Photoprotection Differences between Dominant Tree Species at Mid- and Late-Successional Stages in Subtropical Forests in Different Seasonal Environments
Abstract
1. Introduction
2. Results
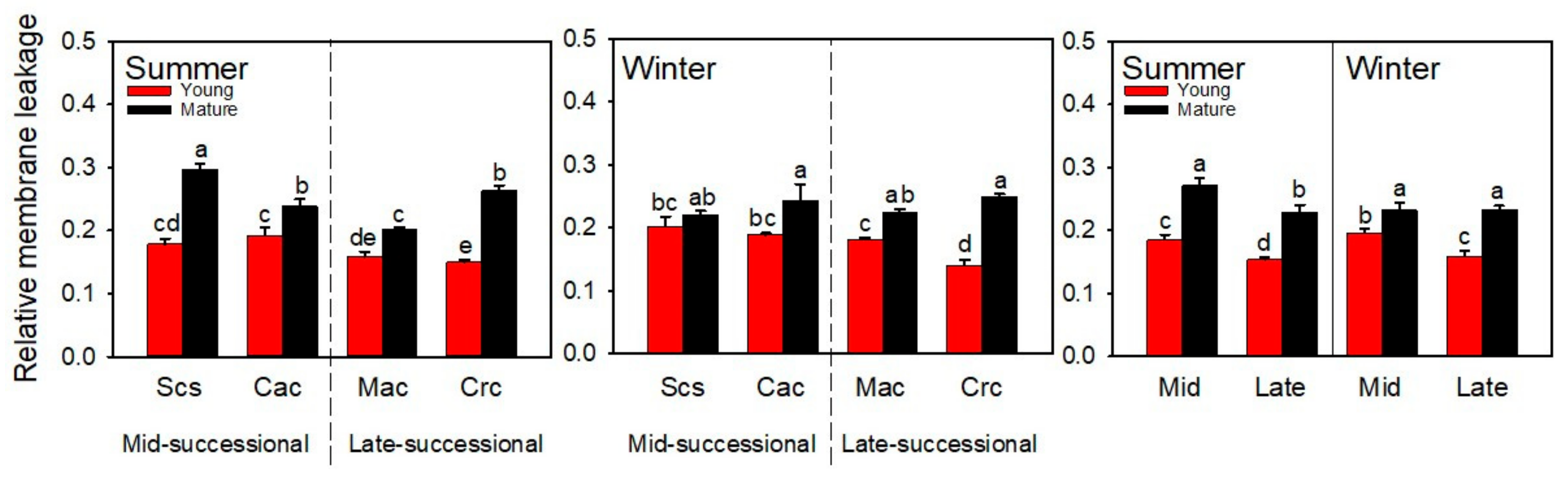
2.1. Relative Membrane Leakage
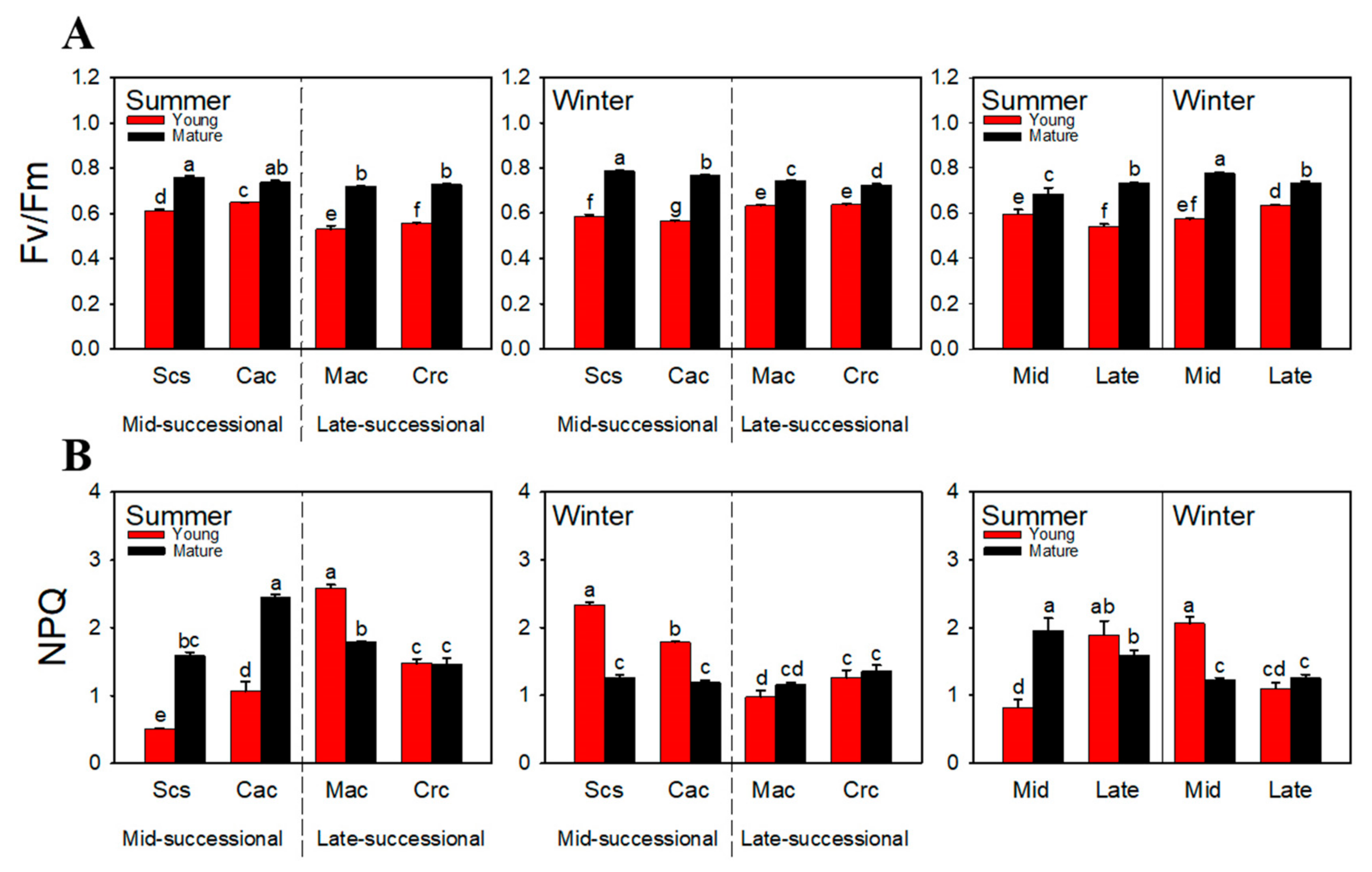
2.2. Chlorophyll Fluorescence
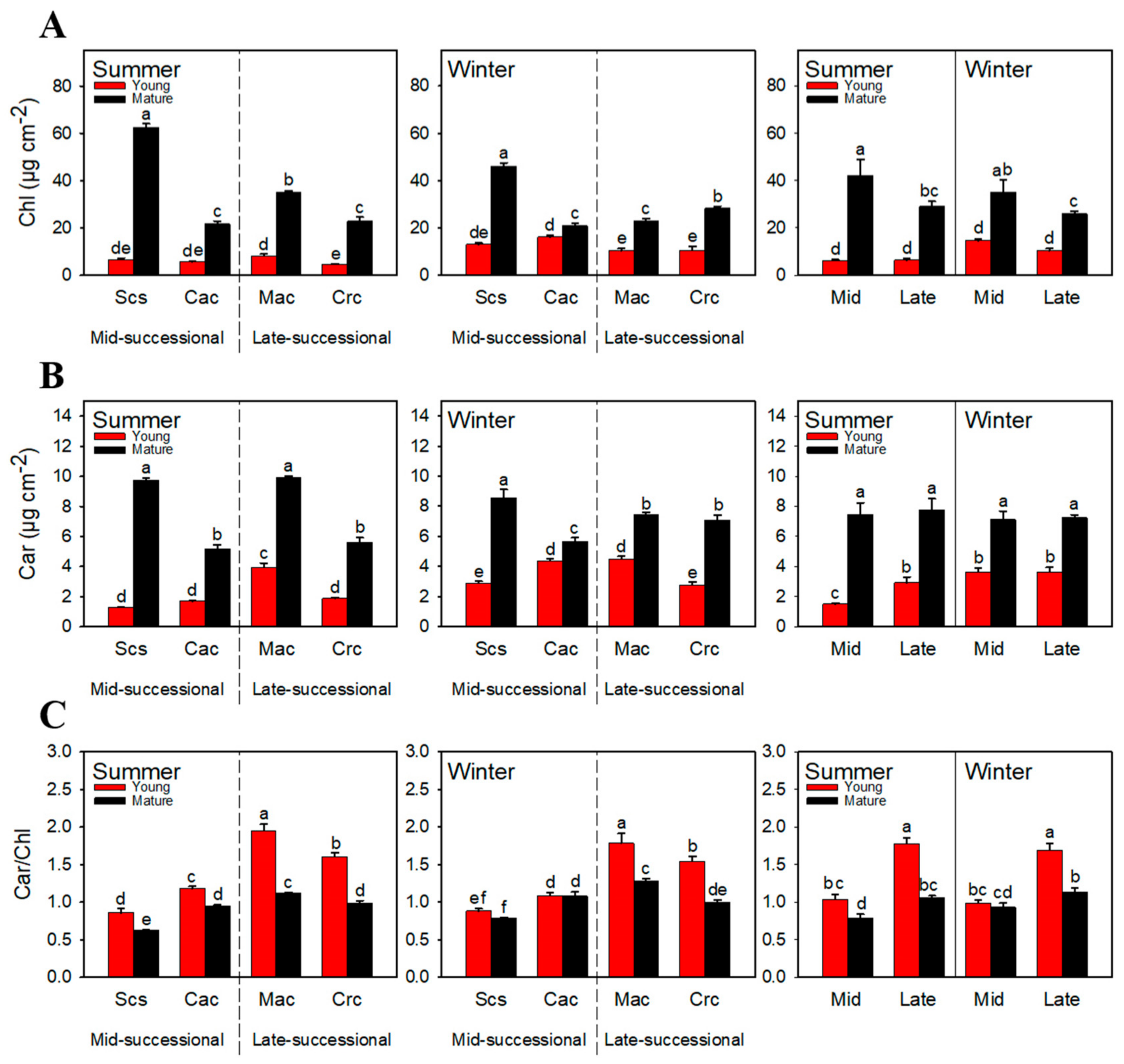
2.3. Pigment Content
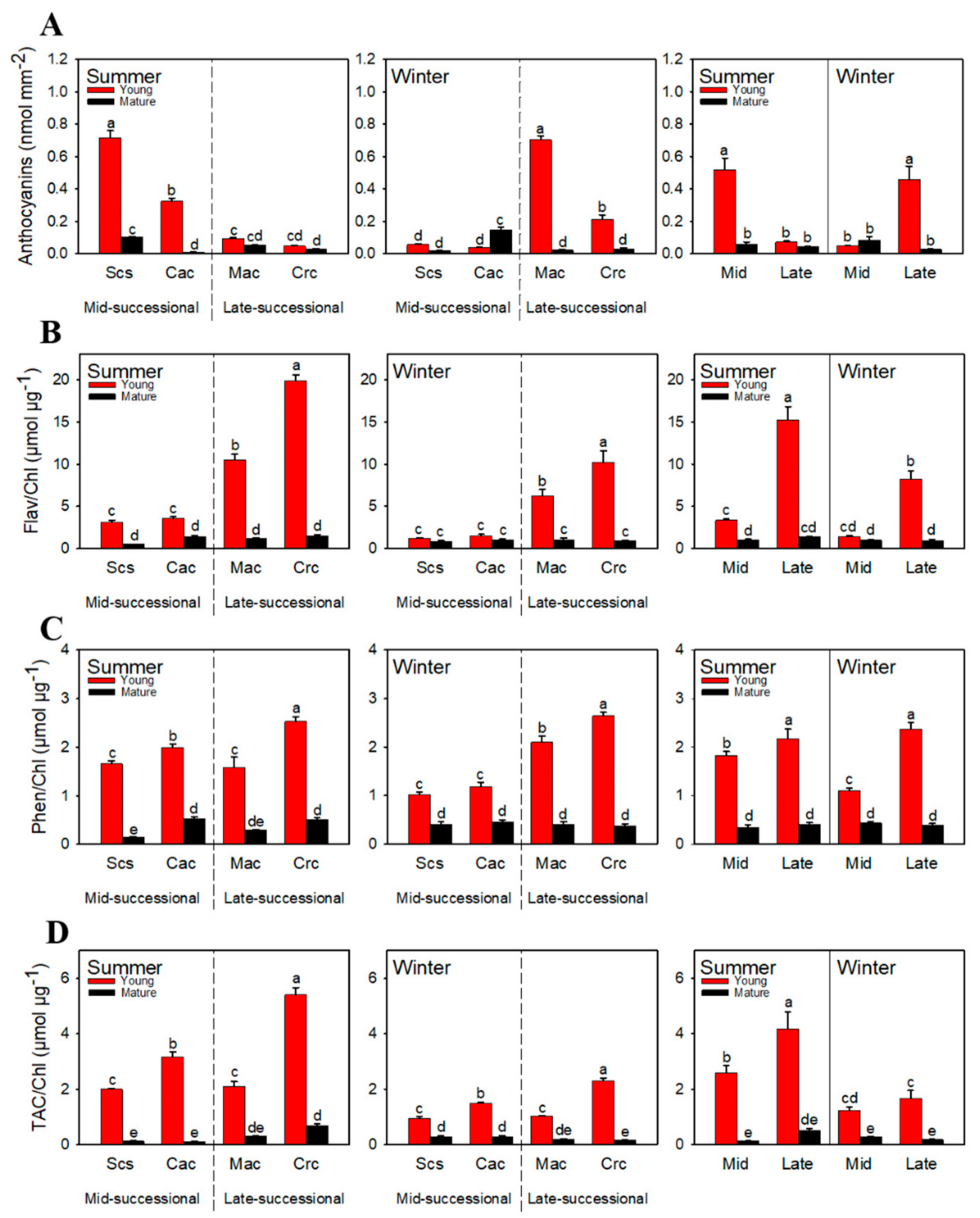
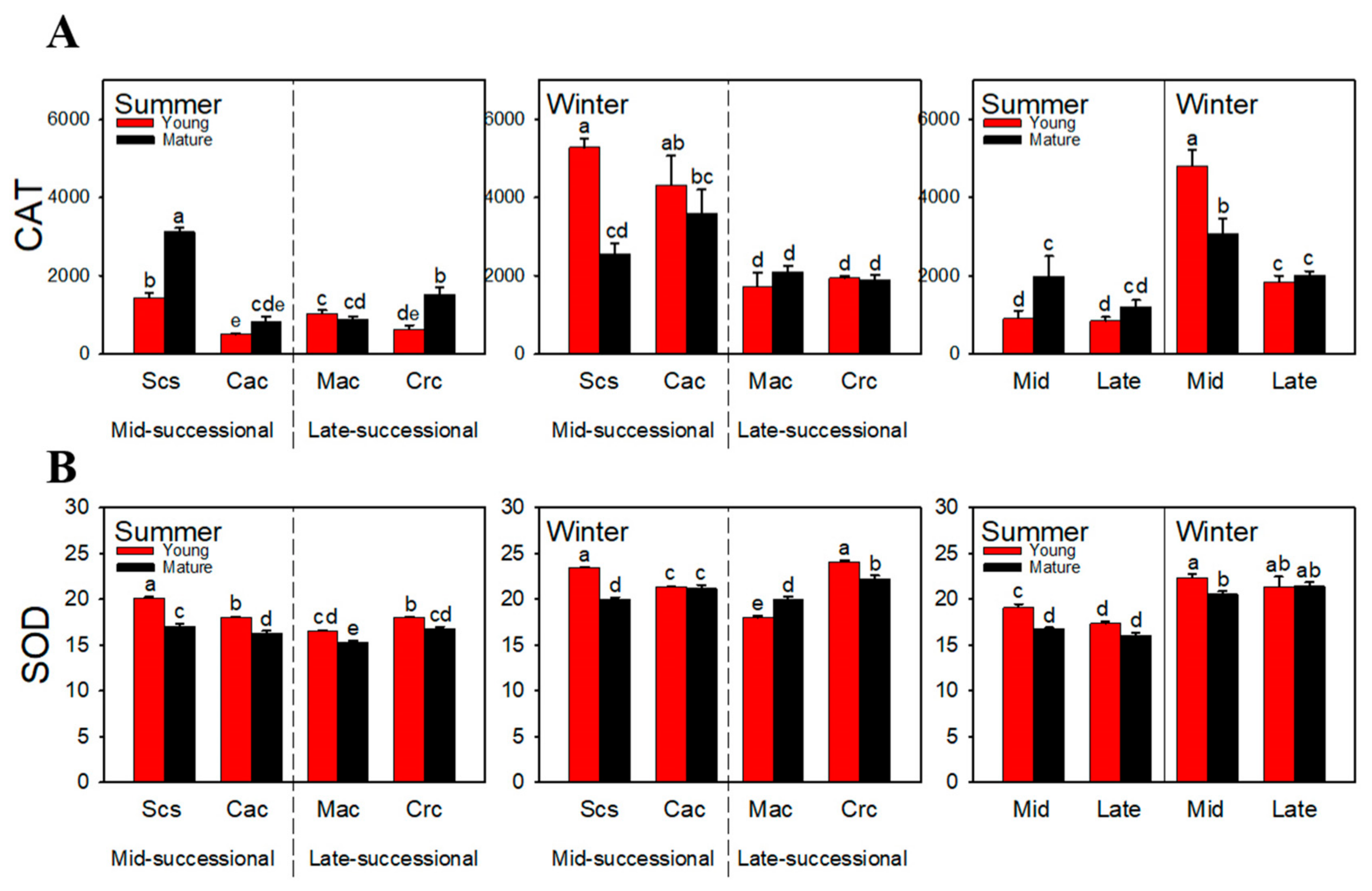
2.4. Total Antioxidant Capacity
2.5. Antioxidant Enzyme Activity
2.6. Structural Equation Modeling
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Relative Membrane Leakage Determination
4.3. Determination of Pigment Content
4.4. Total Antioxidant Capacity Assessment
4.5. Chlorophyll Fluorescence Assay
4.6. Antioxidant Enzyme Activity Assay
4.7. Data Analysis
5. Conclusions
- In summer, dominant species in the mid-successional stage mainly reduced the degree of photoinhibition through light shielding and enzymatic antioxidant systems to achieve photoprotection. In the late-successional stage, the dominant species improved their photoprotective ability by improving their heat dissipation ability and non-enzymatic antioxidant system, which was mainly reflected in their recovery ability after photoinhibition.
- In winter, the photoprotective ability of dominant species in the mid-successional stage came from improved NPQ and antioxidant enzyme activity, while those in the late-successional stage maintained low photoinhibition by a combination of light shielding, heat dissipation, and enzymatic antioxidant systems.
- The environmental transition from summer to winter reduced the light-shielding effect and antioxidant content of tree species in the mid-successional stage, leading to an increased degree of photoinhibition, despite a slight increase in antioxidant enzyme activity. In addition, although the environmental transition from summer to winter reduced the heat dissipation capacity and antioxidant content of tree species at the late-successional stage, their strong photoprotective ability could be maintained by increasing the light-shielding effect and antioxidant enzyme activity.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.Y.; Xie, S.; Yan, S.Q.; Xu, W.G.; Chen, J.W. Light energy partitioning and photoprotection from excess light energy in shade-tolerant plant Amorphophallus xiei under steady-state and fluctuating high light. Acta Physiol. Plant. 2021, 43, 1–17. [Google Scholar] [CrossRef]
- Ding, X.T.; Jiang, Y.P.; Wang, H.; Jin, H.J.; Zhang, H.M.; Chen, C.H.; Yu, J.Z. Effects of cytokinin on photosynthetic gas exchange, chlorophyll fluorescence parameters, antioxidative system and carbohydrate accumulation in cucumber (Cucumis sativus L.) under low light. Acta Physiol. Plant. 2013, 35, 1427–1438. [Google Scholar] [CrossRef]
- Vranová, E.; Inzé, D.; Breusegem, F.V. Signal transduction during oxidative stress. J. Exp. Bot. 2002, 53, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.L.; Huang, F.D.; Cao, Z.Z.; Lei, B.T.; Cheng, F.M. Effects of high temperature stress on PSII function and its relation to D1 protein in chloroplast thylakoid in rice flag leaves. Acta Agron. Sin. 2013, 39, 1060–1068. [Google Scholar] [CrossRef]
- Efeoglu, B.; Terzioglu, S. Photosynthetic responses of two wheat varieties to high temperature. Eurasia J. Biosci. 2009, 3, 97–106. [Google Scholar] [CrossRef]
- Hendrickson, L.; Ball, M.C.; Wood, J.T.; Chow, W.S.; Furbank, R.T. Low temperature effects on photosynthesis and growth of grapevine. Plant Cell Environ. 2010, 27, 795–809. [Google Scholar] [CrossRef]
- Mathur, S.; Agrawal, D.; Jajoo, A. Photosynthesis: Response to high temperature stress. J. Photochem. Photobiol. B 2014, 137, 116–126. [Google Scholar] [CrossRef]
- Chen, L.B.; Jia, H.; Qiu, T.; Gao, L.B.; Miao, Y.L. Protecting effect of phosphorylation on oxidative damage of D1 protein by down-regulating the production of superoxide anion in photosystem II membranes under high light. Photosynth. Res. 2012, 112, 141–148. [Google Scholar] [CrossRef]
- Ghotbi-Ravandi, A.A.; Sedighi, M.; Aghaei, K.; Mohtadi, A. Differential changes in D1 protein content and quantum yield of wild and cultivated barley genotypes caused by moderate and severe drought stress in relation to oxidative stress. Plant Mol. Biol. Rep. 2021, 39, 501–507. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, D.; Fernández-Lorenzo, J.L.; Shen, H.; Yang, L. Reactive oxygen species, nitric oxide and plant cell death associated with caspase-like protease activity during somatic embryogenesis in Fraxinus mandshurica. J. For. Res. 2021, 33, 1–13. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Tattini, M.; Gravano, E.; Pinelli, P.; Mulnacci, N.; Romani, A. Flavonoids accumulate in leaves and glandular trichomes of Phillyrea latifolia exposed to excess solar radiation. New Phytol. 2000, 148, 69–77. [Google Scholar] [CrossRef]
- Martel, A.B.; Taylor, A.E.; Qaderi, M.M. Individual and interactive effects of temperature and light intensity on canola growth, physiological characteristics and methane emissions. Plant Physiol. Biochem. 2020, 157, 160–168. [Google Scholar] [CrossRef]
- Choi, S.; Ye, R.K.; Hong, S.W.; Lee, B.H.; Lee, H. A mutation in ELA1, an age-dependent negative regulator of PAP1/MYB75, causes UV and cold stress-tolerance in Arabidopsis thaliana seedlings. Plant Sci. 2009, 176, 678–686. [Google Scholar] [CrossRef]
- Wang, G.; Cao, F.; Chang, L.; Guo, X.; Wang, J. Temperature has more effects than soil moisture on biosynthesis of flavonoids in Ginkgo (Ginkgo biloba L.) leaves. New For. 2014, 45, 797–812. [Google Scholar] [CrossRef]
- Ballizany, W.L.; Hofmann, R.W.; Jahufer, M.Z.Z.; Barrett, B.A. Genotype × environment analysis of flavonoid accumulation and morphology in white clover under contrasting field conditions. Field Crops Res. 2012, 128, 156–166. [Google Scholar] [CrossRef]
- Esmaeili, A.K.; Taha, R.M.; Mohajer, S.; Banisalam, B. Antioxidant activity and total phenolic and flavonoid content of various solvent extracts from in vivo and in vitro grown Trifolium pratense L. (red clover). Biomed. Res. Int. 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- Zhang, T.J.; Chow, W.S.; Liu, X.T.; Zhang, P.; Liu, N.; Peng, C.L. A magic red coat on the surface of young leaves: Anthocyanins distributed in trichome layer protect Castanopsis fssa leaves from photoinhibition. Tree Physiol. 2016, 36, 1296–1306. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Fang, Z.; Huang, D.; Wang, J. The effect of Malus doumeri leaf flavonoids on oxidative stress injury induced by hydrogen peroxide (H2O2) in human embryonic kidney 293 T cells. BMC Complement. Med. 2020, 20, 276. [Google Scholar] [CrossRef]
- Hano, C. Green Extraction of antioxidant flavonoids from pigeon pea (Cajanus cajan (L.) Millsp.) seeds and its antioxidant potentials using ultrasound-assisted methodology. Molecules 2021, 26, 7557. [Google Scholar] [CrossRef]
- Sasikumar, J.M.; Poulin, R.C.; Meseret, C.E.; Selvakumar, P. In vitro analysis of antioxidant capacity of Indian yellow raspberry (Rubus ellipticus Smith.). Int. Food Res. J. 2015, 22, 1338–1346. [Google Scholar]
- Yildiztugay, E.; Ozfidan-Konakci, C.; Kucukoduk, M.; Tekis, S.A. The impact of selenium application on enzymatic and non-enzymatic antioxidant systems in Zea mays roots treated with combined osmotic and heat stress. Arch. Agron. Soil Sci. 2017, 63, 261–275. [Google Scholar] [CrossRef]
- Ren, R.; Li, Z.; Zhang, L.; Zhou, H.; Liu, Y. Enzymatic and nonenzymatic antioxidant systems impact the viability of cryopreserved Paeonia suffruticosa pollen. Plant Cell Tissue Org. 2021, 144, 233–246. [Google Scholar] [CrossRef]
- Yu, Z.C.; Lin, W.; Zheng, X.T.; Chow, W.S.; Luo, Y.N.; Cai, M.L.; Peng, C.L. The relationship between anthocyanin accumulation and photoprotection in young leaves of two dominant tree species in subtropical forests in different seasons. Photosynth. Res. 2020, 149, 41–55. [Google Scholar] [CrossRef]
- El-Amier, Y.; Elhindi, K.; El-Hendawy, S.; Al-Rashed, S.; Abd-Elgawad, A. Antioxidant system and biomolecules alteration in Pisum sativum under heavy metal stress and possible alleviation by 5-aminolevulinic acid. Molecules 2019, 24, 4194. [Google Scholar] [CrossRef]
- Goharrizi, K.J.; Moosavi, S.S.; Amirmahani, F.; Salehi, F.; Nazari, M. Assessment of changes in growth traits, oxidative stress parameters, and enzymatic and non-enzymatic antioxidant defense mechanisms in Lepidium drabaplant under osmotic stress induced by polyethylene glycol. Protoplasma 2019, 257, 459–473. [Google Scholar] [CrossRef]
- Moustafa-Farag, M.; Mohamed, H.I.; Mahmoud, A.; Mahmoud, A.; Elkelish, A.; Misra, A.N.; Guy, K.M.; Kamran, M.; Ai, S.; Zhang, M. Salicylic acid stimulates antioxidant defense and osmolyte metabolism to alleviate oxidative stress in watermelons under excess boron. Plants 2020, 9, 724. [Google Scholar] [CrossRef]
- Singh, R.; Misra, A.N.; Sharma, P. Effect of arsenate toxicity on antioxidant enzymes and expression of nicotianamine synthase in contrasting genotypes of bioenergy crop Ricinus communis. Environ. Sci. Pollut. Res. 2021, 28, 31421–31430. [Google Scholar] [CrossRef]
- Mishra, A.N. Chlorophyll fluorescence: A practical approach to study ecophysiology of green plants. Adv. Plant Ecophysiol. Tech. 2018, 5, 77–97. [Google Scholar] [CrossRef]
- Müller, P.; Li, X.P.; Niyogi, K.K. Non-photochemical quenching a response to excess light energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, T.J.; Zheng, J.; Huang, X.D.; Yu, Z.C.; Peng, C.L.; Chow, W.S. Anthocyanins function as a light attenuator to compensate for insufficient photoprotection mediated by nonphotochemical quenching in young leaves of Acmena acuminatissima in winter. Photosynthetica 2018, 56, 445–454. [Google Scholar] [CrossRef]
- Hamdani, S.; Khan, N.; Perveen, S.; Qu, M.; Jiang, J.; Govindjee; Zhu, X. Changes in the photosynthesis properties and photoprotection capacity in rice (Oryza sativa) grown under red, blue, or white light. Photosynth. Res. 2018, 139, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Li, Y.; Fu, H. Effect of high temperature on the balance between photosynthetic light absorption and energy utilization in Chlorella pyrenoidosa (Chlorophyceae). J. Oceanol. Limnol. 2019, 38, 186–194. [Google Scholar] [CrossRef]
- Kato, M.C.; Kouki, H.; Naoki, H.; Amane, M.; Tadaki, H. The excess light energy that is neither utilized in photosynthesis nor dissipated by photoprotective mechanisms determines the rate of photoinactivation in photosystem II. Plant Cell Physiol. 2003, 44, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Dmytro, K.; Logan, B.A.; Tissue, D.T.; Allen, R.D.; Scott, H.A. Compensation for PSII photoinactivation by regulated non-photochemical dissipation influences the impact of photoinactivation on electron transport and CO2 assimilation. Plant Cell Physiol. 2006, 47, 437–446. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, S.B.; Cao, K.F. Evidence for leaf fold to remedy the deficiency of physiological photoprotection for photosystem II. Photosynth. Res. 2012, 110, 185–191. [Google Scholar] [CrossRef]
- Yang, Y.; Yin, C.; Li, W.; Xu, X.; Yang, Y.; Yin, C.; Li, W.; Xu, X. Alpha-tocopherol is essential for acquired chill-light tolerance in the cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 2008, 190, 1554–1560. [Google Scholar] [CrossRef]
- Amel, L.; Marion, R.; Zhang, C.C. Oxidative stress in cyanobacteria. FEMS Microbiol. Rev. 2010, 33, 258–278. [Google Scholar] [CrossRef]
- Montillet, J.; Chamnongpol, S.; Rustérucci, C.; Dat, J.F.; Triantaphylidès, C. Fatty acid hydroperoxides and H2O2 in the execution of hypersensitive cell death in tobacco leaves. Plant Physiol. 2005, 138, 1516–1526. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Krause, G.H.; Gallé, A.; Virgo, A.; García, M.; Bucic, P.; Jahns, P.; Winter, K. High-light stress does not impair biomass accumulation of sun-acclimated tropical tree seedlings (Calophyllum longifolium Willd. and Tectona grandis L. f.). Plant Biol. 2006, 8, 31–41. [Google Scholar] [CrossRef][Green Version]
- Tait, L.W.; Schiel, D.R.; Tasman, C. Impacts of temperature on primary productivity and respiration in naturally structured macroalgal assemblages. PLoS ONE 2013, 8, e74413. [Google Scholar] [CrossRef]
- Richardson, S.J.; Bonner, K.I.; Bickford, C.P. Cold tolerance of photosynthesis as a determinant of tree species regeneration patterns in an evergreen temperate forest. Plant Ecol. 2013, 214, 787–798. [Google Scholar] [CrossRef]
- Perera-Castro, A.V.; Flexas, J.; González-Rodríguez, G.M.; Fernández-Marín, B. Photosynthesis on the edge: Photoinhibition, desiccation and freezing tolerance of Antarctic bryophytes. Photosynth. Res. 2020, 149, 135–153. [Google Scholar] [CrossRef]
- Hájek, J.; Barták, M.; Hazdrová, J.; Forbelská, M. Sensitivity of photosynthetic processes to freezing temperature in extremophilic lichens evaluated by linear cooling and chlorophyll fluorescence. Cryobiology 2016, 73, 329–334. [Google Scholar] [CrossRef]
- Poudyal, D.; Rosenqvist, E.; Ottosen, C.O. Phenotyping from lab to field—Tomato lines screened for heat stress using Fv/Fm maintain high fruit yield during thermal stress in the field. Funct. Plant Biol. 2019, 46, 44–55. [Google Scholar] [CrossRef]
- Zhang, T.J.; Zheng, J.; Yu, Z.C.; Gu, X.Q.; Tian, X.S.; Peng, C.L.; Chow, W.S. Variations in photoprotective potential along gradients of leaf development and plant succession in subtropical forests under contrasting irradiances. Environ. Exp. Bot. 2018, 154, 23–32. [Google Scholar] [CrossRef]
- Yu, Z.C.; Zheng, X.T.; Lin, W.; Cai, M.L.; Peng, C.L. Different photoprotection strategies for mid- and late-successional dominant tree species in a high-light environment in summer. Environ. Exp. Bot. 2019, 171, 103927. [Google Scholar] [CrossRef]
- Manetas, K.Y. Mesophyll versus epidermal anthocyanins as potential in vivo antioxidants: Evidence linking the putative antioxidant role to the proximity of oxy-radical source. J. Exp. Bot. 2006, 57, 2203–2210. [Google Scholar] [CrossRef]
- Nichelmann, L.; Bilger, W. Quantification of light screening by anthocyanins in leaves of Berberis thunbergii. Planta 2017, 246, 1069–1082. [Google Scholar] [CrossRef]
- Landi, M.; Tattini, M.; Gould, K.S. Multiple functional roles of anthocyanins in plant-environment interactions. Environ. Exp. Bot. 2015, 119, 4–17. [Google Scholar] [CrossRef]
- Zhang, T.J. Photoprotection of Anthocyanins in Juvenile Leaves of Dominant Tree Species in Mid- and Late-Successional Stages of Low Subtropical Forest; South China Normal University: Guangzhou, China, 2017. [Google Scholar]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Wang, H.; Gao, G.; Prior, R.L. Oxygen radical absorbing capacity of anthocyanins. J. Agric. Food Chem. 1997, 45, 304–309. [Google Scholar] [CrossRef]
- Zhang, T.J.; Zheng, J.; Yu, Z.C.; Huang, X.D.; Zhang, Q.L.; Tian, X.S.; Peng, C.L. Functional characteristics of phenolic compounds accumulated in young leaves of two subtropical forest tree species of different successional stages. Tree Physiol. 2018, 38, 1486–1501. [Google Scholar] [CrossRef]
- Popova, A.V.; Dobrev, K.; Velitchkova, M.; Ivanov, A.G. Differential temperature effects on dissipation of excess light energy and energy partitioning in lut2 mutant of Arabidopsis thaliana under photoinhibitory conditions. Photosynth. Res. 2018, 139, 367–385. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, W.; Che, Y.; Huang, D.; Sun, G. Atmospheric nitrogen dioxide improves photosynthesis in mulberry leaves via effective utilization of excess absorbed light Energy. Forests 2019, 10, 312. [Google Scholar] [CrossRef]
- Yu, C.W.; Guan, Z.Q.; Hong, Y.L.; Wu, Y.J.; Wang, C.; Liu, G.F.; Yang, C.P. Enhanced salt tolerance of transgenic poplar plants expressing a manganese superoxide dismutase from Tamarix androssowii. Mol. Biol. Rep. 2010, 37, 1119–1124. [Google Scholar] [CrossRef]
- Dadkhah-Aghdash, H.; Heydari, M.; Zare-Maivan, H.; Sharifi, M.; Miralles, I.; Lucas-Borja, M.E. Variation in Brant’s oak (Quercus brantii Lindl.) leaf traits in response to pollution from a gas refinery in semiarid forests of western Iran. Environ. Sci. Pollut. Res. 2022, 29, 10366–10379. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Heimler, D.; Vignolini, P.; Dini, M.G.; Romani, A. Rapid tests to assess the antioxidant activity of Phaseolus vulgaris L. dry beans. J. Agric. Food Chem. 2005, 53, 3053–3056. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Saha, M.R.; Hasan, S.M.R.; Akter, R.; Hossain, M.; Alam, M.; Alam, M.; Mazumder, M. Free radical scavenging activity of methanol extract of the leaves of the leaves of Mimusops elengi Linn. Bangladesh J. Vet. Med. 2008, 6, 197–202. [Google Scholar] [CrossRef]
- Oxborough, K.; Baker, N.R. Resolving chlorophyll a fluorescence images of photosynthetic efciency into photochemical and nonphotochemical components–calculation of qP and Fv’/Fm’; without measuring Fo’. Photosynth. Res. 1997, 54, 135–142. [Google Scholar] [CrossRef]
- Bilger, W.; Björkman, O. Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth. Res. 1990, 25, 173–185. [Google Scholar] [CrossRef]
- Luo, H.; Li, H.; Zhang, X.; Fu, J. Antioxidant responses and gene expression in perennial ryegrass (Lolium perenne L.) under cadmium stress. Ecotoxicology 2011, 20, 770–778. [Google Scholar] [CrossRef]
- Fu, Y.; Guo, C.; Wu, H.; Chen, C. Arginine decarboxylase ADC2 enhances salt tolerance through increasing ROS scavenging enzyme activity in Arabidopsis thaliana. Plant Growth Regul. 2017, 83, 253–263. [Google Scholar] [CrossRef]
- Hameed, A.; Iqbal, N.; Malik, S.A. Mannose-induced modulations in antioxidants, protease activity, lipid peroxidation, and total phenolics in etiolated wheat leaves. J. Plant Growth Regul. 2009, 28, 58–65. [Google Scholar] [CrossRef]
- Grace, J.B. Structural Equation Modeling and Natural Systems; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, W.; Yu, Z.; Luo, Y.; He, W.; Yan, G.; Peng, C. Photoprotection Differences between Dominant Tree Species at Mid- and Late-Successional Stages in Subtropical Forests in Different Seasonal Environments. Int. J. Mol. Sci. 2022, 23, 5417. https://doi.org/10.3390/ijms23105417
Lin W, Yu Z, Luo Y, He W, Yan G, Peng C. Photoprotection Differences between Dominant Tree Species at Mid- and Late-Successional Stages in Subtropical Forests in Different Seasonal Environments. International Journal of Molecular Sciences. 2022; 23(10):5417. https://doi.org/10.3390/ijms23105417
Chicago/Turabian StyleLin, Wei, Zhengchao Yu, Yanna Luo, Wei He, Guanzhao Yan, and Changlian Peng. 2022. "Photoprotection Differences between Dominant Tree Species at Mid- and Late-Successional Stages in Subtropical Forests in Different Seasonal Environments" International Journal of Molecular Sciences 23, no. 10: 5417. https://doi.org/10.3390/ijms23105417
APA StyleLin, W., Yu, Z., Luo, Y., He, W., Yan, G., & Peng, C. (2022). Photoprotection Differences between Dominant Tree Species at Mid- and Late-Successional Stages in Subtropical Forests in Different Seasonal Environments. International Journal of Molecular Sciences, 23(10), 5417. https://doi.org/10.3390/ijms23105417




