Nanoparticles Formulation Improves the Antifibrogenic Effect of Quercetin on an Adenine-Induced Model of Chronic Kidney Disease
Abstract
1. Introduction
2. Results
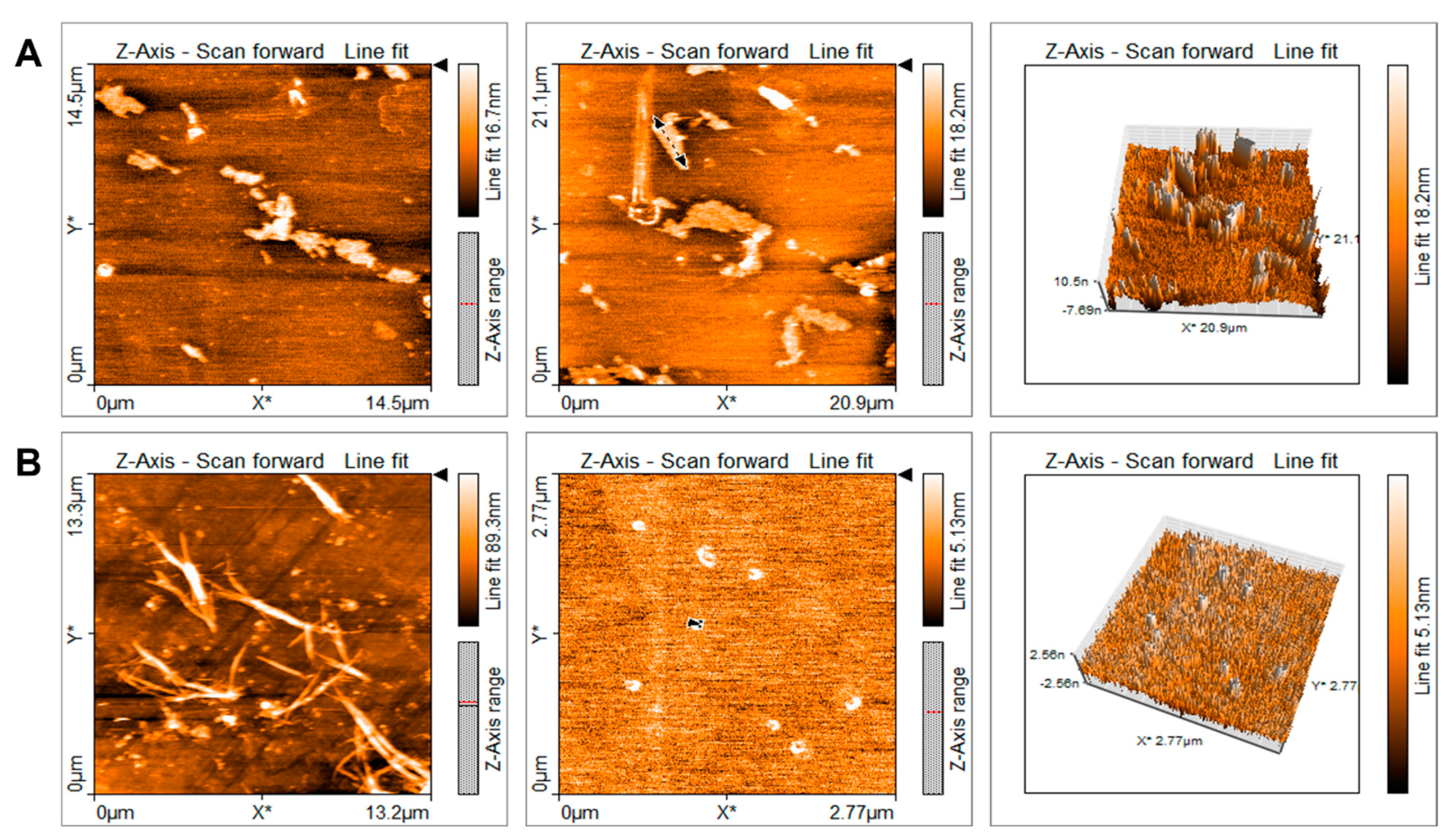
2.1. Characterization of Quercetin Particles
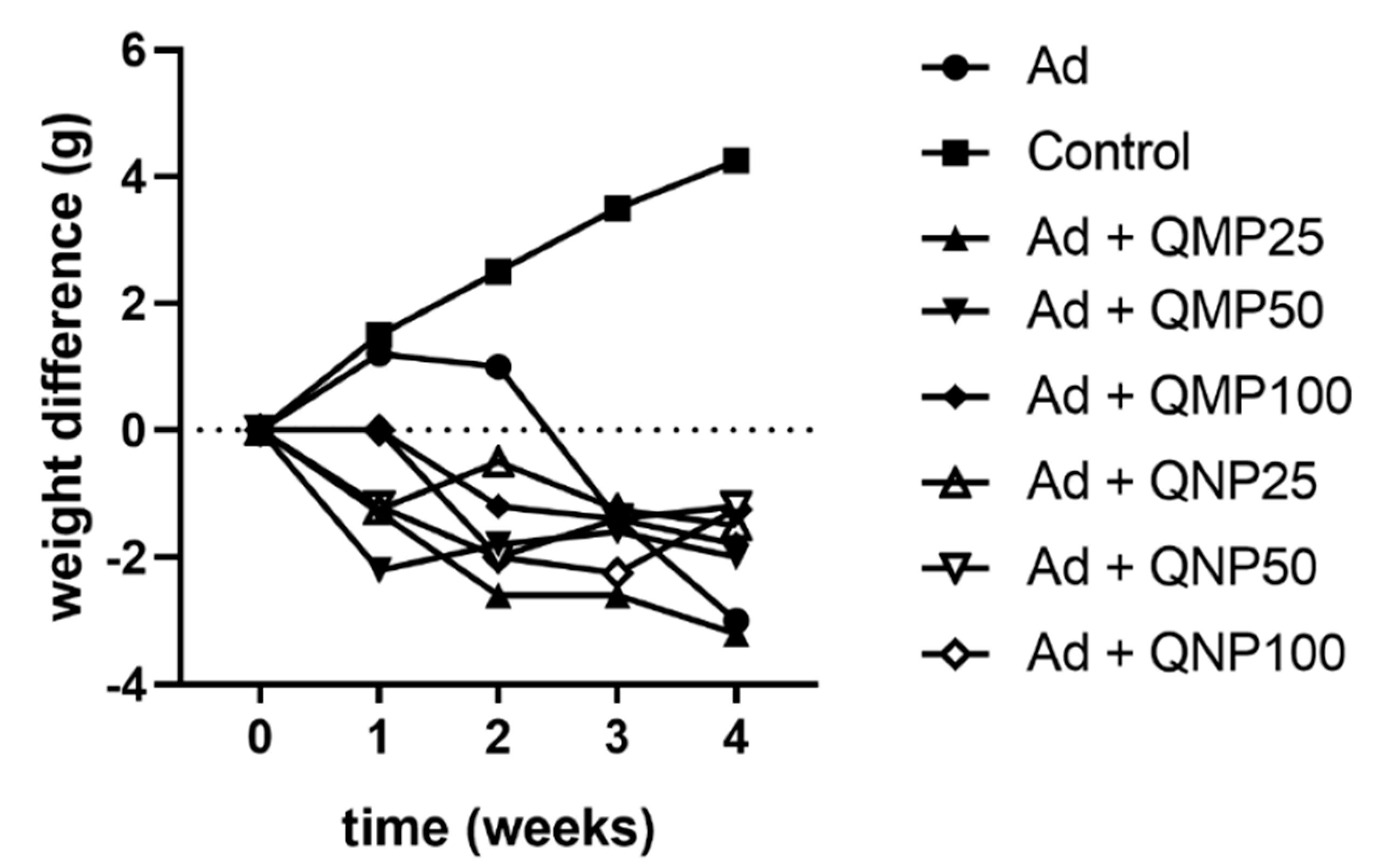
2.2. Effect of Quercetin on Animal Body Weight
2.3. Biochemical Markers of Kidney Damage Are Diminished Using Quercetin
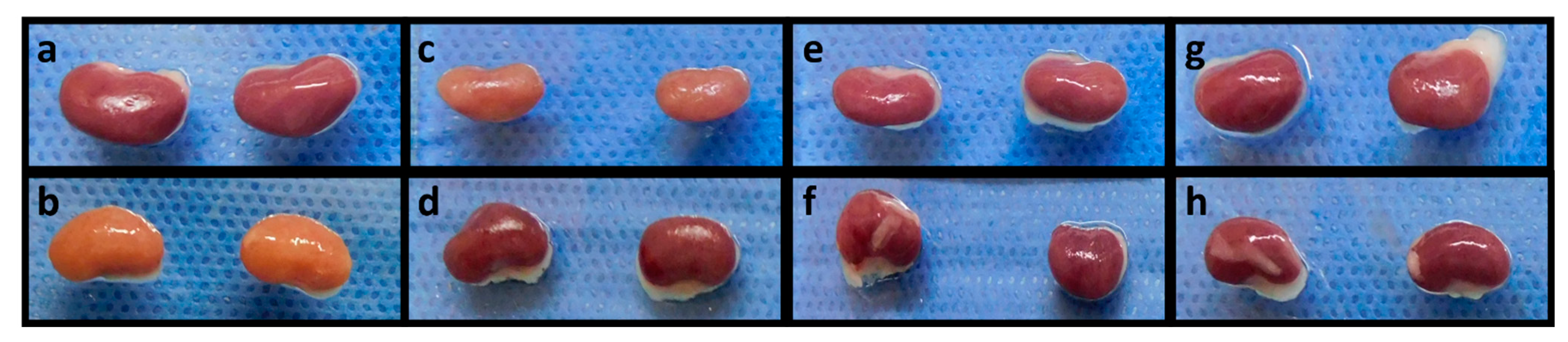
2.4. Quercetin Inhibits Kidney Damage
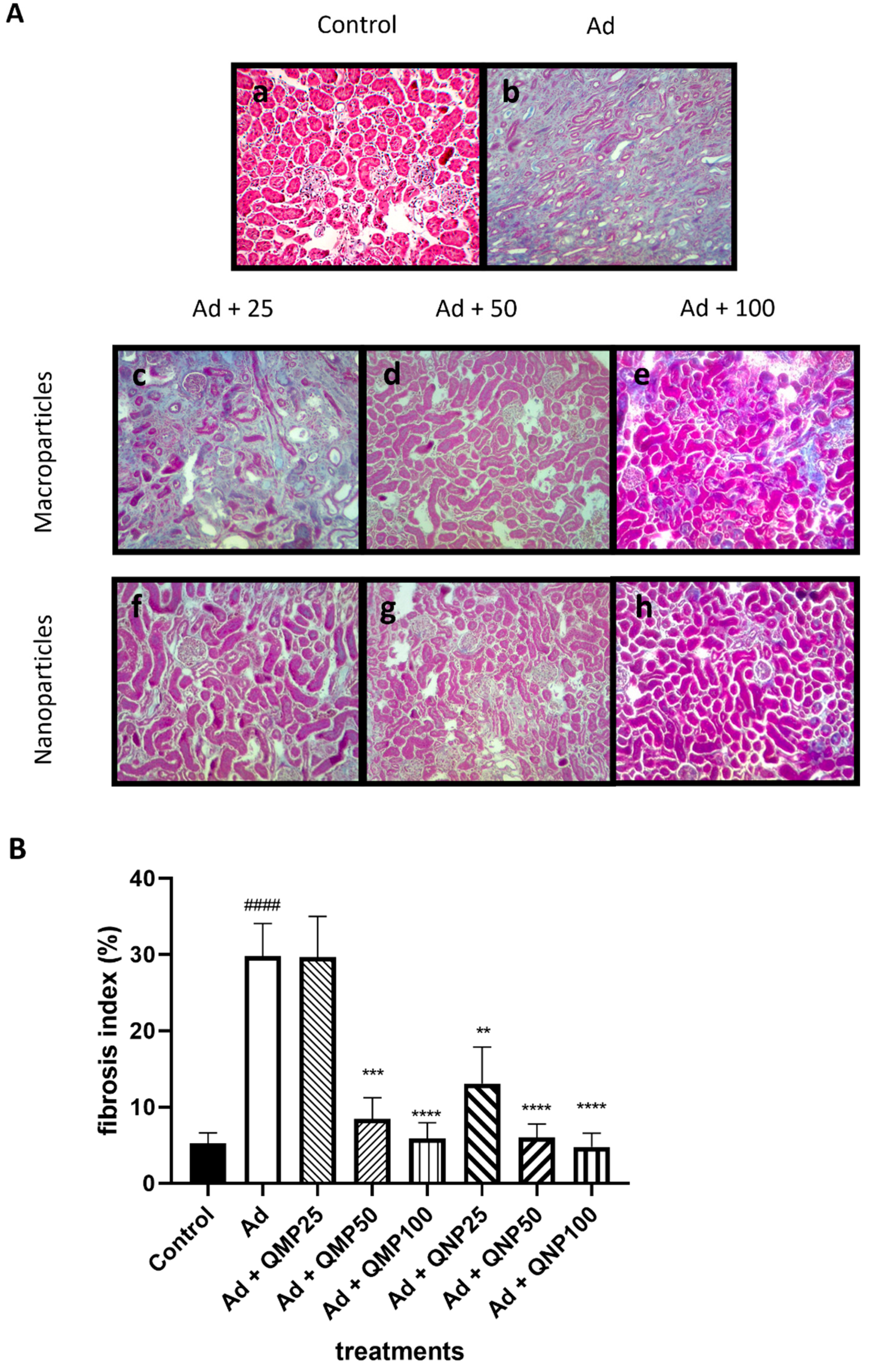
2.5. Quercetin Prevents Kidney Fibrosis
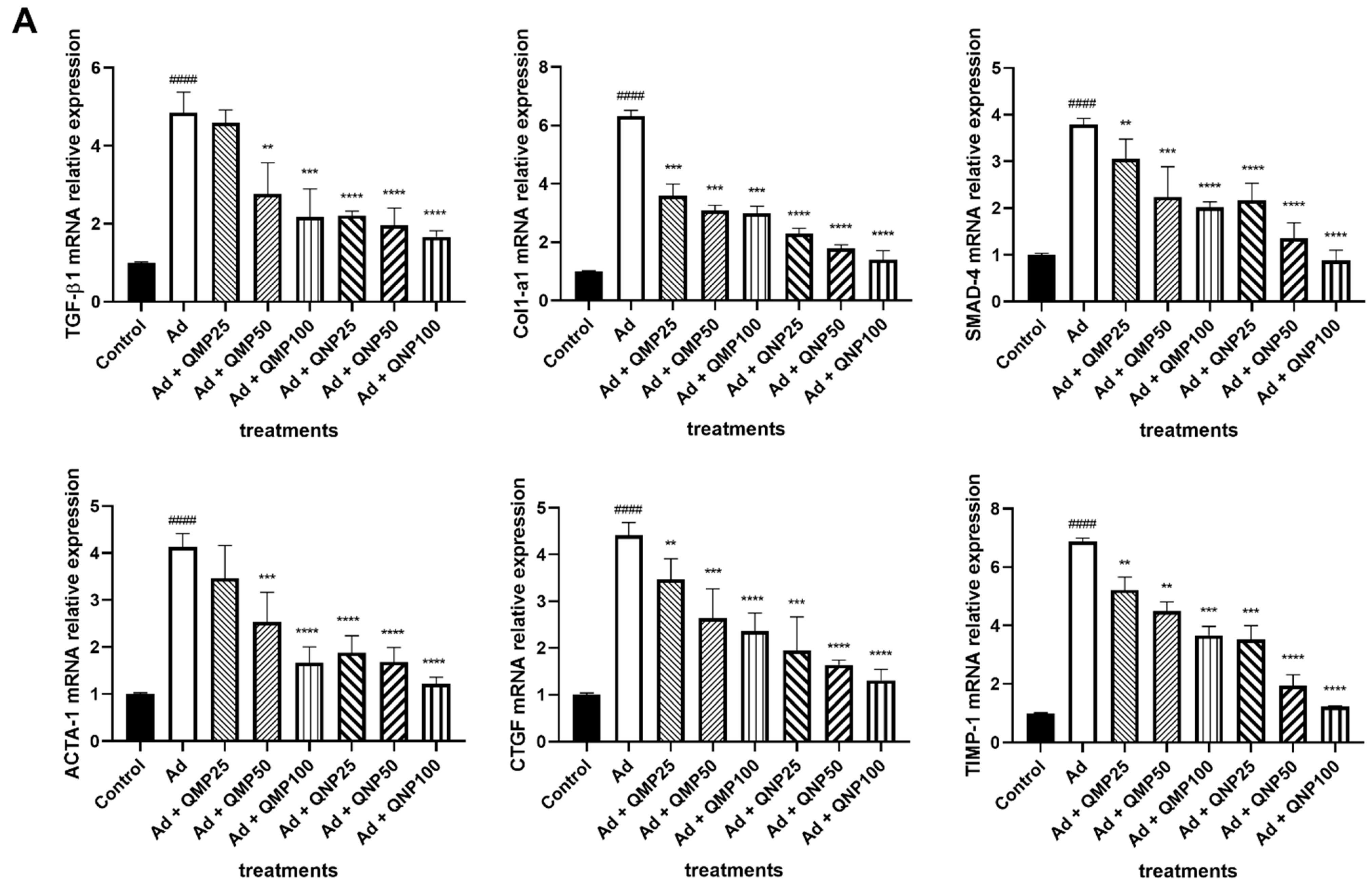
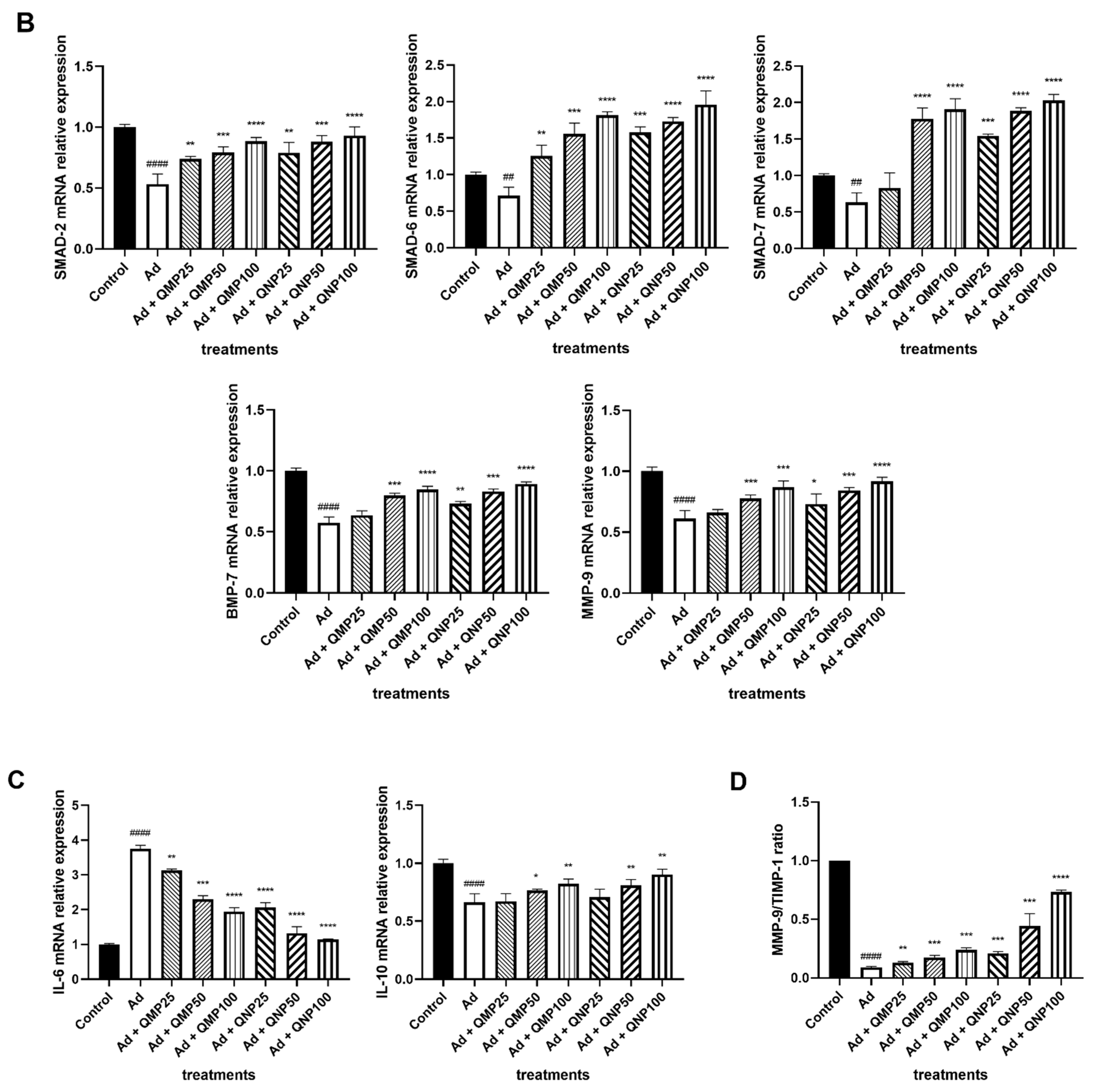
2.6. Quercetin Decreases Profibrogenic and Proinflammatory Cytokine Gene Expression and Increases Antifibrogenic and Anti-Inflammatory Cytokines Gene Expression
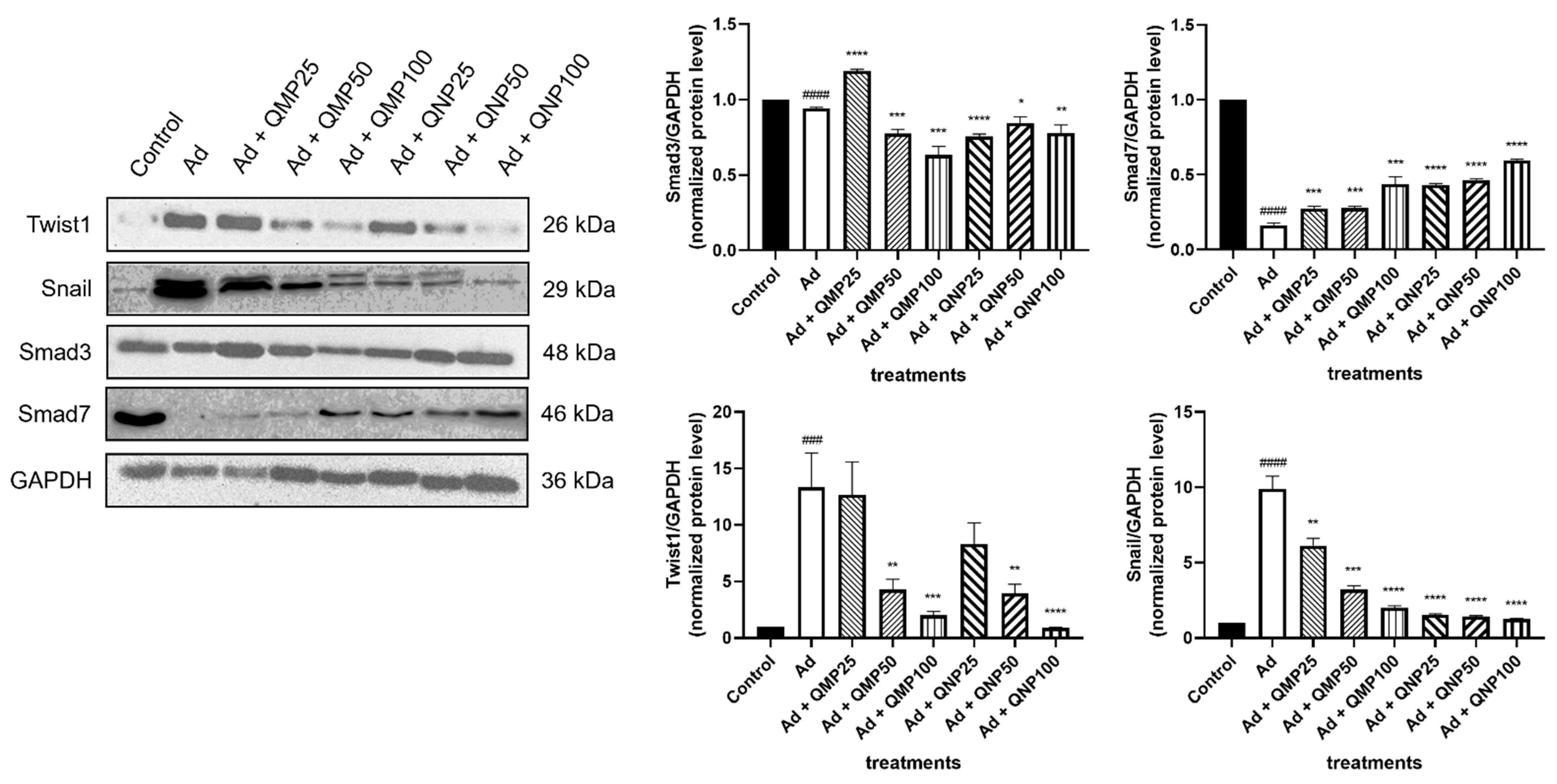
2.7. Quercetin Inhibits the Epithelial–Mesenchymal Transition (EMT) and the TGF-β/Smad Fibrogenic Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Quercetin Nanoparticles Preparation
4.3. Quercetin Particle Imaging and Length Measurement
4.4. Animal Model
4.5. Biochemical Assays
4.6. Histopathological Analysis
4.7. Real-Time Polymerase Chain Reaction (RT-qPCR) Assays
4.8. Western Blot Assays
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y. Renal fibrosis: New insights into the pathogenesis and therapeutics. Kidney Int. 2006, 69, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Border, W.A.; Noble, N.A. TGF-beta in kidney fibrosis: A target for gene therapy. Kidney Int. 1997, 51, 1388–1396. [Google Scholar] [CrossRef] [PubMed]
- Grabias, B.M.; Konstantopoulos, K. The physical basis of renal fibrosis: Effects of altered hydrodynamic forces on kidney homeostasis. Am. J. Physiol. Ren. Physiol. 2014, 306, F473–F485. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.Y.; Liu, X.S.; Huang, X.R.; Yu, X.Q.; Lan, H.Y. Diverse Role of TGF-beta in Kidney Disease. Front. Cell Dev. Biol. 2020, 8, 123. [Google Scholar] [CrossRef]
- Karunaweera, N.; Raju, R.; Gyengesi, E.; Munch, G. Plant polyphenols as inhibitors of NF-kappaB induced cytokine production-a potential anti-inflammatory treatment for Alzheimer’s disease? Front. Mol. Neurosci. 2015, 8, 24. [Google Scholar] [CrossRef]
- Nijveldt, R.J.; van Nood, E.; van Hoorn, D.E.; Boelens, P.G.; van Norren, K.; van Leeuwen, P.A. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef]
- Hernandez-Ortega, L.D.; Alcantar-Diaz, B.E.; Ruiz-Corro, L.A.; Sandoval-Rodriguez, A.; Bueno-Topete, M.; Armendariz-Borunda, J.; Salazar-Montes, A.M. Quercetin improves hepatic fibrosis reducing hepatic stellate cells and regulating pro-fibrogenic/anti-fibrogenic molecules balance. J. Gastroenterol. Hepatol. 2012, 27, 1865–1872. [Google Scholar] [CrossRef]
- Verma, R.; Kushwah, L.; Gohel, D.; Patel, M.; Marvania, T.; Balakrishnan, S. Evaluating the Ameliorative Potential of Quercetin against the Bleomycin-Induced Pulmonary Fibrosis in Wistar Rats. Pulm. Med. 2013, 2013, 921724. [Google Scholar] [CrossRef]
- Malayeri, A.; Hemmati, A.A.; Arzi, A.; Rezaie, A.; Ghafurian-Boroojerdnia, M.; Khalili, H.R. A comparison of the effects of quercetin hydrate with those of vitamin E on the levels of IL-13, PDGF, TNF-α, and INF-γ in bleomycin-induced pulmonary fibrosis in rats. J. Nat. Pharm Prod. 2016, 11, 2. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Q.; Mo, W.; Feng, J.; Li, S.; Li, J.; Liu, T.; Xu, S.; Wang, W.; Lu, X.; et al. Quercetin prevents hepatic fibrosis by inhibiting hepatic stellate cell activation and reducing autophagy via the TGF-beta1/Smads and PI3K/Akt pathways. Sci. Rep. 2017, 7, 9289. [Google Scholar] [CrossRef]
- Ren, J.; Li, J.; Liu, X.; Feng, Y.; Gui, Y.; Yang, J.; He, W.; Dai, C. Quercetin Inhibits Fibroblast Activation and Kidney Fibrosis Involving the Suppression of Mammalian Target of Rapamycin and beta-catenin Signaling. Sci. Rep. 2016, 6, 23968. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Matsushima, M.; Hayashi, Y.; Shibasaki, M.; Imaizumi, K.; Hashimoto, N.; Shimokata, K.; Hasegawa, Y.; Kawabe, T. Attenuation of transforming growth factor-beta-stimulated collagen production in fibroblasts by quercetin-induced heme oxygenase-1. Am. J. Respir. Cell Mol. Biol. 2011, 44, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.; van Trijp, J.M.; Mengelers, M.J.; de Vries, J.H.; Katan, M.B. Bioavailability of the dietary antioxidant flavonol quercetin in man. Cancer Lett. 1997, 114, 139–140. [Google Scholar] [CrossRef]
- Khaled, K.A.; El-Sayed, Y.M.; Al-Hadiya, B.M. Disposition of the flavonoid quercetin in rats after single intravenous and oral doses. Drug Dev. Ind. Pharm. 2003, 29, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Gugler, R.; Leschik, M.; Dengler, H.J. Disposition of quercetin in man after single oral and intravenous doses. Eur. J. Clin. Pharmacol. 1975, 9, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, X.; Ma, Y.; Zhai, G.; Li, L.; Lou, H. Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. J. Control. Release Off. J. Control. Release Soc. 2009, 133, 238–244. [Google Scholar] [CrossRef]
- Jia, L.; Wong, H.; Cerna, C.; Weitman, S.D. Effect of nanonization on absorption of 301029: Ex vivo and in vivo pharmacokinetic correlations determined by liquid chromatography/mass spectrometry. Pharm. Res. 2002, 19, 1091–1096. [Google Scholar] [CrossRef]
- Jia, L.; Wong, H.; Wang, Y.; Garza, M.; Weitman, S.D. Carbendazim: Disposition, cellular permeability, metabolite identification, and pharmacokinetic comparison with its nanoparticle. J. Pharm. Sci. 2003, 92, 161–172. [Google Scholar] [CrossRef]
- Jia, L. Nanoparticle Formulation Increases Oral Bioavailability of Poorly Soluble Drugs: Approaches Experimental Evidences and Theory. Curr. Nanosci. 2005, 1, 237–243. [Google Scholar] [CrossRef]
- Kakran, M.; Sahoo, N.G.; Li, L. Dissolution enhancement of quercetin through nanofabrication, complexation, and solid dispersion. Colloids Surf. B Biointerfaces 2011, 88, 121–130. [Google Scholar] [CrossRef]
- Tousif, S.; Singh, D.K.; Mukherjee, S.; Ahmad, S.; Arya, R.; Nanda, R.; Ranganathan, A.; Bhattacharyya, M.; Van Kaer, L.; Kar, S.K.; et al. Nanoparticle-Formulated Curcumin Prevents Posttherapeutic Disease Reactivation and Reinfection with Mycobacterium tuberculosis following Isoniazid Therapy. Front. Immunol. 2017, 8, 739. [Google Scholar] [CrossRef] [PubMed]
- Amanzadeh, E.; Esmaeili, A.; Rahgozar, S.; Nourbakhshnia, M. Application of quercetin in neurological disorders: From nutrition to nanomedicine. Rev. Neurosci. 2019, 30, 555–572. [Google Scholar] [CrossRef] [PubMed]
- Kakran, M.; Sahoo, N.G.; Li, L.; Judeh, Z. Fabrication of quercetin nanoparticles by anti-solvent precipitation method for enhanced dissolution. Powder Technol. 2012, 223, 59–64. [Google Scholar] [CrossRef]
- Pinheiro, R.G.R.; Pinheiro, M.; Neves, A.R. Nanotechnology Innovations to Enhance the Therapeutic Efficacy of Quercetin. Nanomaterials 2021, 11, 2658. [Google Scholar] [CrossRef]
- Seo, M.J.; Lee, Y.J.; Hwang, J.H.; Kim, K.J.; Lee, B.Y. The inhibitory effects of quercetin on obesity and obesity-induced inflammation by regulation of MAPK signaling. J. Nutr. Biochem. 2015, 26, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, B.; Shen, J.; Wan, L.; Zhu, Y.; Yi, T.; Xiao, Z. The Beneficial Effects of Quercetin, Curcumin, and Resveratrol in Obesity. Oxidative Med. Cell. Longev. 2017, 2017, 1459497. [Google Scholar] [CrossRef] [PubMed]
- Elbe, H.; Vardi, N.; Esrefoglu, M.; Ates, B.; Yologlu, S.; Taskapan, C. Amelioration of streptozotocin-induced diabetic nephropathy by melatonin, quercetin, and resveratrol in rats. Hum. Exp. Toxicol. 2015, 34, 100–113. [Google Scholar] [CrossRef]
- Eid, H.M.; Haddad, P.S. The Antidiabetic Potential of Quercetin: Underlying Mechanisms. Curr. Med. Chem. 2017, 24, 355–364. [Google Scholar] [CrossRef]
- Khan, A.; Ali, T.; Rehman, S.U.; Khan, M.S.; Alam, S.I.; Ikram, M.; Muhammad, T.; Saeed, K.; Badshah, H.; Kim, M.O. Neuroprotective Effect of Quercetin Against the Detrimental Effects of LPS in the Adult Mouse Brain. Front. Pharmacol. 2018, 9, 1383. [Google Scholar] [CrossRef]
- Rifaai, R.A.; Mokhemer, S.A.; Saber, E.A.; El-Aleem, S.A.A.; El-Tahawy, N.F.G. Neuroprotective effect of quercetin nanoparticles: A possible prophylactic and therapeutic role in alzheimer’s disease. J. Chem. Neuroanat. 2020, 107, 101795. [Google Scholar] [CrossRef]
- Ren, K.; Jiang, T.; Zhao, G.J. Quercetin induces the selective uptake of HDL-cholesterol via promoting SR-BI expression and the activation of the PPARgamma/LXRalpha pathway. Food Funct. 2018, 9, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Li, X.X.; Fang, Y.; Chen, X.; Xue, J. Therapeutic Potential of Quercetin as an Antiatherosclerotic Agent in Atherosclerotic Cardiovascular Disease: A Review. Evid. Based Complementary Altern. Med. eCAM 2020, 2020, 5926381. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, C. Quercetin Has Antimetastatic Effects on Gastric Cancer Cells via the Interruption of uPA/uPAR Function by Modulating NF-kappab, PKC-delta, ERK1/2, and AMPKalpha. Integr. Cancer Ther. 2018, 17, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhou, S.; Chen, J.; Zheng, J.; Ren, J.; Qi, P.; Zhu, Z.; Li, Z. Quercetin Nanoparticle Ameliorates Lipopolysaccharide-Triggered Renal Inflammatory Impairment by Regulation of Sirt1/NF-KB Pathway. J. Biomed. Nanotechnol. 2021, 17, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Gomes, I.B.; Porto, M.L.; Santos, M.C.; Campagnaro, B.P.; Pereira, T.M.; Meyrelles, S.S.; Vasquez, E.C. Renoprotective, anti-oxidative and anti-apoptotic effects of oral low-dose quercetin in the C57BL/6J model of diabetic nephropathy. Lipids Health Dis. 2014, 13, 184. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Shen, Y.; Huang, L.; Liu, C.; Wang, J. Senolytic therapy ameliorates renal fibrosis postacute kidney injury by alleviating renal senescence. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2021, 35, e21229. [Google Scholar] [CrossRef]
- Tu, H.; Ma, D.; Luo, Y.; Tang, S.; Li, Y.; Chen, G.; Wang, L.; Hou, Z.; Shen, C.; Lu, H.; et al. Quercetin alleviates chronic renal failure by targeting the PI3k/Akt pathway. Bioengineered 2021, 12, 6538–6558. [Google Scholar] [CrossRef]
- Guan, T.; Xin, Y.; Zheng, K.; Wang, R.; Zhang, X.; Jia, S.; Li, S.; Cao, C.; Zhao, X. Metabolomics analysis of the effects of quercetin on renal toxicity induced by cadmium exposure in rats. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2021, 34, 33–48. [Google Scholar] [CrossRef]
- Diniz, L.R.L.; Souza, M.T.S.; Duarte, A.B.S.; Sousa, D.P. Mechanistic Aspects and Therapeutic Potential of Quercetin against COVID-19-Associated Acute Kidney Injury. Molecules 2020, 25, 5772. [Google Scholar] [CrossRef]
- Sahoo, N.G.; Kakran, M.; Shaal, L.A.; Li, L.; Muller, R.H.; Pal, M.; Tan, L.P. Preparation and characterization of quercetin nanocrystals. J. Pharm. Sci. 2011, 100, 2379–2390. [Google Scholar] [CrossRef]
- Kakran, M.; Shegokar, R.; Sahoo, N.G.; Shaal, L.A.; Li, L.; Muller, R.H. Fabrication of quercetin nanocrystals: Comparison of different methods. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. E.V 2012, 80, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Marcato, P.D. Pharmacokinetics and Pharmacodynamics of Nanomaterials. In Nanotoxicology. Materials, Methodologies and Assessments; Durán, N., Guterres, S., Alves, O., Zuculotto, V., Eds.; Nanomedicine and Nanotoxicology; Springer: New York, NY, USA, 2014; pp. 97–110. [Google Scholar]
- Valdivielso, J.M.; Jacobs-Cacha, C.; Soler, M.J. Sex hormones and their influence on chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2019, 28, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yanes, L.L.; Sartori-Valinotti, J.C.; Reckelhoff, J.F. Sex steroids and renal disease: Lessons from animal studies. Hypertension 2008, 51, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Hodeify, R.; Megyesi, J.; Tarcsafalvi, A.; Mustafa, H.I.; Hti Lar Seng, N.S.; Price, P.M. Gender differences control the susceptibility to ER stress-induced acute kidney injury. Am. J. Physiol. Ren. Physiol. 2013, 304, F875–F882. [Google Scholar] [CrossRef]
- Kang, K.P.; Lee, J.E.; Lee, A.S.; Jung, Y.J.; Kim, D.; Lee, S.; Hwang, H.P.; Kim, W.; Park, S.K. Effect of gender differences on the regulation of renal ischemia-reperfusion-induced inflammation in mice. Mol. Med. Rep. 2014, 9, 2061–2068. [Google Scholar] [CrossRef]
- Diwan, V.; Small, D.; Kauter, K.; Gobe, G.C.; Brown, L. Gender differences in adenine-induced chronic kidney disease and cardiovascular complications in rats. Am. J. Physiol. Ren. Physiol. 2014, 307, F1169–F1178. [Google Scholar] [CrossRef]
- Rahman, A.; Yamazaki, D.; Sufiun, A.; Kitada, K.; Hitomi, H.; Nakano, D.; Nishiyama, A. A novel approach to adenine-induced chronic kidney disease associated anemia in rodents. PLoS ONE 2018, 13, e0192531. [Google Scholar] [CrossRef]
- Musial, K.; Zwolinska, D. Matrix metalloproteinases (MMP-2,9) and their tissue inhibitors (TIMP-1,2) as novel markers of stress response and atherogenesis in children with chronic kidney disease (CKD) on conservative treatment. Cell Stress Chaperones 2011, 16, 97–103. [Google Scholar] [CrossRef]
- Pawlak, K.; Mysliwiec, M.; Pawlak, D. Peripheral blood level alterations of MMP-2 and MMP-9 in patients with chronic kidney disease on conservative treatment and on hemodialysis. Clin. Biochem. 2011, 44, 838–843. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Wang, Y.; Fu, Q.; Lei, Y.H.; Nie, Z.Y.; Qiu, J.; Bao, T.Y. Protective effect of exogenous matrix metalloproteinase-9 on chronic renal failure. Exp. Ther. Med. 2014, 7, 329–334. [Google Scholar] [CrossRef][Green Version]
- Zakiyanov, O.; Kalousova, M.; Zima, T.; Tesar, V. Matrix Metalloproteinases in Renal Diseases: A Critical Appraisal. Kidney Blood Press. Res. 2019, 44, 298–330. [Google Scholar] [CrossRef] [PubMed]
- Ulusoy, H.G.; Sanlier, N. A minireview of quercetin: From its metabolism to possible mechanisms of its biological activities. Crit. Rev. Food Sci. Nutr. 2020, 60, 3290–3303. [Google Scholar] [CrossRef] [PubMed]
- Serban, M.C.; Sahebkar, A.; Zanchetti, A.; Mikhailidis, D.P.; Howard, G.; Antal, D.; Andrica, F.; Ahmed, A.; Aronow, W.S.; Muntner, P.; et al. Effects of Quercetin on Blood Pressure: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2016, 5, e002713. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Miniscalco, A.; Lundahl, J.; Regardh, C.G.; Edgar, B.; Eriksson, U.G. Inhibition of dihydropyridine metabolism in rat and human liver microsomes by flavonoids found in grapefruit juice. J. Pharmacol. Exp. Ther. 1992, 261, 1195–1199. [Google Scholar]
- Scambia, G.; Ranelletti, F.O.; Panici, P.B.; De Vincenzo, R.; Bonanno, G.; Ferrandina, G.; Piantelli, M.; Bussa, S.; Rumi, C.; Cianfriglia, M.; et al. Quercetin potentiates the effect of adriamycin in a multidrug-resistant MCF-7 human breast-cancer cell line: P-glycoprotein as a possible target. Cancer Chemother. Pharmacol. 1994, 34, 459–464. [Google Scholar] [CrossRef]
- Choi, J.S.; Piao, Y.J.; Kang, K.W. Effects of quercetin on the bioavailability of doxorubicin in rats: Role of CYP3A4 and P-gp inhibition by quercetin. Arch. Pharmacal Res. 2011, 34, 607–613. [Google Scholar] [CrossRef]
- Bahadar, H.; Maqbool, F.; Niaz, K.; Abdollahi, M. Toxicity of Nanoparticles and an Overview of Current Experimental Models. Iran. Biomed. J. 2016, 20, 1–11. [Google Scholar] [CrossRef]
- Buchman, J.T.; Hudson-Smith, N.V.; Landy, K.M.; Haynes, C.L. Understanding Nanoparticle Toxicity Mechanisms to Inform Redesign Strategies To Reduce Environmental Impact. Acc. Chem. Res. 2019, 52, 1632–1642. [Google Scholar] [CrossRef]
- Egbuna, C.; Parmar, V.K.; Jeevanandam, J.; Ezzat, S.M.; Patrick-Iwuanyanwu, K.C.; Adetunji, C.O.; Khan, J.; Onyeike, E.N.; Uche, C.Z.; Akram, M.; et al. Toxicity of Nanoparticles in Biomedical Application: Nanotoxicology. J. Toxicol. 2021, 2021, 9954443. [Google Scholar] [CrossRef]
- Lamprecht, M.R.; Sabatini, D.M.; Carpenter, A.E. CellProfiler: Free, versatile software for automated biological image analysis. BioTechniques 2007, 42, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Biosystems, A. User Bulletin #5. Available online: https://assets.thermofisher.com/TFS-Assets/LSG/manuals/cms_041019.pdf (accessed on 23 February 2022).


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Jaramillo, E.A.; Gasca-Lozano, L.E.; Vera-Cruz, J.M.; Hernández-Ortega, L.D.; Gurrola-Díaz, C.M.; Bastidas-Ramírez, B.E.; Vargas-Guerrero, B.; Mena-Enríquez, M.; Martínez-Limón, F.d.J.; Salazar-Montes, A.M. Nanoparticles Formulation Improves the Antifibrogenic Effect of Quercetin on an Adenine-Induced Model of Chronic Kidney Disease. Int. J. Mol. Sci. 2022, 23, 5392. https://doi.org/10.3390/ijms23105392
Sánchez-Jaramillo EA, Gasca-Lozano LE, Vera-Cruz JM, Hernández-Ortega LD, Gurrola-Díaz CM, Bastidas-Ramírez BE, Vargas-Guerrero B, Mena-Enríquez M, Martínez-Limón FdJ, Salazar-Montes AM. Nanoparticles Formulation Improves the Antifibrogenic Effect of Quercetin on an Adenine-Induced Model of Chronic Kidney Disease. International Journal of Molecular Sciences. 2022; 23(10):5392. https://doi.org/10.3390/ijms23105392
Chicago/Turabian StyleSánchez-Jaramillo, Esteban Andrés, Luz Elena Gasca-Lozano, José María Vera-Cruz, Luis Daniel Hernández-Ortega, Carmen Magdalena Gurrola-Díaz, Blanca Estela Bastidas-Ramírez, Belinda Vargas-Guerrero, Mayra Mena-Enríquez, Felipe de Jesús Martínez-Limón, and Adriana María Salazar-Montes. 2022. "Nanoparticles Formulation Improves the Antifibrogenic Effect of Quercetin on an Adenine-Induced Model of Chronic Kidney Disease" International Journal of Molecular Sciences 23, no. 10: 5392. https://doi.org/10.3390/ijms23105392
APA StyleSánchez-Jaramillo, E. A., Gasca-Lozano, L. E., Vera-Cruz, J. M., Hernández-Ortega, L. D., Gurrola-Díaz, C. M., Bastidas-Ramírez, B. E., Vargas-Guerrero, B., Mena-Enríquez, M., Martínez-Limón, F. d. J., & Salazar-Montes, A. M. (2022). Nanoparticles Formulation Improves the Antifibrogenic Effect of Quercetin on an Adenine-Induced Model of Chronic Kidney Disease. International Journal of Molecular Sciences, 23(10), 5392. https://doi.org/10.3390/ijms23105392







