3’ | ||
| IL-17 | Forward | 5’-CTGTCCCCATCCAGCAAGAG-3’ | 60 |
| Reverse | 5’-AGGCCACATGGTGGACAATC-3’ | ||
| INSIG1 | Forward | 5’-GGCAGCTTCCCAAGTATTCG-3’ | 55 |
| Reverse | 5’-AGCACCATCAACCTACCTCCT-3’ | ||
| INSIG2 | Forward | 5’-TCACACTGGCTGCACTATCC-3’ | 60 |
| Reverse | 5’-ACAGTTGCCAAGAAGGCAAT-3’ | ||
| IGF-1 | Forward | 5’-CATGTCCTCCTCGCATCTCT-3’ | 50 |
| Reverse | 5’-GGTGCGCAATACATCTCGAG-3’ | ||
| IGF-1R | Forward | 5’-AGAAGGAGGAGGCTGAATAC-3’ | 55 |
| Reverse | 5’-GGTCGGTGATGTTGTAGGT-3’ | ||
| TGFβ1 | Forward | 5’-CTGGACACCAACTATTGC-3’ | 50 |
| Reverse | 5’-CTTCCACCCGAGGTCCTT-3’ | ||
| TGFβ2 | Forward | 5’-ATTGCCCTCCTACAGACTTGAG-3’ | 60 |
| Reverse | 5’-CAGCACAGAAGTTGGCCATTGTA-3’ | ||
| TNFα | Forward | 5’-GGTGATCGGTGCCAACAAGGA-3’ | 60 |
| Reverse | 5’-CACGCTGGCTCAGCCACTG-3’ | ||
| IL-6 | Forward | 5’-TGAGAAAGGAGACATGTAACAAGAGT-3’ | 60 |
| Reverse | 5’-TTGTTCCTCACTACTCTCAAATCTGT-3’ | ||
| IL-8 | Forward | 5’-ACTCCAAACCTTTCCACC-3’ | 60 |
| Reverse | 5’-CTTCTCCACAACCCTCTG-3’ | ||
| ICAM-1 | Forward | 5’-CAGTCACCTATGGCAACGACT-3’ | 50 |
| Reverse | 5’-CTCTGGCTTCGTCAGAATCAC-3’ | ||
| IL-1β | Forward | 5’-ATGAGTGCTCCTTCCAGGA-3’ | 60 |
| Reverse | 5’-GATAGGTTCTTCAAAGATG-3’ | ||
| GAPDH | Forward | 5’-TCCTTCTGCATCCTGTCAGCA-3’ | 60 |
| Reverse | 5’-CAGGAGATGGCCACTGCCGCA-3’ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, H.-A.; Park, M.; Banga, J.P.; Lew, H. TGFβ-Treated Placenta-Derived Mesenchymal Stem Cells Selectively Promote Anti-Adipogenesis in Thyroid-Associated Ophthalmopathy. Int. J. Mol. Sci. 2022, 23, 5603. https://doi.org/10.3390/ijms23105603
Shin H-A, Park M, Banga JP, Lew H. TGFβ-Treated Placenta-Derived Mesenchymal Stem Cells Selectively Promote Anti-Adipogenesis in Thyroid-Associated Ophthalmopathy. International Journal of Molecular Sciences. 2022; 23(10):5603. https://doi.org/10.3390/ijms23105603
Chicago/Turabian StyleShin, Hyun-Ah, Mira Park, Jasvinder Paul Banga, and Helen Lew. 2022. "TGFβ-Treated Placenta-Derived Mesenchymal Stem Cells Selectively Promote Anti-Adipogenesis in Thyroid-Associated Ophthalmopathy" International Journal of Molecular Sciences 23, no. 10: 5603. https://doi.org/10.3390/ijms23105603
APA StyleShin, H.-A., Park, M., Banga, J. P., & Lew, H. (2022). TGFβ-Treated Placenta-Derived Mesenchymal Stem Cells Selectively Promote Anti-Adipogenesis in Thyroid-Associated Ophthalmopathy. International Journal of Molecular Sciences, 23(10), 5603. https://doi.org/10.3390/ijms23105603






